User login
Federal Health Care Data Trends 2023
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
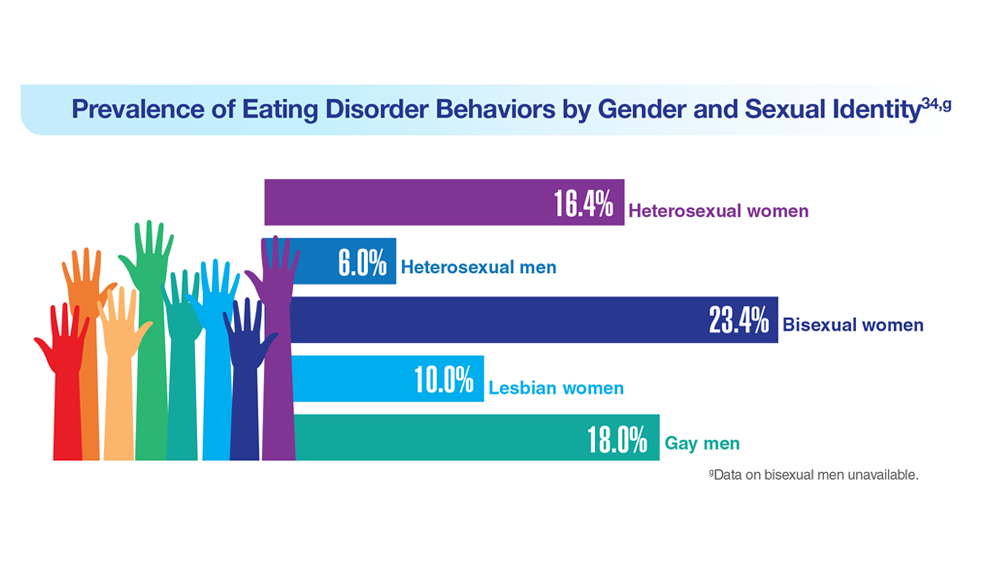
Data Trends 2023: Eating Disorders
29. Touma DA et al. Mil Med. 2022;usac180. doi:10.1093/milmed/usac180
30. Flatt RE et al. Eat Behav. 2021;43:101562. doi:10.1016/j.eatbeh.2021.101562
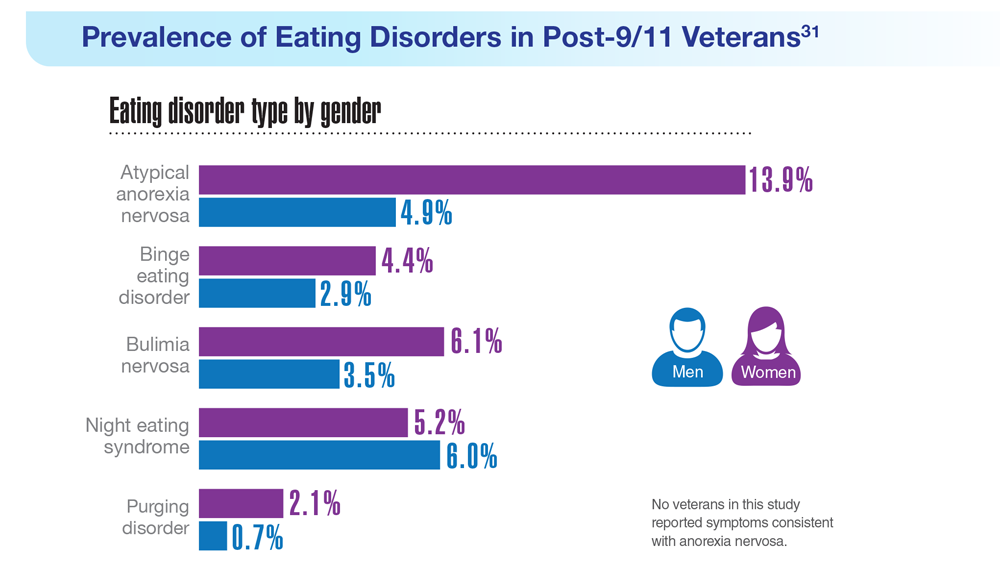
31. Masheb RM et al. Int J Eat Disord. 2021;54(7):1171-1180. doi:10.1002/eat.23501
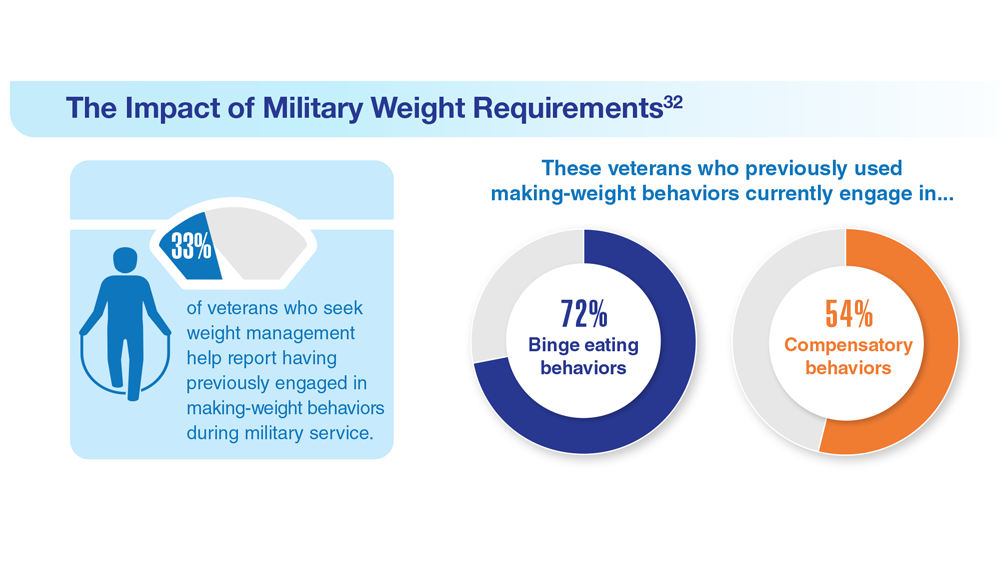
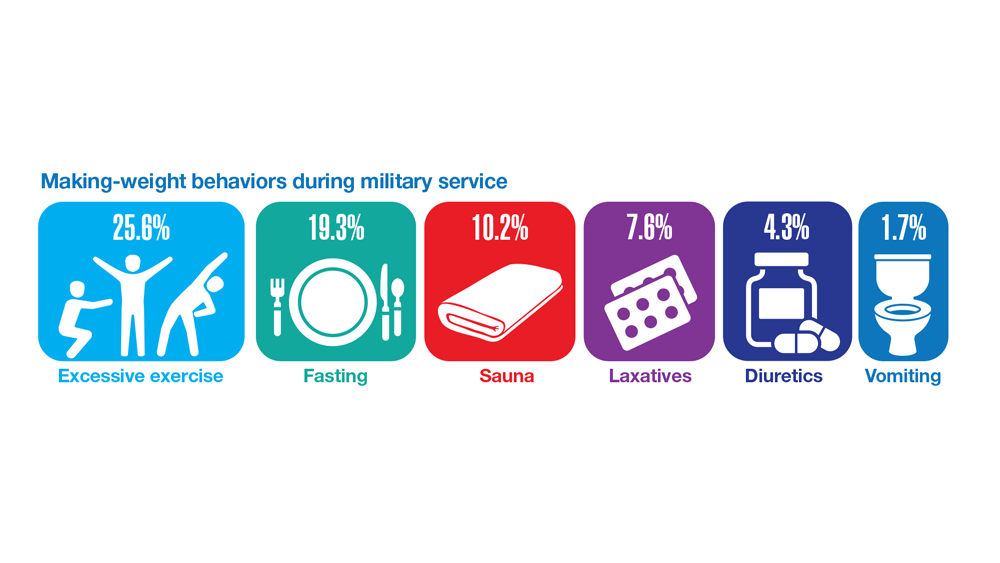
32. Masheb RM et al. Eat Weight Disord. 2019;24(6):1063-1070. doi:10.1007/s40519-019-00766-w
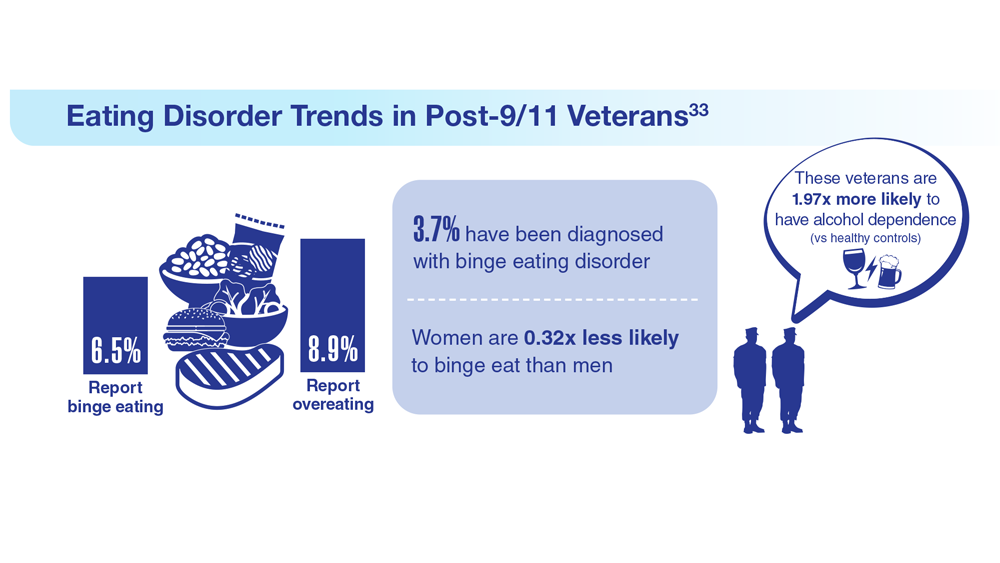
33. Etuk R et al. Mil Med. 2022;187(3-4):297-303. doi:10.1093/milmed/usab533
34. Serier KN et al. Int J Eat Disord. 2022;55(4):470-480. doi:10.1002/eat.23680
29. Touma DA et al. Mil Med. 2022;usac180. doi:10.1093/milmed/usac180
30. Flatt RE et al. Eat Behav. 2021;43:101562. doi:10.1016/j.eatbeh.2021.101562
31. Masheb RM et al. Int J Eat Disord. 2021;54(7):1171-1180. doi:10.1002/eat.23501
32. Masheb RM et al. Eat Weight Disord. 2019;24(6):1063-1070. doi:10.1007/s40519-019-00766-w
33. Etuk R et al. Mil Med. 2022;187(3-4):297-303. doi:10.1093/milmed/usab533
34. Serier KN et al. Int J Eat Disord. 2022;55(4):470-480. doi:10.1002/eat.23680
29. Touma DA et al. Mil Med. 2022;usac180. doi:10.1093/milmed/usac180
30. Flatt RE et al. Eat Behav. 2021;43:101562. doi:10.1016/j.eatbeh.2021.101562
31. Masheb RM et al. Int J Eat Disord. 2021;54(7):1171-1180. doi:10.1002/eat.23501
32. Masheb RM et al. Eat Weight Disord. 2019;24(6):1063-1070. doi:10.1007/s40519-019-00766-w
33. Etuk R et al. Mil Med. 2022;187(3-4):297-303. doi:10.1093/milmed/usab533
34. Serier KN et al. Int J Eat Disord. 2022;55(4):470-480. doi:10.1002/eat.23680
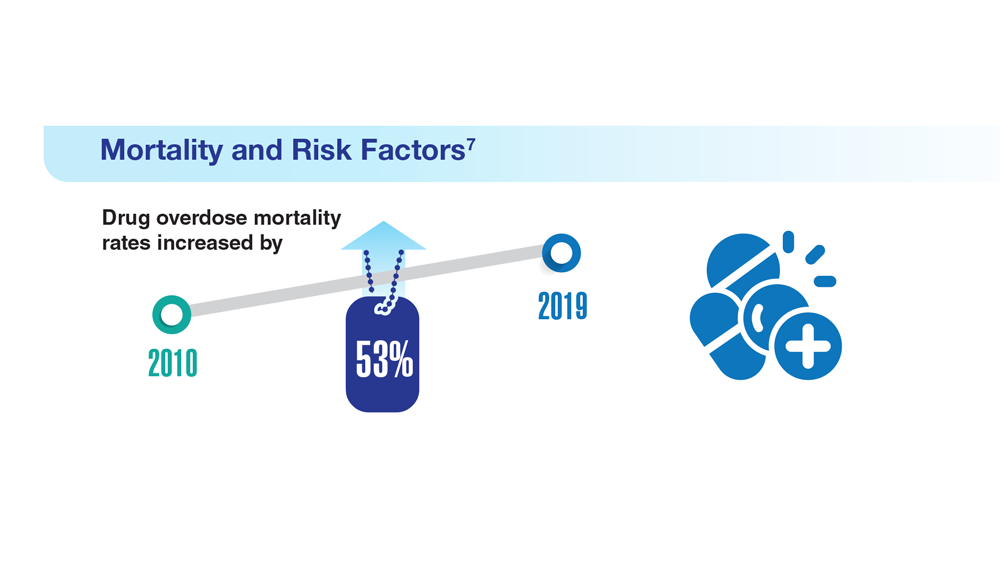
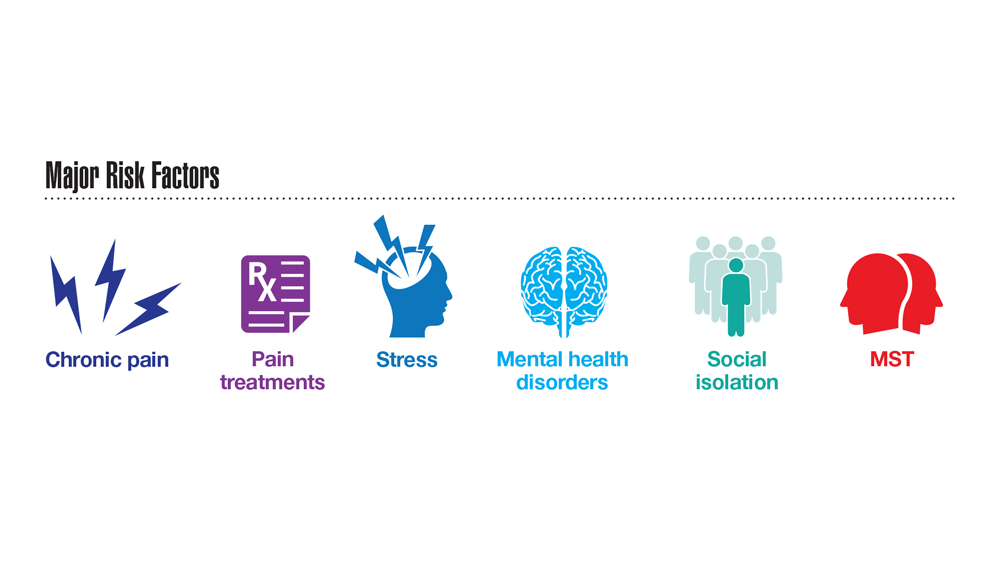
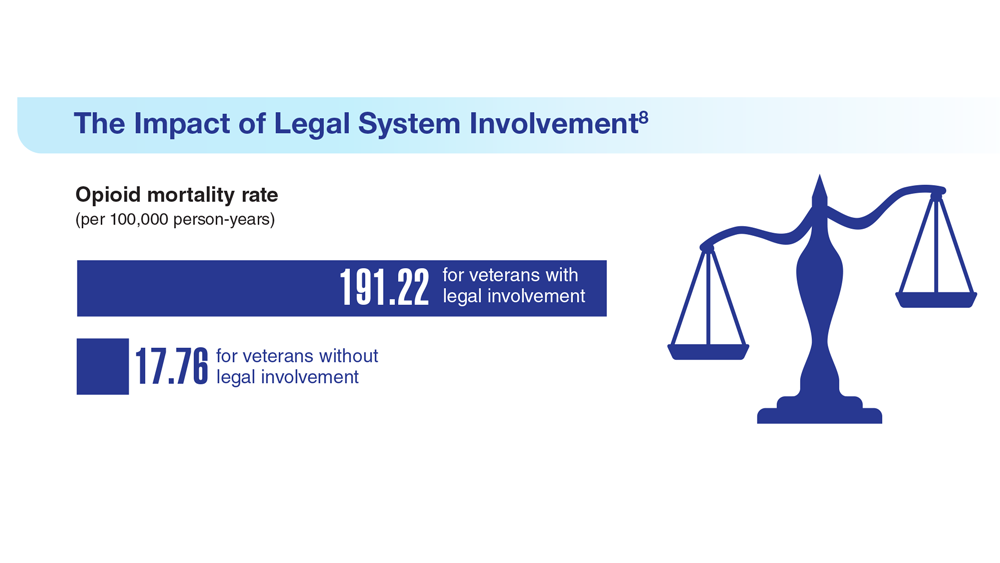
Data Trends 2023: Opioid Use Disorder
7. Bennett AS et al. Ann Med. 2022;54(1):1826-1838. doi:10.1080/07853890.2022.2092896
8. Finlay AK et al. Am J Prev Med. 2022;62(1):e29-e37. doi:10.1016/j.amepre.2021.06.014
9. Peltier MR et al. J Dual Diagn. 2021;17(2):124-134. doi:10.1080/15504263.2021.1904162
10. Beckman KL et al. Am J Prev Med. 2022;62(3):377-386. doi:10.1016/j.amepre.2021.08.020
11. Boyer TL et al. Am J Prev Med. 2022;63(2):168-177. doi:10.1016/j.amepre.2022.02.011
12. Jasuja GK et al. Med Care. 2021;59(suppl 2):S165-S169. doi:10.1097/MLR.0000000000001437
13. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA’s Rapid Naloxone Initiative recognized in fight against opioid overdose deaths. Published June 8, 2021. Accessed April 21, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5679
14. Chen EF, et. al. Fed Pract. 2022;39(3):136-141. doi:10.12788/fp.0236
7. Bennett AS et al. Ann Med. 2022;54(1):1826-1838. doi:10.1080/07853890.2022.2092896
8. Finlay AK et al. Am J Prev Med. 2022;62(1):e29-e37. doi:10.1016/j.amepre.2021.06.014
9. Peltier MR et al. J Dual Diagn. 2021;17(2):124-134. doi:10.1080/15504263.2021.1904162
10. Beckman KL et al. Am J Prev Med. 2022;62(3):377-386. doi:10.1016/j.amepre.2021.08.020
11. Boyer TL et al. Am J Prev Med. 2022;63(2):168-177. doi:10.1016/j.amepre.2022.02.011
12. Jasuja GK et al. Med Care. 2021;59(suppl 2):S165-S169. doi:10.1097/MLR.0000000000001437
13. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA’s Rapid Naloxone Initiative recognized in fight against opioid overdose deaths. Published June 8, 2021. Accessed April 21, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5679
14. Chen EF, et. al. Fed Pract. 2022;39(3):136-141. doi:10.12788/fp.0236
7. Bennett AS et al. Ann Med. 2022;54(1):1826-1838. doi:10.1080/07853890.2022.2092896
8. Finlay AK et al. Am J Prev Med. 2022;62(1):e29-e37. doi:10.1016/j.amepre.2021.06.014
9. Peltier MR et al. J Dual Diagn. 2021;17(2):124-134. doi:10.1080/15504263.2021.1904162
10. Beckman KL et al. Am J Prev Med. 2022;62(3):377-386. doi:10.1016/j.amepre.2021.08.020
11. Boyer TL et al. Am J Prev Med. 2022;63(2):168-177. doi:10.1016/j.amepre.2022.02.011
12. Jasuja GK et al. Med Care. 2021;59(suppl 2):S165-S169. doi:10.1097/MLR.0000000000001437
13. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA’s Rapid Naloxone Initiative recognized in fight against opioid overdose deaths. Published June 8, 2021. Accessed April 21, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5679
14. Chen EF, et. al. Fed Pract. 2022;39(3):136-141. doi:10.12788/fp.0236
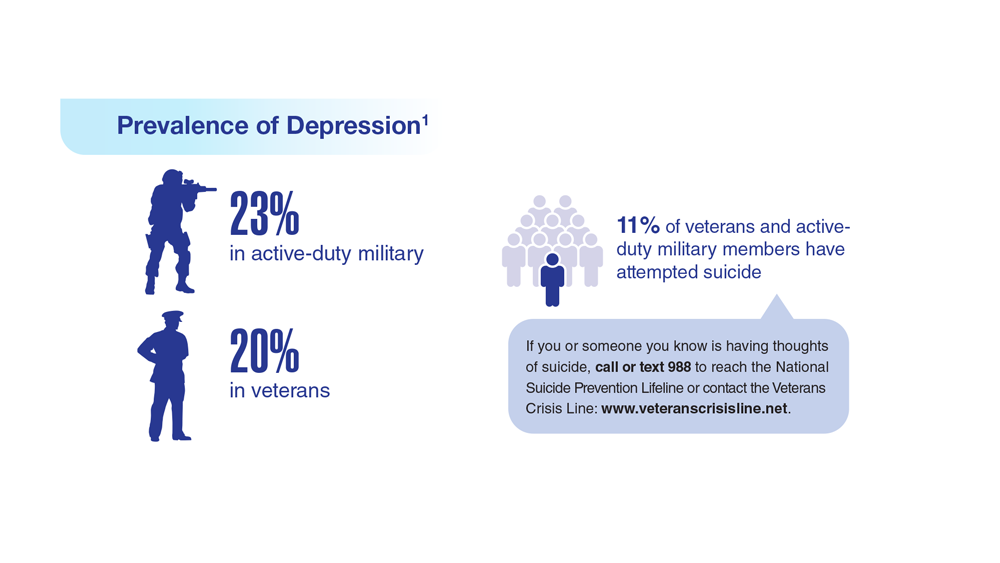
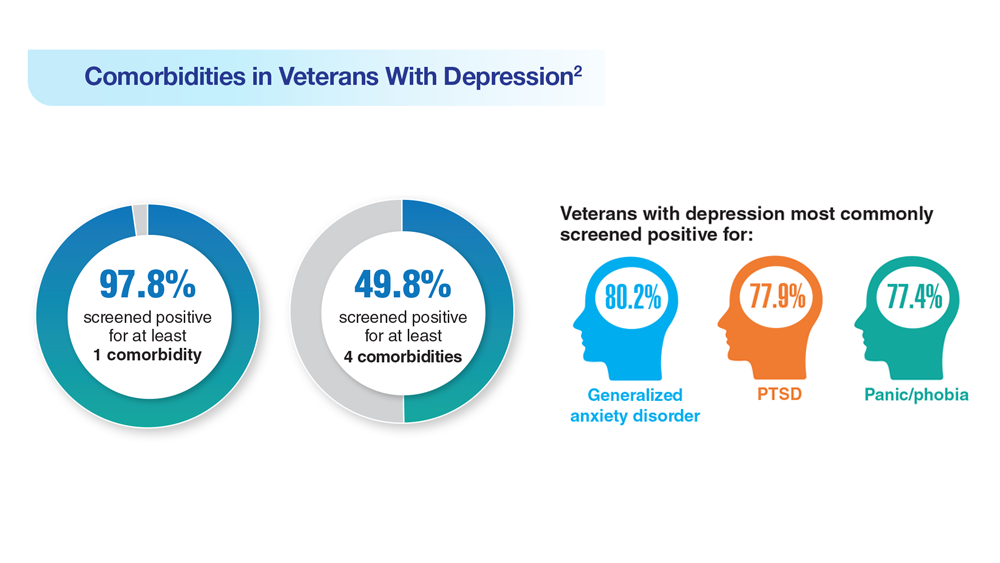
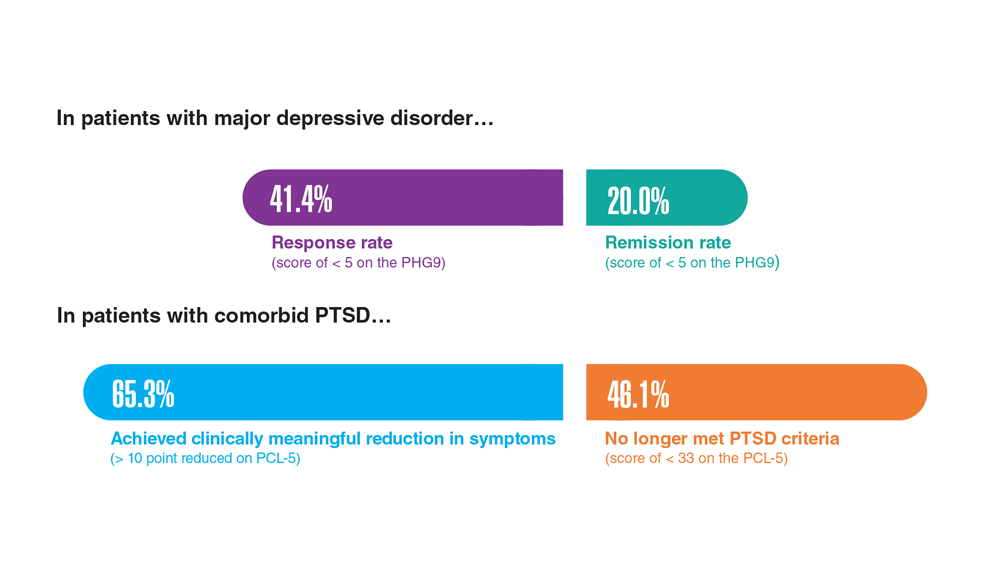
Data Trends 2023: Depression
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
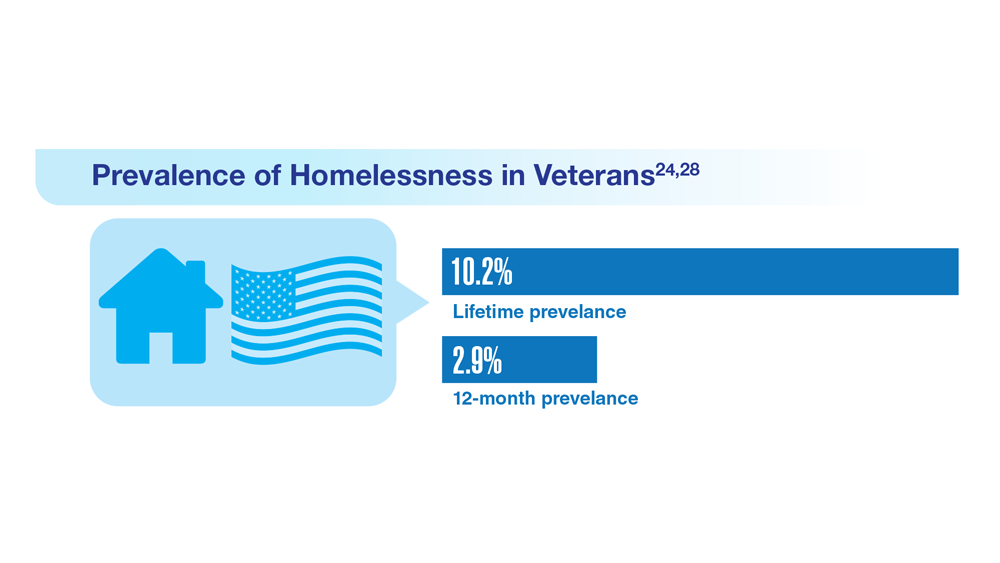
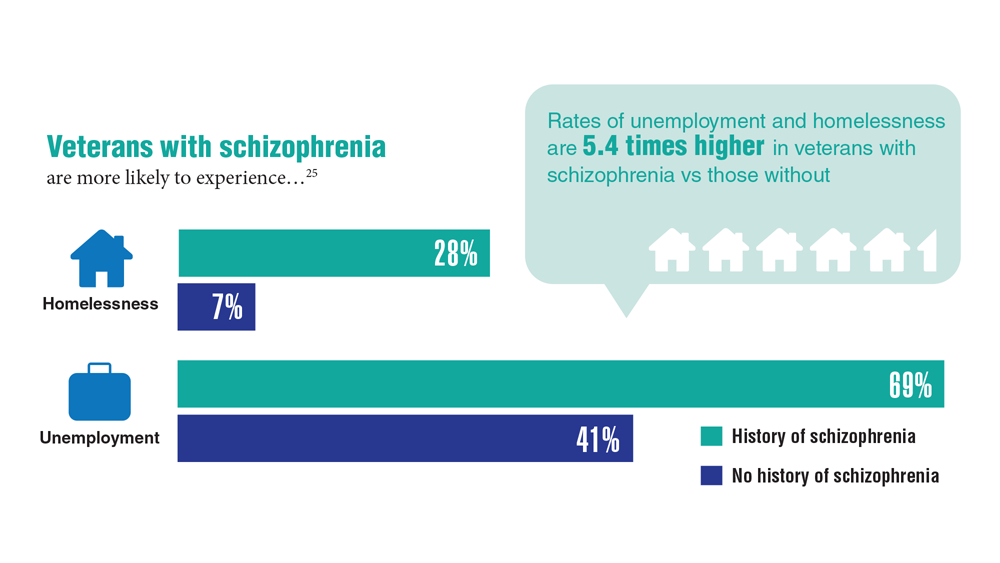
Data Trends 2023: Homelessness
24. Nichter B et al. Psychol Med. 2022;1-11. doi:10.1017/S0033291722000617
25. Lin D et al. BMC Psychiatry. 2022;22(1):458. doi:10.1186/s12888-022-04022-x
26. Jutkowitz E et al. R I Med J (2013). 2021;104(4):20-25. Published 2021 May 3.
27. Holliday R et al. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
28. Koh KA et al. Am J Prev Med. 2022;63(1):13-23. doi:10.1016/j.amepre.2021.12.028
24. Nichter B et al. Psychol Med. 2022;1-11. doi:10.1017/S0033291722000617
25. Lin D et al. BMC Psychiatry. 2022;22(1):458. doi:10.1186/s12888-022-04022-x
26. Jutkowitz E et al. R I Med J (2013). 2021;104(4):20-25. Published 2021 May 3.
27. Holliday R et al. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
28. Koh KA et al. Am J Prev Med. 2022;63(1):13-23. doi:10.1016/j.amepre.2021.12.028
24. Nichter B et al. Psychol Med. 2022;1-11. doi:10.1017/S0033291722000617
25. Lin D et al. BMC Psychiatry. 2022;22(1):458. doi:10.1186/s12888-022-04022-x
