User login
Home Treatment of Presumed Melanocytic Nevus With Frankincense
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
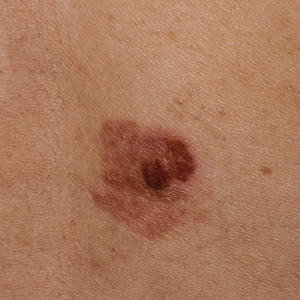
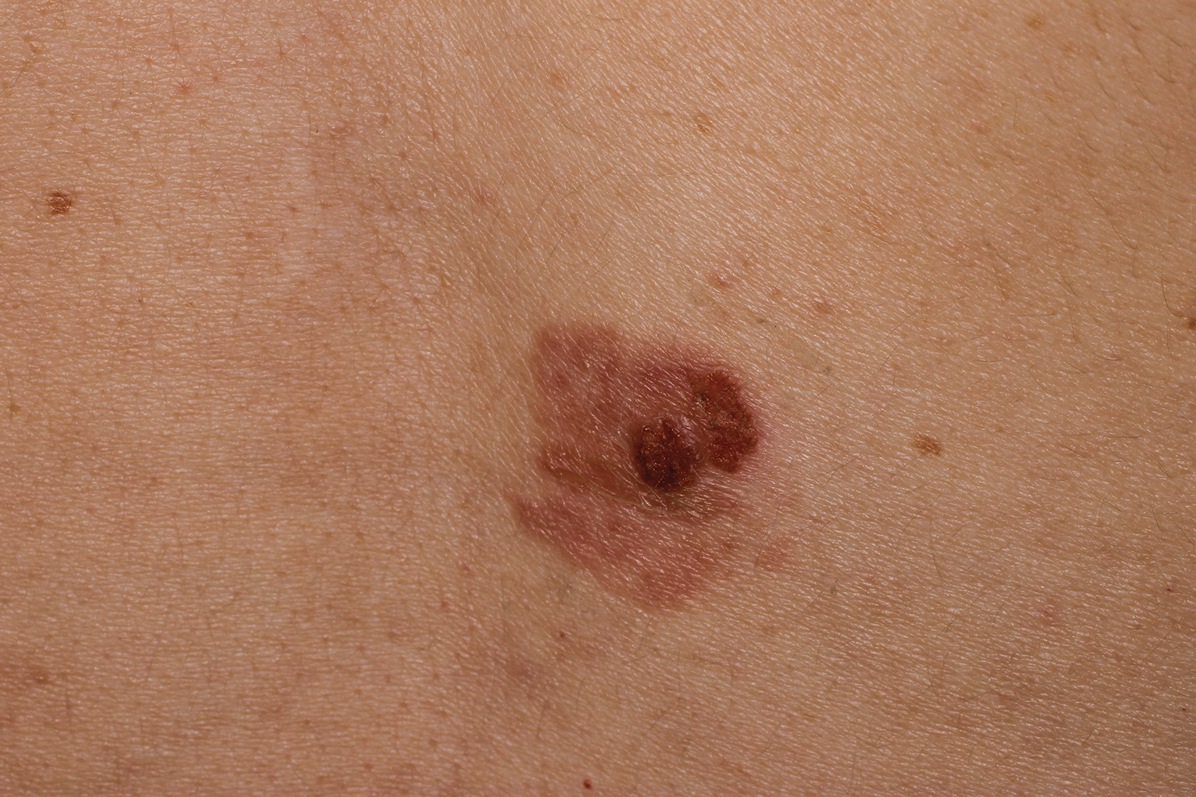
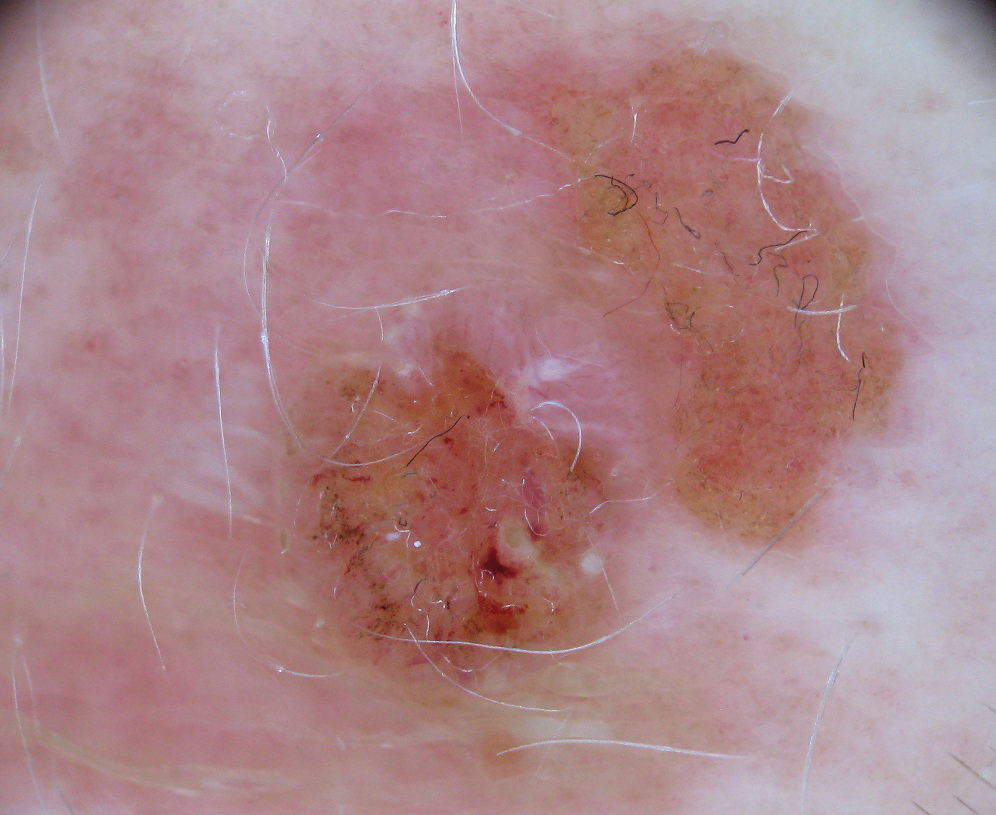
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.


Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.


Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
Practice Points
- Many patients seek natural methods of home mole removal online, including topical application of essential oils such as frankincense.
- These agents often are unregulated and can be potentially harmful when used without appropriate supervision.
- Dermatologists should be aware of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
For diagnosing skin lesions, AI risks failing in skin of color
In the analysis of images for detecting potential pathology, if training does not specifically address these skin types, according to Adewole S. Adamson, MD, who outlined this issue at the American Academy of Dermatology Virtual Meeting Experience.
“Machine learning algorithms are only as good as the inputs through which they learn. Without representation from individuals with skin of color, we are at risk of creating a new source of racial disparity in patient care,” Dr. Adamson, assistant professor in the division of dermatology, department of internal medicine, University of Texas at Austin, said at the meeting.
Diagnostic algorithms using AI are typically based on deep learning, a subset of machine learning that depends on artificial neural networks. In the case of image processing, neural networks can “learn” to recognize objects, faces, or, in the realm of health care, disease, from exposure to multiple images.
There are many other variables that affect the accuracy of deep learning for diagnostic algorithms, including the depth of the layering through which the process distills multiple inputs of information, but the number of inputs is critical. In the case of skin lesions, machines cannot learn to recognize features of different skin types without exposure.
“There are studies demonstrating that dermatologists can be outperformed for detection of skin cancers by AI, so this is going to be an increasingly powerful tool,” Dr. Adamson said. The problem is that “there has been very little representation in darker skin types” in the algorithms developed so far.
The risk is that AI will exacerbate an existing problem. Skin cancer in darker skin is less common but already underdiagnosed, independent of AI. Per 100,000 males in the United States, the rate of melanoma is about 30-fold greater in White men than in Black men (33.0 vs. 1.0). Among females, the racial difference is smaller but still enormous (20.2 vs. 1.2 per 100,000 females), according to U.S. data.
For the low representation of darker skin in studies so far with AI, “one of the arguments is that skin cancer is not a big deal in darker skin types,” Dr. Adamson said.
It might be the other way around. The relative infrequency with which skin cancer occurs in the Black population in the United States might explain a low level of suspicion and ultimately delays in diagnosis, which, in turn, leads to worse outcomes. According to one analysis drawn from the Surveillance, Epidemiology and End-Result (SEER) database (1998-2011), the proportion of patients with regionally advanced or distant disease was nearly twice as great (11.6% vs. 6.0%; P < .05) in Black patients, relative to White patients.
Not surprisingly, given the importance of early diagnosis of cancers overall and skin cancer specifically, the mean survival for malignant melanoma in Black patients was almost 4 years lower than in White patients (10.8 vs. 14.6 years; P < .001) for nodular melanoma, the same study found.
In humans, bias is reasonably attributed in many cases to judgments made on a small sample size. The problem in AI is analogous. Dr. Adamson, who has published research on the potential for machine learning to contribute to health care disparities in dermatology, cited work done by Joy Buolamwini, a graduate researcher in the media lab at the Massachusetts Institute of Technology. In one study she conducted, the rate of AI facial recognition failure was 1% in White males, 7% in White females, 12% in skin-of-color males, and 35% in skin-of-color females. Fewer inputs of skin of color is the likely explanation, Dr. Adamson said.
The potential for racial bias from AI in the diagnosis of disease increases and becomes more complex when inputs beyond imaging, such as past medical history, are included. Dr. Adamson warned of the potential for “bias to creep in” when there is failure to account for societal, cultural, or other differences that distinguish one patient group from another. However, for skin cancer or other diseases based on images alone, he said there are solutions.
“We are in the early days, and there is time to change this,” Dr. Adamson said, referring to the low representation of skin of color in AI training sets. In addition to including more skin types to train recognition, creating AI algorithms specifically for dark skin is another potential approach.
However, his key point was the importance of recognizing the need for solutions.
“AI is the future, but we must apply the same rigor to AI as to other medical interventions to ensure that the technology is not applied in a biased fashion,” he said.
Susan M. Swetter, MD, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, agreed. As someone who has been following the progress of AI in the diagnosis of skin cancer, Dr. Swetter recognizes the potential for this technology to increase diagnostic efficiency and accuracy, but she also called for studies specific to skin of color.
The algorithms “have not yet been adequately evaluated in people of color, particularly Black patients in whom dermoscopic criteria for benign versus malignant melanocytic neoplasms differ from those with lighter skin types,” Dr. Swetter said in an interview.
She sees the same fix as that proposed by Dr. Adamson.
“Efforts to include skin of color in AI algorithms for validation and further training are needed to prevent potential harms of over- or underdiagnosis in darker skin patients,” she pointed out.
Dr. Adamson reports no potential conflicts of interest relevant to this topic. Dr. Swetter had no relevant disclosures.
In the analysis of images for detecting potential pathology, if training does not specifically address these skin types, according to Adewole S. Adamson, MD, who outlined this issue at the American Academy of Dermatology Virtual Meeting Experience.
“Machine learning algorithms are only as good as the inputs through which they learn. Without representation from individuals with skin of color, we are at risk of creating a new source of racial disparity in patient care,” Dr. Adamson, assistant professor in the division of dermatology, department of internal medicine, University of Texas at Austin, said at the meeting.
Diagnostic algorithms using AI are typically based on deep learning, a subset of machine learning that depends on artificial neural networks. In the case of image processing, neural networks can “learn” to recognize objects, faces, or, in the realm of health care, disease, from exposure to multiple images.
There are many other variables that affect the accuracy of deep learning for diagnostic algorithms, including the depth of the layering through which the process distills multiple inputs of information, but the number of inputs is critical. In the case of skin lesions, machines cannot learn to recognize features of different skin types without exposure.
“There are studies demonstrating that dermatologists can be outperformed for detection of skin cancers by AI, so this is going to be an increasingly powerful tool,” Dr. Adamson said. The problem is that “there has been very little representation in darker skin types” in the algorithms developed so far.
The risk is that AI will exacerbate an existing problem. Skin cancer in darker skin is less common but already underdiagnosed, independent of AI. Per 100,000 males in the United States, the rate of melanoma is about 30-fold greater in White men than in Black men (33.0 vs. 1.0). Among females, the racial difference is smaller but still enormous (20.2 vs. 1.2 per 100,000 females), according to U.S. data.
For the low representation of darker skin in studies so far with AI, “one of the arguments is that skin cancer is not a big deal in darker skin types,” Dr. Adamson said.
It might be the other way around. The relative infrequency with which skin cancer occurs in the Black population in the United States might explain a low level of suspicion and ultimately delays in diagnosis, which, in turn, leads to worse outcomes. According to one analysis drawn from the Surveillance, Epidemiology and End-Result (SEER) database (1998-2011), the proportion of patients with regionally advanced or distant disease was nearly twice as great (11.6% vs. 6.0%; P < .05) in Black patients, relative to White patients.
Not surprisingly, given the importance of early diagnosis of cancers overall and skin cancer specifically, the mean survival for malignant melanoma in Black patients was almost 4 years lower than in White patients (10.8 vs. 14.6 years; P < .001) for nodular melanoma, the same study found.
In humans, bias is reasonably attributed in many cases to judgments made on a small sample size. The problem in AI is analogous. Dr. Adamson, who has published research on the potential for machine learning to contribute to health care disparities in dermatology, cited work done by Joy Buolamwini, a graduate researcher in the media lab at the Massachusetts Institute of Technology. In one study she conducted, the rate of AI facial recognition failure was 1% in White males, 7% in White females, 12% in skin-of-color males, and 35% in skin-of-color females. Fewer inputs of skin of color is the likely explanation, Dr. Adamson said.
The potential for racial bias from AI in the diagnosis of disease increases and becomes more complex when inputs beyond imaging, such as past medical history, are included. Dr. Adamson warned of the potential for “bias to creep in” when there is failure to account for societal, cultural, or other differences that distinguish one patient group from another. However, for skin cancer or other diseases based on images alone, he said there are solutions.
“We are in the early days, and there is time to change this,” Dr. Adamson said, referring to the low representation of skin of color in AI training sets. In addition to including more skin types to train recognition, creating AI algorithms specifically for dark skin is another potential approach.
However, his key point was the importance of recognizing the need for solutions.
“AI is the future, but we must apply the same rigor to AI as to other medical interventions to ensure that the technology is not applied in a biased fashion,” he said.
Susan M. Swetter, MD, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, agreed. As someone who has been following the progress of AI in the diagnosis of skin cancer, Dr. Swetter recognizes the potential for this technology to increase diagnostic efficiency and accuracy, but she also called for studies specific to skin of color.
The algorithms “have not yet been adequately evaluated in people of color, particularly Black patients in whom dermoscopic criteria for benign versus malignant melanocytic neoplasms differ from those with lighter skin types,” Dr. Swetter said in an interview.
She sees the same fix as that proposed by Dr. Adamson.
“Efforts to include skin of color in AI algorithms for validation and further training are needed to prevent potential harms of over- or underdiagnosis in darker skin patients,” she pointed out.
Dr. Adamson reports no potential conflicts of interest relevant to this topic. Dr. Swetter had no relevant disclosures.
In the analysis of images for detecting potential pathology, if training does not specifically address these skin types, according to Adewole S. Adamson, MD, who outlined this issue at the American Academy of Dermatology Virtual Meeting Experience.
“Machine learning algorithms are only as good as the inputs through which they learn. Without representation from individuals with skin of color, we are at risk of creating a new source of racial disparity in patient care,” Dr. Adamson, assistant professor in the division of dermatology, department of internal medicine, University of Texas at Austin, said at the meeting.
Diagnostic algorithms using AI are typically based on deep learning, a subset of machine learning that depends on artificial neural networks. In the case of image processing, neural networks can “learn” to recognize objects, faces, or, in the realm of health care, disease, from exposure to multiple images.
There are many other variables that affect the accuracy of deep learning for diagnostic algorithms, including the depth of the layering through which the process distills multiple inputs of information, but the number of inputs is critical. In the case of skin lesions, machines cannot learn to recognize features of different skin types without exposure.
“There are studies demonstrating that dermatologists can be outperformed for detection of skin cancers by AI, so this is going to be an increasingly powerful tool,” Dr. Adamson said. The problem is that “there has been very little representation in darker skin types” in the algorithms developed so far.
The risk is that AI will exacerbate an existing problem. Skin cancer in darker skin is less common but already underdiagnosed, independent of AI. Per 100,000 males in the United States, the rate of melanoma is about 30-fold greater in White men than in Black men (33.0 vs. 1.0). Among females, the racial difference is smaller but still enormous (20.2 vs. 1.2 per 100,000 females), according to U.S. data.
For the low representation of darker skin in studies so far with AI, “one of the arguments is that skin cancer is not a big deal in darker skin types,” Dr. Adamson said.
It might be the other way around. The relative infrequency with which skin cancer occurs in the Black population in the United States might explain a low level of suspicion and ultimately delays in diagnosis, which, in turn, leads to worse outcomes. According to one analysis drawn from the Surveillance, Epidemiology and End-Result (SEER) database (1998-2011), the proportion of patients with regionally advanced or distant disease was nearly twice as great (11.6% vs. 6.0%; P < .05) in Black patients, relative to White patients.
Not surprisingly, given the importance of early diagnosis of cancers overall and skin cancer specifically, the mean survival for malignant melanoma in Black patients was almost 4 years lower than in White patients (10.8 vs. 14.6 years; P < .001) for nodular melanoma, the same study found.
In humans, bias is reasonably attributed in many cases to judgments made on a small sample size. The problem in AI is analogous. Dr. Adamson, who has published research on the potential for machine learning to contribute to health care disparities in dermatology, cited work done by Joy Buolamwini, a graduate researcher in the media lab at the Massachusetts Institute of Technology. In one study she conducted, the rate of AI facial recognition failure was 1% in White males, 7% in White females, 12% in skin-of-color males, and 35% in skin-of-color females. Fewer inputs of skin of color is the likely explanation, Dr. Adamson said.
The potential for racial bias from AI in the diagnosis of disease increases and becomes more complex when inputs beyond imaging, such as past medical history, are included. Dr. Adamson warned of the potential for “bias to creep in” when there is failure to account for societal, cultural, or other differences that distinguish one patient group from another. However, for skin cancer or other diseases based on images alone, he said there are solutions.
“We are in the early days, and there is time to change this,” Dr. Adamson said, referring to the low representation of skin of color in AI training sets. In addition to including more skin types to train recognition, creating AI algorithms specifically for dark skin is another potential approach.
However, his key point was the importance of recognizing the need for solutions.
“AI is the future, but we must apply the same rigor to AI as to other medical interventions to ensure that the technology is not applied in a biased fashion,” he said.
Susan M. Swetter, MD, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, agreed. As someone who has been following the progress of AI in the diagnosis of skin cancer, Dr. Swetter recognizes the potential for this technology to increase diagnostic efficiency and accuracy, but she also called for studies specific to skin of color.
The algorithms “have not yet been adequately evaluated in people of color, particularly Black patients in whom dermoscopic criteria for benign versus malignant melanocytic neoplasms differ from those with lighter skin types,” Dr. Swetter said in an interview.
She sees the same fix as that proposed by Dr. Adamson.
“Efforts to include skin of color in AI algorithms for validation and further training are needed to prevent potential harms of over- or underdiagnosis in darker skin patients,” she pointed out.
Dr. Adamson reports no potential conflicts of interest relevant to this topic. Dr. Swetter had no relevant disclosures.
FROM AAD VMX 2021
The power and promise of social media in oncology
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM COSMO 2021
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
LGBTQ patients face unique skin risks
Dermatologists cautioned colleagues to in transgender people, who are especially vulnerable to acne because of hormone therapy.
The identities of sexual minorities “have a significant influence on many facets of health,” dermatologist Matthew Mansh, MD, of the University of Minnesota, Minneapolis, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
In regard to skin cancer, he said, “there seems to be consistently higher rates of skin cancer and certain preventable risk behaviors like indoor tanning among sexual minority men.”
Dr. Mansh, codirector of the high-risk nonmelanoma skin cancer clinic at the University of Minnesota, highlighted a report, published in JAMA Dermatology in 2020, that used 2014-2018 U.S. survey data of over 870,000 adults to look at the association between sexual orientation and lifetime prevalence of skin cancer. The investigators found that gay and bisexual men had a higher lifetime prevalence of skin cancer compared with heterosexual men (adjusted odds ratio [aOR], 1.25; 95% confidence interval, 1.03-1.50; P = .02; and aOR, 1.46; 95% CI, 1.01-2.10; P = .04; for gay and bisexual men, respectively).
When compared with heterosexual women, risk among bisexual women was lower (aOR, 0.75; 95% CI, 0.60-0.95; P = .02), but not among lesbian women (aOR, 1.01; 95% CI, 0.77-1.33; P = .95, respectively).
Other studies have reached similar conclusions, Dr. Mansh said, although there’s been fairly little research in this area. What could explain these differences? Factors such as smoking, age, and alcohol use affect skin cancer risk, he said, but these studies control for those variables. Instead, he noted, it’s useful to look at studies of ultraviolet exposure.
