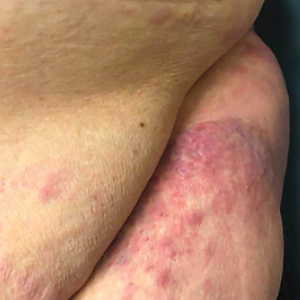
User login
Florida Legislature Passes Free Skin Cancer Screening Requirement
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
Few Childhood Cancer Survivors Get Recommended Screenings
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
AI in Clinical Dermatology: Consider Limitations, Current Issues
SAN DIEGO — Just a day before the annual meeting of the American Academy of Dermatology (AAD) began, .
Not least of the problems among the 41 apps evaluated, the majority offered no supporting evidence, no information about whether the app performance had been validated, and no information about how user privacy would be managed, reported Shannon Wongvibulsin, MD, PhD, a resident in the dermatology program at the University of California, Los Angeles, and her coauthors.
The findings from this report were also summarized in a poster at the AAD meeting, and the major themes were reiterated in several AAD symposia devoted to AI at the meeting. Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York City, was one of those who weighed in on the future of AI. Although she was the senior author of the report, she did not address the report or poster directly, but her presentation on the practical aspects of incorporating AI into dermatology practice revisited several of its themes.
Of the different themes, perhaps the most important were the concept that the source of AI data matters and the point that practicing clinicians should be familiar with the data source.
To date, “there is not much transparency in what data AI models are using,” Dr. Rotemberg said at the meeting. Based on the expectation that dermatologists will be purchasing rather than developing their own AI-based systems, she reiterated more than once that “transparency of data is critical,” even if vendors are often reluctant to reveal how their proprietary systems have been developed.
Few Dermatology Apps Are Vetted for Accuracy
In the poster and in the more detailed JAMA Dermatology paper, Dr. Wongvibulsin and her coinvestigators evaluated direct-to-consumer downloadable apps that claim to help with the assessment and management of skin conditions. Very few provided any supporting evidence of accuracy or even information about how they functioned.
The 41 apps were drawn from more than 300 apps; the others were excluded for failing to meet such criteria as failing to employ AI, not being available in English, or not addressing clinical management of dermatologic diseases. Dr. Wongvibulsin pointed out that none of the apps had been granted regulatory approval even though only two provided a disclaimer to that effect.
In all, just 5 of the 41 provided supporting evidence from a peer-reviewed journal, and less than 40% were created with any input from a dermatologist, Dr. Wongvibulsin reported. The result is that the utility and accuracy of these apps were, for the most part, difficult to judge.
“At a minimum, app developers should provide details on what AI algorithms are used, what data sets were used for training, testing, and validation, whether there was any clinician input, whether there are any supporting publications, how user-submitted images are used, and if there are any measures used to ensure data privacy,” Dr. Wongvibulsin wrote in the poster.
For AI-based apps or systems designed for use by dermatologists, Dr. Rotemberg made similar assertions in her overview of what clinicians should be considering for proprietary AI systems, whether to help with diagnosis or improve office efficiency.
Only One Dermatology App Cleared By the FDA
Currently, the only FDA-cleared app for dermatologic use is the DermaSensor, an AI-driven device. It was cleared for use in January 2024 for the evaluation of skin lesions “suggestive” of melanoma, basal cell carcinoma, and/or squamous cell carcinoma in patients aged ≥ 40 years “to assist health care providers in determining whether to refer a patient to a dermatologist,” according to an FDA announcement.
Using elastic scattering spectroscopy to analyze light reflecting off the skin to detect malignancy, the manufacturer’s promotional material claims a 96% sensitivity and a 97% specificity.
While Dr. Rotemberg did not comment on these claims, she cautioned that AI models differ with regards to how they were trained and the relative heterogeneity of the training dataset defined by types of patients, types of skin, and types of AI learning processes. All of these variables are relevant in whether the AI will perform in a given clinical setting at the level it performed during development.
“The most accurate models employ narrow datasets, but these do not necessarily mimic what we see in practice,” she said.
In addition, even when an AI-based system is working for a given task, it must be monitored over time. Dr. Rotemberg warned about the potential for “data drift,” which describes the slow evolution in how diseases present, their prevalence by age, or other factors that might affect AI performance. She explained that repeated validation is needed to ensure that the AI-based models remain as accurate over time as they were when first used.
Many of these concepts were explored in a consensus statement from the International Skin Imaging Collaboration AI Working Group, published in JAMA Dermatology in December 2021. The statement, of which Dr. Rotemberg was a coauthor, provided recommendations for the principles of AI algorithm development specific to dermatologic considerations.
