User login
Severe maternal morbidity increasing in California
The according to a study covering almost 8.3 million births in California.
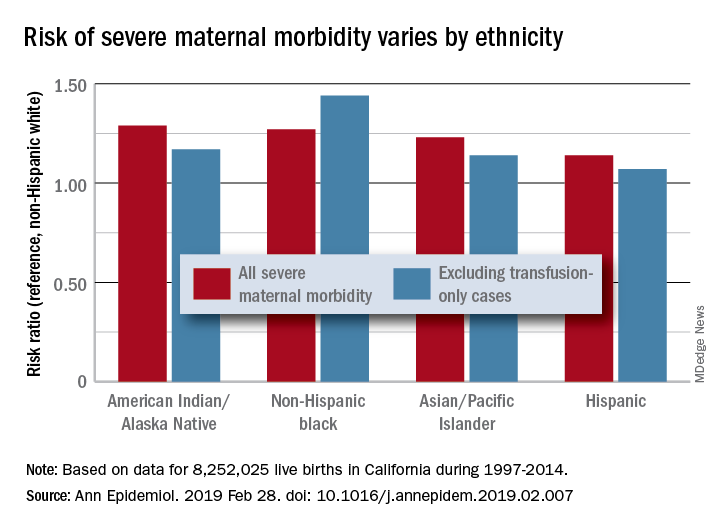
Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
The according to a study covering almost 8.3 million births in California.

Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
The according to a study covering almost 8.3 million births in California.

Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
FROM ANNALS OF EPIDEMIOLOGY
New SOFA version could streamline outcomes research
SAN DIEGO – The new method replaces some of SOFA’s more subjective criteria with objective measures.
eSOFA relies on electronic health records to reduce reliance on administrative records, which suffer from cross-hospital variability in diagnosis and coding practices, as well as changes in these practices over time. The diagnosis of sepsis itself is also highly subjective. Instead, eSOFA determines dysfunction in six organ systems, indicated by use of vasopressors and mechanical ventilation, and the presence of abnormal laboratory values.
“The SOFA score includes measures like the Glasgow Coma Scale, which undoubtedly at the bedside is a very important clinical sign, but when trying to implement something that is objective for purposes of retrospective case counting and standardization, it can be problematic. The measures we chose [for eSOFA] are concrete, important maneuvers that were initiated by clinicians,” Chanu Rhee, MD, said in an interview.
Dr. Rhee is assistant professor of population medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. He presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine, and the work was simultaneously published online in Critical Care Medicine.
Key elements of SOFA that pose challenges for administrative data include: PaO2/FiO2, which are not routinely measured, and can be difficult to assign to arterial or venous samples; inconsistency in blood pressure and transient increases in vasopressor dose; the subjectivity of the Glasgow Coma Scale, which is also difficult to assess in sedated patients; and inconsistent urine output.
eSOFA introduced new measures for various organ functions, including cardiovascular (vasopressor initiation), pulmonary (mechanical ventilation initiation), renal (doubling of creatinine levels or a 50% or greater decrease in estimated glomerular filtration rate, compared with baseline), hepatic (bilirubin levels greater than or equal to 2.0 mg/dL and at least doubled from baseline), coagulation (platelet count less than 100 cells/mcL and at least a 50% decrease from a baseline of at least 100 cells/mcL), and neurological (lactate greater than or equal to 2.0 mmol/L).
“[eSOFA] opens a window into inter-facility comparisons that has not been possible to do. It’s really critical to ask, ‘How am I doing compared to my peer institutions?’ If you’re doing worse, you can look at the whole spectrum of things to try to drive improvements in care,” said Dr. Rhee.
The new tool isn’t just limited to quality improvement research. Shaeesta Khan, MD, assistant professor of critical care medicine at Geisinger Medical Center,Danville, Pa., has found eSOFA to be useful in her research into how genetic polymorphisms play a role in sepsis outcomes. Geisinger has a large population of patients with completed whole genome sequencing, and Dr. Khan began by trying to glean sepsis outcomes from administrative data.
“I explained SOFA scores to our data broker, and he pulled up 3,000 patients and gave everybody a SOFA score based on the algorithm he created, and it was all over the chart. Once I started doing chart review and phenotype verification, it was just a nightmare,” Dr. Khan said in an interview.
After struggling with the project, one of her mentors put her in touch with one of Dr. Rhee’s colleagues, and she asked the data broker to modify the eSOFA algorithm to fit her specific criteria. “It was a blessing,” she said.
Now, she has data from 5,000 patients with sepsis and sequenced DNA, and can begin comparing outcomes and genetic variants. About 20 candidate genes for sepsis outcomes have been identified to date, but she has a particular interest in PCSK9, which is an innate immune system regulator. She hopes to present results at CCC49 in 2020.
Validating mortality prediction
The researchers compared eSOFA and SOFA in a sample from 111 U.S. acute care hospitals to see if eSOFA had a comparable predictive validity for mortality. The analysis included 942,360 adults seen between 2013 and 2015. A total of 11.1% (104,903) had a presumed serious infection based on a blood culture order and at least 4 consecutive days of antibiotic use.
The analysis showed that 6.1% of those with infections had a sepsis event based on at least a 2-point increase in SOFA score from baseline (Sepsis-3 criteria), compared with 4.4% identified by at least a 1-point increase in eSOFA score. A total of 34,174 patients (3.6%) overlapped between SOFA and eSOFA, which represented good agreement (Cronbach’s alpha, 0.81). Compared with SOFA/Sepsis-3, eSOFA had a sensitivity of 60%, and a positive predictive value of 82%.
Patients identified by eSOFA were slightly more ill, with more requiring ICU admission (41% vs. 35%), and a greater frequency of in-hospital mortality (17% vs. 14%). Those patients who were identified by SOFA/Sepsis-3, but missed by eSOFA, had an overall lower mortality (6%).
There was a similar risk of mortality across deciles between SOFA- and eSOFA-identified sepsis patients. In an independent analysis of four hospitals from the Emory system, the area under the receiver operating characteristics was 0.77 for eSOFA and 0.76 for SOFA (P less than .001).
The Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality funded the study. Dr. Rhee and Dr. Khan have no relevant financial conflicts.
SOURCE: Rhee C et al. Crit Care Med. 2019;47(3):307-14.
SAN DIEGO – The new method replaces some of SOFA’s more subjective criteria with objective measures.
eSOFA relies on electronic health records to reduce reliance on administrative records, which suffer from cross-hospital variability in diagnosis and coding practices, as well as changes in these practices over time. The diagnosis of sepsis itself is also highly subjective. Instead, eSOFA determines dysfunction in six organ systems, indicated by use of vasopressors and mechanical ventilation, and the presence of abnormal laboratory values.
“The SOFA score includes measures like the Glasgow Coma Scale, which undoubtedly at the bedside is a very important clinical sign, but when trying to implement something that is objective for purposes of retrospective case counting and standardization, it can be problematic. The measures we chose [for eSOFA] are concrete, important maneuvers that were initiated by clinicians,” Chanu Rhee, MD, said in an interview.
Dr. Rhee is assistant professor of population medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. He presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine, and the work was simultaneously published online in Critical Care Medicine.
Key elements of SOFA that pose challenges for administrative data include: PaO2/FiO2, which are not routinely measured, and can be difficult to assign to arterial or venous samples; inconsistency in blood pressure and transient increases in vasopressor dose; the subjectivity of the Glasgow Coma Scale, which is also difficult to assess in sedated patients; and inconsistent urine output.
eSOFA introduced new measures for various organ functions, including cardiovascular (vasopressor initiation), pulmonary (mechanical ventilation initiation), renal (doubling of creatinine levels or a 50% or greater decrease in estimated glomerular filtration rate, compared with baseline), hepatic (bilirubin levels greater than or equal to 2.0 mg/dL and at least doubled from baseline), coagulation (platelet count less than 100 cells/mcL and at least a 50% decrease from a baseline of at least 100 cells/mcL), and neurological (lactate greater than or equal to 2.0 mmol/L).
“[eSOFA] opens a window into inter-facility comparisons that has not been possible to do. It’s really critical to ask, ‘How am I doing compared to my peer institutions?’ If you’re doing worse, you can look at the whole spectrum of things to try to drive improvements in care,” said Dr. Rhee.
The new tool isn’t just limited to quality improvement research. Shaeesta Khan, MD, assistant professor of critical care medicine at Geisinger Medical Center,Danville, Pa., has found eSOFA to be useful in her research into how genetic polymorphisms play a role in sepsis outcomes. Geisinger has a large population of patients with completed whole genome sequencing, and Dr. Khan began by trying to glean sepsis outcomes from administrative data.
“I explained SOFA scores to our data broker, and he pulled up 3,000 patients and gave everybody a SOFA score based on the algorithm he created, and it was all over the chart. Once I started doing chart review and phenotype verification, it was just a nightmare,” Dr. Khan said in an interview.
After struggling with the project, one of her mentors put her in touch with one of Dr. Rhee’s colleagues, and she asked the data broker to modify the eSOFA algorithm to fit her specific criteria. “It was a blessing,” she said.
Now, she has data from 5,000 patients with sepsis and sequenced DNA, and can begin comparing outcomes and genetic variants. About 20 candidate genes for sepsis outcomes have been identified to date, but she has a particular interest in PCSK9, which is an innate immune system regulator. She hopes to present results at CCC49 in 2020.
Validating mortality prediction
The researchers compared eSOFA and SOFA in a sample from 111 U.S. acute care hospitals to see if eSOFA had a comparable predictive validity for mortality. The analysis included 942,360 adults seen between 2013 and 2015. A total of 11.1% (104,903) had a presumed serious infection based on a blood culture order and at least 4 consecutive days of antibiotic use.
The analysis showed that 6.1% of those with infections had a sepsis event based on at least a 2-point increase in SOFA score from baseline (Sepsis-3 criteria), compared with 4.4% identified by at least a 1-point increase in eSOFA score. A total of 34,174 patients (3.6%) overlapped between SOFA and eSOFA, which represented good agreement (Cronbach’s alpha, 0.81). Compared with SOFA/Sepsis-3, eSOFA had a sensitivity of 60%, and a positive predictive value of 82%.
Patients identified by eSOFA were slightly more ill, with more requiring ICU admission (41% vs. 35%), and a greater frequency of in-hospital mortality (17% vs. 14%). Those patients who were identified by SOFA/Sepsis-3, but missed by eSOFA, had an overall lower mortality (6%).
There was a similar risk of mortality across deciles between SOFA- and eSOFA-identified sepsis patients. In an independent analysis of four hospitals from the Emory system, the area under the receiver operating characteristics was 0.77 for eSOFA and 0.76 for SOFA (P less than .001).
The Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality funded the study. Dr. Rhee and Dr. Khan have no relevant financial conflicts.
SOURCE: Rhee C et al. Crit Care Med. 2019;47(3):307-14.
SAN DIEGO – The new method replaces some of SOFA’s more subjective criteria with objective measures.
eSOFA relies on electronic health records to reduce reliance on administrative records, which suffer from cross-hospital variability in diagnosis and coding practices, as well as changes in these practices over time. The diagnosis of sepsis itself is also highly subjective. Instead, eSOFA determines dysfunction in six organ systems, indicated by use of vasopressors and mechanical ventilation, and the presence of abnormal laboratory values.
“The SOFA score includes measures like the Glasgow Coma Scale, which undoubtedly at the bedside is a very important clinical sign, but when trying to implement something that is objective for purposes of retrospective case counting and standardization, it can be problematic. The measures we chose [for eSOFA] are concrete, important maneuvers that were initiated by clinicians,” Chanu Rhee, MD, said in an interview.
Dr. Rhee is assistant professor of population medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. He presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine, and the work was simultaneously published online in Critical Care Medicine.
Key elements of SOFA that pose challenges for administrative data include: PaO2/FiO2, which are not routinely measured, and can be difficult to assign to arterial or venous samples; inconsistency in blood pressure and transient increases in vasopressor dose; the subjectivity of the Glasgow Coma Scale, which is also difficult to assess in sedated patients; and inconsistent urine output.
eSOFA introduced new measures for various organ functions, including cardiovascular (vasopressor initiation), pulmonary (mechanical ventilation initiation), renal (doubling of creatinine levels or a 50% or greater decrease in estimated glomerular filtration rate, compared with baseline), hepatic (bilirubin levels greater than or equal to 2.0 mg/dL and at least doubled from baseline), coagulation (platelet count less than 100 cells/mcL and at least a 50% decrease from a baseline of at least 100 cells/mcL), and neurological (lactate greater than or equal to 2.0 mmol/L).
“[eSOFA] opens a window into inter-facility comparisons that has not been possible to do. It’s really critical to ask, ‘How am I doing compared to my peer institutions?’ If you’re doing worse, you can look at the whole spectrum of things to try to drive improvements in care,” said Dr. Rhee.
The new tool isn’t just limited to quality improvement research. Shaeesta Khan, MD, assistant professor of critical care medicine at Geisinger Medical Center,Danville, Pa., has found eSOFA to be useful in her research into how genetic polymorphisms play a role in sepsis outcomes. Geisinger has a large population of patients with completed whole genome sequencing, and Dr. Khan began by trying to glean sepsis outcomes from administrative data.
“I explained SOFA scores to our data broker, and he pulled up 3,000 patients and gave everybody a SOFA score based on the algorithm he created, and it was all over the chart. Once I started doing chart review and phenotype verification, it was just a nightmare,” Dr. Khan said in an interview.
After struggling with the project, one of her mentors put her in touch with one of Dr. Rhee’s colleagues, and she asked the data broker to modify the eSOFA algorithm to fit her specific criteria. “It was a blessing,” she said.
Now, she has data from 5,000 patients with sepsis and sequenced DNA, and can begin comparing outcomes and genetic variants. About 20 candidate genes for sepsis outcomes have been identified to date, but she has a particular interest in PCSK9, which is an innate immune system regulator. She hopes to present results at CCC49 in 2020.
Validating mortality prediction
The researchers compared eSOFA and SOFA in a sample from 111 U.S. acute care hospitals to see if eSOFA had a comparable predictive validity for mortality. The analysis included 942,360 adults seen between 2013 and 2015. A total of 11.1% (104,903) had a presumed serious infection based on a blood culture order and at least 4 consecutive days of antibiotic use.
The analysis showed that 6.1% of those with infections had a sepsis event based on at least a 2-point increase in SOFA score from baseline (Sepsis-3 criteria), compared with 4.4% identified by at least a 1-point increase in eSOFA score. A total of 34,174 patients (3.6%) overlapped between SOFA and eSOFA, which represented good agreement (Cronbach’s alpha, 0.81). Compared with SOFA/Sepsis-3, eSOFA had a sensitivity of 60%, and a positive predictive value of 82%.
Patients identified by eSOFA were slightly more ill, with more requiring ICU admission (41% vs. 35%), and a greater frequency of in-hospital mortality (17% vs. 14%). Those patients who were identified by SOFA/Sepsis-3, but missed by eSOFA, had an overall lower mortality (6%).
There was a similar risk of mortality across deciles between SOFA- and eSOFA-identified sepsis patients. In an independent analysis of four hospitals from the Emory system, the area under the receiver operating characteristics was 0.77 for eSOFA and 0.76 for SOFA (P less than .001).
The Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality funded the study. Dr. Rhee and Dr. Khan have no relevant financial conflicts.
SOURCE: Rhee C et al. Crit Care Med. 2019;47(3):307-14.
REPORTING FROM CCC48
Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
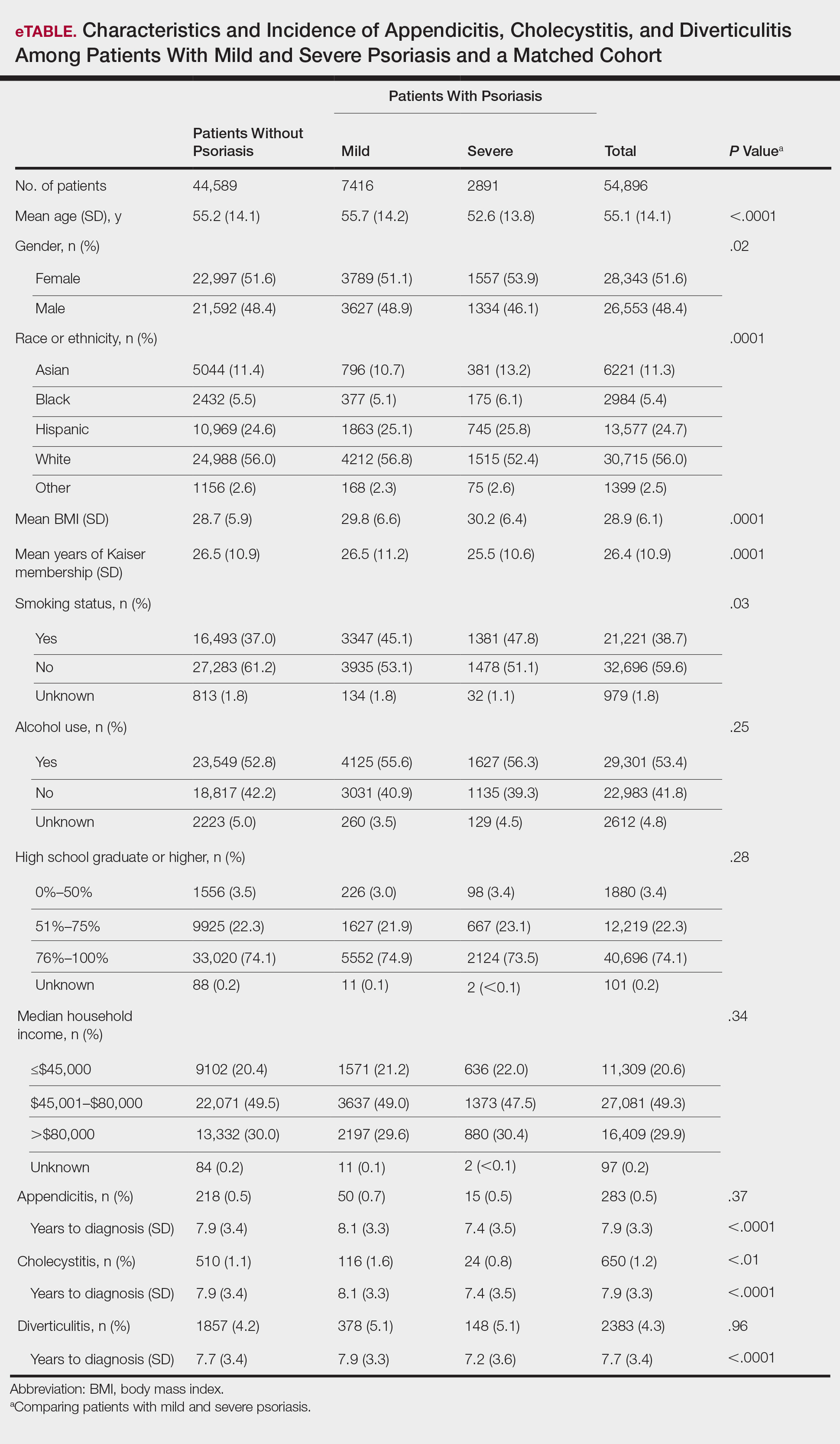
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
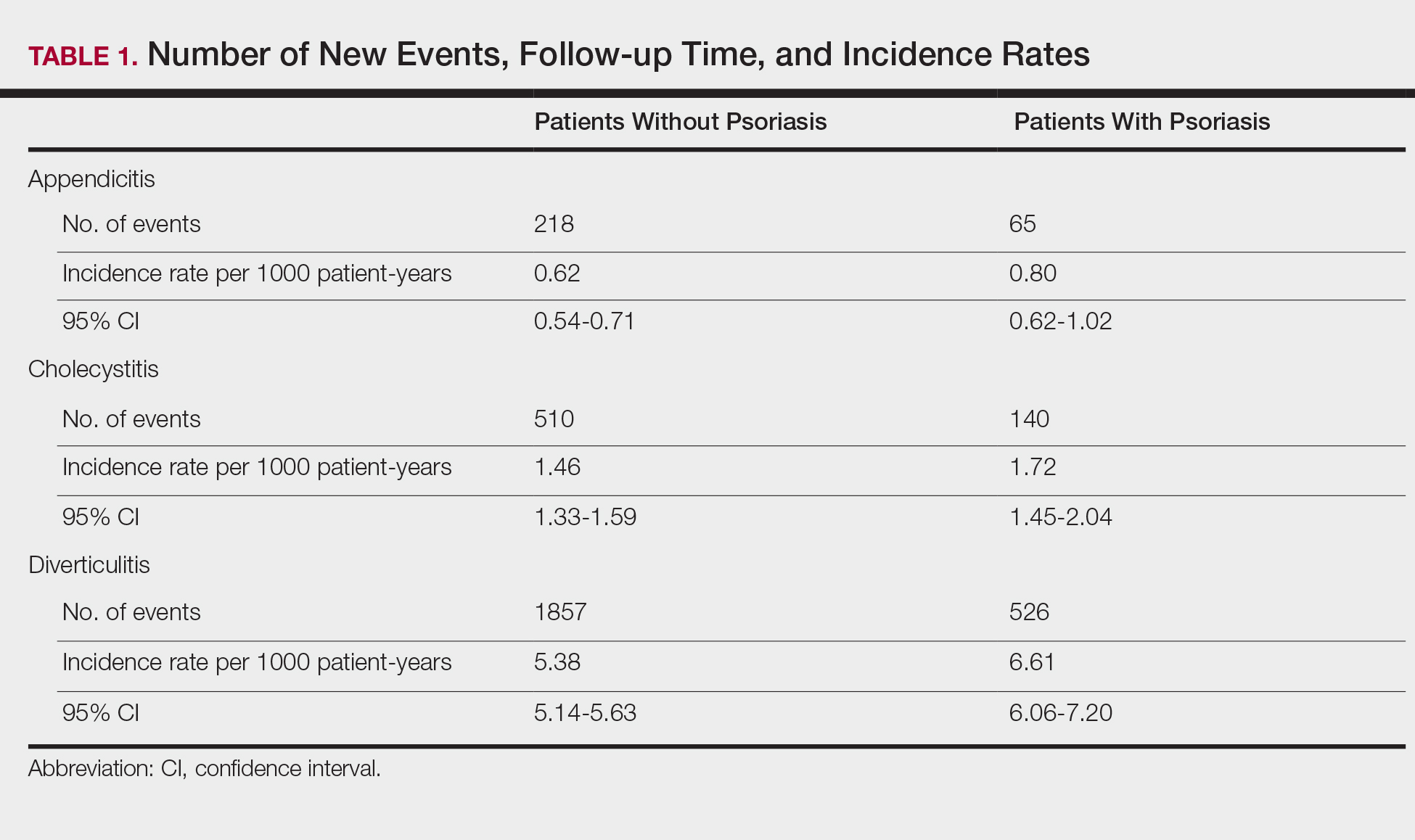
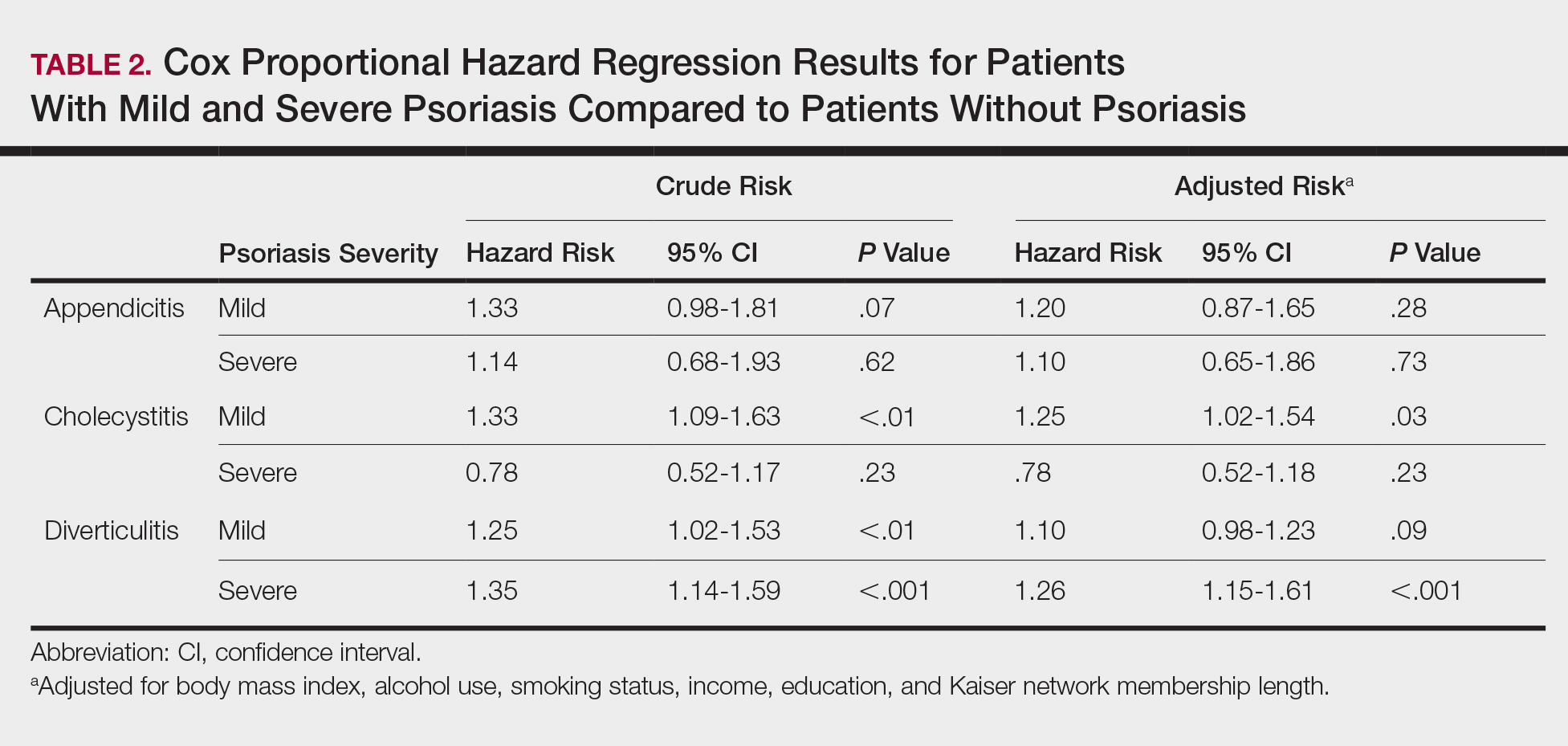
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
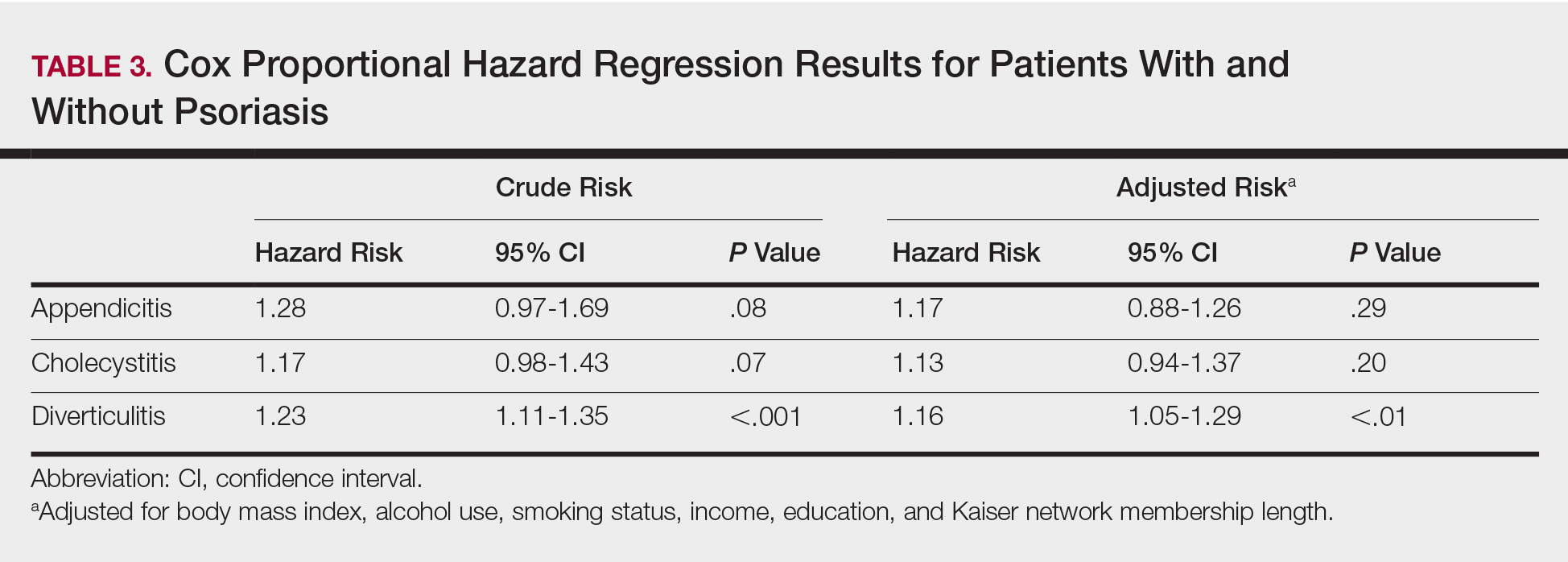
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Practice Points
- Patients with psoriasis may have elevated risk of diverticulitis compared to healthy patients. However, psoriasis patients do not appear to have increased risk of appendicitis or cholecystitis.
- Clinicians treating psoriasis patients should consider assessing for other risk factors of diverticulitis at regular intervals.
Flu or strep? Rapid tests can mislead
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
Norwegian scabies
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
Does early repolarization on ECG increase the risk of cardiac death in healthy people?
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
Today’s Care Must Extend Beyond the Exam Room
In May 2014, a 70-year-old retiree underwent repair of a fracture of her left ankle. The procedure was performed at a local hospital. A splint was applied to the ankle, and a nurse provided crutches.
Following discharge from the hospital, the patient hailed a taxi to take her home. As she was exiting the taxi at her residence, the patient fell and sustained comminuted fractures to the distal radius and distal ulna of her right (dominant) wrist and a trimalleolar fracture to her repaired left ankle.
The plaintiff was transported back to the hospital via ambulance. She underwent closed reduction of her wrist fractures and 11 days later was transferred to another facility for open reduction and internal fixation of her left ankle fracture. Her hospitalizations totaled 13 days and were followed by a course of inpatient rehabilitative therapy; the latter lasted until late August 2014, with a brief interruption in June when she underwent open reduction and internal fixation of her wrist fractures. When she returned home in August, the patient required the assistance of visiting aides and 3 additional months of rehabilitative therapy.
At trial, the plaintiff claimed that her left ankle and her right wrist remained painful, that she sustained a mild residual diminution of each area’s range of motion, and that these residual effects hindered her performance of basic physical activities (eg, cleaning and cooking).
The plaintiff alleged that her fall while exiting the taxi resulted from unsteadiness, which was a lingering effect of morphine that was administered during the repair of her fracture. She sought recovery of damages for past and future pain and suffering from the hospital’s operator. The lawsuit alleged that the nurse had failed to provide instructions on the proper use of crutches, that the nurse had failed to undertake measures that would have diminished the plaintiff’s likelihood of falling, that the nurse’s failures constituted malpractice and negligence, and that the hospital operator was vicariously liable for the nurse’s actions.
The plaintiff claimed that she repeatedly warned that she did not believe that she could safely use the crutches provided by the nurse. She claimed that she was unsteady and lightheaded, and that when she requested a wheelchair, an escort, or an ambulance, the nurse rejected the request. The nursing standards expert for the plaintiff opined that the request should have been satisfied or alternatively, that the nurse should have explained the manner in which a crutch-dependent person could safely enter and exit a vehicle.
Defense counsel claimed that the nurse explained proper use of the crutches, the plaintiff indicated that she understood the explanation, and the plaintiff demonstrated proper use and did not express concern. The defense’s expert contended that the nurse did not have to explain how a crutch-dependent person could safely enter and exit a vehicle and that the plaintiff’s fall resulted from her own failure to exercise appropriate caution. The defense further contended that the plaintiff achieved an excellent recovery.
Continue to: After a 7-day trial...
After a 7-day trial and 3 hours and 45 minutes’ deliberation, the jury found in favor of the plaintiff. It found that the nurse was negligent in her provision of crutches and that the act was a substantial cause of the plaintiff’s injuries. The jury also found that the nurse did not properly explain the use of crutches but determined that the error was not a substantial cause of the plaintiff’s injuries.
VERDICT
The jury awarded the plaintiff a total of $850,000 in damages. The plaintiff also recovered stipulated medical expenses.
COMMENTARY
Medical malpractice litigation involves recovery for acts or omissions that constitute a departure from the standard of care. We all recognize injurious acts—improper esophageal intubation in the emergency department, transection of a nerve in the operating room, or prescription of a contraindicated medication to an allergic patient—and acknowledge damaging omissions, such as failure to screen for colon cancer or recognize treatable diabetes.
However, some cases are disposition related; they arise from how patients are discharged, what instructions they are given, where they go, and what they do after discharge. These cases involve the patient’s medical issues engrafted on his or her transportation, job, and more generally, living environment.
The lay public expects patients to have a right of self-determination, to control the nature and course of their medical care. Yet, the modern lay public also expects the medical profession to act as an authority figure—exercising a degree of paternalism to safeguard patients from harm. This expectation is commonly articulated in retrospect, after something has gone wrong. Consequently, clinicians must be aware of what will happen to the patient after discharge.
Continue to: With all interventions...
With all interventions, weigh the post-discharge consequences. If you give an injection of hydromorphone, you cannot discharge the patient to drive home 45 minutes later. If you have diagnosed vertigo in a patient, you cannot prescribe meclizine and return that patient to her job working on scaffolding 50 ft above ground. If a frail patient lives alone and cannot safely walk, and you’ve started him on furosemide, you cannot discharge him without considering how he will get to the bathroom. Other concerns are even more difficult—for example, the homeless patient who does not have the environment or resources to follow your instructions.
It is tempting to view these concerns as not our responsibility or dismiss them as “not medicine.” Clinicians can feel frustrated at being pulled into the realm of social work, where we are ill equipped to deal with and sort out the patient’s “life problems.” For one thing, we don’t often have the resources to deal with these issues. And for another, addressing the patient’s postdischarge living situation takes time—something in short supply and intangible to the other patients in the waiting room, who are expecting your attention and wondering, “What’s the holdup?”
In the case presented, the plaintiff was a 70-year-old retiree. She was discharged from the hospital with crutches. Crutches are age-old and familiar devices. Nevertheless, crutches are for people who are able to use their arms for weight bearing and propulsion and require a fair amount of physical strength, timing, and dexterity. While a potentially debatable point, an assumption that a 70-year-old patient has the arm strength and dexterity to properly propel herself with crutches may be faulty. There was disagreement between the patient, who claimed she could not safely use the crutches, and the nurse, who said the patient accepted the crutches without concern. The safest course of action would be for discharge personnel to demonstrate the use of crutches, observe the patient using the crutches, and document that in the record.
In this case, it is unclear if the nurse demonstrated how to use the crutches or witnessed the plaintiff demonstrating she could safely use them. The jury found the nurse was negligent “in her provision” of crutches—an act they deemed a substantial cause of the plaintiff’s injuries. Interestingly, the jury did not consider the lack of explanation on the crutches’ use to be a substantial cause of injury. But the bottom line is, they faulted the nurse for the act of giving this patient crutches and awarded $850,000 in damages.
Society is changing. Fifty years ago, jurors would expect people to be familiar with crutches, and if you fell while using them, that was your own fault. Modern jurors expect hospitals and providers to get more involved in what happens to a patient after discharge. The news media has heavily publicized cases of alleged “patient dumping.”
Continue to: As a result...
As a result, we see legislative changes, such as the recently passed California Senate Bill 1152, which requires that homeless patients be fed; provided weather-appropriate clothing, filled prescriptions, and vaccinations; given medical screening, examination, and evaluation that requires the “treating physician” to arrange behavioral health care; and enrolled in “any affordable health insurance coverage for which he or she is eligible.”
Whether it is appropriate to ask hospitals and clinicians to get this involved is beyond the scope of this column. What is clear is that society increasingly expects clinicians and hospitals to take responsibility for patients. This societal change has an impact on the lay public’s perception of what is expected of health care providers. Tomorrow’s juror comes to court with a belief that hospitals and clinicians owe a duty of care that extends beyond the walls of the exam room.
IN SUMMARY
Reality test your post-treatment instructions to be sure they will work for the patient and are not grossly incompatible with his or her known postdischarge environment. To the extent possible, involve discharge planning personnel in your practice. Let your record reflect that you are acting in the patient’s best interest, and evade the temptation to squint narrowly to avoid seeing circumstances in the patient’s life that prevent safe implementation of your plan.
In May 2014, a 70-year-old retiree underwent repair of a fracture of her left ankle. The procedure was performed at a local hospital. A splint was applied to the ankle, and a nurse provided crutches.
Following discharge from the hospital, the patient hailed a taxi to take her home. As she was exiting the taxi at her residence, the patient fell and sustained comminuted fractures to the distal radius and distal ulna of her right (dominant) wrist and a trimalleolar fracture to her repaired left ankle.
The plaintiff was transported back to the hospital via ambulance. She underwent closed reduction of her wrist fractures and 11 days later was transferred to another facility for open reduction and internal fixation of her left ankle fracture. Her hospitalizations totaled 13 days and were followed by a course of inpatient rehabilitative therapy; the latter lasted until late August 2014, with a brief interruption in June when she underwent open reduction and internal fixation of her wrist fractures. When she returned home in August, the patient required the assistance of visiting aides and 3 additional months of rehabilitative therapy.
At trial, the plaintiff claimed that her left ankle and her right wrist remained painful, that she sustained a mild residual diminution of each area’s range of motion, and that these residual effects hindered her performance of basic physical activities (eg, cleaning and cooking).
The plaintiff alleged that her fall while exiting the taxi resulted from unsteadiness, which was a lingering effect of morphine that was administered during the repair of her fracture. She sought recovery of damages for past and future pain and suffering from the hospital’s operator. The lawsuit alleged that the nurse had failed to provide instructions on the proper use of crutches, that the nurse had failed to undertake measures that would have diminished the plaintiff’s likelihood of falling, that the nurse’s failures constituted malpractice and negligence, and that the hospital operator was vicariously liable for the nurse’s actions.
The plaintiff claimed that she repeatedly warned that she did not believe that she could safely use the crutches provided by the nurse. She claimed that she was unsteady and lightheaded, and that when she requested a wheelchair, an escort, or an ambulance, the nurse rejected the request. The nursing standards expert for the plaintiff opined that the request should have been satisfied or alternatively, that the nurse should have explained the manner in which a crutch-dependent person could safely enter and exit a vehicle.
Defense counsel claimed that the nurse explained proper use of the crutches, the plaintiff indicated that she understood the explanation, and the plaintiff demonstrated proper use and did not express concern. The defense’s expert contended that the nurse did not have to explain how a crutch-dependent person could safely enter and exit a vehicle and that the plaintiff’s fall resulted from her own failure to exercise appropriate caution. The defense further contended that the plaintiff achieved an excellent recovery.
Continue to: After a 7-day trial...
After a 7-day trial and 3 hours and 45 minutes’ deliberation, the jury found in favor of the plaintiff. It found that the nurse was negligent in her provision of crutches and that the act was a substantial cause of the plaintiff’s injuries. The jury also found that the nurse did not properly explain the use of crutches but determined that the error was not a substantial cause of the plaintiff’s injuries.
VERDICT
The jury awarded the plaintiff a total of $850,000 in damages. The plaintiff also recovered stipulated medical expenses.
COMMENTARY
Medical malpractice litigation involves recovery for acts or omissions that constitute a departure from the standard of care. We all recognize injurious acts—improper esophageal intubation in the emergency department, transection of a nerve in the operating room, or prescription of a contraindicated medication to an allergic patient—and acknowledge damaging omissions, such as failure to screen for colon cancer or recognize treatable diabetes.
However, some cases are disposition related; they arise from how patients are discharged, what instructions they are given, where they go, and what they do after discharge. These cases involve the patient’s medical issues engrafted on his or her transportation, job, and more generally, living environment.
The lay public expects patients to have a right of self-determination, to control the nature and course of their medical care. Yet, the modern lay public also expects the medical profession to act as an authority figure—exercising a degree of paternalism to safeguard patients from harm. This expectation is commonly articulated in retrospect, after something has gone wrong. Consequently, clinicians must be aware of what will happen to the patient after discharge.
Continue to: With all interventions...
With all interventions, weigh the post-discharge consequences. If you give an injection of hydromorphone, you cannot discharge the patient to drive home 45 minutes later. If you have diagnosed vertigo in a patient, you cannot prescribe meclizine and return that patient to her job working on scaffolding 50 ft above ground. If a frail patient lives alone and cannot safely walk, and you’ve started him on furosemide, you cannot discharge him without considering how he will get to the bathroom. Other concerns are even more difficult—for example, the homeless patient who does not have the environment or resources to follow your instructions.
It is tempting to view these concerns as not our responsibility or dismiss them as “not medicine.” Clinicians can feel frustrated at being pulled into the realm of social work, where we are ill equipped to deal with and sort out the patient’s “life problems.” For one thing, we don’t often have the resources to deal with these issues. And for another, addressing the patient’s postdischarge living situation takes time—something in short supply and intangible to the other patients in the waiting room, who are expecting your attention and wondering, “What’s the holdup?”
In the case presented, the plaintiff was a 70-year-old retiree. She was discharged from the hospital with crutches. Crutches are age-old and familiar devices. Nevertheless, crutches are for people who are able to use their arms for weight bearing and propulsion and require a fair amount of physical strength, timing, and dexterity. While a potentially debatable point, an assumption that a 70-year-old patient has the arm strength and dexterity to properly propel herself with crutches may be faulty. There was disagreement between the patient, who claimed she could not safely use the crutches, and the nurse, who said the patient accepted the crutches without concern. The safest course of action would be for discharge personnel to demonstrate the use of crutches, observe the patient using the crutches, and document that in the record.
In this case, it is unclear if the nurse demonstrated how to use the crutches or witnessed the plaintiff demonstrating she could safely use them. The jury found the nurse was negligent “in her provision” of crutches—an act they deemed a substantial cause of the plaintiff’s injuries. Interestingly, the jury did not consider the lack of explanation on the crutches’ use to be a substantial cause of injury. But the bottom line is, they faulted the nurse for the act of giving this patient crutches and awarded $850,000 in damages.
Society is changing. Fifty years ago, jurors would expect people to be familiar with crutches, and if you fell while using them, that was your own fault. Modern jurors expect hospitals and providers to get more involved in what happens to a patient after discharge. The news media has heavily publicized cases of alleged “patient dumping.”
Continue to: As a result...
As a result, we see legislative changes, such as the recently passed California Senate Bill 1152, which requires that homeless patients be fed; provided weather-appropriate clothing, filled prescriptions, and vaccinations; given medical screening, examination, and evaluation that requires the “treating physician” to arrange behavioral health care; and enrolled in “any affordable health insurance coverage for which he or she is eligible.”
Whether it is appropriate to ask hospitals and clinicians to get this involved is beyond the scope of this column. What is clear is that society increasingly expects clinicians and hospitals to take responsibility for patients. This societal change has an impact on the lay public’s perception of what is expected of health care providers. Tomorrow’s juror comes to court with a belief that hospitals and clinicians owe a duty of care that extends beyond the walls of the exam room.
IN SUMMARY
Reality test your post-treatment instructions to be sure they will work for the patient and are not grossly incompatible with his or her known postdischarge environment. To the extent possible, involve discharge planning personnel in your practice. Let your record reflect that you are acting in the patient’s best interest, and evade the temptation to squint narrowly to avoid seeing circumstances in the patient’s life that prevent safe implementation of your plan.
In May 2014, a 70-year-old retiree underwent repair of a fracture of her left ankle. The procedure was performed at a local hospital. A splint was applied to the ankle, and a nurse provided crutches.
Following discharge from the hospital, the patient hailed a taxi to take her home. As she was exiting the taxi at her residence, the patient fell and sustained comminuted fractures to the distal radius and distal ulna of her right (dominant) wrist and a trimalleolar fracture to her repaired left ankle.
The plaintiff was transported back to the hospital via ambulance. She underwent closed reduction of her wrist fractures and 11 days later was transferred to another facility for open reduction and internal fixation of her left ankle fracture. Her hospitalizations totaled 13 days and were followed by a course of inpatient rehabilitative therapy; the latter lasted until late August 2014, with a brief interruption in June when she underwent open reduction and internal fixation of her wrist fractures. When she returned home in August, the patient required the assistance of visiting aides and 3 additional months of rehabilitative therapy.
At trial, the plaintiff claimed that her left ankle and her right wrist remained painful, that she sustained a mild residual diminution of each area’s range of motion, and that these residual effects hindered her performance of basic physical activities (eg, cleaning and cooking).
The plaintiff alleged that her fall while exiting the taxi resulted from unsteadiness, which was a lingering effect of morphine that was administered during the repair of her fracture. She sought recovery of damages for past and future pain and suffering from the hospital’s operator. The lawsuit alleged that the nurse had failed to provide instructions on the proper use of crutches, that the nurse had failed to undertake measures that would have diminished the plaintiff’s likelihood of falling, that the nurse’s failures constituted malpractice and negligence, and that the hospital operator was vicariously liable for the nurse’s actions.
The plaintiff claimed that she repeatedly warned that she did not believe that she could safely use the crutches provided by the nurse. She claimed that she was unsteady and lightheaded, and that when she requested a wheelchair, an escort, or an ambulance, the nurse rejected the request. The nursing standards expert for the plaintiff opined that the request should have been satisfied or alternatively, that the nurse should have explained the manner in which a crutch-dependent person could safely enter and exit a vehicle.
Defense counsel claimed that the nurse explained proper use of the crutches, the plaintiff indicated that she understood the explanation, and the plaintiff demonstrated proper use and did not express concern. The defense’s expert contended that the nurse did not have to explain how a crutch-dependent person could safely enter and exit a vehicle and that the plaintiff’s fall resulted from her own failure to exercise appropriate caution. The defense further contended that the plaintiff achieved an excellent recovery.
Continue to: After a 7-day trial...
After a 7-day trial and 3 hours and 45 minutes’ deliberation, the jury found in favor of the plaintiff. It found that the nurse was negligent in her provision of crutches and that the act was a substantial cause of the plaintiff’s injuries. The jury also found that the nurse did not properly explain the use of crutches but determined that the error was not a substantial cause of the plaintiff’s injuries.
VERDICT
The jury awarded the plaintiff a total of $850,000 in damages. The plaintiff also recovered stipulated medical expenses.
COMMENTARY
Medical malpractice litigation involves recovery for acts or omissions that constitute a departure from the standard of care. We all recognize injurious acts—improper esophageal intubation in the emergency department, transection of a nerve in the operating room, or prescription of a contraindicated medication to an allergic patient—and acknowledge damaging omissions, such as failure to screen for colon cancer or recognize treatable diabetes.
However, some cases are disposition related; they arise from how patients are discharged, what instructions they are given, where they go, and what they do after discharge. These cases involve the patient’s medical issues engrafted on his or her transportation, job, and more generally, living environment.
The lay public expects patients to have a right of self-determination, to control the nature and course of their medical care. Yet, the modern lay public also expects the medical profession to act as an authority figure—exercising a degree of paternalism to safeguard patients from harm. This expectation is commonly articulated in retrospect, after something has gone wrong. Consequently, clinicians must be aware of what will happen to the patient after discharge.
Continue to: With all interventions...
With all interventions, weigh the post-discharge consequences. If you give an injection of hydromorphone, you cannot discharge the patient to drive home 45 minutes later. If you have diagnosed vertigo in a patient, you cannot prescribe meclizine and return that patient to her job working on scaffolding 50 ft above ground. If a frail patient lives alone and cannot safely walk, and you’ve started him on furosemide, you cannot discharge him without considering how he will get to the bathroom. Other concerns are even more difficult—for example, the homeless patient who does not have the environment or resources to follow your instructions.
It is tempting to view these concerns as not our responsibility or dismiss them as “not medicine.” Clinicians can feel frustrated at being pulled into the realm of social work, where we are ill equipped to deal with and sort out the patient’s “life problems.” For one thing, we don’t often have the resources to deal with these issues. And for another, addressing the patient’s postdischarge living situation takes time—something in short supply and intangible to the other patients in the waiting room, who are expecting your attention and wondering, “What’s the holdup?”
In the case presented, the plaintiff was a 70-year-old retiree. She was discharged from the hospital with crutches. Crutches are age-old and familiar devices. Nevertheless, crutches are for people who are able to use their arms for weight bearing and propulsion and require a fair amount of physical strength, timing, and dexterity. While a potentially debatable point, an assumption that a 70-year-old patient has the arm strength and dexterity to properly propel herself with crutches may be faulty. There was disagreement between the patient, who claimed she could not safely use the crutches, and the nurse, who said the patient accepted the crutches without concern. The safest course of action would be for discharge personnel to demonstrate the use of crutches, observe the patient using the crutches, and document that in the record.
In this case, it is unclear if the nurse demonstrated how to use the crutches or witnessed the plaintiff demonstrating she could safely use them. The jury found the nurse was negligent “in her provision” of crutches—an act they deemed a substantial cause of the plaintiff’s injuries. Interestingly, the jury did not consider the lack of explanation on the crutches’ use to be a substantial cause of injury. But the bottom line is, they faulted the nurse for the act of giving this patient crutches and awarded $850,000 in damages.
Society is changing. Fifty years ago, jurors would expect people to be familiar with crutches, and if you fell while using them, that was your own fault. Modern jurors expect hospitals and providers to get more involved in what happens to a patient after discharge. The news media has heavily publicized cases of alleged “patient dumping.”
Continue to: As a result...
As a result, we see legislative changes, such as the recently passed California Senate Bill 1152, which requires that homeless patients be fed; provided weather-appropriate clothing, filled prescriptions, and vaccinations; given medical screening, examination, and evaluation that requires the “treating physician” to arrange behavioral health care; and enrolled in “any affordable health insurance coverage for which he or she is eligible.”
Whether it is appropriate to ask hospitals and clinicians to get this involved is beyond the scope of this column. What is clear is that society increasingly expects clinicians and hospitals to take responsibility for patients. This societal change has an impact on the lay public’s perception of what is expected of health care providers. Tomorrow’s juror comes to court with a belief that hospitals and clinicians owe a duty of care that extends beyond the walls of the exam room.
IN SUMMARY
Reality test your post-treatment instructions to be sure they will work for the patient and are not grossly incompatible with his or her known postdischarge environment. To the extent possible, involve discharge planning personnel in your practice. Let your record reflect that you are acting in the patient’s best interest, and evade the temptation to squint narrowly to avoid seeing circumstances in the patient’s life that prevent safe implementation of your plan.
Most U.S. tPA-eligible stroke patients now get treated within an hour
HONOLULU – The speed at which eligible U.S. patients with acute ischemic stroke receive thrombolytic therapy has surged in recent years, and .
By the second half of last year, 75% of acute ischemic stroke patients treated at any of the 913 U.S. hospitals in the Get With The Guidelines-Stroke program received intravenous tissue plasminogen activator (tPA; Alteplase) within 60 minutes of their hospital arrival (their door-to-needle time (DTN), and 52% received tPA with a DTN time of 45 minutes or less. These levels met the treatment-speed goals set by the second phase of the Target: Stroke program, which called for delivering tPA to 75% of appropriate stroke patients within a DTN time of 60 minutes, and within 45 minutes in at least 50% of patients, Gregg C. Fonarow, MD, and his associates reported at the International Stroke Conference, sponsored by the American Heart Association.
The analyses they reported also documented how these most recent gains in thrombolytic speed played out in improved patient outcomes. During phase 2 of Target: Stroke, which ran from January 2014 to September 2018, 85,078 U.S. patients received tPA at one of the participating hospitals. During those 4 years, the rate of in-hospital mortality was 6.0%, half the patients were discharged home, 53% could ambulate independently, and the rate of intracerebral hemorrhage (ICH) was 3.5%. The researchers compared these clinical event rates with the rates from 24,603 tPA-treated patients during 2003-2009, before the Target: Stroke campaign began. After adjustment for many potential confounders, the more recently treated cohort had a 31% relative risk reduction in in-hospital mortality, a 43% relative increase in being discharged home, a 40% relative increase in independent ambulation, and a 32% relative risk reduction in the rate of symptomatic ICH. All these between-group differences were statistically significant.
“We were hoping that, by improving DTN times we could achieve improved outcomes, but often in quality-improvement research – even when the process of care improves – the gains in outcomes don’t necessarily match expectations. Fortunately, with Target: Stroke, the remarkable improvements in timely treatment translated to remarkable improvements in clinical outcomes,” Dr. Fonarow said in an interview. “These are substantial, clinically relevant improvements in clinical outcomes for patients with acute ischemic stroke. As a result of the program, more than 100,000 acute ischemic stroke patients received much more timely acute ischemic stroke care and achieved far better clinical outcomes.”
During the 2003-2018 period reviewed, the percentage of presenting acute ischemic stroke patients who received tPA treatment at the 913 Get With The Guidelines hospitals that participated in the Target: Stroke program (and so had reviewable data) throughout all three periods rose from 6% during 2003-2009 (prestudy) to 8% during 2010-2013 (phase 1), and to 12% during 2014-2018 (phase 2). The percentages of these patients who received the drug within 60 minutes were 27% during 2003-2009, 43% during 2010-2013, and 68% during the entire 2014-2018 period, culminating in the 75% rate during July-September 2018, reported Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Fonarow attributed the drop in the rate of ICH – from 5.7% during 2003-2009, to 4.4% during 2010-2013, and down to 3.5% during 2014-2018 – to the faster delivery of tPA. “With faster treatment, there is less ischemic brain and vascular damage and thus a lower likelihood of ICH as a complication of tPA,” he explained.
The Target: Stroke program achieved these gains in speedier thrombolytic treatment (and better recognition of eligible patients) through educational and promotional activities including dissemination of best practices. Notable best practices have included EMS prenotification of hospitals before they arrive with a stroke patient, direct transport of patients to a brain imaging scanner, premix of tPA, initiation of tPA in the brain imaging suite, and prompt data feedback, Dr. Fonarow said.
The Get With The Guidelines-Stroke and Target: Stroke programs now involve more than 2,100 U.S. hospitals, and they are able to deliver emergency care to roughly 70% of U.S. acute ischemic stroke patients, he noted.
With achievement of Target: Stroke’s phase 2 goals, the program announced its launch of a third phase, with new treatment goals: Initiation of thrombolytic treatment to 85% of eligible patients within 60 minutes, to 75% within 45 minutes, and to 50% within 30 minutes. The phase 3 Target: Stroke program also for the first time includes treatment goals for delivery of endovascular thrombectomy treatment.
SOURCE: Fonarow GC et al. ISC 2019, Abstract LBP9.
The Target: Stroke and Get With The Guidelines-Stroke programs should be commended for the very impressive achievements they have made in improved delivery of thrombolytic therapy to acute ischemic stroke patients. What’s happened over the past decade in the speed of delivery of tissue plasminogen activator for treating U.S. stroke patients has been a real success story.
Programs like Get With The Guidelines and Target: Stroke have proven their value, but a significant barrier remains to bringing this program to all U.S. stroke patients and to all U.S. hospitals that treat stroke patients. That barrier is resources. Participating hospitals need to meet certain data-collection standards, but some U.S. hospitals do not have the resources to do this.
Bruce Ovbiagele, MD , is a neurologist and chief of staff for the San Francisco Veterans Affairs Health Care System. He had no disclosures. He made these comments in an interview.
The Target: Stroke and Get With The Guidelines-Stroke programs should be commended for the very impressive achievements they have made in improved delivery of thrombolytic therapy to acute ischemic stroke patients. What’s happened over the past decade in the speed of delivery of tissue plasminogen activator for treating U.S. stroke patients has been a real success story.
Programs like Get With The Guidelines and Target: Stroke have proven their value, but a significant barrier remains to bringing this program to all U.S. stroke patients and to all U.S. hospitals that treat stroke patients. That barrier is resources. Participating hospitals need to meet certain data-collection standards, but some U.S. hospitals do not have the resources to do this.
Bruce Ovbiagele, MD , is a neurologist and chief of staff for the San Francisco Veterans Affairs Health Care System. He had no disclosures. He made these comments in an interview.
The Target: Stroke and Get With The Guidelines-Stroke programs should be commended for the very impressive achievements they have made in improved delivery of thrombolytic therapy to acute ischemic stroke patients. What’s happened over the past decade in the speed of delivery of tissue plasminogen activator for treating U.S. stroke patients has been a real success story.
Programs like Get With The Guidelines and Target: Stroke have proven their value, but a significant barrier remains to bringing this program to all U.S. stroke patients and to all U.S. hospitals that treat stroke patients. That barrier is resources. Participating hospitals need to meet certain data-collection standards, but some U.S. hospitals do not have the resources to do this.
Bruce Ovbiagele, MD , is a neurologist and chief of staff for the San Francisco Veterans Affairs Health Care System. He had no disclosures. He made these comments in an interview.
HONOLULU – The speed at which eligible U.S. patients with acute ischemic stroke receive thrombolytic therapy has surged in recent years, and .
By the second half of last year, 75% of acute ischemic stroke patients treated at any of the 913 U.S. hospitals in the Get With The Guidelines-Stroke program received intravenous tissue plasminogen activator (tPA; Alteplase) within 60 minutes of their hospital arrival (their door-to-needle time (DTN), and 52% received tPA with a DTN time of 45 minutes or less. These levels met the treatment-speed goals set by the second phase of the Target: Stroke program, which called for delivering tPA to 75% of appropriate stroke patients within a DTN time of 60 minutes, and within 45 minutes in at least 50% of patients, Gregg C. Fonarow, MD, and his associates reported at the International Stroke Conference, sponsored by the American Heart Association.
The analyses they reported also documented how these most recent gains in thrombolytic speed played out in improved patient outcomes. During phase 2 of Target: Stroke, which ran from January 2014 to September 2018, 85,078 U.S. patients received tPA at one of the participating hospitals. During those 4 years, the rate of in-hospital mortality was 6.0%, half the patients were discharged home, 53% could ambulate independently, and the rate of intracerebral hemorrhage (ICH) was 3.5%. The researchers compared these clinical event rates with the rates from 24,603 tPA-treated patients during 2003-2009, before the Target: Stroke campaign began. After adjustment for many potential confounders, the more recently treated cohort had a 31% relative risk reduction in in-hospital mortality, a 43% relative increase in being discharged home, a 40% relative increase in independent ambulation, and a 32% relative risk reduction in the rate of symptomatic ICH. All these between-group differences were statistically significant.
“We were hoping that, by improving DTN times we could achieve improved outcomes, but often in quality-improvement research – even when the process of care improves – the gains in outcomes don’t necessarily match expectations. Fortunately, with Target: Stroke, the remarkable improvements in timely treatment translated to remarkable improvements in clinical outcomes,” Dr. Fonarow said in an interview. “These are substantial, clinically relevant improvements in clinical outcomes for patients with acute ischemic stroke. As a result of the program, more than 100,000 acute ischemic stroke patients received much more timely acute ischemic stroke care and achieved far better clinical outcomes.”
During the 2003-2018 period reviewed, the percentage of presenting acute ischemic stroke patients who received tPA treatment at the 913 Get With The Guidelines hospitals that participated in the Target: Stroke program (and so had reviewable data) throughout all three periods rose from 6% during 2003-2009 (prestudy) to 8% during 2010-2013 (phase 1), and to 12% during 2014-2018 (phase 2). The percentages of these patients who received the drug within 60 minutes were 27% during 2003-2009, 43% during 2010-2013, and 68% during the entire 2014-2018 period, culminating in the 75% rate during July-September 2018, reported Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Fonarow attributed the drop in the rate of ICH – from 5.7% during 2003-2009, to 4.4% during 2010-2013, and down to 3.5% during 2014-2018 – to the faster delivery of tPA. “With faster treatment, there is less ischemic brain and vascular damage and thus a lower likelihood of ICH as a complication of tPA,” he explained.
The Target: Stroke program achieved these gains in speedier thrombolytic treatment (and better recognition of eligible patients) through educational and promotional activities including dissemination of best practices. Notable best practices have included EMS prenotification of hospitals before they arrive with a stroke patient, direct transport of patients to a brain imaging scanner, premix of tPA, initiation of tPA in the brain imaging suite, and prompt data feedback, Dr. Fonarow said.
The Get With The Guidelines-Stroke and Target: Stroke programs now involve more than 2,100 U.S. hospitals, and they are able to deliver emergency care to roughly 70% of U.S. acute ischemic stroke patients, he noted.
With achievement of Target: Stroke’s phase 2 goals, the program announced its launch of a third phase, with new treatment goals: Initiation of thrombolytic treatment to 85% of eligible patients within 60 minutes, to 75% within 45 minutes, and to 50% within 30 minutes. The phase 3 Target: Stroke program also for the first time includes treatment goals for delivery of endovascular thrombectomy treatment.
SOURCE: Fonarow GC et al. ISC 2019, Abstract LBP9.
HONOLULU – The speed at which eligible U.S. patients with acute ischemic stroke receive thrombolytic therapy has surged in recent years, and .
By the second half of last year, 75% of acute ischemic stroke patients treated at any of the 913 U.S. hospitals in the Get With The Guidelines-Stroke program received intravenous tissue plasminogen activator (tPA; Alteplase) within 60 minutes of their hospital arrival (their door-to-needle time (DTN), and 52% received tPA with a DTN time of 45 minutes or less. These levels met the treatment-speed goals set by the second phase of the Target: Stroke program, which called for delivering tPA to 75% of appropriate stroke patients within a DTN time of 60 minutes, and within 45 minutes in at least 50% of patients, Gregg C. Fonarow, MD, and his associates reported at the International Stroke Conference, sponsored by the American Heart Association.
The analyses they reported also documented how these most recent gains in thrombolytic speed played out in improved patient outcomes. During phase 2 of Target: Stroke, which ran from January 2014 to September 2018, 85,078 U.S. patients received tPA at one of the participating hospitals. During those 4 years, the rate of in-hospital mortality was 6.0%, half the patients were discharged home, 53% could ambulate independently, and the rate of intracerebral hemorrhage (ICH) was 3.5%. The researchers compared these clinical event rates with the rates from 24,603 tPA-treated patients during 2003-2009, before the Target: Stroke campaign began. After adjustment for many potential confounders, the more recently treated cohort had a 31% relative risk reduction in in-hospital mortality, a 43% relative increase in being discharged home, a 40% relative increase in independent ambulation, and a 32% relative risk reduction in the rate of symptomatic ICH. All these between-group differences were statistically significant.
“We were hoping that, by improving DTN times we could achieve improved outcomes, but often in quality-improvement research – even when the process of care improves – the gains in outcomes don’t necessarily match expectations. Fortunately, with Target: Stroke, the remarkable improvements in timely treatment translated to remarkable improvements in clinical outcomes,” Dr. Fonarow said in an interview. “These are substantial, clinically relevant improvements in clinical outcomes for patients with acute ischemic stroke. As a result of the program, more than 100,000 acute ischemic stroke patients received much more timely acute ischemic stroke care and achieved far better clinical outcomes.”
During the 2003-2018 period reviewed, the percentage of presenting acute ischemic stroke patients who received tPA treatment at the 913 Get With The Guidelines hospitals that participated in the Target: Stroke program (and so had reviewable data) throughout all three periods rose from 6% during 2003-2009 (prestudy) to 8% during 2010-2013 (phase 1), and to 12% during 2014-2018 (phase 2). The percentages of these patients who received the drug within 60 minutes were 27% during 2003-2009, 43% during 2010-2013, and 68% during the entire 2014-2018 period, culminating in the 75% rate during July-September 2018, reported Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Fonarow attributed the drop in the rate of ICH – from 5.7% during 2003-2009, to 4.4% during 2010-2013, and down to 3.5% during 2014-2018 – to the faster delivery of tPA. “With faster treatment, there is less ischemic brain and vascular damage and thus a lower likelihood of ICH as a complication of tPA,” he explained.
The Target: Stroke program achieved these gains in speedier thrombolytic treatment (and better recognition of eligible patients) through educational and promotional activities including dissemination of best practices. Notable best practices have included EMS prenotification of hospitals before they arrive with a stroke patient, direct transport of patients to a brain imaging scanner, premix of tPA, initiation of tPA in the brain imaging suite, and prompt data feedback, Dr. Fonarow said.
The Get With The Guidelines-Stroke and Target: Stroke programs now involve more than 2,100 U.S. hospitals, and they are able to deliver emergency care to roughly 70% of U.S. acute ischemic stroke patients, he noted.
With achievement of Target: Stroke’s phase 2 goals, the program announced its launch of a third phase, with new treatment goals: Initiation of thrombolytic treatment to 85% of eligible patients within 60 minutes, to 75% within 45 minutes, and to 50% within 30 minutes. The phase 3 Target: Stroke program also for the first time includes treatment goals for delivery of endovascular thrombectomy treatment.
SOURCE: Fonarow GC et al. ISC 2019, Abstract LBP9.
REPORTING FROM ISC 2019
Key clinical point: In late 2018, the Target: Stroke program met its phase 2 goal for timely delivery of thrombolytic therapy to acute ischemic stroke patients.
Major finding: In September 2018, 75% of eligible stroke patients underwent thrombolysis within 60 minutes of hospital arrival, and 52% within 45 minutes.
Study details: Review of data collected from 154,221 U.S. stroke patients treated with thrombolysis during 2003-2018.
Disclosures: Target: Stroke has received funding from Boehringer Ingelheim, Janssen, Bristol-Myers Squibb/Sanofi, and Merck. Dr. Fonarow had no relevant disclosures.
Source: Fonarow GC et al. ISC 2019, Abstract LBP9.
Medical advice prompts unneeded emergency visits by AF patients
BOSTON – Patients with atrial fibrillation who present to emergency departments, despite being asymptomatic, often go based on of their understanding of advice they had previously received from their physicians, according to results from a prospective study of 356 Canadian atrial arrhythmia patients seen in emergency settings.
One way to deal with potentially inappropriate emergency department use is to have concerned patients with atrial fibrillation (AF) record their heart rhythm data with a handheld device or watch, transfer the records to their smartphones, and transmit the information to a remote physician for interpretation and advice, Benedict M. Glover, MD, said at the annual International AF Symposium.
Dr. Glover and his associates are in the process of developing a prototype system of this design to address the need they identified in a recent registry of 356 patients with a primary diagnosis of AF who sought care in the emergency department (ED) of any of seven participating Canadian medical centers, including five academic centers and two community hospitals. The survey results showed that 71% of the patients were symptomatic and 29% were asymptomatic then they first presented to an emergency department.
Case reviews of the 356 patients showed that 152 (43%) came to the EDs for what were classified as inappropriate reasons. The most common cause by far of an inappropriate emergency presentation was prior medical advice the patient had received, cited in 62% of the inappropriate cases, compared with 9% of the appropriate cases, said Dr. Glover, an electrophysiologist at Sunnybrook Health Sciences Centre in Toronto.
The inappropriate ED use by AF patients could be addressed in at least two ways, he said. One solution might be to give patients an alternative destination, so that instead of going to an emergency department they could go to an outpatient AF clinic. A second solution is to give patients a way to have their heart rhythm assessed remotely at the time of their concern. Dr. Glover said that his center had the staff capacity to deal with the potential influx of rhythm data from a pilot-sized program of remote heart-rhythm monitoring, but he conceded that scaling up to deal with the data that could come from the entire panel of AF patients managed by Sunnybrook physicians would be a huge challenge.
“The issue is what do we do with the data after we get it,” Dr. Glover said. “It’s a lot of information.”
Dr. Glover had no disclosures.
BOSTON – Patients with atrial fibrillation who present to emergency departments, despite being asymptomatic, often go based on of their understanding of advice they had previously received from their physicians, according to results from a prospective study of 356 Canadian atrial arrhythmia patients seen in emergency settings.
One way to deal with potentially inappropriate emergency department use is to have concerned patients with atrial fibrillation (AF) record their heart rhythm data with a handheld device or watch, transfer the records to their smartphones, and transmit the information to a remote physician for interpretation and advice, Benedict M. Glover, MD, said at the annual International AF Symposium.
Dr. Glover and his associates are in the process of developing a prototype system of this design to address the need they identified in a recent registry of 356 patients with a primary diagnosis of AF who sought care in the emergency department (ED) of any of seven participating Canadian medical centers, including five academic centers and two community hospitals. The survey results showed that 71% of the patients were symptomatic and 29% were asymptomatic then they first presented to an emergency department.
Case reviews of the 356 patients showed that 152 (43%) came to the EDs for what were classified as inappropriate reasons. The most common cause by far of an inappropriate emergency presentation was prior medical advice the patient had received, cited in 62% of the inappropriate cases, compared with 9% of the appropriate cases, said Dr. Glover, an electrophysiologist at Sunnybrook Health Sciences Centre in Toronto.
The inappropriate ED use by AF patients could be addressed in at least two ways, he said. One solution might be to give patients an alternative destination, so that instead of going to an emergency department they could go to an outpatient AF clinic. A second solution is to give patients a way to have their heart rhythm assessed remotely at the time of their concern. Dr. Glover said that his center had the staff capacity to deal with the potential influx of rhythm data from a pilot-sized program of remote heart-rhythm monitoring, but he conceded that scaling up to deal with the data that could come from the entire panel of AF patients managed by Sunnybrook physicians would be a huge challenge.
“The issue is what do we do with the data after we get it,” Dr. Glover said. “It’s a lot of information.”
Dr. Glover had no disclosures.
BOSTON – Patients with atrial fibrillation who present to emergency departments, despite being asymptomatic, often go based on of their understanding of advice they had previously received from their physicians, according to results from a prospective study of 356 Canadian atrial arrhythmia patients seen in emergency settings.
One way to deal with potentially inappropriate emergency department use is to have concerned patients with atrial fibrillation (AF) record their heart rhythm data with a handheld device or watch, transfer the records to their smartphones, and transmit the information to a remote physician for interpretation and advice, Benedict M. Glover, MD, said at the annual International AF Symposium.
Dr. Glover and his associates are in the process of developing a prototype system of this design to address the need they identified in a recent registry of 356 patients with a primary diagnosis of AF who sought care in the emergency department (ED) of any of seven participating Canadian medical centers, including five academic centers and two community hospitals. The survey results showed that 71% of the patients were symptomatic and 29% were asymptomatic then they first presented to an emergency department.
Case reviews of the 356 patients showed that 152 (43%) came to the EDs for what were classified as inappropriate reasons. The most common cause by far of an inappropriate emergency presentation was prior medical advice the patient had received, cited in 62% of the inappropriate cases, compared with 9% of the appropriate cases, said Dr. Glover, an electrophysiologist at Sunnybrook Health Sciences Centre in Toronto.
The inappropriate ED use by AF patients could be addressed in at least two ways, he said. One solution might be to give patients an alternative destination, so that instead of going to an emergency department they could go to an outpatient AF clinic. A second solution is to give patients a way to have their heart rhythm assessed remotely at the time of their concern. Dr. Glover said that his center had the staff capacity to deal with the potential influx of rhythm data from a pilot-sized program of remote heart-rhythm monitoring, but he conceded that scaling up to deal with the data that could come from the entire panel of AF patients managed by Sunnybrook physicians would be a huge challenge.
“The issue is what do we do with the data after we get it,” Dr. Glover said. “It’s a lot of information.”
Dr. Glover had no disclosures.
REPORTING FROM THE AF SYMPOSIUM 2019
Key clinical point:
Major finding: Among 152 AF patients who made an inappropriate ED visit, 62% cited their prior medical advice.
Study details: Prospective study of 356 AF patients who sought ED care at any of seven Canadian hospitals.
Disclosures: Dr. Glover had no disclosures.
Stryker issues voluntary field action for Lifepak 15 defibrillators
Stryker has announced a voluntary field action for its Lifepak 15 monitor/defibrillators, according to a safety alert from the Food and Drug Administration.
The company is notifying certain Lifepak 15 customers of an issue causing the device to lock up after a defibrillation shock is delivered. The lockup displays as a blank monitor with the LED lights on, indicating that the power is on, but the keypad and device become nonfunctional, the FDA said. This lockup can delay delivery of therapy, which can cause injury or death.
Since the introduction of the device in 2009, 58 complaints regarding the issue have been reported, including 6 that resulted in death. In all, 13,003 devices are included in the field action.
Customers should continue to use their devices if they have been affected until a correction can be completed. If the lockup occurs, the user should press and hold the “on” button until the LED turns off, then hit the “on” button again. If this does not reset the device, the batteries should be removed and reinserted, or the device should be removed and reconnected to its power adapter, the FDA said.
Find the full press release on the FDA website.
Stryker has announced a voluntary field action for its Lifepak 15 monitor/defibrillators, according to a safety alert from the Food and Drug Administration.
The company is notifying certain Lifepak 15 customers of an issue causing the device to lock up after a defibrillation shock is delivered. The lockup displays as a blank monitor with the LED lights on, indicating that the power is on, but the keypad and device become nonfunctional, the FDA said. This lockup can delay delivery of therapy, which can cause injury or death.
Since the introduction of the device in 2009, 58 complaints regarding the issue have been reported, including 6 that resulted in death. In all, 13,003 devices are included in the field action.
Customers should continue to use their devices if they have been affected until a correction can be completed. If the lockup occurs, the user should press and hold the “on” button until the LED turns off, then hit the “on” button again. If this does not reset the device, the batteries should be removed and reinserted, or the device should be removed and reconnected to its power adapter, the FDA said.
Find the full press release on the FDA website.
Stryker has announced a voluntary field action for its Lifepak 15 monitor/defibrillators, according to a safety alert from the Food and Drug Administration.
The company is notifying certain Lifepak 15 customers of an issue causing the device to lock up after a defibrillation shock is delivered. The lockup displays as a blank monitor with the LED lights on, indicating that the power is on, but the keypad and device become nonfunctional, the FDA said. This lockup can delay delivery of therapy, which can cause injury or death.
Since the introduction of the device in 2009, 58 complaints regarding the issue have been reported, including 6 that resulted in death. In all, 13,003 devices are included in the field action.
Customers should continue to use their devices if they have been affected until a correction can be completed. If the lockup occurs, the user should press and hold the “on” button until the LED turns off, then hit the “on” button again. If this does not reset the device, the batteries should be removed and reinserted, or the device should be removed and reconnected to its power adapter, the FDA said.
Find the full press release on the FDA website.