26. Jutkowitz E et al. R I Med J (2013). 2021;104(4):20-25. Published 2021 May 3.
27. Holliday R et al. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
28. Koh KA et al. Am J Prev Med. 2022;63(1):13-23. doi:10.1016/j.amepre.2021.12.028
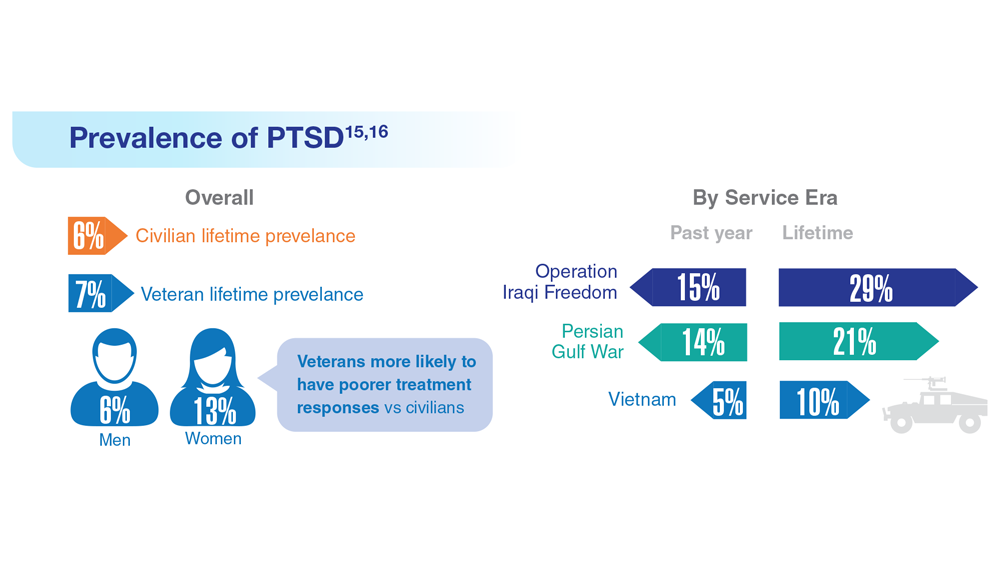
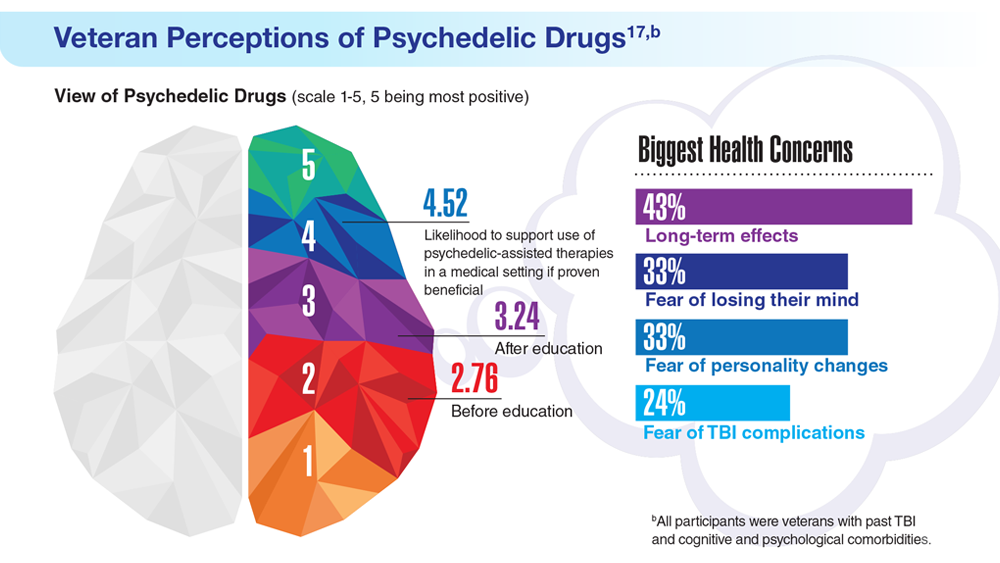
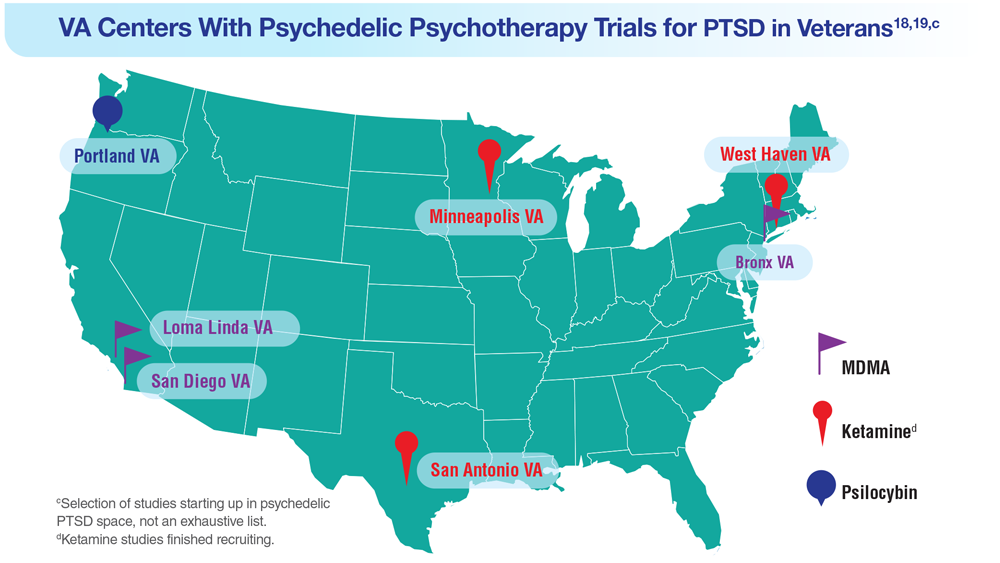
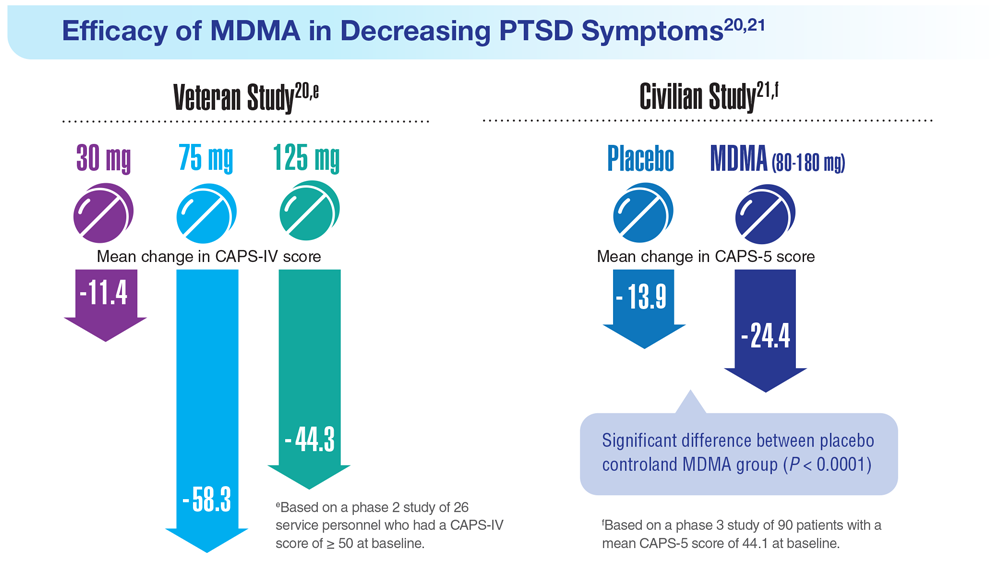
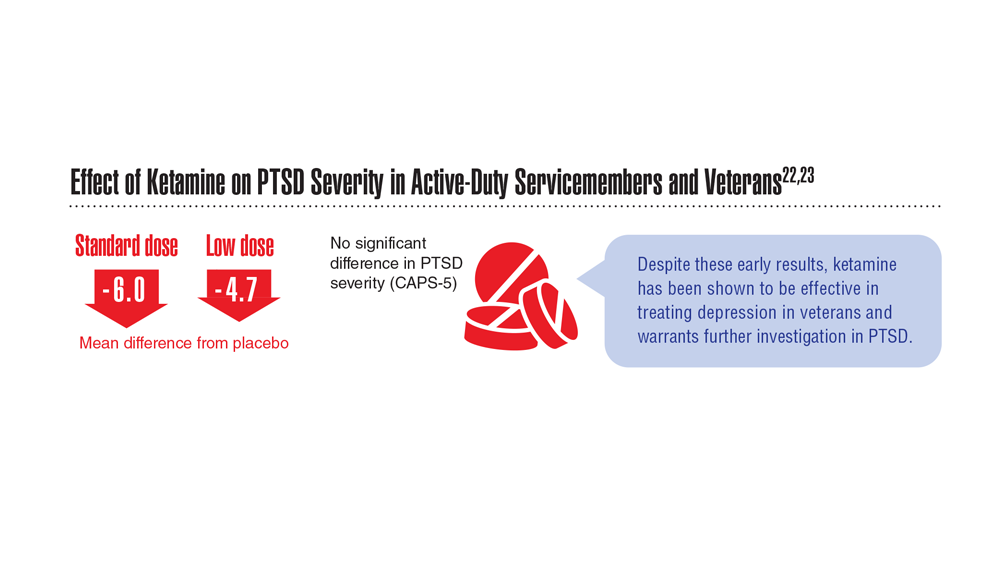
Data Trends 2023: PTSD and Psychedelic Treatments
15. US Department of Veterans Affairs. How common is PTSD in veterans? Updated February 3, 2023. Accessed April 21, 2023. https://www.ptsd.va.gov/understand/common/common_veterans.asp
16. Murphy D, Smith KV. J Trauma Stress. 2018;31(5):753-763. doi:10.1002/jts.22333
17. Gray JC et al. Mil Med. 2022;usac400. doi:10.1093/milmed/usac400
18. Herrington AJ. VA studying psychedelics as mental health treatment for veterans. Forbes. Published June 24, 2022. Accessed April 21, 2023. https://www.forbes.com/sites/ajherrington/2022/06/24/va-studying-psychedelics-as-mental-health-treatment-for-veterans/?sh=149266f6c0d4
19. Search of: Veterans: Ketamine - list results. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=ketamine&term=veterans&cntry=&state=&city=&dist=. Accessed March 23, 2023.
20. Mithoefer MC et al. Lancet Psychiatry. 2018;5(6):486-497. doi:10.1016/S2215-0366(18)30135-4
21. Mitchell JM et al. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
22. Abdallah CG et al. Neuropsychopharmacology. 2022;47(8):1574-1581. doi:10.1038/s41386-022-01266-9
23. Artin H et al. EClinicalMedicine. 2022;48:101439. doi:10.1016/j.eclinm.2022.101439
15. US Department of Veterans Affairs. How common is PTSD in veterans? Updated February 3, 2023. Accessed April 21, 2023. https://www.ptsd.va.gov/understand/common/common_veterans.asp
16. Murphy D, Smith KV. J Trauma Stress. 2018;31(5):753-763. doi:10.1002/jts.22333
17. Gray JC et al. Mil Med. 2022;usac400. doi:10.1093/milmed/usac400
18. Herrington AJ. VA studying psychedelics as mental health treatment for veterans. Forbes. Published June 24, 2022. Accessed April 21, 2023. https://www.forbes.com/sites/ajherrington/2022/06/24/va-studying-psychedelics-as-mental-health-treatment-for-veterans/?sh=149266f6c0d4
19. Search of: Veterans: Ketamine - list results. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=ketamine&term=veterans&cntry=&state=&city=&dist=. Accessed March 23, 2023.
20. Mithoefer MC et al. Lancet Psychiatry. 2018;5(6):486-497. doi:10.1016/S2215-0366(18)30135-4
21. Mitchell JM et al. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
22. Abdallah CG et al. Neuropsychopharmacology. 2022;47(8):1574-1581. doi:10.1038/s41386-022-01266-9
23. Artin H et al. EClinicalMedicine. 2022;48:101439. doi:10.1016/j.eclinm.2022.101439
15. US Department of Veterans Affairs. How common is PTSD in veterans? Updated February 3, 2023. Accessed April 21, 2023. https://www.ptsd.va.gov/understand/common/common_veterans.asp
16. Murphy D, Smith KV. J Trauma Stress. 2018;31(5):753-763. doi:10.1002/jts.22333
17. Gray JC et al. Mil Med. 2022;usac400. doi:10.1093/milmed/usac400
18. Herrington AJ. VA studying psychedelics as mental health treatment for veterans. Forbes. Published June 24, 2022. Accessed April 21, 2023. https://www.forbes.com/sites/ajherrington/2022/06/24/va-studying-psychedelics-as-mental-health-treatment-for-veterans/?sh=149266f6c0d4
19. Search of: Veterans: Ketamine - list results. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=ketamine&term=veterans&cntry=&state=&city=&dist=. Accessed March 23, 2023.
20. Mithoefer MC et al. Lancet Psychiatry. 2018;5(6):486-497. doi:10.1016/S2215-0366(18)30135-4
21. Mitchell JM et al. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
22. Abdallah CG et al. Neuropsychopharmacology. 2022;47(8):1574-1581. doi:10.1038/s41386-022-01266-9
23. Artin H et al. EClinicalMedicine. 2022;48:101439. doi:10.1016/j.eclinm.2022.101439
The unappreciated healing power of awe
I’m standing atop the Klein Matterhorn, staring out at the Alps, their moonscape peaks forming a jagged, terrifying, glorious white horizon.
I am small. But the emotions are huge. The joy: I get to be a part of all this today. The fear: It could kill me. More than kill me, it could consume me.
That’s what I always used to feel when training in Zermatt, Switzerland.
I was lucky. As a former U.S. Ski Team athlete, I was regularly able to experience such magnificent scenescapes – and feel the tactile insanity of it, too, the rise and fall of helicopters or trams taking us up the mountains, the slicing, frigid air at the summit, and the lurking on-edge feeling that you, tiny human, really aren’t meant to be standing where you are standing.
“Awe puts things in perspective,” said Craig Anderson, PhD, postdoctoral scholar at Washington University at St. Louis, and researcher of emotions and behavior. “It’s about feeling connected with people and part of the larger collective – and that makes it okay to feel small.”
Our modern world is at odds with awe. We tend to shrink into our daily lives, our problems, our devices, and the real-time emotional reactions to those things, especially anger.
It doesn’t have to be that way.
‘In the upper reaches of pleasure and on the boundary of fear’
That’s how New York University ethical leadership professor Jonathan Haidt, PhD, and psychology professor Dacher Keltner, PhD, of the University of California, Berkeley, defined awe in a seminal report from 2003.
The feeling is composed of two elements: perceived vastness (sensing something larger than ourselves) and accommodation (our need to process and understand that vastness). The researchers also wrote that awe could “change the course of life in profound and permanent ways.”
“There’s a correlation between people who are happier and those who report more feelings of awe,” said David Yaden, PhD, assistant professor in the department of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, and coauthor of “The Varieties of Spiritual Experience.” “It’s unclear, though, which way the causality runs. Is it that having more awe experiences makes people happier? Or that happy people have more awe. But there is a correlation.”
One aspect about awe that’s clear: When people experience it, they report feeling more connected. And that sense of connection can lead to prosocial behavior – such as serving others and engaging with one’s community.
“Feelings of isolation are quite difficult, and we’re social creatures, so when we feel connected, we can benefit from it,” Dr. Yaden said.
A 2022 study published in the Journal of Personality and Social Psychology revealed that awe “awakens self-transcendence, which in turn invigorates pursuit of the authentic self.”
While these effects can be seen as one individual’s benefits, the researchers posited that they also lead to prosocial behaviors. Another study conducted by the same scientists showed that awe led to greater-good behavior during the pandemic, to the tune of an increased willingness to donate blood. In this study, researchers also cited a correlation between feelings of awe and increased empathy.
The awe experience
Dr. Yaden joined Dr. Keltner and other researchers in creating a scale for the “awe experience,” and found six related factors: a feeling that time momentarily slows; a sense of self-diminishment (your sense of self becomes smaller); a sense of connectedness; feeling in the presence of something grand; the need to mentally process the experience; and physical changes, like goosebumps or feeling your jaw slightly drop.
“Any of these factors can be large or small,” Dr. Yaden noted, adding that awe can also feel positive or negative. A hurricane can instill awe, for example, and the experience might not be pleasant.
However, “it’s more common for the awe experience to be positive,” Dr. Yaden said.
How your brain processes awe
Functional MRI, by which brain activity is measured through blood flow, allows researchers to see what’s happening in the brain after an awe experience.
One study that was conducted in the Netherlands and was published in the journal Human Brain Mapping suggested that certain parts of the brain that are responsible for self-reflection were less “activated” when participants watched awe-inspiring videos.
The researchers posit that the “captivating nature of awe stimuli” could be responsible for such reductions, meaning participants’ brains were geared more toward feelings of connection with others or something greater – and a smaller sense of self.
Another study published in the journal Emotion revealed a link between awe and lower levels of inflammatory cytokines, so awe could have positive and potentially protective health benefits, as well.
And of course there are the physical and emotional benefits of nature, as dozens of studies reveal. Dr. Anderson’s research in the journal Emotion showed that nature “experiences” led to more feelings of awe and that the effects of nature also reduced stress and increased well-being.
Why we turn away from awe
The world we inhabit day to day isn’t conducive to experiencing awe – indoors, seated, reacting negatively to work or social media. The mentalities we forge because of this sometimes work against experiencing any form of awe.
Example: Some people don’t like to feel small. That requires a capacity for humility.
“That [feeling] can be threatening,” noted Dr. Anderson, who earned his doctorate studying as part of Dr. Keltner’s “Project Awe” research team at UC Berkeley.
The pandemic and politics and rise in angry Internet culture also contribute. And if you didn’t know, humans have a “negativity bias.”
“Our responses to stress tend to be stronger in magnitude than responses to positive things,” Dr. Anderson said. “Browsing the Internet and seeing negative things can hijack our responses. Anger really narrows our attention on what makes us angry.”
In that sense, anger is the antithesis of awe. As Dr. Anderson puts it: Awe broadens our attention to the world and “opens us up to other people and possibilities,” he said. “When we’re faced with daily hassles, when we experience something vast and awe-inspiring, those other problems aren’t as big of a deal.”
We crave awe in spite of ourselves
An awful lot of us are out there seeking awe, knowingly or not.
People have been stopping at scenic overlooks and climbing local peaks since forever, but let’s start with record-setting attendance at the most basic and accessible source of natural awe we have in the United States: national parks.
In 2022, 68% of the 312 million visitors sought out nature-based or recreational park activities (as opposed to historical or cultural activities). Even though a rise in national park visits in 2021 and 2022 could be attributed to pandemic-related behavior (the need for social distancing and/or the desire to get outside), people were flocking to parks prior to COVID-19. In fact, 33 parks set visitation records in 2019; 12 did so in 2022.
We also seek awe in man-made spectacle. Consider annual visitor numbers for the following:
- Golden Gate Bridge: 10 million
- : 4 million
- : 1.62 million
And what about the most awe-inducing experience ever manufactured: Space tourism. While catering to the wealthy for now, flying to space allows untrained people to enjoy something only a chosen few astronauts have been able to feel: the “overview effect,” a term coined by author Frank White for the shift in perspective that occurs in people who see Earth from space.
Upon his return from his Blue Origin flight, actor William Shatner was candid about his emotional experience. “I was crying,” he told NPR. “I didn’t know what I was crying about. It was the death that I saw in space and the lifeforce that I saw coming from the planet – the blue, the beige, and the white. And I realized one was death and the other was life.”
We want awe. We want to feel this way.
Adding everyday awe to your life
It may seem counterintuitive: Most awe-inspiring places are special occasion destinations, but in truth it’s possible to find awe each day. Outdoors and indoors.
Park Rx America, led by Robert Zarr, MD, MPH, boasts a network of nearly 1500 healthcare providers ready to “prescribe” walks or time in nature as part of healing. “Our growing community of ‘nature prescribers’ incorporate nature as a treatment option for their willing clients and patients,” Dr. Zarr said.
He also noted that awe is all about where you look, including in small places.
“Something as simple as going for a walk and stopping to notice the complexity of fractal patterns in the leaves, for example, leaves me with a sense of awe,” he said. “Although difficult to measure, there is no doubt that an important part of our health is intricately linked to these daily awe-filled moments.”
Nature is not the only way. Dr. Yaden suggested that going to a museum to see art or sporting events is also a way to experience the feeling.
An unexpected source of man-made awe: Screens. A study published in Nature showed that immersive video experiences (in this case, one achieved by virtual reality) were effective in eliciting an awe response in participants.
While virtual reality isn’t ubiquitous, immersive film experiences are. IMAX screens were created for just this purpose (as anyone who saw the Avatar films in this format can attest).
Is it perfect? No. But whether you’re witnessing a birth, hiking an autumn trail bathed in orange, or letting off a little gasp when you see Oppenheimer’s nuclear explosion in 70 mm, it all counts.
Because it’s not about the thing. It’s about your openness to be awed by the thing.
I’m a little like Dr. Zarr in that I can find wonder in the crystalline structures of a snowflake. And I also love to hike and inhale expansive views. If you can get to Switzerland, and specifically Zermatt, take the old red tram to the top. I highly recommend it.
A version of this article appeared on Medscape.com.
I’m standing atop the Klein Matterhorn, staring out at the Alps, their moonscape peaks forming a jagged, terrifying, glorious white horizon.
I am small. But the emotions are huge. The joy: I get to be a part of all this today. The fear: It could kill me. More than kill me, it could consume me.
That’s what I always used to feel when training in Zermatt, Switzerland.
I was lucky. As a former U.S. Ski Team athlete, I was regularly able to experience such magnificent scenescapes – and feel the tactile insanity of it, too, the rise and fall of helicopters or trams taking us up the mountains, the slicing, frigid air at the summit, and the lurking on-edge feeling that you, tiny human, really aren’t meant to be standing where you are standing.
“Awe puts things in perspective,” said Craig Anderson, PhD, postdoctoral scholar at Washington University at St. Louis, and researcher of emotions and behavior. “It’s about feeling connected with people and part of the larger collective – and that makes it okay to feel small.”
Our modern world is at odds with awe. We tend to shrink into our daily lives, our problems, our devices, and the real-time emotional reactions to those things, especially anger.
It doesn’t have to be that way.
‘In the upper reaches of pleasure and on the boundary of fear’
That’s how New York University ethical leadership professor Jonathan Haidt, PhD, and psychology professor Dacher Keltner, PhD, of the University of California, Berkeley, defined awe in a seminal report from 2003.
The feeling is composed of two elements: perceived vastness (sensing something larger than ourselves) and accommodation (our need to process and understand that vastness). The researchers also wrote that awe could “change the course of life in profound and permanent ways.”
“There’s a correlation between people who are happier and those who report more feelings of awe,” said David Yaden, PhD, assistant professor in the department of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, and coauthor of “The Varieties of Spiritual Experience.” “It’s unclear, though, which way the causality runs. Is it that having more awe experiences makes people happier? Or that happy people have more awe. But there is a correlation.”
One aspect about awe that’s clear: When people experience it, they report feeling more connected. And that sense of connection can lead to prosocial behavior – such as serving others and engaging with one’s community.
“Feelings of isolation are quite difficult, and we’re social creatures, so when we feel connected, we can benefit from it,” Dr. Yaden said.
A 2022 study published in the Journal of Personality and Social Psychology revealed that awe “awakens self-transcendence, which in turn invigorates pursuit of the authentic self.”
While these effects can be seen as one individual’s benefits, the researchers posited that they also lead to prosocial behaviors. Another study conducted by the same scientists showed that awe led to greater-good behavior during the pandemic, to the tune of an increased willingness to donate blood. In this study, researchers also cited a correlation between feelings of awe and increased empathy.
The awe experience
Dr. Yaden joined Dr. Keltner and other researchers in creating a scale for the “awe experience,” and found six related factors: a feeling that time momentarily slows; a sense of self-diminishment (your sense of self becomes smaller); a sense of connectedness; feeling in the presence of something grand; the need to mentally process the experience; and physical changes, like goosebumps or feeling your jaw slightly drop.
“Any of these factors can be large or small,” Dr. Yaden noted, adding that awe can also feel positive or negative. A hurricane can instill awe, for example, and the experience might not be pleasant.
However, “it’s more common for the awe experience to be positive,” Dr. Yaden said.
How your brain processes awe
Functional MRI, by which brain activity is measured through blood flow, allows researchers to see what’s happening in the brain after an awe experience.
One study that was conducted in the Netherlands and was published in the journal Human Brain Mapping suggested that certain parts of the brain that are responsible for self-reflection were less “activated” when participants watched awe-inspiring videos.
The researchers posit that the “captivating nature of awe stimuli” could be responsible for such reductions, meaning participants’ brains were geared more toward feelings of connection with others or something greater – and a smaller sense of self.
Another study published in the journal Emotion revealed a link between awe and lower levels of inflammatory cytokines, so awe could have positive and potentially protective health benefits, as well.
And of course there are the physical and emotional benefits of nature, as dozens of studies reveal. Dr. Anderson’s research in the journal Emotion showed that nature “experiences” led to more feelings of awe and that the effects of nature also reduced stress and increased well-being.
Why we turn away from awe
The world we inhabit day to day isn’t conducive to experiencing awe – indoors, seated, reacting negatively to work or social media. The mentalities we forge because of this sometimes work against experiencing any form of awe.
Example: Some people don’t like to feel small. That requires a capacity for humility.
“That [feeling] can be threatening,” noted Dr. Anderson, who earned his doctorate studying as part of Dr. Keltner’s “Project Awe” research team at UC Berkeley.
The pandemic and politics and rise in angry Internet culture also contribute. And if you didn’t know, humans have a “negativity bias.”
“Our responses to stress tend to be stronger in magnitude than responses to positive things,” Dr. Anderson said. “Browsing the Internet and seeing negative things can hijack our responses. Anger really narrows our attention on what makes us angry.”
In that sense, anger is the antithesis of awe. As Dr. Anderson puts it: Awe broadens our attention to the world and “opens us up to other people and possibilities,” he said. “When we’re faced with daily hassles, when we experience something vast and awe-inspiring, those other problems aren’t as big of a deal.”
We crave awe in spite of ourselves
An awful lot of us are out there seeking awe, knowingly or not.
People have been stopping at scenic overlooks and climbing local peaks since forever, but let’s start with record-setting attendance at the most basic and accessible source of natural awe we have in the United States: national parks.
In 2022, 68% of the 312 million visitors sought out nature-based or recreational park activities (as opposed to historical or cultural activities). Even though a rise in national park visits in 2021 and 2022 could be attributed to pandemic-related behavior (the need for social distancing and/or the desire to get outside), people were flocking to parks prior to COVID-19. In fact, 33 parks set visitation records in 2019; 12 did so in 2022.
We also seek awe in man-made spectacle. Consider annual visitor numbers for the following:
- Golden Gate Bridge: 10 million
- : 4 million
- : 1.62 million
And what about the most awe-inducing experience ever manufactured: Space tourism. While catering to the wealthy for now, flying to space allows untrained people to enjoy something only a chosen few astronauts have been able to feel: the “overview effect,” a term coined by author Frank White for the shift in perspective that occurs in people who see Earth from space.
Upon his return from his Blue Origin flight, actor William Shatner was candid about his emotional experience. “I was crying,” he told NPR. “I didn’t know what I was crying about. It was the death that I saw in space and the lifeforce that I saw coming from the planet – the blue, the beige, and the white. And I realized one was death and the other was life.”
We want awe. We want to feel this way.
Adding everyday awe to your life
It may seem counterintuitive: Most awe-inspiring places are special occasion destinations, but in truth it’s possible to find awe each day. Outdoors and indoors.
Park Rx America, led by Robert Zarr, MD, MPH, boasts a network of nearly 1500 healthcare providers ready to “prescribe” walks or time in nature as part of healing. “Our growing community of ‘nature prescribers’ incorporate nature as a treatment option for their willing clients and patients,” Dr. Zarr said.
He also noted that awe is all about where you look, including in small places.
“Something as simple as going for a walk and stopping to notice the complexity of fractal patterns in the leaves, for example, leaves me with a sense of awe,” he said. “Although difficult to measure, there is no doubt that an important part of our health is intricately linked to these daily awe-filled moments.”
Nature is not the only way. Dr. Yaden suggested that going to a museum to see art or sporting events is also a way to experience the feeling.
An unexpected source of man-made awe: Screens. A study published in Nature showed that immersive video experiences (in this case, one achieved by virtual reality) were effective in eliciting an awe response in participants.
While virtual reality isn’t ubiquitous, immersive film experiences are. IMAX screens were created for just this purpose (as anyone who saw the Avatar films in this format can attest).
Is it perfect? No. But whether you’re witnessing a birth, hiking an autumn trail bathed in orange, or letting off a little gasp when you see Oppenheimer’s nuclear explosion in 70 mm, it all counts.
Because it’s not about the thing. It’s about your openness to be awed by the thing.
I’m a little like Dr. Zarr in that I can find wonder in the crystalline structures of a snowflake. And I also love to hike and inhale expansive views. If you can get to Switzerland, and specifically Zermatt, take the old red tram to the top. I highly recommend it.
A version of this article appeared on Medscape.com.
I’m standing atop the Klein Matterhorn, staring out at the Alps, their moonscape peaks forming a jagged, terrifying, glorious white horizon.
I am small. But the emotions are huge. The joy: I get to be a part of all this today. The fear: It could kill me. More than kill me, it could consume me.
That’s what I always used to feel when training in Zermatt, Switzerland.
I was lucky. As a former U.S. Ski Team athlete, I was regularly able to experience such magnificent scenescapes – and feel the tactile insanity of it, too, the rise and fall of helicopters or trams taking us up the mountains, the slicing, frigid air at the summit, and the lurking on-edge feeling that you, tiny human, really aren’t meant to be standing where you are standing.
“Awe puts things in perspective,” said Craig Anderson, PhD, postdoctoral scholar at Washington University at St. Louis, and researcher of emotions and behavior. “It’s about feeling connected with people and part of the larger collective – and that makes it okay to feel small.”
Our modern world is at odds with awe. We tend to shrink into our daily lives, our problems, our devices, and the real-time emotional reactions to those things, especially anger.
It doesn’t have to be that way.
‘In the upper reaches of pleasure and on the boundary of fear’
That’s how New York University ethical leadership professor Jonathan Haidt, PhD, and psychology professor Dacher Keltner, PhD, of the University of California, Berkeley, defined awe in a seminal report from 2003.
The feeling is composed of two elements: perceived vastness (sensing something larger than ourselves) and accommodation (our need to process and understand that vastness). The researchers also wrote that awe could “change the course of life in profound and permanent ways.”
“There’s a correlation between people who are happier and those who report more feelings of awe,” said David Yaden, PhD, assistant professor in the department of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, and coauthor of “The Varieties of Spiritual Experience.” “It’s unclear, though, which way the causality runs. Is it that having more awe experiences makes people happier? Or that happy people have more awe. But there is a correlation.”
One aspect about awe that’s clear: When people experience it, they report feeling more connected. And that sense of connection can lead to prosocial behavior – such as serving others and engaging with one’s community.
“Feelings of isolation are quite difficult, and we’re social creatures, so when we feel connected, we can benefit from it,” Dr. Yaden said.
A 2022 study published in the Journal of Personality and Social Psychology revealed that awe “awakens self-transcendence, which in turn invigorates pursuit of the authentic self.”
While these effects can be seen as one individual’s benefits, the researchers posited that they also lead to prosocial behaviors. Another study conducted by the same scientists showed that awe led to greater-good behavior during the pandemic, to the tune of an increased willingness to donate blood. In this study, researchers also cited a correlation between feelings of awe and increased empathy.
The awe experience
Dr. Yaden joined Dr. Keltner and other researchers in creating a scale for the “awe experience,” and found six related factors: a feeling that time momentarily slows; a sense of self-diminishment (your sense of self becomes smaller); a sense of connectedness; feeling in the presence of something grand; the need to mentally process the experience; and physical changes, like goosebumps or feeling your jaw slightly drop.
“Any of these factors can be large or small,” Dr. Yaden noted, adding that awe can also feel positive or negative. A hurricane can instill awe, for example, and the experience might not be pleasant.
However, “it’s more common for the awe experience to be positive,” Dr. Yaden said.
How your brain processes awe
Functional MRI, by which brain activity is measured through blood flow, allows researchers to see what’s happening in the brain after an awe experience.
One study that was conducted in the Netherlands and was published in the journal Human Brain Mapping suggested that certain parts of the brain that are responsible for self-reflection were less “activated” when participants watched awe-inspiring videos.
The researchers posit that the “captivating nature of awe stimuli” could be responsible for such reductions, meaning participants’ brains were geared more toward feelings of connection with others or something greater – and a smaller sense of self.
Another study published in the journal Emotion revealed a link between awe and lower levels of inflammatory cytokines, so awe could have positive and potentially protective health benefits, as well.
And of course there are the physical and emotional benefits of nature, as dozens of studies reveal. Dr. Anderson’s research in the journal Emotion showed that nature “experiences” led to more feelings of awe and that the effects of nature also reduced stress and increased well-being.
Why we turn away from awe
The world we inhabit day to day isn’t conducive to experiencing awe – indoors, seated, reacting negatively to work or social media. The mentalities we forge because of this sometimes work against experiencing any form of awe.
Example: Some people don’t like to feel small. That requires a capacity for humility.
“That [feeling] can be threatening,” noted Dr. Anderson, who earned his doctorate studying as part of Dr. Keltner’s “Project Awe” research team at UC Berkeley.
The pandemic and politics and rise in angry Internet culture also contribute. And if you didn’t know, humans have a “negativity bias.”
“Our responses to stress tend to be stronger in magnitude than responses to positive things,” Dr. Anderson said. “Browsing the Internet and seeing negative things can hijack our responses. Anger really narrows our attention on what makes us angry.”
In that sense, anger is the antithesis of awe. As Dr. Anderson puts it: Awe broadens our attention to the world and “opens us up to other people and possibilities,” he said. “When we’re faced with daily hassles, when we experience something vast and awe-inspiring, those other problems aren’t as big of a deal.”
We crave awe in spite of ourselves
An awful lot of us are out there seeking awe, knowingly or not.
People have been stopping at scenic overlooks and climbing local peaks since forever, but let’s start with record-setting attendance at the most basic and accessible source of natural awe we have in the United States: national parks.
In 2022, 68% of the 312 million visitors sought out nature-based or recreational park activities (as opposed to historical or cultural activities). Even though a rise in national park visits in 2021 and 2022 could be attributed to pandemic-related behavior (the need for social distancing and/or the desire to get outside), people were flocking to parks prior to COVID-19. In fact, 33 parks set visitation records in 2019; 12 did so in 2022.
We also seek awe in man-made spectacle. Consider annual visitor numbers for the following:
- Golden Gate Bridge: 10 million
- : 4 million
- : 1.62 million
And what about the most awe-inducing experience ever manufactured: Space tourism. While catering to the wealthy for now, flying to space allows untrained people to enjoy something only a chosen few astronauts have been able to feel: the “overview effect,” a term coined by author Frank White for the shift in perspective that occurs in people who see Earth from space.
Upon his return from his Blue Origin flight, actor William Shatner was candid about his emotional experience. “I was crying,” he told NPR. “I didn’t know what I was crying about. It was the death that I saw in space and the lifeforce that I saw coming from the planet – the blue, the beige, and the white. And I realized one was death and the other was life.”
We want awe. We want to feel this way.
Adding everyday awe to your life
It may seem counterintuitive: Most awe-inspiring places are special occasion destinations, but in truth it’s possible to find awe each day. Outdoors and indoors.
Park Rx America, led by Robert Zarr, MD, MPH, boasts a network of nearly 1500 healthcare providers ready to “prescribe” walks or time in nature as part of healing. “Our growing community of ‘nature prescribers’ incorporate nature as a treatment option for their willing clients and patients,” Dr. Zarr said.
He also noted that awe is all about where you look, including in small places.
“Something as simple as going for a walk and stopping to notice the complexity of fractal patterns in the leaves, for example, leaves me with a sense of awe,” he said. “Although difficult to measure, there is no doubt that an important part of our health is intricately linked to these daily awe-filled moments.”
Nature is not the only way. Dr. Yaden suggested that going to a museum to see art or sporting events is also a way to experience the feeling.
An unexpected source of man-made awe: Screens. A study published in Nature showed that immersive video experiences (in this case, one achieved by virtual reality) were effective in eliciting an awe response in participants.
While virtual reality isn’t ubiquitous, immersive film experiences are. IMAX screens were created for just this purpose (as anyone who saw the Avatar films in this format can attest).
Is it perfect? No. But whether you’re witnessing a birth, hiking an autumn trail bathed in orange, or letting off a little gasp when you see Oppenheimer’s nuclear explosion in 70 mm, it all counts.
Because it’s not about the thing. It’s about your openness to be awed by the thing.
I’m a little like Dr. Zarr in that I can find wonder in the crystalline structures of a snowflake. And I also love to hike and inhale expansive views. If you can get to Switzerland, and specifically Zermatt, take the old red tram to the top. I highly recommend it.
A version of this article appeared on Medscape.com.
ADHD underappreciated in older adults
Negative consequences can develop from not treating ADHD, including job loss, suppressed income levels, low educational attainment, and difficulty maintaining relationships. Perhaps as many as 5% of adults have ADHD, which is highly hereditary.
Primary care physicians or family medicine clinicians are often best placed to diagnose ADHD in older adults, according to experts interviewed for this article. But first these providers need to tease out whether a patient has ADHD or another condition that is causing difficulties for these older adults.
However, clinicians may have a hard time discerning the symptoms of ADHD from that of other conditions of aging, such as mild cognitive impairment. Literature is lacking on best practices for screening and treating the condition in this population, according to a recent review published in the Expert Review of Neurotherapeutics, and a 2020 study by the same authors, epidemiologists, and psychiatrists based in Europe.
“If you don’t manage it, who will? It can be very hard to get psychiatric consults, almost impossible in some cases,” said Brendan Montano, MD, an internist in Cromwell, Conn., who specializes in treating ADHD.
A critical role for primary care and family medicine
Although no formal U.S. guidelines exist on diagnosis of adult ADHD, Dr. Montano stressed that adults usually present with symptoms such as chronic forgetfulness, distractibility, or procrastination. Hyperactivity is more internalized as a feeling of internal restlessness in this age group, Dr. Montano said, rather than the more visible hyper behavior seen in children.
“The two big ways that people present are thinking they have it or [being] in chaos of some sort,” perhaps because they have been fired or their spouse has asked for a divorce, Dr. Montano explained.
To assess the possibility of ADHD, a clinician could give a patient a six-item screening test that asks questions such as how often over the previous 6 months have they had trouble completing assignments or remembering appointments. If they report difficulty in four or more of the six areas in the brief screener, clinicians can move on to a longer 18-item screening tool developed by the World Health Organization, Dr. Montano said.
To make an ADHD diagnosis definitively, however, a patient needs to report sustained difficulties in at least two aspects of life: work, home, or social situations. And they have to indicate to a clinician that these troubles began before age 12.
“You have to really sit down and paint the picture with the patient: There are no shortcuts here,” said Lenard Adler, MD, a psychiatrist who directs the Adult ADHD program at New York University. Dr. Adler helped develop the six-item screener and is currently on a committee developing formal screening guidelines for adult ADHD in the United States.
He said that often, “ADHD cotravels with other mental health disorders like depression, bipolar disorders, anxiety disorders, and substance use disorders at least half the time.”
To tease out if a patient really has ADHD, clinicians can start with a more general screening for anxiety and hone in on the possibility of the condition if the anxiety screening reveals challenges with distractibility and forgetfulness, according to Karen Smith, MD, a family physician in Raeford, N.C.
“We can’t expect patients to walk through the door with the diagnosis stamped on their foreheads,” said Dr. Smith, who is a member of the board of directors for the American Academy of Family Physicians.
But a patient’s apparent signs of ADHD could be due to another condition such as hyperthyroidism or diabetes, Dr. Smith said. The driving principle is to take enough time to figure out exactly what is going on, she said, and then to treat accordingly. “If you ask the right questions, patients will give you good answers.”
Medications are often prescribed to help manage ADHD symptoms. These can include stimulants, controlled substances that include methylphenidate or amphetamine, or nonstimulants, such as atomoxetine and bupropion. Dr. Montano starts his patients on a lower dose and titrates them up rapidly until they reach the optimum dose that allows them to sustain focus and feel more productive. Patients complete frequent self-reports during this process.
“Any diversion is inappropriate,” said Dr. Montano, who writes a contract with his patients saying that stimulant medications will not be refilled if they are used more quickly than prescribed.
For older adults, physicians should make sure that stimulants do not exacerbate any underlying cardiovascular conditions, Dr. Montano said, and consult a cardiologist when needed.
The benefits of proper ADHD treatment can be profound, Dr. Adler noted, no matter when it begins.
“Older adults have lived their lives feeling they’ve been disorganized and a burden to their significant others, and only when they’ve taken medication have they really been able to organize their days and feel effective in their social interactions and with their families,” Dr. Adler said.
Dr. Montano has reported relationships with AbbVie, Axsome, Corium, Idorsia, Intracellular, Otsuka, Supernus, Sage/Biogen, Biohaven, Boehringer Ingelheim, Cassava, Eli Lilly, Intracellular, Janssen, Jazz, Sage, Supernus, Otsuka, and Tonix. Dr. Adler has reported relationships with Shire/Takeda Pharmaceuticals, National Football League, Major League Baseball, Corium, Otsuka, Neurocentria, Bracket/Signant, and SUNY; and is receiving royalty payments for developing adult ADHD assessments. Dr. Smith has reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Negative consequences can develop from not treating ADHD, including job loss, suppressed income levels, low educational attainment, and difficulty maintaining relationships. Perhaps as many as 5% of adults have ADHD, which is highly hereditary.
Primary care physicians or family medicine clinicians are often best placed to diagnose ADHD in older adults, according to experts interviewed for this article. But first these providers need to tease out whether a patient has ADHD or another condition that is causing difficulties for these older adults.
However, clinicians may have a hard time discerning the symptoms of ADHD from that of other conditions of aging, such as mild cognitive impairment. Literature is lacking on best practices for screening and treating the condition in this population, according to a recent review published in the Expert Review of Neurotherapeutics, and a 2020 study by the same authors, epidemiologists, and psychiatrists based in Europe.
“If you don’t manage it, who will? It can be very hard to get psychiatric consults, almost impossible in some cases,” said Brendan Montano, MD, an internist in Cromwell, Conn., who specializes in treating ADHD.
A critical role for primary care and family medicine
Although no formal U.S. guidelines exist on diagnosis of adult ADHD, Dr. Montano stressed that adults usually present with symptoms such as chronic forgetfulness, distractibility, or procrastination. Hyperactivity is more internalized as a feeling of internal restlessness in this age group, Dr. Montano said, rather than the more visible hyper behavior seen in children.
“The two big ways that people present are thinking they have it or [being] in chaos of some sort,” perhaps because they have been fired or their spouse has asked for a divorce, Dr. Montano explained.
To assess the possibility of ADHD, a clinician could give a patient a six-item screening test that asks questions such as how often over the previous 6 months have they had trouble completing assignments or remembering appointments. If they report difficulty in four or more of the six areas in the brief screener, clinicians can move on to a longer 18-item screening tool developed by the World Health Organization, Dr. Montano said.
To make an ADHD diagnosis definitively, however, a patient needs to report sustained difficulties in at least two aspects of life: work, home, or social situations. And they have to indicate to a clinician that these troubles began before age 12.
“You have to really sit down and paint the picture with the patient: There are no shortcuts here,” said Lenard Adler, MD, a psychiatrist who directs the Adult ADHD program at New York University. Dr. Adler helped develop the six-item screener and is currently on a committee developing formal screening guidelines for adult ADHD in the United States.
He said that often, “ADHD cotravels with other mental health disorders like depression, bipolar disorders, anxiety disorders, and substance use disorders at least half the time.”
To tease out if a patient really has ADHD, clinicians can start with a more general screening for anxiety and hone in on the possibility of the condition if the anxiety screening reveals challenges with distractibility and forgetfulness, according to Karen Smith, MD, a family physician in Raeford, N.C.
“We can’t expect patients to walk through the door with the diagnosis stamped on their foreheads,” said Dr. Smith, who is a member of the board of directors for the American Academy of Family Physicians.
But a patient’s apparent signs of ADHD could be due to another condition such as hyperthyroidism or diabetes, Dr. Smith said. The driving principle is to take enough time to figure out exactly what is going on, she said, and then to treat accordingly. “If you ask the right questions, patients will give you good answers.”
Medications are often prescribed to help manage ADHD symptoms. These can include stimulants, controlled substances that include methylphenidate or amphetamine, or nonstimulants, such as atomoxetine and bupropion. Dr. Montano starts his patients on a lower dose and titrates them up rapidly until they reach the optimum dose that allows them to sustain focus and feel more productive. Patients complete frequent self-reports during this process.
“Any diversion is inappropriate,” said Dr. Montano, who writes a contract with his patients saying that stimulant medications will not be refilled if they are used more quickly than prescribed.
For older adults, physicians should make sure that stimulants do not exacerbate any underlying cardiovascular conditions, Dr. Montano said, and consult a cardiologist when needed.
The benefits of proper ADHD treatment can be profound, Dr. Adler noted, no matter when it begins.
“Older adults have lived their lives feeling they’ve been disorganized and a burden to their significant others, and only when they’ve taken medication have they really been able to organize their days and feel effective in their social interactions and with their families,” Dr. Adler said.
Dr. Montano has reported relationships with AbbVie, Axsome, Corium, Idorsia, Intracellular, Otsuka, Supernus, Sage/Biogen, Biohaven, Boehringer Ingelheim, Cassava, Eli Lilly, Intracellular, Janssen, Jazz, Sage, Supernus, Otsuka, and Tonix. Dr. Adler has reported relationships with Shire/Takeda Pharmaceuticals, National Football League, Major League Baseball, Corium, Otsuka, Neurocentria, Bracket/Signant, and SUNY; and is receiving royalty payments for developing adult ADHD assessments. Dr. Smith has reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Negative consequences can develop from not treating ADHD, including job loss, suppressed income levels, low educational attainment, and difficulty maintaining relationships. Perhaps as many as 5% of adults have ADHD, which is highly hereditary.
Primary care physicians or family medicine clinicians are often best placed to diagnose ADHD in older adults, according to experts interviewed for this article. But first these providers need to tease out whether a patient has ADHD or another condition that is causing difficulties for these older adults.
However, clinicians may have a hard time discerning the symptoms of ADHD from that of other conditions of aging, such as mild cognitive impairment. Literature is lacking on best practices for screening and treating the condition in this population, according to a recent review published in the Expert Review of Neurotherapeutics, and a 2020 study by the same authors, epidemiologists, and psychiatrists based in Europe.
“If you don’t manage it, who will? It can be very hard to get psychiatric consults, almost impossible in some cases,” said Brendan Montano, MD, an internist in Cromwell, Conn., who specializes in treating ADHD.
A critical role for primary care and family medicine
Although no formal U.S. guidelines exist on diagnosis of adult ADHD, Dr. Montano stressed that adults usually present with symptoms such as chronic forgetfulness, distractibility, or procrastination. Hyperactivity is more internalized as a feeling of internal restlessness in this age group, Dr. Montano said, rather than the more visible hyper behavior seen in children.
“The two big ways that people present are thinking they have it or [being] in chaos of some sort,” perhaps because they have been fired or their spouse has asked for a divorce, Dr. Montano explained.
To assess the possibility of ADHD, a clinician could give a patient a six-item screening test that asks questions such as how often over the previous 6 months have they had trouble completing assignments or remembering appointments. If they report difficulty in four or more of the six areas in the brief screener, clinicians can move on to a longer 18-item screening tool developed by the World Health Organization, Dr. Montano said.
To make an ADHD diagnosis definitively, however, a patient needs to report sustained difficulties in at least two aspects of life: work, home, or social situations. And they have to indicate to a clinician that these troubles began before age 12.
“You have to really sit down and paint the picture with the patient: There are no shortcuts here,” said Lenard Adler, MD, a psychiatrist who directs the Adult ADHD program at New York University. Dr. Adler helped develop the six-item screener and is currently on a committee developing formal screening guidelines for adult ADHD in the United States.
He said that often, “ADHD cotravels with other mental health disorders like depression, bipolar disorders, anxiety disorders, and substance use disorders at least half the time.”
To tease out if a patient really has ADHD, clinicians can start with a more general screening for anxiety and hone in on the possibility of the condition if the anxiety screening reveals challenges with distractibility and forgetfulness, according to Karen Smith, MD, a family physician in Raeford, N.C.
“We can’t expect patients to walk through the door with the diagnosis stamped on their foreheads,” said Dr. Smith, who is a member of the board of directors for the American Academy of Family Physicians.
But a patient’s apparent signs of ADHD could be due to another condition such as hyperthyroidism or diabetes, Dr. Smith said. The driving principle is to take enough time to figure out exactly what is going on, she said, and then to treat accordingly. “If you ask the right questions, patients will give you good answers.”
Medications are often prescribed to help manage ADHD symptoms. These can include stimulants, controlled substances that include methylphenidate or amphetamine, or nonstimulants, such as atomoxetine and bupropion. Dr. Montano starts his patients on a lower dose and titrates them up rapidly until they reach the optimum dose that allows them to sustain focus and feel more productive. Patients complete frequent self-reports during this process.
“Any diversion is inappropriate,” said Dr. Montano, who writes a contract with his patients saying that stimulant medications will not be refilled if they are used more quickly than prescribed.
For older adults, physicians should make sure that stimulants do not exacerbate any underlying cardiovascular conditions, Dr. Montano said, and consult a cardiologist when needed.
The benefits of proper ADHD treatment can be profound, Dr. Adler noted, no matter when it begins.
“Older adults have lived their lives feeling they’ve been disorganized and a burden to their significant others, and only when they’ve taken medication have they really been able to organize their days and feel effective in their social interactions and with their families,” Dr. Adler said.
Dr. Montano has reported relationships with AbbVie, Axsome, Corium, Idorsia, Intracellular, Otsuka, Supernus, Sage/Biogen, Biohaven, Boehringer Ingelheim, Cassava, Eli Lilly, Intracellular, Janssen, Jazz, Sage, Supernus, Otsuka, and Tonix. Dr. Adler has reported relationships with Shire/Takeda Pharmaceuticals, National Football League, Major League Baseball, Corium, Otsuka, Neurocentria, Bracket/Signant, and SUNY; and is receiving royalty payments for developing adult ADHD assessments. Dr. Smith has reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Substance use in pregnancy linked to adverse CVD outcomes
TOPLINE:
, including more than double the risk of maternal mortality, a new study shows.
METHODOLOGY:
- Using the National Inpatient Sample database to identify hospital deliveries between 2004 and 2018 and diagnostic codes to identify maternal substance use, researchers compared 955,531 pregnancies with accompanying substance use – the most common substances being cannabis and opioids, followed by stimulants – to over 60 million pregnancies in which there was no substance use.
- The primary outcome was any CV event, including acute myocardial infarction, stroke, arrhythmia, endocarditis, any acute cardiomyopathy or heart failure, or cardiac arrest; other outcomes included maternal mortality and major adverse cardiac events (MACE).
TAKEAWAY:
- Deliveries complicated by substance use increased from 1,126 per 100,000 deliveries in 2004 to 1,547 per 100,000 in 2018, peaking at 2,187 per 100,000 in 2014.
- After the researchers controlled for patient demographics and CVD risk factors, results showed that pregnant women who used any substance (cannabis, opioids, methamphetamine, alcohol, tobacco, or cocaine) were more likely to experience a CVD event (adjusted odds ratio [aOR], 1.61; 95% confidence interval [CI], 1.53-1.70; P < .001), MACE (aOR, 1.53; 95% CI, 1.46-1.61; P < .001), or maternal mortality (aOR, 2.65; 95% CI, 2.15-3.25; P < .001) during hospitalization for delivery.
- Those using amphetamine/methamphetamine had ninefold higher odds of cardiomyopathy or heart failure and more than sevenfold higher odds of cardiac arrest.
IN PRACTICE:
“For the wellbeing of pregnant women and their children, substance use needs to be considered an independent risk factor for CV events in pregnancy,” the authors wrote. They called for prenatal assessments by a multidisciplinary cardio-obstetrics team to try to decrease cardiac complications.
In an accompanying editorial by Abha Khandelwal, MD, department of medicine, Stanford (Calif.) University, and others, the authors said the findings “highlight the critical support required during pregnancy and postpartum” for substance users, which should include comprehensive medical care and social services as well as access to addiction medicine and treatment of co-occurring mental health disorders.
SOURCE:
The study was carried out by Kari Evans, MD, division of maternal fetal medicine, department of obstetrics and gynecology, University of Arizona, Phoenix. It was published online in the Journal of the American College of Cardiology: Advances.
LIMITATIONS:
Use of administrative databases may have resulted in underreporting of diagnoses. The researchers could not assess the association of dose, duration, method, or timing of use for any substance with CV events. They also could not examine the effect of vaping on maternal CV events or differentiate hospitalizations for delivery that were complicated by CV events from hospitalizations for CV events that prompted delivery. The data did not reflect the postpartum period, during which a high rate of adverse CV events occurs.
DISCLOSURES:
The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, including more than double the risk of maternal mortality, a new study shows.
METHODOLOGY:
- Using the National Inpatient Sample database to identify hospital deliveries between 2004 and 2018 and diagnostic codes to identify maternal substance use, researchers compared 955,531 pregnancies with accompanying substance use – the most common substances being cannabis and opioids, followed by stimulants – to over 60 million pregnancies in which there was no substance use.
- The primary outcome was any CV event, including acute myocardial infarction, stroke, arrhythmia, endocarditis, any acute cardiomyopathy or heart failure, or cardiac arrest; other outcomes included maternal mortality and major adverse cardiac events (MACE).
TAKEAWAY:
- Deliveries complicated by substance use increased from 1,126 per 100,000 deliveries in 2004 to 1,547 per 100,000 in 2018, peaking at 2,187 per 100,000 in 2014.
- After the researchers controlled for patient demographics and CVD risk factors, results showed that pregnant women who used any substance (cannabis, opioids, methamphetamine, alcohol, tobacco, or cocaine) were more likely to experience a CVD event (adjusted odds ratio [aOR], 1.61; 95% confidence interval [CI], 1.53-1.70; P < .001), MACE (aOR, 1.53; 95% CI, 1.46-1.61; P < .001), or maternal mortality (aOR, 2.65; 95% CI, 2.15-3.25; P < .001) during hospitalization for delivery.
- Those using amphetamine/methamphetamine had ninefold higher odds of cardiomyopathy or heart failure and more than sevenfold higher odds of cardiac arrest.
IN PRACTICE:
“For the wellbeing of pregnant women and their children, substance use needs to be considered an independent risk factor for CV events in pregnancy,” the authors wrote. They called for prenatal assessments by a multidisciplinary cardio-obstetrics team to try to decrease cardiac complications.
In an accompanying editorial by Abha Khandelwal, MD, department of medicine, Stanford (Calif.) University, and others, the authors said the findings “highlight the critical support required during pregnancy and postpartum” for substance users, which should include comprehensive medical care and social services as well as access to addiction medicine and treatment of co-occurring mental health disorders.
SOURCE:
The study was carried out by Kari Evans, MD, division of maternal fetal medicine, department of obstetrics and gynecology, University of Arizona, Phoenix. It was published online in the Journal of the American College of Cardiology: Advances.
LIMITATIONS:
Use of administrative databases may have resulted in underreporting of diagnoses. The researchers could not assess the association of dose, duration, method, or timing of use for any substance with CV events. They also could not examine the effect of vaping on maternal CV events or differentiate hospitalizations for delivery that were complicated by CV events from hospitalizations for CV events that prompted delivery. The data did not reflect the postpartum period, during which a high rate of adverse CV events occurs.
DISCLOSURES:
The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, including more than double the risk of maternal mortality, a new study shows.
METHODOLOGY:
- Using the National Inpatient Sample database to identify hospital deliveries between 2004 and 2018 and diagnostic codes to identify maternal substance use, researchers compared 955,531 pregnancies with accompanying substance use – the most common substances being cannabis and opioids, followed by stimulants – to over 60 million pregnancies in which there was no substance use.
- The primary outcome was any CV event, including acute myocardial infarction, stroke, arrhythmia, endocarditis, any acute cardiomyopathy or heart failure, or cardiac arrest; other outcomes included maternal mortality and major adverse cardiac events (MACE).
TAKEAWAY:
- Deliveries complicated by substance use increased from 1,126 per 100,000 deliveries in 2004 to 1,547 per 100,000 in 2018, peaking at 2,187 per 100,000 in 2014.
- After the researchers controlled for patient demographics and CVD risk factors, results showed that pregnant women who used any substance (cannabis, opioids, methamphetamine, alcohol, tobacco, or cocaine) were more likely to experience a CVD event (adjusted odds ratio [aOR], 1.61; 95% confidence interval [CI], 1.53-1.70; P < .001), MACE (aOR, 1.53; 95% CI, 1.46-1.61; P < .001), or maternal mortality (aOR, 2.65; 95% CI, 2.15-3.25; P < .001) during hospitalization for delivery.
- Those using amphetamine/methamphetamine had ninefold higher odds of cardiomyopathy or heart failure and more than sevenfold higher odds of cardiac arrest.
IN PRACTICE:
“For the wellbeing of pregnant women and their children, substance use needs to be considered an independent risk factor for CV events in pregnancy,” the authors wrote. They called for prenatal assessments by a multidisciplinary cardio-obstetrics team to try to decrease cardiac complications.
In an accompanying editorial by Abha Khandelwal, MD, department of medicine, Stanford (Calif.) University, and others, the authors said the findings “highlight the critical support required during pregnancy and postpartum” for substance users, which should include comprehensive medical care and social services as well as access to addiction medicine and treatment of co-occurring mental health disorders.
SOURCE:
The study was carried out by Kari Evans, MD, division of maternal fetal medicine, department of obstetrics and gynecology, University of Arizona, Phoenix. It was published online in the Journal of the American College of Cardiology: Advances.
LIMITATIONS:
Use of administrative databases may have resulted in underreporting of diagnoses. The researchers could not assess the association of dose, duration, method, or timing of use for any substance with CV events. They also could not examine the effect of vaping on maternal CV events or differentiate hospitalizations for delivery that were complicated by CV events from hospitalizations for CV events that prompted delivery. The data did not reflect the postpartum period, during which a high rate of adverse CV events occurs.
DISCLOSURES:
The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JACC: ADVANCES
Artificial sweeteners in processed foods tied to increased depression risk
new data from the Nurses Health Study II (NHS II) suggest.
Nurses who consumed more than eight servings daily had about a 50% higher risk of developing depression than nurses who consumed four or fewer servings daily.
However, in a secondary analysis, in which the researchers tried to tease out specific foods that may be associated with increased risk, only artificial sweeteners and artificially sweetened beverages were associated with an increased risk of depression.
“Animal studies have shown that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” study investigator Andrew T. Chan, MD, MPH, of the clinical and translational epidemiology unit at Massachusetts General Hospital, Boston, said in an interview.
“Given this potential association between ultraprocessed food and multiple adverse health conditions, wherever possible individuals may wish to limit their intake of such foods. This may be a lifestyle change that could have important benefits, particularly for those who struggle with mental health,” Dr. Chan said.
The study was published online in JAMA Network Open.
Multiple potential mechanisms
The findings are based on 31,712 mostly non-Hispanic White women who were free of depression at baseline. The mean age of the patients at baseline was 52 years. As part of the NHS II, the women provided information on diet every 4 years using validated food frequency questionnaires.
Compared with women with low UPF intake, those with high UPF intake had greater body mass index (BMI). In addition, they were apt to smoke and have diabetes, hypertension, and dyslipidemia, and they were less apt to exercise regularly.
During the study period, there were 2,122 incident cases of depression, as determined using a strict definition that required self-reported clinician-diagnosed depression and regular antidepressant use. There were 4,840 incident cases, as determined using a broad definition that required clinical diagnosis and/or antidepressant use.
Compared with women in the lowest quintile of UPF consumption (fewer than four daily servings), those in the highest quintile (more than 8.8 daily servings) had an increased risk of depression.
This was noted for both the strict depression definition (hazard ratio, 1.49; 95% confidence interval, 1.26-1.76; P < .001) and the broad one (HR, 1.34; 95% CI, 1.20-1.50; P < .001).
“Models were not materially altered after inclusion of potential confounders. We did not observe differential associations in subgroups defined by age, BMI, physical activity, or smoking,” the researchers reported.
In secondary analyses, they classified UPF into their components, including ultraprocessed grain foods, sweet snacks, ready-to-eat meals, fats, sauces, ultraprocessed dairy products, savory snacks, processed meat, beverages, and artificial sweeteners.
Comparing the highest with the lowest quintiles, only high intake of artificially sweetened beverages (HR, 1.37; 95% CI, 1.19-1.57; P < .001) and artificial sweeteners (HR, 1.26; 95% CI, 1.10-1.43; P < .001) was associated with greater risk of depression and after multivariable regression.
In an exploratory analysis, women who reduced their UPF intake by at least three servings per day were at lower risk of depression (strict definition: HR, 0.84; 95% CI, 0.71-0.99), compared with those with relatively stable intake in each 4-year period.
“Ultraprocessed foods have been associated with several different health outcomes which may reflect an effect on common pathways that underlie chronic conditions,” said Dr. Chan.
For example, UPF intake has been associated with chronic inflammation, which in turns leads to multiple potential adverse health effects, including depression, he explained.
There is also a link between UPF and disruption of the gut microbiome.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” Dr. Chan said.
Association, not causation
Several experts weighed in on the study results in a statement from the U.K. nonprofit organization, Science Media Centre.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England), cautioned that the study only offers information on association – not causation.
“It is very possible that people with depression change their diet and might decide to consume foods that are easier to prepare – which would often be foods considered to be ultraprocessed,” Dr. Kuhnle said.
What’s most interesting is that the association between UPF intake and depression was driven by a single factor – artificial sweeteners.
“This supports one of the main criticisms of the UPF concept, that it combines a wide range of different foods and thereby makes it difficult to identify underlying causes,” Dr. Kuhnle added.
“There are currently no data that link artificial sweetener use to mental health, despite most of them having been available for some time. It is also important to note that there are a wide range of different artificial sweeteners that are metabolized very differently and that there might be reverse causality,” Dr. Kuhnle commented.
Paul Keedwell, MBChB, PhD, consultant psychiatrist and fellow of the Royal College of Psychiatrists, said this is an “interesting and important finding, but one that raises more questions. At this stage, we cannot say how big an effect diet has on depression risk compared to other risk factors, like family history of depression, stress levels, and having a supportive social network.”
Dr. Keedwell noted that the investigators carefully excluded the possibility that the effect is mediated by obesity or lack of exercise.
“However, an important consideration is that a diet based on ready meals and artificially sweetened drinks might indicate a hectic lifestyle or one with shift work. In other words, a fast-food diet could be an indirect marker of chronic stress. Prolonged stress probably remains the main risk factor for depression,” Dr. Keedwell said.
Keith Frayn, PhD, professor emeritus of human metabolism, University of Oxford (England), noted that the relationship between artificial sweeteners and depression “stands out clearly” even after adjusting for multiple confounding factors, including BMI, smoking, and exercise.
“This adds to growing concerns about artificial sweeteners and cardiometabolic health. The link with depression needs confirmation and further research to suggest how it might be brought about,” Dr. Frayn cautioned.
The NHS II was funded by a grant from the National Cancer Institute. Dr. Chan reported receiving grants from Bayer and Zoe and personal fees from Boehringer Ingelheim, Pfizer, and Freenome outside this work. Dr. Keedwell and Dr. Kuhnle disclosed no relevant financial relationships. Dr. Frayn is an author of books on nutrition and metabolism.
A version of this article first appeared on Medscape.com.
new data from the Nurses Health Study II (NHS II) suggest.
Nurses who consumed more than eight servings daily had about a 50% higher risk of developing depression than nurses who consumed four or fewer servings daily.
However, in a secondary analysis, in which the researchers tried to tease out specific foods that may be associated with increased risk, only artificial sweeteners and artificially sweetened beverages were associated with an increased risk of depression.
“Animal studies have shown that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” study investigator Andrew T. Chan, MD, MPH, of the clinical and translational epidemiology unit at Massachusetts General Hospital, Boston, said in an interview.
“Given this potential association between ultraprocessed food and multiple adverse health conditions, wherever possible individuals may wish to limit their intake of such foods. This may be a lifestyle change that could have important benefits, particularly for those who struggle with mental health,” Dr. Chan said.
The study was published online in JAMA Network Open.
Multiple potential mechanisms
The findings are based on 31,712 mostly non-Hispanic White women who were free of depression at baseline. The mean age of the patients at baseline was 52 years. As part of the NHS II, the women provided information on diet every 4 years using validated food frequency questionnaires.
Compared with women with low UPF intake, those with high UPF intake had greater body mass index (BMI). In addition, they were apt to smoke and have diabetes, hypertension, and dyslipidemia, and they were less apt to exercise regularly.
During the study period, there were 2,122 incident cases of depression, as determined using a strict definition that required self-reported clinician-diagnosed depression and regular antidepressant use. There were 4,840 incident cases, as determined using a broad definition that required clinical diagnosis and/or antidepressant use.
Compared with women in the lowest quintile of UPF consumption (fewer than four daily servings), those in the highest quintile (more than 8.8 daily servings) had an increased risk of depression.
This was noted for both the strict depression definition (hazard ratio, 1.49; 95% confidence interval, 1.26-1.76; P < .001) and the broad one (HR, 1.34; 95% CI, 1.20-1.50; P < .001).
“Models were not materially altered after inclusion of potential confounders. We did not observe differential associations in subgroups defined by age, BMI, physical activity, or smoking,” the researchers reported.
In secondary analyses, they classified UPF into their components, including ultraprocessed grain foods, sweet snacks, ready-to-eat meals, fats, sauces, ultraprocessed dairy products, savory snacks, processed meat, beverages, and artificial sweeteners.
Comparing the highest with the lowest quintiles, only high intake of artificially sweetened beverages (HR, 1.37; 95% CI, 1.19-1.57; P < .001) and artificial sweeteners (HR, 1.26; 95% CI, 1.10-1.43; P < .001) was associated with greater risk of depression and after multivariable regression.
In an exploratory analysis, women who reduced their UPF intake by at least three servings per day were at lower risk of depression (strict definition: HR, 0.84; 95% CI, 0.71-0.99), compared with those with relatively stable intake in each 4-year period.
“Ultraprocessed foods have been associated with several different health outcomes which may reflect an effect on common pathways that underlie chronic conditions,” said Dr. Chan.
For example, UPF intake has been associated with chronic inflammation, which in turns leads to multiple potential adverse health effects, including depression, he explained.
There is also a link between UPF and disruption of the gut microbiome.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” Dr. Chan said.
Association, not causation
Several experts weighed in on the study results in a statement from the U.K. nonprofit organization, Science Media Centre.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England), cautioned that the study only offers information on association – not causation.
“It is very possible that people with depression change their diet and might decide to consume foods that are easier to prepare – which would often be foods considered to be ultraprocessed,” Dr. Kuhnle said.
What’s most interesting is that the association between UPF intake and depression was driven by a single factor – artificial sweeteners.
“This supports one of the main criticisms of the UPF concept, that it combines a wide range of different foods and thereby makes it difficult to identify underlying causes,” Dr. Kuhnle added.
“There are currently no data that link artificial sweetener use to mental health, despite most of them having been available for some time. It is also important to note that there are a wide range of different artificial sweeteners that are metabolized very differently and that there might be reverse causality,” Dr. Kuhnle commented.
Paul Keedwell, MBChB, PhD, consultant psychiatrist and fellow of the Royal College of Psychiatrists, said this is an “interesting and important finding, but one that raises more questions. At this stage, we cannot say how big an effect diet has on depression risk compared to other risk factors, like family history of depression, stress levels, and having a supportive social network.”
Dr. Keedwell noted that the investigators carefully excluded the possibility that the effect is mediated by obesity or lack of exercise.
“However, an important consideration is that a diet based on ready meals and artificially sweetened drinks might indicate a hectic lifestyle or one with shift work. In other words, a fast-food diet could be an indirect marker of chronic stress. Prolonged stress probably remains the main risk factor for depression,” Dr. Keedwell said.
Keith Frayn, PhD, professor emeritus of human metabolism, University of Oxford (England), noted that the relationship between artificial sweeteners and depression “stands out clearly” even after adjusting for multiple confounding factors, including BMI, smoking, and exercise.
“This adds to growing concerns about artificial sweeteners and cardiometabolic health. The link with depression needs confirmation and further research to suggest how it might be brought about,” Dr. Frayn cautioned.
The NHS II was funded by a grant from the National Cancer Institute. Dr. Chan reported receiving grants from Bayer and Zoe and personal fees from Boehringer Ingelheim, Pfizer, and Freenome outside this work. Dr. Keedwell and Dr. Kuhnle disclosed no relevant financial relationships. Dr. Frayn is an author of books on nutrition and metabolism.
A version of this article first appeared on Medscape.com.
new data from the Nurses Health Study II (NHS II) suggest.
Nurses who consumed more than eight servings daily had about a 50% higher risk of developing depression than nurses who consumed four or fewer servings daily.
However, in a secondary analysis, in which the researchers tried to tease out specific foods that may be associated with increased risk, only artificial sweeteners and artificially sweetened beverages were associated with an increased risk of depression.
“Animal studies have shown that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” study investigator Andrew T. Chan, MD, MPH, of the clinical and translational epidemiology unit at Massachusetts General Hospital, Boston, said in an interview.
“Given this potential association between ultraprocessed food and multiple adverse health conditions, wherever possible individuals may wish to limit their intake of such foods. This may be a lifestyle change that could have important benefits, particularly for those who struggle with mental health,” Dr. Chan said.
The study was published online in JAMA Network Open.
Multiple potential mechanisms
The findings are based on 31,712 mostly non-Hispanic White women who were free of depression at baseline. The mean age of the patients at baseline was 52 years. As part of the NHS II, the women provided information on diet every 4 years using validated food frequency questionnaires.
Compared with women with low UPF intake, those with high UPF intake had greater body mass index (BMI). In addition, they were apt to smoke and have diabetes, hypertension, and dyslipidemia, and they were less apt to exercise regularly.
During the study period, there were 2,122 incident cases of depression, as determined using a strict definition that required self-reported clinician-diagnosed depression and regular antidepressant use. There were 4,840 incident cases, as determined using a broad definition that required clinical diagnosis and/or antidepressant use.
Compared with women in the lowest quintile of UPF consumption (fewer than four daily servings), those in the highest quintile (more than 8.8 daily servings) had an increased risk of depression.
This was noted for both the strict depression definition (hazard ratio, 1.49; 95% confidence interval, 1.26-1.76; P < .001) and the broad one (HR, 1.34; 95% CI, 1.20-1.50; P < .001).
“Models were not materially altered after inclusion of potential confounders. We did not observe differential associations in subgroups defined by age, BMI, physical activity, or smoking,” the researchers reported.
In secondary analyses, they classified UPF into their components, including ultraprocessed grain foods, sweet snacks, ready-to-eat meals, fats, sauces, ultraprocessed dairy products, savory snacks, processed meat, beverages, and artificial sweeteners.
Comparing the highest with the lowest quintiles, only high intake of artificially sweetened beverages (HR, 1.37; 95% CI, 1.19-1.57; P < .001) and artificial sweeteners (HR, 1.26; 95% CI, 1.10-1.43; P < .001) was associated with greater risk of depression and after multivariable regression.
In an exploratory analysis, women who reduced their UPF intake by at least three servings per day were at lower risk of depression (strict definition: HR, 0.84; 95% CI, 0.71-0.99), compared with those with relatively stable intake in each 4-year period.
“Ultraprocessed foods have been associated with several different health outcomes which may reflect an effect on common pathways that underlie chronic conditions,” said Dr. Chan.
For example, UPF intake has been associated with chronic inflammation, which in turns leads to multiple potential adverse health effects, including depression, he explained.
There is also a link between UPF and disruption of the gut microbiome.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” Dr. Chan said.
Association, not causation
Several experts weighed in on the study results in a statement from the U.K. nonprofit organization, Science Media Centre.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England), cautioned that the study only offers information on association – not causation.
“It is very possible that people with depression change their diet and might decide to consume foods that are easier to prepare – which would often be foods considered to be ultraprocessed,” Dr. Kuhnle said.
What’s most interesting is that the association between UPF intake and depression was driven by a single factor – artificial sweeteners.
“This supports one of the main criticisms of the UPF concept, that it combines a wide range of different foods and thereby makes it difficult to identify underlying causes,” Dr. Kuhnle added.
“There are currently no data that link artificial sweetener use to mental health, despite most of them having been available for some time. It is also important to note that there are a wide range of different artificial sweeteners that are metabolized very differently and that there might be reverse causality,” Dr. Kuhnle commented.
Paul Keedwell, MBChB, PhD, consultant psychiatrist and fellow of the Royal College of Psychiatrists, said this is an “interesting and important finding, but one that raises more questions. At this stage, we cannot say how big an effect diet has on depression risk compared to other risk factors, like family history of depression, stress levels, and having a supportive social network.”
Dr. Keedwell noted that the investigators carefully excluded the possibility that the effect is mediated by obesity or lack of exercise.
“However, an important consideration is that a diet based on ready meals and artificially sweetened drinks might indicate a hectic lifestyle or one with shift work. In other words, a fast-food diet could be an indirect marker of chronic stress. Prolonged stress probably remains the main risk factor for depression,” Dr. Keedwell said.
Keith Frayn, PhD, professor emeritus of human metabolism, University of Oxford (England), noted that the relationship between artificial sweeteners and depression “stands out clearly” even after adjusting for multiple confounding factors, including BMI, smoking, and exercise.
“This adds to growing concerns about artificial sweeteners and cardiometabolic health. The link with depression needs confirmation and further research to suggest how it might be brought about,” Dr. Frayn cautioned.
The NHS II was funded by a grant from the National Cancer Institute. Dr. Chan reported receiving grants from Bayer and Zoe and personal fees from Boehringer Ingelheim, Pfizer, and Freenome outside this work. Dr. Keedwell and Dr. Kuhnle disclosed no relevant financial relationships. Dr. Frayn is an author of books on nutrition and metabolism.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN