User login
Vulvar melanoma is increasing in older women
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
FROM AAD 2020
Combo exhibits activity in metastatic mucosal melanoma
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
FROM ASCO 2020
Anti–PD1 Immune Checkpoint Inhibitor–Induced Bullous Pemphigoid in Metastatic Melanoma and Non–Small Cell Lung Cancer
Immune checkpoint inhibitors are used for a variety of advanced malignancies, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma. Anti–programmed cell death 1 (PD1) targeted therapies, such as pembrolizumab and nivolumab, are improving patient survival. This class of immunotherapy is revolutionary but is associated with autoimmune adverse effects. A rare but increasingly reported adverse effect of anti-PD1 therapy is bullous pemphigoid (BP), an autoimmune blistering disease directed against
High clinical suspicion, early diagnosis, and proper management of immunotherapy-related BP are imperative for keeping patients on life-prolonging treatment. We present 3 cases of BP secondary to anti-PD1 immunotherapy in patients with melanoma or non–small cell lung cancer to highlight the diagnosis and treatment of BP as well as emphasize the importance of the dermatologist in the care of patients with immunotherapy-related skin disease.
Case Reports
Patient 1
A 72-year-old woman with metastatic BRAF-mutated melanoma from an unknown primary site presented with intensely pruritic papules on the back, chest, and extremities of 4 months’ duration. She described her symptoms as insidious in onset and refractory to clobetasol ointment, oral diphenhydramine, and over-the-counter anti-itch creams. The patient had been treated with oral dabrafenib 150 mg twice daily and trametinib 2 mg/d but was switched to pembrolizumab when the disease progressed. After 8 months, she had a complete radiologic response to pembrolizumab 2 mg/kg every 3 weeks, which was discontinued in favor of observation 3 months prior to presentation to dermatology.
