User login
For MD-IQ use only
Clinical Research in Early Career Academic Medicine
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
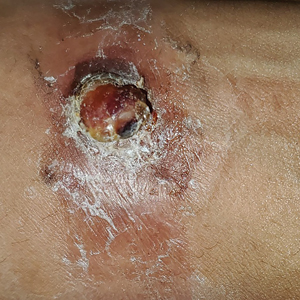
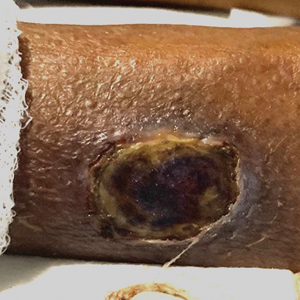
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
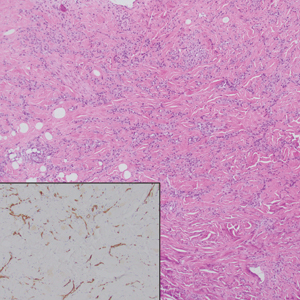
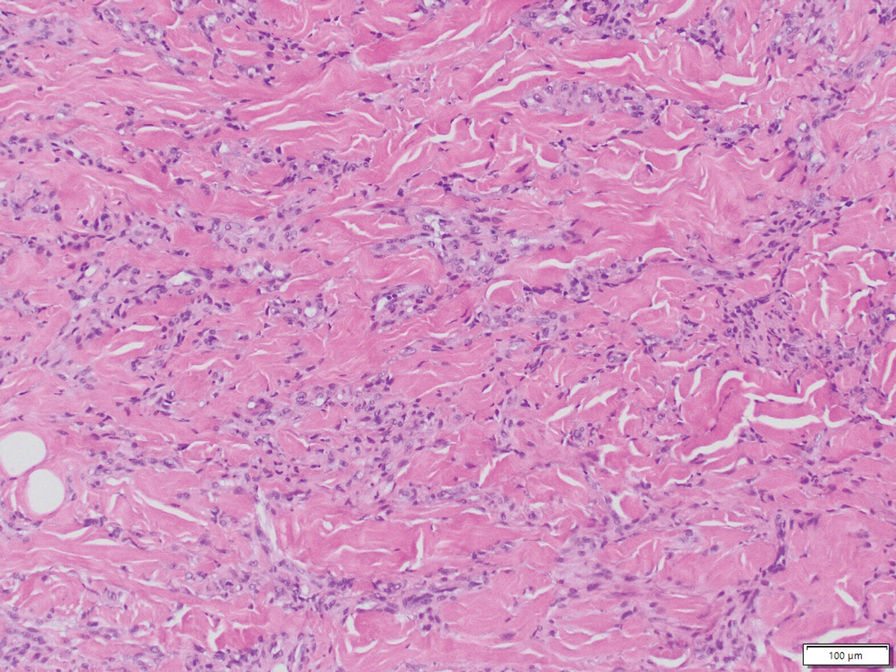
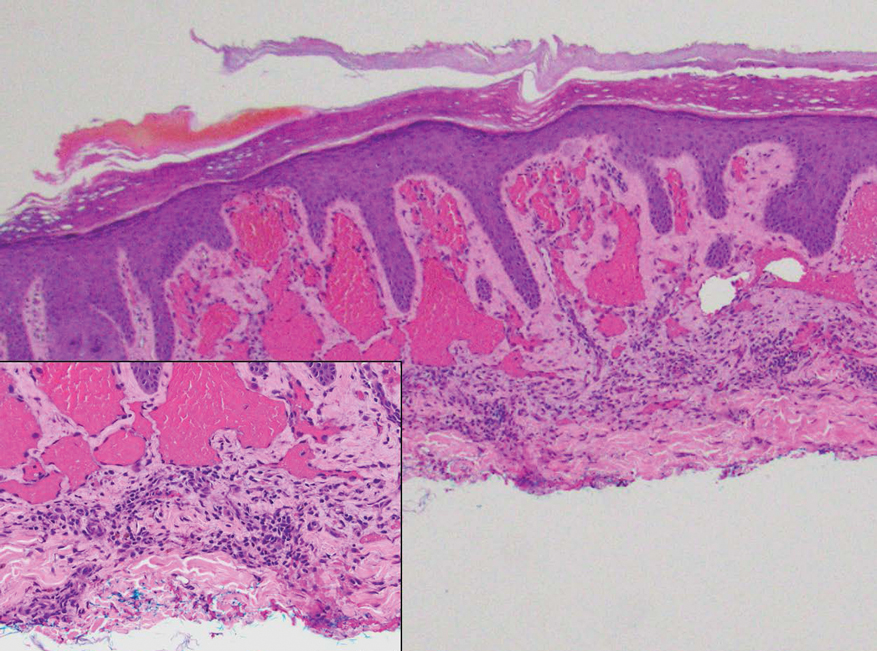
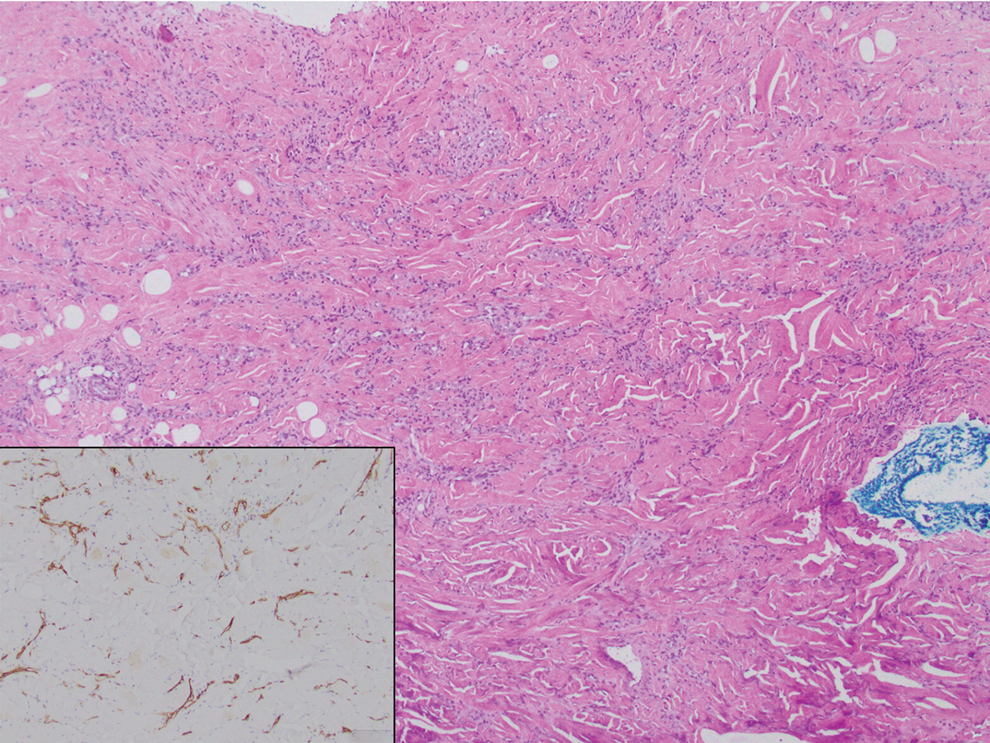
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.
The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
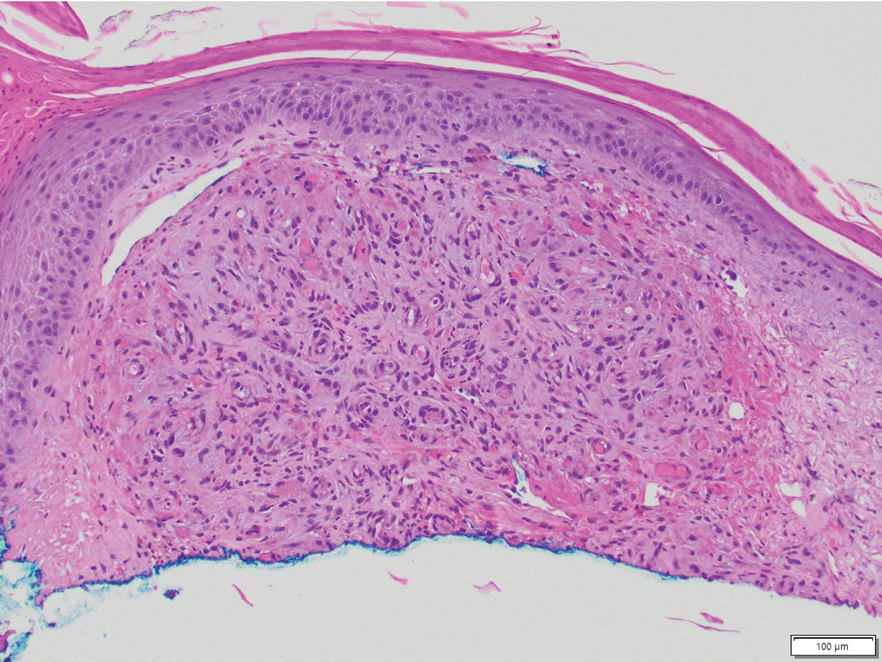
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6
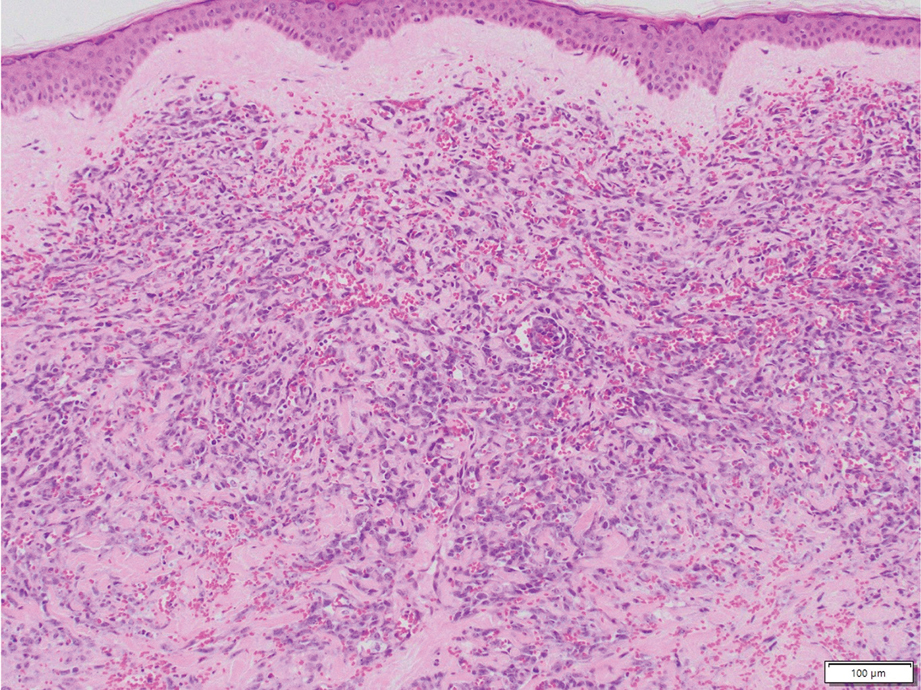
Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9
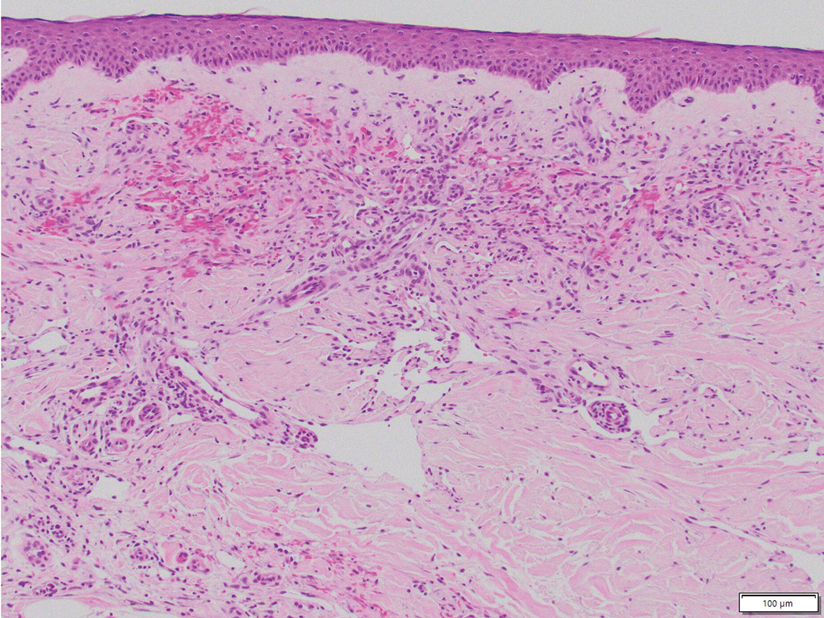
Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
A 46-year-old woman with a history of systemic lupus erythematosus and end-stage renal disease presented to the dermatology department with painful ulcers on the extensor surfaces of the elbows, knees, and ankles of 2 months’ duration. Physical examination revealed angulated ulcers with surrounding pink erythema. A 4-mm punch biopsy and CD31 immunostaining of the left knee revealed dystrophic elastic fibers and purplish calciumlike depositions on connective tissue fibers in the mid to deep dermis.
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
To the Editor:
Idiopathic gingival papillokeratosis with crypt formation (IGPC) is an uncommon benign condition that first was reported in 1967.1 The condition manifests as white plaques with a papillary appearance on the gingival tissue. While data on the prevalence of IGPC are limited, it is known to occur more frequently in younger patients (ie, 9-24 years1-3) and has been linked to use of orthodontic appliances.3,4 The lesions typically are asymptomatic with a bilateral appearance along the mucogingival junction. Research on IGPC has not identified the underlying mechanisms that trigger the hyperkeratinization and papillary alterations within the gingival tissue.
Management of IGPC can be challenging due to the rarity of the condition and its uncertain pathogenesis. Wiping or brushing the affected area offers only temporary improvement of symptoms and the appearance of the lesions. Surgical excision is another option; however, it can result in aesthetic and/or functional periodontal defects.2 Alternately, employing methods such as wiping or brushing the affected area offers only transient and temporary results in managing the condition. Additional investigative approaches and clinical studies are needed to identify more effective therapeutic modalities for the management of IGPC, particularly in pediatric patients, in whom aesthetic results may take on a heightened importance.1-3 We report a case of IGPC in which cryotherapy yielded satisfactory results with no recurrence of the lesions.
