User login
What would you do if ... ?
A few weeks ago we went to Phoenix Theater’s production of “A Chorus Line.” As with all their shows, it was excellent.
The penultimate scene is where one of the auditioning dancers suffers a career-ending injury, forcing the others to consider what they’d do if they couldn’t dance anymore, and facing the fact that sooner or later it will happen to all of them.
Let’s flip it onto us: What if tomorrow you couldn’t practice medicine anymore? To keep it from getting too depressing, let’s say it was because of paperwork. Your medical license expired and you weren’t warned in advance, and because of some legal glitch you can’t ever renew it now.
It’s a good question. I mean, I’ve wanted to be doctor as long as I can remember. (Actually I wanted to be Batman, then a scientist, then a doctor. Though I’d still rather be Batman. I’m even the same age as he was in The Dark Knight Returns.)
For all the paperwork and insurance fights and aggravations the job brings, I still love doing it. I get up on weekday mornings and feel good about going to the office. I generally feel good about what I’ve done to help people (or at least did my best to try) at the end of the day.
During my first year of residency (30 years ago) I remember telling my parents that, if even if I were phenomenally wealthy, I’d still do this job for free. Well, I’m not phenomenally wealthy, but I still enjoy the job.
If I couldn’t do it anymore, I’d be pretty sad. I mean, it’s not like I couldn’t find something else – consulting, research, writing, joining my daughter at her bakery – but I doubt I’d like it as much. Even if money weren’t an issue, there’s only so many jigsaw puzzles to do and books to read.
What about you?
Realistically, most of us won’t do this for the rest of our lives. Our expiration date may be longer than that of a professional dancer, but we still have one. Even if the mind stays sharp, sooner or later we all reach a point where it’s time to move on and leave the field in the capable hands of the next generation, just as a prior group of physicians left it to us. As the line in the song states, “the gift was ours to borrow.” And yes, I still see being able to do this for a living as a privilege and gift. But inevitably we all have to pass it on to the next ones, as will they someday.
But I’ll miss it. An oncologist I know was retired for a few months before he signed up for a nonmedical volunteer job at his old hospital, helping people find the rooms and departments they need to go to. He’s happy with it.
But when you do, you need to do your best to do it without regret. After all, you got to do something that many only dream of. Helping others and (I hope) having a job you enjoy.
I have dancers, and retired dancers, in my practice. The retired ones still miss it, but very few of them leave. They do volunteer teaching at community theaters, or just keep dancing on their own in groups of like-minded friends, as best they can. While medicine has made us one of the longer-lived mammals, it doesn’t stop the years.
When it’s time to walk away and point to tomorrow, do it without regrets, and remember that, even with the sweetness and the sorrow, it was what you did for love.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A few weeks ago we went to Phoenix Theater’s production of “A Chorus Line.” As with all their shows, it was excellent.
The penultimate scene is where one of the auditioning dancers suffers a career-ending injury, forcing the others to consider what they’d do if they couldn’t dance anymore, and facing the fact that sooner or later it will happen to all of them.
Let’s flip it onto us: What if tomorrow you couldn’t practice medicine anymore? To keep it from getting too depressing, let’s say it was because of paperwork. Your medical license expired and you weren’t warned in advance, and because of some legal glitch you can’t ever renew it now.
It’s a good question. I mean, I’ve wanted to be doctor as long as I can remember. (Actually I wanted to be Batman, then a scientist, then a doctor. Though I’d still rather be Batman. I’m even the same age as he was in The Dark Knight Returns.)
For all the paperwork and insurance fights and aggravations the job brings, I still love doing it. I get up on weekday mornings and feel good about going to the office. I generally feel good about what I’ve done to help people (or at least did my best to try) at the end of the day.
During my first year of residency (30 years ago) I remember telling my parents that, if even if I were phenomenally wealthy, I’d still do this job for free. Well, I’m not phenomenally wealthy, but I still enjoy the job.
If I couldn’t do it anymore, I’d be pretty sad. I mean, it’s not like I couldn’t find something else – consulting, research, writing, joining my daughter at her bakery – but I doubt I’d like it as much. Even if money weren’t an issue, there’s only so many jigsaw puzzles to do and books to read.
What about you?
Realistically, most of us won’t do this for the rest of our lives. Our expiration date may be longer than that of a professional dancer, but we still have one. Even if the mind stays sharp, sooner or later we all reach a point where it’s time to move on and leave the field in the capable hands of the next generation, just as a prior group of physicians left it to us. As the line in the song states, “the gift was ours to borrow.” And yes, I still see being able to do this for a living as a privilege and gift. But inevitably we all have to pass it on to the next ones, as will they someday.
But I’ll miss it. An oncologist I know was retired for a few months before he signed up for a nonmedical volunteer job at his old hospital, helping people find the rooms and departments they need to go to. He’s happy with it.
But when you do, you need to do your best to do it without regret. After all, you got to do something that many only dream of. Helping others and (I hope) having a job you enjoy.
I have dancers, and retired dancers, in my practice. The retired ones still miss it, but very few of them leave. They do volunteer teaching at community theaters, or just keep dancing on their own in groups of like-minded friends, as best they can. While medicine has made us one of the longer-lived mammals, it doesn’t stop the years.
When it’s time to walk away and point to tomorrow, do it without regrets, and remember that, even with the sweetness and the sorrow, it was what you did for love.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A few weeks ago we went to Phoenix Theater’s production of “A Chorus Line.” As with all their shows, it was excellent.
The penultimate scene is where one of the auditioning dancers suffers a career-ending injury, forcing the others to consider what they’d do if they couldn’t dance anymore, and facing the fact that sooner or later it will happen to all of them.
Let’s flip it onto us: What if tomorrow you couldn’t practice medicine anymore? To keep it from getting too depressing, let’s say it was because of paperwork. Your medical license expired and you weren’t warned in advance, and because of some legal glitch you can’t ever renew it now.
It’s a good question. I mean, I’ve wanted to be doctor as long as I can remember. (Actually I wanted to be Batman, then a scientist, then a doctor. Though I’d still rather be Batman. I’m even the same age as he was in The Dark Knight Returns.)
For all the paperwork and insurance fights and aggravations the job brings, I still love doing it. I get up on weekday mornings and feel good about going to the office. I generally feel good about what I’ve done to help people (or at least did my best to try) at the end of the day.
During my first year of residency (30 years ago) I remember telling my parents that, if even if I were phenomenally wealthy, I’d still do this job for free. Well, I’m not phenomenally wealthy, but I still enjoy the job.
If I couldn’t do it anymore, I’d be pretty sad. I mean, it’s not like I couldn’t find something else – consulting, research, writing, joining my daughter at her bakery – but I doubt I’d like it as much. Even if money weren’t an issue, there’s only so many jigsaw puzzles to do and books to read.
What about you?
Realistically, most of us won’t do this for the rest of our lives. Our expiration date may be longer than that of a professional dancer, but we still have one. Even if the mind stays sharp, sooner or later we all reach a point where it’s time to move on and leave the field in the capable hands of the next generation, just as a prior group of physicians left it to us. As the line in the song states, “the gift was ours to borrow.” And yes, I still see being able to do this for a living as a privilege and gift. But inevitably we all have to pass it on to the next ones, as will they someday.
But I’ll miss it. An oncologist I know was retired for a few months before he signed up for a nonmedical volunteer job at his old hospital, helping people find the rooms and departments they need to go to. He’s happy with it.
But when you do, you need to do your best to do it without regret. After all, you got to do something that many only dream of. Helping others and (I hope) having a job you enjoy.
