User login
Enzalutamide Improves Progression-Free and Overall Survival in Metastatic Hormone-Sensitive Prostate Cancer
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
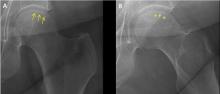
Osteonecrosis of the femoral head with subchondral collapse
A 45-year-old woman with a history of multiple organ transplants presented with a 1-month history of anterior left hip pain with insidious onset. Although she was able to perform activities of daily living, she reported increasing difficulty with weight-bearing activities.
RISK FACTORS
Osteonecrosis of the hip is caused by prolonged interruption of blood flow to the femoral head.2 While idiopathic osteonecrosis is not uncommon, the condition is often associated with alcohol abuse or, as in our patient, long-term corticosteroid use after organ transplant.3 Corticosteroid use is also the most frequently reported risk factor for multifocal osteonecrosis.
Less common risk factors include systemic lupus erythematosus, antiphospholipid antibodies, coagulopathies, sickle cell disease, Gaucher disease, trauma, and external-beam therapy.
Young age is also associated with osteonecrosis, as nearly 75% of patients are between age 30 and 60.4
APPROACH TO DIAGNOSIS
Our patient had a typical clinical presentation of this disease: she was relatively young, was on long-term corticosteroids, and had acute anterior groin pain followed by progressive functional impairment.
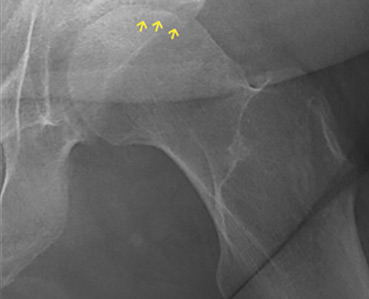
The diagnostic evaluation consists of a detailed history, with attention to specific risk factors, and a thorough clinical examination followed by imaging, usually with plain radiography. However, plain radiographs are often unremarkable when the condition is in the early stages. In such cases, magnetic resonance imaging is recommended if clinical suspicion for osteonecrosis is high. It is far more sensitive (> 99%) and specific (> 99%) than plain radiography, and it detects early changes in the femoral head such as focal lesions and bone marrow edema.5
TREATMENT OPTIONS
Treatment of osteonecrosis is surgical and depends on the stage of disease.6
Joint preservation may be an option for small to medium-sized lesions before subchondral collapse has occurred; options include core decompression, bone grafting, and femoral osteotomy to preserve the native femoral head. These procedures have a higher success rate in young patients.
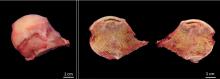
Subchondral collapse usually warrants hip replacement.
OUR PATIENT’S TREATMENT
- Pappas JN. The musculoskeletal crescent sign. Radiology 2000; 217(1):213–214. doi:10.1148/radiology.217.1.r00oc22213
- Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 2015; 8(3):201–209. doi:10.1007/s12178-015-9277-8
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590–601. doi:10.5312/wjo.v6.i8.590
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002; 32(2):94–124. pmid:12430099
- Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015; 8(3):221–227. doi:10.1007/s12178-015-9279-6
- Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B(10):1267–1279. doi:10.1302/0301-620X.99B10.BJJ-2017-0233.R2
A 45-year-old woman with a history of multiple organ transplants presented with a 1-month history of anterior left hip pain with insidious onset. Although she was able to perform activities of daily living, she reported increasing difficulty with weight-bearing activities.
RISK FACTORS
Osteonecrosis of the hip is caused by prolonged interruption of blood flow to the femoral head.2 While idiopathic osteonecrosis is not uncommon, the condition is often associated with alcohol abuse or, as in our patient, long-term corticosteroid use after organ transplant.3 Corticosteroid use is also the most frequently reported risk factor for multifocal osteonecrosis.
Less common risk factors include systemic lupus erythematosus, antiphospholipid antibodies, coagulopathies, sickle cell disease, Gaucher disease, trauma, and external-beam therapy.
Young age is also associated with osteonecrosis, as nearly 75% of patients are between age 30 and 60.4
APPROACH TO DIAGNOSIS
Our patient had a typical clinical presentation of this disease: she was relatively young, was on long-term corticosteroids, and had acute anterior groin pain followed by progressive functional impairment.
The diagnostic evaluation consists of a detailed history, with attention to specific risk factors, and a thorough clinical examination followed by imaging, usually with plain radiography. However, plain radiographs are often unremarkable when the condition is in the early stages. In such cases, magnetic resonance imaging is recommended if clinical suspicion for osteonecrosis is high. It is far more sensitive (> 99%) and specific (> 99%) than plain radiography, and it detects early changes in the femoral head such as focal lesions and bone marrow edema.5
TREATMENT OPTIONS
Treatment of osteonecrosis is surgical and depends on the stage of disease.6
Joint preservation may be an option for small to medium-sized lesions before subchondral collapse has occurred; options include core decompression, bone grafting, and femoral osteotomy to preserve the native femoral head. These procedures have a higher success rate in young patients.
Subchondral collapse usually warrants hip replacement.
OUR PATIENT’S TREATMENT
A 45-year-old woman with a history of multiple organ transplants presented with a 1-month history of anterior left hip pain with insidious onset. Although she was able to perform activities of daily living, she reported increasing difficulty with weight-bearing activities.
RISK FACTORS
Osteonecrosis of the hip is caused by prolonged interruption of blood flow to the femoral head.2 While idiopathic osteonecrosis is not uncommon, the condition is often associated with alcohol abuse or, as in our patient, long-term corticosteroid use after organ transplant.3 Corticosteroid use is also the most frequently reported risk factor for multifocal osteonecrosis.
Less common risk factors include systemic lupus erythematosus, antiphospholipid antibodies, coagulopathies, sickle cell disease, Gaucher disease, trauma, and external-beam therapy.
Young age is also associated with osteonecrosis, as nearly 75% of patients are between age 30 and 60.4
APPROACH TO DIAGNOSIS
Our patient had a typical clinical presentation of this disease: she was relatively young, was on long-term corticosteroids, and had acute anterior groin pain followed by progressive functional impairment.
The diagnostic evaluation consists of a detailed history, with attention to specific risk factors, and a thorough clinical examination followed by imaging, usually with plain radiography. However, plain radiographs are often unremarkable when the condition is in the early stages. In such cases, magnetic resonance imaging is recommended if clinical suspicion for osteonecrosis is high. It is far more sensitive (> 99%) and specific (> 99%) than plain radiography, and it detects early changes in the femoral head such as focal lesions and bone marrow edema.5
TREATMENT OPTIONS
Treatment of osteonecrosis is surgical and depends on the stage of disease.6
Joint preservation may be an option for small to medium-sized lesions before subchondral collapse has occurred; options include core decompression, bone grafting, and femoral osteotomy to preserve the native femoral head. These procedures have a higher success rate in young patients.
Subchondral collapse usually warrants hip replacement.
