User login
Combination therapy may benefit patients with migraine
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
, according to a large retrospective analysis. The results lend hope that the combination may be synergistic, according to Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad. Dr. Blumenfeld presented at the American Headache Society’s 2021 annual meeting. The study was published online April 21 in Pain Therapy.
The retrospective analysis showed a 4-day reduction in headache days per month. In contrast, in the pivotal study for erenumab, the most commonly used anti-CGRP antibody among subjects in the study, showed a 2-day benefit in a subanalysis of patients who had failed at least two oral preventives.
There is mechanistic evidence to suggest the two therapies could be synergistic. OnabotulinumtoxinA is believed to inhibit the release of CGRP, and antibodies reduce CGRP levels. OnabotulinumtoxinA prevents activation of C-fibers in the trigeminal sensory afferents, but does not affect A-delta fibers.
On the other hand, most data indicate that the anti-CGRP antibody fremanezumab prevents activation of A-delta but not C-fibers, and a recent review argues that CGRP antibody nonresponders may have migraines driven by C-fibers or other pathways. “Thus, concomitant use of medications blocking the activation of meningeal C-fibers may provide a synergistic effect on the trigeminal nociceptive pathway,” the authors wrote.
Study finding match clinical practice
The results of the new study strengthen the case that the combination is effective, though proof would require prospective, randomized trials. “I think that it really gives credibility to what we are seeing in practice, which is that combined therapy often is much better than therapy with onabotulinumtoxinA alone, said Deborah Friedman, MD, MPH, who was asked to comment on the findings. Dr. Friedman is professor of neurology and ophthalmology at the University of Texas, Dallas.
The extra 4 migraine-free days per month is a significant benefit, said Stewart Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H. “It’s an extra month and a half of no disability per year, and that’s on top of what onabotulinumtoxinA does. So it’s really a very important clinical finding,” Dr. Tepper said in an interview.
Many insurance companies refuse to pay for the combination therapy, despite the fact that relatively few migraine patients would likely seek it out, according to Dr. Friedman. “It’s just kind of a shame,” she said.
Insurance companies often object that the combination therapy is experimental, despite the widespread use of combination therapies in migraine. “It’s no more experimental in my opinion than any other combination of medications that we use. For people that have severe migraine, we use combination therapy all the time,” said Dr. Friedman.
Improvements with combination therapy
The study was a chart review of 257 patients who started on onabotulinumtoxinA and later initiated anti-CGRP antibody therapy. A total of 104 completed four visits after initiation of anti-CGRP antibody therapy (completers). Before starting any therapy, patients reported an average of 21 headache days per month in the overall group, and 22 among completers. That frequency dropped to 12 in both groups after onabotulinumtoxinA therapy (overall group difference, –9 days; 95% confidence interval, –8 to –11 days; completers group difference, –10; 95% CI, –7 to –12 days).
A total of 77.8% of subjects in the overall cohort took erenumab, 16.3% took galcanezumab, and 5.8% took fremanezumab. In the completers cohort, the percentages were 84.5%, 10.7%, and 4.9%, respectively.
Compared with baseline, both completers and noncompleters had clinically significant improvements in disability, as measured by at least a 5-point improvement in Migraine Disability Assessment (MIDAS) score at the 3-month visit (–5.8 for completers and –6.3 for the overall cohort group), the 6-month visit (–6.6 and –11.1), the 9-month visit (–8.3 and –6.1), and 1 year (–12.7 and –8.4).
At the first visit, 33.0% of completers had at least a 5-point reduction in MIDAS, as did 36.0% of the overall cohort group, and the trend continued at 6 months (39.8% and 45.1%), 9 months (43.7% and 43.7%), and at 1 year (45.3% and 44.8%).
The study was funded by Allergan. Dr. Blumenfeld has served on advisory boards for Aeon, AbbVie, Amgen, Alder, Biohaven, Teva, Supernus, Promius, Eaglet, and Lilly, and has received funding for speaking from AbbVie, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, Eli Lilly, Lundbeck, Novartis, and Theranica. Dr. Tepper has consulted for Teva. Dr. Friedman has been on the advisory board for Allergan, Amgen, Lundbeck, Eli Lilly, and Teva Pharmaceuticals, and has received grant support from Allergan and Eli Lilly.
FROM AHS 2021
Almost half of patients with migraine are reluctant to seek care
, new research shows. A survey of nearly 18,000 participants with migraine showed that 46% were reluctant to consult a physician about their condition. Among those who hesitated, 58% ultimately consulted a physician, but 42% did not.
Common reasons for failure to seek treatment included believing that migraine was not severe enough to warrant a consultation, worries about cost and health insurance, and concern that the health care professional would not take the disorder seriously.
This is the first study to query patients with migraine regarding whether and why they have hesitated to seek care, said coinvestigator Robert E. Shapiro, MD, PhD, professor of neurologic sciences and director of the division of headache medicine at the University of Vermont, Burlington. “Previous studies have noted differences in care seeking by demographic or other distinguishing characteristics but have not asked people with migraine whether they actually intended to seek or not seek such care,” he said.
Dr. Shapiro presented the findings at the American Headache Society’s 2021 annual meeting.
Delays prevent diagnosis and care
For patients with migraine, hesitating to consult a physician causes delays in, and sometimes prevents, receiving a diagnosis and appropriate care.
To assess the proportion of patients who hesitate to seek a consultation for migraine care, as well as reasons for doing so, the investigators examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study. OVERCOME incorporated a prospective web-based survey that was administered to a representative sample of 41,925 individuals in the United States.
Eligible participants who completed the study’s baseline assessment had had at least one migraine attack in the previous year and either met criteria for migraine on the basis of a validated diagnostic screen or provided a self-report of a migraine diagnosis by a health care practitioner. In all, 39,494 participants reported whether they had hesitated to seek a consultation from a physician for migraine care. Of these, 17,951 were included in the analysis.
Among the 46% who hesitated to seek care, 58% ultimately sought migraine care, and 42% did not.
The investigators also examined sociodemographic characteristics and migraine-related data, including the number of monthly headache days and information regarding nausea, photophobia, and phonophobia.
Patient-reported outcomes included days with migraine-related disability during the past 3 months, treatment optimization, and the degree to which migraine limited regular activities. Investigators also examined participants’ health care use in the previous 12 months and reasons for hesitating to seek migraine care.
Reasons for hesitancy
A total of 17,920 participants provided reasons for hesitating to seek a migraine consultation. These included a desire to take care of migraine attacks on one’s own (45%), the belief that migraine would not be taken seriously (35%), the belief that the migraine attacks were not serious or painful enough (29%), inability to afford or unwillingness to spend money on care (29%), lack of or inadequate health insurance (21%), and fear of receiving a serious diagnosis (19%).
Reasons for hesitation differed between participants who ultimately sought a consultation with a physician and those who did not. Those who did not receive a consultation (n = 7,495) were more likely to want to take care of the migraine attacks on their own (48% vs. 43%) and to believe the attacks were not serious or painful enough (36% vs. 25%).
Participants who hesitated but later sought a consultation were more likely to report concerns that migraine would not be taken seriously (38% vs. 31%) and fear of receiving a serious diagnosis (22% vs. 15%).
Among those who did not seek a consultation versus those who did, a significantly higher proportion were women (76% vs. 73%; P < .001).
“This is an interesting finding, since prior studies have indicated that, overall, women with migraine are more likely to have consulted a doctor for it – and also more likely to have been diagnosed with it,” Dr. Shapiro said.
On the other hand, women were 30% more likely to visit emergency departments or urgent care clinics for migraine care than men, he noted.
“These findings suggest some women may be experiencing particular barriers to receiving successful consultation care and that they may persistently hesitate to seek it,” said Dr. Shapiro. He noted that these barriers might be financial or attitudinal.
“Women are reported to be less likely to receive treatment for pain conditions, and furthermore, stigma toward migraine in particular may limit its perceived seriousness,” he said.
‘Equitable access’ needed
Those with full-time employment were significantly more likely to seek a migraine consultation than were those who were not employed full time (46% vs. 42%; P < .001). Patients who sought care were more likely to have health insurance (87% vs. 78%; P < .001).
Having health insurance (odds ratio [OR], 1.99), having previously received a migraine diagnosis (OR, 2.71), and degree of disability (severe vs. none: OR, 2.76; moderate vs. none: OR, 2.04) were associated with increased likelihood of seeking a migraine consultation among those who initially hesitated. Other factors included being male (OR, 1.49), having nausea (OR, 1.15), or being employed full time (OR, 1.24).
“Taken together, our findings suggest consultation rates may be limited by financial barriers and pervasive attitudes that migraine is either not serious or is untreatable,” said Dr. Shapiro. Consistent with this hypothesis is the finding that individuals with migraine who had received an appropriate diagnosis and were therefore better informed about the condition were more likely to continue to seek care for it, he noted.
Because most outpatient medical encounters for migraine are with primary care practitioners, it may make sense to ensure that such clinicians are “well trained in diagnosing and treating common presentations of migraine,” Dr. Shapiro said. It is equally important to ensure “equitable access to health insurance to pay for these consultations,” he added.
‘Take migraine more seriously’
Commenting on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said the study was well designed.
Potential weaknesses include the fact that patients were required only to have one migraine attack per year and that not all were diagnosed by a headache specialist using ICHD-3 criteria.
Still, “online, validated, patient-reported data is quite acceptable,” said Dr. Rapoport, who was not involved in the research.
He noted that there is a clear message from the findings for all physicians who see patients with headache disorders: “You will increase the chance of patients consulting and continuing to consult when you make an accurate migraine diagnosis, take migraine more seriously, and understand the stigmas attached to it – and when there are reduced institutional barriers and costs of health care.”
The findings suggest that neurologists should strive to provide patients with ongoing care and medication, he added. In addition, there is a need for further education about the stigma associated with migraine and about how others view this disabling disease, Dr. Rapoport concluded.
The study was funded by Eli Lilly. Dr. Shapiro has consulted for Eli Lilly and Lundbeck. Dr. Rapoport has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. A survey of nearly 18,000 participants with migraine showed that 46% were reluctant to consult a physician about their condition. Among those who hesitated, 58% ultimately consulted a physician, but 42% did not.
Common reasons for failure to seek treatment included believing that migraine was not severe enough to warrant a consultation, worries about cost and health insurance, and concern that the health care professional would not take the disorder seriously.
This is the first study to query patients with migraine regarding whether and why they have hesitated to seek care, said coinvestigator Robert E. Shapiro, MD, PhD, professor of neurologic sciences and director of the division of headache medicine at the University of Vermont, Burlington. “Previous studies have noted differences in care seeking by demographic or other distinguishing characteristics but have not asked people with migraine whether they actually intended to seek or not seek such care,” he said.
Dr. Shapiro presented the findings at the American Headache Society’s 2021 annual meeting.
Delays prevent diagnosis and care
For patients with migraine, hesitating to consult a physician causes delays in, and sometimes prevents, receiving a diagnosis and appropriate care.
To assess the proportion of patients who hesitate to seek a consultation for migraine care, as well as reasons for doing so, the investigators examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study. OVERCOME incorporated a prospective web-based survey that was administered to a representative sample of 41,925 individuals in the United States.
Eligible participants who completed the study’s baseline assessment had had at least one migraine attack in the previous year and either met criteria for migraine on the basis of a validated diagnostic screen or provided a self-report of a migraine diagnosis by a health care practitioner. In all, 39,494 participants reported whether they had hesitated to seek a consultation from a physician for migraine care. Of these, 17,951 were included in the analysis.
Among the 46% who hesitated to seek care, 58% ultimately sought migraine care, and 42% did not.
The investigators also examined sociodemographic characteristics and migraine-related data, including the number of monthly headache days and information regarding nausea, photophobia, and phonophobia.
Patient-reported outcomes included days with migraine-related disability during the past 3 months, treatment optimization, and the degree to which migraine limited regular activities. Investigators also examined participants’ health care use in the previous 12 months and reasons for hesitating to seek migraine care.
Reasons for hesitancy
A total of 17,920 participants provided reasons for hesitating to seek a migraine consultation. These included a desire to take care of migraine attacks on one’s own (45%), the belief that migraine would not be taken seriously (35%), the belief that the migraine attacks were not serious or painful enough (29%), inability to afford or unwillingness to spend money on care (29%), lack of or inadequate health insurance (21%), and fear of receiving a serious diagnosis (19%).
Reasons for hesitation differed between participants who ultimately sought a consultation with a physician and those who did not. Those who did not receive a consultation (n = 7,495) were more likely to want to take care of the migraine attacks on their own (48% vs. 43%) and to believe the attacks were not serious or painful enough (36% vs. 25%).
Participants who hesitated but later sought a consultation were more likely to report concerns that migraine would not be taken seriously (38% vs. 31%) and fear of receiving a serious diagnosis (22% vs. 15%).
Among those who did not seek a consultation versus those who did, a significantly higher proportion were women (76% vs. 73%; P < .001).
“This is an interesting finding, since prior studies have indicated that, overall, women with migraine are more likely to have consulted a doctor for it – and also more likely to have been diagnosed with it,” Dr. Shapiro said.
On the other hand, women were 30% more likely to visit emergency departments or urgent care clinics for migraine care than men, he noted.
“These findings suggest some women may be experiencing particular barriers to receiving successful consultation care and that they may persistently hesitate to seek it,” said Dr. Shapiro. He noted that these barriers might be financial or attitudinal.
“Women are reported to be less likely to receive treatment for pain conditions, and furthermore, stigma toward migraine in particular may limit its perceived seriousness,” he said.
‘Equitable access’ needed
Those with full-time employment were significantly more likely to seek a migraine consultation than were those who were not employed full time (46% vs. 42%; P < .001). Patients who sought care were more likely to have health insurance (87% vs. 78%; P < .001).
Having health insurance (odds ratio [OR], 1.99), having previously received a migraine diagnosis (OR, 2.71), and degree of disability (severe vs. none: OR, 2.76; moderate vs. none: OR, 2.04) were associated with increased likelihood of seeking a migraine consultation among those who initially hesitated. Other factors included being male (OR, 1.49), having nausea (OR, 1.15), or being employed full time (OR, 1.24).
