User login
Cutaneous Manifestation as Initial Presentation of Metastatic Breast Cancer: A Systematic Review
Breast cancer is the second most common malignancy in women (after primary skin cancer) and is the second leading cause of cancer-related death in this population. In 2020, the American Cancer Society reported an estimated 276,480 new breast cancer diagnoses and 42,170 breast cancer–related deaths.1 Despite the fact that routine screening with mammography and sonography is standard, the incidence of advanced breast cancer at the time of diagnosis has remained stable over time, suggesting that life-threatening breast cancers are not being caught at an earlier stage. The number of breast cancers with distant metastases at the time of diagnosis also has not decreased.2 Therefore, although screening tests are valuable, they are imperfect and not without limitations.
Cutaneous metastasis is defined as the spread of malignant cells from an internal neoplasm to the skin, which can occur either by contiguous invasion or by distant metastasis through hematogenous or lymphatic routes.3 The diagnosis of cutaneous metastasis requires a high index of suspicion on the part of the clinician.4 Of the various internal malignancies in women, breast cancer most frequently results in metastasis to the skin,5 with up to 24% of patients with metastatic breast cancer developing cutaneous lesions.6
In recent years, there have been multiple reports of skin lesions prompting the diagnosis of a previously unknown breast cancer. In a study by Lookingbill et al,6 6.3% of patients with breast cancer presented with cutaneous involvement at the time of diagnosis, with 3.5% having skin symptoms as the presenting sign. Although there have been studies analyzing cutaneous metastasis from various internal malignancies, none thus far have focused on cutaneous metastasis as a presenting sign of breast cancer. This systematic review aimed to highlight the diverse clinical presentations of cutaneous metastatic breast cancer and their clinical implications.
Methods
Study Selection
This study utilized the PRISMA guidelines for systematic reviews.7 A review of the literature was conducted using the following databases: MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO.
Search Strategy and Analysis
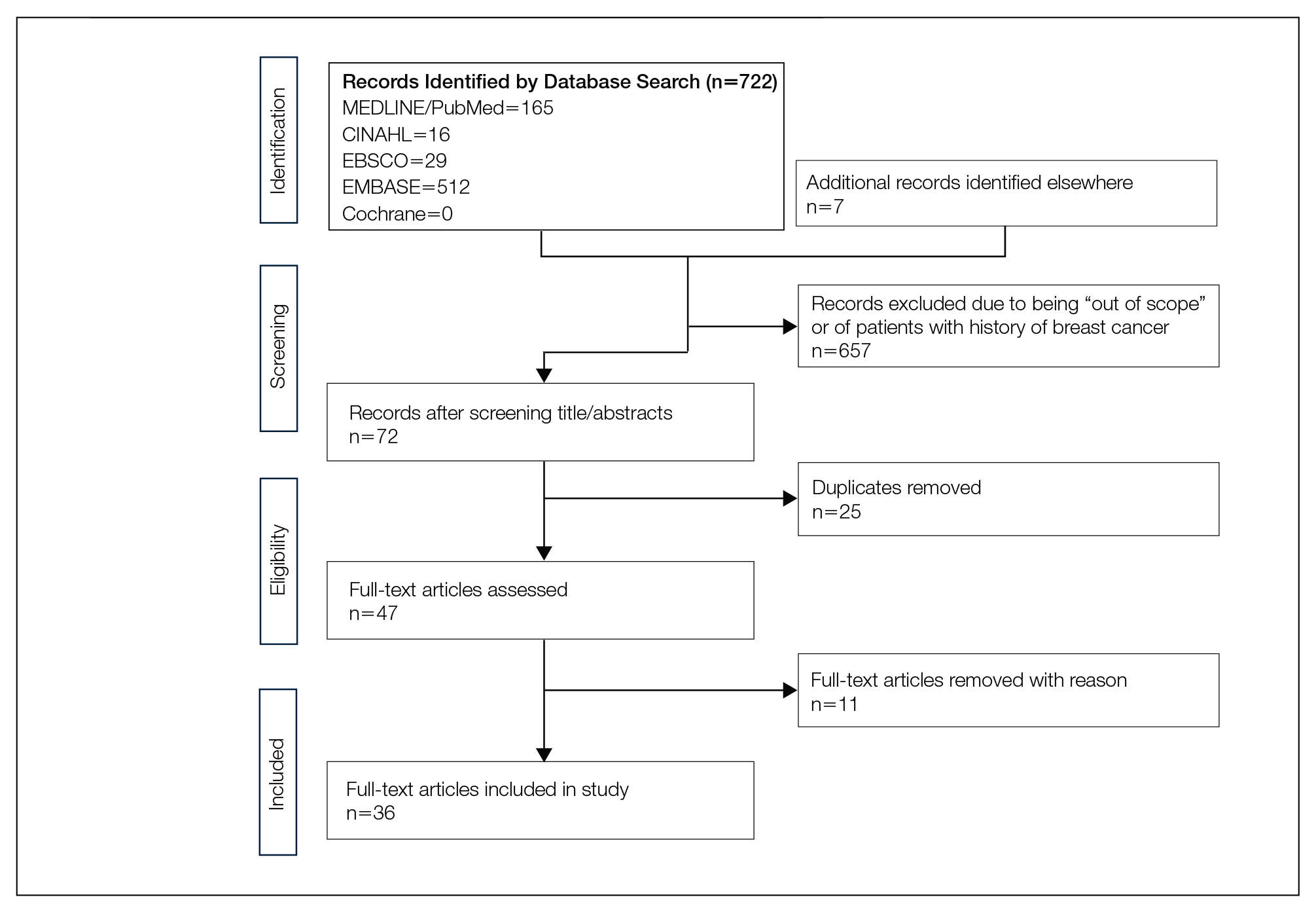
We completed our search of each of the databases on December 16, 2017, using the phrases cutaneous metastasis and breast cancer to find relevant case reports and retrospective studies. Three authors (C.J., S.R., and M.A.) manually reviewed the resulting abstracts. If an abstract did not include enough information to determine inclusion, the full-text version was reviewed by 2 of the authors (C.J. and S.R.). Two of the authors (C.J. and M.A.) also assessed each source for relevancy and included the articles deemed eligible (Figure 1).
Inclusion criteria were the following: case reports and retrospective studies published in the prior 10 years (January 1, 2007, to December 16, 2017) with human female patients who developed metastatic cutaneous lesions due to a previously unknown primary breast malignancy. Studies published in other languages were included; these articles were translated into English using a human translator or computer translation program (Google Translate). Exclusion criteria were the following: male patients, patients with a known diagnosis of primary breast malignancy prior to the appearance of a metastatic cutaneous lesion, articles focusing on the treatment of breast cancer, and articles without enough details to draw meaningful conclusions.
For a retrospective review to be included, it must have specified the number of breast cancer cases and the number of cutaneous metastases presenting initially or simultaneously to the breast cancer diagnosis. Bansal et al8 defined a simultaneous diagnosis as a skin lesion presenting with other concerns associated with the primary malignancy.
Results
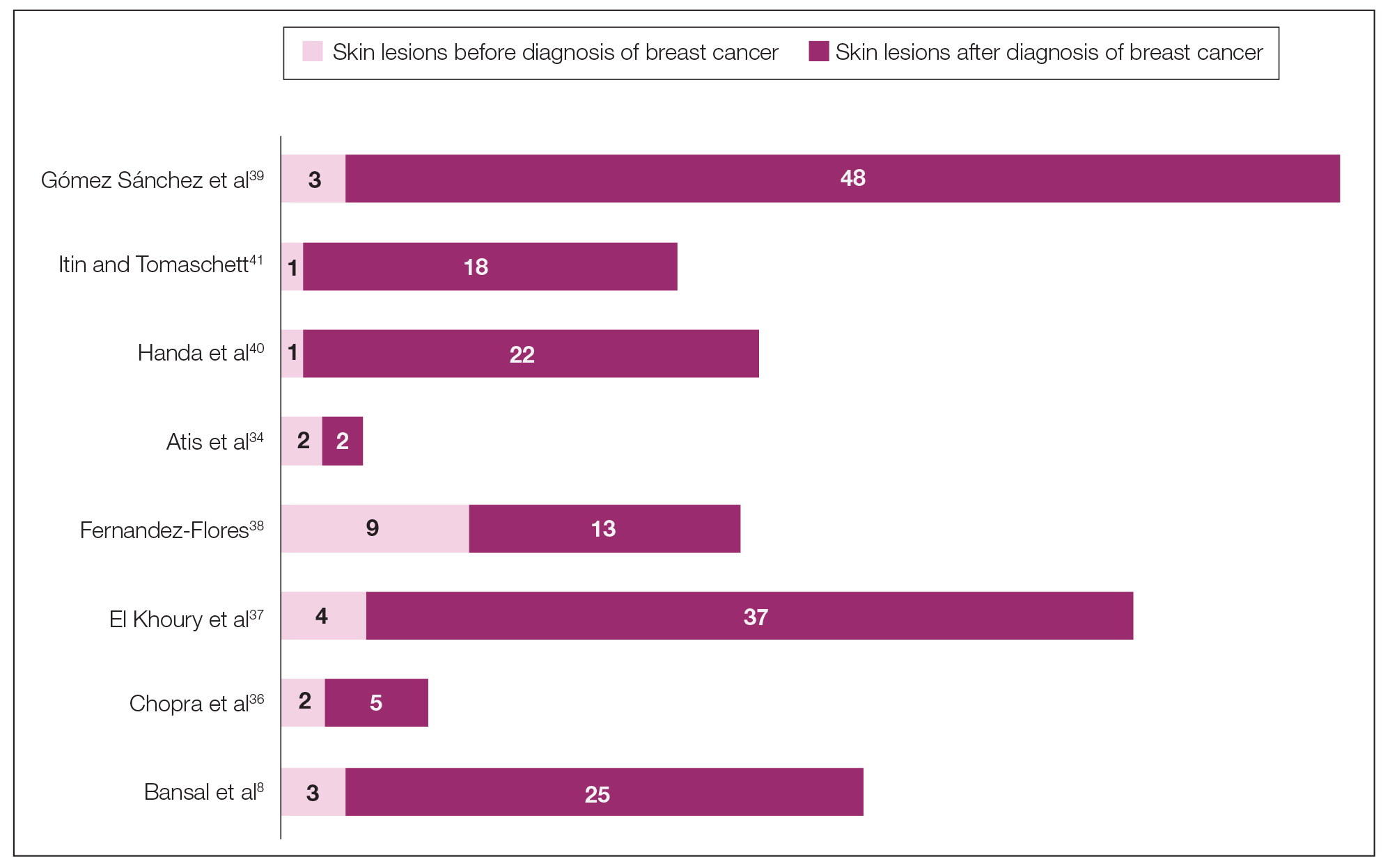
The initial search of MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO yielded a total of 722 articles. Seven other articles found separately while undergoing our initial research were added to this total. Abstracts were manually screened, with 657 articles discarded after failing to meet the predetermined inclusion criteria. After removal of 25 duplicate articles, the full text of the remaining 47 articles were reviewed, leading to the elimination of an additional 11 articles that did not meet the necessary criteria. This resulted in 36 articles (Figure 1), including 27 individual case reports (Table 1) and 9 retrospective reviews (Table 2). Approximately 13.7% of patients in the 9 retrospective reviews presented with a skin lesion before or simultaneous to the diagnosis of breast cancer (Figure 2).
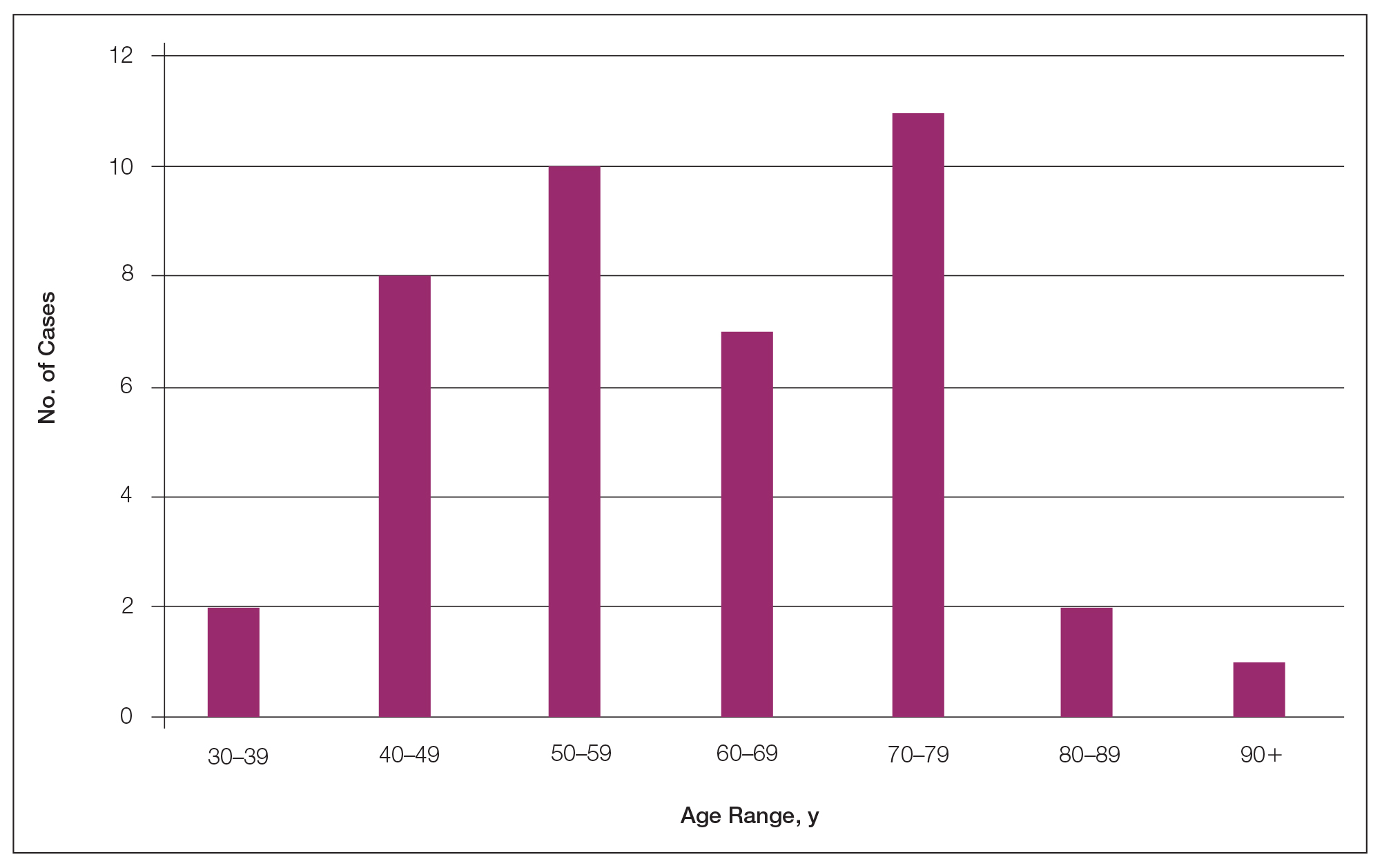
Forty-one percent (17/41) of the patients with cutaneous metastasis as a presenting feature of their breast cancer fell outside the age range for breast cancer screening recommended by the US Preventive Services Task Force,42 with 24% of the patients younger than 50 years and 17% older than 74 years (Figure 3).
Lesion Characteristics
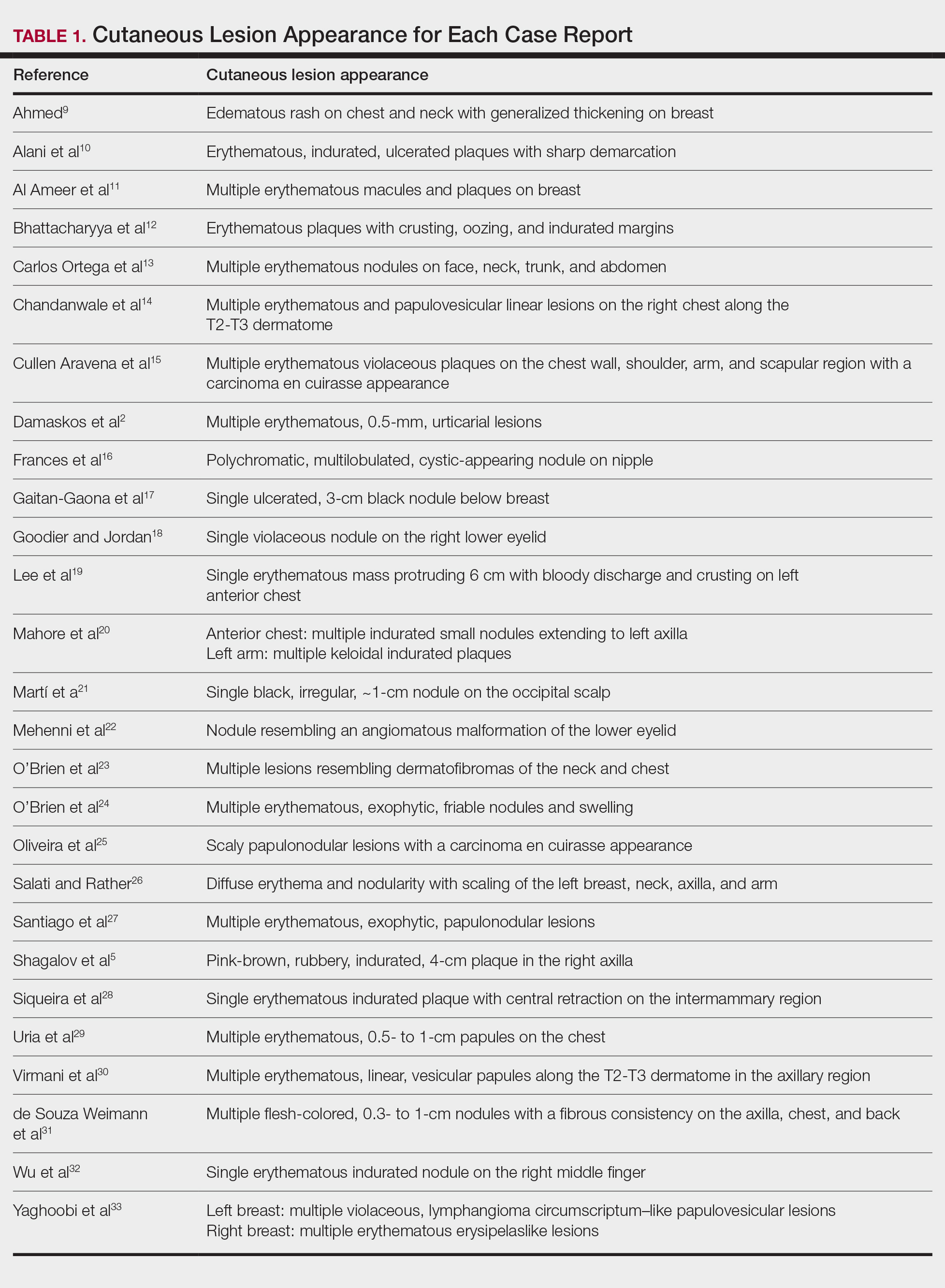
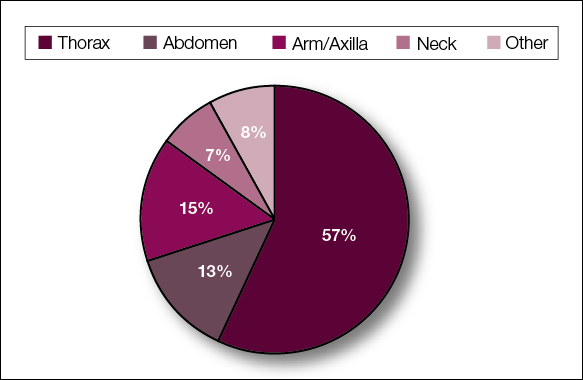
The most common cutaneous lesions were erythematous nodules and plaques, with a few reports of black17,21 or flesh-colored5,20,31 lesions, as well as ulceration.8,17,32 The most common location for skin lesions was on the thorax (chest or breast), accounting for 57% of the cutaneous metastases, with the arms and axillae being second most commonly involved (15%)(Figure 4). Some cases presented with skin lesions extending to multiple regions. In these cases, each location of the lesion was recorded separately when analyzing the data. An additional 5 cases, shown as “Other” in Figure 4, included the eyelids, occiput, and finger. Eight case reports described symptoms associated with the cutaneous lesions, with painful or tender lesions reported in 7 cases5,9,14,17,20,30,32 and pruritus in 2 cases.12,20 Moreover, 6 case reports presented patients denying any systemic or associated symptoms with their skin lesions.2,5,9,16,17,28 Multiple cases were initially treated as other conditions due to misdiagnosis, including herpes zoster14,30 and dermatitis.11,12
Diagnostic Data
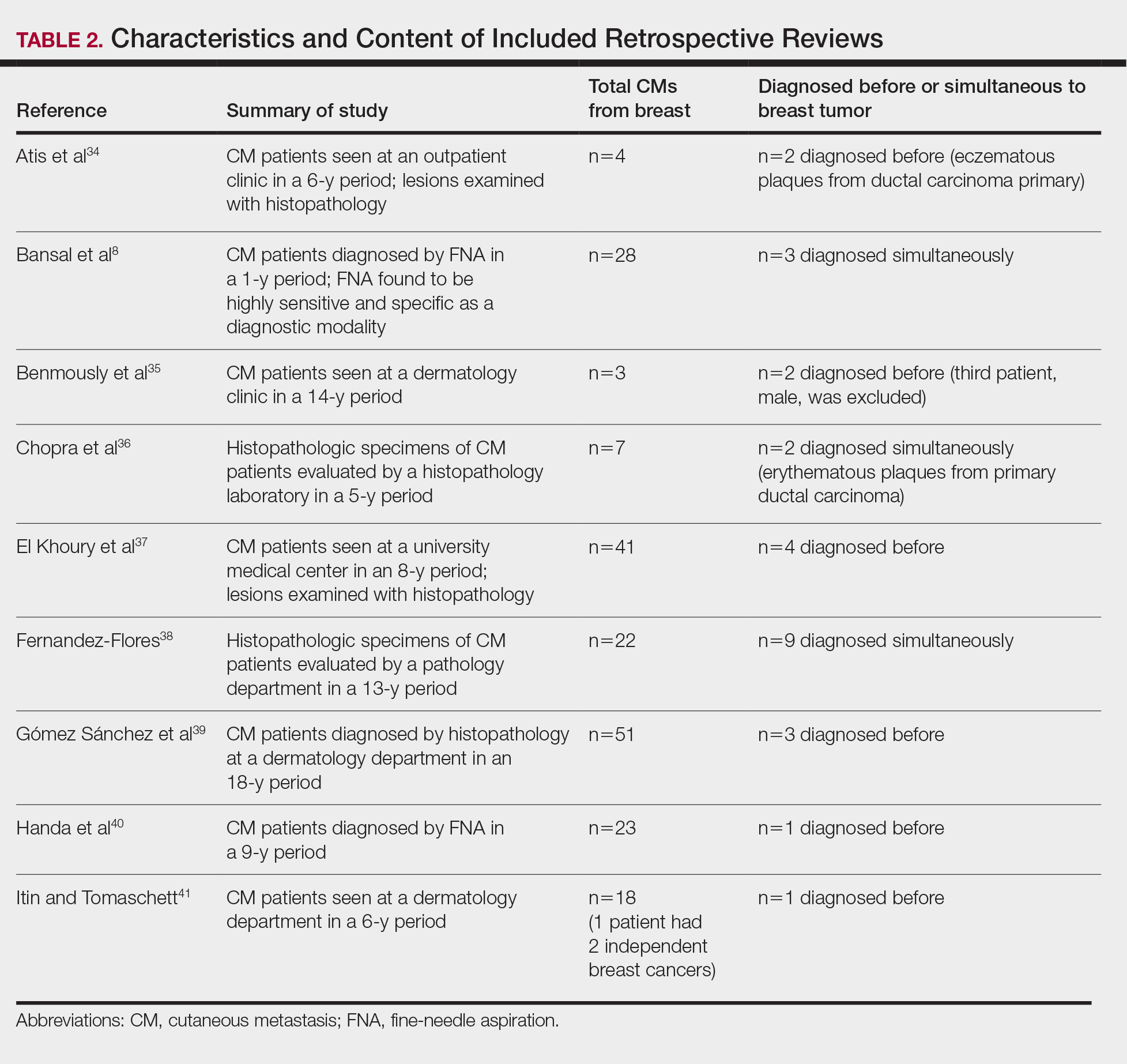
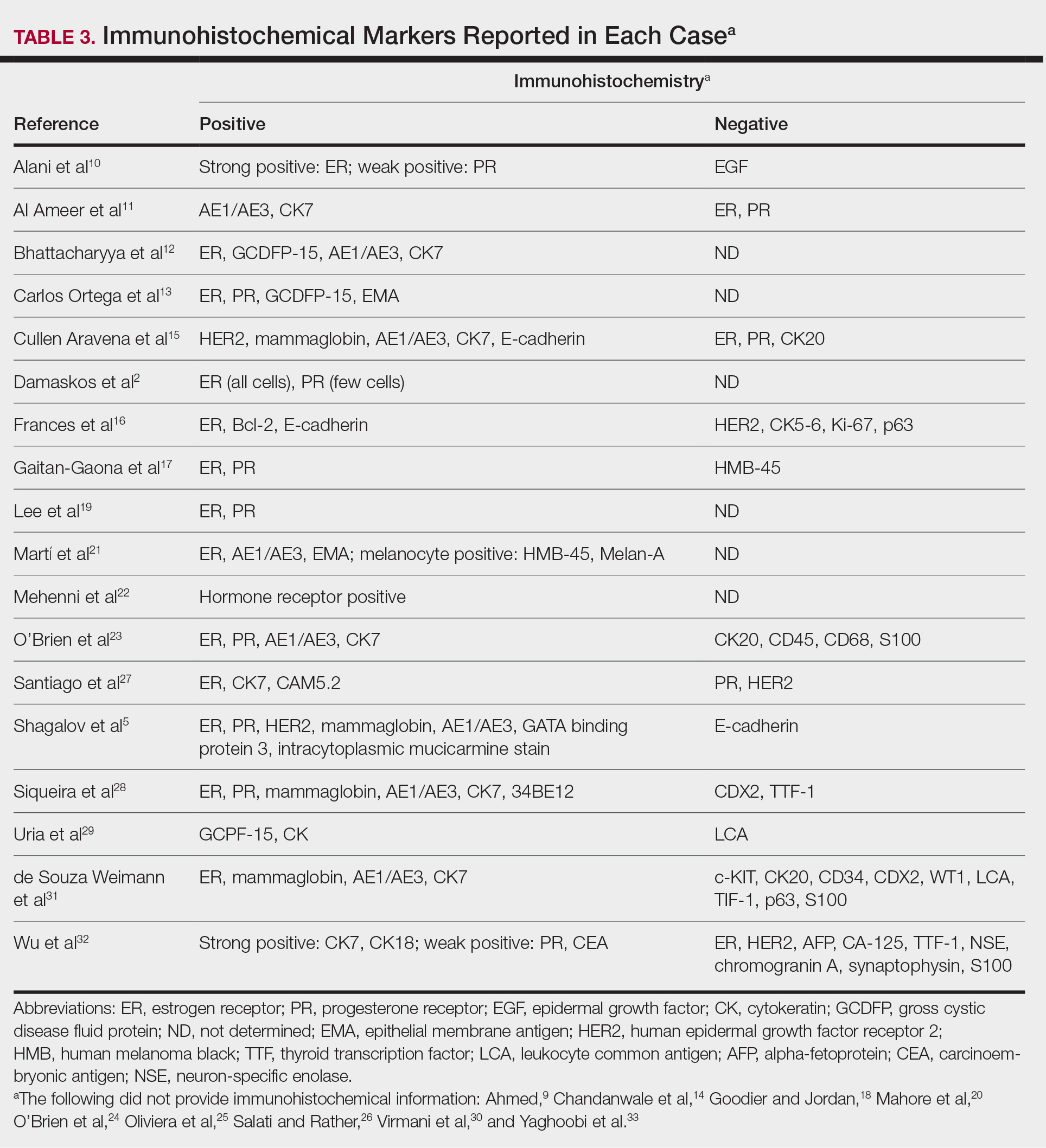
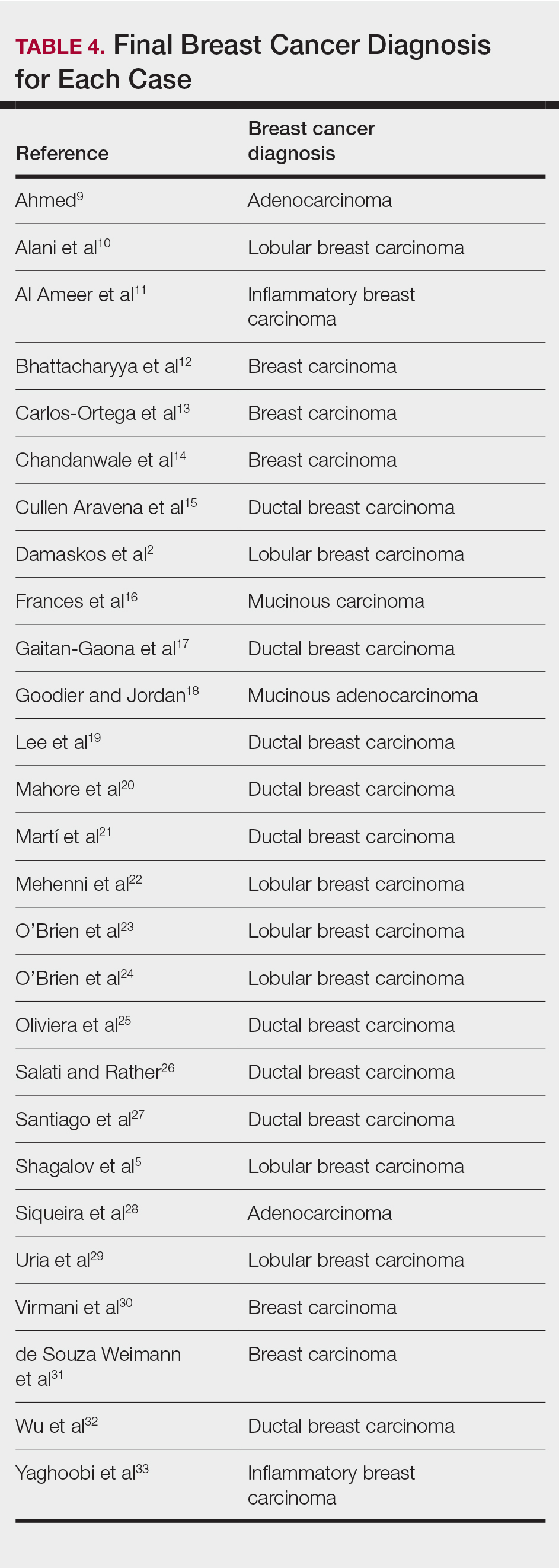
Eighteen cases reported positive immunohistochemistry from cutaneous biopsy (Table 3), given its high specificity in determining the origin of cutaneous metastases, while 8 case reports only performed hematoxylin and eosin staining. One case did not report hematoxylin and eosin or immunohistochemical staining. Table 4 lists the final breast cancer diagnosis for each case.
As per the standard of care, patients were evaluated with mammography or ultrasonography, combined with fine-needle aspiration of a suspected primary tumor, to give a definitive diagnosis of breast cancer. However, 4 cases reported negative mammography and ultrasonography.13,22,28,31 In 3 of these cases, no primary tumor was ever found.13,22,31
Comment
Our systematic review demonstrated that cutaneous lesions may be the first clinical manifestation of an undetected primary malignancy.40 These lesions often occur on the chest but may involve the face, abdomen, or extremities. Although asymptomatic erythematous nodules and plaques are the most common clinical presentations, lesions may be tender or pruritic or may even resemble benign skin conditions, including dermatitis, cellulitis, urticaria, and papulovesicular eruptions, causing them to go unrecognized.
Nevertheless, cutaneous metastasis of a visceral malignancy generally is observed late in the disease course, often following the diagnosis of a primary malignancy.14 Breast cancer is the most common internal malignancy to feature cutaneous spread, with the largest case series revealing a 23.9% rate of cutaneous metastases in females with breast carcinoma.6 Because of its proximity, the chest wall is the most common location for cutaneous lesions of metastatic breast cancer.
Malignant cells from a primary breast tumor may spread to the skin via lymphatic, hematogenous, or contiguous tissue dissemination, as well as iatrogenically through direct implantation during surgical procedures.3 The mechanism of neoplasm spread may likewise influence the clinical appearance of the resulting lesions. The localized lymphedema with a peau d’orange appearance of inflammatory metastatic breast carcinoma or the erythematous plaques of carcinoma erysipeloides are caused by embolized tumor cells obstructing dermal lymphatic vessels.3,11 On the other hand, the indurated erythematous plaques of carcinoma en cuirasse are caused by diffuse cutaneous and subcutaneous infiltration of tumor cells that also may be associated with marked reduction in breast volume.3
