User login
Heart failure guidelines: What you need to know about the 2017 focused update
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
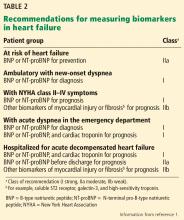
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
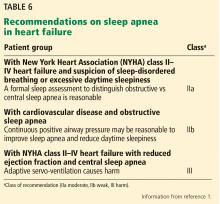
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
KEY POINTS
- Despite advances in treatment, heart failure remains highly morbid, common, and costly. Prevention is key.
- Strategies to prevent progression to clinical heart failure in high-risk patients include new blood pressure targets (< 130/80 mm Hg) and B-type natriuretic peptide screening to prompt referral to a cardiovascular specialist.
- An aldosterone receptor antagonist might be considered to decrease hospitalizations in appropriately selected stage C HFpEF patients. Routine use of nitrates or phosphodiesterase-5 inhibitors in such patients is not recommended.
- Outpatient intravenous iron infusions are reasonable in persistently symptomatic New York Heart Association stage II to III heart failure with reduced ejection fraction (HFrEF) to improve functional capacity and quality of life.
- The new systolic blood pressure target is less than 130 mm Hg for stage A heart failure, stage C HFrEF, and stage C HFpEF.
Who needs to carry an epinephrine autoinjector?
Anaphylaxis is potentially fatal but can be prevented if the trigger is identified and avoided, and death can be avoided if episodes are treated promptly.
A consensus definition of anaphylaxis has been difficult to achieve, with slight variations among international guidelines. The World Allergy Organization classifies anaphylaxis as immunologic, nonimmunologic, or idiopathic.1 The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network highlight clinical symptoms and criteria.2 The International Consensus on Food Allergy describes reactions as being immunoglobulin E (IgE)-mediated, cell-mediated, or a combination of the 2 mechanisms.3
Despite the subtle differences in these definitions, all 3 international organizations have a common recommendation for anaphylaxis: once it is diagnosed, epinephrine is the treatment of choice.
EPINEPHRINE IS THE TREATMENT OF CHOICE FOR ANAPHYLAXIS
Anaphylaxis commonly results from exposure to foods, medications, and Hymenoptera venom.4 Avoiding triggers is key in preventing anaphylaxis but is not always possible.
Although epinephrine is the cornerstone of the emergency treatment of anaphylaxis, many patients instead receive antihistamines and corticosteroids as initial therapy. Some take these medications on their own, and some receive them in emergency departments and outpatient clinics.5
Diphenhydramine, a histamine 1 receptor antagonist, is often used as a first-line medication. But diphenhydramine has a slow onset of action, taking 80 minutes after an oral dose to suppress a histamine-induced cutaneous flare by 50%, and taking 52 minutes with intramuscular administration.6 Corticosteroids also have a slow onset of action. These drugs cannot prevent death in anaphylaxis, a condition in which the median time to respiratory or cardiac arrest is 30 minutes after ingestion of food, 15 minutes after envenomation, and 5 minutes after iatrogenic reactions.7
Combination therapy with diphenhydramine and a histamine 2 receptor antagonist (eg, cimetidine, famotidine) is also commonly used,8 but this combination offers no advantage in terms of onset of action, and a Cochrane review could find no definitive evidence for or against the use of histamine 2 receptor antagonists.9
Because of their slow onset of action, all of these should be second-line therapies, given after epinephrine. Epinephrine is the first line of treatment because it has a maximal pharmacokinetic effect (time to maximal peak serum level) within 10 minutes of intramuscular injection into the thigh.10,11
In addition, epinephrine acts on numerous receptors to antagonize the multiple pathologic effects of the mediators released during an anaphylactic episode. In contrast, antihistamines block only 1 mediator, while mediators other than histamine can be responsible for severe events and deaths.12,13
It is crucial that epinephrine be given immediately, as delay has been associated with fatalities.14 In addition, guidelines recommend repeating epinephrine dosing after 5 to 15 minutes if the response to the first dose is suboptimal.1,2 From 16% to 36% of patients may need a second dose.15–18 Therefore, many physicians recommend that patients at risk of anaphylaxis keep not 1 but 2 epinephrine autoinjectors on hand at all times, and so say the US guidelines for the management of anaphylaxis.19
WHO SHOULD CARRY AN EPINEPHRINE AUTOINJECTOR?
All published guidelines recommend epinephrine as the drug of choice for anaphylaxis. And an epinephrine autoinjector is indicated for anyone who has experienced an anaphylactic event or is at risk of one, and these patients should carry it with them at all times. Such individuals include those with food allergy or Hymenoptera hypersensitivity.
Food allergy
The foods that most often cause anaphylaxis are peanuts, tree nuts, fish, shellfish, milk, and eggs, but any food can cause a reaction.
The prevalence of food allergy has increased over time, and treatments are limited. Some food desensitization protocols look promising but are still in the research stages. The best treatment at this time is to avoid the offending food, but there are accidental exposures.
Hymenoptera hypersensitivity
Patients who have had anaphylaxis after being stung by insects such as bees, wasps, yellow-faced hornets, white-faced hornets, yellow jackets, and fire ants should be evaluated by an allergist. Skin testing and serum IgE testing helps properly diagnose Hymenoptera hypersensitivity.
Once the diagnosis is confirmed, venom immunotherapy should be considered. Some patients choose only to carry an epinephrine autoinjector and to avoid these insects as much as possible. However, most patients also choose to receive venom immunotherapy, because 80% to 90% of those who receive this treatment for 3 to 5 years do not have a systemic reaction if they are stung again.20
Regardless of whether they choose to undergo immunotherapy, sensitive patients should always carry an epinephrine autoinjector. This is also the case after treatment ends, since the therapy is not 100% effective.
PATIENTS FOR WHOM THE NEED MAY BE LESS CLEAR
In other patients who may be at increased risk, the mandate for an epinephrine autoinjector is less clear, and the decision to carry one is determined on an individual basis. Such individuals are those receiving allergen immunotherapy, with large local reactions to insect stings, with oral allergy syndrome, with mastocytosis, and with drug allergy. In these cases, the benefit vs the burden of carrying an autoinjector should be discussed with the patient.
Patients on allergen immunotherapy
National guidelines recommend that all patients who receive allergen immunotherapy be monitored in the clinic under a physician’s supervision for 30 minutes after the injection. Fortunately, life-threatening reactions occurring after 30 minutes are rare. But delayed systemic reactions can occur and may account for up to 50% of such events.21
Therefore, many physicians consider it prudent for patients on immunotherapy to carry an epinephrine autoinjector, but there is no consensus. A survey22 found that 13.5% of allergists did not prescribe the autoinjector for patients on immunotherapy, while 33.3% prescribed it for all their patients on immunotherapy, and the rest prescribed based on risk.
Since there are no national guidelines on epinephrine autoinjectors for patients on immunotherapy, the decision should be based on the patient’s risks and comorbidities and informed by discussion between the individual patient and his or her allergist.
Patients with large local reactions to insect stings
From 5% to 10% of patients who have large local reactions to insect stings are at risk of systemic reactions.20
Patients with oral allergy syndrome
Oral allergy syndrome, also known as pollen-food allergy, causes itching and mild swelling of the mouth, lips, and throat after eating fresh fruits and vegetables. The prevalence ranges from 2% to 10% of patients with allergies.23
A survey of allergists found that 20% of patients with oral allergy syndrome had experienced systemic symptoms.24 The survey also showed that the decision to prescribe an epinephrine autoinjector to these patients was highly variable. Only about 30% of allergists recommend epinephrine autoinjectors to patients with oral allergy syndrome, while most believe that the decision should be based on the individual’s symptoms and risk.
More research is needed in the area of food allergy. Because data are limited, there are no national guidelines on whether these patients should carry an epinephrine autoinjector. We agree with the Joint Task Force on Practice Parameters14 recommendation that the decision be made on an individual basis following discussion between the patient and physician.
Patients with mastocytosis
Patients with mastocytosis and a history of anaphylaxis are at increased risk for systemic reactions to Hymenoptera venom.
Patients with medication allergy
Once medication allergy has been diagnosed, avoidance is usually effective, obviating the need for an epinephrine autoinjector, although the physician has the option of prescribing one.
CAUTIONS, NOT CONTRAINDICATIONS
Physicians may be reluctant to prescribe an epinephrine autoinjector because of the risk of an adverse reaction in patients with hypertension, coronary artery disease, or arrhythmias, and in elderly patients taking multiple drugs, especially drugs that can interact with epinephrine. Nevertheless, there is no absolute contraindication to the use of epinephrine in anaphylaxis.
In patients with atherosclerosis and cardiovascular disease
Epinephrine increases vasoconstriction, heart rate, and cardiac force of contraction. These effects are beneficial during anaphylaxis, but in rare cases patients have experienced myocardial infarction and acute coronary syndrome after receiving intravenous epinephrine.25 These incidents have naturally prompted reluctance to prescribe it in susceptible patients with coronary disease during anaphylaxis.
Yet epinephrine may not be solely to blame for these adverse responses. Mast cells are abundant in the heart, and their release of mediators can also result in adverse cardiac manifestations, including myocardial infarction.26
Conversely, some drugs used to treat cardiovascular disease can worsen anaphylaxis.
Beta-blockers can cause bronchospasm and decrease cardiac contractility. They can also blunt the pharmacologic effects of epinephrine. There is concern that epinephrine may produce dangerous elevations of blood pressure in patients taking beta-blockers by unopposed alpha-adrenergic stimulation and reflex vagotonic effects.27 And there is evidence that beta-blockers may increase the risk and severity of reactions. One study reported that patients taking beta-blockers are more than 8 times more likely to be hospitalized due to anaphylactoid reaction with bronchospasm.28
Beta-blockers and, to a lesser extent, angiotensin-converting enzyme inhibitors have been shown to increase the risk of anaphylaxis in the emergency department.29,30 However, some investigators have not found beta-blockers to be a risk factor. A study evaluating anaphylactoid reactions from contrast media found no statistically significant higher risk in patients taking beta-blockers.31 Similarly, a study of 3,178 patients on beta-blockers receiving venom immunotherapy or allergen immunotherapy found no increase in the frequency of systemic reactions.32 Nevertheless, overall, more studies support the hypothesis that beta-blockers may be an additional risk factor in anaphylaxis.33
Thus, clinicians treating patients with cardiovascular disease and anaphylaxis face a dilemma. Although there is concern in this population, epinephrine should not be withheld in patients with cardiovascular disease who are experiencing an anaphylactic event.33 If epinephrine is not administered, the patient could die.
Elderly patients on multiple medications
Older patients are also at risk of anaphylaxis. But clinicians are reluctant to treat older patients with epinephrine because of concerns about adverse effects.
