User login
Discrepancies in Skin Cancer Screening Reporting Among Patients, Primary Care Physicians, and Patient Medical Records
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
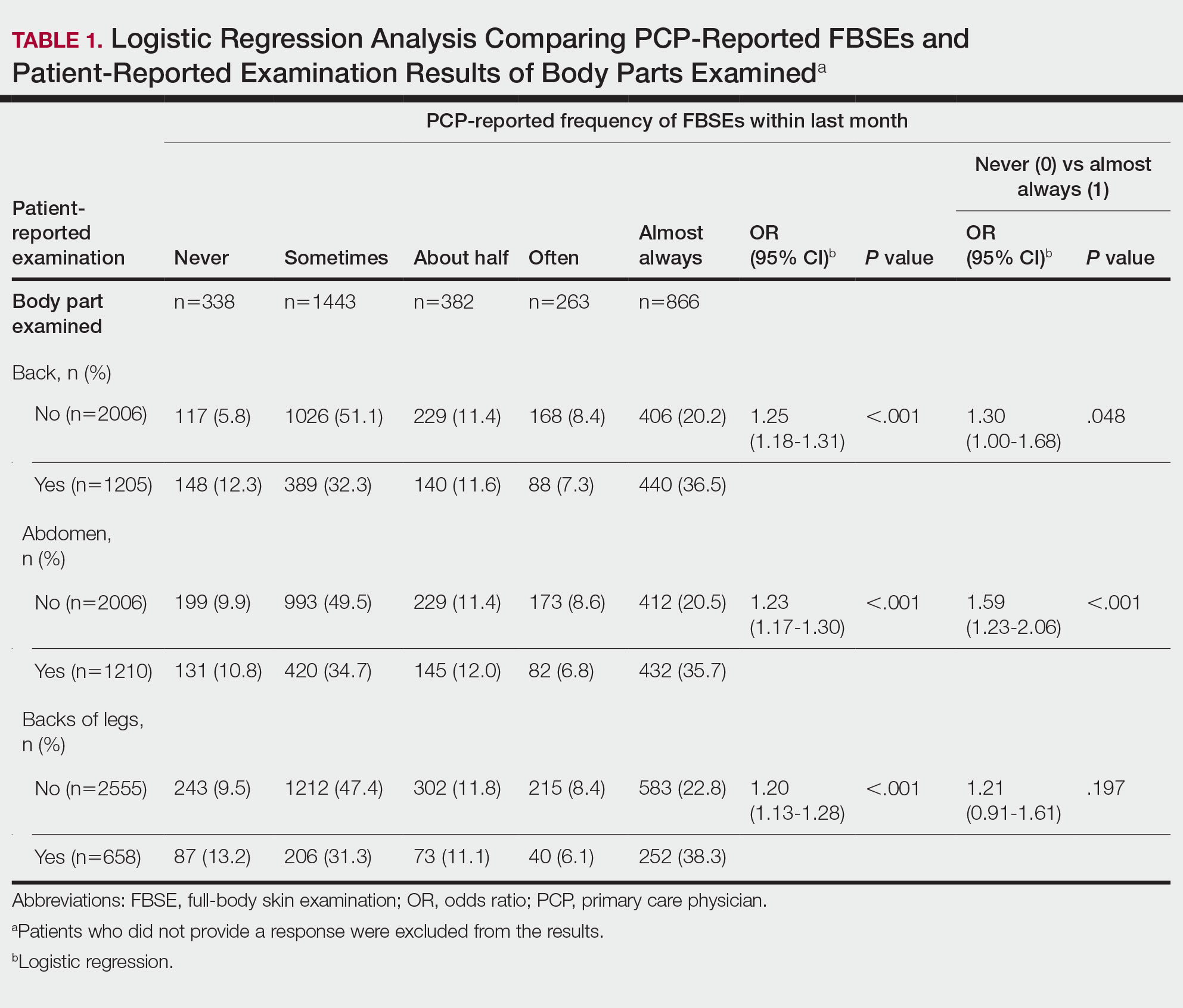
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”
Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).
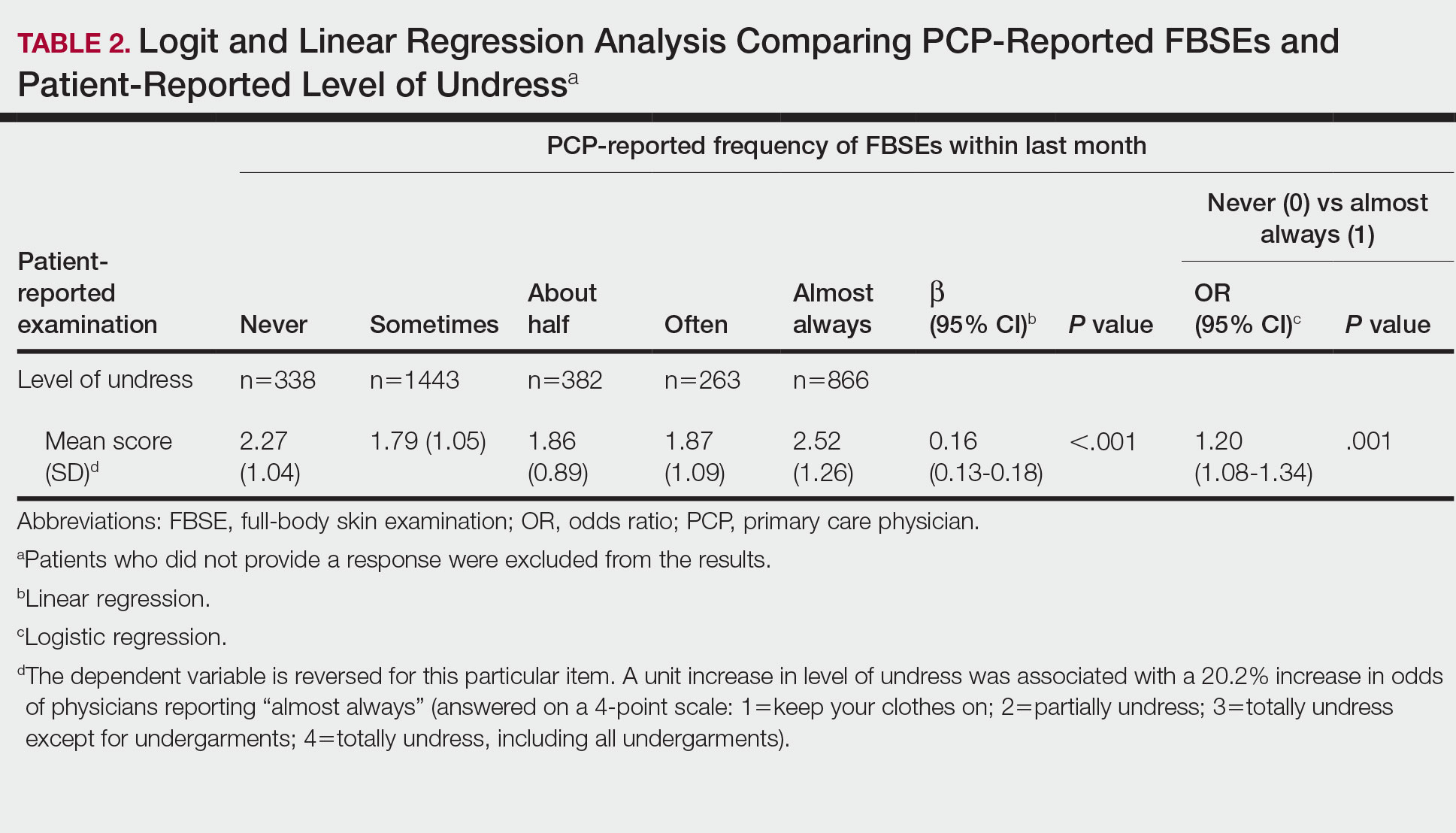
Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
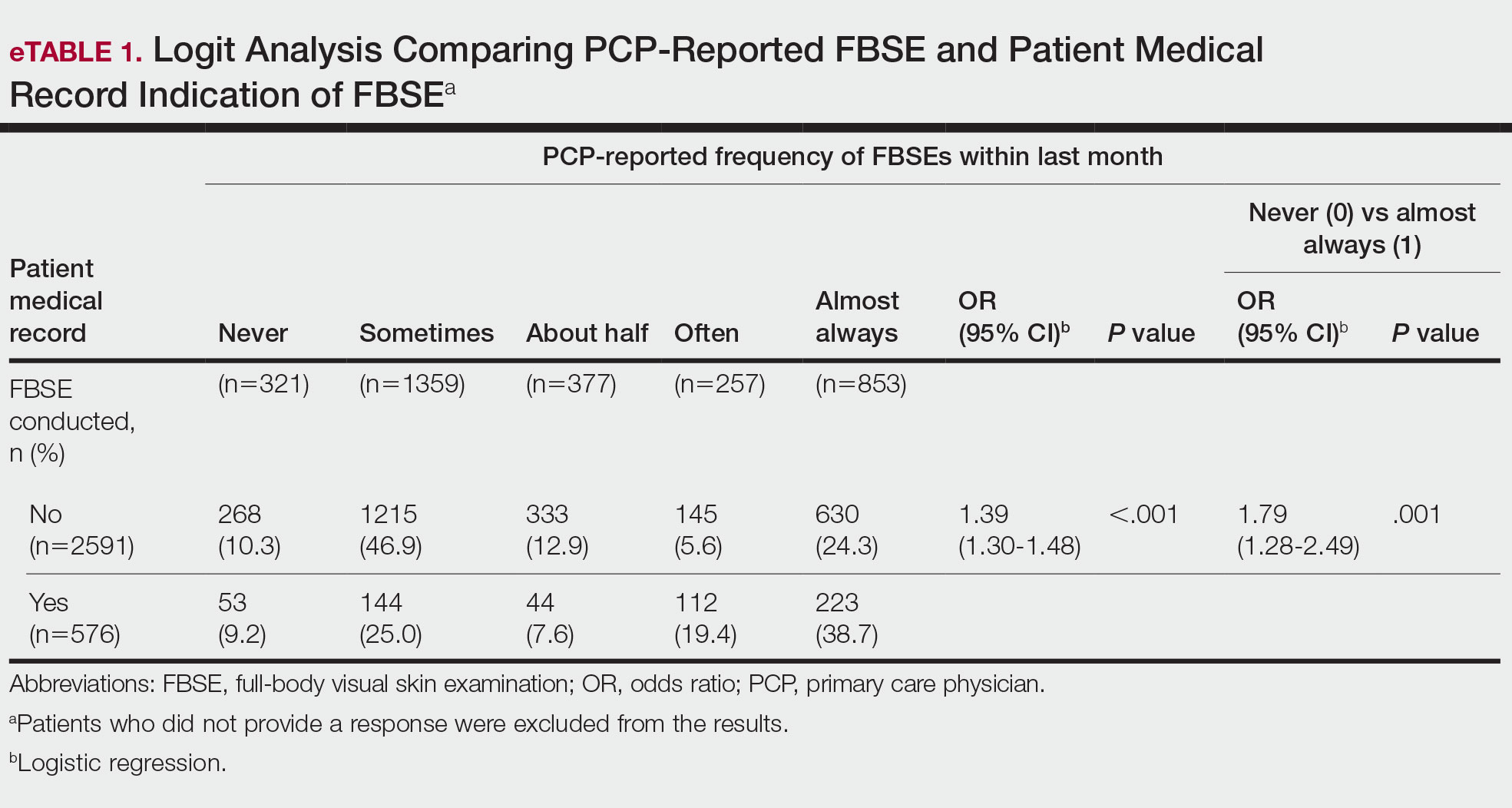
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.
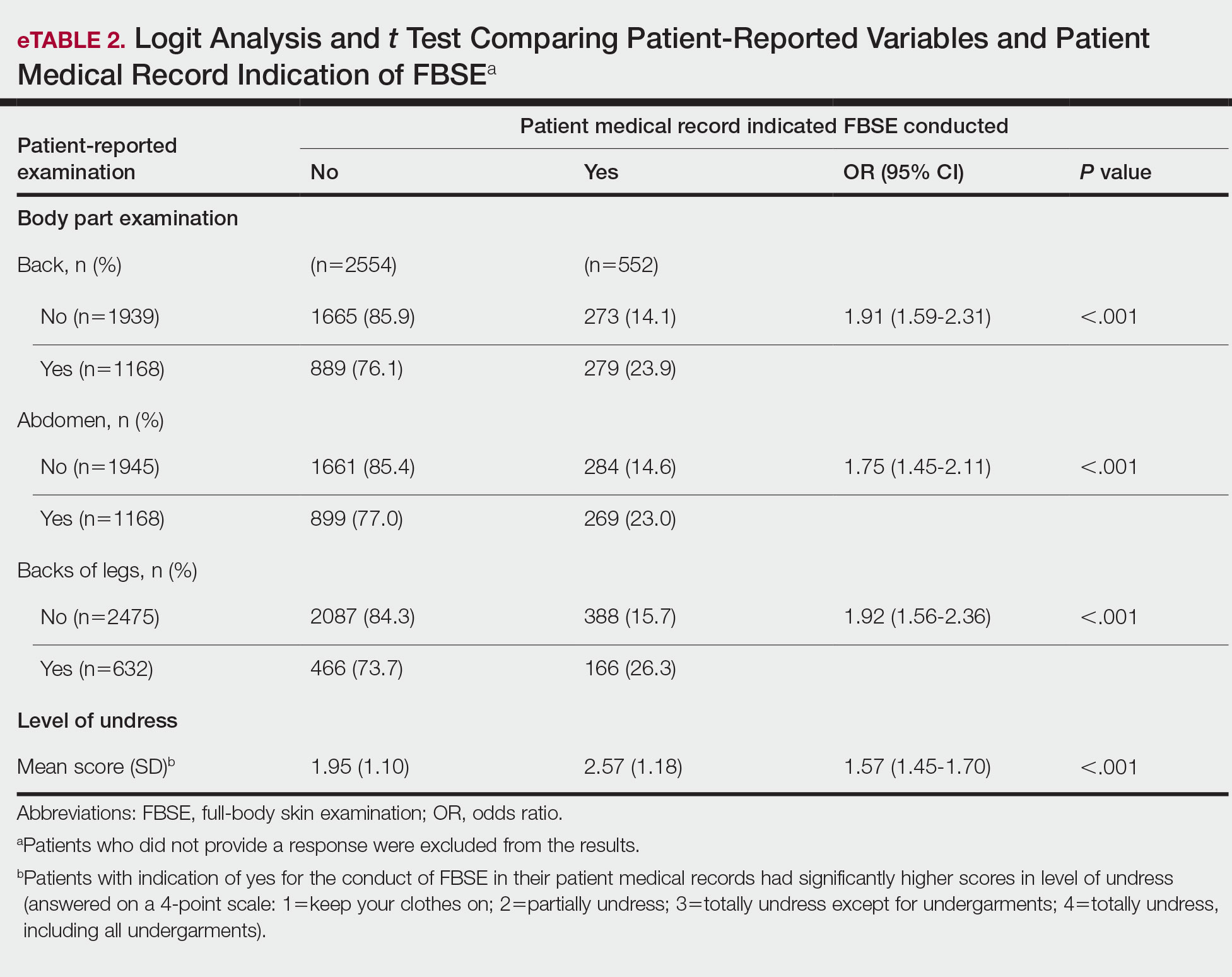
When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).
COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”

Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).

Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.

When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).

COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”

Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).

Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.

When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).

COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
PRACTICE POINTS
- Dermatologists should be aware of the variability in practice and execution of full-body skin examinations (FBSEs) among primary care providers and offer comprehensive examinations for every patient.
- Variability in reporting and execution of FBSEs may impact the continued US Preventive Services Task Force I rating in their guidelines and promotion of skin cancer screening in the primary care setting.
Devices to detect skin cancer: FDA advisers offer mixed views
.
So far, the U.S. Food and Drug Administration has cleared two devices. Both are computer-aided skin lesion classification devices meant to help clinicians assess cases of suspected melanoma.
Both were given a class III designation. That classification is intended for products that are considered to have a high risk of harm because of flawed design or implementation. Many such devices are under development, and there has been a proposal to include these devices in class II, which is less restrictive.
The FDA turned to one of its expert panels for advice. At a meeting held on Aug. 29, experts on the panel offered differing views and expressed concerns about the accuracy of these devices.
This was the second day of meetings of the general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee. On the previous day, the panel held a wide-ranging discussion about expanding use of skin lesion analyzer devices.
The FDA sought the expert panel’s advice concerning a field that appears to be heating up quickly after relatively quiet times.
Two devices have been approved by the FDA so far, but only one is still being promoted – SciBase AB’s Nevisense. The Swedish company announced in May 2020 that it had received FDA approval for Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection, an AI-based point-of-care system for the noninvasive evaluation of irregular moles.
The other device, known as MelaFind, was acquired by Strata Skin Sciences, but the company said in 2017 that it discontinued research and development, sales, and support activity related to the device, according to a filing with the Securities and Exchange Commission.
But there’s been a swell in recent years in the number of publications related to the use of AI and machine learning, which could give rise to new tools for aiding in the diagnosis of skin conditions, including cancer. Google is among the companies that are involved in these efforts.
So, the FDA asked the expert panel to discuss a series of questions related to how the agency should weigh the risks of computer-aided devices for melanoma diagnosis. The agency also asked the panel to provide feedback about how well risks associated with such devices and tools might be managed and to offer suggestions.
The discussion at the July 29 meeting spun beyond narrow questions about reclassification of the current class III devices to topics involving emerging technology, such as efforts to apply AI to dermatology.
“Innovation continues. Medical device developers are anxious to plan how they might be able to develop the level of evidence that would meet your expectations” for future products, Binita Ashar, MD, a senior official in FDA’s Center for Devices and Radiological Health, told the panel.
Company CEO backs tougher regulation
Simon Grant, the chief executive of SciBase, which markets Nevisense, the first and only skin cancer–detecting device currently on the U.S. market, sought to make a case for sticking with the tougher class III regulations.
Speaking during the public comment session, Mr. Grant said switching to class II designations would weaken the standards used in clearing products that analyze skin lesions so as to put patients at risk.
Under the FDA’s rules, the agency designates as class III devices that present potential unreasonable risk of illness or injury. Only about 10% of devices fall into this category. Such devices include implantable pacemakers and breast implants, as well as SciBase’s Nevisense.
About 43% of medical devices fall into the class II category, which includes powered wheelchairs and some pregnancy test kits, the FDA website says.
Class I medical devices pose minimal potential for harm and tend to be simpler in design. These include enema kits and elastic bandages, the FDA says.
Mr. Grant told the meeting that in his career he has worked on two class III products and about 20 class II products. (He had previously worked at medical startups Synectics Medical and Neoventa, as well as established multinationals such as Medtronic.)
“I can tell you that – practically – the FDA has many fewer sticks and much less control when it comes to class II devices,” he said. He offered an example of a manufacturer of a class II device having more latitude in making small changes to products without notifying the FDA.
In his hypothetical example, such a change could have unintended consequences, and “with AI systems, small changes can result in large and nonlinear or even random effects,” Mr. Grant said. “But it’s too late if the product is on the market and the harm has already occurred,” he said.
The American Society for Dermatologic Surgery Association also protested the reclassifying of approved computer-aided melanoma detection class III devices.
In a statement posted on the FDA website as part of the materials for the meeting, the ASDSA raised a series of concerns about the prospects of expanded U.S. use of tools for assisting in diagnosing melanoma, including ones that would be marketed to consumers.
“To the extent that algorithms and devices for patient self-diagnosis of skin lesions are already widely available, they should be required to include detailed disclaimers that include that they are for entertainment and educational purposes and not a diagnostic device, that they are not approved by dermatologists or a recognized medical regulatory authority for self-diagnosis,” the ASDSA said.
Devices and algorithms in screening tools “are not highly regulated and remain unproven. They may result in wrong diagnoses, missed diagnoses, or over- or underdiagnosis,” the ASDSA added. “Both patients at low risk and those at high risk are better served by scheduling an in-person examination with a board-certified dermatologist, who can also help them determine the appropriate future skin screening schedule that is most appropriate for them.”
‘Stepping stone’
However, there is strong consumer demand for better information about skin conditions, and many patients face hurdles in going to dermatologists.
Google research has shown that consumers are seeking “a stepping stone” between the information they can easily find online and what they could get from a medical professional, said Lily Peng, MD, PhD, a director of product management for the health AI team at Google. Dr. Peng was a scheduled presenter at the July 29 meeting.
Consumers often are looking for more information on common conditions such as acne and poison ivy, and they sometimes face challenges in getting access to clinicians, she said.
“There are many unmet needs for consumers experiencing skin issues, many of which are lower-acuity conditions. There’s a big opportunity to increase accessibility and relevance of health journeys for consumers,” Dr. Peng said. “We have heard from consumers that they would like to have a self-help tool for nonserious conditions so they can decide when to seek medical attention.”
Dr. Peng’s presentation was not directly related to the question of class II or class III designation for existing products. Instead, her talk served as a glimpse into the work already underway in creating apps and tools for consumers.
Google researchers have published a number of studies in recent years about the use of AI to improve dermatology diagnosis.
A 2020 article reported on Google’s test of a form of AI known as deep learning system (DLS) to provide a differential diagnosis of skin conditions. On 963 validation cases, where a rotating panel of three board-certified dermatologists defined the reference standard, the DLS was noninferior to six other dermatologists and was superior to six primary care physicians (PCPs) and six nurse practitioners (NPs), according to a summary of the article.
A 2021 report published in JAMA Network Open said that use of an AI tool was associated with a higher agreement rate with dermatologists’ reference diagnoses for both PCPs and NPs.
In a 2021 blog post, Google scientists wrote that their AI model that powers a tool for checking skin conditions had earned European clearance, known as a CE mark, as a class I medical device.
SkinVision has an app that the company says “is available worldwide (with the exception of the USA and Canada).” The firm’s website includes a link where people in the United States and Canada can sign up for notifications about when SkinVision will be available in these nations.
‘Not ready for prime time’
The FDA panel did not cast formal votes at the July 29 meeting. Rather, the members engaged in broad discussions about risks and potential benefits of new tools for aiding in the detection of skin cancer.
Among the key issues discussed was a question of whether the FDA could impose requirements and restrictions, known as special controls, to provide “reasonable assurance of safety and effectiveness” for computer-aided devices that provide adjunctive diagnostic information to dermatologists about lesions suspicious for melanoma.
Among the potential special controls would be clinical performance testing in regards to rates of the sensitivity (true-positive rate) and specificity (true-negative rate).
The FDA could also look at requirements on software validation and verification and cybersecurity testing, as well as directions on labeling so as to mitigate risk.
Dermatologists serving on the panel called for caution in proceeding with steps that would make it easier for companies to market tools for aiding in melanoma diagnosis than it would be within the class III framework used for MelaFind and Nevisense.
Many expressed concerns about the need to design studies that would answer questions about how well new tools could accurately identify concerning lesions.
The phrase “not ready for prime time” was used at least three times during the discussion.
FDA panelist Maral Skelsey, MD, a skin cancer specialist from Chevy Chase, Maryland, said that over the years, she had used both Nevisense and MelaFind.
She said she had found MelaFind “unusable,” owing in large part to the high number of false positives it generated. The device also was limited as to where on patients’ bodies it could be used.
However, she spoke with enthusiasm about the prospects for better devices to aid in diagnosis of skin lesions. “It’s an area where we’re on the verge, and we really need these devices. There’s a need for patients to be able to examine themselves, for nondermatologists to be able to assess lesions,” Dr. Skelsey said.
But this field is “just not ready for prime time” yet, even with special controls, Dr. Skelsey said. To loosen approval standards too quickly could be a “detriment to what’s coming down the pipeline,” she said.
“It’s harmful to things that are likely to be around the corner,” she said.
FDA panelist Renata Block, PA-C, who works in a Chicago dermatology practice, pressed for maintaining a class III designation. “We are not ready for prime time yet, though the data that is coming down the pipeline on what we have is quite exciting,” Ms. Block said.
FDA panelist Karla V. Ballman, PhD, a statistician from Weill Cornell Medicine, New York, said there would need to be a clear standard for clinical performance before proceeding toward reclassification of devices for aid in detecting melanoma. “I just don’t think it’s ready for prime time at this point and should remain in class III,” she said.
But there was support from some panelists for the idea of a lower bar for clearance, combined with special controls to ensure patient safety.
In expressing her view, FDA panelist Katalin Roth, MD, JD, professor of medicine, George Washington University, Washington, said she was an outlier in her support for the agency’s view that these risks could be managed and that future tools could allow more patients to take a step on the pathway toward critical diagnoses.
“I deal with a lot of people with cancer as a palliative care physician,” Dr. Roth said. “I think what we’re missing here is the issue of time. Melanoma is a terrible disease, and missing the diagnosis is a terrible thing, but I think special controls would be sufficient to counter the concerns of my colleagues on the committee.”
The FDA’s Dr. Ashar ended the meeting with questions posed to one panelist, Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center in New York.
Dr. Rotemberg has for years been working in the field of research on developing AI and other computer-based tools for detecting and diagnosing melanoma, the deadliest form of skin cancer.
She has been publicly skeptical of the performance of commercial apps that scan moles and other lesions and that claim to identify which are cancerous. A May blog post on the Memorial Sloan Kettering website highlighted a recent British Journal of Dermatology article in which Dr. Rotemberg and coauthors reported on their evaluations of commercial apps. They judged them to be on average only 59% accurate, the blog post said.
However, during an earlier discussion at the meeting, she had spoken more positively about the prospects for using special controls in the near term to mitigate risk, although she said she would have a “very long list” of these requirements.
In the closing exchange with Dr. Ashar, Dr. Rotemberg outlined steps that could potentially ensure the safe use of tools to aid in melanoma screening. These included a need for postmarketing surveillance, which would require evaluation over time of algorithms used in tools meant to detect skin cancer.
“We need to have a mechanism for sampling,” Dr. Rotemberg said. “Most of our data is electronic now anyway, so comparing an algorithm and performance with biopsy results should not be that challenging.”
A version of this article first appeared on Medscape.com.
.
So far, the U.S. Food and Drug Administration has cleared two devices. Both are computer-aided skin lesion classification devices meant to help clinicians assess cases of suspected melanoma.
Both were given a class III designation. That classification is intended for products that are considered to have a high risk of harm because of flawed design or implementation. Many such devices are under development, and there has been a proposal to include these devices in class II, which is less restrictive.
The FDA turned to one of its expert panels for advice. At a meeting held on Aug. 29, experts on the panel offered differing views and expressed concerns about the accuracy of these devices.
This was the second day of meetings of the general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee. On the previous day, the panel held a wide-ranging discussion about expanding use of skin lesion analyzer devices.
The FDA sought the expert panel’s advice concerning a field that appears to be heating up quickly after relatively quiet times.
Two devices have been approved by the FDA so far, but only one is still being promoted – SciBase AB’s Nevisense. The Swedish company announced in May 2020 that it had received FDA approval for Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection, an AI-based point-of-care system for the noninvasive evaluation of irregular moles.
The other device, known as MelaFind, was acquired by Strata Skin Sciences, but the company said in 2017 that it discontinued research and development, sales, and support activity related to the device, according to a filing with the Securities and Exchange Commission.
But there’s been a swell in recent years in the number of publications related to the use of AI and machine learning, which could give rise to new tools for aiding in the diagnosis of skin conditions, including cancer. Google is among the companies that are involved in these efforts.
So, the FDA asked the expert panel to discuss a series of questions related to how the agency should weigh the risks of computer-aided devices for melanoma diagnosis. The agency also asked the panel to provide feedback about how well risks associated with such devices and tools might be managed and to offer suggestions.
The discussion at the July 29 meeting spun beyond narrow questions about reclassification of the current class III devices to topics involving emerging technology, such as efforts to apply AI to dermatology.
“Innovation continues. Medical device developers are anxious to plan how they might be able to develop the level of evidence that would meet your expectations” for future products, Binita Ashar, MD, a senior official in FDA’s Center for Devices and Radiological Health, told the panel.
Company CEO backs tougher regulation
Simon Grant, the chief executive of SciBase, which markets Nevisense, the first and only skin cancer–detecting device currently on the U.S. market, sought to make a case for sticking with the tougher class III regulations.
Speaking during the public comment session, Mr. Grant said switching to class II designations would weaken the standards used in clearing products that analyze skin lesions so as to put patients at risk.
Under the FDA’s rules, the agency designates as class III devices that present potential unreasonable risk of illness or injury. Only about 10% of devices fall into this category. Such devices include implantable pacemakers and breast implants, as well as SciBase’s Nevisense.
About 43% of medical devices fall into the class II category, which includes powered wheelchairs and some pregnancy test kits, the FDA website says.
Class I medical devices pose minimal potential for harm and tend to be simpler in design. These include enema kits and elastic bandages, the FDA says.
Mr. Grant told the meeting that in his career he has worked on two class III products and about 20 class II products. (He had previously worked at medical startups Synectics Medical and Neoventa, as well as established multinationals such as Medtronic.)
“I can tell you that – practically – the FDA has many fewer sticks and much less control when it comes to class II devices,” he said. He offered an example of a manufacturer of a class II device having more latitude in making small changes to products without notifying the FDA.
In his hypothetical example, such a change could have unintended consequences, and “with AI systems, small changes can result in large and nonlinear or even random effects,” Mr. Grant said. “But it’s too late if the product is on the market and the harm has already occurred,” he said.
The American Society for Dermatologic Surgery Association also protested the reclassifying of approved computer-aided melanoma detection class III devices.
In a statement posted on the FDA website as part of the materials for the meeting, the ASDSA raised a series of concerns about the prospects of expanded U.S. use of tools for assisting in diagnosing melanoma, including ones that would be marketed to consumers.
“To the extent that algorithms and devices for patient self-diagnosis of skin lesions are already widely available, they should be required to include detailed disclaimers that include that they are for entertainment and educational purposes and not a diagnostic device, that they are not approved by dermatologists or a recognized medical regulatory authority for self-diagnosis,” the ASDSA said.
Devices and algorithms in screening tools “are not highly regulated and remain unproven. They may result in wrong diagnoses, missed diagnoses, or over- or underdiagnosis,” the ASDSA added. “Both patients at low risk and those at high risk are better served by scheduling an in-person examination with a board-certified dermatologist, who can also help them determine the appropriate future skin screening schedule that is most appropriate for them.”
‘Stepping stone’
However, there is strong consumer demand for better information about skin conditions, and many patients face hurdles in going to dermatologists.
Google research has shown that consumers are seeking “a stepping stone” between the information they can easily find online and what they could get from a medical professional, said Lily Peng, MD, PhD, a director of product management for the health AI team at Google. Dr. Peng was a scheduled presenter at the July 29 meeting.
Consumers often are looking for more information on common conditions such as acne and poison ivy, and they sometimes face challenges in getting access to clinicians, she said.
“There are many unmet needs for consumers experiencing skin issues, many of which are lower-acuity conditions. There’s a big opportunity to increase accessibility and relevance of health journeys for consumers,” Dr. Peng said. “We have heard from consumers that they would like to have a self-help tool for nonserious conditions so they can decide when to seek medical attention.”
Dr. Peng’s presentation was not directly related to the question of class II or class III designation for existing products. Instead, her talk served as a glimpse into the work already underway in creating apps and tools for consumers.
Google researchers have published a number of studies in recent years about the use of AI to improve dermatology diagnosis.
A 2020 article reported on Google’s test of a form of AI known as deep learning system (DLS) to provide a differential diagnosis of skin conditions. On 963 validation cases, where a rotating panel of three board-certified dermatologists defined the reference standard, the DLS was noninferior to six other dermatologists and was superior to six primary care physicians (PCPs) and six nurse practitioners (NPs), according to a summary of the article.
A 2021 report published in JAMA Network Open said that use of an AI tool was associated with a higher agreement rate with dermatologists’ reference diagnoses for both PCPs and NPs.
In a 2021 blog post, Google scientists wrote that their AI model that powers a tool for checking skin conditions had earned European clearance, known as a CE mark, as a class I medical device.
SkinVision has an app that the company says “is available worldwide (with the exception of the USA and Canada).” The firm’s website includes a link where people in the United States and Canada can sign up for notifications about when SkinVision will be available in these nations.
‘Not ready for prime time’
The FDA panel did not cast formal votes at the July 29 meeting. Rather, the members engaged in broad discussions about risks and potential benefits of new tools for aiding in the detection of skin cancer.
Among the key issues discussed was a question of whether the FDA could impose requirements and restrictions, known as special controls, to provide “reasonable assurance of safety and effectiveness” for computer-aided devices that provide adjunctive diagnostic information to dermatologists about lesions suspicious for melanoma.
Among the potential special controls would be clinical performance testing in regards to rates of the sensitivity (true-positive rate) and specificity (true-negative rate).
The FDA could also look at requirements on software validation and verification and cybersecurity testing, as well as directions on labeling so as to mitigate risk.
Dermatologists serving on the panel called for caution in proceeding with steps that would make it easier for companies to market tools for aiding in melanoma diagnosis than it would be within the class III framework used for MelaFind and Nevisense.
Many expressed concerns about the need to design studies that would answer questions about how well new tools could accurately identify concerning lesions.
The phrase “not ready for prime time” was used at least three times during the discussion.
FDA panelist Maral Skelsey, MD, a skin cancer specialist from Chevy Chase, Maryland, said that over the years, she had used both Nevisense and MelaFind.
She said she had found MelaFind “unusable,” owing in large part to the high number of false positives it generated. The device also was limited as to where on patients’ bodies it could be used.
However, she spoke with enthusiasm about the prospects for better devices to aid in diagnosis of skin lesions. “It’s an area where we’re on the verge, and we really need these devices. There’s a need for patients to be able to examine themselves, for nondermatologists to be able to assess lesions,” Dr. Skelsey said.
But this field is “just not ready for prime time” yet, even with special controls, Dr. Skelsey said. To loosen approval standards too quickly could be a “detriment to what’s coming down the pipeline,” she said.
“It’s harmful to things that are likely to be around the corner,” she said.
FDA panelist Renata Block, PA-C, who works in a Chicago dermatology practice, pressed for maintaining a class III designation. “We are not ready for prime time yet, though the data that is coming down the pipeline on what we have is quite exciting,” Ms. Block said.
FDA panelist Karla V. Ballman, PhD, a statistician from Weill Cornell Medicine, New York, said there would need to be a clear standard for clinical performance before proceeding toward reclassification of devices for aid in detecting melanoma. “I just don’t think it’s ready for prime time at this point and should remain in class III,” she said.
But there was support from some panelists for the idea of a lower bar for clearance, combined with special controls to ensure patient safety.
In expressing her view, FDA panelist Katalin Roth, MD, JD, professor of medicine, George Washington University, Washington, said she was an outlier in her support for the agency’s view that these risks could be managed and that future tools could allow more patients to take a step on the pathway toward critical diagnoses.
“I deal with a lot of people with cancer as a palliative care physician,” Dr. Roth said. “I think what we’re missing here is the issue of time. Melanoma is a terrible disease, and missing the diagnosis is a terrible thing, but I think special controls would be sufficient to counter the concerns of my colleagues on the committee.”
The FDA’s Dr. Ashar ended the meeting with questions posed to one panelist, Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center in New York.
Dr. Rotemberg has for years been working in the field of research on developing AI and other computer-based tools for detecting and diagnosing melanoma, the deadliest form of skin cancer.
She has been publicly skeptical of the performance of commercial apps that scan moles and other lesions and that claim to identify which are cancerous. A May blog post on the Memorial Sloan Kettering website highlighted a recent British Journal of Dermatology article in which Dr. Rotemberg and coauthors reported on their evaluations of commercial apps. They judged them to be on average only 59% accurate, the blog post said.
However, during an earlier discussion at the meeting, she had spoken more positively about the prospects for using special controls in the near term to mitigate risk, although she said she would have a “very long list” of these requirements.
In the closing exchange with Dr. Ashar, Dr. Rotemberg outlined steps that could potentially ensure the safe use of tools to aid in melanoma screening. These included a need for postmarketing surveillance, which would require evaluation over time of algorithms used in tools meant to detect skin cancer.
“We need to have a mechanism for sampling,” Dr. Rotemberg said. “Most of our data is electronic now anyway, so comparing an algorithm and performance with biopsy results should not be that challenging.”
A version of this article first appeared on Medscape.com.
.
So far, the U.S. Food and Drug Administration has cleared two devices. Both are computer-aided skin lesion classification devices meant to help clinicians assess cases of suspected melanoma.
Both were given a class III designation. That classification is intended for products that are considered to have a high risk of harm because of flawed design or implementation. Many such devices are under development, and there has been a proposal to include these devices in class II, which is less restrictive.
The FDA turned to one of its expert panels for advice. At a meeting held on Aug. 29, experts on the panel offered differing views and expressed concerns about the accuracy of these devices.
This was the second day of meetings of the general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee. On the previous day, the panel held a wide-ranging discussion about expanding use of skin lesion analyzer devices.
The FDA sought the expert panel’s advice concerning a field that appears to be heating up quickly after relatively quiet times.
Two devices have been approved by the FDA so far, but only one is still being promoted – SciBase AB’s Nevisense. The Swedish company announced in May 2020 that it had received FDA approval for Nevisense 3.0, the third generation of their Nevisense system for early melanoma detection, an AI-based point-of-care system for the noninvasive evaluation of irregular moles.
The other device, known as MelaFind, was acquired by Strata Skin Sciences, but the company said in 2017 that it discontinued research and development, sales, and support activity related to the device, according to a filing with the Securities and Exchange Commission.
But there’s been a swell in recent years in the number of publications related to the use of AI and machine learning, which could give rise to new tools for aiding in the diagnosis of skin conditions, including cancer. Google is among the companies that are involved in these efforts.
So, the FDA asked the expert panel to discuss a series of questions related to how the agency should weigh the risks of computer-aided devices for melanoma diagnosis. The agency also asked the panel to provide feedback about how well risks associated with such devices and tools might be managed and to offer suggestions.
The discussion at the July 29 meeting spun beyond narrow questions about reclassification of the current class III devices to topics involving emerging technology, such as efforts to apply AI to dermatology.
“Innovation continues. Medical device developers are anxious to plan how they might be able to develop the level of evidence that would meet your expectations” for future products, Binita Ashar, MD, a senior official in FDA’s Center for Devices and Radiological Health, told the panel.
Company CEO backs tougher regulation
Simon Grant, the chief executive of SciBase, which markets Nevisense, the first and only skin cancer–detecting device currently on the U.S. market, sought to make a case for sticking with the tougher class III regulations.
Speaking during the public comment session, Mr. Grant said switching to class II designations would weaken the standards used in clearing products that analyze skin lesions so as to put patients at risk.
Under the FDA’s rules, the agency designates as class III devices that present potential unreasonable risk of illness or injury. Only about 10% of devices fall into this category. Such devices include implantable pacemakers and breast implants, as well as SciBase’s Nevisense.
About 43% of medical devices fall into the class II category, which includes powered wheelchairs and some pregnancy test kits, the FDA website says.
Class I medical devices pose minimal potential for harm and tend to be simpler in design. These include enema kits and elastic bandages, the FDA says.
Mr. Grant told the meeting that in his career he has worked on two class III products and about 20 class II products. (He had previously worked at medical startups Synectics Medical and Neoventa, as well as established multinationals such as Medtronic.)
“I can tell you that – practically – the FDA has many fewer sticks and much less control when it comes to class II devices,” he said. He offered an example of a manufacturer of a class II device having more latitude in making small changes to products without notifying the FDA.
In his hypothetical example, such a change could have unintended consequences, and “with AI systems, small changes can result in large and nonlinear or even random effects,” Mr. Grant said. “But it’s too late if the product is on the market and the harm has already occurred,” he said.
The American Society for Dermatologic Surgery Association also protested the reclassifying of approved computer-aided melanoma detection class III devices.
In a statement posted on the FDA website as part of the materials for the meeting, the ASDSA raised a series of concerns about the prospects of expanded U.S. use of tools for assisting in diagnosing melanoma, including ones that would be marketed to consumers.
“To the extent that algorithms and devices for patient self-diagnosis of skin lesions are already widely available, they should be required to include detailed disclaimers that include that they are for entertainment and educational purposes and not a diagnostic device, that they are not approved by dermatologists or a recognized medical regulatory authority for self-diagnosis,” the ASDSA said.
Devices and algorithms in screening tools “are not highly regulated and remain unproven. They may result in wrong diagnoses, missed diagnoses, or over- or underdiagnosis,” the ASDSA added. “Both patients at low risk and those at high risk are better served by scheduling an in-person examination with a board-certified dermatologist, who can also help them determine the appropriate future skin screening schedule that is most appropriate for them.”
‘Stepping stone’
However, there is strong consumer demand for better information about skin conditions, and many patients face hurdles in going to dermatologists.
Google research has shown that consumers are seeking “a stepping stone” between the information they can easily find online and what they could get from a medical professional, said Lily Peng, MD, PhD, a director of product management for the health AI team at Google. Dr. Peng was a scheduled presenter at the July 29 meeting.
Consumers often are looking for more information on common conditions such as acne and poison ivy, and they sometimes face challenges in getting access to clinicians, she said.
“There are many unmet needs for consumers experiencing skin issues, many of which are lower-acuity conditions. There’s a big opportunity to increase accessibility and relevance of health journeys for consumers,” Dr. Peng said. “We have heard from consumers that they would like to have a self-help tool for nonserious conditions so they can decide when to seek medical attention.”
Dr. Peng’s presentation was not directly related to the question of class II or class III designation for existing products. Instead, her talk served as a glimpse into the work already underway in creating apps and tools for consumers.
Google researchers have published a number of studies in recent years about the use of AI to improve dermatology diagnosis.
A 2020 article reported on Google’s test of a form of AI known as deep learning system (DLS) to provide a differential diagnosis of skin conditions. On 963 validation cases, where a rotating panel of three board-certified dermatologists defined the reference standard, the DLS was noninferior to six other dermatologists and was superior to six primary care physicians (PCPs) and six nurse practitioners (NPs), according to a summary of the article.
A 2021 report published in JAMA Network Open said that use of an AI tool was associated with a higher agreement rate with dermatologists’ reference diagnoses for both PCPs and NPs.
In a 2021 blog post, Google scientists wrote that their AI model that powers a tool for checking skin conditions had earned European clearance, known as a CE mark, as a class I medical device.
SkinVision has an app that the company says “is available worldwide (with the exception of the USA and Canada).” The firm’s website includes a link where people in the United States and Canada can sign up for notifications about when SkinVision will be available in these nations.
‘Not ready for prime time’
The FDA panel did not cast formal votes at the July 29 meeting. Rather, the members engaged in broad discussions about risks and potential benefits of new tools for aiding in the detection of skin cancer.
Among the key issues discussed was a question of whether the FDA could impose requirements and restrictions, known as special controls, to provide “reasonable assurance of safety and effectiveness” for computer-aided devices that provide adjunctive diagnostic information to dermatologists about lesions suspicious for melanoma.
Among the potential special controls would be clinical performance testing in regards to rates of the sensitivity (true-positive rate) and specificity (true-negative rate).
The FDA could also look at requirements on software validation and verification and cybersecurity testing, as well as directions on labeling so as to mitigate risk.
Dermatologists serving on the panel called for caution in proceeding with steps that would make it easier for companies to market tools for aiding in melanoma diagnosis than it would be within the class III framework used for MelaFind and Nevisense.
Many expressed concerns about the need to design studies that would answer questions about how well new tools could accurately identify concerning lesions.
The phrase “not ready for prime time” was used at least three times during the discussion.
FDA panelist Maral Skelsey, MD, a skin cancer specialist from Chevy Chase, Maryland, said that over the years, she had used both Nevisense and MelaFind.
She said she had found MelaFind “unusable,” owing in large part to the high number of false positives it generated. The device also was limited as to where on patients’ bodies it could be used.
However, she spoke with enthusiasm about the prospects for better devices to aid in diagnosis of skin lesions. “It’s an area where we’re on the verge, and we really need these devices. There’s a need for patients to be able to examine themselves, for nondermatologists to be able to assess lesions,” Dr. Skelsey said.
But this field is “just not ready for prime time” yet, even with special controls, Dr. Skelsey said. To loosen approval standards too quickly could be a “detriment to what’s coming down the pipeline,” she said.
“It’s harmful to things that are likely to be around the corner,” she said.
FDA panelist Renata Block, PA-C, who works in a Chicago dermatology practice, pressed for maintaining a class III designation. “We are not ready for prime time yet, though the data that is coming down the pipeline on what we have is quite exciting,” Ms. Block said.
FDA panelist Karla V. Ballman, PhD, a statistician from Weill Cornell Medicine, New York, said there would need to be a clear standard for clinical performance before proceeding toward reclassification of devices for aid in detecting melanoma. “I just don’t think it’s ready for prime time at this point and should remain in class III,” she said.
But there was support from some panelists for the idea of a lower bar for clearance, combined with special controls to ensure patient safety.
In expressing her view, FDA panelist Katalin Roth, MD, JD, professor of medicine, George Washington University, Washington, said she was an outlier in her support for the agency’s view that these risks could be managed and that future tools could allow more patients to take a step on the pathway toward critical diagnoses.
“I deal with a lot of people with cancer as a palliative care physician,” Dr. Roth said. “I think what we’re missing here is the issue of time. Melanoma is a terrible disease, and missing the diagnosis is a terrible thing, but I think special controls would be sufficient to counter the concerns of my colleagues on the committee.”
The FDA’s Dr. Ashar ended the meeting with questions posed to one panelist, Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center in New York.
Dr. Rotemberg has for years been working in the field of research on developing AI and other computer-based tools for detecting and diagnosing melanoma, the deadliest form of skin cancer.
She has been publicly skeptical of the performance of commercial apps that scan moles and other lesions and that claim to identify which are cancerous. A May blog post on the Memorial Sloan Kettering website highlighted a recent British Journal of Dermatology article in which Dr. Rotemberg and coauthors reported on their evaluations of commercial apps. They judged them to be on average only 59% accurate, the blog post said.
However, during an earlier discussion at the meeting, she had spoken more positively about the prospects for using special controls in the near term to mitigate risk, although she said she would have a “very long list” of these requirements.
In the closing exchange with Dr. Ashar, Dr. Rotemberg outlined steps that could potentially ensure the safe use of tools to aid in melanoma screening. These included a need for postmarketing surveillance, which would require evaluation over time of algorithms used in tools meant to detect skin cancer.
“We need to have a mechanism for sampling,” Dr. Rotemberg said. “Most of our data is electronic now anyway, so comparing an algorithm and performance with biopsy results should not be that challenging.”
A version of this article first appeared on Medscape.com.
Banana Boat recalls scalp sunscreen spray
.
The company announced a voluntary recall for three batches of the Banana Boat Hair & Scalp Spray SPF 30, which came in 6-ounce bottles and was sold across the U.S. through various retailers and online, according to a recall alert by the Food and Drug Administration.
The three batches have a UPC label of 0-79656-04041-8 and fall under the lot codes 20016AF, 20084BF, and 21139AF, with the expiration dates of December 2022, February 2023, and April 2024, respectively.
“An internal review found that some samples of the product contained trace levels of benzene. While benzene is not an ingredient in any Banana Boat products, the review showed the unexpected levels of benzene came from the propellant that sprays the product out of the can,” according to the recall notice.
“Importantly, no other batches of Hair & Scalp (either before or after these batch codes) and no other Banana Boat products are in the scope of this recall and may continue to be used by consumers safely and as intended,” the company wrote.
Benzene is classified as a human carcinogen, the FDA wrote. Exposure to benzene can occur through the nose, mouth, and skin, and it can result in serious conditions such as leukemia, bone marrow cancer, and blood disorders.
“Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources,” the company said. “Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines.”
Edgewell said it hasn’t received any reports of bad events related to the recall. The company has told retailers to remove the affected batches from shelves.
Banana Boat will reimburse consumers who purchased a product with one of the affected lot codes, which are on the bottom of the can. In the meantime, consumers should stop using the affected product right away and discard it.
The recall comes a little over a year after Johnson & Johnson recalled five sunscreens due to low levels of benzene, according to The Associated Press. That recall included Aveeno and Neutrogena products in spray cans.
Consumers with questions about the recall can contact Edgewell Personal Care at 888-686-3988 Monday through Friday, 9 a.m. to 6 p.m. ET. People can also read more at the Banana Boat FAQ page or file for a refund directly on the Banana Boat Recall page.
A version of this article first appeared on WebMD.com.
.
The company announced a voluntary recall for three batches of the Banana Boat Hair & Scalp Spray SPF 30, which came in 6-ounce bottles and was sold across the U.S. through various retailers and online, according to a recall alert by the Food and Drug Administration.
The three batches have a UPC label of 0-79656-04041-8 and fall under the lot codes 20016AF, 20084BF, and 21139AF, with the expiration dates of December 2022, February 2023, and April 2024, respectively.
“An internal review found that some samples of the product contained trace levels of benzene. While benzene is not an ingredient in any Banana Boat products, the review showed the unexpected levels of benzene came from the propellant that sprays the product out of the can,” according to the recall notice.
“Importantly, no other batches of Hair & Scalp (either before or after these batch codes) and no other Banana Boat products are in the scope of this recall and may continue to be used by consumers safely and as intended,” the company wrote.
Benzene is classified as a human carcinogen, the FDA wrote. Exposure to benzene can occur through the nose, mouth, and skin, and it can result in serious conditions such as leukemia, bone marrow cancer, and blood disorders.
“Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources,” the company said. “Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines.”
Edgewell said it hasn’t received any reports of bad events related to the recall. The company has told retailers to remove the affected batches from shelves.
Banana Boat will reimburse consumers who purchased a product with one of the affected lot codes, which are on the bottom of the can. In the meantime, consumers should stop using the affected product right away and discard it.
The recall comes a little over a year after Johnson & Johnson recalled five sunscreens due to low levels of benzene, according to The Associated Press. That recall included Aveeno and Neutrogena products in spray cans.
Consumers with questions about the recall can contact Edgewell Personal Care at 888-686-3988 Monday through Friday, 9 a.m. to 6 p.m. ET. People can also read more at the Banana Boat FAQ page or file for a refund directly on the Banana Boat Recall page.
A version of this article first appeared on WebMD.com.
.
The company announced a voluntary recall for three batches of the Banana Boat Hair & Scalp Spray SPF 30, which came in 6-ounce bottles and was sold across the U.S. through various retailers and online, according to a recall alert by the Food and Drug Administration.
The three batches have a UPC label of 0-79656-04041-8 and fall under the lot codes 20016AF, 20084BF, and 21139AF, with the expiration dates of December 2022, February 2023, and April 2024, respectively.
“An internal review found that some samples of the product contained trace levels of benzene. While benzene is not an ingredient in any Banana Boat products, the review showed the unexpected levels of benzene came from the propellant that sprays the product out of the can,” according to the recall notice.
“Importantly, no other batches of Hair & Scalp (either before or after these batch codes) and no other Banana Boat products are in the scope of this recall and may continue to be used by consumers safely and as intended,” the company wrote.
Benzene is classified as a human carcinogen, the FDA wrote. Exposure to benzene can occur through the nose, mouth, and skin, and it can result in serious conditions such as leukemia, bone marrow cancer, and blood disorders.
“Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources,” the company said. “Daily exposure to benzene in the recalled products would not be expected to cause adverse health consequences according to an independent health assessment using established exposure modeling guidelines.”
Edgewell said it hasn’t received any reports of bad events related to the recall. The company has told retailers to remove the affected batches from shelves.
Banana Boat will reimburse consumers who purchased a product with one of the affected lot codes, which are on the bottom of the can. In the meantime, consumers should stop using the affected product right away and discard it.
The recall comes a little over a year after Johnson & Johnson recalled five sunscreens due to low levels of benzene, according to The Associated Press. That recall included Aveeno and Neutrogena products in spray cans.
Consumers with questions about the recall can contact Edgewell Personal Care at 888-686-3988 Monday through Friday, 9 a.m. to 6 p.m. ET. People can also read more at the Banana Boat FAQ page or file for a refund directly on the Banana Boat Recall page.
A version of this article first appeared on WebMD.com.
FDA panel urges caution with skin cancer–detecting tools
A and how to address longstanding issues of racial equity in this field of medicine.
The Food and Drug Administration has scheduled two meetings to gather expert feedback about managing an expected expansion in the use of skin lesion apps and devices. Outside of the United States, there are apps promoted as being able to help spot skin lesions that should trigger a medical visit.
The general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee began work on this topic on July 28, with a wide-ranging discussion about potential expanded use of computer-aided, skin lesion analyzer (SLA) devices. On Friday, the panel is considering an FDA proposal to shift the designation for an approved device for aiding dermatologists in skin cancer diagnoses from the most stringent regulatory category, class III, to the less restrictive class II.
The FDA called the meeting amid growing interest in using technology to aid in finding cancers, with some of these products already marketed to consumers outside of the United States. There are presently no legally marketed, FDA-cleared or FDA-approved SLA devices indicated for use by clinicians other than dermatologists or the lay public, the agency said in a briefing memo for the meeting. There are two devices with FDA approval, though, for aiding dermatologists. The FDA approved SciBase’s Nevisense in 2017 and Mela Sciences’ MelaFind, which has fallen out of use, in 2012. Both are class III devices.
But some companies intend to offer products for consumers in the United States. The company SkinVision, for example, has developed an app of the same name, which is intended to detect suspicious-looking skin spots via smartphone photos. SkinVision’s website says the product has been offered to consumers in Australia for remote skin checks since 2015. People in the Netherlands and United Kingdom also can use SkinVision, according to the company’s website. SkinVision says the company is working on providing the app for U.S. customers, “but we are not quite there yet.”
During the meeting, FDA panelists repeatedly emphasized the potential risks of these devices in terms of sensitivity (how often a test correctly generates a positive result) and of specificity (how often a test correctly generates a negative result).
New tools intended to aid in detection of skin cancer might produce too many false positives and thus trigger floods of worried patients seeking care and often facing unnecessary biopsies, the FDA panelists said. But more worrisome would be FDA clearance of tools that delivered too many false negative results, leaving people unaware of their cancers.
The standards would have to be set very high for new products, especially those intended for consumers, said FDA panelist Murad Alam, MD, a dermatologist and vice chair of the department of dermatology at Northwestern University, Chicago. Current technologies for analyzing skin lesions are not yet up to that task. Dr. Alam likened the situation to the hopes for self-driving cars.
“It sounds great in principle. If you read the predictions from 20 years ago, it should already have happened,” Dr. Alam said. “But we’re still struggling with that because there are serious points of failure.”
FDA panelist Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York, also argued for well-designed studies to understand how consumers and clinicians would react to new tools.
“We have to define what prospective information, in the intended use setting, we need to feel comfortable saying that these tools could be in a layperson’s hand or a primary care person’s hand,” Dr. Rotemberg said.
The studies would not need to be large, especially in the case of nonmelanoma skin cancer, which is common, she added.
“There’s too much nuance here for us to be able to say: ‘This is what would happen,’ without testing it,” Dr. Rotemberg said. “I do not think these prospective studies would be very burdensome, but they would help us understand what the burden would be and what the costs would be and what the potential harms would be.”
Because of rules against disclosing corporate information, the FDA cannot tell the public about the kinds of inquiries it already may have fielded from companies interested in selling skin cancer detection tools.
But in response to a question during the FDA meeting, Binita Ashar, MD, a top official in the FDA’s Center for Devices and Radiological Health, said there is interest in having these kinds of products sold in the United States as well.
“I can tell you that this a very timely discussion and questions that we’re posing to you are the questions that we’re encountering or that we have been grappling with,” Dr. Ashar said.
FDA panelists noted that many patients cannot get access easily to dermatology visits.
Companies seeking to develop SLA devices likely will market their tools as attempts to fill a gap that now exists in medical care.
But there will be challenges ahead in explaining to patients how to interpret readings from these tools, the FDA panelists said. Consumers should know these tools are meant to assist in diagnosis, and not to make it.
“I’m not sure the layperson will hear that,” said FDA panelist Paula E. Bourelly, MD, a dermatologist from Olney, Md.
As a result, use of SLA tools could create tension between physicians and patients, with consumers demanding biopsies after seeing readings they don’t understand.
“I do have great concerns about the layperson feeling overly confident and reducing the provider to a technician,” she said.
The FDA panelists were not asked to cast formal votes on any issues discussed during the meeting They instead engaged in broad discussions around questions posed by the FDA in three key areas:
- What standards should be used to confirm lesion diagnosis in clinical testing of the accuracy of SLA devices?
- What would be acceptable true false-positive and false-negative results (sensitivity and specificity) for different diagnoses and users?
- How can the FDA address health equity considerations based on variable incidence of skin lesions?
Developing standards
The FDA asked the panel to consider several scenarios for SLA devices and to discuss how standards might vary depending on the user of the device, whether it would be dermatologists, other clinicians, or consumers.
The agency sought comments in particular about using histological diagnosis (core specimen processing with a consensus diagnosis from an expert dermatopathologist panel). In the briefing document for the meeting, the FDA argued that this approach provides the greatest certainty in the diagnosis.
“Device developers, however, cite concerns, both practical and ethical, in requiring biopsy of all lesions, particularly those that appear benign,” the FDA said. “They have proposed alternate means of defining ground truth, including consensus opinion of experts (of visual or dermoscopic examination of the lesion[s]), opinion of one expert (visual or dermoscopic examination), or other methods.”
In summarizing the discussion on this question, the FDA panel chairman, Hobart W. Harris, MD, MPH, a surgeon from the University of California, San Francisco, noted that there was broad support for histological data in clinical trials of SLA devices, with some allowance for cases where more hybrid approaches would be used.
There were also suggestions offered about designing trials and the need for biopsies of lesions that are clearly benign, as this would help gather data to help in developing algorithms.
Dr. Alam said care should be taken in explaining to study participants that they might have to undergo biopsies that they didn’t need, as part of the larger effort to gather data. This should be detailed in the consent form, he said.
“But I also think this is a relatively minor risk,” Dr. Alam said, comparing these biopsies to the blood samples that patients in many clinical studies routinely give.
“Are all of those blood draws necessary to track the change in whatever parameters that are being tracked? Probably not,” Dr. Alam said. “I think it would be possible to explain to a reasonable patient what this entails.”
Dr. Alam noted that companies might face extra hurdles in enrolling study participants and keeping them in the trials if the FDA seeks this kind of biopsy data. “But I don’t think inconvenience to the study sponsor is a good argument” for not seeking this kind of data, he added.
Leaving a loophole where certain kinds of clearly benign lesions don’t require a biopsy would eventually erode the quality of the research done on these devices. “That bar will be moved to accommodate the convenience of the sponsor, to make the study feasible,” Dr. Alam said. “And pretty soon, you’ll be missing a lot of patients that really should have biopsies.”
Acceptable rates of false positives, false negatives
The FDA panel chair noted that his colleagues had strongly urged review standards that would require that the devices improve on the rates of successful catches of suspicious lesions and lower false positives. But they did not endorse specific targets regarding the sensitivity and specificity rates.
“No one seems to be comfortable with providing or preordaining” these targets, Dr. Harris said.
Panelist Deneen Hesser, MSHSA, RN, urged a deep recognition of the power of a FDA clearance in the view of consumers.
“We need to be cognizant of what the term ‘FDA approved’ means to the lay individual,” said Ms. Hesser, who served as the patient representative on the panel. “A patient who sees that those tools are FDA approved will assume that each of those is the gold standard” in terms of expectations for delivering accurate results.
Like many of the panelists, Dr. Rotemberg urged the FDA to gather data about how patients would react to different messages encoded in consumer-oriented products.
“If the device says: ‘You should see a dermatologist for this’ and no other information, that’s very different from [saying]: ‘That lesion is suspicious for melanoma,’ ” Dr. Rotemberg said.
Despite the likely difficulties in conducting trials, the FDA needs to have the data to answer key questions about patient and physician reactions to readings from new tools, Dr. Rotemberg said.
“We don’t know how many additional biopsies we would cause with a specificity of 80%” for a new SLA tool, Dr. Rotemberg said, giving an example. “We don’t know how confident a dermatologist might be to say: ‘Actually, I’m not suspicious about that lesion and we can just fudge it or not biopsy it.’ We don’t know any of that until we study it in real life.”
The panelists also urged the FDA to seek to ensure that new tools used in analyzing skin lesions improve the quality of diagnosis.
Addressing equity
The FDA also asked the panel to weigh in on whether the agency should clear SLA tools in cases where the existing study data is drawn heavily from people considered to be at higher risk for skin cancer.
“To ensure generalizability across the entire U.S. population, should FDA require SLAs indicated for use beyond cancerous lesions be tested in a representative U.S. population?” the FDA asked.
The three most common skin cancers – melanoma, basal cell carcinoma, and squamous cell carcinoma – are more prevalent in people with Fitzpatrick I and II skin types, who tend to get sunburns, not tans. But people of color are more likely to develop melanoma in areas that are not sun exposed, such as the sole of the foot or under fingernails or toenails.
“Due in part to lower expected risk and screening, these melanomas are often detected late,” the FDA said in the briefing document.
There was broad consensus among panelists that the FDA should encourage companies to enroll people with all skin types and tones.
But they also looked for ways that the FDA could clear devices based on initial studies conducted largely with people considered to be at higher risk, with the agency then requiring follow-up trials to see how these products would work for the general U.S. population.
A version of this article first appeared on Medscape.com.
A and how to address longstanding issues of racial equity in this field of medicine.
The Food and Drug Administration has scheduled two meetings to gather expert feedback about managing an expected expansion in the use of skin lesion apps and devices. Outside of the United States, there are apps promoted as being able to help spot skin lesions that should trigger a medical visit.
The general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee began work on this topic on July 28, with a wide-ranging discussion about potential expanded use of computer-aided, skin lesion analyzer (SLA) devices. On Friday, the panel is considering an FDA proposal to shift the designation for an approved device for aiding dermatologists in skin cancer diagnoses from the most stringent regulatory category, class III, to the less restrictive class II.
The FDA called the meeting amid growing interest in using technology to aid in finding cancers, with some of these products already marketed to consumers outside of the United States. There are presently no legally marketed, FDA-cleared or FDA-approved SLA devices indicated for use by clinicians other than dermatologists or the lay public, the agency said in a briefing memo for the meeting. There are two devices with FDA approval, though, for aiding dermatologists. The FDA approved SciBase’s Nevisense in 2017 and Mela Sciences’ MelaFind, which has fallen out of use, in 2012. Both are class III devices.
But some companies intend to offer products for consumers in the United States. The company SkinVision, for example, has developed an app of the same name, which is intended to detect suspicious-looking skin spots via smartphone photos. SkinVision’s website says the product has been offered to consumers in Australia for remote skin checks since 2015. People in the Netherlands and United Kingdom also can use SkinVision, according to the company’s website. SkinVision says the company is working on providing the app for U.S. customers, “but we are not quite there yet.”
During the meeting, FDA panelists repeatedly emphasized the potential risks of these devices in terms of sensitivity (how often a test correctly generates a positive result) and of specificity (how often a test correctly generates a negative result).
New tools intended to aid in detection of skin cancer might produce too many false positives and thus trigger floods of worried patients seeking care and often facing unnecessary biopsies, the FDA panelists said. But more worrisome would be FDA clearance of tools that delivered too many false negative results, leaving people unaware of their cancers.
The standards would have to be set very high for new products, especially those intended for consumers, said FDA panelist Murad Alam, MD, a dermatologist and vice chair of the department of dermatology at Northwestern University, Chicago. Current technologies for analyzing skin lesions are not yet up to that task. Dr. Alam likened the situation to the hopes for self-driving cars.
“It sounds great in principle. If you read the predictions from 20 years ago, it should already have happened,” Dr. Alam said. “But we’re still struggling with that because there are serious points of failure.”
FDA panelist Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York, also argued for well-designed studies to understand how consumers and clinicians would react to new tools.
“We have to define what prospective information, in the intended use setting, we need to feel comfortable saying that these tools could be in a layperson’s hand or a primary care person’s hand,” Dr. Rotemberg said.
The studies would not need to be large, especially in the case of nonmelanoma skin cancer, which is common, she added.
“There’s too much nuance here for us to be able to say: ‘This is what would happen,’ without testing it,” Dr. Rotemberg said. “I do not think these prospective studies would be very burdensome, but they would help us understand what the burden would be and what the costs would be and what the potential harms would be.”
Because of rules against disclosing corporate information, the FDA cannot tell the public about the kinds of inquiries it already may have fielded from companies interested in selling skin cancer detection tools.
But in response to a question during the FDA meeting, Binita Ashar, MD, a top official in the FDA’s Center for Devices and Radiological Health, said there is interest in having these kinds of products sold in the United States as well.
“I can tell you that this a very timely discussion and questions that we’re posing to you are the questions that we’re encountering or that we have been grappling with,” Dr. Ashar said.
FDA panelists noted that many patients cannot get access easily to dermatology visits.
Companies seeking to develop SLA devices likely will market their tools as attempts to fill a gap that now exists in medical care.
But there will be challenges ahead in explaining to patients how to interpret readings from these tools, the FDA panelists said. Consumers should know these tools are meant to assist in diagnosis, and not to make it.
“I’m not sure the layperson will hear that,” said FDA panelist Paula E. Bourelly, MD, a dermatologist from Olney, Md.
As a result, use of SLA tools could create tension between physicians and patients, with consumers demanding biopsies after seeing readings they don’t understand.
“I do have great concerns about the layperson feeling overly confident and reducing the provider to a technician,” she said.
The FDA panelists were not asked to cast formal votes on any issues discussed during the meeting They instead engaged in broad discussions around questions posed by the FDA in three key areas:
- What standards should be used to confirm lesion diagnosis in clinical testing of the accuracy of SLA devices?
- What would be acceptable true false-positive and false-negative results (sensitivity and specificity) for different diagnoses and users?
- How can the FDA address health equity considerations based on variable incidence of skin lesions?
Developing standards
The FDA asked the panel to consider several scenarios for SLA devices and to discuss how standards might vary depending on the user of the device, whether it would be dermatologists, other clinicians, or consumers.
The agency sought comments in particular about using histological diagnosis (core specimen processing with a consensus diagnosis from an expert dermatopathologist panel). In the briefing document for the meeting, the FDA argued that this approach provides the greatest certainty in the diagnosis.
“Device developers, however, cite concerns, both practical and ethical, in requiring biopsy of all lesions, particularly those that appear benign,” the FDA said. “They have proposed alternate means of defining ground truth, including consensus opinion of experts (of visual or dermoscopic examination of the lesion[s]), opinion of one expert (visual or dermoscopic examination), or other methods.”
In summarizing the discussion on this question, the FDA panel chairman, Hobart W. Harris, MD, MPH, a surgeon from the University of California, San Francisco, noted that there was broad support for histological data in clinical trials of SLA devices, with some allowance for cases where more hybrid approaches would be used.
There were also suggestions offered about designing trials and the need for biopsies of lesions that are clearly benign, as this would help gather data to help in developing algorithms.
Dr. Alam said care should be taken in explaining to study participants that they might have to undergo biopsies that they didn’t need, as part of the larger effort to gather data. This should be detailed in the consent form, he said.
“But I also think this is a relatively minor risk,” Dr. Alam said, comparing these biopsies to the blood samples that patients in many clinical studies routinely give.
“Are all of those blood draws necessary to track the change in whatever parameters that are being tracked? Probably not,” Dr. Alam said. “I think it would be possible to explain to a reasonable patient what this entails.”
Dr. Alam noted that companies might face extra hurdles in enrolling study participants and keeping them in the trials if the FDA seeks this kind of biopsy data. “But I don’t think inconvenience to the study sponsor is a good argument” for not seeking this kind of data, he added.
Leaving a loophole where certain kinds of clearly benign lesions don’t require a biopsy would eventually erode the quality of the research done on these devices. “That bar will be moved to accommodate the convenience of the sponsor, to make the study feasible,” Dr. Alam said. “And pretty soon, you’ll be missing a lot of patients that really should have biopsies.”
Acceptable rates of false positives, false negatives
The FDA panel chair noted that his colleagues had strongly urged review standards that would require that the devices improve on the rates of successful catches of suspicious lesions and lower false positives. But they did not endorse specific targets regarding the sensitivity and specificity rates.
“No one seems to be comfortable with providing or preordaining” these targets, Dr. Harris said.
Panelist Deneen Hesser, MSHSA, RN, urged a deep recognition of the power of a FDA clearance in the view of consumers.
“We need to be cognizant of what the term ‘FDA approved’ means to the lay individual,” said Ms. Hesser, who served as the patient representative on the panel. “A patient who sees that those tools are FDA approved will assume that each of those is the gold standard” in terms of expectations for delivering accurate results.
Like many of the panelists, Dr. Rotemberg urged the FDA to gather data about how patients would react to different messages encoded in consumer-oriented products.
“If the device says: ‘You should see a dermatologist for this’ and no other information, that’s very different from [saying]: ‘That lesion is suspicious for melanoma,’ ” Dr. Rotemberg said.
Despite the likely difficulties in conducting trials, the FDA needs to have the data to answer key questions about patient and physician reactions to readings from new tools, Dr. Rotemberg said.
“We don’t know how many additional biopsies we would cause with a specificity of 80%” for a new SLA tool, Dr. Rotemberg said, giving an example. “We don’t know how confident a dermatologist might be to say: ‘Actually, I’m not suspicious about that lesion and we can just fudge it or not biopsy it.’ We don’t know any of that until we study it in real life.”
The panelists also urged the FDA to seek to ensure that new tools used in analyzing skin lesions improve the quality of diagnosis.
Addressing equity
The FDA also asked the panel to weigh in on whether the agency should clear SLA tools in cases where the existing study data is drawn heavily from people considered to be at higher risk for skin cancer.
“To ensure generalizability across the entire U.S. population, should FDA require SLAs indicated for use beyond cancerous lesions be tested in a representative U.S. population?” the FDA asked.
The three most common skin cancers – melanoma, basal cell carcinoma, and squamous cell carcinoma – are more prevalent in people with Fitzpatrick I and II skin types, who tend to get sunburns, not tans. But people of color are more likely to develop melanoma in areas that are not sun exposed, such as the sole of the foot or under fingernails or toenails.
“Due in part to lower expected risk and screening, these melanomas are often detected late,” the FDA said in the briefing document.
There was broad consensus among panelists that the FDA should encourage companies to enroll people with all skin types and tones.
But they also looked for ways that the FDA could clear devices based on initial studies conducted largely with people considered to be at higher risk, with the agency then requiring follow-up trials to see how these products would work for the general U.S. population.
A version of this article first appeared on Medscape.com.
A and how to address longstanding issues of racial equity in this field of medicine.
The Food and Drug Administration has scheduled two meetings to gather expert feedback about managing an expected expansion in the use of skin lesion apps and devices. Outside of the United States, there are apps promoted as being able to help spot skin lesions that should trigger a medical visit.
The general and plastic surgery devices panel of the FDA’s Medical Devices Advisory Committee began work on this topic on July 28, with a wide-ranging discussion about potential expanded use of computer-aided, skin lesion analyzer (SLA) devices. On Friday, the panel is considering an FDA proposal to shift the designation for an approved device for aiding dermatologists in skin cancer diagnoses from the most stringent regulatory category, class III, to the less restrictive class II.
The FDA called the meeting amid growing interest in using technology to aid in finding cancers, with some of these products already marketed to consumers outside of the United States. There are presently no legally marketed, FDA-cleared or FDA-approved SLA devices indicated for use by clinicians other than dermatologists or the lay public, the agency said in a briefing memo for the meeting. There are two devices with FDA approval, though, for aiding dermatologists. The FDA approved SciBase’s Nevisense in 2017 and Mela Sciences’ MelaFind, which has fallen out of use, in 2012. Both are class III devices.
But some companies intend to offer products for consumers in the United States. The company SkinVision, for example, has developed an app of the same name, which is intended to detect suspicious-looking skin spots via smartphone photos. SkinVision’s website says the product has been offered to consumers in Australia for remote skin checks since 2015. People in the Netherlands and United Kingdom also can use SkinVision, according to the company’s website. SkinVision says the company is working on providing the app for U.S. customers, “but we are not quite there yet.”
During the meeting, FDA panelists repeatedly emphasized the potential risks of these devices in terms of sensitivity (how often a test correctly generates a positive result) and of specificity (how often a test correctly generates a negative result).
New tools intended to aid in detection of skin cancer might produce too many false positives and thus trigger floods of worried patients seeking care and often facing unnecessary biopsies, the FDA panelists said. But more worrisome would be FDA clearance of tools that delivered too many false negative results, leaving people unaware of their cancers.
The standards would have to be set very high for new products, especially those intended for consumers, said FDA panelist Murad Alam, MD, a dermatologist and vice chair of the department of dermatology at Northwestern University, Chicago. Current technologies for analyzing skin lesions are not yet up to that task. Dr. Alam likened the situation to the hopes for self-driving cars.
“It sounds great in principle. If you read the predictions from 20 years ago, it should already have happened,” Dr. Alam said. “But we’re still struggling with that because there are serious points of failure.”
FDA panelist Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York, also argued for well-designed studies to understand how consumers and clinicians would react to new tools.
“We have to define what prospective information, in the intended use setting, we need to feel comfortable saying that these tools could be in a layperson’s hand or a primary care person’s hand,” Dr. Rotemberg said.
The studies would not need to be large, especially in the case of nonmelanoma skin cancer, which is common, she added.
“There’s too much nuance here for us to be able to say: ‘This is what would happen,’ without testing it,” Dr. Rotemberg said. “I do not think these prospective studies would be very burdensome, but they would help us understand what the burden would be and what the costs would be and what the potential harms would be.”
Because of rules against disclosing corporate information, the FDA cannot tell the public about the kinds of inquiries it already may have fielded from companies interested in selling skin cancer detection tools.
But in response to a question during the FDA meeting, Binita Ashar, MD, a top official in the FDA’s Center for Devices and Radiological Health, said there is interest in having these kinds of products sold in the United States as well.
“I can tell you that this a very timely discussion and questions that we’re posing to you are the questions that we’re encountering or that we have been grappling with,” Dr. Ashar said.
FDA panelists noted that many patients cannot get access easily to dermatology visits.
Companies seeking to develop SLA devices likely will market their tools as attempts to fill a gap that now exists in medical care.
But there will be challenges ahead in explaining to patients how to interpret readings from these tools, the FDA panelists said. Consumers should know these tools are meant to assist in diagnosis, and not to make it.
“I’m not sure the layperson will hear that,” said FDA panelist Paula E. Bourelly, MD, a dermatologist from Olney, Md.
As a result, use of SLA tools could create tension between physicians and patients, with consumers demanding biopsies after seeing readings they don’t understand.
“I do have great concerns about the layperson feeling overly confident and reducing the provider to a technician,” she said.
The FDA panelists were not asked to cast formal votes on any issues discussed during the meeting They instead engaged in broad discussions around questions posed by the FDA in three key areas:
- What standards should be used to confirm lesion diagnosis in clinical testing of the accuracy of SLA devices?
- What would be acceptable true false-positive and false-negative results (sensitivity and specificity) for different diagnoses and users?
- How can the FDA address health equity considerations based on variable incidence of skin lesions?
Developing standards
The FDA asked the panel to consider several scenarios for SLA devices and to discuss how standards might vary depending on the user of the device, whether it would be dermatologists, other clinicians, or consumers.
The agency sought comments in particular about using histological diagnosis (core specimen processing with a consensus diagnosis from an expert dermatopathologist panel). In the briefing document for the meeting, the FDA argued that this approach provides the greatest certainty in the diagnosis.
“Device developers, however, cite concerns, both practical and ethical, in requiring biopsy of all lesions, particularly those that appear benign,” the FDA said. “They have proposed alternate means of defining ground truth, including consensus opinion of experts (of visual or dermoscopic examination of the lesion[s]), opinion of one expert (visual or dermoscopic examination), or other methods.”
In summarizing the discussion on this question, the FDA panel chairman, Hobart W. Harris, MD, MPH, a surgeon from the University of California, San Francisco, noted that there was broad support for histological data in clinical trials of SLA devices, with some allowance for cases where more hybrid approaches would be used.
There were also suggestions offered about designing trials and the need for biopsies of lesions that are clearly benign, as this would help gather data to help in developing algorithms.
Dr. Alam said care should be taken in explaining to study participants that they might have to undergo biopsies that they didn’t need, as part of the larger effort to gather data. This should be detailed in the consent form, he said.
“But I also think this is a relatively minor risk,” Dr. Alam said, comparing these biopsies to the blood samples that patients in many clinical studies routinely give.
“Are all of those blood draws necessary to track the change in whatever parameters that are being tracked? Probably not,” Dr. Alam said. “I think it would be possible to explain to a reasonable patient what this entails.”
Dr. Alam noted that companies might face extra hurdles in enrolling study participants and keeping them in the trials if the FDA seeks this kind of biopsy data. “But I don’t think inconvenience to the study sponsor is a good argument” for not seeking this kind of data, he added.
Leaving a loophole where certain kinds of clearly benign lesions don’t require a biopsy would eventually erode the quality of the research done on these devices. “That bar will be moved to accommodate the convenience of the sponsor, to make the study feasible,” Dr. Alam said. “And pretty soon, you’ll be missing a lot of patients that really should have biopsies.”
Acceptable rates of false positives, false negatives
The FDA panel chair noted that his colleagues had strongly urged review standards that would require that the devices improve on the rates of successful catches of suspicious lesions and lower false positives. But they did not endorse specific targets regarding the sensitivity and specificity rates.
“No one seems to be comfortable with providing or preordaining” these targets, Dr. Harris said.
Panelist Deneen Hesser, MSHSA, RN, urged a deep recognition of the power of a FDA clearance in the view of consumers.
“We need to be cognizant of what the term ‘FDA approved’ means to the lay individual,” said Ms. Hesser, who served as the patient representative on the panel. “A patient who sees that those tools are FDA approved will assume that each of those is the gold standard” in terms of expectations for delivering accurate results.
Like many of the panelists, Dr. Rotemberg urged the FDA to gather data about how patients would react to different messages encoded in consumer-oriented products.
“If the device says: ‘You should see a dermatologist for this’ and no other information, that’s very different from [saying]: ‘That lesion is suspicious for melanoma,’ ” Dr. Rotemberg said.
Despite the likely difficulties in conducting trials, the FDA needs to have the data to answer key questions about patient and physician reactions to readings from new tools, Dr. Rotemberg said.
“We don’t know how many additional biopsies we would cause with a specificity of 80%” for a new SLA tool, Dr. Rotemberg said, giving an example. “We don’t know how confident a dermatologist might be to say: ‘Actually, I’m not suspicious about that lesion and we can just fudge it or not biopsy it.’ We don’t know any of that until we study it in real life.”
The panelists also urged the FDA to seek to ensure that new tools used in analyzing skin lesions improve the quality of diagnosis.
Addressing equity
The FDA also asked the panel to weigh in on whether the agency should clear SLA tools in cases where the existing study data is drawn heavily from people considered to be at higher risk for skin cancer.
“To ensure generalizability across the entire U.S. population, should FDA require SLAs indicated for use beyond cancerous lesions be tested in a representative U.S. population?” the FDA asked.
The three most common skin cancers – melanoma, basal cell carcinoma, and squamous cell carcinoma – are more prevalent in people with Fitzpatrick I and II skin types, who tend to get sunburns, not tans. But people of color are more likely to develop melanoma in areas that are not sun exposed, such as the sole of the foot or under fingernails or toenails.
“Due in part to lower expected risk and screening, these melanomas are often detected late,” the FDA said in the briefing document.
There was broad consensus among panelists that the FDA should encourage companies to enroll people with all skin types and tones.
But they also looked for ways that the FDA could clear devices based on initial studies conducted largely with people considered to be at higher risk, with the agency then requiring follow-up trials to see how these products would work for the general U.S. population.
A version of this article first appeared on Medscape.com.
Amazon involved with new cancer vaccine clinical trial
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
Pembrolizumab for melanoma bittersweet, doctor says
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
AT ASCO 2022
Study explores gender differences in pediatric melanoma
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Quality of life benefit exaggerated in some cancer studies
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
FROM JAMA ONCOLOGY
Melanoma incidence is up, but death rates are down
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
AT ASCO 2022
Evidence still lacking that vitamins prevent CVD, cancer: USPSTF
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
FROM JAMA











