User login
Tanning Attitudes and Behaviors in Adolescents and Young Adults
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.

Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
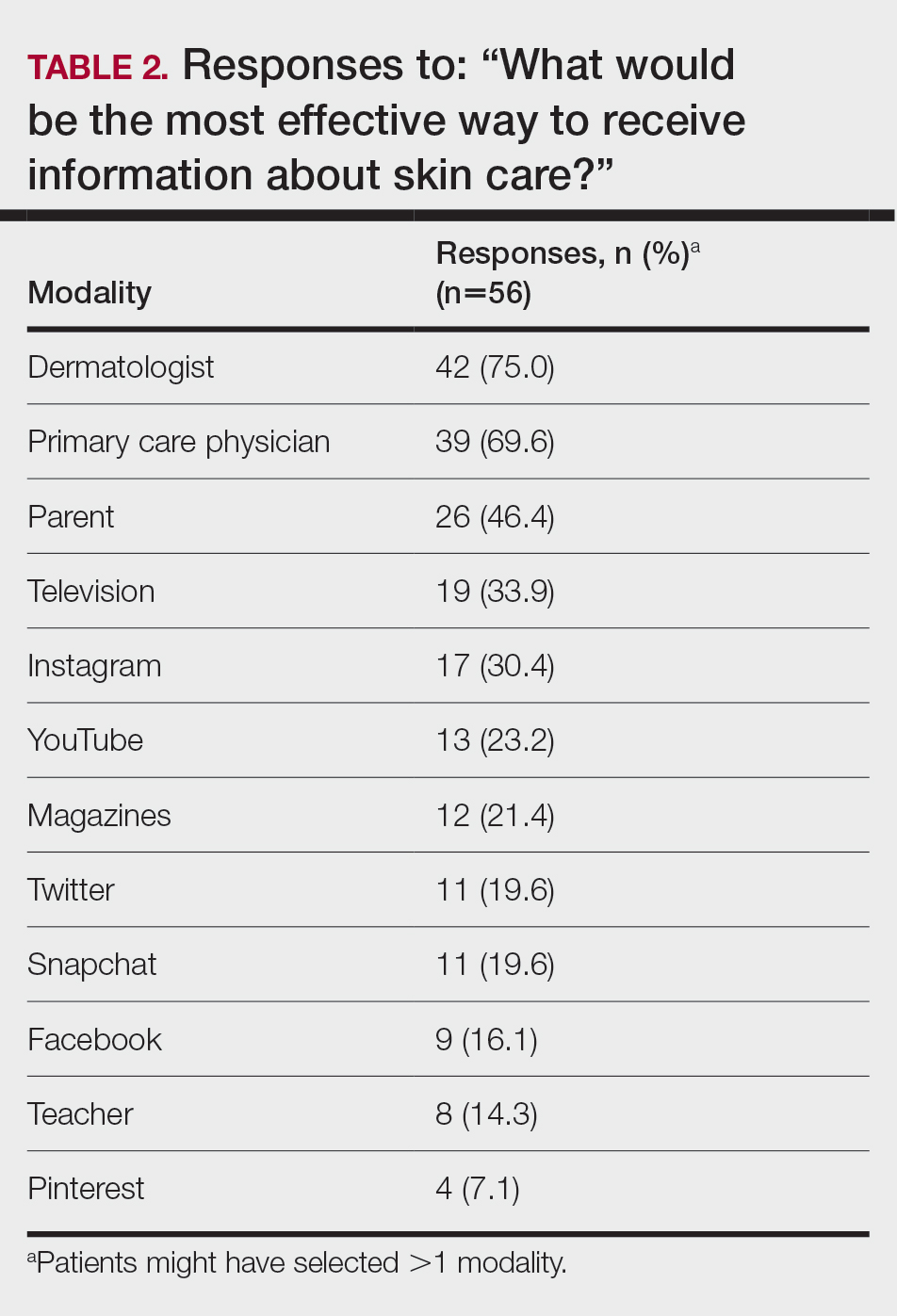
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.

Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).

Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.

Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).

Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
PRACTICE POINTS
- Dermatologists are the preferred educators of skin care for adolescents and young adults.
- Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist.
- Education of young people focusing on their concerns about maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Communication Strategies in Mohs Micrographic Surgery: A Survey of Methods, Time Savings, and Perceived Patient Satisfaction
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
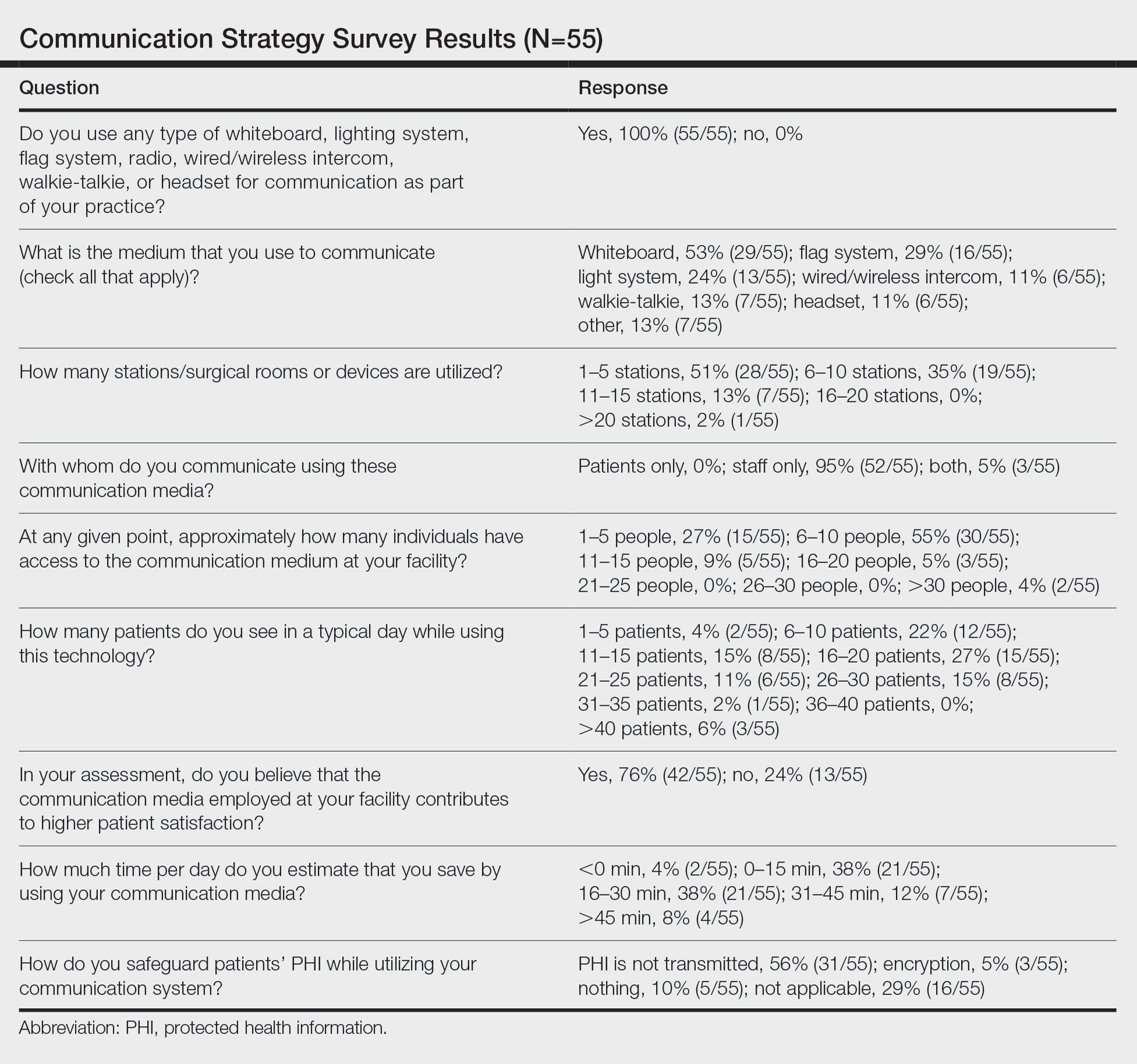
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
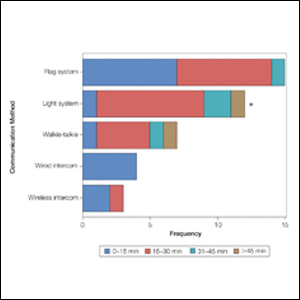
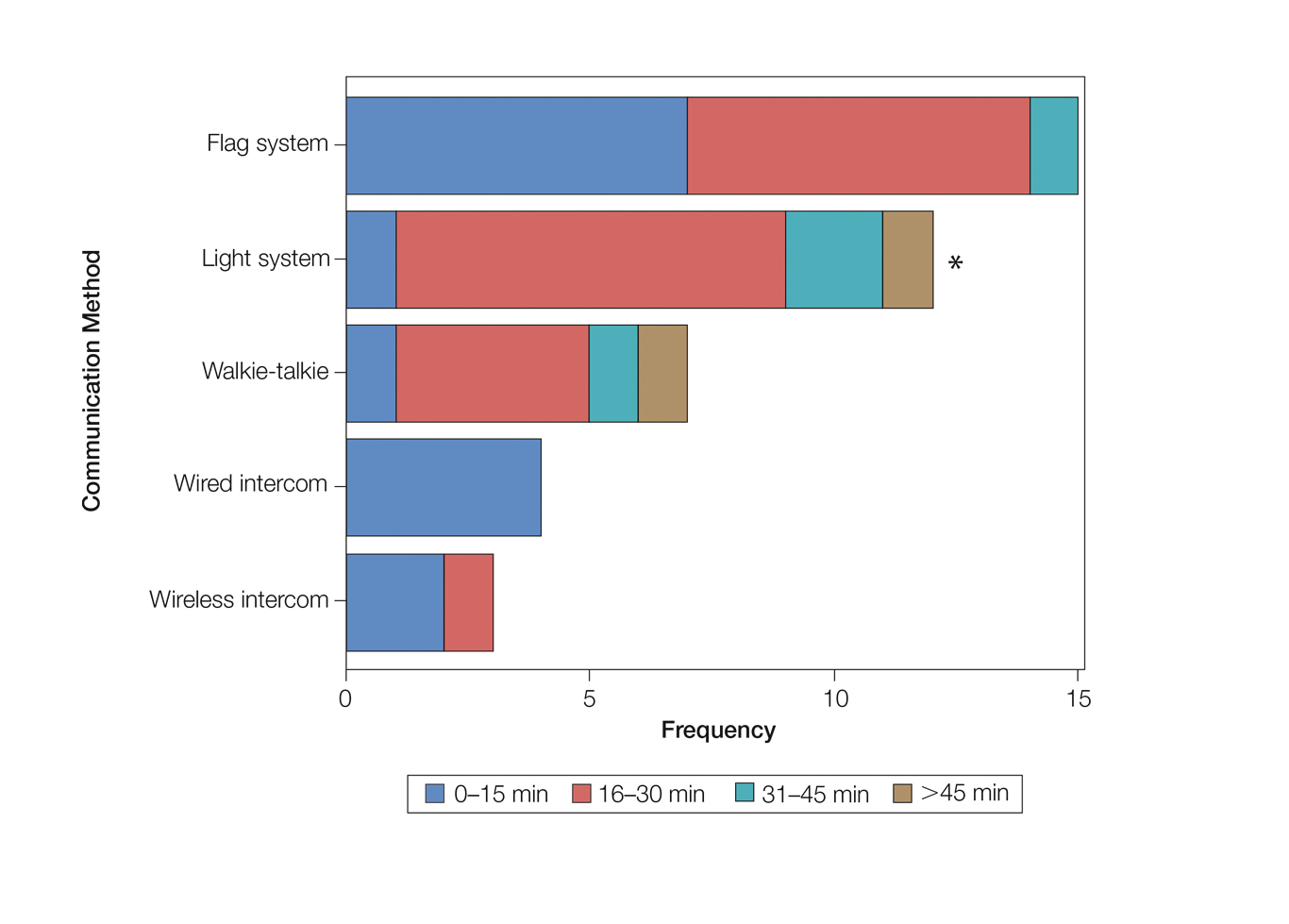
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Practice Points
- There are limited studies evaluating the efficacy of different communication methods in Mohs micrographic surgery (MMS) clinics.
- This study suggests that incorporation of a light-based system into an MMS clinic improves workplace efficiency.
Checkpoint inhibitor skin side effects more common in women
of 235 patients at Dana Farber Cancer Center, Boston.
Overall, 62.4% of the 93 women in the review and 48.6% of the 142 men experienced confirmed skin reactions, for an odds ratio (OR) of 2.11 for women compared with men (P = .01).
“Clinicians should consider these results in counseling female patients regarding an elevated risk of dermatologic adverse events” when taking checkpoint inhibitors, said investigators led by Harvard University medical student Jordan Said, who presented the results at the American Academy of Dermatology Virtual Meeting Experience.
Autoimmune-like adverse events are common with checkpoint inhibitors. Dermatologic side effects occur in about half of people receiving monotherapy and more than that among patients receiving combination therapy.
Skin reactions can include psoriasiform dermatitis, lichenoid reactions, vitiligo, and bullous pemphigoid and may require hospitalization and prolonged steroid treatment.
Not much is known about risk factors for these reactions. A higher incidence among women has been previously reported. A 2019 study found a higher risk for pneumonitis and endocrinopathy, including hypophysitis, among women who underwent treatment for non–small cell lung cancer or metastatic melanoma.
The 2019 study found that the risk was higher among premenopausal women than postmenopausal women, which led some to suggest that estrogen may play a role.
The results of the Dana Farber review argue against that notion. In their review, the investigators found that the risk was similarly elevated among the 27 premenopausal women (OR, 1.97; P = .40) and the 66 postmenopausal women (OR, 2.17, P = .05). In the study, women who were aged 52 years or older at the start of treatment were considered to be postmenopausal.
“This suggests that factors beyond sex hormones are likely contributory” to the difference in risk between men and women. It’s known that women are at higher risk for autoimmune disease overall, which might be related to the increased odds of autoimmune-like reactions, and it may be that sex-related differences in innate and adoptive immunity are at work, Mr. Said noted.
When asked for comment, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn., said that although some studies have reported a greater risk for side effects among women, others have not. “Additional research is needed to determine the interactions between sex and effects of immune checkpoint inhibitors, as well as many other possible triggers of immune-related adverse events,” he said.
“Continued work in this area will be so important to help determine how to best counsel women and to ensure early recognition and intervention for dermatologic side effects,” said Bernice Kwong, MD, director of the Supportive Dermato-Oncology Program at Stanford (Calif.) University.
The patients in the review were treated from 2011 to 2016 and underwent at least monthly evaluations by their medical teams. They were taking either nivolumab, pembrolizumab, or ipilimumab or a nivolumab/ipilimumab combination.
The median age of the men in the study was 65 years; the median age of women was 60 years. Almost 98% of the participants were White. The majority received one to three infusions, most commonly with pembrolizumab monotherapy.
No funding for the study was reported. Mr. Said has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
of 235 patients at Dana Farber Cancer Center, Boston.
Overall, 62.4% of the 93 women in the review and 48.6% of the 142 men experienced confirmed skin reactions, for an odds ratio (OR) of 2.11 for women compared with men (P = .01).
“Clinicians should consider these results in counseling female patients regarding an elevated risk of dermatologic adverse events” when taking checkpoint inhibitors, said investigators led by Harvard University medical student Jordan Said, who presented the results at the American Academy of Dermatology Virtual Meeting Experience.
Autoimmune-like adverse events are common with checkpoint inhibitors. Dermatologic side effects occur in about half of people receiving monotherapy and more than that among patients receiving combination therapy.
Skin reactions can include psoriasiform dermatitis, lichenoid reactions, vitiligo, and bullous pemphigoid and may require hospitalization and prolonged steroid treatment.
Not much is known about risk factors for these reactions. A higher incidence among women has been previously reported. A 2019 study found a higher risk for pneumonitis and endocrinopathy, including hypophysitis, among women who underwent treatment for non–small cell lung cancer or metastatic melanoma.
The 2019 study found that the risk was higher among premenopausal women than postmenopausal women, which led some to suggest that estrogen may play a role.
The results of the Dana Farber review argue against that notion. In their review, the investigators found that the risk was similarly elevated among the 27 premenopausal women (OR, 1.97; P = .40) and the 66 postmenopausal women (OR, 2.17, P = .05). In the study, women who were aged 52 years or older at the start of treatment were considered to be postmenopausal.
“This suggests that factors beyond sex hormones are likely contributory” to the difference in risk between men and women. It’s known that women are at higher risk for autoimmune disease overall, which might be related to the increased odds of autoimmune-like reactions, and it may be that sex-related differences in innate and adoptive immunity are at work, Mr. Said noted.
When asked for comment, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn., said that although some studies have reported a greater risk for side effects among women, others have not. “Additional research is needed to determine the interactions between sex and effects of immune checkpoint inhibitors, as well as many other possible triggers of immune-related adverse events,” he said.
“Continued work in this area will be so important to help determine how to best counsel women and to ensure early recognition and intervention for dermatologic side effects,” said Bernice Kwong, MD, director of the Supportive Dermato-Oncology Program at Stanford (Calif.) University.
The patients in the review were treated from 2011 to 2016 and underwent at least monthly evaluations by their medical teams. They were taking either nivolumab, pembrolizumab, or ipilimumab or a nivolumab/ipilimumab combination.
The median age of the men in the study was 65 years; the median age of women was 60 years. Almost 98% of the participants were White. The majority received one to three infusions, most commonly with pembrolizumab monotherapy.
No funding for the study was reported. Mr. Said has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
of 235 patients at Dana Farber Cancer Center, Boston.
Overall, 62.4% of the 93 women in the review and 48.6% of the 142 men experienced confirmed skin reactions, for an odds ratio (OR) of 2.11 for women compared with men (P = .01).
“Clinicians should consider these results in counseling female patients regarding an elevated risk of dermatologic adverse events” when taking checkpoint inhibitors, said investigators led by Harvard University medical student Jordan Said, who presented the results at the American Academy of Dermatology Virtual Meeting Experience.
Autoimmune-like adverse events are common with checkpoint inhibitors. Dermatologic side effects occur in about half of people receiving monotherapy and more than that among patients receiving combination therapy.
Skin reactions can include psoriasiform dermatitis, lichenoid reactions, vitiligo, and bullous pemphigoid and may require hospitalization and prolonged steroid treatment.
Not much is known about risk factors for these reactions. A higher incidence among women has been previously reported. A 2019 study found a higher risk for pneumonitis and endocrinopathy, including hypophysitis, among women who underwent treatment for non–small cell lung cancer or metastatic melanoma.
The 2019 study found that the risk was higher among premenopausal women than postmenopausal women, which led some to suggest that estrogen may play a role.
The results of the Dana Farber review argue against that notion. In their review, the investigators found that the risk was similarly elevated among the 27 premenopausal women (OR, 1.97; P = .40) and the 66 postmenopausal women (OR, 2.17, P = .05). In the study, women who were aged 52 years or older at the start of treatment were considered to be postmenopausal.
“This suggests that factors beyond sex hormones are likely contributory” to the difference in risk between men and women. It’s known that women are at higher risk for autoimmune disease overall, which might be related to the increased odds of autoimmune-like reactions, and it may be that sex-related differences in innate and adoptive immunity are at work, Mr. Said noted.
When asked for comment, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn., said that although some studies have reported a greater risk for side effects among women, others have not. “Additional research is needed to determine the interactions between sex and effects of immune checkpoint inhibitors, as well as many other possible triggers of immune-related adverse events,” he said.
“Continued work in this area will be so important to help determine how to best counsel women and to ensure early recognition and intervention for dermatologic side effects,” said Bernice Kwong, MD, director of the Supportive Dermato-Oncology Program at Stanford (Calif.) University.
The patients in the review were treated from 2011 to 2016 and underwent at least monthly evaluations by their medical teams. They were taking either nivolumab, pembrolizumab, or ipilimumab or a nivolumab/ipilimumab combination.
The median age of the men in the study was 65 years; the median age of women was 60 years. Almost 98% of the participants were White. The majority received one to three infusions, most commonly with pembrolizumab monotherapy.
No funding for the study was reported. Mr. Said has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment Delay in Melanoma: A Risk Factor Analysis of an Impending Crisis
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.

Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.

Statistical Methods
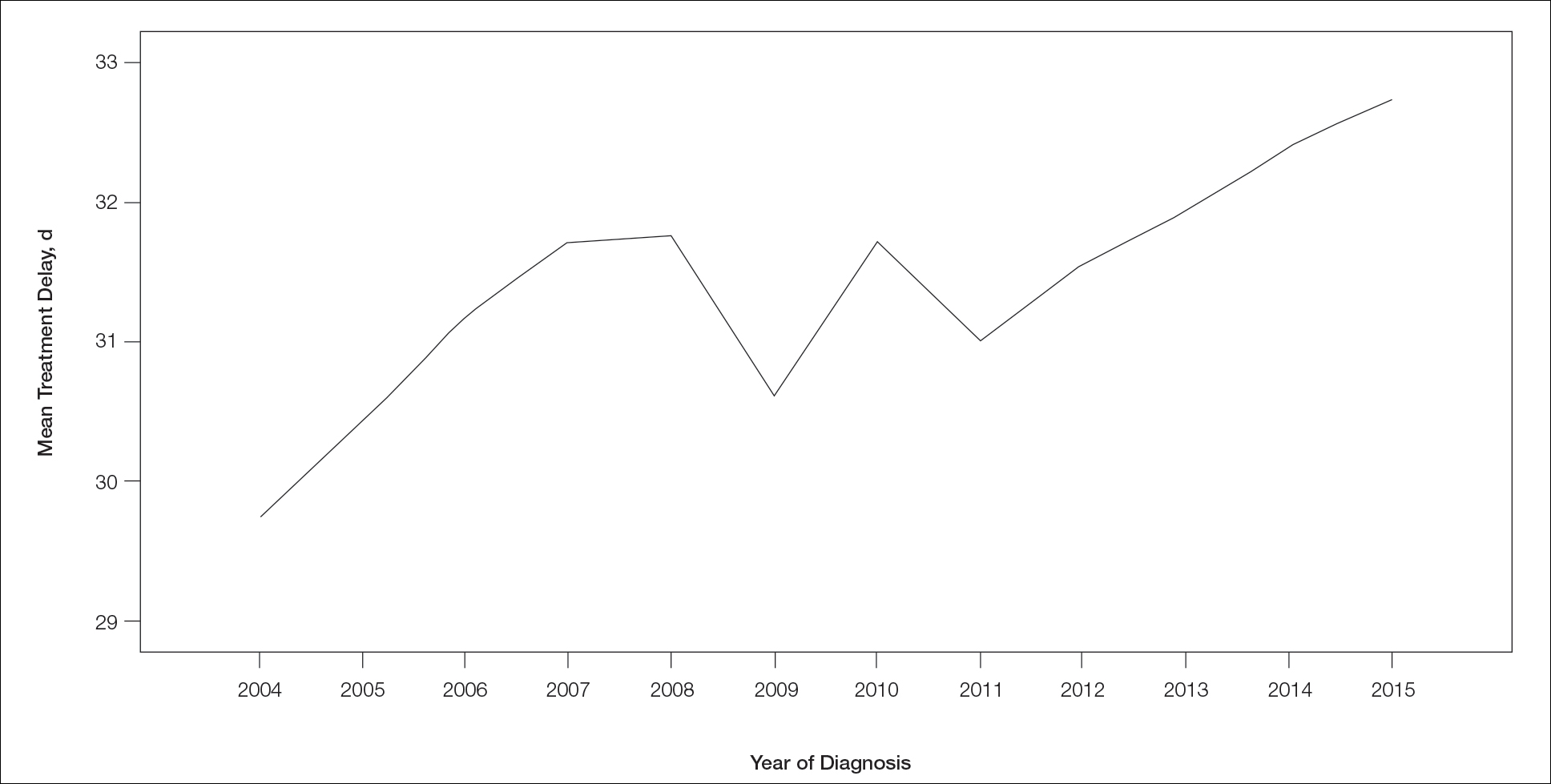
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
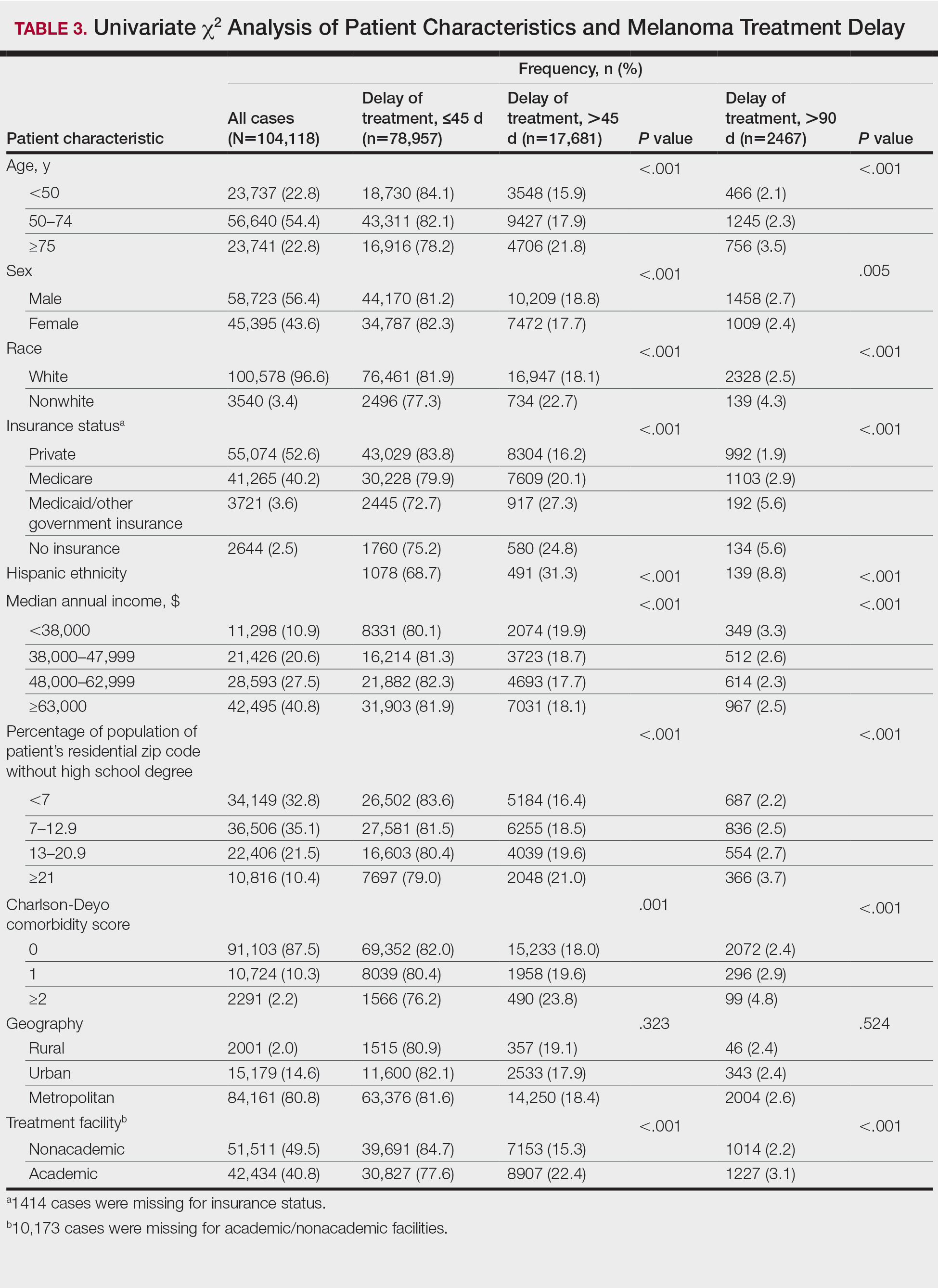
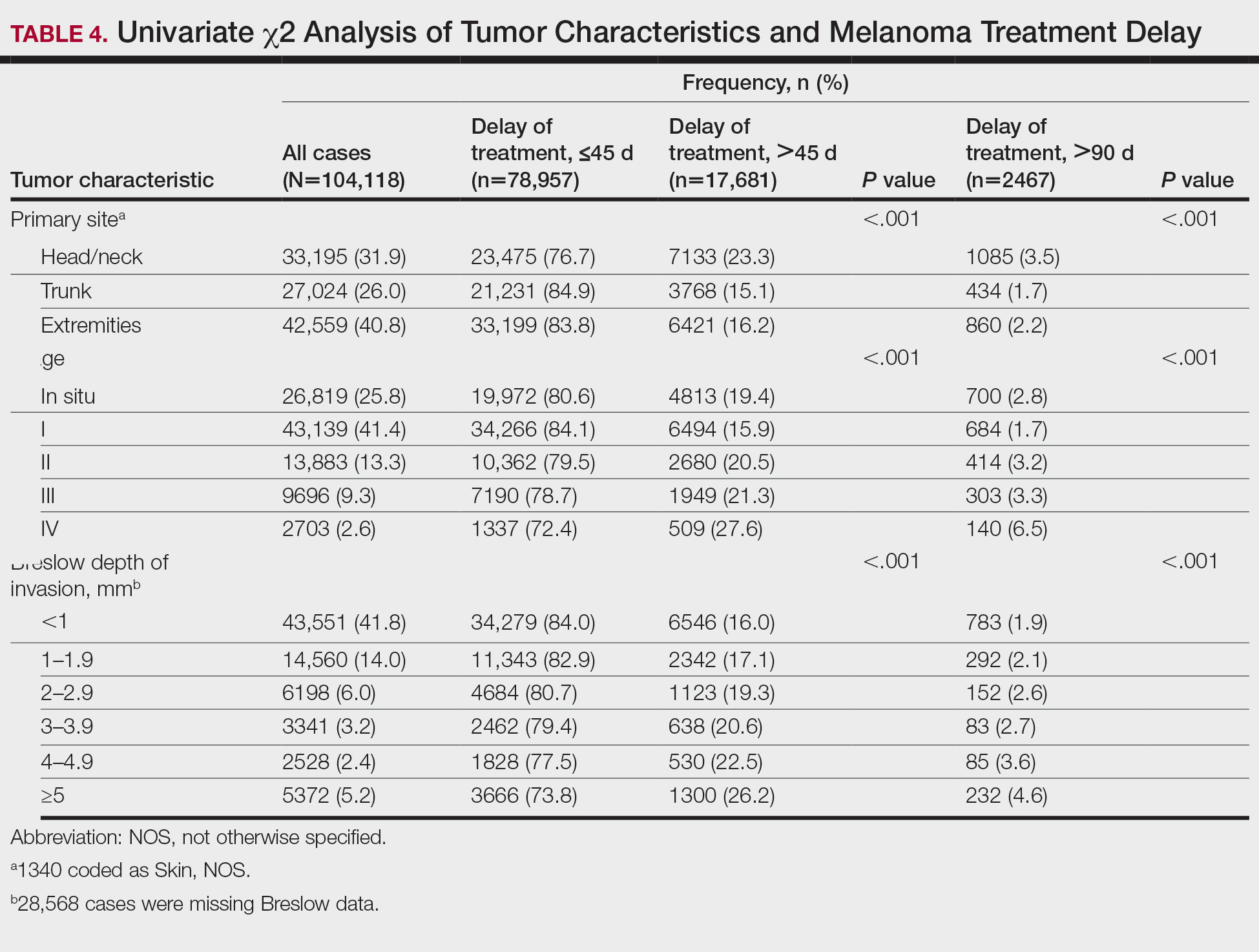
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
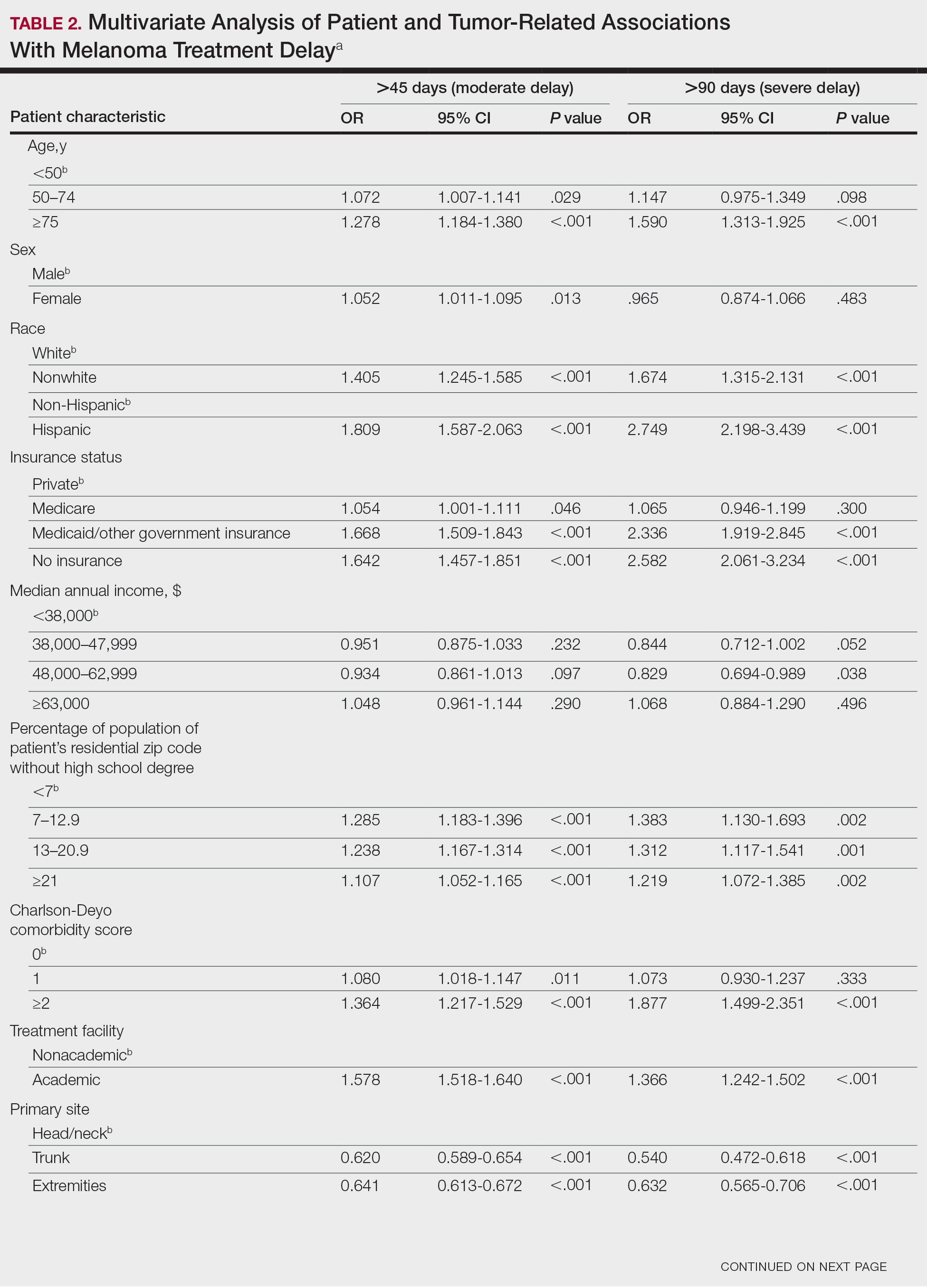
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.

Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.


Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).

Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).


On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.

Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.


Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).

Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).


On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Practice Points
- Melanoma treatment delays (MTDs) have been linked to poor outcomes.
- Based on the National Cancer Database, the mean MTD has increased significantly from 2004 to 2015 (P11<.001).
- More delays are seen in patients who are older than 50 years, female, nonwhite, not privately insured, and treated at an academic facility and who have more advanced tumor stage and head/neck primaries.
For diagnosing skin lesions, AI risks failing in skin of color
In the analysis of images for detecting potential pathology, if training does not specifically address these skin types, according to Adewole S. Adamson, MD, who outlined this issue at the American Academy of Dermatology Virtual Meeting Experience.
“Machine learning algorithms are only as good as the inputs through which they learn. Without representation from individuals with skin of color, we are at risk of creating a new source of racial disparity in patient care,” Dr. Adamson, assistant professor in the division of dermatology, department of internal medicine, University of Texas at Austin, said at the meeting.
Diagnostic algorithms using AI are typically based on deep learning, a subset of machine learning that depends on artificial neural networks. In the case of image processing, neural networks can “learn” to recognize objects, faces, or, in the realm of health care, disease, from exposure to multiple images.
There are many other variables that affect the accuracy of deep learning for diagnostic algorithms, including the depth of the layering through which the process distills multiple inputs of information, but the number of inputs is critical. In the case of skin lesions, machines cannot learn to recognize features of different skin types without exposure.
“There are studies demonstrating that dermatologists can be outperformed for detection of skin cancers by AI, so this is going to be an increasingly powerful tool,” Dr. Adamson said. The problem is that “there has been very little representation in darker skin types” in the algorithms developed so far.
The risk is that AI will exacerbate an existing problem. Skin cancer in darker skin is less common but already underdiagnosed, independent of AI. Per 100,000 males in the United States, the rate of melanoma is about 30-fold greater in White men than in Black men (33.0 vs. 1.0). Among females, the racial difference is smaller but still enormous (20.2 vs. 1.2 per 100,000 females), according to U.S. data.
For the low representation of darker skin in studies so far with AI, “one of the arguments is that skin cancer is not a big deal in darker skin types,” Dr. Adamson said.
It might be the other way around. The relative infrequency with which skin cancer occurs in the Black population in the United States might explain a low level of suspicion and ultimately delays in diagnosis, which, in turn, leads to worse outcomes. According to one analysis drawn from the Surveillance, Epidemiology and End-Result (SEER) database (1998-2011), the proportion of patients with regionally advanced or distant disease was nearly twice as great (11.6% vs. 6.0%; P < .05) in Black patients, relative to White patients.
Not surprisingly, given the importance of early diagnosis of cancers overall and skin cancer specifically, the mean survival for malignant melanoma in Black patients was almost 4 years lower than in White patients (10.8 vs. 14.6 years; P < .001) for nodular melanoma, the same study found.
In humans, bias is reasonably attributed in many cases to judgments made on a small sample size. The problem in AI is analogous. Dr. Adamson, who has published research on the potential for machine learning to contribute to health care disparities in dermatology, cited work done by Joy Buolamwini, a graduate researcher in the media lab at the Massachusetts Institute of Technology. In one study she conducted, the rate of AI facial recognition failure was 1% in White males, 7% in White females, 12% in skin-of-color males, and 35% in skin-of-color females. Fewer inputs of skin of color is the likely explanation, Dr. Adamson said.
The potential for racial bias from AI in the diagnosis of disease increases and becomes more complex when inputs beyond imaging, such as past medical history, are included. Dr. Adamson warned of the potential for “bias to creep in” when there is failure to account for societal, cultural, or other differences that distinguish one patient group from another. However, for skin cancer or other diseases based on images alone, he said there are solutions.
“We are in the early days, and there is time to change this,” Dr. Adamson said, referring to the low representation of skin of color in AI training sets. In addition to including more skin types to train recognition, creating AI algorithms specifically for dark skin is another potential approach.
However, his key point was the importance of recognizing the need for solutions.
“AI is the future, but we must apply the same rigor to AI as to other medical interventions to ensure that the technology is not applied in a biased fashion,” he said.
Susan M. Swetter, MD, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, agreed. As someone who has been following the progress of AI in the diagnosis of skin cancer, Dr. Swetter recognizes the potential for this technology to increase diagnostic efficiency and accuracy, but she also called for studies specific to skin of color.
The algorithms “have not yet been adequately evaluated in people of color, particularly Black patients in whom dermoscopic criteria for benign versus malignant melanocytic neoplasms differ from those with lighter skin types,” Dr. Swetter said in an interview.
She sees the same fix as that proposed by Dr. Adamson.
“Efforts to include skin of color in AI algorithms for validation and further training are needed to prevent potential harms of over- or underdiagnosis in darker skin patients,” she pointed out.
Dr. Adamson reports no potential conflicts of interest relevant to this topic. Dr. Swetter had no relevant disclosures.
In the analysis of images for detecting potential pathology, if training does not specifically address these skin types, according to Adewole S. Adamson, MD, who outlined this issue at the American Academy of Dermatology Virtual Meeting Experience.
“Machine learning algorithms are only as good as the inputs through which they learn. Without representation from individuals with skin of color, we are at risk of creating a new source of racial disparity in patient care,” Dr. Adamson, assistant professor in the division of dermatology, department of internal medicine, University of Texas at Austin, said at the meeting.
Diagnostic algorithms using AI are typically based on deep learning, a subset of machine learning that depends on artificial neural networks. In the case of image processing, neural networks can “learn” to recognize objects, faces, or, in the realm of health care, disease, from exposure to multiple images.
There are many other variables that affect the accuracy of deep learning for diagnostic algorithms, including the depth of the layering through which the process distills multiple inputs of information, but the number of inputs is critical. In the case of skin lesions, machines cannot learn to recognize features of different skin types without exposure.
“There are studies demonstrating that dermatologists can be outperformed for detection of skin cancers by AI, so this is going to be an increasingly powerful tool,” Dr. Adamson said. The problem is that “there has been very little representation in darker skin types” in the algorithms developed so far.
The risk is that AI will exacerbate an existing problem. Skin cancer in darker skin is less common but already underdiagnosed, independent of AI. Per 100,000 males in the United States, the rate of melanoma is about 30-fold greater in White men than in Black men (33.0 vs. 1.0). Among females, the racial difference is smaller but still enormous (20.2 vs. 1.2 per 100,000 females), according to U.S. data.
For the low representation of darker skin in studies so far with AI, “one of the arguments is that skin cancer is not a big deal in darker skin types,” Dr. Adamson said.
It might be the other way around. The relative infrequency with which skin cancer occurs in the Black population in the United States might explain a low level of suspicion and ultimately delays in diagnosis, which, in turn, leads to worse outcomes. According to one analysis drawn from the Surveillance, Epidemiology and End-Result (SEER) database (1998-2011), the proportion of patients with regionally advanced or distant disease was nearly twice as great (11.6% vs. 6.0%; P < .05) in Black patients, relative to White patients.
Not surprisingly, given the importance of early diagnosis of cancers overall and skin cancer specifically, the mean survival for malignant melanoma in Black patients was almost 4 years lower than in White patients (10.8 vs. 14.6 years; P < .001) for nodular melanoma, the same study found.
In humans, bias is reasonably attributed in many cases to judgments made on a small sample size. The problem in AI is analogous. Dr. Adamson, who has published research on the potential for machine learning to contribute to health care disparities in dermatology, cited work done by Joy Buolamwini, a graduate researcher in the media lab at the Massachusetts Institute of Technology. In one study she conducted, the rate of AI facial recognition failure was 1% in White males, 7% in White females, 12% in skin-of-color males, and 35% in skin-of-color females. Fewer inputs of skin of color is the likely explanation, Dr. Adamson said.
The potential for racial bias from AI in the diagnosis of disease increases and becomes more complex when inputs beyond imaging, such as past medical history, are included. Dr. Adamson warned of the potential for “bias to creep in” when there is failure to account for societal, cultural, or other differences that distinguish one patient group from another. However, for skin cancer or other diseases based on images alone, he said there are solutions.
“We are in the early days, and there is time to change this,” Dr. Adamson said, referring to the low representation of skin of color in AI training sets. In addition to including more skin types to train recognition, creating AI algorithms specifically for dark skin is another potential approach.
However, his key point was the importance of recognizing the need for solutions.
“AI is the future, but we must apply the same rigor to AI as to other medical interventions to ensure that the technology is not applied in a biased fashion,” he said.
Susan M. Swetter, MD, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, agreed. As someone who has been following the progress of AI in the diagnosis of skin cancer, Dr. Swetter recognizes the potential for this technology to increase diagnostic efficiency and accuracy, but she also called for studies specific to skin of color.
The algorithms “have not yet been adequately evaluated in people of color, particularly Black patients in whom dermoscopic criteria for benign versus malignant melanocytic neoplasms differ from those with lighter skin types,” Dr. Swetter said in an interview.
She sees the same fix as that proposed by Dr. Adamson.
“Efforts to include skin of color in AI algorithms for validation and further training are needed to prevent potential harms of over- or underdiagnosis in darker skin patients,” she pointed out.
Dr. Adamson reports no potential conflicts of interest relevant to this topic. Dr. Swetter had no relevant disclosures.
In the analysis of images for detecting potential pathology, if training does not specifically address these skin types, according to Adewole S. Adamson, MD, who outlined this issue at the American Academy of Dermatology Virtual Meeting Experience.
“Machine learning algorithms are only as good as the inputs through which they learn. Without representation from individuals with skin of color, we are at risk of creating a new source of racial disparity in patient care,” Dr. Adamson, assistant professor in the division of dermatology, department of internal medicine, University of Texas at Austin, said at the meeting.
Diagnostic algorithms using AI are typically based on deep learning, a subset of machine learning that depends on artificial neural networks. In the case of image processing, neural networks can “learn” to recognize objects, faces, or, in the realm of health care, disease, from exposure to multiple images.
There are many other variables that affect the accuracy of deep learning for diagnostic algorithms, including the depth of the layering through which the process distills multiple inputs of information, but the number of inputs is critical. In the case of skin lesions, machines cannot learn to recognize features of different skin types without exposure.
“There are studies demonstrating that dermatologists can be outperformed for detection of skin cancers by AI, so this is going to be an increasingly powerful tool,” Dr. Adamson said. The problem is that “there has been very little representation in darker skin types” in the algorithms developed so far.
The risk is that AI will exacerbate an existing problem. Skin cancer in darker skin is less common but already underdiagnosed, independent of AI. Per 100,000 males in the United States, the rate of melanoma is about 30-fold greater in White men than in Black men (33.0 vs. 1.0). Among females, the racial difference is smaller but still enormous (20.2 vs. 1.2 per 100,000 females), according to U.S. data.
For the low representation of darker skin in studies so far with AI, “one of the arguments is that skin cancer is not a big deal in darker skin types,” Dr. Adamson said.
It might be the other way around. The relative infrequency with which skin cancer occurs in the Black population in the United States might explain a low level of suspicion and ultimately delays in diagnosis, which, in turn, leads to worse outcomes. According to one analysis drawn from the Surveillance, Epidemiology and End-Result (SEER) database (1998-2011), the proportion of patients with regionally advanced or distant disease was nearly twice as great (11.6% vs. 6.0%; P < .05) in Black patients, relative to White patients.
Not surprisingly, given the importance of early diagnosis of cancers overall and skin cancer specifically, the mean survival for malignant melanoma in Black patients was almost 4 years lower than in White patients (10.8 vs. 14.6 years; P < .001) for nodular melanoma, the same study found.
In humans, bias is reasonably attributed in many cases to judgments made on a small sample size. The problem in AI is analogous. Dr. Adamson, who has published research on the potential for machine learning to contribute to health care disparities in dermatology, cited work done by Joy Buolamwini, a graduate researcher in the media lab at the Massachusetts Institute of Technology. In one study she conducted, the rate of AI facial recognition failure was 1% in White males, 7% in White females, 12% in skin-of-color males, and 35% in skin-of-color females. Fewer inputs of skin of color is the likely explanation, Dr. Adamson said.
The potential for racial bias from AI in the diagnosis of disease increases and becomes more complex when inputs beyond imaging, such as past medical history, are included. Dr. Adamson warned of the potential for “bias to creep in” when there is failure to account for societal, cultural, or other differences that distinguish one patient group from another. However, for skin cancer or other diseases based on images alone, he said there are solutions.
“We are in the early days, and there is time to change this,” Dr. Adamson said, referring to the low representation of skin of color in AI training sets. In addition to including more skin types to train recognition, creating AI algorithms specifically for dark skin is another potential approach.
However, his key point was the importance of recognizing the need for solutions.
“AI is the future, but we must apply the same rigor to AI as to other medical interventions to ensure that the technology is not applied in a biased fashion,” he said.
Susan M. Swetter, MD, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, agreed. As someone who has been following the progress of AI in the diagnosis of skin cancer, Dr. Swetter recognizes the potential for this technology to increase diagnostic efficiency and accuracy, but she also called for studies specific to skin of color.
The algorithms “have not yet been adequately evaluated in people of color, particularly Black patients in whom dermoscopic criteria for benign versus malignant melanocytic neoplasms differ from those with lighter skin types,” Dr. Swetter said in an interview.
She sees the same fix as that proposed by Dr. Adamson.
“Efforts to include skin of color in AI algorithms for validation and further training are needed to prevent potential harms of over- or underdiagnosis in darker skin patients,” she pointed out.
Dr. Adamson reports no potential conflicts of interest relevant to this topic. Dr. Swetter had no relevant disclosures.
FROM AAD VMX 2021
The power and promise of social media in oncology
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM COSMO 2021
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Teen tanning bed ban would prevent more than 15,000 melanoma cases
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.
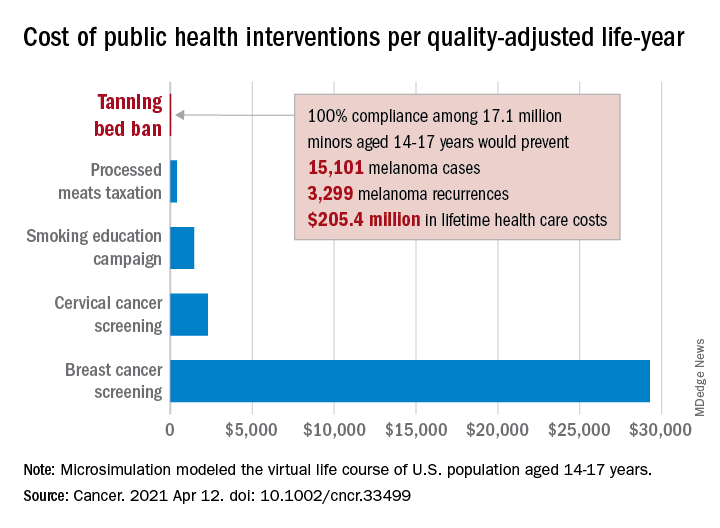
“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.

“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.

“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
FROM CANCER
Made-to-order TILs effective against metastatic melanoma
In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents, objective clinical responses occurred with a customized cell therapy based on T cells extracted directly from tumor tissue.
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies that utilize T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” said Jason Alan Chesney, MD, PhD, of the James Graham Brown Cancer Center at the University of Louisville (Ky.)
He presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT008).
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the clinical research division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, where the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2.
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The had received a mean of 3.3 prior lines of therapy. All patients had received prior anti–PD-1/PD-L1 agents; 53 had received a CTLA4 inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
In all, 24 patients (36.4%) had an objective response, 3 patients had a complete response, and 21 had a partial response. There were 29 patients who had stable disease and 9 who progressed. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to more than 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade. All but two patients experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, hypophosphatemia, and lymphopenia.
“The adverse-event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloablative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg said one of the study’s limitations is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stem-like T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents, objective clinical responses occurred with a customized cell therapy based on T cells extracted directly from tumor tissue.
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies that utilize T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” said Jason Alan Chesney, MD, PhD, of the James Graham Brown Cancer Center at the University of Louisville (Ky.)
He presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT008).
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the clinical research division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, where the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2.
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The had received a mean of 3.3 prior lines of therapy. All patients had received prior anti–PD-1/PD-L1 agents; 53 had received a CTLA4 inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
In all, 24 patients (36.4%) had an objective response, 3 patients had a complete response, and 21 had a partial response. There were 29 patients who had stable disease and 9 who progressed. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to more than 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade. All but two patients experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, hypophosphatemia, and lymphopenia.
“The adverse-event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloablative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg said one of the study’s limitations is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stem-like T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents, objective clinical responses occurred with a customized cell therapy based on T cells extracted directly from tumor tissue.
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies that utilize T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” said Jason Alan Chesney, MD, PhD, of the James Graham Brown Cancer Center at the University of Louisville (Ky.)
He presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT008).
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the clinical research division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, where the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2.
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The had received a mean of 3.3 prior lines of therapy. All patients had received prior anti–PD-1/PD-L1 agents; 53 had received a CTLA4 inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
In all, 24 patients (36.4%) had an objective response, 3 patients had a complete response, and 21 had a partial response. There were 29 patients who had stable disease and 9 who progressed. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to more than 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade. All but two patients experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, hypophosphatemia, and lymphopenia.
“The adverse-event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloablative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg said one of the study’s limitations is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stem-like T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
Leveraging the microbiome to enhance cancer treatment
Andrea Facciabene, PhD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a preclinical study in which vancomycin enhanced the efficacy of radiotherapy against melanoma and lung cancer. Now, researchers are conducting a clinical trial to determine if vancomycin can have the same effect in patients with non–small cell lung cancer.
Dr. Facciabene reviewed this research at the AACR Virtual Special Conference: Radiation Science and Medicine.
According to Dr. Facciabene, “gut microbiota” includes the more than 1,000 different strains of bacteria living in human intestines. He indicated that the average human has 10 times more bacteria than cells in the body and 150 times more genes in the gut microbiome than in the human genome.
In healthy individuals, the gut microbiota play a key role in intestinal function and digestive processes, modulation of hormones and vitamin secretion, energy extraction from food, and development and maintenance of a balanced immune system.
“Dysbiosis” is the term applied to a change in the composition, diversity, or metabolites of the microbiome from a healthy pattern to one associated with disease. Antibiotic therapy is a classic cause of dysbiosis, and dysbiosis has been implicated in a variety of inflammatory diseases.
The mechanisms by which the gut microbiome could influence systemic immunity is not known but is relevant to cancer therapy response. Augmenting the frequency and durability of response to immune-targeted treatments – potentially by manipulating the influence of gut microbiota on the immune system – could be highly impactful.
Gut microbiota and radiation-induced cell death
Immunogenic cell death – a process by which tumors die and release their intracellular molecular contents – is one of the mechanisms by which radiotherapy kills cancer cells.
Tumor cells succumbing to immunogenic cell death stimulate antigen presenting cells, such as dendritic cells, that engulf tumor antigens and cross-present them to CD8+ cytotoxic T lymphocytes. This process culminates in the generation of a specific immune response capable of killing the malignant cells in the irradiated area, but it also impacts distant nonirradiated tumors – an abscopal effect.
Dr. Facciabene and colleagues hypothesized that alterations of the gut microbiota could have an impact on the effect of radiotherapy. To investigate this, they studied mouse models of melanoma.
The team allowed B16-OVA tumors to grow for 9-12 days, then delivered a single dose of radiotherapy (21 Gy) to one – but not all – tumors. Simultaneously with the delivery of radiotherapy, the investigators started some animals on oral vancomycin. The team chose vancomycin because its effects are localized and impact the gut microbiota directly, without any known systemic effects.
Results showed that vancomycin significantly augmented the impact of radiotherapy in the irradiated area and was associated with regression of remote tumors.
The effects of the combination treatment on tumor volume were significantly greater than the effects of either treatment alone. Since manipulation of the gut microbiome potentiated radiotherapy effects both locally and distantly, the investigators concluded that immunogenic cell death may be involved in both the local and abscopal effects of radiotherapy.
When the experiment was repeated with a lung tumor model, similar findings were observed.
Involvement of cytotoxic T cells and interferon-gamma
Dr. Facciabene and colleagues found that the irradiated and unirradiated B16 OVA melanoma tumors treated with the radiotherapy-vancomycin combination were infiltrated by CD3+ and CD8+ T cells.
The investigators selectively depleted CD8+ T cells by pretreating the mice with an anti-CD8 monoclonal antibody. Depletion of CD8+ cells prior to administering radiotherapy plus vancomycin abrogated the antitumor effects of the combination treatment, demonstrating that the CD8+ T cells were required.
To characterize the antigen specificity of the tumor-infiltrating CD8+ T cells, Dr. Facciabene and colleagues used OVA MHC class 1 tetramer. Tumors from mice treated with vancomycin alone, radiotherapy alone, or the combination were dissected. Individual dendritic cells were assayed for OVA tetramer by flow cytometry.
The investigators found that tumors from mice treated with radiotherapy plus vancomycin had a significantly higher number of OVA-specific CD8+ T cells, in comparison with untreated tumors or tumors treated with either vancomycin alone or radiotherapy alone. Since antibody that impaired recognition of MHC class I peptides by T cells ablated the effect, it was clear that antigen recognition was vital.
Interferon-gamma (IFN-gamma) is known to play a critical role in both differentiation and effector functions of CD8+ cytolytic T cells in the antitumor immune response. To determine whether IFN-gamma is involved in the antitumor effects of the radiotherapy-vancomycin combination, the investigators measured intratumoral expression of IFN-gamma in the tumors 5 days after radiotherapy.
IFN-gamma messenger RNA expression levels were significantly elevated in the combination treatment group when compared with either treatment alone. In B16-OVA melanoma–challenged knockout mice, the enhancement of the radiotherapy effects by vancomycin was ablated.
The investigators concluded that vancomycin remodels the tumor microenvironment and increases the functionality of tumor-infiltrating, tumor-specific, CD8+ T cells. Furthermore, IFN-gamma is required to augment the radiotherapy-induced immune effect against the tumor.
Potential biochemical mediators of immune effects
The gut microbiota aid host digestion and generate a large repertoire of metabolites after defermentation of fiber. Short-chain fatty acids (SCFAs) constitute the major products of bacterial fermentation.
Acetic acid, propionic acid, and butyric acid represent 95% of total SCFAs present in the intestine. SCFAs are known to directly modulate cytokine production and dendritic cell function.
In their study, Dr. Facciabene and colleagues focused on butyric acid. Using mass spectroscopy, they demonstrated that vancomycin treatment reduces butyrate concentrations in tumor and tumor-draining lymph nodes by eradicating the major families of SCFA-producing Clostridia species.
To test whether supplementing butyrate could influence the synergy of the radiotherapy-vancomycin combination in vivo, the investigators added sodium butyrate to the mice’s drinking water when starting vancomycin treatment. The team then challenged the mice with B16-OVA tumors and treated them with radiotherapy.
In agreement with the group’s prior findings, vancomycin enhanced the tumor-inhibitory effects of radiotherapy, but dietary butyrate inhibited the benefit. The investigators found a significant decrease in the population of B16-OVA–presenting dendritic cells in the lymph nodes of mice receiving the supplemental butyrate.
Dr. Facciabene said these findings were supported by a recent publication. The authors observed that butyrate inhibited type I IFN expression in dendritic cells and radiotherapy-induced, tumor-specific cytotoxic T-cell immune responses without directly protecting tumor cells from the cytotoxic effects of radiotherapy.
Wide-ranging implications
Overall, Dr. Facciabene’s research has shown that:
- Vancomycin significantly enhances the tumor inhibitory effect of targeted radiation, including abscopal effects.
- The synergistic effects are dependent upon IFN-gamma and CD8+ cells.
- Depletion of some gut microbiome species increases antigen presentation by dendritic cells. This is mediated by SCFAs produced by certain bacterial families.
- There are promising new strategies to improve responses to radiotherapy, including targeting gut microbiota.
A clinical trial (NCT03546829) of vancomycin plus stereotactic body radiation in patients with locally advanced non–small cell lung cancer has been launched to investigate these findings further. Early data analysis has shown a significant impact of vancomycin on several species of gut microbiota, according to Dr. Facciabene.
Revolutionary results from immune-targeted therapy in the recent past have highlighted the important role the immune system can play in fighting cancer. Still, up to one-third of cancer patients fail to respond to overtly immune-targeted therapy.
The ability to inhibit cancer cells from evading immune surveillance by using new adjuvants – including those acting on non-traditional targets like gut microbiota – could herald the next major advances in cancer therapy. During his presentation, Dr. Facciabene gave participants an enticing hint of what could be coming for cancer patients in the years ahead.
Dr. Facciabene reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Andrea Facciabene, PhD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a preclinical study in which vancomycin enhanced the efficacy of radiotherapy against melanoma and lung cancer. Now, researchers are conducting a clinical trial to determine if vancomycin can have the same effect in patients with non–small cell lung cancer.
Dr. Facciabene reviewed this research at the AACR Virtual Special Conference: Radiation Science and Medicine.
According to Dr. Facciabene, “gut microbiota” includes the more than 1,000 different strains of bacteria living in human intestines. He indicated that the average human has 10 times more bacteria than cells in the body and 150 times more genes in the gut microbiome than in the human genome.
In healthy individuals, the gut microbiota play a key role in intestinal function and digestive processes, modulation of hormones and vitamin secretion, energy extraction from food, and development and maintenance of a balanced immune system.
“Dysbiosis” is the term applied to a change in the composition, diversity, or metabolites of the microbiome from a healthy pattern to one associated with disease. Antibiotic therapy is a classic cause of dysbiosis, and dysbiosis has been implicated in a variety of inflammatory diseases.
The mechanisms by which the gut microbiome could influence systemic immunity is not known but is relevant to cancer therapy response. Augmenting the frequency and durability of response to immune-targeted treatments – potentially by manipulating the influence of gut microbiota on the immune system – could be highly impactful.
Gut microbiota and radiation-induced cell death
Immunogenic cell death – a process by which tumors die and release their intracellular molecular contents – is one of the mechanisms by which radiotherapy kills cancer cells.
Tumor cells succumbing to immunogenic cell death stimulate antigen presenting cells, such as dendritic cells, that engulf tumor antigens and cross-present them to CD8+ cytotoxic T lymphocytes. This process culminates in the generation of a specific immune response capable of killing the malignant cells in the irradiated area, but it also impacts distant nonirradiated tumors – an abscopal effect.
Dr. Facciabene and colleagues hypothesized that alterations of the gut microbiota could have an impact on the effect of radiotherapy. To investigate this, they studied mouse models of melanoma.
The team allowed B16-OVA tumors to grow for 9-12 days, then delivered a single dose of radiotherapy (21 Gy) to one – but not all – tumors. Simultaneously with the delivery of radiotherapy, the investigators started some animals on oral vancomycin. The team chose vancomycin because its effects are localized and impact the gut microbiota directly, without any known systemic effects.
Results showed that vancomycin significantly augmented the impact of radiotherapy in the irradiated area and was associated with regression of remote tumors.
The effects of the combination treatment on tumor volume were significantly greater than the effects of either treatment alone. Since manipulation of the gut microbiome potentiated radiotherapy effects both locally and distantly, the investigators concluded that immunogenic cell death may be involved in both the local and abscopal effects of radiotherapy.
When the experiment was repeated with a lung tumor model, similar findings were observed.
Involvement of cytotoxic T cells and interferon-gamma
Dr. Facciabene and colleagues found that the irradiated and unirradiated B16 OVA melanoma tumors treated with the radiotherapy-vancomycin combination were infiltrated by CD3+ and CD8+ T cells.
The investigators selectively depleted CD8+ T cells by pretreating the mice with an anti-CD8 monoclonal antibody. Depletion of CD8+ cells prior to administering radiotherapy plus vancomycin abrogated the antitumor effects of the combination treatment, demonstrating that the CD8+ T cells were required.
To characterize the antigen specificity of the tumor-infiltrating CD8+ T cells, Dr. Facciabene and colleagues used OVA MHC class 1 tetramer. Tumors from mice treated with vancomycin alone, radiotherapy alone, or the combination were dissected. Individual dendritic cells were assayed for OVA tetramer by flow cytometry.
The investigators found that tumors from mice treated with radiotherapy plus vancomycin had a significantly higher number of OVA-specific CD8+ T cells, in comparison with untreated tumors or tumors treated with either vancomycin alone or radiotherapy alone. Since antibody that impaired recognition of MHC class I peptides by T cells ablated the effect, it was clear that antigen recognition was vital.
Interferon-gamma (IFN-gamma) is known to play a critical role in both differentiation and effector functions of CD8+ cytolytic T cells in the antitumor immune response. To determine whether IFN-gamma is involved in the antitumor effects of the radiotherapy-vancomycin combination, the investigators measured intratumoral expression of IFN-gamma in the tumors 5 days after radiotherapy.
IFN-gamma messenger RNA expression levels were significantly elevated in the combination treatment group when compared with either treatment alone. In B16-OVA melanoma–challenged knockout mice, the enhancement of the radiotherapy effects by vancomycin was ablated.
The investigators concluded that vancomycin remodels the tumor microenvironment and increases the functionality of tumor-infiltrating, tumor-specific, CD8+ T cells. Furthermore, IFN-gamma is required to augment the radiotherapy-induced immune effect against the tumor.
Potential biochemical mediators of immune effects
The gut microbiota aid host digestion and generate a large repertoire of metabolites after defermentation of fiber. Short-chain fatty acids (SCFAs) constitute the major products of bacterial fermentation.
Acetic acid, propionic acid, and butyric acid represent 95% of total SCFAs present in the intestine. SCFAs are known to directly modulate cytokine production and dendritic cell function.
In their study, Dr. Facciabene and colleagues focused on butyric acid. Using mass spectroscopy, they demonstrated that vancomycin treatment reduces butyrate concentrations in tumor and tumor-draining lymph nodes by eradicating the major families of SCFA-producing Clostridia species.
To test whether supplementing butyrate could influence the synergy of the radiotherapy-vancomycin combination in vivo, the investigators added sodium butyrate to the mice’s drinking water when starting vancomycin treatment. The team then challenged the mice with B16-OVA tumors and treated them with radiotherapy.
In agreement with the group’s prior findings, vancomycin enhanced the tumor-inhibitory effects of radiotherapy, but dietary butyrate inhibited the benefit. The investigators found a significant decrease in the population of B16-OVA–presenting dendritic cells in the lymph nodes of mice receiving the supplemental butyrate.
Dr. Facciabene said these findings were supported by a recent publication. The authors observed that butyrate inhibited type I IFN expression in dendritic cells and radiotherapy-induced, tumor-specific cytotoxic T-cell immune responses without directly protecting tumor cells from the cytotoxic effects of radiotherapy.
Wide-ranging implications
Overall, Dr. Facciabene’s research has shown that:
- Vancomycin significantly enhances the tumor inhibitory effect of targeted radiation, including abscopal effects.
- The synergistic effects are dependent upon IFN-gamma and CD8+ cells.
- Depletion of some gut microbiome species increases antigen presentation by dendritic cells. This is mediated by SCFAs produced by certain bacterial families.
- There are promising new strategies to improve responses to radiotherapy, including targeting gut microbiota.
A clinical trial (NCT03546829) of vancomycin plus stereotactic body radiation in patients with locally advanced non–small cell lung cancer has been launched to investigate these findings further. Early data analysis has shown a significant impact of vancomycin on several species of gut microbiota, according to Dr. Facciabene.
Revolutionary results from immune-targeted therapy in the recent past have highlighted the important role the immune system can play in fighting cancer. Still, up to one-third of cancer patients fail to respond to overtly immune-targeted therapy.
The ability to inhibit cancer cells from evading immune surveillance by using new adjuvants – including those acting on non-traditional targets like gut microbiota – could herald the next major advances in cancer therapy. During his presentation, Dr. Facciabene gave participants an enticing hint of what could be coming for cancer patients in the years ahead.
Dr. Facciabene reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Andrea Facciabene, PhD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a preclinical study in which vancomycin enhanced the efficacy of radiotherapy against melanoma and lung cancer. Now, researchers are conducting a clinical trial to determine if vancomycin can have the same effect in patients with non–small cell lung cancer.
Dr. Facciabene reviewed this research at the AACR Virtual Special Conference: Radiation Science and Medicine.
According to Dr. Facciabene, “gut microbiota” includes the more than 1,000 different strains of bacteria living in human intestines. He indicated that the average human has 10 times more bacteria than cells in the body and 150 times more genes in the gut microbiome than in the human genome.
In healthy individuals, the gut microbiota play a key role in intestinal function and digestive processes, modulation of hormones and vitamin secretion, energy extraction from food, and development and maintenance of a balanced immune system.
“Dysbiosis” is the term applied to a change in the composition, diversity, or metabolites of the microbiome from a healthy pattern to one associated with disease. Antibiotic therapy is a classic cause of dysbiosis, and dysbiosis has been implicated in a variety of inflammatory diseases.
The mechanisms by which the gut microbiome could influence systemic immunity is not known but is relevant to cancer therapy response. Augmenting the frequency and durability of response to immune-targeted treatments – potentially by manipulating the influence of gut microbiota on the immune system – could be highly impactful.
Gut microbiota and radiation-induced cell death
Immunogenic cell death – a process by which tumors die and release their intracellular molecular contents – is one of the mechanisms by which radiotherapy kills cancer cells.
Tumor cells succumbing to immunogenic cell death stimulate antigen presenting cells, such as dendritic cells, that engulf tumor antigens and cross-present them to CD8+ cytotoxic T lymphocytes. This process culminates in the generation of a specific immune response capable of killing the malignant cells in the irradiated area, but it also impacts distant nonirradiated tumors – an abscopal effect.
Dr. Facciabene and colleagues hypothesized that alterations of the gut microbiota could have an impact on the effect of radiotherapy. To investigate this, they studied mouse models of melanoma.
The team allowed B16-OVA tumors to grow for 9-12 days, then delivered a single dose of radiotherapy (21 Gy) to one – but not all – tumors. Simultaneously with the delivery of radiotherapy, the investigators started some animals on oral vancomycin. The team chose vancomycin because its effects are localized and impact the gut microbiota directly, without any known systemic effects.
Results showed that vancomycin significantly augmented the impact of radiotherapy in the irradiated area and was associated with regression of remote tumors.
The effects of the combination treatment on tumor volume were significantly greater than the effects of either treatment alone. Since manipulation of the gut microbiome potentiated radiotherapy effects both locally and distantly, the investigators concluded that immunogenic cell death may be involved in both the local and abscopal effects of radiotherapy.
When the experiment was repeated with a lung tumor model, similar findings were observed.
Involvement of cytotoxic T cells and interferon-gamma
Dr. Facciabene and colleagues found that the irradiated and unirradiated B16 OVA melanoma tumors treated with the radiotherapy-vancomycin combination were infiltrated by CD3+ and CD8+ T cells.
The investigators selectively depleted CD8+ T cells by pretreating the mice with an anti-CD8 monoclonal antibody. Depletion of CD8+ cells prior to administering radiotherapy plus vancomycin abrogated the antitumor effects of the combination treatment, demonstrating that the CD8+ T cells were required.
To characterize the antigen specificity of the tumor-infiltrating CD8+ T cells, Dr. Facciabene and colleagues used OVA MHC class 1 tetramer. Tumors from mice treated with vancomycin alone, radiotherapy alone, or the combination were dissected. Individual dendritic cells were assayed for OVA tetramer by flow cytometry.
The investigators found that tumors from mice treated with radiotherapy plus vancomycin had a significantly higher number of OVA-specific CD8+ T cells, in comparison with untreated tumors or tumors treated with either vancomycin alone or radiotherapy alone. Since antibody that impaired recognition of MHC class I peptides by T cells ablated the effect, it was clear that antigen recognition was vital.
Interferon-gamma (IFN-gamma) is known to play a critical role in both differentiation and effector functions of CD8+ cytolytic T cells in the antitumor immune response. To determine whether IFN-gamma is involved in the antitumor effects of the radiotherapy-vancomycin combination, the investigators measured intratumoral expression of IFN-gamma in the tumors 5 days after radiotherapy.
IFN-gamma messenger RNA expression levels were significantly elevated in the combination treatment group when compared with either treatment alone. In B16-OVA melanoma–challenged knockout mice, the enhancement of the radiotherapy effects by vancomycin was ablated.
The investigators concluded that vancomycin remodels the tumor microenvironment and increases the functionality of tumor-infiltrating, tumor-specific, CD8+ T cells. Furthermore, IFN-gamma is required to augment the radiotherapy-induced immune effect against the tumor.
Potential biochemical mediators of immune effects
The gut microbiota aid host digestion and generate a large repertoire of metabolites after defermentation of fiber. Short-chain fatty acids (SCFAs) constitute the major products of bacterial fermentation.
Acetic acid, propionic acid, and butyric acid represent 95% of total SCFAs present in the intestine. SCFAs are known to directly modulate cytokine production and dendritic cell function.
In their study, Dr. Facciabene and colleagues focused on butyric acid. Using mass spectroscopy, they demonstrated that vancomycin treatment reduces butyrate concentrations in tumor and tumor-draining lymph nodes by eradicating the major families of SCFA-producing Clostridia species.
To test whether supplementing butyrate could influence the synergy of the radiotherapy-vancomycin combination in vivo, the investigators added sodium butyrate to the mice’s drinking water when starting vancomycin treatment. The team then challenged the mice with B16-OVA tumors and treated them with radiotherapy.
In agreement with the group’s prior findings, vancomycin enhanced the tumor-inhibitory effects of radiotherapy, but dietary butyrate inhibited the benefit. The investigators found a significant decrease in the population of B16-OVA–presenting dendritic cells in the lymph nodes of mice receiving the supplemental butyrate.
Dr. Facciabene said these findings were supported by a recent publication. The authors observed that butyrate inhibited type I IFN expression in dendritic cells and radiotherapy-induced, tumor-specific cytotoxic T-cell immune responses without directly protecting tumor cells from the cytotoxic effects of radiotherapy.
Wide-ranging implications
Overall, Dr. Facciabene’s research has shown that:
- Vancomycin significantly enhances the tumor inhibitory effect of targeted radiation, including abscopal effects.
- The synergistic effects are dependent upon IFN-gamma and CD8+ cells.
- Depletion of some gut microbiome species increases antigen presentation by dendritic cells. This is mediated by SCFAs produced by certain bacterial families.
- There are promising new strategies to improve responses to radiotherapy, including targeting gut microbiota.
A clinical trial (NCT03546829) of vancomycin plus stereotactic body radiation in patients with locally advanced non–small cell lung cancer has been launched to investigate these findings further. Early data analysis has shown a significant impact of vancomycin on several species of gut microbiota, according to Dr. Facciabene.
Revolutionary results from immune-targeted therapy in the recent past have highlighted the important role the immune system can play in fighting cancer. Still, up to one-third of cancer patients fail to respond to overtly immune-targeted therapy.
The ability to inhibit cancer cells from evading immune surveillance by using new adjuvants – including those acting on non-traditional targets like gut microbiota – could herald the next major advances in cancer therapy. During his presentation, Dr. Facciabene gave participants an enticing hint of what could be coming for cancer patients in the years ahead.
Dr. Facciabene reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: RADIATION SCIENCE AND MEDICINE