User login
HIV, HBV, and HCV Increase Risks After Joint Replacement
As patients with HIV, hepatitis B (HBV), and hepatitis C (HCV) live longer, more active lives with the help of antiviral treatments, they are now more often having joint replacement surgeries. But HIV, HBV, and HCV can increase the risk of postoperative complications, say researchers from Duke University in North Carolina.
The researchers studied 16,338 patients in 4 cohorts: those with HIV, HBV, HCV, and HIV plus HBV or HCV. They evaluated the patients at 30 days, 90 days, and 2 years after total joint arthroplasty (TJA), comparing their progress with that of a control group.
Patients with HBV and HCV were at risk of both acute and long-term medical and surgical complications. At 90 days and 2 years, the participants had increased risk of pneumonia, sepsis, joint infection, and revision surgery. They also had a greater risk of complications than did patients with HIV, especially for infection, within the first 90 days postsurgery.
Notably, after TJA, patients with HCV had increased risk of acute kidney injury, sepsis, and transfusion at 30 and 90 days. After hip replacement, patients with HBV had a higher risk of acute kidney injury, pneumonia, and transfusion at 30 and 90 days.
Only 364 patients in the study had both HIV and HBV or HBC, but they did have a greater risk of transfusion at 30 and 90 days following both knee and hip surgery.
Following total hip arthroplasty, patients with HIV were more at risk for deep vein thrombosis and transfusion rather than infection. The lower incidence of infection is likely to be related to effective highly active antiretroviral therapy, the researchers say. HIV patients also were more likely to have mechanical complications, such as loosening, periprosthetic fracture, and revision at 90 days, but not at 2 years. Patients with HIV who underwent total knee arthroplasty (TKA) had a higher risk of death at 30 days and medical complications at 90 days. At 2 years, they had a higher risk of periprosthetic joint infection, irrigation and debridement, and revision. The researchers say the higher incidence of mechanical complications can be explained by the younger, more active, and healthy HIV patients in the cohort—the majority were aged younger than 65 years. They also emphasize that the only risk of infection following TJA in their study was 2 years after TKA.
The researchers suggest that their findings could help prompt future guidelines supporting routine screening prior to elective TJA.
Source:
Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. J Arthroplasty. 2018;33(suppl 7):S86-S92.
doi: 10.1016/j.arth.2017.10.061.
As patients with HIV, hepatitis B (HBV), and hepatitis C (HCV) live longer, more active lives with the help of antiviral treatments, they are now more often having joint replacement surgeries. But HIV, HBV, and HCV can increase the risk of postoperative complications, say researchers from Duke University in North Carolina.
The researchers studied 16,338 patients in 4 cohorts: those with HIV, HBV, HCV, and HIV plus HBV or HCV. They evaluated the patients at 30 days, 90 days, and 2 years after total joint arthroplasty (TJA), comparing their progress with that of a control group.
Patients with HBV and HCV were at risk of both acute and long-term medical and surgical complications. At 90 days and 2 years, the participants had increased risk of pneumonia, sepsis, joint infection, and revision surgery. They also had a greater risk of complications than did patients with HIV, especially for infection, within the first 90 days postsurgery.
Notably, after TJA, patients with HCV had increased risk of acute kidney injury, sepsis, and transfusion at 30 and 90 days. After hip replacement, patients with HBV had a higher risk of acute kidney injury, pneumonia, and transfusion at 30 and 90 days.
Only 364 patients in the study had both HIV and HBV or HBC, but they did have a greater risk of transfusion at 30 and 90 days following both knee and hip surgery.
Following total hip arthroplasty, patients with HIV were more at risk for deep vein thrombosis and transfusion rather than infection. The lower incidence of infection is likely to be related to effective highly active antiretroviral therapy, the researchers say. HIV patients also were more likely to have mechanical complications, such as loosening, periprosthetic fracture, and revision at 90 days, but not at 2 years. Patients with HIV who underwent total knee arthroplasty (TKA) had a higher risk of death at 30 days and medical complications at 90 days. At 2 years, they had a higher risk of periprosthetic joint infection, irrigation and debridement, and revision. The researchers say the higher incidence of mechanical complications can be explained by the younger, more active, and healthy HIV patients in the cohort—the majority were aged younger than 65 years. They also emphasize that the only risk of infection following TJA in their study was 2 years after TKA.