Epinephrine dispensing rates vary substantially in different age groups: 1.44% for patients under age 17, 0.9% for those ages 17 to 64, and 0.32% for those age 65 or older.34 A Canadian study of 492 patients with anaphylaxis in the emergency department showed that those over age 50 received epinephrine less often than younger patients (36.1% vs 60.5%).35 Cardiovascular complications were more frequent in the older group, occurring in 4 (9.1%) of the 44 older patients who received epinephrine compared with 1 (0.4%) of the 225 younger patients who received it. On the other hand, the rate of adverse effects from subcutaneous epinephrine was no different in older asthma patients compared with younger patients.36
Many older patients take multiple medications, raising concern about adverse effects. Commonly prescribed medications in the elderly can affect the actions of epinephrine. Monoamine oxidase inhibitors retard the catabolism of epinephrine. Tricyclic antidepressants may decrease the reuptake of catecholamines by neurons and thus interfere with the degradation of epinephrine. Digoxin has a narrow therapeutic window and can potentially increase the risk of arrhythmias when given with epinephrine.
Although the clinician must be cautious in treating older patients who have comorbidities, these are not sufficient to withhold prescribing an epinephrine autoinjector to elderly patients at risk of anaphylaxis.
INJECTOR OPTIONS
Epinephrine autoinjectors come preloaded for prompt delivery of the drug. They are intended primarily for use by patients themselves in unsupervised settings in suspected anaphylaxis. Simplicity of use and safety must be considered in such a setting so that patients can use the device correctly and are not incorrectly dosed.
Several models are commercially available, with different ergonomic designs and sizes. EpiPen, the first one marketed in the United States, was introduced in 1987. One device (Auvi-Q) contains an audio chip that gives step-by-step instructions at the time of use. It is hoped that this device will reduce errors in usage during this stressful time for patients and caregivers.
In the United States, epinephrine autoinjectors contain either 0.15 or 0.30 mg of the drug, but some clinicians believe this may not be enough. The UK Resuscitation Council recommends 0.50 mg for patients over age 12,37 and an epinephrine autoinjector with that dose is available in Europe.
Subcutaneous vs intramuscular delivery
The package insert for some epinephrine autoinjectors says the injector can be used to treat anaphylaxis by both subcutaneous and intramuscular administration. However, the routes are not equivalent.
The goal in anaphylaxis is to quickly achieve high tissue and plasma epinephrine concentrations, and studies have found that injection into the vastus lateralis muscle, but not the deltoid muscle, results in faster time to peak plasma concentration: 8 minutes for injection in the vastus lateralis muscle and 34 minutes for subcutaneous delivery.10,11 In addition, injection in the vastus lateralis muscle results in a higher peak plasma concentration than the subcutaneous or deltoid route. Based on these data, intramuscular injection into the vastus lateralis muscle in the thigh appears to be the preferred route of administration of epinephrine.
Obese patients may need a longer needle
Research on the original autoinjector was conducted by the US military, which wanted a rapidly effective and easy-to-use antidote for battlefield exposure to poison gas. The resulting device had 2 separate spring-loaded syringes, 1 containing pralidoxime chloride and the other atropine sulfate. To enable its use through the thick fabric of a chemical warfare suit, the needles were 2.2 cm long.
The first commercial autoinjector to contain epinephrine was made by Survival Technology (Bethesda, MD) in the mid-1970s. The manufacturer considered a 2.2-cm needle to be too long, and the first commercially available epinephrine autoinjector, EpiPen, had a 1.43-cm needle for adult use.
Since then, needle lengths have ranged from 1.17 to 2.5 cm to accommodate different skin-to-muscle depths, with shorter needles for children and longer needles for obese adults.38
However, the prevalence of obesity is high and continues to rise.39 Obesity raises concern that the needles in epinephrine autoinjectors may be too short for the preferred intramuscular delivery, resulting in subcutaneous deposition.
A study that used computed tomography of the thigh found that 1 (2%) of 50 men and 21 (42%) of 50 women studied had a subcutaneous tissue depth greater than 1.43 cm, the needle length in EpiPen. These were not anaphylaxis patients, but the findings suggest that many patients—especially women—may be getting subcutaneous instead of intramuscular delivery with this device.40
Another study that used ultrasonography showed that the 1.43-cm EpiPen needle was too short for 36 (31%) of 116 adults.41 Women were 6.4 times more likely than men to encounter this problem. Other risk factors include higher body mass index, short height, and thicker thighs.
Emerade, an injector with a 2.5-cm needle, is available in some European countries. A longer needle may be helpful in some cases. but we do not yet have enough data to determine the optimal needle length.
Conversely, some children may need shorter needles and may in fact be at risk of having the needle penetrate bone.42 The US Food and Drug Administration recently approved a shorter needle for an epinephrine autoinjector (Auvi-Q) to be used in children weighing 7.5 kg to 15 kg.
BARRIERS TO USING EPINEPHRINE AUTOINJECTORS
Many patients do not use their epinephrine autoinjector in times of anaphylaxis or do not have one with them. Common reasons cited by respondents in a survey43 of 1,385 patients included the following:
They took an oral antihistamine instead (38%).
They never received a prescription for an epinephrine autoinjector (28%).
They thought their symptoms were mild and would resolve with time (13%).
They were afraid (6%). There are reports of accidental injection, typically into fingers, hands, and thumbs. Fortunately, most accidental injections do not require a hand surgeon evaluation or surgery.44 Conservative therapy and monitoring of the injection site are sufficient in most cases.
They could not afford an epinephrine autoinjector (1%).43 Mylan Pharmaceuticals infamously increased the price of its EpiPen to more than $600 for a package of 2 pens. Generic devices are available in the United States but are still too expensive for some patients and are cumbersome to carry.
However, even expensive epinephrine autoinjectors may be cost-effective. Epidemiologic studies have found that patients who did not use an epinephrine autoinjector incurred a higher burden of cost due to emergency department visits and inpatient hospitalizations.45
As a do-it-yourself option, some resourceful patients are obtaining autoinjectors intended for insulin injection, replacing the needle, and filling the injector with epinephrine, at a cost of about $30. (The manufacturer does not endorse this off-label use of their device—www.owenmumford.com/us/patients/if-you-need-to-inject.) Least costly of all is to prescribe multidose vials of epinephrine and regular syringes and teach patients and their caregivers how to draw up the proper dose and give themselves an injection—in essence going back to what was done before 1987.
It was past its expiration date (2%).43 Failure to refill the prescription is common. A California Kaiser Permanente study46 showed that only 46% of patients refilled their epinephrine autoinjector prescription at least once, and the refill rate decreased over time: 43% at 1 to 2 year follow-up, 35% at 3 to 4 years, and 30% at 5 years or longer. Based on these data, it is imperative to educate patients regarding the importance of replacing the epinephrine autoinjector when the old one expires.
NEED FOR PATIENT EDUCATION
Even though prompt treatment with epinephrine decreases fatalities, it continues to be underused in the community. In addition, it is often prescribed without adequate training in its use and appropriate emphasis on the need to keep the device on hand at all times and to replace it in a timely manner if it is used or has expired. Physicians need to educate patients on how to avoid triggers and how to recognize symptoms of anaphylaxis whenever they prescribe an epinephrine autoinjector.
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014; 7(1):9. doi:10.1186/1939-4551-7-9
- NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6 suppl):S1–S58. doi:10.1016/j.jaci.2010.10.007
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129(4):906–920. doi:10.1016/j.jaci.2012.02.001
- Lieberman P, Carmago CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma, and Immunology. Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97(5):596–602. doi:10.1016/S1081-1206(10)61086-1
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(suppl 7):S18–S26. doi:10.1097/WOX.0b013e31817c9338
- Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol 2008; 100(5):452–456. doi:10.1016/S1081-1206(10)60470-X
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allerg 2000; 30(8):1144–1150. pmid:10931122
- Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med 1992; 21:237–242. pmid:1536481
- Sheikh A, Simons FE, Barbour V, Worth A. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev 2012; (8):CD008935. doi:10.1002/14651858.CD008935.pub2
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108(5):871–873. doi:10.1067/mai.2001.119409
- Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101(1 pt 1):33–37. doi:10.1016/S0091-6749(98)70190-3
- Vadas P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2016; 117(5):455–457. doi:10.1016/j.anai.2016.05.003
- Stone SF, Brown SG. Mediators released during human anaphylaxis. Curr Allergy Asthma Rep 2012; 12(1):33–41. doi:10.1007/s11882-011-0231-6
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126(3):477–480.e1–e42. doi:10.1016/j.jaci.2010.06.022
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008; 63(8):1061–1070. doi:10.1111/j.1398-9995.2008.01733.x
- Oren E, Banderji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol 2007; 99(5):429–432. doi:10.1016/S1081-1206(10)60568-6
- Uguz A, Lack G, Pumphrey R, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy 2005; 35(6):746–750. doi:10.1111/j.1365-2222.2005.02257.x
- Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117(2):464–465. doi:10.1016/j.jaci.2005.11.015
- Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015; 115(5):341–384. doi:10.1016/j.anai.2015.07.019
- Golden BK, Demain J, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol 2017; 118(1):28–54. doi:10.1016/j.anai.2016.10.031
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Gupta P, Gerrish PK, Silverman B, Schneider A. Current practices among allergists on writing self-injectable epinephrine prescriptions for immunotherapy patients. J Allergy Clin Immunol 2012; 129(2):571–572.e1-e2. doi:10.1016/j.jaci.2011.09.033
- Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy 1993; 71:470–476. pmid: 8250353
- Ma S, Shcherer SH, Nowak-Wegrzyn A. A survey on the management of pollen food allergy syndrome in allergy practices. J Allergy Clin Immunol 2003;112:784–788. doi:10.1016/S0091-6749(03)02008-6
- Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low dose intravenous epinephrine for anaphylaxis. CJEM 2006; 8(4):289–294. pmid:17324313
- Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol 2008; 153(suppl 1):7–11. doi:10.1111/j.1365-2249.2008.03714.x
- Gilman AG, Rail TW, Nies AS, Taylor P, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press; 1990.
- Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 1991; 115(14):270–276. pmid:1677239
- Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015; 135(2):491–499. doi:10.1016/j.jaci.2014.09.004
- Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol 2013; 131(4):1103–1108. doi:10.1016/j.jaci.2013.01.011
- Greenberger PA, Meyers SN, Kramer BL, Kramer BL. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 1987; 80(5):698–702. pmid:2890682
- Hepner MJ, Ownby DR, Anderson JA, Rowe MS, Sears-Ewald D, Brown EB. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 1990; 86(3 pt 1):407–411. pmid:1976666
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy 2015; 45(8):1288–1295. doi:10.1111/cea.12520
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol 2002; 110(4):647–651. pmid:12373275
- Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation 2017; 112:53–58. doi:10.1016/j.resuscitation.2016.12.020
- Cydulka R, Davison R, Grammer L, Parker M, Mathews J 4th. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med 1988; 17(4):322–326. pmid:3354935
- Soar J, Pumphrey R, Cant A, et al; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77(2):157–169. doi:10.1016/j.resuscitation.2008.02.001
- Dreborg S, Wen X, Kim L, et al. Do epinephrine auto-injectors have an unsuitable needle length in children and adolescents at risk for anaphylaxis from food allergy? Allergy Asthma Clin Immunol 2016; 12:11. doi:10.1186/s13223-016-0110-8
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311(8):806–814. doi:10.1001/jama.2014.732
- Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94(5):539–542. doi:10.1016/S1081-1206(10)61130-1
- Bhalla MC, Gable BD, Frey JA, Reichenbach MR, Wilber ST. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med 2013; 31(12):1671–1676. doi:10.1016/j.ajem.2013.09.001
- Kim H, Dinakar C, McInnis P, et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118(6):719–725.e1. doi:10.1016/j.anai.2017.03.017
- Simons FE, Clark S, Camargo CA Jr. Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009; 124(2):301–306. doi:10.1016/j.jaci.2009.03.050
- Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med 2010; 56(3):270–274. doi:10.1016/j.annemergmed.2010.02.019
- Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015; 3(1):57–62. doi:10.1016/j.jaip.2014.07.004
- Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep 2011; 11(1):65–70. doi:10.1007/s11882-010-0155-6
Anaphylaxis is potentially fatal but can be prevented if the trigger is identified and avoided, and death can be avoided if episodes are treated promptly.
A consensus definition of anaphylaxis has been difficult to achieve, with slight variations among international guidelines. The World Allergy Organization classifies anaphylaxis as immunologic, nonimmunologic, or idiopathic.1 The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network highlight clinical symptoms and criteria.2 The International Consensus on Food Allergy describes reactions as being immunoglobulin E (IgE)-mediated, cell-mediated, or a combination of the 2 mechanisms.3
Despite the subtle differences in these definitions, all 3 international organizations have a common recommendation for anaphylaxis: once it is diagnosed, epinephrine is the treatment of choice.
EPINEPHRINE IS THE TREATMENT OF CHOICE FOR ANAPHYLAXIS
Anaphylaxis commonly results from exposure to foods, medications, and Hymenoptera venom.4 Avoiding triggers is key in preventing anaphylaxis but is not always possible.
Although epinephrine is the cornerstone of the emergency treatment of anaphylaxis, many patients instead receive antihistamines and corticosteroids as initial therapy. Some take these medications on their own, and some receive them in emergency departments and outpatient clinics.5
Diphenhydramine, a histamine 1 receptor antagonist, is often used as a first-line medication. But diphenhydramine has a slow onset of action, taking 80 minutes after an oral dose to suppress a histamine-induced cutaneous flare by 50%, and taking 52 minutes with intramuscular administration.6 Corticosteroids also have a slow onset of action. These drugs cannot prevent death in anaphylaxis, a condition in which the median time to respiratory or cardiac arrest is 30 minutes after ingestion of food, 15 minutes after envenomation, and 5 minutes after iatrogenic reactions.7
Combination therapy with diphenhydramine and a histamine 2 receptor antagonist (eg, cimetidine, famotidine) is also commonly used,8 but this combination offers no advantage in terms of onset of action, and a Cochrane review could find no definitive evidence for or against the use of histamine 2 receptor antagonists.9
Because of their slow onset of action, all of these should be second-line therapies, given after epinephrine. Epinephrine is the first line of treatment because it has a maximal pharmacokinetic effect (time to maximal peak serum level) within 10 minutes of intramuscular injection into the thigh.10,11
In addition, epinephrine acts on numerous receptors to antagonize the multiple pathologic effects of the mediators released during an anaphylactic episode. In contrast, antihistamines block only 1 mediator, while mediators other than histamine can be responsible for severe events and deaths.12,13
It is crucial that epinephrine be given immediately, as delay has been associated with fatalities.14 In addition, guidelines recommend repeating epinephrine dosing after 5 to 15 minutes if the response to the first dose is suboptimal.1,2 From 16% to 36% of patients may need a second dose.15–18 Therefore, many physicians recommend that patients at risk of anaphylaxis keep not 1 but 2 epinephrine autoinjectors on hand at all times, and so say the US guidelines for the management of anaphylaxis.19
WHO SHOULD CARRY AN EPINEPHRINE AUTOINJECTOR?
All published guidelines recommend epinephrine as the drug of choice for anaphylaxis. And an epinephrine autoinjector is indicated for anyone who has experienced an anaphylactic event or is at risk of one, and these patients should carry it with them at all times. Such individuals include those with food allergy or Hymenoptera hypersensitivity.
Food allergy
The foods that most often cause anaphylaxis are peanuts, tree nuts, fish, shellfish, milk, and eggs, but any food can cause a reaction.
The prevalence of food allergy has increased over time, and treatments are limited. Some food desensitization protocols look promising but are still in the research stages. The best treatment at this time is to avoid the offending food, but there are accidental exposures.
Hymenoptera hypersensitivity
Patients who have had anaphylaxis after being stung by insects such as bees, wasps, yellow-faced hornets, white-faced hornets, yellow jackets, and fire ants should be evaluated by an allergist. Skin testing and serum IgE testing helps properly diagnose Hymenoptera hypersensitivity.
Once the diagnosis is confirmed, venom immunotherapy should be considered. Some patients choose only to carry an epinephrine autoinjector and to avoid these insects as much as possible. However, most patients also choose to receive venom immunotherapy, because 80% to 90% of those who receive this treatment for 3 to 5 years do not have a systemic reaction if they are stung again.20
Regardless of whether they choose to undergo immunotherapy, sensitive patients should always carry an epinephrine autoinjector. This is also the case after treatment ends, since the therapy is not 100% effective.
PATIENTS FOR WHOM THE NEED MAY BE LESS CLEAR
In other patients who may be at increased risk, the mandate for an epinephrine autoinjector is less clear, and the decision to carry one is determined on an individual basis. Such individuals are those receiving allergen immunotherapy, with large local reactions to insect stings, with oral allergy syndrome, with mastocytosis, and with drug allergy. In these cases, the benefit vs the burden of carrying an autoinjector should be discussed with the patient.
Patients on allergen immunotherapy
National guidelines recommend that all patients who receive allergen immunotherapy be monitored in the clinic under a physician’s supervision for 30 minutes after the injection. Fortunately, life-threatening reactions occurring after 30 minutes are rare. But delayed systemic reactions can occur and may account for up to 50% of such events.21
Therefore, many physicians consider it prudent for patients on immunotherapy to carry an epinephrine autoinjector, but there is no consensus. A survey22 found that 13.5% of allergists did not prescribe the autoinjector for patients on immunotherapy, while 33.3% prescribed it for all their patients on immunotherapy, and the rest prescribed based on risk.
Since there are no national guidelines on epinephrine autoinjectors for patients on immunotherapy, the decision should be based on the patient’s risks and comorbidities and informed by discussion between the individual patient and his or her allergist.
Patients with large local reactions to insect stings
From 5% to 10% of patients who have large local reactions to insect stings are at risk of systemic reactions.20
Patients with oral allergy syndrome
Oral allergy syndrome, also known as pollen-food allergy, causes itching and mild swelling of the mouth, lips, and throat after eating fresh fruits and vegetables. The prevalence ranges from 2% to 10% of patients with allergies.23
A survey of allergists found that 20% of patients with oral allergy syndrome had experienced systemic symptoms.24 The survey also showed that the decision to prescribe an epinephrine autoinjector to these patients was highly variable. Only about 30% of allergists recommend epinephrine autoinjectors to patients with oral allergy syndrome, while most believe that the decision should be based on the individual’s symptoms and risk.
More research is needed in the area of food allergy. Because data are limited, there are no national guidelines on whether these patients should carry an epinephrine autoinjector. We agree with the Joint Task Force on Practice Parameters14 recommendation that the decision be made on an individual basis following discussion between the patient and physician.
Patients with mastocytosis
Patients with mastocytosis and a history of anaphylaxis are at increased risk for systemic reactions to Hymenoptera venom.
Patients with medication allergy
Once medication allergy has been diagnosed, avoidance is usually effective, obviating the need for an epinephrine autoinjector, although the physician has the option of prescribing one.
CAUTIONS, NOT CONTRAINDICATIONS
Physicians may be reluctant to prescribe an epinephrine autoinjector because of the risk of an adverse reaction in patients with hypertension, coronary artery disease, or arrhythmias, and in elderly patients taking multiple drugs, especially drugs that can interact with epinephrine. Nevertheless, there is no absolute contraindication to the use of epinephrine in anaphylaxis.
In patients with atherosclerosis and cardiovascular disease
Epinephrine increases vasoconstriction, heart rate, and cardiac force of contraction. These effects are beneficial during anaphylaxis, but in rare cases patients have experienced myocardial infarction and acute coronary syndrome after receiving intravenous epinephrine.25 These incidents have naturally prompted reluctance to prescribe it in susceptible patients with coronary disease during anaphylaxis.
Yet epinephrine may not be solely to blame for these adverse responses. Mast cells are abundant in the heart, and their release of mediators can also result in adverse cardiac manifestations, including myocardial infarction.26
Conversely, some drugs used to treat cardiovascular disease can worsen anaphylaxis.
Beta-blockers can cause bronchospasm and decrease cardiac contractility. They can also blunt the pharmacologic effects of epinephrine. There is concern that epinephrine may produce dangerous elevations of blood pressure in patients taking beta-blockers by unopposed alpha-adrenergic stimulation and reflex vagotonic effects.27 And there is evidence that beta-blockers may increase the risk and severity of reactions. One study reported that patients taking beta-blockers are more than 8 times more likely to be hospitalized due to anaphylactoid reaction with bronchospasm.28
Beta-blockers and, to a lesser extent, angiotensin-converting enzyme inhibitors have been shown to increase the risk of anaphylaxis in the emergency department.29,30 However, some investigators have not found beta-blockers to be a risk factor. A study evaluating anaphylactoid reactions from contrast media found no statistically significant higher risk in patients taking beta-blockers.31 Similarly, a study of 3,178 patients on beta-blockers receiving venom immunotherapy or allergen immunotherapy found no increase in the frequency of systemic reactions.32 Nevertheless, overall, more studies support the hypothesis that beta-blockers may be an additional risk factor in anaphylaxis.33
Thus, clinicians treating patients with cardiovascular disease and anaphylaxis face a dilemma. Although there is concern in this population, epinephrine should not be withheld in patients with cardiovascular disease who are experiencing an anaphylactic event.33 If epinephrine is not administered, the patient could die.
Elderly patients on multiple medications
Older patients are also at risk of anaphylaxis. But clinicians are reluctant to treat older patients with epinephrine because of concerns about adverse effects.
