User login
Radiotherapy for early breast cancer: Sharp cutoff at age 70
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF RADIATION ONCOLOGY: BIOLOGY, PHYSICS
Physician opinions vary on surveillance colonoscopies in older adults with prior adenomas, survey finds
Physician recommendations for surveillance colonoscopies in older adults with prior adenomas vary based on several factors, including patient age, health, adenoma risk, and physician specialty, according to a national survey.
In general, physicians were more likely to recommend surveillance for patients at a younger age, with better health, and with prior high-risk adenomas. Additionally, a large proportion of physicians reported uncertainty about whether the benefits of continued surveillance outweighed the risk of harm in older adults.
“There are no existing surveillance colonoscopy guidelines that integrate patient age, health, and adenoma risk, and physicians report significant decisional uncertainty,” Nancy Schoenborn, MD, MHS, associate professor of medicine at Johns Hopkins University, Baltimore, and colleagues wrote.
“Developing the evidence base to evaluate the risks and benefits of surveillance colonoscopy in older adults and decisional support tools that help physicians and patients incorporate available data and weigh risks and benefits are needed to address current gaps in care for older adults with prior adenomas,” the authors wrote.
The study was published online in the American Journal of Gastroenterology.
Surveying physicians
National guidelines recommend surveillance colonoscopy after adenoma removal at more frequent intervals than screening colonoscopy because of a higher risk of colorectal cancer among patients with adenomas. The high quality of screening colonoscopies coupled with an aging population means that many older adults have a history of adenomas and continue to undergo surveillance colonoscopies, the authors wrote.
they wrote.
Dr. Schoenborn and colleagues conducted a national cross-sectional survey of 1,800 primary care physicians and 600 gastroenterologists between April and November 2021. The primary care group included internal medicine, family medicine, general practice, and geriatric medicine physicians.
The research team asked whether physicians would recommend surveillance colonoscopy in a series of 12 vignettes that varied by patient age (75 or 85), patient health (good, medium, or poor), and prior adenoma risk (low or high).
Good health was described as well-controlled hypertension and living independently, whereas moderate health was described as moderate heart failure and has difficulty walking, and poor health was described as severe chronic obstructive pulmonary disease on oxygenand requires help with self-care.
For prior adenomas, high risk involved five tubular adenomas, one of which was 15 mm, and low risk involved two tubular adenomas, both of which were less than 10 mm. The survey also noted that the recommended surveillance intervals were 3 years in the high-risk scenario and 7 years in the low-risk scenario.
Researchers mailed 2,400 surveys and received 1,040 responses. They included 874 in the analysis because the physician respondents provided care to patients ages 65 and older and spent time seeing patients in clinic. Decisions about surveillance colonoscopies for adenomas in the absence of symptoms almost always occur in the outpatient setting, rather than acute or urgent care, the authors wrote.
Large variations found
Overall, physicians were less likely to recommend surveillance colonoscopies if the patient was older, had poor health, and had lower-risk adenomas. Patient age and health had larger effects on decision-making than adenoma risk, with health status having the largest effect.
About 20.6% of physicians recommended surveillance if the patient was 85, compared with 49.8% if the patient was 75. In addition, 7.1% of physicians recommended surveillance if the patient was in poor health, compared with 28.8% for those in moderate health, and 67.7% for patients in good health.
If the prior adenoma was low risk, 29.7% of physicians recommended surveillance, compared with 41.6% if the prior adenoma was high risk.
In general, family medicine and general practice physicians were most likely to recommend surveillance, at 40%, and gastroenterologists were least likely to recommend surveillance, at 30.9%. Patient age and health had larger effects among gastroenterologists than among primary care physicians, and adenoma risk had similar effects between the two groups.
“The importance of patient age and health status found in our study mirrors study results on physician decision-making regarding screening colonoscopies in older adults and makes intuitive sense,” the authors wrote. “Whether the priorities reflected in our findings are supported by evidence is not clear, and our results highlight important knowledge gaps in the field that warrant future research.”
Physician uncertainty
Additional guidance would be helpful, the authors wrote. In the survey, about 52.3% of primary care physicians and 35.4% of gastroenterologists reported uncertainty about the benefit–harm balance of surveillance in older adults.
“Current guidelines on surveillance colonoscopies are solely based on prior adenoma characteristics,” the authors wrote. “Guidelines need to incorporate guidance that considers patient age and health status, as well as adenoma risk, and explicitly considers when surveillance should stop in older adults.”
In addition, most physicians in the survey – 85.9% of primary care physicians and 77% of gastroenterologists – said they would find a decision support tool helpful. At the same time, 32.8% of primary care physicians and 71.5% of gastroenterologists perceived it as the gastroenterologist’s role to decide about surveillance colonoscopies.
“Developing patient-facing materials, communication tools for clinicians, and tools to support shared decision-making about surveillance colonoscopies that engage both physicians and patients are all important next steps,” the authors wrote. “To our knowledge, there is no existing patient decision aid about surveillance colonoscopies; developing such a tool may be valuable.”
The study was supported by Dr. Schoenborn’s career development award from the National Institute on Aging. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Physician recommendations for surveillance colonoscopies in older adults with prior adenomas vary based on several factors, including patient age, health, adenoma risk, and physician specialty, according to a national survey.
In general, physicians were more likely to recommend surveillance for patients at a younger age, with better health, and with prior high-risk adenomas. Additionally, a large proportion of physicians reported uncertainty about whether the benefits of continued surveillance outweighed the risk of harm in older adults.
“There are no existing surveillance colonoscopy guidelines that integrate patient age, health, and adenoma risk, and physicians report significant decisional uncertainty,” Nancy Schoenborn, MD, MHS, associate professor of medicine at Johns Hopkins University, Baltimore, and colleagues wrote.
“Developing the evidence base to evaluate the risks and benefits of surveillance colonoscopy in older adults and decisional support tools that help physicians and patients incorporate available data and weigh risks and benefits are needed to address current gaps in care for older adults with prior adenomas,” the authors wrote.
The study was published online in the American Journal of Gastroenterology.
Surveying physicians
National guidelines recommend surveillance colonoscopy after adenoma removal at more frequent intervals than screening colonoscopy because of a higher risk of colorectal cancer among patients with adenomas. The high quality of screening colonoscopies coupled with an aging population means that many older adults have a history of adenomas and continue to undergo surveillance colonoscopies, the authors wrote.
they wrote.
Dr. Schoenborn and colleagues conducted a national cross-sectional survey of 1,800 primary care physicians and 600 gastroenterologists between April and November 2021. The primary care group included internal medicine, family medicine, general practice, and geriatric medicine physicians.
The research team asked whether physicians would recommend surveillance colonoscopy in a series of 12 vignettes that varied by patient age (75 or 85), patient health (good, medium, or poor), and prior adenoma risk (low or high).
Good health was described as well-controlled hypertension and living independently, whereas moderate health was described as moderate heart failure and has difficulty walking, and poor health was described as severe chronic obstructive pulmonary disease on oxygenand requires help with self-care.
For prior adenomas, high risk involved five tubular adenomas, one of which was 15 mm, and low risk involved two tubular adenomas, both of which were less than 10 mm. The survey also noted that the recommended surveillance intervals were 3 years in the high-risk scenario and 7 years in the low-risk scenario.
Researchers mailed 2,400 surveys and received 1,040 responses. They included 874 in the analysis because the physician respondents provided care to patients ages 65 and older and spent time seeing patients in clinic. Decisions about surveillance colonoscopies for adenomas in the absence of symptoms almost always occur in the outpatient setting, rather than acute or urgent care, the authors wrote.
Large variations found
Overall, physicians were less likely to recommend surveillance colonoscopies if the patient was older, had poor health, and had lower-risk adenomas. Patient age and health had larger effects on decision-making than adenoma risk, with health status having the largest effect.
About 20.6% of physicians recommended surveillance if the patient was 85, compared with 49.8% if the patient was 75. In addition, 7.1% of physicians recommended surveillance if the patient was in poor health, compared with 28.8% for those in moderate health, and 67.7% for patients in good health.
If the prior adenoma was low risk, 29.7% of physicians recommended surveillance, compared with 41.6% if the prior adenoma was high risk.
In general, family medicine and general practice physicians were most likely to recommend surveillance, at 40%, and gastroenterologists were least likely to recommend surveillance, at 30.9%. Patient age and health had larger effects among gastroenterologists than among primary care physicians, and adenoma risk had similar effects between the two groups.
“The importance of patient age and health status found in our study mirrors study results on physician decision-making regarding screening colonoscopies in older adults and makes intuitive sense,” the authors wrote. “Whether the priorities reflected in our findings are supported by evidence is not clear, and our results highlight important knowledge gaps in the field that warrant future research.”
Physician uncertainty
Additional guidance would be helpful, the authors wrote. In the survey, about 52.3% of primary care physicians and 35.4% of gastroenterologists reported uncertainty about the benefit–harm balance of surveillance in older adults.
“Current guidelines on surveillance colonoscopies are solely based on prior adenoma characteristics,” the authors wrote. “Guidelines need to incorporate guidance that considers patient age and health status, as well as adenoma risk, and explicitly considers when surveillance should stop in older adults.”
In addition, most physicians in the survey – 85.9% of primary care physicians and 77% of gastroenterologists – said they would find a decision support tool helpful. At the same time, 32.8% of primary care physicians and 71.5% of gastroenterologists perceived it as the gastroenterologist’s role to decide about surveillance colonoscopies.
“Developing patient-facing materials, communication tools for clinicians, and tools to support shared decision-making about surveillance colonoscopies that engage both physicians and patients are all important next steps,” the authors wrote. “To our knowledge, there is no existing patient decision aid about surveillance colonoscopies; developing such a tool may be valuable.”
The study was supported by Dr. Schoenborn’s career development award from the National Institute on Aging. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Physician recommendations for surveillance colonoscopies in older adults with prior adenomas vary based on several factors, including patient age, health, adenoma risk, and physician specialty, according to a national survey.
In general, physicians were more likely to recommend surveillance for patients at a younger age, with better health, and with prior high-risk adenomas. Additionally, a large proportion of physicians reported uncertainty about whether the benefits of continued surveillance outweighed the risk of harm in older adults.
“There are no existing surveillance colonoscopy guidelines that integrate patient age, health, and adenoma risk, and physicians report significant decisional uncertainty,” Nancy Schoenborn, MD, MHS, associate professor of medicine at Johns Hopkins University, Baltimore, and colleagues wrote.
“Developing the evidence base to evaluate the risks and benefits of surveillance colonoscopy in older adults and decisional support tools that help physicians and patients incorporate available data and weigh risks and benefits are needed to address current gaps in care for older adults with prior adenomas,” the authors wrote.
The study was published online in the American Journal of Gastroenterology.
Surveying physicians
National guidelines recommend surveillance colonoscopy after adenoma removal at more frequent intervals than screening colonoscopy because of a higher risk of colorectal cancer among patients with adenomas. The high quality of screening colonoscopies coupled with an aging population means that many older adults have a history of adenomas and continue to undergo surveillance colonoscopies, the authors wrote.
they wrote.
Dr. Schoenborn and colleagues conducted a national cross-sectional survey of 1,800 primary care physicians and 600 gastroenterologists between April and November 2021. The primary care group included internal medicine, family medicine, general practice, and geriatric medicine physicians.
The research team asked whether physicians would recommend surveillance colonoscopy in a series of 12 vignettes that varied by patient age (75 or 85), patient health (good, medium, or poor), and prior adenoma risk (low or high).
Good health was described as well-controlled hypertension and living independently, whereas moderate health was described as moderate heart failure and has difficulty walking, and poor health was described as severe chronic obstructive pulmonary disease on oxygenand requires help with self-care.
For prior adenomas, high risk involved five tubular adenomas, one of which was 15 mm, and low risk involved two tubular adenomas, both of which were less than 10 mm. The survey also noted that the recommended surveillance intervals were 3 years in the high-risk scenario and 7 years in the low-risk scenario.
Researchers mailed 2,400 surveys and received 1,040 responses. They included 874 in the analysis because the physician respondents provided care to patients ages 65 and older and spent time seeing patients in clinic. Decisions about surveillance colonoscopies for adenomas in the absence of symptoms almost always occur in the outpatient setting, rather than acute or urgent care, the authors wrote.
Large variations found
Overall, physicians were less likely to recommend surveillance colonoscopies if the patient was older, had poor health, and had lower-risk adenomas. Patient age and health had larger effects on decision-making than adenoma risk, with health status having the largest effect.
About 20.6% of physicians recommended surveillance if the patient was 85, compared with 49.8% if the patient was 75. In addition, 7.1% of physicians recommended surveillance if the patient was in poor health, compared with 28.8% for those in moderate health, and 67.7% for patients in good health.
If the prior adenoma was low risk, 29.7% of physicians recommended surveillance, compared with 41.6% if the prior adenoma was high risk.
In general, family medicine and general practice physicians were most likely to recommend surveillance, at 40%, and gastroenterologists were least likely to recommend surveillance, at 30.9%. Patient age and health had larger effects among gastroenterologists than among primary care physicians, and adenoma risk had similar effects between the two groups.
“The importance of patient age and health status found in our study mirrors study results on physician decision-making regarding screening colonoscopies in older adults and makes intuitive sense,” the authors wrote. “Whether the priorities reflected in our findings are supported by evidence is not clear, and our results highlight important knowledge gaps in the field that warrant future research.”
Physician uncertainty
Additional guidance would be helpful, the authors wrote. In the survey, about 52.3% of primary care physicians and 35.4% of gastroenterologists reported uncertainty about the benefit–harm balance of surveillance in older adults.
“Current guidelines on surveillance colonoscopies are solely based on prior adenoma characteristics,” the authors wrote. “Guidelines need to incorporate guidance that considers patient age and health status, as well as adenoma risk, and explicitly considers when surveillance should stop in older adults.”
In addition, most physicians in the survey – 85.9% of primary care physicians and 77% of gastroenterologists – said they would find a decision support tool helpful. At the same time, 32.8% of primary care physicians and 71.5% of gastroenterologists perceived it as the gastroenterologist’s role to decide about surveillance colonoscopies.
“Developing patient-facing materials, communication tools for clinicians, and tools to support shared decision-making about surveillance colonoscopies that engage both physicians and patients are all important next steps,” the authors wrote. “To our knowledge, there is no existing patient decision aid about surveillance colonoscopies; developing such a tool may be valuable.”
The study was supported by Dr. Schoenborn’s career development award from the National Institute on Aging. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Cognitive testing for older drivers: Is there a benefit?
, according to results from a large population-based study using data from Japan.
But the same study, published in the Journal of the American Geriatrics Society, also reported a concurrent increase in pedestrian and cycling injuries, possibly because more older former drivers were getting around by alternative means. That finding echoed a 2012 study from Denmark, which also looked at the effects of an age-based cognitive screening policy for older drivers, and saw more fatal road injuries among older people who were not driving.
While some governments, including those of Denmark, Taiwan, and Japan, have implemented age-based cognitive screening for older drivers, there has been little evidence to date that such policies improve road safety. Guidelines issued in 2010 by the American Academy of Neurology discourage age-based screening, advising instead that people diagnosed with cognitive disorders be carefully evaluated for driving fitness and recommending one widely used scale, the Clinical Dementia Rating, as useful in identifying potentially unsafe drivers.
Japan’s national screening policy: Did it work?
The new study, led by Haruhiko Inada, MD, PhD, an epidemiologist at Johns Hopkins University in Baltimore, used national crash data from Japan, where since 2017 all drivers 75 and older not only must take cognitive tests measuring temporal orientation and memory at license renewal, but are also referred for medical evaluation if they fail them. People receiving a subsequent dementia diagnosis can have their licenses suspended or revoked.
Dr. Inada and his colleagues looked at national data from nearly 603,000 police-reported vehicle collisions and nearly 197,000 pedestrian or cyclist road injuries between March 2012 and December 2019, all involving people aged 70 and older. To assess the screening policy’s impact, the researchers calculated estimated monthly collision or injury incidence rates per 100,000 person-years. This way, they could “control for secular trends that were unaffected by the policy, such as the decreasing incidence of motor vehicle collisions year by year,” the researchers explained.
After the screening was implemented, cumulative estimated collisions among drivers 75 or older decreased by 3,670 (95% confidence interval, 5,125-2,104), while reported pedestrian or cyclist injuries increased by an estimated 959 (95% CI, 24-1,834). Dr. Inada and colleagues found that crashes declined among men but not women, noting also that more older men than women are licensed to drive in Japan. Pedestrian and cyclist injuries were highest among men aged 80-84, and women aged 80 and older.
“Cognitively screening older drivers at license renewal and promoting voluntary surrender of licenses may prevent motor vehicle collisions,” Dr. Inada and his colleagues concluded. “However, they are associated with an increase in road injuries for older pedestrians and cyclists. Future studies should examine the effectiveness of mitigation measures, such as alternative, safe transportation, and accommodations for pedestrians and cyclists.”
No definitive answers
Two investigators who have studied cognitive screening related to road safety were contacted for commentary on the study findings.
Anu Siren, PhD, professor of gerontology at Tampere (Finland) University, who in 2012 reported higher injuries after implementation of older-driver cognitive screening in Denmark, commented that the new study, while benefiting from a much larger data set than earlier studies, still “fails to show that decrease in collisions is because ‘unfit’ drivers were removed from the road. But it does confirm previous findings about how strict screening policies make people shift from cars to unprotected modes of transportation,” which are riskier.
In studies measuring driving safety, the usual definition of risk is incidents per exposure, Dr. Siren noted. In Dr. Inada and colleagues’ study, “the incident measure, or numerator, is the number of collisions. The exposure measure or denominator is population. Because the study uses population and not driver licenses (or distance traveled) as an exposure measure, the observed decrease in collisions does not say much about how the collision risk develops after the implementation of screening.”
Older driver screening “is likely to cause some older persons to cease from driving and probably continue to travel as unprotected road users,” Dr. Siren continued. “Similar to what we found [in 2012], the injury rates for pedestrians and cyclists went up after the introduction of screening, which suggests that screening indirectly causes increasing number of injuries among older unprotected road users.”
Matthew Rizzo, MD, professor and chair of the department of neurological sciences at the University of Nebraska Medical Center and codirector of the Nebraska Neuroscience Alliance in Omaha, Neb., and the lead author of the 2010 AAN guidelines on cognitive impairment and driving risk, cautioned against ageism in designing policies meant to protect motorists.
“We find some erratic/weak effects of age here and there, but the big effects we consistently find are from cognitive and visual decline – which is somewhat correlated with age, but with huge variance,” Dr. Rizzo said. “It is hard to say what an optimal age threshold for risk would be, and if 75 is it.”
U.S. crash data from the last decade points to drivers 80 and older as significantly more accident-prone than those in their 70s, or even late 70s, Dr. Rizzo noted. Moreover, “willingness to get on the road, number of miles driven, type of road (urban, rural, highway, commercial, residential), type of vehicle driven, traffic, and environment (day, night, weather), et cetera, are all factors to consider in driving risk and restriction,” he said.
Dr. Rizzo added that the 2010 AAN guidelines might need to be revisited in light of newer vehicle safety systems and automation.
Dr. Inada and colleagues’ study was funded by Japanese government grants, and Dr. Inada and his coauthors reported no financial conflicts of interest. Dr. Siren and Dr. Rizzo reported no financial conflicts of interest.
, according to results from a large population-based study using data from Japan.
But the same study, published in the Journal of the American Geriatrics Society, also reported a concurrent increase in pedestrian and cycling injuries, possibly because more older former drivers were getting around by alternative means. That finding echoed a 2012 study from Denmark, which also looked at the effects of an age-based cognitive screening policy for older drivers, and saw more fatal road injuries among older people who were not driving.
While some governments, including those of Denmark, Taiwan, and Japan, have implemented age-based cognitive screening for older drivers, there has been little evidence to date that such policies improve road safety. Guidelines issued in 2010 by the American Academy of Neurology discourage age-based screening, advising instead that people diagnosed with cognitive disorders be carefully evaluated for driving fitness and recommending one widely used scale, the Clinical Dementia Rating, as useful in identifying potentially unsafe drivers.
Japan’s national screening policy: Did it work?
The new study, led by Haruhiko Inada, MD, PhD, an epidemiologist at Johns Hopkins University in Baltimore, used national crash data from Japan, where since 2017 all drivers 75 and older not only must take cognitive tests measuring temporal orientation and memory at license renewal, but are also referred for medical evaluation if they fail them. People receiving a subsequent dementia diagnosis can have their licenses suspended or revoked.
Dr. Inada and his colleagues looked at national data from nearly 603,000 police-reported vehicle collisions and nearly 197,000 pedestrian or cyclist road injuries between March 2012 and December 2019, all involving people aged 70 and older. To assess the screening policy’s impact, the researchers calculated estimated monthly collision or injury incidence rates per 100,000 person-years. This way, they could “control for secular trends that were unaffected by the policy, such as the decreasing incidence of motor vehicle collisions year by year,” the researchers explained.
After the screening was implemented, cumulative estimated collisions among drivers 75 or older decreased by 3,670 (95% confidence interval, 5,125-2,104), while reported pedestrian or cyclist injuries increased by an estimated 959 (95% CI, 24-1,834). Dr. Inada and colleagues found that crashes declined among men but not women, noting also that more older men than women are licensed to drive in Japan. Pedestrian and cyclist injuries were highest among men aged 80-84, and women aged 80 and older.
“Cognitively screening older drivers at license renewal and promoting voluntary surrender of licenses may prevent motor vehicle collisions,” Dr. Inada and his colleagues concluded. “However, they are associated with an increase in road injuries for older pedestrians and cyclists. Future studies should examine the effectiveness of mitigation measures, such as alternative, safe transportation, and accommodations for pedestrians and cyclists.”
No definitive answers
Two investigators who have studied cognitive screening related to road safety were contacted for commentary on the study findings.
Anu Siren, PhD, professor of gerontology at Tampere (Finland) University, who in 2012 reported higher injuries after implementation of older-driver cognitive screening in Denmark, commented that the new study, while benefiting from a much larger data set than earlier studies, still “fails to show that decrease in collisions is because ‘unfit’ drivers were removed from the road. But it does confirm previous findings about how strict screening policies make people shift from cars to unprotected modes of transportation,” which are riskier.
In studies measuring driving safety, the usual definition of risk is incidents per exposure, Dr. Siren noted. In Dr. Inada and colleagues’ study, “the incident measure, or numerator, is the number of collisions. The exposure measure or denominator is population. Because the study uses population and not driver licenses (or distance traveled) as an exposure measure, the observed decrease in collisions does not say much about how the collision risk develops after the implementation of screening.”
Older driver screening “is likely to cause some older persons to cease from driving and probably continue to travel as unprotected road users,” Dr. Siren continued. “Similar to what we found [in 2012], the injury rates for pedestrians and cyclists went up after the introduction of screening, which suggests that screening indirectly causes increasing number of injuries among older unprotected road users.”
Matthew Rizzo, MD, professor and chair of the department of neurological sciences at the University of Nebraska Medical Center and codirector of the Nebraska Neuroscience Alliance in Omaha, Neb., and the lead author of the 2010 AAN guidelines on cognitive impairment and driving risk, cautioned against ageism in designing policies meant to protect motorists.
“We find some erratic/weak effects of age here and there, but the big effects we consistently find are from cognitive and visual decline – which is somewhat correlated with age, but with huge variance,” Dr. Rizzo said. “It is hard to say what an optimal age threshold for risk would be, and if 75 is it.”
U.S. crash data from the last decade points to drivers 80 and older as significantly more accident-prone than those in their 70s, or even late 70s, Dr. Rizzo noted. Moreover, “willingness to get on the road, number of miles driven, type of road (urban, rural, highway, commercial, residential), type of vehicle driven, traffic, and environment (day, night, weather), et cetera, are all factors to consider in driving risk and restriction,” he said.
Dr. Rizzo added that the 2010 AAN guidelines might need to be revisited in light of newer vehicle safety systems and automation.
Dr. Inada and colleagues’ study was funded by Japanese government grants, and Dr. Inada and his coauthors reported no financial conflicts of interest. Dr. Siren and Dr. Rizzo reported no financial conflicts of interest.
, according to results from a large population-based study using data from Japan.
But the same study, published in the Journal of the American Geriatrics Society, also reported a concurrent increase in pedestrian and cycling injuries, possibly because more older former drivers were getting around by alternative means. That finding echoed a 2012 study from Denmark, which also looked at the effects of an age-based cognitive screening policy for older drivers, and saw more fatal road injuries among older people who were not driving.
While some governments, including those of Denmark, Taiwan, and Japan, have implemented age-based cognitive screening for older drivers, there has been little evidence to date that such policies improve road safety. Guidelines issued in 2010 by the American Academy of Neurology discourage age-based screening, advising instead that people diagnosed with cognitive disorders be carefully evaluated for driving fitness and recommending one widely used scale, the Clinical Dementia Rating, as useful in identifying potentially unsafe drivers.
Japan’s national screening policy: Did it work?
The new study, led by Haruhiko Inada, MD, PhD, an epidemiologist at Johns Hopkins University in Baltimore, used national crash data from Japan, where since 2017 all drivers 75 and older not only must take cognitive tests measuring temporal orientation and memory at license renewal, but are also referred for medical evaluation if they fail them. People receiving a subsequent dementia diagnosis can have their licenses suspended or revoked.
Dr. Inada and his colleagues looked at national data from nearly 603,000 police-reported vehicle collisions and nearly 197,000 pedestrian or cyclist road injuries between March 2012 and December 2019, all involving people aged 70 and older. To assess the screening policy’s impact, the researchers calculated estimated monthly collision or injury incidence rates per 100,000 person-years. This way, they could “control for secular trends that were unaffected by the policy, such as the decreasing incidence of motor vehicle collisions year by year,” the researchers explained.
After the screening was implemented, cumulative estimated collisions among drivers 75 or older decreased by 3,670 (95% confidence interval, 5,125-2,104), while reported pedestrian or cyclist injuries increased by an estimated 959 (95% CI, 24-1,834). Dr. Inada and colleagues found that crashes declined among men but not women, noting also that more older men than women are licensed to drive in Japan. Pedestrian and cyclist injuries were highest among men aged 80-84, and women aged 80 and older.
“Cognitively screening older drivers at license renewal and promoting voluntary surrender of licenses may prevent motor vehicle collisions,” Dr. Inada and his colleagues concluded. “However, they are associated with an increase in road injuries for older pedestrians and cyclists. Future studies should examine the effectiveness of mitigation measures, such as alternative, safe transportation, and accommodations for pedestrians and cyclists.”
No definitive answers
Two investigators who have studied cognitive screening related to road safety were contacted for commentary on the study findings.
Anu Siren, PhD, professor of gerontology at Tampere (Finland) University, who in 2012 reported higher injuries after implementation of older-driver cognitive screening in Denmark, commented that the new study, while benefiting from a much larger data set than earlier studies, still “fails to show that decrease in collisions is because ‘unfit’ drivers were removed from the road. But it does confirm previous findings about how strict screening policies make people shift from cars to unprotected modes of transportation,” which are riskier.
In studies measuring driving safety, the usual definition of risk is incidents per exposure, Dr. Siren noted. In Dr. Inada and colleagues’ study, “the incident measure, or numerator, is the number of collisions. The exposure measure or denominator is population. Because the study uses population and not driver licenses (or distance traveled) as an exposure measure, the observed decrease in collisions does not say much about how the collision risk develops after the implementation of screening.”
Older driver screening “is likely to cause some older persons to cease from driving and probably continue to travel as unprotected road users,” Dr. Siren continued. “Similar to what we found [in 2012], the injury rates for pedestrians and cyclists went up after the introduction of screening, which suggests that screening indirectly causes increasing number of injuries among older unprotected road users.”
Matthew Rizzo, MD, professor and chair of the department of neurological sciences at the University of Nebraska Medical Center and codirector of the Nebraska Neuroscience Alliance in Omaha, Neb., and the lead author of the 2010 AAN guidelines on cognitive impairment and driving risk, cautioned against ageism in designing policies meant to protect motorists.
“We find some erratic/weak effects of age here and there, but the big effects we consistently find are from cognitive and visual decline – which is somewhat correlated with age, but with huge variance,” Dr. Rizzo said. “It is hard to say what an optimal age threshold for risk would be, and if 75 is it.”
U.S. crash data from the last decade points to drivers 80 and older as significantly more accident-prone than those in their 70s, or even late 70s, Dr. Rizzo noted. Moreover, “willingness to get on the road, number of miles driven, type of road (urban, rural, highway, commercial, residential), type of vehicle driven, traffic, and environment (day, night, weather), et cetera, are all factors to consider in driving risk and restriction,” he said.
Dr. Rizzo added that the 2010 AAN guidelines might need to be revisited in light of newer vehicle safety systems and automation.
Dr. Inada and colleagues’ study was funded by Japanese government grants, and Dr. Inada and his coauthors reported no financial conflicts of interest. Dr. Siren and Dr. Rizzo reported no financial conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Similar brain atrophy in obesity and Alzheimer’s disease
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Six healthy lifestyle habits linked to slowed memory decline
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.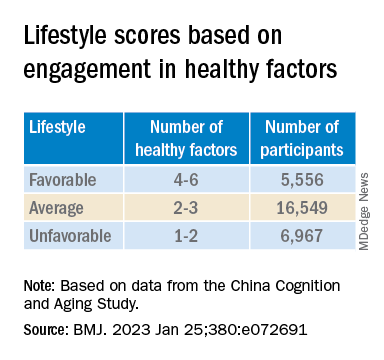
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.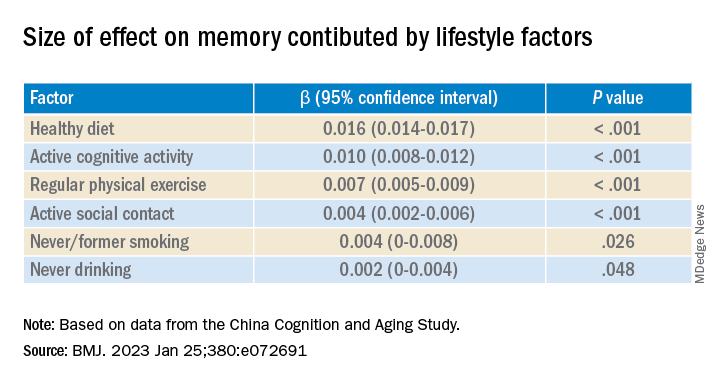
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Nine more minutes a day of vigorous exercise tied to better cognition
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH
Geriatrician advises on use of vitamin D supplementation, lecanemab, and texting for her patients
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Social isolation hikes dementia risk in older adults
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a longitudinal study that included more than 5,000 United States–based seniors showed that nearly one-quarter were socially isolated.
