User login
For MD-IQ use only
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
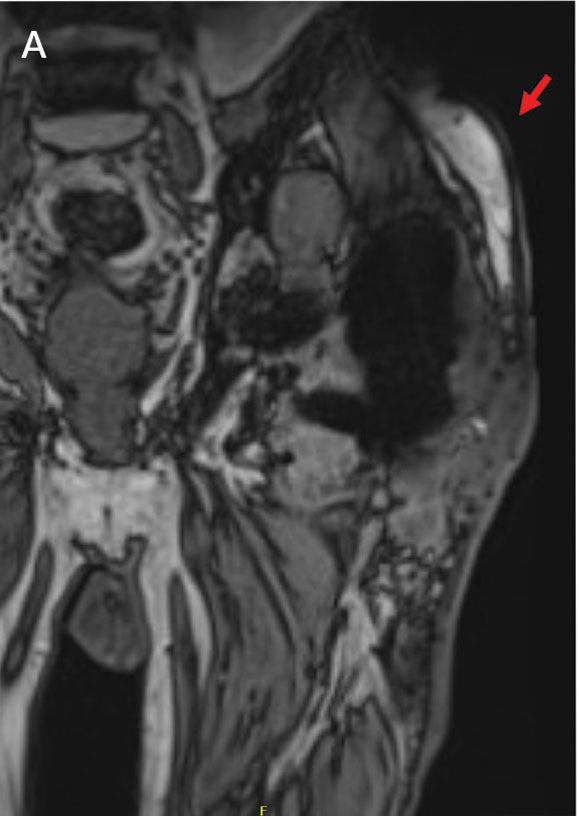
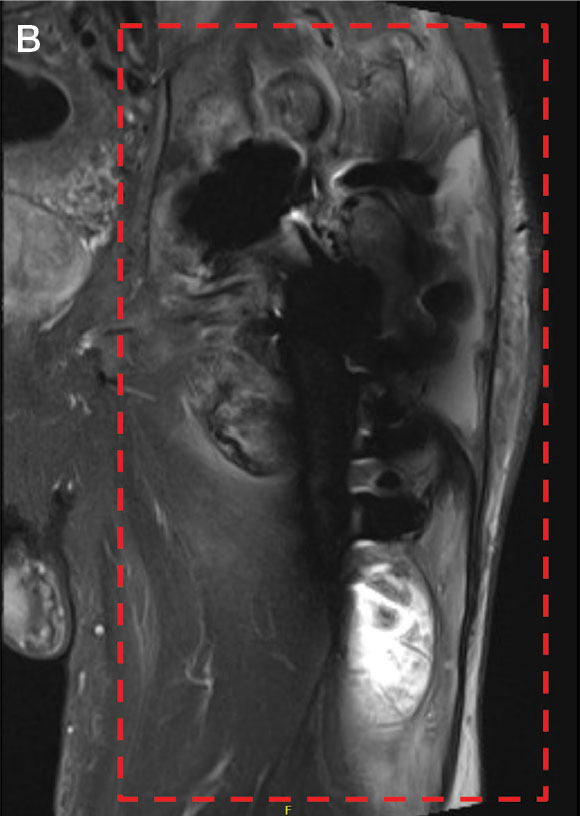
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).
Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
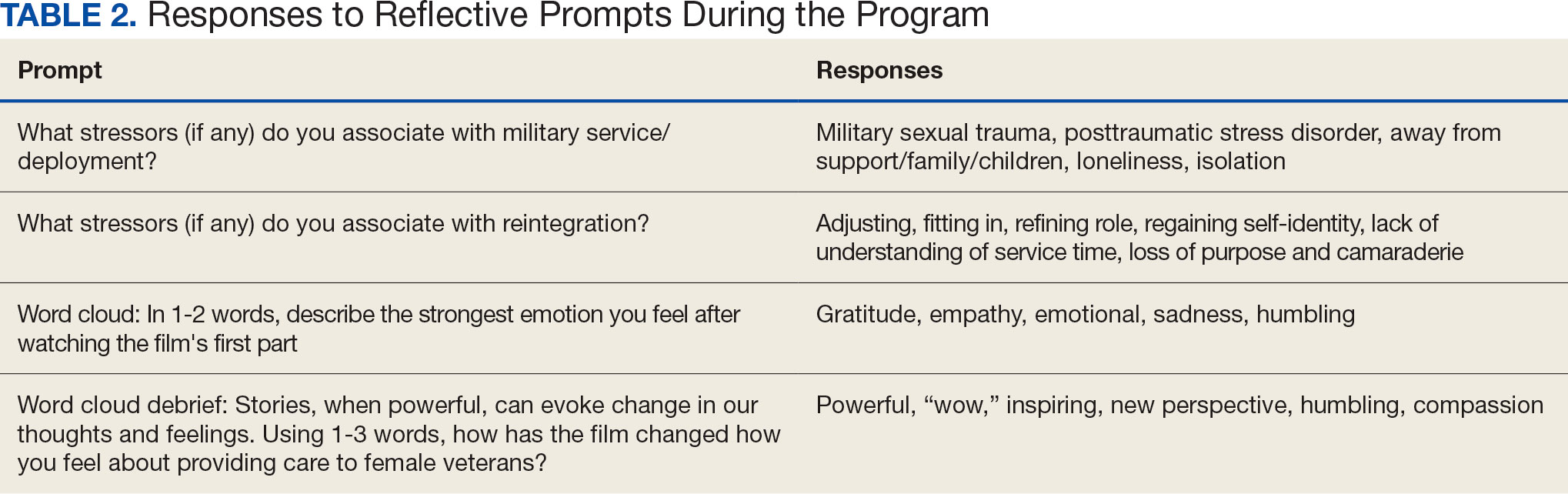
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.
DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.

DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.

DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Where Are All the Nurses? Data Show That Some States Have a Far Higher Number of Nurses Per Capita Than Others
During their 12-hour shifts, registered nurses (RNs) in Arizona and Arkansas perform many of the same tasks as RNs in Wisconsin and Wyoming: Assessing patients, monitoring vital signs, administering medications, and charting records to provide the best patient care. The work might be similar, but there are vast differences in the number of RNs in each state.
In states like Idaho, Utah, and Nevada, which have the lowest number of nurses per capita, there are as few as 7 nurses per 1000 residents, compared with South Dakota and the District of Columbia, which have double the number of nurses than underserved states — giving them the highest number of nurses per capita.
Even states with the largest number of nurses per capita are not immune to the nursing shortage. The National Bureau of Labor Statistics estimates that there will be 195,400 job openings for RNs from 2021 to 2031.
So, what makes it easier for some states to recruit and retain RNs than others?
States With the Highest Number of Nurses Per Capita
South Dakota
RNs per 1000 residents: 15.79
Average wage: $67,030 or $32.23 per hour
Average rent in Sioux Falls: $1192 per month
The Midwestern state has more miles of shoreline than Florida, herds of wild buffalo, the highest summit east of the Rockies, and more nurses per capita than all other states . Healthcare is one of the major industries in the Mount Rushmore State.
Haifa Abou Samra, dean and professor at the University of South Dakota School of Health Sciences, Vermillion, isn’t surprised that RNs want to call the state home.
“South Dakota is a nice place to live,” Samra said. “[The] schools are wonderful. If people are growing families, there is support; neighbors support their neighbors, and it’s a relatively safe community.”
South Dakota has 19 approved nursing education programs that graduated 878 RNs in 2022. Scholarships and student loan forgiveness programs have helped attract qualified RNs, and collaborations between education and industry have been instrumental in addressing the nursing shortage, Samra told this news organization.
Even though RNs earn less than the median wage ($87,070 per year/41.38 per hour), South Dakota has a low cost of living and no individual income tax, which helps stretch those earnings.
District of Columbia
RNs per 1000 residents: 15.39
Average wage: $105,220 or $50.59 per hour
Average rent in Washington, DC: $2485 per month
After a shift at some of the top-ranking hospitals in the nation, RNs working in the compact capital region can explore museums, monuments, and cultural sites; walk along the banks of the Potomac River; or grab a bite at award-winning restaurants.
Washington, a top-ranking metro area because of its growth, high wages, and access to economic opportunities, is also home to several top-tier hospitals and some of the best healthcare in the nation, and RNs who want to pursue continuing education have access to top-tier universities.
Nurses in Washington, DC, might make some of the highest wages in the nation, but the region also has the second-highest cost of living in the United States, with average rents topping $2400 per month and an average home price of $594,337.
North Dakota
RNs per 1000 residents: 12.99
Average wage: $74,930 or $36.03 per hour
Average rent in Fargo: $1051 per month
North Dakota projects a 10.4% increase in employment for RNs, which is higher than the national average, and the state has implemented several strategies to address chronic nursing shortages. The Nurse Staffing Clearinghouse connects nursing school graduates with local employers and created a statewide nursing staffing pool for in-state recruitment of travel nurses.
But it’s not just plentiful job opportunities and a low cost of living that attract nurses to the Peace Garden State. The state and its largest cities, Bismarck and Fargo, hold several “best of” accolades, including nods for the safest places to live and among the Best Places to Raise a Family, giving it high marks for quality of life.
Sure, the winters are cold, but the outdoor recreation can’t be beaten. RNs can bundle up and see the bighorn sheep in the Badlands at Theodore Roosevelt National Park or explore expansive terrain for skiing, snowboarding, and snowmobile trails.
States With the Lowest Number of Nurses Per Capita
Nevada
RNs per 1000 residents: 7.92
Average wage: $96,201 or $46.25 per hour
Average rent in Las Vegas: $1478 per month
Despite a projected 23% job growth for RNs between 2020 and 2030, the state has struggled to fill open positions. It might be the higher-than-average cost of living (9.7% higher than the US average) or higher-than-average crime rates that make RNs reluctant to gamble on a job in the Silver State. But there are some big wins for nurses in the state.
Salaries are higher than the national average, there is no state income tax, and some of the lowest property taxes in the nation. Thanks to new legislation, RNs with student loan debt won’t have to bet on black at the casino to make their payments. The Health Equity and Loan Assistance Program is a new initiative that offers up to $120,000 in loan repayment assistance to providers, including RNs, who commit to working in underserved and rural areas across the state for 5 years.
The state also has incredible attractions, from the neon lights and over-the-top architecture in Las Vegas to iconic red rock canyons, stunning state parks, and landmarks like Hoover Dam and Lake Tahoe.
Utah
RNs per 1000 residents: 7.05
Average wage: $79,790 or $38.36 per hour
Average rent in Salt Lake City: $1611 per month
Healthcare is one of the biggest employers in Utah, and nurses are the most in-demand healthcare workers in the state. But below-average wages and a cost of living that is a whopping 28% higher than the national average could be some reasons that the Beehive State is struggling to attract nurses.
A high number of job vacancies mean higher patient-to-nurse ratios, creating additional stress for a workforce prone to burnout. Much of the state is rural, public transportation is inadequate, and poor air quality causes frequent haze and smog.