The researchers suggest that their findings could help prompt future guidelines supporting routine screening prior to elective TJA.
Source:
Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. J Arthroplasty. 2018;33(suppl 7):S86-S92.
doi: 10.1016/j.arth.2017.10.061.
As patients with HIV, hepatitis B (HBV), and hepatitis C (HCV) live longer, more active lives with the help of antiviral treatments, they are now more often having joint replacement surgeries. But HIV, HBV, and HCV can increase the risk of postoperative complications, say researchers from Duke University in North Carolina.
The researchers studied 16,338 patients in 4 cohorts: those with HIV, HBV, HCV, and HIV plus HBV or HCV. They evaluated the patients at 30 days, 90 days, and 2 years after total joint arthroplasty (TJA), comparing their progress with that of a control group.
Patients with HBV and HCV were at risk of both acute and long-term medical and surgical complications. At 90 days and 2 years, the participants had increased risk of pneumonia, sepsis, joint infection, and revision surgery. They also had a greater risk of complications than did patients with HIV, especially for infection, within the first 90 days postsurgery.
Notably, after TJA, patients with HCV had increased risk of acute kidney injury, sepsis, and transfusion at 30 and 90 days. After hip replacement, patients with HBV had a higher risk of acute kidney injury, pneumonia, and transfusion at 30 and 90 days.
Only 364 patients in the study had both HIV and HBV or HBC, but they did have a greater risk of transfusion at 30 and 90 days following both knee and hip surgery.
Following total hip arthroplasty, patients with HIV were more at risk for deep vein thrombosis and transfusion rather than infection. The lower incidence of infection is likely to be related to effective highly active antiretroviral therapy, the researchers say. HIV patients also were more likely to have mechanical complications, such as loosening, periprosthetic fracture, and revision at 90 days, but not at 2 years. Patients with HIV who underwent total knee arthroplasty (TKA) had a higher risk of death at 30 days and medical complications at 90 days. At 2 years, they had a higher risk of periprosthetic joint infection, irrigation and debridement, and revision. The researchers say the higher incidence of mechanical complications can be explained by the younger, more active, and healthy HIV patients in the cohort—the majority were aged younger than 65 years. They also emphasize that the only risk of infection following TJA in their study was 2 years after TKA.
The researchers suggest that their findings could help prompt future guidelines supporting routine screening prior to elective TJA.
Source:
Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. J Arthroplasty. 2018;33(suppl 7):S86-S92.
doi: 10.1016/j.arth.2017.10.061.
Acute Aortic Occlusion With Spinal Cord Infarction
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
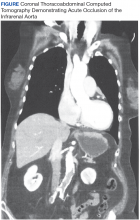
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
High Body Mass Index is Related to Increased Perioperative Complications After Periacetabular Osteotomy
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1
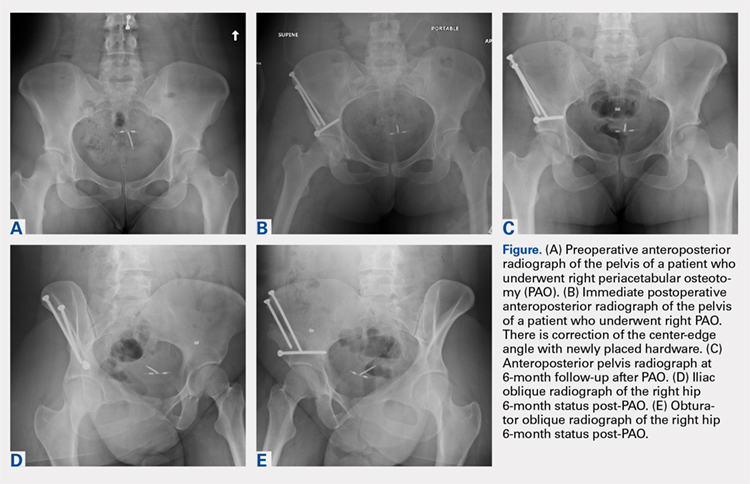
There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1

There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1

There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
TAKE-HOME POINTS
- PAO is an effective procedure to treat symptomatic hip dysplasia in patients without degenerative changes.