For example, he highlighted a study published in JAMA Dermatology in 2015, which examined 12-month indoor-tanning rates and skin cancer prevalence by sexual orientation, using data from California and national health interview surveys. The study found that compared with heterosexual men, “sexual minority men had higher rates of indoor tanning by roughly three- to sixfold,” said Dr. Mansh, the lead author. “And this was among respondents who were adults over age 18. People between the ages of 18 and 34 years are important from a skin cancer perspective as it’s well established that exposure to tanning beds at a younger age is most associated with an increased risk of skin cancer.”
Sexual minority men were also significantly more likely to report having skin cancer, compared with heterosexual men.
In the study, sexual minority women had about half the odds of engaging in indoor tanning compared with heterosexual women, and were less likely to report having been diagnosed with nonmelanoma skin cancer, he added.
Other studies suggest that gay and bisexual men live in neighborhoods with more indoor tanning salons and that they may spend more time in the sun outside too, he said. Some research suggests motivations for tanning include social pressure and the desire to improve appearance, he added.
Overall, “we may be able to use these data to add more appropriate screening and recommendations for these patients, which are sorely lacking in dermatology,” and to design targeted behavioral interventions, said Dr. Mansh, codirector of the dermatology gender care clinic at the University of Minnesota.
What can dermatologists do now? In an interview, dermatologist Jon Klint Peebles, MD, of the mid-Atlantic Permanente Medical Group, in Largo, Md., suggested that colleagues ask patients questions about indoor tanning frequency, the motivations for tanning, exposure to outdoor ultraviolet radiation, sunscreen use, and use of photoprotective clothing.
Hormone therapy and acne
In a related presentation at the meeting, Howa Yeung, MD, of the department of dermatology, Emory University, Atlanta, said that in transgender people, estrogen therapy can actually reduce sebum production and often improves acne, while testosterone therapy frequently has the opposite effect.
“We’ve seen some pretty tough cases of acne in transmasculine patients in my practice,” said Dr. Yeung, who highlighted a recently published study that tracked 988 transgender patients in Boston who underwent testosterone therapy. Nearly a third were diagnosed with acne, compared with 6% prior to hormone therapy, and those at the highest risk were aged 18-21.
The prevalence of acne was 25% 2 years after initiation of hormone therapy. “Acne remains a very common issue and not just at the beginning of treatment,” he said.
In 2020, Dr. Yeung and colleagues reported the results of a survey of 696 transgender patients in California and Georgia; most were treated with hormone therapy. They found that 14% of transmasculine patients reported currently having moderate to severe acne diagnosed by a physician, compared with 1% of transfeminine patients.
Dr. Yeung noted that another survey of transmasculine persons who had received testosterone found that those who had moderate to severe acne were more likely to suffer from depression and anxiety than were those who had never had acne (aOR, 2.4; 95% CI, 1.1-5.4; P = .001, for depression; and aOR, 2.7; 95% CI, 1.2-6.3; P = .002, for anxiety).
Acne treatments in transmasculine patients are complicated by the fact that hormone treatments for acne can have feminizing effects, Dr. Yeung said, adding that it’s not clear how clascoterone, a new anti-androgen topical therapy for acne, will affect them. For now, many patients will require isotretinoin for treating acne.
Dr. Peebles cautioned that with isotretinoin, “we still do not yet have solid data on the optimal dosing or duration in the context of testosterone-induced acne, as well as what individual factors may be predictive of treatment success or failure. It is also important to be aware of any planned surgical procedures, whether as part of gender-affirming care or otherwise, given that some surgeons may view isotretinoin as a barrier for some procedures, despite limited data to support this.”
Both Dr. Peebles and Dr. Yeung noted that the iPledge risk management program for isotretinoin patients who may become pregnant is problematic. “A trans man who is assigned female at birth and identifies as a man and has a uterus and ovaries must be registered as a female with reproductive potential,” Dr. Yeung said.
“While the program remains inherently discriminatory, it is important to have an honest conversation with patients about these issues in a sensitive way,” Dr. Peebles noted. “Luckily, there is substantial momentum building around modifying iPLEDGE to become more inclusive. While the mechanics are complicated and involve a variety of entities and advocacy initiatives, we are optimistic that major changes are in the pipeline.”
Dr. Mansh, Dr. Yeung, and Dr. Peebles reported no disclosures.
Dermatologists cautioned colleagues to in transgender people, who are especially vulnerable to acne because of hormone therapy.
The identities of sexual minorities “have a significant influence on many facets of health,” dermatologist Matthew Mansh, MD, of the University of Minnesota, Minneapolis, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
In regard to skin cancer, he said, “there seems to be consistently higher rates of skin cancer and certain preventable risk behaviors like indoor tanning among sexual minority men.”
Dr. Mansh, codirector of the high-risk nonmelanoma skin cancer clinic at the University of Minnesota, highlighted a report, published in JAMA Dermatology in 2020, that used 2014-2018 U.S. survey data of over 870,000 adults to look at the association between sexual orientation and lifetime prevalence of skin cancer. The investigators found that gay and bisexual men had a higher lifetime prevalence of skin cancer compared with heterosexual men (adjusted odds ratio [aOR], 1.25; 95% confidence interval, 1.03-1.50; P = .02; and aOR, 1.46; 95% CI, 1.01-2.10; P = .04; for gay and bisexual men, respectively).
When compared with heterosexual women, risk among bisexual women was lower (aOR, 0.75; 95% CI, 0.60-0.95; P = .02), but not among lesbian women (aOR, 1.01; 95% CI, 0.77-1.33; P = .95, respectively).
Other studies have reached similar conclusions, Dr. Mansh said, although there’s been fairly little research in this area. What could explain these differences? Factors such as smoking, age, and alcohol use affect skin cancer risk, he said, but these studies control for those variables. Instead, he noted, it’s useful to look at studies of ultraviolet exposure.
For example, he highlighted a study published in JAMA Dermatology in 2015, which examined 12-month indoor-tanning rates and skin cancer prevalence by sexual orientation, using data from California and national health interview surveys. The study found that compared with heterosexual men, “sexual minority men had higher rates of indoor tanning by roughly three- to sixfold,” said Dr. Mansh, the lead author. “And this was among respondents who were adults over age 18. People between the ages of 18 and 34 years are important from a skin cancer perspective as it’s well established that exposure to tanning beds at a younger age is most associated with an increased risk of skin cancer.”
Sexual minority men were also significantly more likely to report having skin cancer, compared with heterosexual men.
In the study, sexual minority women had about half the odds of engaging in indoor tanning compared with heterosexual women, and were less likely to report having been diagnosed with nonmelanoma skin cancer, he added.
Other studies suggest that gay and bisexual men live in neighborhoods with more indoor tanning salons and that they may spend more time in the sun outside too, he said. Some research suggests motivations for tanning include social pressure and the desire to improve appearance, he added.
Overall, “we may be able to use these data to add more appropriate screening and recommendations for these patients, which are sorely lacking in dermatology,” and to design targeted behavioral interventions, said Dr. Mansh, codirector of the dermatology gender care clinic at the University of Minnesota.
What can dermatologists do now? In an interview, dermatologist Jon Klint Peebles, MD, of the mid-Atlantic Permanente Medical Group, in Largo, Md., suggested that colleagues ask patients questions about indoor tanning frequency, the motivations for tanning, exposure to outdoor ultraviolet radiation, sunscreen use, and use of photoprotective clothing.
Hormone therapy and acne
In a related presentation at the meeting, Howa Yeung, MD, of the department of dermatology, Emory University, Atlanta, said that in transgender people, estrogen therapy can actually reduce sebum production and often improves acne, while testosterone therapy frequently has the opposite effect.
“We’ve seen some pretty tough cases of acne in transmasculine patients in my practice,” said Dr. Yeung, who highlighted a recently published study that tracked 988 transgender patients in Boston who underwent testosterone therapy. Nearly a third were diagnosed with acne, compared with 6% prior to hormone therapy, and those at the highest risk were aged 18-21.
The prevalence of acne was 25% 2 years after initiation of hormone therapy. “Acne remains a very common issue and not just at the beginning of treatment,” he said.
In 2020, Dr. Yeung and colleagues reported the results of a survey of 696 transgender patients in California and Georgia; most were treated with hormone therapy. They found that 14% of transmasculine patients reported currently having moderate to severe acne diagnosed by a physician, compared with 1% of transfeminine patients.
Dr. Yeung noted that another survey of transmasculine persons who had received testosterone found that those who had moderate to severe acne were more likely to suffer from depression and anxiety than were those who had never had acne (aOR, 2.4; 95% CI, 1.1-5.4; P = .001, for depression; and aOR, 2.7; 95% CI, 1.2-6.3; P = .002, for anxiety).
Acne treatments in transmasculine patients are complicated by the fact that hormone treatments for acne can have feminizing effects, Dr. Yeung said, adding that it’s not clear how clascoterone, a new anti-androgen topical therapy for acne, will affect them. For now, many patients will require isotretinoin for treating acne.
Dr. Peebles cautioned that with isotretinoin, “we still do not yet have solid data on the optimal dosing or duration in the context of testosterone-induced acne, as well as what individual factors may be predictive of treatment success or failure. It is also important to be aware of any planned surgical procedures, whether as part of gender-affirming care or otherwise, given that some surgeons may view isotretinoin as a barrier for some procedures, despite limited data to support this.”
Both Dr. Peebles and Dr. Yeung noted that the iPledge risk management program for isotretinoin patients who may become pregnant is problematic. “A trans man who is assigned female at birth and identifies as a man and has a uterus and ovaries must be registered as a female with reproductive potential,” Dr. Yeung said.
“While the program remains inherently discriminatory, it is important to have an honest conversation with patients about these issues in a sensitive way,” Dr. Peebles noted. “Luckily, there is substantial momentum building around modifying iPLEDGE to become more inclusive. While the mechanics are complicated and involve a variety of entities and advocacy initiatives, we are optimistic that major changes are in the pipeline.”
Dr. Mansh, Dr. Yeung, and Dr. Peebles reported no disclosures.
Dermatologists cautioned colleagues to in transgender people, who are especially vulnerable to acne because of hormone therapy.
The identities of sexual minorities “have a significant influence on many facets of health,” dermatologist Matthew Mansh, MD, of the University of Minnesota, Minneapolis, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
In regard to skin cancer, he said, “there seems to be consistently higher rates of skin cancer and certain preventable risk behaviors like indoor tanning among sexual minority men.”
Dr. Mansh, codirector of the high-risk nonmelanoma skin cancer clinic at the University of Minnesota, highlighted a report, published in JAMA Dermatology in 2020, that used 2014-2018 U.S. survey data of over 870,000 adults to look at the association between sexual orientation and lifetime prevalence of skin cancer. The investigators found that gay and bisexual men had a higher lifetime prevalence of skin cancer compared with heterosexual men (adjusted odds ratio [aOR], 1.25; 95% confidence interval, 1.03-1.50; P = .02; and aOR, 1.46; 95% CI, 1.01-2.10; P = .04; for gay and bisexual men, respectively).
When compared with heterosexual women, risk among bisexual women was lower (aOR, 0.75; 95% CI, 0.60-0.95; P = .02), but not among lesbian women (aOR, 1.01; 95% CI, 0.77-1.33; P = .95, respectively).
Other studies have reached similar conclusions, Dr. Mansh said, although there’s been fairly little research in this area. What could explain these differences? Factors such as smoking, age, and alcohol use affect skin cancer risk, he said, but these studies control for those variables. Instead, he noted, it’s useful to look at studies of ultraviolet exposure.
For example, he highlighted a study published in JAMA Dermatology in 2015, which examined 12-month indoor-tanning rates and skin cancer prevalence by sexual orientation, using data from California and national health interview surveys. The study found that compared with heterosexual men, “sexual minority men had higher rates of indoor tanning by roughly three- to sixfold,” said Dr. Mansh, the lead author. “And this was among respondents who were adults over age 18. People between the ages of 18 and 34 years are important from a skin cancer perspective as it’s well established that exposure to tanning beds at a younger age is most associated with an increased risk of skin cancer.”
Sexual minority men were also significantly more likely to report having skin cancer, compared with heterosexual men.
In the study, sexual minority women had about half the odds of engaging in indoor tanning compared with heterosexual women, and were less likely to report having been diagnosed with nonmelanoma skin cancer, he added.
Other studies suggest that gay and bisexual men live in neighborhoods with more indoor tanning salons and that they may spend more time in the sun outside too, he said. Some research suggests motivations for tanning include social pressure and the desire to improve appearance, he added.
Overall, “we may be able to use these data to add more appropriate screening and recommendations for these patients, which are sorely lacking in dermatology,” and to design targeted behavioral interventions, said Dr. Mansh, codirector of the dermatology gender care clinic at the University of Minnesota.
What can dermatologists do now? In an interview, dermatologist Jon Klint Peebles, MD, of the mid-Atlantic Permanente Medical Group, in Largo, Md., suggested that colleagues ask patients questions about indoor tanning frequency, the motivations for tanning, exposure to outdoor ultraviolet radiation, sunscreen use, and use of photoprotective clothing.
Hormone therapy and acne
In a related presentation at the meeting, Howa Yeung, MD, of the department of dermatology, Emory University, Atlanta, said that in transgender people, estrogen therapy can actually reduce sebum production and often improves acne, while testosterone therapy frequently has the opposite effect.
“We’ve seen some pretty tough cases of acne in transmasculine patients in my practice,” said Dr. Yeung, who highlighted a recently published study that tracked 988 transgender patients in Boston who underwent testosterone therapy. Nearly a third were diagnosed with acne, compared with 6% prior to hormone therapy, and those at the highest risk were aged 18-21.
The prevalence of acne was 25% 2 years after initiation of hormone therapy. “Acne remains a very common issue and not just at the beginning of treatment,” he said.
In 2020, Dr. Yeung and colleagues reported the results of a survey of 696 transgender patients in California and Georgia; most were treated with hormone therapy. They found that 14% of transmasculine patients reported currently having moderate to severe acne diagnosed by a physician, compared with 1% of transfeminine patients.
Dr. Yeung noted that another survey of transmasculine persons who had received testosterone found that those who had moderate to severe acne were more likely to suffer from depression and anxiety than were those who had never had acne (aOR, 2.4; 95% CI, 1.1-5.4; P = .001, for depression; and aOR, 2.7; 95% CI, 1.2-6.3; P = .002, for anxiety).
Acne treatments in transmasculine patients are complicated by the fact that hormone treatments for acne can have feminizing effects, Dr. Yeung said, adding that it’s not clear how clascoterone, a new anti-androgen topical therapy for acne, will affect them. For now, many patients will require isotretinoin for treating acne.
Dr. Peebles cautioned that with isotretinoin, “we still do not yet have solid data on the optimal dosing or duration in the context of testosterone-induced acne, as well as what individual factors may be predictive of treatment success or failure. It is also important to be aware of any planned surgical procedures, whether as part of gender-affirming care or otherwise, given that some surgeons may view isotretinoin as a barrier for some procedures, despite limited data to support this.”
Both Dr. Peebles and Dr. Yeung noted that the iPledge risk management program for isotretinoin patients who may become pregnant is problematic. “A trans man who is assigned female at birth and identifies as a man and has a uterus and ovaries must be registered as a female with reproductive potential,” Dr. Yeung said.
“While the program remains inherently discriminatory, it is important to have an honest conversation with patients about these issues in a sensitive way,” Dr. Peebles noted. “Luckily, there is substantial momentum building around modifying iPLEDGE to become more inclusive. While the mechanics are complicated and involve a variety of entities and advocacy initiatives, we are optimistic that major changes are in the pipeline.”
Dr. Mansh, Dr. Yeung, and Dr. Peebles reported no disclosures.
FROM AAD VMX 2021
Survey finds Mohs surgeons favor nicotinamide for chemoprevention
, in a survey of members of the American College of Mohs Surgeons.
Although nicotinamide, a vitamin B3 derivative, has been shown to reduce keratinocyte carcinoma (KC) in high-risk patients, it is not approved by the Food and Drug Administration for chemoprevention, and no safe upper limit has been established in clinical trials to date, wrote Sheena Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues.
The investigators emailed an anonymous 12-question survey to 1,500 members of the American College of Mohs Surgeons. Of the 170 who responded, 10 were excluded for discordant responses, leaving 160 participants whose replies were included in a multiple logistic regression analysis. The respondents were mainly U.S. board-certified dermatologists and Mohs surgeons (99.4% for both); 86.9% were in clinical practice, including 78.8% in private practice, according to the report of the results, published in Dermatologic Surgery.
Overall, 76.9% of the respondents said they recommended nicotinamide for preventing KC, and 20% said they had recommended nicotinamide to more than 100 patients in the past year. In addition, 45% of respondents reported patients who had been taking nicotinamide for 2 years or more. Overall, 63.8% of the respondents expressed no concerns about long-term safety of nicotinamide, compared with 28.1% who said they were uncertain about long-term safety. Those who expressed concern or uncertainty about long-term safety were significantly less likely to recommend nicotinamide for KC prevention in the past year (odds ratio, 0.30; 95% confidence interval [CI] 0.13-0.71). Clinicians with more than 10 years in practice were significantly less likely to recommend nicotinamide for chemoprevention (OR, 0.20; 95% CI 0.05-0.82).
The study findings were limited by several factors, including the low number of responses and the potential lack of generalizability to clinicians other than Mohs surgeons, the researchers noted. “Additional studies on nicotinamide safety and use patterns, including cost-effectiveness analyses, are needed given the widespread use identified in this study,” they concluded.
Limited safety data highlight research gaps
The study is particularly important at this time because nicotinamide has been increasingly used for KC chemoprevention since a randomized, controlled trial published in 2015 in the New England Journal of Medicine showed benefits, corresponding author Rebecca I. Hartman, MD, of the department of dermatology, Brigham and Women’s Hospital and Harvard University, Boston, said in an interview. That study of high-risk patients found that nicotinamide, 500 mg twice a day, was safe and effective in lowering the rates of new nonmelanoma skin cancers and AKs after 12 months .
“However, because this is not a prescription medication, but rather an OTC vitamin supplement, data on its use are not available,” she said.
Dr. Hartman said she was not surprised that nicotinamide is being used frequently by a majority of the survey respondents. “Most are using this if someone has two KCs over 2 years, which is a quite common occurrence,” she noted. However, “I was a bit surprised that nearly two-thirds had no safety concerns with long-term use, even though this has not been well-studied,” she added.
“Like anything we recommend, we must consider the risks and benefits,” Dr. Hartman said of nicotinamide. “Unfortunately, we don’t know the risks well, since this hasn’t been well-characterized with regular long-term use in these doses,” and more research is needed, she said. “The risks are likely low, as this is a vitamin that has been used for years in various OTC supplements,” she added. “However, there are some data showing slightly increased all-cause mortality with similar doses of a related medicine, niacin, in cardiovascular patients. For this reason, I recommend the medication when a patient’s KCs are really becoming burdensome – several KCs in a year or two – or when they are high-risk due to immunosuppression,” she explained.
“We also must consider the individual patient. For a healthy younger patient who has a public-facing job and as a result is very averse to developing any KCs on his or her face and very motivated to try prevention, it may make sense to try nicotinamide,” Dr. Hartman said. But for an older patient with cardiovascular comorbidities who is not bothered by a KC on his or her back or extremities, “this medication may not have a favorable risk-benefit profile.”
To address safety concerns, “researchers need to examine whether there are any harms in long-term regular nicotinamide use for KC prevention,” Dr. Hartman said. “This is something we hope to do in our patients; however, it is challenging to study in a retrospective way since the harm is likely small and there are so many other features that influence mortality as an outcome,” she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, in a survey of members of the American College of Mohs Surgeons.
Although nicotinamide, a vitamin B3 derivative, has been shown to reduce keratinocyte carcinoma (KC) in high-risk patients, it is not approved by the Food and Drug Administration for chemoprevention, and no safe upper limit has been established in clinical trials to date, wrote Sheena Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues.
The investigators emailed an anonymous 12-question survey to 1,500 members of the American College of Mohs Surgeons. Of the 170 who responded, 10 were excluded for discordant responses, leaving 160 participants whose replies were included in a multiple logistic regression analysis. The respondents were mainly U.S. board-certified dermatologists and Mohs surgeons (99.4% for both); 86.9% were in clinical practice, including 78.8% in private practice, according to the report of the results, published in Dermatologic Surgery.
Overall, 76.9% of the respondents said they recommended nicotinamide for preventing KC, and 20% said they had recommended nicotinamide to more than 100 patients in the past year. In addition, 45% of respondents reported patients who had been taking nicotinamide for 2 years or more. Overall, 63.8% of the respondents expressed no concerns about long-term safety of nicotinamide, compared with 28.1% who said they were uncertain about long-term safety. Those who expressed concern or uncertainty about long-term safety were significantly less likely to recommend nicotinamide for KC prevention in the past year (odds ratio, 0.30; 95% confidence interval [CI] 0.13-0.71). Clinicians with more than 10 years in practice were significantly less likely to recommend nicotinamide for chemoprevention (OR, 0.20; 95% CI 0.05-0.82).
The study findings were limited by several factors, including the low number of responses and the potential lack of generalizability to clinicians other than Mohs surgeons, the researchers noted. “Additional studies on nicotinamide safety and use patterns, including cost-effectiveness analyses, are needed given the widespread use identified in this study,” they concluded.
Limited safety data highlight research gaps
The study is particularly important at this time because nicotinamide has been increasingly used for KC chemoprevention since a randomized, controlled trial published in 2015 in the New England Journal of Medicine showed benefits, corresponding author Rebecca I. Hartman, MD, of the department of dermatology, Brigham and Women’s Hospital and Harvard University, Boston, said in an interview. That study of high-risk patients found that nicotinamide, 500 mg twice a day, was safe and effective in lowering the rates of new nonmelanoma skin cancers and AKs after 12 months .