At the AAD symposium, Dr. Rotemberg asked the audience for suggestions about the needs they hoped AI might address for in office care or efficiency. Their responses included generating prior authorizations for prescriptions, triaging email for importance, and helping to improve efficiency for common front desk tasks. She liked all of these suggestions, but she warned that as powerful as it can be, AI is not likely to provide technology that will fit seamlessly into workflows without adjustment.
“Our current systems do not allow human integration of AI models,” Dr. Rotemberg said. Rather than counting on AI to adapt to current practices, she cautioned that “we may have to redesign our entire structure to actually be able to accommodate AI-based” systems. The risk for users is tasks that become more challenging before they become easier.
AI Should Not Be a Black Box
AI is promising, but it is not magic, according to other investigators, including Tofunmi A. Omiye, PhD, a postdoctoral scholar in dermatology at Stanford University, California. First author of a recent review of AI in dermatology published in Frontiers in Medicine, Dr. Omiye agreed that clinicians who want to employ AI should be able to understand basic principles if they want the technology to perform as expected.
“I totally agree that physicians should at least have a basic understanding of the data sources for training AI models as we have found that to be important to the performance of these models in the clinical setting,” he told this news organization.
“Beyond understanding the data sources, I believe physicians can also try to have a comprehensive understanding of what AI means, its training process, and evaluation as this will help them to evaluate its utility in their practice,” he added. He also reinforced the relevance of data drift.
“Concepts like distribution shift — where models perform less well over time due to changes in the patient population — are also important to keep in mind,” Dr. Omiye said.
Dr. Wongvibulsin, Dr. Rotemberg, and Dr. Omiye reported no potential financial conflicts of interest relevant to this topic.
A version of this article appeared on Medscape.com .
SAN DIEGO — Just a day before the annual meeting of the American Academy of Dermatology (AAD) began, .
Not least of the problems among the 41 apps evaluated, the majority offered no supporting evidence, no information about whether the app performance had been validated, and no information about how user privacy would be managed, reported Shannon Wongvibulsin, MD, PhD, a resident in the dermatology program at the University of California, Los Angeles, and her coauthors.
The findings from this report were also summarized in a poster at the AAD meeting, and the major themes were reiterated in several AAD symposia devoted to AI at the meeting. Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York City, was one of those who weighed in on the future of AI. Although she was the senior author of the report, she did not address the report or poster directly, but her presentation on the practical aspects of incorporating AI into dermatology practice revisited several of its themes.
Of the different themes, perhaps the most important were the concept that the source of AI data matters and the point that practicing clinicians should be familiar with the data source.
To date, “there is not much transparency in what data AI models are using,” Dr. Rotemberg said at the meeting. Based on the expectation that dermatologists will be purchasing rather than developing their own AI-based systems, she reiterated more than once that “transparency of data is critical,” even if vendors are often reluctant to reveal how their proprietary systems have been developed.
Few Dermatology Apps Are Vetted for Accuracy
In the poster and in the more detailed JAMA Dermatology paper, Dr. Wongvibulsin and her coinvestigators evaluated direct-to-consumer downloadable apps that claim to help with the assessment and management of skin conditions. Very few provided any supporting evidence of accuracy or even information about how they functioned.
The 41 apps were drawn from more than 300 apps; the others were excluded for failing to meet such criteria as failing to employ AI, not being available in English, or not addressing clinical management of dermatologic diseases. Dr. Wongvibulsin pointed out that none of the apps had been granted regulatory approval even though only two provided a disclaimer to that effect.
In all, just 5 of the 41 provided supporting evidence from a peer-reviewed journal, and less than 40% were created with any input from a dermatologist, Dr. Wongvibulsin reported. The result is that the utility and accuracy of these apps were, for the most part, difficult to judge.
“At a minimum, app developers should provide details on what AI algorithms are used, what data sets were used for training, testing, and validation, whether there was any clinician input, whether there are any supporting publications, how user-submitted images are used, and if there are any measures used to ensure data privacy,” Dr. Wongvibulsin wrote in the poster.
For AI-based apps or systems designed for use by dermatologists, Dr. Rotemberg made similar assertions in her overview of what clinicians should be considering for proprietary AI systems, whether to help with diagnosis or improve office efficiency.
Only One Dermatology App Cleared By the FDA
Currently, the only FDA-cleared app for dermatologic use is the DermaSensor, an AI-driven device. It was cleared for use in January 2024 for the evaluation of skin lesions “suggestive” of melanoma, basal cell carcinoma, and/or squamous cell carcinoma in patients aged ≥ 40 years “to assist health care providers in determining whether to refer a patient to a dermatologist,” according to an FDA announcement.
Using elastic scattering spectroscopy to analyze light reflecting off the skin to detect malignancy, the manufacturer’s promotional material claims a 96% sensitivity and a 97% specificity.
While Dr. Rotemberg did not comment on these claims, she cautioned that AI models differ with regards to how they were trained and the relative heterogeneity of the training dataset defined by types of patients, types of skin, and types of AI learning processes. All of these variables are relevant in whether the AI will perform in a given clinical setting at the level it performed during development.
“The most accurate models employ narrow datasets, but these do not necessarily mimic what we see in practice,” she said.
In addition, even when an AI-based system is working for a given task, it must be monitored over time. Dr. Rotemberg warned about the potential for “data drift,” which describes the slow evolution in how diseases present, their prevalence by age, or other factors that might affect AI performance. She explained that repeated validation is needed to ensure that the AI-based models remain as accurate over time as they were when first used.
Many of these concepts were explored in a consensus statement from the International Skin Imaging Collaboration AI Working Group, published in JAMA Dermatology in December 2021. The statement, of which Dr. Rotemberg was a coauthor, provided recommendations for the principles of AI algorithm development specific to dermatologic considerations.
At the AAD symposium, Dr. Rotemberg asked the audience for suggestions about the needs they hoped AI might address for in office care or efficiency. Their responses included generating prior authorizations for prescriptions, triaging email for importance, and helping to improve efficiency for common front desk tasks. She liked all of these suggestions, but she warned that as powerful as it can be, AI is not likely to provide technology that will fit seamlessly into workflows without adjustment.
“Our current systems do not allow human integration of AI models,” Dr. Rotemberg said. Rather than counting on AI to adapt to current practices, she cautioned that “we may have to redesign our entire structure to actually be able to accommodate AI-based” systems. The risk for users is tasks that become more challenging before they become easier.
AI Should Not Be a Black Box
AI is promising, but it is not magic, according to other investigators, including Tofunmi A. Omiye, PhD, a postdoctoral scholar in dermatology at Stanford University, California. First author of a recent review of AI in dermatology published in Frontiers in Medicine, Dr. Omiye agreed that clinicians who want to employ AI should be able to understand basic principles if they want the technology to perform as expected.
“I totally agree that physicians should at least have a basic understanding of the data sources for training AI models as we have found that to be important to the performance of these models in the clinical setting,” he told this news organization.
“Beyond understanding the data sources, I believe physicians can also try to have a comprehensive understanding of what AI means, its training process, and evaluation as this will help them to evaluate its utility in their practice,” he added. He also reinforced the relevance of data drift.
“Concepts like distribution shift — where models perform less well over time due to changes in the patient population — are also important to keep in mind,” Dr. Omiye said.
Dr. Wongvibulsin, Dr. Rotemberg, and Dr. Omiye reported no potential financial conflicts of interest relevant to this topic.
A version of this article appeared on Medscape.com .
SAN DIEGO — Just a day before the annual meeting of the American Academy of Dermatology (AAD) began, .
Not least of the problems among the 41 apps evaluated, the majority offered no supporting evidence, no information about whether the app performance had been validated, and no information about how user privacy would be managed, reported Shannon Wongvibulsin, MD, PhD, a resident in the dermatology program at the University of California, Los Angeles, and her coauthors.
The findings from this report were also summarized in a poster at the AAD meeting, and the major themes were reiterated in several AAD symposia devoted to AI at the meeting. Veronica Rotemberg, MD, PhD, a dermatologist at Memorial Sloan Kettering Cancer Center, New York City, was one of those who weighed in on the future of AI. Although she was the senior author of the report, she did not address the report or poster directly, but her presentation on the practical aspects of incorporating AI into dermatology practice revisited several of its themes.
Of the different themes, perhaps the most important were the concept that the source of AI data matters and the point that practicing clinicians should be familiar with the data source.
To date, “there is not much transparency in what data AI models are using,” Dr. Rotemberg said at the meeting. Based on the expectation that dermatologists will be purchasing rather than developing their own AI-based systems, she reiterated more than once that “transparency of data is critical,” even if vendors are often reluctant to reveal how their proprietary systems have been developed.