The challenges are offset by some big benefits: Utah has been ranked as the “best state” thanks to the strong economy, infrastructure, and quality education — and it doesn’t hurt that Utah is home to myriad outdoor recreation opportunities and the stunning scenery at landmarks like Bryce Canyon and Arches National Park.
Moreover, Utah is hustling to boost its RN workforce. The University of Utah, Salt Lake City, has increased enrollment by 25% and hired additional faculty to help boost the nursing workforce — and those who work in hospitals and health clinics across the state benefit from a flat 4.55% individual income tax rate.
Idaho
RNs per 1000 residents: 7.02
Average wage: $80,130 or $38.53 per hour
Average rent in Boise: $1646 per month
Although the nursing workforce in Idaho has increased, it still ranks as the lowest in the nation. Teresa Stanfill, DNP, RN, executive director for the Idaho Center for Nursing, said that the number of new nurses is too low to replace the number of retiring nurses.
The state introduced loan repayment programs that award up to $25,000 to cover student loan debt, and hospitals and health systems often offer sign-on bonuses and relocation packages to attract RNs. But long winters, an isolated location, and limited cultural options can make it harder to attract nurses to the state.
It’s easier to recruit RNs to suburban areas like Boise, Meridian, and Nampa, but rural parts of the state struggle, Stanfill added. The nursing shortage is among the reasons that 11 hospitals and emergency departments closed in 2024, and healthcare organizations slashed services across the state.
Idaho has a lot to offer RNs, from small-town charm, reasonable cost of living, and gorgeous landscapes that make it one of the top 10 fastest-growing states in the nation. Collaboration between industry leaders and nursing programs is focused on finding creative solutions to boost the nursing workforce in Idaho.
A version of this article first appeared on Medscape.com.
During their 12-hour shifts, registered nurses (RNs) in Arizona and Arkansas perform many of the same tasks as RNs in Wisconsin and Wyoming: Assessing patients, monitoring vital signs, administering medications, and charting records to provide the best patient care. The work might be similar, but there are vast differences in the number of RNs in each state.
In states like Idaho, Utah, and Nevada, which have the lowest number of nurses per capita, there are as few as 7 nurses per 1000 residents, compared with South Dakota and the District of Columbia, which have double the number of nurses than underserved states — giving them the highest number of nurses per capita.
Even states with the largest number of nurses per capita are not immune to the nursing shortage. The National Bureau of Labor Statistics estimates that there will be 195,400 job openings for RNs from 2021 to 2031.
So, what makes it easier for some states to recruit and retain RNs than others?
States With the Highest Number of Nurses Per Capita
South Dakota
RNs per 1000 residents: 15.79
Average wage: $67,030 or $32.23 per hour
Average rent in Sioux Falls: $1192 per month
The Midwestern state has more miles of shoreline than Florida, herds of wild buffalo, the highest summit east of the Rockies, and more nurses per capita than all other states . Healthcare is one of the major industries in the Mount Rushmore State.
Haifa Abou Samra, dean and professor at the University of South Dakota School of Health Sciences, Vermillion, isn’t surprised that RNs want to call the state home.
“South Dakota is a nice place to live,” Samra said. “[The] schools are wonderful. If people are growing families, there is support; neighbors support their neighbors, and it’s a relatively safe community.”
South Dakota has 19 approved nursing education programs that graduated 878 RNs in 2022. Scholarships and student loan forgiveness programs have helped attract qualified RNs, and collaborations between education and industry have been instrumental in addressing the nursing shortage, Samra told this news organization.
Even though RNs earn less than the median wage ($87,070 per year/41.38 per hour), South Dakota has a low cost of living and no individual income tax, which helps stretch those earnings.
District of Columbia
RNs per 1000 residents: 15.39
Average wage: $105,220 or $50.59 per hour
Average rent in Washington, DC: $2485 per month
After a shift at some of the top-ranking hospitals in the nation, RNs working in the compact capital region can explore museums, monuments, and cultural sites; walk along the banks of the Potomac River; or grab a bite at award-winning restaurants.
Washington, a top-ranking metro area because of its growth, high wages, and access to economic opportunities, is also home to several top-tier hospitals and some of the best healthcare in the nation, and RNs who want to pursue continuing education have access to top-tier universities.
Nurses in Washington, DC, might make some of the highest wages in the nation, but the region also has the second-highest cost of living in the United States, with average rents topping $2400 per month and an average home price of $594,337.
North Dakota
RNs per 1000 residents: 12.99
Average wage: $74,930 or $36.03 per hour
Average rent in Fargo: $1051 per month
North Dakota projects a 10.4% increase in employment for RNs, which is higher than the national average, and the state has implemented several strategies to address chronic nursing shortages. The Nurse Staffing Clearinghouse connects nursing school graduates with local employers and created a statewide nursing staffing pool for in-state recruitment of travel nurses.
But it’s not just plentiful job opportunities and a low cost of living that attract nurses to the Peace Garden State. The state and its largest cities, Bismarck and Fargo, hold several “best of” accolades, including nods for the safest places to live and among the Best Places to Raise a Family, giving it high marks for quality of life.
Sure, the winters are cold, but the outdoor recreation can’t be beaten. RNs can bundle up and see the bighorn sheep in the Badlands at Theodore Roosevelt National Park or explore expansive terrain for skiing, snowboarding, and snowmobile trails.
States With the Lowest Number of Nurses Per Capita
Nevada
RNs per 1000 residents: 7.92
Average wage: $96,201 or $46.25 per hour
Average rent in Las Vegas: $1478 per month
Despite a projected 23% job growth for RNs between 2020 and 2030, the state has struggled to fill open positions. It might be the higher-than-average cost of living (9.7% higher than the US average) or higher-than-average crime rates that make RNs reluctant to gamble on a job in the Silver State. But there are some big wins for nurses in the state.
Salaries are higher than the national average, there is no state income tax, and some of the lowest property taxes in the nation. Thanks to new legislation, RNs with student loan debt won’t have to bet on black at the casino to make their payments. The Health Equity and Loan Assistance Program is a new initiative that offers up to $120,000 in loan repayment assistance to providers, including RNs, who commit to working in underserved and rural areas across the state for 5 years.
The state also has incredible attractions, from the neon lights and over-the-top architecture in Las Vegas to iconic red rock canyons, stunning state parks, and landmarks like Hoover Dam and Lake Tahoe.
Utah
RNs per 1000 residents: 7.05
Average wage: $79,790 or $38.36 per hour
Average rent in Salt Lake City: $1611 per month
Healthcare is one of the biggest employers in Utah, and nurses are the most in-demand healthcare workers in the state. But below-average wages and a cost of living that is a whopping 28% higher than the national average could be some reasons that the Beehive State is struggling to attract nurses.
A high number of job vacancies mean higher patient-to-nurse ratios, creating additional stress for a workforce prone to burnout. Much of the state is rural, public transportation is inadequate, and poor air quality causes frequent haze and smog.
The challenges are offset by some big benefits: Utah has been ranked as the “best state” thanks to the strong economy, infrastructure, and quality education — and it doesn’t hurt that Utah is home to myriad outdoor recreation opportunities and the stunning scenery at landmarks like Bryce Canyon and Arches National Park.
Moreover, Utah is hustling to boost its RN workforce. The University of Utah, Salt Lake City, has increased enrollment by 25% and hired additional faculty to help boost the nursing workforce — and those who work in hospitals and health clinics across the state benefit from a flat 4.55% individual income tax rate.
Idaho
RNs per 1000 residents: 7.02
Average wage: $80,130 or $38.53 per hour
Average rent in Boise: $1646 per month
Although the nursing workforce in Idaho has increased, it still ranks as the lowest in the nation. Teresa Stanfill, DNP, RN, executive director for the Idaho Center for Nursing, said that the number of new nurses is too low to replace the number of retiring nurses.
The state introduced loan repayment programs that award up to $25,000 to cover student loan debt, and hospitals and health systems often offer sign-on bonuses and relocation packages to attract RNs. But long winters, an isolated location, and limited cultural options can make it harder to attract nurses to the state.
It’s easier to recruit RNs to suburban areas like Boise, Meridian, and Nampa, but rural parts of the state struggle, Stanfill added. The nursing shortage is among the reasons that 11 hospitals and emergency departments closed in 2024, and healthcare organizations slashed services across the state.
Idaho has a lot to offer RNs, from small-town charm, reasonable cost of living, and gorgeous landscapes that make it one of the top 10 fastest-growing states in the nation. Collaboration between industry leaders and nursing programs is focused on finding creative solutions to boost the nursing workforce in Idaho.
A version of this article first appeared on Medscape.com.
During their 12-hour shifts, registered nurses (RNs) in Arizona and Arkansas perform many of the same tasks as RNs in Wisconsin and Wyoming: Assessing patients, monitoring vital signs, administering medications, and charting records to provide the best patient care. The work might be similar, but there are vast differences in the number of RNs in each state.
In states like Idaho, Utah, and Nevada, which have the lowest number of nurses per capita, there are as few as 7 nurses per 1000 residents, compared with South Dakota and the District of Columbia, which have double the number of nurses than underserved states — giving them the highest number of nurses per capita.
Even states with the largest number of nurses per capita are not immune to the nursing shortage. The National Bureau of Labor Statistics estimates that there will be 195,400 job openings for RNs from 2021 to 2031.
So, what makes it easier for some states to recruit and retain RNs than others?
States With the Highest Number of Nurses Per Capita
South Dakota
RNs per 1000 residents: 15.79
Average wage: $67,030 or $32.23 per hour
Average rent in Sioux Falls: $1192 per month
The Midwestern state has more miles of shoreline than Florida, herds of wild buffalo, the highest summit east of the Rockies, and more nurses per capita than all other states . Healthcare is one of the major industries in the Mount Rushmore State.
Haifa Abou Samra, dean and professor at the University of South Dakota School of Health Sciences, Vermillion, isn’t surprised that RNs want to call the state home.
“South Dakota is a nice place to live,” Samra said. “[The] schools are wonderful. If people are growing families, there is support; neighbors support their neighbors, and it’s a relatively safe community.”
South Dakota has 19 approved nursing education programs that graduated 878 RNs in 2022. Scholarships and student loan forgiveness programs have helped attract qualified RNs, and collaborations between education and industry have been instrumental in addressing the nursing shortage, Samra told this news organization.
Even though RNs earn less than the median wage ($87,070 per year/41.38 per hour), South Dakota has a low cost of living and no individual income tax, which helps stretch those earnings.
District of Columbia
RNs per 1000 residents: 15.39
Average wage: $105,220 or $50.59 per hour
Average rent in Washington, DC: $2485 per month
After a shift at some of the top-ranking hospitals in the nation, RNs working in the compact capital region can explore museums, monuments, and cultural sites; walk along the banks of the Potomac River; or grab a bite at award-winning restaurants.
Washington, a top-ranking metro area because of its growth, high wages, and access to economic opportunities, is also home to several top-tier hospitals and some of the best healthcare in the nation, and RNs who want to pursue continuing education have access to top-tier universities.
Nurses in Washington, DC, might make some of the highest wages in the nation, but the region also has the second-highest cost of living in the United States, with average rents topping $2400 per month and an average home price of $594,337.
North Dakota
RNs per 1000 residents: 12.99
Average wage: $74,930 or $36.03 per hour
Average rent in Fargo: $1051 per month
North Dakota projects a 10.4% increase in employment for RNs, which is higher than the national average, and the state has implemented several strategies to address chronic nursing shortages. The Nurse Staffing Clearinghouse connects nursing school graduates with local employers and created a statewide nursing staffing pool for in-state recruitment of travel nurses.
But it’s not just plentiful job opportunities and a low cost of living that attract nurses to the Peace Garden State. The state and its largest cities, Bismarck and Fargo, hold several “best of” accolades, including nods for the safest places to live and among the Best Places to Raise a Family, giving it high marks for quality of life.
Sure, the winters are cold, but the outdoor recreation can’t be beaten. RNs can bundle up and see the bighorn sheep in the Badlands at Theodore Roosevelt National Park or explore expansive terrain for skiing, snowboarding, and snowmobile trails.
States With the Lowest Number of Nurses Per Capita
Nevada
RNs per 1000 residents: 7.92
Average wage: $96,201 or $46.25 per hour
Average rent in Las Vegas: $1478 per month
Despite a projected 23% job growth for RNs between 2020 and 2030, the state has struggled to fill open positions. It might be the higher-than-average cost of living (9.7% higher than the US average) or higher-than-average crime rates that make RNs reluctant to gamble on a job in the Silver State. But there are some big wins for nurses in the state.
Salaries are higher than the national average, there is no state income tax, and some of the lowest property taxes in the nation. Thanks to new legislation, RNs with student loan debt won’t have to bet on black at the casino to make their payments. The Health Equity and Loan Assistance Program is a new initiative that offers up to $120,000 in loan repayment assistance to providers, including RNs, who commit to working in underserved and rural areas across the state for 5 years.
The state also has incredible attractions, from the neon lights and over-the-top architecture in Las Vegas to iconic red rock canyons, stunning state parks, and landmarks like Hoover Dam and Lake Tahoe.
Utah
RNs per 1000 residents: 7.05
Average wage: $79,790 or $38.36 per hour
Average rent in Salt Lake City: $1611 per month
Healthcare is one of the biggest employers in Utah, and nurses are the most in-demand healthcare workers in the state. But below-average wages and a cost of living that is a whopping 28% higher than the national average could be some reasons that the Beehive State is struggling to attract nurses.
A high number of job vacancies mean higher patient-to-nurse ratios, creating additional stress for a workforce prone to burnout. Much of the state is rural, public transportation is inadequate, and poor air quality causes frequent haze and smog.
The challenges are offset by some big benefits: Utah has been ranked as the “best state” thanks to the strong economy, infrastructure, and quality education — and it doesn’t hurt that Utah is home to myriad outdoor recreation opportunities and the stunning scenery at landmarks like Bryce Canyon and Arches National Park.
Moreover, Utah is hustling to boost its RN workforce. The University of Utah, Salt Lake City, has increased enrollment by 25% and hired additional faculty to help boost the nursing workforce — and those who work in hospitals and health clinics across the state benefit from a flat 4.55% individual income tax rate.
Idaho
RNs per 1000 residents: 7.02
Average wage: $80,130 or $38.53 per hour
Average rent in Boise: $1646 per month
Although the nursing workforce in Idaho has increased, it still ranks as the lowest in the nation. Teresa Stanfill, DNP, RN, executive director for the Idaho Center for Nursing, said that the number of new nurses is too low to replace the number of retiring nurses.
The state introduced loan repayment programs that award up to $25,000 to cover student loan debt, and hospitals and health systems often offer sign-on bonuses and relocation packages to attract RNs. But long winters, an isolated location, and limited cultural options can make it harder to attract nurses to the state.
It’s easier to recruit RNs to suburban areas like Boise, Meridian, and Nampa, but rural parts of the state struggle, Stanfill added. The nursing shortage is among the reasons that 11 hospitals and emergency departments closed in 2024, and healthcare organizations slashed services across the state.
Idaho has a lot to offer RNs, from small-town charm, reasonable cost of living, and gorgeous landscapes that make it one of the top 10 fastest-growing states in the nation. Collaboration between industry leaders and nursing programs is focused on finding creative solutions to boost the nursing workforce in Idaho.
A version of this article first appeared on Medscape.com.
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.
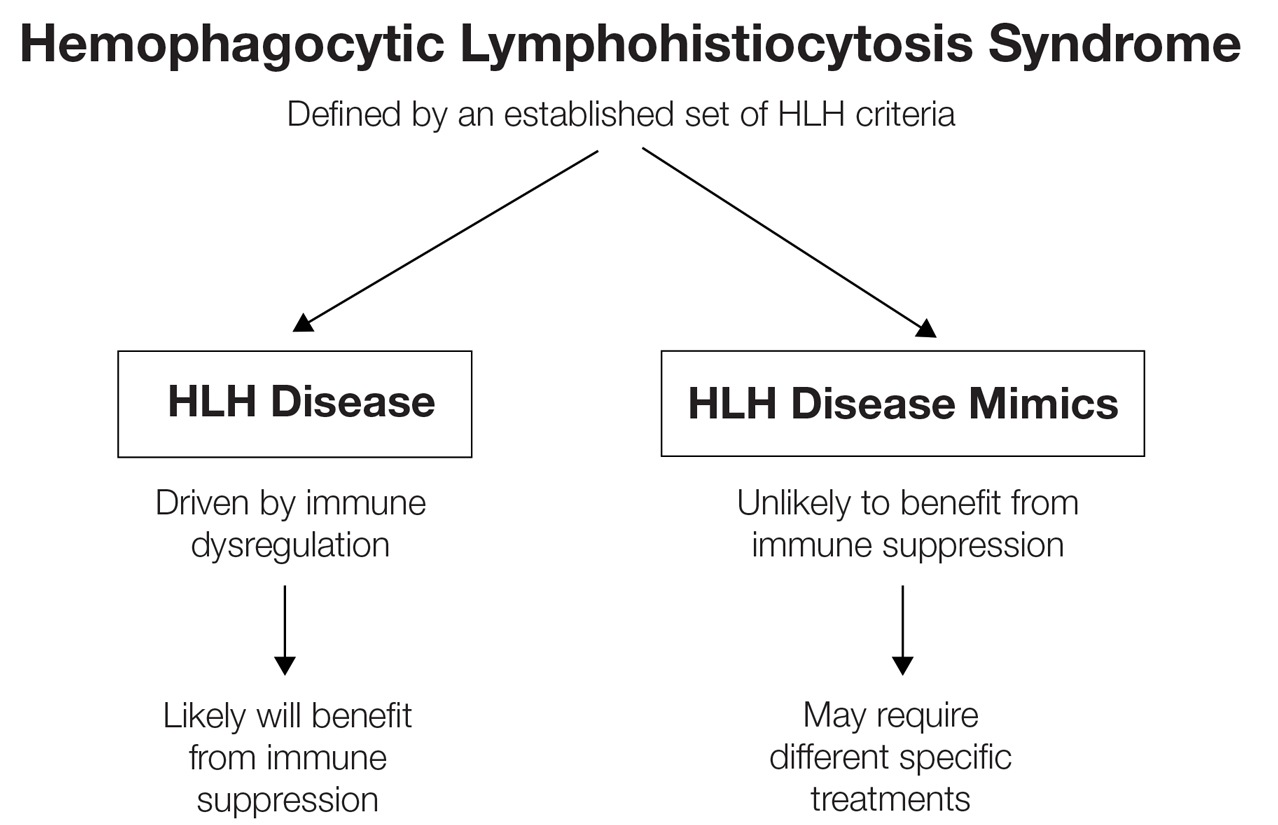
Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.
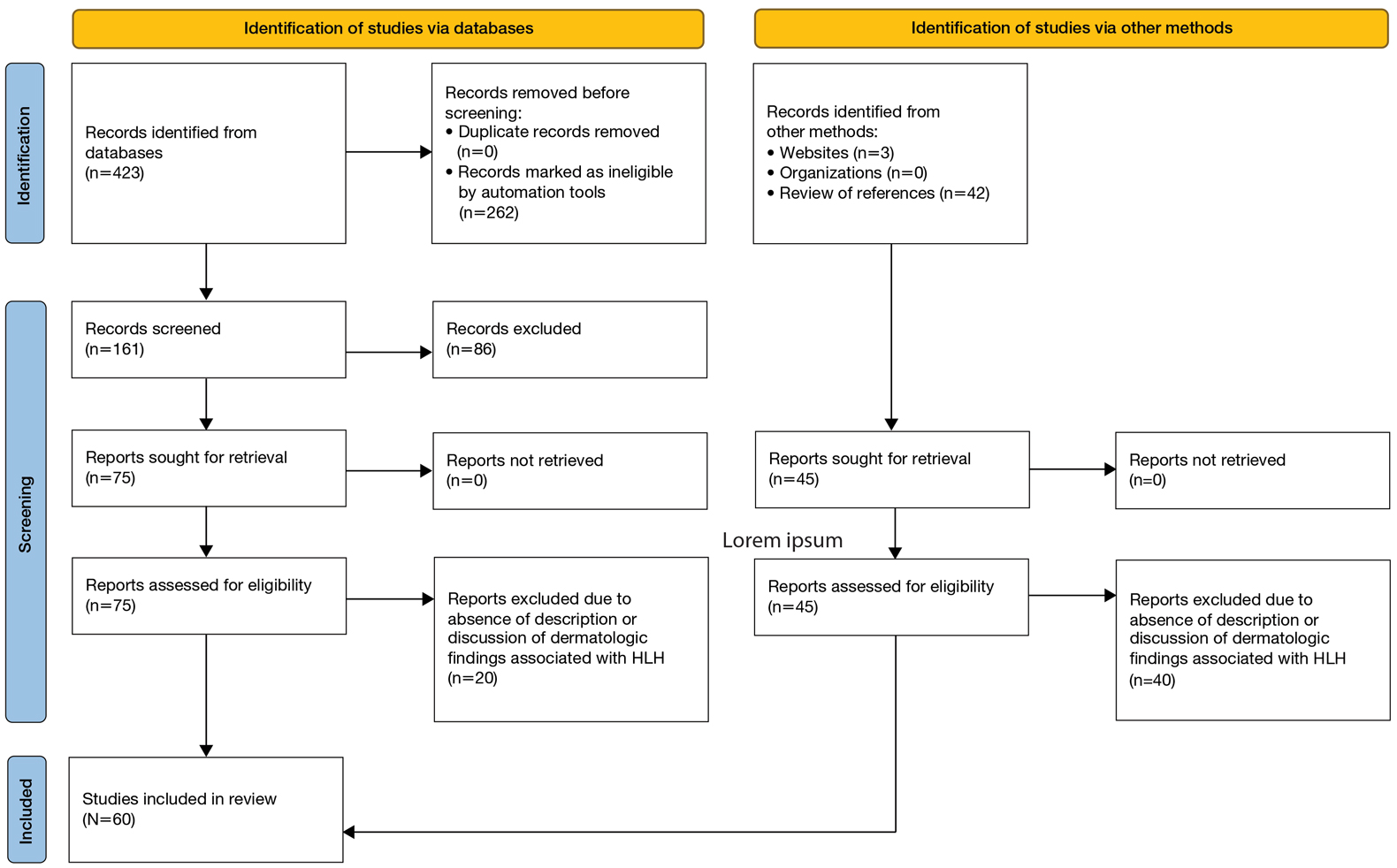
Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.
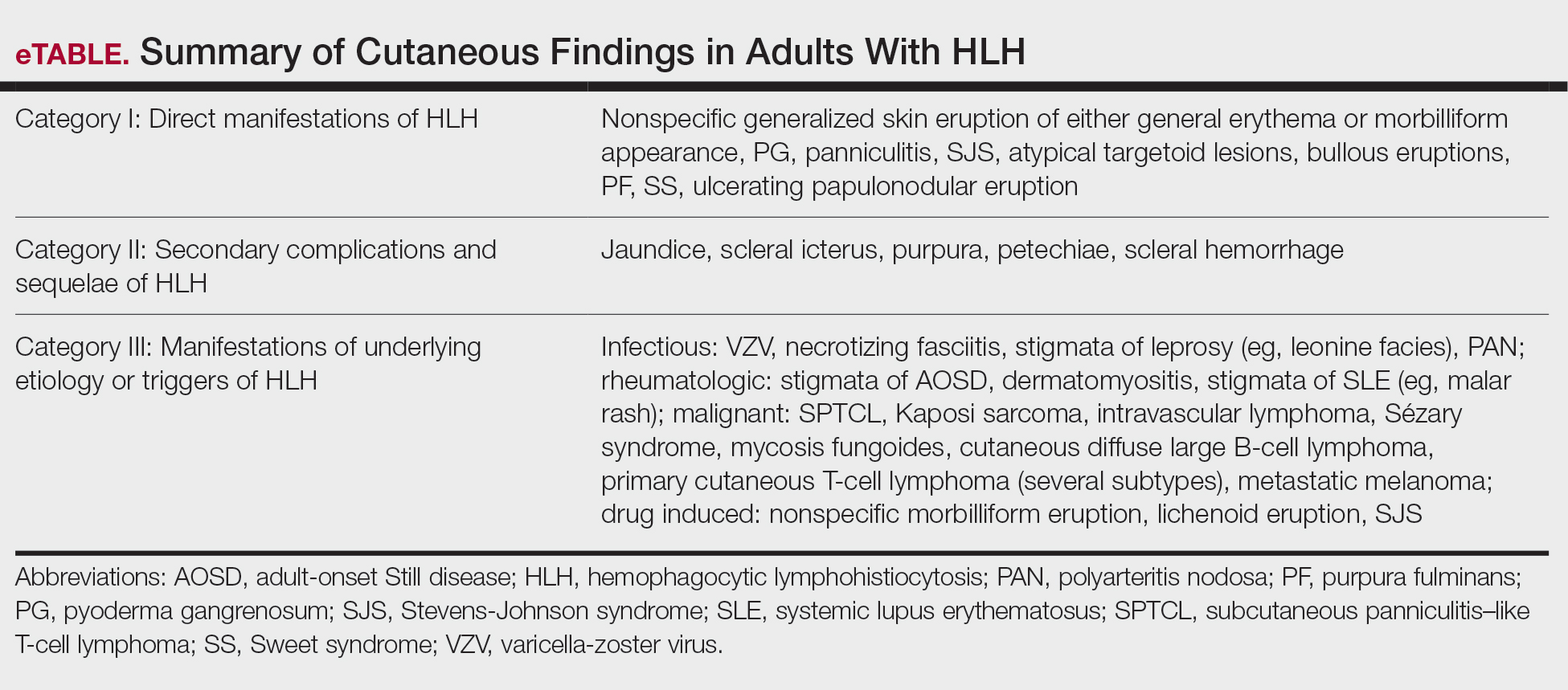
Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.

Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.

Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.

Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.

Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.

Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.

Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
PRACTICE POINTS
- Hemophagocytic lymphohistiocytosis (HLH) is a complex, life-threatening immunologic condition that is associated with various diagnostic tools.
- Physicians who care for patients with HLH should know that skin findings are not uncommon but are largely nonspecific and can be a direct result of HLH itself, systemic complications, or the underlying etiology of the condition.
- There is a myriad of cutaneous findings that can manifest in adult patients with HLH. Awareness of HLH-associated dermatologic conditions and available diagnostic tools among multidisciplinary teams will aid in diagnosis.
A Veteran Presenting With Symptomatic Postprandial Episodes
A Veteran Presenting With Symptomatic Postprandial Episodes
Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.
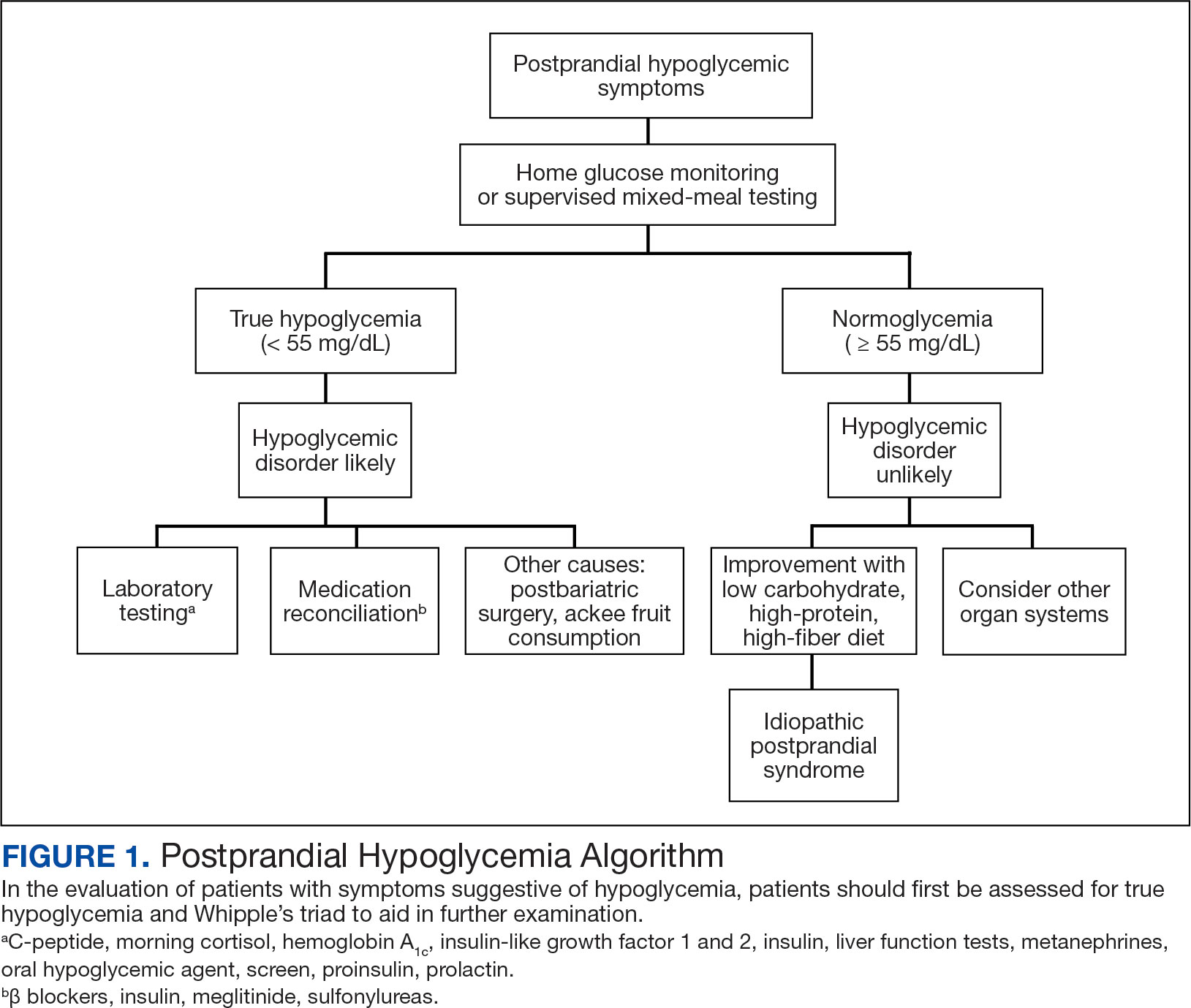
The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).
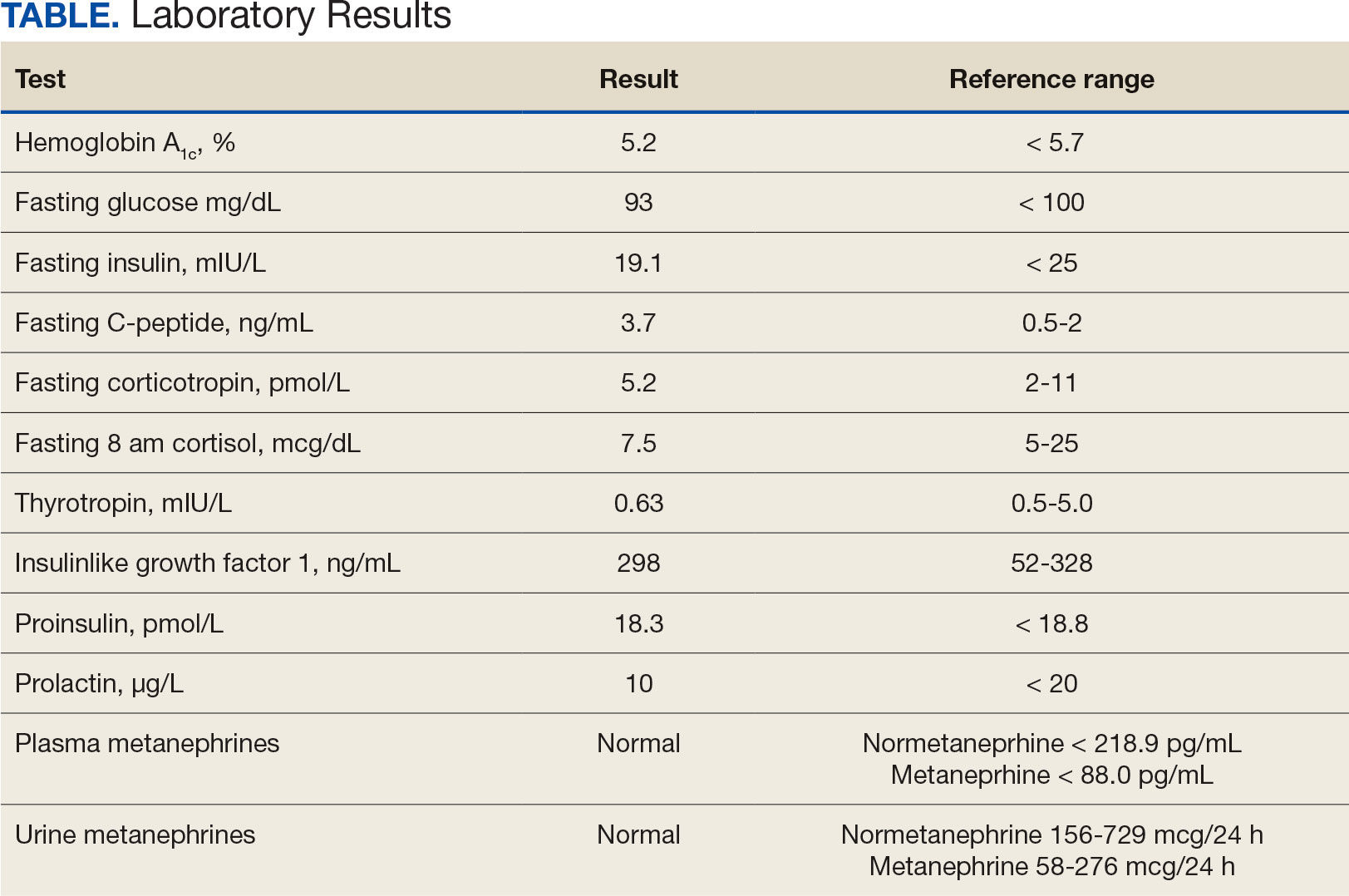
The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1
The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.
Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.

The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).

The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1

The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.

Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.

The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).

The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1

The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.

Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
A Veteran Presenting With Symptomatic Postprandial Episodes
A Veteran Presenting With Symptomatic Postprandial Episodes
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2
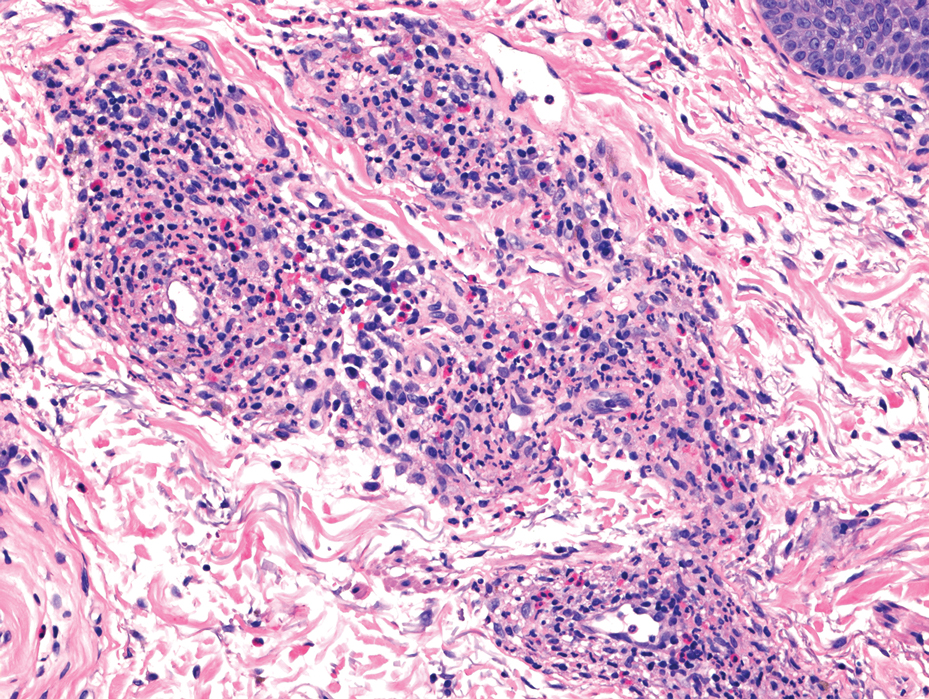
Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2



Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2



Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
A 44-year-old man presented to the dermatology clinic with a facial rash of 2 years’ duration. The patient reported associated pruritus but no systemic symptoms. His medical history was relevant for childhood eczema. He had tried various over-the-counter treatments for the facial rash, including topical hydrocortisone, neomycin/bacitracin/polymyxin antibiotic ointment, moisturizers, and antihistamines, with no success. Physical examination demonstrated symmetric, well-circumscribed, circinate, brownish-red, indurated plaques without scaling on the cheeks. A 4-mm punch biopsy was obtained from a plaque on the left cheek.
Finding and Following Your Passion
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
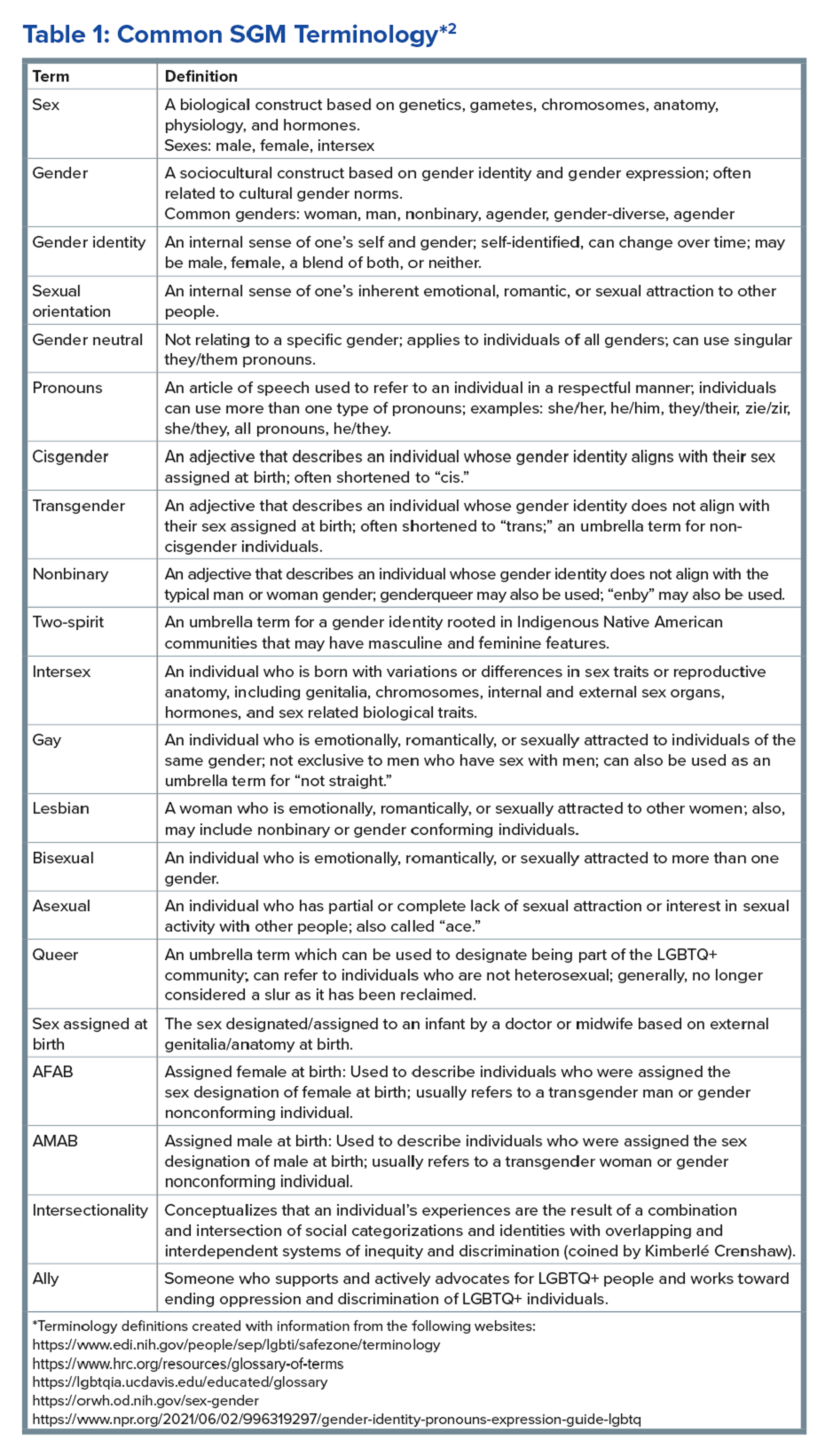
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
The Value of Public Service
Former Secretary of State Condoleezza Rice once said: “There is no greater challenge and there is no greater honor than to be in public service.” It has been a challenging few months for public servants, including the thousands of federal healthcare and public health workers who care for our veterans, provide critical services to underserved communities, work to fund high-impact biomedical research that improves health outcomes, and otherwise further important public health goals.
From the VA to the Department of Health & Human Services and its operating divisions, including the Centers for Disease Control and Prevention, National Institutes of Health, Centers for Medicare & Medicaid Services, and others, dedicated federal civil servants have had their work ethic, commitment, and productivity questioned in late-night emails from anonymous authors. They have been encouraged indiscriminately to resign and “move from [their] lower-productivity jobs in the public sector to higher-productivity jobs in the private sector,” and been subjected to vague threats of future job loss regardless of role, duration of service, performance, or political persuasion. This includes the roughly 30% of federal employees who are themselves US military veterans.
In essence, the message is that their work does not matter, and their service and sacrifice is not valued (which, of course, could not be further from the truth). These actions, along with a plethora of other divisive policies, not only threaten our democratic principles, but also serve to degrade our collective values and norms. We are at a “fork in the road” as a nation. I hope for the greater good that we can work together to uphold the value of public service, of community, of civility — both for the sake of our democracy and to preserve our nation’s health.
In our March issue, This month’s Member Spotlight features Dr. Pooja Singhal (Oklahoma Gastro Health and Wellness), who describes how she integrates wellness principles into her clinical practice, discusses the evolution of her interest in women’s digestive health, and shares how she serves her community outside of medicine.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Former Secretary of State Condoleezza Rice once said: “There is no greater challenge and there is no greater honor than to be in public service.” It has been a challenging few months for public servants, including the thousands of federal healthcare and public health workers who care for our veterans, provide critical services to underserved communities, work to fund high-impact biomedical research that improves health outcomes, and otherwise further important public health goals.
From the VA to the Department of Health & Human Services and its operating divisions, including the Centers for Disease Control and Prevention, National Institutes of Health, Centers for Medicare & Medicaid Services, and others, dedicated federal civil servants have had their work ethic, commitment, and productivity questioned in late-night emails from anonymous authors. They have been encouraged indiscriminately to resign and “move from [their] lower-productivity jobs in the public sector to higher-productivity jobs in the private sector,” and been subjected to vague threats of future job loss regardless of role, duration of service, performance, or political persuasion. This includes the roughly 30% of federal employees who are themselves US military veterans.
In essence, the message is that their work does not matter, and their service and sacrifice is not valued (which, of course, could not be further from the truth). These actions, along with a plethora of other divisive policies, not only threaten our democratic principles, but also serve to degrade our collective values and norms. We are at a “fork in the road” as a nation. I hope for the greater good that we can work together to uphold the value of public service, of community, of civility — both for the sake of our democracy and to preserve our nation’s health.
In our March issue, This month’s Member Spotlight features Dr. Pooja Singhal (Oklahoma Gastro Health and Wellness), who describes how she integrates wellness principles into her clinical practice, discusses the evolution of her interest in women’s digestive health, and shares how she serves her community outside of medicine.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Former Secretary of State Condoleezza Rice once said: “There is no greater challenge and there is no greater honor than to be in public service.” It has been a challenging few months for public servants, including the thousands of federal healthcare and public health workers who care for our veterans, provide critical services to underserved communities, work to fund high-impact biomedical research that improves health outcomes, and otherwise further important public health goals.
From the VA to the Department of Health & Human Services and its operating divisions, including the Centers for Disease Control and Prevention, National Institutes of Health, Centers for Medicare & Medicaid Services, and others, dedicated federal civil servants have had their work ethic, commitment, and productivity questioned in late-night emails from anonymous authors. They have been encouraged indiscriminately to resign and “move from [their] lower-productivity jobs in the public sector to higher-productivity jobs in the private sector,” and been subjected to vague threats of future job loss regardless of role, duration of service, performance, or political persuasion. This includes the roughly 30% of federal employees who are themselves US military veterans.
In essence, the message is that their work does not matter, and their service and sacrifice is not valued (which, of course, could not be further from the truth). These actions, along with a plethora of other divisive policies, not only threaten our democratic principles, but also serve to degrade our collective values and norms. We are at a “fork in the road” as a nation. I hope for the greater good that we can work together to uphold the value of public service, of community, of civility — both for the sake of our democracy and to preserve our nation’s health.
In our March issue, This month’s Member Spotlight features Dr. Pooja Singhal (Oklahoma Gastro Health and Wellness), who describes how she integrates wellness principles into her clinical practice, discusses the evolution of her interest in women’s digestive health, and shares how she serves her community outside of medicine.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Walter Reed National Military Medical Center Recovering After Flood
A burst sprinkler pipe and broken steam system caused significant infrastructure failures and wreaked havoc on patient care at Walter Reed National Military Medical Center in January.
An email sent to Walter Reed staff from the medical center’s director, Navy Capt. Melissa C. Austin, said 60,000 gallons of water, or enough “to fill a 25x50 foot swimming pool” flooded throughout the facility on Jan. 20 before it was contained, damaging 50 rooms and 6 elevators.
Frozen pipes burst due to extreme cold, and the issues were exacerbated by aging infrastructure and “deferred maintenance due to underfunding,” the Defense Health Agency (DHA), which oversees Walter Reed, said in a public statement.
The damage was severe enough to impact patient care. The facility had to evacuate the neonatal intensive care unit as well as several clinics. The steam system outages also meant operating rooms had fewer clean surgical tools available and had to send them to regional hospitals for sterilization, staffers told The Washington Post. Health care workers could not “flash sterilize” equipment in emergencies, further risking patient safety.
Rick McNamara, a spokesperson for the Defense Health Network National Capital Region, confirmed other hospitals are “sharing the burden” to sterilize equipment. McNamara said it could take 6 weeks to complete the immediate repairs, which will cost between $1 million and $2 million.
Patient appointments were delayed, and nonemergency procedures were canceled or delayed. Overall, 212 patients were “deferred or rescheduled,” and 56 other patients were sent to other hospitals to receive care.
Defense Secretary Pete Hegseth said on Jan. 31 the problem was “real and unacceptable” in response to a video circulating on social media that showed flooding.
Acknowledging that the water damage “temporarily impacted health care operations,” the Defense Department says DHA and Walter Reed staff were “working diligently around the clock” to find and implement solutions while minimizing disruptions to patient care: “High waters and loss of steam pressure impacted the capacity of services delivered, but the ability to deliver the hospital’s core capabilities of safe, quality care was never compromised,” the agency said.
In response to the flooding, the hospital moved quickly to provide the required urgent care: “We are utilizing all the hospitals and clinics in the National Capital Region Network from Malcom Grow at Joint Base Andrews to Kimbrough Ambulatory Care Center at Fort Meade to the Alexander T. Augusta Military Medical Center at Fort Belvoir,” Capt. Austin said.
DHA is also funding emergency work orders and contract modifications required to return Walter Reed to full operational capability. It is prioritizing resources for repairs and is collaborating with the Naval Installations Command and Naval Support Activity Bethesda to implement necessary repairs.
“This acute issue is being managed aggressively to ensure patient care continues to be delivered safely,” DHA said
A burst sprinkler pipe and broken steam system caused significant infrastructure failures and wreaked havoc on patient care at Walter Reed National Military Medical Center in January.
An email sent to Walter Reed staff from the medical center’s director, Navy Capt. Melissa C. Austin, said 60,000 gallons of water, or enough “to fill a 25x50 foot swimming pool” flooded throughout the facility on Jan. 20 before it was contained, damaging 50 rooms and 6 elevators.
Frozen pipes burst due to extreme cold, and the issues were exacerbated by aging infrastructure and “deferred maintenance due to underfunding,” the Defense Health Agency (DHA), which oversees Walter Reed, said in a public statement.
The damage was severe enough to impact patient care. The facility had to evacuate the neonatal intensive care unit as well as several clinics. The steam system outages also meant operating rooms had fewer clean surgical tools available and had to send them to regional hospitals for sterilization, staffers told The Washington Post. Health care workers could not “flash sterilize” equipment in emergencies, further risking patient safety.
Rick McNamara, a spokesperson for the Defense Health Network National Capital Region, confirmed other hospitals are “sharing the burden” to sterilize equipment. McNamara said it could take 6 weeks to complete the immediate repairs, which will cost between $1 million and $2 million.
Patient appointments were delayed, and nonemergency procedures were canceled or delayed. Overall, 212 patients were “deferred or rescheduled,” and 56 other patients were sent to other hospitals to receive care.
Defense Secretary Pete Hegseth said on Jan. 31 the problem was “real and unacceptable” in response to a video circulating on social media that showed flooding.
Acknowledging that the water damage “temporarily impacted health care operations,” the Defense Department says DHA and Walter Reed staff were “working diligently around the clock” to find and implement solutions while minimizing disruptions to patient care: “High waters and loss of steam pressure impacted the capacity of services delivered, but the ability to deliver the hospital’s core capabilities of safe, quality care was never compromised,” the agency said.
In response to the flooding, the hospital moved quickly to provide the required urgent care: “We are utilizing all the hospitals and clinics in the National Capital Region Network from Malcom Grow at Joint Base Andrews to Kimbrough Ambulatory Care Center at Fort Meade to the Alexander T. Augusta Military Medical Center at Fort Belvoir,” Capt. Austin said.
DHA is also funding emergency work orders and contract modifications required to return Walter Reed to full operational capability. It is prioritizing resources for repairs and is collaborating with the Naval Installations Command and Naval Support Activity Bethesda to implement necessary repairs.
“This acute issue is being managed aggressively to ensure patient care continues to be delivered safely,” DHA said
A burst sprinkler pipe and broken steam system caused significant infrastructure failures and wreaked havoc on patient care at Walter Reed National Military Medical Center in January.
An email sent to Walter Reed staff from the medical center’s director, Navy Capt. Melissa C. Austin, said 60,000 gallons of water, or enough “to fill a 25x50 foot swimming pool” flooded throughout the facility on Jan. 20 before it was contained, damaging 50 rooms and 6 elevators.
Frozen pipes burst due to extreme cold, and the issues were exacerbated by aging infrastructure and “deferred maintenance due to underfunding,” the Defense Health Agency (DHA), which oversees Walter Reed, said in a public statement.
The damage was severe enough to impact patient care. The facility had to evacuate the neonatal intensive care unit as well as several clinics. The steam system outages also meant operating rooms had fewer clean surgical tools available and had to send them to regional hospitals for sterilization, staffers told The Washington Post. Health care workers could not “flash sterilize” equipment in emergencies, further risking patient safety.
Rick McNamara, a spokesperson for the Defense Health Network National Capital Region, confirmed other hospitals are “sharing the burden” to sterilize equipment. McNamara said it could take 6 weeks to complete the immediate repairs, which will cost between $1 million and $2 million.
Patient appointments were delayed, and nonemergency procedures were canceled or delayed. Overall, 212 patients were “deferred or rescheduled,” and 56 other patients were sent to other hospitals to receive care.
Defense Secretary Pete Hegseth said on Jan. 31 the problem was “real and unacceptable” in response to a video circulating on social media that showed flooding.
Acknowledging that the water damage “temporarily impacted health care operations,” the Defense Department says DHA and Walter Reed staff were “working diligently around the clock” to find and implement solutions while minimizing disruptions to patient care: “High waters and loss of steam pressure impacted the capacity of services delivered, but the ability to deliver the hospital’s core capabilities of safe, quality care was never compromised,” the agency said.
In response to the flooding, the hospital moved quickly to provide the required urgent care: “We are utilizing all the hospitals and clinics in the National Capital Region Network from Malcom Grow at Joint Base Andrews to Kimbrough Ambulatory Care Center at Fort Meade to the Alexander T. Augusta Military Medical Center at Fort Belvoir,” Capt. Austin said.
DHA is also funding emergency work orders and contract modifications required to return Walter Reed to full operational capability. It is prioritizing resources for repairs and is collaborating with the Naval Installations Command and Naval Support Activity Bethesda to implement necessary repairs.
“This acute issue is being managed aggressively to ensure patient care continues to be delivered safely,” DHA said