Epinephrine dispensing rates vary substantially in different age groups: 1.44% for patients under age 17, 0.9% for those ages 17 to 64, and 0.32% for those age 65 or older.34 A Canadian study of 492 patients with anaphylaxis in the emergency department showed that those over age 50 received epinephrine less often than younger patients (36.1% vs 60.5%).35 Cardiovascular complications were more frequent in the older group, occurring in 4 (9.1%) of the 44 older patients who received epinephrine compared with 1 (0.4%) of the 225 younger patients who received it. On the other hand, the rate of adverse effects from subcutaneous epinephrine was no different in older asthma patients compared with younger patients.36
Many older patients take multiple medications, raising concern about adverse effects. Commonly prescribed medications in the elderly can affect the actions of epinephrine. Monoamine oxidase inhibitors retard the catabolism of epinephrine. Tricyclic antidepressants may decrease the reuptake of catecholamines by neurons and thus interfere with the degradation of epinephrine. Digoxin has a narrow therapeutic window and can potentially increase the risk of arrhythmias when given with epinephrine.
Although the clinician must be cautious in treating older patients who have comorbidities, these are not sufficient to withhold prescribing an epinephrine autoinjector to elderly patients at risk of anaphylaxis.
INJECTOR OPTIONS
Epinephrine autoinjectors come preloaded for prompt delivery of the drug. They are intended primarily for use by patients themselves in unsupervised settings in suspected anaphylaxis. Simplicity of use and safety must be considered in such a setting so that patients can use the device correctly and are not incorrectly dosed.
Several models are commercially available, with different ergonomic designs and sizes. EpiPen, the first one marketed in the United States, was introduced in 1987. One device (Auvi-Q) contains an audio chip that gives step-by-step instructions at the time of use. It is hoped that this device will reduce errors in usage during this stressful time for patients and caregivers.
In the United States, epinephrine autoinjectors contain either 0.15 or 0.30 mg of the drug, but some clinicians believe this may not be enough. The UK Resuscitation Council recommends 0.50 mg for patients over age 12,37 and an epinephrine autoinjector with that dose is available in Europe.
Subcutaneous vs intramuscular delivery
The package insert for some epinephrine autoinjectors says the injector can be used to treat anaphylaxis by both subcutaneous and intramuscular administration. However, the routes are not equivalent.
The goal in anaphylaxis is to quickly achieve high tissue and plasma epinephrine concentrations, and studies have found that injection into the vastus lateralis muscle, but not the deltoid muscle, results in faster time to peak plasma concentration: 8 minutes for injection in the vastus lateralis muscle and 34 minutes for subcutaneous delivery.10,11 In addition, injection in the vastus lateralis muscle results in a higher peak plasma concentration than the subcutaneous or deltoid route. Based on these data, intramuscular injection into the vastus lateralis muscle in the thigh appears to be the preferred route of administration of epinephrine.
Obese patients may need a longer needle
Research on the original autoinjector was conducted by the US military, which wanted a rapidly effective and easy-to-use antidote for battlefield exposure to poison gas. The resulting device had 2 separate spring-loaded syringes, 1 containing pralidoxime chloride and the other atropine sulfate. To enable its use through the thick fabric of a chemical warfare suit, the needles were 2.2 cm long.
The first commercial autoinjector to contain epinephrine was made by Survival Technology (Bethesda, MD) in the mid-1970s. The manufacturer considered a 2.2-cm needle to be too long, and the first commercially available epinephrine autoinjector, EpiPen, had a 1.43-cm needle for adult use.
Since then, needle lengths have ranged from 1.17 to 2.5 cm to accommodate different skin-to-muscle depths, with shorter needles for children and longer needles for obese adults.38
However, the prevalence of obesity is high and continues to rise.39 Obesity raises concern that the needles in epinephrine autoinjectors may be too short for the preferred intramuscular delivery, resulting in subcutaneous deposition.
A study that used computed tomography of the thigh found that 1 (2%) of 50 men and 21 (42%) of 50 women studied had a subcutaneous tissue depth greater than 1.43 cm, the needle length in EpiPen. These were not anaphylaxis patients, but the findings suggest that many patients—especially women—may be getting subcutaneous instead of intramuscular delivery with this device.40
Another study that used ultrasonography showed that the 1.43-cm EpiPen needle was too short for 36 (31%) of 116 adults.41 Women were 6.4 times more likely than men to encounter this problem. Other risk factors include higher body mass index, short height, and thicker thighs.
Emerade, an injector with a 2.5-cm needle, is available in some European countries. A longer needle may be helpful in some cases. but we do not yet have enough data to determine the optimal needle length.
Conversely, some children may need shorter needles and may in fact be at risk of having the needle penetrate bone.42 The US Food and Drug Administration recently approved a shorter needle for an epinephrine autoinjector (Auvi-Q) to be used in children weighing 7.5 kg to 15 kg.
BARRIERS TO USING EPINEPHRINE AUTOINJECTORS
Many patients do not use their epinephrine autoinjector in times of anaphylaxis or do not have one with them. Common reasons cited by respondents in a survey43 of 1,385 patients included the following:
They took an oral antihistamine instead (38%).
They never received a prescription for an epinephrine autoinjector (28%).
They thought their symptoms were mild and would resolve with time (13%).
They were afraid (6%). There are reports of accidental injection, typically into fingers, hands, and thumbs. Fortunately, most accidental injections do not require a hand surgeon evaluation or surgery.44 Conservative therapy and monitoring of the injection site are sufficient in most cases.
They could not afford an epinephrine autoinjector (1%).43 Mylan Pharmaceuticals infamously increased the price of its EpiPen to more than $600 for a package of 2 pens. Generic devices are available in the United States but are still too expensive for some patients and are cumbersome to carry.
However, even expensive epinephrine autoinjectors may be cost-effective. Epidemiologic studies have found that patients who did not use an epinephrine autoinjector incurred a higher burden of cost due to emergency department visits and inpatient hospitalizations.45
As a do-it-yourself option, some resourceful patients are obtaining autoinjectors intended for insulin injection, replacing the needle, and filling the injector with epinephrine, at a cost of about $30. (The manufacturer does not endorse this off-label use of their device—www.owenmumford.com/us/patients/if-you-need-to-inject.) Least costly of all is to prescribe multidose vials of epinephrine and regular syringes and teach patients and their caregivers how to draw up the proper dose and give themselves an injection—in essence going back to what was done before 1987.
It was past its expiration date (2%).43 Failure to refill the prescription is common. A California Kaiser Permanente study46 showed that only 46% of patients refilled their epinephrine autoinjector prescription at least once, and the refill rate decreased over time: 43% at 1 to 2 year follow-up, 35% at 3 to 4 years, and 30% at 5 years or longer. Based on these data, it is imperative to educate patients regarding the importance of replacing the epinephrine autoinjector when the old one expires.
NEED FOR PATIENT EDUCATION
Even though prompt treatment with epinephrine decreases fatalities, it continues to be underused in the community. In addition, it is often prescribed without adequate training in its use and appropriate emphasis on the need to keep the device on hand at all times and to replace it in a timely manner if it is used or has expired. Physicians need to educate patients on how to avoid triggers and how to recognize symptoms of anaphylaxis whenever they prescribe an epinephrine autoinjector.
Anaphylaxis is potentially fatal but can be prevented if the trigger is identified and avoided, and death can be avoided if episodes are treated promptly.
A consensus definition of anaphylaxis has been difficult to achieve, with slight variations among international guidelines. The World Allergy Organization classifies anaphylaxis as immunologic, nonimmunologic, or idiopathic.1 The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network highlight clinical symptoms and criteria.2 The International Consensus on Food Allergy describes reactions as being immunoglobulin E (IgE)-mediated, cell-mediated, or a combination of the 2 mechanisms.3
Despite the subtle differences in these definitions, all 3 international organizations have a common recommendation for anaphylaxis: once it is diagnosed, epinephrine is the treatment of choice.
EPINEPHRINE IS THE TREATMENT OF CHOICE FOR ANAPHYLAXIS
Anaphylaxis commonly results from exposure to foods, medications, and Hymenoptera venom.4 Avoiding triggers is key in preventing anaphylaxis but is not always possible.
Although epinephrine is the cornerstone of the emergency treatment of anaphylaxis, many patients instead receive antihistamines and corticosteroids as initial therapy. Some take these medications on their own, and some receive them in emergency departments and outpatient clinics.5
Diphenhydramine, a histamine 1 receptor antagonist, is often used as a first-line medication. But diphenhydramine has a slow onset of action, taking 80 minutes after an oral dose to suppress a histamine-induced cutaneous flare by 50%, and taking 52 minutes with intramuscular administration.6 Corticosteroids also have a slow onset of action. These drugs cannot prevent death in anaphylaxis, a condition in which the median time to respiratory or cardiac arrest is 30 minutes after ingestion of food, 15 minutes after envenomation, and 5 minutes after iatrogenic reactions.7
Combination therapy with diphenhydramine and a histamine 2 receptor antagonist (eg, cimetidine, famotidine) is also commonly used,8 but this combination offers no advantage in terms of onset of action, and a Cochrane review could find no definitive evidence for or against the use of histamine 2 receptor antagonists.9
Because of their slow onset of action, all of these should be second-line therapies, given after epinephrine. Epinephrine is the first line of treatment because it has a maximal pharmacokinetic effect (time to maximal peak serum level) within 10 minutes of intramuscular injection into the thigh.10,11
In addition, epinephrine acts on numerous receptors to antagonize the multiple pathologic effects of the mediators released during an anaphylactic episode. In contrast, antihistamines block only 1 mediator, while mediators other than histamine can be responsible for severe events and deaths.12,13
It is crucial that epinephrine be given immediately, as delay has been associated with fatalities.14 In addition, guidelines recommend repeating epinephrine dosing after 5 to 15 minutes if the response to the first dose is suboptimal.1,2 From 16% to 36% of patients may need a second dose.15–18 Therefore, many physicians recommend that patients at risk of anaphylaxis keep not 1 but 2 epinephrine autoinjectors on hand at all times, and so say the US guidelines for the management of anaphylaxis.19
WHO SHOULD CARRY AN EPINEPHRINE AUTOINJECTOR?
All published guidelines recommend epinephrine as the drug of choice for anaphylaxis. And an epinephrine autoinjector is indicated for anyone who has experienced an anaphylactic event or is at risk of one, and these patients should carry it with them at all times. Such individuals include those with food allergy or Hymenoptera hypersensitivity.
Food allergy
The foods that most often cause anaphylaxis are peanuts, tree nuts, fish, shellfish, milk, and eggs, but any food can cause a reaction.
The prevalence of food allergy has increased over time, and treatments are limited. Some food desensitization protocols look promising but are still in the research stages. The best treatment at this time is to avoid the offending food, but there are accidental exposures.
Hymenoptera hypersensitivity
Patients who have had anaphylaxis after being stung by insects such as bees, wasps, yellow-faced hornets, white-faced hornets, yellow jackets, and fire ants should be evaluated by an allergist. Skin testing and serum IgE testing helps properly diagnose Hymenoptera hypersensitivity.