Few Dermatology Apps Are Vetted for Accuracy
In the poster and in the more detailed JAMA Dermatology paper, Dr. Wongvibulsin and her coinvestigators evaluated direct-to-consumer downloadable apps that claim to help with the assessment and management of skin conditions. Very few provided any supporting evidence of accuracy or even information about how they functioned.
The 41 apps were drawn from more than 300 apps; the others were excluded for failing to meet such criteria as failing to employ AI, not being available in English, or not addressing clinical management of dermatologic diseases. Dr. Wongvibulsin pointed out that none of the apps had been granted regulatory approval even though only two provided a disclaimer to that effect.
In all, just 5 of the 41 provided supporting evidence from a peer-reviewed journal, and less than 40% were created with any input from a dermatologist, Dr. Wongvibulsin reported. The result is that the utility and accuracy of these apps were, for the most part, difficult to judge.
“At a minimum, app developers should provide details on what AI algorithms are used, what data sets were used for training, testing, and validation, whether there was any clinician input, whether there are any supporting publications, how user-submitted images are used, and if there are any measures used to ensure data privacy,” Dr. Wongvibulsin wrote in the poster.
For AI-based apps or systems designed for use by dermatologists, Dr. Rotemberg made similar assertions in her overview of what clinicians should be considering for proprietary AI systems, whether to help with diagnosis or improve office efficiency.
Only One Dermatology App Cleared By the FDA
Currently, the only FDA-cleared app for dermatologic use is the DermaSensor, an AI-driven device. It was cleared for use in January 2024 for the evaluation of skin lesions “suggestive” of melanoma, basal cell carcinoma, and/or squamous cell carcinoma in patients aged ≥ 40 years “to assist health care providers in determining whether to refer a patient to a dermatologist,” according to an FDA announcement.
Using elastic scattering spectroscopy to analyze light reflecting off the skin to detect malignancy, the manufacturer’s promotional material claims a 96% sensitivity and a 97% specificity.
While Dr. Rotemberg did not comment on these claims, she cautioned that AI models differ with regards to how they were trained and the relative heterogeneity of the training dataset defined by types of patients, types of skin, and types of AI learning processes. All of these variables are relevant in whether the AI will perform in a given clinical setting at the level it performed during development.
“The most accurate models employ narrow datasets, but these do not necessarily mimic what we see in practice,” she said.
In addition, even when an AI-based system is working for a given task, it must be monitored over time. Dr. Rotemberg warned about the potential for “data drift,” which describes the slow evolution in how diseases present, their prevalence by age, or other factors that might affect AI performance. She explained that repeated validation is needed to ensure that the AI-based models remain as accurate over time as they were when first used.
Many of these concepts were explored in a consensus statement from the International Skin Imaging Collaboration AI Working Group, published in JAMA Dermatology in December 2021. The statement, of which Dr. Rotemberg was a coauthor, provided recommendations for the principles of AI algorithm development specific to dermatologic considerations.
At the AAD symposium, Dr. Rotemberg asked the audience for suggestions about the needs they hoped AI might address for in office care or efficiency. Their responses included generating prior authorizations for prescriptions, triaging email for importance, and helping to improve efficiency for common front desk tasks. She liked all of these suggestions, but she warned that as powerful as it can be, AI is not likely to provide technology that will fit seamlessly into workflows without adjustment.
“Our current systems do not allow human integration of AI models,” Dr. Rotemberg said. Rather than counting on AI to adapt to current practices, she cautioned that “we may have to redesign our entire structure to actually be able to accommodate AI-based” systems. The risk for users is tasks that become more challenging before they become easier.
AI Should Not Be a Black Box
AI is promising, but it is not magic, according to other investigators, including Tofunmi A. Omiye, PhD, a postdoctoral scholar in dermatology at Stanford University, California. First author of a recent review of AI in dermatology published in Frontiers in Medicine, Dr. Omiye agreed that clinicians who want to employ AI should be able to understand basic principles if they want the technology to perform as expected.
“I totally agree that physicians should at least have a basic understanding of the data sources for training AI models as we have found that to be important to the performance of these models in the clinical setting,” he told this news organization.
“Beyond understanding the data sources, I believe physicians can also try to have a comprehensive understanding of what AI means, its training process, and evaluation as this will help them to evaluate its utility in their practice,” he added. He also reinforced the relevance of data drift.
“Concepts like distribution shift — where models perform less well over time due to changes in the patient population — are also important to keep in mind,” Dr. Omiye said.
Dr. Wongvibulsin, Dr. Rotemberg, and Dr. Omiye reported no potential financial conflicts of interest relevant to this topic.