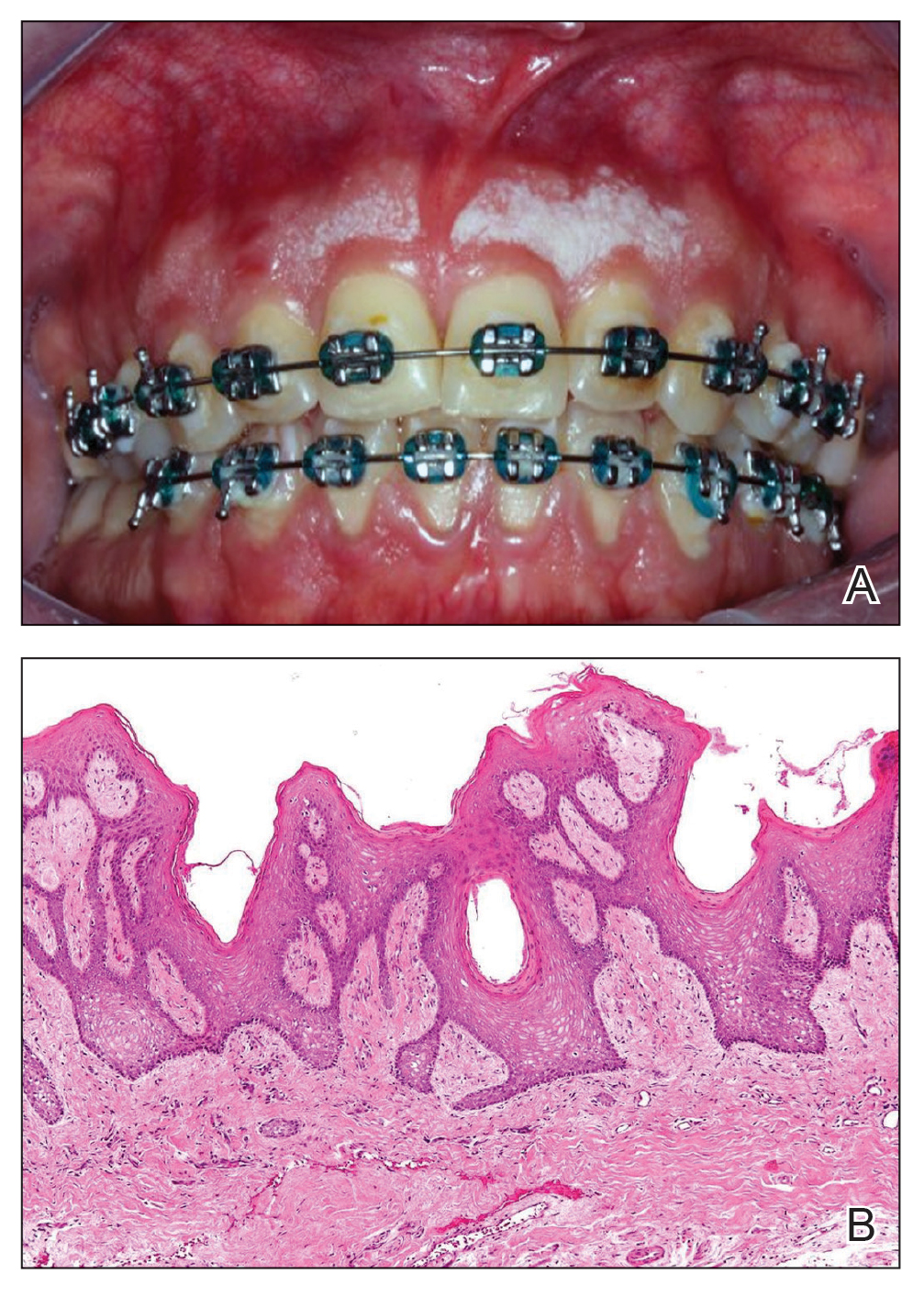
A 32-year-old woman presented to the dental clinic with white spots on the gingiva of 5 months’ duration. The patient reported a history of smoking cigarettes (3 packs per year) and drinking alcohol in social situations; her medical history was otherwise unremarkable. Clinical examination of the oral cavity revealed a bilateral, irregular, verrucouslike plaque throughout the vestibular upper attached gingiva. An incisional biopsy from the attached gingiva between teeth 13 and 23 was performed. Histopathologic analysis revealed parakeratosis and papillary acanthosis of the gingival mucosa associated with multifocal epithelial invaginations resembling crypts as well as long tapered epithelial ridges with no inflammation in the lamina propria. Based on the histopathologic findings, a diagnosis of IGPC was made (Figure 1).
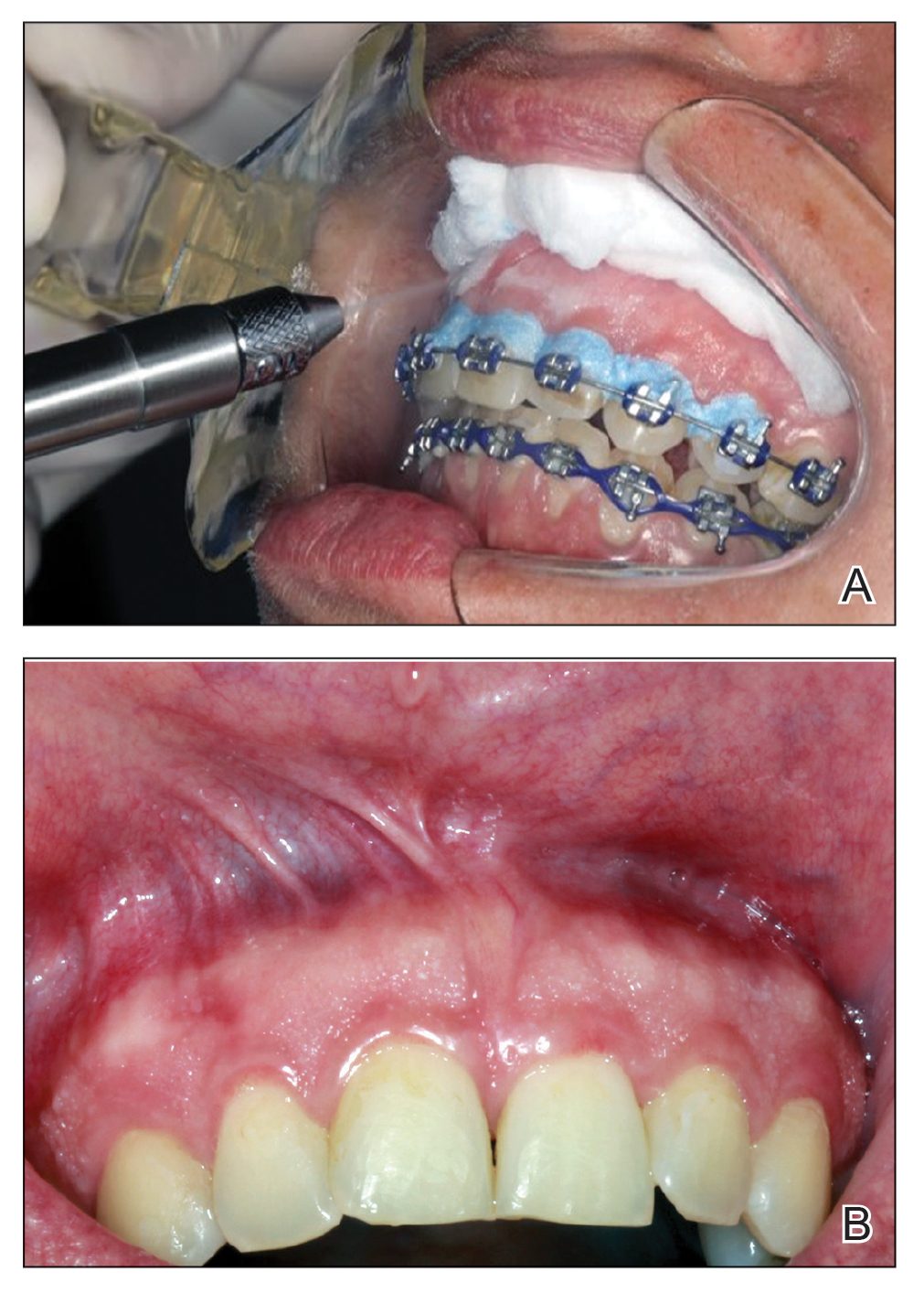
Given the patient’s clinical presentation, we suggested treatment with cryotherapy as a minimally invasive option that would preserve the gingival architecture and aesthetics while avoiding the potential complications of surgical excision. The patient consented to the procedure, and liquid nitrogen was administered through a handheld device using a 0.6-mm aperture spray tip. During application, the spray tip was positioned at a distance of 0.5 to 1.0 cm from the labial marginal gingiva at about a 45° angle. The freeze/thaw cycle involved a continuous one-way spray application of liquid nitrogen onto the lesion until solid ice formed over the entire area, followed by a waiting period until gradual thawing occurred.
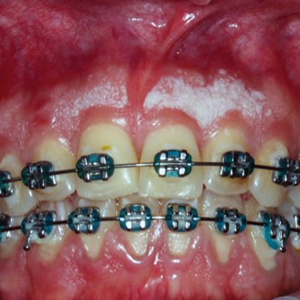
A total of 5 cryotherapy sessions were conducted over an 8-week period; no recurrence of the lesions was observed during a 2-year follow-up period (Figure 2).
We present our case to add to the body of knowledge regarding management options for IGPC, specifically cryotherapy. Historically, brushing with a toothbrush and surgical excision have been the most commonly used interventions.2 Gently brushing the affected areas can help stimulate local blood circulation, which can improve the health of the gingival tissue, promote oxygenation and delivery of nutrients to the cells, and aid in the removal of metabolic waste. Surgical excision is the most commonly used treatment method for IGPC to ensure that the lesions are safely and completely removed; however, this option can result in aesthetic and/or functional periodontal defects. There also is a risk for recurrence, although Noonan et al2 reported no recurrence 4 years after performing a surgical excision for IGPC.
Cryotherapy reduces tissue sensitivity, provides local anesthesia, and reduces inflammation in the oral mucosa. Moreover, cryotherapy accelerates healing by stimulating vasoconstriction and reactive vasodilation, thus enhancing blood flow, oxygenation, and nutrient delivery for faster cell regeneration of the oral mucosa.4,5 Cryotherapy generally is regarded as a simple noninvasive procedure that is relatively safe when performed by qualified professionals.4,5 It can provide benefits such as minimal patient discomfort, rapid recovery, and potential reduction of complications associated with more invasive procedures.5
The efficacy of cryotherapy for IGPC may vary based on lesion severity, individual patient response, and the need for repeated treatment sessions. Robust scientific evidence concerning the long-term efficacy of cryotherapy as a treatment for IGPC is limited due to the rarity of this condition.
The etiopathogenesis of IGPC has been hypothesized to involve both genetic and environmental factors with equal significance. This suggestion is based on reports of IGPC occurring in multiple members of the same family and animal model studies indicating that gingival tissue is sensitive to environmental influences, such as nutritional factors.1,6 However, it is important to emphasize that these hypotheses remain speculative, and the true etiopathogenesis of IGPC remains uncertain.6 Microscopically, biopsy fragments from suspected cases of IGPC reveal gingival mucosa characterized by parakeratosis and papillary acanthosis accompanied by multifocal epithelial invaginations resembling crypts.2 Additionally, elongated and tapered epithelial ridges without inflammation in the lamina propria may be observed (as in our case), favoring the diagnosis of IGPC.3 The absence of inflammation is noteworthy because it suggests that the observed alterations are not attributed to typical inflammatory processes seen in some gingival conditions.
The limited number of studies reporting successful treatment outcomes with long-term follow-up for IGPC cases underscores the need for further exploration of effective treatment options. Cryotherapy emerges as a promising minimally invasive therapeutic approach, with our case offering support for its potential application. Additional research and clinical trials are essential to validate its efficacy and improve our understanding of cryotherapy as a treatment modality for IGPC lesions.
- Bennett JS, Grupe HE. Epithelial adnexal formations in human gingiva. Oral Surg Oral Med Oral Pathol. 1967;23:789-795. doi:10.1016/0030-4220(67)90371-4
- Noonan VL, Woo SB, Sundararajan D, et al. Idiopathic gingival papillokeratosis with crypt formation, a report of 7 cases of a previously undescribed entity: possible unusual oral epithelial nevus? Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:358-364. doi:10.1016/j.oooo.2016.10.018
- Romo SA, de Arruda JAA, Nava FJT, et al. Idiopathic gingival papillokeratosis with crypt formation: a clinicopathological entity in the young population? Int J Dermatol. 2023;62:E291-E293. doi: 10.1111/ijd.16579
- Farah CS, Savage NW. Cryotherapy for treatment of oral lesions. Aust Dent J. 2006;51:2-5. doi:10.1111/j.1834-7819.2006.tb00392.x
- Nogueira VKC, Fernandes D, Navarro CM, et al. Cryotherapy for localized juvenile spongiotic gingival hyperplasia: preliminary findings on two cases. Int J Paediatr Dent. 2017;27:231-235. doi:10.1111/ipd.12278
- Bernick S, Bavetta LA. The development of gingival sebaceous-like glands and cysts in rats of the Holtzman strain. Oral Surg Oral Med Oral Pathol Oral Radiol. 1962;15:351-354. doi:10.1016/0030-4220(62)90116-0
To the Editor:
Idiopathic gingival papillokeratosis with crypt formation (IGPC) is an uncommon benign condition that first was reported in 1967.1 The condition manifests as white plaques with a papillary appearance on the gingival tissue. While data on the prevalence of IGPC are limited, it is known to occur more frequently in younger patients (ie, 9-24 years1-3) and has been linked to use of orthodontic appliances.3,4 The lesions typically are asymptomatic with a bilateral appearance along the mucogingival junction. Research on IGPC has not identified the underlying mechanisms that trigger the hyperkeratinization and papillary alterations within the gingival tissue.
Management of IGPC can be challenging due to the rarity of the condition and its uncertain pathogenesis. Wiping or brushing the affected area offers only temporary improvement of symptoms and the appearance of the lesions. Surgical excision is another option; however, it can result in aesthetic and/or functional periodontal defects.2 Alternately, employing methods such as wiping or brushing the affected area offers only transient and temporary results in managing the condition. Additional investigative approaches and clinical studies are needed to identify more effective therapeutic modalities for the management of IGPC, particularly in pediatric patients, in whom aesthetic results may take on a heightened importance.1-3 We report a case of IGPC in which cryotherapy yielded satisfactory results with no recurrence of the lesions.
A 32-year-old woman presented to the dental clinic with white spots on the gingiva of 5 months’ duration. The patient reported a history of smoking cigarettes (3 packs per year) and drinking alcohol in social situations; her medical history was otherwise unremarkable. Clinical examination of the oral cavity revealed a bilateral, irregular, verrucouslike plaque throughout the vestibular upper attached gingiva. An incisional biopsy from the attached gingiva between teeth 13 and 23 was performed. Histopathologic analysis revealed parakeratosis and papillary acanthosis of the gingival mucosa associated with multifocal epithelial invaginations resembling crypts as well as long tapered epithelial ridges with no inflammation in the lamina propria. Based on the histopathologic findings, a diagnosis of IGPC was made (Figure 1).

Given the patient’s clinical presentation, we suggested treatment with cryotherapy as a minimally invasive option that would preserve the gingival architecture and aesthetics while avoiding the potential complications of surgical excision. The patient consented to the procedure, and liquid nitrogen was administered through a handheld device using a 0.6-mm aperture spray tip. During application, the spray tip was positioned at a distance of 0.5 to 1.0 cm from the labial marginal gingiva at about a 45° angle. The freeze/thaw cycle involved a continuous one-way spray application of liquid nitrogen onto the lesion until solid ice formed over the entire area, followed by a waiting period until gradual thawing occurred.