“However, because this is not a prescription medication, but rather an OTC vitamin supplement, data on its use are not available,” she said.
Dr. Hartman said she was not surprised that nicotinamide is being used frequently by a majority of the survey respondents. “Most are using this if someone has two KCs over 2 years, which is a quite common occurrence,” she noted. However, “I was a bit surprised that nearly two-thirds had no safety concerns with long-term use, even though this has not been well-studied,” she added.
“Like anything we recommend, we must consider the risks and benefits,” Dr. Hartman said of nicotinamide. “Unfortunately, we don’t know the risks well, since this hasn’t been well-characterized with regular long-term use in these doses,” and more research is needed, she said. “The risks are likely low, as this is a vitamin that has been used for years in various OTC supplements,” she added. “However, there are some data showing slightly increased all-cause mortality with similar doses of a related medicine, niacin, in cardiovascular patients. For this reason, I recommend the medication when a patient’s KCs are really becoming burdensome – several KCs in a year or two – or when they are high-risk due to immunosuppression,” she explained.
“We also must consider the individual patient. For a healthy younger patient who has a public-facing job and as a result is very averse to developing any KCs on his or her face and very motivated to try prevention, it may make sense to try nicotinamide,” Dr. Hartman said. But for an older patient with cardiovascular comorbidities who is not bothered by a KC on his or her back or extremities, “this medication may not have a favorable risk-benefit profile.”
To address safety concerns, “researchers need to examine whether there are any harms in long-term regular nicotinamide use for KC prevention,” Dr. Hartman said. “This is something we hope to do in our patients; however, it is challenging to study in a retrospective way since the harm is likely small and there are so many other features that influence mortality as an outcome,” she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, in a survey of members of the American College of Mohs Surgeons.
Although nicotinamide, a vitamin B3 derivative, has been shown to reduce keratinocyte carcinoma (KC) in high-risk patients, it is not approved by the Food and Drug Administration for chemoprevention, and no safe upper limit has been established in clinical trials to date, wrote Sheena Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues.
The investigators emailed an anonymous 12-question survey to 1,500 members of the American College of Mohs Surgeons. Of the 170 who responded, 10 were excluded for discordant responses, leaving 160 participants whose replies were included in a multiple logistic regression analysis. The respondents were mainly U.S. board-certified dermatologists and Mohs surgeons (99.4% for both); 86.9% were in clinical practice, including 78.8% in private practice, according to the report of the results, published in Dermatologic Surgery.
Overall, 76.9% of the respondents said they recommended nicotinamide for preventing KC, and 20% said they had recommended nicotinamide to more than 100 patients in the past year. In addition, 45% of respondents reported patients who had been taking nicotinamide for 2 years or more. Overall, 63.8% of the respondents expressed no concerns about long-term safety of nicotinamide, compared with 28.1% who said they were uncertain about long-term safety. Those who expressed concern or uncertainty about long-term safety were significantly less likely to recommend nicotinamide for KC prevention in the past year (odds ratio, 0.30; 95% confidence interval [CI] 0.13-0.71). Clinicians with more than 10 years in practice were significantly less likely to recommend nicotinamide for chemoprevention (OR, 0.20; 95% CI 0.05-0.82).
The study findings were limited by several factors, including the low number of responses and the potential lack of generalizability to clinicians other than Mohs surgeons, the researchers noted. “Additional studies on nicotinamide safety and use patterns, including cost-effectiveness analyses, are needed given the widespread use identified in this study,” they concluded.
Limited safety data highlight research gaps
The study is particularly important at this time because nicotinamide has been increasingly used for KC chemoprevention since a randomized, controlled trial published in 2015 in the New England Journal of Medicine showed benefits, corresponding author Rebecca I. Hartman, MD, of the department of dermatology, Brigham and Women’s Hospital and Harvard University, Boston, said in an interview. That study of high-risk patients found that nicotinamide, 500 mg twice a day, was safe and effective in lowering the rates of new nonmelanoma skin cancers and AKs after 12 months .
“However, because this is not a prescription medication, but rather an OTC vitamin supplement, data on its use are not available,” she said.
Dr. Hartman said she was not surprised that nicotinamide is being used frequently by a majority of the survey respondents. “Most are using this if someone has two KCs over 2 years, which is a quite common occurrence,” she noted. However, “I was a bit surprised that nearly two-thirds had no safety concerns with long-term use, even though this has not been well-studied,” she added.
“Like anything we recommend, we must consider the risks and benefits,” Dr. Hartman said of nicotinamide. “Unfortunately, we don’t know the risks well, since this hasn’t been well-characterized with regular long-term use in these doses,” and more research is needed, she said. “The risks are likely low, as this is a vitamin that has been used for years in various OTC supplements,” she added. “However, there are some data showing slightly increased all-cause mortality with similar doses of a related medicine, niacin, in cardiovascular patients. For this reason, I recommend the medication when a patient’s KCs are really becoming burdensome – several KCs in a year or two – or when they are high-risk due to immunosuppression,” she explained.
“We also must consider the individual patient. For a healthy younger patient who has a public-facing job and as a result is very averse to developing any KCs on his or her face and very motivated to try prevention, it may make sense to try nicotinamide,” Dr. Hartman said. But for an older patient with cardiovascular comorbidities who is not bothered by a KC on his or her back or extremities, “this medication may not have a favorable risk-benefit profile.”
To address safety concerns, “researchers need to examine whether there are any harms in long-term regular nicotinamide use for KC prevention,” Dr. Hartman said. “This is something we hope to do in our patients; however, it is challenging to study in a retrospective way since the harm is likely small and there are so many other features that influence mortality as an outcome,” she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM DERMATOLOGIC SURGERY
High-Potency Topical Steroid Treatment of Multiple Keratoacanthomas Associated With Prurigo Nodularis
Practice Gap
Multiple keratoacanthomas (KAs) of the legs often are a challenge to treat, especially when these lesions appear within a field of prurigo nodules. Multiple KAs associated with prurigo nodularis is a rarer finding; more often, the condition is reported on the lower limbs of elderly women with actinically damaged skin.1,2 At times, it can be difficult to distinguish between KA and prurigo nodularis in these patients, who often report notable pruritus and might have associated eczematous dermatitis.2
Keratoacanthomas often are treated with aggressive modalities, such as Mohs micrographic surgery, excision, and electrodesiccation and curettage. Some patients are hesitant to undergo surgical treatment, however, preferring a less invasive approach. Trauma from these aggressive modalities also can be associated with recurrence of existing lesions or development of new KAs, possibly related to stimulation of a local inflammatory response and upregulation of helper T cells.2-4
Acitretin and other systemic retinoids often are considered first-line therapy for multiple KAs. Cyclosporine has been added as adjunctive treatment in cases associated with prurigo nodularis or eczematous dermatitis1,2; however, these treatments have a high rate of discontinuation because of adverse effects, including transaminitis, xerostomia, alopecia (acitretin), and renal toxicity (cyclosporine).2
Another treatment option for patients with coexisting KA and prurigo nodularis is intralesional corticosteroids, often administered in combination with systemic retinoids.3 Topical 5-fluorouracil (5-FU) has been used successfully for KA, but topical treatment options are limited if 5-FU fails. Topical imiquimod and cryotherapy are thought to be of little benefit, and the appearance of new KA within imiquimod and cryotherapy treatment fields has been reported.1,2 Topical corticosteroids have been used as an adjuvant therapy for multiple KAs associated with prurigo nodularis; however, a PubMed search of articles indexed for MEDLINE using the terms keratoacanthoma and steroid and keratoacanthoma and prurigo nodularis yielded no published reports of successful use of topical corticosteroids as monotherapy.2
The Technique
For patients who want to continue topical treatment of coexisting KA and prurigo nodularis after topical 5-FU fails, we have found success applying a high-potency topical corticosteroid to affected areas under occlusion nightly for 6 to 8 weeks. This treatment not only leads to resolution of KA but also simultaneously treats prurigo nodules that might be clinically difficult to distinguish from KA in some presentations. This regimen has been implemented in our practice with remarkable reduction of KA burden and relief of pruritus.
In a 68-year-old woman who was treated with this technique, multiple biopsies had shown KA (or well-differentiated squamous cell carcinoma that appeared clinically as KA) on the shin (Figure, A) arising amid many lesions consistent with prurigo nodules. Topical 5-FU had failed, but the patient did not want to be treated with a more invasive modality, such as excision or injection.
Instead, we treated the patient with clobetasol propionate ointment 0.05% under occlusion nightly for 6 weeks. This strategy produced resolution of both KA and prurigo nodules (Figure, B). When lesions recurred after a few months, they were successfully re-treated with topical clobetasol under occlusion in a second 6-week course.
Practical Implications
Treatment of multiple KAs associated with prurigo nodularis can present a distinct challenge. For the subset of patients who want to pursue topical treatment, options reported in the literature are limited. We have found success treating multiple KAs and associated prurigo nodules with a high-potency topical corticosteroid under occlusion, with minimal or no adverse effects. We believe that a topical corticosteroid can be implemented easily in clinical practice before a more invasive surgical or intralesional modality is considered.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Wu TP, Miller K, Cohen DE, et al. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J Am Acad Dermatol. 2013;69:426-430. doi:10.1016/J.JAAD.2013.03.035
- Sanders S, Busam KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958. doi:10.1046/j.1524-4725.2002.02069.x
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:E117-E119. doi:10.1111/ajd.12501
Practice Gap
Multiple keratoacanthomas (KAs) of the legs often are a challenge to treat, especially when these lesions appear within a field of prurigo nodules. Multiple KAs associated with prurigo nodularis is a rarer finding; more often, the condition is reported on the lower limbs of elderly women with actinically damaged skin.1,2 At times, it can be difficult to distinguish between KA and prurigo nodularis in these patients, who often report notable pruritus and might have associated eczematous dermatitis.2
Keratoacanthomas often are treated with aggressive modalities, such as Mohs micrographic surgery, excision, and electrodesiccation and curettage. Some patients are hesitant to undergo surgical treatment, however, preferring a less invasive approach. Trauma from these aggressive modalities also can be associated with recurrence of existing lesions or development of new KAs, possibly related to stimulation of a local inflammatory response and upregulation of helper T cells.2-4
Acitretin and other systemic retinoids often are considered first-line therapy for multiple KAs. Cyclosporine has been added as adjunctive treatment in cases associated with prurigo nodularis or eczematous dermatitis1,2; however, these treatments have a high rate of discontinuation because of adverse effects, including transaminitis, xerostomia, alopecia (acitretin), and renal toxicity (cyclosporine).2
Another treatment option for patients with coexisting KA and prurigo nodularis is intralesional corticosteroids, often administered in combination with systemic retinoids.3 Topical 5-fluorouracil (5-FU) has been used successfully for KA, but topical treatment options are limited if 5-FU fails. Topical imiquimod and cryotherapy are thought to be of little benefit, and the appearance of new KA within imiquimod and cryotherapy treatment fields has been reported.1,2 Topical corticosteroids have been used as an adjuvant therapy for multiple KAs associated with prurigo nodularis; however, a PubMed search of articles indexed for MEDLINE using the terms keratoacanthoma and steroid and keratoacanthoma and prurigo nodularis yielded no published reports of successful use of topical corticosteroids as monotherapy.2
The Technique
For patients who want to continue topical treatment of coexisting KA and prurigo nodularis after topical 5-FU fails, we have found success applying a high-potency topical corticosteroid to affected areas under occlusion nightly for 6 to 8 weeks. This treatment not only leads to resolution of KA but also simultaneously treats prurigo nodules that might be clinically difficult to distinguish from KA in some presentations. This regimen has been implemented in our practice with remarkable reduction of KA burden and relief of pruritus.
In a 68-year-old woman who was treated with this technique, multiple biopsies had shown KA (or well-differentiated squamous cell carcinoma that appeared clinically as KA) on the shin (Figure, A) arising amid many lesions consistent with prurigo nodules. Topical 5-FU had failed, but the patient did not want to be treated with a more invasive modality, such as excision or injection.

Instead, we treated the patient with clobetasol propionate ointment 0.05% under occlusion nightly for 6 weeks. This strategy produced resolution of both KA and prurigo nodules (Figure, B). When lesions recurred after a few months, they were successfully re-treated with topical clobetasol under occlusion in a second 6-week course.
Practical Implications
Treatment of multiple KAs associated with prurigo nodularis can present a distinct challenge. For the subset of patients who want to pursue topical treatment, options reported in the literature are limited. We have found success treating multiple KAs and associated prurigo nodules with a high-potency topical corticosteroid under occlusion, with minimal or no adverse effects. We believe that a topical corticosteroid can be implemented easily in clinical practice before a more invasive surgical or intralesional modality is considered.
Practice Gap
Multiple keratoacanthomas (KAs) of the legs often are a challenge to treat, especially when these lesions appear within a field of prurigo nodules. Multiple KAs associated with prurigo nodularis is a rarer finding; more often, the condition is reported on the lower limbs of elderly women with actinically damaged skin.1,2 At times, it can be difficult to distinguish between KA and prurigo nodularis in these patients, who often report notable pruritus and might have associated eczematous dermatitis.2
Keratoacanthomas often are treated with aggressive modalities, such as Mohs micrographic surgery, excision, and electrodesiccation and curettage. Some patients are hesitant to undergo surgical treatment, however, preferring a less invasive approach. Trauma from these aggressive modalities also can be associated with recurrence of existing lesions or development of new KAs, possibly related to stimulation of a local inflammatory response and upregulation of helper T cells.2-4
Acitretin and other systemic retinoids often are considered first-line therapy for multiple KAs. Cyclosporine has been added as adjunctive treatment in cases associated with prurigo nodularis or eczematous dermatitis1,2; however, these treatments have a high rate of discontinuation because of adverse effects, including transaminitis, xerostomia, alopecia (acitretin), and renal toxicity (cyclosporine).2
Another treatment option for patients with coexisting KA and prurigo nodularis is intralesional corticosteroids, often administered in combination with systemic retinoids.3 Topical 5-fluorouracil (5-FU) has been used successfully for KA, but topical treatment options are limited if 5-FU fails. Topical imiquimod and cryotherapy are thought to be of little benefit, and the appearance of new KA within imiquimod and cryotherapy treatment fields has been reported.1,2 Topical corticosteroids have been used as an adjuvant therapy for multiple KAs associated with prurigo nodularis; however, a PubMed search of articles indexed for MEDLINE using the terms keratoacanthoma and steroid and keratoacanthoma and prurigo nodularis yielded no published reports of successful use of topical corticosteroids as monotherapy.2
The Technique
For patients who want to continue topical treatment of coexisting KA and prurigo nodularis after topical 5-FU fails, we have found success applying a high-potency topical corticosteroid to affected areas under occlusion nightly for 6 to 8 weeks. This treatment not only leads to resolution of KA but also simultaneously treats prurigo nodules that might be clinically difficult to distinguish from KA in some presentations. This regimen has been implemented in our practice with remarkable reduction of KA burden and relief of pruritus.
In a 68-year-old woman who was treated with this technique, multiple biopsies had shown KA (or well-differentiated squamous cell carcinoma that appeared clinically as KA) on the shin (Figure, A) arising amid many lesions consistent with prurigo nodules. Topical 5-FU had failed, but the patient did not want to be treated with a more invasive modality, such as excision or injection.

Instead, we treated the patient with clobetasol propionate ointment 0.05% under occlusion nightly for 6 weeks. This strategy produced resolution of both KA and prurigo nodules (Figure, B). When lesions recurred after a few months, they were successfully re-treated with topical clobetasol under occlusion in a second 6-week course.
Practical Implications
Treatment of multiple KAs associated with prurigo nodularis can present a distinct challenge. For the subset of patients who want to pursue topical treatment, options reported in the literature are limited. We have found success treating multiple KAs and associated prurigo nodules with a high-potency topical corticosteroid under occlusion, with minimal or no adverse effects. We believe that a topical corticosteroid can be implemented easily in clinical practice before a more invasive surgical or intralesional modality is considered.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Wu TP, Miller K, Cohen DE, et al. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J Am Acad Dermatol. 2013;69:426-430. doi:10.1016/J.JAAD.2013.03.035
- Sanders S, Busam KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958. doi:10.1046/j.1524-4725.2002.02069.x
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:E117-E119. doi:10.1111/ajd.12501
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Wu TP, Miller K, Cohen DE, et al. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J Am Acad Dermatol. 2013;69:426-430. doi:10.1016/J.JAAD.2013.03.035
- Sanders S, Busam KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958. doi:10.1046/j.1524-4725.2002.02069.x
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:E117-E119. doi:10.1111/ajd.12501
Steroid-refractory pneumonitis from ICIs: Experience at major centers
Pneumonitis is an uncommon and potentially life-threatening complication of immune checkpoint inhibitor (ICI) therapy. A fraction of patients with ICI-related pneumonitis fail to respond to initial therapy with high-dose systemic steroids.
The recently published experiences at two major cancer centers shed light on the outcomes from treatment and can provide some advice to clinicians for dealing with affected patients.
The Johns Hopkins experience
Because ICI-related pneumonitis typically improves within 48-72 hours of steroid therapy, at Johns Hopkins University, Baltimore, steroid-refractory pneumonitis is defined as pneumonitis that demonstrates no clinical improvement after high-dose corticosteroids for 2-14 days. If the immune toxicity–specialized, multidisciplinary management team implements additional immunosuppressive therapy, that is regarded as confirmatory evidence.
Aanika Balaji, a medical student at Johns Hopkins University, and colleagues retrospectively summarized the clinical course of 12 patients with ICI-related pneumonitis between 2011 and 2020. Clinical improvement with subsequent treatment was evidenced by reduction in either level of care or oxygen requirements.
Three-quarters of the patients were current or former smokers, and the same proportion had lung cancer. Most patients (91.6%) had received chemotherapy, 58.3% had prior chest radiotherapy, and 58.3% had achieved partial response or stable disease with an ICI.
Steroid-refractory ICI-related pneumonitis developed between 40 and 127 days (median, 85 days) after the first dose of ICI therapy. Subsequent immunosuppressive management included IVIg, infliximab, or the combination, in addition to ICU-level supportive care.
Among the seven patients who received IVIg alone, two patients (29%) achieved clinical improvement and hospital discharge. The remainder died.
The two patients treated with infliximab and the three patients treated with sequential IVIg and infliximab died. All deaths were attributed to ICI-related pneumonitis or infectious complications.
Overall, clinically relevant findings were:
- Steroid-refractory ICI-related pneumonitis was seen in 18.5% of patients referred for multidisciplinary care.
- Steroid-refractory ICI-related pneumonitis occurred at a median of 85 days into a patient’s ICI treatment.
- Some patients improved clinically after IVIg therapy, but mortality was high overall.
- Infliximab therapy, alone or in combination with IVIg, was ineffective.
The Memorial Sloan Kettering experience
Jason Beattie, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues performed a retrospective study of patients who had pneumonitis after ICI therapy and/or received immune modulator therapy after corticosteroids in the setting of ICI cancer treatment.
Manual record review was performed to exclude cases of pneumonitis from other causes. The period reviewed was roughly contemporaneous with the Johns Hopkins series.
Patients with ICI-related pneumonitis were divided into “steroid refractory” (i.e., no response to high-dose corticosteroids) or “steroid resistant” (i.e., initial response, followed by worsening) categories.
The researchers identified 26 patients with ICI-related pneumonitis, all of whom had advanced malignancy (8 lung cancer, 4 malignant melanoma, 4 renal cell cancer, and 10 “other” cancers).
A majority of patients (85%) were current or former smokers, 73% had received ICI monotherapy, 35% had received prior chest radiation at a median interval of 4.9 months prior to pneumonitis onset, and 27% had preexisting pulmonary disease.
Twelve patients (46%) had steroid-refractory ICI-related pneumonitis, and 14 (54%) had steroid-resistant ICI-related pneumonitis.
The two groups differed in time to pneumonitis onset (a median of 68 days in the refractory group and 182 days in the resistant group) and time to immune modulator therapy after beginning steroids (median 7 days and 2.9 months, respectively). In the steroid-refractory cases, pneumonitis was more severe.
In addition to corticosteroids, most patients received infliximab monotherapy or infliximab with mycophenolate mofetil. In contrast to the Johns Hopkins series, IVIg was not used in the Memorial Sloan Kettering cases.
Outcomes from immune modulators were graded based on clinical evidence (progress notes, oxygen requirements, level of care, radiologic information, etc.) of resolution of pneumonitis on imaging at least 8 weeks after cessation of steroids and immune modulator therapy, durable improvement for at least 8 weeks after immune modulator therapy, transient improvement followed by pneumonitis relapse or inadequate follow-up because of death or hospice referral, or no improvement.
Ten patients (38%) had durable improvement of ICI-related pneumonitis, of whom three (12%) had complete resolution. Two of the patients with complete resolution had steroid-refractory pneumonitis, both of whom had received infliximab followed by mycophenolate mofetil.
Among the seven patients with durable improvement, four remained alive on immune modulators. Time to resolution of pneumonitis was protracted, ranging from 2.3 months to 8.4 months in the steroid-refractory patients.
Durable response was less common with steroid-refractory (25%) than steroid-resistant (50%) disease, with a significant difference in 90-day survival of 25% and 71%, respectively.
Among the 13 (50%) patients with transient improvement in ICI-related pneumonitis, 8 ultimately died, either because of recurrent ICI-related pneumonitis or infection. All three patients with no improvement from immune modulators died.
The 90-day all-cause mortality was 50%, with durable pneumonitis improvement and freedom from severe infectious complications occurring in only about a third of patients.
Lessons for clinicians
The National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the European Society of Medical Oncology have all published guidelines and recommendations for immunosuppression for steroid-refractory adverse events from ICIs.
Unfortunately, there is little experience with steroid-unresponsive ICI-related pneumonitis. The ideal sequence, dose, and duration of additional immune modulator therapy for ICI-related pneumonitis are unclear and may differ from the approaches to other immune-related toxicities.
This is important because, as suggested in an editorial by Margaret Gatti-Mays, MD, and James L. Gulley, MD, PhD, it is likely that ICI-related pneumonitis will be seen more in routine practice than in clinical trial populations. In addition, across all tumor types, ICI-related pneumonitis is the most common cause of ICI-associated death from toxicity.
The retrospective studies from Johns Hopkins and Memorial Sloan Kettering constitute the largest published experience with ICI-related pneumonitis and yield important clinical insights.
Uniform definitions of potentially important patient subgroups (e.g., steroid refractory vs. steroid resistant) are needed. The steroid-refractory and steroid-resistant subgroups have distinctly different clinical features and outcomes. Uniformity in the subgroup definitions would be a useful starting point from both clinical and research perspectives.
Preferred treatment choices need to be tested systematically in multi-institutional studies. Any potential impact of treatment for ICI-related pneumonitis on antitumor immune control should be identified.
Endpoints of interest need to be defined and measured prospectively. All-cause mortality after 90 days is important, but, as the authors of both reviews noted, there are vitally important narratives and differences in functionality that are completely concealed by restricting the focus to mortality.
Potential causal relationships with antecedent exposure to tobacco, radiation, intrathoracic tumor burden, or other factors need to be defined.
Clinicians need predictive biomarkers for ICI-related pneumonitis (e.g., in peripheral blood, pulmonary function testing, or bronchoscopy specimens). At-risk patients may benefit from early intervention.
The limitations of single-institution record reviews in guiding real-world patient management notwithstanding, these reviews illustrate the value of registries and prospective studies to guide the path forward. Taking these next steps will ensure for our patients that the success of immune-targeted therapy against their cancer never becomes a Pyrrhic victory.
The Johns Hopkins investigators and the editorialists reported having no disclosures. The Memorial Sloan Kettering investigators disclosed relationships with Targeted Oncology, Merck, Array BioPharma, Novartis, and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Pneumonitis is an uncommon and potentially life-threatening complication of immune checkpoint inhibitor (ICI) therapy. A fraction of patients with ICI-related pneumonitis fail to respond to initial therapy with high-dose systemic steroids.
The recently published experiences at two major cancer centers shed light on the outcomes from treatment and can provide some advice to clinicians for dealing with affected patients.
The Johns Hopkins experience
Because ICI-related pneumonitis typically improves within 48-72 hours of steroid therapy, at Johns Hopkins University, Baltimore, steroid-refractory pneumonitis is defined as pneumonitis that demonstrates no clinical improvement after high-dose corticosteroids for 2-14 days. If the immune toxicity–specialized, multidisciplinary management team implements additional immunosuppressive therapy, that is regarded as confirmatory evidence.
Aanika Balaji, a medical student at Johns Hopkins University, and colleagues retrospectively summarized the clinical course of 12 patients with ICI-related pneumonitis between 2011 and 2020. Clinical improvement with subsequent treatment was evidenced by reduction in either level of care or oxygen requirements.
Three-quarters of the patients were current or former smokers, and the same proportion had lung cancer. Most patients (91.6%) had received chemotherapy, 58.3% had prior chest radiotherapy, and 58.3% had achieved partial response or stable disease with an ICI.
Steroid-refractory ICI-related pneumonitis developed between 40 and 127 days (median, 85 days) after the first dose of ICI therapy. Subsequent immunosuppressive management included IVIg, infliximab, or the combination, in addition to ICU-level supportive care.
Among the seven patients who received IVIg alone, two patients (29%) achieved clinical improvement and hospital discharge. The remainder died.
The two patients treated with infliximab and the three patients treated with sequential IVIg and infliximab died. All deaths were attributed to ICI-related pneumonitis or infectious complications.
Overall, clinically relevant findings were:
- Steroid-refractory ICI-related pneumonitis was seen in 18.5% of patients referred for multidisciplinary care.
- Steroid-refractory ICI-related pneumonitis occurred at a median of 85 days into a patient’s ICI treatment.
- Some patients improved clinically after IVIg therapy, but mortality was high overall.
- Infliximab therapy, alone or in combination with IVIg, was ineffective.
The Memorial Sloan Kettering experience
Jason Beattie, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues performed a retrospective study of patients who had pneumonitis after ICI therapy and/or received immune modulator therapy after corticosteroids in the setting of ICI cancer treatment.
Manual record review was performed to exclude cases of pneumonitis from other causes. The period reviewed was roughly contemporaneous with the Johns Hopkins series.
Patients with ICI-related pneumonitis were divided into “steroid refractory” (i.e., no response to high-dose corticosteroids) or “steroid resistant” (i.e., initial response, followed by worsening) categories.
The researchers identified 26 patients with ICI-related pneumonitis, all of whom had advanced malignancy (8 lung cancer, 4 malignant melanoma, 4 renal cell cancer, and 10 “other” cancers).
A majority of patients (85%) were current or former smokers, 73% had received ICI monotherapy, 35% had received prior chest radiation at a median interval of 4.9 months prior to pneumonitis onset, and 27% had preexisting pulmonary disease.
Twelve patients (46%) had steroid-refractory ICI-related pneumonitis, and 14 (54%) had steroid-resistant ICI-related pneumonitis.
The two groups differed in time to pneumonitis onset (a median of 68 days in the refractory group and 182 days in the resistant group) and time to immune modulator therapy after beginning steroids (median 7 days and 2.9 months, respectively). In the steroid-refractory cases, pneumonitis was more severe.
In addition to corticosteroids, most patients received infliximab monotherapy or infliximab with mycophenolate mofetil. In contrast to the Johns Hopkins series, IVIg was not used in the Memorial Sloan Kettering cases.
Outcomes from immune modulators were graded based on clinical evidence (progress notes, oxygen requirements, level of care, radiologic information, etc.) of resolution of pneumonitis on imaging at least 8 weeks after cessation of steroids and immune modulator therapy, durable improvement for at least 8 weeks after immune modulator therapy, transient improvement followed by pneumonitis relapse or inadequate follow-up because of death or hospice referral, or no improvement.
Ten patients (38%) had durable improvement of ICI-related pneumonitis, of whom three (12%) had complete resolution. Two of the patients with complete resolution had steroid-refractory pneumonitis, both of whom had received infliximab followed by mycophenolate mofetil.
Among the seven patients with durable improvement, four remained alive on immune modulators. Time to resolution of pneumonitis was protracted, ranging from 2.3 months to 8.4 months in the steroid-refractory patients.
Durable response was less common with steroid-refractory (25%) than steroid-resistant (50%) disease, with a significant difference in 90-day survival of 25% and 71%, respectively.
Among the 13 (50%) patients with transient improvement in ICI-related pneumonitis, 8 ultimately died, either because of recurrent ICI-related pneumonitis or infection. All three patients with no improvement from immune modulators died.
The 90-day all-cause mortality was 50%, with durable pneumonitis improvement and freedom from severe infectious complications occurring in only about a third of patients.
Lessons for clinicians
The National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the European Society of Medical Oncology have all published guidelines and recommendations for immunosuppression for steroid-refractory adverse events from ICIs.
Unfortunately, there is little experience with steroid-unresponsive ICI-related pneumonitis. The ideal sequence, dose, and duration of additional immune modulator therapy for ICI-related pneumonitis are unclear and may differ from the approaches to other immune-related toxicities.
This is important because, as suggested in an editorial by Margaret Gatti-Mays, MD, and James L. Gulley, MD, PhD, it is likely that ICI-related pneumonitis will be seen more in routine practice than in clinical trial populations. In addition, across all tumor types, ICI-related pneumonitis is the most common cause of ICI-associated death from toxicity.
The retrospective studies from Johns Hopkins and Memorial Sloan Kettering constitute the largest published experience with ICI-related pneumonitis and yield important clinical insights.
Uniform definitions of potentially important patient subgroups (e.g., steroid refractory vs. steroid resistant) are needed. The steroid-refractory and steroid-resistant subgroups have distinctly different clinical features and outcomes. Uniformity in the subgroup definitions would be a useful starting point from both clinical and research perspectives.
Preferred treatment choices need to be tested systematically in multi-institutional studies. Any potential impact of treatment for ICI-related pneumonitis on antitumor immune control should be identified.
Endpoints of interest need to be defined and measured prospectively. All-cause mortality after 90 days is important, but, as the authors of both reviews noted, there are vitally important narratives and differences in functionality that are completely concealed by restricting the focus to mortality.
Potential causal relationships with antecedent exposure to tobacco, radiation, intrathoracic tumor burden, or other factors need to be defined.
Clinicians need predictive biomarkers for ICI-related pneumonitis (e.g., in peripheral blood, pulmonary function testing, or bronchoscopy specimens). At-risk patients may benefit from early intervention.
The limitations of single-institution record reviews in guiding real-world patient management notwithstanding, these reviews illustrate the value of registries and prospective studies to guide the path forward. Taking these next steps will ensure for our patients that the success of immune-targeted therapy against their cancer never becomes a Pyrrhic victory.
The Johns Hopkins investigators and the editorialists reported having no disclosures. The Memorial Sloan Kettering investigators disclosed relationships with Targeted Oncology, Merck, Array BioPharma, Novartis, and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Pneumonitis is an uncommon and potentially life-threatening complication of immune checkpoint inhibitor (ICI) therapy. A fraction of patients with ICI-related pneumonitis fail to respond to initial therapy with high-dose systemic steroids.
The recently published experiences at two major cancer centers shed light on the outcomes from treatment and can provide some advice to clinicians for dealing with affected patients.
The Johns Hopkins experience
Because ICI-related pneumonitis typically improves within 48-72 hours of steroid therapy, at Johns Hopkins University, Baltimore, steroid-refractory pneumonitis is defined as pneumonitis that demonstrates no clinical improvement after high-dose corticosteroids for 2-14 days. If the immune toxicity–specialized, multidisciplinary management team implements additional immunosuppressive therapy, that is regarded as confirmatory evidence.