A primary breast cancer is classically diagnosed with a combination of clinical breast examination, radiologic imaging (ultrasound, mammogram, breast magnetic resonance imaging, or computed tomography), and fine-needle aspiration or lesional biopsy with histopathology.9 Given that in 20% of metastasized breast cancers the primary tumor may not be identified, a negative breast examination and imaging do not rule out breast cancer, especially if cutaneous biopsy reveals a primary malignancy.43 Histopathology and immunohistochemistry can thereby confirm the presence of metastatic cutaneous lesions and help characterize the breast cancer type involved, with adenocarcinomas being most commonly implicated.28 Although both ductal and lobular adenocarcinomas stain positive for cytokeratin 7, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15, carcinoembryonic antigen, and mammaglobin, only the former shows positivity for e-cadherin markers.3 Conversely, inflammatory carcinoma stains positive for CD31 and podoplanin, telangiectatic carcinoma stains positive for CD31, and mammary Paget disease stains positive for cytokeratin 7 and mucin 1, cell surface associated.3 Apart from cutaneous biopsy, fine-needle aspiration cytology can likewise provide a simple and rapid method of diagnosis with high sensitivity and specificity.14
Conclusion
Although cutaneous metastasis as the presenting sign of a breast malignancy is rare, a high index of suspicion should be exercised when encountering rapid-onset, out-of-place nodules or plaques in female patients, particularly nodules or plaques presenting on the chest.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2020 [published online January 8, 2020]. CA Cancer J Clin. 2020;70:7-30.
- Damaskos C, Dimitroulis D, Pergialiotis V, et al. An unexpected metastasis of breast cancer mimicking wheal rush. G Chir. 2016;37:136-138.
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
- Shagalov D, Xu M, Liebman T, et al. Unilateral indurated plaque in the axilla: a case of metastatic breast carcinoma. Dermatol Online J. 2016;22:13030/qt8vw382nx.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol. 2011;39:882-887.
- Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011:bcr0620114398.
- Alani A, Roberts G, Kerr O. Carcinoma en cuirasse. BMJ Case Rep. 2017;2017:bcr2017222121.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Bhattacharyya A, Gangopadhyay M, Ghosh K, et al. Wolf in sheep’s clothing: a case of carcinoma erysipeloides. Oxf Med Case Rep. 2016;2016:97-100.
- Carlos Ortega B, Alfaro Mejia A, Gómez-Campos G, et al. Metástasis de carcinoma de mama que simula prototecosis. Dermatol Rev Mex. 2012;56:55-61.
- Chandanwale SS, Gore CR, Buch AC, et al. Zosteriform cutaneous metastasis: a primary manifestation of carcinoma breast, rare case report. Indian J Pathol Microbiol. 2011;54:863-864.
- Cullen Aravena R, Cullen Aravena D, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hm3z850.
- Frances L, Cuesta L, Leiva-Salinas M, et al. Secondary mucinous carcinoma of the skin. Dermatol Online J. 2014;20:22361.
- Gaitan-Gaona F, Said MC, Valdes-Rodriguez R. Cutaneous metastatic pigmented breast carcinoma. Dermatol Online J. 2016;22:13030/qt0sv018ck.
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope. 2010;120(suppl 4):S129.
- Lee H-J, Kim J-M, Kim G-W, et al. A unique cutaneous presentation of breast cancer: a red apple stuck in the breast. Ann Dermatol. 2016;28:499-501.
- Mahore SD, Bothale KA, Patrikar AD, et al. Carcinoma en cuirasse : a rare presentation of breast cancer. Indian J Pathol Microbiol. 2010;53:351-358.
- Martí N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Mehenni NN, Gamaz-Bensaou M, Bouzid K. Metastatic breast carcinoma to the gallbladder and the lower eyelid with no malignant lesion in the breast: an unusual case report with a short review of the literature [abstract]. Ann Oncol. 2013;24(suppl 3):iii49.
- O’Brien OA, AboGhaly E, Heffron C. An unusual presentation of a common malignancy [abstract]. J Pathol. 2013;231:S33.
- O’Brien R, Porto DA, Friedman BJ, et al. Elderly female with swelling of the right breast. Ann Emerg Med. 2016;67:e25-e26.
- Oliveira GM de, Zachetti DBC, Barros HR, et al. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88:608-610.
- Salati SA, Rather AA. Carcinoma en cuirasse. J Pak Assoc Derma. 2013;23:452-454.
- Santiago F, Saleiro S, Brites MM, et al. A remarkable case of cutaneous metastatic breast carcinoma. Dermatol Online J. 2009;15:10.
- Siqueira VR, Frota AS, Maia IL, et al. Cutaneous involvement as the initial presentation of metastatic breast adenocarcinoma - case report. An Bras Dermatol. 2014;89:960-963.
- Uria M, Chirino C, Rivas D. Inusual clinical presentation of cutaneous metastasis from breast carcinoma. A case report. Rev Argent Dermatol. 2009;90:230-236.
- Virmani NC, Sharma YK, Panicker NK, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56:726-727.
- de Souza Weimann ET, Botero EB, Mendes C, et al. Cutaneous metastasis as the first manifestation of occult malignant breast neoplasia. An Bras Dermatol. 2016;91(5 suppl 1):105-107.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:e409-e410.
- Yaghoobi R, Talaizade A, Lal K, et al. Inflammatory breast carcinoma presenting with two different patterns of cutaneous metastases: carcinoma telangiectaticum and carcinoma erysipeloides. J Clin Aesthet Dermatol. 2015;8:47-51.
- Atis G, Demirci GT, Atunay IK, et al. The clinical characteristics and the frequency of metastatic cutaneous tumors among primary skin tumors. Turkderm. 2013;47:166-169.
- Benmously R, Souissi A, Badri T, et al. Cutaneous metastases from internal cancers. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:167-170.
- Chopra R, Chhabra S, Samra SG, et al. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010;76:125-131.
- El Khoury J, Khalifeh I, Kibbi AG, et al. Cutaneous metastasis: clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int J Dermatol. 2014;53:147-158.
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222-239.
- Gómez Sánchez ME, Martinez Martinez ML, Martín De Hijas MC, et al. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel. 2014;29:207-212
- Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47-54.
- Itin P, Tomaschett S. Cutaneous metastases from malignancies which do not originate from the skin. An epidemiological study. Article in German. Internist (Berl). 2009;50:179-186.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Torres HA, Bodey GP, Tarrand JJ, et al. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9:786-792.
Breast cancer is the second most common malignancy in women (after primary skin cancer) and is the second leading cause of cancer-related death in this population. In 2020, the American Cancer Society reported an estimated 276,480 new breast cancer diagnoses and 42,170 breast cancer–related deaths.1 Despite the fact that routine screening with mammography and sonography is standard, the incidence of advanced breast cancer at the time of diagnosis has remained stable over time, suggesting that life-threatening breast cancers are not being caught at an earlier stage. The number of breast cancers with distant metastases at the time of diagnosis also has not decreased.2 Therefore, although screening tests are valuable, they are imperfect and not without limitations.
Cutaneous metastasis is defined as the spread of malignant cells from an internal neoplasm to the skin, which can occur either by contiguous invasion or by distant metastasis through hematogenous or lymphatic routes.3 The diagnosis of cutaneous metastasis requires a high index of suspicion on the part of the clinician.4 Of the various internal malignancies in women, breast cancer most frequently results in metastasis to the skin,5 with up to 24% of patients with metastatic breast cancer developing cutaneous lesions.6
In recent years, there have been multiple reports of skin lesions prompting the diagnosis of a previously unknown breast cancer. In a study by Lookingbill et al,6 6.3% of patients with breast cancer presented with cutaneous involvement at the time of diagnosis, with 3.5% having skin symptoms as the presenting sign. Although there have been studies analyzing cutaneous metastasis from various internal malignancies, none thus far have focused on cutaneous metastasis as a presenting sign of breast cancer. This systematic review aimed to highlight the diverse clinical presentations of cutaneous metastatic breast cancer and their clinical implications.
Methods
Study Selection
This study utilized the PRISMA guidelines for systematic reviews.7 A review of the literature was conducted using the following databases: MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO.
Search Strategy and Analysis
We completed our search of each of the databases on December 16, 2017, using the phrases cutaneous metastasis and breast cancer to find relevant case reports and retrospective studies. Three authors (C.J., S.R., and M.A.) manually reviewed the resulting abstracts. If an abstract did not include enough information to determine inclusion, the full-text version was reviewed by 2 of the authors (C.J. and S.R.). Two of the authors (C.J. and M.A.) also assessed each source for relevancy and included the articles deemed eligible (Figure 1).

Inclusion criteria were the following: case reports and retrospective studies published in the prior 10 years (January 1, 2007, to December 16, 2017) with human female patients who developed metastatic cutaneous lesions due to a previously unknown primary breast malignancy. Studies published in other languages were included; these articles were translated into English using a human translator or computer translation program (Google Translate). Exclusion criteria were the following: male patients, patients with a known diagnosis of primary breast malignancy prior to the appearance of a metastatic cutaneous lesion, articles focusing on the treatment of breast cancer, and articles without enough details to draw meaningful conclusions.
For a retrospective review to be included, it must have specified the number of breast cancer cases and the number of cutaneous metastases presenting initially or simultaneously to the breast cancer diagnosis. Bansal et al8 defined a simultaneous diagnosis as a skin lesion presenting with other concerns associated with the primary malignancy.
Results
The initial search of MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO yielded a total of 722 articles. Seven other articles found separately while undergoing our initial research were added to this total. Abstracts were manually screened, with 657 articles discarded after failing to meet the predetermined inclusion criteria. After removal of 25 duplicate articles, the full text of the remaining 47 articles were reviewed, leading to the elimination of an additional 11 articles that did not meet the necessary criteria. This resulted in 36 articles (Figure 1), including 27 individual case reports (Table 1) and 9 retrospective reviews (Table 2). Approximately 13.7% of patients in the 9 retrospective reviews presented with a skin lesion before or simultaneous to the diagnosis of breast cancer (Figure 2).