OUR PATIENT’S TREATMENT
- Pappas JN. The musculoskeletal crescent sign. Radiology 2000; 217(1):213–214. doi:10.1148/radiology.217.1.r00oc22213
- Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 2015; 8(3):201–209. doi:10.1007/s12178-015-9277-8
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590–601. doi:10.5312/wjo.v6.i8.590
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002; 32(2):94–124. pmid:12430099
- Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015; 8(3):221–227. doi:10.1007/s12178-015-9279-6
- Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B(10):1267–1279. doi:10.1302/0301-620X.99B10.BJJ-2017-0233.R2
- Pappas JN. The musculoskeletal crescent sign. Radiology 2000; 217(1):213–214. doi:10.1148/radiology.217.1.r00oc22213
- Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 2015; 8(3):201–209. doi:10.1007/s12178-015-9277-8
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590–601. doi:10.5312/wjo.v6.i8.590
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002; 32(2):94–124. pmid:12430099
- Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015; 8(3):221–227. doi:10.1007/s12178-015-9279-6
- Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B(10):1267–1279. doi:10.1302/0301-620X.99B10.BJJ-2017-0233.R2
Should we stop aspirin before noncardiac surgery?
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
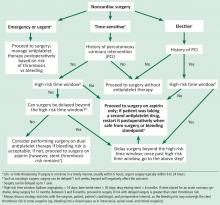
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
Aspirin: 4,000 years and still learning
Aspirin (acetylsalicylic acid) and its progenitors are valuable medications with a history spanning at least 4 millennia. An enormous number of patients take aspirin for a variety of reasons, and managing their therapy around the time of surgery can be challenging, as Drs. Prabhakaran and Whinney discuss in this issue.1 Even after 4,000 years, we are still learning about these remarkable drugs.
LEARNING WHAT SALICYLATES ARE
LEARNING (AND IGNORING) WHAT ASPIRIN CAN DO
In the 1940s, a general practitioner in California named Lawrence Craven recognized that many of his post-tonsillectomy patients had to be hospitalized for bleeding after he started recommending they use aspirin-containing chewing gum for pain relief.4 Under the then-debated hypothesis that myocardial infarction (MI) involves thrombosis, he recommended that adult men should take aspirin daily. He believed that women had lower rates of MI because they were more likely to take aspirin, something that men did not view as a “masculine” thing to do.
In a series of letters in journals such as the Mississippi Valley Medical Journal,5 Craven reported his observations of very low rates of MI and no strokes in aspirin users. Given the nonrigorous nature of his research and the obscure journals in which he published, his findings languished for many years. Ironically, he died of an MI in 1957.
LEARNING HOW ASPIRIN WORKS (AND A FEW OTHER THINGS)
The history of aspirin research illustrates how the fields of hemostasis and inflammation are now linked.
In the late 1960s, Weiss et al6 reported that aspirin rapidly and irreversibly inhibits platelet aggregation. In parallel, using biological assays in work that eventually led to the Nobel Prize, Vane7 discovered that inflammation involves the de novo synthesis of prostaglandins and that aspirin directly inhibits this synthesis. Further work connecting these lines of investigation led us to understand that platelet aggregation is enhanced by the prostaglandin derivative thromboxane A2, produced by cyclooxygenase-1, and that aspirin irreversibly inhibits this enzyme by acetylation.
LEARNING WHEN TO USE ASPIRIN
After decades of research ranging from the Physicians’ Health Study to well-named trials such as ARRIVE, ASCEND, and ASPREE, we now know that taking daily low doses of aspirin for primary prevention can reduce the risk of cardiovascular events and may reduce the risk of colorectal cancer—but at the cost of an increased risk of bleeding.8
Which patients will gain the most benefit and incur the least risk is still debated. What is certain, however, is that aspirin has an important role in acute coronary syndromes, secondary prevention of MI and stroke, and prevention of thrombosis after coronary stent placement. In the perioperative setting, we are learning that aspirin may benefit patients with myocardial injury after noncardiac surgery, a recently described clinical entity associated with surprisingly high mortality rates.9,10
LEARNING WHEN NOT TO USE ASPIRIN
The perioperative period is a dangerous time—surgical stress, hypercoagulability, inflammation, pain, and hemodynamic changes predispose to plaque rupture and supply-demand imbalance. It is therefore logical to hope aspirin would provide protection for at-risk patients in this context.
Unfortunately, results from the second Perioperative Ischemic Evaluation trial have dampened enthusiasm.11 Aspirin has now joined clonidine and beta-blockers on the list of interventions that probably do not reduce perioperative cardiovascular mortality rates. Other than protecting against stent thrombosis, aspirin’s main perioperative effect is to increase bleeding. Consequently, some surgical procedures mandate withdrawal of aspirin.
WHAT WE STILL NEED TO LEARN
Over the years, we have learned the broad outlines of using aspirin to prevent and treat cardiovascular disease, to relieve pain and inflammation (its original purpose), and to prevent stent thrombosis.
However, many details remain to be filled in. We need to better define groups who should and should not take aspirin for primary prevention. We also need to understand aspirin’s role in cancer chemoprevention, to find better ways to mitigate its undesirable effects, and to study its role in treating myocardial injury after noncardiac surgery.
Finally, we need to determine which (if any) patients without coronary stents will benefit from continuing their aspirin perioperatively or even initiating aspirin therapy preoperatively.
Will humanity still be using salicylates 4,000 years from now? Probably not. But what we have learned and will continue to learn from this remarkable group of medications will certainly inform new and better therapies in the years to come.
- Prabhakaran A, Whinney C. Should we stop aspirin before noncardiac surgery? Cleve Clin J Med 2019; 86(8):518–521. doi:10.3949/ccjm.86a.19036
- Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York: Bloomsbury; 2008.
- Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. New York: Alfred A. Knopf; 1991.
- Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J 2007; 34(2):179–186. pmid:17622365
- Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956; 78(5):213–215. pmid:13358612
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47(9):2169–2180. doi:10.1172/JCI105903
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25):232–235. pmid:5284360
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150(6):396–404. pmid:19293072
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
Aspirin (acetylsalicylic acid) and its progenitors are valuable medications with a history spanning at least 4 millennia. An enormous number of patients take aspirin for a variety of reasons, and managing their therapy around the time of surgery can be challenging, as Drs. Prabhakaran and Whinney discuss in this issue.1 Even after 4,000 years, we are still learning about these remarkable drugs.
LEARNING WHAT SALICYLATES ARE
LEARNING (AND IGNORING) WHAT ASPIRIN CAN DO
In the 1940s, a general practitioner in California named Lawrence Craven recognized that many of his post-tonsillectomy patients had to be hospitalized for bleeding after he started recommending they use aspirin-containing chewing gum for pain relief.4 Under the then-debated hypothesis that myocardial infarction (MI) involves thrombosis, he recommended that adult men should take aspirin daily. He believed that women had lower rates of MI because they were more likely to take aspirin, something that men did not view as a “masculine” thing to do.
In a series of letters in journals such as the Mississippi Valley Medical Journal,5 Craven reported his observations of very low rates of MI and no strokes in aspirin users. Given the nonrigorous nature of his research and the obscure journals in which he published, his findings languished for many years. Ironically, he died of an MI in 1957.
LEARNING HOW ASPIRIN WORKS (AND A FEW OTHER THINGS)
The history of aspirin research illustrates how the fields of hemostasis and inflammation are now linked.
In the late 1960s, Weiss et al6 reported that aspirin rapidly and irreversibly inhibits platelet aggregation. In parallel, using biological assays in work that eventually led to the Nobel Prize, Vane7 discovered that inflammation involves the de novo synthesis of prostaglandins and that aspirin directly inhibits this synthesis. Further work connecting these lines of investigation led us to understand that platelet aggregation is enhanced by the prostaglandin derivative thromboxane A2, produced by cyclooxygenase-1, and that aspirin irreversibly inhibits this enzyme by acetylation.