“Taken together, our findings suggest consultation rates may be limited by financial barriers and pervasive attitudes that migraine is either not serious or is untreatable,” said Dr. Shapiro. Consistent with this hypothesis is the finding that individuals with migraine who had received an appropriate diagnosis and were therefore better informed about the condition were more likely to continue to seek care for it, he noted.
Because most outpatient medical encounters for migraine are with primary care practitioners, it may make sense to ensure that such clinicians are “well trained in diagnosing and treating common presentations of migraine,” Dr. Shapiro said. It is equally important to ensure “equitable access to health insurance to pay for these consultations,” he added.
‘Take migraine more seriously’
Commenting on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said the study was well designed.
Potential weaknesses include the fact that patients were required only to have one migraine attack per year and that not all were diagnosed by a headache specialist using ICHD-3 criteria.
Still, “online, validated, patient-reported data is quite acceptable,” said Dr. Rapoport, who was not involved in the research.
He noted that there is a clear message from the findings for all physicians who see patients with headache disorders: “You will increase the chance of patients consulting and continuing to consult when you make an accurate migraine diagnosis, take migraine more seriously, and understand the stigmas attached to it – and when there are reduced institutional barriers and costs of health care.”
The findings suggest that neurologists should strive to provide patients with ongoing care and medication, he added. In addition, there is a need for further education about the stigma associated with migraine and about how others view this disabling disease, Dr. Rapoport concluded.
The study was funded by Eli Lilly. Dr. Shapiro has consulted for Eli Lilly and Lundbeck. Dr. Rapoport has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. A survey of nearly 18,000 participants with migraine showed that 46% were reluctant to consult a physician about their condition. Among those who hesitated, 58% ultimately consulted a physician, but 42% did not.
Common reasons for failure to seek treatment included believing that migraine was not severe enough to warrant a consultation, worries about cost and health insurance, and concern that the health care professional would not take the disorder seriously.
This is the first study to query patients with migraine regarding whether and why they have hesitated to seek care, said coinvestigator Robert E. Shapiro, MD, PhD, professor of neurologic sciences and director of the division of headache medicine at the University of Vermont, Burlington. “Previous studies have noted differences in care seeking by demographic or other distinguishing characteristics but have not asked people with migraine whether they actually intended to seek or not seek such care,” he said.
Dr. Shapiro presented the findings at the American Headache Society’s 2021 annual meeting.
Delays prevent diagnosis and care
For patients with migraine, hesitating to consult a physician causes delays in, and sometimes prevents, receiving a diagnosis and appropriate care.
To assess the proportion of patients who hesitate to seek a consultation for migraine care, as well as reasons for doing so, the investigators examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study. OVERCOME incorporated a prospective web-based survey that was administered to a representative sample of 41,925 individuals in the United States.
Eligible participants who completed the study’s baseline assessment had had at least one migraine attack in the previous year and either met criteria for migraine on the basis of a validated diagnostic screen or provided a self-report of a migraine diagnosis by a health care practitioner. In all, 39,494 participants reported whether they had hesitated to seek a consultation from a physician for migraine care. Of these, 17,951 were included in the analysis.
Among the 46% who hesitated to seek care, 58% ultimately sought migraine care, and 42% did not.
The investigators also examined sociodemographic characteristics and migraine-related data, including the number of monthly headache days and information regarding nausea, photophobia, and phonophobia.
Patient-reported outcomes included days with migraine-related disability during the past 3 months, treatment optimization, and the degree to which migraine limited regular activities. Investigators also examined participants’ health care use in the previous 12 months and reasons for hesitating to seek migraine care.
Reasons for hesitancy
A total of 17,920 participants provided reasons for hesitating to seek a migraine consultation. These included a desire to take care of migraine attacks on one’s own (45%), the belief that migraine would not be taken seriously (35%), the belief that the migraine attacks were not serious or painful enough (29%), inability to afford or unwillingness to spend money on care (29%), lack of or inadequate health insurance (21%), and fear of receiving a serious diagnosis (19%).
Reasons for hesitation differed between participants who ultimately sought a consultation with a physician and those who did not. Those who did not receive a consultation (n = 7,495) were more likely to want to take care of the migraine attacks on their own (48% vs. 43%) and to believe the attacks were not serious or painful enough (36% vs. 25%).
Participants who hesitated but later sought a consultation were more likely to report concerns that migraine would not be taken seriously (38% vs. 31%) and fear of receiving a serious diagnosis (22% vs. 15%).
Among those who did not seek a consultation versus those who did, a significantly higher proportion were women (76% vs. 73%; P < .001).
“This is an interesting finding, since prior studies have indicated that, overall, women with migraine are more likely to have consulted a doctor for it – and also more likely to have been diagnosed with it,” Dr. Shapiro said.
On the other hand, women were 30% more likely to visit emergency departments or urgent care clinics for migraine care than men, he noted.
“These findings suggest some women may be experiencing particular barriers to receiving successful consultation care and that they may persistently hesitate to seek it,” said Dr. Shapiro. He noted that these barriers might be financial or attitudinal.
“Women are reported to be less likely to receive treatment for pain conditions, and furthermore, stigma toward migraine in particular may limit its perceived seriousness,” he said.
‘Equitable access’ needed
Those with full-time employment were significantly more likely to seek a migraine consultation than were those who were not employed full time (46% vs. 42%; P < .001). Patients who sought care were more likely to have health insurance (87% vs. 78%; P < .001).
Having health insurance (odds ratio [OR], 1.99), having previously received a migraine diagnosis (OR, 2.71), and degree of disability (severe vs. none: OR, 2.76; moderate vs. none: OR, 2.04) were associated with increased likelihood of seeking a migraine consultation among those who initially hesitated. Other factors included being male (OR, 1.49), having nausea (OR, 1.15), or being employed full time (OR, 1.24).
“Taken together, our findings suggest consultation rates may be limited by financial barriers and pervasive attitudes that migraine is either not serious or is untreatable,” said Dr. Shapiro. Consistent with this hypothesis is the finding that individuals with migraine who had received an appropriate diagnosis and were therefore better informed about the condition were more likely to continue to seek care for it, he noted.
Because most outpatient medical encounters for migraine are with primary care practitioners, it may make sense to ensure that such clinicians are “well trained in diagnosing and treating common presentations of migraine,” Dr. Shapiro said. It is equally important to ensure “equitable access to health insurance to pay for these consultations,” he added.
‘Take migraine more seriously’
Commenting on the findings, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said the study was well designed.
Potential weaknesses include the fact that patients were required only to have one migraine attack per year and that not all were diagnosed by a headache specialist using ICHD-3 criteria.
Still, “online, validated, patient-reported data is quite acceptable,” said Dr. Rapoport, who was not involved in the research.
He noted that there is a clear message from the findings for all physicians who see patients with headache disorders: “You will increase the chance of patients consulting and continuing to consult when you make an accurate migraine diagnosis, take migraine more seriously, and understand the stigmas attached to it – and when there are reduced institutional barriers and costs of health care.”
The findings suggest that neurologists should strive to provide patients with ongoing care and medication, he added. In addition, there is a need for further education about the stigma associated with migraine and about how others view this disabling disease, Dr. Rapoport concluded.
The study was funded by Eli Lilly. Dr. Shapiro has consulted for Eli Lilly and Lundbeck. Dr. Rapoport has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHS 2021
Telemedicine for headache visits had high patient satisfaction
according to a study presented at the American Headache Society’s 2021 annual meeting. Most patients who used telemedicine said they would like to continue using it after the pandemic, though the study also revealed barriers to care for a small percentage of respondents.
“Telemedicine minimizes the physical and geographic barriers to health care, preserves personal protective equipment, and prevents the spread of COVID-19 by allowing encounters to happen in a socially distanced way,” said Chia-Chun Chiang, MD, assistant professor of neurology at Mayo Clinic in Rochester, Minn. “Telemedicine provides patients with opportunities to gain better control of their headache disorders while not having to commit to the time to travel and risk of exposure to COVID-19.” If insurance coverage for virtual care were rolled back, “patients and multiple levels of health care providers would be significantly affected,” she said.
The research relied on findings from a 15-question survey distributed by the nonprofit American Migraine Foundation through email and social media to more than 100,000 people. Among the 1,172 patients who responded to the survey, 1,098 had complete responses, and 86.6% were female.
The vast majority of these patients (93.8%) had had a previous diagnosis of a headache. Just over half (57.5%) said they used telemedicine during the study period, with most of those visits (85.5%) being follow-up care and 14.5% involving a new patient visit.
Among those who did not use telemedicine, most (56.1%) said they didn’t need a visit. However, a quarter of these respondents (25.2%) said they didn’t know telemedicine was an option, and 12.9% said they would have preferred telemedicine but it wasn’t offered by their doctors. A smaller proportion (3.5%) said they wanted to use virtual care but that their insurance did not cover it, and nearly as many (2.2%) said they wanted telemedicine but didn’t have the technology needed to use it.
“The COVID-19 pandemic has highlighted that reliable Internet service has contributed to disparities in access in many ways, including health care via telemedicine,” Dr. Chiang said. “Those who are not able to afford Internet, lack proficiency in the use of technology, or have cognitive impairment might not be able to utilize telemedicine.”
Among those who did receive telemedicine care for headache, about a third (34.4%) received care from a general neurologist while 43.7% saw a headache specialist and nearly a third (30.7%) saw a primary care provider. The remaining visits included 11.3% who saw headache nurse practitioners and 3.2% who saw headache nurses.
Most patients did not have a new or changed diagnosis at their visit; only 7.4% received a new headache diagnosis during their telemedicine appointment. Though 43.7% had no change to their therapy, a little more than half of patients (52.4%) received a new treatment, a finding that caught the interest of Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and past president of the International Headache Society.
“The techniques used [in virtual visits] were good enough for the caregiver to make critical decisions about how the patient was doing and what new treatment might be better for them,” said Dr. Rapoport, who was not involved in the research. “I believe that most headache specialists will gradually resume in office visits,” he said, but “this study shows it would be okay for some or most of the revisits to continue to be done virtually.”
The vast majority of patients rated their care as “very good” (62.1%) or “good” (20.7%). Less satisfied responses included 10.5% who felt their experience was “fair,” 3.6% who said it was “poor,” and 3.1% who gave other responses.
These results fit with the experience of Dr. Rapoport and of Paul B. Rizzoli, MD, associate professor of neurology at Harvard Medical School and clinical director of the John R. Graham Headache Center at Brigham and Women’s Faulkner Hospital, both in Boston.
“Telemedicine worked better than we anticipated,” said Dr. Rizzoli when asked for comment. “I was especially surprised how comfortable I became with its use for many, but not all, new patients. While I don’t expect it to replace in-person visits, I do expect that it will and should be a permanent part of our care going forward, especially for follow-up visits.”
The findings supported that expectation as well: An overwhelming majority of those who responded to the survey (89.8%) also said they would like to keep receiving telemedicine care for their headache care and treatment. This percentage was split evenly between those who said they would like to always receive care virtually and those who would only want to use it for some appointments. A smaller proportion said they did not want to keep using virtual care (7.1%) or weren’t sure (3.1%).
“Telemedicine has become an essential tool for patients and a wide variety of clinicians,” Dr. Chiang reported during her presentation. “Telemedicine facilitated headache care for many patients during the COVID-19 pandemic, resulting in high patient satisfaction rates and a desire to continue to utilize telemedicine for future headache care for those who responded to the online survey.”
Dr. Rapoport noted that a particular benefit of telemedicine in his practice is avoiding transportation issues.
“In Santa Monica and Los Angeles, my patients coming from 10 or more miles away usually have to contend with difficult traffic, which created stress and often made them late and upset the office schedule,” Dr. Rapoport said. “I found that virtual visits were almost always shorter, on time, and were as effective for the patient as an in-person visit.”
Dr. Chiang drew attention, however, to the barriers to care found in the study, including not having or knowing of telemedicine as an option, and not having access to the technology or insurance coverage needed to take advantage of it. She listed three ways to address those challenges and increase health care accessibility to patients:
- Expand insurance coverage to reimburse telemedicine even after the pandemic.
- Widely promote and broadcast the use of virtual care.
- Make Internet access a priority as a necessity in society and expand access.
Dr. Rizzoli also noted some ways to improve telemedicine. “We could easily develop improved means of delivering vital signs and other bio-information over telemedicine to improve decision-making,” he said. “A difficult task going forward will be to fix legal questions associated with virtual visits across state lines which, especially in the small New England states, come up frequently and are currently illegal.”
Dr. Rapoport noted ways that patients can facilitate effective telemedicine visits. “Doctors should insist that patients keep careful records of their headaches, triggers, medicines, etc., either on paper or preferably via an app on their smartphones, which is usually always accessible,” Dr. Rapoport said. “With good data and a good electronic connection, the visit should go well.”
Among the study’s limitations were a comparatively small response rate (1.11% of those invited to participate) and ascertainment bias.
“The take-home message from the experience is that this turns out to be an effective, efficient and accepted means of delivering care that should be developed further,” Dr. Rizzoli said.
No external funding was noted. Dr. Chiang and Dr. Rizzoli had no disclosures. Dr. Rapoport has advised AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano, and is on the speakers bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries.
according to a study presented at the American Headache Society’s 2021 annual meeting. Most patients who used telemedicine said they would like to continue using it after the pandemic, though the study also revealed barriers to care for a small percentage of respondents.
“Telemedicine minimizes the physical and geographic barriers to health care, preserves personal protective equipment, and prevents the spread of COVID-19 by allowing encounters to happen in a socially distanced way,” said Chia-Chun Chiang, MD, assistant professor of neurology at Mayo Clinic in Rochester, Minn. “Telemedicine provides patients with opportunities to gain better control of their headache disorders while not having to commit to the time to travel and risk of exposure to COVID-19.” If insurance coverage for virtual care were rolled back, “patients and multiple levels of health care providers would be significantly affected,” she said.
The research relied on findings from a 15-question survey distributed by the nonprofit American Migraine Foundation through email and social media to more than 100,000 people. Among the 1,172 patients who responded to the survey, 1,098 had complete responses, and 86.6% were female.
The vast majority of these patients (93.8%) had had a previous diagnosis of a headache. Just over half (57.5%) said they used telemedicine during the study period, with most of those visits (85.5%) being follow-up care and 14.5% involving a new patient visit.
Among those who did not use telemedicine, most (56.1%) said they didn’t need a visit. However, a quarter of these respondents (25.2%) said they didn’t know telemedicine was an option, and 12.9% said they would have preferred telemedicine but it wasn’t offered by their doctors. A smaller proportion (3.5%) said they wanted to use virtual care but that their insurance did not cover it, and nearly as many (2.2%) said they wanted telemedicine but didn’t have the technology needed to use it.
“The COVID-19 pandemic has highlighted that reliable Internet service has contributed to disparities in access in many ways, including health care via telemedicine,” Dr. Chiang said. “Those who are not able to afford Internet, lack proficiency in the use of technology, or have cognitive impairment might not be able to utilize telemedicine.”
Among those who did receive telemedicine care for headache, about a third (34.4%) received care from a general neurologist while 43.7% saw a headache specialist and nearly a third (30.7%) saw a primary care provider. The remaining visits included 11.3% who saw headache nurse practitioners and 3.2% who saw headache nurses.
Most patients did not have a new or changed diagnosis at their visit; only 7.4% received a new headache diagnosis during their telemedicine appointment. Though 43.7% had no change to their therapy, a little more than half of patients (52.4%) received a new treatment, a finding that caught the interest of Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and past president of the International Headache Society.
“The techniques used [in virtual visits] were good enough for the caregiver to make critical decisions about how the patient was doing and what new treatment might be better for them,” said Dr. Rapoport, who was not involved in the research. “I believe that most headache specialists will gradually resume in office visits,” he said, but “this study shows it would be okay for some or most of the revisits to continue to be done virtually.”
The vast majority of patients rated their care as “very good” (62.1%) or “good” (20.7%). Less satisfied responses included 10.5% who felt their experience was “fair,” 3.6% who said it was “poor,” and 3.1% who gave other responses.
These results fit with the experience of Dr. Rapoport and of Paul B. Rizzoli, MD, associate professor of neurology at Harvard Medical School and clinical director of the John R. Graham Headache Center at Brigham and Women’s Faulkner Hospital, both in Boston.
“Telemedicine worked better than we anticipated,” said Dr. Rizzoli when asked for comment. “I was especially surprised how comfortable I became with its use for many, but not all, new patients. While I don’t expect it to replace in-person visits, I do expect that it will and should be a permanent part of our care going forward, especially for follow-up visits.”
The findings supported that expectation as well: An overwhelming majority of those who responded to the survey (89.8%) also said they would like to keep receiving telemedicine care for their headache care and treatment. This percentage was split evenly between those who said they would like to always receive care virtually and those who would only want to use it for some appointments. A smaller proportion said they did not want to keep using virtual care (7.1%) or weren’t sure (3.1%).
“Telemedicine has become an essential tool for patients and a wide variety of clinicians,” Dr. Chiang reported during her presentation. “Telemedicine facilitated headache care for many patients during the COVID-19 pandemic, resulting in high patient satisfaction rates and a desire to continue to utilize telemedicine for future headache care for those who responded to the online survey.”
Dr. Rapoport noted that a particular benefit of telemedicine in his practice is avoiding transportation issues.
“In Santa Monica and Los Angeles, my patients coming from 10 or more miles away usually have to contend with difficult traffic, which created stress and often made them late and upset the office schedule,” Dr. Rapoport said. “I found that virtual visits were almost always shorter, on time, and were as effective for the patient as an in-person visit.”
Dr. Chiang drew attention, however, to the barriers to care found in the study, including not having or knowing of telemedicine as an option, and not having access to the technology or insurance coverage needed to take advantage of it. She listed three ways to address those challenges and increase health care accessibility to patients:
- Expand insurance coverage to reimburse telemedicine even after the pandemic.
- Widely promote and broadcast the use of virtual care.
- Make Internet access a priority as a necessity in society and expand access.
Dr. Rizzoli also noted some ways to improve telemedicine. “We could easily develop improved means of delivering vital signs and other bio-information over telemedicine to improve decision-making,” he said. “A difficult task going forward will be to fix legal questions associated with virtual visits across state lines which, especially in the small New England states, come up frequently and are currently illegal.”
Dr. Rapoport noted ways that patients can facilitate effective telemedicine visits. “Doctors should insist that patients keep careful records of their headaches, triggers, medicines, etc., either on paper or preferably via an app on their smartphones, which is usually always accessible,” Dr. Rapoport said. “With good data and a good electronic connection, the visit should go well.”
Among the study’s limitations were a comparatively small response rate (1.11% of those invited to participate) and ascertainment bias.
“The take-home message from the experience is that this turns out to be an effective, efficient and accepted means of delivering care that should be developed further,” Dr. Rizzoli said.
No external funding was noted. Dr. Chiang and Dr. Rizzoli had no disclosures. Dr. Rapoport has advised AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano, and is on the speakers bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries.
according to a study presented at the American Headache Society’s 2021 annual meeting. Most patients who used telemedicine said they would like to continue using it after the pandemic, though the study also revealed barriers to care for a small percentage of respondents.
“Telemedicine minimizes the physical and geographic barriers to health care, preserves personal protective equipment, and prevents the spread of COVID-19 by allowing encounters to happen in a socially distanced way,” said Chia-Chun Chiang, MD, assistant professor of neurology at Mayo Clinic in Rochester, Minn. “Telemedicine provides patients with opportunities to gain better control of their headache disorders while not having to commit to the time to travel and risk of exposure to COVID-19.” If insurance coverage for virtual care were rolled back, “patients and multiple levels of health care providers would be significantly affected,” she said.
The research relied on findings from a 15-question survey distributed by the nonprofit American Migraine Foundation through email and social media to more than 100,000 people. Among the 1,172 patients who responded to the survey, 1,098 had complete responses, and 86.6% were female.
The vast majority of these patients (93.8%) had had a previous diagnosis of a headache. Just over half (57.5%) said they used telemedicine during the study period, with most of those visits (85.5%) being follow-up care and 14.5% involving a new patient visit.
Among those who did not use telemedicine, most (56.1%) said they didn’t need a visit. However, a quarter of these respondents (25.2%) said they didn’t know telemedicine was an option, and 12.9% said they would have preferred telemedicine but it wasn’t offered by their doctors. A smaller proportion (3.5%) said they wanted to use virtual care but that their insurance did not cover it, and nearly as many (2.2%) said they wanted telemedicine but didn’t have the technology needed to use it.
“The COVID-19 pandemic has highlighted that reliable Internet service has contributed to disparities in access in many ways, including health care via telemedicine,” Dr. Chiang said. “Those who are not able to afford Internet, lack proficiency in the use of technology, or have cognitive impairment might not be able to utilize telemedicine.”
Among those who did receive telemedicine care for headache, about a third (34.4%) received care from a general neurologist while 43.7% saw a headache specialist and nearly a third (30.7%) saw a primary care provider. The remaining visits included 11.3% who saw headache nurse practitioners and 3.2% who saw headache nurses.
Most patients did not have a new or changed diagnosis at their visit; only 7.4% received a new headache diagnosis during their telemedicine appointment. Though 43.7% had no change to their therapy, a little more than half of patients (52.4%) received a new treatment, a finding that caught the interest of Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and past president of the International Headache Society.
“The techniques used [in virtual visits] were good enough for the caregiver to make critical decisions about how the patient was doing and what new treatment might be better for them,” said Dr. Rapoport, who was not involved in the research. “I believe that most headache specialists will gradually resume in office visits,” he said, but “this study shows it would be okay for some or most of the revisits to continue to be done virtually.”
The vast majority of patients rated their care as “very good” (62.1%) or “good” (20.7%). Less satisfied responses included 10.5% who felt their experience was “fair,” 3.6% who said it was “poor,” and 3.1% who gave other responses.
These results fit with the experience of Dr. Rapoport and of Paul B. Rizzoli, MD, associate professor of neurology at Harvard Medical School and clinical director of the John R. Graham Headache Center at Brigham and Women’s Faulkner Hospital, both in Boston.
“Telemedicine worked better than we anticipated,” said Dr. Rizzoli when asked for comment. “I was especially surprised how comfortable I became with its use for many, but not all, new patients. While I don’t expect it to replace in-person visits, I do expect that it will and should be a permanent part of our care going forward, especially for follow-up visits.”
The findings supported that expectation as well: An overwhelming majority of those who responded to the survey (89.8%) also said they would like to keep receiving telemedicine care for their headache care and treatment. This percentage was split evenly between those who said they would like to always receive care virtually and those who would only want to use it for some appointments. A smaller proportion said they did not want to keep using virtual care (7.1%) or weren’t sure (3.1%).
“Telemedicine has become an essential tool for patients and a wide variety of clinicians,” Dr. Chiang reported during her presentation. “Telemedicine facilitated headache care for many patients during the COVID-19 pandemic, resulting in high patient satisfaction rates and a desire to continue to utilize telemedicine for future headache care for those who responded to the online survey.”
Dr. Rapoport noted that a particular benefit of telemedicine in his practice is avoiding transportation issues.
“In Santa Monica and Los Angeles, my patients coming from 10 or more miles away usually have to contend with difficult traffic, which created stress and often made them late and upset the office schedule,” Dr. Rapoport said. “I found that virtual visits were almost always shorter, on time, and were as effective for the patient as an in-person visit.”
Dr. Chiang drew attention, however, to the barriers to care found in the study, including not having or knowing of telemedicine as an option, and not having access to the technology or insurance coverage needed to take advantage of it. She listed three ways to address those challenges and increase health care accessibility to patients:
- Expand insurance coverage to reimburse telemedicine even after the pandemic.
- Widely promote and broadcast the use of virtual care.
- Make Internet access a priority as a necessity in society and expand access.
Dr. Rizzoli also noted some ways to improve telemedicine. “We could easily develop improved means of delivering vital signs and other bio-information over telemedicine to improve decision-making,” he said. “A difficult task going forward will be to fix legal questions associated with virtual visits across state lines which, especially in the small New England states, come up frequently and are currently illegal.”
Dr. Rapoport noted ways that patients can facilitate effective telemedicine visits. “Doctors should insist that patients keep careful records of their headaches, triggers, medicines, etc., either on paper or preferably via an app on their smartphones, which is usually always accessible,” Dr. Rapoport said. “With good data and a good electronic connection, the visit should go well.”
Among the study’s limitations were a comparatively small response rate (1.11% of those invited to participate) and ascertainment bias.
“The take-home message from the experience is that this turns out to be an effective, efficient and accepted means of delivering care that should be developed further,” Dr. Rizzoli said.
No external funding was noted. Dr. Chiang and Dr. Rizzoli had no disclosures. Dr. Rapoport has advised AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano, and is on the speakers bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries.
FROM AHS 2021
FDA approves controversial Alzheimer’s drug aducanumab (Aduhelm)
In November, the Peripheral and Central Nervous System Drugs Advisory Committee voted eight to one against approving the drug because, based on clinical trial results, evidence of efficacy was not strong enough. Two other members said they were uncertain on the issue of efficacy.
In a company release Michel Vounatsos, Biogen’s Chief Executive Officer, said, “this historic moment is the culmination of more than a decade of groundbreaking research in the complex field of Alzheimer’s disease. We believe this first-in-class medicine will transform the treatment of people living with Alzheimer’s disease and spark continuous innovation in the years to come.
Rocky road
The road to approval has been extremely rocky for aducanumab, an anti-amyloid-beta human monoclonal antibody, previously known as BIIB037.
As reported by this news organization, two phase 3 trials evaluating the drug were initially scrapped in March 2019 because of interim futility analysis. At the time, Biogen released a statement saying that aducanumab was unlikely to meet primary endpoints in the ENGAGE and EMERGE randomized controlled trials.
However, in an about-face 7 months later, Biogen and Eisai announced that a new analysis showed the drug met its primary endpoint of reduction in clinical decline, including cognition and function, in the EMERGE trial.
Although ENGAGE still didn’t meet its primary endpoint, data from its new analysis “supported” the EMERGE findings, the drug companies said at the time.
However, 1 year later, a majority of the members of the FDA’s advisory panel were against the drug’s approval. Details of that decision were published online March 30 in the Journal of the American Medical Association.
As reported by this news organization, a Viewpoint written by three of the committee members notes that results from the drug’s only large positive clinical trial fell short.
“There is no persuasive evidence to support approval of aducanumab at this time,” they write.
Groups such as Public Citizen’s Health Research Group not only agree with the Viewpoint’s authors, they also criticized the FDA for its collaboration with the drug’s manufacturers on briefing documents and more.
On April 1, Health Research Group members sent a letter to the U.S. Secretary of Health and Human Services requesting the temporary suspension of the FDA’s neuroscience chief, Bill Dunn, MD, because of his role in supervising the collaboration.
Alzheimer association weighs in
The Alzheimer’s Association has been a proponent of the drug throughout its development.
Ahead of today’s news, the organization noted in a statement that a decision to approve “would be historic” because it would make aducanumab “the first drug to slow Alzheimer’s disease” and would mark the beginning of a new future for AD treatments.
“The Alzheimer’s Association urgently supports FDA approval of the treatment based on clinical trial results that showed a 22% reduction in cognitive and function decline — something that could make a meaningful difference” for patients with AD, it said.
Kristen Clifford, chief program officer for the Alzheimer’s Association, said in an interview at the time that approval would be considered a “victory” for patients with AD and for the field overall.
“For individuals who would potentially be eligible for the treatment, this drug could mean more quality time. Slowing decline, particularly in early diagnosis, could add weeks or months or maybe even years of active life,” Clifford said.
“If approved, this would really be a landmark moment. And it could provide hope for those living with Alzheimer’s and their families,” she added.
Clifford noted that approval of this type of drug would also underscore the importance of early detection for AD. “This treatment would encourage earlier diagnosis of the disease,” she said.
In a new statement released just after approval for aducanumab was announced, the organization said that today’s news is a win-win for all patients with AD and their families.
A version of this article first appeared on Medscape.com.
In November, the Peripheral and Central Nervous System Drugs Advisory Committee voted eight to one against approving the drug because, based on clinical trial results, evidence of efficacy was not strong enough. Two other members said they were uncertain on the issue of efficacy.
In a company release Michel Vounatsos, Biogen’s Chief Executive Officer, said, “this historic moment is the culmination of more than a decade of groundbreaking research in the complex field of Alzheimer’s disease. We believe this first-in-class medicine will transform the treatment of people living with Alzheimer’s disease and spark continuous innovation in the years to come.
Rocky road
The road to approval has been extremely rocky for aducanumab, an anti-amyloid-beta human monoclonal antibody, previously known as BIIB037.
As reported by this news organization, two phase 3 trials evaluating the drug were initially scrapped in March 2019 because of interim futility analysis. At the time, Biogen released a statement saying that aducanumab was unlikely to meet primary endpoints in the ENGAGE and EMERGE randomized controlled trials.
However, in an about-face 7 months later, Biogen and Eisai announced that a new analysis showed the drug met its primary endpoint of reduction in clinical decline, including cognition and function, in the EMERGE trial.
Although ENGAGE still didn’t meet its primary endpoint, data from its new analysis “supported” the EMERGE findings, the drug companies said at the time.
However, 1 year later, a majority of the members of the FDA’s advisory panel were against the drug’s approval. Details of that decision were published online March 30 in the Journal of the American Medical Association.
As reported by this news organization, a Viewpoint written by three of the committee members notes that results from the drug’s only large positive clinical trial fell short.
“There is no persuasive evidence to support approval of aducanumab at this time,” they write.
Groups such as Public Citizen’s Health Research Group not only agree with the Viewpoint’s authors, they also criticized the FDA for its collaboration with the drug’s manufacturers on briefing documents and more.
On April 1, Health Research Group members sent a letter to the U.S. Secretary of Health and Human Services requesting the temporary suspension of the FDA’s neuroscience chief, Bill Dunn, MD, because of his role in supervising the collaboration.
Alzheimer association weighs in
The Alzheimer’s Association has been a proponent of the drug throughout its development.
Ahead of today’s news, the organization noted in a statement that a decision to approve “would be historic” because it would make aducanumab “the first drug to slow Alzheimer’s disease” and would mark the beginning of a new future for AD treatments.
“The Alzheimer’s Association urgently supports FDA approval of the treatment based on clinical trial results that showed a 22% reduction in cognitive and function decline — something that could make a meaningful difference” for patients with AD, it said.
Kristen Clifford, chief program officer for the Alzheimer’s Association, said in an interview at the time that approval would be considered a “victory” for patients with AD and for the field overall.
“For individuals who would potentially be eligible for the treatment, this drug could mean more quality time. Slowing decline, particularly in early diagnosis, could add weeks or months or maybe even years of active life,” Clifford said.
“If approved, this would really be a landmark moment. And it could provide hope for those living with Alzheimer’s and their families,” she added.
Clifford noted that approval of this type of drug would also underscore the importance of early detection for AD. “This treatment would encourage earlier diagnosis of the disease,” she said.
In a new statement released just after approval for aducanumab was announced, the organization said that today’s news is a win-win for all patients with AD and their families.
A version of this article first appeared on Medscape.com.
In November, the Peripheral and Central Nervous System Drugs Advisory Committee voted eight to one against approving the drug because, based on clinical trial results, evidence of efficacy was not strong enough. Two other members said they were uncertain on the issue of efficacy.
In a company release Michel Vounatsos, Biogen’s Chief Executive Officer, said, “this historic moment is the culmination of more than a decade of groundbreaking research in the complex field of Alzheimer’s disease. We believe this first-in-class medicine will transform the treatment of people living with Alzheimer’s disease and spark continuous innovation in the years to come.
Rocky road
The road to approval has been extremely rocky for aducanumab, an anti-amyloid-beta human monoclonal antibody, previously known as BIIB037.
As reported by this news organization, two phase 3 trials evaluating the drug were initially scrapped in March 2019 because of interim futility analysis. At the time, Biogen released a statement saying that aducanumab was unlikely to meet primary endpoints in the ENGAGE and EMERGE randomized controlled trials.
However, in an about-face 7 months later, Biogen and Eisai announced that a new analysis showed the drug met its primary endpoint of reduction in clinical decline, including cognition and function, in the EMERGE trial.
Although ENGAGE still didn’t meet its primary endpoint, data from its new analysis “supported” the EMERGE findings, the drug companies said at the time.
However, 1 year later, a majority of the members of the FDA’s advisory panel were against the drug’s approval. Details of that decision were published online March 30 in the Journal of the American Medical Association.
As reported by this news organization, a Viewpoint written by three of the committee members notes that results from the drug’s only large positive clinical trial fell short.
“There is no persuasive evidence to support approval of aducanumab at this time,” they write.
Groups such as Public Citizen’s Health Research Group not only agree with the Viewpoint’s authors, they also criticized the FDA for its collaboration with the drug’s manufacturers on briefing documents and more.
On April 1, Health Research Group members sent a letter to the U.S. Secretary of Health and Human Services requesting the temporary suspension of the FDA’s neuroscience chief, Bill Dunn, MD, because of his role in supervising the collaboration.
Alzheimer association weighs in
The Alzheimer’s Association has been a proponent of the drug throughout its development.
Ahead of today’s news, the organization noted in a statement that a decision to approve “would be historic” because it would make aducanumab “the first drug to slow Alzheimer’s disease” and would mark the beginning of a new future for AD treatments.
“The Alzheimer’s Association urgently supports FDA approval of the treatment based on clinical trial results that showed a 22% reduction in cognitive and function decline — something that could make a meaningful difference” for patients with AD, it said.
Kristen Clifford, chief program officer for the Alzheimer’s Association, said in an interview at the time that approval would be considered a “victory” for patients with AD and for the field overall.
“For individuals who would potentially be eligible for the treatment, this drug could mean more quality time. Slowing decline, particularly in early diagnosis, could add weeks or months or maybe even years of active life,” Clifford said.
“If approved, this would really be a landmark moment. And it could provide hope for those living with Alzheimer’s and their families,” she added.
Clifford noted that approval of this type of drug would also underscore the importance of early detection for AD. “This treatment would encourage earlier diagnosis of the disease,” she said.
In a new statement released just after approval for aducanumab was announced, the organization said that today’s news is a win-win for all patients with AD and their families.
A version of this article first appeared on Medscape.com.
Not your ordinary neuropathy
She has had a diagnosis of type 2 diabetes for the past 4 years. She initially presented with polyuria/polydipsia and a hemoglobin A1c level of 9.5. She has previously not tolerated metformin, and did not want to take any subsequent medications. She was seen 4 months ago and at that time had an A1c level of 12.5. She decided she wanted to really treat her diabetes as well as she could. She started consuming a low carbohydrate diet, restarted metformin and began using a continuous glucose monitor. She also started taking nighttime glargine insulin, and mealtime insulin apart. She reports she lost 20 pounds over the past 4 months, her blood sugars now run between 100-120 fasting, and up to 180 before meals. She has had a severe, sharp pain in both of her feet over the past month that is interfering with sleep and makes walking painful for her. An exam reveals hyperesthesia of both feet, and her A1c level is 7.5. What is the most likely cause of her neuropathic symptoms?
A. Vitamin B12 deficiency
B. Diabetic neuropathy
C. Insulin neuritis
D. Charcot-Marie-Tooth disease
The most likely cause
In this case, certainly considering vitamin B12 deficiency is reasonable. It is highly unlikely though, given the rapidity of onset of symptoms, and that the patient has been on metformin for a very short period of time. Chronic metformin use is associated with low B12 levels, and the American Diabetes Association has advised that regular monitoring of vitamin B12 levels should be done on patients who are on long-term metformin.1
Diabetic neuropathy is also unlikely, given the rapidity of symptoms in this patient. What is most likely in this patient is treatment-induced neuropathy (TIN), first described with the name “insulin neuritis”.
Research on TIN
Gibbons and colleagues evaluated 16 patients with diabetes with recent marked, rapid improvement in glycemic control who developed a sudden, painful neuropathy.2 All developed symptoms within 8 weeks of intensive glucose control, with 69% having autonomic dysfunction as well, and all developing worsening retinopathy.
Gibbons and Freeman did a retrospective study of patients referred to a diabetic neuropathy clinic over a 5-year period to try to understand how prevalent TIN is.3
A total of 954 patients were evaluated for diabetic neuropathy. Treatment induced neuropathy was defined as a painful neuropathy and/or autonomic dysfunction occurring within 8 weeks of intensified treatment and a drop of the A1c level greater than 2 over a 3-month period.
A total of 104 patients (10.9%) met the criteria for treatment induced neuropathy. Patients who had a decrease in A1c had a much greater chance of developing a painful or autonomic neuropathy than patients who had no change in A1c (P < .0001). The same patients had a much higher risk of developing retinopathy (P < .001). The greater the reduction in A1c, the greater the risk. Patients whose A1c decreased by 2%-3% over 3 months had an absolute risk of 20%, whereas those with a A1c decease of greater than 4% had an 80% absolute risk.
Siddique and colleagues reported on three cases with very different clinical presentations of TIN.4 One patient had an acute third nerve palsy, another patient had a lumbosacral radiculoplexus neuropathy, and the third patient presented with a diffuse painful sensory neuropathy and postural hypotension.
Most patients improve over time from their neuropathic symptoms, with better recovery in patients with type 1 diabetes.2
Pearl
Strongly consider treatment induced neuropathy in your patients with diabetes who present with acute painful neuropathy and/or autonomic dysfunction in the setting of rapid improvement of glucose control.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. American Diabetes Association. Diabetes Care. 2019 Jan;42(Suppl 1):S90-102.
2. Gibbons CH and Freeman R. Ann Neurol 2010; 67:534–41.
3. Gibbons CH and Freeman R. Brain. 2015;138:43-52.
4. Siddique N et al. Endocrinol Diabetes Metab Case Rep. 2020 Feb 26;2020:19-0140.
She has had a diagnosis of type 2 diabetes for the past 4 years. She initially presented with polyuria/polydipsia and a hemoglobin A1c level of 9.5. She has previously not tolerated metformin, and did not want to take any subsequent medications. She was seen 4 months ago and at that time had an A1c level of 12.5. She decided she wanted to really treat her diabetes as well as she could. She started consuming a low carbohydrate diet, restarted metformin and began using a continuous glucose monitor. She also started taking nighttime glargine insulin, and mealtime insulin apart. She reports she lost 20 pounds over the past 4 months, her blood sugars now run between 100-120 fasting, and up to 180 before meals. She has had a severe, sharp pain in both of her feet over the past month that is interfering with sleep and makes walking painful for her. An exam reveals hyperesthesia of both feet, and her A1c level is 7.5. What is the most likely cause of her neuropathic symptoms?
A. Vitamin B12 deficiency
B. Diabetic neuropathy
C. Insulin neuritis
D. Charcot-Marie-Tooth disease
The most likely cause
In this case, certainly considering vitamin B12 deficiency is reasonable. It is highly unlikely though, given the rapidity of onset of symptoms, and that the patient has been on metformin for a very short period of time. Chronic metformin use is associated with low B12 levels, and the American Diabetes Association has advised that regular monitoring of vitamin B12 levels should be done on patients who are on long-term metformin.1
Diabetic neuropathy is also unlikely, given the rapidity of symptoms in this patient. What is most likely in this patient is treatment-induced neuropathy (TIN), first described with the name “insulin neuritis”.
Research on TIN
Gibbons and colleagues evaluated 16 patients with diabetes with recent marked, rapid improvement in glycemic control who developed a sudden, painful neuropathy.2 All developed symptoms within 8 weeks of intensive glucose control, with 69% having autonomic dysfunction as well, and all developing worsening retinopathy.
Gibbons and Freeman did a retrospective study of patients referred to a diabetic neuropathy clinic over a 5-year period to try to understand how prevalent TIN is.3
A total of 954 patients were evaluated for diabetic neuropathy. Treatment induced neuropathy was defined as a painful neuropathy and/or autonomic dysfunction occurring within 8 weeks of intensified treatment and a drop of the A1c level greater than 2 over a 3-month period.
A total of 104 patients (10.9%) met the criteria for treatment induced neuropathy. Patients who had a decrease in A1c had a much greater chance of developing a painful or autonomic neuropathy than patients who had no change in A1c (P < .0001). The same patients had a much higher risk of developing retinopathy (P < .001). The greater the reduction in A1c, the greater the risk. Patients whose A1c decreased by 2%-3% over 3 months had an absolute risk of 20%, whereas those with a A1c decease of greater than 4% had an 80% absolute risk.
Siddique and colleagues reported on three cases with very different clinical presentations of TIN.4 One patient had an acute third nerve palsy, another patient had a lumbosacral radiculoplexus neuropathy, and the third patient presented with a diffuse painful sensory neuropathy and postural hypotension.
Most patients improve over time from their neuropathic symptoms, with better recovery in patients with type 1 diabetes.2
Pearl
Strongly consider treatment induced neuropathy in your patients with diabetes who present with acute painful neuropathy and/or autonomic dysfunction in the setting of rapid improvement of glucose control.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. American Diabetes Association. Diabetes Care. 2019 Jan;42(Suppl 1):S90-102.
2. Gibbons CH and Freeman R. Ann Neurol 2010; 67:534–41.
3. Gibbons CH and Freeman R. Brain. 2015;138:43-52.
4. Siddique N et al. Endocrinol Diabetes Metab Case Rep. 2020 Feb 26;2020:19-0140.
She has had a diagnosis of type 2 diabetes for the past 4 years. She initially presented with polyuria/polydipsia and a hemoglobin A1c level of 9.5. She has previously not tolerated metformin, and did not want to take any subsequent medications. She was seen 4 months ago and at that time had an A1c level of 12.5. She decided she wanted to really treat her diabetes as well as she could. She started consuming a low carbohydrate diet, restarted metformin and began using a continuous glucose monitor. She also started taking nighttime glargine insulin, and mealtime insulin apart. She reports she lost 20 pounds over the past 4 months, her blood sugars now run between 100-120 fasting, and up to 180 before meals. She has had a severe, sharp pain in both of her feet over the past month that is interfering with sleep and makes walking painful for her. An exam reveals hyperesthesia of both feet, and her A1c level is 7.5. What is the most likely cause of her neuropathic symptoms?
A. Vitamin B12 deficiency
B. Diabetic neuropathy
C. Insulin neuritis
D. Charcot-Marie-Tooth disease
The most likely cause
In this case, certainly considering vitamin B12 deficiency is reasonable. It is highly unlikely though, given the rapidity of onset of symptoms, and that the patient has been on metformin for a very short period of time. Chronic metformin use is associated with low B12 levels, and the American Diabetes Association has advised that regular monitoring of vitamin B12 levels should be done on patients who are on long-term metformin.1
Diabetic neuropathy is also unlikely, given the rapidity of symptoms in this patient. What is most likely in this patient is treatment-induced neuropathy (TIN), first described with the name “insulin neuritis”.
Research on TIN
Gibbons and colleagues evaluated 16 patients with diabetes with recent marked, rapid improvement in glycemic control who developed a sudden, painful neuropathy.2 All developed symptoms within 8 weeks of intensive glucose control, with 69% having autonomic dysfunction as well, and all developing worsening retinopathy.
Gibbons and Freeman did a retrospective study of patients referred to a diabetic neuropathy clinic over a 5-year period to try to understand how prevalent TIN is.3
A total of 954 patients were evaluated for diabetic neuropathy. Treatment induced neuropathy was defined as a painful neuropathy and/or autonomic dysfunction occurring within 8 weeks of intensified treatment and a drop of the A1c level greater than 2 over a 3-month period.
A total of 104 patients (10.9%) met the criteria for treatment induced neuropathy. Patients who had a decrease in A1c had a much greater chance of developing a painful or autonomic neuropathy than patients who had no change in A1c (P < .0001). The same patients had a much higher risk of developing retinopathy (P < .001). The greater the reduction in A1c, the greater the risk. Patients whose A1c decreased by 2%-3% over 3 months had an absolute risk of 20%, whereas those with a A1c decease of greater than 4% had an 80% absolute risk.
Siddique and colleagues reported on three cases with very different clinical presentations of TIN.4 One patient had an acute third nerve palsy, another patient had a lumbosacral radiculoplexus neuropathy, and the third patient presented with a diffuse painful sensory neuropathy and postural hypotension.
Most patients improve over time from their neuropathic symptoms, with better recovery in patients with type 1 diabetes.2
Pearl
Strongly consider treatment induced neuropathy in your patients with diabetes who present with acute painful neuropathy and/or autonomic dysfunction in the setting of rapid improvement of glucose control.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. American Diabetes Association. Diabetes Care. 2019 Jan;42(Suppl 1):S90-102.
2. Gibbons CH and Freeman R. Ann Neurol 2010; 67:534–41.
3. Gibbons CH and Freeman R. Brain. 2015;138:43-52.
4. Siddique N et al. Endocrinol Diabetes Metab Case Rep. 2020 Feb 26;2020:19-0140.
FDA approves diagnostic device for autism spectrum disorder
The Food and Drug Administration has approved marketing for a device that will help diagnose autism spectrum disorder (ASD) in children between the ages of 18 months and 5 years old who exhibit potential symptoms.
Cognoa ASD Diagnosis Aid is a machine learning–based software program that receives information from parents or caregivers, video analysts, and health care providers to assist physicians in evaluating whether a child is at risk of having autism.
Autism is a developmental disorder that can cause social, communication, and behavioral challenges, according to the Centers for Disease Control and Prevention. The disorder affects about 1 in 54 children. The disorder is difficult to diagnose because there isn’t a medical test to diagnose the it. Instead, physicians have to look at a child’s developmental history and behavior to make a diagnosis.
Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the FDA.
“[ASD] can delay a child’s physical, cognitive, and social development, including motor skill development, learning, communication, and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s marketing authorization provides a new tool for helping diagnose children with ASD.”
The safety and efficacy of the Cognoa ASD Diagnosis Aid was assessed in a study of 425 patients between the ages of 18 months and 5 years old. For the study, researchers compared the diagnostic assessments made by the device to those made by a panel of clinical experts who used the current standard ASD diagnostic process. The device diagnosed 32% of the children with either a “Positive for ASD” or a “Negative for ASD” result. Researchers found that the device matched the panel’s conclusions for 81% of the patients who received a positive diagnosis. For those who received a negative diagnosis, the device matched the panel’s conclusions for 98% of the patients. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Cognoa ASD Diagnosis Aid has three main components. One component includes a mobile app for caregivers to answer questions about the child’s behavioral problems and to upload videos of the child. The next component is a video analysis portal for specialists to view and analyze uploaded videos of patients. Another component is a portal for health care providers that allows them to enter answers to preloaded questions about behavior problems, track the information provided by parents, and review a report of the results.
After the machine learning–based device processes the information provided by parents and health care providers, it reports either a positive or a negative diagnosis. If there is insufficient information to make either a positive or a negative diagnosis, the ASD Diagnostic AID will report that no result can be generated.
Some of the risks associated with this device include misdiagnosis and delayed diagnosis of ASD because of a false-positive or false-negative result, or when no result is generated. Researchers said a false-positive result occurred in 15 out of 303 study subjects without ASD and a false-negative result occurred in 1 out of 122 study subjects with ASD.
The FDA emphasized that the device is indicated to aid physicians in the process of diagnosing ASD in children. This means it shouldn’t be treated as a standalone diagnostic device, but as an adjunct to the diagnostic process.
The Food and Drug Administration has approved marketing for a device that will help diagnose autism spectrum disorder (ASD) in children between the ages of 18 months and 5 years old who exhibit potential symptoms.
Cognoa ASD Diagnosis Aid is a machine learning–based software program that receives information from parents or caregivers, video analysts, and health care providers to assist physicians in evaluating whether a child is at risk of having autism.
Autism is a developmental disorder that can cause social, communication, and behavioral challenges, according to the Centers for Disease Control and Prevention. The disorder affects about 1 in 54 children. The disorder is difficult to diagnose because there isn’t a medical test to diagnose the it. Instead, physicians have to look at a child’s developmental history and behavior to make a diagnosis.
Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the FDA.
“[ASD] can delay a child’s physical, cognitive, and social development, including motor skill development, learning, communication, and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s marketing authorization provides a new tool for helping diagnose children with ASD.”
The safety and efficacy of the Cognoa ASD Diagnosis Aid was assessed in a study of 425 patients between the ages of 18 months and 5 years old. For the study, researchers compared the diagnostic assessments made by the device to those made by a panel of clinical experts who used the current standard ASD diagnostic process. The device diagnosed 32% of the children with either a “Positive for ASD” or a “Negative for ASD” result. Researchers found that the device matched the panel’s conclusions for 81% of the patients who received a positive diagnosis. For those who received a negative diagnosis, the device matched the panel’s conclusions for 98% of the patients. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Cognoa ASD Diagnosis Aid has three main components. One component includes a mobile app for caregivers to answer questions about the child’s behavioral problems and to upload videos of the child. The next component is a video analysis portal for specialists to view and analyze uploaded videos of patients. Another component is a portal for health care providers that allows them to enter answers to preloaded questions about behavior problems, track the information provided by parents, and review a report of the results.
After the machine learning–based device processes the information provided by parents and health care providers, it reports either a positive or a negative diagnosis. If there is insufficient information to make either a positive or a negative diagnosis, the ASD Diagnostic AID will report that no result can be generated.
Some of the risks associated with this device include misdiagnosis and delayed diagnosis of ASD because of a false-positive or false-negative result, or when no result is generated. Researchers said a false-positive result occurred in 15 out of 303 study subjects without ASD and a false-negative result occurred in 1 out of 122 study subjects with ASD.
The FDA emphasized that the device is indicated to aid physicians in the process of diagnosing ASD in children. This means it shouldn’t be treated as a standalone diagnostic device, but as an adjunct to the diagnostic process.
The Food and Drug Administration has approved marketing for a device that will help diagnose autism spectrum disorder (ASD) in children between the ages of 18 months and 5 years old who exhibit potential symptoms.
Cognoa ASD Diagnosis Aid is a machine learning–based software program that receives information from parents or caregivers, video analysts, and health care providers to assist physicians in evaluating whether a child is at risk of having autism.
Autism is a developmental disorder that can cause social, communication, and behavioral challenges, according to the Centers for Disease Control and Prevention. The disorder affects about 1 in 54 children. The disorder is difficult to diagnose because there isn’t a medical test to diagnose the it. Instead, physicians have to look at a child’s developmental history and behavior to make a diagnosis.
Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the FDA.
“[ASD] can delay a child’s physical, cognitive, and social development, including motor skill development, learning, communication, and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s marketing authorization provides a new tool for helping diagnose children with ASD.”
The safety and efficacy of the Cognoa ASD Diagnosis Aid was assessed in a study of 425 patients between the ages of 18 months and 5 years old. For the study, researchers compared the diagnostic assessments made by the device to those made by a panel of clinical experts who used the current standard ASD diagnostic process. The device diagnosed 32% of the children with either a “Positive for ASD” or a “Negative for ASD” result. Researchers found that the device matched the panel’s conclusions for 81% of the patients who received a positive diagnosis. For those who received a negative diagnosis, the device matched the panel’s conclusions for 98% of the patients. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Cognoa ASD Diagnosis Aid has three main components. One component includes a mobile app for caregivers to answer questions about the child’s behavioral problems and to upload videos of the child. The next component is a video analysis portal for specialists to view and analyze uploaded videos of patients. Another component is a portal for health care providers that allows them to enter answers to preloaded questions about behavior problems, track the information provided by parents, and review a report of the results.
After the machine learning–based device processes the information provided by parents and health care providers, it reports either a positive or a negative diagnosis. If there is insufficient information to make either a positive or a negative diagnosis, the ASD Diagnostic AID will report that no result can be generated.
Some of the risks associated with this device include misdiagnosis and delayed diagnosis of ASD because of a false-positive or false-negative result, or when no result is generated. Researchers said a false-positive result occurred in 15 out of 303 study subjects without ASD and a false-negative result occurred in 1 out of 122 study subjects with ASD.
The FDA emphasized that the device is indicated to aid physicians in the process of diagnosing ASD in children. This means it shouldn’t be treated as a standalone diagnostic device, but as an adjunct to the diagnostic process.
Differences in Palliative Care Delivery Among Adults With Cancer and With Terminal Noncancer Illness in Their Last Year of Life
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
A Service Evaluation of Acute Neurological Patients Managed on Clinically Inappropriate Wards
From Western Sussex Hospitals NHS Foundation Trust, Physiotherapy Department, Chichester, UK (Richard J. Holmes), and Western Sussex Hospitals NHS Foundation Trust, Department of Occupational Therapy, Chichester, UK (Sophie Stratford).
Objective: Despite the benefits of early and frequent input from a neurologist, there is wide variation in the availability of this service, especially in district general hospitals, with many patients managed on clinically inappropriate wards. The purpose of this service evaluation was to explore the impact this had on patient care.
Methods: A retrospective service evaluation was undertaken at a National Health Service hospital by reviewing patient records over a 6-month period. Data related to demographics, processes within the patient’s care, and secondary complications were recorded. Findings were compared with those of stroke patients managed on a specialist stroke ward.
Results: A total of 63 patients were identified, with a mean age of 72 years. The mean length of stay was 25.9 days, with a readmission rate of 16.7%. Only 15.9% of patients were reviewed by a neurologist. There was a high rate of secondary complications, with a number of patients experiencing falls (11.1%), pressure ulcers (14.3%), and health care–acquired infections (33.3%) during their admission.
Conclusions: The lack of specialist input from a neurologist and the management of patients on clinically inappropriate wards may have negatively impacted length of stay, readmission rates, and the frequency of secondary complications.
Keywords: evaluation; clinical safety; neurology; patient-centered care; clinical outcomes; length of stay.
It is estimated that 10% of acute admissions to district general hospitals (DGHs) of the National Health Service (NHS) in the United Kingdom are due to a neurological problem other than stroke.1 In 2011, a joint report from the Royal College of Physicians and the Association of British Neurologists (ABN) recommended that all of these patients should be admitted under the care of a neurologist and be regularly reviewed by a neurologist during their admission.2 The rationale for this recommendation is clear. The involvement of a neurologist has been shown to improve accuracy of the diagnosis3 and significantly reduce length of stay.4,5 Studies have also shown that the involvement of a neurologist has led to a change in the management plan in as high as 79%6 to 89%3 of cases, suggesting that a high proportion of neurological patients not seen by a neurologist are being managed suboptimally.
Despite this, a recent ABN survey of acute neurology services found ongoing wide variations in the availability of this specialist care, with a large proportion of DGHs having limited or no access to a neurologist and very few having dedicated neurology beds.7 While it is recognized that services have been structured in response to the reduced numbers of neurologists within the United Kingdom,8 it is prudent to assess the impact that such services have on patient care.
With this in mind, we planned to evaluate the current provision of care provided to neurological patients in a real-world setting. This was conducted in the context of a neurology liaison service at a DGH with no dedicated neurology beds.
Methods
A retrospective service evaluation was undertaken at a DGH in the southeast of England. The NHS hospital has neurologists on site who provide diagnostic and therapeutic consultations on the wards, but there are no dedicated beds for patients with neurological conditions. Patients requiring neurosurgical input are referred to a tertiary neurosciences center.
Patients were selected from the neurotherapy database if they were referred into the service between August 1, 2019, and January 31, 2020. The neurotherapy database was used as this was the only source that held thorough data on this patient group and allowed for the identification of patients who were not referred into the neurologist’s service. Patients were included if they had a new neurological condition as their primary diagnosis or if they had an exacerbation of an already established neurological condition. If a patient was admitted with more than 1 neurological diagnosis then the primary diagnosis for the admission was to be used in the analysis, though this did not occur during this evaluation. Patients with a primary diagnosis of a stroke were included if they were not managed on the acute stroke ward. Those managed on the stroke ward were excluded so that an analysis of patients managed on wards that were deemed clinically inappropriate could be undertaken. Patients were not included if they had a pre-existing neurological condition (ie, dementia, multiple sclerosis) but were admitted due to a non-neurological cause such as a fall or infection. All patients who met the criteria were included.
A team member independently reviewed each set of patient notes. Demographic data extracted from the medical notes included the patient’s age (on admission), gender, and diagnosis. Medical, nursing, and therapy notes were reviewed to identify secondary complications that arose during the patient’s admission. The secondary complications reviewed were falls (defined as the patient unexpectedly coming to the ground or other lower level), health care–acquired infections (HAIs) (defined as any infection acquired during the hospital admission), and pressure ulcers (defined as injuries to the skin or underlying tissue during the hospital admission). Other details, obtained from the patient administration system, included the length of stay (days), the number of ward moves the patient experienced, the speciality of the consultant responsible for the patient’s care, the discharge destination, and whether the patient was readmitted for any cause within 30 days. All data collected were stored on a password-protected computer and no patient-identifiable data were included.
The results were collated using descriptive statistics. The χ2 test was used to compare categorical data between those patients who were and were not reviewed by a neurologist, and the Mann-Whitney U test was used to compare differences in the length of stay between these 2 groups.
No national data relating to this specific patient group were available within the literature. Therefore, to provide a comparator of neurological patients within the same hospital, data were collected on stroke patients managed on the stroke ward. This group was deemed most appropriate for comparison as they present with similar neurological symptoms but are cared for on a specialist ward. During the evaluation period, 284 stroke patients were admitted to the stroke ward. A sample of 75 patients was randomly selected using a random number generator, and the procedure for data collection was repeated. It was not appropriate to make direct comparative analysis on these 2 groups due to the inherent differences, but it was felt important to provide context with regards to what usual care was like on a specialist ward within the same hospital.
Ethical approval was not required as this was a service evaluation of routinely collected data within a single hospital site.
Results
In total, 63 patients were identified: 26 females and 37 males. The median age of patients was 74 years (range, 39-92 years). These demographic details and comparisons to stroke patients managed on a specialist ward can be seen in Table 1. To quantify the range of diagnoses, the condition groups defined by GIRFT Neurology Methodology9 were used. The most common diagnoses were tumors of the nervous system (25.4%) and traumatic brain and spine injury (23.8%). The other conditions included in the analysis can be seen in Table 2.
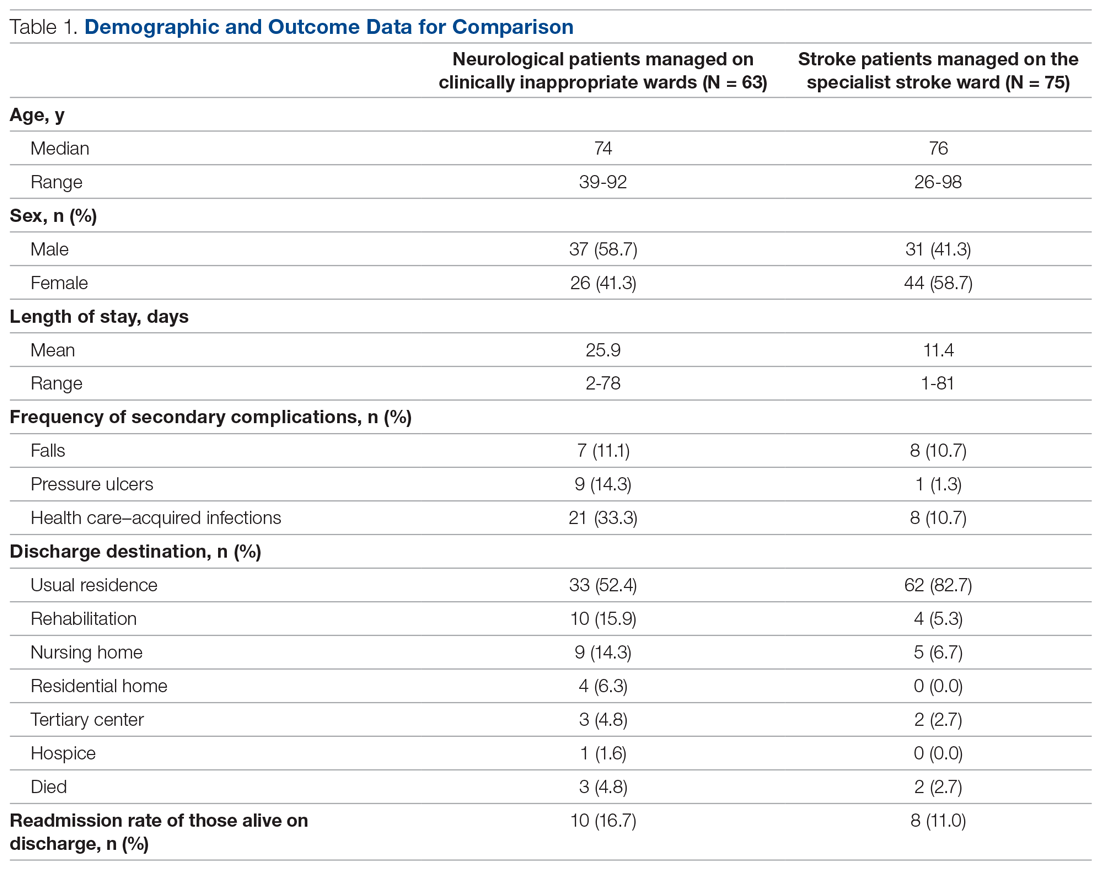
Despite having a neurological condition as their primary diagnosis, only 15.9% of patients were reviewed by a neurologist during their hospital admission. Patients were most commonly under the care of a geriatrician (60.3%), but they were also managed by orthopedics (12.6%), acute medicine (7.9%), respiratory (6.3%), cardiology (4.8%), gastroenterology (3.2%), and surgery (3.2%). One patient (1.6%) was managed by intensivists.
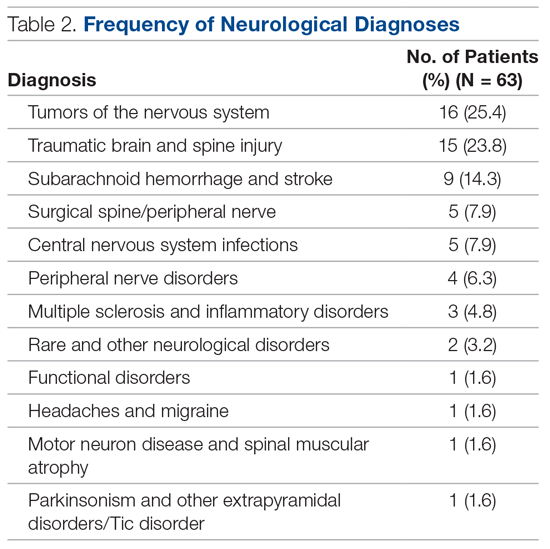
The average length of stay was 25.9 days (range, 2-78 days). This was more than double the average length of stay on the stroke ward (11.4 days) (Table 1) and the national average for patients with neurological conditions (9.78 days).10 During their stay, 33% had 2 or more ward moves, with 1 patient moving wards a total of 6 times. Just over half (52.4%) of the patients returned to their usual residence on discharge. The remainder were discharged to rehabilitation units (15.9%), nursing homes (14.3%), residential homes (6.3%), tertiary centers (4.8%), and hospice (1.6%). Unfortunately, 3 patients (4.8%) passed away. Of those still alive (n = 60), 16.7% were readmitted to the hospital within 30 days, compared to a readmission rate of 11% on the stroke ward. None of the patients who were readmitted were seen by a neurologist during their initial admission.
The frequency of secondary complications was reviewed as a measure of the multidisciplinary management of this patient group. It was noted that 11.1% had a fall on the ward, which was similar to a rate of 10.7% on the stroke ward. More striking was the fact that 14.3% of patients developed a pressure ulcer and 33.3% developed an HAI during their admission, compared with rates of 1.3% and 10.7%, respectively, on the stroke ward (Table 1).
There were no significant differences found in length of stay between those who were and were not reviewed by a neurologist (P = .73). This was also true for categorical data, whereby readmission rate (P = .13), frequency of falls (P = .22), frequency of pressure ulcers (P = .67), and HAIs (P = .81) all failed to show a significant difference between groups.
Discussion
The findings of this service evaluation show markedly poorer outcomes for neurological patients compared to stroke patients managed on a specialist stroke ward. It is suggested that these results are in part due to the lack of specialist input from a neurologist in the majority of cases and the fact that all were managed on clinically inappropriate wards. Only 15.9% of neurological patients were seen by a neurologist. This is a slight improvement compared to previous studies in DGHs that showed rates of 10%1 and 11%,11 but it is still a far cry from the goal of 100% set out in recommendations.2 In addition, the increased readmission rate may be suggestive of suboptimal management, especially given that none of those readmitted had been reviewed by a neurologist. There are undoubtedly other factors that may influence readmissions, such as comorbidities, the severity/complexity of the condition, and the strength of community services. However, the impact of a lack of input from a specialist should not be underestimated, and further evaluation of this factor (with confounding factors controlled) would be beneficial.
The result of an extended length of stay was also a predictable outcome based on previous evidence.4,5 With the potential for suboptimal management plans and inaccurate diagnoses, it is inevitable that the patient’s movement through the hospital system will be impeded. In our example, it is possible that the extended length of stay was influenced by the fact that patients included in the evaluation were managed on nonspecialist wards and a large proportion had multiple ward changes.
Given that the evidence clearly shows that stroke patients are most effectively managed by a multidisciplinary team (MDT) with specialist skills,12 it is likely that other neurological patients, who have similar multifactorial needs, would also benefit. The patients in our evaluation were cared for by nursing staff who lacked specific skills and experience in neurology. The allied health professionals involved were specialists in neurotherapy but were not based on the ward and not directly linked to the ward MDT. A review by Epstein found that the benefits of having a MDT, in any speciality, working together on a ward included improved communication, reduced adverse events, and a reduced length of stay.13 This lack of an effective MDT approach may provide some explanation as to why the average length of stay and the rates of some secondary complications were at such elevated levels.
A systematic review exploring the impact of patients admitted to clinically inappropriate wards in a range of specialities found that these patients were associated with worse outcomes.14 This is supported by our findings, in which a higher rate of pressure ulcers and HAIs were observed when compared to rates in the specialist stroke ward. Again, a potential explanation for this is the impact of patients being managed by clinicians who lack the specialist knowledge of the patient group and the risks they face. Another explanation could be due to the high number of ward moves the patients experienced. Blay et al found that ward moves increased length of stay and carried an associated clinical risk, with the odds of falls and HAIs increasing with each move.15 A case example of this is apparent within our analysis in that the patient who experienced 6 ward moves not only had the longest length of stay (78 days), but also developed a pressure ulcer and 2 HAIs during their admission.
This service evaluation had a number of limitations that should be considered when interpreting the results. First, despite including all patients who met the criteria within the stipulated time frame, the sample size was relatively small, making it difficult to identify consistent patterns of behavior within the data.
Furthermore, caution should be applied when interpreting the comparators used, as the patient groups are not equivalent. The use of comparison against a standard is not a prerequisite in a service evaluation of this nature, but comparators were included to help frame the context for the reader. As such, they should only be used in this way rather than to make any firm conclusions.
Finally, as the evaluation was limited to the use of routinely collected data, there are several variables, other than those reported, which may have influenced the results. For example, it was not possible to ascertain certain demographic details, such as body mass index and socioeconomic factors, nor lifestyle factors such as smoking status, alcohol consumption, and exercise levels, all of which could impact negatively on the outcomes of interest. Furthermore, data were not collected on follow-up services after discharge to evaluate whether these had any impact on readmission rates.
Conclusion
This service evaluation highlights the potential impact of managing neurological patients on clinically inappropriate wards with limited input from a neurologist. There is the potential to ameliorate these impacts by cohorting these patients in neurologist-led beds with a specialist MDT. While there are limitations in the design of our study, including the lack of a controlled comparison, the small sample size, and the fact that this is an evaluation of a single service, the negative impacts to patients are concerning and warrant further investigation.
Corresponding author: Richard J. Holmes, MSc, Physiotherapy Department, St. Richard’s Hospital, Chichester, West Sussex, PO19 6SE; [email protected].
Financial disclosures: None.
1. Kanagaratnam M, Boodhoo A, MacDonald BK, Nitkunan A. Prevalence of acute neurology: a 2-week snapshot in a district general hospital. Clin Med (Lond). 2020;20(2):169-173.
2. Royal College of Physicians. Local adult neurology services for the next decade. Report of a working party. June 2011. Accessed October 29, 2020. https://www.mstrust.org.uk/sites/default/files/files/Local%20adult%20neurology%20services%20for%20the%20next%20decade.pdf
3. McColgan P, Carr AS, McCarron MO. The value of a liaison neurology service in a district general hospital. Postgrad Med J. 2011;87(1025):166-169.
4. Forbes R, Craig J, Callender M, Patterson V. Liaison neurology for acute medical admissions. Clin Med (Lond). 2004;4(3):290.
5. Craig J, Chua R, Russell C, et al. A cohort study of early neurological consultation by telemedicine on the care of neurological inpatients. J Neurol Neurosurg Psychiatry. 2004;75(7):1031-1035.
6. Ali E, Chaila E, Hutchinson M, Tubridy N. The ‘hidden work’ of a hospital neurologist: 1000 consults later. Eur J Neurol. 2010;17(4):e28-e32.
7. Association of British Neurologists. Acute Neurology services survey 2017. Accessed October 29, 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/ABN_2017_Acute_Neurology_Survey.pdf
8. Nitkunan A, Lawrence J, Reilly MM. Neurology Workforce Survey. January 28, 2020. Accessed October 28, 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/2020_ABN_Neurology_Workforce_Survey_2018-19_28_Jan_2020.pdf
9. Fuller G, Connolly M, Mummery C, Williams A. GIRT Neurology Methodology and Initial Summary of Regional Data. September 2019. Accessed October 26, 2020. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2017/07/GIRFT-neurology-methodology-090919-FINAL.pdf
10. The Neurological Alliance. Neuro Numbers 2019. Accessed October 28, 2020. https://www.neural.org.uk/wp-content/uploads/2019/07/neuro-numbers-2019.pdf
11. Cai A, Brex P. A survey of acute neurology at a general hospital in the UK. Clin Med (Lond). 2010;10(6):642-643.
12. Langhorne P, Ramachandra S; Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. 2020;4(4):CD000197.
13. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
14. La Regina M, Guarneri F, Romano E, et al. What Quality and Safety of Care for Patients Admitted to Clinically Inappropriate Wards: a Systematic Review. J Gen Intern Med. 2019;34(7):1314-1321.
15. Blay N, Roche M, Duffield C, Xu X. Intrahospital transfers and adverse patient outcomes: An analysis of administrative health data. J Clin Nurs. 2017;26(23-24):4927-4935.
From Western Sussex Hospitals NHS Foundation Trust, Physiotherapy Department, Chichester, UK (Richard J. Holmes), and Western Sussex Hospitals NHS Foundation Trust, Department of Occupational Therapy, Chichester, UK (Sophie Stratford).
Objective: Despite the benefits of early and frequent input from a neurologist, there is wide variation in the availability of this service, especially in district general hospitals, with many patients managed on clinically inappropriate wards. The purpose of this service evaluation was to explore the impact this had on patient care.
Methods: A retrospective service evaluation was undertaken at a National Health Service hospital by reviewing patient records over a 6-month period. Data related to demographics, processes within the patient’s care, and secondary complications were recorded. Findings were compared with those of stroke patients managed on a specialist stroke ward.
Results: A total of 63 patients were identified, with a mean age of 72 years. The mean length of stay was 25.9 days, with a readmission rate of 16.7%. Only 15.9% of patients were reviewed by a neurologist. There was a high rate of secondary complications, with a number of patients experiencing falls (11.1%), pressure ulcers (14.3%), and health care–acquired infections (33.3%) during their admission.
Conclusions: The lack of specialist input from a neurologist and the management of patients on clinically inappropriate wards may have negatively impacted length of stay, readmission rates, and the frequency of secondary complications.
Keywords: evaluation; clinical safety; neurology; patient-centered care; clinical outcomes; length of stay.
It is estimated that 10% of acute admissions to district general hospitals (DGHs) of the National Health Service (NHS) in the United Kingdom are due to a neurological problem other than stroke.1 In 2011, a joint report from the Royal College of Physicians and the Association of British Neurologists (ABN) recommended that all of these patients should be admitted under the care of a neurologist and be regularly reviewed by a neurologist during their admission.2 The rationale for this recommendation is clear. The involvement of a neurologist has been shown to improve accuracy of the diagnosis3 and significantly reduce length of stay.4,5 Studies have also shown that the involvement of a neurologist has led to a change in the management plan in as high as 79%6 to 89%3 of cases, suggesting that a high proportion of neurological patients not seen by a neurologist are being managed suboptimally.
Despite this, a recent ABN survey of acute neurology services found ongoing wide variations in the availability of this specialist care, with a large proportion of DGHs having limited or no access to a neurologist and very few having dedicated neurology beds.7 While it is recognized that services have been structured in response to the reduced numbers of neurologists within the United Kingdom,8 it is prudent to assess the impact that such services have on patient care.
With this in mind, we planned to evaluate the current provision of care provided to neurological patients in a real-world setting. This was conducted in the context of a neurology liaison service at a DGH with no dedicated neurology beds.
Methods
A retrospective service evaluation was undertaken at a DGH in the southeast of England. The NHS hospital has neurologists on site who provide diagnostic and therapeutic consultations on the wards, but there are no dedicated beds for patients with neurological conditions. Patients requiring neurosurgical input are referred to a tertiary neurosciences center.
Patients were selected from the neurotherapy database if they were referred into the service between August 1, 2019, and January 31, 2020. The neurotherapy database was used as this was the only source that held thorough data on this patient group and allowed for the identification of patients who were not referred into the neurologist’s service. Patients were included if they had a new neurological condition as their primary diagnosis or if they had an exacerbation of an already established neurological condition. If a patient was admitted with more than 1 neurological diagnosis then the primary diagnosis for the admission was to be used in the analysis, though this did not occur during this evaluation. Patients with a primary diagnosis of a stroke were included if they were not managed on the acute stroke ward. Those managed on the stroke ward were excluded so that an analysis of patients managed on wards that were deemed clinically inappropriate could be undertaken. Patients were not included if they had a pre-existing neurological condition (ie, dementia, multiple sclerosis) but were admitted due to a non-neurological cause such as a fall or infection. All patients who met the criteria were included.
A team member independently reviewed each set of patient notes. Demographic data extracted from the medical notes included the patient’s age (on admission), gender, and diagnosis. Medical, nursing, and therapy notes were reviewed to identify secondary complications that arose during the patient’s admission. The secondary complications reviewed were falls (defined as the patient unexpectedly coming to the ground or other lower level), health care–acquired infections (HAIs) (defined as any infection acquired during the hospital admission), and pressure ulcers (defined as injuries to the skin or underlying tissue during the hospital admission). Other details, obtained from the patient administration system, included the length of stay (days), the number of ward moves the patient experienced, the speciality of the consultant responsible for the patient’s care, the discharge destination, and whether the patient was readmitted for any cause within 30 days. All data collected were stored on a password-protected computer and no patient-identifiable data were included.
The results were collated using descriptive statistics. The χ2 test was used to compare categorical data between those patients who were and were not reviewed by a neurologist, and the Mann-Whitney U test was used to compare differences in the length of stay between these 2 groups.
No national data relating to this specific patient group were available within the literature. Therefore, to provide a comparator of neurological patients within the same hospital, data were collected on stroke patients managed on the stroke ward. This group was deemed most appropriate for comparison as they present with similar neurological symptoms but are cared for on a specialist ward. During the evaluation period, 284 stroke patients were admitted to the stroke ward. A sample of 75 patients was randomly selected using a random number generator, and the procedure for data collection was repeated. It was not appropriate to make direct comparative analysis on these 2 groups due to the inherent differences, but it was felt important to provide context with regards to what usual care was like on a specialist ward within the same hospital.
Ethical approval was not required as this was a service evaluation of routinely collected data within a single hospital site.
Results
In total, 63 patients were identified: 26 females and 37 males. The median age of patients was 74 years (range, 39-92 years). These demographic details and comparisons to stroke patients managed on a specialist ward can be seen in Table 1. To quantify the range of diagnoses, the condition groups defined by GIRFT Neurology Methodology9 were used. The most common diagnoses were tumors of the nervous system (25.4%) and traumatic brain and spine injury (23.8%). The other conditions included in the analysis can be seen in Table 2.

Despite having a neurological condition as their primary diagnosis, only 15.9% of patients were reviewed by a neurologist during their hospital admission. Patients were most commonly under the care of a geriatrician (60.3%), but they were also managed by orthopedics (12.6%), acute medicine (7.9%), respiratory (6.3%), cardiology (4.8%), gastroenterology (3.2%), and surgery (3.2%). One patient (1.6%) was managed by intensivists.

The average length of stay was 25.9 days (range, 2-78 days). This was more than double the average length of stay on the stroke ward (11.4 days) (Table 1) and the national average for patients with neurological conditions (9.78 days).10 During their stay, 33% had 2 or more ward moves, with 1 patient moving wards a total of 6 times. Just over half (52.4%) of the patients returned to their usual residence on discharge. The remainder were discharged to rehabilitation units (15.9%), nursing homes (14.3%), residential homes (6.3%), tertiary centers (4.8%), and hospice (1.6%). Unfortunately, 3 patients (4.8%) passed away. Of those still alive (n = 60), 16.7% were readmitted to the hospital within 30 days, compared to a readmission rate of 11% on the stroke ward. None of the patients who were readmitted were seen by a neurologist during their initial admission.
The frequency of secondary complications was reviewed as a measure of the multidisciplinary management of this patient group. It was noted that 11.1% had a fall on the ward, which was similar to a rate of 10.7% on the stroke ward. More striking was the fact that 14.3% of patients developed a pressure ulcer and 33.3% developed an HAI during their admission, compared with rates of 1.3% and 10.7%, respectively, on the stroke ward (Table 1).
There were no significant differences found in length of stay between those who were and were not reviewed by a neurologist (P = .73). This was also true for categorical data, whereby readmission rate (P = .13), frequency of falls (P = .22), frequency of pressure ulcers (P = .67), and HAIs (P = .81) all failed to show a significant difference between groups.
Discussion
The findings of this service evaluation show markedly poorer outcomes for neurological patients compared to stroke patients managed on a specialist stroke ward. It is suggested that these results are in part due to the lack of specialist input from a neurologist in the majority of cases and the fact that all were managed on clinically inappropriate wards. Only 15.9% of neurological patients were seen by a neurologist. This is a slight improvement compared to previous studies in DGHs that showed rates of 10%1 and 11%,11 but it is still a far cry from the goal of 100% set out in recommendations.2 In addition, the increased readmission rate may be suggestive of suboptimal management, especially given that none of those readmitted had been reviewed by a neurologist. There are undoubtedly other factors that may influence readmissions, such as comorbidities, the severity/complexity of the condition, and the strength of community services. However, the impact of a lack of input from a specialist should not be underestimated, and further evaluation of this factor (with confounding factors controlled) would be beneficial.
The result of an extended length of stay was also a predictable outcome based on previous evidence.4,5 With the potential for suboptimal management plans and inaccurate diagnoses, it is inevitable that the patient’s movement through the hospital system will be impeded. In our example, it is possible that the extended length of stay was influenced by the fact that patients included in the evaluation were managed on nonspecialist wards and a large proportion had multiple ward changes.
Given that the evidence clearly shows that stroke patients are most effectively managed by a multidisciplinary team (MDT) with specialist skills,12 it is likely that other neurological patients, who have similar multifactorial needs, would also benefit. The patients in our evaluation were cared for by nursing staff who lacked specific skills and experience in neurology. The allied health professionals involved were specialists in neurotherapy but were not based on the ward and not directly linked to the ward MDT. A review by Epstein found that the benefits of having a MDT, in any speciality, working together on a ward included improved communication, reduced adverse events, and a reduced length of stay.13 This lack of an effective MDT approach may provide some explanation as to why the average length of stay and the rates of some secondary complications were at such elevated levels.
A systematic review exploring the impact of patients admitted to clinically inappropriate wards in a range of specialities found that these patients were associated with worse outcomes.14 This is supported by our findings, in which a higher rate of pressure ulcers and HAIs were observed when compared to rates in the specialist stroke ward. Again, a potential explanation for this is the impact of patients being managed by clinicians who lack the specialist knowledge of the patient group and the risks they face. Another explanation could be due to the high number of ward moves the patients experienced. Blay et al found that ward moves increased length of stay and carried an associated clinical risk, with the odds of falls and HAIs increasing with each move.15 A case example of this is apparent within our analysis in that the patient who experienced 6 ward moves not only had the longest length of stay (78 days), but also developed a pressure ulcer and 2 HAIs during their admission.
This service evaluation had a number of limitations that should be considered when interpreting the results. First, despite including all patients who met the criteria within the stipulated time frame, the sample size was relatively small, making it difficult to identify consistent patterns of behavior within the data.
Furthermore, caution should be applied when interpreting the comparators used, as the patient groups are not equivalent. The use of comparison against a standard is not a prerequisite in a service evaluation of this nature, but comparators were included to help frame the context for the reader. As such, they should only be used in this way rather than to make any firm conclusions.
Finally, as the evaluation was limited to the use of routinely collected data, there are several variables, other than those reported, which may have influenced the results. For example, it was not possible to ascertain certain demographic details, such as body mass index and socioeconomic factors, nor lifestyle factors such as smoking status, alcohol consumption, and exercise levels, all of which could impact negatively on the outcomes of interest. Furthermore, data were not collected on follow-up services after discharge to evaluate whether these had any impact on readmission rates.
Conclusion
This service evaluation highlights the potential impact of managing neurological patients on clinically inappropriate wards with limited input from a neurologist. There is the potential to ameliorate these impacts by cohorting these patients in neurologist-led beds with a specialist MDT. While there are limitations in the design of our study, including the lack of a controlled comparison, the small sample size, and the fact that this is an evaluation of a single service, the negative impacts to patients are concerning and warrant further investigation.
Corresponding author: Richard J. Holmes, MSc, Physiotherapy Department, St. Richard’s Hospital, Chichester, West Sussex, PO19 6SE; [email protected].
Financial disclosures: None.
From Western Sussex Hospitals NHS Foundation Trust, Physiotherapy Department, Chichester, UK (Richard J. Holmes), and Western Sussex Hospitals NHS Foundation Trust, Department of Occupational Therapy, Chichester, UK (Sophie Stratford).
Objective: Despite the benefits of early and frequent input from a neurologist, there is wide variation in the availability of this service, especially in district general hospitals, with many patients managed on clinically inappropriate wards. The purpose of this service evaluation was to explore the impact this had on patient care.
Methods: A retrospective service evaluation was undertaken at a National Health Service hospital by reviewing patient records over a 6-month period. Data related to demographics, processes within the patient’s care, and secondary complications were recorded. Findings were compared with those of stroke patients managed on a specialist stroke ward.
Results: A total of 63 patients were identified, with a mean age of 72 years. The mean length of stay was 25.9 days, with a readmission rate of 16.7%. Only 15.9% of patients were reviewed by a neurologist. There was a high rate of secondary complications, with a number of patients experiencing falls (11.1%), pressure ulcers (14.3%), and health care–acquired infections (33.3%) during their admission.
Conclusions: The lack of specialist input from a neurologist and the management of patients on clinically inappropriate wards may have negatively impacted length of stay, readmission rates, and the frequency of secondary complications.
Keywords: evaluation; clinical safety; neurology; patient-centered care; clinical outcomes; length of stay.
It is estimated that 10% of acute admissions to district general hospitals (DGHs) of the National Health Service (NHS) in the United Kingdom are due to a neurological problem other than stroke.1 In 2011, a joint report from the Royal College of Physicians and the Association of British Neurologists (ABN) recommended that all of these patients should be admitted under the care of a neurologist and be regularly reviewed by a neurologist during their admission.2 The rationale for this recommendation is clear. The involvement of a neurologist has been shown to improve accuracy of the diagnosis3 and significantly reduce length of stay.4,5 Studies have also shown that the involvement of a neurologist has led to a change in the management plan in as high as 79%6 to 89%3 of cases, suggesting that a high proportion of neurological patients not seen by a neurologist are being managed suboptimally.
Despite this, a recent ABN survey of acute neurology services found ongoing wide variations in the availability of this specialist care, with a large proportion of DGHs having limited or no access to a neurologist and very few having dedicated neurology beds.7 While it is recognized that services have been structured in response to the reduced numbers of neurologists within the United Kingdom,8 it is prudent to assess the impact that such services have on patient care.
With this in mind, we planned to evaluate the current provision of care provided to neurological patients in a real-world setting. This was conducted in the context of a neurology liaison service at a DGH with no dedicated neurology beds.
Methods
A retrospective service evaluation was undertaken at a DGH in the southeast of England. The NHS hospital has neurologists on site who provide diagnostic and therapeutic consultations on the wards, but there are no dedicated beds for patients with neurological conditions. Patients requiring neurosurgical input are referred to a tertiary neurosciences center.
Patients were selected from the neurotherapy database if they were referred into the service between August 1, 2019, and January 31, 2020. The neurotherapy database was used as this was the only source that held thorough data on this patient group and allowed for the identification of patients who were not referred into the neurologist’s service. Patients were included if they had a new neurological condition as their primary diagnosis or if they had an exacerbation of an already established neurological condition. If a patient was admitted with more than 1 neurological diagnosis then the primary diagnosis for the admission was to be used in the analysis, though this did not occur during this evaluation. Patients with a primary diagnosis of a stroke were included if they were not managed on the acute stroke ward. Those managed on the stroke ward were excluded so that an analysis of patients managed on wards that were deemed clinically inappropriate could be undertaken. Patients were not included if they had a pre-existing neurological condition (ie, dementia, multiple sclerosis) but were admitted due to a non-neurological cause such as a fall or infection. All patients who met the criteria were included.
A team member independently reviewed each set of patient notes. Demographic data extracted from the medical notes included the patient’s age (on admission), gender, and diagnosis. Medical, nursing, and therapy notes were reviewed to identify secondary complications that arose during the patient’s admission. The secondary complications reviewed were falls (defined as the patient unexpectedly coming to the ground or other lower level), health care–acquired infections (HAIs) (defined as any infection acquired during the hospital admission), and pressure ulcers (defined as injuries to the skin or underlying tissue during the hospital admission). Other details, obtained from the patient administration system, included the length of stay (days), the number of ward moves the patient experienced, the speciality of the consultant responsible for the patient’s care, the discharge destination, and whether the patient was readmitted for any cause within 30 days. All data collected were stored on a password-protected computer and no patient-identifiable data were included.
The results were collated using descriptive statistics. The χ2 test was used to compare categorical data between those patients who were and were not reviewed by a neurologist, and the Mann-Whitney U test was used to compare differences in the length of stay between these 2 groups.
No national data relating to this specific patient group were available within the literature. Therefore, to provide a comparator of neurological patients within the same hospital, data were collected on stroke patients managed on the stroke ward. This group was deemed most appropriate for comparison as they present with similar neurological symptoms but are cared for on a specialist ward. During the evaluation period, 284 stroke patients were admitted to the stroke ward. A sample of 75 patients was randomly selected using a random number generator, and the procedure for data collection was repeated. It was not appropriate to make direct comparative analysis on these 2 groups due to the inherent differences, but it was felt important to provide context with regards to what usual care was like on a specialist ward within the same hospital.
Ethical approval was not required as this was a service evaluation of routinely collected data within a single hospital site.
Results
In total, 63 patients were identified: 26 females and 37 males. The median age of patients was 74 years (range, 39-92 years). These demographic details and comparisons to stroke patients managed on a specialist ward can be seen in Table 1. To quantify the range of diagnoses, the condition groups defined by GIRFT Neurology Methodology9 were used. The most common diagnoses were tumors of the nervous system (25.4%) and traumatic brain and spine injury (23.8%). The other conditions included in the analysis can be seen in Table 2.

Despite having a neurological condition as their primary diagnosis, only 15.9% of patients were reviewed by a neurologist during their hospital admission. Patients were most commonly under the care of a geriatrician (60.3%), but they were also managed by orthopedics (12.6%), acute medicine (7.9%), respiratory (6.3%), cardiology (4.8%), gastroenterology (3.2%), and surgery (3.2%). One patient (1.6%) was managed by intensivists.

The average length of stay was 25.9 days (range, 2-78 days). This was more than double the average length of stay on the stroke ward (11.4 days) (Table 1) and the national average for patients with neurological conditions (9.78 days).10 During their stay, 33% had 2 or more ward moves, with 1 patient moving wards a total of 6 times. Just over half (52.4%) of the patients returned to their usual residence on discharge. The remainder were discharged to rehabilitation units (15.9%), nursing homes (14.3%), residential homes (6.3%), tertiary centers (4.8%), and hospice (1.6%). Unfortunately, 3 patients (4.8%) passed away. Of those still alive (n = 60), 16.7% were readmitted to the hospital within 30 days, compared to a readmission rate of 11% on the stroke ward. None of the patients who were readmitted were seen by a neurologist during their initial admission.
The frequency of secondary complications was reviewed as a measure of the multidisciplinary management of this patient group. It was noted that 11.1% had a fall on the ward, which was similar to a rate of 10.7% on the stroke ward. More striking was the fact that 14.3% of patients developed a pressure ulcer and 33.3% developed an HAI during their admission, compared with rates of 1.3% and 10.7%, respectively, on the stroke ward (Table 1).
There were no significant differences found in length of stay between those who were and were not reviewed by a neurologist (P = .73). This was also true for categorical data, whereby readmission rate (P = .13), frequency of falls (P = .22), frequency of pressure ulcers (P = .67), and HAIs (P = .81) all failed to show a significant difference between groups.
Discussion
The findings of this service evaluation show markedly poorer outcomes for neurological patients compared to stroke patients managed on a specialist stroke ward. It is suggested that these results are in part due to the lack of specialist input from a neurologist in the majority of cases and the fact that all were managed on clinically inappropriate wards. Only 15.9% of neurological patients were seen by a neurologist. This is a slight improvement compared to previous studies in DGHs that showed rates of 10%1 and 11%,11 but it is still a far cry from the goal of 100% set out in recommendations.2 In addition, the increased readmission rate may be suggestive of suboptimal management, especially given that none of those readmitted had been reviewed by a neurologist. There are undoubtedly other factors that may influence readmissions, such as comorbidities, the severity/complexity of the condition, and the strength of community services. However, the impact of a lack of input from a specialist should not be underestimated, and further evaluation of this factor (with confounding factors controlled) would be beneficial.
The result of an extended length of stay was also a predictable outcome based on previous evidence.4,5 With the potential for suboptimal management plans and inaccurate diagnoses, it is inevitable that the patient’s movement through the hospital system will be impeded. In our example, it is possible that the extended length of stay was influenced by the fact that patients included in the evaluation were managed on nonspecialist wards and a large proportion had multiple ward changes.
Given that the evidence clearly shows that stroke patients are most effectively managed by a multidisciplinary team (MDT) with specialist skills,12 it is likely that other neurological patients, who have similar multifactorial needs, would also benefit. The patients in our evaluation were cared for by nursing staff who lacked specific skills and experience in neurology. The allied health professionals involved were specialists in neurotherapy but were not based on the ward and not directly linked to the ward MDT. A review by Epstein found that the benefits of having a MDT, in any speciality, working together on a ward included improved communication, reduced adverse events, and a reduced length of stay.13 This lack of an effective MDT approach may provide some explanation as to why the average length of stay and the rates of some secondary complications were at such elevated levels.
A systematic review exploring the impact of patients admitted to clinically inappropriate wards in a range of specialities found that these patients were associated with worse outcomes.14 This is supported by our findings, in which a higher rate of pressure ulcers and HAIs were observed when compared to rates in the specialist stroke ward. Again, a potential explanation for this is the impact of patients being managed by clinicians who lack the specialist knowledge of the patient group and the risks they face. Another explanation could be due to the high number of ward moves the patients experienced. Blay et al found that ward moves increased length of stay and carried an associated clinical risk, with the odds of falls and HAIs increasing with each move.15 A case example of this is apparent within our analysis in that the patient who experienced 6 ward moves not only had the longest length of stay (78 days), but also developed a pressure ulcer and 2 HAIs during their admission.
This service evaluation had a number of limitations that should be considered when interpreting the results. First, despite including all patients who met the criteria within the stipulated time frame, the sample size was relatively small, making it difficult to identify consistent patterns of behavior within the data.
Furthermore, caution should be applied when interpreting the comparators used, as the patient groups are not equivalent. The use of comparison against a standard is not a prerequisite in a service evaluation of this nature, but comparators were included to help frame the context for the reader. As such, they should only be used in this way rather than to make any firm conclusions.
Finally, as the evaluation was limited to the use of routinely collected data, there are several variables, other than those reported, which may have influenced the results. For example, it was not possible to ascertain certain demographic details, such as body mass index and socioeconomic factors, nor lifestyle factors such as smoking status, alcohol consumption, and exercise levels, all of which could impact negatively on the outcomes of interest. Furthermore, data were not collected on follow-up services after discharge to evaluate whether these had any impact on readmission rates.
Conclusion
This service evaluation highlights the potential impact of managing neurological patients on clinically inappropriate wards with limited input from a neurologist. There is the potential to ameliorate these impacts by cohorting these patients in neurologist-led beds with a specialist MDT. While there are limitations in the design of our study, including the lack of a controlled comparison, the small sample size, and the fact that this is an evaluation of a single service, the negative impacts to patients are concerning and warrant further investigation.
Corresponding author: Richard J. Holmes, MSc, Physiotherapy Department, St. Richard’s Hospital, Chichester, West Sussex, PO19 6SE; [email protected].
Financial disclosures: None.
1. Kanagaratnam M, Boodhoo A, MacDonald BK, Nitkunan A. Prevalence of acute neurology: a 2-week snapshot in a district general hospital. Clin Med (Lond). 2020;20(2):169-173.
2. Royal College of Physicians. Local adult neurology services for the next decade. Report of a working party. June 2011. Accessed October 29, 2020. https://www.mstrust.org.uk/sites/default/files/files/Local%20adult%20neurology%20services%20for%20the%20next%20decade.pdf
3. McColgan P, Carr AS, McCarron MO. The value of a liaison neurology service in a district general hospital. Postgrad Med J. 2011;87(1025):166-169.
4. Forbes R, Craig J, Callender M, Patterson V. Liaison neurology for acute medical admissions. Clin Med (Lond). 2004;4(3):290.
5. Craig J, Chua R, Russell C, et al. A cohort study of early neurological consultation by telemedicine on the care of neurological inpatients. J Neurol Neurosurg Psychiatry. 2004;75(7):1031-1035.
6. Ali E, Chaila E, Hutchinson M, Tubridy N. The ‘hidden work’ of a hospital neurologist: 1000 consults later. Eur J Neurol. 2010;17(4):e28-e32.
7. Association of British Neurologists. Acute Neurology services survey 2017. Accessed October 29, 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/ABN_2017_Acute_Neurology_Survey.pdf
8. Nitkunan A, Lawrence J, Reilly MM. Neurology Workforce Survey. January 28, 2020. Accessed October 28, 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/2020_ABN_Neurology_Workforce_Survey_2018-19_28_Jan_2020.pdf
9. Fuller G, Connolly M, Mummery C, Williams A. GIRT Neurology Methodology and Initial Summary of Regional Data. September 2019. Accessed October 26, 2020. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2017/07/GIRFT-neurology-methodology-090919-FINAL.pdf
10. The Neurological Alliance. Neuro Numbers 2019. Accessed October 28, 2020. https://www.neural.org.uk/wp-content/uploads/2019/07/neuro-numbers-2019.pdf
11. Cai A, Brex P. A survey of acute neurology at a general hospital in the UK. Clin Med (Lond). 2010;10(6):642-643.
12. Langhorne P, Ramachandra S; Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. 2020;4(4):CD000197.
13. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
14. La Regina M, Guarneri F, Romano E, et al. What Quality and Safety of Care for Patients Admitted to Clinically Inappropriate Wards: a Systematic Review. J Gen Intern Med. 2019;34(7):1314-1321.
15. Blay N, Roche M, Duffield C, Xu X. Intrahospital transfers and adverse patient outcomes: An analysis of administrative health data. J Clin Nurs. 2017;26(23-24):4927-4935.
1. Kanagaratnam M, Boodhoo A, MacDonald BK, Nitkunan A. Prevalence of acute neurology: a 2-week snapshot in a district general hospital. Clin Med (Lond). 2020;20(2):169-173.
2. Royal College of Physicians. Local adult neurology services for the next decade. Report of a working party. June 2011. Accessed October 29, 2020. https://www.mstrust.org.uk/sites/default/files/files/Local%20adult%20neurology%20services%20for%20the%20next%20decade.pdf
3. McColgan P, Carr AS, McCarron MO. The value of a liaison neurology service in a district general hospital. Postgrad Med J. 2011;87(1025):166-169.
4. Forbes R, Craig J, Callender M, Patterson V. Liaison neurology for acute medical admissions. Clin Med (Lond). 2004;4(3):290.
5. Craig J, Chua R, Russell C, et al. A cohort study of early neurological consultation by telemedicine on the care of neurological inpatients. J Neurol Neurosurg Psychiatry. 2004;75(7):1031-1035.
6. Ali E, Chaila E, Hutchinson M, Tubridy N. The ‘hidden work’ of a hospital neurologist: 1000 consults later. Eur J Neurol. 2010;17(4):e28-e32.
7. Association of British Neurologists. Acute Neurology services survey 2017. Accessed October 29, 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/ABN_2017_Acute_Neurology_Survey.pdf
8. Nitkunan A, Lawrence J, Reilly MM. Neurology Workforce Survey. January 28, 2020. Accessed October 28, 2020. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/2020_ABN_Neurology_Workforce_Survey_2018-19_28_Jan_2020.pdf
9. Fuller G, Connolly M, Mummery C, Williams A. GIRT Neurology Methodology and Initial Summary of Regional Data. September 2019. Accessed October 26, 2020. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2017/07/GIRFT-neurology-methodology-090919-FINAL.pdf
10. The Neurological Alliance. Neuro Numbers 2019. Accessed October 28, 2020. https://www.neural.org.uk/wp-content/uploads/2019/07/neuro-numbers-2019.pdf
11. Cai A, Brex P. A survey of acute neurology at a general hospital in the UK. Clin Med (Lond). 2010;10(6):642-643.
12. Langhorne P, Ramachandra S; Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. 2020;4(4):CD000197.
13. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
14. La Regina M, Guarneri F, Romano E, et al. What Quality and Safety of Care for Patients Admitted to Clinically Inappropriate Wards: a Systematic Review. J Gen Intern Med. 2019;34(7):1314-1321.
15. Blay N, Roche M, Duffield C, Xu X. Intrahospital transfers and adverse patient outcomes: An analysis of administrative health data. J Clin Nurs. 2017;26(23-24):4927-4935.
New AHA/ASA guideline on secondary stroke prevention
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.