Once the diagnosis is confirmed, venom immunotherapy should be considered. Some patients choose only to carry an epinephrine autoinjector and to avoid these insects as much as possible. However, most patients also choose to receive venom immunotherapy, because 80% to 90% of those who receive this treatment for 3 to 5 years do not have a systemic reaction if they are stung again.20
Regardless of whether they choose to undergo immunotherapy, sensitive patients should always carry an epinephrine autoinjector. This is also the case after treatment ends, since the therapy is not 100% effective.
PATIENTS FOR WHOM THE NEED MAY BE LESS CLEAR
In other patients who may be at increased risk, the mandate for an epinephrine autoinjector is less clear, and the decision to carry one is determined on an individual basis. Such individuals are those receiving allergen immunotherapy, with large local reactions to insect stings, with oral allergy syndrome, with mastocytosis, and with drug allergy. In these cases, the benefit vs the burden of carrying an autoinjector should be discussed with the patient.
Patients on allergen immunotherapy
National guidelines recommend that all patients who receive allergen immunotherapy be monitored in the clinic under a physician’s supervision for 30 minutes after the injection. Fortunately, life-threatening reactions occurring after 30 minutes are rare. But delayed systemic reactions can occur and may account for up to 50% of such events.21
Therefore, many physicians consider it prudent for patients on immunotherapy to carry an epinephrine autoinjector, but there is no consensus. A survey22 found that 13.5% of allergists did not prescribe the autoinjector for patients on immunotherapy, while 33.3% prescribed it for all their patients on immunotherapy, and the rest prescribed based on risk.
Since there are no national guidelines on epinephrine autoinjectors for patients on immunotherapy, the decision should be based on the patient’s risks and comorbidities and informed by discussion between the individual patient and his or her allergist.
Patients with large local reactions to insect stings
From 5% to 10% of patients who have large local reactions to insect stings are at risk of systemic reactions.20
Patients with oral allergy syndrome
Oral allergy syndrome, also known as pollen-food allergy, causes itching and mild swelling of the mouth, lips, and throat after eating fresh fruits and vegetables. The prevalence ranges from 2% to 10% of patients with allergies.23
A survey of allergists found that 20% of patients with oral allergy syndrome had experienced systemic symptoms.24 The survey also showed that the decision to prescribe an epinephrine autoinjector to these patients was highly variable. Only about 30% of allergists recommend epinephrine autoinjectors to patients with oral allergy syndrome, while most believe that the decision should be based on the individual’s symptoms and risk.
More research is needed in the area of food allergy. Because data are limited, there are no national guidelines on whether these patients should carry an epinephrine autoinjector. We agree with the Joint Task Force on Practice Parameters14 recommendation that the decision be made on an individual basis following discussion between the patient and physician.
Patients with mastocytosis
Patients with mastocytosis and a history of anaphylaxis are at increased risk for systemic reactions to Hymenoptera venom.
Patients with medication allergy
Once medication allergy has been diagnosed, avoidance is usually effective, obviating the need for an epinephrine autoinjector, although the physician has the option of prescribing one.
CAUTIONS, NOT CONTRAINDICATIONS
Physicians may be reluctant to prescribe an epinephrine autoinjector because of the risk of an adverse reaction in patients with hypertension, coronary artery disease, or arrhythmias, and in elderly patients taking multiple drugs, especially drugs that can interact with epinephrine. Nevertheless, there is no absolute contraindication to the use of epinephrine in anaphylaxis.
In patients with atherosclerosis and cardiovascular disease
Epinephrine increases vasoconstriction, heart rate, and cardiac force of contraction. These effects are beneficial during anaphylaxis, but in rare cases patients have experienced myocardial infarction and acute coronary syndrome after receiving intravenous epinephrine.25 These incidents have naturally prompted reluctance to prescribe it in susceptible patients with coronary disease during anaphylaxis.
Yet epinephrine may not be solely to blame for these adverse responses. Mast cells are abundant in the heart, and their release of mediators can also result in adverse cardiac manifestations, including myocardial infarction.26
Conversely, some drugs used to treat cardiovascular disease can worsen anaphylaxis.
Beta-blockers can cause bronchospasm and decrease cardiac contractility. They can also blunt the pharmacologic effects of epinephrine. There is concern that epinephrine may produce dangerous elevations of blood pressure in patients taking beta-blockers by unopposed alpha-adrenergic stimulation and reflex vagotonic effects.27 And there is evidence that beta-blockers may increase the risk and severity of reactions. One study reported that patients taking beta-blockers are more than 8 times more likely to be hospitalized due to anaphylactoid reaction with bronchospasm.28
Beta-blockers and, to a lesser extent, angiotensin-converting enzyme inhibitors have been shown to increase the risk of anaphylaxis in the emergency department.29,30 However, some investigators have not found beta-blockers to be a risk factor. A study evaluating anaphylactoid reactions from contrast media found no statistically significant higher risk in patients taking beta-blockers.31 Similarly, a study of 3,178 patients on beta-blockers receiving venom immunotherapy or allergen immunotherapy found no increase in the frequency of systemic reactions.32 Nevertheless, overall, more studies support the hypothesis that beta-blockers may be an additional risk factor in anaphylaxis.33
Thus, clinicians treating patients with cardiovascular disease and anaphylaxis face a dilemma. Although there is concern in this population, epinephrine should not be withheld in patients with cardiovascular disease who are experiencing an anaphylactic event.33 If epinephrine is not administered, the patient could die.
Elderly patients on multiple medications
Older patients are also at risk of anaphylaxis. But clinicians are reluctant to treat older patients with epinephrine because of concerns about adverse effects.
Epinephrine dispensing rates vary substantially in different age groups: 1.44% for patients under age 17, 0.9% for those ages 17 to 64, and 0.32% for those age 65 or older.34 A Canadian study of 492 patients with anaphylaxis in the emergency department showed that those over age 50 received epinephrine less often than younger patients (36.1% vs 60.5%).35 Cardiovascular complications were more frequent in the older group, occurring in 4 (9.1%) of the 44 older patients who received epinephrine compared with 1 (0.4%) of the 225 younger patients who received it. On the other hand, the rate of adverse effects from subcutaneous epinephrine was no different in older asthma patients compared with younger patients.36
Many older patients take multiple medications, raising concern about adverse effects. Commonly prescribed medications in the elderly can affect the actions of epinephrine. Monoamine oxidase inhibitors retard the catabolism of epinephrine. Tricyclic antidepressants may decrease the reuptake of catecholamines by neurons and thus interfere with the degradation of epinephrine. Digoxin has a narrow therapeutic window and can potentially increase the risk of arrhythmias when given with epinephrine.
Although the clinician must be cautious in treating older patients who have comorbidities, these are not sufficient to withhold prescribing an epinephrine autoinjector to elderly patients at risk of anaphylaxis.
INJECTOR OPTIONS
Epinephrine autoinjectors come preloaded for prompt delivery of the drug. They are intended primarily for use by patients themselves in unsupervised settings in suspected anaphylaxis. Simplicity of use and safety must be considered in such a setting so that patients can use the device correctly and are not incorrectly dosed.
Several models are commercially available, with different ergonomic designs and sizes. EpiPen, the first one marketed in the United States, was introduced in 1987. One device (Auvi-Q) contains an audio chip that gives step-by-step instructions at the time of use. It is hoped that this device will reduce errors in usage during this stressful time for patients and caregivers.
In the United States, epinephrine autoinjectors contain either 0.15 or 0.30 mg of the drug, but some clinicians believe this may not be enough. The UK Resuscitation Council recommends 0.50 mg for patients over age 12,37 and an epinephrine autoinjector with that dose is available in Europe.
Subcutaneous vs intramuscular delivery
The package insert for some epinephrine autoinjectors says the injector can be used to treat anaphylaxis by both subcutaneous and intramuscular administration. However, the routes are not equivalent.
The goal in anaphylaxis is to quickly achieve high tissue and plasma epinephrine concentrations, and studies have found that injection into the vastus lateralis muscle, but not the deltoid muscle, results in faster time to peak plasma concentration: 8 minutes for injection in the vastus lateralis muscle and 34 minutes for subcutaneous delivery.10,11 In addition, injection in the vastus lateralis muscle results in a higher peak plasma concentration than the subcutaneous or deltoid route. Based on these data, intramuscular injection into the vastus lateralis muscle in the thigh appears to be the preferred route of administration of epinephrine.
Obese patients may need a longer needle
Research on the original autoinjector was conducted by the US military, which wanted a rapidly effective and easy-to-use antidote for battlefield exposure to poison gas. The resulting device had 2 separate spring-loaded syringes, 1 containing pralidoxime chloride and the other atropine sulfate. To enable its use through the thick fabric of a chemical warfare suit, the needles were 2.2 cm long.
The first commercial autoinjector to contain epinephrine was made by Survival Technology (Bethesda, MD) in the mid-1970s. The manufacturer considered a 2.2-cm needle to be too long, and the first commercially available epinephrine autoinjector, EpiPen, had a 1.43-cm needle for adult use.
Since then, needle lengths have ranged from 1.17 to 2.5 cm to accommodate different skin-to-muscle depths, with shorter needles for children and longer needles for obese adults.38
However, the prevalence of obesity is high and continues to rise.39 Obesity raises concern that the needles in epinephrine autoinjectors may be too short for the preferred intramuscular delivery, resulting in subcutaneous deposition.
A study that used computed tomography of the thigh found that 1 (2%) of 50 men and 21 (42%) of 50 women studied had a subcutaneous tissue depth greater than 1.43 cm, the needle length in EpiPen. These were not anaphylaxis patients, but the findings suggest that many patients—especially women—may be getting subcutaneous instead of intramuscular delivery with this device.40
Another study that used ultrasonography showed that the 1.43-cm EpiPen needle was too short for 36 (31%) of 116 adults.41 Women were 6.4 times more likely than men to encounter this problem. Other risk factors include higher body mass index, short height, and thicker thighs.
Emerade, an injector with a 2.5-cm needle, is available in some European countries. A longer needle may be helpful in some cases. but we do not yet have enough data to determine the optimal needle length.
Conversely, some children may need shorter needles and may in fact be at risk of having the needle penetrate bone.42 The US Food and Drug Administration recently approved a shorter needle for an epinephrine autoinjector (Auvi-Q) to be used in children weighing 7.5 kg to 15 kg.
BARRIERS TO USING EPINEPHRINE AUTOINJECTORS
Many patients do not use their epinephrine autoinjector in times of anaphylaxis or do not have one with them. Common reasons cited by respondents in a survey43 of 1,385 patients included the following:
They took an oral antihistamine instead (38%).
They never received a prescription for an epinephrine autoinjector (28%).
They thought their symptoms were mild and would resolve with time (13%).
They were afraid (6%). There are reports of accidental injection, typically into fingers, hands, and thumbs. Fortunately, most accidental injections do not require a hand surgeon evaluation or surgery.44 Conservative therapy and monitoring of the injection site are sufficient in most cases.
They could not afford an epinephrine autoinjector (1%).43 Mylan Pharmaceuticals infamously increased the price of its EpiPen to more than $600 for a package of 2 pens. Generic devices are available in the United States but are still too expensive for some patients and are cumbersome to carry.
However, even expensive epinephrine autoinjectors may be cost-effective. Epidemiologic studies have found that patients who did not use an epinephrine autoinjector incurred a higher burden of cost due to emergency department visits and inpatient hospitalizations.45
As a do-it-yourself option, some resourceful patients are obtaining autoinjectors intended for insulin injection, replacing the needle, and filling the injector with epinephrine, at a cost of about $30. (The manufacturer does not endorse this off-label use of their device—www.owenmumford.com/us/patients/if-you-need-to-inject.) Least costly of all is to prescribe multidose vials of epinephrine and regular syringes and teach patients and their caregivers how to draw up the proper dose and give themselves an injection—in essence going back to what was done before 1987.
It was past its expiration date (2%).43 Failure to refill the prescription is common. A California Kaiser Permanente study46 showed that only 46% of patients refilled their epinephrine autoinjector prescription at least once, and the refill rate decreased over time: 43% at 1 to 2 year follow-up, 35% at 3 to 4 years, and 30% at 5 years or longer. Based on these data, it is imperative to educate patients regarding the importance of replacing the epinephrine autoinjector when the old one expires.
NEED FOR PATIENT EDUCATION
Even though prompt treatment with epinephrine decreases fatalities, it continues to be underused in the community. In addition, it is often prescribed without adequate training in its use and appropriate emphasis on the need to keep the device on hand at all times and to replace it in a timely manner if it is used or has expired. Physicians need to educate patients on how to avoid triggers and how to recognize symptoms of anaphylaxis whenever they prescribe an epinephrine autoinjector.
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014; 7(1):9. doi:10.1186/1939-4551-7-9
- NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6 suppl):S1–S58. doi:10.1016/j.jaci.2010.10.007
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129(4):906–920. doi:10.1016/j.jaci.2012.02.001
- Lieberman P, Carmago CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma, and Immunology. Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97(5):596–602. doi:10.1016/S1081-1206(10)61086-1
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(suppl 7):S18–S26. doi:10.1097/WOX.0b013e31817c9338
- Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol 2008; 100(5):452–456. doi:10.1016/S1081-1206(10)60470-X
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allerg 2000; 30(8):1144–1150. pmid:10931122
- Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med 1992; 21:237–242. pmid:1536481
- Sheikh A, Simons FE, Barbour V, Worth A. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev 2012; (8):CD008935. doi:10.1002/14651858.CD008935.pub2
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108(5):871–873. doi:10.1067/mai.2001.119409
- Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101(1 pt 1):33–37. doi:10.1016/S0091-6749(98)70190-3
- Vadas P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2016; 117(5):455–457. doi:10.1016/j.anai.2016.05.003
- Stone SF, Brown SG. Mediators released during human anaphylaxis. Curr Allergy Asthma Rep 2012; 12(1):33–41. doi:10.1007/s11882-011-0231-6
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126(3):477–480.e1–e42. doi:10.1016/j.jaci.2010.06.022
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008; 63(8):1061–1070. doi:10.1111/j.1398-9995.2008.01733.x
- Oren E, Banderji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol 2007; 99(5):429–432. doi:10.1016/S1081-1206(10)60568-6
- Uguz A, Lack G, Pumphrey R, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy 2005; 35(6):746–750. doi:10.1111/j.1365-2222.2005.02257.x
- Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117(2):464–465. doi:10.1016/j.jaci.2005.11.015
- Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015; 115(5):341–384. doi:10.1016/j.anai.2015.07.019
- Golden BK, Demain J, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol 2017; 118(1):28–54. doi:10.1016/j.anai.2016.10.031
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Gupta P, Gerrish PK, Silverman B, Schneider A. Current practices among allergists on writing self-injectable epinephrine prescriptions for immunotherapy patients. J Allergy Clin Immunol 2012; 129(2):571–572.e1-e2. doi:10.1016/j.jaci.2011.09.033
- Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy 1993; 71:470–476. pmid: 8250353
- Ma S, Shcherer SH, Nowak-Wegrzyn A. A survey on the management of pollen food allergy syndrome in allergy practices. J Allergy Clin Immunol 2003;112:784–788. doi:10.1016/S0091-6749(03)02008-6
- Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low dose intravenous epinephrine for anaphylaxis. CJEM 2006; 8(4):289–294. pmid:17324313
- Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol 2008; 153(suppl 1):7–11. doi:10.1111/j.1365-2249.2008.03714.x
- Gilman AG, Rail TW, Nies AS, Taylor P, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press; 1990.
- Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 1991; 115(14):270–276. pmid:1677239
- Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015; 135(2):491–499. doi:10.1016/j.jaci.2014.09.004
- Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol 2013; 131(4):1103–1108. doi:10.1016/j.jaci.2013.01.011
- Greenberger PA, Meyers SN, Kramer BL, Kramer BL. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 1987; 80(5):698–702. pmid:2890682
- Hepner MJ, Ownby DR, Anderson JA, Rowe MS, Sears-Ewald D, Brown EB. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 1990; 86(3 pt 1):407–411. pmid:1976666
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy 2015; 45(8):1288–1295. doi:10.1111/cea.12520
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol 2002; 110(4):647–651. pmid:12373275
- Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation 2017; 112:53–58. doi:10.1016/j.resuscitation.2016.12.020
- Cydulka R, Davison R, Grammer L, Parker M, Mathews J 4th. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med 1988; 17(4):322–326. pmid:3354935
- Soar J, Pumphrey R, Cant A, et al; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77(2):157–169. doi:10.1016/j.resuscitation.2008.02.001
- Dreborg S, Wen X, Kim L, et al. Do epinephrine auto-injectors have an unsuitable needle length in children and adolescents at risk for anaphylaxis from food allergy? Allergy Asthma Clin Immunol 2016; 12:11. doi:10.1186/s13223-016-0110-8
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311(8):806–814. doi:10.1001/jama.2014.732
- Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94(5):539–542. doi:10.1016/S1081-1206(10)61130-1
- Bhalla MC, Gable BD, Frey JA, Reichenbach MR, Wilber ST. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med 2013; 31(12):1671–1676. doi:10.1016/j.ajem.2013.09.001
- Kim H, Dinakar C, McInnis P, et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118(6):719–725.e1. doi:10.1016/j.anai.2017.03.017
- Simons FE, Clark S, Camargo CA Jr. Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009; 124(2):301–306. doi:10.1016/j.jaci.2009.03.050
- Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med 2010; 56(3):270–274. doi:10.1016/j.annemergmed.2010.02.019
- Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015; 3(1):57–62. doi:10.1016/j.jaip.2014.07.004
- Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep 2011; 11(1):65–70. doi:10.1007/s11882-010-0155-6
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014; 7(1):9. doi:10.1186/1939-4551-7-9
- NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6 suppl):S1–S58. doi:10.1016/j.jaci.2010.10.007
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129(4):906–920. doi:10.1016/j.jaci.2012.02.001
- Lieberman P, Carmago CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma, and Immunology. Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97(5):596–602. doi:10.1016/S1081-1206(10)61086-1
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(suppl 7):S18–S26. doi:10.1097/WOX.0b013e31817c9338
- Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol 2008; 100(5):452–456. doi:10.1016/S1081-1206(10)60470-X
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allerg 2000; 30(8):1144–1150. pmid:10931122
- Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med 1992; 21:237–242. pmid:1536481
- Sheikh A, Simons FE, Barbour V, Worth A. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev 2012; (8):CD008935. doi:10.1002/14651858.CD008935.pub2
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108(5):871–873. doi:10.1067/mai.2001.119409
- Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101(1 pt 1):33–37. doi:10.1016/S0091-6749(98)70190-3
- Vadas P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2016; 117(5):455–457. doi:10.1016/j.anai.2016.05.003
- Stone SF, Brown SG. Mediators released during human anaphylaxis. Curr Allergy Asthma Rep 2012; 12(1):33–41. doi:10.1007/s11882-011-0231-6
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126(3):477–480.e1–e42. doi:10.1016/j.jaci.2010.06.022
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008; 63(8):1061–1070. doi:10.1111/j.1398-9995.2008.01733.x
- Oren E, Banderji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol 2007; 99(5):429–432. doi:10.1016/S1081-1206(10)60568-6
- Uguz A, Lack G, Pumphrey R, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy 2005; 35(6):746–750. doi:10.1111/j.1365-2222.2005.02257.x
- Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117(2):464–465. doi:10.1016/j.jaci.2005.11.015
- Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015; 115(5):341–384. doi:10.1016/j.anai.2015.07.019
- Golden BK, Demain J, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol 2017; 118(1):28–54. doi:10.1016/j.anai.2016.10.031
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Gupta P, Gerrish PK, Silverman B, Schneider A. Current practices among allergists on writing self-injectable epinephrine prescriptions for immunotherapy patients. J Allergy Clin Immunol 2012; 129(2):571–572.e1-e2. doi:10.1016/j.jaci.2011.09.033
- Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy 1993; 71:470–476. pmid: 8250353
- Ma S, Shcherer SH, Nowak-Wegrzyn A. A survey on the management of pollen food allergy syndrome in allergy practices. J Allergy Clin Immunol 2003;112:784–788. doi:10.1016/S0091-6749(03)02008-6
- Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low dose intravenous epinephrine for anaphylaxis. CJEM 2006; 8(4):289–294. pmid:17324313
- Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol 2008; 153(suppl 1):7–11. doi:10.1111/j.1365-2249.2008.03714.x
- Gilman AG, Rail TW, Nies AS, Taylor P, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press; 1990.
- Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 1991; 115(14):270–276. pmid:1677239
- Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015; 135(2):491–499. doi:10.1016/j.jaci.2014.09.004
- Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol 2013; 131(4):1103–1108. doi:10.1016/j.jaci.2013.01.011
- Greenberger PA, Meyers SN, Kramer BL, Kramer BL. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 1987; 80(5):698–702. pmid:2890682
- Hepner MJ, Ownby DR, Anderson JA, Rowe MS, Sears-Ewald D, Brown EB. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 1990; 86(3 pt 1):407–411. pmid:1976666
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy 2015; 45(8):1288–1295. doi:10.1111/cea.12520
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol 2002; 110(4):647–651. pmid:12373275
- Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation 2017; 112:53–58. doi:10.1016/j.resuscitation.2016.12.020
- Cydulka R, Davison R, Grammer L, Parker M, Mathews J 4th. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med 1988; 17(4):322–326. pmid:3354935
- Soar J, Pumphrey R, Cant A, et al; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77(2):157–169. doi:10.1016/j.resuscitation.2008.02.001
- Dreborg S, Wen X, Kim L, et al. Do epinephrine auto-injectors have an unsuitable needle length in children and adolescents at risk for anaphylaxis from food allergy? Allergy Asthma Clin Immunol 2016; 12:11. doi:10.1186/s13223-016-0110-8
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311(8):806–814. doi:10.1001/jama.2014.732
- Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94(5):539–542. doi:10.1016/S1081-1206(10)61130-1
- Bhalla MC, Gable BD, Frey JA, Reichenbach MR, Wilber ST. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med 2013; 31(12):1671–1676. doi:10.1016/j.ajem.2013.09.001
- Kim H, Dinakar C, McInnis P, et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118(6):719–725.e1. doi:10.1016/j.anai.2017.03.017
- Simons FE, Clark S, Camargo CA Jr. Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009; 124(2):301–306. doi:10.1016/j.jaci.2009.03.050
- Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med 2010; 56(3):270–274. doi:10.1016/j.annemergmed.2010.02.019
- Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015; 3(1):57–62. doi:10.1016/j.jaip.2014.07.004
- Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep 2011; 11(1):65–70. doi:10.1007/s11882-010-0155-6
KEY POINTS
- Based on current data, there is no absolute contraindication to epinephrine for anaphylaxis. And failure to give epinephrine promptly has resulted in deaths.
- Clinicians concerned about adverse effects of epinephrine may be reluctant to give it during anaphylaxis.
- Education about anaphylaxis and its prompt treatment with epinephrine is critical for patients and their caregivers.
Acute-onset quadriplegia with hyperreflexia
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
Emphysematous cystitis
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
Early intervention initiative cut oncology patient hospitalizations
, according to a report from a large, independent, community-based oncology practice.
The program saved nearly $3.2 million in Medicare costs over the course of the year, said Molly Mendenhall, RN, of Oncology Hematology Care in Cincinnati.
“By keeping those patients out of the hospital, we were able to improve patient quality of life, and increase patient satisfaction by treating them in their home clinic instead of the hospital,” Ms. Mendenhall said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
The campaign was developed in anticipation of participating in the Oncology Care Model (OCM), a program focused on providing coordinated and high-quality care for Medicare oncology patients at the same or lower cost.
Prior to participating in OCM, Ms. Mendenhall and her colleagues set up an after-hours phone triage system, proactive chemotherapy follow-up calls, and a Saturday-Sunday urgent care clinic designed to help avoid any unnecessary hospitalizations over the weekend.
They also set up a 2-hour structured OCM treatment planning visit to prioritize shared decision making between the patient and the clinical team regarding diagnosis, symptom management, financial assistance, and other aspects of care.
The most influential part of the initiative, according to Ms. Mendenhall, was a patient-directed “Call Us Early – Call Us First” campaign that included symptom management teaching sheets, a 34-page teaching book, and branded buttons, pens, and magnets all designed to emphasize the patient responsibility to use the phone.
“All of those previous steps really wouldn’t make a difference if the patients still weren’t calling us,” Ms. Mendenhall explained.
Over the first year of participation in the OCM program, the oncology practice saw a 16% statistically significant reduction in hospital admissions (P = .005). The number of inpatient admissions per 100 patients dropped from 26.8 at baseline to 22.6 at the most recent follow-up in a report published simultaneously in the Journal of Oncology Practice.
Reduced admissions translated into a drop of $798,000 in inpatient costs per quarter over 1,600 patients, or $3.129 million in savings for the Centers for Medicare & Medicaid Services over the first year of participation in OCM, according to the researchers.
Patient satisfaction scores trended positively over the course of that year based on a review of blinded surveys that asked patients to rate clinical care, communication, access, information exchange, and other aspects of their experience.
Scores on those surveys were 8.03 on a scale of 0-10 (low to high) during the baseline period of January to September 2016, the researchers said. Scores were 8.29 and 8.26 for two follow-up surveys.
Ms. Mendenhall had no disclosures to report. Coauthors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, and Lilly. Two coauthors reported leadership, stock, or other ownership interests in Oncology Hematology Care/US Oncology.
SOURCE: Mendenhall M et al. Quality Care Symposium, Abstract 30.
, according to a report from a large, independent, community-based oncology practice.
The program saved nearly $3.2 million in Medicare costs over the course of the year, said Molly Mendenhall, RN, of Oncology Hematology Care in Cincinnati.
“By keeping those patients out of the hospital, we were able to improve patient quality of life, and increase patient satisfaction by treating them in their home clinic instead of the hospital,” Ms. Mendenhall said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
The campaign was developed in anticipation of participating in the Oncology Care Model (OCM), a program focused on providing coordinated and high-quality care for Medicare oncology patients at the same or lower cost.
Prior to participating in OCM, Ms. Mendenhall and her colleagues set up an after-hours phone triage system, proactive chemotherapy follow-up calls, and a Saturday-Sunday urgent care clinic designed to help avoid any unnecessary hospitalizations over the weekend.
They also set up a 2-hour structured OCM treatment planning visit to prioritize shared decision making between the patient and the clinical team regarding diagnosis, symptom management, financial assistance, and other aspects of care.
The most influential part of the initiative, according to Ms. Mendenhall, was a patient-directed “Call Us Early – Call Us First” campaign that included symptom management teaching sheets, a 34-page teaching book, and branded buttons, pens, and magnets all designed to emphasize the patient responsibility to use the phone.
“All of those previous steps really wouldn’t make a difference if the patients still weren’t calling us,” Ms. Mendenhall explained.
Over the first year of participation in the OCM program, the oncology practice saw a 16% statistically significant reduction in hospital admissions (P = .005). The number of inpatient admissions per 100 patients dropped from 26.8 at baseline to 22.6 at the most recent follow-up in a report published simultaneously in the Journal of Oncology Practice.
Reduced admissions translated into a drop of $798,000 in inpatient costs per quarter over 1,600 patients, or $3.129 million in savings for the Centers for Medicare & Medicaid Services over the first year of participation in OCM, according to the researchers.
Patient satisfaction scores trended positively over the course of that year based on a review of blinded surveys that asked patients to rate clinical care, communication, access, information exchange, and other aspects of their experience.
Scores on those surveys were 8.03 on a scale of 0-10 (low to high) during the baseline period of January to September 2016, the researchers said. Scores were 8.29 and 8.26 for two follow-up surveys.
Ms. Mendenhall had no disclosures to report. Coauthors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, and Lilly. Two coauthors reported leadership, stock, or other ownership interests in Oncology Hematology Care/US Oncology.
SOURCE: Mendenhall M et al. Quality Care Symposium, Abstract 30.
, according to a report from a large, independent, community-based oncology practice.
The program saved nearly $3.2 million in Medicare costs over the course of the year, said Molly Mendenhall, RN, of Oncology Hematology Care in Cincinnati.
“By keeping those patients out of the hospital, we were able to improve patient quality of life, and increase patient satisfaction by treating them in their home clinic instead of the hospital,” Ms. Mendenhall said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
The campaign was developed in anticipation of participating in the Oncology Care Model (OCM), a program focused on providing coordinated and high-quality care for Medicare oncology patients at the same or lower cost.
Prior to participating in OCM, Ms. Mendenhall and her colleagues set up an after-hours phone triage system, proactive chemotherapy follow-up calls, and a Saturday-Sunday urgent care clinic designed to help avoid any unnecessary hospitalizations over the weekend.
They also set up a 2-hour structured OCM treatment planning visit to prioritize shared decision making between the patient and the clinical team regarding diagnosis, symptom management, financial assistance, and other aspects of care.
The most influential part of the initiative, according to Ms. Mendenhall, was a patient-directed “Call Us Early – Call Us First” campaign that included symptom management teaching sheets, a 34-page teaching book, and branded buttons, pens, and magnets all designed to emphasize the patient responsibility to use the phone.
“All of those previous steps really wouldn’t make a difference if the patients still weren’t calling us,” Ms. Mendenhall explained.
Over the first year of participation in the OCM program, the oncology practice saw a 16% statistically significant reduction in hospital admissions (P = .005). The number of inpatient admissions per 100 patients dropped from 26.8 at baseline to 22.6 at the most recent follow-up in a report published simultaneously in the Journal of Oncology Practice.
Reduced admissions translated into a drop of $798,000 in inpatient costs per quarter over 1,600 patients, or $3.129 million in savings for the Centers for Medicare & Medicaid Services over the first year of participation in OCM, according to the researchers.
Patient satisfaction scores trended positively over the course of that year based on a review of blinded surveys that asked patients to rate clinical care, communication, access, information exchange, and other aspects of their experience.
Scores on those surveys were 8.03 on a scale of 0-10 (low to high) during the baseline period of January to September 2016, the researchers said. Scores were 8.29 and 8.26 for two follow-up surveys.
Ms. Mendenhall had no disclosures to report. Coauthors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, and Lilly. Two coauthors reported leadership, stock, or other ownership interests in Oncology Hematology Care/US Oncology.
SOURCE: Mendenhall M et al. Quality Care Symposium, Abstract 30.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: An initiative designed to reduce avoidable emergency room visits and hospitalizations reduced both admissions and inpatient costs.
Major finding: The program cut admissions by 16% and saved nearly $3.2 million in Medicare costs savings over the course of a year.
Study details: Analysis of first-year experience including 1,600 patients per quarter for a large, independent, community-based oncology practice participating in the Oncology Care Model (OCM) of the Centers for Medicare and Medicaid Services.
Disclosures: Authors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, Lilly, and Oncology Hematology Care/US Oncology.
Source: Mendenhall M et al. Quality Care Symposium, Abstract 30.
Effectiveness of Epinephrine in Out-of-Hospital Cardiac Arrest
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
Study Overview
Objective. To assess the safety and effectiveness of the use of epinephrine in out-of-hospital cardiac arrest patients.
Design. Randomized, double-blind placebo-controlled trial in the United Kingdom.
Setting and participants. Patients aged 16 years or older who had sustained an out-of-hospital cardiac arrest for which advanced life support was provided by trial-trained paramedics were eligible for inclusion. Exclusion criteria included apparent pregnancy, arrest from anaphylaxis or asthma, or the administration of epinephrine before the arrival of the trial-trained paramedic. In 1 of the 5 ambulance services, traumatic cardiac arrests were also excluded in accordance with local protocol.
Main outcome measures. The primary outcome was the rate of survival at 30 days. Secondary outcomes included rate of survival until hospital admission, length of stay in the hospital and intensive care unit (ICU), rates of survival at hospital discharge and at 3 months, and neurologic outcomes at hospital discharge and at 3 months.
Main results. Between December 2014 and October 2017, 10,623 patients were screened for eligibility in 5 National Health Service ambulance services in the United Kingdom. Of these, 8103 were eligible, and 8014 patients were assigned to either the epinephrine group (4015 patients) or the placebo group (3999 patients).
