User login
CDC recommends screening all adults for hepatitis B
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
Are early childhood viral infections linked with asthma?
MARSEILLE, France – It is well known that viral infections, especially respiratory syncytial virus (RSV) and rhinovirus (RV), exacerbate symptoms of asthma. But could they also play a part in triggering the onset of asthma?
The link between RSV and RV infections in early childhood and the development of asthma symptoms is well established, said Camille Taillé, MD, PhD, of the department of respiratory medicine and the rare diseases center of excellence at Bichat Hospital, Paris. But getting asthma is probably not just a matter of having a viral infection at a young age or of having a severe form of it. Gene polymorphisms, immune system disorders, and preexisting atopy are also associated with the risk of asthma. This was the focus of the 27th French-language respiratory medicine conference, held in Marseille, France.
RV and RSV
Persons with asthma are vulnerable to certain viral respiratory infections, in particular the flu and RV, which can exacerbate asthma symptoms. Inhaled corticosteroids have an overall protective effect against viral-induced exacerbations. For worsening asthma symptoms during an epidemic or pandemic, there is no contraindication to inhaled or oral corticosteroids.
Young children from the time of birth to 4 years of age are particularly susceptible to viral respiratory infections. According to data from France’s clinical surveillance network, Sentinelles, from the period covering winter 2021-2022, the rate of incidence per 100,000 inhabitants was systematically greater for the 0 to 4-year age range than for older age ranges.
Of the most common viruses that infect young children, RV, the virus that causes the common cold, is a nonenveloped RNA virus from the enterovirus family. There are 160 types, which are classified into three strains (A, B, and C). Of those strains, A and C confer the most severe infections. The virus is highly variable, which makes developing a vaccine challenging. The virus circulates year round, usually peaking in the fall and at the end of spring. RSV is an RNA virus that is classed as a respiratory virus. It comprises two serotypes: type A and B. Almost all children will have been infected with RSV by the time they are 2 years old. Epidemics occur each year during winter or in early spring in temperate climates. Vaccines are currently being developed and will soon be marketed. A monoclonal antibody (palivizumab), which targets fusion proteins of the virus, is available as prophylactic treatment for at-risk children.
RSV infection
During an RSV infection, the severe inflammation of the bronchial and alveolar wall causes acute respiratory distress. “But not all infants will develop severe forms of bronchiolitis,” said Dr. Taillé. “The risk factors for the severe form of the illness are well known: being under 6 months of age, prematurity, comorbidities (neurovascular, cardiovascular, respiratory, etc.), history of a stay in a neonatal intensive care unit at birth, living in low socioeconomic status towns, and exposure to smoking.”
Asthma development
The issue of whether or not viral diseases cause asthma has been the subject of intense debate. The studies are starting to stack up, however. They seem to show that RSV or RV infections are associated with the risk of subsequent asthma development. “For example, in a study published in 2022,” said Dr. Taillé, “in children admitted with an RSV infection, 60% of those who had been admitted to neonatal intensive care presented with symptoms of asthma between 3 and 6 years of age, compared with 18% of those who had had a milder case of RSV (admitted to nonintensive care settings). A serious RSV infection is a risk factor for later development of asthma.”
However, the link between RSV and later onset of asthma is also seen in milder cases of the infection. The American COAST study was designed to examine the effect of childhood respiratory infections on the risk of developing asthma. Researchers followed 259 newborns prospectively for 1, 3, and 6 years. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) or a history of physician-diagnosed asthma. Regular samples taken during infectious episodes identified a virus in 90% of cases.
“We now know that RSV is not the only pathogen responsible for bronchiolitis. RV is often found, now that it can routinely be detected by PCR tests,” said Dr. Taillé. In the COAST study, the onset of wheezing during an RSV or RV infection in children aged 0-3 years was associated with an increased risk of asthma at 6 years of age. Globally, 28% of children infected by either virus were deemed to have asthma at 6 years of age. “There is clearly a link between having had a respiratory virus like RV or RSV and getting asthma symptoms at 6 years of age,” said Dr. Taillé. “What’s more, the effect of RV is not changed in this study by allergic sensitization.”
Many articles have been published on this topic. The results of cohort studies, from Japan to Finland and the United States, Italy, and Australia, are consistent with each other. Persons who have contracted RV or RSV are more likely to suffer from recurrent wheezing or asthma, especially if the infection is contracted in infancy or if it is severe. “Some studies even suggest that viral-induced asthma is more severe,” said Dr. Taillé. “For example, a Scottish study ... showed that children with a previous history of RSV infection had more hospital admissions and required more medication than asthmatics with no history of an RSV infection, suggesting the link between a previous history of RSV infection and the development of a more severe form of asthma.”
Reaching adulthood
Few longitudinal cohorts explore this issue in adulthood. A relatively old study reported an increased rate of asthma among adults who had required hospital admission for bronchiolitis in early childhood, as well as the effect on respiratory function. A 2023 study of the effects of respiratory illnesses in childhood reported similar findings. The authors evaluated lung structure and function via CT scans of 39 patients aged 26 years and concluded that participants who had been infected with RSV in childhood presented with increased air trapping, which is suggestive of airway abnormalities, possibly linked to a direct effect of viruses on lung development.
Mechanisms of action
“The real question is understanding if it’s the virus itself that causes asthma, or if the virus is simply uncovering underlying asthma in predisposed children,” said Dr. Taillé. From 30% to 40% of children who have had RSV will go on to develop wheezing or asthma in childhood. This observation suggests that there are factors favoring the development of asthma after infection with RSV. It has been shown that there is a genetic predisposition for RV. The roles of cigarette smoke, air pollution, environmental exposures to allergens, rapid urbanization, low vitamin D levels, low maternal omega-3 long-chain polyunsaturated fatty acid levels, maternal stress, and depression have also been highlighted.
It would seem that RSV and RV are a bit different. RV is thought to be associated with the development of asthma and wheezing, especially in people with a preexisting atopy or a reduced interferon immune response, while RSV, which occurs at a younger age and among the most vulnerable populations, seems to act independently of a person’s predisposition to allergies. RV stands out from other viral factors, owing to its tendency to create a Th2-biased inflammatory environment and its association with specific risk genes in people predisposed to asthma development (CDHR3).
Dr. Taillé has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MARSEILLE, France – It is well known that viral infections, especially respiratory syncytial virus (RSV) and rhinovirus (RV), exacerbate symptoms of asthma. But could they also play a part in triggering the onset of asthma?
The link between RSV and RV infections in early childhood and the development of asthma symptoms is well established, said Camille Taillé, MD, PhD, of the department of respiratory medicine and the rare diseases center of excellence at Bichat Hospital, Paris. But getting asthma is probably not just a matter of having a viral infection at a young age or of having a severe form of it. Gene polymorphisms, immune system disorders, and preexisting atopy are also associated with the risk of asthma. This was the focus of the 27th French-language respiratory medicine conference, held in Marseille, France.
RV and RSV
Persons with asthma are vulnerable to certain viral respiratory infections, in particular the flu and RV, which can exacerbate asthma symptoms. Inhaled corticosteroids have an overall protective effect against viral-induced exacerbations. For worsening asthma symptoms during an epidemic or pandemic, there is no contraindication to inhaled or oral corticosteroids.
Young children from the time of birth to 4 years of age are particularly susceptible to viral respiratory infections. According to data from France’s clinical surveillance network, Sentinelles, from the period covering winter 2021-2022, the rate of incidence per 100,000 inhabitants was systematically greater for the 0 to 4-year age range than for older age ranges.
Of the most common viruses that infect young children, RV, the virus that causes the common cold, is a nonenveloped RNA virus from the enterovirus family. There are 160 types, which are classified into three strains (A, B, and C). Of those strains, A and C confer the most severe infections. The virus is highly variable, which makes developing a vaccine challenging. The virus circulates year round, usually peaking in the fall and at the end of spring. RSV is an RNA virus that is classed as a respiratory virus. It comprises two serotypes: type A and B. Almost all children will have been infected with RSV by the time they are 2 years old. Epidemics occur each year during winter or in early spring in temperate climates. Vaccines are currently being developed and will soon be marketed. A monoclonal antibody (palivizumab), which targets fusion proteins of the virus, is available as prophylactic treatment for at-risk children.
RSV infection
During an RSV infection, the severe inflammation of the bronchial and alveolar wall causes acute respiratory distress. “But not all infants will develop severe forms of bronchiolitis,” said Dr. Taillé. “The risk factors for the severe form of the illness are well known: being under 6 months of age, prematurity, comorbidities (neurovascular, cardiovascular, respiratory, etc.), history of a stay in a neonatal intensive care unit at birth, living in low socioeconomic status towns, and exposure to smoking.”
Asthma development
The issue of whether or not viral diseases cause asthma has been the subject of intense debate. The studies are starting to stack up, however. They seem to show that RSV or RV infections are associated with the risk of subsequent asthma development. “For example, in a study published in 2022,” said Dr. Taillé, “in children admitted with an RSV infection, 60% of those who had been admitted to neonatal intensive care presented with symptoms of asthma between 3 and 6 years of age, compared with 18% of those who had had a milder case of RSV (admitted to nonintensive care settings). A serious RSV infection is a risk factor for later development of asthma.”
However, the link between RSV and later onset of asthma is also seen in milder cases of the infection. The American COAST study was designed to examine the effect of childhood respiratory infections on the risk of developing asthma. Researchers followed 259 newborns prospectively for 1, 3, and 6 years. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) or a history of physician-diagnosed asthma. Regular samples taken during infectious episodes identified a virus in 90% of cases.
“We now know that RSV is not the only pathogen responsible for bronchiolitis. RV is often found, now that it can routinely be detected by PCR tests,” said Dr. Taillé. In the COAST study, the onset of wheezing during an RSV or RV infection in children aged 0-3 years was associated with an increased risk of asthma at 6 years of age. Globally, 28% of children infected by either virus were deemed to have asthma at 6 years of age. “There is clearly a link between having had a respiratory virus like RV or RSV and getting asthma symptoms at 6 years of age,” said Dr. Taillé. “What’s more, the effect of RV is not changed in this study by allergic sensitization.”
Many articles have been published on this topic. The results of cohort studies, from Japan to Finland and the United States, Italy, and Australia, are consistent with each other. Persons who have contracted RV or RSV are more likely to suffer from recurrent wheezing or asthma, especially if the infection is contracted in infancy or if it is severe. “Some studies even suggest that viral-induced asthma is more severe,” said Dr. Taillé. “For example, a Scottish study ... showed that children with a previous history of RSV infection had more hospital admissions and required more medication than asthmatics with no history of an RSV infection, suggesting the link between a previous history of RSV infection and the development of a more severe form of asthma.”
Reaching adulthood
Few longitudinal cohorts explore this issue in adulthood. A relatively old study reported an increased rate of asthma among adults who had required hospital admission for bronchiolitis in early childhood, as well as the effect on respiratory function. A 2023 study of the effects of respiratory illnesses in childhood reported similar findings. The authors evaluated lung structure and function via CT scans of 39 patients aged 26 years and concluded that participants who had been infected with RSV in childhood presented with increased air trapping, which is suggestive of airway abnormalities, possibly linked to a direct effect of viruses on lung development.
Mechanisms of action
“The real question is understanding if it’s the virus itself that causes asthma, or if the virus is simply uncovering underlying asthma in predisposed children,” said Dr. Taillé. From 30% to 40% of children who have had RSV will go on to develop wheezing or asthma in childhood. This observation suggests that there are factors favoring the development of asthma after infection with RSV. It has been shown that there is a genetic predisposition for RV. The roles of cigarette smoke, air pollution, environmental exposures to allergens, rapid urbanization, low vitamin D levels, low maternal omega-3 long-chain polyunsaturated fatty acid levels, maternal stress, and depression have also been highlighted.
It would seem that RSV and RV are a bit different. RV is thought to be associated with the development of asthma and wheezing, especially in people with a preexisting atopy or a reduced interferon immune response, while RSV, which occurs at a younger age and among the most vulnerable populations, seems to act independently of a person’s predisposition to allergies. RV stands out from other viral factors, owing to its tendency to create a Th2-biased inflammatory environment and its association with specific risk genes in people predisposed to asthma development (CDHR3).
Dr. Taillé has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MARSEILLE, France – It is well known that viral infections, especially respiratory syncytial virus (RSV) and rhinovirus (RV), exacerbate symptoms of asthma. But could they also play a part in triggering the onset of asthma?
The link between RSV and RV infections in early childhood and the development of asthma symptoms is well established, said Camille Taillé, MD, PhD, of the department of respiratory medicine and the rare diseases center of excellence at Bichat Hospital, Paris. But getting asthma is probably not just a matter of having a viral infection at a young age or of having a severe form of it. Gene polymorphisms, immune system disorders, and preexisting atopy are also associated with the risk of asthma. This was the focus of the 27th French-language respiratory medicine conference, held in Marseille, France.
RV and RSV
Persons with asthma are vulnerable to certain viral respiratory infections, in particular the flu and RV, which can exacerbate asthma symptoms. Inhaled corticosteroids have an overall protective effect against viral-induced exacerbations. For worsening asthma symptoms during an epidemic or pandemic, there is no contraindication to inhaled or oral corticosteroids.
Young children from the time of birth to 4 years of age are particularly susceptible to viral respiratory infections. According to data from France’s clinical surveillance network, Sentinelles, from the period covering winter 2021-2022, the rate of incidence per 100,000 inhabitants was systematically greater for the 0 to 4-year age range than for older age ranges.
Of the most common viruses that infect young children, RV, the virus that causes the common cold, is a nonenveloped RNA virus from the enterovirus family. There are 160 types, which are classified into three strains (A, B, and C). Of those strains, A and C confer the most severe infections. The virus is highly variable, which makes developing a vaccine challenging. The virus circulates year round, usually peaking in the fall and at the end of spring. RSV is an RNA virus that is classed as a respiratory virus. It comprises two serotypes: type A and B. Almost all children will have been infected with RSV by the time they are 2 years old. Epidemics occur each year during winter or in early spring in temperate climates. Vaccines are currently being developed and will soon be marketed. A monoclonal antibody (palivizumab), which targets fusion proteins of the virus, is available as prophylactic treatment for at-risk children.
RSV infection
During an RSV infection, the severe inflammation of the bronchial and alveolar wall causes acute respiratory distress. “But not all infants will develop severe forms of bronchiolitis,” said Dr. Taillé. “The risk factors for the severe form of the illness are well known: being under 6 months of age, prematurity, comorbidities (neurovascular, cardiovascular, respiratory, etc.), history of a stay in a neonatal intensive care unit at birth, living in low socioeconomic status towns, and exposure to smoking.”
Asthma development
The issue of whether or not viral diseases cause asthma has been the subject of intense debate. The studies are starting to stack up, however. They seem to show that RSV or RV infections are associated with the risk of subsequent asthma development. “For example, in a study published in 2022,” said Dr. Taillé, “in children admitted with an RSV infection, 60% of those who had been admitted to neonatal intensive care presented with symptoms of asthma between 3 and 6 years of age, compared with 18% of those who had had a milder case of RSV (admitted to nonintensive care settings). A serious RSV infection is a risk factor for later development of asthma.”
However, the link between RSV and later onset of asthma is also seen in milder cases of the infection. The American COAST study was designed to examine the effect of childhood respiratory infections on the risk of developing asthma. Researchers followed 259 newborns prospectively for 1, 3, and 6 years. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) or a history of physician-diagnosed asthma. Regular samples taken during infectious episodes identified a virus in 90% of cases.
“We now know that RSV is not the only pathogen responsible for bronchiolitis. RV is often found, now that it can routinely be detected by PCR tests,” said Dr. Taillé. In the COAST study, the onset of wheezing during an RSV or RV infection in children aged 0-3 years was associated with an increased risk of asthma at 6 years of age. Globally, 28% of children infected by either virus were deemed to have asthma at 6 years of age. “There is clearly a link between having had a respiratory virus like RV or RSV and getting asthma symptoms at 6 years of age,” said Dr. Taillé. “What’s more, the effect of RV is not changed in this study by allergic sensitization.”
Many articles have been published on this topic. The results of cohort studies, from Japan to Finland and the United States, Italy, and Australia, are consistent with each other. Persons who have contracted RV or RSV are more likely to suffer from recurrent wheezing or asthma, especially if the infection is contracted in infancy or if it is severe. “Some studies even suggest that viral-induced asthma is more severe,” said Dr. Taillé. “For example, a Scottish study ... showed that children with a previous history of RSV infection had more hospital admissions and required more medication than asthmatics with no history of an RSV infection, suggesting the link between a previous history of RSV infection and the development of a more severe form of asthma.”
Reaching adulthood
Few longitudinal cohorts explore this issue in adulthood. A relatively old study reported an increased rate of asthma among adults who had required hospital admission for bronchiolitis in early childhood, as well as the effect on respiratory function. A 2023 study of the effects of respiratory illnesses in childhood reported similar findings. The authors evaluated lung structure and function via CT scans of 39 patients aged 26 years and concluded that participants who had been infected with RSV in childhood presented with increased air trapping, which is suggestive of airway abnormalities, possibly linked to a direct effect of viruses on lung development.
Mechanisms of action
“The real question is understanding if it’s the virus itself that causes asthma, or if the virus is simply uncovering underlying asthma in predisposed children,” said Dr. Taillé. From 30% to 40% of children who have had RSV will go on to develop wheezing or asthma in childhood. This observation suggests that there are factors favoring the development of asthma after infection with RSV. It has been shown that there is a genetic predisposition for RV. The roles of cigarette smoke, air pollution, environmental exposures to allergens, rapid urbanization, low vitamin D levels, low maternal omega-3 long-chain polyunsaturated fatty acid levels, maternal stress, and depression have also been highlighted.
It would seem that RSV and RV are a bit different. RV is thought to be associated with the development of asthma and wheezing, especially in people with a preexisting atopy or a reduced interferon immune response, while RSV, which occurs at a younger age and among the most vulnerable populations, seems to act independently of a person’s predisposition to allergies. RV stands out from other viral factors, owing to its tendency to create a Th2-biased inflammatory environment and its association with specific risk genes in people predisposed to asthma development (CDHR3).
Dr. Taillé has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Kaposi Varicelliform Eruption of Mpox in a Peeling Sunburn
To the Editor:
The recent global mpox (monkeypox) outbreak that started in May 2022 has distinctive risk factors, clinical features, and patient attributes that can portend dissemination of infection. We report a case of Kaposi varicelliform eruption (KVE) over a peeling sunburn after mpox infection. Dermatologists should recognize cutaneous risk factors for dissemination of mpox.
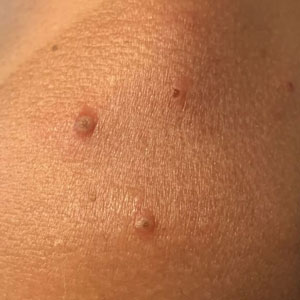
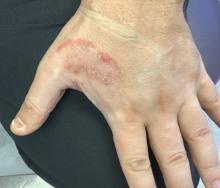
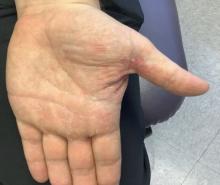
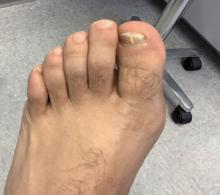
A 35-year-old man who was otherwise healthy presented with a papulopustular eruption that began on the shoulders in an area that had been sunburned 24 to 48 hours earlier. He experienced fever (temperature, 38.6 °C)[101.5 °F]), chills, malaise, and the appearance of a painful penile ulcer. He reported a recent male sexual partner a week prior to the eruption during travel to eastern Asia and a subsequent male partner in the United States 5 days prior to eruption. Physical examination revealed a peeling sunburn with sharp clothing demarcation. Locations with the most notable desquamation—the superior shoulders, dorsal arms, upper chest, and ventral thighs—positively correlated with the highest density of scattered, discrete, erythematous-based pustules and pink papules, some with crusted umbilication (Figures 1 and 2). Lesions spared sun-protected locations except a punctate painful ulcer on the buccal mucosa and a tender well-demarcated ulcer with elevated borders on the ventral penile shaft. HIV antigen/antibody testing was negative; syphilis antibody testing was positive due to a prior infection 1 year earlier with titers down to 1:1. A penile ulcer swab did not detect herpes simplex virus types 1/2 DNA. Pharyngeal, penile, and rectal swabs were negative for chlamydia or gonorrhea DNA. A polymerase chain reaction assay of a pustule was positive for orthopoxvirus, and the Centers for Disease Control and Prevention confirmed Monkeypox virus. On day 12, a penile ulcer biopsy was nonspecific with dense mixed inflammation; immunohistochemical stains for Treponema pallidum and herpes simplex virus types 1/2 were negative. Consideration was given to starting antiviral treatment with tecovirimat, which is approved by the US Food and Drug Administration for smallpox caused by variola virus, through the Centers for Disease Control and Prevention expanded access protocol, but the patient’s symptoms and lesions cleared quickly without intervention. The patient’s recent sexual contact in the United States later tested positive for mpox. Given that the density of our patient’s mpox lesions positively correlated with areas of peeling sunburn with rapid spread during the period of desquamation, he was diagnosed with KVE due to mpox in the setting of a peeling sunburn.
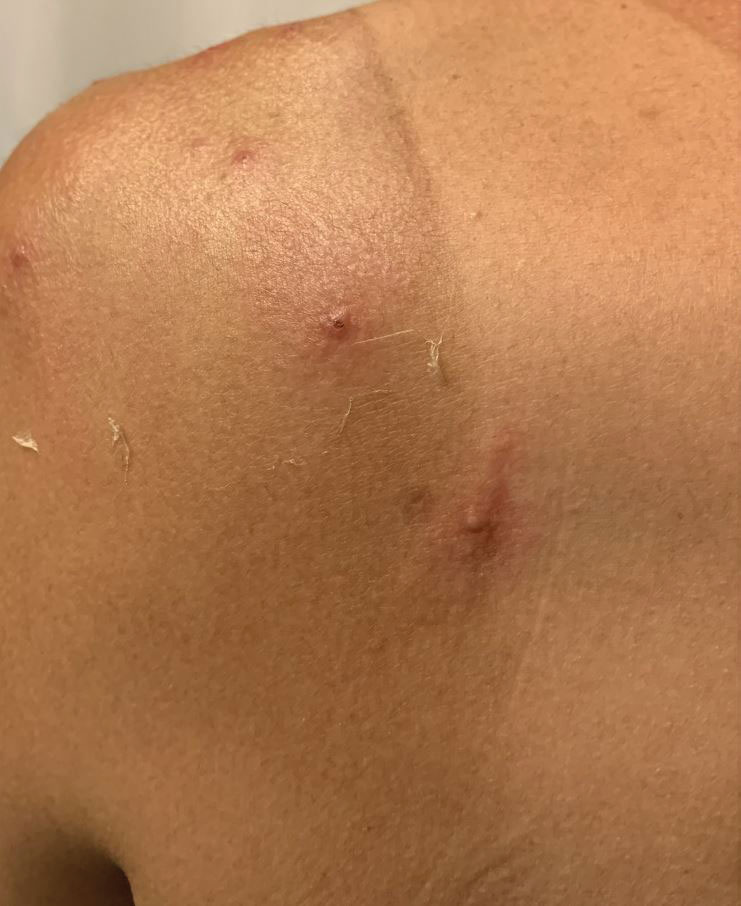
The recent mpox outbreak began in May 2022, and within 3 months there were more than 31,000 confirmed mpox cases worldwide, with more than 11,000 of those cases within the United States across 49 states and Puerto Rico.1 Gay, bisexual, and other men who have sex with men have constituted the majority of cases. Although prior outbreaks have exhibited cases of classic mpox lesions, the current cases are clinically distinctive from classic mpox due to prevalent orogenital involvement and generalized symptoms that often are mild, nonexistent, or can occur after the cutaneous lesions.2
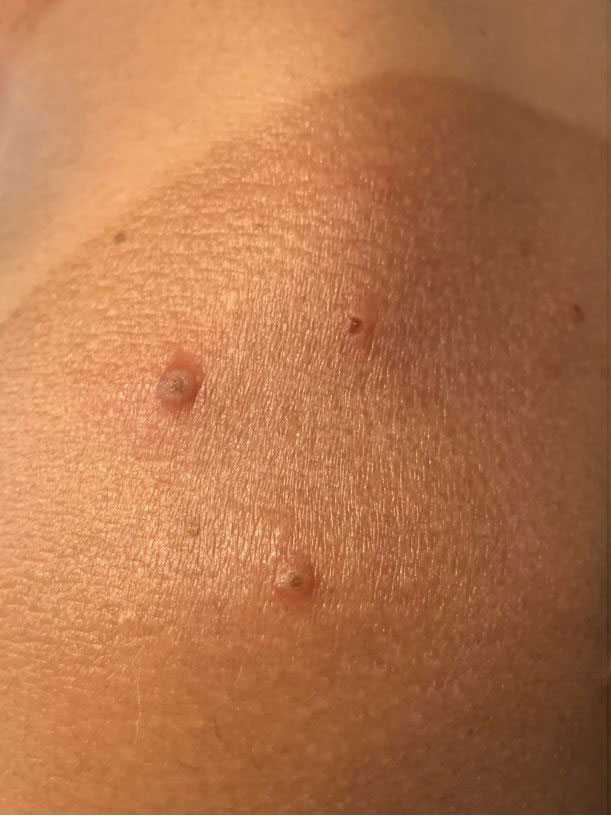
Although most current cases of mpox have been mildly symptomatic, several patients have been ill enough to require hospital admission, including patients with severe anogenital ulcerative lesions or bacterial superinfection.3 Antiviral treatment with tecovirimat may be warranted for patients with severe disease or those at risk of becoming severe due to immunosuppression, pregnancy/breastfeeding, complications (as determined by the provider), younger age (ie, pediatric patients), or skin barrier disruption. Dermatologists play a particularly important role in identifying cutaneous risk factors that may indicate progression of infection (eg, atopic dermatitis, severe acne, intertrigo, Darier disease). Kaposi varicelliform eruption is the phenomenon where a more typically localized vesicular infection is disseminated to areas with a defective skin barrier.2 Eczema herpeticum refers to the most common type of KVE due to herpes simplex virus, but other known etiologies of KVE include coxsackievirus A16, vaccinia virus, varicella-zoster virus, and smallpox.2 Although classic mpox previously had only the theoretical potential to lead to a secondary KVE, we expect the literature to evolve as cases spread, with one recent report of eczema monkeypoxicum in the setting of atopic dermatitis.4
At the time of publication, mpox cases have notably dropped globally due to public health interventions; however, mpox infections are ongoing in areas previously identified as nonendemic. Given the distinctive risk factors and clinical presentations of this most recent outbreak, clinicians will need to be adept at identifying not only infection but also risk for dissemination, including skin barrier disruption.
- Centers for Disease Control and Prevention. Mpox: 2022 US map & case count. Updated February 15, 2023. Accessed February 23, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls. Updated September 12, 2022. Accessed February 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;S1473-3099(22)00411-X. doi:10.1016/S1473-3099(22)00411-X
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: report of monkeypox transmission in patients with atopic dermatitis. JAAD Case Reports. 2022;29:95-99.
To the Editor:
The recent global mpox (monkeypox) outbreak that started in May 2022 has distinctive risk factors, clinical features, and patient attributes that can portend dissemination of infection. We report a case of Kaposi varicelliform eruption (KVE) over a peeling sunburn after mpox infection. Dermatologists should recognize cutaneous risk factors for dissemination of mpox.
A 35-year-old man who was otherwise healthy presented with a papulopustular eruption that began on the shoulders in an area that had been sunburned 24 to 48 hours earlier. He experienced fever (temperature, 38.6 °C)[101.5 °F]), chills, malaise, and the appearance of a painful penile ulcer. He reported a recent male sexual partner a week prior to the eruption during travel to eastern Asia and a subsequent male partner in the United States 5 days prior to eruption. Physical examination revealed a peeling sunburn with sharp clothing demarcation. Locations with the most notable desquamation—the superior shoulders, dorsal arms, upper chest, and ventral thighs—positively correlated with the highest density of scattered, discrete, erythematous-based pustules and pink papules, some with crusted umbilication (Figures 1 and 2). Lesions spared sun-protected locations except a punctate painful ulcer on the buccal mucosa and a tender well-demarcated ulcer with elevated borders on the ventral penile shaft. HIV antigen/antibody testing was negative; syphilis antibody testing was positive due to a prior infection 1 year earlier with titers down to 1:1. A penile ulcer swab did not detect herpes simplex virus types 1/2 DNA. Pharyngeal, penile, and rectal swabs were negative for chlamydia or gonorrhea DNA. A polymerase chain reaction assay of a pustule was positive for orthopoxvirus, and the Centers for Disease Control and Prevention confirmed Monkeypox virus. On day 12, a penile ulcer biopsy was nonspecific with dense mixed inflammation; immunohistochemical stains for Treponema pallidum and herpes simplex virus types 1/2 were negative. Consideration was given to starting antiviral treatment with tecovirimat, which is approved by the US Food and Drug Administration for smallpox caused by variola virus, through the Centers for Disease Control and Prevention expanded access protocol, but the patient’s symptoms and lesions cleared quickly without intervention. The patient’s recent sexual contact in the United States later tested positive for mpox. Given that the density of our patient’s mpox lesions positively correlated with areas of peeling sunburn with rapid spread during the period of desquamation, he was diagnosed with KVE due to mpox in the setting of a peeling sunburn.

The recent mpox outbreak began in May 2022, and within 3 months there were more than 31,000 confirmed mpox cases worldwide, with more than 11,000 of those cases within the United States across 49 states and Puerto Rico.1 Gay, bisexual, and other men who have sex with men have constituted the majority of cases. Although prior outbreaks have exhibited cases of classic mpox lesions, the current cases are clinically distinctive from classic mpox due to prevalent orogenital involvement and generalized symptoms that often are mild, nonexistent, or can occur after the cutaneous lesions.2

Although most current cases of mpox have been mildly symptomatic, several patients have been ill enough to require hospital admission, including patients with severe anogenital ulcerative lesions or bacterial superinfection.3 Antiviral treatment with tecovirimat may be warranted for patients with severe disease or those at risk of becoming severe due to immunosuppression, pregnancy/breastfeeding, complications (as determined by the provider), younger age (ie, pediatric patients), or skin barrier disruption. Dermatologists play a particularly important role in identifying cutaneous risk factors that may indicate progression of infection (eg, atopic dermatitis, severe acne, intertrigo, Darier disease). Kaposi varicelliform eruption is the phenomenon where a more typically localized vesicular infection is disseminated to areas with a defective skin barrier.2 Eczema herpeticum refers to the most common type of KVE due to herpes simplex virus, but other known etiologies of KVE include coxsackievirus A16, vaccinia virus, varicella-zoster virus, and smallpox.2 Although classic mpox previously had only the theoretical potential to lead to a secondary KVE, we expect the literature to evolve as cases spread, with one recent report of eczema monkeypoxicum in the setting of atopic dermatitis.4
At the time of publication, mpox cases have notably dropped globally due to public health interventions; however, mpox infections are ongoing in areas previously identified as nonendemic. Given the distinctive risk factors and clinical presentations of this most recent outbreak, clinicians will need to be adept at identifying not only infection but also risk for dissemination, including skin barrier disruption.
To the Editor:
The recent global mpox (monkeypox) outbreak that started in May 2022 has distinctive risk factors, clinical features, and patient attributes that can portend dissemination of infection. We report a case of Kaposi varicelliform eruption (KVE) over a peeling sunburn after mpox infection. Dermatologists should recognize cutaneous risk factors for dissemination of mpox.
A 35-year-old man who was otherwise healthy presented with a papulopustular eruption that began on the shoulders in an area that had been sunburned 24 to 48 hours earlier. He experienced fever (temperature, 38.6 °C)[101.5 °F]), chills, malaise, and the appearance of a painful penile ulcer. He reported a recent male sexual partner a week prior to the eruption during travel to eastern Asia and a subsequent male partner in the United States 5 days prior to eruption. Physical examination revealed a peeling sunburn with sharp clothing demarcation. Locations with the most notable desquamation—the superior shoulders, dorsal arms, upper chest, and ventral thighs—positively correlated with the highest density of scattered, discrete, erythematous-based pustules and pink papules, some with crusted umbilication (Figures 1 and 2). Lesions spared sun-protected locations except a punctate painful ulcer on the buccal mucosa and a tender well-demarcated ulcer with elevated borders on the ventral penile shaft. HIV antigen/antibody testing was negative; syphilis antibody testing was positive due to a prior infection 1 year earlier with titers down to 1:1. A penile ulcer swab did not detect herpes simplex virus types 1/2 DNA. Pharyngeal, penile, and rectal swabs were negative for chlamydia or gonorrhea DNA. A polymerase chain reaction assay of a pustule was positive for orthopoxvirus, and the Centers for Disease Control and Prevention confirmed Monkeypox virus. On day 12, a penile ulcer biopsy was nonspecific with dense mixed inflammation; immunohistochemical stains for Treponema pallidum and herpes simplex virus types 1/2 were negative. Consideration was given to starting antiviral treatment with tecovirimat, which is approved by the US Food and Drug Administration for smallpox caused by variola virus, through the Centers for Disease Control and Prevention expanded access protocol, but the patient’s symptoms and lesions cleared quickly without intervention. The patient’s recent sexual contact in the United States later tested positive for mpox. Given that the density of our patient’s mpox lesions positively correlated with areas of peeling sunburn with rapid spread during the period of desquamation, he was diagnosed with KVE due to mpox in the setting of a peeling sunburn.

The recent mpox outbreak began in May 2022, and within 3 months there were more than 31,000 confirmed mpox cases worldwide, with more than 11,000 of those cases within the United States across 49 states and Puerto Rico.1 Gay, bisexual, and other men who have sex with men have constituted the majority of cases. Although prior outbreaks have exhibited cases of classic mpox lesions, the current cases are clinically distinctive from classic mpox due to prevalent orogenital involvement and generalized symptoms that often are mild, nonexistent, or can occur after the cutaneous lesions.2

Although most current cases of mpox have been mildly symptomatic, several patients have been ill enough to require hospital admission, including patients with severe anogenital ulcerative lesions or bacterial superinfection.3 Antiviral treatment with tecovirimat may be warranted for patients with severe disease or those at risk of becoming severe due to immunosuppression, pregnancy/breastfeeding, complications (as determined by the provider), younger age (ie, pediatric patients), or skin barrier disruption. Dermatologists play a particularly important role in identifying cutaneous risk factors that may indicate progression of infection (eg, atopic dermatitis, severe acne, intertrigo, Darier disease). Kaposi varicelliform eruption is the phenomenon where a more typically localized vesicular infection is disseminated to areas with a defective skin barrier.2 Eczema herpeticum refers to the most common type of KVE due to herpes simplex virus, but other known etiologies of KVE include coxsackievirus A16, vaccinia virus, varicella-zoster virus, and smallpox.2 Although classic mpox previously had only the theoretical potential to lead to a secondary KVE, we expect the literature to evolve as cases spread, with one recent report of eczema monkeypoxicum in the setting of atopic dermatitis.4
At the time of publication, mpox cases have notably dropped globally due to public health interventions; however, mpox infections are ongoing in areas previously identified as nonendemic. Given the distinctive risk factors and clinical presentations of this most recent outbreak, clinicians will need to be adept at identifying not only infection but also risk for dissemination, including skin barrier disruption.
- Centers for Disease Control and Prevention. Mpox: 2022 US map & case count. Updated February 15, 2023. Accessed February 23, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls. Updated September 12, 2022. Accessed February 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;S1473-3099(22)00411-X. doi:10.1016/S1473-3099(22)00411-X
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: report of monkeypox transmission in patients with atopic dermatitis. JAAD Case Reports. 2022;29:95-99.
- Centers for Disease Control and Prevention. Mpox: 2022 US map & case count. Updated February 15, 2023. Accessed February 23, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls. Updated September 12, 2022. Accessed February 24, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;S1473-3099(22)00411-X. doi:10.1016/S1473-3099(22)00411-X
- Xia J, Huang CL, Chu P, et al. Eczema monkeypoxicum: report of monkeypox transmission in patients with atopic dermatitis. JAAD Case Reports. 2022;29:95-99.
Practice Points
- Desquamation can be associated with dissemination and higher severity course in the setting of mpox (monkeypox) viral infection.
- Antiviral treatment with tecovirimat is warranted in those with severe mpox infection or those at risk of severe infection including skin barrier disruption.
- Kaposi varicelliform–like eruptions can happen in the setting of barrier disruption from peeling sunburns, atopic dermatitis, severe acne, and other dermatologic conditions.
'Zombie viruses': Fascinating and a little frightening
Of all the consequences of climate change, here’s one nobody counted on.
A team of European researchers digging into Siberian permafrost discovered and revived 13 types of prehistoric viruses.
The researchers coined the isn’t-that-just-great term “zombie viruses” to describe previously dormant viruses that had been frozen in ice for tens of thousands of years – 27,000 to 48,500 years, in fact.
The first question is obvious: This is fascinating, but is it a good idea? We’re still dealing with a certain mutating virus our immune systems have never encountered before.
The second question: What does it mean?
No humans were harmed in this study
The quick answer: The viruses observed here were only able to infect amoebae. But viruses that can infect humans do indeed exist in environments like permafrost.
The possibility that an unearthed, unknown virus will one day appear from seemingly nowhere and result in another pandemic is not necessarily zero.
“There is an objective risk, and it is increasing,” says Jean-Michel Claverie, PhD, the lead researcher and an emeritus professor of genomics and bioinformatics at Aix-Marseille University in France. “However, we cannot put a number on this probability, specifically because we refuse to work with and revive human- and animal-infecting viruses. It would be much too dangerous.”
Based on Dr. Claverie and his team’s results, human- and animal-infecting viruses can indeed survive deep within the permafrost for extended periods of time.
“From our research, we can deduce that other viruses present in the permafrost are likely still infectious,” says Dr. Claverie. “By sequencing the total DNA, we can detect the presence of viruses similar to those infecting animals or humans today.”
That said, the chances of something catastrophic happening from, say, humans exposed to thawed permafrost are slim. “[The microbes] would be quick to decay once they’re exposed to heat, UV light, and oxygen,” he says.
Also, in places like Siberia where permafrost exists, people generally do not. So, some science fiction-inspired fears (we see you, fans of John Carpenter’s “The Thing”) are pretty unfounded. But if more people or companies begin to migrate toward the areas where these microbes are being released, the chances of a virus successfully infecting a host could be greater.
But what if ...
So, what would happen – hypothetically – if the next deadly virus to overtake our planet came from the Arctic permafrost? Would we even be remotely prepared?
“There is a small risk that a frozen virus that gets unearthed is able to start an infection chain that ends up in humans,” says Adrian Liston, PhD, an immunologist and senior group leader at the Babraham Institute, a life sciences research institute at the University of Cambridge in England. Dr. Liston was not involved in the research discussed here. “On the one hand, we would not have preexisting immunity against it, so the initial ability to combat the infection is low. On the other hand, the virus would not be adapted to infect (modern-day) humans, so the chance of an initial infection being successful for the virus is extremely low.”
That’s something a lot of folks don’t understand: Today’s viruses and other infectious microbes are infectious only because they exist today. They have evolved to work within our modern immune systems – for either good or ill.
“ ‘Entry events’ do happen, very rarely, and they can shape human evolution,” says Dr. Liston. “Major examples would be smallpox (a virus) and tuberculosis (a bacteria), which strongly influenced human evolution when they entered our species, selecting for the type of immune system that was able to fight them and killing off individuals with the ‘wrong’ type of immune system.”
And not all organisms are harmful.
“There are many, many microbes that are beneficial to humans,” Dr. Liston says. “But generally speaking, these are microbes that have evolved for millions of years to work in harmony with our body, such as our microbiome, or have been selected for thousands of years to do beneficial chores for us, like yeast in making bread or brewing beer.”
Some random frozen microbe is unlikely to affect us directly, but if it does, it is far more likely to be bad, Dr. Liston says.
For now, at least, we can rest easy knowing that Dr. Claverie and his team have no plans to revive dangerous viruses or retrieve more samples. “Because of the Russian-Ukrainian war, all of our collaborations have stopped. We are now focused on studying the viruses already in our lab and understanding how they replicate and interact with their cellular hosts,” he says.
If anything, zombie viruses can at least remind us about the constant increasing effects that climate change will have on our lives and planet in the near future.
“The most important take-home message is that climate change is going to create unexpected problems,” says Dr. Liston. “It isn’t simply changes to weather, climate events, and sea levels rising. A whole cascade of secondary problems will be generated. New infections, some of which could go pandemic, are almost certainly going to happen because of climate change.”
A version of this article first appeared on WebMD.com.
Of all the consequences of climate change, here’s one nobody counted on.
A team of European researchers digging into Siberian permafrost discovered and revived 13 types of prehistoric viruses.
The researchers coined the isn’t-that-just-great term “zombie viruses” to describe previously dormant viruses that had been frozen in ice for tens of thousands of years – 27,000 to 48,500 years, in fact.
The first question is obvious: This is fascinating, but is it a good idea? We’re still dealing with a certain mutating virus our immune systems have never encountered before.
The second question: What does it mean?
No humans were harmed in this study
The quick answer: The viruses observed here were only able to infect amoebae. But viruses that can infect humans do indeed exist in environments like permafrost.
The possibility that an unearthed, unknown virus will one day appear from seemingly nowhere and result in another pandemic is not necessarily zero.
“There is an objective risk, and it is increasing,” says Jean-Michel Claverie, PhD, the lead researcher and an emeritus professor of genomics and bioinformatics at Aix-Marseille University in France. “However, we cannot put a number on this probability, specifically because we refuse to work with and revive human- and animal-infecting viruses. It would be much too dangerous.”
Based on Dr. Claverie and his team’s results, human- and animal-infecting viruses can indeed survive deep within the permafrost for extended periods of time.
“From our research, we can deduce that other viruses present in the permafrost are likely still infectious,” says Dr. Claverie. “By sequencing the total DNA, we can detect the presence of viruses similar to those infecting animals or humans today.”
That said, the chances of something catastrophic happening from, say, humans exposed to thawed permafrost are slim. “[The microbes] would be quick to decay once they’re exposed to heat, UV light, and oxygen,” he says.
Also, in places like Siberia where permafrost exists, people generally do not. So, some science fiction-inspired fears (we see you, fans of John Carpenter’s “The Thing”) are pretty unfounded. But if more people or companies begin to migrate toward the areas where these microbes are being released, the chances of a virus successfully infecting a host could be greater.
But what if ...
So, what would happen – hypothetically – if the next deadly virus to overtake our planet came from the Arctic permafrost? Would we even be remotely prepared?
“There is a small risk that a frozen virus that gets unearthed is able to start an infection chain that ends up in humans,” says Adrian Liston, PhD, an immunologist and senior group leader at the Babraham Institute, a life sciences research institute at the University of Cambridge in England. Dr. Liston was not involved in the research discussed here. “On the one hand, we would not have preexisting immunity against it, so the initial ability to combat the infection is low. On the other hand, the virus would not be adapted to infect (modern-day) humans, so the chance of an initial infection being successful for the virus is extremely low.”
That’s something a lot of folks don’t understand: Today’s viruses and other infectious microbes are infectious only because they exist today. They have evolved to work within our modern immune systems – for either good or ill.
“ ‘Entry events’ do happen, very rarely, and they can shape human evolution,” says Dr. Liston. “Major examples would be smallpox (a virus) and tuberculosis (a bacteria), which strongly influenced human evolution when they entered our species, selecting for the type of immune system that was able to fight them and killing off individuals with the ‘wrong’ type of immune system.”
And not all organisms are harmful.
“There are many, many microbes that are beneficial to humans,” Dr. Liston says. “But generally speaking, these are microbes that have evolved for millions of years to work in harmony with our body, such as our microbiome, or have been selected for thousands of years to do beneficial chores for us, like yeast in making bread or brewing beer.”
Some random frozen microbe is unlikely to affect us directly, but if it does, it is far more likely to be bad, Dr. Liston says.
For now, at least, we can rest easy knowing that Dr. Claverie and his team have no plans to revive dangerous viruses or retrieve more samples. “Because of the Russian-Ukrainian war, all of our collaborations have stopped. We are now focused on studying the viruses already in our lab and understanding how they replicate and interact with their cellular hosts,” he says.
If anything, zombie viruses can at least remind us about the constant increasing effects that climate change will have on our lives and planet in the near future.
“The most important take-home message is that climate change is going to create unexpected problems,” says Dr. Liston. “It isn’t simply changes to weather, climate events, and sea levels rising. A whole cascade of secondary problems will be generated. New infections, some of which could go pandemic, are almost certainly going to happen because of climate change.”
A version of this article first appeared on WebMD.com.
Of all the consequences of climate change, here’s one nobody counted on.
A team of European researchers digging into Siberian permafrost discovered and revived 13 types of prehistoric viruses.
The researchers coined the isn’t-that-just-great term “zombie viruses” to describe previously dormant viruses that had been frozen in ice for tens of thousands of years – 27,000 to 48,500 years, in fact.
The first question is obvious: This is fascinating, but is it a good idea? We’re still dealing with a certain mutating virus our immune systems have never encountered before.
The second question: What does it mean?
No humans were harmed in this study
The quick answer: The viruses observed here were only able to infect amoebae. But viruses that can infect humans do indeed exist in environments like permafrost.
The possibility that an unearthed, unknown virus will one day appear from seemingly nowhere and result in another pandemic is not necessarily zero.
“There is an objective risk, and it is increasing,” says Jean-Michel Claverie, PhD, the lead researcher and an emeritus professor of genomics and bioinformatics at Aix-Marseille University in France. “However, we cannot put a number on this probability, specifically because we refuse to work with and revive human- and animal-infecting viruses. It would be much too dangerous.”
Based on Dr. Claverie and his team’s results, human- and animal-infecting viruses can indeed survive deep within the permafrost for extended periods of time.
“From our research, we can deduce that other viruses present in the permafrost are likely still infectious,” says Dr. Claverie. “By sequencing the total DNA, we can detect the presence of viruses similar to those infecting animals or humans today.”
That said, the chances of something catastrophic happening from, say, humans exposed to thawed permafrost are slim. “[The microbes] would be quick to decay once they’re exposed to heat, UV light, and oxygen,” he says.
Also, in places like Siberia where permafrost exists, people generally do not. So, some science fiction-inspired fears (we see you, fans of John Carpenter’s “The Thing”) are pretty unfounded. But if more people or companies begin to migrate toward the areas where these microbes are being released, the chances of a virus successfully infecting a host could be greater.
But what if ...
So, what would happen – hypothetically – if the next deadly virus to overtake our planet came from the Arctic permafrost? Would we even be remotely prepared?
“There is a small risk that a frozen virus that gets unearthed is able to start an infection chain that ends up in humans,” says Adrian Liston, PhD, an immunologist and senior group leader at the Babraham Institute, a life sciences research institute at the University of Cambridge in England. Dr. Liston was not involved in the research discussed here. “On the one hand, we would not have preexisting immunity against it, so the initial ability to combat the infection is low. On the other hand, the virus would not be adapted to infect (modern-day) humans, so the chance of an initial infection being successful for the virus is extremely low.”
That’s something a lot of folks don’t understand: Today’s viruses and other infectious microbes are infectious only because they exist today. They have evolved to work within our modern immune systems – for either good or ill.
“ ‘Entry events’ do happen, very rarely, and they can shape human evolution,” says Dr. Liston. “Major examples would be smallpox (a virus) and tuberculosis (a bacteria), which strongly influenced human evolution when they entered our species, selecting for the type of immune system that was able to fight them and killing off individuals with the ‘wrong’ type of immune system.”
And not all organisms are harmful.
“There are many, many microbes that are beneficial to humans,” Dr. Liston says. “But generally speaking, these are microbes that have evolved for millions of years to work in harmony with our body, such as our microbiome, or have been selected for thousands of years to do beneficial chores for us, like yeast in making bread or brewing beer.”
Some random frozen microbe is unlikely to affect us directly, but if it does, it is far more likely to be bad, Dr. Liston says.
For now, at least, we can rest easy knowing that Dr. Claverie and his team have no plans to revive dangerous viruses or retrieve more samples. “Because of the Russian-Ukrainian war, all of our collaborations have stopped. We are now focused on studying the viruses already in our lab and understanding how they replicate and interact with their cellular hosts,” he says.
If anything, zombie viruses can at least remind us about the constant increasing effects that climate change will have on our lives and planet in the near future.
“The most important take-home message is that climate change is going to create unexpected problems,” says Dr. Liston. “It isn’t simply changes to weather, climate events, and sea levels rising. A whole cascade of secondary problems will be generated. New infections, some of which could go pandemic, are almost certainly going to happen because of climate change.”
A version of this article first appeared on WebMD.com.
‘Breakthrough’ study: Diabetes drug helps prevent long COVID
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
White male presents with pruritic, scaly, erythematous patches on his feet and left hand
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Measles exposures in Kentucky have CDC on alert
The Centers for Disease Control and Prevention has issued a Health Alert Network (HAN) health advisory notifying clinicians and public health officials of a confirmed measles case in an individual who for 2 days (February 17-18) attended a large religious gathering that was attended by an estimated 20,000 people at Asbury University in Wilmore, Ky.
Given that large numbers of people might have been exposed to the attendee (who was not vaccinated) and that the individual had a history of recent international travel, the CDC has encouraged clinicians to be vigilant for patients presenting with symptoms that meet the measles case definition. A steady increase in measles cases from 49 in 2021 to 121 in 2022 in children who were not fully vaccinated – coupled with outbreaks in Ohio and Minnesota – underscores the potential gravity of the CDC advisory as well as the need to mitigate the risk of ongoing or secondary transmission.
Currently, little is known about the individual who contracted measles other than the fact that he is a resident of Jessamine County, Ky., according to a news release issued by the Kentucky Department of Public Health. It is the third confirmed case in Kentucky over the past 3 months. State and national health officials are concerned that the individual might have transmitted measles to attendees visiting from other states.
David Sugerman, MD, MPH, a medical officer in CDC’s division of viral diseases and lead for the measles, rubella, and cytomegalovirus team, noted that the timing of the alert coincides with the period in which persons who had had contact with the initial case patient might be expected to develop symptoms.
For clinicians, “It’s really about considering measles in any un- or undervaccinated patient that arrives at a clinic and recently traveled internationally,” Dr. Sugerman told this news organization. He explained that “when doctors are seeing patients, they’re not going to necessarily share that information off the bat when they present with fever or rash, or if their child has fever and rash, or that they traveled internationally. So, eliciting that history from the patient or their parents is really critical.”
The CDC recommends that measles be considered in anyone presenting with a febrile illness and symptoms that are clinically compatible with measles (that is, rash, cough, coryza, or conjunctivitis), as well as in patients who have recently traveled abroad, especially to countries with ongoing outbreaks, including India, Somalia, and Yemen.
“In general, if they’ve traveled internationally and they are undervaccinated, measles should be part of the differential diagnosis,” Sugerman said. He also emphasized the need to follow airborne isolation precautions in addition to general infection control measures.
Immediate triage is critical, especially since overcrowded waiting rooms might be filled with patients who are not yet eligible for vaccination or are not up to date or fully vaccinated.
“Measles is under airborne isolation criteria and precautions, and therefore, [patients] need to be placed as soon as possible into a negative pressure or airborne infection isolation room – and that should be a single room,” he explained. He noted, “In some settings, there may not be a negative pressure room, e.g., an outpatient pediatrics or family medicine office.”
Dr. Sugerman said that in these circumstances, patients should be placed in a room with masked health care providers who have received two doses of measles, mumps, and rubella (MMR) vaccine and that they should wear an N95 mask when entering the room and interviewing the patient.
Clinicians should follow CDC’s testing recommendations and collect a nasopharyngeal or throat swab or a urine specimen for PCR testing and a blood specimen for serology. In addition, they should immediately report cases to local and state public health authorities.
For all patients, it’s critical to be up to date on MMR vaccines, especially persons who are going to be traveling internationally. “We recommend that when they’ve got infants traveling with them who are 6-11 months of age, that they get a first dose (which we consider a zero dose), because they need a routine dose at 12-15 months, and then 4-6 years,” said Dr. Sugerman. He said that it’s safe for adults who are unsure of their status to receive an MMR dose as well.
Dr. Sugerman stressed that despite major strides, “we just don’t have enough coverage in all individuals in this country. Because people are traveling as often as they are, it can be imported. Until measles is eliminated globally, there’s going to be an ongoing risk of importation and potential spread amongst others in their household or community, especially amongst individuals who are not fully vaccinated and, in particular, amongst those who are unvaccinated,” he said.
Dr. Sugerman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued a Health Alert Network (HAN) health advisory notifying clinicians and public health officials of a confirmed measles case in an individual who for 2 days (February 17-18) attended a large religious gathering that was attended by an estimated 20,000 people at Asbury University in Wilmore, Ky.
Given that large numbers of people might have been exposed to the attendee (who was not vaccinated) and that the individual had a history of recent international travel, the CDC has encouraged clinicians to be vigilant for patients presenting with symptoms that meet the measles case definition. A steady increase in measles cases from 49 in 2021 to 121 in 2022 in children who were not fully vaccinated – coupled with outbreaks in Ohio and Minnesota – underscores the potential gravity of the CDC advisory as well as the need to mitigate the risk of ongoing or secondary transmission.
Currently, little is known about the individual who contracted measles other than the fact that he is a resident of Jessamine County, Ky., according to a news release issued by the Kentucky Department of Public Health. It is the third confirmed case in Kentucky over the past 3 months. State and national health officials are concerned that the individual might have transmitted measles to attendees visiting from other states.
David Sugerman, MD, MPH, a medical officer in CDC’s division of viral diseases and lead for the measles, rubella, and cytomegalovirus team, noted that the timing of the alert coincides with the period in which persons who had had contact with the initial case patient might be expected to develop symptoms.
For clinicians, “It’s really about considering measles in any un- or undervaccinated patient that arrives at a clinic and recently traveled internationally,” Dr. Sugerman told this news organization. He explained that “when doctors are seeing patients, they’re not going to necessarily share that information off the bat when they present with fever or rash, or if their child has fever and rash, or that they traveled internationally. So, eliciting that history from the patient or their parents is really critical.”
The CDC recommends that measles be considered in anyone presenting with a febrile illness and symptoms that are clinically compatible with measles (that is, rash, cough, coryza, or conjunctivitis), as well as in patients who have recently traveled abroad, especially to countries with ongoing outbreaks, including India, Somalia, and Yemen.
“In general, if they’ve traveled internationally and they are undervaccinated, measles should be part of the differential diagnosis,” Sugerman said. He also emphasized the need to follow airborne isolation precautions in addition to general infection control measures.
Immediate triage is critical, especially since overcrowded waiting rooms might be filled with patients who are not yet eligible for vaccination or are not up to date or fully vaccinated.
“Measles is under airborne isolation criteria and precautions, and therefore, [patients] need to be placed as soon as possible into a negative pressure or airborne infection isolation room – and that should be a single room,” he explained. He noted, “In some settings, there may not be a negative pressure room, e.g., an outpatient pediatrics or family medicine office.”
Dr. Sugerman said that in these circumstances, patients should be placed in a room with masked health care providers who have received two doses of measles, mumps, and rubella (MMR) vaccine and that they should wear an N95 mask when entering the room and interviewing the patient.
Clinicians should follow CDC’s testing recommendations and collect a nasopharyngeal or throat swab or a urine specimen for PCR testing and a blood specimen for serology. In addition, they should immediately report cases to local and state public health authorities.
For all patients, it’s critical to be up to date on MMR vaccines, especially persons who are going to be traveling internationally. “We recommend that when they’ve got infants traveling with them who are 6-11 months of age, that they get a first dose (which we consider a zero dose), because they need a routine dose at 12-15 months, and then 4-6 years,” said Dr. Sugerman. He said that it’s safe for adults who are unsure of their status to receive an MMR dose as well.
Dr. Sugerman stressed that despite major strides, “we just don’t have enough coverage in all individuals in this country. Because people are traveling as often as they are, it can be imported. Until measles is eliminated globally, there’s going to be an ongoing risk of importation and potential spread amongst others in their household or community, especially amongst individuals who are not fully vaccinated and, in particular, amongst those who are unvaccinated,” he said.
Dr. Sugerman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued a Health Alert Network (HAN) health advisory notifying clinicians and public health officials of a confirmed measles case in an individual who for 2 days (February 17-18) attended a large religious gathering that was attended by an estimated 20,000 people at Asbury University in Wilmore, Ky.
Given that large numbers of people might have been exposed to the attendee (who was not vaccinated) and that the individual had a history of recent international travel, the CDC has encouraged clinicians to be vigilant for patients presenting with symptoms that meet the measles case definition. A steady increase in measles cases from 49 in 2021 to 121 in 2022 in children who were not fully vaccinated – coupled with outbreaks in Ohio and Minnesota – underscores the potential gravity of the CDC advisory as well as the need to mitigate the risk of ongoing or secondary transmission.
Currently, little is known about the individual who contracted measles other than the fact that he is a resident of Jessamine County, Ky., according to a news release issued by the Kentucky Department of Public Health. It is the third confirmed case in Kentucky over the past 3 months. State and national health officials are concerned that the individual might have transmitted measles to attendees visiting from other states.
David Sugerman, MD, MPH, a medical officer in CDC’s division of viral diseases and lead for the measles, rubella, and cytomegalovirus team, noted that the timing of the alert coincides with the period in which persons who had had contact with the initial case patient might be expected to develop symptoms.
For clinicians, “It’s really about considering measles in any un- or undervaccinated patient that arrives at a clinic and recently traveled internationally,” Dr. Sugerman told this news organization. He explained that “when doctors are seeing patients, they’re not going to necessarily share that information off the bat when they present with fever or rash, or if their child has fever and rash, or that they traveled internationally. So, eliciting that history from the patient or their parents is really critical.”
The CDC recommends that measles be considered in anyone presenting with a febrile illness and symptoms that are clinically compatible with measles (that is, rash, cough, coryza, or conjunctivitis), as well as in patients who have recently traveled abroad, especially to countries with ongoing outbreaks, including India, Somalia, and Yemen.
“In general, if they’ve traveled internationally and they are undervaccinated, measles should be part of the differential diagnosis,” Sugerman said. He also emphasized the need to follow airborne isolation precautions in addition to general infection control measures.
Immediate triage is critical, especially since overcrowded waiting rooms might be filled with patients who are not yet eligible for vaccination or are not up to date or fully vaccinated.
“Measles is under airborne isolation criteria and precautions, and therefore, [patients] need to be placed as soon as possible into a negative pressure or airborne infection isolation room – and that should be a single room,” he explained. He noted, “In some settings, there may not be a negative pressure room, e.g., an outpatient pediatrics or family medicine office.”
Dr. Sugerman said that in these circumstances, patients should be placed in a room with masked health care providers who have received two doses of measles, mumps, and rubella (MMR) vaccine and that they should wear an N95 mask when entering the room and interviewing the patient.
Clinicians should follow CDC’s testing recommendations and collect a nasopharyngeal or throat swab or a urine specimen for PCR testing and a blood specimen for serology. In addition, they should immediately report cases to local and state public health authorities.
For all patients, it’s critical to be up to date on MMR vaccines, especially persons who are going to be traveling internationally. “We recommend that when they’ve got infants traveling with them who are 6-11 months of age, that they get a first dose (which we consider a zero dose), because they need a routine dose at 12-15 months, and then 4-6 years,” said Dr. Sugerman. He said that it’s safe for adults who are unsure of their status to receive an MMR dose as well.
Dr. Sugerman stressed that despite major strides, “we just don’t have enough coverage in all individuals in this country. Because people are traveling as often as they are, it can be imported. Until measles is eliminated globally, there’s going to be an ongoing risk of importation and potential spread amongst others in their household or community, especially amongst individuals who are not fully vaccinated and, in particular, amongst those who are unvaccinated,” he said.
Dr. Sugerman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One in four parents lied about kids’ COVID status: Survey
More than 1 in 4 parents lied to school officials about their children’s COVID-19 status or refused to comply with public health rules during the height of the pandemic, a new study found. Researchers said they suspected the 26% of parents who misrepresented their children’s health status may have undercounted the actual figure.
“If anything, 26% is probably the minimum” of parents who misled school officials, said Angela Fagerlin, PhD, a researcher at the University of Utah Medical School, Salt Lake City.
In the survey, many parents said they considered it their right as parents to make their own decision about their children’s health status, said Dr. Fagerlin, who is also the chair of the department of population health sciences at the University of Utah School of Medicine.
“It appears that many parents were concerned about their children missing school,” she said. “At the same time, they’re potentially exposing other kids to a serious illness.”
In the survey, parents were asked whether they lied or misrepresented information about their children on seven different COVID-19 topics, including illness and vaccination status and if they followed quarantine protocols. Researchers tallied survey responses collected in December 2021 from 580 parents, whose average age was 36 and of whom 70% were women. Results were published in the journal JAMA Network Open.
Overall, 24% of parents said they lied to people that their children were with while knowing or suspecting the children had COVID. About half of parents cited at least one of the following reasons for doing so: parental freedom, child did not feel very sick, or wanted the child’s life to feel “normal.”
About 20% of parents said they avoided testing when they thought their child had COVID, and parents also reported allowing children to break quarantine rules at a similar rate. More than half of parents who avoided testing said they were worried testing would hurt or feel uncomfortable.
About 4 in 10 parents who lied about their child’s illness status or who lied about whether their child should be in quarantine said they did so because of guidance from a public figure such as a celebrity or politician. At least 3 in 10 said they lied because they could not miss work to stay home with their child.
“We need to do a better job of providing support mechanisms like paid sick leave for family illness so that parents don’t feel like their only option is to engage in misrepresentation or non-adherence to public health guidelines during a future infectious disease outbreak that matches or exceeds the magnitude of COVID-19,” says researcher Andrea Gurmankin Levy, PhD, of Middlesex (Conn.) Community College.
A version of this article first appeared on WebMD.com.
More than 1 in 4 parents lied to school officials about their children’s COVID-19 status or refused to comply with public health rules during the height of the pandemic, a new study found. Researchers said they suspected the 26% of parents who misrepresented their children’s health status may have undercounted the actual figure.
“If anything, 26% is probably the minimum” of parents who misled school officials, said Angela Fagerlin, PhD, a researcher at the University of Utah Medical School, Salt Lake City.
In the survey, many parents said they considered it their right as parents to make their own decision about their children’s health status, said Dr. Fagerlin, who is also the chair of the department of population health sciences at the University of Utah School of Medicine.
“It appears that many parents were concerned about their children missing school,” she said. “At the same time, they’re potentially exposing other kids to a serious illness.”
In the survey, parents were asked whether they lied or misrepresented information about their children on seven different COVID-19 topics, including illness and vaccination status and if they followed quarantine protocols. Researchers tallied survey responses collected in December 2021 from 580 parents, whose average age was 36 and of whom 70% were women. Results were published in the journal JAMA Network Open.
Overall, 24% of parents said they lied to people that their children were with while knowing or suspecting the children had COVID. About half of parents cited at least one of the following reasons for doing so: parental freedom, child did not feel very sick, or wanted the child’s life to feel “normal.”
About 20% of parents said they avoided testing when they thought their child had COVID, and parents also reported allowing children to break quarantine rules at a similar rate. More than half of parents who avoided testing said they were worried testing would hurt or feel uncomfortable.
About 4 in 10 parents who lied about their child’s illness status or who lied about whether their child should be in quarantine said they did so because of guidance from a public figure such as a celebrity or politician. At least 3 in 10 said they lied because they could not miss work to stay home with their child.
“We need to do a better job of providing support mechanisms like paid sick leave for family illness so that parents don’t feel like their only option is to engage in misrepresentation or non-adherence to public health guidelines during a future infectious disease outbreak that matches or exceeds the magnitude of COVID-19,” says researcher Andrea Gurmankin Levy, PhD, of Middlesex (Conn.) Community College.
A version of this article first appeared on WebMD.com.
More than 1 in 4 parents lied to school officials about their children’s COVID-19 status or refused to comply with public health rules during the height of the pandemic, a new study found. Researchers said they suspected the 26% of parents who misrepresented their children’s health status may have undercounted the actual figure.
“If anything, 26% is probably the minimum” of parents who misled school officials, said Angela Fagerlin, PhD, a researcher at the University of Utah Medical School, Salt Lake City.
In the survey, many parents said they considered it their right as parents to make their own decision about their children’s health status, said Dr. Fagerlin, who is also the chair of the department of population health sciences at the University of Utah School of Medicine.
“It appears that many parents were concerned about their children missing school,” she said. “At the same time, they’re potentially exposing other kids to a serious illness.”
In the survey, parents were asked whether they lied or misrepresented information about their children on seven different COVID-19 topics, including illness and vaccination status and if they followed quarantine protocols. Researchers tallied survey responses collected in December 2021 from 580 parents, whose average age was 36 and of whom 70% were women. Results were published in the journal JAMA Network Open.
Overall, 24% of parents said they lied to people that their children were with while knowing or suspecting the children had COVID. About half of parents cited at least one of the following reasons for doing so: parental freedom, child did not feel very sick, or wanted the child’s life to feel “normal.”
About 20% of parents said they avoided testing when they thought their child had COVID, and parents also reported allowing children to break quarantine rules at a similar rate. More than half of parents who avoided testing said they were worried testing would hurt or feel uncomfortable.
About 4 in 10 parents who lied about their child’s illness status or who lied about whether their child should be in quarantine said they did so because of guidance from a public figure such as a celebrity or politician. At least 3 in 10 said they lied because they could not miss work to stay home with their child.
“We need to do a better job of providing support mechanisms like paid sick leave for family illness so that parents don’t feel like their only option is to engage in misrepresentation or non-adherence to public health guidelines during a future infectious disease outbreak that matches or exceeds the magnitude of COVID-19,” says researcher Andrea Gurmankin Levy, PhD, of Middlesex (Conn.) Community College.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
FDA accepts application for topical molluscum treatment
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
Be vigilant about suspected cases of measles, expert advises
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR