User login
Synthetic drugs pose regulatory, diagnostic challenges
SAN FRANCISCO – Designer drugs, especially synthetic opioids and cannabinoids, are presenting increasing challenges to psychiatrists treating patients with overdoses or psychiatric adverse effects. In 2017, synthetic opioids caused more than 28,000 deaths in the United States, more than any other type. Some of these drugs are technically legal, because their modified chemical structures aren’t covered as legal definitions struggle to keep up with street drug identities.
... people are using drugs and they don’t even know what they’re using,” Vanessa Torres-Llenza, MD, assistant professor of psychiatry at George Washington University, Washington, said in an interview. Dr. Torres-Llenza moderated a session on synthetic opioids at the annual meeting of the American Psychiatric Association.
Of particular concern is the synthetic opioid fentanyl, which has a potency about 50 times that of heroin, and 100 times that of morphine. It is a legal pharmaceutical drug for use in severe pain, but it can be made illicitly, and it is frequently mixed with heroin or cocaine and put into counterfeit pills. The user often is not even aware of its presence. Another derivative, carfentanil, is even more dangerous. Used as a large-animal tranquilizer, and illegal for human use, carfentanil is about 100 times more potent than fentanyl.
These developments may require reconsideration of treatment using the opioid antagonist naloxone and similar drugs. The current guidance for naloxone is a 0.4- to 2-mg dose, followed by repeat dose at 2- to 3-minute intervals as needed. Considering the increasing presence of more potent drugs, “there may not be time to wait,” Dr. Torres-Llenza said.
Another concern is illicit manufacturing: By making even slight modifications to legal drugs, illegal operations can stay a step ahead of regulators because these derivatives are completely legal until legislation is passed to ban them. Estimates peg the number of such new derivatives at about 250 per year.
The recent history of the Food Drug Administration’s regulation of synthetic opioids, presented during the session by Gowri Ramachandran, MD, a resident at George Washington University, illustrates the challenges. The Controlled Substances Act of 1970 assigned every regulated drug into one of five classes based on medical use, and potential for abuse and dependence. Schedule I substances are flagged for a high potential of abuse, having no medical use in the United States, and a lack of accepted safety data for use under medical supervision. Schedule II substances have accepted medical uses.
In 2012, the Synthetic Drug Abuse Prevention Act amended the earlier legislation, declaring that any chemical or related derivative with cannabimimetic properties, as well as some other hallucinogenic molecules and their close relatives, were included as schedule I controlled substances.
The amended legislation also extended the potential length of temporary schedule I status, from 1 year with a 6-month extension, to 2 years with a 1-year extension, to give regulators more time to catch up with both legal and illegal synthetic changes to determine if a drug should be schedule I or II.
A recent example of this problem is bath salts, which are far more powerful, synthetic versions of a stimulant derived from the khat plant that is grown in East Africa and southern Arabia. Bath salts can produce hallucinogenic and euphoric effects similar to methamphetamine and ecstasy, but they are readily available online and in retail stores, labeled as “not for human use” and marketed as “bath salts,” “plant food,” “jewelry cleaner,” or “phone screen cleaner.”
Another concern is synthetic cannabinoids, which resemble the 100 or so cannabinoids found in marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD) being the most well-known examples. These began to appear in recreational use in 2005, representing legal forms of marijuana and sold with names like K2, Spice, and Kronic. They are sold in tobacco shops, again labeled “not for human consumption,” trumpeted instead as a “harmless incense blend” or “natural herbs.” Manufacture and content of these derivatives are completely unregulated, according to Dr. Ramachandran.
Like other drug classes, synthetic cannabinoids – many related to THC – have been structurally altered in recent years, posing challenges to regulation and even detection. This is especially concerning because a synthetic cannabinoid product could contain a potpourri of other drugs such as opioids or herbs, leading to unpredictable effects. It’s also nearly impossible to identify everything in a patient’s system, Dr. Torrez-Llenza said.
That makes diagnosis challenging given that synthetic cannabinoids can cause a wide range of symptoms, commonly violence, agitation, panic attacks, hallucinations, hyperglycemia, hyperkalemia, and tachycardia.
Synthetic cannabinoids usually do not contain CBD, which has some antipsychotic and anxiolytic effects. Instead they are generally derived from THC, which is associated with psychosis, and they are 40-660 times more potent than natural THC. This suggests that synthetic versions may pose a greater psychosis risk than natural cannabis. However, only case reports have examined the existence of an association between synthetic cannabinoids and psychosis, and it is difficult to distinguish a toxic syndrome from exacerbation of a previous prodromal syndrome, or new-onset illness.
Acute reactions can occur within minutes of use and last 2-5 hours or more. But this is all very unpredictable as it depends on the specific mixture used.
In the emergency department, agitation, aggression, and impulsive behaviors may signal exposure to synthetic cannabinoids. Most patients can be treated in the ED with antipsychotics or benzodiazepines to manage symptoms. There could be regional toxidromes that arise from local distribution of specific synthetic cannabinoid combinations.
While testing for synthetic cannabinoids remains challenging, Quest Diagnostics has a urine-based panel that includes them, and the company says it is working with information from the National Forensic Laboratory Information System, the Drug Enforcement Agency, industry sources, and the scientific literature to periodically update its standard panel.
Dr. Torres-Llenza had no relevant financial disclosures.
SAN FRANCISCO – Designer drugs, especially synthetic opioids and cannabinoids, are presenting increasing challenges to psychiatrists treating patients with overdoses or psychiatric adverse effects. In 2017, synthetic opioids caused more than 28,000 deaths in the United States, more than any other type. Some of these drugs are technically legal, because their modified chemical structures aren’t covered as legal definitions struggle to keep up with street drug identities.
... people are using drugs and they don’t even know what they’re using,” Vanessa Torres-Llenza, MD, assistant professor of psychiatry at George Washington University, Washington, said in an interview. Dr. Torres-Llenza moderated a session on synthetic opioids at the annual meeting of the American Psychiatric Association.
Of particular concern is the synthetic opioid fentanyl, which has a potency about 50 times that of heroin, and 100 times that of morphine. It is a legal pharmaceutical drug for use in severe pain, but it can be made illicitly, and it is frequently mixed with heroin or cocaine and put into counterfeit pills. The user often is not even aware of its presence. Another derivative, carfentanil, is even more dangerous. Used as a large-animal tranquilizer, and illegal for human use, carfentanil is about 100 times more potent than fentanyl.
These developments may require reconsideration of treatment using the opioid antagonist naloxone and similar drugs. The current guidance for naloxone is a 0.4- to 2-mg dose, followed by repeat dose at 2- to 3-minute intervals as needed. Considering the increasing presence of more potent drugs, “there may not be time to wait,” Dr. Torres-Llenza said.
Another concern is illicit manufacturing: By making even slight modifications to legal drugs, illegal operations can stay a step ahead of regulators because these derivatives are completely legal until legislation is passed to ban them. Estimates peg the number of such new derivatives at about 250 per year.
The recent history of the Food Drug Administration’s regulation of synthetic opioids, presented during the session by Gowri Ramachandran, MD, a resident at George Washington University, illustrates the challenges. The Controlled Substances Act of 1970 assigned every regulated drug into one of five classes based on medical use, and potential for abuse and dependence. Schedule I substances are flagged for a high potential of abuse, having no medical use in the United States, and a lack of accepted safety data for use under medical supervision. Schedule II substances have accepted medical uses.
In 2012, the Synthetic Drug Abuse Prevention Act amended the earlier legislation, declaring that any chemical or related derivative with cannabimimetic properties, as well as some other hallucinogenic molecules and their close relatives, were included as schedule I controlled substances.
The amended legislation also extended the potential length of temporary schedule I status, from 1 year with a 6-month extension, to 2 years with a 1-year extension, to give regulators more time to catch up with both legal and illegal synthetic changes to determine if a drug should be schedule I or II.
A recent example of this problem is bath salts, which are far more powerful, synthetic versions of a stimulant derived from the khat plant that is grown in East Africa and southern Arabia. Bath salts can produce hallucinogenic and euphoric effects similar to methamphetamine and ecstasy, but they are readily available online and in retail stores, labeled as “not for human use” and marketed as “bath salts,” “plant food,” “jewelry cleaner,” or “phone screen cleaner.”