Forty-one percent (17/41) of the patients with cutaneous metastasis as a presenting feature of their breast cancer fell outside the age range for breast cancer screening recommended by the US Preventive Services Task Force,42 with 24% of the patients younger than 50 years and 17% older than 74 years (Figure 3).

Lesion Characteristics
The most common cutaneous lesions were erythematous nodules and plaques, with a few reports of black17,21 or flesh-colored5,20,31 lesions, as well as ulceration.8,17,32 The most common location for skin lesions was on the thorax (chest or breast), accounting for 57% of the cutaneous metastases, with the arms and axillae being second most commonly involved (15%)(Figure 4). Some cases presented with skin lesions extending to multiple regions. In these cases, each location of the lesion was recorded separately when analyzing the data. An additional 5 cases, shown as “Other” in Figure 4, included the eyelids, occiput, and finger. Eight case reports described symptoms associated with the cutaneous lesions, with painful or tender lesions reported in 7 cases5,9,14,17,20,30,32 and pruritus in 2 cases.12,20 Moreover, 6 case reports presented patients denying any systemic or associated symptoms with their skin lesions.2,5,9,16,17,28 Multiple cases were initially treated as other conditions due to misdiagnosis, including herpes zoster14,30 and dermatitis.11,12

Diagnostic Data
Eighteen cases reported positive immunohistochemistry from cutaneous biopsy (Table 3), given its high specificity in determining the origin of cutaneous metastases, while 8 case reports only performed hematoxylin and eosin staining. One case did not report hematoxylin and eosin or immunohistochemical staining. Table 4 lists the final breast cancer diagnosis for each case.


As per the standard of care, patients were evaluated with mammography or ultrasonography, combined with fine-needle aspiration of a suspected primary tumor, to give a definitive diagnosis of breast cancer. However, 4 cases reported negative mammography and ultrasonography.13,22,28,31 In 3 of these cases, no primary tumor was ever found.13,22,31
Comment
Our systematic review demonstrated that cutaneous lesions may be the first clinical manifestation of an undetected primary malignancy.40 These lesions often occur on the chest but may involve the face, abdomen, or extremities. Although asymptomatic erythematous nodules and plaques are the most common clinical presentations, lesions may be tender or pruritic or may even resemble benign skin conditions, including dermatitis, cellulitis, urticaria, and papulovesicular eruptions, causing them to go unrecognized.
Nevertheless, cutaneous metastasis of a visceral malignancy generally is observed late in the disease course, often following the diagnosis of a primary malignancy.14 Breast cancer is the most common internal malignancy to feature cutaneous spread, with the largest case series revealing a 23.9% rate of cutaneous metastases in females with breast carcinoma.6 Because of its proximity, the chest wall is the most common location for cutaneous lesions of metastatic breast cancer.
Malignant cells from a primary breast tumor may spread to the skin via lymphatic, hematogenous, or contiguous tissue dissemination, as well as iatrogenically through direct implantation during surgical procedures.3 The mechanism of neoplasm spread may likewise influence the clinical appearance of the resulting lesions. The localized lymphedema with a peau d’orange appearance of inflammatory metastatic breast carcinoma or the erythematous plaques of carcinoma erysipeloides are caused by embolized tumor cells obstructing dermal lymphatic vessels.3,11 On the other hand, the indurated erythematous plaques of carcinoma en cuirasse are caused by diffuse cutaneous and subcutaneous infiltration of tumor cells that also may be associated with marked reduction in breast volume.3
A primary breast cancer is classically diagnosed with a combination of clinical breast examination, radiologic imaging (ultrasound, mammogram, breast magnetic resonance imaging, or computed tomography), and fine-needle aspiration or lesional biopsy with histopathology.9 Given that in 20% of metastasized breast cancers the primary tumor may not be identified, a negative breast examination and imaging do not rule out breast cancer, especially if cutaneous biopsy reveals a primary malignancy.43 Histopathology and immunohistochemistry can thereby confirm the presence of metastatic cutaneous lesions and help characterize the breast cancer type involved, with adenocarcinomas being most commonly implicated.28 Although both ductal and lobular adenocarcinomas stain positive for cytokeratin 7, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15, carcinoembryonic antigen, and mammaglobin, only the former shows positivity for e-cadherin markers.3 Conversely, inflammatory carcinoma stains positive for CD31 and podoplanin, telangiectatic carcinoma stains positive for CD31, and mammary Paget disease stains positive for cytokeratin 7 and mucin 1, cell surface associated.3 Apart from cutaneous biopsy, fine-needle aspiration cytology can likewise provide a simple and rapid method of diagnosis with high sensitivity and specificity.14
Conclusion
Although cutaneous metastasis as the presenting sign of a breast malignancy is rare, a high index of suspicion should be exercised when encountering rapid-onset, out-of-place nodules or plaques in female patients, particularly nodules or plaques presenting on the chest.
Breast cancer is the second most common malignancy in women (after primary skin cancer) and is the second leading cause of cancer-related death in this population. In 2020, the American Cancer Society reported an estimated 276,480 new breast cancer diagnoses and 42,170 breast cancer–related deaths.1 Despite the fact that routine screening with mammography and sonography is standard, the incidence of advanced breast cancer at the time of diagnosis has remained stable over time, suggesting that life-threatening breast cancers are not being caught at an earlier stage. The number of breast cancers with distant metastases at the time of diagnosis also has not decreased.2 Therefore, although screening tests are valuable, they are imperfect and not without limitations.
Cutaneous metastasis is defined as the spread of malignant cells from an internal neoplasm to the skin, which can occur either by contiguous invasion or by distant metastasis through hematogenous or lymphatic routes.3 The diagnosis of cutaneous metastasis requires a high index of suspicion on the part of the clinician.4 Of the various internal malignancies in women, breast cancer most frequently results in metastasis to the skin,5 with up to 24% of patients with metastatic breast cancer developing cutaneous lesions.6
In recent years, there have been multiple reports of skin lesions prompting the diagnosis of a previously unknown breast cancer. In a study by Lookingbill et al,6 6.3% of patients with breast cancer presented with cutaneous involvement at the time of diagnosis, with 3.5% having skin symptoms as the presenting sign. Although there have been studies analyzing cutaneous metastasis from various internal malignancies, none thus far have focused on cutaneous metastasis as a presenting sign of breast cancer. This systematic review aimed to highlight the diverse clinical presentations of cutaneous metastatic breast cancer and their clinical implications.
Methods
Study Selection
This study utilized the PRISMA guidelines for systematic reviews.7 A review of the literature was conducted using the following databases: MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO.
Search Strategy and Analysis
We completed our search of each of the databases on December 16, 2017, using the phrases cutaneous metastasis and breast cancer to find relevant case reports and retrospective studies. Three authors (C.J., S.R., and M.A.) manually reviewed the resulting abstracts. If an abstract did not include enough information to determine inclusion, the full-text version was reviewed by 2 of the authors (C.J. and S.R.). Two of the authors (C.J. and M.A.) also assessed each source for relevancy and included the articles deemed eligible (Figure 1).

Inclusion criteria were the following: case reports and retrospective studies published in the prior 10 years (January 1, 2007, to December 16, 2017) with human female patients who developed metastatic cutaneous lesions due to a previously unknown primary breast malignancy. Studies published in other languages were included; these articles were translated into English using a human translator or computer translation program (Google Translate). Exclusion criteria were the following: male patients, patients with a known diagnosis of primary breast malignancy prior to the appearance of a metastatic cutaneous lesion, articles focusing on the treatment of breast cancer, and articles without enough details to draw meaningful conclusions.
For a retrospective review to be included, it must have specified the number of breast cancer cases and the number of cutaneous metastases presenting initially or simultaneously to the breast cancer diagnosis. Bansal et al8 defined a simultaneous diagnosis as a skin lesion presenting with other concerns associated with the primary malignancy.
Results
The initial search of MEDLINE/PubMed, EMBASE, Cochrane library, CINAHL, and EBSCO yielded a total of 722 articles. Seven other articles found separately while undergoing our initial research were added to this total. Abstracts were manually screened, with 657 articles discarded after failing to meet the predetermined inclusion criteria. After removal of 25 duplicate articles, the full text of the remaining 47 articles were reviewed, leading to the elimination of an additional 11 articles that did not meet the necessary criteria. This resulted in 36 articles (Figure 1), including 27 individual case reports (Table 1) and 9 retrospective reviews (Table 2). Approximately 13.7% of patients in the 9 retrospective reviews presented with a skin lesion before or simultaneous to the diagnosis of breast cancer (Figure 2).



Forty-one percent (17/41) of the patients with cutaneous metastasis as a presenting feature of their breast cancer fell outside the age range for breast cancer screening recommended by the US Preventive Services Task Force,42 with 24% of the patients younger than 50 years and 17% older than 74 years (Figure 3).