After adjusting for demographic and health factors, social isolation was found to be associated with a 28% higher risk for developing dementia over a 9-year period, compared with non-isolation. In addition, this finding held true regardless of race or ethnicity.
“Social connections are increasingly understood as a critical factor for the health of individuals as they age,” senior study author Thomas K.M. Cudjoe, MD, Robert and Jane Meyerhoff Endowed Professor and assistant professor of medicine, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, said in a press release. “Our study expands our understanding of the deleterious impact of social isolation on one’s risk for dementia over time,” Dr. Cudjoe added.
The findings were published online in the Journal of the American Geriatric Society.
Upstream resources, downstream outcomes
Social isolation is a “multidimensional construct” characterized by factors such as social connections, social support, resource sharing, and relationship strain. It also affects approximately a quarter of older adults, the investigators noted.
Although prior studies have pointed to an association between socially isolated older adults and increased risk for incident dementia, no study has described this longitudinal association in a nationally representative cohort of U.S. seniors.
Dr. Cudjoe said he was motivated to conduct the current study because he wondered whether or not older adults throughout the United States were similar to some of his patients “who might be at risk for worse cognitive outcomes because they lacked social contact with friends, family, or neighbors.”
The study was also “informed by conceptual foundation that upstream social and personal resources are linked to downstream health outcomes, including cognitive health and function,” the researchers added.
They turned to 2011-2020 data from the National Health and Aging Trends Study, a nationally representative, longitudinal cohort of U.S. Medicare beneficiaries. The sample was drawn from the Medicare enrollment file and incorporated 95 counties and 655 zip codes.
Participants (n = 5,022; mean age, 76.4 years; 57.2% women; 71.7% White, non-Hispanic; 42.4% having more than a college education) were community-dwelling older adults who completed annual 2-hour interviews that included assessment of function, economic health status, and well-being. To be included, they had to attend at least the baseline and first follow-up visits.
NHATS “includes domains that are relevant for the characterization of social isolation,” the investigators wrote. It used a typology of structural social isolation that is informed by the Berkman-Syme Social Network Index.
Included domains were living arrangements, discussion networks, and participation. All are “clinically relevant, practical, and components of a comprehensive social history,” the researchers noted.
They added that individuals classified as “socially isolated” often live alone, have no one or only one person that they can rely upon to discuss important matters, and have limited or no engagement in social or religious groups.
Social isolation in the study was characterized using questions about living with at least one other person, talking to two or more other people about “important matters” in the past year, attending religious services in the past month, and participating in the past month in such things as clubs, meetings, group activities, or volunteer work.
Wake-up call
Study participants received 1 point for each item/domain, with a sum score of 0 or 1 classified as “socially isolated” and 2 or more points considered “not socially isolated.” They were classified as having probable dementia based either on self-report or lower-than-mean performance in 2 or more cognitive domains, or a score indicating probable dementia on the AD8 Dementia Screening Interview.
Covariates included demographic factors, education, and health factors. Mean follow-up was 5.1 years.
Results showed close to one-quarter (23.3%) of the study population was classified as socially isolated, with one-fifth (21.1%) developing dementia by the end of the follow-up period.
Compared with non-isolated older adults, those who were socially isolated were more likely to develop dementia during the follow-up period (19.6% vs. 25.9%, respectively).
After adjusting for demographic factors, social isolation was significantly associated with a higher risk for incident dementia (hazard ratio, 1.33; 95% confidence interval, 1.13-1.56). This association persisted after further adjustment for health factors (HR, 1.27; 95% CI, 1.08-1.49). Race and ethnicity had no bearing on the association.
In addition to the association between social isolation and dementia, the researchers also estimated the cause-specific hazard of death before dementia and found that, overall, 18% of participants died prior to dementia over the follow-up period. In particular, the social isolation–associated cause-specific HR of death before dementia was 1.28 (95% CI, 1.2-1.5).
Dr. Cudjoe noted that the mechanism behind the association between social isolation and dementia in this population needs further study. Still, he hopes that the findings will “serve as a wake-up call for all of us to be more thoughtful of the role of social connections on our cognitive health.”
Clinicians “should be thinking about and assessing the presence or absence of social connections in their patients,” Dr. Cudjoe added.
‘Instrumental role’
Commenting on the study, Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, said the study “contributes to the growing body of evidence that finds social isolation is a serious public health risk for many seniors living in the United States, increasing their risk for dementia and other serious mental conditions.”
Dr. Purcell, who was not involved with the study, added that “health care systems and medical professionals can play an instrumental role in identifying individuals at risk for social isolation.”
She noted that for those experiencing social isolation, “interaction with health care providers may be one of the few opportunities those individuals have for social engagement, [so] using these interactions to identify individuals at risk for social isolation and referring them to local resources and groups that promote engagement, well-being, and access to senior services may help decrease dementia risk for vulnerable seniors.”
Dr. Purcell added that the Alzheimer’s Association offers early-stage programs throughout the country, including support groups, education, art, music, and other socially engaging activities.
The study was funded by the National Institute on Aging, National Institute on Minority Health and Health Disparities, and Secunda Family Foundation. The investigators and Dr. Purcell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer in women 65+ often deadly: so why not screen?