- The postoperative correction of dysplasia as measured by center-edge angles were similar in low and high BMI groups.
- Patients with obesity (BMI >30) have a higher incidence of postoperative complications following PAO.
- There were too few patients with diabetes or smoking to determine a significantly increased rate of complications. However, we believe based on the literature these patient populations are at higher risk for complications in the early postoperative period.
- Patients with BMI >30 can have a successful outcome with a PAO procedure. However, this patient population should have counseling about their increased risk of complications, and be given opportunity to lose weight when possible preoperatively.
Screw Fixation Without Bone Grafting for Delayed Unions and Nonunions of Minimally Displaced Scaphoids
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.
Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.
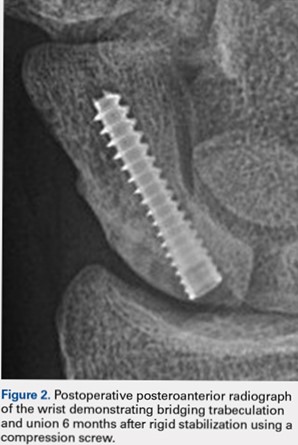
DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.

Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.

DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.

Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.

DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
TAKE-HOME POINTS
- Scaphoid nonunions can occur in minimally displaced fractures.
- If there is no deformity of the scaphoid delayed or nonunion, then a percutaneous screw fixation without bone grafting can reliably lead to bony union.
- Not all scaphoid delayed unions and nonunions require bone grafting.
Hip fracture outcomes are the next ERAS improvement goal
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
REPORTING FROM ACSQSC 2018
Key clinical point: Fractured hip patients managed with the ERAS protocol had improved outcomes.
Major finding: After implementation of the ERAS protocol, 43% of fractured hip patients were discharged to home, which is up from 20% before the project.
Study details: More than 200 patients treated for hip fracture during 2016-2017 at the Langley (B.C.) Memorial Hospital.
Disclosures: The investigator had no disclosures. .
Everything’s Fine … Except His Spine
ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).

ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).

ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
A 25-year-old man is admitted to your facility for a possible infection in his spine. He reports a two-week history of severe back pain with no history of injury or trauma. Imaging performed at an outside facility suggested compression and erosion of his vertebral bodies at the thoracolumbar junction, and the radiologist raised concern for possible osteomyelitis and diskitis.
The patient is otherwise healthy and denies any medical problems. He denies drug use of any form. Review of systems is significant for a three-month history of anorexia and night sweats but no fever.
Physical exam reveals a healthy-appearing male with normal vital signs. His heart and lung sounds are normal.
A chest radiograph is obtained (shown). What is your impression?
Tranexamic Acid Reduces Perioperative Blood Loss and Hemarthrosis in Total Ankle Arthroplasty
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).
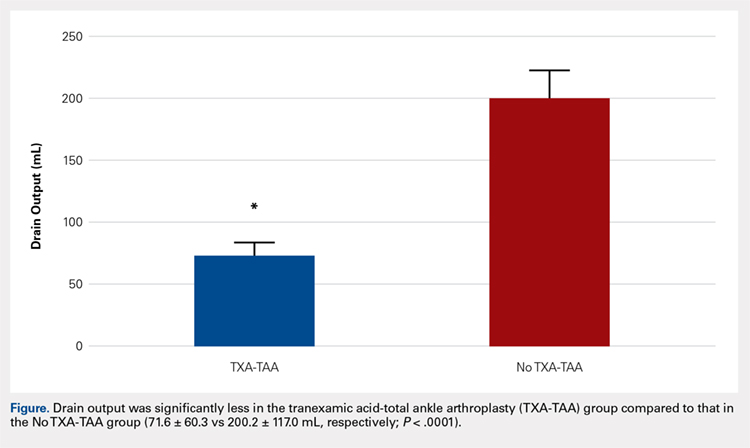
RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
TAKE-HOME POINTS
- TXA is an inexpensive and effective hemostatic agent used during TAA.
- The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of bone during TAA are not as extensive when compared to TKA and THA, but the intra-articular volume is smaller and surrounding soft tissues may be less yielding when blood accumulation occurs.
- If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management during TAA.
- TXA decreases postoperative hemarthrosis and helps to reduce the risk of postoperative wound complications.
- The administration of TXA in the appropriate patient has the potential to decrease hospital cost by controlling postoperative pain and swelling allowing for earlier discharge.
Osteochondritis Dissecans Lesion of the Radial Head
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).
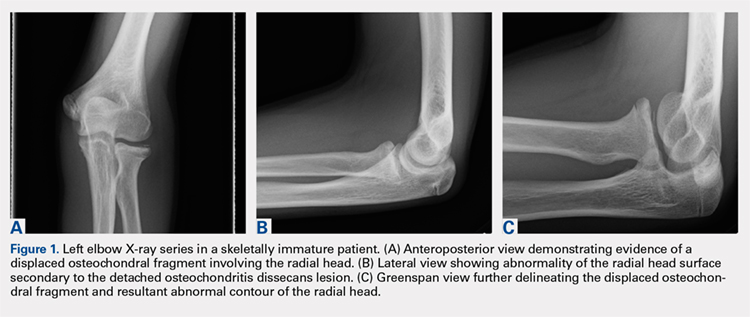
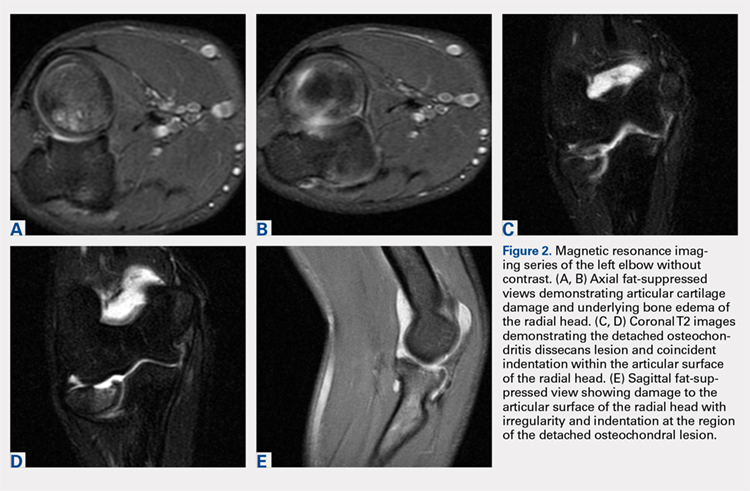
Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).
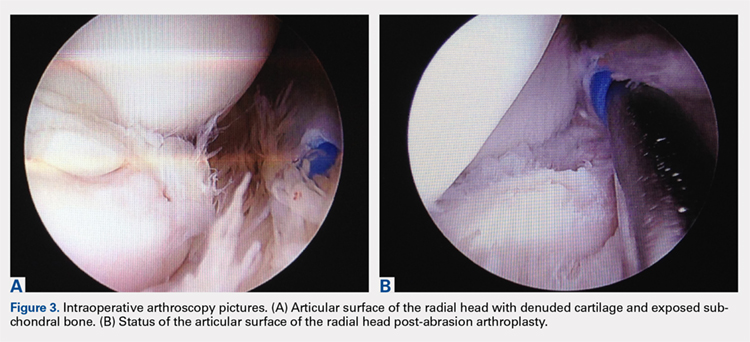
The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
TAKE-HOME POINTS
- Radial Head OCD lesions are uncommon.
- Typically present in athletes that engage in repetitive trauma to elbow (throwers, gymnasts).
- MRI is the best modality for making diagnosis.
- Attempt nonsurgical treatment initially, especially in skeletally immature patients.
- If nonsurgical fails or there is an unstable lesion, consider operative intervention.
Volumetric Considerations for Valving Long-Arm Casts: The Utility of the Cast Spacer
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.
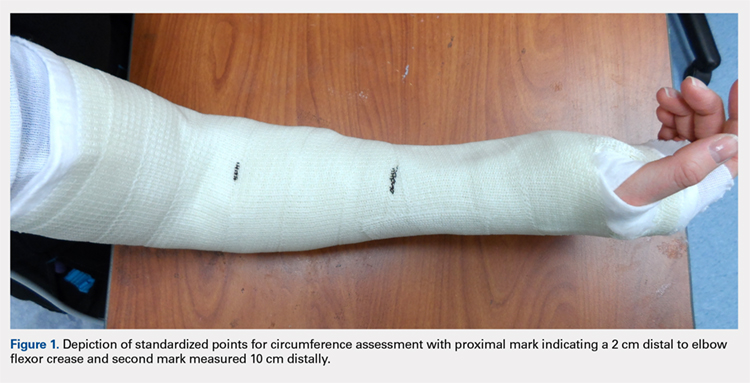
Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).
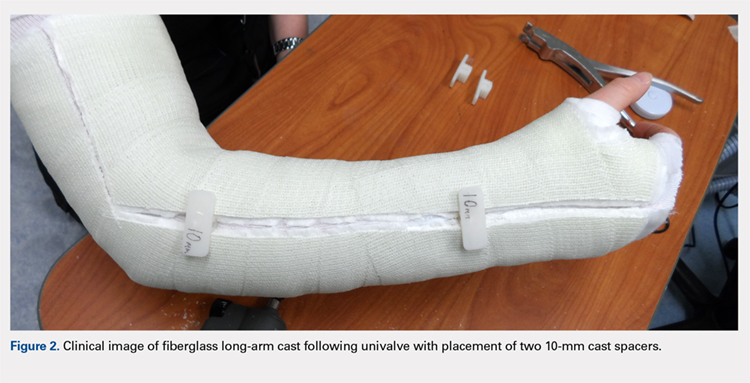
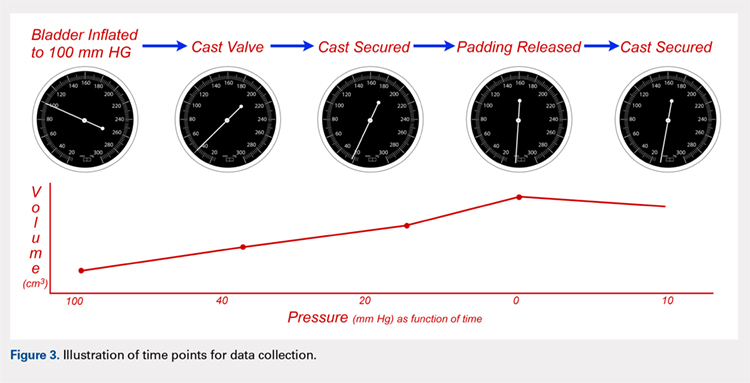
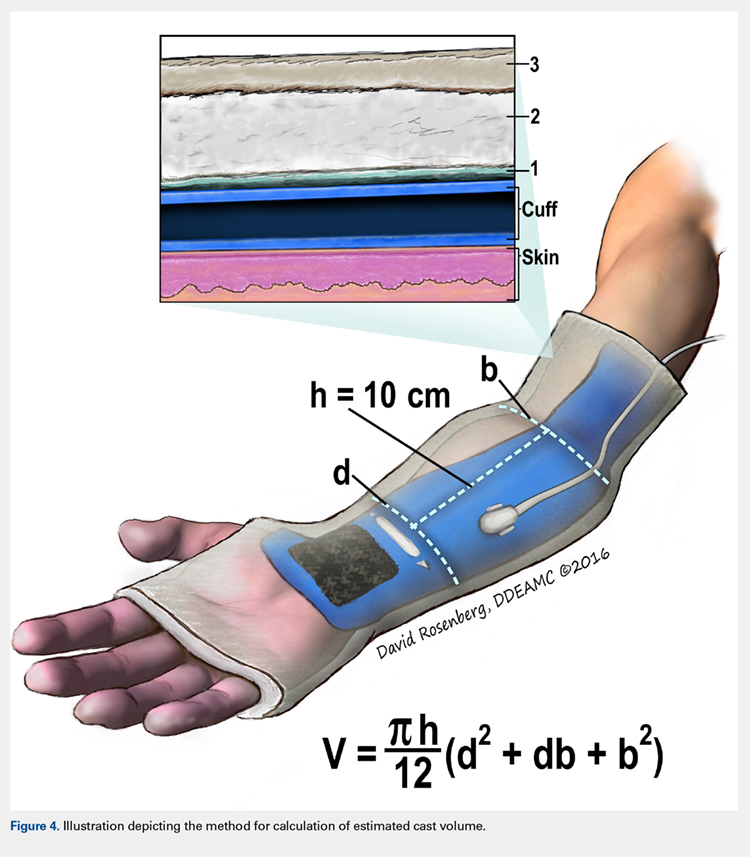
We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |
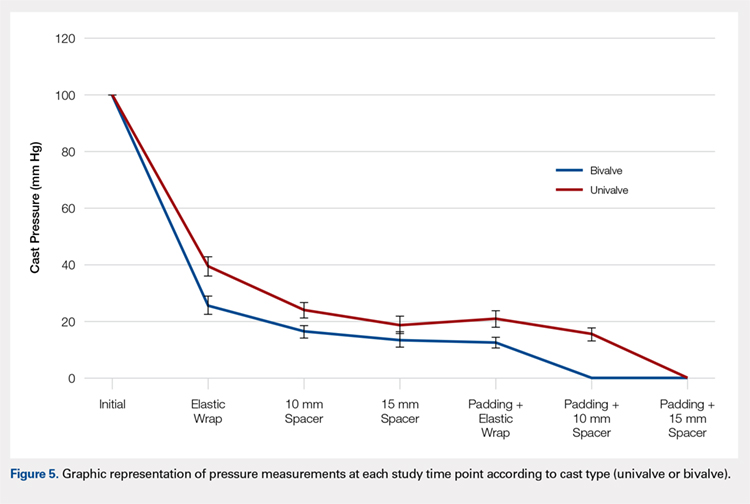
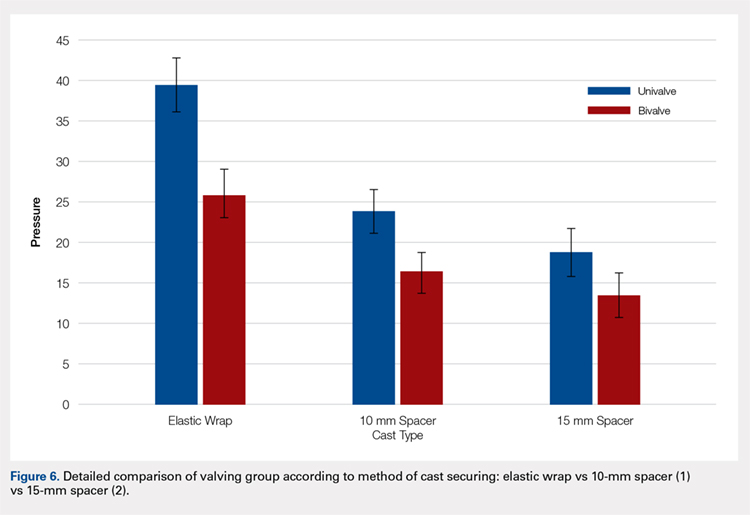
Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |
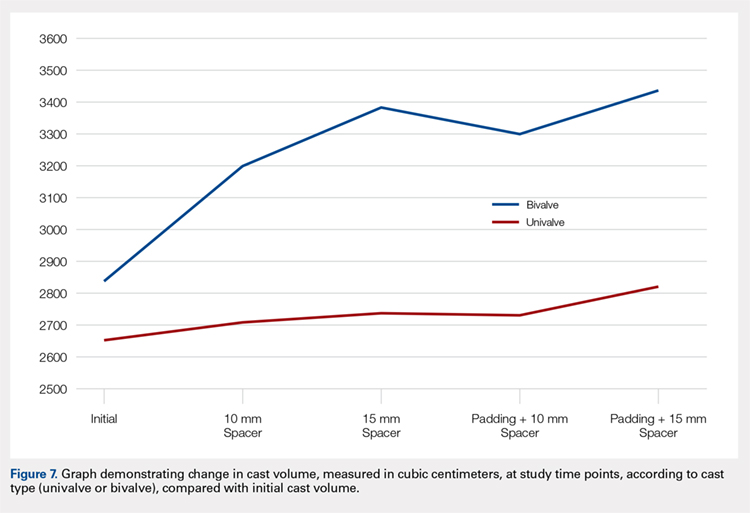
Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
TAKE-HOME POINTS
- Valving a long-arm cast results in decreased cast pressures.
- Univalving can produce a 60% reduction in cast pressure.
- Bivalving produces a 75% reduction in cast pressure.
- Release of the underlying cast padding produces an additional pressure reduction.
- Adding a cast spacer to a univalved cast obtains similar pressure reduction to a bivalved cast.
Steroid injection prior to rotator cuff surgery elevates risk of revision repair
SAN DIEGO – Patients who received a corticosteroid injection within 6 months prior to rotator cuff repair were more likely to undergo a revision rotator cuff surgery within the following 3 years, results from a large database study show.
“Corticosteroid injections are frequently utilized in the nonoperative management of rotator cuff tears,” researchers led by Sophia A. Traven, MD, wrote in an abstract presented during a poster session at the annual meeting of the American Orthopaedic Society for Sports Medicine. “However, recent literature suggests that injections may reduce biomechanical strengths of tendons and ligaments in animal models.”
In an effort to examine the effect of preoperative shoulder injections on the rate of revision cuff repair following arthroscopic rotator cuff repair, the researchers retrospectively reviewed MarketScan claims data between 2010 and 2014 to identify 4,959 patients with an ICD-9 diagnosis of a rotator cuff tear with subsequent arthroscopic rotator cuff repair (CPT 29827).
They used multivariable logistic regression to compare the odds of reoperation between groups, while controlling for certain demographic and comorbid variables, including age and gender, tobacco use, diabetes, and the Charlson comorbidity index score.
Dr. Traven, an orthopedic surgeon at the Medical University of South Carolina, Charleston, and her associates reported that 392 of the 4,959 patients required rotator cuff repair revision within the following 3 years. Compared with those who did not require revision, those who did were older (a mean age of 53 vs. 49 years, respectively), more likely to be smokers (7% vs. 4%), and more likely to receive any injection prior to rotator cuff repair (36% vs 25%; P less than .0001 for all associations).
(odds ratio, 1.822), followed by those who received an injection 0-3 months before the primary repair (OR, 1.375), and those who received an injection 6-12 months before the primary repair (OR, 1.237).
“The risk of revision rotator cuff repair remains elevated for 6 months following a shoulder injection,” the researchers concluded in their poster. “Consideration should therefore be given to minimizing preoperative injections in patients who may require rotator cuff repair.”
They reported having no financial disclosures.
SAN DIEGO – Patients who received a corticosteroid injection within 6 months prior to rotator cuff repair were more likely to undergo a revision rotator cuff surgery within the following 3 years, results from a large database study show.
“Corticosteroid injections are frequently utilized in the nonoperative management of rotator cuff tears,” researchers led by Sophia A. Traven, MD, wrote in an abstract presented during a poster session at the annual meeting of the American Orthopaedic Society for Sports Medicine. “However, recent literature suggests that injections may reduce biomechanical strengths of tendons and ligaments in animal models.”
In an effort to examine the effect of preoperative shoulder injections on the rate of revision cuff repair following arthroscopic rotator cuff repair, the researchers retrospectively reviewed MarketScan claims data between 2010 and 2014 to identify 4,959 patients with an ICD-9 diagnosis of a rotator cuff tear with subsequent arthroscopic rotator cuff repair (CPT 29827).
They used multivariable logistic regression to compare the odds of reoperation between groups, while controlling for certain demographic and comorbid variables, including age and gender, tobacco use, diabetes, and the Charlson comorbidity index score.
Dr. Traven, an orthopedic surgeon at the Medical University of South Carolina, Charleston, and her associates reported that 392 of the 4,959 patients required rotator cuff repair revision within the following 3 years. Compared with those who did not require revision, those who did were older (a mean age of 53 vs. 49 years, respectively), more likely to be smokers (7% vs. 4%), and more likely to receive any injection prior to rotator cuff repair (36% vs 25%; P less than .0001 for all associations).
(odds ratio, 1.822), followed by those who received an injection 0-3 months before the primary repair (OR, 1.375), and those who received an injection 6-12 months before the primary repair (OR, 1.237).
“The risk of revision rotator cuff repair remains elevated for 6 months following a shoulder injection,” the researchers concluded in their poster. “Consideration should therefore be given to minimizing preoperative injections in patients who may require rotator cuff repair.”
They reported having no financial disclosures.
SAN DIEGO – Patients who received a corticosteroid injection within 6 months prior to rotator cuff repair were more likely to undergo a revision rotator cuff surgery within the following 3 years, results from a large database study show.
“Corticosteroid injections are frequently utilized in the nonoperative management of rotator cuff tears,” researchers led by Sophia A. Traven, MD, wrote in an abstract presented during a poster session at the annual meeting of the American Orthopaedic Society for Sports Medicine. “However, recent literature suggests that injections may reduce biomechanical strengths of tendons and ligaments in animal models.”
In an effort to examine the effect of preoperative shoulder injections on the rate of revision cuff repair following arthroscopic rotator cuff repair, the researchers retrospectively reviewed MarketScan claims data between 2010 and 2014 to identify 4,959 patients with an ICD-9 diagnosis of a rotator cuff tear with subsequent arthroscopic rotator cuff repair (CPT 29827).
They used multivariable logistic regression to compare the odds of reoperation between groups, while controlling for certain demographic and comorbid variables, including age and gender, tobacco use, diabetes, and the Charlson comorbidity index score.
Dr. Traven, an orthopedic surgeon at the Medical University of South Carolina, Charleston, and her associates reported that 392 of the 4,959 patients required rotator cuff repair revision within the following 3 years. Compared with those who did not require revision, those who did were older (a mean age of 53 vs. 49 years, respectively), more likely to be smokers (7% vs. 4%), and more likely to receive any injection prior to rotator cuff repair (36% vs 25%; P less than .0001 for all associations).
(odds ratio, 1.822), followed by those who received an injection 0-3 months before the primary repair (OR, 1.375), and those who received an injection 6-12 months before the primary repair (OR, 1.237).
“The risk of revision rotator cuff repair remains elevated for 6 months following a shoulder injection,” the researchers concluded in their poster. “Consideration should therefore be given to minimizing preoperative injections in patients who may require rotator cuff repair.”
They reported having no financial disclosures.
REPORTING FROM AOSSM 2018
Key clinical point: Consideration should be given to minimizing preoperative injections in patients who may require rotator cuff repair.
Major finding: The risk for revision rotator cuff repair was highest for patients who received an injection 3-6 months before the primary rotator cuff repair (odds ratio, 1.822).
Study details: A retrospective analysis of 4,959 patients with an ICD-9 diagnosis of a rotator cuff tear with subsequent arthroscopic rotator cuff repair.
Disclosures: The researchers reported having no financial disclosures.