Lesion Characteristics
The most common cutaneous lesions were erythematous nodules and plaques, with a few reports of black17,21 or flesh-colored5,20,31 lesions, as well as ulceration.8,17,32 The most common location for skin lesions was on the thorax (chest or breast), accounting for 57% of the cutaneous metastases, with the arms and axillae being second most commonly involved (15%)(Figure 4). Some cases presented with skin lesions extending to multiple regions. In these cases, each location of the lesion was recorded separately when analyzing the data. An additional 5 cases, shown as “Other” in Figure 4, included the eyelids, occiput, and finger. Eight case reports described symptoms associated with the cutaneous lesions, with painful or tender lesions reported in 7 cases5,9,14,17,20,30,32 and pruritus in 2 cases.12,20 Moreover, 6 case reports presented patients denying any systemic or associated symptoms with their skin lesions.2,5,9,16,17,28 Multiple cases were initially treated as other conditions due to misdiagnosis, including herpes zoster14,30 and dermatitis.11,12

Diagnostic Data
Eighteen cases reported positive immunohistochemistry from cutaneous biopsy (Table 3), given its high specificity in determining the origin of cutaneous metastases, while 8 case reports only performed hematoxylin and eosin staining. One case did not report hematoxylin and eosin or immunohistochemical staining. Table 4 lists the final breast cancer diagnosis for each case.


