User login
Skin Cancer Screening and Prevention During the COVID-19 Pandemic
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
Practice Points
- It is important for dermatologists to maintain skin cancer screening and prevention efforts during the coronavirus disease 2019 pandemic.
- Patient populations at increased risk for skin cancer should be prioritized for in-person evaluations, but teledermatology should be considered for initial examination in new patients and patients at average risk for skin cancer.
- Teledermatology presents a learning curve for dermatologists and patients, but the confidence level will increase, and evidence-based data will pave the way to enhance this experience.
Skin Cancer in the US Military
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
Practice Points
- An increased risk for melanoma and keratinocyte carcinomas has been identified in those who have served in the US Military.
- UV radiation exposure, low utilization of sun-protective strategies, and low overall education regarding the risks of UV exposure appear to be the primary contributors to increased risks of skin cancer in this population.
- Improving education for military servicemembers on the risks of UV exposure, increasing utilization of sun-protective clothing, and improving access and utilization of sunscreen are viable options to decrease the risk for cutaneous malignancies in US Military servicemembers.
Why a mycosis fungoides diagnosis takes so long
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Should all skin cancer patients be taking nicotinamide?
In 2014, I began taking care of a patient (see photo) who had developed over 25 basal cell carcinomas on her lower legs, which were surgically removed. She has been clear of any skin cancers in the last 2 years since starting supplementation.
Nicotinamide, also known as niacinamide, is a water soluble form of vitamin B3 that has been shown to enhance the repair of UV-induced DNA damage. Nicotinamide is found naturally in meat, fish, nuts, grains, and legumes, and is a key component of the glycolysis pathway, by generating nicotinamide adenine dinucleotide for adenosine triphosphate production. Nicotinamide deficiency causes photosensitive dermatitis, diarrhea, and dementia. It has been studied for its anti-inflammatory benefits as an adjunct treatment for rosacea, bullous diseases, acne, and melasma.
Nonmelanoma skin cancers are known to be caused primarily by UV radiation. The supplementation of nicotinamide orally twice daily has been shown to reduce the rate of actinic keratoses and new nonmelanoma skin cancers compared with placebo after 1 year in patients who previously had skin cancer. In the phase 3 study published in 2015, a randomized, controlled trial of 386 patients who had at least two nonmelanoma skin cancers within the previous 5-year period, oral nicotinamide 500 mg given twice daily for a 12-month period significantly reduced the number of new nonmelanoma skin cancers by 23% versus those on placebo.
The recommended dose for nicotinamide, which is available over the counter as Vitamin B3, is 500 mg twice a day. Nicotinamide should not be confused with niacin (nicotinic acid), which has been used to treat high cholesterol and cardiovascular disease. There are no significant side effects from long-term use; however nicotinamide should not be used in patients with end-stage kidney disease or chronic kidney disease. (Niacin, however, can cause elevation of liver enzymes, headache, flushing, and increased blood pressure.) Nicotinamide crosses the placenta and should not be used in pregnancy as it has not been studied in pregnant populations.
We should counsel patients that this is not an oral sunscreen, and that sun avoidance, sunscreen, and yearly skin cancer checks are still the mainstay of skin cancer prevention. However, given the safety profile of nicotinamide and the protective effects, should all of our skin cancer patients be taking nicotinamide daily? In my practice they are, all of whom swear by it and have had significant reductions of both actinic keratoses and nonmelanoma skin cancers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 2014, I began taking care of a patient (see photo) who had developed over 25 basal cell carcinomas on her lower legs, which were surgically removed. She has been clear of any skin cancers in the last 2 years since starting supplementation.
Nicotinamide, also known as niacinamide, is a water soluble form of vitamin B3 that has been shown to enhance the repair of UV-induced DNA damage. Nicotinamide is found naturally in meat, fish, nuts, grains, and legumes, and is a key component of the glycolysis pathway, by generating nicotinamide adenine dinucleotide for adenosine triphosphate production. Nicotinamide deficiency causes photosensitive dermatitis, diarrhea, and dementia. It has been studied for its anti-inflammatory benefits as an adjunct treatment for rosacea, bullous diseases, acne, and melasma.
Nonmelanoma skin cancers are known to be caused primarily by UV radiation. The supplementation of nicotinamide orally twice daily has been shown to reduce the rate of actinic keratoses and new nonmelanoma skin cancers compared with placebo after 1 year in patients who previously had skin cancer. In the phase 3 study published in 2015, a randomized, controlled trial of 386 patients who had at least two nonmelanoma skin cancers within the previous 5-year period, oral nicotinamide 500 mg given twice daily for a 12-month period significantly reduced the number of new nonmelanoma skin cancers by 23% versus those on placebo.
The recommended dose for nicotinamide, which is available over the counter as Vitamin B3, is 500 mg twice a day. Nicotinamide should not be confused with niacin (nicotinic acid), which has been used to treat high cholesterol and cardiovascular disease. There are no significant side effects from long-term use; however nicotinamide should not be used in patients with end-stage kidney disease or chronic kidney disease. (Niacin, however, can cause elevation of liver enzymes, headache, flushing, and increased blood pressure.) Nicotinamide crosses the placenta and should not be used in pregnancy as it has not been studied in pregnant populations.