Aanika Balaji, a medical student at Johns Hopkins University, and colleagues retrospectively summarized the clinical course of 12 patients with ICI-related pneumonitis between 2011 and 2020. Clinical improvement with subsequent treatment was evidenced by reduction in either level of care or oxygen requirements.
Three-quarters of the patients were current or former smokers, and the same proportion had lung cancer. Most patients (91.6%) had received chemotherapy, 58.3% had prior chest radiotherapy, and 58.3% had achieved partial response or stable disease with an ICI.
Steroid-refractory ICI-related pneumonitis developed between 40 and 127 days (median, 85 days) after the first dose of ICI therapy. Subsequent immunosuppressive management included IVIg, infliximab, or the combination, in addition to ICU-level supportive care.
Among the seven patients who received IVIg alone, two patients (29%) achieved clinical improvement and hospital discharge. The remainder died.
The two patients treated with infliximab and the three patients treated with sequential IVIg and infliximab died. All deaths were attributed to ICI-related pneumonitis or infectious complications.
Overall, clinically relevant findings were:
- Steroid-refractory ICI-related pneumonitis was seen in 18.5% of patients referred for multidisciplinary care.
- Steroid-refractory ICI-related pneumonitis occurred at a median of 85 days into a patient’s ICI treatment.
- Some patients improved clinically after IVIg therapy, but mortality was high overall.
- Infliximab therapy, alone or in combination with IVIg, was ineffective.
The Memorial Sloan Kettering experience
Jason Beattie, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues performed a retrospective study of patients who had pneumonitis after ICI therapy and/or received immune modulator therapy after corticosteroids in the setting of ICI cancer treatment.
Manual record review was performed to exclude cases of pneumonitis from other causes. The period reviewed was roughly contemporaneous with the Johns Hopkins series.
Patients with ICI-related pneumonitis were divided into “steroid refractory” (i.e., no response to high-dose corticosteroids) or “steroid resistant” (i.e., initial response, followed by worsening) categories.
The researchers identified 26 patients with ICI-related pneumonitis, all of whom had advanced malignancy (8 lung cancer, 4 malignant melanoma, 4 renal cell cancer, and 10 “other” cancers).
A majority of patients (85%) were current or former smokers, 73% had received ICI monotherapy, 35% had received prior chest radiation at a median interval of 4.9 months prior to pneumonitis onset, and 27% had preexisting pulmonary disease.
Twelve patients (46%) had steroid-refractory ICI-related pneumonitis, and 14 (54%) had steroid-resistant ICI-related pneumonitis.
The two groups differed in time to pneumonitis onset (a median of 68 days in the refractory group and 182 days in the resistant group) and time to immune modulator therapy after beginning steroids (median 7 days and 2.9 months, respectively). In the steroid-refractory cases, pneumonitis was more severe.
In addition to corticosteroids, most patients received infliximab monotherapy or infliximab with mycophenolate mofetil. In contrast to the Johns Hopkins series, IVIg was not used in the Memorial Sloan Kettering cases.
Outcomes from immune modulators were graded based on clinical evidence (progress notes, oxygen requirements, level of care, radiologic information, etc.) of resolution of pneumonitis on imaging at least 8 weeks after cessation of steroids and immune modulator therapy, durable improvement for at least 8 weeks after immune modulator therapy, transient improvement followed by pneumonitis relapse or inadequate follow-up because of death or hospice referral, or no improvement.
Ten patients (38%) had durable improvement of ICI-related pneumonitis, of whom three (12%) had complete resolution. Two of the patients with complete resolution had steroid-refractory pneumonitis, both of whom had received infliximab followed by mycophenolate mofetil.
Among the seven patients with durable improvement, four remained alive on immune modulators. Time to resolution of pneumonitis was protracted, ranging from 2.3 months to 8.4 months in the steroid-refractory patients.
Durable response was less common with steroid-refractory (25%) than steroid-resistant (50%) disease, with a significant difference in 90-day survival of 25% and 71%, respectively.
Among the 13 (50%) patients with transient improvement in ICI-related pneumonitis, 8 ultimately died, either because of recurrent ICI-related pneumonitis or infection. All three patients with no improvement from immune modulators died.
The 90-day all-cause mortality was 50%, with durable pneumonitis improvement and freedom from severe infectious complications occurring in only about a third of patients.
Lessons for clinicians
The National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the European Society of Medical Oncology have all published guidelines and recommendations for immunosuppression for steroid-refractory adverse events from ICIs.
Unfortunately, there is little experience with steroid-unresponsive ICI-related pneumonitis. The ideal sequence, dose, and duration of additional immune modulator therapy for ICI-related pneumonitis are unclear and may differ from the approaches to other immune-related toxicities.
This is important because, as suggested in an editorial by Margaret Gatti-Mays, MD, and James L. Gulley, MD, PhD, it is likely that ICI-related pneumonitis will be seen more in routine practice than in clinical trial populations. In addition, across all tumor types, ICI-related pneumonitis is the most common cause of ICI-associated death from toxicity.
The retrospective studies from Johns Hopkins and Memorial Sloan Kettering constitute the largest published experience with ICI-related pneumonitis and yield important clinical insights.
Uniform definitions of potentially important patient subgroups (e.g., steroid refractory vs. steroid resistant) are needed. The steroid-refractory and steroid-resistant subgroups have distinctly different clinical features and outcomes. Uniformity in the subgroup definitions would be a useful starting point from both clinical and research perspectives.
Preferred treatment choices need to be tested systematically in multi-institutional studies. Any potential impact of treatment for ICI-related pneumonitis on antitumor immune control should be identified.
Endpoints of interest need to be defined and measured prospectively. All-cause mortality after 90 days is important, but, as the authors of both reviews noted, there are vitally important narratives and differences in functionality that are completely concealed by restricting the focus to mortality.
Potential causal relationships with antecedent exposure to tobacco, radiation, intrathoracic tumor burden, or other factors need to be defined.
Clinicians need predictive biomarkers for ICI-related pneumonitis (e.g., in peripheral blood, pulmonary function testing, or bronchoscopy specimens). At-risk patients may benefit from early intervention.
The limitations of single-institution record reviews in guiding real-world patient management notwithstanding, these reviews illustrate the value of registries and prospective studies to guide the path forward. Taking these next steps will ensure for our patients that the success of immune-targeted therapy against their cancer never becomes a Pyrrhic victory.
The Johns Hopkins investigators and the editorialists reported having no disclosures. The Memorial Sloan Kettering investigators disclosed relationships with Targeted Oncology, Merck, Array BioPharma, Novartis, and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Squamous Cell Carcinoma in Hidradenitis Suppurativa Lesions Following Tumor Necrosis Factor α Inhibitors
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
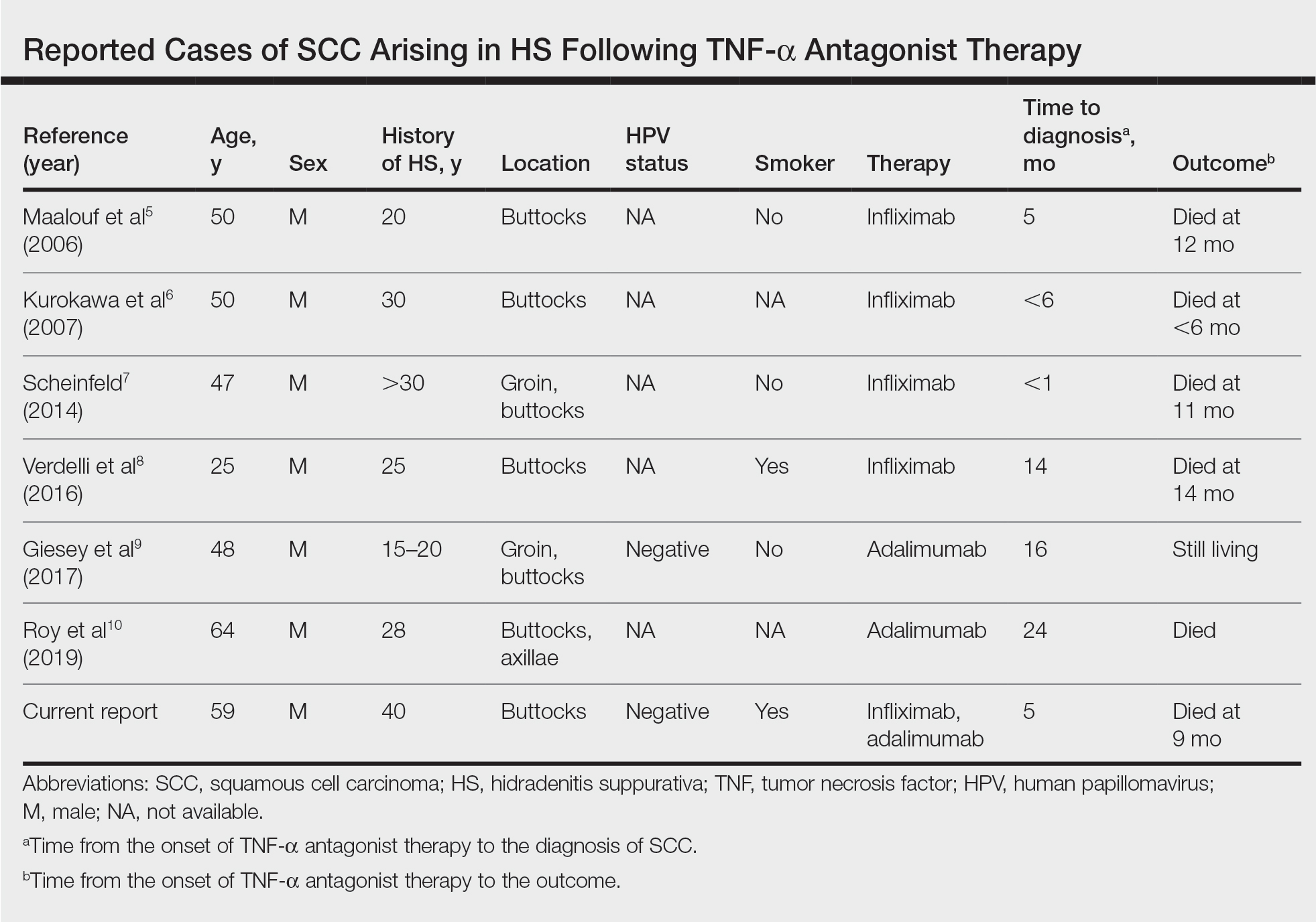
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
Practice Points
- Consider biopsy of representative lesions in men older than 20 years with moderate to severe disease of the groin and/or buttocks prior to initiation of tumor necrosis factor inhibitors.
- Consider more frequent clinical monitoring with a decrease in threshold to perform biopsy of any new or ulcerating lesions.
Hedgehog inhibitor alternative dosing advantageous for BCC
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY