User login
Abbreviated Delirium Screening Instruments: Plausible Tool to Improve Delirium Detection in Hospitalized Older Patients
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
Walking intensity and step count are linked to health benefits
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
FROM JAMA INTERNAL MEDICINE
Hip fractures likely to double by 2050 as population ages
The annual incidence of hip fractures declined in most countries from 2005 to 2018, but this rate is projected to roughly double by 2050, according to a new study of 19 countries/regions.
The study by Chor-Wing Sing, PhD, and colleagues was presented at the annual meeting of the American Society of Bone and Mineral Research. The predicted increase in hip fractures is being driven by the aging population, with the population of those age 85 and older projected to increase 4.5-fold from 2010 to 2050, they note.
The researchers also estimate that from 2018 to 2050 the incidence of fractures will increase by 1.9-fold overall – more in men (2.4-fold) than in women (1.7-fold).
In addition, rates of use of osteoporosis drugs 1 year after a hip fracture were less than 50%, with less treatment in men. Men were also more likely than women to die within 1 year of a hip fracture.
The researchers conclude that “larger and more collaborative efforts among health care providers, policymakers, and patients are needed to prevent hip fractures and improve the treatment gap and post-fracture care, especially in men and the oldest old.”
Aging will fuel rise in hip fractures; more preventive treatment needed
“Even though there is a decreasing trend of hip fracture incidence in some countries, such a percentage decrease is insufficient to offset the percentage increase in the aging population,” senior co-author Ching-Lung Cheung, PhD, associate professor in the department of pharmacology and pharmacy at the University of Hong Kong, explained to this news organization.
The takeaways from the study are that “a greater effort on fracture prevention should be made to avoid the continuous increase in the number of hip fractures,” he said.
In addition, “although initiation of anti-osteoporosis medication after hip fracture is recommended in international guidelines, the 1-year treatment rate [was] well below 50% in most of the countries and regions studied. This indicates the treatment rate is far from optimal.”
“Our study also showed that the use of anti-osteoporosis medications following a hip fracture is lower in men than in women by 30% to 67%,” he said. “Thus, more attention should be paid to preventing and treating hip fractures in men.”
“The greater increase in the projected number of hip fractures in men than in women “could be [because] osteoporosis is commonly perceived as a ‘woman’s disease,’ ” he speculated.
Invited to comment, Juliet Compston, MD, who selected the study as one of the top clinical science highlight abstracts at the ASBMR meeting, agrees that “there is substantial room for improvement” in osteoporosis treatment rates following a hip fracture “in all the regions covered by the study.”
“In addition,” she continues, “the wide variations in treatment rates can provide important lessons about the most effective models of care for people who sustain a hip fracture: for example, fracture liaison services.”
Men suffer as osteoporosis perceived to be a ‘woman’s disease’
The even lower treatment rate in men than women is “concerning and likely reflects the mistaken perception that osteoporosis is predominantly a disease affecting women,” notes Dr. Compston, emeritus professor of bone medicine, University of Cambridge, United Kingdom.
Also invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, said that the projected doubling of hip fractures “is likely mainly due to aging of the population, with increasing lifespan for males in particular. However, increasing urbanization and decreasing weight-bearing exercise as a result are likely to also contribute in developing countries.”
“Unfortunately, despite the advances in treatments for osteoporosis over the last 25 years, osteoporosis treatment rates remain low, and osteoporosis remains undiagnosed in postmenopausal women and older men,” added Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research.
“More targeted screening for osteoporosis would help,” he said, “as would treating patients for it following other minimal trauma fractures (vertebral, distal radius, and humerus, etc.), since if left untreated, about 50% of these patients will have hip fractures later in life.”
“Some countries may be doing better because they have health quality standards for hip fracture (for example, surgery within 24 hours, investigation, and treatment for osteoporosis). In other countries like Australia, bone density tests and treatment for osteoporosis are reimbursed, increasing their uptake.”
The public health implications of this study are “substantial” according to Dr. Compston. “People who have sustained a hip fracture are at high risk of subsequent fractures if untreated. There is a range of safe, cost-effective pharmacological therapies to reduce fracture rate, and wider use of these would have a major impact on the current and future burden imposed by hip fractures in the elderly population.”
Similarly, Dr. Ebeling noted that “prevention is important to save a huge health burden for patients and costs for society.”
“Patients with minimal trauma fractures (particularly hip or spinal fractures) should be investigated and treated for osteoporosis with care pathways established in the hospitals, reaching out to the community [fracture liaison services],” he said.
Support for these is being sought under Medicare in the United States, he noted, and bone densitometry reimbursement rates also need to be higher in the United States.
Projections for number of hip fractures to 2050
Previous international reviews of hip fractures have been based on heterogeneous data from more than 10 to 30 years ago, the researchers note.
They performed a retrospective cohort study using a common protocol across 19 countries/regions, as described in an article about the protocol published in BMJ Open.
They analyzed data from adults aged 50 and older who were hospitalized with a hip fracture to determine 1) the annual incidence of hip fractures in 2008-2015; 2) the uptake of drugs to treat osteoporosis at 1 year after a hip fracture; and 3) all-cause mortality at 1 year after a hip fracture.
In a second step, they estimated the number of hip fractures that would occur from 2030 to 2050, using World Bank population growth projections.
The data are from 20 health care databases from 19 countries/regions: Oceania (Australia, New Zealand), Asia (Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand), Northern Europe (Denmark, Finland, and U.K.), Western Europe (France, Germany, Italy, The Netherlands, and Spain), and North and South America (Canada, United States, and Brazil).
The population in Japan was under age 75. U.S. data are from two databases: Medicare (age ≥ 65) and Optum.
Most databases (13) covered 90%-100% of the national population, and the rest covered 5%-70% of the population.
From 2008 to 2015, the annual incidence of hip fractures declined in 11 countries/regions (Singapore, Denmark, Hong Kong, Taiwan, Finland, U.K., Italy, Spain, United States [Medicare], Canada, and New Zealand).
“One potential reason that some countries have seen relatively large declines in hip fractures is better osteoporosis management and post-fracture care,” said Dr. Sing in a press release issued by ASBMR. “Better fall-prevention programs and clearer guidelines for clinical care have likely made a difference.”
Hip fracture incidence increased in five countries (The Netherlands, South Korea, France, Germany, and Brazil) and was stable in four countries (Australia, Japan, Thailand, and United States [Optum]).
The United Kingdom had the highest rate of osteoporosis treatment at 1-year after a hip fracture (50.3%). Rates in the other countries/regions ranged from 11.5% to 37%.
Fewer men than women were receiving drugs for osteoporosis at 1 year (range 5.1% to 38.2% versus 15.0% to 54.7%).
From 2005 to 2018, rates of osteoporosis treatment at 1 year after a hip fracture declined in six countries, increased in four countries, and were stable in five countries.
All-cause mortality within 1 year of hip fracture was higher in men than in women (range 19.2% to 35.8% versus 12.1% to 25.4%).
“Among the studied countries and regions, the U.S. ranks fifth with the highest hip fracture incidence,” Dr. Cheung replied when specifically asked about this. “The risk of hip fracture is determined by multiple factors: for example, lifestyle, diet, genetics, as well as management of osteoporosis,” he noted.
“Denmark is the only country showing no projected increase, and it is because Denmark had a continuous and remarkable decrease in the incidence of hip fractures,” he added, which “can offset the number of hip fractures contributed by the population aging.”
The study was funded by Amgen. Dr. Sing and Dr. Cheung have reported no relevant financial relationships. One of the study authors is employed by Amgen.
A version of this article first appeared on Medscape.com.
The annual incidence of hip fractures declined in most countries from 2005 to 2018, but this rate is projected to roughly double by 2050, according to a new study of 19 countries/regions.
The study by Chor-Wing Sing, PhD, and colleagues was presented at the annual meeting of the American Society of Bone and Mineral Research. The predicted increase in hip fractures is being driven by the aging population, with the population of those age 85 and older projected to increase 4.5-fold from 2010 to 2050, they note.
The researchers also estimate that from 2018 to 2050 the incidence of fractures will increase by 1.9-fold overall – more in men (2.4-fold) than in women (1.7-fold).
In addition, rates of use of osteoporosis drugs 1 year after a hip fracture were less than 50%, with less treatment in men. Men were also more likely than women to die within 1 year of a hip fracture.
The researchers conclude that “larger and more collaborative efforts among health care providers, policymakers, and patients are needed to prevent hip fractures and improve the treatment gap and post-fracture care, especially in men and the oldest old.”
Aging will fuel rise in hip fractures; more preventive treatment needed
“Even though there is a decreasing trend of hip fracture incidence in some countries, such a percentage decrease is insufficient to offset the percentage increase in the aging population,” senior co-author Ching-Lung Cheung, PhD, associate professor in the department of pharmacology and pharmacy at the University of Hong Kong, explained to this news organization.
The takeaways from the study are that “a greater effort on fracture prevention should be made to avoid the continuous increase in the number of hip fractures,” he said.
In addition, “although initiation of anti-osteoporosis medication after hip fracture is recommended in international guidelines, the 1-year treatment rate [was] well below 50% in most of the countries and regions studied. This indicates the treatment rate is far from optimal.”
“Our study also showed that the use of anti-osteoporosis medications following a hip fracture is lower in men than in women by 30% to 67%,” he said. “Thus, more attention should be paid to preventing and treating hip fractures in men.”
“The greater increase in the projected number of hip fractures in men than in women “could be [because] osteoporosis is commonly perceived as a ‘woman’s disease,’ ” he speculated.
Invited to comment, Juliet Compston, MD, who selected the study as one of the top clinical science highlight abstracts at the ASBMR meeting, agrees that “there is substantial room for improvement” in osteoporosis treatment rates following a hip fracture “in all the regions covered by the study.”
“In addition,” she continues, “the wide variations in treatment rates can provide important lessons about the most effective models of care for people who sustain a hip fracture: for example, fracture liaison services.”
Men suffer as osteoporosis perceived to be a ‘woman’s disease’
The even lower treatment rate in men than women is “concerning and likely reflects the mistaken perception that osteoporosis is predominantly a disease affecting women,” notes Dr. Compston, emeritus professor of bone medicine, University of Cambridge, United Kingdom.
Also invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, said that the projected doubling of hip fractures “is likely mainly due to aging of the population, with increasing lifespan for males in particular. However, increasing urbanization and decreasing weight-bearing exercise as a result are likely to also contribute in developing countries.”
“Unfortunately, despite the advances in treatments for osteoporosis over the last 25 years, osteoporosis treatment rates remain low, and osteoporosis remains undiagnosed in postmenopausal women and older men,” added Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research.
“More targeted screening for osteoporosis would help,” he said, “as would treating patients for it following other minimal trauma fractures (vertebral, distal radius, and humerus, etc.), since if left untreated, about 50% of these patients will have hip fractures later in life.”
“Some countries may be doing better because they have health quality standards for hip fracture (for example, surgery within 24 hours, investigation, and treatment for osteoporosis). In other countries like Australia, bone density tests and treatment for osteoporosis are reimbursed, increasing their uptake.”
The public health implications of this study are “substantial” according to Dr. Compston. “People who have sustained a hip fracture are at high risk of subsequent fractures if untreated. There is a range of safe, cost-effective pharmacological therapies to reduce fracture rate, and wider use of these would have a major impact on the current and future burden imposed by hip fractures in the elderly population.”
Similarly, Dr. Ebeling noted that “prevention is important to save a huge health burden for patients and costs for society.”
“Patients with minimal trauma fractures (particularly hip or spinal fractures) should be investigated and treated for osteoporosis with care pathways established in the hospitals, reaching out to the community [fracture liaison services],” he said.
Support for these is being sought under Medicare in the United States, he noted, and bone densitometry reimbursement rates also need to be higher in the United States.
Projections for number of hip fractures to 2050
Previous international reviews of hip fractures have been based on heterogeneous data from more than 10 to 30 years ago, the researchers note.
They performed a retrospective cohort study using a common protocol across 19 countries/regions, as described in an article about the protocol published in BMJ Open.
They analyzed data from adults aged 50 and older who were hospitalized with a hip fracture to determine 1) the annual incidence of hip fractures in 2008-2015; 2) the uptake of drugs to treat osteoporosis at 1 year after a hip fracture; and 3) all-cause mortality at 1 year after a hip fracture.
In a second step, they estimated the number of hip fractures that would occur from 2030 to 2050, using World Bank population growth projections.
The data are from 20 health care databases from 19 countries/regions: Oceania (Australia, New Zealand), Asia (Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand), Northern Europe (Denmark, Finland, and U.K.), Western Europe (France, Germany, Italy, The Netherlands, and Spain), and North and South America (Canada, United States, and Brazil).
The population in Japan was under age 75. U.S. data are from two databases: Medicare (age ≥ 65) and Optum.
Most databases (13) covered 90%-100% of the national population, and the rest covered 5%-70% of the population.
From 2008 to 2015, the annual incidence of hip fractures declined in 11 countries/regions (Singapore, Denmark, Hong Kong, Taiwan, Finland, U.K., Italy, Spain, United States [Medicare], Canada, and New Zealand).
“One potential reason that some countries have seen relatively large declines in hip fractures is better osteoporosis management and post-fracture care,” said Dr. Sing in a press release issued by ASBMR. “Better fall-prevention programs and clearer guidelines for clinical care have likely made a difference.”
Hip fracture incidence increased in five countries (The Netherlands, South Korea, France, Germany, and Brazil) and was stable in four countries (Australia, Japan, Thailand, and United States [Optum]).
The United Kingdom had the highest rate of osteoporosis treatment at 1-year after a hip fracture (50.3%). Rates in the other countries/regions ranged from 11.5% to 37%.
Fewer men than women were receiving drugs for osteoporosis at 1 year (range 5.1% to 38.2% versus 15.0% to 54.7%).
From 2005 to 2018, rates of osteoporosis treatment at 1 year after a hip fracture declined in six countries, increased in four countries, and were stable in five countries.
All-cause mortality within 1 year of hip fracture was higher in men than in women (range 19.2% to 35.8% versus 12.1% to 25.4%).
“Among the studied countries and regions, the U.S. ranks fifth with the highest hip fracture incidence,” Dr. Cheung replied when specifically asked about this. “The risk of hip fracture is determined by multiple factors: for example, lifestyle, diet, genetics, as well as management of osteoporosis,” he noted.
“Denmark is the only country showing no projected increase, and it is because Denmark had a continuous and remarkable decrease in the incidence of hip fractures,” he added, which “can offset the number of hip fractures contributed by the population aging.”
The study was funded by Amgen. Dr. Sing and Dr. Cheung have reported no relevant financial relationships. One of the study authors is employed by Amgen.
A version of this article first appeared on Medscape.com.
The annual incidence of hip fractures declined in most countries from 2005 to 2018, but this rate is projected to roughly double by 2050, according to a new study of 19 countries/regions.
The study by Chor-Wing Sing, PhD, and colleagues was presented at the annual meeting of the American Society of Bone and Mineral Research. The predicted increase in hip fractures is being driven by the aging population, with the population of those age 85 and older projected to increase 4.5-fold from 2010 to 2050, they note.
The researchers also estimate that from 2018 to 2050 the incidence of fractures will increase by 1.9-fold overall – more in men (2.4-fold) than in women (1.7-fold).
In addition, rates of use of osteoporosis drugs 1 year after a hip fracture were less than 50%, with less treatment in men. Men were also more likely than women to die within 1 year of a hip fracture.
The researchers conclude that “larger and more collaborative efforts among health care providers, policymakers, and patients are needed to prevent hip fractures and improve the treatment gap and post-fracture care, especially in men and the oldest old.”
Aging will fuel rise in hip fractures; more preventive treatment needed
“Even though there is a decreasing trend of hip fracture incidence in some countries, such a percentage decrease is insufficient to offset the percentage increase in the aging population,” senior co-author Ching-Lung Cheung, PhD, associate professor in the department of pharmacology and pharmacy at the University of Hong Kong, explained to this news organization.
The takeaways from the study are that “a greater effort on fracture prevention should be made to avoid the continuous increase in the number of hip fractures,” he said.
In addition, “although initiation of anti-osteoporosis medication after hip fracture is recommended in international guidelines, the 1-year treatment rate [was] well below 50% in most of the countries and regions studied. This indicates the treatment rate is far from optimal.”
“Our study also showed that the use of anti-osteoporosis medications following a hip fracture is lower in men than in women by 30% to 67%,” he said. “Thus, more attention should be paid to preventing and treating hip fractures in men.”
“The greater increase in the projected number of hip fractures in men than in women “could be [because] osteoporosis is commonly perceived as a ‘woman’s disease,’ ” he speculated.
Invited to comment, Juliet Compston, MD, who selected the study as one of the top clinical science highlight abstracts at the ASBMR meeting, agrees that “there is substantial room for improvement” in osteoporosis treatment rates following a hip fracture “in all the regions covered by the study.”
“In addition,” she continues, “the wide variations in treatment rates can provide important lessons about the most effective models of care for people who sustain a hip fracture: for example, fracture liaison services.”
Men suffer as osteoporosis perceived to be a ‘woman’s disease’
The even lower treatment rate in men than women is “concerning and likely reflects the mistaken perception that osteoporosis is predominantly a disease affecting women,” notes Dr. Compston, emeritus professor of bone medicine, University of Cambridge, United Kingdom.
Also invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, said that the projected doubling of hip fractures “is likely mainly due to aging of the population, with increasing lifespan for males in particular. However, increasing urbanization and decreasing weight-bearing exercise as a result are likely to also contribute in developing countries.”
“Unfortunately, despite the advances in treatments for osteoporosis over the last 25 years, osteoporosis treatment rates remain low, and osteoporosis remains undiagnosed in postmenopausal women and older men,” added Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research.
“More targeted screening for osteoporosis would help,” he said, “as would treating patients for it following other minimal trauma fractures (vertebral, distal radius, and humerus, etc.), since if left untreated, about 50% of these patients will have hip fractures later in life.”
“Some countries may be doing better because they have health quality standards for hip fracture (for example, surgery within 24 hours, investigation, and treatment for osteoporosis). In other countries like Australia, bone density tests and treatment for osteoporosis are reimbursed, increasing their uptake.”
The public health implications of this study are “substantial” according to Dr. Compston. “People who have sustained a hip fracture are at high risk of subsequent fractures if untreated. There is a range of safe, cost-effective pharmacological therapies to reduce fracture rate, and wider use of these would have a major impact on the current and future burden imposed by hip fractures in the elderly population.”
Similarly, Dr. Ebeling noted that “prevention is important to save a huge health burden for patients and costs for society.”
“Patients with minimal trauma fractures (particularly hip or spinal fractures) should be investigated and treated for osteoporosis with care pathways established in the hospitals, reaching out to the community [fracture liaison services],” he said.
Support for these is being sought under Medicare in the United States, he noted, and bone densitometry reimbursement rates also need to be higher in the United States.
Projections for number of hip fractures to 2050
Previous international reviews of hip fractures have been based on heterogeneous data from more than 10 to 30 years ago, the researchers note.
They performed a retrospective cohort study using a common protocol across 19 countries/regions, as described in an article about the protocol published in BMJ Open.
They analyzed data from adults aged 50 and older who were hospitalized with a hip fracture to determine 1) the annual incidence of hip fractures in 2008-2015; 2) the uptake of drugs to treat osteoporosis at 1 year after a hip fracture; and 3) all-cause mortality at 1 year after a hip fracture.
In a second step, they estimated the number of hip fractures that would occur from 2030 to 2050, using World Bank population growth projections.
The data are from 20 health care databases from 19 countries/regions: Oceania (Australia, New Zealand), Asia (Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand), Northern Europe (Denmark, Finland, and U.K.), Western Europe (France, Germany, Italy, The Netherlands, and Spain), and North and South America (Canada, United States, and Brazil).
The population in Japan was under age 75. U.S. data are from two databases: Medicare (age ≥ 65) and Optum.
Most databases (13) covered 90%-100% of the national population, and the rest covered 5%-70% of the population.
From 2008 to 2015, the annual incidence of hip fractures declined in 11 countries/regions (Singapore, Denmark, Hong Kong, Taiwan, Finland, U.K., Italy, Spain, United States [Medicare], Canada, and New Zealand).
“One potential reason that some countries have seen relatively large declines in hip fractures is better osteoporosis management and post-fracture care,” said Dr. Sing in a press release issued by ASBMR. “Better fall-prevention programs and clearer guidelines for clinical care have likely made a difference.”
Hip fracture incidence increased in five countries (The Netherlands, South Korea, France, Germany, and Brazil) and was stable in four countries (Australia, Japan, Thailand, and United States [Optum]).
The United Kingdom had the highest rate of osteoporosis treatment at 1-year after a hip fracture (50.3%). Rates in the other countries/regions ranged from 11.5% to 37%.
Fewer men than women were receiving drugs for osteoporosis at 1 year (range 5.1% to 38.2% versus 15.0% to 54.7%).
From 2005 to 2018, rates of osteoporosis treatment at 1 year after a hip fracture declined in six countries, increased in four countries, and were stable in five countries.
All-cause mortality within 1 year of hip fracture was higher in men than in women (range 19.2% to 35.8% versus 12.1% to 25.4%).
“Among the studied countries and regions, the U.S. ranks fifth with the highest hip fracture incidence,” Dr. Cheung replied when specifically asked about this. “The risk of hip fracture is determined by multiple factors: for example, lifestyle, diet, genetics, as well as management of osteoporosis,” he noted.
“Denmark is the only country showing no projected increase, and it is because Denmark had a continuous and remarkable decrease in the incidence of hip fractures,” he added, which “can offset the number of hip fractures contributed by the population aging.”
The study was funded by Amgen. Dr. Sing and Dr. Cheung have reported no relevant financial relationships. One of the study authors is employed by Amgen.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
An FP’s guide to exercise counseling for older adults
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)
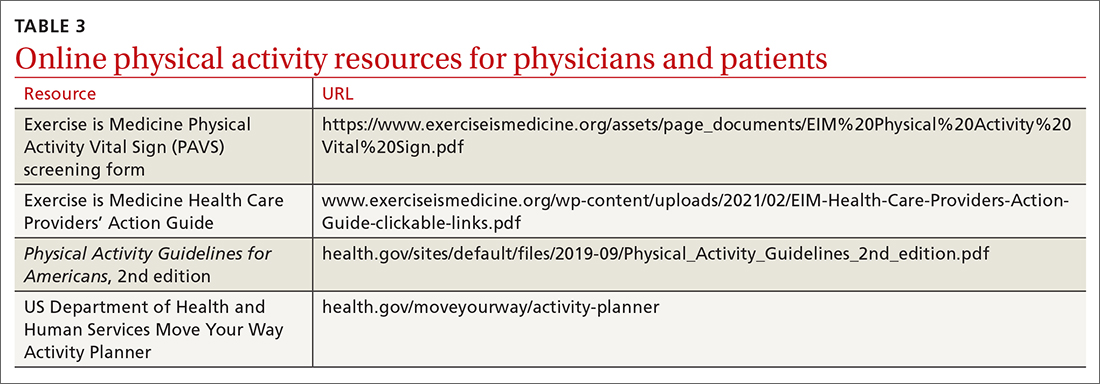
Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2
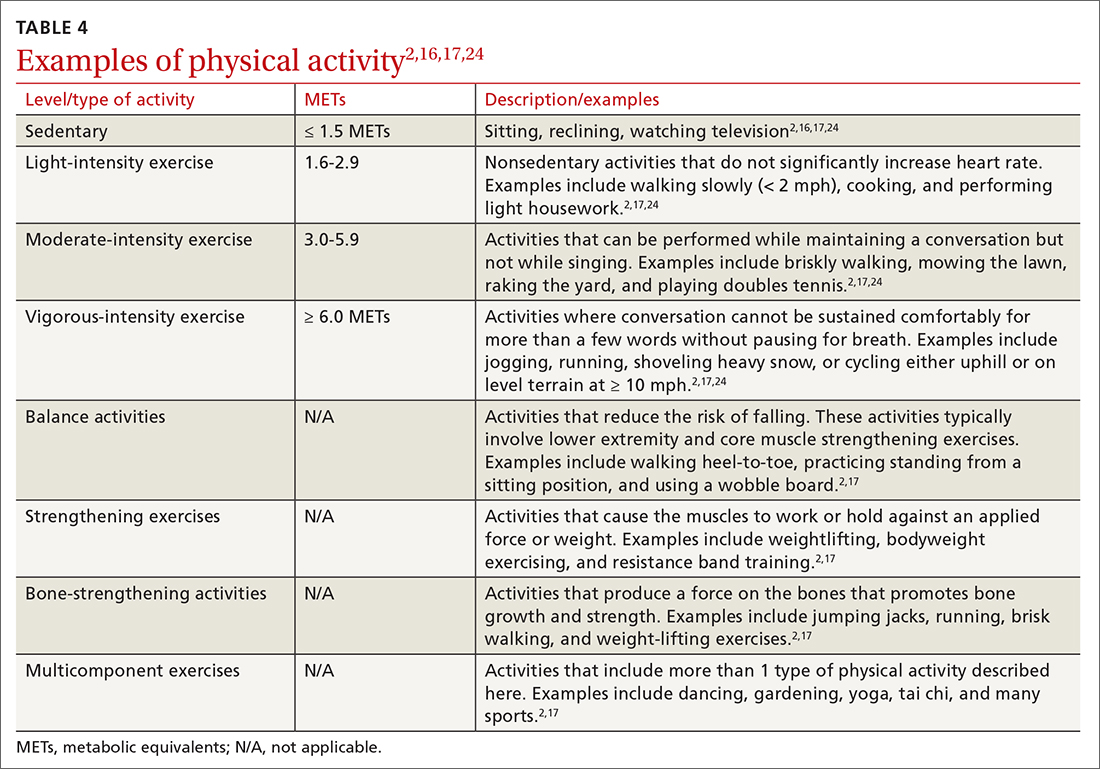
The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
PRACTICE RECOMMENDATIONS
› Encourage older adults to engage in at least 150 minutes of moderate-intensity aerobic physical activity throughout the week, OR at least 75 minutes of vigorous-intensity aerobic physical activity throughout the week, OR an equivalent combination of moderate- and vigorous-intensity activity. A
› Recommend older adults perform muscle-strengthening activities involving major muscle groups on 2 or more days per week. A
› Encourage older adults to be as physically active as possible, even when their health conditions and abilities prevent them from reaching their minimum levels of physical activity. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Yoga, CBT provide long-term improvement in insomnia, worry
new research suggests.
The study is the first to compare the long-term effects from the two interventions; and the results offer clinicians and patients two effective choices for reducing worry and anxiety, researchers noted.
“Anxiety can be a really big problem for older adults,” lead investigator Suzanne Danhauer, PhD, professor of social sciences and health policy at Wake Forest University, Winston-Salem, N.C., said in an interview.
“So to find something they can do that lasts ... and has some enduring impact on their quality of life and their mental health, and they’re both nonpharmacologic treatments, I think for a lot of older people that’s really attractive,” Dr. Danhauer said.
The findings are published in the September issue of the American Journal of Geriatric Psychiatry.
Long-term benefits
The two-stage randomized preference trial included 500 community-dwelling individuals over age 60 who scored 26 or above on the Penn State Worry Questionnaire–Abbreviated (PSWQ-A), indicating heightened anxiety and worry.
Half the group took part in a randomized, controlled trial comparing CBT (n = 125) with yoga (n = 125). The other half participated in a preference trial where they were allowed to choose between CBT (n = 120) and yoga (n = 130).
Participants completed 20 yoga sessions over 10 weeks or 10 weekly CBT calls between May 2017 and November 2018.
Measures used included the PSWQ-A, the Insomnia Severity Index (ISI), the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Anxiety 8a, and the PROMIS-29 to assess depression, fatigue, physical function, social participation, and pain.
In 2020, the researchers published results at 11 weeks showing improvements from baseline in all areas. The scores for anxiety and worry were similar between the CBT and yoga groups, but CBT yielded significantly higher improvement in insomnia.
At 37 weeks, about 6 months after the interventions had ended, the investigators found even greater improvements from baseline in all areas measured – except physical function.
However, at that point, there were no significant differences between the two interventions in either the randomized controlled trial or the preference trial. There were also no differences in the results between the two trial designs.
“There were some little differences, but by and large we found both interventions to be efficacious,” Dr. Danhauer said. “This gives clinicians [the] choice to be able to say, ‘you can try either one of these and they’re probably going to help.’ ”
Beyond statistically significant
The researchers also found the improvements were not just statistically significant, but were also clinically meaningful for worry, anxiety, and insomnia.
Meaningful changes were defined as a decrease of at least 5.5 points on the PSWQ-A for worry, a decrease of at least 3 points on the PROMIS Anxiety scale for anxiety, and a decrease of at least 6 points in the ISI for insomnia.
At long-term follow-up, the majority of participants in both the CBT and yoga arms of the randomized, controlled trial demonstrated meaningful change in worry (85.7% and 77.6%, respectively), anxiety (82.1% and 80.8%), and insomnia (52.8% and 44.3%).
The majority of participants also reported meaningful improvements in generalized anxiety symptoms, depressive symptoms, and fatigue, but not for physical function, pain interference, or pain intensity.
“That’s the part to me that’s particularly notable. The improvements weren’t just statistically significant, they were clinically meaningful as well,” Dr. Danhauer said.
“When it comes right down to people’s lives, they want differences they can feel and see and not just what a P value looks like,” she added.
Real-world impact
In an accompanying editorial, Carmen Andreescu, MD, associate professor of psychiatry at the University of Pittsburgh, agreed that the results have “real-world impact.”
“Clinicians can direct their patients toward interventions that may be beneficial, consolidate the results over time and avoid fueling the well-trained worry cognitive loop with concerns related to potential side effects,” Dr. Andreescu wrote.
She adds that interventions such as these “may increase accessibility and provide relief for the immediate suffering of our patients.”
The study was funded by the Patient-Centered Outcomes Research Institute Program. Dr. Danhauer and Dr. Andreescu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The study is the first to compare the long-term effects from the two interventions; and the results offer clinicians and patients two effective choices for reducing worry and anxiety, researchers noted.
“Anxiety can be a really big problem for older adults,” lead investigator Suzanne Danhauer, PhD, professor of social sciences and health policy at Wake Forest University, Winston-Salem, N.C., said in an interview.
“So to find something they can do that lasts ... and has some enduring impact on their quality of life and their mental health, and they’re both nonpharmacologic treatments, I think for a lot of older people that’s really attractive,” Dr. Danhauer said.
The findings are published in the September issue of the American Journal of Geriatric Psychiatry.
Long-term benefits
The two-stage randomized preference trial included 500 community-dwelling individuals over age 60 who scored 26 or above on the Penn State Worry Questionnaire–Abbreviated (PSWQ-A), indicating heightened anxiety and worry.
Half the group took part in a randomized, controlled trial comparing CBT (n = 125) with yoga (n = 125). The other half participated in a preference trial where they were allowed to choose between CBT (n = 120) and yoga (n = 130).
Participants completed 20 yoga sessions over 10 weeks or 10 weekly CBT calls between May 2017 and November 2018.
Measures used included the PSWQ-A, the Insomnia Severity Index (ISI), the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Anxiety 8a, and the PROMIS-29 to assess depression, fatigue, physical function, social participation, and pain.
In 2020, the researchers published results at 11 weeks showing improvements from baseline in all areas. The scores for anxiety and worry were similar between the CBT and yoga groups, but CBT yielded significantly higher improvement in insomnia.
At 37 weeks, about 6 months after the interventions had ended, the investigators found even greater improvements from baseline in all areas measured – except physical function.
However, at that point, there were no significant differences between the two interventions in either the randomized controlled trial or the preference trial. There were also no differences in the results between the two trial designs.
“There were some little differences, but by and large we found both interventions to be efficacious,” Dr. Danhauer said. “This gives clinicians [the] choice to be able to say, ‘you can try either one of these and they’re probably going to help.’ ”
Beyond statistically significant
The researchers also found the improvements were not just statistically significant, but were also clinically meaningful for worry, anxiety, and insomnia.
Meaningful changes were defined as a decrease of at least 5.5 points on the PSWQ-A for worry, a decrease of at least 3 points on the PROMIS Anxiety scale for anxiety, and a decrease of at least 6 points in the ISI for insomnia.
At long-term follow-up, the majority of participants in both the CBT and yoga arms of the randomized, controlled trial demonstrated meaningful change in worry (85.7% and 77.6%, respectively), anxiety (82.1% and 80.8%), and insomnia (52.8% and 44.3%).
The majority of participants also reported meaningful improvements in generalized anxiety symptoms, depressive symptoms, and fatigue, but not for physical function, pain interference, or pain intensity.
“That’s the part to me that’s particularly notable. The improvements weren’t just statistically significant, they were clinically meaningful as well,” Dr. Danhauer said.
“When it comes right down to people’s lives, they want differences they can feel and see and not just what a P value looks like,” she added.
Real-world impact
In an accompanying editorial, Carmen Andreescu, MD, associate professor of psychiatry at the University of Pittsburgh, agreed that the results have “real-world impact.”
“Clinicians can direct their patients toward interventions that may be beneficial, consolidate the results over time and avoid fueling the well-trained worry cognitive loop with concerns related to potential side effects,” Dr. Andreescu wrote.
She adds that interventions such as these “may increase accessibility and provide relief for the immediate suffering of our patients.”
The study was funded by the Patient-Centered Outcomes Research Institute Program. Dr. Danhauer and Dr. Andreescu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The study is the first to compare the long-term effects from the two interventions; and the results offer clinicians and patients two effective choices for reducing worry and anxiety, researchers noted.
“Anxiety can be a really big problem for older adults,” lead investigator Suzanne Danhauer, PhD, professor of social sciences and health policy at Wake Forest University, Winston-Salem, N.C., said in an interview.
“So to find something they can do that lasts ... and has some enduring impact on their quality of life and their mental health, and they’re both nonpharmacologic treatments, I think for a lot of older people that’s really attractive,” Dr. Danhauer said.
The findings are published in the September issue of the American Journal of Geriatric Psychiatry.
Long-term benefits
The two-stage randomized preference trial included 500 community-dwelling individuals over age 60 who scored 26 or above on the Penn State Worry Questionnaire–Abbreviated (PSWQ-A), indicating heightened anxiety and worry.
Half the group took part in a randomized, controlled trial comparing CBT (n = 125) with yoga (n = 125). The other half participated in a preference trial where they were allowed to choose between CBT (n = 120) and yoga (n = 130).
Participants completed 20 yoga sessions over 10 weeks or 10 weekly CBT calls between May 2017 and November 2018.
Measures used included the PSWQ-A, the Insomnia Severity Index (ISI), the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Anxiety 8a, and the PROMIS-29 to assess depression, fatigue, physical function, social participation, and pain.
In 2020, the researchers published results at 11 weeks showing improvements from baseline in all areas. The scores for anxiety and worry were similar between the CBT and yoga groups, but CBT yielded significantly higher improvement in insomnia.
At 37 weeks, about 6 months after the interventions had ended, the investigators found even greater improvements from baseline in all areas measured – except physical function.
However, at that point, there were no significant differences between the two interventions in either the randomized controlled trial or the preference trial. There were also no differences in the results between the two trial designs.
“There were some little differences, but by and large we found both interventions to be efficacious,” Dr. Danhauer said. “This gives clinicians [the] choice to be able to say, ‘you can try either one of these and they’re probably going to help.’ ”
Beyond statistically significant
The researchers also found the improvements were not just statistically significant, but were also clinically meaningful for worry, anxiety, and insomnia.
Meaningful changes were defined as a decrease of at least 5.5 points on the PSWQ-A for worry, a decrease of at least 3 points on the PROMIS Anxiety scale for anxiety, and a decrease of at least 6 points in the ISI for insomnia.
At long-term follow-up, the majority of participants in both the CBT and yoga arms of the randomized, controlled trial demonstrated meaningful change in worry (85.7% and 77.6%, respectively), anxiety (82.1% and 80.8%), and insomnia (52.8% and 44.3%).
The majority of participants also reported meaningful improvements in generalized anxiety symptoms, depressive symptoms, and fatigue, but not for physical function, pain interference, or pain intensity.
“That’s the part to me that’s particularly notable. The improvements weren’t just statistically significant, they were clinically meaningful as well,” Dr. Danhauer said.
“When it comes right down to people’s lives, they want differences they can feel and see and not just what a P value looks like,” she added.
Real-world impact
In an accompanying editorial, Carmen Andreescu, MD, associate professor of psychiatry at the University of Pittsburgh, agreed that the results have “real-world impact.”
“Clinicians can direct their patients toward interventions that may be beneficial, consolidate the results over time and avoid fueling the well-trained worry cognitive loop with concerns related to potential side effects,” Dr. Andreescu wrote.
She adds that interventions such as these “may increase accessibility and provide relief for the immediate suffering of our patients.”
The study was funded by the Patient-Centered Outcomes Research Institute Program. Dr. Danhauer and Dr. Andreescu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Sacubitril/valsartan shows cognitive safety in heart failure: PERSPECTIVE
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
AT ESC CONGRESS 2022
Secondary CV prevention benefit from polypill promises global health benefit
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
FROM ESC CONGRESS 2022
Low-dose edoxaban curbs stroke risk in elderly with AF, despite frailty
Elderly patients with atrial fibrillation (AF) who are at high risk of bleeding may benefit from a low 15-mg dose of edoxaban, regardless of their frailty status, a subanalysis of the ELDERCARE-AF trial suggests.
Major bleeding and major or clinically relevant nonmajor bleeding events were both numerically higher in the edoxaban group than placebo, the authors reported, with no heterogeneity by frailty status.
The subanalysis extends findings of the overall study by teasing out stroke, systemic embolism (SSE) and bleeding events across frailty status among Japanese patients aged 80 and older who were ineligible for oral anticoagulants (OACs) at usual doses.
Findings from the original phase 3 ELDERCARE-AF study were previously reported during the virtual European Society of Cardiology Congress 2020 and simultaneously published in The New England Journal of Medicine. The current study was published online in JAMA Network Open.
All frailty levels benefited
Shintaro Akashi, MD, PhD, of National Hospital Organization Hamada Medical Center, Shimane, Japan, and colleagues analyzed data from 944 patients randomly assigned to edoxaban 15 mg or placebo for about 3 years. The mean age of participants was 86.6 years and 57% were women. Baseline characteristics, including history of bleeding, were similar between groups.
Patient physical condition was assessed via five parameters: weight loss, grip strength, walking speed, exhaustion, and activity level. This yielded a frailty score, with one point given for each parameter: 0 indicated robust; 1 or 2, prefrail; and 3 or higher, frail. For this analysis, robust (6.5% of patients) and prefrail (51%) were combined and categorized as nonfrail.
In the placebo group, estimated event rates for stroke or SSE were 7.1% per patient-year among frail patients and 6.1% per patient-year among those who were nonfrail.
In the edoxaban group, SSE occurred at an estimated event rate of 2.5% of frail patients and 1.5% of nonfrail patients (adjusted HR, 1.41).
The edoxaban group “consistently had fewer SSE events regardless of frailty status including each frailty assessment parameter, and there was no heterogeneity between the groups,” the authors wrote, with similar trends for the association of edoxaban 15 mg for each frailty assessment parameter.
However, major bleeding and major or clinically relevant nonmajor (CRNM) bleeding events were both higher with edoxaban, regardless of frailty status.
More specifically, in the placebo group, the incidence of major bleeding was 2.3% in the frail group and 1.5% in the nonfrail group (adjusted HR, 1.48) versus 3.7% and 2.9%, respectively, in the edoxaban group (adjusted HR, 1.04).
In addition, exhaustion was related to a significantly increased risk of major or CRNM bleeding in frail versus nonfrail patients (16.3% vs. 8.4%; adjusted HR, 1.97). The incidences were all higher in the edoxaban group, irrespective of frailty status.
Furthermore, although both all-cause death and the net clinical composite outcome of stroke or SSE occurred more frequently in frail than in nonfrail patients, there was no association with frailty status between the edoxaban and placebo groups.
Findings unrelated to edoxaban were also noteworthy. “Surprisingly, grip strength showed an association with adverse events,” the authors wrote. Among those with lower grip strength, “there was nearly a 3-fold increase in risk of SSE and major bleeding and a more than 16-fold significant increase in risk of death. In addition, in those with exhaustion, there was nearly a 2-fold significant increase in major or CRNM bleeding.”
Thus, they suggested, in this patient population, “an objective physical assessment of grip strength or exhaustion in addition to the well-known walking speed may more accurately estimate the risks of clinical outcomes than the overall frailty assessment.”
Head-to-head comparisons needed
Commenting on the findings, Richard Kovach, MD, chair of the interventional cardiology division at Deborah Heart and Lung Center, Browns Mills, N.J., said, “It is interesting that the lower dose of edoxaban still appears to have a statistically significant reduction in the incidence of stroke in this subgroup of extremely frail elderly patients, and it may be useful in this highly selected subset.
“That being said,” he added, “the major complication of oral anticoagulants – major bleeding – appears to be similar to other NOACs prescribed more frequently in the U.S., specifically rivaroxaban and apixaban.”
“Furthermore, in the U.S., frail or complex patients who are not candidates for oral anticoagulant therapy are much more likely to receive a left atrial appendage closure device such as a Watchman or Amulet in order to avoid the risk of bleeding complications completely,” he said. “Procedural success with these devices is extremely high and procedural complications are extremely low. With both devices, the long-term reduction in stroke risk is equivalent to the use of anticoagulant therapy.
“Clearly, more research is needed to compare the outcomes with edoxaban against other NOACs,” Dr. Kovach concluded. “A head-to-head comparison of low-dose edoxaban versus left atrial appendage closure in this high-risk group would also be of great clinical value.”
The study was funded by Daiichi Sankyo. Two coauthors are employees of and five have received fees from the company. Dr. Kovach has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Elderly patients with atrial fibrillation (AF) who are at high risk of bleeding may benefit from a low 15-mg dose of edoxaban, regardless of their frailty status, a subanalysis of the ELDERCARE-AF trial suggests.
Major bleeding and major or clinically relevant nonmajor bleeding events were both numerically higher in the edoxaban group than placebo, the authors reported, with no heterogeneity by frailty status.
The subanalysis extends findings of the overall study by teasing out stroke, systemic embolism (SSE) and bleeding events across frailty status among Japanese patients aged 80 and older who were ineligible for oral anticoagulants (OACs) at usual doses.
Findings from the original phase 3 ELDERCARE-AF study were previously reported during the virtual European Society of Cardiology Congress 2020 and simultaneously published in The New England Journal of Medicine. The current study was published online in JAMA Network Open.
All frailty levels benefited
Shintaro Akashi, MD, PhD, of National Hospital Organization Hamada Medical Center, Shimane, Japan, and colleagues analyzed data from 944 patients randomly assigned to edoxaban 15 mg or placebo for about 3 years. The mean age of participants was 86.6 years and 57% were women. Baseline characteristics, including history of bleeding, were similar between groups.
Patient physical condition was assessed via five parameters: weight loss, grip strength, walking speed, exhaustion, and activity level. This yielded a frailty score, with one point given for each parameter: 0 indicated robust; 1 or 2, prefrail; and 3 or higher, frail. For this analysis, robust (6.5% of patients) and prefrail (51%) were combined and categorized as nonfrail.
In the placebo group, estimated event rates for stroke or SSE were 7.1% per patient-year among frail patients and 6.1% per patient-year among those who were nonfrail.
In the edoxaban group, SSE occurred at an estimated event rate of 2.5% of frail patients and 1.5% of nonfrail patients (adjusted HR, 1.41).
The edoxaban group “consistently had fewer SSE events regardless of frailty status including each frailty assessment parameter, and there was no heterogeneity between the groups,” the authors wrote, with similar trends for the association of edoxaban 15 mg for each frailty assessment parameter.
However, major bleeding and major or clinically relevant nonmajor (CRNM) bleeding events were both higher with edoxaban, regardless of frailty status.
More specifically, in the placebo group, the incidence of major bleeding was 2.3% in the frail group and 1.5% in the nonfrail group (adjusted HR, 1.48) versus 3.7% and 2.9%, respectively, in the edoxaban group (adjusted HR, 1.04).
In addition, exhaustion was related to a significantly increased risk of major or CRNM bleeding in frail versus nonfrail patients (16.3% vs. 8.4%; adjusted HR, 1.97). The incidences were all higher in the edoxaban group, irrespective of frailty status.
Furthermore, although both all-cause death and the net clinical composite outcome of stroke or SSE occurred more frequently in frail than in nonfrail patients, there was no association with frailty status between the edoxaban and placebo groups.
Findings unrelated to edoxaban were also noteworthy. “Surprisingly, grip strength showed an association with adverse events,” the authors wrote. Among those with lower grip strength, “there was nearly a 3-fold increase in risk of SSE and major bleeding and a more than 16-fold significant increase in risk of death. In addition, in those with exhaustion, there was nearly a 2-fold significant increase in major or CRNM bleeding.”
Thus, they suggested, in this patient population, “an objective physical assessment of grip strength or exhaustion in addition to the well-known walking speed may more accurately estimate the risks of clinical outcomes than the overall frailty assessment.”
Head-to-head comparisons needed
Commenting on the findings, Richard Kovach, MD, chair of the interventional cardiology division at Deborah Heart and Lung Center, Browns Mills, N.J., said, “It is interesting that the lower dose of edoxaban still appears to have a statistically significant reduction in the incidence of stroke in this subgroup of extremely frail elderly patients, and it may be useful in this highly selected subset.
“That being said,” he added, “the major complication of oral anticoagulants – major bleeding – appears to be similar to other NOACs prescribed more frequently in the U.S., specifically rivaroxaban and apixaban.”
“Furthermore, in the U.S., frail or complex patients who are not candidates for oral anticoagulant therapy are much more likely to receive a left atrial appendage closure device such as a Watchman or Amulet in order to avoid the risk of bleeding complications completely,” he said. “Procedural success with these devices is extremely high and procedural complications are extremely low. With both devices, the long-term reduction in stroke risk is equivalent to the use of anticoagulant therapy.
“Clearly, more research is needed to compare the outcomes with edoxaban against other NOACs,” Dr. Kovach concluded. “A head-to-head comparison of low-dose edoxaban versus left atrial appendage closure in this high-risk group would also be of great clinical value.”
The study was funded by Daiichi Sankyo. Two coauthors are employees of and five have received fees from the company. Dr. Kovach has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Elderly patients with atrial fibrillation (AF) who are at high risk of bleeding may benefit from a low 15-mg dose of edoxaban, regardless of their frailty status, a subanalysis of the ELDERCARE-AF trial suggests.
Major bleeding and major or clinically relevant nonmajor bleeding events were both numerically higher in the edoxaban group than placebo, the authors reported, with no heterogeneity by frailty status.
The subanalysis extends findings of the overall study by teasing out stroke, systemic embolism (SSE) and bleeding events across frailty status among Japanese patients aged 80 and older who were ineligible for oral anticoagulants (OACs) at usual doses.
Findings from the original phase 3 ELDERCARE-AF study were previously reported during the virtual European Society of Cardiology Congress 2020 and simultaneously published in The New England Journal of Medicine. The current study was published online in JAMA Network Open.
All frailty levels benefited
Shintaro Akashi, MD, PhD, of National Hospital Organization Hamada Medical Center, Shimane, Japan, and colleagues analyzed data from 944 patients randomly assigned to edoxaban 15 mg or placebo for about 3 years. The mean age of participants was 86.6 years and 57% were women. Baseline characteristics, including history of bleeding, were similar between groups.
Patient physical condition was assessed via five parameters: weight loss, grip strength, walking speed, exhaustion, and activity level. This yielded a frailty score, with one point given for each parameter: 0 indicated robust; 1 or 2, prefrail; and 3 or higher, frail. For this analysis, robust (6.5% of patients) and prefrail (51%) were combined and categorized as nonfrail.
In the placebo group, estimated event rates for stroke or SSE were 7.1% per patient-year among frail patients and 6.1% per patient-year among those who were nonfrail.
In the edoxaban group, SSE occurred at an estimated event rate of 2.5% of frail patients and 1.5% of nonfrail patients (adjusted HR, 1.41).
The edoxaban group “consistently had fewer SSE events regardless of frailty status including each frailty assessment parameter, and there was no heterogeneity between the groups,” the authors wrote, with similar trends for the association of edoxaban 15 mg for each frailty assessment parameter.
However, major bleeding and major or clinically relevant nonmajor (CRNM) bleeding events were both higher with edoxaban, regardless of frailty status.
More specifically, in the placebo group, the incidence of major bleeding was 2.3% in the frail group and 1.5% in the nonfrail group (adjusted HR, 1.48) versus 3.7% and 2.9%, respectively, in the edoxaban group (adjusted HR, 1.04).
In addition, exhaustion was related to a significantly increased risk of major or CRNM bleeding in frail versus nonfrail patients (16.3% vs. 8.4%; adjusted HR, 1.97). The incidences were all higher in the edoxaban group, irrespective of frailty status.
Furthermore, although both all-cause death and the net clinical composite outcome of stroke or SSE occurred more frequently in frail than in nonfrail patients, there was no association with frailty status between the edoxaban and placebo groups.
Findings unrelated to edoxaban were also noteworthy. “Surprisingly, grip strength showed an association with adverse events,” the authors wrote. Among those with lower grip strength, “there was nearly a 3-fold increase in risk of SSE and major bleeding and a more than 16-fold significant increase in risk of death. In addition, in those with exhaustion, there was nearly a 2-fold significant increase in major or CRNM bleeding.”
Thus, they suggested, in this patient population, “an objective physical assessment of grip strength or exhaustion in addition to the well-known walking speed may more accurately estimate the risks of clinical outcomes than the overall frailty assessment.”
Head-to-head comparisons needed
Commenting on the findings, Richard Kovach, MD, chair of the interventional cardiology division at Deborah Heart and Lung Center, Browns Mills, N.J., said, “It is interesting that the lower dose of edoxaban still appears to have a statistically significant reduction in the incidence of stroke in this subgroup of extremely frail elderly patients, and it may be useful in this highly selected subset.
“That being said,” he added, “the major complication of oral anticoagulants – major bleeding – appears to be similar to other NOACs prescribed more frequently in the U.S., specifically rivaroxaban and apixaban.”
“Furthermore, in the U.S., frail or complex patients who are not candidates for oral anticoagulant therapy are much more likely to receive a left atrial appendage closure device such as a Watchman or Amulet in order to avoid the risk of bleeding complications completely,” he said. “Procedural success with these devices is extremely high and procedural complications are extremely low. With both devices, the long-term reduction in stroke risk is equivalent to the use of anticoagulant therapy.
“Clearly, more research is needed to compare the outcomes with edoxaban against other NOACs,” Dr. Kovach concluded. “A head-to-head comparison of low-dose edoxaban versus left atrial appendage closure in this high-risk group would also be of great clinical value.”
The study was funded by Daiichi Sankyo. Two coauthors are employees of and five have received fees from the company. Dr. Kovach has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One hour of walking per week may boost longevity for octogenarians
Adults aged 85 years and older who logged an hour or more of walking each week had a 40% reduced risk of all-cause mortality compared with less active peers, according to data from more than 7,000 individuals.
“Aging is accompanied by reduced physical activity and increased sedentary behavior, and reduced physical activity is associated with decreased life expectancy,” Moo-Nyun Jin, MD, of Inje University Sanggye Paik Hospital, Seoul, South Korea, said in an interview.
Reduced physical activity was especially likely in the elderly during the COVID-19 pandemic, he added.
“Promoting walking may be a simple way to help older adults avoid inactivity and encourage an active lifestyle for all-cause and cardiovascular mortality risk reduction,” Dr. Jin said.
Although walking is generally an easy form of exercise for the older adult population, the specific benefit of walking on reducing mortality has not been well studied, according to Dr. Jin and colleagues.
For adults of any age, current guidelines recommend at least 150 minutes per week of moderate activity or 75 minutes per week of vigorous activity, but the amount of physical activity tends to decline with age, and activity recommendations are more difficult to meet, the authors wrote in a press release accompanying their study.
In the study, to be presented at the European Society of Cardiology Congress on Aug. 28 (Abstract 85643), the researchers reviewed data from 7,047 adults aged 85 years and older who participated in the Korean National Health Screening Program. The average age of the study population was 87 years, and 68% were women. Participants completed questionnaires about the amount of time spent in leisure time activities each week, including walking at a slow pace, moderate activity (such as cycling or brisk walking), and vigorous activity (such as running).
Those who walked at a slow pace for at least 1 hour per week had a 40% reduced risk of all-cause mortality and a 39% reduced risk of cardiovascular mortality, compared with inactive participants.
The proportions of participants who reported walking, moderate activity, and vigorous intensity physical activity were 42.5%, 14.7%, and 11.0%, respectively. Roughly one-third (33%) of those who reported slow walking each week also reported moderate or vigorous physical activity.
However, walking for 1 hour per week significantly reduced the risk for all-cause mortality and cardiovascular mortality among individuals who reported walking only, without other moderate or vigorous physical activity (hazard ratio, 0.50 and 0.46, respectively).
“Walking was linked with a lower likelihood of dying in older adults, regardless of whether or not they did any moderate to vigorous intensity physical activity,” Dr. Jin told this news organization. “Our study indicates that walking even just 1 hour every week is advantageous to those aged 85 years and older compared to being inactive.”
The hour of walking need not be in long bouts, 10 minutes each day will do, Dr. Jin added.
The participants were divided into five groups based on reported amount of weekly walking. More than half (57.5%) reported no slow walking, 8.5% walked less than 1 hour per week, 12.0% walked 1-2 hours, 8.7% walked 2-3 hours, and 13.3% walked more than 3 hours.
Although the study was limited by the reliance on self-reports, the results were strengthened by the large sample size and support the value of easy walking for adults aged 85 years and older compared to being inactive.
“Walking may present an opportunity for promoting physical activity among the elderly population, offering a simple way to avoid inactivity and increase physical activity,” said Dr. Jin. However, more research is needed to evaluate the association between mortality and walking by objective measurement of walking levels, using a device such as a smart watch, he noted.
Results are preliminary
“This is an observational study, not an experiment, so it means causality cannot be presumed,” said Maria Fiatarone Singh, MD, a geriatrician with a focus on exercise physiology at the University of Sydney, in an interview. “In other words, it is possible that diseases resulting in mortality prevented people from walking rather than the other way around,” she noted. The only published experimental study on exercise and mortality in older adults was conducted by Dr. Fiatarone Singh and colleagues in Norway. In that study, published in the British Medical Journal in 2020, high-intensity training programs were associated with reduced all-cause mortality compared with inactive controls and individuals who engaged in moderate intensity exercise.
The current study “would have needed to control for many factors related to mortality, such as cardiovascular disease, hypertension, diabetes, malnutrition, and dementia to see what residual benefit might be related to walking,” Dr. Fiatarone Singh said.
“Although walking seems easy and safe, in fact people who are frail, sarcopenic, osteoporotic, or have fallen are recommended to do resistance and balance training rather than walking, and add walking later when they are able to do it safely,” she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Fiatarone Singh had no financial conflicts to disclose.
Adults aged 85 years and older who logged an hour or more of walking each week had a 40% reduced risk of all-cause mortality compared with less active peers, according to data from more than 7,000 individuals.
“Aging is accompanied by reduced physical activity and increased sedentary behavior, and reduced physical activity is associated with decreased life expectancy,” Moo-Nyun Jin, MD, of Inje University Sanggye Paik Hospital, Seoul, South Korea, said in an interview.
Reduced physical activity was especially likely in the elderly during the COVID-19 pandemic, he added.
“Promoting walking may be a simple way to help older adults avoid inactivity and encourage an active lifestyle for all-cause and cardiovascular mortality risk reduction,” Dr. Jin said.
Although walking is generally an easy form of exercise for the older adult population, the specific benefit of walking on reducing mortality has not been well studied, according to Dr. Jin and colleagues.
For adults of any age, current guidelines recommend at least 150 minutes per week of moderate activity or 75 minutes per week of vigorous activity, but the amount of physical activity tends to decline with age, and activity recommendations are more difficult to meet, the authors wrote in a press release accompanying their study.
In the study, to be presented at the European Society of Cardiology Congress on Aug. 28 (Abstract 85643), the researchers reviewed data from 7,047 adults aged 85 years and older who participated in the Korean National Health Screening Program. The average age of the study population was 87 years, and 68% were women. Participants completed questionnaires about the amount of time spent in leisure time activities each week, including walking at a slow pace, moderate activity (such as cycling or brisk walking), and vigorous activity (such as running).
Those who walked at a slow pace for at least 1 hour per week had a 40% reduced risk of all-cause mortality and a 39% reduced risk of cardiovascular mortality, compared with inactive participants.
The proportions of participants who reported walking, moderate activity, and vigorous intensity physical activity were 42.5%, 14.7%, and 11.0%, respectively. Roughly one-third (33%) of those who reported slow walking each week also reported moderate or vigorous physical activity.
However, walking for 1 hour per week significantly reduced the risk for all-cause mortality and cardiovascular mortality among individuals who reported walking only, without other moderate or vigorous physical activity (hazard ratio, 0.50 and 0.46, respectively).
“Walking was linked with a lower likelihood of dying in older adults, regardless of whether or not they did any moderate to vigorous intensity physical activity,” Dr. Jin told this news organization. “Our study indicates that walking even just 1 hour every week is advantageous to those aged 85 years and older compared to being inactive.”
The hour of walking need not be in long bouts, 10 minutes each day will do, Dr. Jin added.
The participants were divided into five groups based on reported amount of weekly walking. More than half (57.5%) reported no slow walking, 8.5% walked less than 1 hour per week, 12.0% walked 1-2 hours, 8.7% walked 2-3 hours, and 13.3% walked more than 3 hours.
Although the study was limited by the reliance on self-reports, the results were strengthened by the large sample size and support the value of easy walking for adults aged 85 years and older compared to being inactive.
“Walking may present an opportunity for promoting physical activity among the elderly population, offering a simple way to avoid inactivity and increase physical activity,” said Dr. Jin. However, more research is needed to evaluate the association between mortality and walking by objective measurement of walking levels, using a device such as a smart watch, he noted.
Results are preliminary
“This is an observational study, not an experiment, so it means causality cannot be presumed,” said Maria Fiatarone Singh, MD, a geriatrician with a focus on exercise physiology at the University of Sydney, in an interview. “In other words, it is possible that diseases resulting in mortality prevented people from walking rather than the other way around,” she noted. The only published experimental study on exercise and mortality in older adults was conducted by Dr. Fiatarone Singh and colleagues in Norway. In that study, published in the British Medical Journal in 2020, high-intensity training programs were associated with reduced all-cause mortality compared with inactive controls and individuals who engaged in moderate intensity exercise.
The current study “would have needed to control for many factors related to mortality, such as cardiovascular disease, hypertension, diabetes, malnutrition, and dementia to see what residual benefit might be related to walking,” Dr. Fiatarone Singh said.
“Although walking seems easy and safe, in fact people who are frail, sarcopenic, osteoporotic, or have fallen are recommended to do resistance and balance training rather than walking, and add walking later when they are able to do it safely,” she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Fiatarone Singh had no financial conflicts to disclose.
Adults aged 85 years and older who logged an hour or more of walking each week had a 40% reduced risk of all-cause mortality compared with less active peers, according to data from more than 7,000 individuals.
“Aging is accompanied by reduced physical activity and increased sedentary behavior, and reduced physical activity is associated with decreased life expectancy,” Moo-Nyun Jin, MD, of Inje University Sanggye Paik Hospital, Seoul, South Korea, said in an interview.
Reduced physical activity was especially likely in the elderly during the COVID-19 pandemic, he added.
“Promoting walking may be a simple way to help older adults avoid inactivity and encourage an active lifestyle for all-cause and cardiovascular mortality risk reduction,” Dr. Jin said.
Although walking is generally an easy form of exercise for the older adult population, the specific benefit of walking on reducing mortality has not been well studied, according to Dr. Jin and colleagues.
For adults of any age, current guidelines recommend at least 150 minutes per week of moderate activity or 75 minutes per week of vigorous activity, but the amount of physical activity tends to decline with age, and activity recommendations are more difficult to meet, the authors wrote in a press release accompanying their study.
In the study, to be presented at the European Society of Cardiology Congress on Aug. 28 (Abstract 85643), the researchers reviewed data from 7,047 adults aged 85 years and older who participated in the Korean National Health Screening Program. The average age of the study population was 87 years, and 68% were women. Participants completed questionnaires about the amount of time spent in leisure time activities each week, including walking at a slow pace, moderate activity (such as cycling or brisk walking), and vigorous activity (such as running).
Those who walked at a slow pace for at least 1 hour per week had a 40% reduced risk of all-cause mortality and a 39% reduced risk of cardiovascular mortality, compared with inactive participants.
The proportions of participants who reported walking, moderate activity, and vigorous intensity physical activity were 42.5%, 14.7%, and 11.0%, respectively. Roughly one-third (33%) of those who reported slow walking each week also reported moderate or vigorous physical activity.
However, walking for 1 hour per week significantly reduced the risk for all-cause mortality and cardiovascular mortality among individuals who reported walking only, without other moderate or vigorous physical activity (hazard ratio, 0.50 and 0.46, respectively).
“Walking was linked with a lower likelihood of dying in older adults, regardless of whether or not they did any moderate to vigorous intensity physical activity,” Dr. Jin told this news organization. “Our study indicates that walking even just 1 hour every week is advantageous to those aged 85 years and older compared to being inactive.”
The hour of walking need not be in long bouts, 10 minutes each day will do, Dr. Jin added.
The participants were divided into five groups based on reported amount of weekly walking. More than half (57.5%) reported no slow walking, 8.5% walked less than 1 hour per week, 12.0% walked 1-2 hours, 8.7% walked 2-3 hours, and 13.3% walked more than 3 hours.
Although the study was limited by the reliance on self-reports, the results were strengthened by the large sample size and support the value of easy walking for adults aged 85 years and older compared to being inactive.
“Walking may present an opportunity for promoting physical activity among the elderly population, offering a simple way to avoid inactivity and increase physical activity,” said Dr. Jin. However, more research is needed to evaluate the association between mortality and walking by objective measurement of walking levels, using a device such as a smart watch, he noted.
Results are preliminary
“This is an observational study, not an experiment, so it means causality cannot be presumed,” said Maria Fiatarone Singh, MD, a geriatrician with a focus on exercise physiology at the University of Sydney, in an interview. “In other words, it is possible that diseases resulting in mortality prevented people from walking rather than the other way around,” she noted. The only published experimental study on exercise and mortality in older adults was conducted by Dr. Fiatarone Singh and colleagues in Norway. In that study, published in the British Medical Journal in 2020, high-intensity training programs were associated with reduced all-cause mortality compared with inactive controls and individuals who engaged in moderate intensity exercise.
The current study “would have needed to control for many factors related to mortality, such as cardiovascular disease, hypertension, diabetes, malnutrition, and dementia to see what residual benefit might be related to walking,” Dr. Fiatarone Singh said.
“Although walking seems easy and safe, in fact people who are frail, sarcopenic, osteoporotic, or have fallen are recommended to do resistance and balance training rather than walking, and add walking later when they are able to do it safely,” she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Fiatarone Singh had no financial conflicts to disclose.
FROM ESC CONGRESS 2022
‘Medical Methuselahs’: Treating the growing population of centenarians
For about the past year, Priya Goel, MD, can be seen cruising around the island of Manhattan as she makes her way between visits to some of New York City’s most treasured residents: a small but essential group of patients born before the Empire State Building scraped the sky and the old Yankee Stadium had become the House That Ruth Built.
– the oldest is a 108-year-old man – whom she visits monthly.
The gray wave
Dr. Goel’s charges are among America’s latest baby boom – babies born a century ago, that is.
Between 1980 and 2019, the share of American centenarians, those aged 100 and up, grew faster than the total population. In 2019, 100,322 persons in the United States were at least 100 years old – more than triple the 1980 figure of 32,194, according to the U.S. Administration on Aging. By 2060, experts predict, the U.S. centenarian population will reach nearly 600,000.
Although some of the ultra-aged live in nursing homes, many continue to live independently. They require both routine and acute medical care. So, what does it take to be a physician for a centenarian?
Dr. Goel, who is in her mid-30s and could well be the great-granddaughter of some of her patients, urged her colleagues not to stereotype patients on the basis of age.
“You have to consider their functional and cognitive abilities, their ability to understand disease processes and make decisions for themselves,” Dr. Goel said. “Age is just one factor in the grand scheme of things.”
Visiting patients in their homes provides her with insights into how well they’re doing, including the safety of their environments and the depth of their social networks.
New York City has its peculiar demands. Heal provides Dr. Goel with a driver who chauffeurs her to her patient visits. She takes notes between stops.
“The idea is to have these patients remain in an environment where they’re comfortable, in surroundings where they’ve grown up or lived for many years,” she said. “A lot of them are in elevator buildings and they are wheelchair-bound or bed-bound and they physically can’t leave.”
She said she gets a far different view of the patient than does an office-based physician.
“When you go into their home, it’s very personal. You’re seeing what their daily environment is like, what their diet is like. You can see their food on the counter. You can see the level of hygiene,” Dr. Goel said. “You get to see their social support. Are their kids involved? Are they hoarding? Stuff that they wouldn’t just necessarily disclose but on a visit you get to see going into the home. It’s an extra layer of understanding that patient.”
Dr. Goel contrasted home care from care in a nursing home, where the patients are seen daily. On the basis of her observations, she decides whether to see her patients every month or every 3 months.
She applies this strategy to everyone from age 60 to over 100.
Tracking a growing group
Since 1995, geriatrician Thomas Perls, MD, has directed the New England Centenarian Study at Boston University. The study, largely funded by the National Institute on Aging, has enrolled 2,599 centenarian persons and 700 of their offspring. At any given time in the study, about 10% of the centenarians are alive. The study has a high mortality rate.
The people in Dr. Perls’s study range in age, but they top out at 119, the third oldest person ever in the world. Most centenarians are women.
“When we first began the study in 1995, the prevalence of centenarians in the United States was about 1 per 10,000 in the population,” Perls told this news organization. “And now, that prevalence has doubled to 1 per 5,000.”
Even if no one has achieved the record of Methuselah, the Biblical patriarch who was purported to have lived to the age of 969, some people always have lived into their 90s and beyond. Dr. Perls attributed the increase in longevity to control at the turn of the 20th century of typhoid fever, diphtheria, and other infectious diseases with effective public health measures, including the availability of clean water and improvement in socioeconomic conditions.
“Infant mortality just plummeted. So, come around 1915, 1920, we were no longer losing a quarter of our population to these diseases. That meant a quarter more of the population could age into adulthood and middle age,” he said. “A certain component of that group was, therefore, able to continue to age to a very, very old age.”
Other advances, such as antibiotics and vaccinations in the 1960s; the availability in the 1970s of much better detection and effective treatment of high blood pressure; the recognition of the harms of smoking; and much more effective treatment of cardiovascular disease and cancer have allowed many people who would have otherwise died in their 70s and 80s to live much longer. “I think what this means is that there is a substantial proportion of the population that has the biology to get to 100,” Dr. Perls said.
Perls said the Latino population and Blacks have a better track record than Whites in reaching the 100-year milestone. “The average life expectancy might be lower in these populations because of socioeconomic factors, but if they are able to get to around their early 80s, compared to Whites, their ability to get to 100 is actually better,” he said.
Asians fare best when it comes to longevity. While around 1% of White women in the United States live to 100, 10% of Asian women in Hong Kong hit that mark.
“I think some of that is better environment and health habits in Hong Kong than in the United States,” Dr. Perls said. “I think another piece may be a genetic advantage in East Asians. We’re looking into that.”
Dr. Perls said he agreed with Dr. Goel that health care providers and the lay public should not make assumptions on the basis of age alone as to how a person is doing. “People can age so very differently from one another,” he said.
Up to about age 90, the vast majority of those differences are determined by our health behaviors, such as smoking, alcohol use, exercise, sleep, the effect of our diets on weight, and access to good health care, including regular screening for problems such as high blood pressure, diabetes, and cancer. “People who are able to do everything right generally add healthy years to their lives, while those who do not have shorter life expectancies and longer periods of chronic diseases,” Dr. Perls said.
Paying diligent attention to these behaviors over the long run can have a huge payoff.
Dr. Perls’s team has found that to live beyond age 90 and on into the early 100s, protective genes can play a strong role. These genes help slow aging and decrease one’s risk for aging-related diseases. Centenarians usually have a history of aging very slowly and greatly delaying aging-related diseases and disability toward the ends of their lives.
Centenarians are the antithesis of the misguided belief that the older you get, the sicker you get. Quite the opposite occurs. For Dr. Perls, “the older you get, the healthier you’ve been.”
MD bias against the elderly?
Care of elderly patients is becoming essential in the practice of primary care physicians – but not all of them enjoy the work.
To be effective, physicians who treat centenarians must get a better idea of the individual patient’s functional status and comorbidities. “You absolutely cannot make assumptions on age alone,” Dr. Perls said.
The so-called “normal” temperature, 98.6° F, can spell trouble for centenarians and other very old patients, warned Natalie Baker, DNP, CRNP, an associate professor of nursing at the University of Alabama, Birmingham, and president of the 3,000-member Gerontological Advanced Practice Nurses Association.
“We have to be very cognizant of what we call a typical presentation of disease or illness and that a very subtle change in an older adult can signal a serious infection or illness,” Dr. Baker said. “If your patient has a high fever, that is a potential problem.”
The average temperature of an older adult is lower than the accepted 98.6° F, and their body’s response to an infection is slow to exhibit an increase in temperature, Dr. Baker said. “When treating centenarians, clinicians must be cognizant of other subtle signs of infection, such as decreased appetite or change in mentation,” she cautioned.
A decline in appetite or insomnia may be a subtle sign that these patients need to be evaluated, she added.
COVID-19 and centenarians
Three-quarters of the 1 million U.S. deaths from COVID-19 occurred in people aged 65 and older. However, Dr. Perls said centenarians may be a special subpopulation when it comes to COVID.
The Japanese Health Ministry, which follows the large centenarian population in that country, noted a marked jump in the number of centenarians during the pandemic – although the reasons for the increase aren’t clear.
Centenarians may be a bit different. Dr. Perls said some evidence suggests that the over-100 crowd may have better immune systems than younger people. “Part of the trick of getting to 100 is having a pretty good immune system,” he said.
Don’t mess with success
“There is no need at that point for us to try to alter their diet to what we think it might be,” Dr. Baker said. “There’s no need to start with diabetic education. They may tell you their secret is a shot of vodka every day. Why should we stop it at that age? Accept their lifestyles, because they’ve done something right to get to that age.”
Opinions differ on how to approach screening for centenarians.
Dr. Goel said guidelines for routine screening, such as colonoscopies, mammograms, and PAP smears, drop off for patients starting at 75. Dr. Perls said this strategy stems from the belief that people will die from other things first, so screening is no longer needed. Dr. Perls said he disagrees with this approach.
“Again, we can’t base our screening and health care decisions on age alone. If I have an independently functioning and robust 95-year-old man in my office, you can be sure I am going to continue recommending regular screening for colon cancer and other screenings that are normal for people who are 30 years younger,” he said.
Justin Zaghi, MD, chief medical officer at Heal, said screening patients in their late 90s and 100s for cancer generally doesn’t make sense except in some rare circumstances in which the cancer would be unlikely to be a cause of death. “However, if we are talking about screening for fall risks, hearing difficulties, poor vision, pain, and malnutrition, those screenings still absolutely make sense for patients in their late 90s and 100s,” Dr. Zaghi said.
One high-functioning 104-year-old patient of Dr. Perls underwent a total hip replacement for a hip fracture and is faring well. “Obviously, if she had end-stage dementia, we’d do everything to keep the person comfortable, or if they had medical problems that made surgery too high risk, then you don’t do it,” he said. “But if they’re otherwise, I would proceed.”
Avoid the ED
Dr. Goel said doctors should avoid sending patients to the emergency department, an often chaotic place that is especially unfriendly to centenarians and the very old. “Sometimes I’ve seen older patients who are being rushed to the ER, and I ask, What are the goals of care?” she said.
Clinicians caring for seniors should keep in mind that infections can cause seniors to appear confused – and this may lead the clinician to think the patient has dementia. Or, Dr. Goel said, a patient with dementia may suddenly experience much worse dementia.
“In either case, you want to make sure you’re not dealing with any underlying infection, like urinary tract infection, or pneumonia brewing, or skin infections,” she said. “Their skin is so much frailer. You want to make sure there are no bedsores.”
She has had patients whose children report that their usually placid centenarian parents are suddenly acting out. “We’ll do a urinary test and it definitely shows a urinary tract infection. You want to make sure you’re not missing out on something else before you attribute it to dementia,” she said.
Environmental changes, such as moving a patient to a new room in a hospital setting, can trigger an acute mental status change, such as delirium, she added. Helping older patients feel in control as much as possible is important.
“You want to make sure you’re orienting them to the time of day. Make sure they get up at the same time, go to bed at the same time, have clocks and calendars present – just making sure that they feel like they’re still in control of their body and their day,” she said.
Physicians should be aware of potential depression in these patients, whose experience of loss – an unavoidable consequence of outliving family and friends – can result in problems with sleep and diet, as well as a sense of social isolation.
Neal Flomenbaum, MD, professor and emergency physician-in-chief emeritus, New York–Presbyterian/Weill Cornell Medical Center in New York, said sometimes the best thing for these very elderly patients is to “get them in and out of ED as quickly as possible, and do what you can diagnostically.”
He noted that EDs have been making accommodations to serve the elderly, such as using LEDs that replicate outdoor lighting conditions, as well as providing seniors with separate rooms with glass doors to protect them from noise, separate air handlers to prevent infections, and adequate space for visitors.
These patients often are subject to trauma from falls.
“The bones don’t heal as well as in younger people, and treating their comorbidities is essential. Once they have trouble with one area and they’re lying in bed and can’t move much, they can get bedsores,” Dr. Flomenbaum said. “In the hospital, they are vulnerable to infections. So, you’re thinking of all of these things at the same time and how to treat them appropriately and then get them out of the hospital as soon as possible with whatever care that they need in their own homes if at all possible.”
“I always err on the side of less is more,” Dr. Goel said. “Obviously, if there is something – if they have a cough, they need an x-ray. That’s very basic. We want to take care of that. Give them the antibiotic if they need that. But rushing them in and out of the hospital doesn’t add to their quality of life.”
Dr. Flomenbaum, a pioneer in geriatric emergency medicine, says physicians need to be aware that centenarians and other very old patients don’t present the same way as younger adults.
He began to notice more than 20 years ago that every night, patients would turn up in his ED who were in their late 90s into their 100s. Some would come in with what their children identified as sudden-onset dementia – they didn’t know their own names and couldn’t identify their kids. They didn’t know the time or day. Dr. Flomenbaum said the children often asked whether their parents should enter a nursing home.
“And I’d say, ‘Not so fast. Well, let’s take a look at this.’ You don’t develop that kind of dementia overnight. It usually takes a while,” he said.
He said he ordered complete blood cell counts and oxygen saturation tests that frequently turned out to be abnormal. They didn’t have a fever, and infiltrates initially weren’t seen on chest x-rays.
With rehydration and supplemental oxygen, their symptoms started to improve, and it became obvious that the symptoms were not of dementia but of pneumonia, and that they required antibiotics, Dr. Flomenbaum said.
Dementia dilemma
Too often, on the basis of age, doctors assume patients have dementia or other cognitive impairments.
“What a shock and a surprise when doctors actually talk to folks and do a neurocognitive screen and find they’re just fine,” Dr. Perls said.
The decline in hearing and vision can lead to a misdiagnosis of cognitive impairment because the patients are not able to hear what you’re asking them. “It’s really important that the person can hear you – whether you use an amplifying device or they have hearing aids, that’s critical,” he said. “You just have to be a good doctor.”
Often the physical toll of aging exacerbates social difficulties. Poor hearing, for example, can accelerate cognitive impairment and cause people to interact less often, and less meaningfully, with their environment. For some, wearing hearing aids seems demeaning – until they hear what they’ve been missing.
“I get them to wear their hearing aids and, lo and behold, they’re a whole new person because they’re now able to take in their environment and interact with others,” Dr. Perls said.
Dr. Flomenbaum said alcohol abuse and drug reactions can cause delirium, which, unlike dementia, is potentially reversible. Yet many physicians cannot reliably differentiate between dementia and delirium, he added.
The geriatric specialists talk about the lessons they’ve learned and the gratification they get from caring for centenarians.
“I have come to realize the importance of family, of having a close circle, whether that’s through friends or neighbors,” Dr. Goel said. “This work is very rewarding because, if it wasn’t for homebound organizations, how would these people get care or get access to care?”
For Dr. Baker, a joy of the job is hearing centenarians share their life stories.
“I love to hear their stories about how they’ve overcome adversity, living through the depression and living through different wars,” she said. “I love talking to veterans, and I think that oftentimes, we do not value our older adults in our society as we should. Sometimes they are dismissed because they move slowly or are hard to communicate with due to hearing deficits. But they are, I think, a very important part of our lives.”
‘They’ve already won’
Most centenarians readily offer the secrets to their longevity. Aline Jacobsohn, of Boca Raton, Fla., is no different.
Ms. Jacobsohn, who will be 101 in October, thinks a diet of small portions of fish, vegetables, and fruit, which she has followed since her husband Leo died in 1982, has helped keep her healthy. She eats lots of salmon and herring and is a fan of spinach sautéed with olive oil. “The only thing I don’t eat is meat,” the trim and active Ms. Jacobsohn said in a recent interview over Zoom.
Her other secret: “Doctors. I like to stay away from them as much as possible.”
Shari Rosenbaum, MD, Jacobsohn’s internist, doesn’t dismiss that approach. She uses a version of it when managing her three centenarian patients, the oldest of whom is 103.
“Let them smoke! Let them drink! They’re happy. It’s not causing harm. Let them eat cake! They’ve already won,” said Dr. Rosenbaum, who is affiliated with Boca Raton–based MDVIP, a national membership-based network of 1,100 primary care physicians serving 368,000 patients. Of those, nearly 460 are centenarians.
“You’re not preventing those problems in this population,” she said. “They’re here to enjoy every moment that they have, and they might as well.”
Dr. Rosenbaum sees a divergence in her patients – those who will reach very old age, and those who won’t – starting in their 60s.
“The centenarians don’t have medical problems,” she said. “They don’t get cancer. They don’t get diabetes. Some of them take good care of themselves. Some don’t take such good care of themselves. But they are all optimists. They all see the glass half full. They all participate in life. They all have excellent support systems. They have good genes, a positive attitude toward life, and a strong social network.”
Ms. Jacobsohn – whose surname at the time was Bakst – grew up in Frankfurt am Main, Germany, during the rise of the Nazi regime. The family fled to Columbia in 1938, where she met and eventually married her husband, Leo, who ran a business importing clocks and watches in Cali.
In 1989, the Jacobsohns and their three children moved to south Florida to escape the dangers of kidnappings and ransoms posed by the drug cartels.
Ms. Jacobsohn agreed that she appears to have longevity genes – “good stock,” she calls it. “My mother died 23 days before she was 100. My grandmother lived till 99, almost 100,” she said.
Two years ago, she donated her car to a charity and stopped driving in the interest of her own safety and that of other drivers and pedestrians.
Ms. Jacobsohn has a strong support system. Two of her children live nearby and visit her nearly every day. A live-in companion helps her with the activities of daily life, including preparing meals.
Ms. Jacobsohn plays bridge regularly, and well. “I’m sorry to say that I’m a very good bridge player,” she said, frankly. “How is it possible that I’ve played bridge so well and then I don’t remember what I had for lunch yesterday?”
She reads, mainly a diet of history but occasionally novels, too. “They have to be engaging,” she said.
The loss of loved ones is an inevitable part of very old age. Her husband of 47 years died of emphysema, and one of her sons died in his 70s of prostate cancer.
She knows well the fate that awaits us all and accepts it philosophically.
“It’s a very normal thing that people die. You don’t live forever. So, whenever it comes, it’s okay. Enough is enough. Dayenu,” she said, using the Hebrew word for, “It would have been enough” – a favorite in the Passover Seder celebrating the ancient Jews’ liberation from slavery in Egypt.
Ms. Jacobsohn sang the song and then took a reporter on a Zoom tour of her tidy home and her large flower garden featuring Cattleya orchids from Colombia.
A version of this article first appeared on Medscape.com.
For about the past year, Priya Goel, MD, can be seen cruising around the island of Manhattan as she makes her way between visits to some of New York City’s most treasured residents: a small but essential group of patients born before the Empire State Building scraped the sky and the old Yankee Stadium had become the House That Ruth Built.
– the oldest is a 108-year-old man – whom she visits monthly.
The gray wave
Dr. Goel’s charges are among America’s latest baby boom – babies born a century ago, that is.
Between 1980 and 2019, the share of American centenarians, those aged 100 and up, grew faster than the total population. In 2019, 100,322 persons in the United States were at least 100 years old – more than triple the 1980 figure of 32,194, according to the U.S. Administration on Aging. By 2060, experts predict, the U.S. centenarian population will reach nearly 600,000.
Although some of the ultra-aged live in nursing homes, many continue to live independently. They require both routine and acute medical care. So, what does it take to be a physician for a centenarian?
Dr. Goel, who is in her mid-30s and could well be the great-granddaughter of some of her patients, urged her colleagues not to stereotype patients on the basis of age.
“You have to consider their functional and cognitive abilities, their ability to understand disease processes and make decisions for themselves,” Dr. Goel said. “Age is just one factor in the grand scheme of things.”
Visiting patients in their homes provides her with insights into how well they’re doing, including the safety of their environments and the depth of their social networks.
New York City has its peculiar demands. Heal provides Dr. Goel with a driver who chauffeurs her to her patient visits. She takes notes between stops.
“The idea is to have these patients remain in an environment where they’re comfortable, in surroundings where they’ve grown up or lived for many years,” she said. “A lot of them are in elevator buildings and they are wheelchair-bound or bed-bound and they physically can’t leave.”
She said she gets a far different view of the patient than does an office-based physician.
“When you go into their home, it’s very personal. You’re seeing what their daily environment is like, what their diet is like. You can see their food on the counter. You can see the level of hygiene,” Dr. Goel said. “You get to see their social support. Are their kids involved? Are they hoarding? Stuff that they wouldn’t just necessarily disclose but on a visit you get to see going into the home. It’s an extra layer of understanding that patient.”
Dr. Goel contrasted home care from care in a nursing home, where the patients are seen daily. On the basis of her observations, she decides whether to see her patients every month or every 3 months.
She applies this strategy to everyone from age 60 to over 100.
Tracking a growing group
Since 1995, geriatrician Thomas Perls, MD, has directed the New England Centenarian Study at Boston University. The study, largely funded by the National Institute on Aging, has enrolled 2,599 centenarian persons and 700 of their offspring. At any given time in the study, about 10% of the centenarians are alive. The study has a high mortality rate.
The people in Dr. Perls’s study range in age, but they top out at 119, the third oldest person ever in the world. Most centenarians are women.
“When we first began the study in 1995, the prevalence of centenarians in the United States was about 1 per 10,000 in the population,” Perls told this news organization. “And now, that prevalence has doubled to 1 per 5,000.”
Even if no one has achieved the record of Methuselah, the Biblical patriarch who was purported to have lived to the age of 969, some people always have lived into their 90s and beyond. Dr. Perls attributed the increase in longevity to control at the turn of the 20th century of typhoid fever, diphtheria, and other infectious diseases with effective public health measures, including the availability of clean water and improvement in socioeconomic conditions.
“Infant mortality just plummeted. So, come around 1915, 1920, we were no longer losing a quarter of our population to these diseases. That meant a quarter more of the population could age into adulthood and middle age,” he said. “A certain component of that group was, therefore, able to continue to age to a very, very old age.”
Other advances, such as antibiotics and vaccinations in the 1960s; the availability in the 1970s of much better detection and effective treatment of high blood pressure; the recognition of the harms of smoking; and much more effective treatment of cardiovascular disease and cancer have allowed many people who would have otherwise died in their 70s and 80s to live much longer. “I think what this means is that there is a substantial proportion of the population that has the biology to get to 100,” Dr. Perls said.
Perls said the Latino population and Blacks have a better track record than Whites in reaching the 100-year milestone. “The average life expectancy might be lower in these populations because of socioeconomic factors, but if they are able to get to around their early 80s, compared to Whites, their ability to get to 100 is actually better,” he said.
Asians fare best when it comes to longevity. While around 1% of White women in the United States live to 100, 10% of Asian women in Hong Kong hit that mark.
“I think some of that is better environment and health habits in Hong Kong than in the United States,” Dr. Perls said. “I think another piece may be a genetic advantage in East Asians. We’re looking into that.”
Dr. Perls said he agreed with Dr. Goel that health care providers and the lay public should not make assumptions on the basis of age alone as to how a person is doing. “People can age so very differently from one another,” he said.
Up to about age 90, the vast majority of those differences are determined by our health behaviors, such as smoking, alcohol use, exercise, sleep, the effect of our diets on weight, and access to good health care, including regular screening for problems such as high blood pressure, diabetes, and cancer. “People who are able to do everything right generally add healthy years to their lives, while those who do not have shorter life expectancies and longer periods of chronic diseases,” Dr. Perls said.
Paying diligent attention to these behaviors over the long run can have a huge payoff.
Dr. Perls’s team has found that to live beyond age 90 and on into the early 100s, protective genes can play a strong role. These genes help slow aging and decrease one’s risk for aging-related diseases. Centenarians usually have a history of aging very slowly and greatly delaying aging-related diseases and disability toward the ends of their lives.
Centenarians are the antithesis of the misguided belief that the older you get, the sicker you get. Quite the opposite occurs. For Dr. Perls, “the older you get, the healthier you’ve been.”
MD bias against the elderly?
Care of elderly patients is becoming essential in the practice of primary care physicians – but not all of them enjoy the work.
To be effective, physicians who treat centenarians must get a better idea of the individual patient’s functional status and comorbidities. “You absolutely cannot make assumptions on age alone,” Dr. Perls said.
The so-called “normal” temperature, 98.6° F, can spell trouble for centenarians and other very old patients, warned Natalie Baker, DNP, CRNP, an associate professor of nursing at the University of Alabama, Birmingham, and president of the 3,000-member Gerontological Advanced Practice Nurses Association.
“We have to be very cognizant of what we call a typical presentation of disease or illness and that a very subtle change in an older adult can signal a serious infection or illness,” Dr. Baker said. “If your patient has a high fever, that is a potential problem.”
The average temperature of an older adult is lower than the accepted 98.6° F, and their body’s response to an infection is slow to exhibit an increase in temperature, Dr. Baker said. “When treating centenarians, clinicians must be cognizant of other subtle signs of infection, such as decreased appetite or change in mentation,” she cautioned.
A decline in appetite or insomnia may be a subtle sign that these patients need to be evaluated, she added.
COVID-19 and centenarians
Three-quarters of the 1 million U.S. deaths from COVID-19 occurred in people aged 65 and older. However, Dr. Perls said centenarians may be a special subpopulation when it comes to COVID.
The Japanese Health Ministry, which follows the large centenarian population in that country, noted a marked jump in the number of centenarians during the pandemic – although the reasons for the increase aren’t clear.
Centenarians may be a bit different. Dr. Perls said some evidence suggests that the over-100 crowd may have better immune systems than younger people. “Part of the trick of getting to 100 is having a pretty good immune system,” he said.
Don’t mess with success
“There is no need at that point for us to try to alter their diet to what we think it might be,” Dr. Baker said. “There’s no need to start with diabetic education. They may tell you their secret is a shot of vodka every day. Why should we stop it at that age? Accept their lifestyles, because they’ve done something right to get to that age.”
Opinions differ on how to approach screening for centenarians.
Dr. Goel said guidelines for routine screening, such as colonoscopies, mammograms, and PAP smears, drop off for patients starting at 75. Dr. Perls said this strategy stems from the belief that people will die from other things first, so screening is no longer needed. Dr. Perls said he disagrees with this approach.
“Again, we can’t base our screening and health care decisions on age alone. If I have an independently functioning and robust 95-year-old man in my office, you can be sure I am going to continue recommending regular screening for colon cancer and other screenings that are normal for people who are 30 years younger,” he said.
Justin Zaghi, MD, chief medical officer at Heal, said screening patients in their late 90s and 100s for cancer generally doesn’t make sense except in some rare circumstances in which the cancer would be unlikely to be a cause of death. “However, if we are talking about screening for fall risks, hearing difficulties, poor vision, pain, and malnutrition, those screenings still absolutely make sense for patients in their late 90s and 100s,” Dr. Zaghi said.
One high-functioning 104-year-old patient of Dr. Perls underwent a total hip replacement for a hip fracture and is faring well. “Obviously, if she had end-stage dementia, we’d do everything to keep the person comfortable, or if they had medical problems that made surgery too high risk, then you don’t do it,” he said. “But if they’re otherwise, I would proceed.”
Avoid the ED
Dr. Goel said doctors should avoid sending patients to the emergency department, an often chaotic place that is especially unfriendly to centenarians and the very old. “Sometimes I’ve seen older patients who are being rushed to the ER, and I ask, What are the goals of care?” she said.
Clinicians caring for seniors should keep in mind that infections can cause seniors to appear confused – and this may lead the clinician to think the patient has dementia. Or, Dr. Goel said, a patient with dementia may suddenly experience much worse dementia.
“In either case, you want to make sure you’re not dealing with any underlying infection, like urinary tract infection, or pneumonia brewing, or skin infections,” she said. “Their skin is so much frailer. You want to make sure there are no bedsores.”
She has had patients whose children report that their usually placid centenarian parents are suddenly acting out. “We’ll do a urinary test and it definitely shows a urinary tract infection. You want to make sure you’re not missing out on something else before you attribute it to dementia,” she said.
Environmental changes, such as moving a patient to a new room in a hospital setting, can trigger an acute mental status change, such as delirium, she added. Helping older patients feel in control as much as possible is important.
“You want to make sure you’re orienting them to the time of day. Make sure they get up at the same time, go to bed at the same time, have clocks and calendars present – just making sure that they feel like they’re still in control of their body and their day,” she said.
Physicians should be aware of potential depression in these patients, whose experience of loss – an unavoidable consequence of outliving family and friends – can result in problems with sleep and diet, as well as a sense of social isolation.
Neal Flomenbaum, MD, professor and emergency physician-in-chief emeritus, New York–Presbyterian/Weill Cornell Medical Center in New York, said sometimes the best thing for these very elderly patients is to “get them in and out of ED as quickly as possible, and do what you can diagnostically.”
He noted that EDs have been making accommodations to serve the elderly, such as using LEDs that replicate outdoor lighting conditions, as well as providing seniors with separate rooms with glass doors to protect them from noise, separate air handlers to prevent infections, and adequate space for visitors.
These patients often are subject to trauma from falls.
“The bones don’t heal as well as in younger people, and treating their comorbidities is essential. Once they have trouble with one area and they’re lying in bed and can’t move much, they can get bedsores,” Dr. Flomenbaum said. “In the hospital, they are vulnerable to infections. So, you’re thinking of all of these things at the same time and how to treat them appropriately and then get them out of the hospital as soon as possible with whatever care that they need in their own homes if at all possible.”
“I always err on the side of less is more,” Dr. Goel said. “Obviously, if there is something – if they have a cough, they need an x-ray. That’s very basic. We want to take care of that. Give them the antibiotic if they need that. But rushing them in and out of the hospital doesn’t add to their quality of life.”
Dr. Flomenbaum, a pioneer in geriatric emergency medicine, says physicians need to be aware that centenarians and other very old patients don’t present the same way as younger adults.
He began to notice more than 20 years ago that every night, patients would turn up in his ED who were in their late 90s into their 100s. Some would come in with what their children identified as sudden-onset dementia – they didn’t know their own names and couldn’t identify their kids. They didn’t know the time or day. Dr. Flomenbaum said the children often asked whether their parents should enter a nursing home.
“And I’d say, ‘Not so fast. Well, let’s take a look at this.’ You don’t develop that kind of dementia overnight. It usually takes a while,” he said.
He said he ordered complete blood cell counts and oxygen saturation tests that frequently turned out to be abnormal. They didn’t have a fever, and infiltrates initially weren’t seen on chest x-rays.
With rehydration and supplemental oxygen, their symptoms started to improve, and it became obvious that the symptoms were not of dementia but of pneumonia, and that they required antibiotics, Dr. Flomenbaum said.
Dementia dilemma
Too often, on the basis of age, doctors assume patients have dementia or other cognitive impairments.
“What a shock and a surprise when doctors actually talk to folks and do a neurocognitive screen and find they’re just fine,” Dr. Perls said.
The decline in hearing and vision can lead to a misdiagnosis of cognitive impairment because the patients are not able to hear what you’re asking them. “It’s really important that the person can hear you – whether you use an amplifying device or they have hearing aids, that’s critical,” he said. “You just have to be a good doctor.”
Often the physical toll of aging exacerbates social difficulties. Poor hearing, for example, can accelerate cognitive impairment and cause people to interact less often, and less meaningfully, with their environment. For some, wearing hearing aids seems demeaning – until they hear what they’ve been missing.
“I get them to wear their hearing aids and, lo and behold, they’re a whole new person because they’re now able to take in their environment and interact with others,” Dr. Perls said.
Dr. Flomenbaum said alcohol abuse and drug reactions can cause delirium, which, unlike dementia, is potentially reversible. Yet many physicians cannot reliably differentiate between dementia and delirium, he added.
The geriatric specialists talk about the lessons they’ve learned and the gratification they get from caring for centenarians.
“I have come to realize the importance of family, of having a close circle, whether that’s through friends or neighbors,” Dr. Goel said. “This work is very rewarding because, if it wasn’t for homebound organizations, how would these people get care or get access to care?”
For Dr. Baker, a joy of the job is hearing centenarians share their life stories.
“I love to hear their stories about how they’ve overcome adversity, living through the depression and living through different wars,” she said. “I love talking to veterans, and I think that oftentimes, we do not value our older adults in our society as we should. Sometimes they are dismissed because they move slowly or are hard to communicate with due to hearing deficits. But they are, I think, a very important part of our lives.”
‘They’ve already won’
Most centenarians readily offer the secrets to their longevity. Aline Jacobsohn, of Boca Raton, Fla., is no different.
Ms. Jacobsohn, who will be 101 in October, thinks a diet of small portions of fish, vegetables, and fruit, which she has followed since her husband Leo died in 1982, has helped keep her healthy. She eats lots of salmon and herring and is a fan of spinach sautéed with olive oil. “The only thing I don’t eat is meat,” the trim and active Ms. Jacobsohn said in a recent interview over Zoom.
Her other secret: “Doctors. I like to stay away from them as much as possible.”
Shari Rosenbaum, MD, Jacobsohn’s internist, doesn’t dismiss that approach. She uses a version of it when managing her three centenarian patients, the oldest of whom is 103.
“Let them smoke! Let them drink! They’re happy. It’s not causing harm. Let them eat cake! They’ve already won,” said Dr. Rosenbaum, who is affiliated with Boca Raton–based MDVIP, a national membership-based network of 1,100 primary care physicians serving 368,000 patients. Of those, nearly 460 are centenarians.
“You’re not preventing those problems in this population,” she said. “They’re here to enjoy every moment that they have, and they might as well.”
Dr. Rosenbaum sees a divergence in her patients – those who will reach very old age, and those who won’t – starting in their 60s.
“The centenarians don’t have medical problems,” she said. “They don’t get cancer. They don’t get diabetes. Some of them take good care of themselves. Some don’t take such good care of themselves. But they are all optimists. They all see the glass half full. They all participate in life. They all have excellent support systems. They have good genes, a positive attitude toward life, and a strong social network.”
Ms. Jacobsohn – whose surname at the time was Bakst – grew up in Frankfurt am Main, Germany, during the rise of the Nazi regime. The family fled to Columbia in 1938, where she met and eventually married her husband, Leo, who ran a business importing clocks and watches in Cali.
In 1989, the Jacobsohns and their three children moved to south Florida to escape the dangers of kidnappings and ransoms posed by the drug cartels.
Ms. Jacobsohn agreed that she appears to have longevity genes – “good stock,” she calls it. “My mother died 23 days before she was 100. My grandmother lived till 99, almost 100,” she said.
Two years ago, she donated her car to a charity and stopped driving in the interest of her own safety and that of other drivers and pedestrians.
Ms. Jacobsohn has a strong support system. Two of her children live nearby and visit her nearly every day. A live-in companion helps her with the activities of daily life, including preparing meals.
Ms. Jacobsohn plays bridge regularly, and well. “I’m sorry to say that I’m a very good bridge player,” she said, frankly. “How is it possible that I’ve played bridge so well and then I don’t remember what I had for lunch yesterday?”
She reads, mainly a diet of history but occasionally novels, too. “They have to be engaging,” she said.
The loss of loved ones is an inevitable part of very old age. Her husband of 47 years died of emphysema, and one of her sons died in his 70s of prostate cancer.
She knows well the fate that awaits us all and accepts it philosophically.
“It’s a very normal thing that people die. You don’t live forever. So, whenever it comes, it’s okay. Enough is enough. Dayenu,” she said, using the Hebrew word for, “It would have been enough” – a favorite in the Passover Seder celebrating the ancient Jews’ liberation from slavery in Egypt.
Ms. Jacobsohn sang the song and then took a reporter on a Zoom tour of her tidy home and her large flower garden featuring Cattleya orchids from Colombia.
A version of this article first appeared on Medscape.com.
For about the past year, Priya Goel, MD, can be seen cruising around the island of Manhattan as she makes her way between visits to some of New York City’s most treasured residents: a small but essential group of patients born before the Empire State Building scraped the sky and the old Yankee Stadium had become the House That Ruth Built.
– the oldest is a 108-year-old man – whom she visits monthly.
The gray wave
Dr. Goel’s charges are among America’s latest baby boom – babies born a century ago, that is.
Between 1980 and 2019, the share of American centenarians, those aged 100 and up, grew faster than the total population. In 2019, 100,322 persons in the United States were at least 100 years old – more than triple the 1980 figure of 32,194, according to the U.S. Administration on Aging. By 2060, experts predict, the U.S. centenarian population will reach nearly 600,000.
Although some of the ultra-aged live in nursing homes, many continue to live independently. They require both routine and acute medical care. So, what does it take to be a physician for a centenarian?
Dr. Goel, who is in her mid-30s and could well be the great-granddaughter of some of her patients, urged her colleagues not to stereotype patients on the basis of age.
“You have to consider their functional and cognitive abilities, their ability to understand disease processes and make decisions for themselves,” Dr. Goel said. “Age is just one factor in the grand scheme of things.”
Visiting patients in their homes provides her with insights into how well they’re doing, including the safety of their environments and the depth of their social networks.
New York City has its peculiar demands. Heal provides Dr. Goel with a driver who chauffeurs her to her patient visits. She takes notes between stops.
“The idea is to have these patients remain in an environment where they’re comfortable, in surroundings where they’ve grown up or lived for many years,” she said. “A lot of them are in elevator buildings and they are wheelchair-bound or bed-bound and they physically can’t leave.”
She said she gets a far different view of the patient than does an office-based physician.
“When you go into their home, it’s very personal. You’re seeing what their daily environment is like, what their diet is like. You can see their food on the counter. You can see the level of hygiene,” Dr. Goel said. “You get to see their social support. Are their kids involved? Are they hoarding? Stuff that they wouldn’t just necessarily disclose but on a visit you get to see going into the home. It’s an extra layer of understanding that patient.”
Dr. Goel contrasted home care from care in a nursing home, where the patients are seen daily. On the basis of her observations, she decides whether to see her patients every month or every 3 months.
She applies this strategy to everyone from age 60 to over 100.
Tracking a growing group
Since 1995, geriatrician Thomas Perls, MD, has directed the New England Centenarian Study at Boston University. The study, largely funded by the National Institute on Aging, has enrolled 2,599 centenarian persons and 700 of their offspring. At any given time in the study, about 10% of the centenarians are alive. The study has a high mortality rate.
The people in Dr. Perls’s study range in age, but they top out at 119, the third oldest person ever in the world. Most centenarians are women.
“When we first began the study in 1995, the prevalence of centenarians in the United States was about 1 per 10,000 in the population,” Perls told this news organization. “And now, that prevalence has doubled to 1 per 5,000.”
Even if no one has achieved the record of Methuselah, the Biblical patriarch who was purported to have lived to the age of 969, some people always have lived into their 90s and beyond. Dr. Perls attributed the increase in longevity to control at the turn of the 20th century of typhoid fever, diphtheria, and other infectious diseases with effective public health measures, including the availability of clean water and improvement in socioeconomic conditions.
“Infant mortality just plummeted. So, come around 1915, 1920, we were no longer losing a quarter of our population to these diseases. That meant a quarter more of the population could age into adulthood and middle age,” he said. “A certain component of that group was, therefore, able to continue to age to a very, very old age.”
Other advances, such as antibiotics and vaccinations in the 1960s; the availability in the 1970s of much better detection and effective treatment of high blood pressure; the recognition of the harms of smoking; and much more effective treatment of cardiovascular disease and cancer have allowed many people who would have otherwise died in their 70s and 80s to live much longer. “I think what this means is that there is a substantial proportion of the population that has the biology to get to 100,” Dr. Perls said.
Perls said the Latino population and Blacks have a better track record than Whites in reaching the 100-year milestone. “The average life expectancy might be lower in these populations because of socioeconomic factors, but if they are able to get to around their early 80s, compared to Whites, their ability to get to 100 is actually better,” he said.
Asians fare best when it comes to longevity. While around 1% of White women in the United States live to 100, 10% of Asian women in Hong Kong hit that mark.
“I think some of that is better environment and health habits in Hong Kong than in the United States,” Dr. Perls said. “I think another piece may be a genetic advantage in East Asians. We’re looking into that.”
Dr. Perls said he agreed with Dr. Goel that health care providers and the lay public should not make assumptions on the basis of age alone as to how a person is doing. “People can age so very differently from one another,” he said.
Up to about age 90, the vast majority of those differences are determined by our health behaviors, such as smoking, alcohol use, exercise, sleep, the effect of our diets on weight, and access to good health care, including regular screening for problems such as high blood pressure, diabetes, and cancer. “People who are able to do everything right generally add healthy years to their lives, while those who do not have shorter life expectancies and longer periods of chronic diseases,” Dr. Perls said.
Paying diligent attention to these behaviors over the long run can have a huge payoff.
Dr. Perls’s team has found that to live beyond age 90 and on into the early 100s, protective genes can play a strong role. These genes help slow aging and decrease one’s risk for aging-related diseases. Centenarians usually have a history of aging very slowly and greatly delaying aging-related diseases and disability toward the ends of their lives.
Centenarians are the antithesis of the misguided belief that the older you get, the sicker you get. Quite the opposite occurs. For Dr. Perls, “the older you get, the healthier you’ve been.”
MD bias against the elderly?
Care of elderly patients is becoming essential in the practice of primary care physicians – but not all of them enjoy the work.
To be effective, physicians who treat centenarians must get a better idea of the individual patient’s functional status and comorbidities. “You absolutely cannot make assumptions on age alone,” Dr. Perls said.
The so-called “normal” temperature, 98.6° F, can spell trouble for centenarians and other very old patients, warned Natalie Baker, DNP, CRNP, an associate professor of nursing at the University of Alabama, Birmingham, and president of the 3,000-member Gerontological Advanced Practice Nurses Association.
“We have to be very cognizant of what we call a typical presentation of disease or illness and that a very subtle change in an older adult can signal a serious infection or illness,” Dr. Baker said. “If your patient has a high fever, that is a potential problem.”
The average temperature of an older adult is lower than the accepted 98.6° F, and their body’s response to an infection is slow to exhibit an increase in temperature, Dr. Baker said. “When treating centenarians, clinicians must be cognizant of other subtle signs of infection, such as decreased appetite or change in mentation,” she cautioned.
A decline in appetite or insomnia may be a subtle sign that these patients need to be evaluated, she added.
COVID-19 and centenarians
Three-quarters of the 1 million U.S. deaths from COVID-19 occurred in people aged 65 and older. However, Dr. Perls said centenarians may be a special subpopulation when it comes to COVID.
The Japanese Health Ministry, which follows the large centenarian population in that country, noted a marked jump in the number of centenarians during the pandemic – although the reasons for the increase aren’t clear.
Centenarians may be a bit different. Dr. Perls said some evidence suggests that the over-100 crowd may have better immune systems than younger people. “Part of the trick of getting to 100 is having a pretty good immune system,” he said.
Don’t mess with success
“There is no need at that point for us to try to alter their diet to what we think it might be,” Dr. Baker said. “There’s no need to start with diabetic education. They may tell you their secret is a shot of vodka every day. Why should we stop it at that age? Accept their lifestyles, because they’ve done something right to get to that age.”
Opinions differ on how to approach screening for centenarians.
Dr. Goel said guidelines for routine screening, such as colonoscopies, mammograms, and PAP smears, drop off for patients starting at 75. Dr. Perls said this strategy stems from the belief that people will die from other things first, so screening is no longer needed. Dr. Perls said he disagrees with this approach.
“Again, we can’t base our screening and health care decisions on age alone. If I have an independently functioning and robust 95-year-old man in my office, you can be sure I am going to continue recommending regular screening for colon cancer and other screenings that are normal for people who are 30 years younger,” he said.
Justin Zaghi, MD, chief medical officer at Heal, said screening patients in their late 90s and 100s for cancer generally doesn’t make sense except in some rare circumstances in which the cancer would be unlikely to be a cause of death. “However, if we are talking about screening for fall risks, hearing difficulties, poor vision, pain, and malnutrition, those screenings still absolutely make sense for patients in their late 90s and 100s,” Dr. Zaghi said.
One high-functioning 104-year-old patient of Dr. Perls underwent a total hip replacement for a hip fracture and is faring well. “Obviously, if she had end-stage dementia, we’d do everything to keep the person comfortable, or if they had medical problems that made surgery too high risk, then you don’t do it,” he said. “But if they’re otherwise, I would proceed.”
Avoid the ED
Dr. Goel said doctors should avoid sending patients to the emergency department, an often chaotic place that is especially unfriendly to centenarians and the very old. “Sometimes I’ve seen older patients who are being rushed to the ER, and I ask, What are the goals of care?” she said.
Clinicians caring for seniors should keep in mind that infections can cause seniors to appear confused – and this may lead the clinician to think the patient has dementia. Or, Dr. Goel said, a patient with dementia may suddenly experience much worse dementia.
“In either case, you want to make sure you’re not dealing with any underlying infection, like urinary tract infection, or pneumonia brewing, or skin infections,” she said. “Their skin is so much frailer. You want to make sure there are no bedsores.”
She has had patients whose children report that their usually placid centenarian parents are suddenly acting out. “We’ll do a urinary test and it definitely shows a urinary tract infection. You want to make sure you’re not missing out on something else before you attribute it to dementia,” she said.
Environmental changes, such as moving a patient to a new room in a hospital setting, can trigger an acute mental status change, such as delirium, she added. Helping older patients feel in control as much as possible is important.
“You want to make sure you’re orienting them to the time of day. Make sure they get up at the same time, go to bed at the same time, have clocks and calendars present – just making sure that they feel like they’re still in control of their body and their day,” she said.
Physicians should be aware of potential depression in these patients, whose experience of loss – an unavoidable consequence of outliving family and friends – can result in problems with sleep and diet, as well as a sense of social isolation.
Neal Flomenbaum, MD, professor and emergency physician-in-chief emeritus, New York–Presbyterian/Weill Cornell Medical Center in New York, said sometimes the best thing for these very elderly patients is to “get them in and out of ED as quickly as possible, and do what you can diagnostically.”
He noted that EDs have been making accommodations to serve the elderly, such as using LEDs that replicate outdoor lighting conditions, as well as providing seniors with separate rooms with glass doors to protect them from noise, separate air handlers to prevent infections, and adequate space for visitors.
These patients often are subject to trauma from falls.
“The bones don’t heal as well as in younger people, and treating their comorbidities is essential. Once they have trouble with one area and they’re lying in bed and can’t move much, they can get bedsores,” Dr. Flomenbaum said. “In the hospital, they are vulnerable to infections. So, you’re thinking of all of these things at the same time and how to treat them appropriately and then get them out of the hospital as soon as possible with whatever care that they need in their own homes if at all possible.”
“I always err on the side of less is more,” Dr. Goel said. “Obviously, if there is something – if they have a cough, they need an x-ray. That’s very basic. We want to take care of that. Give them the antibiotic if they need that. But rushing them in and out of the hospital doesn’t add to their quality of life.”
Dr. Flomenbaum, a pioneer in geriatric emergency medicine, says physicians need to be aware that centenarians and other very old patients don’t present the same way as younger adults.
He began to notice more than 20 years ago that every night, patients would turn up in his ED who were in their late 90s into their 100s. Some would come in with what their children identified as sudden-onset dementia – they didn’t know their own names and couldn’t identify their kids. They didn’t know the time or day. Dr. Flomenbaum said the children often asked whether their parents should enter a nursing home.
“And I’d say, ‘Not so fast. Well, let’s take a look at this.’ You don’t develop that kind of dementia overnight. It usually takes a while,” he said.
He said he ordered complete blood cell counts and oxygen saturation tests that frequently turned out to be abnormal. They didn’t have a fever, and infiltrates initially weren’t seen on chest x-rays.
With rehydration and supplemental oxygen, their symptoms started to improve, and it became obvious that the symptoms were not of dementia but of pneumonia, and that they required antibiotics, Dr. Flomenbaum said.
Dementia dilemma
Too often, on the basis of age, doctors assume patients have dementia or other cognitive impairments.
“What a shock and a surprise when doctors actually talk to folks and do a neurocognitive screen and find they’re just fine,” Dr. Perls said.
The decline in hearing and vision can lead to a misdiagnosis of cognitive impairment because the patients are not able to hear what you’re asking them. “It’s really important that the person can hear you – whether you use an amplifying device or they have hearing aids, that’s critical,” he said. “You just have to be a good doctor.”
Often the physical toll of aging exacerbates social difficulties. Poor hearing, for example, can accelerate cognitive impairment and cause people to interact less often, and less meaningfully, with their environment. For some, wearing hearing aids seems demeaning – until they hear what they’ve been missing.
“I get them to wear their hearing aids and, lo and behold, they’re a whole new person because they’re now able to take in their environment and interact with others,” Dr. Perls said.
Dr. Flomenbaum said alcohol abuse and drug reactions can cause delirium, which, unlike dementia, is potentially reversible. Yet many physicians cannot reliably differentiate between dementia and delirium, he added.
The geriatric specialists talk about the lessons they’ve learned and the gratification they get from caring for centenarians.
“I have come to realize the importance of family, of having a close circle, whether that’s through friends or neighbors,” Dr. Goel said. “This work is very rewarding because, if it wasn’t for homebound organizations, how would these people get care or get access to care?”
For Dr. Baker, a joy of the job is hearing centenarians share their life stories.
“I love to hear their stories about how they’ve overcome adversity, living through the depression and living through different wars,” she said. “I love talking to veterans, and I think that oftentimes, we do not value our older adults in our society as we should. Sometimes they are dismissed because they move slowly or are hard to communicate with due to hearing deficits. But they are, I think, a very important part of our lives.”
‘They’ve already won’
Most centenarians readily offer the secrets to their longevity. Aline Jacobsohn, of Boca Raton, Fla., is no different.
Ms. Jacobsohn, who will be 101 in October, thinks a diet of small portions of fish, vegetables, and fruit, which she has followed since her husband Leo died in 1982, has helped keep her healthy. She eats lots of salmon and herring and is a fan of spinach sautéed with olive oil. “The only thing I don’t eat is meat,” the trim and active Ms. Jacobsohn said in a recent interview over Zoom.
Her other secret: “Doctors. I like to stay away from them as much as possible.”
Shari Rosenbaum, MD, Jacobsohn’s internist, doesn’t dismiss that approach. She uses a version of it when managing her three centenarian patients, the oldest of whom is 103.
“Let them smoke! Let them drink! They’re happy. It’s not causing harm. Let them eat cake! They’ve already won,” said Dr. Rosenbaum, who is affiliated with Boca Raton–based MDVIP, a national membership-based network of 1,100 primary care physicians serving 368,000 patients. Of those, nearly 460 are centenarians.
“You’re not preventing those problems in this population,” she said. “They’re here to enjoy every moment that they have, and they might as well.”
Dr. Rosenbaum sees a divergence in her patients – those who will reach very old age, and those who won’t – starting in their 60s.
“The centenarians don’t have medical problems,” she said. “They don’t get cancer. They don’t get diabetes. Some of them take good care of themselves. Some don’t take such good care of themselves. But they are all optimists. They all see the glass half full. They all participate in life. They all have excellent support systems. They have good genes, a positive attitude toward life, and a strong social network.”
Ms. Jacobsohn – whose surname at the time was Bakst – grew up in Frankfurt am Main, Germany, during the rise of the Nazi regime. The family fled to Columbia in 1938, where she met and eventually married her husband, Leo, who ran a business importing clocks and watches in Cali.
In 1989, the Jacobsohns and their three children moved to south Florida to escape the dangers of kidnappings and ransoms posed by the drug cartels.
Ms. Jacobsohn agreed that she appears to have longevity genes – “good stock,” she calls it. “My mother died 23 days before she was 100. My grandmother lived till 99, almost 100,” she said.
Two years ago, she donated her car to a charity and stopped driving in the interest of her own safety and that of other drivers and pedestrians.
Ms. Jacobsohn has a strong support system. Two of her children live nearby and visit her nearly every day. A live-in companion helps her with the activities of daily life, including preparing meals.
Ms. Jacobsohn plays bridge regularly, and well. “I’m sorry to say that I’m a very good bridge player,” she said, frankly. “How is it possible that I’ve played bridge so well and then I don’t remember what I had for lunch yesterday?”
She reads, mainly a diet of history but occasionally novels, too. “They have to be engaging,” she said.
The loss of loved ones is an inevitable part of very old age. Her husband of 47 years died of emphysema, and one of her sons died in his 70s of prostate cancer.
She knows well the fate that awaits us all and accepts it philosophically.
“It’s a very normal thing that people die. You don’t live forever. So, whenever it comes, it’s okay. Enough is enough. Dayenu,” she said, using the Hebrew word for, “It would have been enough” – a favorite in the Passover Seder celebrating the ancient Jews’ liberation from slavery in Egypt.
Ms. Jacobsohn sang the song and then took a reporter on a Zoom tour of her tidy home and her large flower garden featuring Cattleya orchids from Colombia.
A version of this article first appeared on Medscape.com.