We should counsel patients that this is not an oral sunscreen, and that sun avoidance, sunscreen, and yearly skin cancer checks are still the mainstay of skin cancer prevention. However, given the safety profile of nicotinamide and the protective effects, should all of our skin cancer patients be taking nicotinamide daily? In my practice they are, all of whom swear by it and have had significant reductions of both actinic keratoses and nonmelanoma skin cancers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 2014, I began taking care of a patient (see photo) who had developed over 25 basal cell carcinomas on her lower legs, which were surgically removed. She has been clear of any skin cancers in the last 2 years since starting supplementation.
Nicotinamide, also known as niacinamide, is a water soluble form of vitamin B3 that has been shown to enhance the repair of UV-induced DNA damage. Nicotinamide is found naturally in meat, fish, nuts, grains, and legumes, and is a key component of the glycolysis pathway, by generating nicotinamide adenine dinucleotide for adenosine triphosphate production. Nicotinamide deficiency causes photosensitive dermatitis, diarrhea, and dementia. It has been studied for its anti-inflammatory benefits as an adjunct treatment for rosacea, bullous diseases, acne, and melasma.
Nonmelanoma skin cancers are known to be caused primarily by UV radiation. The supplementation of nicotinamide orally twice daily has been shown to reduce the rate of actinic keratoses and new nonmelanoma skin cancers compared with placebo after 1 year in patients who previously had skin cancer. In the phase 3 study published in 2015, a randomized, controlled trial of 386 patients who had at least two nonmelanoma skin cancers within the previous 5-year period, oral nicotinamide 500 mg given twice daily for a 12-month period significantly reduced the number of new nonmelanoma skin cancers by 23% versus those on placebo.
The recommended dose for nicotinamide, which is available over the counter as Vitamin B3, is 500 mg twice a day. Nicotinamide should not be confused with niacin (nicotinic acid), which has been used to treat high cholesterol and cardiovascular disease. There are no significant side effects from long-term use; however nicotinamide should not be used in patients with end-stage kidney disease or chronic kidney disease. (Niacin, however, can cause elevation of liver enzymes, headache, flushing, and increased blood pressure.) Nicotinamide crosses the placenta and should not be used in pregnancy as it has not been studied in pregnant populations.
We should counsel patients that this is not an oral sunscreen, and that sun avoidance, sunscreen, and yearly skin cancer checks are still the mainstay of skin cancer prevention. However, given the safety profile of nicotinamide and the protective effects, should all of our skin cancer patients be taking nicotinamide daily? In my practice they are, all of whom swear by it and have had significant reductions of both actinic keratoses and nonmelanoma skin cancers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Palpation key when evaluating the skin for suspected MCC
“The lack of a pathognomonic appearance is often what precludes an early diagnosis of this cancer,” Dr. Thakuria, a dermatologist at Brigham and Women’s Hospital, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and the Global Academy for Medical Education. “MCCs can vary in appearance in their color, from pink to red to purple, or sometimes they have no color at all. They can be exophytic and obvious, or subtle, deeper tumors. These tumors are generally firm and nontender and are characterized by rapid growth, which is usually but not exclusively the feature that prompts biopsy.”
The typical patient with MCC is elderly, with an average age of 75 years. It affects males more than females by an approximately 2:1 ratio and tends to occur in fair-skinned individuals, although MCC does develop in skin of color. “While the majority of patients with this disease are immunocompetent, immunosuppressed patients are overrepresented in this disease, compared with the general population,” she said.
The clinical differential diagnosis is broad and includes both malignant and benign tumors, which requires a high index of suspicion. Most primary lesions are located on the head and neck, lower limb, and upper limb, but they may appear in non–sun exposed areas, such as the buttocks, as well.
One prospective study of 195 MCC patients found that 56% of clinicians presumed that these tumors were benign at the time of biopsy, and 32% were thought to have a cyst or acneiform lesion. The study authors summarized key clinical features of MCC with the acronym AEIOU: A stands for asymptomatic or nontender; E stands for expanding rapidly, usually over a duration less than 3 months; I stands for immunosuppression; O stands for patient older than age 50 years; and U stands for UV exposed skin location. The researchers found that 89% of the patients studied met three or more of the AEIOU criteria.
Dr. Thakuria, codirector of the Merkel Cell Carcinoma Center of Excellence at the Dana-Farber/Brigham and Women’s Cancer Center and assistant professor of dermatology at Harvard University, both in Boston, shared the following tips for dermatologic evaluation when MCC is suspected:
- Measure and record the clinical diameter of the lesions. “This helps you determine the T staging later, and from there can help you decide on proper treatment,” she said.
- Inspect and palpate the surrounding skin to look for in-transit metastases. “This may actually upstage the patient.”
- For a subcutaneous nodule, hub your punch biopsy. “These tumors can be centered in the deep dermis or fat,” Dr. Thakuria said. “If you really suspect MCC and you don’t get a result on your first biopsy, you may want to consider doing a second deeper biopsy, perhaps even a telescoping biopsy. This is especially true if your first biopsy was via shave technique and showed normal skin.”
- Refer to surgical oncology and radiation oncology ASAP. “You want to call them to ensure speedy consultation, within 1 week if possible,” she said. “Remember that all clinically node-negative MCCs warrant consideration of sentinel lymph node biopsy, regardless of tumor size. Upstaging will occur in 25%-32% of patients.”
Staging workup includes a full skin and lymph node exam to identify in-transit metastases and regional lymphadenopathy. “Palpation is key,” Dr. Thakuria said. “Next, you want to do some form of radiographic examination, so either a scalp to toes PET/CT or CT scan of the chest, abdomen, and pelvis. Finally, sentinel lymph node biopsy is going to be important if you have a clinically node-negative patient but you want to pathologically stage the person appropriately.” Although not formally part of the staging workup, she recommends ordering an AMERK test at diagnosis. AMERK detects antibodies to a Merkel cell polyomavirus oncoprotein, which is a marker of disease status present in about half of MCC patients. It falls with the treatment of cancer and rises with recurrence.
Discussing prognosis with MCC patients “can be challenging and uncomfortable, but even more so if you’re unfamiliar with some of the nuances of the terminology that is used,” Dr. Thakuria said. “Patients who go to Google are often going to encounter overall survival numbers, which are going to be worse than disease-specific numbers in any disease because they take into account death from any cause. This effect is heightened in MCC because this is cancer of predominately older adults, so there are other competing causes of death in this population, which drags down the overall survival estimates.”
Another point to remember when discussing survival with patients is that advances in immunotherapy are not necessarily reflected in national databases. “This is important, because usually in any cancer there’s a 5- to 10-year lag in survival information,” she said. “The last 5 years have brought an incredible change to MCC because of the advent of immunotherapy. Now we’re seeing incredible responses [in the clinic], but we’re not yet seeing those reflected in our survival tables.”
According to an analysis of prognostic factors from 9,387 MCC cases, nodal status is one of most important predictors of lower survival at 5 years, compared with having local disease: 35% versus 51%, respectively. Among patients with macroscopic lymph nodes, having known primary disease is associated with a lower survival at 5 years, compared with having unknown primary disease (27% vs. 42% at five years).
Dr. Thakuria concluded her presentation by recommending a three-step plan for surveillance, starting with a full skin and lymph node exam every 3-6 months for the first 3 years and every 6-12 months thereafter. Second, she advised routine imaging for high risk patients (American Joint Committee on Cancer stage 2 and above) and symptom-directed imaging for low-risk patients. Finally, she recommended the AMERK test every 3 months for the first 2-3 years in patients who were seropositive at diagnosis. A rising titer may be an early indicator of recurrence.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Thakuria reported having no financial disclosures.
“The lack of a pathognomonic appearance is often what precludes an early diagnosis of this cancer,” Dr. Thakuria, a dermatologist at Brigham and Women’s Hospital, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and the Global Academy for Medical Education. “MCCs can vary in appearance in their color, from pink to red to purple, or sometimes they have no color at all. They can be exophytic and obvious, or subtle, deeper tumors. These tumors are generally firm and nontender and are characterized by rapid growth, which is usually but not exclusively the feature that prompts biopsy.”
The typical patient with MCC is elderly, with an average age of 75 years. It affects males more than females by an approximately 2:1 ratio and tends to occur in fair-skinned individuals, although MCC does develop in skin of color. “While the majority of patients with this disease are immunocompetent, immunosuppressed patients are overrepresented in this disease, compared with the general population,” she said.
The clinical differential diagnosis is broad and includes both malignant and benign tumors, which requires a high index of suspicion. Most primary lesions are located on the head and neck, lower limb, and upper limb, but they may appear in non–sun exposed areas, such as the buttocks, as well.
One prospective study of 195 MCC patients found that 56% of clinicians presumed that these tumors were benign at the time of biopsy, and 32% were thought to have a cyst or acneiform lesion. The study authors summarized key clinical features of MCC with the acronym AEIOU: A stands for asymptomatic or nontender; E stands for expanding rapidly, usually over a duration less than 3 months; I stands for immunosuppression; O stands for patient older than age 50 years; and U stands for UV exposed skin location. The researchers found that 89% of the patients studied met three or more of the AEIOU criteria.
Dr. Thakuria, codirector of the Merkel Cell Carcinoma Center of Excellence at the Dana-Farber/Brigham and Women’s Cancer Center and assistant professor of dermatology at Harvard University, both in Boston, shared the following tips for dermatologic evaluation when MCC is suspected:
- Measure and record the clinical diameter of the lesions. “This helps you determine the T staging later, and from there can help you decide on proper treatment,” she said.
- Inspect and palpate the surrounding skin to look for in-transit metastases. “This may actually upstage the patient.”
- For a subcutaneous nodule, hub your punch biopsy. “These tumors can be centered in the deep dermis or fat,” Dr. Thakuria said. “If you really suspect MCC and you don’t get a result on your first biopsy, you may want to consider doing a second deeper biopsy, perhaps even a telescoping biopsy. This is especially true if your first biopsy was via shave technique and showed normal skin.”
- Refer to surgical oncology and radiation oncology ASAP. “You want to call them to ensure speedy consultation, within 1 week if possible,” she said. “Remember that all clinically node-negative MCCs warrant consideration of sentinel lymph node biopsy, regardless of tumor size. Upstaging will occur in 25%-32% of patients.”
Staging workup includes a full skin and lymph node exam to identify in-transit metastases and regional lymphadenopathy. “Palpation is key,” Dr. Thakuria said. “Next, you want to do some form of radiographic examination, so either a scalp to toes PET/CT or CT scan of the chest, abdomen, and pelvis. Finally, sentinel lymph node biopsy is going to be important if you have a clinically node-negative patient but you want to pathologically stage the person appropriately.” Although not formally part of the staging workup, she recommends ordering an AMERK test at diagnosis. AMERK detects antibodies to a Merkel cell polyomavirus oncoprotein, which is a marker of disease status present in about half of MCC patients. It falls with the treatment of cancer and rises with recurrence.
Discussing prognosis with MCC patients “can be challenging and uncomfortable, but even more so if you’re unfamiliar with some of the nuances of the terminology that is used,” Dr. Thakuria said. “Patients who go to Google are often going to encounter overall survival numbers, which are going to be worse than disease-specific numbers in any disease because they take into account death from any cause. This effect is heightened in MCC because this is cancer of predominately older adults, so there are other competing causes of death in this population, which drags down the overall survival estimates.”
Another point to remember when discussing survival with patients is that advances in immunotherapy are not necessarily reflected in national databases. “This is important, because usually in any cancer there’s a 5- to 10-year lag in survival information,” she said. “The last 5 years have brought an incredible change to MCC because of the advent of immunotherapy. Now we’re seeing incredible responses [in the clinic], but we’re not yet seeing those reflected in our survival tables.”
According to an analysis of prognostic factors from 9,387 MCC cases, nodal status is one of most important predictors of lower survival at 5 years, compared with having local disease: 35% versus 51%, respectively. Among patients with macroscopic lymph nodes, having known primary disease is associated with a lower survival at 5 years, compared with having unknown primary disease (27% vs. 42% at five years).
Dr. Thakuria concluded her presentation by recommending a three-step plan for surveillance, starting with a full skin and lymph node exam every 3-6 months for the first 3 years and every 6-12 months thereafter. Second, she advised routine imaging for high risk patients (American Joint Committee on Cancer stage 2 and above) and symptom-directed imaging for low-risk patients. Finally, she recommended the AMERK test every 3 months for the first 2-3 years in patients who were seropositive at diagnosis. A rising titer may be an early indicator of recurrence.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Thakuria reported having no financial disclosures.
“The lack of a pathognomonic appearance is often what precludes an early diagnosis of this cancer,” Dr. Thakuria, a dermatologist at Brigham and Women’s Hospital, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and the Global Academy for Medical Education. “MCCs can vary in appearance in their color, from pink to red to purple, or sometimes they have no color at all. They can be exophytic and obvious, or subtle, deeper tumors. These tumors are generally firm and nontender and are characterized by rapid growth, which is usually but not exclusively the feature that prompts biopsy.”
The typical patient with MCC is elderly, with an average age of 75 years. It affects males more than females by an approximately 2:1 ratio and tends to occur in fair-skinned individuals, although MCC does develop in skin of color. “While the majority of patients with this disease are immunocompetent, immunosuppressed patients are overrepresented in this disease, compared with the general population,” she said.
The clinical differential diagnosis is broad and includes both malignant and benign tumors, which requires a high index of suspicion. Most primary lesions are located on the head and neck, lower limb, and upper limb, but they may appear in non–sun exposed areas, such as the buttocks, as well.
One prospective study of 195 MCC patients found that 56% of clinicians presumed that these tumors were benign at the time of biopsy, and 32% were thought to have a cyst or acneiform lesion. The study authors summarized key clinical features of MCC with the acronym AEIOU: A stands for asymptomatic or nontender; E stands for expanding rapidly, usually over a duration less than 3 months; I stands for immunosuppression; O stands for patient older than age 50 years; and U stands for UV exposed skin location. The researchers found that 89% of the patients studied met three or more of the AEIOU criteria.
Dr. Thakuria, codirector of the Merkel Cell Carcinoma Center of Excellence at the Dana-Farber/Brigham and Women’s Cancer Center and assistant professor of dermatology at Harvard University, both in Boston, shared the following tips for dermatologic evaluation when MCC is suspected:
- Measure and record the clinical diameter of the lesions. “This helps you determine the T staging later, and from there can help you decide on proper treatment,” she said.
- Inspect and palpate the surrounding skin to look for in-transit metastases. “This may actually upstage the patient.”
- For a subcutaneous nodule, hub your punch biopsy. “These tumors can be centered in the deep dermis or fat,” Dr. Thakuria said. “If you really suspect MCC and you don’t get a result on your first biopsy, you may want to consider doing a second deeper biopsy, perhaps even a telescoping biopsy. This is especially true if your first biopsy was via shave technique and showed normal skin.”
- Refer to surgical oncology and radiation oncology ASAP. “You want to call them to ensure speedy consultation, within 1 week if possible,” she said. “Remember that all clinically node-negative MCCs warrant consideration of sentinel lymph node biopsy, regardless of tumor size. Upstaging will occur in 25%-32% of patients.”
Staging workup includes a full skin and lymph node exam to identify in-transit metastases and regional lymphadenopathy. “Palpation is key,” Dr. Thakuria said. “Next, you want to do some form of radiographic examination, so either a scalp to toes PET/CT or CT scan of the chest, abdomen, and pelvis. Finally, sentinel lymph node biopsy is going to be important if you have a clinically node-negative patient but you want to pathologically stage the person appropriately.” Although not formally part of the staging workup, she recommends ordering an AMERK test at diagnosis. AMERK detects antibodies to a Merkel cell polyomavirus oncoprotein, which is a marker of disease status present in about half of MCC patients. It falls with the treatment of cancer and rises with recurrence.
Discussing prognosis with MCC patients “can be challenging and uncomfortable, but even more so if you’re unfamiliar with some of the nuances of the terminology that is used,” Dr. Thakuria said. “Patients who go to Google are often going to encounter overall survival numbers, which are going to be worse than disease-specific numbers in any disease because they take into account death from any cause. This effect is heightened in MCC because this is cancer of predominately older adults, so there are other competing causes of death in this population, which drags down the overall survival estimates.”
Another point to remember when discussing survival with patients is that advances in immunotherapy are not necessarily reflected in national databases. “This is important, because usually in any cancer there’s a 5- to 10-year lag in survival information,” she said. “The last 5 years have brought an incredible change to MCC because of the advent of immunotherapy. Now we’re seeing incredible responses [in the clinic], but we’re not yet seeing those reflected in our survival tables.”
According to an analysis of prognostic factors from 9,387 MCC cases, nodal status is one of most important predictors of lower survival at 5 years, compared with having local disease: 35% versus 51%, respectively. Among patients with macroscopic lymph nodes, having known primary disease is associated with a lower survival at 5 years, compared with having unknown primary disease (27% vs. 42% at five years).
Dr. Thakuria concluded her presentation by recommending a three-step plan for surveillance, starting with a full skin and lymph node exam every 3-6 months for the first 3 years and every 6-12 months thereafter. Second, she advised routine imaging for high risk patients (American Joint Committee on Cancer stage 2 and above) and symptom-directed imaging for low-risk patients. Finally, she recommended the AMERK test every 3 months for the first 2-3 years in patients who were seropositive at diagnosis. A rising titer may be an early indicator of recurrence.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Thakuria reported having no financial disclosures.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Reliability of Biopsy Margin Status for Basal Cell Carcinoma: A Retrospective Study
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).
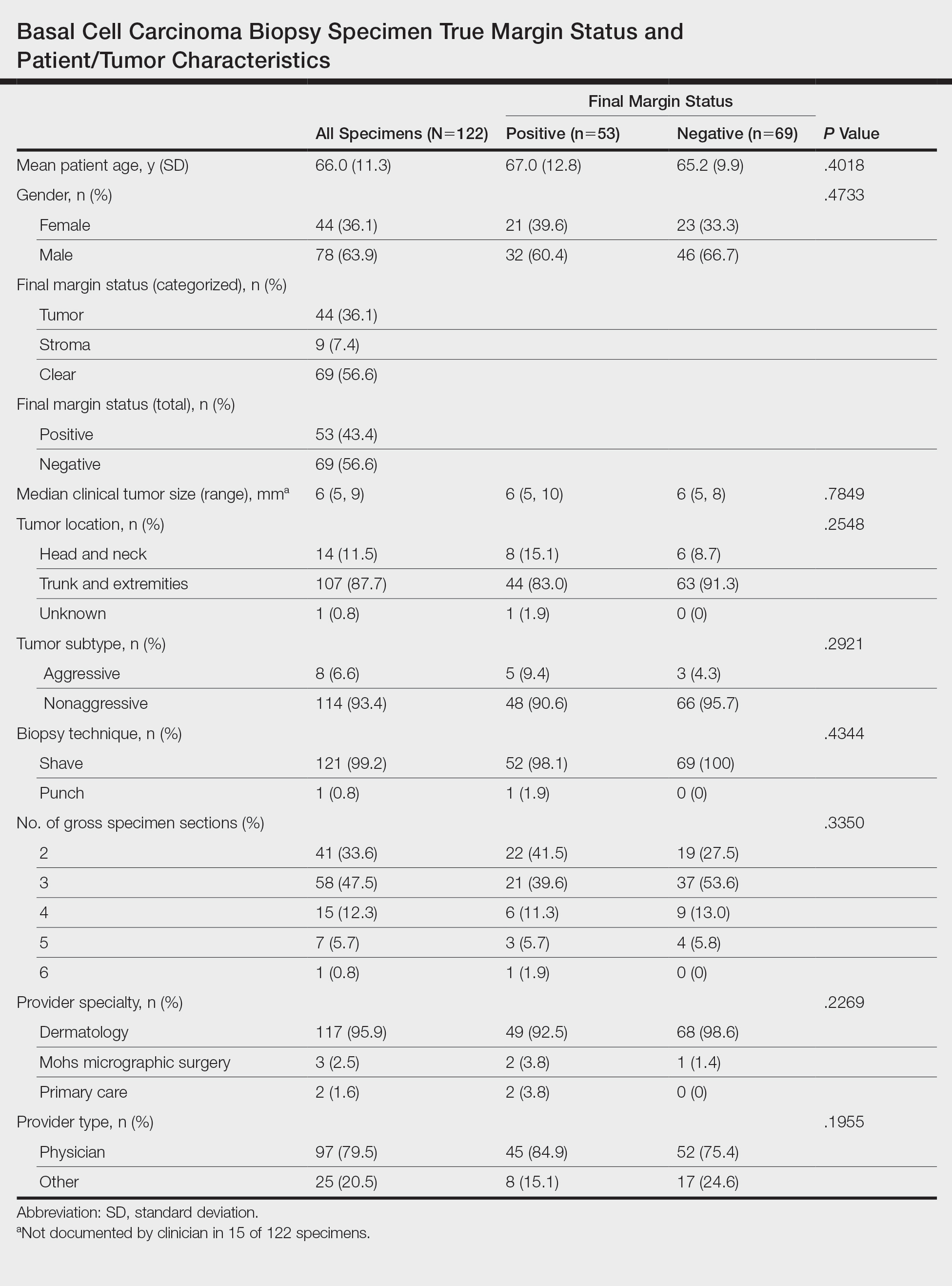
We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).

We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).

We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
Practice Points
- Clinicians must recognize the limitations of margin assessment of biopsy specimens and not rely on margin status to dictate treatment.
- Dermatopathologists should consider modifying how margin status is reported, either by omitting it or clarifying its limitations on the pathology report.
Daily sunscreen use will prevent more melanoma deaths than early detection
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Pigment traits, sun sensitivity associated with risk of non-Hodgkin lymphomas and chronic lymphocytic leukemia
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
FROM CANCER MEDICINE
Sunscreen myths, controversies continue
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Immunotherapy could fill unmet need in leptomeningeal metastases
Results from the trial were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Unfortunately, when patients present with leptomeningeal disease, they usually have a poor prognosis. Their median survival is measured at 6-24 weeks,” commented lead study author Jarushka Naidoo, MBBCh, an adjunct assistant professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, and a consultant medical oncologist at Beaumont Hospital in Dublin.
“While there may be some standard approaches for how we treat leptomeningeal disease, there are no universal standard therapies that are efficacious across solid tumor types,” Dr. Naidoo added.
With this in mind, Dr. Naidoo and colleagues tested systemic pembrolizumab in a trial of patients with leptomeningeal metastases from solid tumors.
The trial closed early because of poor accrual, after enrolling 13 patients: 5 with breast carcinoma, 3 with high-grade glioma, 3 with non–small cell lung cancer, 1 with squamous cell carcinoma of the skin, and 1 with head and neck squamous carcinoma. Nine patients (69%) had received at least two prior lines of systemic therapy.
Response, safety, and biomarkers
Overall, five patients (38%) had a central nervous system response, as ascertained from radiologic response on MRI, cytologic response in cerebrospinal fluid (CSF), and/or clinical response in neurologic symptoms, Dr. Naidoo reported.
Two patients had a complete CNS response: a patient with squamous cell carcinoma of the skin, who was still alive at 3 years, and a patient with non–small cell lung cancer, who survived 9 months but succumbed to metastases elsewhere.
For the entire cohort, median CNS progression-free survival was 2.9 months, and median overall survival was 4.9 months.
“This is consistent with published prospective studies of systemic agents for leptomeningeal disease,” Dr. Naidoo pointed out. “Notably, even though numbers are small, we do see the tail-on-the-curve phenomenon in both of these survival curves, which is consistent with immune checkpoint blockade prospective studies.”
The rate of grade 3 or higher treatment-related adverse events was 15.4%, and there were no grade 3 or higher immune-related adverse events.
The number of patients was too small for formal correlational testing, but both patients who achieved a complete response developed immune-related adverse events.
The trial’s biomarker analyses showed that an aneuploidy assay using CSF tumor-derived DNA performed well at detecting leptomeningeal metastases, with sensitivity of 84.6%, compared with just 53.8% for CSF cytopathology (the current preferred method).
A multiplex assay of CSF cytokines identified similar baseline profiles for patients who went on to have responses and showed similar changes in profile (notably a reduction in proinflammatory cytokines) for the two patients who had complete responses.
Given the trial’s 38% CNS response rate, pembrolizumab “needs to be studied in larger populations of patients to confirm this result, but it could be used as a potential treatment option for patients with leptomeningeal disease from solid tumors,” Dr. Naidoo concluded. “Reassuringly, pembrolizumab was well tolerated, and this is extremely important in a patient population that is traditionally quite frail and in which other standard therapies that are used, such as high-dose methotrexate or intrathecal chemotherapy, are associated with far higher rates of toxicity.”
An unmet need
“Leptomeningeal metastasis is a strong unmet need, although its occurrence is fortunately quite rare,” commented Kim Margolin, MD, a clinical professor and medical oncologist at City of Hope National Medical Center in Duarte, Calif., who was not involved in this study.
The trial is noteworthy for showing activity of programmed death–1 (PD-1) blockade given only systemically and not with additional intrathecal therapy (as has been done in a concurrent study at MD Anderson Cancer Center) and for providing insight into various biomarkers, Dr. Margolin said in an interview.
“I cannot take a stand on author conclusions other than to agree it warrants further evaluation in carefully selected patients, and it would be great to compare something like peripheral PD-1 blockade alone versus in combination with intrathecal therapy versus a combination such as CTLA4 blockade plus PD-1 blockade such as our group and others have shown to have increased activity in CNS metastases over PD-1 block alone,” Dr. Margolin said.
“The drugs in this class are already approved, so there is no reason not to try them,” she noted.
However, patients with leptomeningeal metastases of melanoma, for example, are likely to have already received anti-PD-1 immunotherapy.
“So the settings in which off-the-shelf PD-1 blockade would be useful are extremely limited,” she concluded.
The current trial was funded by Merck, the National Institutes of Health, the Lung Cancer Foundation of America, the International Association for the Study of Lung Cancer, and Johns Hopkins University Seed Grants. Dr. Naidoo disclosed relationships with AstraZeneca, Merck, Bristol Myers Squibb, and Roche/Genentech. Dr. Margolin disclosed no relevant conflicts of interest.
SOURCE: Naidoo J et al. SITC 2020, Abstract 788.
Results from the trial were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Unfortunately, when patients present with leptomeningeal disease, they usually have a poor prognosis. Their median survival is measured at 6-24 weeks,” commented lead study author Jarushka Naidoo, MBBCh, an adjunct assistant professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, and a consultant medical oncologist at Beaumont Hospital in Dublin.
“While there may be some standard approaches for how we treat leptomeningeal disease, there are no universal standard therapies that are efficacious across solid tumor types,” Dr. Naidoo added.
With this in mind, Dr. Naidoo and colleagues tested systemic pembrolizumab in a trial of patients with leptomeningeal metastases from solid tumors.
The trial closed early because of poor accrual, after enrolling 13 patients: 5 with breast carcinoma, 3 with high-grade glioma, 3 with non–small cell lung cancer, 1 with squamous cell carcinoma of the skin, and 1 with head and neck squamous carcinoma. Nine patients (69%) had received at least two prior lines of systemic therapy.
Response, safety, and biomarkers
Overall, five patients (38%) had a central nervous system response, as ascertained from radiologic response on MRI, cytologic response in cerebrospinal fluid (CSF), and/or clinical response in neurologic symptoms, Dr. Naidoo reported.
Two patients had a complete CNS response: a patient with squamous cell carcinoma of the skin, who was still alive at 3 years, and a patient with non–small cell lung cancer, who survived 9 months but succumbed to metastases elsewhere.
For the entire cohort, median CNS progression-free survival was 2.9 months, and median overall survival was 4.9 months.
“This is consistent with published prospective studies of systemic agents for leptomeningeal disease,” Dr. Naidoo pointed out. “Notably, even though numbers are small, we do see the tail-on-the-curve phenomenon in both of these survival curves, which is consistent with immune checkpoint blockade prospective studies.”
The rate of grade 3 or higher treatment-related adverse events was 15.4%, and there were no grade 3 or higher immune-related adverse events.
The number of patients was too small for formal correlational testing, but both patients who achieved a complete response developed immune-related adverse events.
The trial’s biomarker analyses showed that an aneuploidy assay using CSF tumor-derived DNA performed well at detecting leptomeningeal metastases, with sensitivity of 84.6%, compared with just 53.8% for CSF cytopathology (the current preferred method).
A multiplex assay of CSF cytokines identified similar baseline profiles for patients who went on to have responses and showed similar changes in profile (notably a reduction in proinflammatory cytokines) for the two patients who had complete responses.
Given the trial’s 38% CNS response rate, pembrolizumab “needs to be studied in larger populations of patients to confirm this result, but it could be used as a potential treatment option for patients with leptomeningeal disease from solid tumors,” Dr. Naidoo concluded. “Reassuringly, pembrolizumab was well tolerated, and this is extremely important in a patient population that is traditionally quite frail and in which other standard therapies that are used, such as high-dose methotrexate or intrathecal chemotherapy, are associated with far higher rates of toxicity.”
An unmet need
“Leptomeningeal metastasis is a strong unmet need, although its occurrence is fortunately quite rare,” commented Kim Margolin, MD, a clinical professor and medical oncologist at City of Hope National Medical Center in Duarte, Calif., who was not involved in this study.
The trial is noteworthy for showing activity of programmed death–1 (PD-1) blockade given only systemically and not with additional intrathecal therapy (as has been done in a concurrent study at MD Anderson Cancer Center) and for providing insight into various biomarkers, Dr. Margolin said in an interview.
“I cannot take a stand on author conclusions other than to agree it warrants further evaluation in carefully selected patients, and it would be great to compare something like peripheral PD-1 blockade alone versus in combination with intrathecal therapy versus a combination such as CTLA4 blockade plus PD-1 blockade such as our group and others have shown to have increased activity in CNS metastases over PD-1 block alone,” Dr. Margolin said.
“The drugs in this class are already approved, so there is no reason not to try them,” she noted.
However, patients with leptomeningeal metastases of melanoma, for example, are likely to have already received anti-PD-1 immunotherapy.
“So the settings in which off-the-shelf PD-1 blockade would be useful are extremely limited,” she concluded.
The current trial was funded by Merck, the National Institutes of Health, the Lung Cancer Foundation of America, the International Association for the Study of Lung Cancer, and Johns Hopkins University Seed Grants. Dr. Naidoo disclosed relationships with AstraZeneca, Merck, Bristol Myers Squibb, and Roche/Genentech. Dr. Margolin disclosed no relevant conflicts of interest.
SOURCE: Naidoo J et al. SITC 2020, Abstract 788.
Results from the trial were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Unfortunately, when patients present with leptomeningeal disease, they usually have a poor prognosis. Their median survival is measured at 6-24 weeks,” commented lead study author Jarushka Naidoo, MBBCh, an adjunct assistant professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, and a consultant medical oncologist at Beaumont Hospital in Dublin.
“While there may be some standard approaches for how we treat leptomeningeal disease, there are no universal standard therapies that are efficacious across solid tumor types,” Dr. Naidoo added.
With this in mind, Dr. Naidoo and colleagues tested systemic pembrolizumab in a trial of patients with leptomeningeal metastases from solid tumors.
The trial closed early because of poor accrual, after enrolling 13 patients: 5 with breast carcinoma, 3 with high-grade glioma, 3 with non–small cell lung cancer, 1 with squamous cell carcinoma of the skin, and 1 with head and neck squamous carcinoma. Nine patients (69%) had received at least two prior lines of systemic therapy.
Response, safety, and biomarkers
Overall, five patients (38%) had a central nervous system response, as ascertained from radiologic response on MRI, cytologic response in cerebrospinal fluid (CSF), and/or clinical response in neurologic symptoms, Dr. Naidoo reported.
Two patients had a complete CNS response: a patient with squamous cell carcinoma of the skin, who was still alive at 3 years, and a patient with non–small cell lung cancer, who survived 9 months but succumbed to metastases elsewhere.
For the entire cohort, median CNS progression-free survival was 2.9 months, and median overall survival was 4.9 months.
“This is consistent with published prospective studies of systemic agents for leptomeningeal disease,” Dr. Naidoo pointed out. “Notably, even though numbers are small, we do see the tail-on-the-curve phenomenon in both of these survival curves, which is consistent with immune checkpoint blockade prospective studies.”
The rate of grade 3 or higher treatment-related adverse events was 15.4%, and there were no grade 3 or higher immune-related adverse events.
The number of patients was too small for formal correlational testing, but both patients who achieved a complete response developed immune-related adverse events.
The trial’s biomarker analyses showed that an aneuploidy assay using CSF tumor-derived DNA performed well at detecting leptomeningeal metastases, with sensitivity of 84.6%, compared with just 53.8% for CSF cytopathology (the current preferred method).
A multiplex assay of CSF cytokines identified similar baseline profiles for patients who went on to have responses and showed similar changes in profile (notably a reduction in proinflammatory cytokines) for the two patients who had complete responses.
Given the trial’s 38% CNS response rate, pembrolizumab “needs to be studied in larger populations of patients to confirm this result, but it could be used as a potential treatment option for patients with leptomeningeal disease from solid tumors,” Dr. Naidoo concluded. “Reassuringly, pembrolizumab was well tolerated, and this is extremely important in a patient population that is traditionally quite frail and in which other standard therapies that are used, such as high-dose methotrexate or intrathecal chemotherapy, are associated with far higher rates of toxicity.”
An unmet need
“Leptomeningeal metastasis is a strong unmet need, although its occurrence is fortunately quite rare,” commented Kim Margolin, MD, a clinical professor and medical oncologist at City of Hope National Medical Center in Duarte, Calif., who was not involved in this study.
The trial is noteworthy for showing activity of programmed death–1 (PD-1) blockade given only systemically and not with additional intrathecal therapy (as has been done in a concurrent study at MD Anderson Cancer Center) and for providing insight into various biomarkers, Dr. Margolin said in an interview.
“I cannot take a stand on author conclusions other than to agree it warrants further evaluation in carefully selected patients, and it would be great to compare something like peripheral PD-1 blockade alone versus in combination with intrathecal therapy versus a combination such as CTLA4 blockade plus PD-1 blockade such as our group and others have shown to have increased activity in CNS metastases over PD-1 block alone,” Dr. Margolin said.
“The drugs in this class are already approved, so there is no reason not to try them,” she noted.
However, patients with leptomeningeal metastases of melanoma, for example, are likely to have already received anti-PD-1 immunotherapy.
“So the settings in which off-the-shelf PD-1 blockade would be useful are extremely limited,” she concluded.
The current trial was funded by Merck, the National Institutes of Health, the Lung Cancer Foundation of America, the International Association for the Study of Lung Cancer, and Johns Hopkins University Seed Grants. Dr. Naidoo disclosed relationships with AstraZeneca, Merck, Bristol Myers Squibb, and Roche/Genentech. Dr. Margolin disclosed no relevant conflicts of interest.
SOURCE: Naidoo J et al. SITC 2020, Abstract 788.
FROM SITC 2020