LEARNING WHEN TO USE ASPIRIN
After decades of research ranging from the Physicians’ Health Study to well-named trials such as ARRIVE, ASCEND, and ASPREE, we now know that taking daily low doses of aspirin for primary prevention can reduce the risk of cardiovascular events and may reduce the risk of colorectal cancer—but at the cost of an increased risk of bleeding.8
Which patients will gain the most benefit and incur the least risk is still debated. What is certain, however, is that aspirin has an important role in acute coronary syndromes, secondary prevention of MI and stroke, and prevention of thrombosis after coronary stent placement. In the perioperative setting, we are learning that aspirin may benefit patients with myocardial injury after noncardiac surgery, a recently described clinical entity associated with surprisingly high mortality rates.9,10
LEARNING WHEN NOT TO USE ASPIRIN
The perioperative period is a dangerous time—surgical stress, hypercoagulability, inflammation, pain, and hemodynamic changes predispose to plaque rupture and supply-demand imbalance. It is therefore logical to hope aspirin would provide protection for at-risk patients in this context.
Unfortunately, results from the second Perioperative Ischemic Evaluation trial have dampened enthusiasm.11 Aspirin has now joined clonidine and beta-blockers on the list of interventions that probably do not reduce perioperative cardiovascular mortality rates. Other than protecting against stent thrombosis, aspirin’s main perioperative effect is to increase bleeding. Consequently, some surgical procedures mandate withdrawal of aspirin.
WHAT WE STILL NEED TO LEARN
Over the years, we have learned the broad outlines of using aspirin to prevent and treat cardiovascular disease, to relieve pain and inflammation (its original purpose), and to prevent stent thrombosis.
However, many details remain to be filled in. We need to better define groups who should and should not take aspirin for primary prevention. We also need to understand aspirin’s role in cancer chemoprevention, to find better ways to mitigate its undesirable effects, and to study its role in treating myocardial injury after noncardiac surgery.
Finally, we need to determine which (if any) patients without coronary stents will benefit from continuing their aspirin perioperatively or even initiating aspirin therapy preoperatively.
Will humanity still be using salicylates 4,000 years from now? Probably not. But what we have learned and will continue to learn from this remarkable group of medications will certainly inform new and better therapies in the years to come.
Aspirin (acetylsalicylic acid) and its progenitors are valuable medications with a history spanning at least 4 millennia. An enormous number of patients take aspirin for a variety of reasons, and managing their therapy around the time of surgery can be challenging, as Drs. Prabhakaran and Whinney discuss in this issue.1 Even after 4,000 years, we are still learning about these remarkable drugs.
LEARNING WHAT SALICYLATES ARE
LEARNING (AND IGNORING) WHAT ASPIRIN CAN DO
In the 1940s, a general practitioner in California named Lawrence Craven recognized that many of his post-tonsillectomy patients had to be hospitalized for bleeding after he started recommending they use aspirin-containing chewing gum for pain relief.4 Under the then-debated hypothesis that myocardial infarction (MI) involves thrombosis, he recommended that adult men should take aspirin daily. He believed that women had lower rates of MI because they were more likely to take aspirin, something that men did not view as a “masculine” thing to do.
In a series of letters in journals such as the Mississippi Valley Medical Journal,5 Craven reported his observations of very low rates of MI and no strokes in aspirin users. Given the nonrigorous nature of his research and the obscure journals in which he published, his findings languished for many years. Ironically, he died of an MI in 1957.
LEARNING HOW ASPIRIN WORKS (AND A FEW OTHER THINGS)
The history of aspirin research illustrates how the fields of hemostasis and inflammation are now linked.
In the late 1960s, Weiss et al6 reported that aspirin rapidly and irreversibly inhibits platelet aggregation. In parallel, using biological assays in work that eventually led to the Nobel Prize, Vane7 discovered that inflammation involves the de novo synthesis of prostaglandins and that aspirin directly inhibits this synthesis. Further work connecting these lines of investigation led us to understand that platelet aggregation is enhanced by the prostaglandin derivative thromboxane A2, produced by cyclooxygenase-1, and that aspirin irreversibly inhibits this enzyme by acetylation.
LEARNING WHEN TO USE ASPIRIN
After decades of research ranging from the Physicians’ Health Study to well-named trials such as ARRIVE, ASCEND, and ASPREE, we now know that taking daily low doses of aspirin for primary prevention can reduce the risk of cardiovascular events and may reduce the risk of colorectal cancer—but at the cost of an increased risk of bleeding.8
Which patients will gain the most benefit and incur the least risk is still debated. What is certain, however, is that aspirin has an important role in acute coronary syndromes, secondary prevention of MI and stroke, and prevention of thrombosis after coronary stent placement. In the perioperative setting, we are learning that aspirin may benefit patients with myocardial injury after noncardiac surgery, a recently described clinical entity associated with surprisingly high mortality rates.9,10
LEARNING WHEN NOT TO USE ASPIRIN
The perioperative period is a dangerous time—surgical stress, hypercoagulability, inflammation, pain, and hemodynamic changes predispose to plaque rupture and supply-demand imbalance. It is therefore logical to hope aspirin would provide protection for at-risk patients in this context.
Unfortunately, results from the second Perioperative Ischemic Evaluation trial have dampened enthusiasm.11 Aspirin has now joined clonidine and beta-blockers on the list of interventions that probably do not reduce perioperative cardiovascular mortality rates. Other than protecting against stent thrombosis, aspirin’s main perioperative effect is to increase bleeding. Consequently, some surgical procedures mandate withdrawal of aspirin.
WHAT WE STILL NEED TO LEARN
Over the years, we have learned the broad outlines of using aspirin to prevent and treat cardiovascular disease, to relieve pain and inflammation (its original purpose), and to prevent stent thrombosis.
However, many details remain to be filled in. We need to better define groups who should and should not take aspirin for primary prevention. We also need to understand aspirin’s role in cancer chemoprevention, to find better ways to mitigate its undesirable effects, and to study its role in treating myocardial injury after noncardiac surgery.
Finally, we need to determine which (if any) patients without coronary stents will benefit from continuing their aspirin perioperatively or even initiating aspirin therapy preoperatively.
Will humanity still be using salicylates 4,000 years from now? Probably not. But what we have learned and will continue to learn from this remarkable group of medications will certainly inform new and better therapies in the years to come.
- Prabhakaran A, Whinney C. Should we stop aspirin before noncardiac surgery? Cleve Clin J Med 2019; 86(8):518–521. doi:10.3949/ccjm.86a.19036
- Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York: Bloomsbury; 2008.
- Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. New York: Alfred A. Knopf; 1991.
- Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J 2007; 34(2):179–186. pmid:17622365
- Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956; 78(5):213–215. pmid:13358612
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47(9):2169–2180. doi:10.1172/JCI105903
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25):232–235. pmid:5284360
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150(6):396–404. pmid:19293072
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Prabhakaran A, Whinney C. Should we stop aspirin before noncardiac surgery? Cleve Clin J Med 2019; 86(8):518–521. doi:10.3949/ccjm.86a.19036
- Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York: Bloomsbury; 2008.
- Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. New York: Alfred A. Knopf; 1991.
- Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J 2007; 34(2):179–186. pmid:17622365
- Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956; 78(5):213–215. pmid:13358612
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47(9):2169–2180. doi:10.1172/JCI105903
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25):232–235. pmid:5284360
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150(6):396–404. pmid:19293072
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
Is it time to taper that opioid? (And how best to do it)
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
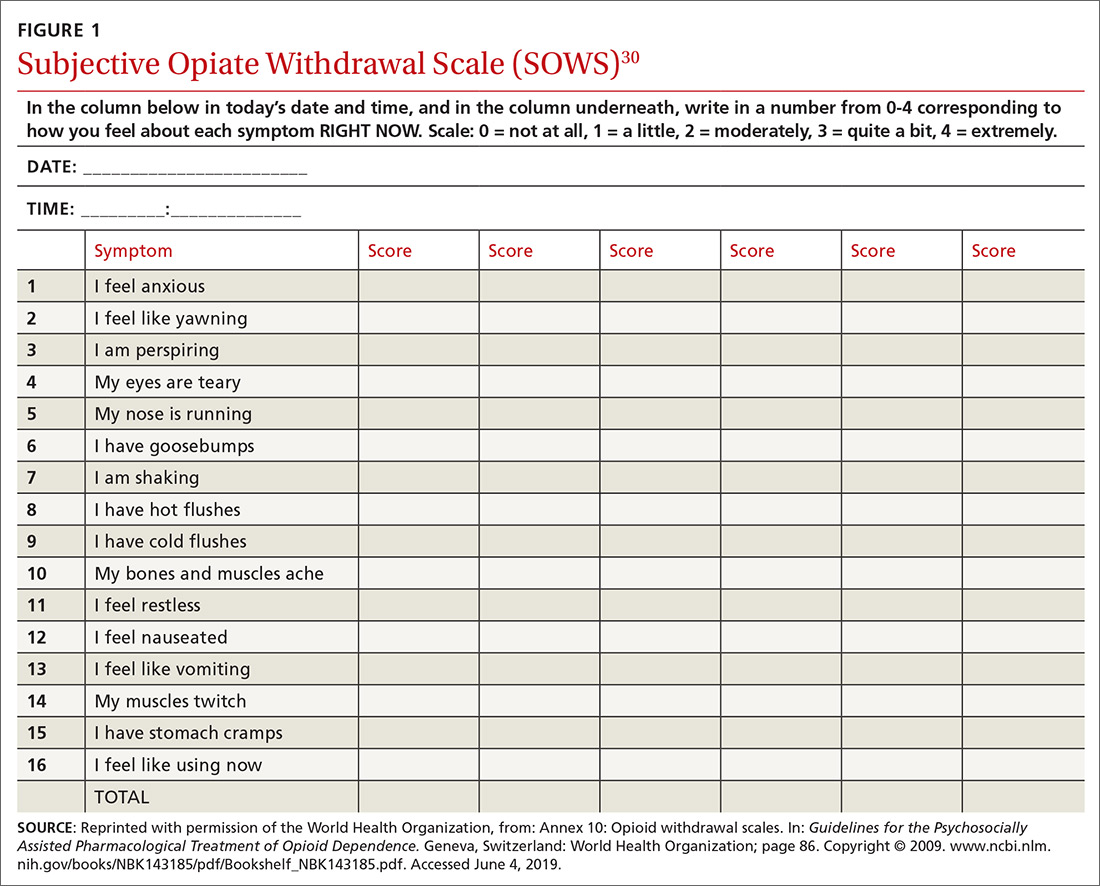
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
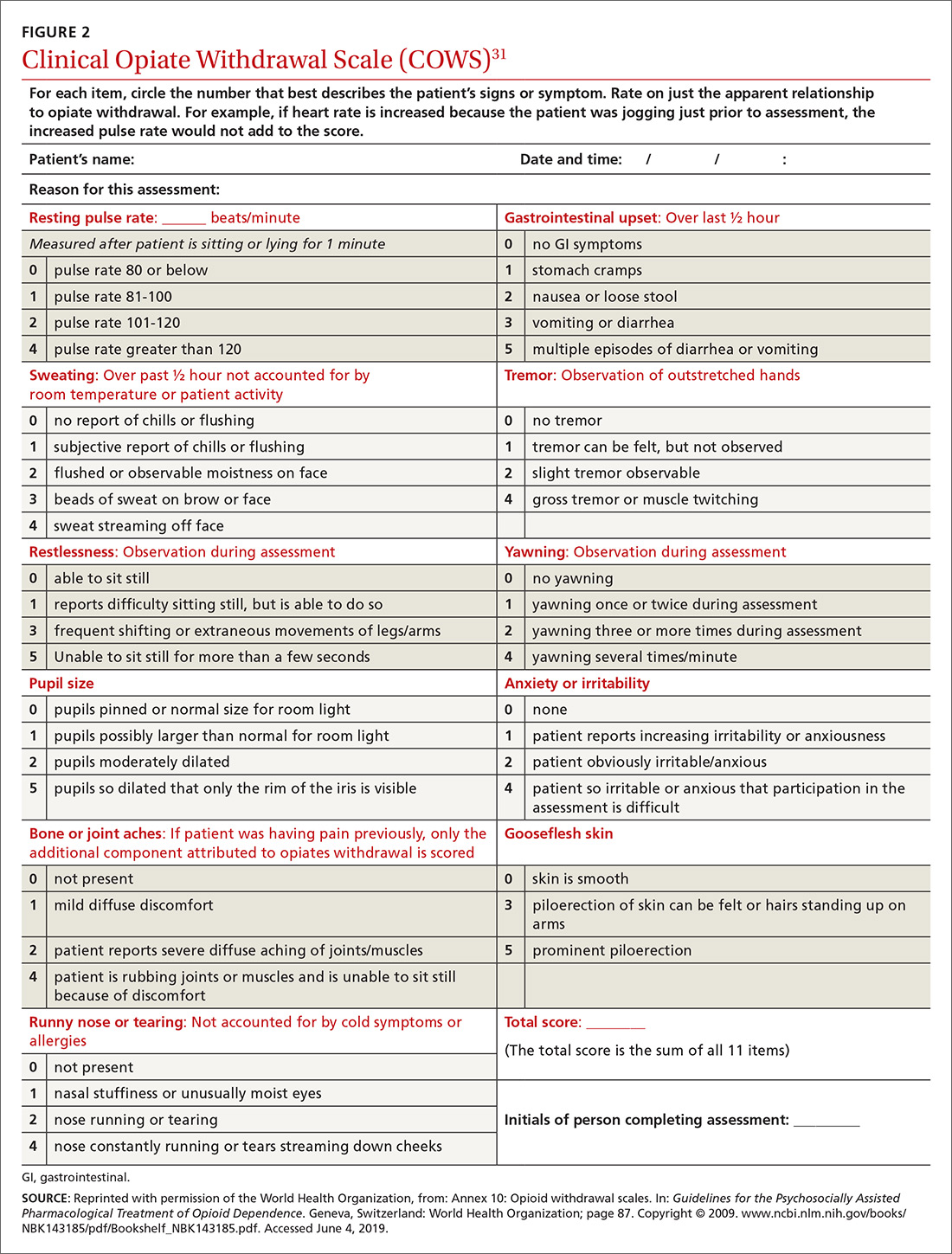
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
PRACTICE RECOMMENDATIONS
› Continue opioid therapy only when it has brought clinically meaningful improvement in pain and function and when the benefits outweigh adverse events or risks. C
› Review the selected opioid tapering plan in detail with the patient and provide close follow-up monitoring of ongoing or emerging risks. C
› Be vigilant: Enacting an opioid-tapering plan can unmask opioid use disorder, which can cause the patient to seek alternative forms of opioids, including illicit, potentially lethal fentanyl analogues. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
HHS proposes pathways for drug importation
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
RNA interference drug fitusiran looks effective in both hemophilia A and B
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
REPORTING FROM 2019 ISTH CONGRESS
Medication overuse prevalent among U.S. migraine patients
PHILADELPHIA – according to findings from an analysis of 16,789 people with migraine.
About 18% of the people identified with migraine in the study cohort reported a drug consumption pattern that met the prespecified definition of “medication overuse,” Todd J. Schwedt, MD, and his associates reported in a poster at the annual meeting of the American Headache Society. Supplying each migraine patient with a “comprehensive treatment plan” along with “improved acute treatment options ... may help reduce the prevalence and associated burden of medication overuse,” said Dr. Schwedt, a professor of neurology at the Mayo Clinic in Phoenix. The analysis also showed that medication overuse (MO) significantly linked with several markers of worse clinical status.
If patients have “an effective preventive treatment that reduces headaches and migraine attacks then they will, in general, use less acute medications. Many people with migraine never even get diagnosed, and patients who qualify for preventive treatment never get it,” Dr. Schwedt noted in an interview. He described a comprehensive treatment plan as a management strategy that includes lifestyle modifications, a migraine-prevention agent, and the availability of an effective acute treatment for a patient to use when a migraine strikes along with clear instructions on how to appropriately self-administer the medication. Only a small fraction of U.S. migraine patients currently receive this complete package of care, he said.
The analysis he ran used data collected in the CaMEO (Chronic Migraine Epidemiology and Outcomes) study, which used an Internet-based survey to collect data from a representative 58,000-person sample of U.S. residents, which included 16,789 who met the applied migraine definition, with 91% having fewer than 15 headaches/month and the remaining 9% with a monthly headache average of 15 or more (Cephalagia. 2015 Jun;35[7]:563-78).
The researchers defined overuse of a single medication as use 15 times or more a month of an NSAID, aspirin, or acetaminophen, or use at least 10 times a month of a triptan, ergotamine, or opioid. They also had a prespecified definition of multidrug overuse that applied similar monthly thresholds. The patients averaged about 41 years old, three-quarters were women, and 85% were white. Patients identified with MO had a substantially higher rate of headaches per month: an average of nearly 12, compared with an average of about 4 per month among those without overuse. Almost two-thirds of the patients with MO reported having been formally diagnosed as having migraine headaches, compared with 41% of those without overuse.
Among the 13,749 patients (82%) on some headache medication, 67% were on a nonopioid analgesic, including 61% on an NSAID. MO among all people on nonopioid analgesics was 16%, and 12% among those who used NSAIDS. The most overused drug in this subgroup were combination analgesics, overused by 18% of those taking these drugs.
The drug class with the biggest MO rate was opioids, used by 12% of those on any medication and overused by 22% of those taking an opioid. Triptans were taken by 11%, with an MO rate of 11% among these users. Ergotamine was used by less than 1% of all patients, and those taking this drug tallied a 19% MO rate.
“Opioids were the class most often overused, more evidence that opioids should rarely if ever be used to treat migraine,” Dr. Schwedt said.
The analysis also showed that patients who had MO has multiple signs of worse clinical status. Patients with MO had a significantly higher rate of diagnosed depression, 54%, compared with 28% in those without MO; anxiety, 49% compared with 26%; migraine-associated disability, 73% compared with 32%; migraine-associated functional impairment (Migraine Interictal Burden Scale), 65% compared with 32%; and emergency department or urgent care use, 13% compared with 3%. All these between-group differences were statistically significant.
CaMEO was funded by Allergan. Dr. Schwedt has been a consultant to Allergan, and also to Alder, Amgen, Cipla, Dr. Reddy’s, Ipsen, Lilly, Novartis, and Teva. He has stock ownership in Aural Analytics, Nocira, and Second Opinion, and he has received research funding from Amgen.
SOURCE: Schwedt TJ et al. Headache. 2019 June;59[S1]:83-4, Abstract P92.
PHILADELPHIA – according to findings from an analysis of 16,789 people with migraine.
About 18% of the people identified with migraine in the study cohort reported a drug consumption pattern that met the prespecified definition of “medication overuse,” Todd J. Schwedt, MD, and his associates reported in a poster at the annual meeting of the American Headache Society. Supplying each migraine patient with a “comprehensive treatment plan” along with “improved acute treatment options ... may help reduce the prevalence and associated burden of medication overuse,” said Dr. Schwedt, a professor of neurology at the Mayo Clinic in Phoenix. The analysis also showed that medication overuse (MO) significantly linked with several markers of worse clinical status.
If patients have “an effective preventive treatment that reduces headaches and migraine attacks then they will, in general, use less acute medications. Many people with migraine never even get diagnosed, and patients who qualify for preventive treatment never get it,” Dr. Schwedt noted in an interview. He described a comprehensive treatment plan as a management strategy that includes lifestyle modifications, a migraine-prevention agent, and the availability of an effective acute treatment for a patient to use when a migraine strikes along with clear instructions on how to appropriately self-administer the medication. Only a small fraction of U.S. migraine patients currently receive this complete package of care, he said.
The analysis he ran used data collected in the CaMEO (Chronic Migraine Epidemiology and Outcomes) study, which used an Internet-based survey to collect data from a representative 58,000-person sample of U.S. residents, which included 16,789 who met the applied migraine definition, with 91% having fewer than 15 headaches/month and the remaining 9% with a monthly headache average of 15 or more (Cephalagia. 2015 Jun;35[7]:563-78).
The researchers defined overuse of a single medication as use 15 times or more a month of an NSAID, aspirin, or acetaminophen, or use at least 10 times a month of a triptan, ergotamine, or opioid. They also had a prespecified definition of multidrug overuse that applied similar monthly thresholds. The patients averaged about 41 years old, three-quarters were women, and 85% were white. Patients identified with MO had a substantially higher rate of headaches per month: an average of nearly 12, compared with an average of about 4 per month among those without overuse. Almost two-thirds of the patients with MO reported having been formally diagnosed as having migraine headaches, compared with 41% of those without overuse.
Among the 13,749 patients (82%) on some headache medication, 67% were on a nonopioid analgesic, including 61% on an NSAID. MO among all people on nonopioid analgesics was 16%, and 12% among those who used NSAIDS. The most overused drug in this subgroup were combination analgesics, overused by 18% of those taking these drugs.
The drug class with the biggest MO rate was opioids, used by 12% of those on any medication and overused by 22% of those taking an opioid. Triptans were taken by 11%, with an MO rate of 11% among these users. Ergotamine was used by less than 1% of all patients, and those taking this drug tallied a 19% MO rate.
“Opioids were the class most often overused, more evidence that opioids should rarely if ever be used to treat migraine,” Dr. Schwedt said.
The analysis also showed that patients who had MO has multiple signs of worse clinical status. Patients with MO had a significantly higher rate of diagnosed depression, 54%, compared with 28% in those without MO; anxiety, 49% compared with 26%; migraine-associated disability, 73% compared with 32%; migraine-associated functional impairment (Migraine Interictal Burden Scale), 65% compared with 32%; and emergency department or urgent care use, 13% compared with 3%. All these between-group differences were statistically significant.
CaMEO was funded by Allergan. Dr. Schwedt has been a consultant to Allergan, and also to Alder, Amgen, Cipla, Dr. Reddy’s, Ipsen, Lilly, Novartis, and Teva. He has stock ownership in Aural Analytics, Nocira, and Second Opinion, and he has received research funding from Amgen.
SOURCE: Schwedt TJ et al. Headache. 2019 June;59[S1]:83-4, Abstract P92.
PHILADELPHIA – according to findings from an analysis of 16,789 people with migraine.
About 18% of the people identified with migraine in the study cohort reported a drug consumption pattern that met the prespecified definition of “medication overuse,” Todd J. Schwedt, MD, and his associates reported in a poster at the annual meeting of the American Headache Society. Supplying each migraine patient with a “comprehensive treatment plan” along with “improved acute treatment options ... may help reduce the prevalence and associated burden of medication overuse,” said Dr. Schwedt, a professor of neurology at the Mayo Clinic in Phoenix. The analysis also showed that medication overuse (MO) significantly linked with several markers of worse clinical status.
If patients have “an effective preventive treatment that reduces headaches and migraine attacks then they will, in general, use less acute medications. Many people with migraine never even get diagnosed, and patients who qualify for preventive treatment never get it,” Dr. Schwedt noted in an interview. He described a comprehensive treatment plan as a management strategy that includes lifestyle modifications, a migraine-prevention agent, and the availability of an effective acute treatment for a patient to use when a migraine strikes along with clear instructions on how to appropriately self-administer the medication. Only a small fraction of U.S. migraine patients currently receive this complete package of care, he said.
The analysis he ran used data collected in the CaMEO (Chronic Migraine Epidemiology and Outcomes) study, which used an Internet-based survey to collect data from a representative 58,000-person sample of U.S. residents, which included 16,789 who met the applied migraine definition, with 91% having fewer than 15 headaches/month and the remaining 9% with a monthly headache average of 15 or more (Cephalagia. 2015 Jun;35[7]:563-78).
The researchers defined overuse of a single medication as use 15 times or more a month of an NSAID, aspirin, or acetaminophen, or use at least 10 times a month of a triptan, ergotamine, or opioid. They also had a prespecified definition of multidrug overuse that applied similar monthly thresholds. The patients averaged about 41 years old, three-quarters were women, and 85% were white. Patients identified with MO had a substantially higher rate of headaches per month: an average of nearly 12, compared with an average of about 4 per month among those without overuse. Almost two-thirds of the patients with MO reported having been formally diagnosed as having migraine headaches, compared with 41% of those without overuse.
Among the 13,749 patients (82%) on some headache medication, 67% were on a nonopioid analgesic, including 61% on an NSAID. MO among all people on nonopioid analgesics was 16%, and 12% among those who used NSAIDS. The most overused drug in this subgroup were combination analgesics, overused by 18% of those taking these drugs.
The drug class with the biggest MO rate was opioids, used by 12% of those on any medication and overused by 22% of those taking an opioid. Triptans were taken by 11%, with an MO rate of 11% among these users. Ergotamine was used by less than 1% of all patients, and those taking this drug tallied a 19% MO rate.
“Opioids were the class most often overused, more evidence that opioids should rarely if ever be used to treat migraine,” Dr. Schwedt said.
The analysis also showed that patients who had MO has multiple signs of worse clinical status. Patients with MO had a significantly higher rate of diagnosed depression, 54%, compared with 28% in those without MO; anxiety, 49% compared with 26%; migraine-associated disability, 73% compared with 32%; migraine-associated functional impairment (Migraine Interictal Burden Scale), 65% compared with 32%; and emergency department or urgent care use, 13% compared with 3%. All these between-group differences were statistically significant.
CaMEO was funded by Allergan. Dr. Schwedt has been a consultant to Allergan, and also to Alder, Amgen, Cipla, Dr. Reddy’s, Ipsen, Lilly, Novartis, and Teva. He has stock ownership in Aural Analytics, Nocira, and Second Opinion, and he has received research funding from Amgen.
SOURCE: Schwedt TJ et al. Headache. 2019 June;59[S1]:83-4, Abstract P92.
REPORTING FROM AHS 2019
Statins crush early seizure risk poststroke
BANGKOK – Statin therapy, even when initiated only upon hospitalization for acute ischemic stroke, was associated with a striking reduction in the risk of early poststroke symptomatic seizure in a large observational study.
Using propensity-score matching to control for potential confounders, use of a statin during acute stroke management was associated with a “robust” 77% reduction in the risk of developing a symptomatic seizure within 7 days after hospital admission, Soichiro Matsubara, MD, reported at the International Epilepsy Congress.
This is an important finding because early symptomatic seizure (ESS) occurs in 2%-7% of patients following an acute ischemic stroke. Moreover, an Italian meta-analysis concluded that ESS was associated with a 4.4-fold increased risk of developing poststroke epilepsy (Epilepsia. 2016 Aug;57[8]:1205-14), noted Dr. Matsubara, a neurologist at the National Cerebral and Cardiovascular Center in Suita, Japan, as well as at Kumamoto (Japan) University.
He presented a study of 2,969 consecutive acute ischemic stroke patients with no history of epilepsy who were admitted to the Japanese comprehensive stroke center, of whom 2.2% experienced ESS. At physician discretion, 19% of the ESS cohort were on a statin during their acute stroke management, as were 55% of the no-ESS group. Four-fifths of patients on a statin initiated the drug only upon hospital admission.
Strokes tended to be more severe in the ESS group, with a median initial National Institutes of Health Stroke Scale score of 12.5, compared with 4 in the seizure-free patients. A cortical stroke lesion was evident upon imaging in 89% of the ESS group and 55% of no-ESS patients. Among ESS patients, 46% had a cardiometabolic stroke, compared with 34% of the no-ESS cohort. Mean C-reactive protein levels and white blood cell counts were significantly higher in the ESS cohort as well. Their median hospital length of stay was 25.5 days, versus 18 days in the no-ESS group, Dr. Matsubara said at the congress sponsored by the International League Against Epilepsy.
Of the 76 ESSs that occurred in 66 patients, 37% were focal awareness seizures, 35% were focal to bilateral tonic-clonic seizures, and 28% were focal impaired awareness seizures.
In a multivariate analysis adjusted for age, sex, body mass index, stroke subtype, and other potential confounders, statin therapy during acute management of stroke was independently associated with a 56% reduction in the relative risk of ESS. In contrast, a cortical stroke lesion was associated with a 2.83-fold increased risk.
Since this wasn’t a randomized trial of statin therapy, Dr. Matsubara and his coinvestigators felt the need to go further in analyzing the data. After extensive propensity score matching for atrial fibrillation, current smoking, systolic blood pressure, the presence or absence of a cortical stroke lesion, large vessel stenosis, and other possible confounders, they were left with two closely comparable groups: 886 statin-treated stroke patients and an equal number who were not on statin therapy during their acute stroke management. The key finding: The risk of ESS was reduced by a whopping 77% in the patients on statin therapy.
The neurologist observed that these new findings in acute ischemic stroke patients are consistent with an earlier study in a U.S. Veterans Affairs population, which demonstrated that statin therapy was associated with a significantly lower risk of new-onset geriatric epilepsy (J Am Geriatr Soc. 2009 Feb;57[2]:237-42).
As to the possible mechanism by which statins may protect against ESS, Dr. Matsubara noted that acute ischemic stroke causes toxic neuronal excitation because of blood-brain barrier disruption, ion channel dysfunction, altered gene expression, and increased release of neurotransmitters. In animal models, statins provide a neuroprotective effect by reducing glutamate levels, activating endothelial nitric oxide synthase, and inhibiting production of interleukin-6, tumor necrosis factor-alpha, and other inflammatory cytokines.
Asked about the intensity of the statin therapy, Dr. Matsubara replied that the target was typically an LDL cholesterol below 100 mg/dL.
He reported having no financial conflicts regarding the study, conducted free of commercial support.
SOURCE: Matsubara S et al. IEC 219, Abstract P002.
BANGKOK – Statin therapy, even when initiated only upon hospitalization for acute ischemic stroke, was associated with a striking reduction in the risk of early poststroke symptomatic seizure in a large observational study.
Using propensity-score matching to control for potential confounders, use of a statin during acute stroke management was associated with a “robust” 77% reduction in the risk of developing a symptomatic seizure within 7 days after hospital admission, Soichiro Matsubara, MD, reported at the International Epilepsy Congress.
This is an important finding because early symptomatic seizure (ESS) occurs in 2%-7% of patients following an acute ischemic stroke. Moreover, an Italian meta-analysis concluded that ESS was associated with a 4.4-fold increased risk of developing poststroke epilepsy (Epilepsia. 2016 Aug;57[8]:1205-14), noted Dr. Matsubara, a neurologist at the National Cerebral and Cardiovascular Center in Suita, Japan, as well as at Kumamoto (Japan) University.
He presented a study of 2,969 consecutive acute ischemic stroke patients with no history of epilepsy who were admitted to the Japanese comprehensive stroke center, of whom 2.2% experienced ESS. At physician discretion, 19% of the ESS cohort were on a statin during their acute stroke management, as were 55% of the no-ESS group. Four-fifths of patients on a statin initiated the drug only upon hospital admission.
Strokes tended to be more severe in the ESS group, with a median initial National Institutes of Health Stroke Scale score of 12.5, compared with 4 in the seizure-free patients. A cortical stroke lesion was evident upon imaging in 89% of the ESS group and 55% of no-ESS patients. Among ESS patients, 46% had a cardiometabolic stroke, compared with 34% of the no-ESS cohort. Mean C-reactive protein levels and white blood cell counts were significantly higher in the ESS cohort as well. Their median hospital length of stay was 25.5 days, versus 18 days in the no-ESS group, Dr. Matsubara said at the congress sponsored by the International League Against Epilepsy.
Of the 76 ESSs that occurred in 66 patients, 37% were focal awareness seizures, 35% were focal to bilateral tonic-clonic seizures, and 28% were focal impaired awareness seizures.
In a multivariate analysis adjusted for age, sex, body mass index, stroke subtype, and other potential confounders, statin therapy during acute management of stroke was independently associated with a 56% reduction in the relative risk of ESS. In contrast, a cortical stroke lesion was associated with a 2.83-fold increased risk.
Since this wasn’t a randomized trial of statin therapy, Dr. Matsubara and his coinvestigators felt the need to go further in analyzing the data. After extensive propensity score matching for atrial fibrillation, current smoking, systolic blood pressure, the presence or absence of a cortical stroke lesion, large vessel stenosis, and other possible confounders, they were left with two closely comparable groups: 886 statin-treated stroke patients and an equal number who were not on statin therapy during their acute stroke management. The key finding: The risk of ESS was reduced by a whopping 77% in the patients on statin therapy.
The neurologist observed that these new findings in acute ischemic stroke patients are consistent with an earlier study in a U.S. Veterans Affairs population, which demonstrated that statin therapy was associated with a significantly lower risk of new-onset geriatric epilepsy (J Am Geriatr Soc. 2009 Feb;57[2]:237-42).
As to the possible mechanism by which statins may protect against ESS, Dr. Matsubara noted that acute ischemic stroke causes toxic neuronal excitation because of blood-brain barrier disruption, ion channel dysfunction, altered gene expression, and increased release of neurotransmitters. In animal models, statins provide a neuroprotective effect by reducing glutamate levels, activating endothelial nitric oxide synthase, and inhibiting production of interleukin-6, tumor necrosis factor-alpha, and other inflammatory cytokines.
Asked about the intensity of the statin therapy, Dr. Matsubara replied that the target was typically an LDL cholesterol below 100 mg/dL.
He reported having no financial conflicts regarding the study, conducted free of commercial support.
SOURCE: Matsubara S et al. IEC 219, Abstract P002.
BANGKOK – Statin therapy, even when initiated only upon hospitalization for acute ischemic stroke, was associated with a striking reduction in the risk of early poststroke symptomatic seizure in a large observational study.
Using propensity-score matching to control for potential confounders, use of a statin during acute stroke management was associated with a “robust” 77% reduction in the risk of developing a symptomatic seizure within 7 days after hospital admission, Soichiro Matsubara, MD, reported at the International Epilepsy Congress.
This is an important finding because early symptomatic seizure (ESS) occurs in 2%-7% of patients following an acute ischemic stroke. Moreover, an Italian meta-analysis concluded that ESS was associated with a 4.4-fold increased risk of developing poststroke epilepsy (Epilepsia. 2016 Aug;57[8]:1205-14), noted Dr. Matsubara, a neurologist at the National Cerebral and Cardiovascular Center in Suita, Japan, as well as at Kumamoto (Japan) University.
He presented a study of 2,969 consecutive acute ischemic stroke patients with no history of epilepsy who were admitted to the Japanese comprehensive stroke center, of whom 2.2% experienced ESS. At physician discretion, 19% of the ESS cohort were on a statin during their acute stroke management, as were 55% of the no-ESS group. Four-fifths of patients on a statin initiated the drug only upon hospital admission.
Strokes tended to be more severe in the ESS group, with a median initial National Institutes of Health Stroke Scale score of 12.5, compared with 4 in the seizure-free patients. A cortical stroke lesion was evident upon imaging in 89% of the ESS group and 55% of no-ESS patients. Among ESS patients, 46% had a cardiometabolic stroke, compared with 34% of the no-ESS cohort. Mean C-reactive protein levels and white blood cell counts were significantly higher in the ESS cohort as well. Their median hospital length of stay was 25.5 days, versus 18 days in the no-ESS group, Dr. Matsubara said at the congress sponsored by the International League Against Epilepsy.
Of the 76 ESSs that occurred in 66 patients, 37% were focal awareness seizures, 35% were focal to bilateral tonic-clonic seizures, and 28% were focal impaired awareness seizures.
In a multivariate analysis adjusted for age, sex, body mass index, stroke subtype, and other potential confounders, statin therapy during acute management of stroke was independently associated with a 56% reduction in the relative risk of ESS. In contrast, a cortical stroke lesion was associated with a 2.83-fold increased risk.
Since this wasn’t a randomized trial of statin therapy, Dr. Matsubara and his coinvestigators felt the need to go further in analyzing the data. After extensive propensity score matching for atrial fibrillation, current smoking, systolic blood pressure, the presence or absence of a cortical stroke lesion, large vessel stenosis, and other possible confounders, they were left with two closely comparable groups: 886 statin-treated stroke patients and an equal number who were not on statin therapy during their acute stroke management. The key finding: The risk of ESS was reduced by a whopping 77% in the patients on statin therapy.
The neurologist observed that these new findings in acute ischemic stroke patients are consistent with an earlier study in a U.S. Veterans Affairs population, which demonstrated that statin therapy was associated with a significantly lower risk of new-onset geriatric epilepsy (J Am Geriatr Soc. 2009 Feb;57[2]:237-42).
As to the possible mechanism by which statins may protect against ESS, Dr. Matsubara noted that acute ischemic stroke causes toxic neuronal excitation because of blood-brain barrier disruption, ion channel dysfunction, altered gene expression, and increased release of neurotransmitters. In animal models, statins provide a neuroprotective effect by reducing glutamate levels, activating endothelial nitric oxide synthase, and inhibiting production of interleukin-6, tumor necrosis factor-alpha, and other inflammatory cytokines.
Asked about the intensity of the statin therapy, Dr. Matsubara replied that the target was typically an LDL cholesterol below 100 mg/dL.
He reported having no financial conflicts regarding the study, conducted free of commercial support.
SOURCE: Matsubara S et al. IEC 219, Abstract P002.
REPORTING FROM IEC 2019
Gum bacteria and Alzheimer’s: A hypothesis inches forward
LOS ANGELES – Scientists testing an unusual hypothesis about the pathogenesis of Alzheimer’s disease say that use of an experimental antimicrobial drug to target a common oral infection was associated with biomarker improvements in people with the disease.
At the Alzheimer’s Association International Conference, Michael Detke, MD, PhD, of Cortexyme in South San Francisco, Calif., presented findings from a small cohort (n = 9) of people with mild to moderate Alzheimer’s disease (AD).
Six patients, mean age 72, were randomized to 4 weeks’ treatment with an agent called COR388, which inhibits toxic proteases produced by Porphyromonas gingivalis, a bacterium that colonizes the mouth and gums and has been found in the brains and cerebrospinal fluid of people with AD more than in non-AD patients. Another three subjects were randomized to placebo.
At 28 days, the CSF levels of an Alzheimer’s-associated apolipoprotein E (ApoE) fragment and serum levels of RANTES, a chemokine associated with chronic inflammation, were reduced from baseline by about one-third in treated subjects, compared with placebo subjects (P less than .05).
“In AD, ApoE is known to be fragmented and the fragments are known to be neurotoxic,” Dr. Detke, the lead study author, said in an interview. “We hypothesized that these P. gingivalis proteases may be acting on ApoE cleavage – so if you bind the proteases you should reduce the fragments. We saw in this study that ApoE fragment was reduced by 30%, or back to about normal levels.”
It is difficult to eradicate P. gingivalis infection using conventional antibiotics. The experimental agent COR388 “does not kill the bacteria but rather neutralizes it by binding to the proteases it produces, making the bacteria benign,” Dr. Detke said.
While hypotheses involving infectious causes of Alzheimer’s remain on the periphery of dementia research, Dr. Detke and his colleagues will soon be able to test theirs in a more meaningful way. At the conference, Dr. Detke presented detailed plans for a phase 2/3, placebo-controlled, clinical trial of COR388 in 570 patients aged 55-80 with mild to moderate AD.
Patients in the study are currently being randomized to 48 weeks of treatment with one of two doses, or placebo, with cognitive and biomarker endpoints planned, including for amyloid-beta, tau, and serum, plasma, and CSF markers of neuroinflammation.
Dr. Detke is an employee of Cortexyme, as are another 8 of the study’s 11 authors.
LOS ANGELES – Scientists testing an unusual hypothesis about the pathogenesis of Alzheimer’s disease say that use of an experimental antimicrobial drug to target a common oral infection was associated with biomarker improvements in people with the disease.
At the Alzheimer’s Association International Conference, Michael Detke, MD, PhD, of Cortexyme in South San Francisco, Calif., presented findings from a small cohort (n = 9) of people with mild to moderate Alzheimer’s disease (AD).
Six patients, mean age 72, were randomized to 4 weeks’ treatment with an agent called COR388, which inhibits toxic proteases produced by Porphyromonas gingivalis, a bacterium that colonizes the mouth and gums and has been found in the brains and cerebrospinal fluid of people with AD more than in non-AD patients. Another three subjects were randomized to placebo.
At 28 days, the CSF levels of an Alzheimer’s-associated apolipoprotein E (ApoE) fragment and serum levels of RANTES, a chemokine associated with chronic inflammation, were reduced from baseline by about one-third in treated subjects, compared with placebo subjects (P less than .05).
“In AD, ApoE is known to be fragmented and the fragments are known to be neurotoxic,” Dr. Detke, the lead study author, said in an interview. “We hypothesized that these P. gingivalis proteases may be acting on ApoE cleavage – so if you bind the proteases you should reduce the fragments. We saw in this study that ApoE fragment was reduced by 30%, or back to about normal levels.”
It is difficult to eradicate P. gingivalis infection using conventional antibiotics. The experimental agent COR388 “does not kill the bacteria but rather neutralizes it by binding to the proteases it produces, making the bacteria benign,” Dr. Detke said.
While hypotheses involving infectious causes of Alzheimer’s remain on the periphery of dementia research, Dr. Detke and his colleagues will soon be able to test theirs in a more meaningful way. At the conference, Dr. Detke presented detailed plans for a phase 2/3, placebo-controlled, clinical trial of COR388 in 570 patients aged 55-80 with mild to moderate AD.
Patients in the study are currently being randomized to 48 weeks of treatment with one of two doses, or placebo, with cognitive and biomarker endpoints planned, including for amyloid-beta, tau, and serum, plasma, and CSF markers of neuroinflammation.
Dr. Detke is an employee of Cortexyme, as are another 8 of the study’s 11 authors.
LOS ANGELES – Scientists testing an unusual hypothesis about the pathogenesis of Alzheimer’s disease say that use of an experimental antimicrobial drug to target a common oral infection was associated with biomarker improvements in people with the disease.
At the Alzheimer’s Association International Conference, Michael Detke, MD, PhD, of Cortexyme in South San Francisco, Calif., presented findings from a small cohort (n = 9) of people with mild to moderate Alzheimer’s disease (AD).
Six patients, mean age 72, were randomized to 4 weeks’ treatment with an agent called COR388, which inhibits toxic proteases produced by Porphyromonas gingivalis, a bacterium that colonizes the mouth and gums and has been found in the brains and cerebrospinal fluid of people with AD more than in non-AD patients. Another three subjects were randomized to placebo.
At 28 days, the CSF levels of an Alzheimer’s-associated apolipoprotein E (ApoE) fragment and serum levels of RANTES, a chemokine associated with chronic inflammation, were reduced from baseline by about one-third in treated subjects, compared with placebo subjects (P less than .05).
“In AD, ApoE is known to be fragmented and the fragments are known to be neurotoxic,” Dr. Detke, the lead study author, said in an interview. “We hypothesized that these P. gingivalis proteases may be acting on ApoE cleavage – so if you bind the proteases you should reduce the fragments. We saw in this study that ApoE fragment was reduced by 30%, or back to about normal levels.”
It is difficult to eradicate P. gingivalis infection using conventional antibiotics. The experimental agent COR388 “does not kill the bacteria but rather neutralizes it by binding to the proteases it produces, making the bacteria benign,” Dr. Detke said.
While hypotheses involving infectious causes of Alzheimer’s remain on the periphery of dementia research, Dr. Detke and his colleagues will soon be able to test theirs in a more meaningful way. At the conference, Dr. Detke presented detailed plans for a phase 2/3, placebo-controlled, clinical trial of COR388 in 570 patients aged 55-80 with mild to moderate AD.
Patients in the study are currently being randomized to 48 weeks of treatment with one of two doses, or placebo, with cognitive and biomarker endpoints planned, including for amyloid-beta, tau, and serum, plasma, and CSF markers of neuroinflammation.
Dr. Detke is an employee of Cortexyme, as are another 8 of the study’s 11 authors.
FROM AAIC 2019