User login
Do patients on biologic drugs for rheumatic disease need PCP prophylaxis?
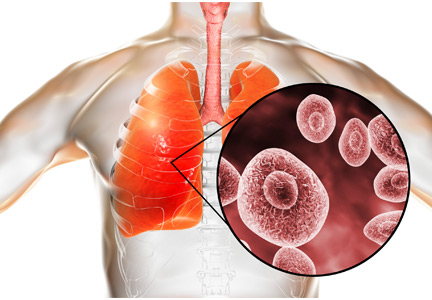
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
Pneumocystis jirovecii (previously carinii) pneumonia (PCP) is rare in patients taking biologic response modifiers for rheumatic disease.1–10 However, prophylaxis should be considered in patients who have granulomatosis with polyangiitis or underlying pulmonary disease, or who are concomitantly receiving glucocorticoids in high doses. There is some risk of adverse reactions to the prophylactic medicine.1,11–21 Until clear guidelines are available, the decision to initiate PCP prophylaxis and the choice of agent should be individualized.
THE BURDEN OF PCP
In a meta-analysis23 of 867 patients who developed PCP and did not have HIV infection, 20.1% had autoimmune or chronic inflammatory disease and the rest were transplant recipients or had malignancies. The mortality rate was 30.6%.
PHARMACOLOGIC RISK FACTORS FOR PCP
Treatment with glucocorticoids
Treatment with glucocorticoids is an important risk factor for PCP, independent of biologic therapy.
Calero-Bernal et al11 reported on 128 patients with non-HIV PCP, of whom 114 (89%) had received a glucocorticoid for more than 4 weeks, and 98 (76%) were currently receiving one. The mean daily dose was equivalent to 27.73 mg of prednisone per day in those on glucocorticoids only, and 21.34 mg in those receiving glucocorticoids in combination with other immunosuppressants.
Park et al,12 in a retrospective study of Korean patients treated for rheumatic disease with high-dose glucocorticoids (≥ 30 mg/day of prednisone or equivalent for more than 4 weeks), reported an incidence rate of PCP of 2.37 per 100 patient-years in those not on prophylaxis.
Other studies13,14 have also found a prednisone dose greater than 15 to 20 mg per day for more than 4 weeks or concomitant use of 2 or more disease-modifying antirheumatic drugs to be a significant risk factor.13,14
Tumor necrosis factor alpha antagonists
A US Food and Drug Administration review1 of voluntary reports of adverse drug events estimated the incidence of PCP to be 2.3 per 100,000 patient-years with infliximab and 1.6 per 100,000 patient-years with etanercept. In most cases, other immunosuppressants were used concomitantly.1
Postmarketing surveillance2 of 5,000 patients with rheumatoid arthritis showed an incidence of suspected PCP of 0.4% within the first 6 months of starting infliximab therapy.
Komano et al,15 in a case-control study of patients with rheumatoid arthritis treated with infliximab, reported that all 21 patients with PCP were also on methotrexate (median dosage 8 mg per week) and prednisolone (median dosage 7.5 mg per day).
PCP has also been reported after adalimumab use in combination with prednisone, azathioprine, and methotrexate, as well as with certolizumab, golimumab, tocilizumab, abatacept, and rituximab.3–6,24–26
Rituximab
Calero-Bernal et al11 reported that 23% of patients with non-HIV PCP who were receiving immunosuppressant drugs were on rituximab.
Alexandre et al16 performed a retrospective review of 11 cases of PCP complicating rituximab therapy for autoimmune disease, in which 10 (91%) of the patients were also on corticosteroids, with a median dosage of 30 mg of prednisone daily. A literature review of an additional 18 cases revealed similar findings.
PATIENT RISK FACTORS FOR PCP
Pulmonary disease, age, other factors
Komano et al,15 in their study of patients with rheumatoid arthritis treated with infliximab, found that 10 (48%) of 21 patients with PCP had preexisting pulmonary disease, compared with 11 (10.8%) of 102 patients without PCP (P < .001). Patients with PCP were older (mean age 64 vs 54, P < .001), were on higher median doses of prednisolone per day (7.5 vs 5 mg, P = .001), and had lower median serum immunoglobulin G (IgG) levels (944 vs 1,394 mg/dL, P < .001).15
Tadros et al13 performed a case-control study that also showed that patients with autoimmune disease who developed PCP had lower lymphocyte counts than controls on admission. Other risk factors included low CD4 counts and age older than 50.
Li et al17 found that patients with autoimmune or inflammatory disease with PCP were more likely to have low CD3, CD4, and CD8 cell counts, as well as albumin levels less than 28 g/L. They therefore suggested that lymphocyte subtyping may be a useful tool to guide PCP prophylaxis.
Granulomatosis with polyangiitis
Patients with granulomatosis with polyangiitis have a significantly higher incidence of PCP than patients with other connective tissue diseases.
Ward and Donald18 reviewed 223 cases of PCP in patients with connective tissue disease. The highest frequency (89 cases per 10,000 hospitalizations per year) was in patients with granulomatosis with polyangiitis, followed by 65 per 10,000 hospitalizations per year for patients with polyarteritis nodosa. The lowest frequency was in rheumatoid arthritis patients, at 2 per 10,000 hospitalizations per year. In decreasing order, diseases with significant associations with PCP were:
- Polyarteritis nodosa (odds ratio [OR] 10.20, 95% confidence interval [CI] 5.69–18.29)
- Granulomatosis with polyangiitis (OR 7.81, 95% CI 4.71–13.05)
- Inflammatory myopathy (OR 4.44, 95% CI 2.67–7.38)
- Systemic lupus erythematosus (OR 2.52, 95% CI 1.66–3.82).
Vallabhaneni and Chiller,26 in a meta-analysis including rheumatoid arthritis patients on biologics, did not find an increased risk of PCP (OR 1.77, 95% CI 0.42–7.47).
Park et al12 found that the highest incidences of PCP were in patients with granulomatosis with polyangiitis, microscopic polyangiitis, and systemic sclerosis. For systemic sclerosis, the main reason for giving high-dose glucocorticoids was interstitial lung disease.
Other studies19,20,28 also found an association with coexisting pulmonary disease in patients with rheumatoid arthritis.
CURRENT GUIDELINES
There are guidelines for primary and secondary prophylaxis of PCP in HIV-positive patients with CD4 counts less than 200/mm3 or a history of acquired immunodeficiency syndrome (AIDS)-defining illness.27 Additionally, patients with a CD4 cell percentage less than 14% should be considered for prophylaxis.27
Unfortunately, there are no guidelines for prophylaxis in patients taking immunosuppressants for rheumatic disease.