A total of 5 cryotherapy sessions were conducted over an 8-week period; no recurrence of the lesions was observed during a 2-year follow-up period (Figure 2).

We present our case to add to the body of knowledge regarding management options for IGPC, specifically cryotherapy. Historically, brushing with a toothbrush and surgical excision have been the most commonly used interventions.2 Gently brushing the affected areas can help stimulate local blood circulation, which can improve the health of the gingival tissue, promote oxygenation and delivery of nutrients to the cells, and aid in the removal of metabolic waste. Surgical excision is the most commonly used treatment method for IGPC to ensure that the lesions are safely and completely removed; however, this option can result in aesthetic and/or functional periodontal defects. There also is a risk for recurrence, although Noonan et al2 reported no recurrence 4 years after performing a surgical excision for IGPC.
Cryotherapy reduces tissue sensitivity, provides local anesthesia, and reduces inflammation in the oral mucosa. Moreover, cryotherapy accelerates healing by stimulating vasoconstriction and reactive vasodilation, thus enhancing blood flow, oxygenation, and nutrient delivery for faster cell regeneration of the oral mucosa.4,5 Cryotherapy generally is regarded as a simple noninvasive procedure that is relatively safe when performed by qualified professionals.4,5 It can provide benefits such as minimal patient discomfort, rapid recovery, and potential reduction of complications associated with more invasive procedures.5
The efficacy of cryotherapy for IGPC may vary based on lesion severity, individual patient response, and the need for repeated treatment sessions. Robust scientific evidence concerning the long-term efficacy of cryotherapy as a treatment for IGPC is limited due to the rarity of this condition.
The etiopathogenesis of IGPC has been hypothesized to involve both genetic and environmental factors with equal significance. This suggestion is based on reports of IGPC occurring in multiple members of the same family and animal model studies indicating that gingival tissue is sensitive to environmental influences, such as nutritional factors.1,6 However, it is important to emphasize that these hypotheses remain speculative, and the true etiopathogenesis of IGPC remains uncertain.6 Microscopically, biopsy fragments from suspected cases of IGPC reveal gingival mucosa characterized by parakeratosis and papillary acanthosis accompanied by multifocal epithelial invaginations resembling crypts.2 Additionally, elongated and tapered epithelial ridges without inflammation in the lamina propria may be observed (as in our case), favoring the diagnosis of IGPC.3 The absence of inflammation is noteworthy because it suggests that the observed alterations are not attributed to typical inflammatory processes seen in some gingival conditions.
The limited number of studies reporting successful treatment outcomes with long-term follow-up for IGPC cases underscores the need for further exploration of effective treatment options. Cryotherapy emerges as a promising minimally invasive therapeutic approach, with our case offering support for its potential application. Additional research and clinical trials are essential to validate its efficacy and improve our understanding of cryotherapy as a treatment modality for IGPC lesions.
To the Editor:
Idiopathic gingival papillokeratosis with crypt formation (IGPC) is an uncommon benign condition that first was reported in 1967.1 The condition manifests as white plaques with a papillary appearance on the gingival tissue. While data on the prevalence of IGPC are limited, it is known to occur more frequently in younger patients (ie, 9-24 years1-3) and has been linked to use of orthodontic appliances.3,4 The lesions typically are asymptomatic with a bilateral appearance along the mucogingival junction. Research on IGPC has not identified the underlying mechanisms that trigger the hyperkeratinization and papillary alterations within the gingival tissue.
Management of IGPC can be challenging due to the rarity of the condition and its uncertain pathogenesis. Wiping or brushing the affected area offers only temporary improvement of symptoms and the appearance of the lesions. Surgical excision is another option; however, it can result in aesthetic and/or functional periodontal defects.2 Alternately, employing methods such as wiping or brushing the affected area offers only transient and temporary results in managing the condition. Additional investigative approaches and clinical studies are needed to identify more effective therapeutic modalities for the management of IGPC, particularly in pediatric patients, in whom aesthetic results may take on a heightened importance.1-3 We report a case of IGPC in which cryotherapy yielded satisfactory results with no recurrence of the lesions.
A 32-year-old woman presented to the dental clinic with white spots on the gingiva of 5 months’ duration. The patient reported a history of smoking cigarettes (3 packs per year) and drinking alcohol in social situations; her medical history was otherwise unremarkable. Clinical examination of the oral cavity revealed a bilateral, irregular, verrucouslike plaque throughout the vestibular upper attached gingiva. An incisional biopsy from the attached gingiva between teeth 13 and 23 was performed. Histopathologic analysis revealed parakeratosis and papillary acanthosis of the gingival mucosa associated with multifocal epithelial invaginations resembling crypts as well as long tapered epithelial ridges with no inflammation in the lamina propria. Based on the histopathologic findings, a diagnosis of IGPC was made (Figure 1).

Given the patient’s clinical presentation, we suggested treatment with cryotherapy as a minimally invasive option that would preserve the gingival architecture and aesthetics while avoiding the potential complications of surgical excision. The patient consented to the procedure, and liquid nitrogen was administered through a handheld device using a 0.6-mm aperture spray tip. During application, the spray tip was positioned at a distance of 0.5 to 1.0 cm from the labial marginal gingiva at about a 45° angle. The freeze/thaw cycle involved a continuous one-way spray application of liquid nitrogen onto the lesion until solid ice formed over the entire area, followed by a waiting period until gradual thawing occurred.
A total of 5 cryotherapy sessions were conducted over an 8-week period; no recurrence of the lesions was observed during a 2-year follow-up period (Figure 2).

We present our case to add to the body of knowledge regarding management options for IGPC, specifically cryotherapy. Historically, brushing with a toothbrush and surgical excision have been the most commonly used interventions.2 Gently brushing the affected areas can help stimulate local blood circulation, which can improve the health of the gingival tissue, promote oxygenation and delivery of nutrients to the cells, and aid in the removal of metabolic waste. Surgical excision is the most commonly used treatment method for IGPC to ensure that the lesions are safely and completely removed; however, this option can result in aesthetic and/or functional periodontal defects. There also is a risk for recurrence, although Noonan et al2 reported no recurrence 4 years after performing a surgical excision for IGPC.
Cryotherapy reduces tissue sensitivity, provides local anesthesia, and reduces inflammation in the oral mucosa. Moreover, cryotherapy accelerates healing by stimulating vasoconstriction and reactive vasodilation, thus enhancing blood flow, oxygenation, and nutrient delivery for faster cell regeneration of the oral mucosa.4,5 Cryotherapy generally is regarded as a simple noninvasive procedure that is relatively safe when performed by qualified professionals.4,5 It can provide benefits such as minimal patient discomfort, rapid recovery, and potential reduction of complications associated with more invasive procedures.5
The efficacy of cryotherapy for IGPC may vary based on lesion severity, individual patient response, and the need for repeated treatment sessions. Robust scientific evidence concerning the long-term efficacy of cryotherapy as a treatment for IGPC is limited due to the rarity of this condition.
The etiopathogenesis of IGPC has been hypothesized to involve both genetic and environmental factors with equal significance. This suggestion is based on reports of IGPC occurring in multiple members of the same family and animal model studies indicating that gingival tissue is sensitive to environmental influences, such as nutritional factors.1,6 However, it is important to emphasize that these hypotheses remain speculative, and the true etiopathogenesis of IGPC remains uncertain.6 Microscopically, biopsy fragments from suspected cases of IGPC reveal gingival mucosa characterized by parakeratosis and papillary acanthosis accompanied by multifocal epithelial invaginations resembling crypts.2 Additionally, elongated and tapered epithelial ridges without inflammation in the lamina propria may be observed (as in our case), favoring the diagnosis of IGPC.3 The absence of inflammation is noteworthy because it suggests that the observed alterations are not attributed to typical inflammatory processes seen in some gingival conditions.
The limited number of studies reporting successful treatment outcomes with long-term follow-up for IGPC cases underscores the need for further exploration of effective treatment options. Cryotherapy emerges as a promising minimally invasive therapeutic approach, with our case offering support for its potential application. Additional research and clinical trials are essential to validate its efficacy and improve our understanding of cryotherapy as a treatment modality for IGPC lesions.
- Bennett JS, Grupe HE. Epithelial adnexal formations in human gingiva. Oral Surg Oral Med Oral Pathol. 1967;23:789-795. doi:10.1016/0030-4220(67)90371-4
- Noonan VL, Woo SB, Sundararajan D, et al. Idiopathic gingival papillokeratosis with crypt formation, a report of 7 cases of a previously undescribed entity: possible unusual oral epithelial nevus? Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:358-364. doi:10.1016/j.oooo.2016.10.018
- Romo SA, de Arruda JAA, Nava FJT, et al. Idiopathic gingival papillokeratosis with crypt formation: a clinicopathological entity in the young population? Int J Dermatol. 2023;62:E291-E293. doi: 10.1111/ijd.16579
- Farah CS, Savage NW. Cryotherapy for treatment of oral lesions. Aust Dent J. 2006;51:2-5. doi:10.1111/j.1834-7819.2006.tb00392.x
- Nogueira VKC, Fernandes D, Navarro CM, et al. Cryotherapy for localized juvenile spongiotic gingival hyperplasia: preliminary findings on two cases. Int J Paediatr Dent. 2017;27:231-235. doi:10.1111/ipd.12278
- Bernick S, Bavetta LA. The development of gingival sebaceous-like glands and cysts in rats of the Holtzman strain. Oral Surg Oral Med Oral Pathol Oral Radiol. 1962;15:351-354. doi:10.1016/0030-4220(62)90116-0
- Bennett JS, Grupe HE. Epithelial adnexal formations in human gingiva. Oral Surg Oral Med Oral Pathol. 1967;23:789-795. doi:10.1016/0030-4220(67)90371-4
- Noonan VL, Woo SB, Sundararajan D, et al. Idiopathic gingival papillokeratosis with crypt formation, a report of 7 cases of a previously undescribed entity: possible unusual oral epithelial nevus? Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:358-364. doi:10.1016/j.oooo.2016.10.018
- Romo SA, de Arruda JAA, Nava FJT, et al. Idiopathic gingival papillokeratosis with crypt formation: a clinicopathological entity in the young population? Int J Dermatol. 2023;62:E291-E293. doi: 10.1111/ijd.16579
- Farah CS, Savage NW. Cryotherapy for treatment of oral lesions. Aust Dent J. 2006;51:2-5. doi:10.1111/j.1834-7819.2006.tb00392.x
- Nogueira VKC, Fernandes D, Navarro CM, et al. Cryotherapy for localized juvenile spongiotic gingival hyperplasia: preliminary findings on two cases. Int J Paediatr Dent. 2017;27:231-235. doi:10.1111/ipd.12278
- Bernick S, Bavetta LA. The development of gingival sebaceous-like glands and cysts in rats of the Holtzman strain. Oral Surg Oral Med Oral Pathol Oral Radiol. 1962;15:351-354. doi:10.1016/0030-4220(62)90116-0
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
PRACTICE POINTS
- Surgical excision is an effective treatment for idiopathic gingival papillokeratosis with crypt formation (IGPC) but may result in periodontal defects that impact the aesthetic outcome.
- Cryotherapy is a novel therapeutic intervention for IGPC.
Exophytic Scaly Nodule on the Wrist
Exophytic Scaly Nodule on the Wrist
THE DIAGNOSIS: Atypical Spitz Tumor
The shave biopsy revealed extensive dermal proliferation with spitzoid cytomorphology containing large, spindled nuclei; prominent nucleoli; and abundant homogenous cytoplasm arranged in haphazard fascicles. The proliferation was associated with prominent pseudoepitheliomatous hyperplasia of the overlying epidermis, and anaplastic lymphoma kinase immunohistochemistry showed diffuse strong positivity. Fluorescence in situ hybridization confirmed fusion of the tropomyosin 3 (TPM3) and anaplastic lymphoma kinase (ALK) genes, which finalized the diagnosis of an ALK-mutated atypical spitz tumor. Due to the location and size of the lesion, Mohs micrographic surgery was performed to excise the tumor and clear the margins.
Spitz nevi are uncommon benign melanocytic neoplasms that typically occur in pediatric populations.1 Atypical spitz nevi comprised fewer than 17% of all childhood melanocytic nevi in the United States and can be considered in the broader category of spitzoid tumors. Spitz nevi are divided into 3 classes: Spitz nevus, atypical Spitz nevus, and spitzoid melanoma. Atypical Spitz nevi have typical Spitz nevus and spitzoid melanoma features and often can be difficult to distinguish on dermoscopy. Malignant Spitz tumors typically occur in the fifth decade of life, though the age distribution can vary widely.1
Black patients are less likely to be diagnosed with Spitz nevi, potentially due to a lower prevalence in this population, thus limiting the clinician’s clinical exposure and leading to increased rates of misdiagnoses.2 Spitz nevi usually manifest as well-circumscribed, dome-shaped papules and frequently are described as pink to red due to increased vascularity and limited melanin content1; however, these lesions may appear more violaceous, dusky, or dark brown in darker skin types. Additionally, approximately 71% of patients in a clinical review of Spitz nevi had a pigmented lesion, ranging from light brown to black.3 It is important for dermatologists to understand that the contrast in color between the nevus and the surrounding skin may not be as striking, prominent, or clinically concerning, particularly in darker skin types, such as in our patient.
Spitz nevi frequently manifest as rapidly growing solitary lesions most frequently developing in the lower legs (shown in 41% of lesions in one report).4 However, a recent retrospective review indicated that Spitz nevi in Black patients most commonly were found on the upper extremities, as was seen in our patient.2 Compared to typical and common Spitz nevi, atypical Spitz nevi often are greater than 10 mm in diameter and have features of ulceration.
Diagnosing atypical spitzoid melanocytic lesions requires adequate clinical suspicion and confirmation via biopsy. Under dermoscopy, typical Spitz nevi often display a starburst or globular pattern with pinpoint vessels, though it can have variable manifestations of both patterns. Atypical Spitz nevi can be challenging to distinguish from melanoma on dermoscopy since both conditions can have atypical pigment networks or structureless homogenous areas.1 Consequently, there often is a lower threshold for biopsy and possible follow-up excision for atypical Spitz nevi. Histopathology of atypical Spitz nevi includes epithelioid and spindle melanocytes but can share features of melanomas, including areas of prominent pagetoid spread, asymmetry, and poor circumscription.5 Furthermore, atypical Spitz nevi with ALK gene fusion, as seen in our patient, have been shown in the literature to demonstrate distinct histopathologic features, such as wedge-shaped extension into the dermis or a bulbous lower border that can resemble pseudoepitheliomatous hyperplasia.6
The differential diagnosis for this rapidly growing scaly nodule also should include pyogenic granuloma, bacillary angiomatosis, Kaposi sarcoma, and amelanotic melanoma. Pyogenic granuloma is a rapidly growing, benign, vascular tumor that often becomes ulcerated and can occur in any age group.7 Pyogenic granuloma frequently appears at sites of trauma as a solitary, bright pink to red, friable, pedunculated papule and often manifests on the arms, hands, and face, similar to atypical Spitz nevi, though they can appear anywhere on the body. Histology shows a lobular capillary network with a central feeder vessel.7
Bacillary angiomatosis is an uncommon cutaneous infection associated with vascular proliferation and neovascularization due to the gram-negative organism Bartonella henselae.8 Bacillary nodules typically are reddish to purple and appear on the arms, sometimes with central ulceration and bleeding. Patients may present with multiple papules and nodules of varying sizes, as the lesions can arise in crops and follow a sporotrichoid pattern. Most patients with bacillary angiomatosis are immunosuppressed, though it rarely can affect immunocompetent patients. Histologically, bacillary angiomatosis is similar to pyogenic granuloma, though Gram or Warthin-Starry stains can help differentiate B henselae.8
Kaposi sarcoma is a malignant vascular neoplasm that often manifests in immunocompromised patients as violaceous, purple, or red patches, plaques, and nodules on the skin or oral mucosa. Histopathology shows spindle cell proliferation of irregular complex vascular channels dissecting through the dermis. Human herpesvirus 8 immunohistochemistry can be used to confirm diagnosis on histopathology.9 In contrast, amelanotic melanoma consists of lack of pigmentation, asymmetry with polymorphous vascular pattern, and high mitotic rate and is commonly found in sun-exposed areas. Dermoscopic features include irregular globules with blue-whitish veil.10
Treatment of atypical Spitz nevi depends mainly on the age of the patient and the histologic features of the nevus. Adults with atypical Spitz nevi frequently require excision, while the preferred choice for treatment in children with common Spitz nevi is regular clinical monitoring when there are no concerning clinical, dermoscopic, or histologic features.8 Compared to common Spitz nevi, atypical Spitz nevi have more melanoma-like features, resulting in a stronger recommendation for excision. Excision allows for a more thorough histologic evaluation and minimizes the likelihood of a recurrent atypical lesion.11 In all cases, close clinical follow-up is recommended to monitor for reoccurrence.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084. doi:10.1016/j.jaad.2011.04.040
- Farid YI, Honda KS. Spitz nevi in African Americans: a retrospective chart review of 11 patients. J Cutan Pathol. 2021;48:511-518. doi:10.1111 /cup.13903
- Dal Pozzo V, Benelli C, Restano L, et al. Clinical review of 247 case records of Spitz nevus (epithelioid cell and/or spindle cell nevus). Dermatology 1997;194:20-25. doi: 10.1159/000246051
- Berlingeri-Ramos AC, Morales-Burgos A, Sanchez JL, et al. Spitz nevus in a Hispanic population: a clinicopathological study of 130 cases. Am J Dermatopathol 2010;32:267-275. doi: 10.1097 /DAD.0b013e3181c52b99
- Brown A, Sawyer JD, Neumeister MW. Spitz nevus: review and update. Clin Plast Surg 2021;48:677-686. doi: 10.1016/j.cps.2021.06.002 [published Online First: 20210818]
- Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581-91. doi: 10.1097/PAS.0000000000000387
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556077/
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary angiomatosis. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 4, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448092/
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/ hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004;150(6):1117-1124. doi: 10.1111/j.1365-2133.2004.05928.x
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part II. natural history and management. J Am Acad Dermatol 2011;65:1087-1092. doi:10.1016/j.jaad.2011.06.045
THE DIAGNOSIS: Atypical Spitz Tumor
The shave biopsy revealed extensive dermal proliferation with spitzoid cytomorphology containing large, spindled nuclei; prominent nucleoli; and abundant homogenous cytoplasm arranged in haphazard fascicles. The proliferation was associated with prominent pseudoepitheliomatous hyperplasia of the overlying epidermis, and anaplastic lymphoma kinase immunohistochemistry showed diffuse strong positivity. Fluorescence in situ hybridization confirmed fusion of the tropomyosin 3 (TPM3) and anaplastic lymphoma kinase (ALK) genes, which finalized the diagnosis of an ALK-mutated atypical spitz tumor. Due to the location and size of the lesion, Mohs micrographic surgery was performed to excise the tumor and clear the margins.
Spitz nevi are uncommon benign melanocytic neoplasms that typically occur in pediatric populations.1 Atypical spitz nevi comprised fewer than 17% of all childhood melanocytic nevi in the United States and can be considered in the broader category of spitzoid tumors. Spitz nevi are divided into 3 classes: Spitz nevus, atypical Spitz nevus, and spitzoid melanoma. Atypical Spitz nevi have typical Spitz nevus and spitzoid melanoma features and often can be difficult to distinguish on dermoscopy. Malignant Spitz tumors typically occur in the fifth decade of life, though the age distribution can vary widely.1
Black patients are less likely to be diagnosed with Spitz nevi, potentially due to a lower prevalence in this population, thus limiting the clinician’s clinical exposure and leading to increased rates of misdiagnoses.2 Spitz nevi usually manifest as well-circumscribed, dome-shaped papules and frequently are described as pink to red due to increased vascularity and limited melanin content1; however, these lesions may appear more violaceous, dusky, or dark brown in darker skin types. Additionally, approximately 71% of patients in a clinical review of Spitz nevi had a pigmented lesion, ranging from light brown to black.3 It is important for dermatologists to understand that the contrast in color between the nevus and the surrounding skin may not be as striking, prominent, or clinically concerning, particularly in darker skin types, such as in our patient.
Spitz nevi frequently manifest as rapidly growing solitary lesions most frequently developing in the lower legs (shown in 41% of lesions in one report).4 However, a recent retrospective review indicated that Spitz nevi in Black patients most commonly were found on the upper extremities, as was seen in our patient.2 Compared to typical and common Spitz nevi, atypical Spitz nevi often are greater than 10 mm in diameter and have features of ulceration.
Diagnosing atypical spitzoid melanocytic lesions requires adequate clinical suspicion and confirmation via biopsy. Under dermoscopy, typical Spitz nevi often display a starburst or globular pattern with pinpoint vessels, though it can have variable manifestations of both patterns. Atypical Spitz nevi can be challenging to distinguish from melanoma on dermoscopy since both conditions can have atypical pigment networks or structureless homogenous areas.1 Consequently, there often is a lower threshold for biopsy and possible follow-up excision for atypical Spitz nevi. Histopathology of atypical Spitz nevi includes epithelioid and spindle melanocytes but can share features of melanomas, including areas of prominent pagetoid spread, asymmetry, and poor circumscription.5 Furthermore, atypical Spitz nevi with ALK gene fusion, as seen in our patient, have been shown in the literature to demonstrate distinct histopathologic features, such as wedge-shaped extension into the dermis or a bulbous lower border that can resemble pseudoepitheliomatous hyperplasia.6
The differential diagnosis for this rapidly growing scaly nodule also should include pyogenic granuloma, bacillary angiomatosis, Kaposi sarcoma, and amelanotic melanoma. Pyogenic granuloma is a rapidly growing, benign, vascular tumor that often becomes ulcerated and can occur in any age group.7 Pyogenic granuloma frequently appears at sites of trauma as a solitary, bright pink to red, friable, pedunculated papule and often manifests on the arms, hands, and face, similar to atypical Spitz nevi, though they can appear anywhere on the body. Histology shows a lobular capillary network with a central feeder vessel.7
Bacillary angiomatosis is an uncommon cutaneous infection associated with vascular proliferation and neovascularization due to the gram-negative organism Bartonella henselae.8 Bacillary nodules typically are reddish to purple and appear on the arms, sometimes with central ulceration and bleeding. Patients may present with multiple papules and nodules of varying sizes, as the lesions can arise in crops and follow a sporotrichoid pattern. Most patients with bacillary angiomatosis are immunosuppressed, though it rarely can affect immunocompetent patients. Histologically, bacillary angiomatosis is similar to pyogenic granuloma, though Gram or Warthin-Starry stains can help differentiate B henselae.8
Kaposi sarcoma is a malignant vascular neoplasm that often manifests in immunocompromised patients as violaceous, purple, or red patches, plaques, and nodules on the skin or oral mucosa. Histopathology shows spindle cell proliferation of irregular complex vascular channels dissecting through the dermis. Human herpesvirus 8 immunohistochemistry can be used to confirm diagnosis on histopathology.9 In contrast, amelanotic melanoma consists of lack of pigmentation, asymmetry with polymorphous vascular pattern, and high mitotic rate and is commonly found in sun-exposed areas. Dermoscopic features include irregular globules with blue-whitish veil.10
Treatment of atypical Spitz nevi depends mainly on the age of the patient and the histologic features of the nevus. Adults with atypical Spitz nevi frequently require excision, while the preferred choice for treatment in children with common Spitz nevi is regular clinical monitoring when there are no concerning clinical, dermoscopic, or histologic features.8 Compared to common Spitz nevi, atypical Spitz nevi have more melanoma-like features, resulting in a stronger recommendation for excision. Excision allows for a more thorough histologic evaluation and minimizes the likelihood of a recurrent atypical lesion.11 In all cases, close clinical follow-up is recommended to monitor for reoccurrence.
THE DIAGNOSIS: Atypical Spitz Tumor
The shave biopsy revealed extensive dermal proliferation with spitzoid cytomorphology containing large, spindled nuclei; prominent nucleoli; and abundant homogenous cytoplasm arranged in haphazard fascicles. The proliferation was associated with prominent pseudoepitheliomatous hyperplasia of the overlying epidermis, and anaplastic lymphoma kinase immunohistochemistry showed diffuse strong positivity. Fluorescence in situ hybridization confirmed fusion of the tropomyosin 3 (TPM3) and anaplastic lymphoma kinase (ALK) genes, which finalized the diagnosis of an ALK-mutated atypical spitz tumor. Due to the location and size of the lesion, Mohs micrographic surgery was performed to excise the tumor and clear the margins.
Spitz nevi are uncommon benign melanocytic neoplasms that typically occur in pediatric populations.1 Atypical spitz nevi comprised fewer than 17% of all childhood melanocytic nevi in the United States and can be considered in the broader category of spitzoid tumors. Spitz nevi are divided into 3 classes: Spitz nevus, atypical Spitz nevus, and spitzoid melanoma. Atypical Spitz nevi have typical Spitz nevus and spitzoid melanoma features and often can be difficult to distinguish on dermoscopy. Malignant Spitz tumors typically occur in the fifth decade of life, though the age distribution can vary widely.1
Black patients are less likely to be diagnosed with Spitz nevi, potentially due to a lower prevalence in this population, thus limiting the clinician’s clinical exposure and leading to increased rates of misdiagnoses.2 Spitz nevi usually manifest as well-circumscribed, dome-shaped papules and frequently are described as pink to red due to increased vascularity and limited melanin content1; however, these lesions may appear more violaceous, dusky, or dark brown in darker skin types. Additionally, approximately 71% of patients in a clinical review of Spitz nevi had a pigmented lesion, ranging from light brown to black.3 It is important for dermatologists to understand that the contrast in color between the nevus and the surrounding skin may not be as striking, prominent, or clinically concerning, particularly in darker skin types, such as in our patient.
Spitz nevi frequently manifest as rapidly growing solitary lesions most frequently developing in the lower legs (shown in 41% of lesions in one report).4 However, a recent retrospective review indicated that Spitz nevi in Black patients most commonly were found on the upper extremities, as was seen in our patient.2 Compared to typical and common Spitz nevi, atypical Spitz nevi often are greater than 10 mm in diameter and have features of ulceration.
Diagnosing atypical spitzoid melanocytic lesions requires adequate clinical suspicion and confirmation via biopsy. Under dermoscopy, typical Spitz nevi often display a starburst or globular pattern with pinpoint vessels, though it can have variable manifestations of both patterns. Atypical Spitz nevi can be challenging to distinguish from melanoma on dermoscopy since both conditions can have atypical pigment networks or structureless homogenous areas.1 Consequently, there often is a lower threshold for biopsy and possible follow-up excision for atypical Spitz nevi. Histopathology of atypical Spitz nevi includes epithelioid and spindle melanocytes but can share features of melanomas, including areas of prominent pagetoid spread, asymmetry, and poor circumscription.5 Furthermore, atypical Spitz nevi with ALK gene fusion, as seen in our patient, have been shown in the literature to demonstrate distinct histopathologic features, such as wedge-shaped extension into the dermis or a bulbous lower border that can resemble pseudoepitheliomatous hyperplasia.6
The differential diagnosis for this rapidly growing scaly nodule also should include pyogenic granuloma, bacillary angiomatosis, Kaposi sarcoma, and amelanotic melanoma. Pyogenic granuloma is a rapidly growing, benign, vascular tumor that often becomes ulcerated and can occur in any age group.7 Pyogenic granuloma frequently appears at sites of trauma as a solitary, bright pink to red, friable, pedunculated papule and often manifests on the arms, hands, and face, similar to atypical Spitz nevi, though they can appear anywhere on the body. Histology shows a lobular capillary network with a central feeder vessel.7
Bacillary angiomatosis is an uncommon cutaneous infection associated with vascular proliferation and neovascularization due to the gram-negative organism Bartonella henselae.8 Bacillary nodules typically are reddish to purple and appear on the arms, sometimes with central ulceration and bleeding. Patients may present with multiple papules and nodules of varying sizes, as the lesions can arise in crops and follow a sporotrichoid pattern. Most patients with bacillary angiomatosis are immunosuppressed, though it rarely can affect immunocompetent patients. Histologically, bacillary angiomatosis is similar to pyogenic granuloma, though Gram or Warthin-Starry stains can help differentiate B henselae.8
Kaposi sarcoma is a malignant vascular neoplasm that often manifests in immunocompromised patients as violaceous, purple, or red patches, plaques, and nodules on the skin or oral mucosa. Histopathology shows spindle cell proliferation of irregular complex vascular channels dissecting through the dermis. Human herpesvirus 8 immunohistochemistry can be used to confirm diagnosis on histopathology.9 In contrast, amelanotic melanoma consists of lack of pigmentation, asymmetry with polymorphous vascular pattern, and high mitotic rate and is commonly found in sun-exposed areas. Dermoscopic features include irregular globules with blue-whitish veil.10
Treatment of atypical Spitz nevi depends mainly on the age of the patient and the histologic features of the nevus. Adults with atypical Spitz nevi frequently require excision, while the preferred choice for treatment in children with common Spitz nevi is regular clinical monitoring when there are no concerning clinical, dermoscopic, or histologic features.8 Compared to common Spitz nevi, atypical Spitz nevi have more melanoma-like features, resulting in a stronger recommendation for excision. Excision allows for a more thorough histologic evaluation and minimizes the likelihood of a recurrent atypical lesion.11 In all cases, close clinical follow-up is recommended to monitor for reoccurrence.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084. doi:10.1016/j.jaad.2011.04.040
- Farid YI, Honda KS. Spitz nevi in African Americans: a retrospective chart review of 11 patients. J Cutan Pathol. 2021;48:511-518. doi:10.1111 /cup.13903
- Dal Pozzo V, Benelli C, Restano L, et al. Clinical review of 247 case records of Spitz nevus (epithelioid cell and/or spindle cell nevus). Dermatology 1997;194:20-25. doi: 10.1159/000246051
- Berlingeri-Ramos AC, Morales-Burgos A, Sanchez JL, et al. Spitz nevus in a Hispanic population: a clinicopathological study of 130 cases. Am J Dermatopathol 2010;32:267-275. doi: 10.1097 /DAD.0b013e3181c52b99
- Brown A, Sawyer JD, Neumeister MW. Spitz nevus: review and update. Clin Plast Surg 2021;48:677-686. doi: 10.1016/j.cps.2021.06.002 [published Online First: 20210818]
- Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581-91. doi: 10.1097/PAS.0000000000000387
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556077/
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary angiomatosis. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 4, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448092/
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/ hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004;150(6):1117-1124. doi: 10.1111/j.1365-2133.2004.05928.x
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part II. natural history and management. J Am Acad Dermatol 2011;65:1087-1092. doi:10.1016/j.jaad.2011.06.045
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084. doi:10.1016/j.jaad.2011.04.040
- Farid YI, Honda KS. Spitz nevi in African Americans: a retrospective chart review of 11 patients. J Cutan Pathol. 2021;48:511-518. doi:10.1111 /cup.13903
- Dal Pozzo V, Benelli C, Restano L, et al. Clinical review of 247 case records of Spitz nevus (epithelioid cell and/or spindle cell nevus). Dermatology 1997;194:20-25. doi: 10.1159/000246051
- Berlingeri-Ramos AC, Morales-Burgos A, Sanchez JL, et al. Spitz nevus in a Hispanic population: a clinicopathological study of 130 cases. Am J Dermatopathol 2010;32:267-275. doi: 10.1097 /DAD.0b013e3181c52b99
- Brown A, Sawyer JD, Neumeister MW. Spitz nevus: review and update. Clin Plast Surg 2021;48:677-686. doi: 10.1016/j.cps.2021.06.002 [published Online First: 20210818]
- Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581-91. doi: 10.1097/PAS.0000000000000387
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556077/
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary angiomatosis. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 4, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448092/
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/ hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004;150(6):1117-1124. doi: 10.1111/j.1365-2133.2004.05928.x
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part II. natural history and management. J Am Acad Dermatol 2011;65:1087-1092. doi:10.1016/j.jaad.2011.06.045
Exophytic Scaly Nodule on the Wrist
Exophytic Scaly Nodule on the Wrist
A 30-year-old Black man presented to the dermatology clinic with a rapidly growing, exophytic, scaly nodule on the right volar wrist of 2 months’ duration. The patient’s medical history was otherwise unremarkable. Physical examination revealed an irregularly bordered, red to violaceous, scaly, eroded, exophytic nodule on the wrist that was 2 cm in diameter with a surrounding adherent white-yellow crust. The patient had presumed the nodule was a wart and had been self-treating with over-the-counter salicylic acid and cryotherapy with no relief. He denied any bleeding or pruritus. The rest of the skin examination was unremarkable. A shave biopsy was performed for further evaluation.
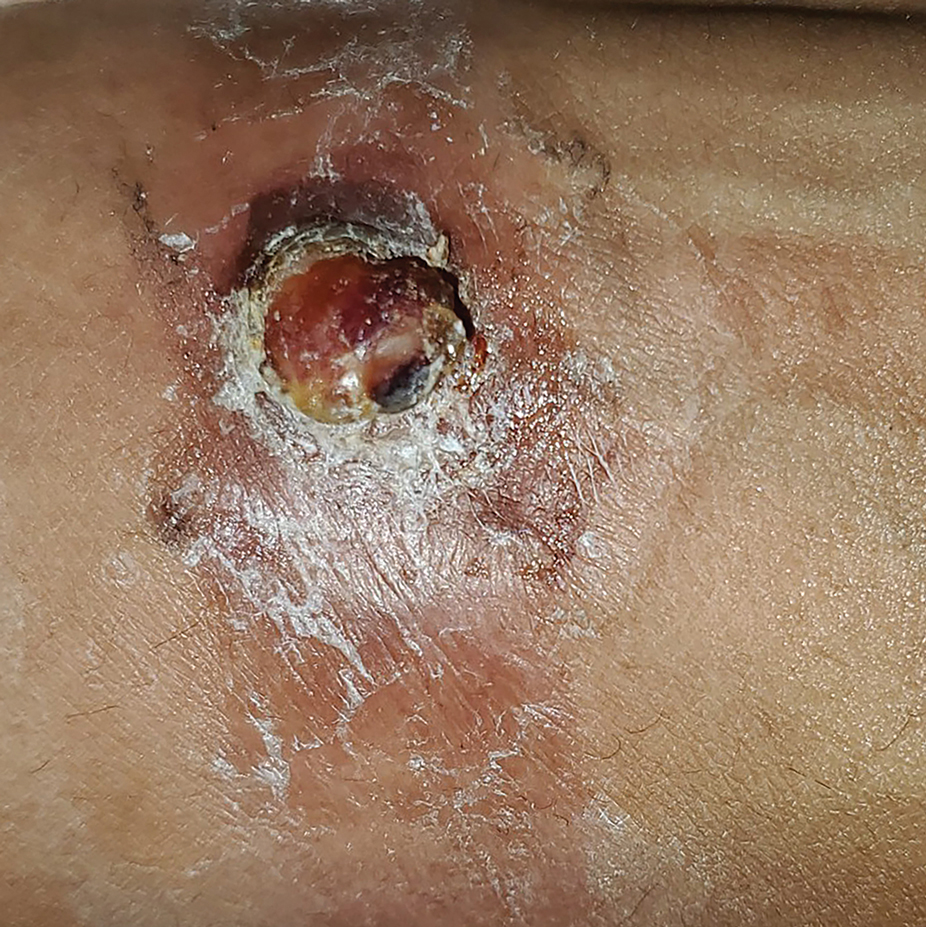
Women Researchers Remain Underrepresented in Pharma-Sponsored IBD Presentations
A recent study found that The study was published in Gastroenterology and also appeared concurrently in Clinical Gastroenterology and Hepatology .
Indeed, among gastrointestinal (GI) subspecialties, IBD was selected by 26.5% of all women GI physicians, compared with 18.9% of all their male counterparts, according to a 2021 study.
Thus, conference organizers and pharmaceutical companies should promote speaker diversity by seeking out women presenters, according to a group led by Maria A. Quintero, MD, of the Division of Gastroenterology at the Leonard Miller School of Medicine at the University of Miami, Florida.
“Seeing more women IBD leaders at the podium will inspire other women to engage in IBD clinical research,” Quintero and associates wrote.
In addition, women investigators should be included at every stage of the study process in industry-sponsored research, both as principal investigators and members of steering committees involved in study design, the authors said. Training more women clinical trial investigators in the IBD setting is another way forward.
In another recommendation, pharmaceutical companies need to be more transparent about the way first and senior authors on IBD studies are chosen because in the past the principal investigator who enrolled the most patients became the first author of the study. “That is no longer the case. However, it remains unclear whether all investigators have an equal opportunity to be the first or senior author,” Quintero and associates wrote.
The Study
The investigators analyzed IBD abstracts of presentations at five conferences for two large GI meetings, Digestive Disease Week (DDW) and United European Gastroenterology (UEG) in the period 2021-2023.
They asked whether women investigators were as likely as their male counterparts to present abstracts based on results from industry-supported clinical trials. As a point of comparison, they also looked for possible gender differences in invited-speaker vs investigator-initiated IBD sessions. To do this, they examined all IBD-related abstracts from the two meetings, identified the lead author of each oral presentation, and divided them into women or men. They also assessed whether the presentation was pharma-sponsored, investigator-initiated, or presented by an invited speaker.
Among the study findings:
- Across categories there were 178 invited lectures, 336 investigator-submitted presentations, and 150 industry-supported presentations for UEG (2021, 2022, and 2023) and DDW (2022 and 2023).
- The gender gap for men vs women was significant for industry-supported oral presentations (78.7% vs 21.3%; P < .0001) and for invited lectures (67.4% vs 32.6%; P < .0001) — but not for investigator-submitted abstracts (49.7% vs 50.3%; P = .91).
- The gender gap for industry-supported abstracts, however, was significantly larger than for investigator-submitted abstracts (57.3% vs 0.6%; P < .0001) and larger than for invited lectures (57.3% vs 34.9%; P = .09).
- The gender gap for invited lectures was significantly larger than for investigator-submitted oral presentations (34.9% vs 0.6%; P = .0009).
Why the Discordance?
This disparity may be due to the paucity of women investigators on steering committees for clinical trials. “Although the number of women doing IBD research continues to increase, then number of women senior investigators is still smaller than the number of men senior investigators,” the researchers wrote. “Ideally, there would be transparency in terms of the metrics used by pharma to choose who will be a presenting author and more intentional recruitment of women investigators to steering committees.”
Commenting on the study but not involved in it, internist Shannon M. Ruzycki, MD, MPH, an assistant professor in the Cumming School of Medicine at the University of Calgary Medical Centre in Alberta, Canada, said the findings are not surprising. “In nearly every setting where gender differences are studied in academic medicine, women are found to be disadvantaged compared to men. These differences are not attributable to skill, merit, or career attainment, but rather appear to be arbitrary and due to biases. They add up across time and likely contribute to the larger differences we see between men and women in promotion, compensation, and awards.”
Ruzycki, lead author of a study of women presenters at medical conferences, noted that differences in gender representation in academia, academic medicine, and clinical trials are similar “because the underlying causes are similar.” On the positive side, she added, conference planning committees are using strategies to reduce bias in how presenters are selected by masking the names and/or institutions of those are submitting abstracts and are being more intentional in inviting a diverse panel of qualified speakers.
“However, one strategy alone is unlikely to address such an insidious problem that affects all parts of selection,” she said. “For example, if pharmaceutical companies believe that men presenters are seen as more authoritative or knowledgeable than women presenters, they will select men to be the first author on submitted abstracts which could deprive these opportunities for deserving women candidates.”
Ruzycki attributed the imbalance to systems (academia, medicine, science) designed by men who lack empathy for the experiences of women. “In the same way you can never really understand how exhausting it is to be a parent until you become a parent or how challenging it can be to have a physical disability until you break a leg and have to navigate the world on crutches, it is really challenging for men to understand how cold and hostile these settings can be for women.”
Many of the things that make conferences, academia, and medicine so challenging for women have straightforward solutions, however, Ruzycki added. Onsite childcare, scrubs that fit women, operating room equipment that is ergonomic for women surgeons — even more washroom stalls would help. “If only we listened and cared about things that didn’t directly impact us.”
This study was supported by the 2023 Travel Grant from the International Organization for the Study of Inflammatory Bowel Diseases. One coauthor serves as a consultant or on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, and Pfizer Pharmaceutical. She is a teacher, lecturer, and speaker for Janssen and Takeda Pharmaceuticals. The remaining authors disclosed no conflicts. Ruzycki had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
A recent study found that The study was published in Gastroenterology and also appeared concurrently in Clinical Gastroenterology and Hepatology .
Indeed, among gastrointestinal (GI) subspecialties, IBD was selected by 26.5% of all women GI physicians, compared with 18.9% of all their male counterparts, according to a 2021 study.
Thus, conference organizers and pharmaceutical companies should promote speaker diversity by seeking out women presenters, according to a group led by Maria A. Quintero, MD, of the Division of Gastroenterology at the Leonard Miller School of Medicine at the University of Miami, Florida.
“Seeing more women IBD leaders at the podium will inspire other women to engage in IBD clinical research,” Quintero and associates wrote.
In addition, women investigators should be included at every stage of the study process in industry-sponsored research, both as principal investigators and members of steering committees involved in study design, the authors said. Training more women clinical trial investigators in the IBD setting is another way forward.
In another recommendation, pharmaceutical companies need to be more transparent about the way first and senior authors on IBD studies are chosen because in the past the principal investigator who enrolled the most patients became the first author of the study. “That is no longer the case. However, it remains unclear whether all investigators have an equal opportunity to be the first or senior author,” Quintero and associates wrote.
The Study
The investigators analyzed IBD abstracts of presentations at five conferences for two large GI meetings, Digestive Disease Week (DDW) and United European Gastroenterology (UEG) in the period 2021-2023.
They asked whether women investigators were as likely as their male counterparts to present abstracts based on results from industry-supported clinical trials. As a point of comparison, they also looked for possible gender differences in invited-speaker vs investigator-initiated IBD sessions. To do this, they examined all IBD-related abstracts from the two meetings, identified the lead author of each oral presentation, and divided them into women or men. They also assessed whether the presentation was pharma-sponsored, investigator-initiated, or presented by an invited speaker.
Among the study findings:
- Across categories there were 178 invited lectures, 336 investigator-submitted presentations, and 150 industry-supported presentations for UEG (2021, 2022, and 2023) and DDW (2022 and 2023).
- The gender gap for men vs women was significant for industry-supported oral presentations (78.7% vs 21.3%; P < .0001) and for invited lectures (67.4% vs 32.6%; P < .0001) — but not for investigator-submitted abstracts (49.7% vs 50.3%; P = .91).
- The gender gap for industry-supported abstracts, however, was significantly larger than for investigator-submitted abstracts (57.3% vs 0.6%; P < .0001) and larger than for invited lectures (57.3% vs 34.9%; P = .09).
- The gender gap for invited lectures was significantly larger than for investigator-submitted oral presentations (34.9% vs 0.6%; P = .0009).
Why the Discordance?
This disparity may be due to the paucity of women investigators on steering committees for clinical trials. “Although the number of women doing IBD research continues to increase, then number of women senior investigators is still smaller than the number of men senior investigators,” the researchers wrote. “Ideally, there would be transparency in terms of the metrics used by pharma to choose who will be a presenting author and more intentional recruitment of women investigators to steering committees.”
Commenting on the study but not involved in it, internist Shannon M. Ruzycki, MD, MPH, an assistant professor in the Cumming School of Medicine at the University of Calgary Medical Centre in Alberta, Canada, said the findings are not surprising. “In nearly every setting where gender differences are studied in academic medicine, women are found to be disadvantaged compared to men. These differences are not attributable to skill, merit, or career attainment, but rather appear to be arbitrary and due to biases. They add up across time and likely contribute to the larger differences we see between men and women in promotion, compensation, and awards.”
Ruzycki, lead author of a study of women presenters at medical conferences, noted that differences in gender representation in academia, academic medicine, and clinical trials are similar “because the underlying causes are similar.” On the positive side, she added, conference planning committees are using strategies to reduce bias in how presenters are selected by masking the names and/or institutions of those are submitting abstracts and are being more intentional in inviting a diverse panel of qualified speakers.
“However, one strategy alone is unlikely to address such an insidious problem that affects all parts of selection,” she said. “For example, if pharmaceutical companies believe that men presenters are seen as more authoritative or knowledgeable than women presenters, they will select men to be the first author on submitted abstracts which could deprive these opportunities for deserving women candidates.”
Ruzycki attributed the imbalance to systems (academia, medicine, science) designed by men who lack empathy for the experiences of women. “In the same way you can never really understand how exhausting it is to be a parent until you become a parent or how challenging it can be to have a physical disability until you break a leg and have to navigate the world on crutches, it is really challenging for men to understand how cold and hostile these settings can be for women.”
Many of the things that make conferences, academia, and medicine so challenging for women have straightforward solutions, however, Ruzycki added. Onsite childcare, scrubs that fit women, operating room equipment that is ergonomic for women surgeons — even more washroom stalls would help. “If only we listened and cared about things that didn’t directly impact us.”
This study was supported by the 2023 Travel Grant from the International Organization for the Study of Inflammatory Bowel Diseases. One coauthor serves as a consultant or on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, and Pfizer Pharmaceutical. She is a teacher, lecturer, and speaker for Janssen and Takeda Pharmaceuticals. The remaining authors disclosed no conflicts. Ruzycki had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
A recent study found that The study was published in Gastroenterology and also appeared concurrently in Clinical Gastroenterology and Hepatology .
Indeed, among gastrointestinal (GI) subspecialties, IBD was selected by 26.5% of all women GI physicians, compared with 18.9% of all their male counterparts, according to a 2021 study.
Thus, conference organizers and pharmaceutical companies should promote speaker diversity by seeking out women presenters, according to a group led by Maria A. Quintero, MD, of the Division of Gastroenterology at the Leonard Miller School of Medicine at the University of Miami, Florida.
“Seeing more women IBD leaders at the podium will inspire other women to engage in IBD clinical research,” Quintero and associates wrote.
In addition, women investigators should be included at every stage of the study process in industry-sponsored research, both as principal investigators and members of steering committees involved in study design, the authors said. Training more women clinical trial investigators in the IBD setting is another way forward.
In another recommendation, pharmaceutical companies need to be more transparent about the way first and senior authors on IBD studies are chosen because in the past the principal investigator who enrolled the most patients became the first author of the study. “That is no longer the case. However, it remains unclear whether all investigators have an equal opportunity to be the first or senior author,” Quintero and associates wrote.
The Study
The investigators analyzed IBD abstracts of presentations at five conferences for two large GI meetings, Digestive Disease Week (DDW) and United European Gastroenterology (UEG) in the period 2021-2023.
They asked whether women investigators were as likely as their male counterparts to present abstracts based on results from industry-supported clinical trials. As a point of comparison, they also looked for possible gender differences in invited-speaker vs investigator-initiated IBD sessions. To do this, they examined all IBD-related abstracts from the two meetings, identified the lead author of each oral presentation, and divided them into women or men. They also assessed whether the presentation was pharma-sponsored, investigator-initiated, or presented by an invited speaker.
Among the study findings:
- Across categories there were 178 invited lectures, 336 investigator-submitted presentations, and 150 industry-supported presentations for UEG (2021, 2022, and 2023) and DDW (2022 and 2023).
- The gender gap for men vs women was significant for industry-supported oral presentations (78.7% vs 21.3%; P < .0001) and for invited lectures (67.4% vs 32.6%; P < .0001) — but not for investigator-submitted abstracts (49.7% vs 50.3%; P = .91).
- The gender gap for industry-supported abstracts, however, was significantly larger than for investigator-submitted abstracts (57.3% vs 0.6%; P < .0001) and larger than for invited lectures (57.3% vs 34.9%; P = .09).
- The gender gap for invited lectures was significantly larger than for investigator-submitted oral presentations (34.9% vs 0.6%; P = .0009).
Why the Discordance?
This disparity may be due to the paucity of women investigators on steering committees for clinical trials. “Although the number of women doing IBD research continues to increase, then number of women senior investigators is still smaller than the number of men senior investigators,” the researchers wrote. “Ideally, there would be transparency in terms of the metrics used by pharma to choose who will be a presenting author and more intentional recruitment of women investigators to steering committees.”
Commenting on the study but not involved in it, internist Shannon M. Ruzycki, MD, MPH, an assistant professor in the Cumming School of Medicine at the University of Calgary Medical Centre in Alberta, Canada, said the findings are not surprising. “In nearly every setting where gender differences are studied in academic medicine, women are found to be disadvantaged compared to men. These differences are not attributable to skill, merit, or career attainment, but rather appear to be arbitrary and due to biases. They add up across time and likely contribute to the larger differences we see between men and women in promotion, compensation, and awards.”
Ruzycki, lead author of a study of women presenters at medical conferences, noted that differences in gender representation in academia, academic medicine, and clinical trials are similar “because the underlying causes are similar.” On the positive side, she added, conference planning committees are using strategies to reduce bias in how presenters are selected by masking the names and/or institutions of those are submitting abstracts and are being more intentional in inviting a diverse panel of qualified speakers.
“However, one strategy alone is unlikely to address such an insidious problem that affects all parts of selection,” she said. “For example, if pharmaceutical companies believe that men presenters are seen as more authoritative or knowledgeable than women presenters, they will select men to be the first author on submitted abstracts which could deprive these opportunities for deserving women candidates.”
Ruzycki attributed the imbalance to systems (academia, medicine, science) designed by men who lack empathy for the experiences of women. “In the same way you can never really understand how exhausting it is to be a parent until you become a parent or how challenging it can be to have a physical disability until you break a leg and have to navigate the world on crutches, it is really challenging for men to understand how cold and hostile these settings can be for women.”
Many of the things that make conferences, academia, and medicine so challenging for women have straightforward solutions, however, Ruzycki added. Onsite childcare, scrubs that fit women, operating room equipment that is ergonomic for women surgeons — even more washroom stalls would help. “If only we listened and cared about things that didn’t directly impact us.”
This study was supported by the 2023 Travel Grant from the International Organization for the Study of Inflammatory Bowel Diseases. One coauthor serves as a consultant or on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, and Pfizer Pharmaceutical. She is a teacher, lecturer, and speaker for Janssen and Takeda Pharmaceuticals. The remaining authors disclosed no conflicts. Ruzycki had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Bilateral Ankle Ulcerations and Gangrene of the Toes
Bilateral Ankle Ulcerations and Gangrene of the Toes
THE DIAGNOSIS: Rheumatoid Vasculitis
A diagnosis of rheumatoid vasculitis (RV) was made based on the clinical features, histopathology, and laboratory results in the setting of rheumatoid arthritis (RA). The distal gangrene was surgically managed with bilateral transmetatarsal amputation followed by ankle collagen graft placement. The patient was started on a prednisone taper for 1 month (40 mg/d for 3 days, then 30 mg/d for 3 days, then 20 mg/d for 24 days) before transitioning to rituximab (375 mg/m2 once weekly for 4 weeks), which improved the size and depth of the ulcers.
Rheumatoid vasculitis is an inflammatory disease that affects small- to medium-sized blood vessels in patients with RA. The pathogenesis involves immune complex deposition and complement system activation, leading to vessel wall destruction.1 Rheumatoid vasculitis is an extra-articular complication of RA that primarily is observed in seropositive patients with long-standing severe disease.1,2 The mean duration between RA diagnosis and RV onset is 10 to 14 years.2 Rheumatoid vasculitis manifests heterogeneously and can affect many organs; however, it most frequently affects the skin. Cutaneous manifestations vary in severity. Palpable purpura, pyoderma gangrenosum, and distal ulcers can be seen in addition to extensive digital ischemia with necrosis, as was present in our patient.1
When RA patients present with skin changes that are concerning for vasculitis, RV should be suspected. Currently, there are no validated diagnostic criteria for RV. Diagnosis is made based on clinical presentation and tissue biopsy. Histopathology shows small- and medium-sized vessel wall destruction with neutrophilic, granulomatous, or lymphocytic infiltration, which may be observed only in the lower dermis sparing superficial vessels.3 Direct immunofluorescence shows IgM, IgA, and C3 deposition within and around vessels.3,4 Laboratory findings including elevated inflammatory markers, positive rheumatoid factor, positive anti–cyclic citrullinated peptide, and hypocomplementemia support a diagnosis of RV.1,2
Mortality rates for RV remain high, necessitating aggressive treatment. High-dose corticosteroids typically are combined with immunosuppressant or biologic agents, frequently cyclophosphamide or rituximab.1 Consistent with other reported cases, our patient’s ulcers improved with rituximab and oral steroids.
The differential diagnosis for our patient included type I cryoglobulinemia, cutaneous polyarteritis nodosa (CPAN), peripheral vascular disease (PVD), and nonuremic calciphylaxis. Type I cryoglobulinemia manifests due to direct occlusion of vessels by precipitation of monoclonal immunoglobulin.5 It commonly is associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and multiple myeloma. While our patient’s history of RA was a risk factor for mixed cryoglobulinemia as opposed to type I cryoglobulinemia, the clinical presentation aligned more closely with type I cryoglobulinemia. The clinical manifestations of type I cryoglobulinemia are related to intravascular obstruction, including Raynaud phenomenon, retiform purpura, ischemic ulcers, distal gangrene, and cold-induced urticaria.6-8 Type I cryoglobulinemia also frequently has neurologic and renal manifestations. Histopathology, along with the detection of serum cryoglobulins, is the gold standard for diagnosing cryoglobulinemia.6 On histopathology, type I cryoglobulinemia typically shows a thrombotic vasculopathy with amorphous eosinophilic periodic acid–Schiff–positive thrombi.7 False-negative results are particularly common with serum cryoglobulins, so repeat testing often is needed. While many clinical features overlap, RV is the most likely diagnosis in a patient with long-standing RA who is negative for cryoglobulins and has no history of lymphoproliferative disorders.
Cutaneous polyarteritis nodosa is a necrotizing vasculitis that similarly affects small- and medium-sized vessels. The exact etiology is unknown, but the high prevalence of anti–phosphatidylserine/prothrombin complex antibodies among patients with CPAN suggests that prothrombin bound to apoptotic endothelial cells may initiate the immune response.9 Underlying infection and inflammatory and autoimmune diseases (including group A beta-hemolytic streptococcus, hepatitis B, inflammatory bowel disease, myasthenia gravis, and RA) also may trigger CPAN.9,10,11 The most common clinical manifestations of CPAN are tender subcutaneous nodules, livedo reticularis, leg ulcers, and cutaneous necrosis. Extracutaneous symptoms such as myalgias and arthralgias also can be associated with CPAN. There is no specific serologic test to diagnose CPAN; the diagnosis is made based on clinicopathologic correlation, with characteristic histopathology showing leukocytoclastic vasculitis in the small- and medium-sized arteries of the deep dermis or hypodermis.9
Peripheral vascular disease is a manifestation of atherosclerosis that affects the legs. Risk factors for atherosclerosis, especially smoking and diabetes mellitus, similarly increase the risk for PVD.12 The most common clinical manifestation of PVD is intermittent claudication, but rarely PVD can progress to critical limb ischemia, which is characterized by pain at rest, nonhealing ulcers, or gangrene of the legs.12 Common findings on physical examination include diminished or absent pedal pulses, abnormal skin color, and skin that is cool to the touch.12 The standard diagnostic test for PVD affecting the legs is evaluation via the ankle-brachial index, with a score of 0.90 or lower being diagnostic of PVD, a score of 0.91 to 1.00 being borderline, and a score of 1.01 to 1.40 being normal.13
Calciphylaxis most frequently is seen in patients with end-stage kidney disease; however, it also has been less commonly reported in patients with normal kidney function, known as nonuremic calciphylaxis. It is characterized by calcification of arteries, arterioles, and soft tissues, which can lead to thrombosis and eventually ischemia and necrosis of the skin.14 Calciphylaxis initially causes tender, indurated, erythematous to purpuric plaques that quickly progress to retiform and stellate ulcers with overlying necrotic eschars.15 Disease typically occurs on the legs and areas that are rich in adipose tissue, such as the abdomen and thighs.16 Skin biopsy is needed for diagnosis of calciphylaxis. Characteristic histopathologic findings include calcification, microvascular thrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles.16
We present a rare case of RV in a patient with well-controlled RA. While the incidence of RV is decreasing in the United States and United Kingdom due to the initiation of earlier and more aggressive RA therapies, mortality remains high.1 Thus, it is important to include RV in the differential diagnosis when there are skin changes concerning vasculitis in patients with seropositive, longstanding RA, even if the RA is well controlled.
- Kishore S, Maher L, Majithia V. Rheumatoid vasculitis: a diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19:39. doi:10.1007/s11926-017-0667-3
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol. 2015;27:63-70. doi:10.1097 /BOR.0000000000000126
- Patterson J. The vasculopathic reaction pattern. In: Patterson J, ed. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:241-301.
- Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243-255. doi:10.23736 /S0392-0488.18.05872-8
- Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. 2021;33:1-7. doi:10.1097/BOR.0000000000000757
- Silva F, Pinto C, Barbosa A, et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi:10.1016 /j.jaut.2019.102313
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j.jbspin.2019.01.016
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756. doi:10.1111/j.1365-4632.2010.04522.
- Criado PR, Marques GF, Morita TC, et al. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558-563. doi:10.1016/j.autrev.2016.02.010
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706-713.
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133-1141. doi:10.1016/j.amjmed.2019.04.043
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013 Jan 1;127:e264]. Circulation. 2012;126:2890-2909. doi:10.1161/CIR.0b013e318276fbcb
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Gomes F, La Feria P, Costa C, et al. Non-uremic calciphylaxis: a rare diagnosis with limited therapeutic strategies. Eur J Case Rep Intern Med.
THE DIAGNOSIS: Rheumatoid Vasculitis
A diagnosis of rheumatoid vasculitis (RV) was made based on the clinical features, histopathology, and laboratory results in the setting of rheumatoid arthritis (RA). The distal gangrene was surgically managed with bilateral transmetatarsal amputation followed by ankle collagen graft placement. The patient was started on a prednisone taper for 1 month (40 mg/d for 3 days, then 30 mg/d for 3 days, then 20 mg/d for 24 days) before transitioning to rituximab (375 mg/m2 once weekly for 4 weeks), which improved the size and depth of the ulcers.
Rheumatoid vasculitis is an inflammatory disease that affects small- to medium-sized blood vessels in patients with RA. The pathogenesis involves immune complex deposition and complement system activation, leading to vessel wall destruction.1 Rheumatoid vasculitis is an extra-articular complication of RA that primarily is observed in seropositive patients with long-standing severe disease.1,2 The mean duration between RA diagnosis and RV onset is 10 to 14 years.2 Rheumatoid vasculitis manifests heterogeneously and can affect many organs; however, it most frequently affects the skin. Cutaneous manifestations vary in severity. Palpable purpura, pyoderma gangrenosum, and distal ulcers can be seen in addition to extensive digital ischemia with necrosis, as was present in our patient.1
When RA patients present with skin changes that are concerning for vasculitis, RV should be suspected. Currently, there are no validated diagnostic criteria for RV. Diagnosis is made based on clinical presentation and tissue biopsy. Histopathology shows small- and medium-sized vessel wall destruction with neutrophilic, granulomatous, or lymphocytic infiltration, which may be observed only in the lower dermis sparing superficial vessels.3 Direct immunofluorescence shows IgM, IgA, and C3 deposition within and around vessels.3,4 Laboratory findings including elevated inflammatory markers, positive rheumatoid factor, positive anti–cyclic citrullinated peptide, and hypocomplementemia support a diagnosis of RV.1,2
Mortality rates for RV remain high, necessitating aggressive treatment. High-dose corticosteroids typically are combined with immunosuppressant or biologic agents, frequently cyclophosphamide or rituximab.1 Consistent with other reported cases, our patient’s ulcers improved with rituximab and oral steroids.
The differential diagnosis for our patient included type I cryoglobulinemia, cutaneous polyarteritis nodosa (CPAN), peripheral vascular disease (PVD), and nonuremic calciphylaxis. Type I cryoglobulinemia manifests due to direct occlusion of vessels by precipitation of monoclonal immunoglobulin.5 It commonly is associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and multiple myeloma. While our patient’s history of RA was a risk factor for mixed cryoglobulinemia as opposed to type I cryoglobulinemia, the clinical presentation aligned more closely with type I cryoglobulinemia. The clinical manifestations of type I cryoglobulinemia are related to intravascular obstruction, including Raynaud phenomenon, retiform purpura, ischemic ulcers, distal gangrene, and cold-induced urticaria.6-8 Type I cryoglobulinemia also frequently has neurologic and renal manifestations. Histopathology, along with the detection of serum cryoglobulins, is the gold standard for diagnosing cryoglobulinemia.6 On histopathology, type I cryoglobulinemia typically shows a thrombotic vasculopathy with amorphous eosinophilic periodic acid–Schiff–positive thrombi.7 False-negative results are particularly common with serum cryoglobulins, so repeat testing often is needed. While many clinical features overlap, RV is the most likely diagnosis in a patient with long-standing RA who is negative for cryoglobulins and has no history of lymphoproliferative disorders.
Cutaneous polyarteritis nodosa is a necrotizing vasculitis that similarly affects small- and medium-sized vessels. The exact etiology is unknown, but the high prevalence of anti–phosphatidylserine/prothrombin complex antibodies among patients with CPAN suggests that prothrombin bound to apoptotic endothelial cells may initiate the immune response.9 Underlying infection and inflammatory and autoimmune diseases (including group A beta-hemolytic streptococcus, hepatitis B, inflammatory bowel disease, myasthenia gravis, and RA) also may trigger CPAN.9,10,11 The most common clinical manifestations of CPAN are tender subcutaneous nodules, livedo reticularis, leg ulcers, and cutaneous necrosis. Extracutaneous symptoms such as myalgias and arthralgias also can be associated with CPAN. There is no specific serologic test to diagnose CPAN; the diagnosis is made based on clinicopathologic correlation, with characteristic histopathology showing leukocytoclastic vasculitis in the small- and medium-sized arteries of the deep dermis or hypodermis.9
Peripheral vascular disease is a manifestation of atherosclerosis that affects the legs. Risk factors for atherosclerosis, especially smoking and diabetes mellitus, similarly increase the risk for PVD.12 The most common clinical manifestation of PVD is intermittent claudication, but rarely PVD can progress to critical limb ischemia, which is characterized by pain at rest, nonhealing ulcers, or gangrene of the legs.12 Common findings on physical examination include diminished or absent pedal pulses, abnormal skin color, and skin that is cool to the touch.12 The standard diagnostic test for PVD affecting the legs is evaluation via the ankle-brachial index, with a score of 0.90 or lower being diagnostic of PVD, a score of 0.91 to 1.00 being borderline, and a score of 1.01 to 1.40 being normal.13
Calciphylaxis most frequently is seen in patients with end-stage kidney disease; however, it also has been less commonly reported in patients with normal kidney function, known as nonuremic calciphylaxis. It is characterized by calcification of arteries, arterioles, and soft tissues, which can lead to thrombosis and eventually ischemia and necrosis of the skin.14 Calciphylaxis initially causes tender, indurated, erythematous to purpuric plaques that quickly progress to retiform and stellate ulcers with overlying necrotic eschars.15 Disease typically occurs on the legs and areas that are rich in adipose tissue, such as the abdomen and thighs.16 Skin biopsy is needed for diagnosis of calciphylaxis. Characteristic histopathologic findings include calcification, microvascular thrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles.16
We present a rare case of RV in a patient with well-controlled RA. While the incidence of RV is decreasing in the United States and United Kingdom due to the initiation of earlier and more aggressive RA therapies, mortality remains high.1 Thus, it is important to include RV in the differential diagnosis when there are skin changes concerning vasculitis in patients with seropositive, longstanding RA, even if the RA is well controlled.
THE DIAGNOSIS: Rheumatoid Vasculitis
A diagnosis of rheumatoid vasculitis (RV) was made based on the clinical features, histopathology, and laboratory results in the setting of rheumatoid arthritis (RA). The distal gangrene was surgically managed with bilateral transmetatarsal amputation followed by ankle collagen graft placement. The patient was started on a prednisone taper for 1 month (40 mg/d for 3 days, then 30 mg/d for 3 days, then 20 mg/d for 24 days) before transitioning to rituximab (375 mg/m2 once weekly for 4 weeks), which improved the size and depth of the ulcers.
Rheumatoid vasculitis is an inflammatory disease that affects small- to medium-sized blood vessels in patients with RA. The pathogenesis involves immune complex deposition and complement system activation, leading to vessel wall destruction.1 Rheumatoid vasculitis is an extra-articular complication of RA that primarily is observed in seropositive patients with long-standing severe disease.1,2 The mean duration between RA diagnosis and RV onset is 10 to 14 years.2 Rheumatoid vasculitis manifests heterogeneously and can affect many organs; however, it most frequently affects the skin. Cutaneous manifestations vary in severity. Palpable purpura, pyoderma gangrenosum, and distal ulcers can be seen in addition to extensive digital ischemia with necrosis, as was present in our patient.1
When RA patients present with skin changes that are concerning for vasculitis, RV should be suspected. Currently, there are no validated diagnostic criteria for RV. Diagnosis is made based on clinical presentation and tissue biopsy. Histopathology shows small- and medium-sized vessel wall destruction with neutrophilic, granulomatous, or lymphocytic infiltration, which may be observed only in the lower dermis sparing superficial vessels.3 Direct immunofluorescence shows IgM, IgA, and C3 deposition within and around vessels.3,4 Laboratory findings including elevated inflammatory markers, positive rheumatoid factor, positive anti–cyclic citrullinated peptide, and hypocomplementemia support a diagnosis of RV.1,2
Mortality rates for RV remain high, necessitating aggressive treatment. High-dose corticosteroids typically are combined with immunosuppressant or biologic agents, frequently cyclophosphamide or rituximab.1 Consistent with other reported cases, our patient’s ulcers improved with rituximab and oral steroids.
The differential diagnosis for our patient included type I cryoglobulinemia, cutaneous polyarteritis nodosa (CPAN), peripheral vascular disease (PVD), and nonuremic calciphylaxis. Type I cryoglobulinemia manifests due to direct occlusion of vessels by precipitation of monoclonal immunoglobulin.5 It commonly is associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and multiple myeloma. While our patient’s history of RA was a risk factor for mixed cryoglobulinemia as opposed to type I cryoglobulinemia, the clinical presentation aligned more closely with type I cryoglobulinemia. The clinical manifestations of type I cryoglobulinemia are related to intravascular obstruction, including Raynaud phenomenon, retiform purpura, ischemic ulcers, distal gangrene, and cold-induced urticaria.6-8 Type I cryoglobulinemia also frequently has neurologic and renal manifestations. Histopathology, along with the detection of serum cryoglobulins, is the gold standard for diagnosing cryoglobulinemia.6 On histopathology, type I cryoglobulinemia typically shows a thrombotic vasculopathy with amorphous eosinophilic periodic acid–Schiff–positive thrombi.7 False-negative results are particularly common with serum cryoglobulins, so repeat testing often is needed. While many clinical features overlap, RV is the most likely diagnosis in a patient with long-standing RA who is negative for cryoglobulins and has no history of lymphoproliferative disorders.
Cutaneous polyarteritis nodosa is a necrotizing vasculitis that similarly affects small- and medium-sized vessels. The exact etiology is unknown, but the high prevalence of anti–phosphatidylserine/prothrombin complex antibodies among patients with CPAN suggests that prothrombin bound to apoptotic endothelial cells may initiate the immune response.9 Underlying infection and inflammatory and autoimmune diseases (including group A beta-hemolytic streptococcus, hepatitis B, inflammatory bowel disease, myasthenia gravis, and RA) also may trigger CPAN.9,10,11 The most common clinical manifestations of CPAN are tender subcutaneous nodules, livedo reticularis, leg ulcers, and cutaneous necrosis. Extracutaneous symptoms such as myalgias and arthralgias also can be associated with CPAN. There is no specific serologic test to diagnose CPAN; the diagnosis is made based on clinicopathologic correlation, with characteristic histopathology showing leukocytoclastic vasculitis in the small- and medium-sized arteries of the deep dermis or hypodermis.9
Peripheral vascular disease is a manifestation of atherosclerosis that affects the legs. Risk factors for atherosclerosis, especially smoking and diabetes mellitus, similarly increase the risk for PVD.12 The most common clinical manifestation of PVD is intermittent claudication, but rarely PVD can progress to critical limb ischemia, which is characterized by pain at rest, nonhealing ulcers, or gangrene of the legs.12 Common findings on physical examination include diminished or absent pedal pulses, abnormal skin color, and skin that is cool to the touch.12 The standard diagnostic test for PVD affecting the legs is evaluation via the ankle-brachial index, with a score of 0.90 or lower being diagnostic of PVD, a score of 0.91 to 1.00 being borderline, and a score of 1.01 to 1.40 being normal.13
Calciphylaxis most frequently is seen in patients with end-stage kidney disease; however, it also has been less commonly reported in patients with normal kidney function, known as nonuremic calciphylaxis. It is characterized by calcification of arteries, arterioles, and soft tissues, which can lead to thrombosis and eventually ischemia and necrosis of the skin.14 Calciphylaxis initially causes tender, indurated, erythematous to purpuric plaques that quickly progress to retiform and stellate ulcers with overlying necrotic eschars.15 Disease typically occurs on the legs and areas that are rich in adipose tissue, such as the abdomen and thighs.16 Skin biopsy is needed for diagnosis of calciphylaxis. Characteristic histopathologic findings include calcification, microvascular thrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles.16
We present a rare case of RV in a patient with well-controlled RA. While the incidence of RV is decreasing in the United States and United Kingdom due to the initiation of earlier and more aggressive RA therapies, mortality remains high.1 Thus, it is important to include RV in the differential diagnosis when there are skin changes concerning vasculitis in patients with seropositive, longstanding RA, even if the RA is well controlled.
- Kishore S, Maher L, Majithia V. Rheumatoid vasculitis: a diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19:39. doi:10.1007/s11926-017-0667-3
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol. 2015;27:63-70. doi:10.1097 /BOR.0000000000000126
- Patterson J. The vasculopathic reaction pattern. In: Patterson J, ed. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:241-301.
- Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243-255. doi:10.23736 /S0392-0488.18.05872-8
- Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. 2021;33:1-7. doi:10.1097/BOR.0000000000000757
- Silva F, Pinto C, Barbosa A, et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi:10.1016 /j.jaut.2019.102313
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j.jbspin.2019.01.016
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756. doi:10.1111/j.1365-4632.2010.04522.
- Criado PR, Marques GF, Morita TC, et al. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558-563. doi:10.1016/j.autrev.2016.02.010
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706-713.
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133-1141. doi:10.1016/j.amjmed.2019.04.043
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013 Jan 1;127:e264]. Circulation. 2012;126:2890-2909. doi:10.1161/CIR.0b013e318276fbcb
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Gomes F, La Feria P, Costa C, et al. Non-uremic calciphylaxis: a rare diagnosis with limited therapeutic strategies. Eur J Case Rep Intern Med.
- Kishore S, Maher L, Majithia V. Rheumatoid vasculitis: a diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19:39. doi:10.1007/s11926-017-0667-3
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol. 2015;27:63-70. doi:10.1097 /BOR.0000000000000126
- Patterson J. The vasculopathic reaction pattern. In: Patterson J, ed. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:241-301.
- Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243-255. doi:10.23736 /S0392-0488.18.05872-8
- Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. 2021;33:1-7. doi:10.1097/BOR.0000000000000757
- Silva F, Pinto C, Barbosa A, et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi:10.1016 /j.jaut.2019.102313
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j.jbspin.2019.01.016
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756. doi:10.1111/j.1365-4632.2010.04522.
- Criado PR, Marques GF, Morita TC, et al. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558-563. doi:10.1016/j.autrev.2016.02.010
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706-713.
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133-1141. doi:10.1016/j.amjmed.2019.04.043
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013 Jan 1;127:e264]. Circulation. 2012;126:2890-2909. doi:10.1161/CIR.0b013e318276fbcb
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Gomes F, La Feria P, Costa C, et al. Non-uremic calciphylaxis: a rare diagnosis with limited therapeutic strategies. Eur J Case Rep Intern Med.
Bilateral Ankle Ulcerations and Gangrene of the Toes
Bilateral Ankle Ulcerations and Gangrene of the Toes
A 74-year-old woman presented to the hospital with large tender ulcerations on both ankles as well as gangrene of the toes of 6 to 8 weeks’ duration. The patient had a history of hypertension as well as seropositive nonerosive rheumatoid arthritis that had been diagnosed 8 years prior and was well controlled with leflunomide and prednisone as needed for flares. She denied any history of similar ulcers as well as any recent illnesses, medication changes, or joint pain or swelling. She was evaluated by vascular surgery 1 week prior to the current presentation, at which time her ankle-brachial index score was normal. Skin examination revealed noninflammatory retiform purpura surrounding ulcerations on both ankles (top) and necrosis of all toes (bottom) with peripheral retiform purpura. Joint examination revealed swan neck deformities of multiple fingers with normal range of motion, and there was no effusion or tenderness of the joints of the fingers on palpation. No rheumatoid nodules were present. Laboratory testing revealed elevated rheumatoid factor, anti–cyclic citrullinated peptide, C-reactive protein, and anti–Sjögren syndrome–related antigen A levels and low C4 levels. Cryoglobulins, antineutrophil cytoplasmic antibodies, and serum protein electrophoresis were negative. Biopsy of an ulcer on the right ankle showed medium-sized vessel vasculitis with fibrinoid necrosis, including endothelium necrosis and a perivascular lymphocytic infiltrate. Direct immunofluorescence demonstrated dense, granular, intraperivascular deposition of IgM and IgG with slightly weaker deposition of IgA, C3, and C5b-9 in the dermis and subcutis with a greater effect on medium-sized vessels.
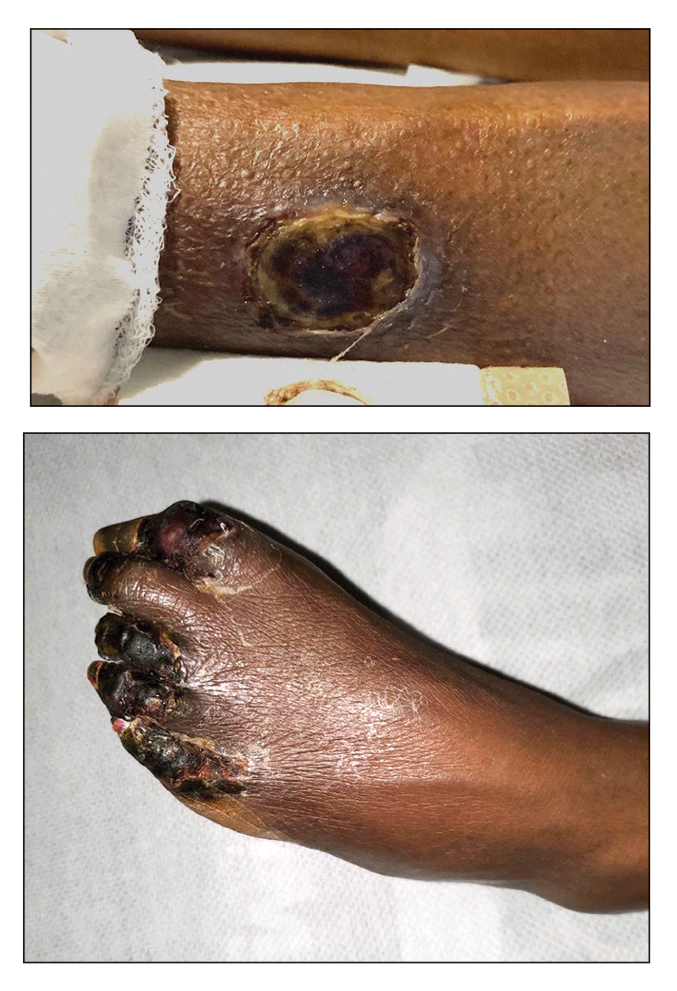
Healthcare AI: Balancing Safety and Innovation
Artificial intelligence (AI) applications are expanding rapidly in healthcare. AI powered tools are increasingly used in everyday medical practice, assisting clinicians with tasks such as diagnosis, treatment planning, data analysis, and patient monitoring, effectively integrating AI into routine clinical decision making. Despite its potential to fundamentally transform the practice of medicine and healthcare delivery, AI in healthcare remains largely unregulated, with a lack of common standards to guide responsible design, development, and adoption of AI-based tools to guide clinical care.
But this is changing. In mid-January, the US Department of Health & Human Services released its Strategic Plan for the Use of AI in Health, Human Services, and Public Health (available at www.healthit.gov), presenting an approach to catalyze innovation, promote trustworthy AI development, democratize technologies and resources, and cultivate AI-empowered workforces and organizational cultures. While there is no immediate regulatory impact, the plan does provide important insights into how the federal government thinks about AI, which will be a part of driving regulations in the future. As crucial stakeholders in the health AI universe and advocates for its responsible use in clinical practice, it is critical that we as clinicians keep abreast of developments in this rapidly evolving space.
In this month’s issue of GI & Hepatology News, we summarize a recent systematic review and meta-analysis highlighting worsening health disparities for Hispanic adults with MASLD. We also report the results of an industry-sponsored study comparing the real-world clinical effectiveness of GI Genius (an AI-driven tool) with that of standard colonoscopy.
In February’s Member Spotlight, we introduce you to international AGA member Dr. Tossapol Kerdsirichairat (clinical associate professor of gastroenterology at Bumrungrad International Hospital in Bangkok, Thailand), who shares his insights regarding the challenges and rewards of practicing gastroenterology at one of the largest private hospitals in Southeast Asia. ‘Tos’ is one of roughly 25% of AGA members who live and work outside the United States.
Finally, this month’s In Focus column from The New Gastroenterologist focuses on management of chronic constipation, a highly prevalent condition that significantly impacts the quality of life of many of our patients. We hope you enjoy this and all the exciting content in our February issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Artificial intelligence (AI) applications are expanding rapidly in healthcare. AI powered tools are increasingly used in everyday medical practice, assisting clinicians with tasks such as diagnosis, treatment planning, data analysis, and patient monitoring, effectively integrating AI into routine clinical decision making. Despite its potential to fundamentally transform the practice of medicine and healthcare delivery, AI in healthcare remains largely unregulated, with a lack of common standards to guide responsible design, development, and adoption of AI-based tools to guide clinical care.
But this is changing. In mid-January, the US Department of Health & Human Services released its Strategic Plan for the Use of AI in Health, Human Services, and Public Health (available at www.healthit.gov), presenting an approach to catalyze innovation, promote trustworthy AI development, democratize technologies and resources, and cultivate AI-empowered workforces and organizational cultures. While there is no immediate regulatory impact, the plan does provide important insights into how the federal government thinks about AI, which will be a part of driving regulations in the future. As crucial stakeholders in the health AI universe and advocates for its responsible use in clinical practice, it is critical that we as clinicians keep abreast of developments in this rapidly evolving space.
In this month’s issue of GI & Hepatology News, we summarize a recent systematic review and meta-analysis highlighting worsening health disparities for Hispanic adults with MASLD. We also report the results of an industry-sponsored study comparing the real-world clinical effectiveness of GI Genius (an AI-driven tool) with that of standard colonoscopy.
In February’s Member Spotlight, we introduce you to international AGA member Dr. Tossapol Kerdsirichairat (clinical associate professor of gastroenterology at Bumrungrad International Hospital in Bangkok, Thailand), who shares his insights regarding the challenges and rewards of practicing gastroenterology at one of the largest private hospitals in Southeast Asia. ‘Tos’ is one of roughly 25% of AGA members who live and work outside the United States.
Finally, this month’s In Focus column from The New Gastroenterologist focuses on management of chronic constipation, a highly prevalent condition that significantly impacts the quality of life of many of our patients. We hope you enjoy this and all the exciting content in our February issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Artificial intelligence (AI) applications are expanding rapidly in healthcare. AI powered tools are increasingly used in everyday medical practice, assisting clinicians with tasks such as diagnosis, treatment planning, data analysis, and patient monitoring, effectively integrating AI into routine clinical decision making. Despite its potential to fundamentally transform the practice of medicine and healthcare delivery, AI in healthcare remains largely unregulated, with a lack of common standards to guide responsible design, development, and adoption of AI-based tools to guide clinical care.
But this is changing. In mid-January, the US Department of Health & Human Services released its Strategic Plan for the Use of AI in Health, Human Services, and Public Health (available at www.healthit.gov), presenting an approach to catalyze innovation, promote trustworthy AI development, democratize technologies and resources, and cultivate AI-empowered workforces and organizational cultures. While there is no immediate regulatory impact, the plan does provide important insights into how the federal government thinks about AI, which will be a part of driving regulations in the future. As crucial stakeholders in the health AI universe and advocates for its responsible use in clinical practice, it is critical that we as clinicians keep abreast of developments in this rapidly evolving space.
In this month’s issue of GI & Hepatology News, we summarize a recent systematic review and meta-analysis highlighting worsening health disparities for Hispanic adults with MASLD. We also report the results of an industry-sponsored study comparing the real-world clinical effectiveness of GI Genius (an AI-driven tool) with that of standard colonoscopy.
In February’s Member Spotlight, we introduce you to international AGA member Dr. Tossapol Kerdsirichairat (clinical associate professor of gastroenterology at Bumrungrad International Hospital in Bangkok, Thailand), who shares his insights regarding the challenges and rewards of practicing gastroenterology at one of the largest private hospitals in Southeast Asia. ‘Tos’ is one of roughly 25% of AGA members who live and work outside the United States.
Finally, this month’s In Focus column from The New Gastroenterologist focuses on management of chronic constipation, a highly prevalent condition that significantly impacts the quality of life of many of our patients. We hope you enjoy this and all the exciting content in our February issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Valaciclovir Shows Promise in Preventing Herpes Zoster During Anifrolumab Treatment for Lupus
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
High-Dose Atropine Curbs Myopia in Kids Despite Side Effects
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of the 3-year results of the MOSAIC trial to investigate the efficacy and safety of different atropine regimens in treatment-naive children aged 6-16 years with a spherical equivalent ≤ −0.50 diopters (D).
- They analyzed data of 199 children in Europe with myopia (mean age, 13.9 years; 60.8% girls) who were randomly assigned to either group 1 (nightly placebo for 2 years followed by 0.05% atropine eye drops for 1 year; n = 66) or group 2 (nightly 0.01% atropine eye drops for 2 years followed by another random assignment to nightly placebo, tapering placebo, or tapering of 0.01% atropine eye drops for 1 year; n = 133).
- The nightly and tapered placebo groups were combined as a single treatment group for the sake of analysis.
- The primary outcome measures included observed changes in the progression of myopia, assessed using cycloplegic spherical equivalent refraction and axial length from month 24 to month 36.
TAKEAWAY:
- Children in the 0.01% atropine then placebo groups showed greater spherical equivalent progression (adjusted difference, –0.13 D; P = .01) and axial elongation (adjusted difference, 0.06 mm; P = .008) than those in the placebo then 0.05% atropine group.
- Children in the placebo then 0.05% atropine group also experienced less axial elongation (P = .04) than those in the 0.01% atropine then tapering 0.01% atropine group.
- Among participants using 0.05% atropine, 15% reported blurred near vision and 8% reported photophobia, whereas 3% reported blurred near vision and 0% reported photophobia in the 0.01% atropine then tapering 0.01% atropine group.
- Despite experiencing adverse events, no participants in the placebo then 0.05% atropine group discontinued treatment, with 92% completing the 36-month visit and 81% adhering to the treatment regimen.
IN PRACTICE:
“Recognizing a 2-year delay in treatment initiation in the group of children originally assigned to placebo, 0.05% atropine eyedrops slowed both myopia progression and axial eye growth over the course of a 1-year period,” the authors of the study wrote.
SOURCE:
This study was led by James Loughman, PhD, of the Centre for Eye Research Ireland, Dublin. It was published online in JAMA Ophthalmology.
LIMITATIONS:
Limitations included smaller sample sizes across treatment groups in year 3 and potential carry-over effects for participants transitioning from 0.01% atropine to placebo or tapered dosing. Because the study lacked an untreated control group, rebound myopia progression could be measured based only on the expected third-year results from the 0.01% atropine then placebo groups. The age of participants during the third year may have affected the ability to detect rebound progression.
DISCLOSURES:
This study was supported partly by a grant from the Health Research Board; Fighting Blindness, Ireland; and Vyluma. Some authors reported receiving grants, nonfinancial support, or consultant fees or having several other ties with Vyluma and other sources.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of the 3-year results of the MOSAIC trial to investigate the efficacy and safety of different atropine regimens in treatment-naive children aged 6-16 years with a spherical equivalent ≤ −0.50 diopters (D).
- They analyzed data of 199 children in Europe with myopia (mean age, 13.9 years; 60.8% girls) who were randomly assigned to either group 1 (nightly placebo for 2 years followed by 0.05% atropine eye drops for 1 year; n = 66) or group 2 (nightly 0.01% atropine eye drops for 2 years followed by another random assignment to nightly placebo, tapering placebo, or tapering of 0.01% atropine eye drops for 1 year; n = 133).
- The nightly and tapered placebo groups were combined as a single treatment group for the sake of analysis.
- The primary outcome measures included observed changes in the progression of myopia, assessed using cycloplegic spherical equivalent refraction and axial length from month 24 to month 36.
TAKEAWAY:
- Children in the 0.01% atropine then placebo groups showed greater spherical equivalent progression (adjusted difference, –0.13 D; P = .01) and axial elongation (adjusted difference, 0.06 mm; P = .008) than those in the placebo then 0.05% atropine group.
- Children in the placebo then 0.05% atropine group also experienced less axial elongation (P = .04) than those in the 0.01% atropine then tapering 0.01% atropine group.
- Among participants using 0.05% atropine, 15% reported blurred near vision and 8% reported photophobia, whereas 3% reported blurred near vision and 0% reported photophobia in the 0.01% atropine then tapering 0.01% atropine group.
- Despite experiencing adverse events, no participants in the placebo then 0.05% atropine group discontinued treatment, with 92% completing the 36-month visit and 81% adhering to the treatment regimen.
IN PRACTICE:
“Recognizing a 2-year delay in treatment initiation in the group of children originally assigned to placebo, 0.05% atropine eyedrops slowed both myopia progression and axial eye growth over the course of a 1-year period,” the authors of the study wrote.
SOURCE:
This study was led by James Loughman, PhD, of the Centre for Eye Research Ireland, Dublin. It was published online in JAMA Ophthalmology.
LIMITATIONS:
Limitations included smaller sample sizes across treatment groups in year 3 and potential carry-over effects for participants transitioning from 0.01% atropine to placebo or tapered dosing. Because the study lacked an untreated control group, rebound myopia progression could be measured based only on the expected third-year results from the 0.01% atropine then placebo groups. The age of participants during the third year may have affected the ability to detect rebound progression.
DISCLOSURES:
This study was supported partly by a grant from the Health Research Board; Fighting Blindness, Ireland; and Vyluma. Some authors reported receiving grants, nonfinancial support, or consultant fees or having several other ties with Vyluma and other sources.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of the 3-year results of the MOSAIC trial to investigate the efficacy and safety of different atropine regimens in treatment-naive children aged 6-16 years with a spherical equivalent ≤ −0.50 diopters (D).
- They analyzed data of 199 children in Europe with myopia (mean age, 13.9 years; 60.8% girls) who were randomly assigned to either group 1 (nightly placebo for 2 years followed by 0.05% atropine eye drops for 1 year; n = 66) or group 2 (nightly 0.01% atropine eye drops for 2 years followed by another random assignment to nightly placebo, tapering placebo, or tapering of 0.01% atropine eye drops for 1 year; n = 133).
- The nightly and tapered placebo groups were combined as a single treatment group for the sake of analysis.
- The primary outcome measures included observed changes in the progression of myopia, assessed using cycloplegic spherical equivalent refraction and axial length from month 24 to month 36.
TAKEAWAY:
- Children in the 0.01% atropine then placebo groups showed greater spherical equivalent progression (adjusted difference, –0.13 D; P = .01) and axial elongation (adjusted difference, 0.06 mm; P = .008) than those in the placebo then 0.05% atropine group.
- Children in the placebo then 0.05% atropine group also experienced less axial elongation (P = .04) than those in the 0.01% atropine then tapering 0.01% atropine group.
- Among participants using 0.05% atropine, 15% reported blurred near vision and 8% reported photophobia, whereas 3% reported blurred near vision and 0% reported photophobia in the 0.01% atropine then tapering 0.01% atropine group.
- Despite experiencing adverse events, no participants in the placebo then 0.05% atropine group discontinued treatment, with 92% completing the 36-month visit and 81% adhering to the treatment regimen.
IN PRACTICE:
“Recognizing a 2-year delay in treatment initiation in the group of children originally assigned to placebo, 0.05% atropine eyedrops slowed both myopia progression and axial eye growth over the course of a 1-year period,” the authors of the study wrote.
SOURCE:
This study was led by James Loughman, PhD, of the Centre for Eye Research Ireland, Dublin. It was published online in JAMA Ophthalmology.
LIMITATIONS:
Limitations included smaller sample sizes across treatment groups in year 3 and potential carry-over effects for participants transitioning from 0.01% atropine to placebo or tapered dosing. Because the study lacked an untreated control group, rebound myopia progression could be measured based only on the expected third-year results from the 0.01% atropine then placebo groups. The age of participants during the third year may have affected the ability to detect rebound progression.
DISCLOSURES:
This study was supported partly by a grant from the Health Research Board; Fighting Blindness, Ireland; and Vyluma. Some authors reported receiving grants, nonfinancial support, or consultant fees or having several other ties with Vyluma and other sources.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
MRI-Invisible Prostate Lesions: Are They Dangerous?
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.