User login
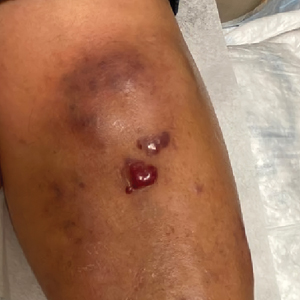
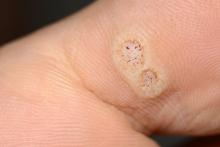
Catheterized urine color change
Physical examination revealed an older man whose vital signs were normal and who had a regular heart rate and rhythm. He denied any pain, and his abdomen was soft and nontender with normal bowel sounds. There was no suprapubic or costovertebral angle tenderness, and his urinary catheter was correctly placed. His urine output was within normal limits, but the urine in the catheter and collection bag was purple.
The patient’s medical history was remarkable for mild cognitive impairment, BPH, and hypertension. A urine culture was significant for > 100,000 CFU/mL pan-sensitive Pseudomonas aeruginosa.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Purple urine bag syndrome
The diagnosis of purple urine bag syndrome (PUBS) was made based on the patient’s clinical presentation and medical history. PUBS is generally a benign condition that can occur in patients who have urinary catheters for prolonged periods of time and urinary tract infections (UTIs), often with constipation.1
PUBS was first described in the literature in 1978.2 Its prevalence has been estimated to be 9.8% in long-term wards and higher in patients with chronic catheters.3-5 PUBS is reported more often in institutionalized older women, although it has been documented in men as well.1 Risk factors include having a chronic indwelling urinary catheter; alkaline urine; the use of plastic, polyvinylchloride urine bags3; chronic constipation6; renal failure4,5; and dementia.1 In many cases, patients with PUBS have been found to have stable vitals and lack systemic symptoms, such as fever, that could indicate an infection.1,5
The pathogenesis of PUBS has been associated with tryptophan.3 Gut bacteria metabolize tryptophan to indole, which is converted to indoxyl sulfate in the liver.3,7 Then certain bacteria associated with UTIs, including Pseudomonas, Escherichia coli, Proteus mirabilis, Providencia spp, Enterococcus faecalis, and Klebsiella,5-7 which contain indoxyl phosphatase and sulfatase enzymes, can convert indoxyl sulfate into indirubin (red) and indigo (blue) compounds; this results in a purple hue in the urine seen in a Foley catheter and bag.
Differential is generally limited to medication and food consumption
Clinical presentation and a detailed history and review of medication and/or food ingestion may distinguish PUBS from other conditions.
Medications and foods, such as rifampicin or beets, may discolor urine and need to be ruled out as a cause with a thorough history.3
Cyanide toxicity in those taking vitamin B12 can result in purple-tinged urine.8 Signs and symptoms can also include reddening of the skin, dyspnea, nausea, headache, erythema at the injection site, and a modest increase in blood pressure.8
Identify the infection and treat as needed
There have been some case reports regarding the progression of PUBS to Fournier gangrene,4 but such cases are rare and associated with immunocompromised patients.9 PUBS is generally a benign condition associated with UTIs. Management involves identifying the underlying infection, treating with antibiotics if indicated (ie, patient is symptomatic or immunocompromised),3 providing proper treatment of constipation if needed, and replacing the Foley catheter.4 Some studies suggest that simply exchanging the catheter may resolve PUBS, particularly in asymptomatic patients.5
In light of his complicated urologic history, our patient was treated with a 10-day course of renally dosed intravenous cefepime (500 mg every 24 hours based on calculated creatine clearance of 21 mL/min) and Foley exchange. The patient’s urine color returned to normal after Foley exchange and 24 hours of antibiotics. His kidney function continued to improve and normalized by the time he was discharged from the facility approximately 2 weeks later.
1. Goyal A, Vikas G, Jindal J. Purple urine bag syndrome: series of nine cases and review of literature. J Clin Diagn Res. 2018;12:PR01-PR03. doi: 10.7860/JCDR/2018/34951.12202
2. Barlow GB, Dickson JAS. Purple urine bags. Lancet. 1978;28:220-221. doi: 10.1016/S0140-6736(78)90667-0
3. Richardson-May J. Single case of purple urine bag syndrome in an elderly woman with stroke. BMJ Case Rep. 2016;2016:bcr2016215465. doi: 10.1136/bcr-2016-215465
4. Khan F, Chaudhry MA, Qureshi N, et al. Purple urine bag syndrome: an alarming hue? A brief review of the literature. Int J Nephrol. 2011;2011:419213. doi: 10.4061/2011/419213
5. Ben-Chetrit E, Munter G. Purple urine. JAMA. 2012;307:193-194. doi: 10.1001/jama.2011.1997
6. Al Montasir A, Al Mustaque A. Purple urine bag syndrome. J Family Med Prim Care. 2013;2:104-105. doi: 10.4103/2249-4863.109970
7. Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988;26:2152-2156. doi: 10.1128/jcm.26.10.2152-2156.1988
8. Hudson M, Cashin BV, Matlock AG, et al. A man with purple urine. Hydroxocobalamin-induced chromaturia. Clin Toxicol (Phila). 2012;50:77. doi: 10.3109/15563650.2011.626782
9. Tasi Y-M, Huang M-S, Yang C-J, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895-897. doi: 10.1016/j.ajem.2009.01.030
Physical examination revealed an older man whose vital signs were normal and who had a regular heart rate and rhythm. He denied any pain, and his abdomen was soft and nontender with normal bowel sounds. There was no suprapubic or costovertebral angle tenderness, and his urinary catheter was correctly placed. His urine output was within normal limits, but the urine in the catheter and collection bag was purple.
The patient’s medical history was remarkable for mild cognitive impairment, BPH, and hypertension. A urine culture was significant for > 100,000 CFU/mL pan-sensitive Pseudomonas aeruginosa.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Purple urine bag syndrome
The diagnosis of purple urine bag syndrome (PUBS) was made based on the patient’s clinical presentation and medical history. PUBS is generally a benign condition that can occur in patients who have urinary catheters for prolonged periods of time and urinary tract infections (UTIs), often with constipation.1
PUBS was first described in the literature in 1978.2 Its prevalence has been estimated to be 9.8% in long-term wards and higher in patients with chronic catheters.3-5 PUBS is reported more often in institutionalized older women, although it has been documented in men as well.1 Risk factors include having a chronic indwelling urinary catheter; alkaline urine; the use of plastic, polyvinylchloride urine bags3; chronic constipation6; renal failure4,5; and dementia.1 In many cases, patients with PUBS have been found to have stable vitals and lack systemic symptoms, such as fever, that could indicate an infection.1,5
The pathogenesis of PUBS has been associated with tryptophan.3 Gut bacteria metabolize tryptophan to indole, which is converted to indoxyl sulfate in the liver.3,7 Then certain bacteria associated with UTIs, including Pseudomonas, Escherichia coli, Proteus mirabilis, Providencia spp, Enterococcus faecalis, and Klebsiella,5-7 which contain indoxyl phosphatase and sulfatase enzymes, can convert indoxyl sulfate into indirubin (red) and indigo (blue) compounds; this results in a purple hue in the urine seen in a Foley catheter and bag.
Differential is generally limited to medication and food consumption
Clinical presentation and a detailed history and review of medication and/or food ingestion may distinguish PUBS from other conditions.
Medications and foods, such as rifampicin or beets, may discolor urine and need to be ruled out as a cause with a thorough history.3
Cyanide toxicity in those taking vitamin B12 can result in purple-tinged urine.8 Signs and symptoms can also include reddening of the skin, dyspnea, nausea, headache, erythema at the injection site, and a modest increase in blood pressure.8
Identify the infection and treat as needed
There have been some case reports regarding the progression of PUBS to Fournier gangrene,4 but such cases are rare and associated with immunocompromised patients.9 PUBS is generally a benign condition associated with UTIs. Management involves identifying the underlying infection, treating with antibiotics if indicated (ie, patient is symptomatic or immunocompromised),3 providing proper treatment of constipation if needed, and replacing the Foley catheter.4 Some studies suggest that simply exchanging the catheter may resolve PUBS, particularly in asymptomatic patients.5
In light of his complicated urologic history, our patient was treated with a 10-day course of renally dosed intravenous cefepime (500 mg every 24 hours based on calculated creatine clearance of 21 mL/min) and Foley exchange. The patient’s urine color returned to normal after Foley exchange and 24 hours of antibiotics. His kidney function continued to improve and normalized by the time he was discharged from the facility approximately 2 weeks later.