For the primary outcome, 130 patients (3.2%) in the epinephrine group were alive at 30 days in comparison to 94 patients (2.4%) in the placebo group (unadjusted odds ratio [OR] for survival, 1.39; 95% confidence interval [CI], 1.06-1.82; P = 0.02). The number needed to treat for a 30-day survival was 112 patients (95% CI, 63-500).
For the secondary outcomes, the epinephrine group had a higher survival until hospital admission: 947 patients (23.8%) as compared to 319 (8.0%) patients in the placebo group (unadjusted OR, 3.59). Otherwise, there were no difference between the 2 groups in the hospital and ICU LOS. There also was not a significant difference between the epinephrine group and the placebo group in the proportion of patients who survived until hospital discharge: 87 of 4007 patients (2.2%) in the epinephrine group and 74 of 3994 patients (1.9%) in the placebo group, with an unadjusted OR of 1.18 (95% CI, 0.85-1.61). Patients in the epinephrine group had a higher rate of severe neurologic impairment at discharge: 39 of 126 patients (31.0%) versus 16 of 90 patients (17.8%).
Conclusion. Among adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a higher rate of 30-day survival as compared with the use of placebo; however, there was no difference in the rate of a favorable neurologic outcome as more survivors in the epinephrine group had severe neurologic impairment.
Commentary
Epinephrine has been used as part of the resuscitation of patients with cardiac arrest since the 1960s. Epinephrine increases vasomotor tone during circulatory collapse, shunts more blood to the heart, and increases the likelihood of restoring spontaneous circulation.1 However, epinephrine also decreases microvascular blood flow and can result in long-term organ dysfunction or hypoperfusion of the heart and brain.2 The current study, the PARAMEDIC2 trial, by Perkins and colleagues is the largest randomized controlled trial to date to address the question of patient-centered benefit of the use of epinephrine during out-of-hospital cardiac arrest.
Similar to prior studies, patients who received epinephrine had a higher rate of 30-day survival than those who received placebo. However, there was no clear improvement in functional recovery among patients who survived, and the proportion of survivors with severe neurologic impairment was higher in the epinephrine group as compared to the placebo group. These results demonstrate that despite its ability to restore spontaneous circulation after out-of-hospital cardiac arrest, epinephrine produced only a small absolute increase in survival with worse functional recovery as compared with placebo.
One major limitation of this study is that the protocol did not control for or measure in-hospital treatments. In a prior study, the most common cause of in-hospital death was iatrogenic limitation of life support, which may result in the death of potentially viable patients.3 Another limitation of the study was the timing to administration of epinephrine. In the current study, paramedics administered the trial agent within a median of 21 minutes after the emergency call, which is a longer duration than previous out-of-hospital trials.4 In addition, this time to administration is much longer than that of in-hospital cardiac arrest, where epinephrine is administered a median of 3 minutes after resuscitation starts.5 Therefore, the results from this study cannot be extrapolated to patients with in-hospital cardiac arrest.
Applications for Clinical Practice
The current study by Perkins et al demonstrated the powerful effect of epinephrine in restoring spontaneous circulation after out-of-hospital cardiac arrest. However, epinephrine produced only a small absolute increase in survival with worse functional recovery, as compared to placebo. While further studies regarding dosage of epinephrine as well as administration based on the basis of cardiac rhythm are needed, we should question our tradition of using epinephrine in out-of-hospital cardiac arrest if meaningful neurological function is our priority.
—Ka Ming Gordon Ngai, MD, MPH, FACEP
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
1. Paradis NA, Martin GB, Rosenberg J, et al. The effect of standard- ad high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139-1144.
2. Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149.
3. Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135.
4. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711-1722.
5. Donnino MW, Salciccioli JD, Howell MD, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028l.
FDA approves sufentanil for adults with acute pain
The Food and Drug Administration on Nov. 2 approved sufentanil (Dsuvia) for managing acute pain in adult patients in certified, medically supervised health care settings.
Sufentanil, an opioid analgesic manufactured by AcelRx Pharmaceuticals, was approved as a 30-mcg sublingual tablet. The efficacy of Dsuvia was shown in a randomized, clinical trial where patients who received the drug demonstrated significantly greater pain relief after both 15 minutes and 12 hours, compared with placebo.
“As a single-dose, noninvasive medication with a rapid reduction in pain intensity, Dsuvia represents an important alternative for health care providers to offer patients for acute pain management,” David Leiman, MD, of the department of surgery at the University of Texas, Houston, said in the AcelRx press statement.
FDA Commissioner Scott Gottlieb, MD, commented on the approval amid concerns expressed by some, such as the advocacy group Public Citizen, that the drug is “more than 1,000 times more potent than morphine,” and that approval could lead to diversion and abuse – particularly in light of the U.S. opioid epidemic.
In his statement, Dr. Gottlieb identified one broad, significant issue. “Why do we need an oral formulation of sufentanil – a more potent form of fentanyl that’s been approved for intravenous and epidural use in the U.S. since 1984 – on the market?”
In particular, he focused on the needs of the military. The Department of Defense has taken interest in sufentanil as it fulfills a small but specific battlefield need, namely as a means of pain relief in battlefield situations where soldiers cannot swallow oral medication and access to intravenous medication is limited.
Dr. Gottlieb made clear that sufentanil was meant only to be taken in controlled settings and will have strong limitations on its use. It cannot be prescribed for home use, and treatment should be limited to 72 hours. It can only be delivered by health care professionals using a single-dose applicator and will not be available in pharmacies. It is only to be used in patients who have not tolerated or are expected not to tolerate alternative methods of pain management.
“The FDA has implemented a REMS [Risk Evaluation and Mitigation Strategy] that reflects the potential risks associated with this product and mandates that Dsuvia will only be made available for use in a certified medically supervised heath care setting, including its use on the battlefield,” Dr. Gottlieb said.
However, he recognized that the debate runs deeper than how the FDA should mitigate risk over a new drug, and “as a public health agency, we have an obligation to address this question openly and directly. As a physician and regulator, I won’t bypass legitimate questions and concerns related to our role in addressing the opioid crisis,” he said.
Find Dr. Gottlieb’s full statement on the FDA website.
The Food and Drug Administration on Nov. 2 approved sufentanil (Dsuvia) for managing acute pain in adult patients in certified, medically supervised health care settings.
Sufentanil, an opioid analgesic manufactured by AcelRx Pharmaceuticals, was approved as a 30-mcg sublingual tablet. The efficacy of Dsuvia was shown in a randomized, clinical trial where patients who received the drug demonstrated significantly greater pain relief after both 15 minutes and 12 hours, compared with placebo.
“As a single-dose, noninvasive medication with a rapid reduction in pain intensity, Dsuvia represents an important alternative for health care providers to offer patients for acute pain management,” David Leiman, MD, of the department of surgery at the University of Texas, Houston, said in the AcelRx press statement.
FDA Commissioner Scott Gottlieb, MD, commented on the approval amid concerns expressed by some, such as the advocacy group Public Citizen, that the drug is “more than 1,000 times more potent than morphine,” and that approval could lead to diversion and abuse – particularly in light of the U.S. opioid epidemic.
In his statement, Dr. Gottlieb identified one broad, significant issue. “Why do we need an oral formulation of sufentanil – a more potent form of fentanyl that’s been approved for intravenous and epidural use in the U.S. since 1984 – on the market?”
In particular, he focused on the needs of the military. The Department of Defense has taken interest in sufentanil as it fulfills a small but specific battlefield need, namely as a means of pain relief in battlefield situations where soldiers cannot swallow oral medication and access to intravenous medication is limited.
Dr. Gottlieb made clear that sufentanil was meant only to be taken in controlled settings and will have strong limitations on its use. It cannot be prescribed for home use, and treatment should be limited to 72 hours. It can only be delivered by health care professionals using a single-dose applicator and will not be available in pharmacies. It is only to be used in patients who have not tolerated or are expected not to tolerate alternative methods of pain management.
“The FDA has implemented a REMS [Risk Evaluation and Mitigation Strategy] that reflects the potential risks associated with this product and mandates that Dsuvia will only be made available for use in a certified medically supervised heath care setting, including its use on the battlefield,” Dr. Gottlieb said.
However, he recognized that the debate runs deeper than how the FDA should mitigate risk over a new drug, and “as a public health agency, we have an obligation to address this question openly and directly. As a physician and regulator, I won’t bypass legitimate questions and concerns related to our role in addressing the opioid crisis,” he said.
Find Dr. Gottlieb’s full statement on the FDA website.
The Food and Drug Administration on Nov. 2 approved sufentanil (Dsuvia) for managing acute pain in adult patients in certified, medically supervised health care settings.
Sufentanil, an opioid analgesic manufactured by AcelRx Pharmaceuticals, was approved as a 30-mcg sublingual tablet. The efficacy of Dsuvia was shown in a randomized, clinical trial where patients who received the drug demonstrated significantly greater pain relief after both 15 minutes and 12 hours, compared with placebo.
“As a single-dose, noninvasive medication with a rapid reduction in pain intensity, Dsuvia represents an important alternative for health care providers to offer patients for acute pain management,” David Leiman, MD, of the department of surgery at the University of Texas, Houston, said in the AcelRx press statement.
FDA Commissioner Scott Gottlieb, MD, commented on the approval amid concerns expressed by some, such as the advocacy group Public Citizen, that the drug is “more than 1,000 times more potent than morphine,” and that approval could lead to diversion and abuse – particularly in light of the U.S. opioid epidemic.
In his statement, Dr. Gottlieb identified one broad, significant issue. “Why do we need an oral formulation of sufentanil – a more potent form of fentanyl that’s been approved for intravenous and epidural use in the U.S. since 1984 – on the market?”
In particular, he focused on the needs of the military. The Department of Defense has taken interest in sufentanil as it fulfills a small but specific battlefield need, namely as a means of pain relief in battlefield situations where soldiers cannot swallow oral medication and access to intravenous medication is limited.
Dr. Gottlieb made clear that sufentanil was meant only to be taken in controlled settings and will have strong limitations on its use. It cannot be prescribed for home use, and treatment should be limited to 72 hours. It can only be delivered by health care professionals using a single-dose applicator and will not be available in pharmacies. It is only to be used in patients who have not tolerated or are expected not to tolerate alternative methods of pain management.
“The FDA has implemented a REMS [Risk Evaluation and Mitigation Strategy] that reflects the potential risks associated with this product and mandates that Dsuvia will only be made available for use in a certified medically supervised heath care setting, including its use on the battlefield,” Dr. Gottlieb said.
However, he recognized that the debate runs deeper than how the FDA should mitigate risk over a new drug, and “as a public health agency, we have an obligation to address this question openly and directly. As a physician and regulator, I won’t bypass legitimate questions and concerns related to our role in addressing the opioid crisis,” he said.
Find Dr. Gottlieb’s full statement on the FDA website.
Renal vein thrombosis and pulmonary embolism
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
Which patients with pulmonary embolism need echocardiography?
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20