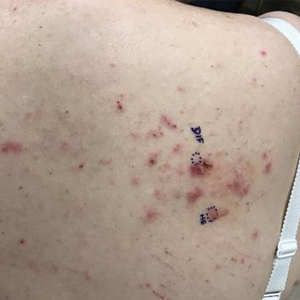
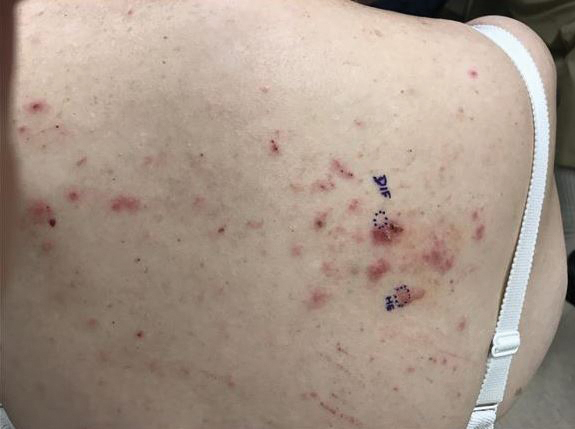
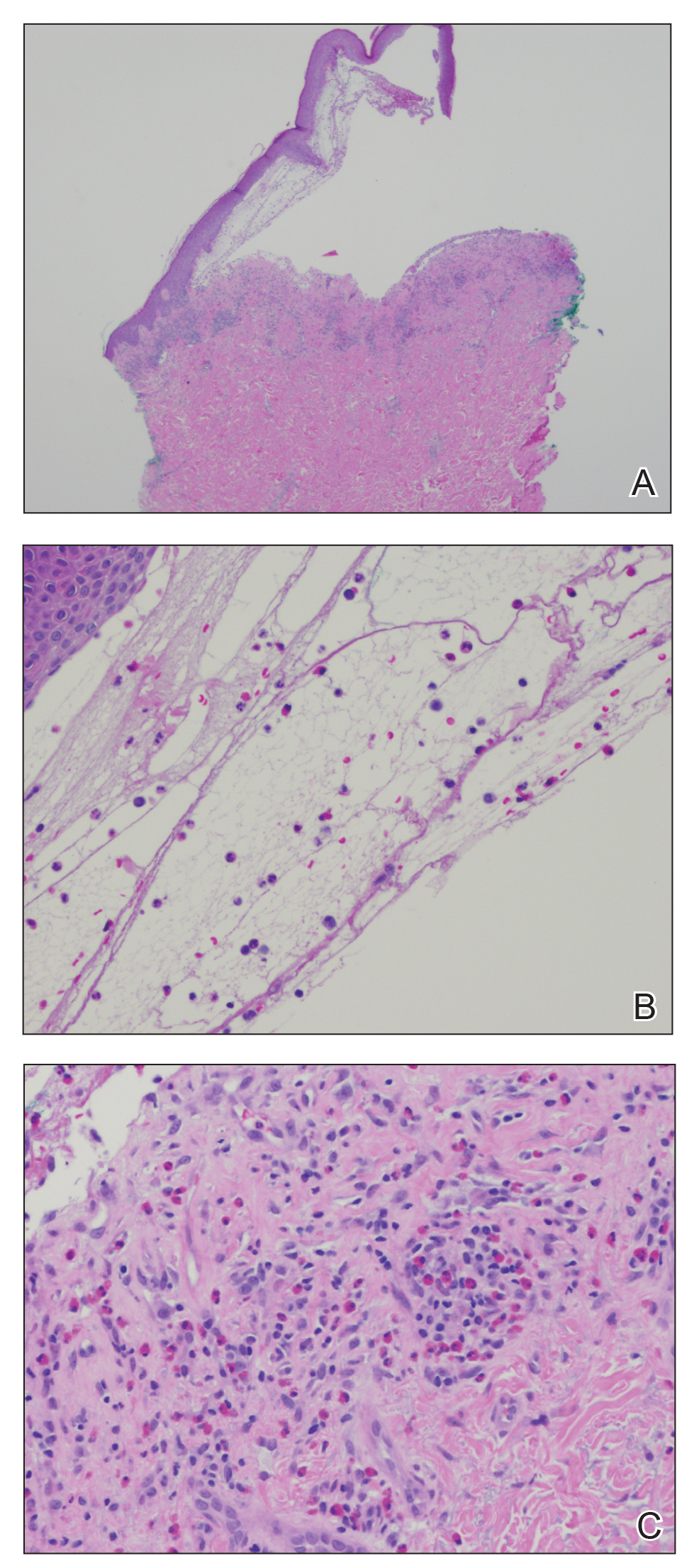
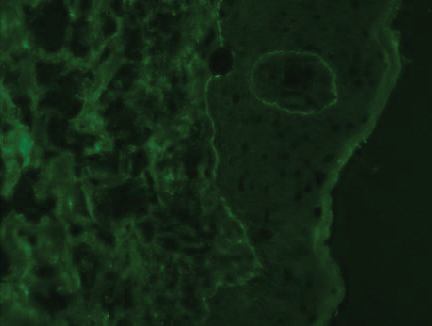
At the current presentation, physical examination revealed innumerable erythematous, excoriated, 2- to 4-mm, red papules diffusely scattered on the upper back, chest, abdomen, and thighs, with one 8×4-mm vesicle on the right side of the upper back (Figure 1). Discrete areas of depigmented macules, consistent with vitiligo, coalesced into patches on the legs, thighs, arms, and back. The patient was started on a 3-week oral prednisone taper for symptom relief. A hematoxylin and eosin (H&E)–stained punch biopsy of the back revealed a subepidermal split with eosinophils and a dense eosinophilic infiltrate in the dermis (Figure 2). Direct immunofluorescence (DIF) studies from a specimen adjacent to the biopsy collected for H&E staining showed linear deposition of IgA, IgG, and C3 along the dermoepidermal junction (Figure 3). Histologic findings were consistent with BP.
The patient was started on doxycycline 100 mg twice daily and clobetasol ointment 0.05% once daily to supplement the prednisone taper. At 3-week follow-up, she reported pruritus and a few erythematous macules but no new bullae. At 12 weeks, some papules persisted; however, the patient was averse to using systemic agents and decided that symptoms were adequately controlled with clobetasol ointment and oral doxycycline.
Because the patient currently remains in clinical and radiologic remission, anti-PD1 immune checkpoint inhibitors have not been restarted but remain an option for the future if disease recurs
Patient 2
An 82-year-old man with a history of stage IIC desmoplastic melanoma presented to dermatology with an intensely pruritic eruption on the legs, arms, waist, upper torso, and scalp of 3 weeks’ duration. Clobetasol ointment had provided minimal relief.