Physical examination revealed an older man whose vital signs were normal and who had a regular heart rate and rhythm. He denied any pain, and his abdomen was soft and nontender with normal bowel sounds. There was no suprapubic or costovertebral angle tenderness, and his urinary catheter was correctly placed. His urine output was within normal limits, but the urine in the catheter and collection bag was purple.
The patient’s medical history was remarkable for mild cognitive impairment, BPH, and hypertension. A urine culture was significant for > 100,000 CFU/mL pan-sensitive Pseudomonas aeruginosa.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Purple urine bag syndrome
The diagnosis of purple urine bag syndrome (PUBS) was made based on the patient’s clinical presentation and medical history. PUBS is generally a benign condition that can occur in patients who have urinary catheters for prolonged periods of time and urinary tract infections (UTIs), often with constipation.1
PUBS was first described in the literature in 1978.2 Its prevalence has been estimated to be 9.8% in long-term wards and higher in patients with chronic catheters.3-5 PUBS is reported more often in institutionalized older women, although it has been documented in men as well.1 Risk factors include having a chronic indwelling urinary catheter; alkaline urine; the use of plastic, polyvinylchloride urine bags3; chronic constipation6; renal failure4,5; and dementia.1 In many cases, patients with PUBS have been found to have stable vitals and lack systemic symptoms, such as fever, that could indicate an infection.1,5
The pathogenesis of PUBS has been associated with tryptophan.3 Gut bacteria metabolize tryptophan to indole, which is converted to indoxyl sulfate in the liver.3,7 Then certain bacteria associated with UTIs, including Pseudomonas, Escherichia coli, Proteus mirabilis, Providencia spp, Enterococcus faecalis, and Klebsiella,5-7 which contain indoxyl phosphatase and sulfatase enzymes, can convert indoxyl sulfate into indirubin (red) and indigo (blue) compounds; this results in a purple hue in the urine seen in a Foley catheter and bag.
Differential is generally limited to medication and food consumption
Clinical presentation and a detailed history and review of medication and/or food ingestion may distinguish PUBS from other conditions.
Medications and foods, such as rifampicin or beets, may discolor urine and need to be ruled out as a cause with a thorough history.3
Cyanide toxicity in those taking vitamin B12 can result in purple-tinged urine.8 Signs and symptoms can also include reddening of the skin, dyspnea, nausea, headache, erythema at the injection site, and a modest increase in blood pressure.8
Identify the infection and treat as needed
There have been some case reports regarding the progression of PUBS to Fournier gangrene,4 but such cases are rare and associated with immunocompromised patients.9 PUBS is generally a benign condition associated with UTIs. Management involves identifying the underlying infection, treating with antibiotics if indicated (ie, patient is symptomatic or immunocompromised),3 providing proper treatment of constipation if needed, and replacing the Foley catheter.4 Some studies suggest that simply exchanging the catheter may resolve PUBS, particularly in asymptomatic patients.5
In light of his complicated urologic history, our patient was treated with a 10-day course of renally dosed intravenous cefepime (500 mg every 24 hours based on calculated creatine clearance of 21 mL/min) and Foley exchange. The patient’s urine color returned to normal after Foley exchange and 24 hours of antibiotics. His kidney function continued to improve and normalized by the time he was discharged from the facility approximately 2 weeks later.
1. Goyal A, Vikas G, Jindal J. Purple urine bag syndrome: series of nine cases and review of literature. J Clin Diagn Res. 2018;12:PR01-PR03. doi: 10.7860/JCDR/2018/34951.12202
2. Barlow GB, Dickson JAS. Purple urine bags. Lancet. 1978;28:220-221. doi: 10.1016/S0140-6736(78)90667-0
3. Richardson-May J. Single case of purple urine bag syndrome in an elderly woman with stroke. BMJ Case Rep. 2016;2016:bcr2016215465. doi: 10.1136/bcr-2016-215465
4. Khan F, Chaudhry MA, Qureshi N, et al. Purple urine bag syndrome: an alarming hue? A brief review of the literature. Int J Nephrol. 2011;2011:419213. doi: 10.4061/2011/419213
5. Ben-Chetrit E, Munter G. Purple urine. JAMA. 2012;307:193-194. doi: 10.1001/jama.2011.1997
6. Al Montasir A, Al Mustaque A. Purple urine bag syndrome. J Family Med Prim Care. 2013;2:104-105. doi: 10.4103/2249-4863.109970
7. Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988;26:2152-2156. doi: 10.1128/jcm.26.10.2152-2156.1988
8. Hudson M, Cashin BV, Matlock AG, et al. A man with purple urine. Hydroxocobalamin-induced chromaturia. Clin Toxicol (Phila). 2012;50:77. doi: 10.3109/15563650.2011.626782
9. Tasi Y-M, Huang M-S, Yang C-J, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895-897. doi: 10.1016/j.ajem.2009.01.030
1. Goyal A, Vikas G, Jindal J. Purple urine bag syndrome: series of nine cases and review of literature. J Clin Diagn Res. 2018;12:PR01-PR03. doi: 10.7860/JCDR/2018/34951.12202
2. Barlow GB, Dickson JAS. Purple urine bags. Lancet. 1978;28:220-221. doi: 10.1016/S0140-6736(78)90667-0
3. Richardson-May J. Single case of purple urine bag syndrome in an elderly woman with stroke. BMJ Case Rep. 2016;2016:bcr2016215465. doi: 10.1136/bcr-2016-215465
4. Khan F, Chaudhry MA, Qureshi N, et al. Purple urine bag syndrome: an alarming hue? A brief review of the literature. Int J Nephrol. 2011;2011:419213. doi: 10.4061/2011/419213
5. Ben-Chetrit E, Munter G. Purple urine. JAMA. 2012;307:193-194. doi: 10.1001/jama.2011.1997
6. Al Montasir A, Al Mustaque A. Purple urine bag syndrome. J Family Med Prim Care. 2013;2:104-105. doi: 10.4103/2249-4863.109970
7. Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988;26:2152-2156. doi: 10.1128/jcm.26.10.2152-2156.1988
8. Hudson M, Cashin BV, Matlock AG, et al. A man with purple urine. Hydroxocobalamin-induced chromaturia. Clin Toxicol (Phila). 2012;50:77. doi: 10.3109/15563650.2011.626782
9. Tasi Y-M, Huang M-S, Yang C-J, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895-897. doi: 10.1016/j.ajem.2009.01.030
Shaved costs, high risk, maximum profits: Regulators worry about Florida’s butt lift boom
The office in Miami where she scheduled what’s known as a Brazilian butt lift had closed and transferred her records to a different facility, she said. The price she was quoted – and paid upfront – increased the day of the procedure, and she said she did not meet her surgeon until she was about to be placed under general anesthesia.
“I was ready to walk out,” said Ms. Ruston, 44, of Lake Alfred in Central Florida. “But I had paid everything.”
A few days after the July procedure, Ms. Ruston was hospitalized because of infection, blood loss, and nausea, her medical records show.
“I went cheap. That’s what I did,” Ms. Ruston recalled recently. “I looked for the lowest price, and I found him on Instagram.”

People like Ms. Ruston are commonly lured to office-based surgery centers in South Florida through social media marketing that makes Brazilian butt lifts and other cosmetic surgery look deceptively painless, safe, and affordable, say researchers, patient advocates, and surgeon groups.
Unlike ambulatory surgery centers and hospitals, where a patient might stay overnight for observation after treatment, office-based surgery centers offer procedures that don’t typically require an inpatient stay and are regulated as an extension of a doctor’s private practice.

But such surgical offices are often owned by corporations that can offer discount prices by contracting with surgeons who are incentivized to work on as many patients per day as possible, in as little time as possible, according to state regulators and physicians critical of the facilities.
After a rash of deaths, and in the absence of national standards, Florida regulators were the first in the nation to enact rules in 2019 meant to make the procedures safer. More than 3 years later, data shows deaths still occur.

Patient advocates and some surgeons – including those who perform the procedure themselves – anticipate the problem will only get worse. Emergency restrictions imposed by the state’s medical board in June expired in September, and the corporate business model popularized in Miami is spreading to other cities.
“We’re seeing entities that have a strong footprint in low-cost, high-volume cosmetic surgery, based in South Florida, manifesting in other parts of the country,” said Bob Basu, MD, MPH, a vice president of the American Society of Plastic Surgeons and a practicing physician in Houston.
During a Brazilian butt lift, fat is taken via liposuction from other areas of the body – such as the torso, back, or thighs – and injected into the buttocks. More than 61,000 buttock augmentation procedures, both butt lifts and implants, were performed nationwide in 2021, a 37% increase from the previous year, according to data from the Aesthetic Society, a trade group of plastic surgeons.
As with all surgery, complications can occur. Miami-Dade County’s medical examiner has documented nearly three dozen cosmetic surgery patient deaths since 2009, of which 26 resulted from a Brazilian butt lift. In each case, the person died from a pulmonary fat embolism, when fat entered the bloodstream through veins in the gluteal muscles and stopped blood from flowing to the lungs.
No national reporting system or insurance code tracks outcomes and patient demographics for a Brazilian butt lift. About 3% of surgeons worldwide had a patient die as a result of the procedure, according to a 2017 report from an Aesthetic Surgery Education and Research Foundation task force.
Medical experts said the problem is driven, in part, by having medical professionals like physician assistants and nurse practitioners perform key parts of the butt lift instead of doctors. It’s also driven by a business model that is motivated by profit, not safety, and incentivizes surgeons to exceed the number of surgeries outlined in their contracts.
In May, after a fifth patient in as many months died of complications in Miami-Dade County, Kevin Cairns, MD, proposed the state’s emergency rule to limit the number of butt lifts a surgeon could perform each day.
“I was getting sick of reading about women dying and seeing cases come before the board,” said Dr. Cairns, a physician and former member of the Florida Board of Medicine.
Some doctors performed as many as seven, according to disciplinary cases against surgeons prosecuted by the Florida Department of Health. The emergency rule limited them to no more than three, and required the use of an ultrasound to help surgeons lower the risk of a pulmonary fat clot.
But a group of physicians who perform Brazilian butt lifts in South Florida clapped back and formed Surgeons for Safety. They argued the new requirements would make the situation worse. Qualified doctors would have to do fewer procedures, they said, thus driving patients to dangerous medical professionals who don’t follow rules.
The group has since donated more than $350,000 to the state’s Republican Party, Republican candidates, and Republican political action committees, according to campaign contribution data from the Florida Department of State.
Surgeons for Safety declined KHN’s repeated interview requests. Although the group’s president, Constantino Mendieta, MD, wrote in an August editorial that he agreed not all surgeons have followed the standard of care, he called the limits put on surgeons “arbitrary.” The rule sets “a historic precedent of controlling surgeons,” he said during a meeting with Florida’s medical board.
In January, Florida state Sen. Ileana Garcia, a Republican, filed a draft bill with the state legislature that proposes no limit on the number of Brazilian butt lifts a surgeon can perform in a day. Instead, it requires office surgery centers where the procedures are performed to staff one physician per patient and prohibits surgeons from working on more than one person at a time.
The bill would also allow surgeons to delegate some parts of the procedure to other clinicians under their direct supervision.
Florida’s legislature convenes on March 7.
Consumers considering cosmetic procedures are urged to be cautious. Like Ms. Ruston, many people base their expectations on before-and-after photos and marketing videos posted on social media platforms such as Facebook, Snapchat, and Instagram.
“That’s very dangerous,” said Dr. Basu, of the American Society of Plastic Surgeons. “They’re excited about a low price and they forget about doing their homework,” he said.
The average price of a buttocks augmentation in 2021 was $4,000, according to data from the Aesthetic Society. But that’s only for the physician’s fee and does not cover anesthesia, operating room fees, prescriptions, or other expenses. A “safe” Brazilian butt lift, performed in an accredited facility and with proper aftercare, costs between $12,000 and $18,000, according to a recent article on the American Society of Plastic Surgeons’ website.
Although Florida requires a physician’s license to perform liposuction on patients who are under general anesthesia, it’s common in the medical field for midlevel medical practitioners, such as physician assistants and nurse practitioners, to do the procedure in office settings, according to Mark Mofid, MD, who coauthored the 2017 Aesthetic Surgery Education and Research Foundation task force study.
By relying on staffers who don’t have the same specialty training and get paid less, office-based surgeons can complete more butt lifts per day and charge a lower price.
“They’re doing all of them simultaneously in three or four different rooms, and it’s being staffed by one surgeon,” said Dr. Mofid, a plastic surgeon in San Diego, who added that he does not perform more than one Brazilian butt lift in a day. “The surgeon isn’t doing the actual case. It’s assistants.”
Dr. Basu said patients should ask whether their doctor holds privileges to perform the same procedure at a hospital or ambulatory surgery center, which have stricter rules than office surgery centers in terms of who can perform butt lifts and how they should be done.
People in search of bargains are reminded that cosmetic surgery can have other serious risks beyond the deadly fat clots, such as infection and organ puncture, plus problems with the kidneys, heart, and lungs.
Ms. Ruston’s surgery was performed by a board-certified plastic surgeon she said she found on Instagram. She was originally quoted $4,995, which she said she paid in full before surgery. But when she arrived in Miami, she said, the clinic tacked on fees for liposuction and for postsurgical garments and devices.
“I ended up having to pay, like, $8,000,” Ms. Ruston said. A few days after Ms. Ruston returned home to Lake Alfred, she said, she started to feel dizzy and weak and called 911.
Paramedics took her to an emergency room, where doctors diagnosed her with anemia due to blood loss, and blood and abdominal infections, her medical records show.
“If I could go back in time,” she said, “I wouldn’t have had it done.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The office in Miami where she scheduled what’s known as a Brazilian butt lift had closed and transferred her records to a different facility, she said. The price she was quoted – and paid upfront – increased the day of the procedure, and she said she did not meet her surgeon until she was about to be placed under general anesthesia.
“I was ready to walk out,” said Ms. Ruston, 44, of Lake Alfred in Central Florida. “But I had paid everything.”
A few days after the July procedure, Ms. Ruston was hospitalized because of infection, blood loss, and nausea, her medical records show.
“I went cheap. That’s what I did,” Ms. Ruston recalled recently. “I looked for the lowest price, and I found him on Instagram.”

People like Ms. Ruston are commonly lured to office-based surgery centers in South Florida through social media marketing that makes Brazilian butt lifts and other cosmetic surgery look deceptively painless, safe, and affordable, say researchers, patient advocates, and surgeon groups.
Unlike ambulatory surgery centers and hospitals, where a patient might stay overnight for observation after treatment, office-based surgery centers offer procedures that don’t typically require an inpatient stay and are regulated as an extension of a doctor’s private practice.

But such surgical offices are often owned by corporations that can offer discount prices by contracting with surgeons who are incentivized to work on as many patients per day as possible, in as little time as possible, according to state regulators and physicians critical of the facilities.
After a rash of deaths, and in the absence of national standards, Florida regulators were the first in the nation to enact rules in 2019 meant to make the procedures safer. More than 3 years later, data shows deaths still occur.

Patient advocates and some surgeons – including those who perform the procedure themselves – anticipate the problem will only get worse. Emergency restrictions imposed by the state’s medical board in June expired in September, and the corporate business model popularized in Miami is spreading to other cities.
“We’re seeing entities that have a strong footprint in low-cost, high-volume cosmetic surgery, based in South Florida, manifesting in other parts of the country,” said Bob Basu, MD, MPH, a vice president of the American Society of Plastic Surgeons and a practicing physician in Houston.
During a Brazilian butt lift, fat is taken via liposuction from other areas of the body – such as the torso, back, or thighs – and injected into the buttocks. More than 61,000 buttock augmentation procedures, both butt lifts and implants, were performed nationwide in 2021, a 37% increase from the previous year, according to data from the Aesthetic Society, a trade group of plastic surgeons.
As with all surgery, complications can occur. Miami-Dade County’s medical examiner has documented nearly three dozen cosmetic surgery patient deaths since 2009, of which 26 resulted from a Brazilian butt lift. In each case, the person died from a pulmonary fat embolism, when fat entered the bloodstream through veins in the gluteal muscles and stopped blood from flowing to the lungs.
No national reporting system or insurance code tracks outcomes and patient demographics for a Brazilian butt lift. About 3% of surgeons worldwide had a patient die as a result of the procedure, according to a 2017 report from an Aesthetic Surgery Education and Research Foundation task force.
Medical experts said the problem is driven, in part, by having medical professionals like physician assistants and nurse practitioners perform key parts of the butt lift instead of doctors. It’s also driven by a business model that is motivated by profit, not safety, and incentivizes surgeons to exceed the number of surgeries outlined in their contracts.
In May, after a fifth patient in as many months died of complications in Miami-Dade County, Kevin Cairns, MD, proposed the state’s emergency rule to limit the number of butt lifts a surgeon could perform each day.
“I was getting sick of reading about women dying and seeing cases come before the board,” said Dr. Cairns, a physician and former member of the Florida Board of Medicine.
Some doctors performed as many as seven, according to disciplinary cases against surgeons prosecuted by the Florida Department of Health. The emergency rule limited them to no more than three, and required the use of an ultrasound to help surgeons lower the risk of a pulmonary fat clot.
But a group of physicians who perform Brazilian butt lifts in South Florida clapped back and formed Surgeons for Safety. They argued the new requirements would make the situation worse. Qualified doctors would have to do fewer procedures, they said, thus driving patients to dangerous medical professionals who don’t follow rules.
The group has since donated more than $350,000 to the state’s Republican Party, Republican candidates, and Republican political action committees, according to campaign contribution data from the Florida Department of State.
Surgeons for Safety declined KHN’s repeated interview requests. Although the group’s president, Constantino Mendieta, MD, wrote in an August editorial that he agreed not all surgeons have followed the standard of care, he called the limits put on surgeons “arbitrary.” The rule sets “a historic precedent of controlling surgeons,” he said during a meeting with Florida’s medical board.
In January, Florida state Sen. Ileana Garcia, a Republican, filed a draft bill with the state legislature that proposes no limit on the number of Brazilian butt lifts a surgeon can perform in a day. Instead, it requires office surgery centers where the procedures are performed to staff one physician per patient and prohibits surgeons from working on more than one person at a time.
The bill would also allow surgeons to delegate some parts of the procedure to other clinicians under their direct supervision.
Florida’s legislature convenes on March 7.
Consumers considering cosmetic procedures are urged to be cautious. Like Ms. Ruston, many people base their expectations on before-and-after photos and marketing videos posted on social media platforms such as Facebook, Snapchat, and Instagram.
“That’s very dangerous,” said Dr. Basu, of the American Society of Plastic Surgeons. “They’re excited about a low price and they forget about doing their homework,” he said.
The average price of a buttocks augmentation in 2021 was $4,000, according to data from the Aesthetic Society. But that’s only for the physician’s fee and does not cover anesthesia, operating room fees, prescriptions, or other expenses. A “safe” Brazilian butt lift, performed in an accredited facility and with proper aftercare, costs between $12,000 and $18,000, according to a recent article on the American Society of Plastic Surgeons’ website.
Although Florida requires a physician’s license to perform liposuction on patients who are under general anesthesia, it’s common in the medical field for midlevel medical practitioners, such as physician assistants and nurse practitioners, to do the procedure in office settings, according to Mark Mofid, MD, who coauthored the 2017 Aesthetic Surgery Education and Research Foundation task force study.
By relying on staffers who don’t have the same specialty training and get paid less, office-based surgeons can complete more butt lifts per day and charge a lower price.
“They’re doing all of them simultaneously in three or four different rooms, and it’s being staffed by one surgeon,” said Dr. Mofid, a plastic surgeon in San Diego, who added that he does not perform more than one Brazilian butt lift in a day. “The surgeon isn’t doing the actual case. It’s assistants.”
Dr. Basu said patients should ask whether their doctor holds privileges to perform the same procedure at a hospital or ambulatory surgery center, which have stricter rules than office surgery centers in terms of who can perform butt lifts and how they should be done.
People in search of bargains are reminded that cosmetic surgery can have other serious risks beyond the deadly fat clots, such as infection and organ puncture, plus problems with the kidneys, heart, and lungs.
Ms. Ruston’s surgery was performed by a board-certified plastic surgeon she said she found on Instagram. She was originally quoted $4,995, which she said she paid in full before surgery. But when she arrived in Miami, she said, the clinic tacked on fees for liposuction and for postsurgical garments and devices.
“I ended up having to pay, like, $8,000,” Ms. Ruston said. A few days after Ms. Ruston returned home to Lake Alfred, she said, she started to feel dizzy and weak and called 911.
Paramedics took her to an emergency room, where doctors diagnosed her with anemia due to blood loss, and blood and abdominal infections, her medical records show.
“If I could go back in time,” she said, “I wouldn’t have had it done.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The office in Miami where she scheduled what’s known as a Brazilian butt lift had closed and transferred her records to a different facility, she said. The price she was quoted – and paid upfront – increased the day of the procedure, and she said she did not meet her surgeon until she was about to be placed under general anesthesia.
“I was ready to walk out,” said Ms. Ruston, 44, of Lake Alfred in Central Florida. “But I had paid everything.”
A few days after the July procedure, Ms. Ruston was hospitalized because of infection, blood loss, and nausea, her medical records show.
“I went cheap. That’s what I did,” Ms. Ruston recalled recently. “I looked for the lowest price, and I found him on Instagram.”

People like Ms. Ruston are commonly lured to office-based surgery centers in South Florida through social media marketing that makes Brazilian butt lifts and other cosmetic surgery look deceptively painless, safe, and affordable, say researchers, patient advocates, and surgeon groups.
Unlike ambulatory surgery centers and hospitals, where a patient might stay overnight for observation after treatment, office-based surgery centers offer procedures that don’t typically require an inpatient stay and are regulated as an extension of a doctor’s private practice.

But such surgical offices are often owned by corporations that can offer discount prices by contracting with surgeons who are incentivized to work on as many patients per day as possible, in as little time as possible, according to state regulators and physicians critical of the facilities.
After a rash of deaths, and in the absence of national standards, Florida regulators were the first in the nation to enact rules in 2019 meant to make the procedures safer. More than 3 years later, data shows deaths still occur.

Patient advocates and some surgeons – including those who perform the procedure themselves – anticipate the problem will only get worse. Emergency restrictions imposed by the state’s medical board in June expired in September, and the corporate business model popularized in Miami is spreading to other cities.
“We’re seeing entities that have a strong footprint in low-cost, high-volume cosmetic surgery, based in South Florida, manifesting in other parts of the country,” said Bob Basu, MD, MPH, a vice president of the American Society of Plastic Surgeons and a practicing physician in Houston.
During a Brazilian butt lift, fat is taken via liposuction from other areas of the body – such as the torso, back, or thighs – and injected into the buttocks. More than 61,000 buttock augmentation procedures, both butt lifts and implants, were performed nationwide in 2021, a 37% increase from the previous year, according to data from the Aesthetic Society, a trade group of plastic surgeons.
As with all surgery, complications can occur. Miami-Dade County’s medical examiner has documented nearly three dozen cosmetic surgery patient deaths since 2009, of which 26 resulted from a Brazilian butt lift. In each case, the person died from a pulmonary fat embolism, when fat entered the bloodstream through veins in the gluteal muscles and stopped blood from flowing to the lungs.
No national reporting system or insurance code tracks outcomes and patient demographics for a Brazilian butt lift. About 3% of surgeons worldwide had a patient die as a result of the procedure, according to a 2017 report from an Aesthetic Surgery Education and Research Foundation task force.
Medical experts said the problem is driven, in part, by having medical professionals like physician assistants and nurse practitioners perform key parts of the butt lift instead of doctors. It’s also driven by a business model that is motivated by profit, not safety, and incentivizes surgeons to exceed the number of surgeries outlined in their contracts.
In May, after a fifth patient in as many months died of complications in Miami-Dade County, Kevin Cairns, MD, proposed the state’s emergency rule to limit the number of butt lifts a surgeon could perform each day.
“I was getting sick of reading about women dying and seeing cases come before the board,” said Dr. Cairns, a physician and former member of the Florida Board of Medicine.
Some doctors performed as many as seven, according to disciplinary cases against surgeons prosecuted by the Florida Department of Health. The emergency rule limited them to no more than three, and required the use of an ultrasound to help surgeons lower the risk of a pulmonary fat clot.
But a group of physicians who perform Brazilian butt lifts in South Florida clapped back and formed Surgeons for Safety. They argued the new requirements would make the situation worse. Qualified doctors would have to do fewer procedures, they said, thus driving patients to dangerous medical professionals who don’t follow rules.
The group has since donated more than $350,000 to the state’s Republican Party, Republican candidates, and Republican political action committees, according to campaign contribution data from the Florida Department of State.
Surgeons for Safety declined KHN’s repeated interview requests. Although the group’s president, Constantino Mendieta, MD, wrote in an August editorial that he agreed not all surgeons have followed the standard of care, he called the limits put on surgeons “arbitrary.” The rule sets “a historic precedent of controlling surgeons,” he said during a meeting with Florida’s medical board.
In January, Florida state Sen. Ileana Garcia, a Republican, filed a draft bill with the state legislature that proposes no limit on the number of Brazilian butt lifts a surgeon can perform in a day. Instead, it requires office surgery centers where the procedures are performed to staff one physician per patient and prohibits surgeons from working on more than one person at a time.
The bill would also allow surgeons to delegate some parts of the procedure to other clinicians under their direct supervision.
Florida’s legislature convenes on March 7.
Consumers considering cosmetic procedures are urged to be cautious. Like Ms. Ruston, many people base their expectations on before-and-after photos and marketing videos posted on social media platforms such as Facebook, Snapchat, and Instagram.
“That’s very dangerous,” said Dr. Basu, of the American Society of Plastic Surgeons. “They’re excited about a low price and they forget about doing their homework,” he said.
The average price of a buttocks augmentation in 2021 was $4,000, according to data from the Aesthetic Society. But that’s only for the physician’s fee and does not cover anesthesia, operating room fees, prescriptions, or other expenses. A “safe” Brazilian butt lift, performed in an accredited facility and with proper aftercare, costs between $12,000 and $18,000, according to a recent article on the American Society of Plastic Surgeons’ website.
Although Florida requires a physician’s license to perform liposuction on patients who are under general anesthesia, it’s common in the medical field for midlevel medical practitioners, such as physician assistants and nurse practitioners, to do the procedure in office settings, according to Mark Mofid, MD, who coauthored the 2017 Aesthetic Surgery Education and Research Foundation task force study.
By relying on staffers who don’t have the same specialty training and get paid less, office-based surgeons can complete more butt lifts per day and charge a lower price.
“They’re doing all of them simultaneously in three or four different rooms, and it’s being staffed by one surgeon,” said Dr. Mofid, a plastic surgeon in San Diego, who added that he does not perform more than one Brazilian butt lift in a day. “The surgeon isn’t doing the actual case. It’s assistants.”
Dr. Basu said patients should ask whether their doctor holds privileges to perform the same procedure at a hospital or ambulatory surgery center, which have stricter rules than office surgery centers in terms of who can perform butt lifts and how they should be done.
People in search of bargains are reminded that cosmetic surgery can have other serious risks beyond the deadly fat clots, such as infection and organ puncture, plus problems with the kidneys, heart, and lungs.
Ms. Ruston’s surgery was performed by a board-certified plastic surgeon she said she found on Instagram. She was originally quoted $4,995, which she said she paid in full before surgery. But when she arrived in Miami, she said, the clinic tacked on fees for liposuction and for postsurgical garments and devices.
“I ended up having to pay, like, $8,000,” Ms. Ruston said. A few days after Ms. Ruston returned home to Lake Alfred, she said, she started to feel dizzy and weak and called 911.
Paramedics took her to an emergency room, where doctors diagnosed her with anemia due to blood loss, and blood and abdominal infections, her medical records show.
“If I could go back in time,” she said, “I wouldn’t have had it done.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
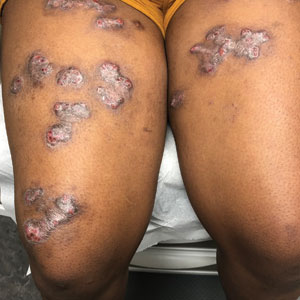
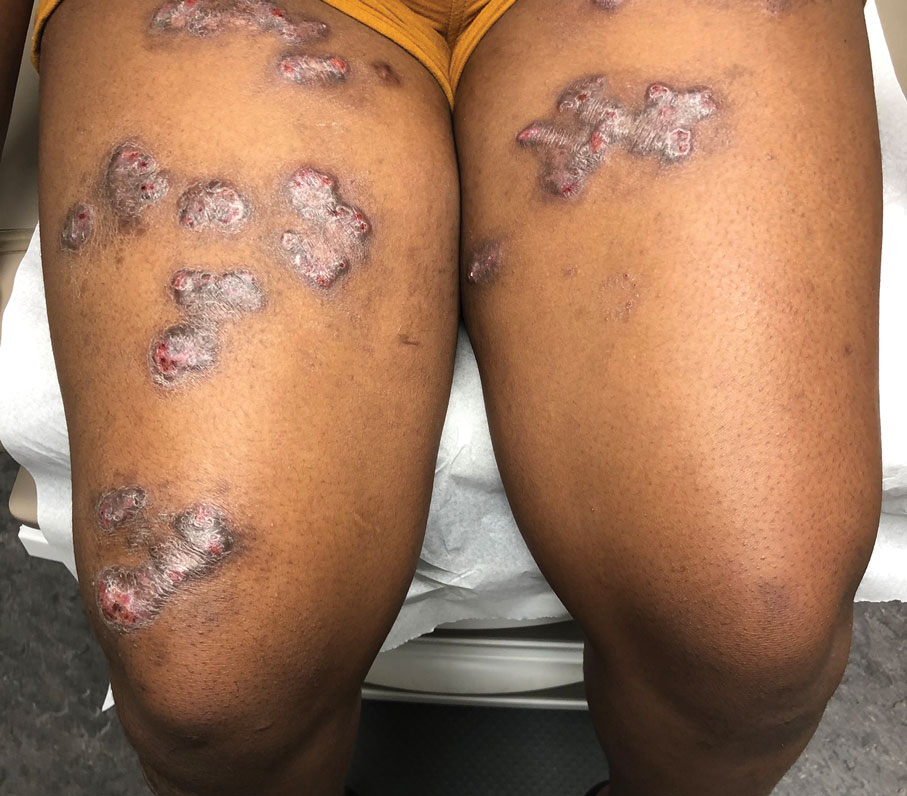
Spreading Painful Lesions on the Legs
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
A 14-year-old adolescent girl presented with spreading painful lesions on the legs and left forearm of 2 years’ duration. Her travel history included several countries in South and Central America, traversing the Colombian jungle on foot. Near the end of the jungle trip, she noted a skin lesion on the left forearm around the site of an insect bite. Within 1 month, the lesions spread to the legs. She was treated with topical corticosteroids without improvement. Physical examination revealed verrucous, reddish-brown plaques on the legs and left forearm. Intranasal examination revealed a red rounded lesion inside the left nostril.
NUDGE-FLU: Electronic ‘nudges’ boost flu shot uptake in seniors
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Multimodal Treatment of Epidermodysplasia Verruciformis in an HIV-Positive Man
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.
A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.
Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.
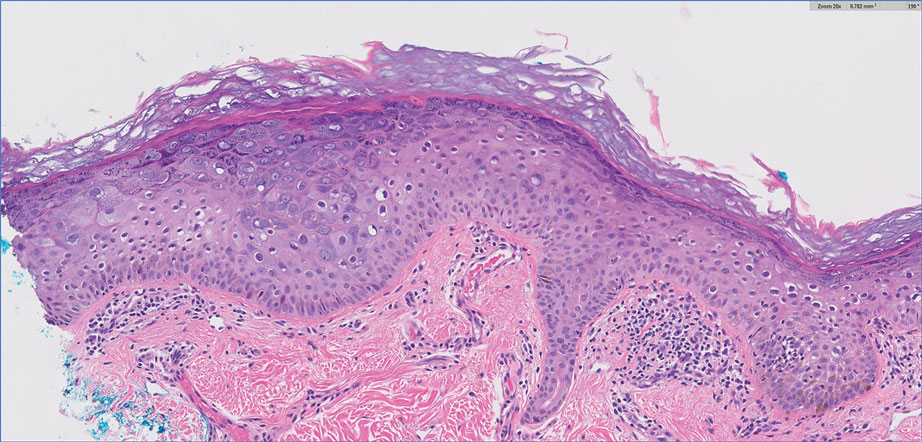
Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is associated with immunocompromised patients with conditions such as HIV.
- Multimodal treatment of HIV-associated acquired EDV with acitretin, intralesional Candida albicans antigen, and cryotherapy may be efficacious for patients with recalcitrant disease.
Drug-resistant stomach bug infections on the rise: CDC
The CDC issued the warning Feb. 24 about the rise in the problematic infections. Most of them have been seen in men who have sex with men, but a small number have also occurred in women and in young children.
The bacteria can be spread in a variety of ways, including changing the diaper of an infected baby, touching your mouth when the bacteria are on your hands, eating or drinking contaminated food or water, or through sexual contact. It’s easily transmitted because just a tiny amount of the bacteria is enough to make someone sick.
Shigella infection causes diarrhea that can be bloody. Other symptoms are a fever, belly cramping, and the feeling that you have to poop but your bowels are already empty. Most people recover on their own with rest and fluids, and severe cases can need antibiotic treatment. But strains of the bacteria that are resistant to treatment are on the rise.
Between 2015 and 2022, cases of antibiotic-resistant Shigella infection rose from 0% to 5% of all Shigella cases in the United States. One analysis showed that 82% of cases were in men, 13% in women, and 5% in children. A small sample of affected people provided information about their sexual activity, and 88% of them reported male-to-male sexual contact.
People at increased risk of infections are young children, people who are homeless, international travelers, people who have weakened immune systems, people living with HIV, and men who have sex with men.
The CDC asked health care workers to be on the lookout for these infections and report them.
A version of this article first appeared on WebMD.com.
The CDC issued the warning Feb. 24 about the rise in the problematic infections. Most of them have been seen in men who have sex with men, but a small number have also occurred in women and in young children.
The bacteria can be spread in a variety of ways, including changing the diaper of an infected baby, touching your mouth when the bacteria are on your hands, eating or drinking contaminated food or water, or through sexual contact. It’s easily transmitted because just a tiny amount of the bacteria is enough to make someone sick.
Shigella infection causes diarrhea that can be bloody. Other symptoms are a fever, belly cramping, and the feeling that you have to poop but your bowels are already empty. Most people recover on their own with rest and fluids, and severe cases can need antibiotic treatment. But strains of the bacteria that are resistant to treatment are on the rise.
Between 2015 and 2022, cases of antibiotic-resistant Shigella infection rose from 0% to 5% of all Shigella cases in the United States. One analysis showed that 82% of cases were in men, 13% in women, and 5% in children. A small sample of affected people provided information about their sexual activity, and 88% of them reported male-to-male sexual contact.
People at increased risk of infections are young children, people who are homeless, international travelers, people who have weakened immune systems, people living with HIV, and men who have sex with men.
The CDC asked health care workers to be on the lookout for these infections and report them.
A version of this article first appeared on WebMD.com.
The CDC issued the warning Feb. 24 about the rise in the problematic infections. Most of them have been seen in men who have sex with men, but a small number have also occurred in women and in young children.
The bacteria can be spread in a variety of ways, including changing the diaper of an infected baby, touching your mouth when the bacteria are on your hands, eating or drinking contaminated food or water, or through sexual contact. It’s easily transmitted because just a tiny amount of the bacteria is enough to make someone sick.
Shigella infection causes diarrhea that can be bloody. Other symptoms are a fever, belly cramping, and the feeling that you have to poop but your bowels are already empty. Most people recover on their own with rest and fluids, and severe cases can need antibiotic treatment. But strains of the bacteria that are resistant to treatment are on the rise.
Between 2015 and 2022, cases of antibiotic-resistant Shigella infection rose from 0% to 5% of all Shigella cases in the United States. One analysis showed that 82% of cases were in men, 13% in women, and 5% in children. A small sample of affected people provided information about their sexual activity, and 88% of them reported male-to-male sexual contact.
People at increased risk of infections are young children, people who are homeless, international travelers, people who have weakened immune systems, people living with HIV, and men who have sex with men.
The CDC asked health care workers to be on the lookout for these infections and report them.
A version of this article first appeared on WebMD.com.
Violaceous Nodules on the Leg in a Patient with HIV
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).
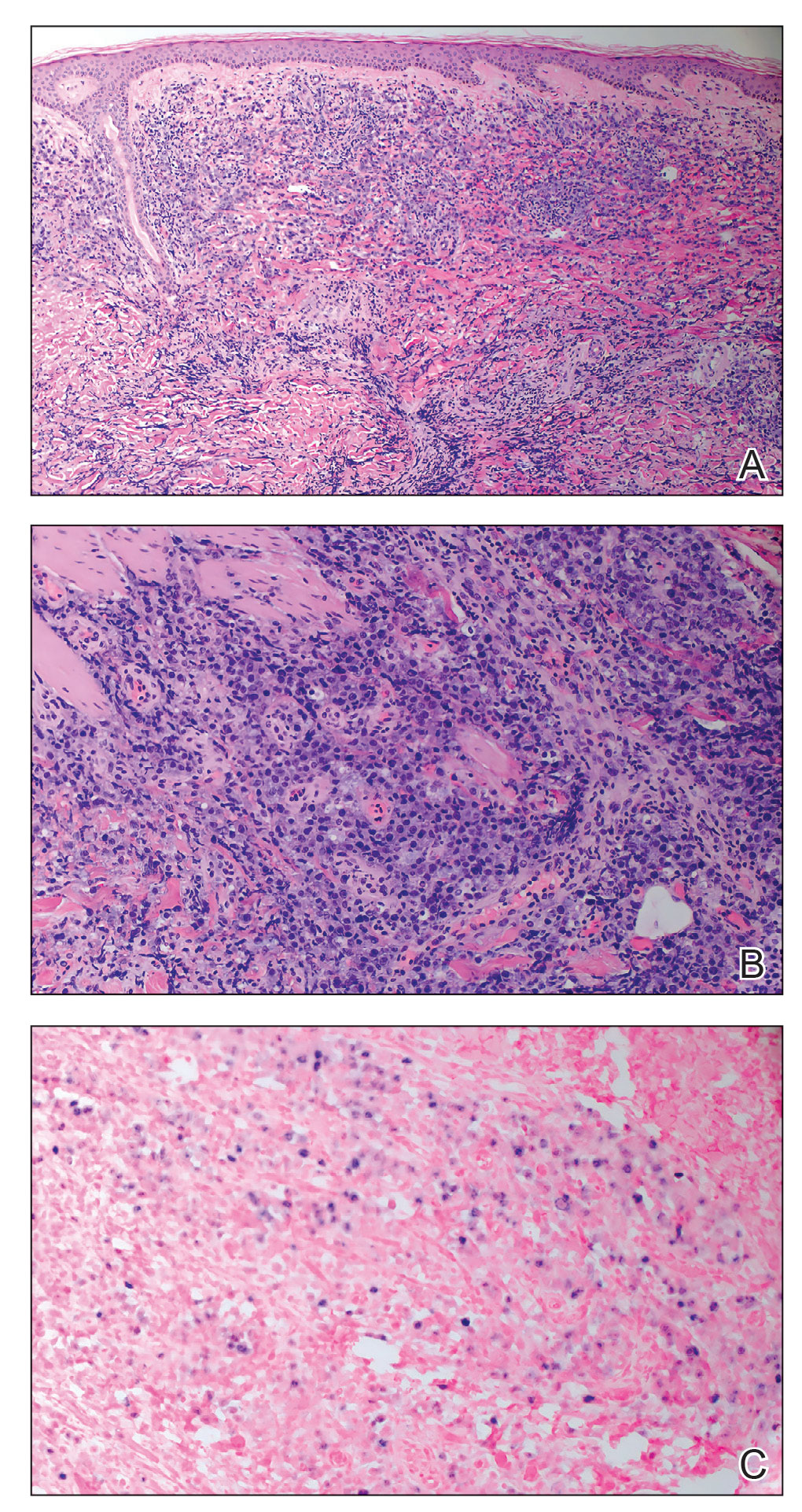
A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
A 67-year-old man with long-standing hepatitis B virus and HIV managed with chronic antiretroviral therapy presented to an urgent care facility with worsening erythema and edema of the legs of 2 weeks’ duration. He was prescribed a 7-day course of cephalexin for presumed cellulitis. Two months later, he developed nodules on the lower extremities. He was seen by podiatry and prescribed a course of amoxicillin–clavulanic acid for presumed infection. Despite 2 courses of antibiotics, his symptoms progressed. The nodules expanded in number and some developed ulceration. Three months into his clinical course, he presented to our dermatology clinic. Physical examination revealed two 2- to 3-cm, violaceous, exophytic, tender nodules. He reported tactile allodynia of the lower extremities and denied fever, chills, night sweats, or weight loss. He also denied exposure to infectious or chemical agents and reported no recent travel. The patient was chronically taking lisinopril/hydrochlorothiazide, escitalopram, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, bupropion, and aspirin with no recent changes. A complete hematologic and biochemical survey largely was unremarkable. His HIV viral load was undetectable with a CD4 count greater than 400/mm3 (reference range, 490–1436/mm3). Lactate dehydrogenase was elevated at 568 IU/L (reference range, 135–225 IU/L). The lower leg lesions were biopsied for confirmatory diagnosis.
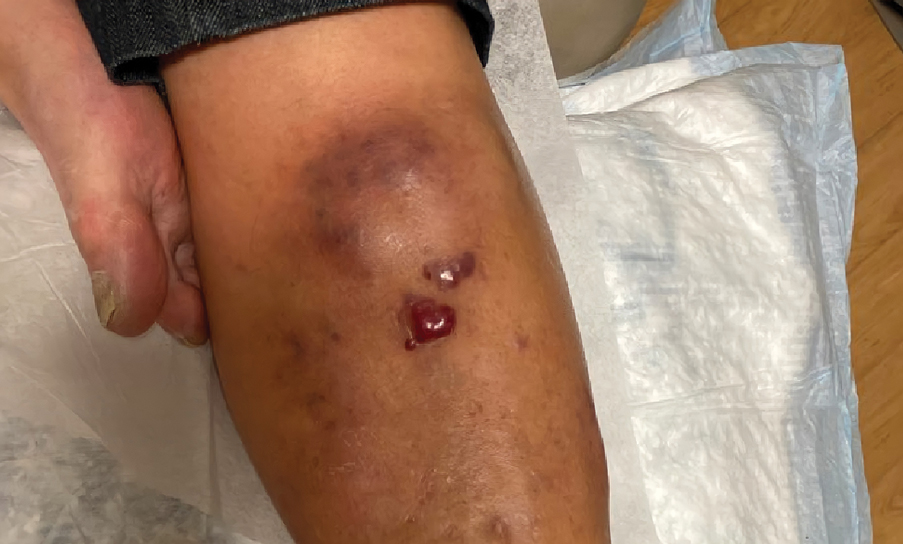
Topical or intralesional cidofovir an option for recalcitrant warts
HONOLULU – Combining or those located in areas that are challenging to treat, according to John S. Barbieri, MD, MBA.
“There are 5 million office visits per year in the United States for warts and molluscum, and they’re most common in pediatrics,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “In fact, some studies have suggested that one in three children in primary school suffers from warts.”
According to a 2012 Cochrane review of topical therapies for warts, first-line treatments such as salicylic acid, cryotherapy, 5-FU, or Candida antigen injection often have modest efficacy when used alone. For example, the authors found that salicylic acid and cryotherapy cleared warts in about 60%-70% of cases, respectively, but clearance rates were improved by combining the two therapies.
In an earlier literature review and meta-analysis, investigators evaluated the effect of 5-FU plus salicylic acid or salicylic acid alone. The therapeutic effect for common warts across all studies was a 63.4% response rate (complete healing) for 5-FU/SA vversus 23.1% for the 5-FU–free controls, respectively. For plantar warts, the response rate was 63% versus 11%, respectively.
“But what about the person with multiple warts or those in challenging locations where you might worry about destructive treatments hurting the nail fold or causing nail dystrophy?” Dr. Barbieri asked. “Maybe they’ve used salicylic acid or intralesional Candida and they’re still not getting better. What can we do for these patients?”
Emerging research suggests that topical cidofovir can be a valuable option for recalcitrant warts or those in sensitive locations. In a case report of a 10-year-old boy with more than 50 severe verrucous papules on his hands and face that were recalcitrant to multiple conventional therapies, topical 1% cidofovir applied daily for 8 weeks was effective, with no adverse side effects. A young female patient who presented to Dr. Barbieri with multiple warts around the nail matrix of several fingers experienced complete clearance after treatment with topical cidofovir, he said. Other researchers found this approach to be effective for plantar warts as well, in a report of two brothers with severe combined immunodeficiency after hematopoietic stem cell transplantation with persistent warts that did not respond to traditional topical treatments.
“Topical cidofovir is typically a painless treatment, which is nice, especially for our pediatric patients who might be afraid of other therapies like or cryotherapy or intralesional injections,” One limitation is that it is “a bit expensive,” Dr. Barbieri said. “To have topical cidofovir compounded is typically $100-$300, depending on the quantity and strength that you ask for.”
Intralesional cidofovir is another treatment option. In a retrospective study of 58 patients, Dr. Barbieri and colleagues evaluated the outcome of intralesional cidofovir treatment of warts in immunocompromised and nonimmunocompromised patients. Rates of improvement ranged from 98.3% to 100%, while resolution rates ranged from 75.9% to 97.6%.
“Most of the patients had warts for more than 5 years and almost half of them had recalcitrant warts,” Dr. Barbieri said. “These were mostly adult patients, but I think this is a treatment that can work in younger populations as well. About 10%-15% had HIV or cancer or diabetes or were transplant recipients, but despite these challenges and despite these recalcitrant warts, about 100% had improvement.”
He pointed out that cidofovir is available as a 75 mg/mL vial that comes with a 5 mL single-use vial. He dilutes this with normal saline to create a 15 mg/mL solution.
“If you want to be efficient you can try to schedule multiple patients together on the same day as a single vial is sufficient to treat about 25 patients,” assuming about 1 mL is injected per patient, he said. “The challenge with intralesional cidofovir is that it’s painful beyond just the needle part of the injection. Sometimes a nerve block can be helpful. But this can be an effective treatment for patients with recalcitrant warts or those with comorbidities.”
Other intralesional therapies to try for recalcitrant warts, he said, include bleomycin (1 U/mL solution, 1-2 mL per treatment, spaced every 2-4 weeks), and 5-FU (a 4:1 mixture of 5-FU [50 mg/mL] and 2% lidocaine).
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Medscape and this news organization are owned by the same parent company.
HONOLULU – Combining or those located in areas that are challenging to treat, according to John S. Barbieri, MD, MBA.
“There are 5 million office visits per year in the United States for warts and molluscum, and they’re most common in pediatrics,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “In fact, some studies have suggested that one in three children in primary school suffers from warts.”
According to a 2012 Cochrane review of topical therapies for warts, first-line treatments such as salicylic acid, cryotherapy, 5-FU, or Candida antigen injection often have modest efficacy when used alone. For example, the authors found that salicylic acid and cryotherapy cleared warts in about 60%-70% of cases, respectively, but clearance rates were improved by combining the two therapies.
In an earlier literature review and meta-analysis, investigators evaluated the effect of 5-FU plus salicylic acid or salicylic acid alone. The therapeutic effect for common warts across all studies was a 63.4% response rate (complete healing) for 5-FU/SA vversus 23.1% for the 5-FU–free controls, respectively. For plantar warts, the response rate was 63% versus 11%, respectively.
“But what about the person with multiple warts or those in challenging locations where you might worry about destructive treatments hurting the nail fold or causing nail dystrophy?” Dr. Barbieri asked. “Maybe they’ve used salicylic acid or intralesional Candida and they’re still not getting better. What can we do for these patients?”
Emerging research suggests that topical cidofovir can be a valuable option for recalcitrant warts or those in sensitive locations. In a case report of a 10-year-old boy with more than 50 severe verrucous papules on his hands and face that were recalcitrant to multiple conventional therapies, topical 1% cidofovir applied daily for 8 weeks was effective, with no adverse side effects. A young female patient who presented to Dr. Barbieri with multiple warts around the nail matrix of several fingers experienced complete clearance after treatment with topical cidofovir, he said. Other researchers found this approach to be effective for plantar warts as well, in a report of two brothers with severe combined immunodeficiency after hematopoietic stem cell transplantation with persistent warts that did not respond to traditional topical treatments.
“Topical cidofovir is typically a painless treatment, which is nice, especially for our pediatric patients who might be afraid of other therapies like or cryotherapy or intralesional injections,” One limitation is that it is “a bit expensive,” Dr. Barbieri said. “To have topical cidofovir compounded is typically $100-$300, depending on the quantity and strength that you ask for.”
Intralesional cidofovir is another treatment option. In a retrospective study of 58 patients, Dr. Barbieri and colleagues evaluated the outcome of intralesional cidofovir treatment of warts in immunocompromised and nonimmunocompromised patients. Rates of improvement ranged from 98.3% to 100%, while resolution rates ranged from 75.9% to 97.6%.
“Most of the patients had warts for more than 5 years and almost half of them had recalcitrant warts,” Dr. Barbieri said. “These were mostly adult patients, but I think this is a treatment that can work in younger populations as well. About 10%-15% had HIV or cancer or diabetes or were transplant recipients, but despite these challenges and despite these recalcitrant warts, about 100% had improvement.”
He pointed out that cidofovir is available as a 75 mg/mL vial that comes with a 5 mL single-use vial. He dilutes this with normal saline to create a 15 mg/mL solution.
“If you want to be efficient you can try to schedule multiple patients together on the same day as a single vial is sufficient to treat about 25 patients,” assuming about 1 mL is injected per patient, he said. “The challenge with intralesional cidofovir is that it’s painful beyond just the needle part of the injection. Sometimes a nerve block can be helpful. But this can be an effective treatment for patients with recalcitrant warts or those with comorbidities.”
Other intralesional therapies to try for recalcitrant warts, he said, include bleomycin (1 U/mL solution, 1-2 mL per treatment, spaced every 2-4 weeks), and 5-FU (a 4:1 mixture of 5-FU [50 mg/mL] and 2% lidocaine).
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Medscape and this news organization are owned by the same parent company.
HONOLULU – Combining or those located in areas that are challenging to treat, according to John S. Barbieri, MD, MBA.
“There are 5 million office visits per year in the United States for warts and molluscum, and they’re most common in pediatrics,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “In fact, some studies have suggested that one in three children in primary school suffers from warts.”
According to a 2012 Cochrane review of topical therapies for warts, first-line treatments such as salicylic acid, cryotherapy, 5-FU, or Candida antigen injection often have modest efficacy when used alone. For example, the authors found that salicylic acid and cryotherapy cleared warts in about 60%-70% of cases, respectively, but clearance rates were improved by combining the two therapies.
In an earlier literature review and meta-analysis, investigators evaluated the effect of 5-FU plus salicylic acid or salicylic acid alone. The therapeutic effect for common warts across all studies was a 63.4% response rate (complete healing) for 5-FU/SA vversus 23.1% for the 5-FU–free controls, respectively. For plantar warts, the response rate was 63% versus 11%, respectively.
“But what about the person with multiple warts or those in challenging locations where you might worry about destructive treatments hurting the nail fold or causing nail dystrophy?” Dr. Barbieri asked. “Maybe they’ve used salicylic acid or intralesional Candida and they’re still not getting better. What can we do for these patients?”
Emerging research suggests that topical cidofovir can be a valuable option for recalcitrant warts or those in sensitive locations. In a case report of a 10-year-old boy with more than 50 severe verrucous papules on his hands and face that were recalcitrant to multiple conventional therapies, topical 1% cidofovir applied daily for 8 weeks was effective, with no adverse side effects. A young female patient who presented to Dr. Barbieri with multiple warts around the nail matrix of several fingers experienced complete clearance after treatment with topical cidofovir, he said. Other researchers found this approach to be effective for plantar warts as well, in a report of two brothers with severe combined immunodeficiency after hematopoietic stem cell transplantation with persistent warts that did not respond to traditional topical treatments.
“Topical cidofovir is typically a painless treatment, which is nice, especially for our pediatric patients who might be afraid of other therapies like or cryotherapy or intralesional injections,” One limitation is that it is “a bit expensive,” Dr. Barbieri said. “To have topical cidofovir compounded is typically $100-$300, depending on the quantity and strength that you ask for.”
Intralesional cidofovir is another treatment option. In a retrospective study of 58 patients, Dr. Barbieri and colleagues evaluated the outcome of intralesional cidofovir treatment of warts in immunocompromised and nonimmunocompromised patients. Rates of improvement ranged from 98.3% to 100%, while resolution rates ranged from 75.9% to 97.6%.
“Most of the patients had warts for more than 5 years and almost half of them had recalcitrant warts,” Dr. Barbieri said. “These were mostly adult patients, but I think this is a treatment that can work in younger populations as well. About 10%-15% had HIV or cancer or diabetes or were transplant recipients, but despite these challenges and despite these recalcitrant warts, about 100% had improvement.”
He pointed out that cidofovir is available as a 75 mg/mL vial that comes with a 5 mL single-use vial. He dilutes this with normal saline to create a 15 mg/mL solution.
“If you want to be efficient you can try to schedule multiple patients together on the same day as a single vial is sufficient to treat about 25 patients,” assuming about 1 mL is injected per patient, he said. “The challenge with intralesional cidofovir is that it’s painful beyond just the needle part of the injection. Sometimes a nerve block can be helpful. But this can be an effective treatment for patients with recalcitrant warts or those with comorbidities.”
Other intralesional therapies to try for recalcitrant warts, he said, include bleomycin (1 U/mL solution, 1-2 mL per treatment, spaced every 2-4 weeks), and 5-FU (a 4:1 mixture of 5-FU [50 mg/mL] and 2% lidocaine).
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Toxic chemicals we consume without knowing it
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Physicians and clinicians should be required to get flu shots: Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.