Approximately one-fifth of cervical cancer cases are diagnosed in women aged 65 years or older, and most of the cases are late-stage disease associated with poor survival rates. The new finding calls into question yet again the many national screening guidelines that advise physicians to halt cervical screening at age 65.
The findings emerged from an analysis of the California Cancer Registry for 2009-2018. The authors, from the University of California, Davis, who manage the registry on behalf of the state, found that 17% of women diagnosed with a first primary cancer were aged 65 years or older.
Up to 71% of these older women had late-stage disease vs. 34%-to 59% of women aged 21-64.
The team also found that older patients, even those with early disease, had much poorer survival after they were diagnosed with cervical cancer than their younger counterparts. For example, patients aged between 65 and 69 with stage I cervical cancer had a 5-year relative survival – that is, survival adjusted for noncancer causes of death – of 82%. By contrast, 94% of women aged 20-39 survived for at least 5 years.
The study was published on January 9 in Cancer Epidemiology, Biomarkers & Prevention.
These new data echo similar findings from other recent cervical cancer studies out of California, Massachusetts, Ohio, and nationally. Those studies show that, in comparison with younger patients, rates of late-stage disease are higher and survival is poorer among women aged 65 and older.
Even so, a coauthor of the present study, Frances Maguire, PhD, who is an epidemiologist at the University of California, Davis, said she and her colleagues were surprised by what they found.
“There are a lot of women in this older-age category who are being diagnosed, and they’re being diagnosed later stage and their survival is worse,” Dr. Maguire said. “That was surprising to all of us,” given that the current recommendations are to stop screening once women reach the age of 65, and yet this age group is “doing quite poorly.”
The American Cancer Society, the U.S. Preventive Services Task Force, and the American College of Obstetricians and Gynecologists all recommend that cervical screening stop at aged 65 for patients with “adequate prior screening.”
Adequate screening is defined as having three consecutive normal Pap tests or two consecutive negative human papillomavirus tests or two consecutive negative cotests within the prior 10 years, with the most recent screening within 5 years and having no precancerous lesions in the past 25 years.
However, as many as 23% of women aged 60-64 report that their last Pap test was administered more than 5 years ago, according to a recent study by Alex Francoeur, MD, and colleagues at the University of California, Los Angeles.
When asked to comment on the new article, Dr. Francoeur said, “There is literature that increasing comorbidities and visits to the doctor [with age] decrease the likelihood of getting a Pap test, which is concerning, as these may be the highest-risk women.”
Said study author Dr. Maguire, “It could be that [the guidelines] are perfectly fine if women were properly screened before they hit 65, so that’s one of our big questions. Perhaps this group are not properly screened before age 65, and then they hit 65, they don’t screen, and this is the result we’re seeing.”
The situation is compounded by the lack of continuity in care at this crucial juncture, said Alexander Olawaiye, MD, a professor in the division of gynecologic oncology at the University of Pittsburgh, who was also approached for comment.
At age 65, many women retire, move across the country, or access new health care providers through Medicare, which kicks in at age 65, so the woman’s new physician doesn’t have access to her screening history, he commented.
This means that a physician needs to rely on the patient’s memory.
This is unrealistic, said Dr. Olawaiye: “Let’s forget about the 65-year-old women for now. Let’s talk about young women with sharp minds. Half of these young adults cannot even remember correctly their last monthly period. And these are the people you want to recollect accurately [at age 65] the number of tests they’ve had over 10 years and the results of those tests? Are you kidding me?” said Dr. Olawaiye. “Is that the kind of verification that you rely on?”
Dr. Olawaiye has consistently advocated for scrapping the 65+ screening moratorium in past and current versions of the cervical screening guidelines. He is puzzled by the national unwillingness to do so and rejects the economic argument, pointing out that a handful of extra tests is a lot cheaper than caring for a patient with advanced cervical cancer.
“Most American women will die around 84-85 years of age,” Dr. Olawaiye commented. “So between 65 and 85, you will need five screens, maybe four. What are you saving by not doing that?”
Dr. Maguire, Dr. Francoeur, and Dr. Olawaiye have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately one-fifth of cervical cancer cases are diagnosed in women aged 65 years or older, and most of the cases are late-stage disease associated with poor survival rates. The new finding calls into question yet again the many national screening guidelines that advise physicians to halt cervical screening at age 65.
The findings emerged from an analysis of the California Cancer Registry for 2009-2018. The authors, from the University of California, Davis, who manage the registry on behalf of the state, found that 17% of women diagnosed with a first primary cancer were aged 65 years or older.
Up to 71% of these older women had late-stage disease vs. 34%-to 59% of women aged 21-64.
The team also found that older patients, even those with early disease, had much poorer survival after they were diagnosed with cervical cancer than their younger counterparts. For example, patients aged between 65 and 69 with stage I cervical cancer had a 5-year relative survival – that is, survival adjusted for noncancer causes of death – of 82%. By contrast, 94% of women aged 20-39 survived for at least 5 years.
The study was published on January 9 in Cancer Epidemiology, Biomarkers & Prevention.
These new data echo similar findings from other recent cervical cancer studies out of California, Massachusetts, Ohio, and nationally. Those studies show that, in comparison with younger patients, rates of late-stage disease are higher and survival is poorer among women aged 65 and older.
Even so, a coauthor of the present study, Frances Maguire, PhD, who is an epidemiologist at the University of California, Davis, said she and her colleagues were surprised by what they found.
“There are a lot of women in this older-age category who are being diagnosed, and they’re being diagnosed later stage and their survival is worse,” Dr. Maguire said. “That was surprising to all of us,” given that the current recommendations are to stop screening once women reach the age of 65, and yet this age group is “doing quite poorly.”
The American Cancer Society, the U.S. Preventive Services Task Force, and the American College of Obstetricians and Gynecologists all recommend that cervical screening stop at aged 65 for patients with “adequate prior screening.”
Adequate screening is defined as having three consecutive normal Pap tests or two consecutive negative human papillomavirus tests or two consecutive negative cotests within the prior 10 years, with the most recent screening within 5 years and having no precancerous lesions in the past 25 years.