Another concern is synthetic cannabinoids, which resemble the 100 or so cannabinoids found in marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD) being the most well-known examples. These began to appear in recreational use in 2005, representing legal forms of marijuana and sold with names like K2, Spice, and Kronic. They are sold in tobacco shops, again labeled “not for human consumption,” trumpeted instead as a “harmless incense blend” or “natural herbs.” Manufacture and content of these derivatives are completely unregulated, according to Dr. Ramachandran.
Like other drug classes, synthetic cannabinoids – many related to THC – have been structurally altered in recent years, posing challenges to regulation and even detection. This is especially concerning because a synthetic cannabinoid product could contain a potpourri of other drugs such as opioids or herbs, leading to unpredictable effects. It’s also nearly impossible to identify everything in a patient’s system, Dr. Torrez-Llenza said.
That makes diagnosis challenging given that synthetic cannabinoids can cause a wide range of symptoms, commonly violence, agitation, panic attacks, hallucinations, hyperglycemia, hyperkalemia, and tachycardia.
Synthetic cannabinoids usually do not contain CBD, which has some antipsychotic and anxiolytic effects. Instead they are generally derived from THC, which is associated with psychosis, and they are 40-660 times more potent than natural THC. This suggests that synthetic versions may pose a greater psychosis risk than natural cannabis. However, only case reports have examined the existence of an association between synthetic cannabinoids and psychosis, and it is difficult to distinguish a toxic syndrome from exacerbation of a previous prodromal syndrome, or new-onset illness.
Acute reactions can occur within minutes of use and last 2-5 hours or more. But this is all very unpredictable as it depends on the specific mixture used.
In the emergency department, agitation, aggression, and impulsive behaviors may signal exposure to synthetic cannabinoids. Most patients can be treated in the ED with antipsychotics or benzodiazepines to manage symptoms. There could be regional toxidromes that arise from local distribution of specific synthetic cannabinoid combinations.
While testing for synthetic cannabinoids remains challenging, Quest Diagnostics has a urine-based panel that includes them, and the company says it is working with information from the National Forensic Laboratory Information System, the Drug Enforcement Agency, industry sources, and the scientific literature to periodically update its standard panel.
Dr. Torres-Llenza had no relevant financial disclosures.
SAN FRANCISCO – Designer drugs, especially synthetic opioids and cannabinoids, are presenting increasing challenges to psychiatrists treating patients with overdoses or psychiatric adverse effects. In 2017, synthetic opioids caused more than 28,000 deaths in the United States, more than any other type. Some of these drugs are technically legal, because their modified chemical structures aren’t covered as legal definitions struggle to keep up with street drug identities.
... people are using drugs and they don’t even know what they’re using,” Vanessa Torres-Llenza, MD, assistant professor of psychiatry at George Washington University, Washington, said in an interview. Dr. Torres-Llenza moderated a session on synthetic opioids at the annual meeting of the American Psychiatric Association.
Of particular concern is the synthetic opioid fentanyl, which has a potency about 50 times that of heroin, and 100 times that of morphine. It is a legal pharmaceutical drug for use in severe pain, but it can be made illicitly, and it is frequently mixed with heroin or cocaine and put into counterfeit pills. The user often is not even aware of its presence. Another derivative, carfentanil, is even more dangerous. Used as a large-animal tranquilizer, and illegal for human use, carfentanil is about 100 times more potent than fentanyl.
These developments may require reconsideration of treatment using the opioid antagonist naloxone and similar drugs. The current guidance for naloxone is a 0.4- to 2-mg dose, followed by repeat dose at 2- to 3-minute intervals as needed. Considering the increasing presence of more potent drugs, “there may not be time to wait,” Dr. Torres-Llenza said.
Another concern is illicit manufacturing: By making even slight modifications to legal drugs, illegal operations can stay a step ahead of regulators because these derivatives are completely legal until legislation is passed to ban them. Estimates peg the number of such new derivatives at about 250 per year.