A version of this article appeared on Medscape.com .
FROM AAD 2024
Most Cancer Trial Centers Located Closer to White, Affluent Populations
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
FROM JAMA ONCOLOGY
New Drug Approvals Are the Wrong Metric for Cancer Policy
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
Extraordinary Patients Inspired Father of Cancer Immunotherapy
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
Rare Cutaneous Presentation of Burkitt Lymphoma
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.
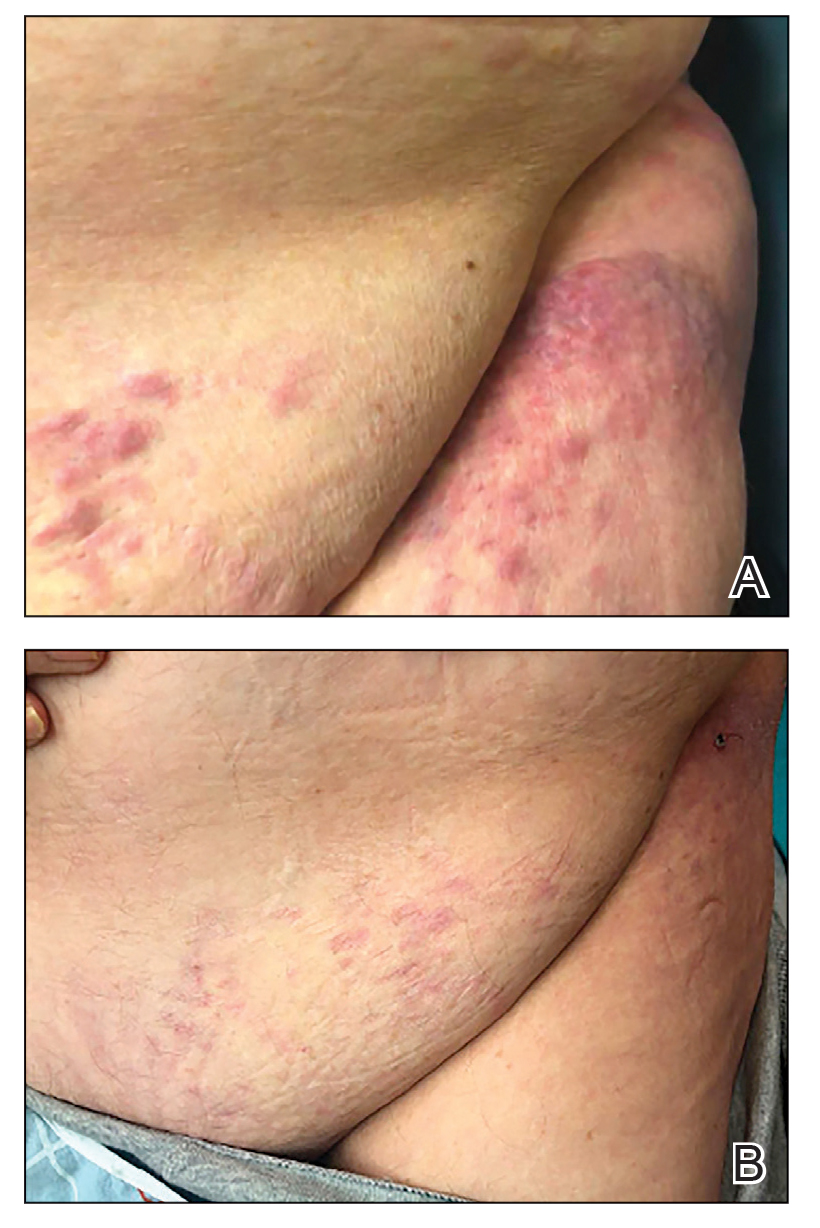
A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.
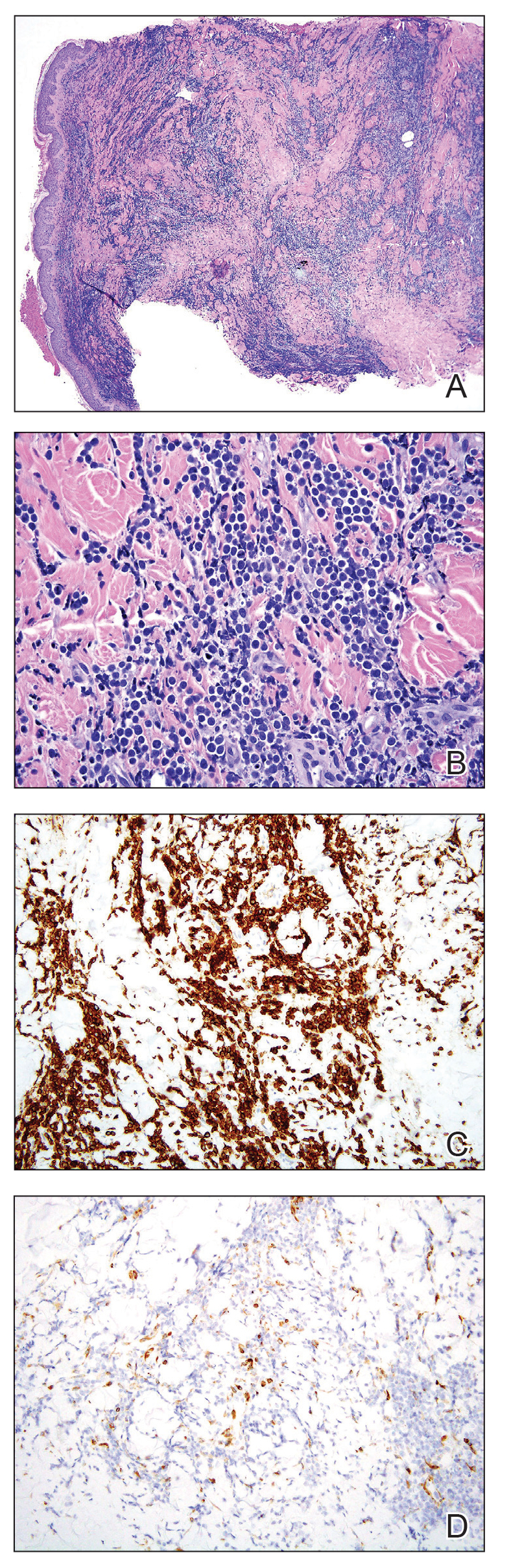
Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.

A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.

Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.

A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.

Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
Practice Points
- Cutaneous metastasis is exceedingly rare in Burkitt lymphoma. When cutaneous involvement does occur, it can represent an uncommon consequence of a surgical procedure, serving as the inciting event for hematogenous spread and local tumor extension into the skin.
- Although cutaneous metasis of Burkitt lymphoma typically is associated with high disease burden and mortality, our case demonstrated that cutaneous spread can be present even in a patient who has a positive outcome. Our patient was able to achieve disease remission and complete resolution of cutaneous lesions with continued chemotherapy, suggesting that cutaneous metastasis does not always portend a poor prognosis.
Consider These Factors in an Academic Radiation Oncology Position
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
Look Beyond BMI: Metabolic Factors’ Link to Cancer Explained
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
FROM CANCER
ASTRO Pushes Return to Direct Supervision in RT: Needed or ‘Babysitting’?
Although serious errors during virtual supervision are rare, ASTRO said radiation treatments (RT) should be done with a radiation oncologist on site to ensure high-quality care. But some radiation oncologists do not agree with the proposal to move back to direct in-person supervision only.
Changes to Direct Supervision
Most radiation oncology treatments are delivered in an outpatient setting under a physician’s direction and control.
During the COVID-19 pandemic when social distancing mandates were in place, CMS temporarily changed the definition of “direct supervision” to include telehealth, specifying that a physician must be immediately available to assist and direct a procedure virtually using real-time audio and video. In other words, a physician did not need to be physically present in the room when the treatment was being performed.
CMS has extended this rule until the end of 2024 and is considering making it a permanent change. In the Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Final Rule, CMS asked for comments on whether to extend the rule.
“We received input from interested parties on potential patient safety or quality concerns when direct supervision occurs virtually, which we will consider for future rulemaking,” a CMS spokesperson told this news organization. “CMS is currently considering the best approach that will protect patient access and safety as well as quality of care and program integrity concerns following CY 2024.”
CMS also noted its concerns that an abrupt transition back to requiring a physician’s physical presence could interrupt care from practitioners who have established new patterns of practice with telehealth.
What Are ASTRO’s Concerns?
Late last month, ASTRO sent CMS a letter, asking the agency to change the rules back to direct in-person supervision for all radiation services, citing that virtual supervision jeopardizes patient safety and quality of care.
Jeff Michalski, MD, MBA, chair of the ASTRO Board of Directors, said in an interview that radiation oncologists should be physically present to supervise the treatments.
“ASTRO is concerned that blanket policies of general or virtual supervision could lead to patients not having direct, in-person access to their doctors’ care,” he said. “While serious errors are rare, real-world experiences of radiation oncologists across practice settings demonstrate how an in-person radiation oncology physician is best suited to ensure high-quality care.”
What Do Radiation Oncologists Think?
According to ASTRO, most radiation oncologists would agree that in-person supervision is best for patients.
But that might not be the case.
Radiation oncologists took to X (formerly Twitter) to voice their opinions about ASTRO’s letter.
Jason Beckta, MD, PhD, of Rutland Regional’s Foley Cancer Center, Vermont, said “the February 26th ASTRO letter reads like an Onion article.”
“I’m struggling to understand the Luddite-level myopia around this topic,” he said in another tweet. “Virtual direct/outpatient general supervision has done nothing but boost my productivity and in particular, face-to-face patient contact.”
Join Y. Luh, MD, with the Providence Medical Network in Eureka, California, said he understands the challenges faced by clinicians working in more isolated rural settings. “For them, it’s either having virtual supervision or closing the center,” Dr. Luh said.
“Virtual care is definitely at my clinic and is not only an option but is critical to my patients who are 2+ snowy, mountainous hours away,” Dr. Luh wrote. “But I’m still in the clinic directly supervising treatments.”
Sidney Roberts, MD, with the CHI St. Luke’s Health-Memorial, Texas, tweeted that supervision does require some face-to-face care but contended that “babysitting trained therapists for every routine treatment is a farce.”
Another issue Dr. Luh brought up is reimbursement for virtual supervision, noting that “the elephant in the room is whether that level of service should be reimbursed at the same rate. Reimbursement has not changed — but will it stay that way?”
ASTRO has acknowledged that radiation oncologists will have varying opinions and says it is working to balance these challenges.
CMS has not reached a decision on whether the change will be implemented permanently. The organization will assess concern, patient safety, and quality of care at the end of the year.
A version of this article first appeared on Medscape.com
Although serious errors during virtual supervision are rare, ASTRO said radiation treatments (RT) should be done with a radiation oncologist on site to ensure high-quality care. But some radiation oncologists do not agree with the proposal to move back to direct in-person supervision only.
Changes to Direct Supervision
Most radiation oncology treatments are delivered in an outpatient setting under a physician’s direction and control.
During the COVID-19 pandemic when social distancing mandates were in place, CMS temporarily changed the definition of “direct supervision” to include telehealth, specifying that a physician must be immediately available to assist and direct a procedure virtually using real-time audio and video. In other words, a physician did not need to be physically present in the room when the treatment was being performed.
CMS has extended this rule until the end of 2024 and is considering making it a permanent change. In the Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Final Rule, CMS asked for comments on whether to extend the rule.
“We received input from interested parties on potential patient safety or quality concerns when direct supervision occurs virtually, which we will consider for future rulemaking,” a CMS spokesperson told this news organization. “CMS is currently considering the best approach that will protect patient access and safety as well as quality of care and program integrity concerns following CY 2024.”
CMS also noted its concerns that an abrupt transition back to requiring a physician’s physical presence could interrupt care from practitioners who have established new patterns of practice with telehealth.
What Are ASTRO’s Concerns?
Late last month, ASTRO sent CMS a letter, asking the agency to change the rules back to direct in-person supervision for all radiation services, citing that virtual supervision jeopardizes patient safety and quality of care.
Jeff Michalski, MD, MBA, chair of the ASTRO Board of Directors, said in an interview that radiation oncologists should be physically present to supervise the treatments.
“ASTRO is concerned that blanket policies of general or virtual supervision could lead to patients not having direct, in-person access to their doctors’ care,” he said. “While serious errors are rare, real-world experiences of radiation oncologists across practice settings demonstrate how an in-person radiation oncology physician is best suited to ensure high-quality care.”
What Do Radiation Oncologists Think?
According to ASTRO, most radiation oncologists would agree that in-person supervision is best for patients.
But that might not be the case.
Radiation oncologists took to X (formerly Twitter) to voice their opinions about ASTRO’s letter.
Jason Beckta, MD, PhD, of Rutland Regional’s Foley Cancer Center, Vermont, said “the February 26th ASTRO letter reads like an Onion article.”
“I’m struggling to understand the Luddite-level myopia around this topic,” he said in another tweet. “Virtual direct/outpatient general supervision has done nothing but boost my productivity and in particular, face-to-face patient contact.”
Join Y. Luh, MD, with the Providence Medical Network in Eureka, California, said he understands the challenges faced by clinicians working in more isolated rural settings. “For them, it’s either having virtual supervision or closing the center,” Dr. Luh said.
“Virtual care is definitely at my clinic and is not only an option but is critical to my patients who are 2+ snowy, mountainous hours away,” Dr. Luh wrote. “But I’m still in the clinic directly supervising treatments.”
Sidney Roberts, MD, with the CHI St. Luke’s Health-Memorial, Texas, tweeted that supervision does require some face-to-face care but contended that “babysitting trained therapists for every routine treatment is a farce.”
Another issue Dr. Luh brought up is reimbursement for virtual supervision, noting that “the elephant in the room is whether that level of service should be reimbursed at the same rate. Reimbursement has not changed — but will it stay that way?”
ASTRO has acknowledged that radiation oncologists will have varying opinions and says it is working to balance these challenges.
CMS has not reached a decision on whether the change will be implemented permanently. The organization will assess concern, patient safety, and quality of care at the end of the year.
A version of this article first appeared on Medscape.com
Although serious errors during virtual supervision are rare, ASTRO said radiation treatments (RT) should be done with a radiation oncologist on site to ensure high-quality care. But some radiation oncologists do not agree with the proposal to move back to direct in-person supervision only.
Changes to Direct Supervision
Most radiation oncology treatments are delivered in an outpatient setting under a physician’s direction and control.
During the COVID-19 pandemic when social distancing mandates were in place, CMS temporarily changed the definition of “direct supervision” to include telehealth, specifying that a physician must be immediately available to assist and direct a procedure virtually using real-time audio and video. In other words, a physician did not need to be physically present in the room when the treatment was being performed.
CMS has extended this rule until the end of 2024 and is considering making it a permanent change. In the Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Final Rule, CMS asked for comments on whether to extend the rule.
“We received input from interested parties on potential patient safety or quality concerns when direct supervision occurs virtually, which we will consider for future rulemaking,” a CMS spokesperson told this news organization. “CMS is currently considering the best approach that will protect patient access and safety as well as quality of care and program integrity concerns following CY 2024.”
CMS also noted its concerns that an abrupt transition back to requiring a physician’s physical presence could interrupt care from practitioners who have established new patterns of practice with telehealth.
What Are ASTRO’s Concerns?
Late last month, ASTRO sent CMS a letter, asking the agency to change the rules back to direct in-person supervision for all radiation services, citing that virtual supervision jeopardizes patient safety and quality of care.
Jeff Michalski, MD, MBA, chair of the ASTRO Board of Directors, said in an interview that radiation oncologists should be physically present to supervise the treatments.
“ASTRO is concerned that blanket policies of general or virtual supervision could lead to patients not having direct, in-person access to their doctors’ care,” he said. “While serious errors are rare, real-world experiences of radiation oncologists across practice settings demonstrate how an in-person radiation oncology physician is best suited to ensure high-quality care.”
What Do Radiation Oncologists Think?
According to ASTRO, most radiation oncologists would agree that in-person supervision is best for patients.
But that might not be the case.
Radiation oncologists took to X (formerly Twitter) to voice their opinions about ASTRO’s letter.
Jason Beckta, MD, PhD, of Rutland Regional’s Foley Cancer Center, Vermont, said “the February 26th ASTRO letter reads like an Onion article.”
“I’m struggling to understand the Luddite-level myopia around this topic,” he said in another tweet. “Virtual direct/outpatient general supervision has done nothing but boost my productivity and in particular, face-to-face patient contact.”
Join Y. Luh, MD, with the Providence Medical Network in Eureka, California, said he understands the challenges faced by clinicians working in more isolated rural settings. “For them, it’s either having virtual supervision or closing the center,” Dr. Luh said.
“Virtual care is definitely at my clinic and is not only an option but is critical to my patients who are 2+ snowy, mountainous hours away,” Dr. Luh wrote. “But I’m still in the clinic directly supervising treatments.”
Sidney Roberts, MD, with the CHI St. Luke’s Health-Memorial, Texas, tweeted that supervision does require some face-to-face care but contended that “babysitting trained therapists for every routine treatment is a farce.”
Another issue Dr. Luh brought up is reimbursement for virtual supervision, noting that “the elephant in the room is whether that level of service should be reimbursed at the same rate. Reimbursement has not changed — but will it stay that way?”
ASTRO has acknowledged that radiation oncologists will have varying opinions and says it is working to balance these challenges.
CMS has not reached a decision on whether the change will be implemented permanently. The organization will assess concern, patient safety, and quality of care at the end of the year.
A version of this article first appeared on Medscape.com