However, as many as 23% of women aged 60-64 report that their last Pap test was administered more than 5 years ago, according to a recent study by Alex Francoeur, MD, and colleagues at the University of California, Los Angeles.
When asked to comment on the new article, Dr. Francoeur said, “There is literature that increasing comorbidities and visits to the doctor [with age] decrease the likelihood of getting a Pap test, which is concerning, as these may be the highest-risk women.”
Said study author Dr. Maguire, “It could be that [the guidelines] are perfectly fine if women were properly screened before they hit 65, so that’s one of our big questions. Perhaps this group are not properly screened before age 65, and then they hit 65, they don’t screen, and this is the result we’re seeing.”
The situation is compounded by the lack of continuity in care at this crucial juncture, said Alexander Olawaiye, MD, a professor in the division of gynecologic oncology at the University of Pittsburgh, who was also approached for comment.
At age 65, many women retire, move across the country, or access new health care providers through Medicare, which kicks in at age 65, so the woman’s new physician doesn’t have access to her screening history, he commented.
This means that a physician needs to rely on the patient’s memory.
This is unrealistic, said Dr. Olawaiye: “Let’s forget about the 65-year-old women for now. Let’s talk about young women with sharp minds. Half of these young adults cannot even remember correctly their last monthly period. And these are the people you want to recollect accurately [at age 65] the number of tests they’ve had over 10 years and the results of those tests? Are you kidding me?” said Dr. Olawaiye. “Is that the kind of verification that you rely on?”
Dr. Olawaiye has consistently advocated for scrapping the 65+ screening moratorium in past and current versions of the cervical screening guidelines. He is puzzled by the national unwillingness to do so and rejects the economic argument, pointing out that a handful of extra tests is a lot cheaper than caring for a patient with advanced cervical cancer.
“Most American women will die around 84-85 years of age,” Dr. Olawaiye commented. “So between 65 and 85, you will need five screens, maybe four. What are you saving by not doing that?”
Dr. Maguire, Dr. Francoeur, and Dr. Olawaiye have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately one-fifth of cervical cancer cases are diagnosed in women aged 65 years or older, and most of the cases are late-stage disease associated with poor survival rates. The new finding calls into question yet again the many national screening guidelines that advise physicians to halt cervical screening at age 65.
The findings emerged from an analysis of the California Cancer Registry for 2009-2018. The authors, from the University of California, Davis, who manage the registry on behalf of the state, found that 17% of women diagnosed with a first primary cancer were aged 65 years or older.
Up to 71% of these older women had late-stage disease vs. 34%-to 59% of women aged 21-64.
The team also found that older patients, even those with early disease, had much poorer survival after they were diagnosed with cervical cancer than their younger counterparts. For example, patients aged between 65 and 69 with stage I cervical cancer had a 5-year relative survival – that is, survival adjusted for noncancer causes of death – of 82%. By contrast, 94% of women aged 20-39 survived for at least 5 years.
The study was published on January 9 in Cancer Epidemiology, Biomarkers & Prevention.
These new data echo similar findings from other recent cervical cancer studies out of California, Massachusetts, Ohio, and nationally. Those studies show that, in comparison with younger patients, rates of late-stage disease are higher and survival is poorer among women aged 65 and older.
Even so, a coauthor of the present study, Frances Maguire, PhD, who is an epidemiologist at the University of California, Davis, said she and her colleagues were surprised by what they found.
“There are a lot of women in this older-age category who are being diagnosed, and they’re being diagnosed later stage and their survival is worse,” Dr. Maguire said. “That was surprising to all of us,” given that the current recommendations are to stop screening once women reach the age of 65, and yet this age group is “doing quite poorly.”
The American Cancer Society, the U.S. Preventive Services Task Force, and the American College of Obstetricians and Gynecologists all recommend that cervical screening stop at aged 65 for patients with “adequate prior screening.”
Adequate screening is defined as having three consecutive normal Pap tests or two consecutive negative human papillomavirus tests or two consecutive negative cotests within the prior 10 years, with the most recent screening within 5 years and having no precancerous lesions in the past 25 years.
However, as many as 23% of women aged 60-64 report that their last Pap test was administered more than 5 years ago, according to a recent study by Alex Francoeur, MD, and colleagues at the University of California, Los Angeles.
When asked to comment on the new article, Dr. Francoeur said, “There is literature that increasing comorbidities and visits to the doctor [with age] decrease the likelihood of getting a Pap test, which is concerning, as these may be the highest-risk women.”
Said study author Dr. Maguire, “It could be that [the guidelines] are perfectly fine if women were properly screened before they hit 65, so that’s one of our big questions. Perhaps this group are not properly screened before age 65, and then they hit 65, they don’t screen, and this is the result we’re seeing.”
The situation is compounded by the lack of continuity in care at this crucial juncture, said Alexander Olawaiye, MD, a professor in the division of gynecologic oncology at the University of Pittsburgh, who was also approached for comment.
At age 65, many women retire, move across the country, or access new health care providers through Medicare, which kicks in at age 65, so the woman’s new physician doesn’t have access to her screening history, he commented.
This means that a physician needs to rely on the patient’s memory.
This is unrealistic, said Dr. Olawaiye: “Let’s forget about the 65-year-old women for now. Let’s talk about young women with sharp minds. Half of these young adults cannot even remember correctly their last monthly period. And these are the people you want to recollect accurately [at age 65] the number of tests they’ve had over 10 years and the results of those tests? Are you kidding me?” said Dr. Olawaiye. “Is that the kind of verification that you rely on?”
Dr. Olawaiye has consistently advocated for scrapping the 65+ screening moratorium in past and current versions of the cervical screening guidelines. He is puzzled by the national unwillingness to do so and rejects the economic argument, pointing out that a handful of extra tests is a lot cheaper than caring for a patient with advanced cervical cancer.
“Most American women will die around 84-85 years of age,” Dr. Olawaiye commented. “So between 65 and 85, you will need five screens, maybe four. What are you saving by not doing that?”
Dr. Maguire, Dr. Francoeur, and Dr. Olawaiye have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Possible bivalent vaccine link to strokes in people over 65
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.