I have dancers, and retired dancers, in my practice. The retired ones still miss it, but very few of them leave. They do volunteer teaching at community theaters, or just keep dancing on their own in groups of like-minded friends, as best they can. While medicine has made us one of the longer-lived mammals, it doesn’t stop the years.
When it’s time to walk away and point to tomorrow, do it without regrets, and remember that, even with the sweetness and the sorrow, it was what you did for love.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The Hospitalist Triage Role for Reducing Admission Delays: Impacts on Throughput, Quality, Interprofessional Practice, and Clinician Experience of Care
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).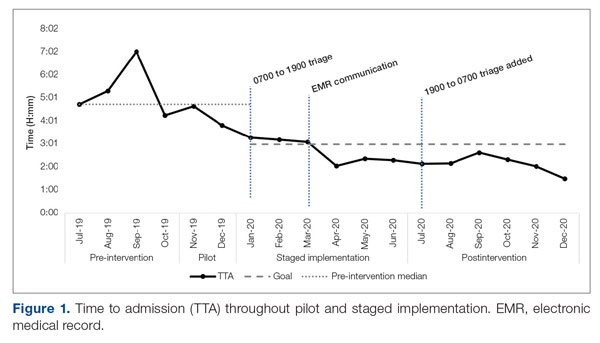
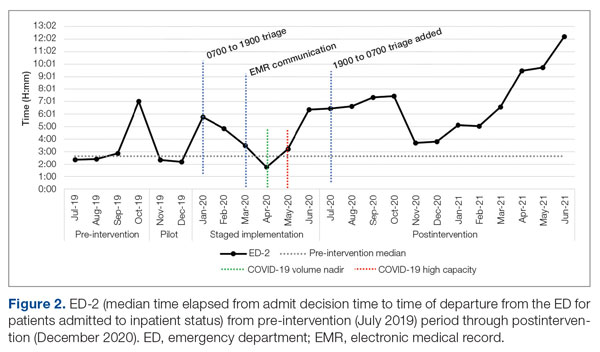
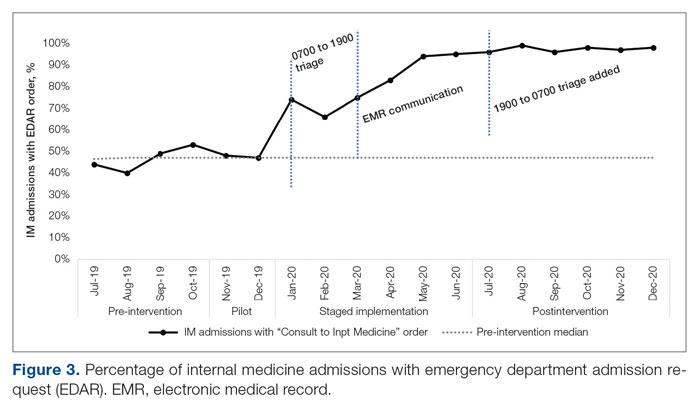
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).
There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.
Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions
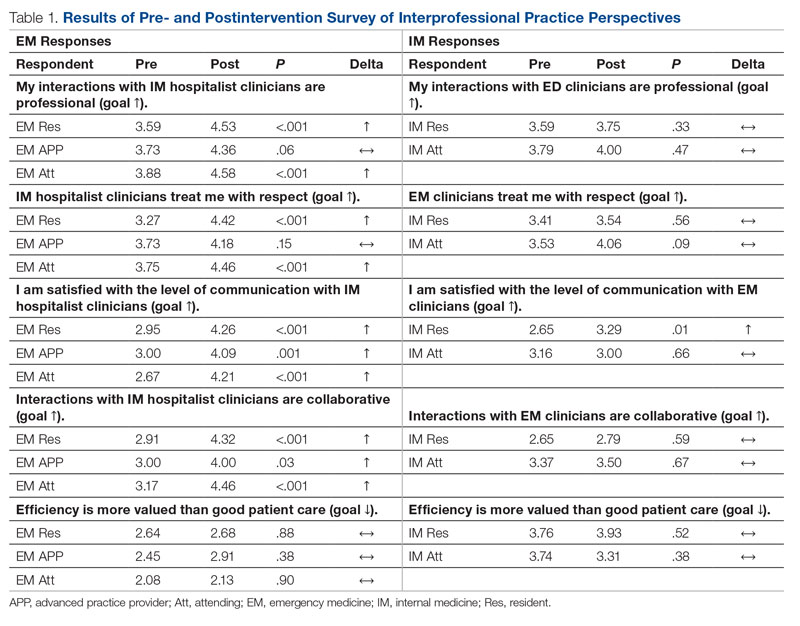
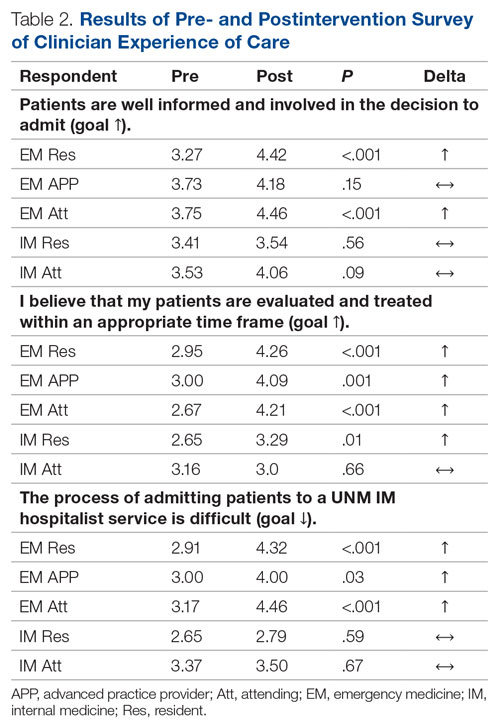
For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.
Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
Glucagon Prescription Rates for Individuals With Type 1 Diabetes Mellitus Following Implementation of an Electronic Health Records Intervention
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.
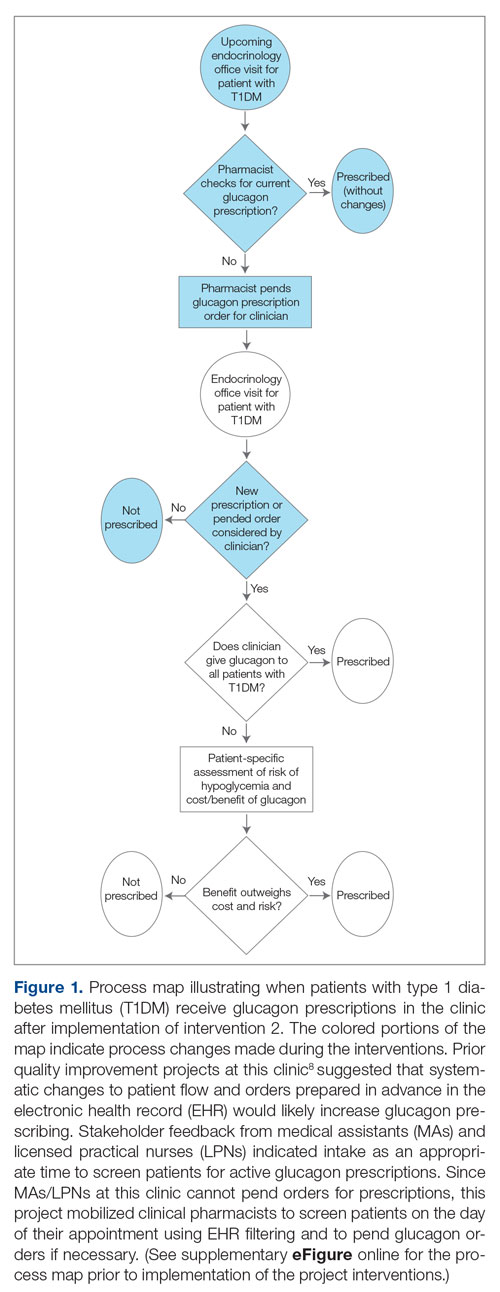
In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).
This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).
Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.
Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
Redesign of Health Care Systems to Reduce Diagnostic Errors: Leveraging Human Experience and Artificial Intelligence
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).
Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
Quality Improvement in Health Care: From Conceptual Frameworks and Definitions to Implementation
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
Differences in 30-Day Readmission Rates in Older Adults With Dementia
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Patient Safety in Transitions of Care: Addressing Discharge Communication Gaps and the Potential of the Teach-Back Method
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
AI at the office: Are clinicians prepared?
AURORA, COLO. – Artificial Intelligence has arrived at medical offices, whether or not clinicians feel ready for it.
AI might result in more accurate, efficient, and cost-effective care. But it’s possible it could cause harm. That’s according to Benjamin Collins, MD, at Vanderbilt University Medical Center, Nashville, Tenn., who spoke on the subject at the annual meeting of the Society of General Internal Medicine.
Understanding the nuances of AI is even more important because of the quick development of the algorithms.
“When I submitted this workshop, there was no ChatGPT,” said Dr. Collins, referring to Chat Generative Pre-trained Transformer, a recently released natural language processing model. “A lot has already changed.”
Biased data
Biased data are perhaps the biggest pitfall of AI algorithms, Dr. Collins said. If garbage data go in, garbage predictions come out.
If the dataset that trains the algorithm underrepresents a particular gender or ethnic group, for example, the algorithm may not respond accurately to prompts. When an AI tool compounds existing inequalities related to socioeconomic status, ethnicity, or sexual orientation, the algorithm is biased, according to Harvard researchers.
“People often assume that artificial intelligence is free of bias due to the use of scientific processes and its development,” he said. “But whatever flaws exist in data collection and old data can lead to poor representation or underrepresentation in the data used to train the AI tool.”
Racial minorities are underrepresented in studies; therefore, data input into an AI tool might skew results for these patients.
The Framingham Heart Study, for example, which began in 1948, examined heart disease in mainly White participants. The findings from the study resulted in the creation of a sex-specific algorithm that was used to estimate the 10-year cardiovascular risk of a patient. While the cardiovascular risk score was accurate for White persons, it was less accurate for Black patients.
A study published in Science in 2019 revealed bias in an algorithm that used health care costs as a proxy for health needs. Because less money was spent on Black patients who had the same level of need as their White counterparts, the output inaccurately showed that Black patients were healthier and thus did not require extra care.
Developers can also be a source of bias, inasmuch as AI often reflects preexisting human biases, Dr. Collins said.
“Algorithmic bias presents a clear risk of harm that clinicians must play against the benefits of using AI,” Dr. Collins said. “That risk of harm is often disproportionately distributed to marginalized populations.”
As clinicians use AI algorithms to diagnose and detect disease, predict outcomes, and guide treatment, trouble comes when those algorithms perform well for some patients and poorly for others. This gap can exacerbate existing disparities in health care outcomes.
Dr. Collins advised clinicians to push to find out what data were used to train AI algorithms to determine how bias could have influenced the model and whether the developers risk-adjusted for bias. If the training data are not available, clinicians should ask their employers and AI developers to know more about the system.
Clinicians may face the so-called black box phenomenon, which occurs when developers cannot or will not explain what data went into an AI model, Dr. Collins said.
According to Stanford (Calif.) University, AI must be trained on large datasets of images that have been annotated by human experts. Those datasets can cost millions of dollars to create, meaning corporations often fund them and do not always share the data publicly.
Some groups, such as Stanford’s Center for Artificial Intelligence in Medicine and Imaging, are working to acquire annotated datasets so researchers who train AI models can know where the data came from.
Paul Haidet, MD, MPH, an internist at Penn State College of Medicine, Hershey, sees the technology as a tool that requires careful handling.
“It takes a while to learn how to use a stethoscope, and AI is like that,” Dr. Haidet said. “The thing about AI, though, is that it can be just dropped into a system and no one knows how it works.”
Dr. Haidet said he likes knowing how the sausage is made, something AI developers are often reticent to make known.
“If you’re just putting blind faith in a tool, that’s scary,” Dr. Haidet said.
Transparency and ‘explainability’
The ability to explain what goes into tools is essential to maintaining trust in the health care system, Dr. Collins said.
“Part of knowing how much trust to place in the system is the transparency of those systems and the ability to audit how well the algorithm is performing,” Dr. Collins said. “The system should also regularly report to users the level of certainty with which it is providing an output rather than providing a simple binary output.”
Dr. Collins recommends that providers develop an understanding of the limits of AI regulations as well, which might including learning how the system was approved and how it is monitored.
“The FDA has oversight over some applications of AI and health care for software as a medical device, but there’s currently no dedicated process to evaluate the systems for the presence of bias,” Dr. Collins said. “The gaps in regulation leave the door open for the use of AI in clinical care that contain significant biases.”
Dr. Haidet likened AI tools to the Global Positioning System: A good GPS system will let users see alternate routes, opt out of toll roads or highways, and will highlight why routes have changed. But users need to understand how to read the map so they can tell when something seems amiss.
Dr. Collins and Dr. Haidet report no relevant financial relationships
A version of this article first appeared on Medscape.com.
AURORA, COLO. – Artificial Intelligence has arrived at medical offices, whether or not clinicians feel ready for it.
AI might result in more accurate, efficient, and cost-effective care. But it’s possible it could cause harm. That’s according to Benjamin Collins, MD, at Vanderbilt University Medical Center, Nashville, Tenn., who spoke on the subject at the annual meeting of the Society of General Internal Medicine.
Understanding the nuances of AI is even more important because of the quick development of the algorithms.
“When I submitted this workshop, there was no ChatGPT,” said Dr. Collins, referring to Chat Generative Pre-trained Transformer, a recently released natural language processing model. “A lot has already changed.”
Biased data
Biased data are perhaps the biggest pitfall of AI algorithms, Dr. Collins said. If garbage data go in, garbage predictions come out.
If the dataset that trains the algorithm underrepresents a particular gender or ethnic group, for example, the algorithm may not respond accurately to prompts. When an AI tool compounds existing inequalities related to socioeconomic status, ethnicity, or sexual orientation, the algorithm is biased, according to Harvard researchers.
“People often assume that artificial intelligence is free of bias due to the use of scientific processes and its development,” he said. “But whatever flaws exist in data collection and old data can lead to poor representation or underrepresentation in the data used to train the AI tool.”
Racial minorities are underrepresented in studies; therefore, data input into an AI tool might skew results for these patients.
The Framingham Heart Study, for example, which began in 1948, examined heart disease in mainly White participants. The findings from the study resulted in the creation of a sex-specific algorithm that was used to estimate the 10-year cardiovascular risk of a patient. While the cardiovascular risk score was accurate for White persons, it was less accurate for Black patients.
A study published in Science in 2019 revealed bias in an algorithm that used health care costs as a proxy for health needs. Because less money was spent on Black patients who had the same level of need as their White counterparts, the output inaccurately showed that Black patients were healthier and thus did not require extra care.
Developers can also be a source of bias, inasmuch as AI often reflects preexisting human biases, Dr. Collins said.
“Algorithmic bias presents a clear risk of harm that clinicians must play against the benefits of using AI,” Dr. Collins said. “That risk of harm is often disproportionately distributed to marginalized populations.”
As clinicians use AI algorithms to diagnose and detect disease, predict outcomes, and guide treatment, trouble comes when those algorithms perform well for some patients and poorly for others. This gap can exacerbate existing disparities in health care outcomes.
Dr. Collins advised clinicians to push to find out what data were used to train AI algorithms to determine how bias could have influenced the model and whether the developers risk-adjusted for bias. If the training data are not available, clinicians should ask their employers and AI developers to know more about the system.
Clinicians may face the so-called black box phenomenon, which occurs when developers cannot or will not explain what data went into an AI model, Dr. Collins said.
According to Stanford (Calif.) University, AI must be trained on large datasets of images that have been annotated by human experts. Those datasets can cost millions of dollars to create, meaning corporations often fund them and do not always share the data publicly.
Some groups, such as Stanford’s Center for Artificial Intelligence in Medicine and Imaging, are working to acquire annotated datasets so researchers who train AI models can know where the data came from.
Paul Haidet, MD, MPH, an internist at Penn State College of Medicine, Hershey, sees the technology as a tool that requires careful handling.
“It takes a while to learn how to use a stethoscope, and AI is like that,” Dr. Haidet said. “The thing about AI, though, is that it can be just dropped into a system and no one knows how it works.”
Dr. Haidet said he likes knowing how the sausage is made, something AI developers are often reticent to make known.
“If you’re just putting blind faith in a tool, that’s scary,” Dr. Haidet said.
Transparency and ‘explainability’
The ability to explain what goes into tools is essential to maintaining trust in the health care system, Dr. Collins said.
“Part of knowing how much trust to place in the system is the transparency of those systems and the ability to audit how well the algorithm is performing,” Dr. Collins said. “The system should also regularly report to users the level of certainty with which it is providing an output rather than providing a simple binary output.”
Dr. Collins recommends that providers develop an understanding of the limits of AI regulations as well, which might including learning how the system was approved and how it is monitored.
“The FDA has oversight over some applications of AI and health care for software as a medical device, but there’s currently no dedicated process to evaluate the systems for the presence of bias,” Dr. Collins said. “The gaps in regulation leave the door open for the use of AI in clinical care that contain significant biases.”
Dr. Haidet likened AI tools to the Global Positioning System: A good GPS system will let users see alternate routes, opt out of toll roads or highways, and will highlight why routes have changed. But users need to understand how to read the map so they can tell when something seems amiss.
Dr. Collins and Dr. Haidet report no relevant financial relationships
A version of this article first appeared on Medscape.com.
AURORA, COLO. – Artificial Intelligence has arrived at medical offices, whether or not clinicians feel ready for it.
AI might result in more accurate, efficient, and cost-effective care. But it’s possible it could cause harm. That’s according to Benjamin Collins, MD, at Vanderbilt University Medical Center, Nashville, Tenn., who spoke on the subject at the annual meeting of the Society of General Internal Medicine.
Understanding the nuances of AI is even more important because of the quick development of the algorithms.
“When I submitted this workshop, there was no ChatGPT,” said Dr. Collins, referring to Chat Generative Pre-trained Transformer, a recently released natural language processing model. “A lot has already changed.”
Biased data
Biased data are perhaps the biggest pitfall of AI algorithms, Dr. Collins said. If garbage data go in, garbage predictions come out.
If the dataset that trains the algorithm underrepresents a particular gender or ethnic group, for example, the algorithm may not respond accurately to prompts. When an AI tool compounds existing inequalities related to socioeconomic status, ethnicity, or sexual orientation, the algorithm is biased, according to Harvard researchers.
“People often assume that artificial intelligence is free of bias due to the use of scientific processes and its development,” he said. “But whatever flaws exist in data collection and old data can lead to poor representation or underrepresentation in the data used to train the AI tool.”
Racial minorities are underrepresented in studies; therefore, data input into an AI tool might skew results for these patients.
The Framingham Heart Study, for example, which began in 1948, examined heart disease in mainly White participants. The findings from the study resulted in the creation of a sex-specific algorithm that was used to estimate the 10-year cardiovascular risk of a patient. While the cardiovascular risk score was accurate for White persons, it was less accurate for Black patients.
A study published in Science in 2019 revealed bias in an algorithm that used health care costs as a proxy for health needs. Because less money was spent on Black patients who had the same level of need as their White counterparts, the output inaccurately showed that Black patients were healthier and thus did not require extra care.
Developers can also be a source of bias, inasmuch as AI often reflects preexisting human biases, Dr. Collins said.
“Algorithmic bias presents a clear risk of harm that clinicians must play against the benefits of using AI,” Dr. Collins said. “That risk of harm is often disproportionately distributed to marginalized populations.”
As clinicians use AI algorithms to diagnose and detect disease, predict outcomes, and guide treatment, trouble comes when those algorithms perform well for some patients and poorly for others. This gap can exacerbate existing disparities in health care outcomes.
Dr. Collins advised clinicians to push to find out what data were used to train AI algorithms to determine how bias could have influenced the model and whether the developers risk-adjusted for bias. If the training data are not available, clinicians should ask their employers and AI developers to know more about the system.
Clinicians may face the so-called black box phenomenon, which occurs when developers cannot or will not explain what data went into an AI model, Dr. Collins said.
According to Stanford (Calif.) University, AI must be trained on large datasets of images that have been annotated by human experts. Those datasets can cost millions of dollars to create, meaning corporations often fund them and do not always share the data publicly.
Some groups, such as Stanford’s Center for Artificial Intelligence in Medicine and Imaging, are working to acquire annotated datasets so researchers who train AI models can know where the data came from.
Paul Haidet, MD, MPH, an internist at Penn State College of Medicine, Hershey, sees the technology as a tool that requires careful handling.
“It takes a while to learn how to use a stethoscope, and AI is like that,” Dr. Haidet said. “The thing about AI, though, is that it can be just dropped into a system and no one knows how it works.”
Dr. Haidet said he likes knowing how the sausage is made, something AI developers are often reticent to make known.
“If you’re just putting blind faith in a tool, that’s scary,” Dr. Haidet said.
Transparency and ‘explainability’
The ability to explain what goes into tools is essential to maintaining trust in the health care system, Dr. Collins said.
“Part of knowing how much trust to place in the system is the transparency of those systems and the ability to audit how well the algorithm is performing,” Dr. Collins said. “The system should also regularly report to users the level of certainty with which it is providing an output rather than providing a simple binary output.”
Dr. Collins recommends that providers develop an understanding of the limits of AI regulations as well, which might including learning how the system was approved and how it is monitored.
“The FDA has oversight over some applications of AI and health care for software as a medical device, but there’s currently no dedicated process to evaluate the systems for the presence of bias,” Dr. Collins said. “The gaps in regulation leave the door open for the use of AI in clinical care that contain significant biases.”
Dr. Haidet likened AI tools to the Global Positioning System: A good GPS system will let users see alternate routes, opt out of toll roads or highways, and will highlight why routes have changed. But users need to understand how to read the map so they can tell when something seems amiss.
Dr. Collins and Dr. Haidet report no relevant financial relationships
A version of this article first appeared on Medscape.com.
AT SGIM 2023
Pop this question to improve medication adherence
AURORA, COLO. – How often do you talk with patients about how to lower their out-of-pocket costs for medical care?
For most clinicians, the answer is: not often enough. But having those conversations can improve medication adherence and strengthen the patient-clinician relationship, according to panelists at the annual meeting of the Society of General Internal Medicine.
The inverse association between out-of-pocket expenditures and fidelity to prescriptions is clear. A 2020 study by the IQVIA Institute for Human Data Science, for example, found that rates of prescription abandonment are less than 5% when a given medication carries no out-of-pocket cost for patients. That figure rises to 45% when the cost is more than $125, and to 60% when it exceeds $500. One in five Americans said cost prevented them from adhering to medication regimens, according to a new study in JAMA Network Open.
The researchers surveyed more than 2,000 men and women, 40.4% of whom were aged 75 or older. They found that nearly 90% of respondents said they would not be uncomfortable being asked about drug costs before a visit with a physician. A similar share (89.5%) said they would welcome the use by their physician of a real-time tool to determine the cost of their medication.
But the survey results contained a note of warning for clinicians: A significant number of respondents said they would be “extremely” upset if the cost of their medication exceeded the estimate from the pricing tool. And many also said they would be “moderately” or “extremely” angry if their physician used a pricing tool but failed to share the results with them.
“Real-time benefit tools may support medication cost conversations and cost-conscious prescribing, and patients are enthusiastic about their use,” the authors write. “However, if disclosed prices are inaccurate, there is potential for harm through loss of confidence in the physician and nonadherence to prescribed medications.”
While having conversations about cost can be difficult for both clinicians and patients, studies have shown that patients who discuss cost concerns with their doctors feel as if they have stronger relationships as a result.
Clinicians often avoid conversations about out-of-pocket expenses because they don’t know specific price information, they lack solutions to address cost, or they are uncomfortable bringing up the issue.
One member of the audience at the SGIM meeting recalled a patient who worked in a warehouse for a large company. The man, who had type 2 diabetes, had medical insurance, but even with insurance, insulin was going to cost him $150 per month. He struggled to afford the necessary treatment.
“He looked at me and said, ‘What do they want me to do? Do they want me to actually not be able to work for them and not manage my diabetes?’ ”
The clinician said he offered empathy in the moment but felt he could do little else.
Panelists acknowledged that clinicians are crunched on time when seeing patients, but being willing to initiate conversations about cost with patients and to offer resources can help patients get necessary treatment.
Start the conversation
Panel member Caroline Sloan, MD, an assistant professor of medicine at Duke University, Durham, N.C., said making patients aware that you know cost can make a big difference.
The American College of Physicians advises clinicians to ask patients whether they are worried about the cost of care and to not assume which patients may have concerns.
The conversation could be started like this: “I’d like to discuss any concerns you might have about the cost of your health care.”
Normalize the concern by making it more general, and reassure your patient that your goal is to get them the best care. Say something like, “I’ve heard from many patients the cost of medications or tests is becoming hard to manage.”
Once a patient’s concerns are clear, you can direct them to resources for assistance in reducing their costs, Dr. Sloan said, such as ClearHealthCosts, FAIR Health, Healthcare Bluebook, New Choice Health, GoodRx, PharmacyChecker, HealthWell Foundation, Patient Advocate Foundation, Good Days, Good Health Will, Mercy Medical Angels, and the American Association of Family Physicians Neighborhood Navigator.
Dr. Sloan said she knows clinicians don’t have time to understand every insurance plan and other issues related to cost. “But at least know to ask about costs,” she said. “Practice, practice, practice. It feels awkward at first, but it gets easier every time.”
A version of this article first appeared on Medscape.com.
AURORA, COLO. – How often do you talk with patients about how to lower their out-of-pocket costs for medical care?
For most clinicians, the answer is: not often enough. But having those conversations can improve medication adherence and strengthen the patient-clinician relationship, according to panelists at the annual meeting of the Society of General Internal Medicine.
The inverse association between out-of-pocket expenditures and fidelity to prescriptions is clear. A 2020 study by the IQVIA Institute for Human Data Science, for example, found that rates of prescription abandonment are less than 5% when a given medication carries no out-of-pocket cost for patients. That figure rises to 45% when the cost is more than $125, and to 60% when it exceeds $500. One in five Americans said cost prevented them from adhering to medication regimens, according to a new study in JAMA Network Open.
The researchers surveyed more than 2,000 men and women, 40.4% of whom were aged 75 or older. They found that nearly 90% of respondents said they would not be uncomfortable being asked about drug costs before a visit with a physician. A similar share (89.5%) said they would welcome the use by their physician of a real-time tool to determine the cost of their medication.
But the survey results contained a note of warning for clinicians: A significant number of respondents said they would be “extremely” upset if the cost of their medication exceeded the estimate from the pricing tool. And many also said they would be “moderately” or “extremely” angry if their physician used a pricing tool but failed to share the results with them.
“Real-time benefit tools may support medication cost conversations and cost-conscious prescribing, and patients are enthusiastic about their use,” the authors write. “However, if disclosed prices are inaccurate, there is potential for harm through loss of confidence in the physician and nonadherence to prescribed medications.”
While having conversations about cost can be difficult for both clinicians and patients, studies have shown that patients who discuss cost concerns with their doctors feel as if they have stronger relationships as a result.
Clinicians often avoid conversations about out-of-pocket expenses because they don’t know specific price information, they lack solutions to address cost, or they are uncomfortable bringing up the issue.
One member of the audience at the SGIM meeting recalled a patient who worked in a warehouse for a large company. The man, who had type 2 diabetes, had medical insurance, but even with insurance, insulin was going to cost him $150 per month. He struggled to afford the necessary treatment.
“He looked at me and said, ‘What do they want me to do? Do they want me to actually not be able to work for them and not manage my diabetes?’ ”
The clinician said he offered empathy in the moment but felt he could do little else.
Panelists acknowledged that clinicians are crunched on time when seeing patients, but being willing to initiate conversations about cost with patients and to offer resources can help patients get necessary treatment.
Start the conversation
Panel member Caroline Sloan, MD, an assistant professor of medicine at Duke University, Durham, N.C., said making patients aware that you know cost can make a big difference.
The American College of Physicians advises clinicians to ask patients whether they are worried about the cost of care and to not assume which patients may have concerns.
The conversation could be started like this: “I’d like to discuss any concerns you might have about the cost of your health care.”
Normalize the concern by making it more general, and reassure your patient that your goal is to get them the best care. Say something like, “I’ve heard from many patients the cost of medications or tests is becoming hard to manage.”
Once a patient’s concerns are clear, you can direct them to resources for assistance in reducing their costs, Dr. Sloan said, such as ClearHealthCosts, FAIR Health, Healthcare Bluebook, New Choice Health, GoodRx, PharmacyChecker, HealthWell Foundation, Patient Advocate Foundation, Good Days, Good Health Will, Mercy Medical Angels, and the American Association of Family Physicians Neighborhood Navigator.
Dr. Sloan said she knows clinicians don’t have time to understand every insurance plan and other issues related to cost. “But at least know to ask about costs,” she said. “Practice, practice, practice. It feels awkward at first, but it gets easier every time.”
A version of this article first appeared on Medscape.com.
AURORA, COLO. – How often do you talk with patients about how to lower their out-of-pocket costs for medical care?
For most clinicians, the answer is: not often enough. But having those conversations can improve medication adherence and strengthen the patient-clinician relationship, according to panelists at the annual meeting of the Society of General Internal Medicine.
The inverse association between out-of-pocket expenditures and fidelity to prescriptions is clear. A 2020 study by the IQVIA Institute for Human Data Science, for example, found that rates of prescription abandonment are less than 5% when a given medication carries no out-of-pocket cost for patients. That figure rises to 45% when the cost is more than $125, and to 60% when it exceeds $500. One in five Americans said cost prevented them from adhering to medication regimens, according to a new study in JAMA Network Open.
The researchers surveyed more than 2,000 men and women, 40.4% of whom were aged 75 or older. They found that nearly 90% of respondents said they would not be uncomfortable being asked about drug costs before a visit with a physician. A similar share (89.5%) said they would welcome the use by their physician of a real-time tool to determine the cost of their medication.
But the survey results contained a note of warning for clinicians: A significant number of respondents said they would be “extremely” upset if the cost of their medication exceeded the estimate from the pricing tool. And many also said they would be “moderately” or “extremely” angry if their physician used a pricing tool but failed to share the results with them.
“Real-time benefit tools may support medication cost conversations and cost-conscious prescribing, and patients are enthusiastic about their use,” the authors write. “However, if disclosed prices are inaccurate, there is potential for harm through loss of confidence in the physician and nonadherence to prescribed medications.”
While having conversations about cost can be difficult for both clinicians and patients, studies have shown that patients who discuss cost concerns with their doctors feel as if they have stronger relationships as a result.
Clinicians often avoid conversations about out-of-pocket expenses because they don’t know specific price information, they lack solutions to address cost, or they are uncomfortable bringing up the issue.
One member of the audience at the SGIM meeting recalled a patient who worked in a warehouse for a large company. The man, who had type 2 diabetes, had medical insurance, but even with insurance, insulin was going to cost him $150 per month. He struggled to afford the necessary treatment.
“He looked at me and said, ‘What do they want me to do? Do they want me to actually not be able to work for them and not manage my diabetes?’ ”
The clinician said he offered empathy in the moment but felt he could do little else.
Panelists acknowledged that clinicians are crunched on time when seeing patients, but being willing to initiate conversations about cost with patients and to offer resources can help patients get necessary treatment.
Start the conversation
Panel member Caroline Sloan, MD, an assistant professor of medicine at Duke University, Durham, N.C., said making patients aware that you know cost can make a big difference.
The American College of Physicians advises clinicians to ask patients whether they are worried about the cost of care and to not assume which patients may have concerns.
The conversation could be started like this: “I’d like to discuss any concerns you might have about the cost of your health care.”
Normalize the concern by making it more general, and reassure your patient that your goal is to get them the best care. Say something like, “I’ve heard from many patients the cost of medications or tests is becoming hard to manage.”
Once a patient’s concerns are clear, you can direct them to resources for assistance in reducing their costs, Dr. Sloan said, such as ClearHealthCosts, FAIR Health, Healthcare Bluebook, New Choice Health, GoodRx, PharmacyChecker, HealthWell Foundation, Patient Advocate Foundation, Good Days, Good Health Will, Mercy Medical Angels, and the American Association of Family Physicians Neighborhood Navigator.
Dr. Sloan said she knows clinicians don’t have time to understand every insurance plan and other issues related to cost. “But at least know to ask about costs,” she said. “Practice, practice, practice. It feels awkward at first, but it gets easier every time.”
A version of this article first appeared on Medscape.com.
AT SGIM 2023
Breast cancer survivors need a comprehensive care plan, says doctor
said Patricia A. Ganz, MD, during a presentation at the European Society for Medical Oncology Breast Cancer annual congress.
Several studies suggest that many breast cancer patients are not well prepared to move forward after a breast cancer diagnosis and subsequent treatments, continued Dr. Ganz, who works at the UCLA Jonsson Comprehensive Cancer Center, Los Angeles.
Meeting the survivorship needs of breast cancer patients requires addressing both their physical and psychosocial needs, Dr. Ganz said. She explained how to achieve that, but first pointed to research elaborating on what's missing from some breast cancer survivors' care and barriers to these patients having their variety of health-related needs met.
In a 2021 study published in the Journal of Cancer Survivorship, Dr. Ganz and colleagues conducted a survey of approximately 200 medical oncologists in the United States. They determined that less than 50% provide survivorship care plans to patients at the end of treatment or communicate with patients’ other physicians about follow-up care.
In a secondary analysis of data from the same survey published in 2022 in Breast Cancer Research and Treatment, Dr. Ganz and colleagues examined medical oncologists’ perceived barriers to addressing both physical and psychosocial long-term effects in breast cancer survivors. For both, lack of time was the greatest perceived barrier, cited by nearly two-thirds of oncologists. Other barriers to addressing physical effects included lack of evidence-based, effective interventions, lack of clinical algorithms to guide care, and ambiguity regarding professional responsibility at the end of treatment. Other top barriers to addressing psychosocial issues included lack of mental health providers, lack of psychosocial resources, and lack of clinician knowledge and skills.
Data from additional studies suggest that, overall, cancer patients with greater physical burdens, such as more complex and lengthy treatment regimens, also have greater psychosocial needs, Dr. Ganz noted. Plus, approximately 15%-20% of cancer survivors have ongoing anxiety and depressive symptoms.
Shift to primary care
As more breast cancer and other cancer patients survive for longer periods, more care will likely occur in general medical settings, Dr. Ganz said. Issues to be addressed will include the potential increased risk of comorbid conditions for these survivors, and whether survivorship interventions earlier in the disease trajectory will impact survivorship. For cancer patients who achieve remission after treatment, the first 5 years after a diagnosis involves treatment and short-term surveillance for late effects. Beyond 5 years, care for cancer survivors mainly involves primary care and management of any comorbid conditions, as well as surveillance for late effects and recurrences, and awareness of new research.
A patient consultation early in the process after diagnosis is the start of a continuum of care, Dr. Ganz said. A patient consultation should address symptoms related to initial treatments, such as neuropathy, pain, fatigue, and insomnia, as well as the psychological symptoms of anxiety and depression. An early consultation also should evaluate adherence to endocrine therapy and management of symptoms, if needed, with the larger goal of preparing patients for recovery and the transition to survivorship, and what to expect for long-term follow-up.
Delivering the three P’s
The “Three P’s” of survivor care for breast cancer patients are palliation, prevention, and promotion of health, according to Dr. Ganz .
The first “P,” for palliative, is a key part of survivorship care, said Dr. Ganz. Palliative care is defined as care that focuses on reducing symptom severity and improving quality of life. The biological effects of cancer treatment can be associated with physical effects, such as functional limitations and frailty, and behavioral/cognitive effects such as depression, fatigue, and cognitive deficits, she said. To manage these effects and provide palliative care, consultation is needed with specialists in relevant areas including mental health, pain management, physical medicine/rehabilitation, endocrinology, cardiology, and neurology.
The second “P,” which is for prevention in survivorship care, refers to ongoing follow-up screening to identify any potentially serious late-onset complications such as osteoporosis or cardiac disease so they can be addressed, said Dr. Ganz. Other considerations include chemoprevention if available and genetic counseling for patients with hereditary cancers. Prevention also includes counseling patients about lifestyle modifications to help prevent additional cancer.
The goal of the third “P,” which is for health promotion, is to promote risk reduction for the health problems associated with accelerated aging that may arise in cancer survivors, said Dr. Ganz.
Health promotion strategies include maintaining a healthy weight, increasing physical activity, and avoiding harmful exposures, she said. Healthy lifestyle interventions can also reduce the risk of other chronic diseases such as diabetes and heart disease.
To that end, Dr. Ganz outlined several behavioral interventions that may mitigate the effects of cancer treatment on the accelerated aging process, including stress reduction in the form of meditation or yoga, cognitive behavioral therapy, improving sleep, increasing physical activity, reducing obesity, and decreasing tobacco and alcohol use. These interventions may help reduce inflammation and promote tissue repair and healing.
For cancer survivors, the life span may be longer than the health span, and these patients may benefit from an integrated model of care, with systematic screening and consolidated appointments, rather than a fragmented model in which departments and referrals are siloed, which may result in conflicting advice or redundancy, said Dr. Ganz.
Looking ahead, more research is needed to explore models of care delivery, as requirements for survivor care will vary among patients and care settings, Dr. Ganz said.
However, regardless of setting, treatment plans and shared decision-making can help reduce potential long-term or late-emerging effects, she said. Developing a survivorship care plan can help patients learn how to enhance their recovery.
During a question and answer session, Dr. Ganz was asked about whether hormone therapy could be used for patients with hormone negative breast cancer. “I think vaginal estrogen can be used if someone is on tamoxifen,” she said. However, “we need to be cautious” in case there are remaining estrogen positive cells, in order to avoid potential metastases, and use of hormone therapy in breast cancer survivors is an individualized decision based in part on quality of life.
Engaging a patient’s partner early can be helpful
If possible, engage the patient’s partner in survivorship discussions, said Luzia Travado, PhD, head of psycho-oncology at the Champalimaud Foundation, Lisbon, who presented on the topic of sexuality and commented on survivorship during the discussion. For those women with partners, engaging the partner early in treatment often means they are more likely to play a larger role in the post treatment and long term by providing stability and emotional support.
“Make sure partners are engaged and understand that they have a role, and that this role is valued,” she said. Unfortunately, there are a lot of divorced women with breast cancer, as the disease can take a toll on relationships. However, remember “sexuality is not just sex; it is caring, loving, and intimacy.”
“To end on a positive note, it is important to empower patients, and to give them self-management skills so they can make things even better in their survivorship,” said Dr. Ganz. In spite of discussing difficulties and challenges, one of the goals of the session was to offer potential solutions and answers.
Dr. Ganz disclosed serving as editor of the cancer survivorship section on Up-to-Date, and serving as a consultant for Blue Note Therapeutics, GRAIL, InformedDNA, and Roche-Genentech. Dr. Travado had no relevant financial conflicts to disclose.
said Patricia A. Ganz, MD, during a presentation at the European Society for Medical Oncology Breast Cancer annual congress.
Several studies suggest that many breast cancer patients are not well prepared to move forward after a breast cancer diagnosis and subsequent treatments, continued Dr. Ganz, who works at the UCLA Jonsson Comprehensive Cancer Center, Los Angeles.
Meeting the survivorship needs of breast cancer patients requires addressing both their physical and psychosocial needs, Dr. Ganz said. She explained how to achieve that, but first pointed to research elaborating on what's missing from some breast cancer survivors' care and barriers to these patients having their variety of health-related needs met.
In a 2021 study published in the Journal of Cancer Survivorship, Dr. Ganz and colleagues conducted a survey of approximately 200 medical oncologists in the United States. They determined that less than 50% provide survivorship care plans to patients at the end of treatment or communicate with patients’ other physicians about follow-up care.
In a secondary analysis of data from the same survey published in 2022 in Breast Cancer Research and Treatment, Dr. Ganz and colleagues examined medical oncologists’ perceived barriers to addressing both physical and psychosocial long-term effects in breast cancer survivors. For both, lack of time was the greatest perceived barrier, cited by nearly two-thirds of oncologists. Other barriers to addressing physical effects included lack of evidence-based, effective interventions, lack of clinical algorithms to guide care, and ambiguity regarding professional responsibility at the end of treatment. Other top barriers to addressing psychosocial issues included lack of mental health providers, lack of psychosocial resources, and lack of clinician knowledge and skills.
Data from additional studies suggest that, overall, cancer patients with greater physical burdens, such as more complex and lengthy treatment regimens, also have greater psychosocial needs, Dr. Ganz noted. Plus, approximately 15%-20% of cancer survivors have ongoing anxiety and depressive symptoms.
Shift to primary care
As more breast cancer and other cancer patients survive for longer periods, more care will likely occur in general medical settings, Dr. Ganz said. Issues to be addressed will include the potential increased risk of comorbid conditions for these survivors, and whether survivorship interventions earlier in the disease trajectory will impact survivorship. For cancer patients who achieve remission after treatment, the first 5 years after a diagnosis involves treatment and short-term surveillance for late effects. Beyond 5 years, care for cancer survivors mainly involves primary care and management of any comorbid conditions, as well as surveillance for late effects and recurrences, and awareness of new research.
A patient consultation early in the process after diagnosis is the start of a continuum of care, Dr. Ganz said. A patient consultation should address symptoms related to initial treatments, such as neuropathy, pain, fatigue, and insomnia, as well as the psychological symptoms of anxiety and depression. An early consultation also should evaluate adherence to endocrine therapy and management of symptoms, if needed, with the larger goal of preparing patients for recovery and the transition to survivorship, and what to expect for long-term follow-up.
Delivering the three P’s
The “Three P’s” of survivor care for breast cancer patients are palliation, prevention, and promotion of health, according to Dr. Ganz .
The first “P,” for palliative, is a key part of survivorship care, said Dr. Ganz. Palliative care is defined as care that focuses on reducing symptom severity and improving quality of life. The biological effects of cancer treatment can be associated with physical effects, such as functional limitations and frailty, and behavioral/cognitive effects such as depression, fatigue, and cognitive deficits, she said. To manage these effects and provide palliative care, consultation is needed with specialists in relevant areas including mental health, pain management, physical medicine/rehabilitation, endocrinology, cardiology, and neurology.
The second “P,” which is for prevention in survivorship care, refers to ongoing follow-up screening to identify any potentially serious late-onset complications such as osteoporosis or cardiac disease so they can be addressed, said Dr. Ganz. Other considerations include chemoprevention if available and genetic counseling for patients with hereditary cancers. Prevention also includes counseling patients about lifestyle modifications to help prevent additional cancer.
The goal of the third “P,” which is for health promotion, is to promote risk reduction for the health problems associated with accelerated aging that may arise in cancer survivors, said Dr. Ganz.
Health promotion strategies include maintaining a healthy weight, increasing physical activity, and avoiding harmful exposures, she said. Healthy lifestyle interventions can also reduce the risk of other chronic diseases such as diabetes and heart disease.
To that end, Dr. Ganz outlined several behavioral interventions that may mitigate the effects of cancer treatment on the accelerated aging process, including stress reduction in the form of meditation or yoga, cognitive behavioral therapy, improving sleep, increasing physical activity, reducing obesity, and decreasing tobacco and alcohol use. These interventions may help reduce inflammation and promote tissue repair and healing.
For cancer survivors, the life span may be longer than the health span, and these patients may benefit from an integrated model of care, with systematic screening and consolidated appointments, rather than a fragmented model in which departments and referrals are siloed, which may result in conflicting advice or redundancy, said Dr. Ganz.
Looking ahead, more research is needed to explore models of care delivery, as requirements for survivor care will vary among patients and care settings, Dr. Ganz said.
However, regardless of setting, treatment plans and shared decision-making can help reduce potential long-term or late-emerging effects, she said. Developing a survivorship care plan can help patients learn how to enhance their recovery.
During a question and answer session, Dr. Ganz was asked about whether hormone therapy could be used for patients with hormone negative breast cancer. “I think vaginal estrogen can be used if someone is on tamoxifen,” she said. However, “we need to be cautious” in case there are remaining estrogen positive cells, in order to avoid potential metastases, and use of hormone therapy in breast cancer survivors is an individualized decision based in part on quality of life.
Engaging a patient’s partner early can be helpful
If possible, engage the patient’s partner in survivorship discussions, said Luzia Travado, PhD, head of psycho-oncology at the Champalimaud Foundation, Lisbon, who presented on the topic of sexuality and commented on survivorship during the discussion. For those women with partners, engaging the partner early in treatment often means they are more likely to play a larger role in the post treatment and long term by providing stability and emotional support.
“Make sure partners are engaged and understand that they have a role, and that this role is valued,” she said. Unfortunately, there are a lot of divorced women with breast cancer, as the disease can take a toll on relationships. However, remember “sexuality is not just sex; it is caring, loving, and intimacy.”
“To end on a positive note, it is important to empower patients, and to give them self-management skills so they can make things even better in their survivorship,” said Dr. Ganz. In spite of discussing difficulties and challenges, one of the goals of the session was to offer potential solutions and answers.
Dr. Ganz disclosed serving as editor of the cancer survivorship section on Up-to-Date, and serving as a consultant for Blue Note Therapeutics, GRAIL, InformedDNA, and Roche-Genentech. Dr. Travado had no relevant financial conflicts to disclose.
said Patricia A. Ganz, MD, during a presentation at the European Society for Medical Oncology Breast Cancer annual congress.
Several studies suggest that many breast cancer patients are not well prepared to move forward after a breast cancer diagnosis and subsequent treatments, continued Dr. Ganz, who works at the UCLA Jonsson Comprehensive Cancer Center, Los Angeles.
Meeting the survivorship needs of breast cancer patients requires addressing both their physical and psychosocial needs, Dr. Ganz said. She explained how to achieve that, but first pointed to research elaborating on what's missing from some breast cancer survivors' care and barriers to these patients having their variety of health-related needs met.
In a 2021 study published in the Journal of Cancer Survivorship, Dr. Ganz and colleagues conducted a survey of approximately 200 medical oncologists in the United States. They determined that less than 50% provide survivorship care plans to patients at the end of treatment or communicate with patients’ other physicians about follow-up care.
In a secondary analysis of data from the same survey published in 2022 in Breast Cancer Research and Treatment, Dr. Ganz and colleagues examined medical oncologists’ perceived barriers to addressing both physical and psychosocial long-term effects in breast cancer survivors. For both, lack of time was the greatest perceived barrier, cited by nearly two-thirds of oncologists. Other barriers to addressing physical effects included lack of evidence-based, effective interventions, lack of clinical algorithms to guide care, and ambiguity regarding professional responsibility at the end of treatment. Other top barriers to addressing psychosocial issues included lack of mental health providers, lack of psychosocial resources, and lack of clinician knowledge and skills.
Data from additional studies suggest that, overall, cancer patients with greater physical burdens, such as more complex and lengthy treatment regimens, also have greater psychosocial needs, Dr. Ganz noted. Plus, approximately 15%-20% of cancer survivors have ongoing anxiety and depressive symptoms.
Shift to primary care
As more breast cancer and other cancer patients survive for longer periods, more care will likely occur in general medical settings, Dr. Ganz said. Issues to be addressed will include the potential increased risk of comorbid conditions for these survivors, and whether survivorship interventions earlier in the disease trajectory will impact survivorship. For cancer patients who achieve remission after treatment, the first 5 years after a diagnosis involves treatment and short-term surveillance for late effects. Beyond 5 years, care for cancer survivors mainly involves primary care and management of any comorbid conditions, as well as surveillance for late effects and recurrences, and awareness of new research.
A patient consultation early in the process after diagnosis is the start of a continuum of care, Dr. Ganz said. A patient consultation should address symptoms related to initial treatments, such as neuropathy, pain, fatigue, and insomnia, as well as the psychological symptoms of anxiety and depression. An early consultation also should evaluate adherence to endocrine therapy and management of symptoms, if needed, with the larger goal of preparing patients for recovery and the transition to survivorship, and what to expect for long-term follow-up.
Delivering the three P’s
The “Three P’s” of survivor care for breast cancer patients are palliation, prevention, and promotion of health, according to Dr. Ganz .
The first “P,” for palliative, is a key part of survivorship care, said Dr. Ganz. Palliative care is defined as care that focuses on reducing symptom severity and improving quality of life. The biological effects of cancer treatment can be associated with physical effects, such as functional limitations and frailty, and behavioral/cognitive effects such as depression, fatigue, and cognitive deficits, she said. To manage these effects and provide palliative care, consultation is needed with specialists in relevant areas including mental health, pain management, physical medicine/rehabilitation, endocrinology, cardiology, and neurology.
The second “P,” which is for prevention in survivorship care, refers to ongoing follow-up screening to identify any potentially serious late-onset complications such as osteoporosis or cardiac disease so they can be addressed, said Dr. Ganz. Other considerations include chemoprevention if available and genetic counseling for patients with hereditary cancers. Prevention also includes counseling patients about lifestyle modifications to help prevent additional cancer.
The goal of the third “P,” which is for health promotion, is to promote risk reduction for the health problems associated with accelerated aging that may arise in cancer survivors, said Dr. Ganz.
Health promotion strategies include maintaining a healthy weight, increasing physical activity, and avoiding harmful exposures, she said. Healthy lifestyle interventions can also reduce the risk of other chronic diseases such as diabetes and heart disease.
To that end, Dr. Ganz outlined several behavioral interventions that may mitigate the effects of cancer treatment on the accelerated aging process, including stress reduction in the form of meditation or yoga, cognitive behavioral therapy, improving sleep, increasing physical activity, reducing obesity, and decreasing tobacco and alcohol use. These interventions may help reduce inflammation and promote tissue repair and healing.
For cancer survivors, the life span may be longer than the health span, and these patients may benefit from an integrated model of care, with systematic screening and consolidated appointments, rather than a fragmented model in which departments and referrals are siloed, which may result in conflicting advice or redundancy, said Dr. Ganz.
Looking ahead, more research is needed to explore models of care delivery, as requirements for survivor care will vary among patients and care settings, Dr. Ganz said.
However, regardless of setting, treatment plans and shared decision-making can help reduce potential long-term or late-emerging effects, she said. Developing a survivorship care plan can help patients learn how to enhance their recovery.
During a question and answer session, Dr. Ganz was asked about whether hormone therapy could be used for patients with hormone negative breast cancer. “I think vaginal estrogen can be used if someone is on tamoxifen,” she said. However, “we need to be cautious” in case there are remaining estrogen positive cells, in order to avoid potential metastases, and use of hormone therapy in breast cancer survivors is an individualized decision based in part on quality of life.
Engaging a patient’s partner early can be helpful
If possible, engage the patient’s partner in survivorship discussions, said Luzia Travado, PhD, head of psycho-oncology at the Champalimaud Foundation, Lisbon, who presented on the topic of sexuality and commented on survivorship during the discussion. For those women with partners, engaging the partner early in treatment often means they are more likely to play a larger role in the post treatment and long term by providing stability and emotional support.
“Make sure partners are engaged and understand that they have a role, and that this role is valued,” she said. Unfortunately, there are a lot of divorced women with breast cancer, as the disease can take a toll on relationships. However, remember “sexuality is not just sex; it is caring, loving, and intimacy.”
“To end on a positive note, it is important to empower patients, and to give them self-management skills so they can make things even better in their survivorship,” said Dr. Ganz. In spite of discussing difficulties and challenges, one of the goals of the session was to offer potential solutions and answers.
Dr. Ganz disclosed serving as editor of the cancer survivorship section on Up-to-Date, and serving as a consultant for Blue Note Therapeutics, GRAIL, InformedDNA, and Roche-Genentech. Dr. Travado had no relevant financial conflicts to disclose.
FROM ESMO BREAST CANCER 2023