The recommended regimen for PCP prophylaxis in HIV-infected patients is trimethoprim-sulfamethoxazole, 1 double-strength or 1 single-strength tablet daily. Alternative regimens include 1 double-strength tablet 3 times per week, dapsone, aerosolized pentamidine, and atovaquone.27
There are also guidelines for prophylaxis in kidney transplant recipients, as well as for patients with hematologic malignancies and solid-organ malignancies, particularly those on chemotherapeutic agents and the T-cell-depleting agent alemtuzumab.29–31
Italian clinical practice guidelines for the use of tumor necrosis factor antagonists in inflammatory bowel disease recommend consideration of PCP prophylaxis in patients who are also on other immunosuppressants, particularly high-dose glucocorticoids.32
Prophylaxis has been shown to increase life expectancy and quality-adjusted life-years and to reduce cost for patients on immunosuppressive therapy for granulomatosis with polyangiitis.21 The European Society of Clinical Microbiology and Infectious Diseases recently produced consensus statements recommending PCP prophylaxis for patients on rituximab with other concomitant immunosuppressants such as the equivalent of prednisone 20 mg daily for more than 4 weeks.33 Prophylaxis was not recommended for other biologic therapies.34,35
THE RISKS OF PROPHYLAXIS
The risk of PCP should be weighed against the risk of prophylaxis in patients with rheumatic disease. Adverse reactions to sulfonamide antibiotics including disease flares have been reported in patients with systemic lupus erythematosus.36,37 Other studies have found no increased risk of flares in patients taking trimethoprim-sulfamethoxazole for PCP prophylaxis.12,38 A retrospective analysis of patients with vasculitis found no increased risk of combining methotrexate and trimethoprim-sulfamethoxazole.39
KEY POINTS
- PCP is an opportunistic infection with a high risk of death.
- PCP has been reported with biologics used as immunomodulators in rheumatic disease.
- PCP prophylaxis should be considered in patients at high risk of PCP, such as those who have granulomatosis with polyangiitis, underlying pulmonary disease or who are concomitantly taking glucocorticoids.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
- US Food and Drug Administration. Safety update on TNF-alpha antagonists: infliximab and etanercept.https://wayback.archive-it.org/7993/20180127041103/https://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2_01_cber_safety_revision2.htm. Accessed May 3, 2019.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67(2):189–194. doi:10.1136/ard.2007.072967
- Koike T, Harigai M, Ishiguro N, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol 2012; 22(4):498–508. doi:10.1007/s10165-011-0541-5
- Bykerk V, Cush J, Winthrop K, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015; 74(1):96–103. doi:10.1136/annrheumdis-2013-203660
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011; 70(12):2148–2151. doi:10.1136/ard.2011.151092
- Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2016; 26(4):491–498. doi:10.3109/14397595.2015.1123211
- Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5):898–906. doi:10.3899/jrheum.080791
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep 2014; 16(10):445. doi:10.1007/s11926-014-0445-4
- US Food and Drug Administration. FDA adverse event reporting system (FAERS) public dashboard. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed May 3, 2019.
- Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57(6):997–1001. doi:10.1093/rheumatology/key023
- Calero-Bernal ML, Martin-Garrido I, Donazar-Ezcurra M, Limper AH, Carmona EM. Intermittent courses of corticosteroids also present a risk for Pneumocystis pneumonia in non-HIV patients. Can Respir J 2016; 2016:2464791. doi:10.1155/2016/2464791
- Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis 2018; 77(5):644–649. doi:10.1136/annrheumdis-2017-211796
- Tadros S, Teichtahl AJ, Ciciriello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum 2017; 46(6):804–809. doi:10.1016/j.semarthrit.2016.09.009
- Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65(2):314–323. doi:10.1002/acr.21857
- Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum 2009; 61(3):305–312. doi:10.1002/art.24283
- Alexandre K, Ingen-Housz-Oro S, Versini M, Sailler L, Benhamou Y. Pneumocystis jirovecii pneumonia in patients treated with rituximab for systemic diseases: report of 11 cases and review of the literature. Eur J Intern Med 2018; 50:e23–e24. doi:10.1016/j.ejim.2017.11.014
- Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis 2017; 57:108–115. doi:10.1016/j.ijid.2017.02.010
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999; 42(4):780–789. doi:10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014; 16(1):R43. doi:10.1186/ar4472
- Tanaka M, Sakai R, Koike R, et al. Pneumocystis jirovecii pneumonia associated with etanercept treatment in patients with rheumatoid arthritis: a retrospective review of 15 cases and analysis of risk factors. Mod Rheumatol 2012; 22(6):849–858. doi:10.1007/s10165-012-0615-z
- Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum 2000; 43(8):1841–1848. doi:10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q
- Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res 2014; 60(2–3):277–288. doi:10.1007/s12026-014-8565-5
- Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8(35):59729–59739. doi:10.18632/oncotarget.19927
- Desales AL, Mendez-Navarro J, Méndez-Tovar LJ, et al. Pneumocystosis in a patient with Crohn's disease treated with combination therapy with adalimumab. J Crohns Colitis 2012; 6(4):483–487. doi:10.1016/j.crohns.2011.10.012
- Kalyoncu U, Karadag O, Akdogan A, et al. Pneumocystis carinii pneumonia in a rheumatoid arthritis patient treated with adalimumab. Scand J Infect Dis 2007; 39(5):475–478. doi:10.1080/00365540601071867
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep 2016; 18(5):29. doi:10.1007/s11926-016-0572-1
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed May 3, 2019.
- Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58(12):1649–1657. doi:10.1093/cid/ciu185
- Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010; 56(2):189–218. doi:10.1053/j.ajkd.2010.04.010
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14(7):882–913. pmid:27407129
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44(12b):1350–1363. doi:10.1111/imj.12599
- Orlando A, Armuzzi A, Papi C, et al; Italian Society of Gastroenterology; Italian Group for the study of Inflammatory Bowel Disease. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43(1):1–20. doi:10.1016/j.dld.2010.07.010
- Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect 2018; 24(suppl 2):S71–S82. doi:10.1016/j.cmi.2018.02.003
- Baddley J, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-alpha agents). Clin Microbiol Infect 2018; 24(suppl 2):S10–S20. doi:10.1016/j.cmi.2017.12.025
- Winthrop K, Mariette X, Silva J, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect 2018; 24(suppl 2):S21–S40. doi:10.1016/j.cmi.2018.02.002
- Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol 1992; 19(2):265–269. pmid:1629825
- Pope J, Jerome D, Fenlon D, Krizova A, Ouimet J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol 2003; 30(3):480–484. pmid:12610805
- Vananuvat P, Suwannalai P, Sungkanuparph S, Limsuwan T, Ngamjanyaporn P, Janwityanujit S. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum 2011; 41(3):497–502. doi:10.1016/j.semarthrit.2011.05.004
- Tamaki H, Butler R, Langford C. Abstract Number: 1755: Safety of methotrexate and low-dose trimethoprim-sulfamethoxazole in patients with ANCA-associated vasculitis. www.acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis. Accessed May 3, 2019.
Polypharmacy: When might it make sense?
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
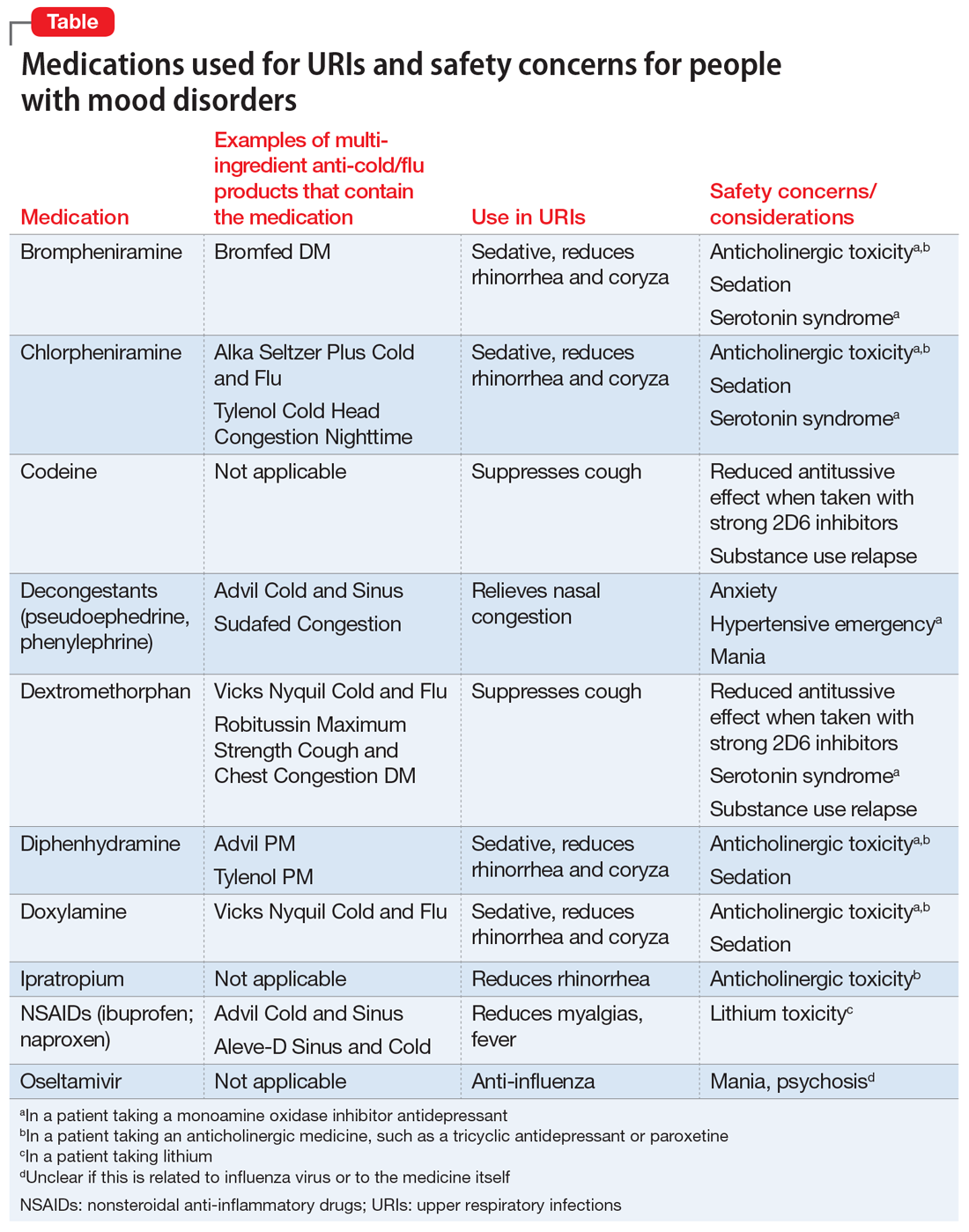
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
What’s new in pediatric sepsis
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ESPID 2019
Anticholinergic drugs linked to dementia in older populations
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
FROM JAMA INTERNAL MEDICINE
EULAR revises its RA management recommendations
MADRID – No change to designating methotrexate the first disease-modifying drug to prescribe, before any biologic drug, and no adoption of imaging criteria to determine whether a patient is in remission.
“Imaging with ultrasound or MRI is out” as a remission criterion. “It’s high risk and a waste of resources,” declared Josef S. Smolen, MD, head of the EULAR writing panel, in the most forceful declaration he made while presenting the pending recommendation revision at the European Congress of Rheumatology.
Dr. Smolen’s strong warning against an imaging parameter when treating RA patients toward a remission target was no surprise, as he had already voiced this opinion in an editorial he coauthored earlier this year (JAMA. 2019 Feb 5;321[5]:457-8). The editorial cited data from three independent studies that compared an RA treatment strategy that used an imaging measure of joint inflammation as a treatment target along with clinical assessment against clinical assessment alone. All three studies found no benefit from ultrasound or MRI for defining a treatment goal, and two of the studies showed evidence for harm. “Using imaging to guide therapy led to prescription of potentially harmful medicines without differences in the primary outcomes, but at high costs and potential burden of unnecessary treatment changes and risks for patients,” noted Dr. Smolen and his coauthor in the editorial.
The report that this editorial addressed (JAMA. 2019 Feb 5;321[5]:461-72) also provided some of the most recent evidence for the second omission from the new revision that Dr. Smolen called out: No change to the recommendation to use methotrexate as initial treatment for any RA patient. “We continue to say that methotrexate is the first treatment strategy. There is no new evidence that any biological treatment is better than methotrexate, so there is no change,” said Dr. Smolen, professor of medicine at the Medical University of Vienna, who also led the EULAR writing panel for the immediately preceding set of RA treatment recommendations first unveiled 3 years before (Ann Rheum Dis. 2017 Jun;76[6]:960-77).
Perhaps the most notable changes to the recommendations are the way they handle targeted-synthetic disease-modifying antirheumatic drugs (tsDMARDs), a class that currently is synonymous with the Janus kinase (JAK) inhibitors. “Because of new evidence we have lifted up the tsDMARDs” so that no preference is given to biologic DMARDs over the ts class as happened in the 2016 version, Dr. Smolen said. Another revision to this recommendation was to change the addition of either a biologic or tsDMARD to a patient not fully responsive to a conventional-synthetic (cs) DMARD and with poor prognostic factors from a “should be considered” to a “should be added” recommendation.
Another way in which the pending revision uplifted tsDMARDs was in the wording for the recommendation that deals with patients who do not respond to a first tumor necrosis factor (TNF) inhibitor plus methotrexate or another csDMARD, and now lists as the first option switching to a biologic or tsDMARD with a different mode of action followed by a different TNF inhibitor, a reversal of order from before when a different TNF inhibitor got first mention. This order change was a modest revision that reflected observational evidence that was modestly persuasive that switching to an agent with a different mechanism of action is often the most effective approach, Dr. Smolen said.
The new recommendations also reaffirmed the eleventh recommendation from the 2016 version, which called for tapering of the biologic or tsDMARD from a patient in remission while retaining the csDMARD, usually methotrexate. Dr. Smolen cited new evidence in favor of this approach (Ann Rheum Dis. 2019 Jun;78[6]:746-53), which allowed the writing panel to upgrade the evidence supporting this recommendation to the A level. The concept of tapering down the biologic or tsDMARD for a patient in sustained remission while maintaining the csDMARD was “fully confirmed” in a recent report, he added. The writing panel also upticked its rating of the evidence in favor of cautiously tapering the csDMARD in patients who maintain remission on just a csDMARD.
One final element in the pending revision called out a newly identified safety signal, an increased risk for venous thromboembolism among patients on certain high dosages of JAK inhibitors, especially in patients with increased risk for venous thromboembolism. This new safety concern adds to the already-described increased risk for herpes zoster from JAK inhibitors, especially in Japanese and Korean populations, Dr. Smolen said. In general, more long-term safety data for JAK inhibitors are needed.
The draft update also added one new overarching principle: “Patients require access to multiple drugs with different modes of action to address the heterogeneity of RA, and patients may require multiple, successive treatments throughout life.” Overall, pending changes to the RA recommendations were limited because “the EULAR recommendations have achieved a steady state of the art” for defining whom to treat, treatment targets, and appropriate treatment strategies, Dr. Smolen said.
Dr. Smolen had been a consultant to or a speaker on behalf of several drug companies.
MADRID – No change to designating methotrexate the first disease-modifying drug to prescribe, before any biologic drug, and no adoption of imaging criteria to determine whether a patient is in remission.
“Imaging with ultrasound or MRI is out” as a remission criterion. “It’s high risk and a waste of resources,” declared Josef S. Smolen, MD, head of the EULAR writing panel, in the most forceful declaration he made while presenting the pending recommendation revision at the European Congress of Rheumatology.
Dr. Smolen’s strong warning against an imaging parameter when treating RA patients toward a remission target was no surprise, as he had already voiced this opinion in an editorial he coauthored earlier this year (JAMA. 2019 Feb 5;321[5]:457-8). The editorial cited data from three independent studies that compared an RA treatment strategy that used an imaging measure of joint inflammation as a treatment target along with clinical assessment against clinical assessment alone. All three studies found no benefit from ultrasound or MRI for defining a treatment goal, and two of the studies showed evidence for harm. “Using imaging to guide therapy led to prescription of potentially harmful medicines without differences in the primary outcomes, but at high costs and potential burden of unnecessary treatment changes and risks for patients,” noted Dr. Smolen and his coauthor in the editorial.
The report that this editorial addressed (JAMA. 2019 Feb 5;321[5]:461-72) also provided some of the most recent evidence for the second omission from the new revision that Dr. Smolen called out: No change to the recommendation to use methotrexate as initial treatment for any RA patient. “We continue to say that methotrexate is the first treatment strategy. There is no new evidence that any biological treatment is better than methotrexate, so there is no change,” said Dr. Smolen, professor of medicine at the Medical University of Vienna, who also led the EULAR writing panel for the immediately preceding set of RA treatment recommendations first unveiled 3 years before (Ann Rheum Dis. 2017 Jun;76[6]:960-77).
Perhaps the most notable changes to the recommendations are the way they handle targeted-synthetic disease-modifying antirheumatic drugs (tsDMARDs), a class that currently is synonymous with the Janus kinase (JAK) inhibitors. “Because of new evidence we have lifted up the tsDMARDs” so that no preference is given to biologic DMARDs over the ts class as happened in the 2016 version, Dr. Smolen said. Another revision to this recommendation was to change the addition of either a biologic or tsDMARD to a patient not fully responsive to a conventional-synthetic (cs) DMARD and with poor prognostic factors from a “should be considered” to a “should be added” recommendation.
Another way in which the pending revision uplifted tsDMARDs was in the wording for the recommendation that deals with patients who do not respond to a first tumor necrosis factor (TNF) inhibitor plus methotrexate or another csDMARD, and now lists as the first option switching to a biologic or tsDMARD with a different mode of action followed by a different TNF inhibitor, a reversal of order from before when a different TNF inhibitor got first mention. This order change was a modest revision that reflected observational evidence that was modestly persuasive that switching to an agent with a different mechanism of action is often the most effective approach, Dr. Smolen said.
The new recommendations also reaffirmed the eleventh recommendation from the 2016 version, which called for tapering of the biologic or tsDMARD from a patient in remission while retaining the csDMARD, usually methotrexate. Dr. Smolen cited new evidence in favor of this approach (Ann Rheum Dis. 2019 Jun;78[6]:746-53), which allowed the writing panel to upgrade the evidence supporting this recommendation to the A level. The concept of tapering down the biologic or tsDMARD for a patient in sustained remission while maintaining the csDMARD was “fully confirmed” in a recent report, he added. The writing panel also upticked its rating of the evidence in favor of cautiously tapering the csDMARD in patients who maintain remission on just a csDMARD.
One final element in the pending revision called out a newly identified safety signal, an increased risk for venous thromboembolism among patients on certain high dosages of JAK inhibitors, especially in patients with increased risk for venous thromboembolism. This new safety concern adds to the already-described increased risk for herpes zoster from JAK inhibitors, especially in Japanese and Korean populations, Dr. Smolen said. In general, more long-term safety data for JAK inhibitors are needed.
The draft update also added one new overarching principle: “Patients require access to multiple drugs with different modes of action to address the heterogeneity of RA, and patients may require multiple, successive treatments throughout life.” Overall, pending changes to the RA recommendations were limited because “the EULAR recommendations have achieved a steady state of the art” for defining whom to treat, treatment targets, and appropriate treatment strategies, Dr. Smolen said.
Dr. Smolen had been a consultant to or a speaker on behalf of several drug companies.
MADRID – No change to designating methotrexate the first disease-modifying drug to prescribe, before any biologic drug, and no adoption of imaging criteria to determine whether a patient is in remission.
“Imaging with ultrasound or MRI is out” as a remission criterion. “It’s high risk and a waste of resources,” declared Josef S. Smolen, MD, head of the EULAR writing panel, in the most forceful declaration he made while presenting the pending recommendation revision at the European Congress of Rheumatology.
Dr. Smolen’s strong warning against an imaging parameter when treating RA patients toward a remission target was no surprise, as he had already voiced this opinion in an editorial he coauthored earlier this year (JAMA. 2019 Feb 5;321[5]:457-8). The editorial cited data from three independent studies that compared an RA treatment strategy that used an imaging measure of joint inflammation as a treatment target along with clinical assessment against clinical assessment alone. All three studies found no benefit from ultrasound or MRI for defining a treatment goal, and two of the studies showed evidence for harm. “Using imaging to guide therapy led to prescription of potentially harmful medicines without differences in the primary outcomes, but at high costs and potential burden of unnecessary treatment changes and risks for patients,” noted Dr. Smolen and his coauthor in the editorial.
The report that this editorial addressed (JAMA. 2019 Feb 5;321[5]:461-72) also provided some of the most recent evidence for the second omission from the new revision that Dr. Smolen called out: No change to the recommendation to use methotrexate as initial treatment for any RA patient. “We continue to say that methotrexate is the first treatment strategy. There is no new evidence that any biological treatment is better than methotrexate, so there is no change,” said Dr. Smolen, professor of medicine at the Medical University of Vienna, who also led the EULAR writing panel for the immediately preceding set of RA treatment recommendations first unveiled 3 years before (Ann Rheum Dis. 2017 Jun;76[6]:960-77).
Perhaps the most notable changes to the recommendations are the way they handle targeted-synthetic disease-modifying antirheumatic drugs (tsDMARDs), a class that currently is synonymous with the Janus kinase (JAK) inhibitors. “Because of new evidence we have lifted up the tsDMARDs” so that no preference is given to biologic DMARDs over the ts class as happened in the 2016 version, Dr. Smolen said. Another revision to this recommendation was to change the addition of either a biologic or tsDMARD to a patient not fully responsive to a conventional-synthetic (cs) DMARD and with poor prognostic factors from a “should be considered” to a “should be added” recommendation.
Another way in which the pending revision uplifted tsDMARDs was in the wording for the recommendation that deals with patients who do not respond to a first tumor necrosis factor (TNF) inhibitor plus methotrexate or another csDMARD, and now lists as the first option switching to a biologic or tsDMARD with a different mode of action followed by a different TNF inhibitor, a reversal of order from before when a different TNF inhibitor got first mention. This order change was a modest revision that reflected observational evidence that was modestly persuasive that switching to an agent with a different mechanism of action is often the most effective approach, Dr. Smolen said.
The new recommendations also reaffirmed the eleventh recommendation from the 2016 version, which called for tapering of the biologic or tsDMARD from a patient in remission while retaining the csDMARD, usually methotrexate. Dr. Smolen cited new evidence in favor of this approach (Ann Rheum Dis. 2019 Jun;78[6]:746-53), which allowed the writing panel to upgrade the evidence supporting this recommendation to the A level. The concept of tapering down the biologic or tsDMARD for a patient in sustained remission while maintaining the csDMARD was “fully confirmed” in a recent report, he added. The writing panel also upticked its rating of the evidence in favor of cautiously tapering the csDMARD in patients who maintain remission on just a csDMARD.
One final element in the pending revision called out a newly identified safety signal, an increased risk for venous thromboembolism among patients on certain high dosages of JAK inhibitors, especially in patients with increased risk for venous thromboembolism. This new safety concern adds to the already-described increased risk for herpes zoster from JAK inhibitors, especially in Japanese and Korean populations, Dr. Smolen said. In general, more long-term safety data for JAK inhibitors are needed.
The draft update also added one new overarching principle: “Patients require access to multiple drugs with different modes of action to address the heterogeneity of RA, and patients may require multiple, successive treatments throughout life.” Overall, pending changes to the RA recommendations were limited because “the EULAR recommendations have achieved a steady state of the art” for defining whom to treat, treatment targets, and appropriate treatment strategies, Dr. Smolen said.
Dr. Smolen had been a consultant to or a speaker on behalf of several drug companies.
EXPERT ANALYSIS FROM EULAR 2019 CONGRESS
FDA approves pembrolizumab for advanced SCLC
The Food and Drug Administration has granted accelerated approval to pembrolizumab for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Approval was based on an overall response rate of 19% among 83 patients with SCLC who had disease progression on or after two or more prior lines of therapy enrolled in two nonrandomized trials, according to the FDA.
SCLC cohorts in KEYNOTE-028 and KEYNOTE-158 received either pembrolizumab 200 mg intravenously every 3 weeks (n = 64) or 10 mg/kg intravenously every 2 weeks (n = 19). Treatment continued until documented disease progression, unacceptable toxicity, or for a maximum of 24 months.
The ORR was 19% (95% confidence interval, 11%-29%), while the complete response rate was 2%. Responses were durable for 6 months or longer in 94% of the 16 responding patients.
Common adverse reactions included fatigue, decreased appetite, cough, nausea, and constipation. The most frequent serious adverse reactions were pneumonia and pleural effusion.
The recommended dosage for SCLC treatment is 200 mg, administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression, the FDA said.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to pembrolizumab for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Approval was based on an overall response rate of 19% among 83 patients with SCLC who had disease progression on or after two or more prior lines of therapy enrolled in two nonrandomized trials, according to the FDA.
SCLC cohorts in KEYNOTE-028 and KEYNOTE-158 received either pembrolizumab 200 mg intravenously every 3 weeks (n = 64) or 10 mg/kg intravenously every 2 weeks (n = 19). Treatment continued until documented disease progression, unacceptable toxicity, or for a maximum of 24 months.
The ORR was 19% (95% confidence interval, 11%-29%), while the complete response rate was 2%. Responses were durable for 6 months or longer in 94% of the 16 responding patients.
Common adverse reactions included fatigue, decreased appetite, cough, nausea, and constipation. The most frequent serious adverse reactions were pneumonia and pleural effusion.
The recommended dosage for SCLC treatment is 200 mg, administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression, the FDA said.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to pembrolizumab for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Approval was based on an overall response rate of 19% among 83 patients with SCLC who had disease progression on or after two or more prior lines of therapy enrolled in two nonrandomized trials, according to the FDA.
SCLC cohorts in KEYNOTE-028 and KEYNOTE-158 received either pembrolizumab 200 mg intravenously every 3 weeks (n = 64) or 10 mg/kg intravenously every 2 weeks (n = 19). Treatment continued until documented disease progression, unacceptable toxicity, or for a maximum of 24 months.
The ORR was 19% (95% confidence interval, 11%-29%), while the complete response rate was 2%. Responses were durable for 6 months or longer in 94% of the 16 responding patients.
Common adverse reactions included fatigue, decreased appetite, cough, nausea, and constipation. The most frequent serious adverse reactions were pneumonia and pleural effusion.
The recommended dosage for SCLC treatment is 200 mg, administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression, the FDA said.
Pembrolizumab is marketed as Keytruda by Merck.
Rapid assay distinguishes viral from bacterial infection
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
REPORTING FROM ESPID 2019
Key clinical point: A novel real-time single-gene–expression PCR test quickly distinguishes viral from bacterial infection in febrile children.
Major finding: The expression signature of the IFI44L gene rapidly distinguished bacterial from viral infection in febrile children with 91% sensitivity and 93% specificity.
Study details: This translational study included 25 febrile children with definite bacterial or viral infections and 10 healthy controls.
Disclosures: The presenter reported having no financial conflicts regarding her study, supported by institutional funding.
Deaths from drugs, alcohol, and suicide increase among millennials
The number of Americans who die each year from alcohol, drugs, or suicide increased to an all-time high in 2017, and the increase was especially pronounced among young adults, according a June 13 report from two public health policy and advocacy organizations. A separate report found that rates of these “deaths of despair” vary widely by state.
The report by Trust for America’s Health and Well Being Trust examined Centers for Disease Control and Prevention (CDC) data with a focus on adults aged 18-34 years. Between 2007 and 2017, alcohol-induced deaths increased by 69%, drug-related deaths by 108%, and suicide by 35% in this age group. These deaths increased in other age groups, too, but often to a lesser extent.
In 1999, there were 7 drug deaths per 100,000 people across age groups, which increased to 22.7 drug deaths per 100,000 people in 2017. Among adults aged 18-34, however, the rate was nearly 31 drug overdose deaths per 100,000 people. Opioid overdoses are largely responsible for the increase in drug-related deaths, and synthetic opioid death rates increased by 6,000% between 1999 and 2017, the report said.
... including the opioid crisis, the skyrocketing costs of education and housing, and entering the job market during the great recession,” according to the report, which was funded with grants from Well Being Trust and the Robert Wood Johnson Foundation.
Screening, treatment, and addressing risk and protective factors are among the measures that the groups recommend to reduce “deaths of despair.”
On June 12, the Commonwealth Fund released a report that examines how drug, alcohol, and suicide death rates across age groups may vary widely by state.
“In Pennsylvania, Maryland, and Ohio, mortality rates from drug overdoses were at least five times higher than rates for alcohol-related deaths and about three times higher than suicide rates,” according to the Commonwealth Fund analysis. “In other states, deaths from suicide and alcohol dominate. In 2017, Montana, Nebraska, the Dakotas, Oregon, and Wyoming saw higher rates of death from suicide and alcohol than from drugs.”
Substance use disorders and suicide might be related, and researchers have suggested that many overdoses may be suicide attempts.
“We assumed that overdoses were accidental ... only to find that many users were actively suicidal, others were playing a version of Russian roulette, and others had passive suicidal ideation,” said Mark S. Gold, MD, adjunct professor of psychiatry at Washington University in St. Louis, in an interview. Opioid use disorders often are treated as “simply opioid deficiency syndromes,” and physicians may miss when patients have physical, sexual, or emotional trauma, anxiety disorders, or major depression, he said.
The number of Americans who die each year from alcohol, drugs, or suicide increased to an all-time high in 2017, and the increase was especially pronounced among young adults, according a June 13 report from two public health policy and advocacy organizations. A separate report found that rates of these “deaths of despair” vary widely by state.
The report by Trust for America’s Health and Well Being Trust examined Centers for Disease Control and Prevention (CDC) data with a focus on adults aged 18-34 years. Between 2007 and 2017, alcohol-induced deaths increased by 69%, drug-related deaths by 108%, and suicide by 35% in this age group. These deaths increased in other age groups, too, but often to a lesser extent.
In 1999, there were 7 drug deaths per 100,000 people across age groups, which increased to 22.7 drug deaths per 100,000 people in 2017. Among adults aged 18-34, however, the rate was nearly 31 drug overdose deaths per 100,000 people. Opioid overdoses are largely responsible for the increase in drug-related deaths, and synthetic opioid death rates increased by 6,000% between 1999 and 2017, the report said.
... including the opioid crisis, the skyrocketing costs of education and housing, and entering the job market during the great recession,” according to the report, which was funded with grants from Well Being Trust and the Robert Wood Johnson Foundation.
Screening, treatment, and addressing risk and protective factors are among the measures that the groups recommend to reduce “deaths of despair.”
On June 12, the Commonwealth Fund released a report that examines how drug, alcohol, and suicide death rates across age groups may vary widely by state.
“In Pennsylvania, Maryland, and Ohio, mortality rates from drug overdoses were at least five times higher than rates for alcohol-related deaths and about three times higher than suicide rates,” according to the Commonwealth Fund analysis. “In other states, deaths from suicide and alcohol dominate. In 2017, Montana, Nebraska, the Dakotas, Oregon, and Wyoming saw higher rates of death from suicide and alcohol than from drugs.”
Substance use disorders and suicide might be related, and researchers have suggested that many overdoses may be suicide attempts.
“We assumed that overdoses were accidental ... only to find that many users were actively suicidal, others were playing a version of Russian roulette, and others had passive suicidal ideation,” said Mark S. Gold, MD, adjunct professor of psychiatry at Washington University in St. Louis, in an interview. Opioid use disorders often are treated as “simply opioid deficiency syndromes,” and physicians may miss when patients have physical, sexual, or emotional trauma, anxiety disorders, or major depression, he said.
The number of Americans who die each year from alcohol, drugs, or suicide increased to an all-time high in 2017, and the increase was especially pronounced among young adults, according a June 13 report from two public health policy and advocacy organizations. A separate report found that rates of these “deaths of despair” vary widely by state.
The report by Trust for America’s Health and Well Being Trust examined Centers for Disease Control and Prevention (CDC) data with a focus on adults aged 18-34 years. Between 2007 and 2017, alcohol-induced deaths increased by 69%, drug-related deaths by 108%, and suicide by 35% in this age group. These deaths increased in other age groups, too, but often to a lesser extent.
In 1999, there were 7 drug deaths per 100,000 people across age groups, which increased to 22.7 drug deaths per 100,000 people in 2017. Among adults aged 18-34, however, the rate was nearly 31 drug overdose deaths per 100,000 people. Opioid overdoses are largely responsible for the increase in drug-related deaths, and synthetic opioid death rates increased by 6,000% between 1999 and 2017, the report said.
... including the opioid crisis, the skyrocketing costs of education and housing, and entering the job market during the great recession,” according to the report, which was funded with grants from Well Being Trust and the Robert Wood Johnson Foundation.
Screening, treatment, and addressing risk and protective factors are among the measures that the groups recommend to reduce “deaths of despair.”
On June 12, the Commonwealth Fund released a report that examines how drug, alcohol, and suicide death rates across age groups may vary widely by state.
“In Pennsylvania, Maryland, and Ohio, mortality rates from drug overdoses were at least five times higher than rates for alcohol-related deaths and about three times higher than suicide rates,” according to the Commonwealth Fund analysis. “In other states, deaths from suicide and alcohol dominate. In 2017, Montana, Nebraska, the Dakotas, Oregon, and Wyoming saw higher rates of death from suicide and alcohol than from drugs.”
Substance use disorders and suicide might be related, and researchers have suggested that many overdoses may be suicide attempts.
“We assumed that overdoses were accidental ... only to find that many users were actively suicidal, others were playing a version of Russian roulette, and others had passive suicidal ideation,” said Mark S. Gold, MD, adjunct professor of psychiatry at Washington University in St. Louis, in an interview. Opioid use disorders often are treated as “simply opioid deficiency syndromes,” and physicians may miss when patients have physical, sexual, or emotional trauma, anxiety disorders, or major depression, he said.
Updated systematic review of aspirin primary prevention shows benefits, risks
Using daily aspirin treatment for the primary prevention of cardiovascular events remains an individualized decision that needs to balance a person’s risks for ischemic events and bleeding, according to results from a new systematic review of 15 randomized, aspirin-prevention trials, including results from 3 major trials that researchers reported during 2018.
“The findings suggest that the decision to use aspirin for primary prevention should be tailored to the individual patients based on estimated atherosclerotic cardiovascular disease risk and perceived bleeding risk, as well as patient preferences regarding the types of event prevented versus potential bleeding caused,” Jawahar L. Mehta, MD, and his associates wrote in an article published on June 10 in the Journal of the American College of Cardiology.
The authors also concluded that if a person decides to use aspirin for primary prevention, then a low dose of 100 mg/day or less is recommended.
This new systematic review follows two reviews published earlier in 2019 that reached roughly similar conclusions after analyzing largely the same randomized trial data, including the same three major trials from 2018. One of these prior reviews included data from 13 trials and a total of 164,225 people (JAMA. 2019 Jan 22;321[3]:277-87). The second review had data from 11 trials with 157,248 people (Eur Heart J. 2019 Feb 14;40[7]:607-17). The newly published review used data collected by 15 trials from 165,502 people.
The three 2018 trials that triggered the updated data assessments were the ARRIVE trial, with 12,546 people randomized (Lancet. 2018 Sep 22;392[10152]:1036-46), the ASPREE trial, with 19,114 people randomized (New Engl J Med. 2018 Oct 18;379[16]:1509-18), and the ASCEND trial, with 15,480 people randomized (New Engl J Med. 2018 Oct 18;379[16]:1529-39).
As stated in the new report from Dr. Mehta, a professor of medicine at the University of Arkansas for Medical Sciences in Little Rock, and his associates, the recent trial results from 2018 added new data from more than 45,000 additional subjects, a development that warranted a reappraisal of the evidence for aspirin’s efficacy and safety for primary prevention in contemporary practice.
The major findings from the analysis by Dr. Mehta and his associates were that in adults without a history of cardiovascular disease, daily aspirin use reduced the incidence of MIs, with a number needed to treat (NNT) of 357; reduced ischemic stroke (NNT, 500), reduced transient ischemic attack (NNT, 370), and reduced the overall, combined rate of all major adverse cardiovascular events (NNT, 263). But on the safety side, daily aspirin led to an increased rate of major bleeding episodes, with a number needed to harm (NNH) of 222, increased intracranial bleeds (NNH, 1,000), and an increase in gastrointestinal bleeds (NNH, 385).
The analysis “demonstrates a potential reduction of net benefit with aspirin in the contemporary era,” the authors concluded. They also noted that the benefits from aspirin prevention were, as expected, “more pronounced” among people with a higher estimated risk from atherosclerotic cardiovascular disease.
The systematic review findings came against the backdrop of a recently released primary prevention guideline from the American College of Cardiology and American Heart Association (J Am Coll Card. 2019 Mar. doi: 10.1016/j.jacc.2019.03.010). The guideline said that aspirin prophylaxis for primary prevention “might be considered” for adults aged 40-70 years, but should not be used for people who are older than 70, and also should not be given to people with an increased risk for bleeding. In general, the experts who produced this guideline said that aspirin prophylaxis should be infrequent.
The new analysis also found no reduction in the incidence of cancer or cancer-related death linked with aspirin use for primary prevention. The systematic review published earlier in 2019 in JAMA also found no link between aspirin use and cancer incidence or mortality. The review from the European Heart Journal did not report on the link between aspirin use and cancer incidence or mortality.
Dr. Mehta has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Medimmune, and Pfizer, and has received grant support from AstraZeneca, Bayer, and Boehringer Ingelheim.
On Twitter @mitchelzoler
SOURCE: Abdelaziz HK et al. J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.501.
The three trials published in 2018 that added important new data on primary prevention for cardiovascular disease with aspirin must ideally be interpreted within the context of the totality of evidence on this subject. This was achieved in the analysis reported by Dr. Mehta and his associates, as well as in other more recent publications.
Making a decision about using aspirin for primary prevention in individuals based on trial data is very challenging because it requires weighing a modest potential benefit that people gain from daily aspirin for preventing a first cardiovascular event against the modest risk of an adverse bleeding event. It does not suffice simply to compare the number of cardiovascular and bleeding events, because those two types of events do not have the same immediate or long-term consequences. Each patient must make a personal choice between the risks and benefits.
The greatest potential benefit from aspirin prophylaxis seems to be in people with increased cardiovascular risk but with no increased bleeding risk. In general, this means people aged 50-59 years old, and also possibly those aged 60-69 years old if their estimated 10-year cardiovascular disease risk is more than 10%. It may make more sense to first focus on other risk-reducing steps, such as smoking cessation, blood pressure control, and statin treatment. After that, prophylactic aspirin may be reasonable for people who retain a 10-year cardiovascular disease risk of more than 10% who are also not at increased bleeding risk. That seems to make it prudent to avoid aspirin for primary prevention once people reach the age of 70 years, although people who have been taking aspirin safely for a period of time before reaching 70 might reasonably consider continuing the prophylaxis for a period of time.
This and similar reviews continue to have major limitations. The duration of the trials they reviewed, a mean of 6.4 years, is insufficient to understand the full effect from aspirin prophylaxis. Also, none of the recent reviews used a patient-level meta-analysis, which could better help us understand aspirin’s action in key subgroups, such as women, patients with diabetes, and patients on treatments such as statins that reduce their cardiovascular risk.
Michael Pignone, MD, is professor and chair of medicine at the University of Texas Dell Medical School in Austin. He had no disclosures. He made these comments in an editorial that accompanied the report (J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.502).
The three trials published in 2018 that added important new data on primary prevention for cardiovascular disease with aspirin must ideally be interpreted within the context of the totality of evidence on this subject. This was achieved in the analysis reported by Dr. Mehta and his associates, as well as in other more recent publications.
Making a decision about using aspirin for primary prevention in individuals based on trial data is very challenging because it requires weighing a modest potential benefit that people gain from daily aspirin for preventing a first cardiovascular event against the modest risk of an adverse bleeding event. It does not suffice simply to compare the number of cardiovascular and bleeding events, because those two types of events do not have the same immediate or long-term consequences. Each patient must make a personal choice between the risks and benefits.
The greatest potential benefit from aspirin prophylaxis seems to be in people with increased cardiovascular risk but with no increased bleeding risk. In general, this means people aged 50-59 years old, and also possibly those aged 60-69 years old if their estimated 10-year cardiovascular disease risk is more than 10%. It may make more sense to first focus on other risk-reducing steps, such as smoking cessation, blood pressure control, and statin treatment. After that, prophylactic aspirin may be reasonable for people who retain a 10-year cardiovascular disease risk of more than 10% who are also not at increased bleeding risk. That seems to make it prudent to avoid aspirin for primary prevention once people reach the age of 70 years, although people who have been taking aspirin safely for a period of time before reaching 70 might reasonably consider continuing the prophylaxis for a period of time.
This and similar reviews continue to have major limitations. The duration of the trials they reviewed, a mean of 6.4 years, is insufficient to understand the full effect from aspirin prophylaxis. Also, none of the recent reviews used a patient-level meta-analysis, which could better help us understand aspirin’s action in key subgroups, such as women, patients with diabetes, and patients on treatments such as statins that reduce their cardiovascular risk.
Michael Pignone, MD, is professor and chair of medicine at the University of Texas Dell Medical School in Austin. He had no disclosures. He made these comments in an editorial that accompanied the report (J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.502).
The three trials published in 2018 that added important new data on primary prevention for cardiovascular disease with aspirin must ideally be interpreted within the context of the totality of evidence on this subject. This was achieved in the analysis reported by Dr. Mehta and his associates, as well as in other more recent publications.
Making a decision about using aspirin for primary prevention in individuals based on trial data is very challenging because it requires weighing a modest potential benefit that people gain from daily aspirin for preventing a first cardiovascular event against the modest risk of an adverse bleeding event. It does not suffice simply to compare the number of cardiovascular and bleeding events, because those two types of events do not have the same immediate or long-term consequences. Each patient must make a personal choice between the risks and benefits.
The greatest potential benefit from aspirin prophylaxis seems to be in people with increased cardiovascular risk but with no increased bleeding risk. In general, this means people aged 50-59 years old, and also possibly those aged 60-69 years old if their estimated 10-year cardiovascular disease risk is more than 10%. It may make more sense to first focus on other risk-reducing steps, such as smoking cessation, blood pressure control, and statin treatment. After that, prophylactic aspirin may be reasonable for people who retain a 10-year cardiovascular disease risk of more than 10% who are also not at increased bleeding risk. That seems to make it prudent to avoid aspirin for primary prevention once people reach the age of 70 years, although people who have been taking aspirin safely for a period of time before reaching 70 might reasonably consider continuing the prophylaxis for a period of time.
This and similar reviews continue to have major limitations. The duration of the trials they reviewed, a mean of 6.4 years, is insufficient to understand the full effect from aspirin prophylaxis. Also, none of the recent reviews used a patient-level meta-analysis, which could better help us understand aspirin’s action in key subgroups, such as women, patients with diabetes, and patients on treatments such as statins that reduce their cardiovascular risk.
Michael Pignone, MD, is professor and chair of medicine at the University of Texas Dell Medical School in Austin. He had no disclosures. He made these comments in an editorial that accompanied the report (J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.502).
Using daily aspirin treatment for the primary prevention of cardiovascular events remains an individualized decision that needs to balance a person’s risks for ischemic events and bleeding, according to results from a new systematic review of 15 randomized, aspirin-prevention trials, including results from 3 major trials that researchers reported during 2018.
“The findings suggest that the decision to use aspirin for primary prevention should be tailored to the individual patients based on estimated atherosclerotic cardiovascular disease risk and perceived bleeding risk, as well as patient preferences regarding the types of event prevented versus potential bleeding caused,” Jawahar L. Mehta, MD, and his associates wrote in an article published on June 10 in the Journal of the American College of Cardiology.
The authors also concluded that if a person decides to use aspirin for primary prevention, then a low dose of 100 mg/day or less is recommended.
This new systematic review follows two reviews published earlier in 2019 that reached roughly similar conclusions after analyzing largely the same randomized trial data, including the same three major trials from 2018. One of these prior reviews included data from 13 trials and a total of 164,225 people (JAMA. 2019 Jan 22;321[3]:277-87). The second review had data from 11 trials with 157,248 people (Eur Heart J. 2019 Feb 14;40[7]:607-17). The newly published review used data collected by 15 trials from 165,502 people.
The three 2018 trials that triggered the updated data assessments were the ARRIVE trial, with 12,546 people randomized (Lancet. 2018 Sep 22;392[10152]:1036-46), the ASPREE trial, with 19,114 people randomized (New Engl J Med. 2018 Oct 18;379[16]:1509-18), and the ASCEND trial, with 15,480 people randomized (New Engl J Med. 2018 Oct 18;379[16]:1529-39).
As stated in the new report from Dr. Mehta, a professor of medicine at the University of Arkansas for Medical Sciences in Little Rock, and his associates, the recent trial results from 2018 added new data from more than 45,000 additional subjects, a development that warranted a reappraisal of the evidence for aspirin’s efficacy and safety for primary prevention in contemporary practice.
The major findings from the analysis by Dr. Mehta and his associates were that in adults without a history of cardiovascular disease, daily aspirin use reduced the incidence of MIs, with a number needed to treat (NNT) of 357; reduced ischemic stroke (NNT, 500), reduced transient ischemic attack (NNT, 370), and reduced the overall, combined rate of all major adverse cardiovascular events (NNT, 263). But on the safety side, daily aspirin led to an increased rate of major bleeding episodes, with a number needed to harm (NNH) of 222, increased intracranial bleeds (NNH, 1,000), and an increase in gastrointestinal bleeds (NNH, 385).
The analysis “demonstrates a potential reduction of net benefit with aspirin in the contemporary era,” the authors concluded. They also noted that the benefits from aspirin prevention were, as expected, “more pronounced” among people with a higher estimated risk from atherosclerotic cardiovascular disease.
The systematic review findings came against the backdrop of a recently released primary prevention guideline from the American College of Cardiology and American Heart Association (J Am Coll Card. 2019 Mar. doi: 10.1016/j.jacc.2019.03.010). The guideline said that aspirin prophylaxis for primary prevention “might be considered” for adults aged 40-70 years, but should not be used for people who are older than 70, and also should not be given to people with an increased risk for bleeding. In general, the experts who produced this guideline said that aspirin prophylaxis should be infrequent.
The new analysis also found no reduction in the incidence of cancer or cancer-related death linked with aspirin use for primary prevention. The systematic review published earlier in 2019 in JAMA also found no link between aspirin use and cancer incidence or mortality. The review from the European Heart Journal did not report on the link between aspirin use and cancer incidence or mortality.
Dr. Mehta has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Medimmune, and Pfizer, and has received grant support from AstraZeneca, Bayer, and Boehringer Ingelheim.
On Twitter @mitchelzoler
SOURCE: Abdelaziz HK et al. J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.501.
Using daily aspirin treatment for the primary prevention of cardiovascular events remains an individualized decision that needs to balance a person’s risks for ischemic events and bleeding, according to results from a new systematic review of 15 randomized, aspirin-prevention trials, including results from 3 major trials that researchers reported during 2018.
“The findings suggest that the decision to use aspirin for primary prevention should be tailored to the individual patients based on estimated atherosclerotic cardiovascular disease risk and perceived bleeding risk, as well as patient preferences regarding the types of event prevented versus potential bleeding caused,” Jawahar L. Mehta, MD, and his associates wrote in an article published on June 10 in the Journal of the American College of Cardiology.
The authors also concluded that if a person decides to use aspirin for primary prevention, then a low dose of 100 mg/day or less is recommended.
This new systematic review follows two reviews published earlier in 2019 that reached roughly similar conclusions after analyzing largely the same randomized trial data, including the same three major trials from 2018. One of these prior reviews included data from 13 trials and a total of 164,225 people (JAMA. 2019 Jan 22;321[3]:277-87). The second review had data from 11 trials with 157,248 people (Eur Heart J. 2019 Feb 14;40[7]:607-17). The newly published review used data collected by 15 trials from 165,502 people.
The three 2018 trials that triggered the updated data assessments were the ARRIVE trial, with 12,546 people randomized (Lancet. 2018 Sep 22;392[10152]:1036-46), the ASPREE trial, with 19,114 people randomized (New Engl J Med. 2018 Oct 18;379[16]:1509-18), and the ASCEND trial, with 15,480 people randomized (New Engl J Med. 2018 Oct 18;379[16]:1529-39).
As stated in the new report from Dr. Mehta, a professor of medicine at the University of Arkansas for Medical Sciences in Little Rock, and his associates, the recent trial results from 2018 added new data from more than 45,000 additional subjects, a development that warranted a reappraisal of the evidence for aspirin’s efficacy and safety for primary prevention in contemporary practice.
The major findings from the analysis by Dr. Mehta and his associates were that in adults without a history of cardiovascular disease, daily aspirin use reduced the incidence of MIs, with a number needed to treat (NNT) of 357; reduced ischemic stroke (NNT, 500), reduced transient ischemic attack (NNT, 370), and reduced the overall, combined rate of all major adverse cardiovascular events (NNT, 263). But on the safety side, daily aspirin led to an increased rate of major bleeding episodes, with a number needed to harm (NNH) of 222, increased intracranial bleeds (NNH, 1,000), and an increase in gastrointestinal bleeds (NNH, 385).
The analysis “demonstrates a potential reduction of net benefit with aspirin in the contemporary era,” the authors concluded. They also noted that the benefits from aspirin prevention were, as expected, “more pronounced” among people with a higher estimated risk from atherosclerotic cardiovascular disease.
The systematic review findings came against the backdrop of a recently released primary prevention guideline from the American College of Cardiology and American Heart Association (J Am Coll Card. 2019 Mar. doi: 10.1016/j.jacc.2019.03.010). The guideline said that aspirin prophylaxis for primary prevention “might be considered” for adults aged 40-70 years, but should not be used for people who are older than 70, and also should not be given to people with an increased risk for bleeding. In general, the experts who produced this guideline said that aspirin prophylaxis should be infrequent.
The new analysis also found no reduction in the incidence of cancer or cancer-related death linked with aspirin use for primary prevention. The systematic review published earlier in 2019 in JAMA also found no link between aspirin use and cancer incidence or mortality. The review from the European Heart Journal did not report on the link between aspirin use and cancer incidence or mortality.
Dr. Mehta has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Medimmune, and Pfizer, and has received grant support from AstraZeneca, Bayer, and Boehringer Ingelheim.
On Twitter @mitchelzoler
SOURCE: Abdelaziz HK et al. J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.501.
FROM JACC
Key clinical point: Cumulative trial results continue to show that aspirin primary prevention cuts CVD events while boosting major bleeds.
Major finding: Aspirin prophylaxis cut cardiovascular events with an NNT of 263, but increased major bleeds with an NNH of 222.
Study details: Systematic review of data from 165,502 people enrolled in 15 randomized trials.
Disclosures: Dr. Mehta has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Medimmune, and Pfizer, and has received grant support from AstraZeneca, Bayer, and Boehringer Ingelheim.
Source: Abdelaziz HK et al. J Am Coll Cardiol. 2019 Jun 10. doi: 10.1016/j.jacc.2019.03.501.