As per the standard of care, patients were evaluated with mammography or ultrasonography, combined with fine-needle aspiration of a suspected primary tumor, to give a definitive diagnosis of breast cancer. However, 4 cases reported negative mammography and ultrasonography.13,22,28,31 In 3 of these cases, no primary tumor was ever found.13,22,31
Comment
Our systematic review demonstrated that cutaneous lesions may be the first clinical manifestation of an undetected primary malignancy.40 These lesions often occur on the chest but may involve the face, abdomen, or extremities. Although asymptomatic erythematous nodules and plaques are the most common clinical presentations, lesions may be tender or pruritic or may even resemble benign skin conditions, including dermatitis, cellulitis, urticaria, and papulovesicular eruptions, causing them to go unrecognized.
Nevertheless, cutaneous metastasis of a visceral malignancy generally is observed late in the disease course, often following the diagnosis of a primary malignancy.14 Breast cancer is the most common internal malignancy to feature cutaneous spread, with the largest case series revealing a 23.9% rate of cutaneous metastases in females with breast carcinoma.6 Because of its proximity, the chest wall is the most common location for cutaneous lesions of metastatic breast cancer.
Malignant cells from a primary breast tumor may spread to the skin via lymphatic, hematogenous, or contiguous tissue dissemination, as well as iatrogenically through direct implantation during surgical procedures.3 The mechanism of neoplasm spread may likewise influence the clinical appearance of the resulting lesions. The localized lymphedema with a peau d’orange appearance of inflammatory metastatic breast carcinoma or the erythematous plaques of carcinoma erysipeloides are caused by embolized tumor cells obstructing dermal lymphatic vessels.3,11 On the other hand, the indurated erythematous plaques of carcinoma en cuirasse are caused by diffuse cutaneous and subcutaneous infiltration of tumor cells that also may be associated with marked reduction in breast volume.3
A primary breast cancer is classically diagnosed with a combination of clinical breast examination, radiologic imaging (ultrasound, mammogram, breast magnetic resonance imaging, or computed tomography), and fine-needle aspiration or lesional biopsy with histopathology.9 Given that in 20% of metastasized breast cancers the primary tumor may not be identified, a negative breast examination and imaging do not rule out breast cancer, especially if cutaneous biopsy reveals a primary malignancy.43 Histopathology and immunohistochemistry can thereby confirm the presence of metastatic cutaneous lesions and help characterize the breast cancer type involved, with adenocarcinomas being most commonly implicated.28 Although both ductal and lobular adenocarcinomas stain positive for cytokeratin 7, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15, carcinoembryonic antigen, and mammaglobin, only the former shows positivity for e-cadherin markers.3 Conversely, inflammatory carcinoma stains positive for CD31 and podoplanin, telangiectatic carcinoma stains positive for CD31, and mammary Paget disease stains positive for cytokeratin 7 and mucin 1, cell surface associated.3 Apart from cutaneous biopsy, fine-needle aspiration cytology can likewise provide a simple and rapid method of diagnosis with high sensitivity and specificity.14
Conclusion
Although cutaneous metastasis as the presenting sign of a breast malignancy is rare, a high index of suspicion should be exercised when encountering rapid-onset, out-of-place nodules or plaques in female patients, particularly nodules or plaques presenting on the chest.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2020 [published online January 8, 2020]. CA Cancer J Clin. 2020;70:7-30.
- Damaskos C, Dimitroulis D, Pergialiotis V, et al. An unexpected metastasis of breast cancer mimicking wheal rush. G Chir. 2016;37:136-138.
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
- Shagalov D, Xu M, Liebman T, et al. Unilateral indurated plaque in the axilla: a case of metastatic breast carcinoma. Dermatol Online J. 2016;22:13030/qt8vw382nx.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol. 2011;39:882-887.
- Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011:bcr0620114398.
- Alani A, Roberts G, Kerr O. Carcinoma en cuirasse. BMJ Case Rep. 2017;2017:bcr2017222121.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Bhattacharyya A, Gangopadhyay M, Ghosh K, et al. Wolf in sheep’s clothing: a case of carcinoma erysipeloides. Oxf Med Case Rep. 2016;2016:97-100.
- Carlos Ortega B, Alfaro Mejia A, Gómez-Campos G, et al. Metástasis de carcinoma de mama que simula prototecosis. Dermatol Rev Mex. 2012;56:55-61.
- Chandanwale SS, Gore CR, Buch AC, et al. Zosteriform cutaneous metastasis: a primary manifestation of carcinoma breast, rare case report. Indian J Pathol Microbiol. 2011;54:863-864.
- Cullen Aravena R, Cullen Aravena D, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hm3z850.
- Frances L, Cuesta L, Leiva-Salinas M, et al. Secondary mucinous carcinoma of the skin. Dermatol Online J. 2014;20:22361.
- Gaitan-Gaona F, Said MC, Valdes-Rodriguez R. Cutaneous metastatic pigmented breast carcinoma. Dermatol Online J. 2016;22:13030/qt0sv018ck.
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope. 2010;120(suppl 4):S129.
- Lee H-J, Kim J-M, Kim G-W, et al. A unique cutaneous presentation of breast cancer: a red apple stuck in the breast. Ann Dermatol. 2016;28:499-501.
- Mahore SD, Bothale KA, Patrikar AD, et al. Carcinoma en cuirasse : a rare presentation of breast cancer. Indian J Pathol Microbiol. 2010;53:351-358.
- Martí N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Mehenni NN, Gamaz-Bensaou M, Bouzid K. Metastatic breast carcinoma to the gallbladder and the lower eyelid with no malignant lesion in the breast: an unusual case report with a short review of the literature [abstract]. Ann Oncol. 2013;24(suppl 3):iii49.
- O’Brien OA, AboGhaly E, Heffron C. An unusual presentation of a common malignancy [abstract]. J Pathol. 2013;231:S33.
- O’Brien R, Porto DA, Friedman BJ, et al. Elderly female with swelling of the right breast. Ann Emerg Med. 2016;67:e25-e26.
- Oliveira GM de, Zachetti DBC, Barros HR, et al. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88:608-610.
- Salati SA, Rather AA. Carcinoma en cuirasse. J Pak Assoc Derma. 2013;23:452-454.
- Santiago F, Saleiro S, Brites MM, et al. A remarkable case of cutaneous metastatic breast carcinoma. Dermatol Online J. 2009;15:10.
- Siqueira VR, Frota AS, Maia IL, et al. Cutaneous involvement as the initial presentation of metastatic breast adenocarcinoma - case report. An Bras Dermatol. 2014;89:960-963.
- Uria M, Chirino C, Rivas D. Inusual clinical presentation of cutaneous metastasis from breast carcinoma. A case report. Rev Argent Dermatol. 2009;90:230-236.
- Virmani NC, Sharma YK, Panicker NK, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56:726-727.
- de Souza Weimann ET, Botero EB, Mendes C, et al. Cutaneous metastasis as the first manifestation of occult malignant breast neoplasia. An Bras Dermatol. 2016;91(5 suppl 1):105-107.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:e409-e410.
- Yaghoobi R, Talaizade A, Lal K, et al. Inflammatory breast carcinoma presenting with two different patterns of cutaneous metastases: carcinoma telangiectaticum and carcinoma erysipeloides. J Clin Aesthet Dermatol. 2015;8:47-51.
- Atis G, Demirci GT, Atunay IK, et al. The clinical characteristics and the frequency of metastatic cutaneous tumors among primary skin tumors. Turkderm. 2013;47:166-169.
- Benmously R, Souissi A, Badri T, et al. Cutaneous metastases from internal cancers. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:167-170.
- Chopra R, Chhabra S, Samra SG, et al. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010;76:125-131.
- El Khoury J, Khalifeh I, Kibbi AG, et al. Cutaneous metastasis: clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int J Dermatol. 2014;53:147-158.
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222-239.
- Gómez Sánchez ME, Martinez Martinez ML, Martín De Hijas MC, et al. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel. 2014;29:207-212
- Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47-54.
- Itin P, Tomaschett S. Cutaneous metastases from malignancies which do not originate from the skin. An epidemiological study. Article in German. Internist (Berl). 2009;50:179-186.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Torres HA, Bodey GP, Tarrand JJ, et al. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9:786-792.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2020 [published online January 8, 2020]. CA Cancer J Clin. 2020;70:7-30.
- Damaskos C, Dimitroulis D, Pergialiotis V, et al. An unexpected metastasis of breast cancer mimicking wheal rush. G Chir. 2016;37:136-138.
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
- Shagalov D, Xu M, Liebman T, et al. Unilateral indurated plaque in the axilla: a case of metastatic breast carcinoma. Dermatol Online J. 2016;22:13030/qt8vw382nx.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol. 2011;39:882-887.
- Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011:bcr0620114398.
- Alani A, Roberts G, Kerr O. Carcinoma en cuirasse. BMJ Case Rep. 2017;2017:bcr2017222121.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Bhattacharyya A, Gangopadhyay M, Ghosh K, et al. Wolf in sheep’s clothing: a case of carcinoma erysipeloides. Oxf Med Case Rep. 2016;2016:97-100.
- Carlos Ortega B, Alfaro Mejia A, Gómez-Campos G, et al. Metástasis de carcinoma de mama que simula prototecosis. Dermatol Rev Mex. 2012;56:55-61.
- Chandanwale SS, Gore CR, Buch AC, et al. Zosteriform cutaneous metastasis: a primary manifestation of carcinoma breast, rare case report. Indian J Pathol Microbiol. 2011;54:863-864.
- Cullen Aravena R, Cullen Aravena D, Velasco MJ, et al. Carcinoma hemorrhagiectoides: case report of an uncommon presentation of cutaneous metastatic breast carcinoma. Dermatol Online J. 2017;23:13030/qt3hm3z850.
- Frances L, Cuesta L, Leiva-Salinas M, et al. Secondary mucinous carcinoma of the skin. Dermatol Online J. 2014;20:22361.
- Gaitan-Gaona F, Said MC, Valdes-Rodriguez R. Cutaneous metastatic pigmented breast carcinoma. Dermatol Online J. 2016;22:13030/qt0sv018ck.
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope. 2010;120(suppl 4):S129.
- Lee H-J, Kim J-M, Kim G-W, et al. A unique cutaneous presentation of breast cancer: a red apple stuck in the breast. Ann Dermatol. 2016;28:499-501.
- Mahore SD, Bothale KA, Patrikar AD, et al. Carcinoma en cuirasse : a rare presentation of breast cancer. Indian J Pathol Microbiol. 2010;53:351-358.
- Martí N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Mehenni NN, Gamaz-Bensaou M, Bouzid K. Metastatic breast carcinoma to the gallbladder and the lower eyelid with no malignant lesion in the breast: an unusual case report with a short review of the literature [abstract]. Ann Oncol. 2013;24(suppl 3):iii49.
- O’Brien OA, AboGhaly E, Heffron C. An unusual presentation of a common malignancy [abstract]. J Pathol. 2013;231:S33.
- O’Brien R, Porto DA, Friedman BJ, et al. Elderly female with swelling of the right breast. Ann Emerg Med. 2016;67:e25-e26.
- Oliveira GM de, Zachetti DBC, Barros HR, et al. Breast carcinoma en Cuirasse—case report. An Bras Dermatol. 2013;88:608-610.
- Salati SA, Rather AA. Carcinoma en cuirasse. J Pak Assoc Derma. 2013;23:452-454.
- Santiago F, Saleiro S, Brites MM, et al. A remarkable case of cutaneous metastatic breast carcinoma. Dermatol Online J. 2009;15:10.
- Siqueira VR, Frota AS, Maia IL, et al. Cutaneous involvement as the initial presentation of metastatic breast adenocarcinoma - case report. An Bras Dermatol. 2014;89:960-963.
- Uria M, Chirino C, Rivas D. Inusual clinical presentation of cutaneous metastasis from breast carcinoma. A case report. Rev Argent Dermatol. 2009;90:230-236.
- Virmani NC, Sharma YK, Panicker NK, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56:726-727.
- de Souza Weimann ET, Botero EB, Mendes C, et al. Cutaneous metastasis as the first manifestation of occult malignant breast neoplasia. An Bras Dermatol. 2016;91(5 suppl 1):105-107.
- Wu CY, Gao HW, Huang WH, et al. Infection-like acral cutaneous metastasis as the presenting sign of an occult breast cancer. Clin Exp Dermatol. 2009;34:e409-e410.
- Yaghoobi R, Talaizade A, Lal K, et al. Inflammatory breast carcinoma presenting with two different patterns of cutaneous metastases: carcinoma telangiectaticum and carcinoma erysipeloides. J Clin Aesthet Dermatol. 2015;8:47-51.
- Atis G, Demirci GT, Atunay IK, et al. The clinical characteristics and the frequency of metastatic cutaneous tumors among primary skin tumors. Turkderm. 2013;47:166-169.
- Benmously R, Souissi A, Badri T, et al. Cutaneous metastases from internal cancers. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:167-170.
- Chopra R, Chhabra S, Samra SG, et al. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010;76:125-131.
- El Khoury J, Khalifeh I, Kibbi AG, et al. Cutaneous metastasis: clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int J Dermatol. 2014;53:147-158.
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222-239.
- Gómez Sánchez ME, Martinez Martinez ML, Martín De Hijas MC, et al. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel. 2014;29:207-212
- Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2017;61:47-54.
- Itin P, Tomaschett S. Cutaneous metastases from malignancies which do not originate from the skin. An epidemiological study. Article in German. Internist (Berl). 2009;50:179-186.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Torres HA, Bodey GP, Tarrand JJ, et al. Protothecosis in patients with cancer: case series and literature review. Clin Microbiol Infect. 2003;9:786-792.
PRACTICE POINTS
- Dermatologists may play a role in diagnosing breast cancer through cutaneous metastasis, even in patients without a history of breast cancer.
- Clinicians should consider breast cancer metastasis in the differential for any erythematous lesion on the trunk.
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The cutaneous benefits of bee venom, Part II: Acupuncture, wound healing, and various potential indications
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
mCODE: Improving data sharing to enhance cancer care
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
An initiative designed to improve sharing of patient data may provide “tremendous benefits” in cancer care and research, according to authors of a review article.
The goals of the initiative, called Minimal Common Oncology Data Elements (mCODE), were to identify the data elements in electronic health records that are “essential” for making treatment decisions and create “a standardized computable data format” that would improve the exchange of data across EHRs, according to the mCODE website.
Travis J. Osterman, DO, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues described the mCODE initiative in a review published in JCO Clinical Cancer Informatics.
At present, commercially available EHRs are poorly designed to support modern oncology workflow, requiring laborious data entry and lacking a common library of oncology-specific discrete data elements. As an example, most EHRs poorly support the needs of precision oncology and clinical genetics, since next-generation sequencing and genetic test results are almost universally reported in PDF files.
In addition, basic, operational oncology data (e.g., cancer staging, adverse event documentation, response to treatment, etc.) are captured in EHRs primarily as an unstructured narrative.
Computable, analytical data are found for only the small percentage of patients in clinical trials. Even then, some degree of manual data abstraction is regularly required.
Interoperability of EHRs between practices and health care institutions is often so poor that the transfer of basic cancer-related information as analyzable data is difficult or even impossible.
Making progress: The 21st Century Cures Act
The American Society of Clinical Oncology has a more than 15-year history of developing oncology data standards. Unfortunately, progress in implementing these standards has been glacially slow. Impediments have included:
- A lack of conformance with clinical workflows.
- Failure to test standards on specific-use cases during pilot testing.
- A focus on data exchange, rather than the practical impediments to data entry.
- Poor engagement with EHR vendors in distributing clinical information modules with an oncology-specific focus
- Instability of data interoperability technologies.
The 21st Century Cures Act, which became law in December 2016, mandated improvement in the interoperability of health information through the development of data standards and application programming interfaces.
In early 2020, final rules for implementation required technology vendors to employ application programming interfaces using a single interoperability resource. In addition, payers were required to use the United States Core Data for Interoperability Standard for data exchange. These requirements were intended to provide patients with access to their own health care data “without special effort.”
As a fortunate byproduct, since EHR vendors are required to implement application program interfaces using the Health Level Seven International (HL7) Fast Healthcare Interoperability Resource (FHIR) Specification, the final rules could enable systems like mCODE to be more easily integrated with existing EHRs.
Lessons from CancerLinQ
ASCO created the health technology platform CancerLinQ in 2014, envisioning that it could become an oncology-focused learning health system – a system in which internal data and experience are systematically integrated with external evidence, allowing knowledge to be put into practice.
CancerLinQ extracts data from EHRs and other sources via direct software connections. CancerLinQ then aggregates, harmonizes, and normalizes the data in a cloud-based environment.
The data are available to participating practices for quality improvement in patient care and secondary research. In 2020, records of cancer patients in the CancerLinQ database surpassed 2 million.
CancerLinQ has been successful. However, because of the nature of the EHR ecosystem and the scope and variability of data capture by clinicians, supporting a true learning health system has proven to be a formidable task. Postprocessing manual review using trained human curators is laborious and unsustainable.
The CancerLinQ experience illustrated that basic cancer-pertinent data should be standardized in the EHR and collected prospectively.
The mCODE model
The mCODE initiative seeks to facilitate progress in care quality, clinical research, and health care policy by developing and maintaining a standard, computable, interoperable data format.
Guiding principles that were adopted early in mCODE’s development included:
- A collaborative, noncommercial, use case–driven developmental model.
- Iterative processes.
- User-driven development, refinement, and maintenance.
- Low ongoing maintenance requirements.
A foundational moment in mCODE’s development involved achieving consensus among stakeholders that the project would fail if EHR vendors required additional data entry by users.
After pilot work, a real-world endpoints project, working-group deliberation, public comment, and refinement, the final data standard included six primary domains: patient, disease, laboratory data/vital signs, genomics, treatment, and outcome.
Each domain is further divided into several concepts with specific associated data elements. The data elements are modeled into value sets that specify the possible values for the data element.
To test mCODE, eight organizations representing oncology EHR vendors, standards developers, and research organizations participated in a cancer interoperability track. The comments helped refine mCODE version 1.0, which was released in March 2020 and is accessible via the mCODE website.
Additions will likely be reviewed by a technical review group after external piloting of new use cases.
Innovation, not regulation
Every interaction between a patient and care provider yields information that could lead to improved safety and better outcomes. To be successful, the information must be collected in a computable format so it can be aggregated with data from other patients, analyzed without manual curation, and shared through interoperable systems. Those data should also be secure enough to protect the privacy of individual patients.
mCODE is a consensus data standard for oncology that provides an infrastructure to share patient data between oncology practices and health care systems while promising little to no additional data entry on the part of clinicians. Adoption by sites will be critical, however.
Publishing the standard through the HL7 FHIR technology demonstrated to EHR vendors and regulatory agencies the stability of HL7, an essential requirement for its incorporation into software.
EHR vendors and others are engaged in the CodeX HL7 FHIR Accelerator to design projects to expand and/or modify mCODE. Their creativity and innovativeness via the external advisory mCODE council and/or CodeX will be encouraged to help mCODE reach its full potential.
As part of CodeX, the Community of Practice, an open forum for end users, was established to provide regular updates about mCODE-related initiatives and use cases to solicit in-progress input, according to Robert S. Miller, MD, medical director of CancerLinQ and an author of the mCODE review.
For mCODE to be embraced by all stakeholders, there should be no additional regulations. By engaging stakeholders in an enterprise that supports innovation and collaboration – without additional regulation – mCODE could maximize the potential of EHRs that, until now, have assisted us only marginally in accomplishing those goals.
mCODE is a joint venture of ASCO/CancerLinQ, the Alliance for Clinical Trials in Oncology Foundation, the MITRE Corporation, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Osterman disclosed a grant from the National Cancer Institute and relationships with Infostratix, eHealth, AstraZeneca, Outcomes Insights, Biodesix, MD Outlook, GenomOncology, Cota Healthcare, GE Healthcare, and Microsoft. Dr. Miller and the third review author disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM JCO CLINICAL CANCER INFORMATICS
The Genital Examination in Dermatologic Practice
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
A casual survey of my dermatology co-residents yielded overwhelmingly unanimous results: A complete skin check goes from head to toe but does not routinely include an examination of the genital area. This observation contrasts starkly with the American Academy of Dermatology’s Basic Dermatology Curriculum, which recommends inspection of the entire skin surface including the mucous membranes (ie, eyes, mouth, anus, genital area) as part of the total-body skin examination (TBSE).1 It even draws attention to so-called hidden areas where lesions easily can be missed, such as the perianal skin. My observation seems far from anecdotal; even a recent attempt at optimizing movements in the TBSE neglected to include examination of the genitalia in the proposed method,2-4 and many practicing dermatologists seem to agree. A survey of international dermatologists at high-risk skin cancer clinics found male and female genitalia were the least frequently examined anatomy sites during the TBSE. Additionally, female genitalia were examined less frequently than male genitalia (labia majora, 28%; penis, 52%; P=.003).5 Another survey of US academic dermatologists (23 dermatologists, 1 nurse practitioner) found that only 4% always visually inspected the vulva during routine annual examinations, and 50% did not think that vulvar examination was the dermatologist’s responsibility.6 Similar findings were reported in a survey of US dermatology residents.7
Why is the genital area routinely omitted from the dermatologic TBSE? Based on the surveys of dermatologists and dermatology residents, the most common reason cited for not examining these sites was patient discomfort, but there also was a dominant belief that other specialties, such as gynecologists, urologists, or primary care providers, routinely examine these areas.5,7 Time constraints also were a concern.
Although examination of sensitive areas can be uncomfortable,8 most patients still expect these locations to be examined during the TBSE. In a survey of 500 adults presenting for TBSE at an academic dermatology clinic, 84% of respondents expected the dermatologist to examine the genital area.9 Similarly, another survey of patient preferences (N=443) for the TBSE found that only 31.3% of women and 12.5% of men preferred not to have their genital area examined.10 As providers, we may be uncomfortable examining the genital area; however, our patients mostly expect it as part of routine practice. There are a number of barriers that may prevent incorporating the genital examination into daily dermatologic practice.
Training in Genital Examinations
Adequate training may be an issue for provider comfort when examining the genital skin. In a survey of dermatology residency program directors (n=38) and residents (n=91), 61.7% reported receiving formal instruction on TBSE technique and 38.3% reported being self-taught. Examination of the genital skin was included only 40% of the time.11 Even vulvar disorder experts have admitted to receiving their training by self-teaching, with only 19% receiving vulvar training during residency and 11% during fellowship.12 Improving this training appears to be an ongoing effort.2
Passing the Buck
It may be easier to think that another provider is routinely examining genital skin based on the relative absence of this area in dermatologic training; however, that does not appear to be the case. In a 1999 survey of primary care providers, only 31% reported performing skin cancer screenings on their adult patients, citing lack of confidence in this clinical skill as the biggest hurdle.13 Similarly, changes in recommendations for the utility of the screening pelvic examination in asymptomatic, average-risk, nonpregnant adult women have decreased the performance of this examination in actual practice.14 Reviews of resident training in vulvovaginal disease also have shown that although dermatology residents receive slightly less formal training hours on vulvar skin disease, they see more than double the number of patients with vulvar disease per year when compared to obstetrics and gynecology residents.15 In practice, dermatologists generally are more confident when evaluating vulvar pigmented lesions than gynecologists.6
The Importance of the Genital Examination
Looking past these barriers seems essential to providing the best dermatologic care, as there are a multitude of neoplastic and inflammatory dermatoses that can affect the genital skin. Furthermore, early diagnosis and treatment of these conditions potentially can limit morbidity and mortality as well as improve quality of life. Genital melanomas are a good example. Although they may be rare, it is well known that genital melanomas are associated with an aggressive disease course and have worse outcomes than melanomas found elsewhere on the body.16,17 Increasing rates of genital and perianal keratinocyte carcinomas make including this as part of the TBSE even more important.18
We also should not forget that inflammatory conditions can routinely involve the genitals.19-21 Although robust data are lacking, chronic vulvar concerns frequently are seen in the primary care setting. In one study in the United Kingdom, 52% of general practitioners surveyed saw more than 3 patients per month with vulvar concerns.22 Even in common dermatologic conditions such as psoriasis and lichen planus, genital involvement often is overlooked despite its relative frequency.23-27 In one study, 60% of psoriasis patients with genital involvement had not had these lesions examined by a physician.28
Theoretically, TBSEs that include genital examination would yield higher and earlier detection rates of neoplasms as well as inflammatory dermatoses.29-32 Thus, there is real value in diagnosing ailments of the genital skin, and dermatologists are well prepared to manage these conditions. Consistently incorporating a genital examination within the TBSE is the first step.
An Approach to the Genital Skin Examination
As with the TBSE, no standardized protocol for the genital skin examination exists, and there is no consensus for how best to perform this evaluation. Ideally, both male and female patients should remove all clothing, including undergarments, though one study found patients preferred to keep undergarments on during the genital examination.10,33,34
In general, adult female genital anatomy is best viewed with the patient in the supine position.6,33,35 There is no clear agreement on the use of stirrups, and the decision to use these may be left to the discretion of the patient. One randomized clinical trial found that women undergoing routine gynecologic examination without stirrups reported less physical discomfort and had a reduced sense of vulnerability than women examined in stirrups.36 During the female genital examination, the head of the bed ideally should be positioned at a 30° to 45° angle to allow the provider to maintain eye contact and face-to-face communication with the patient.33 This positioning also facilitates the use of a handheld mirror to instruct patients on techniques for medication application as well as to point out sites of disease.
For adult males, the genital examination can be performed with the patient standing facing a seated examiner.35 The patient’s gown should be raised to the level of the umbilicus to expose the entire genital region. Good lighting is essential. These recommendations apply mainly to adults, but helpful tips on how to approach evaluating prepubertal children in the dermatology clinic are available.37
The presence of a chaperone also is optional for maximizing patient comfort but also may be helpful for providing medicolegal protection for the provider. It always should be offered regardless of patient gender. A dermatology study found that when patients were examined by a same-gender physician, women and men were more comfortable without a chaperone than with a chaperone, and patients generally preferred fewer bodies in the room during sensitive examinations.9
Educating Patients About the TBSE
The most helpful recommendation for successfully incorporating and performing the genital skin examination as part of the TBSE appears to be patient education. In a randomized double-arm study, patients who received pre-education consisting of written information explaining the need for a TBSE were less likely to be concerned about a genital examination compared to patients who received no information.38 Discussing that skin diseases, including melanoma, can arise in all areas of the body including the genital skin and encouraging patients to perform genital self-examinations is critical.35 In the age of the electronic health record and virtual communication, disseminating this information has become even easier.39 It may be beneficial to explore patients’ TBSE expectations at the outset through these varied avenues to help establish a trusted physician-patient relationship.40
Final Thoughts
Dermatologists should consistently offer a genital examination to all patients who present for a routine TBSE. Patients should be provided with adequate education to assess their comfort level for the skin examination. If a patient declines this examination, the dermatologist should ensure that another physician—be it a gynecologist, primary care provider, or other specialist—is routinely examining the area.6,7
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
- The skin exam. American Academy of Dermatology. https://digital-catalog.aad.org/diweb/catalog/launch/package/4/did/327974/iid/327974
- Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total-body skin exam: an observational cohort study. J Am Acad Dermatol. 2019;81:1115-1119.
- Nielson CB, Grant-Kels JM. Commentary on “optimizing the total-body skin exam: an observational cohort study.” J Am Acad Dermatol. 2019;81:E131.
- Helm MF, Hallock KK, Bisbee E, et al. Reply to: “commentary on ‘optimizing the total-body skin exam: an observational cohort study.’” J Am Acad Dermatol. 2019;81:E133.
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138.
- Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66:697-698.
- Hosking AM, Chapman L, Zachary CB, et al. Anogenital examination practices among U.S. dermatology residents [published online January 9, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.061
- Grundström H, Wallin K, Berterö C. ‘You expose yourself in so many ways’: young women’s experiences of pelvic examination. J Psychosom Obstet Gynaecol. 2011;32:59-64.
- McClatchey Connors T, Reddy P, Weiss E, et al. Patient comfort and expectations for total body skin examinations: a cross-sectional study. J Am Acad Dermatol. 2019;81:615-617.
- Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152:1052-1054.
- Milchak M, Miller J, Dellasega C, et al. Education on total body skin examination in dermatology residency. Poster presented at: Association of Professors of Dermatology Annual Meeting; September 25-26, 2015; Chicago, IL.
- Venkatesan A, Farsani T, O’Sullivan P, et al. Identifying competencies in vulvar disorder management for medical students and residents: a survey of US vulvar disorder experts. J Low Genit Tract Dis. 2012;16:398-402.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:947-953.
- Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6:182-185.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34:E7-E14.
- Vyas R, Thompson CL, Zargar H, et al. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J Am Acad Dermatol. 2016;75:144-150.
- Misitzis A, Beatson M, Weinstock MA. Keratinocyte carcinoma mortality in the United States as reported in death certificates, 2011-2017. Dermatol Surg. 2020;46:1135-1140.
- Sullivan AK, Straughair GJ, Marwood RP, et al. A multidisciplinary vulva clinic: the role of genito-urinary medicine. J Eur Acad Dermatol Venereol. 1999;13:36-40.
- Goncalves DLM, Romero RL, Ferreira PL, et al. Clinical and epidemiological profile of patients attended in a vulvar clinic of the dermatology outpatient unit of a tertiary hospital during a 4-year period. Int J Dermatol. 2019;58:1311-1316.
- Bauer A, Greif C, Vollandt R, et al. Vulval diseases need an interdisciplinary approach. Dermatology. 1999;199:223-226.
- Nunns D, Mandal D. The chronically symptomatic vulva: prevalence in primary health care. Genitourin Med. 1996;72:343-344.
- Meeuwis KA, de Hullu JA, de Jager ME, et al. Genital psoriasis: a questionnaire-based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol. 2010;24:1425-1430.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;(19 suppl 3):2-6.
- Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431-436.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29:754-760.
- Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180:647-656.
- Rigel DS, Friedman RJ, Kopf AW, et al. Importance of complete cutaneous examination for the detection of malignant melanoma. J Am Acad Dermatol. 1986;14(5 pt 1):857-860.
- De Rooij MJ, Rampen FH, Schouten LJ, et al. Total skin examination during screening for malignant melanoma does not increase the detection rate. Br J Dermatol. 1996;135:42-45.
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Mauskar MM, Marathe K, Venkatesan A, et al. Vulvar diseases: approach to the patient. J Am Acad Dermatol. 2020;82:1277-1284.
- Chen C. How full is a full body skin exam? investigation into the practice of the full body skin exam as conducted by board-certified and board-eligibile dermatologists. Michigan State University. Published April 24, 2015. Accessed February 4, 2021. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/2015SpringMeeting/ChenSpr15.pdf
- Zikry J, Chapman LW, Korta DZ, et al. Genital melanoma: are we adequately screening our patients? Dermatol Online J. 2017;23:13030/qt7zk476vn.
- Seehusen DA, Johnson DR, Earwood JS, et al. Improving women’s experience during speculum examinations at routine gynaecological visits: randomised clinical trial [published online June 27, 2006]. BMJ. 2006;333:171.
- Habeshian K, Fowler K, Gomez-Lobo V, et al. Guidelines for pediatric anogenital examination: insights from our vulvar dermatology clinic. Pediatr Dermatol. 2018;35:693-695.
- Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19:660-663.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part II: patient education. J Am Acad Dermatol. 2013;68:364.e361-310.
- Rosamilia LL. The naked truth about total body skin examination: a lesson from Goldilocks and the Three Bears. American Academy of Dermatology. Published November 13, 2019. Accessed February 4, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/november/dwii-11-13-19-the-naked-truth-about-total-body-skin-examination-a-lesson-from-goldilocks-and-the-three-bears
Resident Pearls
- Dermatologists should offer a genital examination to all patients who present for a routine total-body skin examination.
- It is critical to educate patients about the importance of examining the genital skin by discussing that skin diseases can arise in all areas of the body including the genital area. Encouraging genital self-examination also is helpful.
- If a patient declines, the dermatologist should strive to ensure that another provider is examining the genital skin.
Less pain, same gain with tirbanibulin for actinic keratosis
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Eruptive Annular Papules on the Trunk of an Organ Transplant Recipient
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.
Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.

Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.

Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
A 50-year-old Black woman with systemic lupus erythematosus and a renal transplant 15 years prior due to lupus nephritis presented with a nonpruritic rash on the abdomen of 1 year’s duration. Her immunosuppressive regimen consisted of tacrolimus, azathioprine, and prednisone. Physical examination revealed numerous monomorphic, annular, hyperpigmented, and thin papules with central clearing present on the abdomen extending to the flanks and groin. The patient denied any family history of similar lesions. A 4-mm punch biopsy of an abdominal lesion was performed.
How has the pandemic affected rural and urban cancer patients?
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: COVID-19 AND CANCER 2021
Hidden Basal Cell Carcinoma in the Intergluteal Crease
Practice Gap
Basal cell carcinoma (BCC) is the most common cancer, and its incidence is on the rise.1 The risk of this skin cancer is increased when there is a history of squamous cell carcinoma (SCC) or BCC.2 Basal cell carcinoma often is found in sun-exposed areas, most commonly due to a history of intense sunburn.3 Other risk factors include male gender and increased age.4
Eighty percent to 85% of BCCs present on the head and neck5; however, BCC also can occur in unusual locations. When BCC presents in areas such as the perianal region, it is found to be larger than when found in more common areas,6 likely because neoplasms in this sensitive area often are overlooked. Literature on BCC of the intergluteal crease is limited.7 Being educated on the existence of BCC in this sensitive area can aid proper diagnosis.
The Technique and Case
An 83-year-old woman presented to the dermatology clinic for a suspicious lesion in the intergluteal crease that was tender to palpation with drainage. She first noticed this lesion and reported it to her primary care physician at a visit 6 months prior. The primary care physician did not pursue investigation of the lesion. One month later, the patient was seen by a gastroenterologist for the lesion and was referred to dermatology. The patient’s medical history included SCC and BCC on the face, both treated successfully with Mohs micrographic surgery.
Physical examination revealed a 2.6×1.1-cm, erythematous, nodular plaque in the coccygeal area of the intergluteal crease (Figure 1). A shave biopsy disclosed BCC, nodular type, ulcerated. Microscopically, there were nodular aggregates of basaloid cells with hyperchromatic nuclei and peripheral palisading, separated from mucinous stromal surroundings by artefactual clefts.
The initial differential diagnosis for this patient’s lesion included an ulcer or SCC. Basal cell carcinoma was not suspected due to the location and appearance of the lesion. The patient was successfully treated with Mohs micrographic surgery.
Practical Implications
Without thorough examination, this cancerous lesion would not have been seen (Figure 2). Therefore, it is important to practice thorough physical examination skills to avoid missing these cancers, particularly when examining a patient with a history of SCC or BCC. Furthermore, biopsy is recommended for suspicious lesions to rule out BCC.
Be careful not to get caught up in epidemiological or demographic considerations when making a diagnosis of this kind or when assessing the severity of a lesion. This patient, for instance, was female, which makes her less likely to present with BCC.8 Moreover, the cancer presented in a highly unlikely location for BCC, where there had not been significant sunburn.9 Patients and physicians should be educated about the incidence of BCC in unexpected areas; without a second and close look, this BCC could have been missed.
Final Thoughts
The literature continuously demonstrates the rarity of BCC in the intergluteal crease.10 However, when perianal BCC is properly identified and treated with local excision, prognosis is good.11 Basal cell carcinoma has been seen to arise in other sensitive locations; vulvar, nipple, and scrotal BCC neoplasms are among the uncommon locations where BCC has appeared.12 These areas are frequently—and easily—ignored. A total-body skin examination should be performed to ensure that these insidious-onset carcinomas are not overlooked to protect patients from the adverse consequences of untreated cancer.13
- Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case–case–control study. Br J Cancer. 2006;94:743-751.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Lorenzini M, Gatti S, Giannitrapani A. Giant basal cell carcinoma of the thoracic wall: a case report and review of the literature. Br J Plast Surg. 2005;58:1007-1010.
- Lee HS, Kim SK. Basal cell carcinoma presenting as a perianal ulcer and treated with radiotherapy. Ann Dermatol. 2015;27:212-214.
- Salih AM, Kakamad FH, Rauf GM. Basal cell carcinoma mimicking pilonidal sinus: a case report with literature review. Int J Surg Case Rep. 2016;28:121-123.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Park J, Cho Y-S, Song K-H, et al. Basal cell carcinoma on the pubic area: report of a case and review of 19 Korean cases of BCC from non-sun-exposed areas. Ann Dermatol. 2011;23:405-408.
- Damin DC, Rosito MA, Gus P, et al. Perianal basal cell carcinoma. J Cutan Med Surg. 2002;6:26-28.
- Paterson CA, Young-Fadok TM, Dozois RR. Basal cell carcinoma of the perianal region: 20-year experience. Dis Colon Rectum. 1999;42:1200-1202.
- Mulvany NJ, Rayoo M, Allen DG. Basal cell carcinoma of the vulva: a case series. Pathology. 2012;44:528-533.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
Practice Gap
Basal cell carcinoma (BCC) is the most common cancer, and its incidence is on the rise.1 The risk of this skin cancer is increased when there is a history of squamous cell carcinoma (SCC) or BCC.2 Basal cell carcinoma often is found in sun-exposed areas, most commonly due to a history of intense sunburn.3 Other risk factors include male gender and increased age.4
Eighty percent to 85% of BCCs present on the head and neck5; however, BCC also can occur in unusual locations. When BCC presents in areas such as the perianal region, it is found to be larger than when found in more common areas,6 likely because neoplasms in this sensitive area often are overlooked. Literature on BCC of the intergluteal crease is limited.7 Being educated on the existence of BCC in this sensitive area can aid proper diagnosis.
The Technique and Case
An 83-year-old woman presented to the dermatology clinic for a suspicious lesion in the intergluteal crease that was tender to palpation with drainage. She first noticed this lesion and reported it to her primary care physician at a visit 6 months prior. The primary care physician did not pursue investigation of the lesion. One month later, the patient was seen by a gastroenterologist for the lesion and was referred to dermatology. The patient’s medical history included SCC and BCC on the face, both treated successfully with Mohs micrographic surgery.
Physical examination revealed a 2.6×1.1-cm, erythematous, nodular plaque in the coccygeal area of the intergluteal crease (Figure 1). A shave biopsy disclosed BCC, nodular type, ulcerated. Microscopically, there were nodular aggregates of basaloid cells with hyperchromatic nuclei and peripheral palisading, separated from mucinous stromal surroundings by artefactual clefts.

The initial differential diagnosis for this patient’s lesion included an ulcer or SCC. Basal cell carcinoma was not suspected due to the location and appearance of the lesion. The patient was successfully treated with Mohs micrographic surgery.
Practical Implications
Without thorough examination, this cancerous lesion would not have been seen (Figure 2). Therefore, it is important to practice thorough physical examination skills to avoid missing these cancers, particularly when examining a patient with a history of SCC or BCC. Furthermore, biopsy is recommended for suspicious lesions to rule out BCC.

Be careful not to get caught up in epidemiological or demographic considerations when making a diagnosis of this kind or when assessing the severity of a lesion. This patient, for instance, was female, which makes her less likely to present with BCC.8 Moreover, the cancer presented in a highly unlikely location for BCC, where there had not been significant sunburn.9 Patients and physicians should be educated about the incidence of BCC in unexpected areas; without a second and close look, this BCC could have been missed.
Final Thoughts
The literature continuously demonstrates the rarity of BCC in the intergluteal crease.10 However, when perianal BCC is properly identified and treated with local excision, prognosis is good.11 Basal cell carcinoma has been seen to arise in other sensitive locations; vulvar, nipple, and scrotal BCC neoplasms are among the uncommon locations where BCC has appeared.12 These areas are frequently—and easily—ignored. A total-body skin examination should be performed to ensure that these insidious-onset carcinomas are not overlooked to protect patients from the adverse consequences of untreated cancer.13
Practice Gap
Basal cell carcinoma (BCC) is the most common cancer, and its incidence is on the rise.1 The risk of this skin cancer is increased when there is a history of squamous cell carcinoma (SCC) or BCC.2 Basal cell carcinoma often is found in sun-exposed areas, most commonly due to a history of intense sunburn.3 Other risk factors include male gender and increased age.4
Eighty percent to 85% of BCCs present on the head and neck5; however, BCC also can occur in unusual locations. When BCC presents in areas such as the perianal region, it is found to be larger than when found in more common areas,6 likely because neoplasms in this sensitive area often are overlooked. Literature on BCC of the intergluteal crease is limited.7 Being educated on the existence of BCC in this sensitive area can aid proper diagnosis.
The Technique and Case
An 83-year-old woman presented to the dermatology clinic for a suspicious lesion in the intergluteal crease that was tender to palpation with drainage. She first noticed this lesion and reported it to her primary care physician at a visit 6 months prior. The primary care physician did not pursue investigation of the lesion. One month later, the patient was seen by a gastroenterologist for the lesion and was referred to dermatology. The patient’s medical history included SCC and BCC on the face, both treated successfully with Mohs micrographic surgery.
Physical examination revealed a 2.6×1.1-cm, erythematous, nodular plaque in the coccygeal area of the intergluteal crease (Figure 1). A shave biopsy disclosed BCC, nodular type, ulcerated. Microscopically, there were nodular aggregates of basaloid cells with hyperchromatic nuclei and peripheral palisading, separated from mucinous stromal surroundings by artefactual clefts.

The initial differential diagnosis for this patient’s lesion included an ulcer or SCC. Basal cell carcinoma was not suspected due to the location and appearance of the lesion. The patient was successfully treated with Mohs micrographic surgery.
Practical Implications
Without thorough examination, this cancerous lesion would not have been seen (Figure 2). Therefore, it is important to practice thorough physical examination skills to avoid missing these cancers, particularly when examining a patient with a history of SCC or BCC. Furthermore, biopsy is recommended for suspicious lesions to rule out BCC.

Be careful not to get caught up in epidemiological or demographic considerations when making a diagnosis of this kind or when assessing the severity of a lesion. This patient, for instance, was female, which makes her less likely to present with BCC.8 Moreover, the cancer presented in a highly unlikely location for BCC, where there had not been significant sunburn.9 Patients and physicians should be educated about the incidence of BCC in unexpected areas; without a second and close look, this BCC could have been missed.
Final Thoughts
The literature continuously demonstrates the rarity of BCC in the intergluteal crease.10 However, when perianal BCC is properly identified and treated with local excision, prognosis is good.11 Basal cell carcinoma has been seen to arise in other sensitive locations; vulvar, nipple, and scrotal BCC neoplasms are among the uncommon locations where BCC has appeared.12 These areas are frequently—and easily—ignored. A total-body skin examination should be performed to ensure that these insidious-onset carcinomas are not overlooked to protect patients from the adverse consequences of untreated cancer.13
- Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case–case–control study. Br J Cancer. 2006;94:743-751.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Lorenzini M, Gatti S, Giannitrapani A. Giant basal cell carcinoma of the thoracic wall: a case report and review of the literature. Br J Plast Surg. 2005;58:1007-1010.
- Lee HS, Kim SK. Basal cell carcinoma presenting as a perianal ulcer and treated with radiotherapy. Ann Dermatol. 2015;27:212-214.
- Salih AM, Kakamad FH, Rauf GM. Basal cell carcinoma mimicking pilonidal sinus: a case report with literature review. Int J Surg Case Rep. 2016;28:121-123.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Park J, Cho Y-S, Song K-H, et al. Basal cell carcinoma on the pubic area: report of a case and review of 19 Korean cases of BCC from non-sun-exposed areas. Ann Dermatol. 2011;23:405-408.
- Damin DC, Rosito MA, Gus P, et al. Perianal basal cell carcinoma. J Cutan Med Surg. 2002;6:26-28.
- Paterson CA, Young-Fadok TM, Dozois RR. Basal cell carcinoma of the perianal region: 20-year experience. Dis Colon Rectum. 1999;42:1200-1202.
- Mulvany NJ, Rayoo M, Allen DG. Basal cell carcinoma of the vulva: a case series. Pathology. 2012;44:528-533.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case–case–control study. Br J Cancer. 2006;94:743-751.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Lorenzini M, Gatti S, Giannitrapani A. Giant basal cell carcinoma of the thoracic wall: a case report and review of the literature. Br J Plast Surg. 2005;58:1007-1010.
- Lee HS, Kim SK. Basal cell carcinoma presenting as a perianal ulcer and treated with radiotherapy. Ann Dermatol. 2015;27:212-214.
- Salih AM, Kakamad FH, Rauf GM. Basal cell carcinoma mimicking pilonidal sinus: a case report with literature review. Int J Surg Case Rep. 2016;28:121-123.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Park J, Cho Y-S, Song K-H, et al. Basal cell carcinoma on the pubic area: report of a case and review of 19 Korean cases of BCC from non-sun-exposed areas. Ann Dermatol. 2011;23:405-408.
- Damin DC, Rosito MA, Gus P, et al. Perianal basal cell carcinoma. J Cutan Med Surg. 2002;6:26-28.
- Paterson CA, Young-Fadok TM, Dozois RR. Basal cell carcinoma of the perianal region: 20-year experience. Dis Colon Rectum. 1999;42:1200-1202.
- Mulvany NJ, Rayoo M, Allen DG. Basal cell carcinoma of the vulva: a case series. Pathology. 2012;44:528-533.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
Cemiplimab approved for locally advanced, metastatic basal cell carcinoma
The FDA granted full approval for the locally advanced BCC indication and accelerated approval for the metastatic BCC indication, according to a press release from Regeneron and Sanofi, the companies jointly developing cemiplimab.
Cemiplimab is a programmed death–1 inhibitor that was first FDA approved in 2018 for locally advanced or metastatic cutaneous squamous cell carcinoma not eligible for curative surgery or radiation.
The new approval “will change the treatment paradigm for patients with advanced basal cell carcinoma,” according to Karl Lewis, MD, a professor at the University of Colorado at Denver, Aurora, and an investigator on the phase 2 trial of cemiplimab.
“While the primary systemic treatment options are hedgehog inhibitors, many patients will eventually progress on or become intolerant to this therapy,” Dr. Lewis said in the press release. “With Libtayo [cemiplimab], these patients now have a new immunotherapy option.”
The approval of cemiplimab in BCC was based on an open-label, phase 2 trial of 132 patients with advanced BCC. Patients could not tolerate, had progressed on, or had not responded to HHIs after 9 months of treatment.
Cemiplimab was given at 350 mg every 3 weeks. The study was not placebo controlled and has not been published, a Regeneron spokesperson said.
There were 112 patients in the efficacy analysis. The overall response rate was 21% (6/28) in metastatic BCC patients, with no complete responders. In locally advanced BCC patients, the objective response rate was 29% (24/84), with five complete responders.
The median duration of response was not reached in either group but was at least 6 months long in all metastatic patients and in 79% (19/84) of the locally advanced BCC patients.
The most common adverse events among the 132 subjects evaluable for safety were fatigue (49%), musculoskeletal pain (33%), diarrhea (25%), rash (22%), pruritus (20%), and upper respiratory tract infection (15%).
Serious adverse events occurred in 32% of patients, including colitis, acute kidney injury, adrenal insufficiency, and anemia. Adverse events led to discontinuation in 13% of patients, most often for colitis and general physical health deterioration.
For more details on cemiplimab, see the full prescribing information.
The FDA granted full approval for the locally advanced BCC indication and accelerated approval for the metastatic BCC indication, according to a press release from Regeneron and Sanofi, the companies jointly developing cemiplimab.
Cemiplimab is a programmed death–1 inhibitor that was first FDA approved in 2018 for locally advanced or metastatic cutaneous squamous cell carcinoma not eligible for curative surgery or radiation.
The new approval “will change the treatment paradigm for patients with advanced basal cell carcinoma,” according to Karl Lewis, MD, a professor at the University of Colorado at Denver, Aurora, and an investigator on the phase 2 trial of cemiplimab.
“While the primary systemic treatment options are hedgehog inhibitors, many patients will eventually progress on or become intolerant to this therapy,” Dr. Lewis said in the press release. “With Libtayo [cemiplimab], these patients now have a new immunotherapy option.”
The approval of cemiplimab in BCC was based on an open-label, phase 2 trial of 132 patients with advanced BCC. Patients could not tolerate, had progressed on, or had not responded to HHIs after 9 months of treatment.
Cemiplimab was given at 350 mg every 3 weeks. The study was not placebo controlled and has not been published, a Regeneron spokesperson said.
There were 112 patients in the efficacy analysis. The overall response rate was 21% (6/28) in metastatic BCC patients, with no complete responders. In locally advanced BCC patients, the objective response rate was 29% (24/84), with five complete responders.
The median duration of response was not reached in either group but was at least 6 months long in all metastatic patients and in 79% (19/84) of the locally advanced BCC patients.
The most common adverse events among the 132 subjects evaluable for safety were fatigue (49%), musculoskeletal pain (33%), diarrhea (25%), rash (22%), pruritus (20%), and upper respiratory tract infection (15%).
Serious adverse events occurred in 32% of patients, including colitis, acute kidney injury, adrenal insufficiency, and anemia. Adverse events led to discontinuation in 13% of patients, most often for colitis and general physical health deterioration.
For more details on cemiplimab, see the full prescribing information.
The FDA granted full approval for the locally advanced BCC indication and accelerated approval for the metastatic BCC indication, according to a press release from Regeneron and Sanofi, the companies jointly developing cemiplimab.
Cemiplimab is a programmed death–1 inhibitor that was first FDA approved in 2018 for locally advanced or metastatic cutaneous squamous cell carcinoma not eligible for curative surgery or radiation.
The new approval “will change the treatment paradigm for patients with advanced basal cell carcinoma,” according to Karl Lewis, MD, a professor at the University of Colorado at Denver, Aurora, and an investigator on the phase 2 trial of cemiplimab.
“While the primary systemic treatment options are hedgehog inhibitors, many patients will eventually progress on or become intolerant to this therapy,” Dr. Lewis said in the press release. “With Libtayo [cemiplimab], these patients now have a new immunotherapy option.”
The approval of cemiplimab in BCC was based on an open-label, phase 2 trial of 132 patients with advanced BCC. Patients could not tolerate, had progressed on, or had not responded to HHIs after 9 months of treatment.
Cemiplimab was given at 350 mg every 3 weeks. The study was not placebo controlled and has not been published, a Regeneron spokesperson said.
There were 112 patients in the efficacy analysis. The overall response rate was 21% (6/28) in metastatic BCC patients, with no complete responders. In locally advanced BCC patients, the objective response rate was 29% (24/84), with five complete responders.
The median duration of response was not reached in either group but was at least 6 months long in all metastatic patients and in 79% (19/84) of the locally advanced BCC patients.
The most common adverse events among the 132 subjects evaluable for safety were fatigue (49%), musculoskeletal pain (33%), diarrhea (25%), rash (22%), pruritus (20%), and upper respiratory tract infection (15%).
Serious adverse events occurred in 32% of patients, including colitis, acute kidney injury, adrenal insufficiency, and anemia. Adverse events led to discontinuation in 13% of patients, most often for colitis and general physical health deterioration.
For more details on cemiplimab, see the full prescribing information.