The recent history of the Food Drug Administration’s regulation of synthetic opioids, presented during the session by Gowri Ramachandran, MD, a resident at George Washington University, illustrates the challenges. The Controlled Substances Act of 1970 assigned every regulated drug into one of five classes based on medical use, and potential for abuse and dependence. Schedule I substances are flagged for a high potential of abuse, having no medical use in the United States, and a lack of accepted safety data for use under medical supervision. Schedule II substances have accepted medical uses.
In 2012, the Synthetic Drug Abuse Prevention Act amended the earlier legislation, declaring that any chemical or related derivative with cannabimimetic properties, as well as some other hallucinogenic molecules and their close relatives, were included as schedule I controlled substances.
The amended legislation also extended the potential length of temporary schedule I status, from 1 year with a 6-month extension, to 2 years with a 1-year extension, to give regulators more time to catch up with both legal and illegal synthetic changes to determine if a drug should be schedule I or II.
A recent example of this problem is bath salts, which are far more powerful, synthetic versions of a stimulant derived from the khat plant that is grown in East Africa and southern Arabia. Bath salts can produce hallucinogenic and euphoric effects similar to methamphetamine and ecstasy, but they are readily available online and in retail stores, labeled as “not for human use” and marketed as “bath salts,” “plant food,” “jewelry cleaner,” or “phone screen cleaner.”
Another concern is synthetic cannabinoids, which resemble the 100 or so cannabinoids found in marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD) being the most well-known examples. These began to appear in recreational use in 2005, representing legal forms of marijuana and sold with names like K2, Spice, and Kronic. They are sold in tobacco shops, again labeled “not for human consumption,” trumpeted instead as a “harmless incense blend” or “natural herbs.” Manufacture and content of these derivatives are completely unregulated, according to Dr. Ramachandran.
Like other drug classes, synthetic cannabinoids – many related to THC – have been structurally altered in recent years, posing challenges to regulation and even detection. This is especially concerning because a synthetic cannabinoid product could contain a potpourri of other drugs such as opioids or herbs, leading to unpredictable effects. It’s also nearly impossible to identify everything in a patient’s system, Dr. Torrez-Llenza said.
That makes diagnosis challenging given that synthetic cannabinoids can cause a wide range of symptoms, commonly violence, agitation, panic attacks, hallucinations, hyperglycemia, hyperkalemia, and tachycardia.
Synthetic cannabinoids usually do not contain CBD, which has some antipsychotic and anxiolytic effects. Instead they are generally derived from THC, which is associated with psychosis, and they are 40-660 times more potent than natural THC. This suggests that synthetic versions may pose a greater psychosis risk than natural cannabis. However, only case reports have examined the existence of an association between synthetic cannabinoids and psychosis, and it is difficult to distinguish a toxic syndrome from exacerbation of a previous prodromal syndrome, or new-onset illness.
Acute reactions can occur within minutes of use and last 2-5 hours or more. But this is all very unpredictable as it depends on the specific mixture used.
In the emergency department, agitation, aggression, and impulsive behaviors may signal exposure to synthetic cannabinoids. Most patients can be treated in the ED with antipsychotics or benzodiazepines to manage symptoms. There could be regional toxidromes that arise from local distribution of specific synthetic cannabinoid combinations.
While testing for synthetic cannabinoids remains challenging, Quest Diagnostics has a urine-based panel that includes them, and the company says it is working with information from the National Forensic Laboratory Information System, the Drug Enforcement Agency, industry sources, and the scientific literature to periodically update its standard panel.
Dr. Torres-Llenza had no relevant financial disclosures.
REPORTING FROM APA 2019
General neurologists lag on prescribing high-efficacy MS drugs
SEATTLE – It is not clear if the greater reluctance among general neurologists to prescribe the drugs is hurting the health of patients, and the study does not examine whether general neurologists are referring their toughest patients to their subspecialist colleagues.
Still, the findings raise questions because “starting highly effective drugs early can prevent long-term disability,” said study lead author and neurologist Casey V. Farin, MD, a clinical fellow in the department of neurology at Duke University, Durham, N.C., who spoke in an interview prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers. “A lot of our general neurologists are prescribing the traditional platform therapies that have fallen a bit out of favor in the MS community,” she said.
Dr. Farin and colleagues launched their study to better understand whether “therapeutic inertia” is affecting how general neurologists treat MS. The term refers to “staying with one drug just because it is easier not to rock the boat,” she said. For the purposes of their study, the term encompasses reluctance of neurologists to escalate therapy or prescribe high-efficacy drugs.
“There have been small studies comparing subspecialists and general neurologists using surveys of theoretical cases,” she said. “No studies have looked at how people are prescribing disease-modifying therapy.”
In the new age of high-efficacy treatment, guidelines about early MS treatment are lacking. As the study abstract notes, “in the absence of robust head-to-head clinical data, neurologists do not have an accepted algorithm for initiation and escalation of therapy, although recent research indicates a benefit in initiating highly effective therapies early in the disease course.”
For the study, researchers tracked 4,753 patients with MS who were treated at the Duke University Health System from 2016 to 2018.
General neurologists prescribed platform therapies – interferons, glatiramer acetate (Copaxone) and dimethyl fumarate (Tecfidera) more often than did MS subspecialists (16% vs. 5%, P less than .0001, 12% vs. 6%, P = .001 and 31% vs. 11%, P less than .0001, respectively).
In regard to high-efficacy MS drugs, there was no significant difference in prescription rates of fingolimod (Gilenya) and natalizumab (Tysabri). But general neurologists were less likely to prescribe three other types than were general neurologists: Alemtuzumab (Lemtrada), ocrelizumab (Ocrevus) and rituximab (Rituxan) (0 vs. 8%, P = .0001, 3% vs. 27%, P less than .0001, and 2% vs. 7%, P = .0001, respectively).
Why might general neurologists be more resistant to embrace high-efficacy MS drugs? “They are newer and seen as more aggressive, and riskier,” Dr. Farin said. If general neurologists are not seeing many patients with MS and not prescribing these newer drugs very often, they may be more familiar with the older platform drugs, she said. “They may start with the ones that seem safer and are easier to start with.”
It is possible, she cautioned, that the study results may be confounded by general neurologists who refer patients to MS subspecialists when initial disease-modifying therapies fail.
No study funding was reported. Dr. Farin and two of the other four authors disclosed consulting fees from Biogen. No other disclosures were reported.
SOURCE: Farin CV et al. CMSC 2019. Abstract DXT44.
SEATTLE – It is not clear if the greater reluctance among general neurologists to prescribe the drugs is hurting the health of patients, and the study does not examine whether general neurologists are referring their toughest patients to their subspecialist colleagues.
Still, the findings raise questions because “starting highly effective drugs early can prevent long-term disability,” said study lead author and neurologist Casey V. Farin, MD, a clinical fellow in the department of neurology at Duke University, Durham, N.C., who spoke in an interview prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers. “A lot of our general neurologists are prescribing the traditional platform therapies that have fallen a bit out of favor in the MS community,” she said.
Dr. Farin and colleagues launched their study to better understand whether “therapeutic inertia” is affecting how general neurologists treat MS. The term refers to “staying with one drug just because it is easier not to rock the boat,” she said. For the purposes of their study, the term encompasses reluctance of neurologists to escalate therapy or prescribe high-efficacy drugs.
“There have been small studies comparing subspecialists and general neurologists using surveys of theoretical cases,” she said. “No studies have looked at how people are prescribing disease-modifying therapy.”
In the new age of high-efficacy treatment, guidelines about early MS treatment are lacking. As the study abstract notes, “in the absence of robust head-to-head clinical data, neurologists do not have an accepted algorithm for initiation and escalation of therapy, although recent research indicates a benefit in initiating highly effective therapies early in the disease course.”
For the study, researchers tracked 4,753 patients with MS who were treated at the Duke University Health System from 2016 to 2018.
General neurologists prescribed platform therapies – interferons, glatiramer acetate (Copaxone) and dimethyl fumarate (Tecfidera) more often than did MS subspecialists (16% vs. 5%, P less than .0001, 12% vs. 6%, P = .001 and 31% vs. 11%, P less than .0001, respectively).
In regard to high-efficacy MS drugs, there was no significant difference in prescription rates of fingolimod (Gilenya) and natalizumab (Tysabri). But general neurologists were less likely to prescribe three other types than were general neurologists: Alemtuzumab (Lemtrada), ocrelizumab (Ocrevus) and rituximab (Rituxan) (0 vs. 8%, P = .0001, 3% vs. 27%, P less than .0001, and 2% vs. 7%, P = .0001, respectively).
Why might general neurologists be more resistant to embrace high-efficacy MS drugs? “They are newer and seen as more aggressive, and riskier,” Dr. Farin said. If general neurologists are not seeing many patients with MS and not prescribing these newer drugs very often, they may be more familiar with the older platform drugs, she said. “They may start with the ones that seem safer and are easier to start with.”
It is possible, she cautioned, that the study results may be confounded by general neurologists who refer patients to MS subspecialists when initial disease-modifying therapies fail.
No study funding was reported. Dr. Farin and two of the other four authors disclosed consulting fees from Biogen. No other disclosures were reported.
SOURCE: Farin CV et al. CMSC 2019. Abstract DXT44.
SEATTLE – It is not clear if the greater reluctance among general neurologists to prescribe the drugs is hurting the health of patients, and the study does not examine whether general neurologists are referring their toughest patients to their subspecialist colleagues.
Still, the findings raise questions because “starting highly effective drugs early can prevent long-term disability,” said study lead author and neurologist Casey V. Farin, MD, a clinical fellow in the department of neurology at Duke University, Durham, N.C., who spoke in an interview prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers. “A lot of our general neurologists are prescribing the traditional platform therapies that have fallen a bit out of favor in the MS community,” she said.
Dr. Farin and colleagues launched their study to better understand whether “therapeutic inertia” is affecting how general neurologists treat MS. The term refers to “staying with one drug just because it is easier not to rock the boat,” she said. For the purposes of their study, the term encompasses reluctance of neurologists to escalate therapy or prescribe high-efficacy drugs.
“There have been small studies comparing subspecialists and general neurologists using surveys of theoretical cases,” she said. “No studies have looked at how people are prescribing disease-modifying therapy.”
In the new age of high-efficacy treatment, guidelines about early MS treatment are lacking. As the study abstract notes, “in the absence of robust head-to-head clinical data, neurologists do not have an accepted algorithm for initiation and escalation of therapy, although recent research indicates a benefit in initiating highly effective therapies early in the disease course.”
For the study, researchers tracked 4,753 patients with MS who were treated at the Duke University Health System from 2016 to 2018.
General neurologists prescribed platform therapies – interferons, glatiramer acetate (Copaxone) and dimethyl fumarate (Tecfidera) more often than did MS subspecialists (16% vs. 5%, P less than .0001, 12% vs. 6%, P = .001 and 31% vs. 11%, P less than .0001, respectively).
In regard to high-efficacy MS drugs, there was no significant difference in prescription rates of fingolimod (Gilenya) and natalizumab (Tysabri). But general neurologists were less likely to prescribe three other types than were general neurologists: Alemtuzumab (Lemtrada), ocrelizumab (Ocrevus) and rituximab (Rituxan) (0 vs. 8%, P = .0001, 3% vs. 27%, P less than .0001, and 2% vs. 7%, P = .0001, respectively).
Why might general neurologists be more resistant to embrace high-efficacy MS drugs? “They are newer and seen as more aggressive, and riskier,” Dr. Farin said. If general neurologists are not seeing many patients with MS and not prescribing these newer drugs very often, they may be more familiar with the older platform drugs, she said. “They may start with the ones that seem safer and are easier to start with.”
It is possible, she cautioned, that the study results may be confounded by general neurologists who refer patients to MS subspecialists when initial disease-modifying therapies fail.
No study funding was reported. Dr. Farin and two of the other four authors disclosed consulting fees from Biogen. No other disclosures were reported.
SOURCE: Farin CV et al. CMSC 2019. Abstract DXT44.
REPORTING FROM CMSC 2019
Key clinical point: MS subspecialists are more likely than are general neurologists to prescribe newer, high-efficacy MS therapies.
Major finding: General neurologists prescribed platform therapies – interferons, glatiramer acetate (Copaxone), and dimethyl fumarate (Tecfidera), more often than did MS subspecialists (16% vs. 5%, P less than .0001, 12% vs. 6%, P = .001, and 31% vs. 11%, P less than .0001, respectively).
Study details: Retrospective chart review of 4,753 patients with MS seen at the Duke University Health System.
Disclosures: Dr. Farin and two of the other four coauthors reported consulting fees from Biogen.
Source: Farin CV et al. CMSC 2019. Abstract DXT44.
When adolescents visit the ED, 10% leave with an opioid
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.
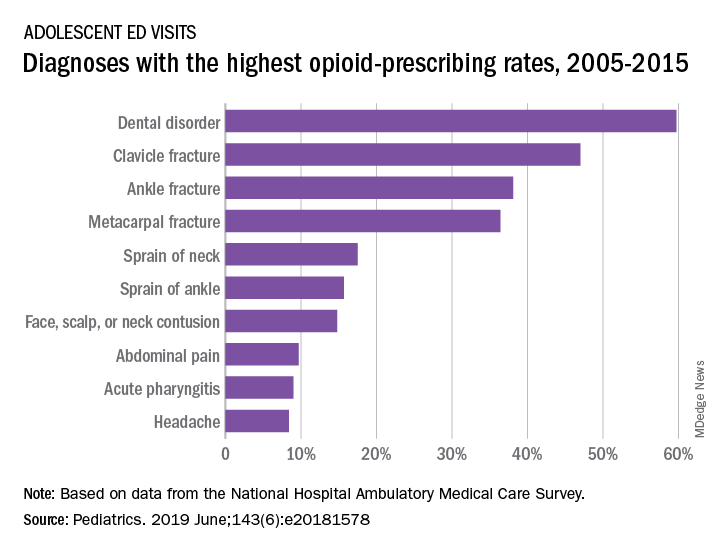
For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
FROM PEDIATRICS
Treatment-resistant GERD reported by more than half of patients
SAN DIEGO – Gastroesophageal reflux disease refractory to proton pump inhibitors may affect nearly half of those treated, according to the findings of a population-based sample of more than 70,000 Americans.
As part of the National Institutes of Health GI Patient Reported Outcomes Measurement Information System (NIH GI-PROMIS) questionnaire, respondents could download a free app called “My GI Health,” which led them through a series of questions about GI diseases. Sean Delshad, MD, MBA, of Cedars-Sinai Medical Center Los Angeles, and his colleagues examined data on symptom responses about GERD and heartburn.
Their somewhat surprising findings were that 44% of respondents had ever had GERD and that 70% of those respondents had symptoms in the past week. GERD seemed to be more common in women than in men, and in non-Hispanic whites more than other demographic groups. The rate of proton pump inhibitor–refractory GERD was reported at 54%.
Dr. Delshad discussed the implications of the study results for treatment and research in a video interview at the annual Digestive Disease Week.
SAN DIEGO – Gastroesophageal reflux disease refractory to proton pump inhibitors may affect nearly half of those treated, according to the findings of a population-based sample of more than 70,000 Americans.
As part of the National Institutes of Health GI Patient Reported Outcomes Measurement Information System (NIH GI-PROMIS) questionnaire, respondents could download a free app called “My GI Health,” which led them through a series of questions about GI diseases. Sean Delshad, MD, MBA, of Cedars-Sinai Medical Center Los Angeles, and his colleagues examined data on symptom responses about GERD and heartburn.
Their somewhat surprising findings were that 44% of respondents had ever had GERD and that 70% of those respondents had symptoms in the past week. GERD seemed to be more common in women than in men, and in non-Hispanic whites more than other demographic groups. The rate of proton pump inhibitor–refractory GERD was reported at 54%.
Dr. Delshad discussed the implications of the study results for treatment and research in a video interview at the annual Digestive Disease Week.
SAN DIEGO – Gastroesophageal reflux disease refractory to proton pump inhibitors may affect nearly half of those treated, according to the findings of a population-based sample of more than 70,000 Americans.
As part of the National Institutes of Health GI Patient Reported Outcomes Measurement Information System (NIH GI-PROMIS) questionnaire, respondents could download a free app called “My GI Health,” which led them through a series of questions about GI diseases. Sean Delshad, MD, MBA, of Cedars-Sinai Medical Center Los Angeles, and his colleagues examined data on symptom responses about GERD and heartburn.
Their somewhat surprising findings were that 44% of respondents had ever had GERD and that 70% of those respondents had symptoms in the past week. GERD seemed to be more common in women than in men, and in non-Hispanic whites more than other demographic groups. The rate of proton pump inhibitor–refractory GERD was reported at 54%.
Dr. Delshad discussed the implications of the study results for treatment and research in a video interview at the annual Digestive Disease Week.
REPORTING FROM DDW 2019
Chronic opioid use linked to low testosterone levels
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
REPORTING FROM ENDO 2019
Elderly concussion patients who used statins had lower dementia risk
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
FROM JAMA NEUROLOGY
Key clinical point: Older adults taking a statin within 90 days after a concussion had a lower rate of dementia.
Major finding: Statin use within 90 days of a concussion in older adults was associated with a 13% reduced risk of dementia (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Study details: A population-based double cohort study of 28,815 elderly patients who had a concussion between April 1993 and April 2013.
Disclosures: This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
Source: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
About one-third of anxiety patients relapse after stopping antidepressants
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
REPORTING FROM APA 2019
High-intensity statins may cut risk of joint replacement
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
REPORTING FROM OARSI 2019
Key clinical point: High-intensity statin therapy may reduce need for joint replacement in arthritis.
Major finding: The risk of knee or hip replacement surgery for rheumatoid arthritis was slashed by 90%, and by 21% for osteoarthritis.
Study details: This study included nearly 180,000 statin users propensity score-matched to an equal number of nonusers and prospectively followed for a mean of 6.5 years.
Disclosures: The study was supported by the National Clinical Research Center of Geriatric Disorders at Central South University in Hunan, China, and by several British universities. The presenter reported having no financial conflicts of interest.
Source: Sarmanova A et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
Peanut contamination risk prompts Promacta recall
Novartis has recalled three lots of 12.5-mg eltrombopag (Promacta) for oral suspension following discovery of possible contamination with peanut flour at a third-party manufacturing site.
Tablets at doses of 12.5 mg, 25 mg, 50 mg, and 75 mg are unaffected by this recall because they are not manufactured in the same facility. The recalled lots of medication were distributed between January and April 2019, but so far, Novartis has not received any reports of adverse events related to the recall.
Oral suspension of eltrombopag is indicated for certain patients with chronic immune thrombocytopenia, hepatitis C–associated thrombocytopenia, and severe aplastic anemia.
More information on the recalled lots and instructions on how to return the product can be found in the full announcement, which is also available through the Food and Drug Administration website.
Novartis has recalled three lots of 12.5-mg eltrombopag (Promacta) for oral suspension following discovery of possible contamination with peanut flour at a third-party manufacturing site.
Tablets at doses of 12.5 mg, 25 mg, 50 mg, and 75 mg are unaffected by this recall because they are not manufactured in the same facility. The recalled lots of medication were distributed between January and April 2019, but so far, Novartis has not received any reports of adverse events related to the recall.
Oral suspension of eltrombopag is indicated for certain patients with chronic immune thrombocytopenia, hepatitis C–associated thrombocytopenia, and severe aplastic anemia.
More information on the recalled lots and instructions on how to return the product can be found in the full announcement, which is also available through the Food and Drug Administration website.
Novartis has recalled three lots of 12.5-mg eltrombopag (Promacta) for oral suspension following discovery of possible contamination with peanut flour at a third-party manufacturing site.
Tablets at doses of 12.5 mg, 25 mg, 50 mg, and 75 mg are unaffected by this recall because they are not manufactured in the same facility. The recalled lots of medication were distributed between January and April 2019, but so far, Novartis has not received any reports of adverse events related to the recall.
Oral suspension of eltrombopag is indicated for certain patients with chronic immune thrombocytopenia, hepatitis C–associated thrombocytopenia, and severe aplastic anemia.
More information on the recalled lots and instructions on how to return the product can be found in the full announcement, which is also available through the Food and Drug Administration website.
CBO predicts more Medicare spending with drug rebate proposal
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.