Six months prior to presenting to dermatology, the patient underwent immunotherapy with 4 cycles of ipilimumab 200 mg intravenous (IV) and nivolumab 240 mg IV every 2 weeks, receiving ipilimumab during the first cycle only because of a lack of availability at the pharmacy. He then received nivolumab 240 mg IV every 2 weeks as maintenance therapy. After the second dose of nivolumab maintenance therapy, however, he developed generalized bullae and pruritus. Dermatology was consulted during an oncology appointment, and his oncologist decided to hold nivolumab.
Physical examination revealed generalized tense and eroded bullae covering more than 50% of the body surface area and affecting the scalp, arms, legs, torso, and buttocks. Two punch biopsies were obtained. Hematoxylin and eosin staining revealed a subepidermal split with predominantly eosinophils and scattered neutrophils. Direct immunofluorescence studies showed linear deposition of IgG, IgA, and C3 along the dermoepidermal junction, consistent with BP.
The patient’s BP was difficult to control, requiring several hospital admissions for wound care, high-dose systemic steroids, and initiation of mycophenolate mofetil. After 4 months of waxing and waning symptoms, the BP was controlled with mycophenolate mofetil 1500 mg/d; clobetasol ointment 0.05%; and diphenhydramine for pruritus. Due to the prolonged recovery and severity of BP, the patient’s oncologist deemed that he was not a candidate for future immunotherapy.
Patient 3
A 68-year-old man with PD1-negative, metastatic, well-differentiated squamous cell carcinoma of the lung presented to dermatology with a pruritic rash of 3 weeks’ duration. He had been receiving nivolumab for 2 years after disease progressed on prior chemotherapies and experienced several grade 1 or grade 2 nivolumab-induced autoimmune reactions including thyroiditis, dermatitis, and nephritis, for which he was taking prednisone 5 mg/d for suppression.
Physical examination revealed psoriasiform pink plaques on the arms, chest, and legs. The differential diagnosis at the time favored psoriasiform dermatitis over lichenoid dermatitis. A punch biopsy revealed psoriasiform dermatitis. The patient was prescribed fluocinonide ointment 0.05% daily. His plaques improved with topical steroids.
The patient returned approximately 1 month later with a report of a new blistering rash on the legs. Physical examination revealed interval improvement of the psoriasiform plaques on the scalp, torso, and extremities, but tense bullae were seen on the thighs, with surrounding superficial erosions at sites of recent bullae. Punch biopsies of the skin for H&E staining and DIF showed BP.
Prednisone was increased to 50 mg/d for a 3-week taper. Doxycycline 100 mg twice daily was started. The patient’s skin disease continued to be difficult to control with therapy; nivolumab was held by his oncologist.
Comment
Immunotherapy with immune checkpoint blockade represents a successful application of immune recognition to treat metastatic cancers, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma.
Anti-PD1 targeted therapies improve survival in solid and hematologic malignancies, with a response rate as high as 40% in melanoma.2 Although these medications can prolong survival, many are associated with loss of self-tolerance and severe autoimmunelike events that can limit therapy.3 An exception is PD1-induced vitiligo, which patient 1 developed and has been associated with a better response to therapy.4
Anti-PD1–induced BP is a newly reported adverse effect. In its early stages, BP can be difficult to differentiate from eczematous or urticarial dermatitis.5-8 Discontinuation of immunotherapy has been reported in more than 70% of patients who develop BP.1 There are reports of successful treatment of BP with a course of a PD1 inhibitor,9 but 2 of our patients had severe BP that led to discontinuation of immunotherapy.
Consider Prescreening
Given that development of BP often leads to cessation of therapy, identifying patients at risk prior to starting an immune checkpoint inhibitor might have clinical utility. Biopsy with DIF is the gold standard for diagnosis, but serologic testing can be a useful adjunct because enzyme-linked immunosorbent assay for BP antigen 1 and BP antigen 2 has a reported sensitivity and specificity of 87% and 98%, respectively.10 Serologic testing prior to starting therapy with an immune checkpoint inhibitor can provide a baseline for patients. A rise in titer, in conjunction with onset of a rash, might aid in earlier diagnosis, particularly because urticarial BP can be difficult to diagnose clinically.
Further study on the utility vs cost-benefit of these screening modalities is warranted. Their predictive utility might be limited, however, and positive serologic test results might have unanticipated consequences, such as hesitation in treating patients, thus leading to a delay in therapy or access to these medications.
Conclusion
The expanding use of immune checkpoint inhibitors is increasing survival in patients with metastatic melanoma and other malignancies. Adverse effects are part of the continuum of immune system stimulation, with overstimulation resulting in dermatitis; thyroiditis; pneumonitis; and less commonly hypophysitis, vitiligo, and colitis.
Rarely, immune checkpoint inhibition induces BP. Development of BP leads to discontinuation of therapy in more than half of reported cases due to lack of adequate treatment for this skin disease and its impact on quality of life. Therefore, quick diagnosis of BP in patients on immunotherapy and successful management techniques can prevent discontinuation of these lifesaving cancer therapies. For that reason, dermatologists play an important role in the management of patients on immune checkpoint inhibitors for cancer.
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669.
- Márquez-Rodas, I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors a review. JAMA Oncol. 2016;2:1346-1353.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Hwang SJE, Carlos G, Chou S, et al. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413-416.
- Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442-444.
- Garje R, Chau JJ, Chung J, et al. Acute flare of bullous pemphigus with pembrolizumab used for treatment of metastatic urothelial cancer. J Immunother. 2018;41:42-44.
- Ito M, Hoashi T, Endo Y, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. 2019;46:e90-e92.
- Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-773.
- Muglia C, Bronsnick T, Kirkorian AY, et al. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99:E27-E30.
Immune checkpoint inhibitors are used for a variety of advanced malignancies, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma. Anti–programmed cell death 1 (PD1) targeted therapies, such as pembrolizumab and nivolumab, are improving patient survival. This class of immunotherapy is revolutionary but is associated with autoimmune adverse effects. A rare but increasingly reported adverse effect of anti-PD1 therapy is bullous pemphigoid (BP), an autoimmune blistering disease directed against
High clinical suspicion, early diagnosis, and proper management of immunotherapy-related BP are imperative for keeping patients on life-prolonging treatment. We present 3 cases of BP secondary to anti-PD1 immunotherapy in patients with melanoma or non–small cell lung cancer to highlight the diagnosis and treatment of BP as well as emphasize the importance of the dermatologist in the care of patients with immunotherapy-related skin disease.
Case Reports
Patient 1
A 72-year-old woman with metastatic BRAF-mutated melanoma from an unknown primary site presented with intensely pruritic papules on the back, chest, and extremities of 4 months’ duration. She described her symptoms as insidious in onset and refractory to clobetasol ointment, oral diphenhydramine, and over-the-counter anti-itch creams. The patient had been treated with oral dabrafenib 150 mg twice daily and trametinib 2 mg/d but was switched to pembrolizumab when the disease progressed. After 8 months, she had a complete radiologic response to pembrolizumab 2 mg/kg every 3 weeks, which was discontinued in favor of observation 3 months prior to presentation to dermatology.
At the current presentation, physical examination revealed innumerable erythematous, excoriated, 2- to 4-mm, red papules diffusely scattered on the upper back, chest, abdomen, and thighs, with one 8×4-mm vesicle on the right side of the upper back (Figure 1). Discrete areas of depigmented macules, consistent with vitiligo, coalesced into patches on the legs, thighs, arms, and back. The patient was started on a 3-week oral prednisone taper for symptom relief. A hematoxylin and eosin (H&E)–stained punch biopsy of the back revealed a subepidermal split with eosinophils and a dense eosinophilic infiltrate in the dermis (Figure 2). Direct immunofluorescence (DIF) studies from a specimen adjacent to the biopsy collected for H&E staining showed linear deposition of IgA, IgG, and C3 along the dermoepidermal junction (Figure 3). Histologic findings were consistent with BP.



The patient was started on doxycycline 100 mg twice daily and clobetasol ointment 0.05% once daily to supplement the prednisone taper. At 3-week follow-up, she reported pruritus and a few erythematous macules but no new bullae. At 12 weeks, some papules persisted; however, the patient was averse to using systemic agents and decided that symptoms were adequately controlled with clobetasol ointment and oral doxycycline.
Because the patient currently remains in clinical and radiologic remission, anti-PD1 immune checkpoint inhibitors have not been restarted but remain an option for the future if disease recurs
Patient 2
An 82-year-old man with a history of stage IIC desmoplastic melanoma presented to dermatology with an intensely pruritic eruption on the legs, arms, waist, upper torso, and scalp of 3 weeks’ duration. Clobetasol ointment had provided minimal relief.
Six months prior to presenting to dermatology, the patient underwent immunotherapy with 4 cycles of ipilimumab 200 mg intravenous (IV) and nivolumab 240 mg IV every 2 weeks, receiving ipilimumab during the first cycle only because of a lack of availability at the pharmacy. He then received nivolumab 240 mg IV every 2 weeks as maintenance therapy. After the second dose of nivolumab maintenance therapy, however, he developed generalized bullae and pruritus. Dermatology was consulted during an oncology appointment, and his oncologist decided to hold nivolumab.
Physical examination revealed generalized tense and eroded bullae covering more than 50% of the body surface area and affecting the scalp, arms, legs, torso, and buttocks. Two punch biopsies were obtained. Hematoxylin and eosin staining revealed a subepidermal split with predominantly eosinophils and scattered neutrophils. Direct immunofluorescence studies showed linear deposition of IgG, IgA, and C3 along the dermoepidermal junction, consistent with BP.
The patient’s BP was difficult to control, requiring several hospital admissions for wound care, high-dose systemic steroids, and initiation of mycophenolate mofetil. After 4 months of waxing and waning symptoms, the BP was controlled with mycophenolate mofetil 1500 mg/d; clobetasol ointment 0.05%; and diphenhydramine for pruritus. Due to the prolonged recovery and severity of BP, the patient’s oncologist deemed that he was not a candidate for future immunotherapy.
Patient 3
A 68-year-old man with PD1-negative, metastatic, well-differentiated squamous cell carcinoma of the lung presented to dermatology with a pruritic rash of 3 weeks’ duration. He had been receiving nivolumab for 2 years after disease progressed on prior chemotherapies and experienced several grade 1 or grade 2 nivolumab-induced autoimmune reactions including thyroiditis, dermatitis, and nephritis, for which he was taking prednisone 5 mg/d for suppression.
Physical examination revealed psoriasiform pink plaques on the arms, chest, and legs. The differential diagnosis at the time favored psoriasiform dermatitis over lichenoid dermatitis. A punch biopsy revealed psoriasiform dermatitis. The patient was prescribed fluocinonide ointment 0.05% daily. His plaques improved with topical steroids.
The patient returned approximately 1 month later with a report of a new blistering rash on the legs. Physical examination revealed interval improvement of the psoriasiform plaques on the scalp, torso, and extremities, but tense bullae were seen on the thighs, with surrounding superficial erosions at sites of recent bullae. Punch biopsies of the skin for H&E staining and DIF showed BP.
Prednisone was increased to 50 mg/d for a 3-week taper. Doxycycline 100 mg twice daily was started. The patient’s skin disease continued to be difficult to control with therapy; nivolumab was held by his oncologist.
Comment
Immunotherapy with immune checkpoint blockade represents a successful application of immune recognition to treat metastatic cancers, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma.
Anti-PD1 targeted therapies improve survival in solid and hematologic malignancies, with a response rate as high as 40% in melanoma.2 Although these medications can prolong survival, many are associated with loss of self-tolerance and severe autoimmunelike events that can limit therapy.3 An exception is PD1-induced vitiligo, which patient 1 developed and has been associated with a better response to therapy.4
Anti-PD1–induced BP is a newly reported adverse effect. In its early stages, BP can be difficult to differentiate from eczematous or urticarial dermatitis.5-8 Discontinuation of immunotherapy has been reported in more than 70% of patients who develop BP.1 There are reports of successful treatment of BP with a course of a PD1 inhibitor,9 but 2 of our patients had severe BP that led to discontinuation of immunotherapy.
Consider Prescreening
Given that development of BP often leads to cessation of therapy, identifying patients at risk prior to starting an immune checkpoint inhibitor might have clinical utility. Biopsy with DIF is the gold standard for diagnosis, but serologic testing can be a useful adjunct because enzyme-linked immunosorbent assay for BP antigen 1 and BP antigen 2 has a reported sensitivity and specificity of 87% and 98%, respectively.10 Serologic testing prior to starting therapy with an immune checkpoint inhibitor can provide a baseline for patients. A rise in titer, in conjunction with onset of a rash, might aid in earlier diagnosis, particularly because urticarial BP can be difficult to diagnose clinically.
Further study on the utility vs cost-benefit of these screening modalities is warranted. Their predictive utility might be limited, however, and positive serologic test results might have unanticipated consequences, such as hesitation in treating patients, thus leading to a delay in therapy or access to these medications.
Conclusion
The expanding use of immune checkpoint inhibitors is increasing survival in patients with metastatic melanoma and other malignancies. Adverse effects are part of the continuum of immune system stimulation, with overstimulation resulting in dermatitis; thyroiditis; pneumonitis; and less commonly hypophysitis, vitiligo, and colitis.
Rarely, immune checkpoint inhibition induces BP. Development of BP leads to discontinuation of therapy in more than half of reported cases due to lack of adequate treatment for this skin disease and its impact on quality of life. Therefore, quick diagnosis of BP in patients on immunotherapy and successful management techniques can prevent discontinuation of these lifesaving cancer therapies. For that reason, dermatologists play an important role in the management of patients on immune checkpoint inhibitors for cancer.
Immune checkpoint inhibitors are used for a variety of advanced malignancies, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma. Anti–programmed cell death 1 (PD1) targeted therapies, such as pembrolizumab and nivolumab, are improving patient survival. This class of immunotherapy is revolutionary but is associated with autoimmune adverse effects. A rare but increasingly reported adverse effect of anti-PD1 therapy is bullous pemphigoid (BP), an autoimmune blistering disease directed against
High clinical suspicion, early diagnosis, and proper management of immunotherapy-related BP are imperative for keeping patients on life-prolonging treatment. We present 3 cases of BP secondary to anti-PD1 immunotherapy in patients with melanoma or non–small cell lung cancer to highlight the diagnosis and treatment of BP as well as emphasize the importance of the dermatologist in the care of patients with immunotherapy-related skin disease.
Case Reports
Patient 1
A 72-year-old woman with metastatic BRAF-mutated melanoma from an unknown primary site presented with intensely pruritic papules on the back, chest, and extremities of 4 months’ duration. She described her symptoms as insidious in onset and refractory to clobetasol ointment, oral diphenhydramine, and over-the-counter anti-itch creams. The patient had been treated with oral dabrafenib 150 mg twice daily and trametinib 2 mg/d but was switched to pembrolizumab when the disease progressed. After 8 months, she had a complete radiologic response to pembrolizumab 2 mg/kg every 3 weeks, which was discontinued in favor of observation 3 months prior to presentation to dermatology.
At the current presentation, physical examination revealed innumerable erythematous, excoriated, 2- to 4-mm, red papules diffusely scattered on the upper back, chest, abdomen, and thighs, with one 8×4-mm vesicle on the right side of the upper back (Figure 1). Discrete areas of depigmented macules, consistent with vitiligo, coalesced into patches on the legs, thighs, arms, and back. The patient was started on a 3-week oral prednisone taper for symptom relief. A hematoxylin and eosin (H&E)–stained punch biopsy of the back revealed a subepidermal split with eosinophils and a dense eosinophilic infiltrate in the dermis (Figure 2). Direct immunofluorescence (DIF) studies from a specimen adjacent to the biopsy collected for H&E staining showed linear deposition of IgA, IgG, and C3 along the dermoepidermal junction (Figure 3). Histologic findings were consistent with BP.



The patient was started on doxycycline 100 mg twice daily and clobetasol ointment 0.05% once daily to supplement the prednisone taper. At 3-week follow-up, she reported pruritus and a few erythematous macules but no new bullae. At 12 weeks, some papules persisted; however, the patient was averse to using systemic agents and decided that symptoms were adequately controlled with clobetasol ointment and oral doxycycline.
Because the patient currently remains in clinical and radiologic remission, anti-PD1 immune checkpoint inhibitors have not been restarted but remain an option for the future if disease recurs
Patient 2
An 82-year-old man with a history of stage IIC desmoplastic melanoma presented to dermatology with an intensely pruritic eruption on the legs, arms, waist, upper torso, and scalp of 3 weeks’ duration. Clobetasol ointment had provided minimal relief.
Six months prior to presenting to dermatology, the patient underwent immunotherapy with 4 cycles of ipilimumab 200 mg intravenous (IV) and nivolumab 240 mg IV every 2 weeks, receiving ipilimumab during the first cycle only because of a lack of availability at the pharmacy. He then received nivolumab 240 mg IV every 2 weeks as maintenance therapy. After the second dose of nivolumab maintenance therapy, however, he developed generalized bullae and pruritus. Dermatology was consulted during an oncology appointment, and his oncologist decided to hold nivolumab.
Physical examination revealed generalized tense and eroded bullae covering more than 50% of the body surface area and affecting the scalp, arms, legs, torso, and buttocks. Two punch biopsies were obtained. Hematoxylin and eosin staining revealed a subepidermal split with predominantly eosinophils and scattered neutrophils. Direct immunofluorescence studies showed linear deposition of IgG, IgA, and C3 along the dermoepidermal junction, consistent with BP.
The patient’s BP was difficult to control, requiring several hospital admissions for wound care, high-dose systemic steroids, and initiation of mycophenolate mofetil. After 4 months of waxing and waning symptoms, the BP was controlled with mycophenolate mofetil 1500 mg/d; clobetasol ointment 0.05%; and diphenhydramine for pruritus. Due to the prolonged recovery and severity of BP, the patient’s oncologist deemed that he was not a candidate for future immunotherapy.
Patient 3
A 68-year-old man with PD1-negative, metastatic, well-differentiated squamous cell carcinoma of the lung presented to dermatology with a pruritic rash of 3 weeks’ duration. He had been receiving nivolumab for 2 years after disease progressed on prior chemotherapies and experienced several grade 1 or grade 2 nivolumab-induced autoimmune reactions including thyroiditis, dermatitis, and nephritis, for which he was taking prednisone 5 mg/d for suppression.
Physical examination revealed psoriasiform pink plaques on the arms, chest, and legs. The differential diagnosis at the time favored psoriasiform dermatitis over lichenoid dermatitis. A punch biopsy revealed psoriasiform dermatitis. The patient was prescribed fluocinonide ointment 0.05% daily. His plaques improved with topical steroids.
The patient returned approximately 1 month later with a report of a new blistering rash on the legs. Physical examination revealed interval improvement of the psoriasiform plaques on the scalp, torso, and extremities, but tense bullae were seen on the thighs, with surrounding superficial erosions at sites of recent bullae. Punch biopsies of the skin for H&E staining and DIF showed BP.
Prednisone was increased to 50 mg/d for a 3-week taper. Doxycycline 100 mg twice daily was started. The patient’s skin disease continued to be difficult to control with therapy; nivolumab was held by his oncologist.
Comment
Immunotherapy with immune checkpoint blockade represents a successful application of immune recognition to treat metastatic cancers, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma.
Anti-PD1 targeted therapies improve survival in solid and hematologic malignancies, with a response rate as high as 40% in melanoma.2 Although these medications can prolong survival, many are associated with loss of self-tolerance and severe autoimmunelike events that can limit therapy.3 An exception is PD1-induced vitiligo, which patient 1 developed and has been associated with a better response to therapy.4
Anti-PD1–induced BP is a newly reported adverse effect. In its early stages, BP can be difficult to differentiate from eczematous or urticarial dermatitis.5-8 Discontinuation of immunotherapy has been reported in more than 70% of patients who develop BP.1 There are reports of successful treatment of BP with a course of a PD1 inhibitor,9 but 2 of our patients had severe BP that led to discontinuation of immunotherapy.
Consider Prescreening
Given that development of BP often leads to cessation of therapy, identifying patients at risk prior to starting an immune checkpoint inhibitor might have clinical utility. Biopsy with DIF is the gold standard for diagnosis, but serologic testing can be a useful adjunct because enzyme-linked immunosorbent assay for BP antigen 1 and BP antigen 2 has a reported sensitivity and specificity of 87% and 98%, respectively.10 Serologic testing prior to starting therapy with an immune checkpoint inhibitor can provide a baseline for patients. A rise in titer, in conjunction with onset of a rash, might aid in earlier diagnosis, particularly because urticarial BP can be difficult to diagnose clinically.
Further study on the utility vs cost-benefit of these screening modalities is warranted. Their predictive utility might be limited, however, and positive serologic test results might have unanticipated consequences, such as hesitation in treating patients, thus leading to a delay in therapy or access to these medications.
Conclusion
The expanding use of immune checkpoint inhibitors is increasing survival in patients with metastatic melanoma and other malignancies. Adverse effects are part of the continuum of immune system stimulation, with overstimulation resulting in dermatitis; thyroiditis; pneumonitis; and less commonly hypophysitis, vitiligo, and colitis.
Rarely, immune checkpoint inhibition induces BP. Development of BP leads to discontinuation of therapy in more than half of reported cases due to lack of adequate treatment for this skin disease and its impact on quality of life. Therefore, quick diagnosis of BP in patients on immunotherapy and successful management techniques can prevent discontinuation of these lifesaving cancer therapies. For that reason, dermatologists play an important role in the management of patients on immune checkpoint inhibitors for cancer.
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669.
- Márquez-Rodas, I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors a review. JAMA Oncol. 2016;2:1346-1353.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Hwang SJE, Carlos G, Chou S, et al. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413-416.
- Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442-444.
- Garje R, Chau JJ, Chung J, et al. Acute flare of bullous pemphigus with pembrolizumab used for treatment of metastatic urothelial cancer. J Immunother. 2018;41:42-44.
- Ito M, Hoashi T, Endo Y, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. 2019;46:e90-e92.
- Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-773.
- Muglia C, Bronsnick T, Kirkorian AY, et al. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99:E27-E30.
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669.
- Márquez-Rodas, I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors a review. JAMA Oncol. 2016;2:1346-1353.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Hwang SJE, Carlos G, Chou S, et al. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413-416.
- Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442-444.
- Garje R, Chau JJ, Chung J, et al. Acute flare of bullous pemphigus with pembrolizumab used for treatment of metastatic urothelial cancer. J Immunother. 2018;41:42-44.
- Ito M, Hoashi T, Endo Y, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. 2019;46:e90-e92.
- Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-773.
- Muglia C, Bronsnick T, Kirkorian AY, et al. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99:E27-E30.
Practice Points
- Anti–programmed cell death 1 (PD1) targeted therapies improve survival in solid and hematologic malignancies but are associated with autoimmune side effects, with bullous pemphigoid (BP) being the newest reported.
- Bullous pemphigoid can develop months into immunotherapy treatment.
- Bullous pemphigoid should be on the differential diagnosis in a patient who is on an anti-PD1 immune checkpoint inhibitor and develops 1 or more of the following: pruritus, dermatitis, and vesicles.
- Early diagnosis of BP is essential for keeping patients on immunotherapy because its severity often results in temporary or permanent discontinuation of treatment.
Adding low-dose ipi to pembro seems safer, still effective for advanced melanoma
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
FROM ASCO 2020
Key clinical point: Low-dose ipilimumab (1 mg/kg) plus pembrolizumab given immediately after progression on a PD-1 antibody alone demonstrated antitumor activity and tolerability in patients with advanced melanoma, according to an investigator.
Major finding: There were 5 complete responses and 14 partial responses, for a response rate of 27%. The rate of grade 3/4 adverse events was 27%.
Study details: Phase 2 study of 70 patients, 61 of whom were evaluable for response.
Disclosures: The study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company.
Source: Olson D et al. ASCO 2020, Abstract 10004.
Can an app guide cancer treatment decisions during the pandemic?
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Biologics may carry melanoma risk for patients with immune-mediated inflammatory diseases
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
FROM JAMA DERMATOLOGY
‘A good and peaceful death’: Cancer hospice during the pandemic
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Germline testing in advanced cancer can lead to targeted treatment
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ASCO 2020
Oncologists’ income and satisfaction are up
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Video coaching may relieve anxiety and distress for long-distance cancer caregivers
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
FROM ASCO 2020