User login
Opioid exposure in early pregnancy linked to congenital anomalies
Exposure to opioid analgesics during the first trimester of pregnancy appears to increase the risk of congenital anomalies diagnosed in the first year of life, researchers report.
While the absolute risk of congenital anomalies was low, these findings add to an increasing body of evidence suggesting that prenatal exposure to opioids may confer harm to infants post partum.
“We undertook a population-based cohort study to estimate associations between opioid analgesic exposure during the first trimester and congenital anomalies using health administrative data capturing all narcotic prescriptions during pregnancy,” lead author Alexa C. Bowie, MPH, of Queen’s University in Kingston, Ont., and colleagues reported in CMAJ.
The researchers retrospectively reviewed administrative health data in a single-payer health care system from 2013 to 2018. They identified parent-infant pair records for all live births and stillbirths that occurred at more than 20 weeks’ gestation.
The exposure of interest was a prescription for any opioid analgesic with a fill date between the estimated date of conception and less than 14 weeks’ gestation. The referent group included any infant not exposed to an opioid analgesic during the index pregnancy period.
Results
The study cohort included a total of 599,579 gestational parent-infant pairs. Of these, 11,903 (2.0%) were exposed to opioid analgesics, and most were exposed during the first trimester only (75.8%).
Overall, 2.0% of these infants developed a congenital anomaly during the first year of life; the prevalence of congenital anomalies was 2.0% in unexposed infants and 2.8% in exposed infants.
Relative to unexposed infants, the researchers observed greater risks among infants who were exposed for some anomaly groups, including many specific anomalies, such as ankyloglossia (any opioid: adjusted risk ratio, 1.88; 95% confidence interval, 1.30-2.72; codeine: aRR, 2.14; 95% CI, 1.35-3.40), as well as gastrointestinal anomalies (any opioid: aRR, 1.46; 95% CI, 1.15-1.85; codeine: aRR, 1.53; 95% CI, 1.12-2.09; tramadol: aRR, 2.69; 95% CI 1.34-5.38).
After sensitivity analyses, which included exposure 4 weeks before conception or excluded individuals with exposure to opioid analgesics before pregnancy, the findings remained unchanged.
“Although the overall risk was low, we observed an increased risk of any congenital anomaly with tramadol, and a previously unreported risk with morphine,” the researchers wrote.
“Previous studies reported elevated risks of heart anomalies with first-trimester exposure to any opioid analgesic, codeine, and tramadol, but others reported no association with any opioid analgesic or codeine,” they explained.
Interpreting the results
Study author Susan Brogly, PhD, of Queen’s University said “Our population-based study confirms evidence of a small increased risk of birth defects from opioid analgesic exposure in the first trimester that was observed in a recent study of private insurance and Medicaid beneficiaries in the U.S. We further show that this small increased risk is not due to other risk factors for fetal harm in women who may take these medications.”
“An opioid prescription dispensed in the first trimester would imply that there was an acute injury or chronic condition also present in the first trimester, which may also be associated with congenital abnormalities,” commented Elisabeth Poorman, MD, MPH, a clinical instructor and primary care physician at the University of Washington in Seattle.
“Opioid use disorder is often diagnosed incorrectly; since the researchers used diagnostic billing codes to exclude individuals with opioid use disorder, some women may have been missed,” Dr. Poorman explained.
Ms. Bowie and colleagues acknowledged that a key limitation of the study was the identification of cases using diagnostic billing codes. As a result, exposure-dependent recording bias could be present and limit the applicability of the findings.
“The diagnosis and documentation of minor anomalies and those with subtle medical significance could be vulnerable to exposure-dependent recording bias,” Ms. Bowie wrote.
Dr. Poorman recommended that these results should be interpreted with caution given these and other limitations. “Overall, results from this study may imply that there is limited evidence to suspect opioids are related to congenital abnormalities due to a very small difference observed in relatively unequal groups,” she concluded.
This study received funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development and was also supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health. One author reported receiving honoraria from the National Institutes of Health and a grant from the Canadian Institute of Health Research, outside the submitted work. No other competing interests were declared.
Exposure to opioid analgesics during the first trimester of pregnancy appears to increase the risk of congenital anomalies diagnosed in the first year of life, researchers report.
While the absolute risk of congenital anomalies was low, these findings add to an increasing body of evidence suggesting that prenatal exposure to opioids may confer harm to infants post partum.
“We undertook a population-based cohort study to estimate associations between opioid analgesic exposure during the first trimester and congenital anomalies using health administrative data capturing all narcotic prescriptions during pregnancy,” lead author Alexa C. Bowie, MPH, of Queen’s University in Kingston, Ont., and colleagues reported in CMAJ.
The researchers retrospectively reviewed administrative health data in a single-payer health care system from 2013 to 2018. They identified parent-infant pair records for all live births and stillbirths that occurred at more than 20 weeks’ gestation.
The exposure of interest was a prescription for any opioid analgesic with a fill date between the estimated date of conception and less than 14 weeks’ gestation. The referent group included any infant not exposed to an opioid analgesic during the index pregnancy period.
Results
The study cohort included a total of 599,579 gestational parent-infant pairs. Of these, 11,903 (2.0%) were exposed to opioid analgesics, and most were exposed during the first trimester only (75.8%).
Overall, 2.0% of these infants developed a congenital anomaly during the first year of life; the prevalence of congenital anomalies was 2.0% in unexposed infants and 2.8% in exposed infants.
Relative to unexposed infants, the researchers observed greater risks among infants who were exposed for some anomaly groups, including many specific anomalies, such as ankyloglossia (any opioid: adjusted risk ratio, 1.88; 95% confidence interval, 1.30-2.72; codeine: aRR, 2.14; 95% CI, 1.35-3.40), as well as gastrointestinal anomalies (any opioid: aRR, 1.46; 95% CI, 1.15-1.85; codeine: aRR, 1.53; 95% CI, 1.12-2.09; tramadol: aRR, 2.69; 95% CI 1.34-5.38).
After sensitivity analyses, which included exposure 4 weeks before conception or excluded individuals with exposure to opioid analgesics before pregnancy, the findings remained unchanged.
“Although the overall risk was low, we observed an increased risk of any congenital anomaly with tramadol, and a previously unreported risk with morphine,” the researchers wrote.
“Previous studies reported elevated risks of heart anomalies with first-trimester exposure to any opioid analgesic, codeine, and tramadol, but others reported no association with any opioid analgesic or codeine,” they explained.
Interpreting the results
Study author Susan Brogly, PhD, of Queen’s University said “Our population-based study confirms evidence of a small increased risk of birth defects from opioid analgesic exposure in the first trimester that was observed in a recent study of private insurance and Medicaid beneficiaries in the U.S. We further show that this small increased risk is not due to other risk factors for fetal harm in women who may take these medications.”
“An opioid prescription dispensed in the first trimester would imply that there was an acute injury or chronic condition also present in the first trimester, which may also be associated with congenital abnormalities,” commented Elisabeth Poorman, MD, MPH, a clinical instructor and primary care physician at the University of Washington in Seattle.
“Opioid use disorder is often diagnosed incorrectly; since the researchers used diagnostic billing codes to exclude individuals with opioid use disorder, some women may have been missed,” Dr. Poorman explained.
Ms. Bowie and colleagues acknowledged that a key limitation of the study was the identification of cases using diagnostic billing codes. As a result, exposure-dependent recording bias could be present and limit the applicability of the findings.
“The diagnosis and documentation of minor anomalies and those with subtle medical significance could be vulnerable to exposure-dependent recording bias,” Ms. Bowie wrote.
Dr. Poorman recommended that these results should be interpreted with caution given these and other limitations. “Overall, results from this study may imply that there is limited evidence to suspect opioids are related to congenital abnormalities due to a very small difference observed in relatively unequal groups,” she concluded.
This study received funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development and was also supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health. One author reported receiving honoraria from the National Institutes of Health and a grant from the Canadian Institute of Health Research, outside the submitted work. No other competing interests were declared.
Exposure to opioid analgesics during the first trimester of pregnancy appears to increase the risk of congenital anomalies diagnosed in the first year of life, researchers report.
While the absolute risk of congenital anomalies was low, these findings add to an increasing body of evidence suggesting that prenatal exposure to opioids may confer harm to infants post partum.
“We undertook a population-based cohort study to estimate associations between opioid analgesic exposure during the first trimester and congenital anomalies using health administrative data capturing all narcotic prescriptions during pregnancy,” lead author Alexa C. Bowie, MPH, of Queen’s University in Kingston, Ont., and colleagues reported in CMAJ.
The researchers retrospectively reviewed administrative health data in a single-payer health care system from 2013 to 2018. They identified parent-infant pair records for all live births and stillbirths that occurred at more than 20 weeks’ gestation.
The exposure of interest was a prescription for any opioid analgesic with a fill date between the estimated date of conception and less than 14 weeks’ gestation. The referent group included any infant not exposed to an opioid analgesic during the index pregnancy period.
Results
The study cohort included a total of 599,579 gestational parent-infant pairs. Of these, 11,903 (2.0%) were exposed to opioid analgesics, and most were exposed during the first trimester only (75.8%).
Overall, 2.0% of these infants developed a congenital anomaly during the first year of life; the prevalence of congenital anomalies was 2.0% in unexposed infants and 2.8% in exposed infants.
Relative to unexposed infants, the researchers observed greater risks among infants who were exposed for some anomaly groups, including many specific anomalies, such as ankyloglossia (any opioid: adjusted risk ratio, 1.88; 95% confidence interval, 1.30-2.72; codeine: aRR, 2.14; 95% CI, 1.35-3.40), as well as gastrointestinal anomalies (any opioid: aRR, 1.46; 95% CI, 1.15-1.85; codeine: aRR, 1.53; 95% CI, 1.12-2.09; tramadol: aRR, 2.69; 95% CI 1.34-5.38).
After sensitivity analyses, which included exposure 4 weeks before conception or excluded individuals with exposure to opioid analgesics before pregnancy, the findings remained unchanged.
“Although the overall risk was low, we observed an increased risk of any congenital anomaly with tramadol, and a previously unreported risk with morphine,” the researchers wrote.
“Previous studies reported elevated risks of heart anomalies with first-trimester exposure to any opioid analgesic, codeine, and tramadol, but others reported no association with any opioid analgesic or codeine,” they explained.
Interpreting the results
Study author Susan Brogly, PhD, of Queen’s University said “Our population-based study confirms evidence of a small increased risk of birth defects from opioid analgesic exposure in the first trimester that was observed in a recent study of private insurance and Medicaid beneficiaries in the U.S. We further show that this small increased risk is not due to other risk factors for fetal harm in women who may take these medications.”
“An opioid prescription dispensed in the first trimester would imply that there was an acute injury or chronic condition also present in the first trimester, which may also be associated with congenital abnormalities,” commented Elisabeth Poorman, MD, MPH, a clinical instructor and primary care physician at the University of Washington in Seattle.
“Opioid use disorder is often diagnosed incorrectly; since the researchers used diagnostic billing codes to exclude individuals with opioid use disorder, some women may have been missed,” Dr. Poorman explained.
Ms. Bowie and colleagues acknowledged that a key limitation of the study was the identification of cases using diagnostic billing codes. As a result, exposure-dependent recording bias could be present and limit the applicability of the findings.
“The diagnosis and documentation of minor anomalies and those with subtle medical significance could be vulnerable to exposure-dependent recording bias,” Ms. Bowie wrote.
Dr. Poorman recommended that these results should be interpreted with caution given these and other limitations. “Overall, results from this study may imply that there is limited evidence to suspect opioids are related to congenital abnormalities due to a very small difference observed in relatively unequal groups,” she concluded.
This study received funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development and was also supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health. One author reported receiving honoraria from the National Institutes of Health and a grant from the Canadian Institute of Health Research, outside the submitted work. No other competing interests were declared.
FROM CMAJ
Naloxone Dispensing in Patients at Risk for Opioid Overdose After Total Knee Arthroplasty Within the Veterans Health Administration
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
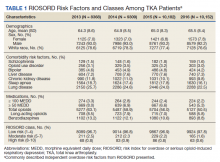
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
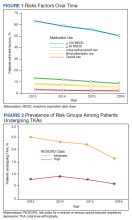
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
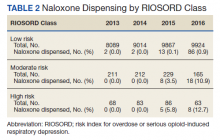
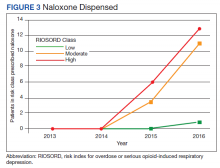
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
Open-label placebo improves symptoms in pediatric IBS and functional abdominal pain
A spoonful of sugar helps the medicine go down – but what if the sugar is the medicine?
Nearly three in four children with irritable bowel syndrome (IBS) or unexplained abdominal pain reported at least a 30% improvement in discomfort after taking a regimen of sugar water they knew had no medicinal properties.
The findings, published online in JAMA Pediatrics on Jan. 31, 2022, also revealed that participants used significantly less rescue medications when taking the so-called “open-label placebo.” The magnitude of the effect was enough to meet one of the criteria from the Food and Drug Administration to approve drugs to treat IBS, which affects between 10% and 15% of U.S. children.
Although open-label placebo is not ready for clinical use, IBS expert Miranda van Tilburg, PhD, said she is “glad we have evidence” of a strong response in this patient population and that the results “may make clinicians rethink how they introduce treatments.
“By emphasizing their belief that a treatment may work, clinicians can harness the placebo effect,” Dr. van Tilburg, professor of medicine and vice chair of research at Marshall University, Huntington, W.Va., told this news organization.
Study leader Samuel Nurko, MD, MPH, the director of the functional abdominal pain program at Harvard Medical School, Boston, said placebo-controlled trials in patients with IBS and functional abdominal pain consistently show a “very high placebo response.” The question his group set out to answer, he said, was: “Can we get the pain symptoms of these children better by giving them placebo with no deception?”
Between 2015 and 2018, Dr. Nurko and colleagues randomly assigned 30 children and adolescents, aged 8-18 years, with IBS or functional abdominal pain to receive either an open-label inert liquid placebo – consisting of 85% sucrose, citric acid, purified water, and the preservative methyl paraben – twice daily for 3 weeks followed by 3 weeks with no placebo, or to follow the reverse sequence. Roughly half (53%) of the children had functional abdominal pain, and 47% had IBS as defined by Rome III criteria.
Researchers at the three participating clinical sites followed a standardized protocol for explaining the nature of placebo (“like sugar pills without medication”), telling participants that adults with conditions like theirs often benefit from placebo when they receive it as part of blinded, randomized clinical trials. Participants in the study were allowed to use hyoscyamine, an anticholinergic medication, as rescue treatment during the trial.
Dr. Nurko’s team reported that patients had a mean pain score of 39.9 on a 100-point visual analogue scale during the open-label placebo phase of the trial and a mean score of 45 during the control period. That difference was statistically significant (P = .03).
Participants took an average of two hyoscyamine pills during the placebo phase, compared with 3.8 pills during the 3-week period when they did not receive placebo (P < .001).
Nearly three-fourths (73.3%) of children in the study reported that open-label placebo improved their pain by over 30%, thus meeting one of the FDA’s criteria for clinical evaluation of drugs for IBS. Half said the placebo liquid cut their pain by more than 50%.
Dr. Nurko said the findings highlight the need to address “mind-body connections” in the management of gut-brain disorders. Like Dr. van Tilburg, he cautioned that open-label placebo “is not ready for widespread use. Placebo is complicated, and we need to understand the mechanism” underlying its efficacy.
“The idea is eventually we will be able to sort out the exact mechanism and harness it for clinical practice,” he added.
However, Dr. van Tilburg expressed that using placebo therapy to treat children and adolescents with these conditions could send the message that “the pain is not real or all in their heads. Children with chronic pain encounter a lot of stigma, and this kind of treatment may increase the feeling of not being believed. We should be careful to avoid this.”
The study was funded by the National Institutes of Health, the Swiss National Science Foundation, the Schwartz family fund, the Foundation for the Science of the Therapeutic Relationship, and the Morgan Family Foundation.
A version of this article first appeared on Medscape.com.
A spoonful of sugar helps the medicine go down – but what if the sugar is the medicine?
Nearly three in four children with irritable bowel syndrome (IBS) or unexplained abdominal pain reported at least a 30% improvement in discomfort after taking a regimen of sugar water they knew had no medicinal properties.
The findings, published online in JAMA Pediatrics on Jan. 31, 2022, also revealed that participants used significantly less rescue medications when taking the so-called “open-label placebo.” The magnitude of the effect was enough to meet one of the criteria from the Food and Drug Administration to approve drugs to treat IBS, which affects between 10% and 15% of U.S. children.
Although open-label placebo is not ready for clinical use, IBS expert Miranda van Tilburg, PhD, said she is “glad we have evidence” of a strong response in this patient population and that the results “may make clinicians rethink how they introduce treatments.
“By emphasizing their belief that a treatment may work, clinicians can harness the placebo effect,” Dr. van Tilburg, professor of medicine and vice chair of research at Marshall University, Huntington, W.Va., told this news organization.
Study leader Samuel Nurko, MD, MPH, the director of the functional abdominal pain program at Harvard Medical School, Boston, said placebo-controlled trials in patients with IBS and functional abdominal pain consistently show a “very high placebo response.” The question his group set out to answer, he said, was: “Can we get the pain symptoms of these children better by giving them placebo with no deception?”
Between 2015 and 2018, Dr. Nurko and colleagues randomly assigned 30 children and adolescents, aged 8-18 years, with IBS or functional abdominal pain to receive either an open-label inert liquid placebo – consisting of 85% sucrose, citric acid, purified water, and the preservative methyl paraben – twice daily for 3 weeks followed by 3 weeks with no placebo, or to follow the reverse sequence. Roughly half (53%) of the children had functional abdominal pain, and 47% had IBS as defined by Rome III criteria.
Researchers at the three participating clinical sites followed a standardized protocol for explaining the nature of placebo (“like sugar pills without medication”), telling participants that adults with conditions like theirs often benefit from placebo when they receive it as part of blinded, randomized clinical trials. Participants in the study were allowed to use hyoscyamine, an anticholinergic medication, as rescue treatment during the trial.
Dr. Nurko’s team reported that patients had a mean pain score of 39.9 on a 100-point visual analogue scale during the open-label placebo phase of the trial and a mean score of 45 during the control period. That difference was statistically significant (P = .03).
Participants took an average of two hyoscyamine pills during the placebo phase, compared with 3.8 pills during the 3-week period when they did not receive placebo (P < .001).
Nearly three-fourths (73.3%) of children in the study reported that open-label placebo improved their pain by over 30%, thus meeting one of the FDA’s criteria for clinical evaluation of drugs for IBS. Half said the placebo liquid cut their pain by more than 50%.
Dr. Nurko said the findings highlight the need to address “mind-body connections” in the management of gut-brain disorders. Like Dr. van Tilburg, he cautioned that open-label placebo “is not ready for widespread use. Placebo is complicated, and we need to understand the mechanism” underlying its efficacy.
“The idea is eventually we will be able to sort out the exact mechanism and harness it for clinical practice,” he added.
However, Dr. van Tilburg expressed that using placebo therapy to treat children and adolescents with these conditions could send the message that “the pain is not real or all in their heads. Children with chronic pain encounter a lot of stigma, and this kind of treatment may increase the feeling of not being believed. We should be careful to avoid this.”
The study was funded by the National Institutes of Health, the Swiss National Science Foundation, the Schwartz family fund, the Foundation for the Science of the Therapeutic Relationship, and the Morgan Family Foundation.
A version of this article first appeared on Medscape.com.
A spoonful of sugar helps the medicine go down – but what if the sugar is the medicine?
Nearly three in four children with irritable bowel syndrome (IBS) or unexplained abdominal pain reported at least a 30% improvement in discomfort after taking a regimen of sugar water they knew had no medicinal properties.
The findings, published online in JAMA Pediatrics on Jan. 31, 2022, also revealed that participants used significantly less rescue medications when taking the so-called “open-label placebo.” The magnitude of the effect was enough to meet one of the criteria from the Food and Drug Administration to approve drugs to treat IBS, which affects between 10% and 15% of U.S. children.
Although open-label placebo is not ready for clinical use, IBS expert Miranda van Tilburg, PhD, said she is “glad we have evidence” of a strong response in this patient population and that the results “may make clinicians rethink how they introduce treatments.
“By emphasizing their belief that a treatment may work, clinicians can harness the placebo effect,” Dr. van Tilburg, professor of medicine and vice chair of research at Marshall University, Huntington, W.Va., told this news organization.
Study leader Samuel Nurko, MD, MPH, the director of the functional abdominal pain program at Harvard Medical School, Boston, said placebo-controlled trials in patients with IBS and functional abdominal pain consistently show a “very high placebo response.” The question his group set out to answer, he said, was: “Can we get the pain symptoms of these children better by giving them placebo with no deception?”
Between 2015 and 2018, Dr. Nurko and colleagues randomly assigned 30 children and adolescents, aged 8-18 years, with IBS or functional abdominal pain to receive either an open-label inert liquid placebo – consisting of 85% sucrose, citric acid, purified water, and the preservative methyl paraben – twice daily for 3 weeks followed by 3 weeks with no placebo, or to follow the reverse sequence. Roughly half (53%) of the children had functional abdominal pain, and 47% had IBS as defined by Rome III criteria.
Researchers at the three participating clinical sites followed a standardized protocol for explaining the nature of placebo (“like sugar pills without medication”), telling participants that adults with conditions like theirs often benefit from placebo when they receive it as part of blinded, randomized clinical trials. Participants in the study were allowed to use hyoscyamine, an anticholinergic medication, as rescue treatment during the trial.
Dr. Nurko’s team reported that patients had a mean pain score of 39.9 on a 100-point visual analogue scale during the open-label placebo phase of the trial and a mean score of 45 during the control period. That difference was statistically significant (P = .03).
Participants took an average of two hyoscyamine pills during the placebo phase, compared with 3.8 pills during the 3-week period when they did not receive placebo (P < .001).
Nearly three-fourths (73.3%) of children in the study reported that open-label placebo improved their pain by over 30%, thus meeting one of the FDA’s criteria for clinical evaluation of drugs for IBS. Half said the placebo liquid cut their pain by more than 50%.
Dr. Nurko said the findings highlight the need to address “mind-body connections” in the management of gut-brain disorders. Like Dr. van Tilburg, he cautioned that open-label placebo “is not ready for widespread use. Placebo is complicated, and we need to understand the mechanism” underlying its efficacy.
“The idea is eventually we will be able to sort out the exact mechanism and harness it for clinical practice,” he added.
However, Dr. van Tilburg expressed that using placebo therapy to treat children and adolescents with these conditions could send the message that “the pain is not real or all in their heads. Children with chronic pain encounter a lot of stigma, and this kind of treatment may increase the feeling of not being believed. We should be careful to avoid this.”
The study was funded by the National Institutes of Health, the Swiss National Science Foundation, the Schwartz family fund, the Foundation for the Science of the Therapeutic Relationship, and the Morgan Family Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Men with hypersexual disorder may have oxytocin overload
Men with hypersexual disorder showed higher levels of oxytocin in their blood than did healthy control men without the disorder, in a study with 102 participants.
Hypersexual disorder (HD) is characterized by “excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control,” John Flanagan, MD, of the Karolinska Institutet in Stockholm and colleagues wrote. Although HD is not included as a separate diagnosis in the current DSM, the similar disorder of compulsive sexual behavior is included in the ICD.
Data on the pathophysiology of HD are limited, although a previous study by corresponding author Andreas Chatzittofis, MD, and colleagues showed evidence of neuroendocrine dysregulation in men with HD, and prompted the current study to explore the possible involvement of the oxytocinergic system in HD.
In the current study, published in the Journal of Clinical Endocrinology & Metabolism, the researchers identified 64 men with HD and 38 healthy male controls. The patients were help-seeking men older than 18 years diagnosed with HD who presented to a single center in Sweden during 2013-2014. The men were included in a randomized clinical trial of cognitive-behavioral therapy for HD, and 30 of them participated in a 7-week CBT program.
Oxytocin, secreted by the pituitary gland, is known to play a role in sexual behavior, but has not been examined in HD men, the researchers said. At baseline, the mean plasma oxytocin was 31.0 pM in the HD patients, which was significantly higher than the mean 16.9 pM in healthy controls (P < .001). However, the 30 HD men who underwent CBT showed significant improvement in oxytocin levels, from a mean pretreatment level of 30.5 to a mean posttreatment level of 20.2 pM (P = .0000019).
The study findings were limited by several factors, including the lack of data on oxytocin for a wait list or control group, as well as the inability to control for confounding factors such as diet, physical activity, ethnicity, and stress, and a lack of data on sexual activity prior to oxytocin measurements, the researchers noted.
However, “although there is no clear consensus at this point, previous studies support the use of oxytocin plasma levels as a surrogate variable for [cerebrospinal fluid] oxytocin activity,” the researchers wrote in their discussion. The current study findings support the potential of oxytocin as a biomarker for HD diagnostics and also as a measure of disease severity. Larger studies to confirm the findings, especially those that exclude potential confounders, would be valuable.
Oxytocin may be treatment target
The study is important because of the lack of knowledge regarding the pathophysiology underlying hypersexual disorder, Dr. Chatzittofis of the University of Cyprus, Nicosia, said in an interview. “This is the first study to indicate a role for oxytocin’s involvement” in hypersexual disorder in men. Dr. Chatzittofis led a team in a previous study that showed an association between HD in men and dysregulation of the hypothalamic pituitary adrenal axis.
In the current study, “we discovered that men with compulsive sexual behavior disorder had higher oxytocin levels, compared with healthy men,” said Dr. Chatzittofis, adding that the take-home message for clinicians is the potential of CBT for treatment. “Cognitive-behavior therapy led to a reduction in both hypersexual behavior and oxytocin levels.” The results suggest that oxytocin plays an important role in sex addiction.
Consequently, oxytocin may be a potential drug target for future pharmacologic treatment of hypersexual disorder, he added.
The study was supported by the Swedish Research Council, the Stockholm County Council, and by a partnership between Umeå University and Västerbotten County Council. The researchers had no financial conflicts to disclose.
Men with hypersexual disorder showed higher levels of oxytocin in their blood than did healthy control men without the disorder, in a study with 102 participants.
Hypersexual disorder (HD) is characterized by “excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control,” John Flanagan, MD, of the Karolinska Institutet in Stockholm and colleagues wrote. Although HD is not included as a separate diagnosis in the current DSM, the similar disorder of compulsive sexual behavior is included in the ICD.
Data on the pathophysiology of HD are limited, although a previous study by corresponding author Andreas Chatzittofis, MD, and colleagues showed evidence of neuroendocrine dysregulation in men with HD, and prompted the current study to explore the possible involvement of the oxytocinergic system in HD.
In the current study, published in the Journal of Clinical Endocrinology & Metabolism, the researchers identified 64 men with HD and 38 healthy male controls. The patients were help-seeking men older than 18 years diagnosed with HD who presented to a single center in Sweden during 2013-2014. The men were included in a randomized clinical trial of cognitive-behavioral therapy for HD, and 30 of them participated in a 7-week CBT program.
Oxytocin, secreted by the pituitary gland, is known to play a role in sexual behavior, but has not been examined in HD men, the researchers said. At baseline, the mean plasma oxytocin was 31.0 pM in the HD patients, which was significantly higher than the mean 16.9 pM in healthy controls (P < .001). However, the 30 HD men who underwent CBT showed significant improvement in oxytocin levels, from a mean pretreatment level of 30.5 to a mean posttreatment level of 20.2 pM (P = .0000019).
The study findings were limited by several factors, including the lack of data on oxytocin for a wait list or control group, as well as the inability to control for confounding factors such as diet, physical activity, ethnicity, and stress, and a lack of data on sexual activity prior to oxytocin measurements, the researchers noted.
However, “although there is no clear consensus at this point, previous studies support the use of oxytocin plasma levels as a surrogate variable for [cerebrospinal fluid] oxytocin activity,” the researchers wrote in their discussion. The current study findings support the potential of oxytocin as a biomarker for HD diagnostics and also as a measure of disease severity. Larger studies to confirm the findings, especially those that exclude potential confounders, would be valuable.
Oxytocin may be treatment target
The study is important because of the lack of knowledge regarding the pathophysiology underlying hypersexual disorder, Dr. Chatzittofis of the University of Cyprus, Nicosia, said in an interview. “This is the first study to indicate a role for oxytocin’s involvement” in hypersexual disorder in men. Dr. Chatzittofis led a team in a previous study that showed an association between HD in men and dysregulation of the hypothalamic pituitary adrenal axis.
In the current study, “we discovered that men with compulsive sexual behavior disorder had higher oxytocin levels, compared with healthy men,” said Dr. Chatzittofis, adding that the take-home message for clinicians is the potential of CBT for treatment. “Cognitive-behavior therapy led to a reduction in both hypersexual behavior and oxytocin levels.” The results suggest that oxytocin plays an important role in sex addiction.
Consequently, oxytocin may be a potential drug target for future pharmacologic treatment of hypersexual disorder, he added.
The study was supported by the Swedish Research Council, the Stockholm County Council, and by a partnership between Umeå University and Västerbotten County Council. The researchers had no financial conflicts to disclose.
Men with hypersexual disorder showed higher levels of oxytocin in their blood than did healthy control men without the disorder, in a study with 102 participants.
Hypersexual disorder (HD) is characterized by “excessive and persistent sexual behaviors in relation to various mood states, with an impulsivity component and experienced loss of control,” John Flanagan, MD, of the Karolinska Institutet in Stockholm and colleagues wrote. Although HD is not included as a separate diagnosis in the current DSM, the similar disorder of compulsive sexual behavior is included in the ICD.
Data on the pathophysiology of HD are limited, although a previous study by corresponding author Andreas Chatzittofis, MD, and colleagues showed evidence of neuroendocrine dysregulation in men with HD, and prompted the current study to explore the possible involvement of the oxytocinergic system in HD.
In the current study, published in the Journal of Clinical Endocrinology & Metabolism, the researchers identified 64 men with HD and 38 healthy male controls. The patients were help-seeking men older than 18 years diagnosed with HD who presented to a single center in Sweden during 2013-2014. The men were included in a randomized clinical trial of cognitive-behavioral therapy for HD, and 30 of them participated in a 7-week CBT program.
Oxytocin, secreted by the pituitary gland, is known to play a role in sexual behavior, but has not been examined in HD men, the researchers said. At baseline, the mean plasma oxytocin was 31.0 pM in the HD patients, which was significantly higher than the mean 16.9 pM in healthy controls (P < .001). However, the 30 HD men who underwent CBT showed significant improvement in oxytocin levels, from a mean pretreatment level of 30.5 to a mean posttreatment level of 20.2 pM (P = .0000019).
The study findings were limited by several factors, including the lack of data on oxytocin for a wait list or control group, as well as the inability to control for confounding factors such as diet, physical activity, ethnicity, and stress, and a lack of data on sexual activity prior to oxytocin measurements, the researchers noted.
However, “although there is no clear consensus at this point, previous studies support the use of oxytocin plasma levels as a surrogate variable for [cerebrospinal fluid] oxytocin activity,” the researchers wrote in their discussion. The current study findings support the potential of oxytocin as a biomarker for HD diagnostics and also as a measure of disease severity. Larger studies to confirm the findings, especially those that exclude potential confounders, would be valuable.
Oxytocin may be treatment target
The study is important because of the lack of knowledge regarding the pathophysiology underlying hypersexual disorder, Dr. Chatzittofis of the University of Cyprus, Nicosia, said in an interview. “This is the first study to indicate a role for oxytocin’s involvement” in hypersexual disorder in men. Dr. Chatzittofis led a team in a previous study that showed an association between HD in men and dysregulation of the hypothalamic pituitary adrenal axis.
In the current study, “we discovered that men with compulsive sexual behavior disorder had higher oxytocin levels, compared with healthy men,” said Dr. Chatzittofis, adding that the take-home message for clinicians is the potential of CBT for treatment. “Cognitive-behavior therapy led to a reduction in both hypersexual behavior and oxytocin levels.” The results suggest that oxytocin plays an important role in sex addiction.
Consequently, oxytocin may be a potential drug target for future pharmacologic treatment of hypersexual disorder, he added.
The study was supported by the Swedish Research Council, the Stockholm County Council, and by a partnership between Umeå University and Västerbotten County Council. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Researchers eye cannabis for gynecologic pain
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
OTC cannabidiol products tied to improved pain, sleep, anxiety
Interim findings from Advancing CBD Education and Science, a 100% virtual, open label, randomized, controlled trial, show study participants experienced various degrees of “clinically meaningful” improvements in sleep quality, anxiety, and pain.
“ACES is the largest clinical trial ever conducted on commercially available CBD products and provides first-of-its-kind real world evidence into what conditions users may experience benefit from CBD usage, whether these benefits are clinically meaningful, what attributes of CBD products may impact health outcomes, and what side effects may occur,” study coinvestigator Jessica Saleska, PhD, MPH, director of research at Radicle Science, the company that conducted the study, told this news organization.
Scant evidence
Despite the growing market size of commercially available CBD products “there is still scant data on the effectiveness of over-the-counter cannabinoid products due to the cost, speed, and scale limitations of the current approach to scientific research,” Jeff Chen, MD, MBA, cofounder and CEO of Radicle Science, told this news organization.
One of the study’s goals, said Ethan Russo, MD, a neurologist, founder/CEO of CReDO Science, and scientific adviser for Radicle, is to help consumers make informed decisions before purchasing and using commercially available oral CBD products.
Designed to eliminate all physical infrastructure, which minimizes costs and facilitates faster execution, ACES was conducted much like a phase 4 clinical trial, collating real-world data gathered over 4 weeks.
“The process that Radicle scientists [have] advanced is sort of a crowdsourcing approach to doing clinical science,” Dr. Russo said. “Hopefully, there is going to be a considerable amount of data generated that [will] affect people’s buying options.”
The study also aimed to evaluate product attributes, including composition, mode of use, dosage, dosage timing and frequency, and their correlation to degrees of outcomes.
Dr. Russo explained why product composition is an important factor, especially when dealing with CBD. “What happens with any given [CBD] preparation is going to be totally a function of other components, if any.
“For example, there’s this mistaken notion that cannabidiol is sedating; it is not. Pure cannabidiol is stimulating in low and moderate amounts. Where the confusion has arisen is that the early chemovars containing cannabidiol were also predominant in myrcene, the sedating terpene, [thereby] creating this misimpression that it is good for sleep,” he added.
However, CBD might also affect sleep by reducing anxiety that interferes with it. “What’s clear is that cannabidiol is an antianxiety agent, if you have a sufficient dose,” Dr. Russo said.
The 4-week study included 2,704 participants aged 21 years and older, self-reporting anxiety, chronic pain, or sleep disturbances as a primary reason for taking CBD. Study participants were randomly assigned to receive 1 of 13 commercially available oral CBD extracts.
Participants were allocated to 1 of 14 cohorts, comprising 13 treatment groups with 208 participants each who received a single CBD product, or a wait-list control group of 296 participants who received product at the study’s end.
The primary outcome focused on “clinically meaningful” changes, which were defined as “distinct and palpable improvements in quality of life through improvements in respective health outcomes.”
Secondary outcomes included changes in sleep, anxiety, and pain based on several validated indices, including the PROMIS (Patient-Reported Outcome Measurement Information System) Sleep Short Form; the PROMIS Anxiety Scale; the Patient Global Impression of Change; the Pain, Enjoyment, General Activity scale; and the General Anxiety Disorder–7 scale.
The interim study results are promising, with participants reporting, on average, a 71% improvement in well-being. Additionally, 63% reported clinically meaningful improvements in anxiety, and 61% in sleep quality. The CBD products provided smaller benefits in pain management, with less than half (47%) experiencing meaningful improvements.
In addition to improvement in sleep, pain, and anxiety, these data highlight how rapidly benefits occurred; most were realized during the first week of the study, with up to 61% of treatment group participants reporting a therapeutic effect within 1-4 hours of taking their assigned product.
Overcoming the placebo effect
Commenting on the research, Justin Strickland, PhD, an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, who was not involved in the research, said without knowing a lot about the pharmacology of the products being tested, early dramatic improvements in these measures, such as sleep impairment, are common.
“There are some data to suggest that there is an expectancy effect when we talk about the therapeutic benefit of cannabinoid products, (i.e., when someone has the expectation that they are going to experience a stronger effect) but this is true of any drug in an open label trial,” Dr. Strickland added.
Dr. Russo took the point a step further. “It’s getting near impossible to look at cannabinoid compounds, even with randomized, controlled trials because of the burgeoning placebo responses. When you couple it with the fact that consumers have the mistaken notion that cannabis-based drugs are miraculous, the expectations are so high that everyone thinks that they’re on the real stuff, even if it’s a placebo group.”
Still, both Dr. Strickland and Dr. Russo highlighted the fact that ACES mirrors real-world experience, which will they hope will inform the use of CBD and CBD-based preparations moving forward. By removing certain barriers like institutional bureaucracy or federal funding restrictions inherent to more traditional randomized controlled trial design, ACES might provide data that bridge the gap between efficacy and effectiveness.
ACES was funded by Radicle Science. Dr. Chen is cofounder and CEO of Radicle Science. Dr. Russo and Dr. Strickland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Interim findings from Advancing CBD Education and Science, a 100% virtual, open label, randomized, controlled trial, show study participants experienced various degrees of “clinically meaningful” improvements in sleep quality, anxiety, and pain.
“ACES is the largest clinical trial ever conducted on commercially available CBD products and provides first-of-its-kind real world evidence into what conditions users may experience benefit from CBD usage, whether these benefits are clinically meaningful, what attributes of CBD products may impact health outcomes, and what side effects may occur,” study coinvestigator Jessica Saleska, PhD, MPH, director of research at Radicle Science, the company that conducted the study, told this news organization.
Scant evidence
Despite the growing market size of commercially available CBD products “there is still scant data on the effectiveness of over-the-counter cannabinoid products due to the cost, speed, and scale limitations of the current approach to scientific research,” Jeff Chen, MD, MBA, cofounder and CEO of Radicle Science, told this news organization.
One of the study’s goals, said Ethan Russo, MD, a neurologist, founder/CEO of CReDO Science, and scientific adviser for Radicle, is to help consumers make informed decisions before purchasing and using commercially available oral CBD products.
Designed to eliminate all physical infrastructure, which minimizes costs and facilitates faster execution, ACES was conducted much like a phase 4 clinical trial, collating real-world data gathered over 4 weeks.
“The process that Radicle scientists [have] advanced is sort of a crowdsourcing approach to doing clinical science,” Dr. Russo said. “Hopefully, there is going to be a considerable amount of data generated that [will] affect people’s buying options.”
The study also aimed to evaluate product attributes, including composition, mode of use, dosage, dosage timing and frequency, and their correlation to degrees of outcomes.
Dr. Russo explained why product composition is an important factor, especially when dealing with CBD. “What happens with any given [CBD] preparation is going to be totally a function of other components, if any.
“For example, there’s this mistaken notion that cannabidiol is sedating; it is not. Pure cannabidiol is stimulating in low and moderate amounts. Where the confusion has arisen is that the early chemovars containing cannabidiol were also predominant in myrcene, the sedating terpene, [thereby] creating this misimpression that it is good for sleep,” he added.
However, CBD might also affect sleep by reducing anxiety that interferes with it. “What’s clear is that cannabidiol is an antianxiety agent, if you have a sufficient dose,” Dr. Russo said.
The 4-week study included 2,704 participants aged 21 years and older, self-reporting anxiety, chronic pain, or sleep disturbances as a primary reason for taking CBD. Study participants were randomly assigned to receive 1 of 13 commercially available oral CBD extracts.
Participants were allocated to 1 of 14 cohorts, comprising 13 treatment groups with 208 participants each who received a single CBD product, or a wait-list control group of 296 participants who received product at the study’s end.
The primary outcome focused on “clinically meaningful” changes, which were defined as “distinct and palpable improvements in quality of life through improvements in respective health outcomes.”
Secondary outcomes included changes in sleep, anxiety, and pain based on several validated indices, including the PROMIS (Patient-Reported Outcome Measurement Information System) Sleep Short Form; the PROMIS Anxiety Scale; the Patient Global Impression of Change; the Pain, Enjoyment, General Activity scale; and the General Anxiety Disorder–7 scale.
The interim study results are promising, with participants reporting, on average, a 71% improvement in well-being. Additionally, 63% reported clinically meaningful improvements in anxiety, and 61% in sleep quality. The CBD products provided smaller benefits in pain management, with less than half (47%) experiencing meaningful improvements.
In addition to improvement in sleep, pain, and anxiety, these data highlight how rapidly benefits occurred; most were realized during the first week of the study, with up to 61% of treatment group participants reporting a therapeutic effect within 1-4 hours of taking their assigned product.
Overcoming the placebo effect
Commenting on the research, Justin Strickland, PhD, an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, who was not involved in the research, said without knowing a lot about the pharmacology of the products being tested, early dramatic improvements in these measures, such as sleep impairment, are common.
“There are some data to suggest that there is an expectancy effect when we talk about the therapeutic benefit of cannabinoid products, (i.e., when someone has the expectation that they are going to experience a stronger effect) but this is true of any drug in an open label trial,” Dr. Strickland added.
Dr. Russo took the point a step further. “It’s getting near impossible to look at cannabinoid compounds, even with randomized, controlled trials because of the burgeoning placebo responses. When you couple it with the fact that consumers have the mistaken notion that cannabis-based drugs are miraculous, the expectations are so high that everyone thinks that they’re on the real stuff, even if it’s a placebo group.”
Still, both Dr. Strickland and Dr. Russo highlighted the fact that ACES mirrors real-world experience, which will they hope will inform the use of CBD and CBD-based preparations moving forward. By removing certain barriers like institutional bureaucracy or federal funding restrictions inherent to more traditional randomized controlled trial design, ACES might provide data that bridge the gap between efficacy and effectiveness.
ACES was funded by Radicle Science. Dr. Chen is cofounder and CEO of Radicle Science. Dr. Russo and Dr. Strickland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Interim findings from Advancing CBD Education and Science, a 100% virtual, open label, randomized, controlled trial, show study participants experienced various degrees of “clinically meaningful” improvements in sleep quality, anxiety, and pain.
“ACES is the largest clinical trial ever conducted on commercially available CBD products and provides first-of-its-kind real world evidence into what conditions users may experience benefit from CBD usage, whether these benefits are clinically meaningful, what attributes of CBD products may impact health outcomes, and what side effects may occur,” study coinvestigator Jessica Saleska, PhD, MPH, director of research at Radicle Science, the company that conducted the study, told this news organization.
Scant evidence
Despite the growing market size of commercially available CBD products “there is still scant data on the effectiveness of over-the-counter cannabinoid products due to the cost, speed, and scale limitations of the current approach to scientific research,” Jeff Chen, MD, MBA, cofounder and CEO of Radicle Science, told this news organization.
One of the study’s goals, said Ethan Russo, MD, a neurologist, founder/CEO of CReDO Science, and scientific adviser for Radicle, is to help consumers make informed decisions before purchasing and using commercially available oral CBD products.
Designed to eliminate all physical infrastructure, which minimizes costs and facilitates faster execution, ACES was conducted much like a phase 4 clinical trial, collating real-world data gathered over 4 weeks.
“The process that Radicle scientists [have] advanced is sort of a crowdsourcing approach to doing clinical science,” Dr. Russo said. “Hopefully, there is going to be a considerable amount of data generated that [will] affect people’s buying options.”
The study also aimed to evaluate product attributes, including composition, mode of use, dosage, dosage timing and frequency, and their correlation to degrees of outcomes.
Dr. Russo explained why product composition is an important factor, especially when dealing with CBD. “What happens with any given [CBD] preparation is going to be totally a function of other components, if any.
“For example, there’s this mistaken notion that cannabidiol is sedating; it is not. Pure cannabidiol is stimulating in low and moderate amounts. Where the confusion has arisen is that the early chemovars containing cannabidiol were also predominant in myrcene, the sedating terpene, [thereby] creating this misimpression that it is good for sleep,” he added.
However, CBD might also affect sleep by reducing anxiety that interferes with it. “What’s clear is that cannabidiol is an antianxiety agent, if you have a sufficient dose,” Dr. Russo said.
The 4-week study included 2,704 participants aged 21 years and older, self-reporting anxiety, chronic pain, or sleep disturbances as a primary reason for taking CBD. Study participants were randomly assigned to receive 1 of 13 commercially available oral CBD extracts.
Participants were allocated to 1 of 14 cohorts, comprising 13 treatment groups with 208 participants each who received a single CBD product, or a wait-list control group of 296 participants who received product at the study’s end.
The primary outcome focused on “clinically meaningful” changes, which were defined as “distinct and palpable improvements in quality of life through improvements in respective health outcomes.”
Secondary outcomes included changes in sleep, anxiety, and pain based on several validated indices, including the PROMIS (Patient-Reported Outcome Measurement Information System) Sleep Short Form; the PROMIS Anxiety Scale; the Patient Global Impression of Change; the Pain, Enjoyment, General Activity scale; and the General Anxiety Disorder–7 scale.
The interim study results are promising, with participants reporting, on average, a 71% improvement in well-being. Additionally, 63% reported clinically meaningful improvements in anxiety, and 61% in sleep quality. The CBD products provided smaller benefits in pain management, with less than half (47%) experiencing meaningful improvements.
In addition to improvement in sleep, pain, and anxiety, these data highlight how rapidly benefits occurred; most were realized during the first week of the study, with up to 61% of treatment group participants reporting a therapeutic effect within 1-4 hours of taking their assigned product.
Overcoming the placebo effect
Commenting on the research, Justin Strickland, PhD, an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, who was not involved in the research, said without knowing a lot about the pharmacology of the products being tested, early dramatic improvements in these measures, such as sleep impairment, are common.
“There are some data to suggest that there is an expectancy effect when we talk about the therapeutic benefit of cannabinoid products, (i.e., when someone has the expectation that they are going to experience a stronger effect) but this is true of any drug in an open label trial,” Dr. Strickland added.
Dr. Russo took the point a step further. “It’s getting near impossible to look at cannabinoid compounds, even with randomized, controlled trials because of the burgeoning placebo responses. When you couple it with the fact that consumers have the mistaken notion that cannabis-based drugs are miraculous, the expectations are so high that everyone thinks that they’re on the real stuff, even if it’s a placebo group.”
Still, both Dr. Strickland and Dr. Russo highlighted the fact that ACES mirrors real-world experience, which will they hope will inform the use of CBD and CBD-based preparations moving forward. By removing certain barriers like institutional bureaucracy or federal funding restrictions inherent to more traditional randomized controlled trial design, ACES might provide data that bridge the gap between efficacy and effectiveness.
ACES was funded by Radicle Science. Dr. Chen is cofounder and CEO of Radicle Science. Dr. Russo and Dr. Strickland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Orally dissolving buprenorphine tied to severe tooth decay, FDA warns
Orally dissolving medications containing buprenorphine are linked to severe dental problems, including total tooth loss, the U.S. Food and Drug Administration warns in a safety communication.
The oral side effects of these medications, which are used to treat opioid use disorder (OUD) and pain, include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss.
Multiple cases have been reported even in patients with no history of dental problems.
The FDA is adding a warning about the risk of dental problems to the prescribing information and the patient medication guide for all buprenorphine-containing medicines dissolved in the mouth.
The FDA emphasizes, however, that buprenorphine remains “an important treatment option for OUD and pain, and the benefits of these medicines clearly outweigh the risks.”
More than 300 reported cases
Buprenorphine was approved in 2002 as a sublingual tablet, and in 2015 as a film to be placed inside the cheek to treat pain. Both delivery methods have been associated with dental problems.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems associated with orally dissolving buprenorphine, including 131 classified as serious.
There may be other cases, the FDA says, as this represents only cases reported to the FDA or published in the medical literature.
, but those as young as 18 years old were also affected.
Most cases occurred in patients using the medicines for OUD; however, 28 cases of dental problems occurred in patients using it to treat pain.
In 26 cases, patients had no prior history of dental problems. Some dental problems developed as soon as 2 weeks after treatment began; the median time to diagnosis was about 2 years after starting treatment.
Among all 305 cases reported, 113 involved two or more teeth.
The most common treatment for the dental problems was tooth extraction/removal, which was reported in 71 cases. Other cases required root canals, dental surgery, and other procedures such as crowns and implants.
Recommendations
The FDA says health care providers should counsel patients that severe and extensive tooth decay, tooth loss, and tooth fracture have been reported with the use of transmucosal buprenorphine-containing medicines and emphasize the importance of visiting their dentist to closely monitor their teeth.
Patients should be counseled to continue taking buprenorphine medications as prescribed and not stop suddenly without first talking to their health care provider, as this could lead to serious consequences, including relapse, misuse or abuse of other opioids, overdose, and death.
Patients are also being advised to take extra steps to help lessen the risk of serious dental problems.
Patients should also be educated on strategies to maintain or improve oral health while taking transmucosal buprenorphine medicines.
Counsel them that after the medicine is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth, as the FDA advises. This will allow time for the mouth to gradually return to oral homeostasis and avoid any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist visit soon after starting the medicine.
Dental problems related to transmucosal buprenorphine-containing medicines should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Orally dissolving medications containing buprenorphine are linked to severe dental problems, including total tooth loss, the U.S. Food and Drug Administration warns in a safety communication.
The oral side effects of these medications, which are used to treat opioid use disorder (OUD) and pain, include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss.
Multiple cases have been reported even in patients with no history of dental problems.
The FDA is adding a warning about the risk of dental problems to the prescribing information and the patient medication guide for all buprenorphine-containing medicines dissolved in the mouth.
The FDA emphasizes, however, that buprenorphine remains “an important treatment option for OUD and pain, and the benefits of these medicines clearly outweigh the risks.”
More than 300 reported cases
Buprenorphine was approved in 2002 as a sublingual tablet, and in 2015 as a film to be placed inside the cheek to treat pain. Both delivery methods have been associated with dental problems.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems associated with orally dissolving buprenorphine, including 131 classified as serious.
There may be other cases, the FDA says, as this represents only cases reported to the FDA or published in the medical literature.
, but those as young as 18 years old were also affected.
Most cases occurred in patients using the medicines for OUD; however, 28 cases of dental problems occurred in patients using it to treat pain.
In 26 cases, patients had no prior history of dental problems. Some dental problems developed as soon as 2 weeks after treatment began; the median time to diagnosis was about 2 years after starting treatment.
Among all 305 cases reported, 113 involved two or more teeth.
The most common treatment for the dental problems was tooth extraction/removal, which was reported in 71 cases. Other cases required root canals, dental surgery, and other procedures such as crowns and implants.
Recommendations
The FDA says health care providers should counsel patients that severe and extensive tooth decay, tooth loss, and tooth fracture have been reported with the use of transmucosal buprenorphine-containing medicines and emphasize the importance of visiting their dentist to closely monitor their teeth.
Patients should be counseled to continue taking buprenorphine medications as prescribed and not stop suddenly without first talking to their health care provider, as this could lead to serious consequences, including relapse, misuse or abuse of other opioids, overdose, and death.
Patients are also being advised to take extra steps to help lessen the risk of serious dental problems.
Patients should also be educated on strategies to maintain or improve oral health while taking transmucosal buprenorphine medicines.
Counsel them that after the medicine is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth, as the FDA advises. This will allow time for the mouth to gradually return to oral homeostasis and avoid any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist visit soon after starting the medicine.
Dental problems related to transmucosal buprenorphine-containing medicines should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Orally dissolving medications containing buprenorphine are linked to severe dental problems, including total tooth loss, the U.S. Food and Drug Administration warns in a safety communication.
The oral side effects of these medications, which are used to treat opioid use disorder (OUD) and pain, include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss.
Multiple cases have been reported even in patients with no history of dental problems.
The FDA is adding a warning about the risk of dental problems to the prescribing information and the patient medication guide for all buprenorphine-containing medicines dissolved in the mouth.
The FDA emphasizes, however, that buprenorphine remains “an important treatment option for OUD and pain, and the benefits of these medicines clearly outweigh the risks.”
More than 300 reported cases
Buprenorphine was approved in 2002 as a sublingual tablet, and in 2015 as a film to be placed inside the cheek to treat pain. Both delivery methods have been associated with dental problems.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems associated with orally dissolving buprenorphine, including 131 classified as serious.
There may be other cases, the FDA says, as this represents only cases reported to the FDA or published in the medical literature.
, but those as young as 18 years old were also affected.
Most cases occurred in patients using the medicines for OUD; however, 28 cases of dental problems occurred in patients using it to treat pain.
In 26 cases, patients had no prior history of dental problems. Some dental problems developed as soon as 2 weeks after treatment began; the median time to diagnosis was about 2 years after starting treatment.
Among all 305 cases reported, 113 involved two or more teeth.
The most common treatment for the dental problems was tooth extraction/removal, which was reported in 71 cases. Other cases required root canals, dental surgery, and other procedures such as crowns and implants.
Recommendations
The FDA says health care providers should counsel patients that severe and extensive tooth decay, tooth loss, and tooth fracture have been reported with the use of transmucosal buprenorphine-containing medicines and emphasize the importance of visiting their dentist to closely monitor their teeth.
Patients should be counseled to continue taking buprenorphine medications as prescribed and not stop suddenly without first talking to their health care provider, as this could lead to serious consequences, including relapse, misuse or abuse of other opioids, overdose, and death.
Patients are also being advised to take extra steps to help lessen the risk of serious dental problems.
Patients should also be educated on strategies to maintain or improve oral health while taking transmucosal buprenorphine medicines.
Counsel them that after the medicine is completely dissolved, the patient should take a large sip of water, swish it gently around the teeth and gums, swallow, and wait at least 1 hour before brushing their teeth, as the FDA advises. This will allow time for the mouth to gradually return to oral homeostasis and avoid any mechanical damage that may occur due to brushing.
The FDA also advises that patients tell their provider about any history of tooth problems, including cavities, and schedule a dentist visit soon after starting the medicine.
Dental problems related to transmucosal buprenorphine-containing medicines should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
One doctor’s psychedelic journey to confront his cancer
Pradeep Bansal considered the five capsules he was about to swallow. Together they made up a 25-mg dose of a substance that, in another setting, could have landed him in federal prison.
The substance was psilocybin, the active ingredient in magic mushrooms. To be more exact, it was a synthetic form of psilocybin called COMP360, made to pharmaceutical standards by a company called COMPASS Pathways. He was taking it as part of an Food and Drug Administration–approved clinical study on mental health therapy for people with cancer.
Dr. Bansal, a New York gastroenterologist, was far more comfortable giving medical treatment than receiving it. But he was getting used to it.
He had already been through surgery and a number of other treatments to address the physical aspects of his cancer. The psilocybin was to address the mental aspects – the crushing anxiety and depression that had stuck with him after his diagnosis.
Dr. Bansal did not arrive at this moment lightly.
“I was extremely skeptical going into this process,” said Dr. Bansal, who during a long medical career had looked with distrust and even disdain at alternative therapies.
“I don’t have much patience for holistic medicine, homeopathy, acupuncture, or alternative medicines with claims of spiritual upliftment or altered states of mind.”
But Bansal had done his homework on psilocybin and was impressed.
according to studies published in 2011, 2014, and 2016.
One study from Johns Hopkins University tracked the effects of a single guided dose of psilocybin in terminal cancer patients with anxiety and depression. More than 80% had a “significant decrease” in symptoms – even 6 months after treatment – with more than 60% of the group remaining in the normal mood range.
For the study Dr. Bansal joined, there had been weeks of screening and consultation and preparation in a strictly controlled scientific trial.
And yet, even with all that he had learned, even with his psychiatrist-guide by his side, he was afraid. Afraid of what he might experience under the powerful effects of psilocybin. And afraid that this was all a misguided waste of time – that his mental angst would still be there when it was all over.
He knew that psilocybin, like other psychedelic substances, could take you on a “trip” – could remove you, at least for a time, from normal conscious experience.
Maybe he would feel “funny,” he thought. Maybe he would have some hallucinations. But how would that change the reality of his cancer? How would it lift the black dread and anxiety he felt about his future?
Stuck in a dark place
Dr. Bansal had first noticed blood in his urine – a lot of it – in September 2019.
Two months later, doctors diagnosed cancer in his right kidney. He would need surgery to remove the kidney and surrounding lymph nodes (an operation called radical nephrectomy).
It was a shock, said Dr. Bansal. But the diagnosis and the surgery happened so quickly that he hardly had time to think. And treatment results seemed good. The cancer was only in stage I and the CT scans showed no signs of cancer after surgery.
“We were so relieved. Everyone was so happy,” Dr. Bansal said. “They didn’t even give me chemotherapy after surgery because it seemed so early.”
But a routine scan in June 2020 revealed more cancer in his lung. Within a couple of months, it was in his bladder too.
“It was devastating,” Dr. Bansal said. “I went from thinking I was healthy again to stage IV cancer.”
As doctors scheduled surgery to remove part of his lung, Dr. Bansal started on painful immunotherapy (BCG therapy) for his bladder.
At this point, from a psychological standpoint, Dr. Bansal was reeling. As a doctor, he knew all too well the meaning of stage IV cancer.
With two adult children and a grandchild on the way, Dr. Bansal had been looking forward to retirement with his wife of almost 40 years. “Suddenly, I wasn’t sure I was going to last that long,” Bansal recalled. “I was in a very dark place. I was very anxious, very depressed from lack of sleep.”
He saw a therapist about his cancer diagnosis and maintained his regular meditation practice at home. He hired a personal trainer and tried to focus on any good news that he got about his treatment.
Those things helped, but not enough.
The basic facts were inescapable. His cancer might end everything. He couldn’t stop thinking about it. And then he couldn’t stop thinking about how he couldn’t stop thinking about it.
If the worst happened, he didn’t want to spend his last days in a state of such relentless existential angst. And it wasn’t just for himself. He wanted to be strong and mentally present for his family and his loved ones and his patients.
As he searched for something to ease his mental anguish, Dr. Bansal recalled some psychedelic research on end-of-life anxiety and depression that he’d read about in Michael Pollan’s book on psychedelics, “How to Change Your Mind” (New York, Penguin Press, 2018).
The studies were small and the research was new, but Dr. Bansal was impressed enough with the results to take a chance. He called a lead researcher of one of the studies, a fellow New York doctor, and eventually found himself accepted into a new study.
Starting the journey
By the time Dr. Bansal arrived at the Bill Richards Center for Healing at the Aquilino Cancer Center in Rockville, Md., he had already been through weeks of screening.
The main requirements for the study were a cancer diagnosis and a measurable level of depression. But study participants also had to be physically fit enough to handle the medication, and psychologically free from a personal or family history of psychosis or schizophrenia. (The study also required participants to slowly wean themselves from medications like SSRIs for depression or antianxiety medications under the strict supervision of a qualified doctor.)
Dr. Bansal’s week of treatment began almost immediately on arrival at Aquilino. Everything was carefully choreographed but not rushed. From Monday to Wednesday, doctors followed his physical health with exams, ECGs, and blood work. And most importantly, they began to prepare him for the “dosing session” on Thursday when he would take the psilocybin.
This is the careful crafting of “set and setting” stressed in so many psychedelic therapies. “Set” refers to your mindset going into the drug experience. “Setting” is the space and people around you when the drug sends you into an altered state of consciousness.
Dr. Bansal met several times with at least three therapists in the days leading up to his dosing. He attended 4-plus hours of therapist-led group sessions with other people who would get a dosing on the same day. Together, they talked about what to expect during the experience and what to do in the face of fear or panic.
He connected with a therapist who would be his personal guide. Dr. Bansal’s therapist was a military psychiatrist with over 30 years’ experience.
“He was there with me from day 1, and so we established a relationship,” Dr. Bansal said.
“He asked me a lot of personal background history – you know, my religious convictions, aspirations, all those things.”
“Trust and let go,” was a kind of mantra for the treatment repeated by his guide and other doctors.
For Dr. Bansal, a doctor and scientist accustomed to using hard facts rather than touchy-feely slogans to navigate the care of patients, it was an adjustment, to say the least.
But he did his best to set aside his doubts and embrace the journey he was about to take.
The day of the trip
Thursday morning finally arrived. The setting of the dosing room was warm and welcoming, more like a cozy home study than a hospital room.
This matters more than you might think. First, because it’s important that you feel safe, open, and comfortable enough to let go and enter into a therapeutic process. But also because though rare, it’s possible – especially with psilocybin – for people to lose track of where they are and what they’re doing and put themselves or others in danger.
The dose, 25 mg, had been carefully calibrated to induce a psychedelic experience sufficient for therapy. Much less than that, say 10 mg, isn’t enough for most people to enter this state. A double dose, 50 mg, though not physically unsafe, may leave you too incoherent to have the useful insights key to therapeutic value.
A doctor, the lead investigator of the study, brought the five capsules into the room in an intricately carved crucible with a small ceremonial cup that held the water with which to take it.
“It was very solemn,” Dr. Bansal said. “He sat down with me in a very calming way.”
The doctor said: “Don’t worry about it. Just trust and let go.”
And that’s just what he did.
Dr. Bansal swallowed the capsules and lay down. The doctor quietly left the room so that Dr. Bansal and his psychiatrist guide could begin their session together.
Special eye shades kept him in the pitch dark whether his eyes were open or closed. Headphones streamed a curated musical playlist – much of it Western classical like Strauss, Bach, Mozart, and Beethoven – but also modern electronica and other music from cultures around the globe.
Dr. Bansal would remain here, with his therapist-guide by his side, in largely this same position, for the next 7-and-a-half hours.
It took about 45 minutes for the medication to kick in.
The investigator
The doctor who brought the capsules into the dosing room was Manish Agrawal, MD, codirector of clinical research at the Aquilino Cancer Center and lead investigator of the study.
Dr. Agrawal trained at the National Cancer Institute and practiced for many years as an oncologist before developing an interest in psychedelic therapies. It was his work with cancer patients that drew him to psychedelics in the first place.
He had seen too many of his patients mentally wrecked by a cancer diagnosis, and he often felt helpless to comfort them.
“You take care of the physical aspects of the cancer, right? You talk about side effects and recommend another scan to look for recurrence.”
“But what about the psychological effects?”
They can be very serious and too often go ignored, said Dr. Agrawal. Your plans for the future suddenly become moot. You may be concerned about your ability to work or worried about the pain and suffering and financial strain that might be ahead for both you and your family. And to top it all off, you’re staring into the face of your own mortality.
So it’s no wonder, said Dr. Agrawal, that many people develop clinical levels of anxiety and depression after a cancer diagnosis.
Like Dr. Bansal, Dr. Agrawal had been impressed by early studies on psilocybin-assisted therapies for end-of-life anxiety and depression. He had tried other approaches – support groups, one-on-one therapy, religious counselors, psychiatrist-prescribed medication – but he was never really happy with the results.
To Dr. Agrawal, psilocybin-assisted therapy was the first thing that looked like it could really make a difference.
And so after his psychedelic certification at the California Institute of Integral Studies, Dr. Agrawal was determined to change his approach.
The result was the Bill Richards Center for Healing at Aquilino Cancer Center, built specifically to study psychedelic-assisted therapies for psychological distress in people with cancer. The mission of the center is to help develop safe, FDA-approved psychedelic therapies for the mental health of cancer patients, and, once approved, provide a state-of-the-art facility and staff to administer those treatments.
A trip into the unknown
Back in the dosing room, Dr. Bansal was starting to feel the effects of the medication. As the psilocybin kicked in, spectacular images swirled.
“It was as if a million stained glass windows had suddenly come to life and were dancing in front of my vision,” Dr. Bansal said.
There were moving landscapes and intricate swirling patterns and massive stages in the sky where he saw orchestras playing the music he was hearing.
Dr. Bansal saw himself being crushed by a huge machine and buried, dead, in the Earth. He died and returned to life several times, glided over the top of New York City with the skyscrapers just below him, and took in the vision of the entire universe.
“I saw this expanse of the sky that was limitless. And there was this prehistoric reptile creature that spanned galaxies in the sky ahead of me who was dying. I said: ‘My God, the universe is dying,’ but then after a few moments, the universe came to life again in a burst of stars exploding.”
All the while, Dr. Bansal said, he was well aware that it was simply his mind creating these images, thoughts, and ideas. He knew he was in a safe room wearing eyeshades and headphones.
And yet, he says, it felt true. “The images and feelings are so powerful that you cannot help but believe they are in some way a part of reality.”
“At one point, I saw this giant Ferris wheel coming towards me and it was full of giant crabs, clicking and clacking their pincers. And my brain told me: ‘That’s my cancer!’ ”
Dr. Bansal was terrified. But he and his therapist had arranged a system of signals before the session. “If I was feeling afraid, I would hold his hand and if I had other issues, I would raise my hand. If I was feeling good, I would give him a thumbs up.”
Dr. Bansal reached out to his therapist and grasped his hand. “I said, ‘My cancer is coming at me!’ ”
His therapist was clear about what to do: Stand firm and walk toward it.
“That’s what they tell you: If you see anything frightening, you face it. And that’s the whole point of this exercise. And so, I stood and walked forward, and it just blew off in a puff of smoke.”
A state of peace
Around 3 hours into the experience, Dr. Bansal started to feel an immense sense of peace, happiness, and even comfort.
“I felt like I was watching a movie or a multidimensional slideshow. I was also a part of the movie. I felt like I could tell my mind what I wanted to see, and it would show it to me. It’s almost like you can mold your own visions. It was mystical.”
After about 8 hours, as the effects of the drug wore off, Dr. Bansal removed his eyeshades and headphones. He was completely drained.
“Even though I was lying down on my back for 7 hours, I felt like I had been run over by a truck. I was exhausted beyond belief physically and mentally.”
This was partly because of the fact that he hadn’t eaten much during the session. But mostly, said Dr. Bansal, it was because of the searing emotional intensity of the experience.
After the journey
It’s hard to put into words, said Dr. Bansal, what this treatment has done for his life. He feels as if he has stumbled onto something very precious that had been right in front of him all along. He wrote of his change in perspective almost obsessively in his journal in the days and weeks after treatment. One passage reads:
“It seems that, as time is passing on, I’m becoming more relaxed and hopeful, more calm, and at peace. Family has become even more important to me now. Money, politics, material gains, alcohol, seem less important.”
And yet there was nothing “easy” about the experience. In fact, in some ways the experience demanded more from him. “I feel I need to be more compassionate and considerate – less irritable and angry, more understanding of others’ needs. I feel I need to be a better human being, a better patient, a better father, and a better doctor for my patients.”
The experience, he said, gave him something far more important than mere ease. It gave him a sense of meaning.
From his journal:
“I died, and I was reborn. If I survived this, then I can face anything and anybody in the cosmic scheme. I can become part of it.
“How many sorrows in the universe? My cancer is nothing. Life does not end with the end of life. What was will be again. Eternally.”
That’s not an unusual response, according to the namesake of the Bill Richards Center for Healing. Bill Richards, PhD, has worked in the world of psychedelic-assisted psychotherapy since 1963.
A psychologist with decades of experience, Dr. Richards and colleagues figure that, with few possible exceptions, he has helped treat more people with psychedelic therapies than anyone alive in Western medicine today. At Aquilino, he works directly with patients and oversees the therapy protocol that goes along with the psilocybin dosing sessions.
“It’s inspiring,” Dr. Richards said.
“You meet someone who’s very depressed and scared and isolating from family and having all kinds of physical complaints. And a few days later, you talk to the same person and they have a whole new lease on life.”
And the positive effects can extend deep into the family system, he said.
After psilocybin treatment, said Dr. Richards, the person with cancer can become a kind of social worker for the family. They’re often far better able to talk about death and loss and even money and family issues than their loved ones. It’s not uncommon after treatment to see the resolution of years-old resentments or grievances that have dogged a family for many years.
Plus, said Dr. Richards, the cancer patient often ends up as a kind model to other family members for how to approach death. “They can demonstrate how to live fully – right to the last breath – which is a real gift because those relatives and loved ones have to die someday too, you know.”
At 80 years old, Dr. Richards is still in active practice and hopes to spend the rest of his days working with people in end-of-life care.
After the experience
Psychedelic-assisted therapy does not end with the dosing session. Integration sessions, where you discuss what happened during the dosing session, are a key part of most treatments.
The goal is to help participants absorb and “integrate” their experience. It typically happens over two or more sessions of 60-90 minutes with a therapist. In some cases, the therapist may invite a significant other to join in the integration process.
Dr. Agrawal’s trial at the Bill Richards center added something new: group therapy. Not only did Dr. Bansal meet with his therapist, he also met with a group of three other people in the trial who had their dosing the same day.
The point, said Dr. Agrawal, is to try and determine the effect of the group on the therapy. After their private dosing sessions, they come back together to discuss their experiences.
“After the psilocybin, they feel like they’ve been to war together,” Dr. Agrawal said. “There is this profound openness and connection. They feel able to share things with each other that they wouldn’t with other people.”
It will take some time to figure out how the group affects the overall outcome, but Dr. Bansal thinks it was integral to the success of his treatment.
In fact, he continues to meet regularly with his therapy group, even though it’s long since past the requirements of the study.
Pradeep 2.0
Dr. Bansal still has tough days with his cancer. Recently, immunotherapy treatment for his bladder caused side effects – pain, bleeding, fever, and chills – for most of the night. He felt like he was “passing razor blades” when he peed.
“And yet it was somehow okay,” he said. “It was only pain.”
“It’s as if there is a part of me that is watching myself objectively, going through the painful process of treatments saying: ‘It’s all right. I will be with you through this journey, through this experience. Don’t worry.’”
Months after taking that one dose, Dr. Bansal still calls it as “the single most powerful experience of my life.”
The change in his mental outlook, Dr. Bansal said, was profound, particularly in regard to his cancer.
“I understood that I still had cancer and that it could kill me in a few weeks, or months, or years. But my perspective had shifted.”
Dr. Bansal was as surprised as anyone. “Had somebody told me going into this that I would come out a transformed being or a person with a completely different perspective on life, I would never have believed it.”
He even named his new outlook. “I call it Pradeep 2.0.”
A version of this article first appeared on WebMD.com.
Pradeep Bansal considered the five capsules he was about to swallow. Together they made up a 25-mg dose of a substance that, in another setting, could have landed him in federal prison.
The substance was psilocybin, the active ingredient in magic mushrooms. To be more exact, it was a synthetic form of psilocybin called COMP360, made to pharmaceutical standards by a company called COMPASS Pathways. He was taking it as part of an Food and Drug Administration–approved clinical study on mental health therapy for people with cancer.
Dr. Bansal, a New York gastroenterologist, was far more comfortable giving medical treatment than receiving it. But he was getting used to it.
He had already been through surgery and a number of other treatments to address the physical aspects of his cancer. The psilocybin was to address the mental aspects – the crushing anxiety and depression that had stuck with him after his diagnosis.
Dr. Bansal did not arrive at this moment lightly.
“I was extremely skeptical going into this process,” said Dr. Bansal, who during a long medical career had looked with distrust and even disdain at alternative therapies.
“I don’t have much patience for holistic medicine, homeopathy, acupuncture, or alternative medicines with claims of spiritual upliftment or altered states of mind.”
But Bansal had done his homework on psilocybin and was impressed.
according to studies published in 2011, 2014, and 2016.
One study from Johns Hopkins University tracked the effects of a single guided dose of psilocybin in terminal cancer patients with anxiety and depression. More than 80% had a “significant decrease” in symptoms – even 6 months after treatment – with more than 60% of the group remaining in the normal mood range.
For the study Dr. Bansal joined, there had been weeks of screening and consultation and preparation in a strictly controlled scientific trial.
And yet, even with all that he had learned, even with his psychiatrist-guide by his side, he was afraid. Afraid of what he might experience under the powerful effects of psilocybin. And afraid that this was all a misguided waste of time – that his mental angst would still be there when it was all over.
He knew that psilocybin, like other psychedelic substances, could take you on a “trip” – could remove you, at least for a time, from normal conscious experience.
Maybe he would feel “funny,” he thought. Maybe he would have some hallucinations. But how would that change the reality of his cancer? How would it lift the black dread and anxiety he felt about his future?
Stuck in a dark place
Dr. Bansal had first noticed blood in his urine – a lot of it – in September 2019.
Two months later, doctors diagnosed cancer in his right kidney. He would need surgery to remove the kidney and surrounding lymph nodes (an operation called radical nephrectomy).
It was a shock, said Dr. Bansal. But the diagnosis and the surgery happened so quickly that he hardly had time to think. And treatment results seemed good. The cancer was only in stage I and the CT scans showed no signs of cancer after surgery.
“We were so relieved. Everyone was so happy,” Dr. Bansal said. “They didn’t even give me chemotherapy after surgery because it seemed so early.”
But a routine scan in June 2020 revealed more cancer in his lung. Within a couple of months, it was in his bladder too.
“It was devastating,” Dr. Bansal said. “I went from thinking I was healthy again to stage IV cancer.”
As doctors scheduled surgery to remove part of his lung, Dr. Bansal started on painful immunotherapy (BCG therapy) for his bladder.
At this point, from a psychological standpoint, Dr. Bansal was reeling. As a doctor, he knew all too well the meaning of stage IV cancer.
With two adult children and a grandchild on the way, Dr. Bansal had been looking forward to retirement with his wife of almost 40 years. “Suddenly, I wasn’t sure I was going to last that long,” Bansal recalled. “I was in a very dark place. I was very anxious, very depressed from lack of sleep.”
He saw a therapist about his cancer diagnosis and maintained his regular meditation practice at home. He hired a personal trainer and tried to focus on any good news that he got about his treatment.
Those things helped, but not enough.
The basic facts were inescapable. His cancer might end everything. He couldn’t stop thinking about it. And then he couldn’t stop thinking about how he couldn’t stop thinking about it.
If the worst happened, he didn’t want to spend his last days in a state of such relentless existential angst. And it wasn’t just for himself. He wanted to be strong and mentally present for his family and his loved ones and his patients.
As he searched for something to ease his mental anguish, Dr. Bansal recalled some psychedelic research on end-of-life anxiety and depression that he’d read about in Michael Pollan’s book on psychedelics, “How to Change Your Mind” (New York, Penguin Press, 2018).
The studies were small and the research was new, but Dr. Bansal was impressed enough with the results to take a chance. He called a lead researcher of one of the studies, a fellow New York doctor, and eventually found himself accepted into a new study.
Starting the journey
By the time Dr. Bansal arrived at the Bill Richards Center for Healing at the Aquilino Cancer Center in Rockville, Md., he had already been through weeks of screening.
The main requirements for the study were a cancer diagnosis and a measurable level of depression. But study participants also had to be physically fit enough to handle the medication, and psychologically free from a personal or family history of psychosis or schizophrenia. (The study also required participants to slowly wean themselves from medications like SSRIs for depression or antianxiety medications under the strict supervision of a qualified doctor.)
Dr. Bansal’s week of treatment began almost immediately on arrival at Aquilino. Everything was carefully choreographed but not rushed. From Monday to Wednesday, doctors followed his physical health with exams, ECGs, and blood work. And most importantly, they began to prepare him for the “dosing session” on Thursday when he would take the psilocybin.
This is the careful crafting of “set and setting” stressed in so many psychedelic therapies. “Set” refers to your mindset going into the drug experience. “Setting” is the space and people around you when the drug sends you into an altered state of consciousness.
Dr. Bansal met several times with at least three therapists in the days leading up to his dosing. He attended 4-plus hours of therapist-led group sessions with other people who would get a dosing on the same day. Together, they talked about what to expect during the experience and what to do in the face of fear or panic.
He connected with a therapist who would be his personal guide. Dr. Bansal’s therapist was a military psychiatrist with over 30 years’ experience.
“He was there with me from day 1, and so we established a relationship,” Dr. Bansal said.
“He asked me a lot of personal background history – you know, my religious convictions, aspirations, all those things.”
“Trust and let go,” was a kind of mantra for the treatment repeated by his guide and other doctors.
For Dr. Bansal, a doctor and scientist accustomed to using hard facts rather than touchy-feely slogans to navigate the care of patients, it was an adjustment, to say the least.
But he did his best to set aside his doubts and embrace the journey he was about to take.
The day of the trip
Thursday morning finally arrived. The setting of the dosing room was warm and welcoming, more like a cozy home study than a hospital room.
This matters more than you might think. First, because it’s important that you feel safe, open, and comfortable enough to let go and enter into a therapeutic process. But also because though rare, it’s possible – especially with psilocybin – for people to lose track of where they are and what they’re doing and put themselves or others in danger.
The dose, 25 mg, had been carefully calibrated to induce a psychedelic experience sufficient for therapy. Much less than that, say 10 mg, isn’t enough for most people to enter this state. A double dose, 50 mg, though not physically unsafe, may leave you too incoherent to have the useful insights key to therapeutic value.
A doctor, the lead investigator of the study, brought the five capsules into the room in an intricately carved crucible with a small ceremonial cup that held the water with which to take it.
“It was very solemn,” Dr. Bansal said. “He sat down with me in a very calming way.”
The doctor said: “Don’t worry about it. Just trust and let go.”
And that’s just what he did.
Dr. Bansal swallowed the capsules and lay down. The doctor quietly left the room so that Dr. Bansal and his psychiatrist guide could begin their session together.
Special eye shades kept him in the pitch dark whether his eyes were open or closed. Headphones streamed a curated musical playlist – much of it Western classical like Strauss, Bach, Mozart, and Beethoven – but also modern electronica and other music from cultures around the globe.
Dr. Bansal would remain here, with his therapist-guide by his side, in largely this same position, for the next 7-and-a-half hours.
It took about 45 minutes for the medication to kick in.
The investigator
The doctor who brought the capsules into the dosing room was Manish Agrawal, MD, codirector of clinical research at the Aquilino Cancer Center and lead investigator of the study.
Dr. Agrawal trained at the National Cancer Institute and practiced for many years as an oncologist before developing an interest in psychedelic therapies. It was his work with cancer patients that drew him to psychedelics in the first place.
He had seen too many of his patients mentally wrecked by a cancer diagnosis, and he often felt helpless to comfort them.
“You take care of the physical aspects of the cancer, right? You talk about side effects and recommend another scan to look for recurrence.”
“But what about the psychological effects?”
They can be very serious and too often go ignored, said Dr. Agrawal. Your plans for the future suddenly become moot. You may be concerned about your ability to work or worried about the pain and suffering and financial strain that might be ahead for both you and your family. And to top it all off, you’re staring into the face of your own mortality.
So it’s no wonder, said Dr. Agrawal, that many people develop clinical levels of anxiety and depression after a cancer diagnosis.
Like Dr. Bansal, Dr. Agrawal had been impressed by early studies on psilocybin-assisted therapies for end-of-life anxiety and depression. He had tried other approaches – support groups, one-on-one therapy, religious counselors, psychiatrist-prescribed medication – but he was never really happy with the results.
To Dr. Agrawal, psilocybin-assisted therapy was the first thing that looked like it could really make a difference.
And so after his psychedelic certification at the California Institute of Integral Studies, Dr. Agrawal was determined to change his approach.
The result was the Bill Richards Center for Healing at Aquilino Cancer Center, built specifically to study psychedelic-assisted therapies for psychological distress in people with cancer. The mission of the center is to help develop safe, FDA-approved psychedelic therapies for the mental health of cancer patients, and, once approved, provide a state-of-the-art facility and staff to administer those treatments.
A trip into the unknown
Back in the dosing room, Dr. Bansal was starting to feel the effects of the medication. As the psilocybin kicked in, spectacular images swirled.
“It was as if a million stained glass windows had suddenly come to life and were dancing in front of my vision,” Dr. Bansal said.
There were moving landscapes and intricate swirling patterns and massive stages in the sky where he saw orchestras playing the music he was hearing.
Dr. Bansal saw himself being crushed by a huge machine and buried, dead, in the Earth. He died and returned to life several times, glided over the top of New York City with the skyscrapers just below him, and took in the vision of the entire universe.
“I saw this expanse of the sky that was limitless. And there was this prehistoric reptile creature that spanned galaxies in the sky ahead of me who was dying. I said: ‘My God, the universe is dying,’ but then after a few moments, the universe came to life again in a burst of stars exploding.”
All the while, Dr. Bansal said, he was well aware that it was simply his mind creating these images, thoughts, and ideas. He knew he was in a safe room wearing eyeshades and headphones.
And yet, he says, it felt true. “The images and feelings are so powerful that you cannot help but believe they are in some way a part of reality.”
“At one point, I saw this giant Ferris wheel coming towards me and it was full of giant crabs, clicking and clacking their pincers. And my brain told me: ‘That’s my cancer!’ ”
Dr. Bansal was terrified. But he and his therapist had arranged a system of signals before the session. “If I was feeling afraid, I would hold his hand and if I had other issues, I would raise my hand. If I was feeling good, I would give him a thumbs up.”
Dr. Bansal reached out to his therapist and grasped his hand. “I said, ‘My cancer is coming at me!’ ”
His therapist was clear about what to do: Stand firm and walk toward it.
“That’s what they tell you: If you see anything frightening, you face it. And that’s the whole point of this exercise. And so, I stood and walked forward, and it just blew off in a puff of smoke.”
A state of peace
Around 3 hours into the experience, Dr. Bansal started to feel an immense sense of peace, happiness, and even comfort.
“I felt like I was watching a movie or a multidimensional slideshow. I was also a part of the movie. I felt like I could tell my mind what I wanted to see, and it would show it to me. It’s almost like you can mold your own visions. It was mystical.”
After about 8 hours, as the effects of the drug wore off, Dr. Bansal removed his eyeshades and headphones. He was completely drained.
“Even though I was lying down on my back for 7 hours, I felt like I had been run over by a truck. I was exhausted beyond belief physically and mentally.”
This was partly because of the fact that he hadn’t eaten much during the session. But mostly, said Dr. Bansal, it was because of the searing emotional intensity of the experience.
After the journey
It’s hard to put into words, said Dr. Bansal, what this treatment has done for his life. He feels as if he has stumbled onto something very precious that had been right in front of him all along. He wrote of his change in perspective almost obsessively in his journal in the days and weeks after treatment. One passage reads:
“It seems that, as time is passing on, I’m becoming more relaxed and hopeful, more calm, and at peace. Family has become even more important to me now. Money, politics, material gains, alcohol, seem less important.”
And yet there was nothing “easy” about the experience. In fact, in some ways the experience demanded more from him. “I feel I need to be more compassionate and considerate – less irritable and angry, more understanding of others’ needs. I feel I need to be a better human being, a better patient, a better father, and a better doctor for my patients.”
The experience, he said, gave him something far more important than mere ease. It gave him a sense of meaning.
From his journal:
“I died, and I was reborn. If I survived this, then I can face anything and anybody in the cosmic scheme. I can become part of it.
“How many sorrows in the universe? My cancer is nothing. Life does not end with the end of life. What was will be again. Eternally.”
That’s not an unusual response, according to the namesake of the Bill Richards Center for Healing. Bill Richards, PhD, has worked in the world of psychedelic-assisted psychotherapy since 1963.
A psychologist with decades of experience, Dr. Richards and colleagues figure that, with few possible exceptions, he has helped treat more people with psychedelic therapies than anyone alive in Western medicine today. At Aquilino, he works directly with patients and oversees the therapy protocol that goes along with the psilocybin dosing sessions.
“It’s inspiring,” Dr. Richards said.
“You meet someone who’s very depressed and scared and isolating from family and having all kinds of physical complaints. And a few days later, you talk to the same person and they have a whole new lease on life.”
And the positive effects can extend deep into the family system, he said.
After psilocybin treatment, said Dr. Richards, the person with cancer can become a kind of social worker for the family. They’re often far better able to talk about death and loss and even money and family issues than their loved ones. It’s not uncommon after treatment to see the resolution of years-old resentments or grievances that have dogged a family for many years.
Plus, said Dr. Richards, the cancer patient often ends up as a kind model to other family members for how to approach death. “They can demonstrate how to live fully – right to the last breath – which is a real gift because those relatives and loved ones have to die someday too, you know.”
At 80 years old, Dr. Richards is still in active practice and hopes to spend the rest of his days working with people in end-of-life care.
After the experience
Psychedelic-assisted therapy does not end with the dosing session. Integration sessions, where you discuss what happened during the dosing session, are a key part of most treatments.
The goal is to help participants absorb and “integrate” their experience. It typically happens over two or more sessions of 60-90 minutes with a therapist. In some cases, the therapist may invite a significant other to join in the integration process.
Dr. Agrawal’s trial at the Bill Richards center added something new: group therapy. Not only did Dr. Bansal meet with his therapist, he also met with a group of three other people in the trial who had their dosing the same day.
The point, said Dr. Agrawal, is to try and determine the effect of the group on the therapy. After their private dosing sessions, they come back together to discuss their experiences.
“After the psilocybin, they feel like they’ve been to war together,” Dr. Agrawal said. “There is this profound openness and connection. They feel able to share things with each other that they wouldn’t with other people.”
It will take some time to figure out how the group affects the overall outcome, but Dr. Bansal thinks it was integral to the success of his treatment.
In fact, he continues to meet regularly with his therapy group, even though it’s long since past the requirements of the study.
Pradeep 2.0
Dr. Bansal still has tough days with his cancer. Recently, immunotherapy treatment for his bladder caused side effects – pain, bleeding, fever, and chills – for most of the night. He felt like he was “passing razor blades” when he peed.
“And yet it was somehow okay,” he said. “It was only pain.”
“It’s as if there is a part of me that is watching myself objectively, going through the painful process of treatments saying: ‘It’s all right. I will be with you through this journey, through this experience. Don’t worry.’”
Months after taking that one dose, Dr. Bansal still calls it as “the single most powerful experience of my life.”
The change in his mental outlook, Dr. Bansal said, was profound, particularly in regard to his cancer.
“I understood that I still had cancer and that it could kill me in a few weeks, or months, or years. But my perspective had shifted.”
Dr. Bansal was as surprised as anyone. “Had somebody told me going into this that I would come out a transformed being or a person with a completely different perspective on life, I would never have believed it.”
He even named his new outlook. “I call it Pradeep 2.0.”
A version of this article first appeared on WebMD.com.
Pradeep Bansal considered the five capsules he was about to swallow. Together they made up a 25-mg dose of a substance that, in another setting, could have landed him in federal prison.
The substance was psilocybin, the active ingredient in magic mushrooms. To be more exact, it was a synthetic form of psilocybin called COMP360, made to pharmaceutical standards by a company called COMPASS Pathways. He was taking it as part of an Food and Drug Administration–approved clinical study on mental health therapy for people with cancer.
Dr. Bansal, a New York gastroenterologist, was far more comfortable giving medical treatment than receiving it. But he was getting used to it.
He had already been through surgery and a number of other treatments to address the physical aspects of his cancer. The psilocybin was to address the mental aspects – the crushing anxiety and depression that had stuck with him after his diagnosis.
Dr. Bansal did not arrive at this moment lightly.
“I was extremely skeptical going into this process,” said Dr. Bansal, who during a long medical career had looked with distrust and even disdain at alternative therapies.
“I don’t have much patience for holistic medicine, homeopathy, acupuncture, or alternative medicines with claims of spiritual upliftment or altered states of mind.”
But Bansal had done his homework on psilocybin and was impressed.
according to studies published in 2011, 2014, and 2016.
One study from Johns Hopkins University tracked the effects of a single guided dose of psilocybin in terminal cancer patients with anxiety and depression. More than 80% had a “significant decrease” in symptoms – even 6 months after treatment – with more than 60% of the group remaining in the normal mood range.
For the study Dr. Bansal joined, there had been weeks of screening and consultation and preparation in a strictly controlled scientific trial.
And yet, even with all that he had learned, even with his psychiatrist-guide by his side, he was afraid. Afraid of what he might experience under the powerful effects of psilocybin. And afraid that this was all a misguided waste of time – that his mental angst would still be there when it was all over.
He knew that psilocybin, like other psychedelic substances, could take you on a “trip” – could remove you, at least for a time, from normal conscious experience.
Maybe he would feel “funny,” he thought. Maybe he would have some hallucinations. But how would that change the reality of his cancer? How would it lift the black dread and anxiety he felt about his future?
Stuck in a dark place
Dr. Bansal had first noticed blood in his urine – a lot of it – in September 2019.
Two months later, doctors diagnosed cancer in his right kidney. He would need surgery to remove the kidney and surrounding lymph nodes (an operation called radical nephrectomy).
It was a shock, said Dr. Bansal. But the diagnosis and the surgery happened so quickly that he hardly had time to think. And treatment results seemed good. The cancer was only in stage I and the CT scans showed no signs of cancer after surgery.
“We were so relieved. Everyone was so happy,” Dr. Bansal said. “They didn’t even give me chemotherapy after surgery because it seemed so early.”
But a routine scan in June 2020 revealed more cancer in his lung. Within a couple of months, it was in his bladder too.
“It was devastating,” Dr. Bansal said. “I went from thinking I was healthy again to stage IV cancer.”
As doctors scheduled surgery to remove part of his lung, Dr. Bansal started on painful immunotherapy (BCG therapy) for his bladder.
At this point, from a psychological standpoint, Dr. Bansal was reeling. As a doctor, he knew all too well the meaning of stage IV cancer.
With two adult children and a grandchild on the way, Dr. Bansal had been looking forward to retirement with his wife of almost 40 years. “Suddenly, I wasn’t sure I was going to last that long,” Bansal recalled. “I was in a very dark place. I was very anxious, very depressed from lack of sleep.”
He saw a therapist about his cancer diagnosis and maintained his regular meditation practice at home. He hired a personal trainer and tried to focus on any good news that he got about his treatment.
Those things helped, but not enough.
The basic facts were inescapable. His cancer might end everything. He couldn’t stop thinking about it. And then he couldn’t stop thinking about how he couldn’t stop thinking about it.
If the worst happened, he didn’t want to spend his last days in a state of such relentless existential angst. And it wasn’t just for himself. He wanted to be strong and mentally present for his family and his loved ones and his patients.
As he searched for something to ease his mental anguish, Dr. Bansal recalled some psychedelic research on end-of-life anxiety and depression that he’d read about in Michael Pollan’s book on psychedelics, “How to Change Your Mind” (New York, Penguin Press, 2018).
The studies were small and the research was new, but Dr. Bansal was impressed enough with the results to take a chance. He called a lead researcher of one of the studies, a fellow New York doctor, and eventually found himself accepted into a new study.
Starting the journey
By the time Dr. Bansal arrived at the Bill Richards Center for Healing at the Aquilino Cancer Center in Rockville, Md., he had already been through weeks of screening.
The main requirements for the study were a cancer diagnosis and a measurable level of depression. But study participants also had to be physically fit enough to handle the medication, and psychologically free from a personal or family history of psychosis or schizophrenia. (The study also required participants to slowly wean themselves from medications like SSRIs for depression or antianxiety medications under the strict supervision of a qualified doctor.)
Dr. Bansal’s week of treatment began almost immediately on arrival at Aquilino. Everything was carefully choreographed but not rushed. From Monday to Wednesday, doctors followed his physical health with exams, ECGs, and blood work. And most importantly, they began to prepare him for the “dosing session” on Thursday when he would take the psilocybin.
This is the careful crafting of “set and setting” stressed in so many psychedelic therapies. “Set” refers to your mindset going into the drug experience. “Setting” is the space and people around you when the drug sends you into an altered state of consciousness.
Dr. Bansal met several times with at least three therapists in the days leading up to his dosing. He attended 4-plus hours of therapist-led group sessions with other people who would get a dosing on the same day. Together, they talked about what to expect during the experience and what to do in the face of fear or panic.
He connected with a therapist who would be his personal guide. Dr. Bansal’s therapist was a military psychiatrist with over 30 years’ experience.
“He was there with me from day 1, and so we established a relationship,” Dr. Bansal said.
“He asked me a lot of personal background history – you know, my religious convictions, aspirations, all those things.”
“Trust and let go,” was a kind of mantra for the treatment repeated by his guide and other doctors.
For Dr. Bansal, a doctor and scientist accustomed to using hard facts rather than touchy-feely slogans to navigate the care of patients, it was an adjustment, to say the least.
But he did his best to set aside his doubts and embrace the journey he was about to take.
The day of the trip
Thursday morning finally arrived. The setting of the dosing room was warm and welcoming, more like a cozy home study than a hospital room.
This matters more than you might think. First, because it’s important that you feel safe, open, and comfortable enough to let go and enter into a therapeutic process. But also because though rare, it’s possible – especially with psilocybin – for people to lose track of where they are and what they’re doing and put themselves or others in danger.
The dose, 25 mg, had been carefully calibrated to induce a psychedelic experience sufficient for therapy. Much less than that, say 10 mg, isn’t enough for most people to enter this state. A double dose, 50 mg, though not physically unsafe, may leave you too incoherent to have the useful insights key to therapeutic value.
A doctor, the lead investigator of the study, brought the five capsules into the room in an intricately carved crucible with a small ceremonial cup that held the water with which to take it.
“It was very solemn,” Dr. Bansal said. “He sat down with me in a very calming way.”
The doctor said: “Don’t worry about it. Just trust and let go.”
And that’s just what he did.
Dr. Bansal swallowed the capsules and lay down. The doctor quietly left the room so that Dr. Bansal and his psychiatrist guide could begin their session together.
Special eye shades kept him in the pitch dark whether his eyes were open or closed. Headphones streamed a curated musical playlist – much of it Western classical like Strauss, Bach, Mozart, and Beethoven – but also modern electronica and other music from cultures around the globe.
Dr. Bansal would remain here, with his therapist-guide by his side, in largely this same position, for the next 7-and-a-half hours.
It took about 45 minutes for the medication to kick in.
The investigator
The doctor who brought the capsules into the dosing room was Manish Agrawal, MD, codirector of clinical research at the Aquilino Cancer Center and lead investigator of the study.
Dr. Agrawal trained at the National Cancer Institute and practiced for many years as an oncologist before developing an interest in psychedelic therapies. It was his work with cancer patients that drew him to psychedelics in the first place.
He had seen too many of his patients mentally wrecked by a cancer diagnosis, and he often felt helpless to comfort them.
“You take care of the physical aspects of the cancer, right? You talk about side effects and recommend another scan to look for recurrence.”
“But what about the psychological effects?”
They can be very serious and too often go ignored, said Dr. Agrawal. Your plans for the future suddenly become moot. You may be concerned about your ability to work or worried about the pain and suffering and financial strain that might be ahead for both you and your family. And to top it all off, you’re staring into the face of your own mortality.
So it’s no wonder, said Dr. Agrawal, that many people develop clinical levels of anxiety and depression after a cancer diagnosis.
Like Dr. Bansal, Dr. Agrawal had been impressed by early studies on psilocybin-assisted therapies for end-of-life anxiety and depression. He had tried other approaches – support groups, one-on-one therapy, religious counselors, psychiatrist-prescribed medication – but he was never really happy with the results.
To Dr. Agrawal, psilocybin-assisted therapy was the first thing that looked like it could really make a difference.
And so after his psychedelic certification at the California Institute of Integral Studies, Dr. Agrawal was determined to change his approach.
The result was the Bill Richards Center for Healing at Aquilino Cancer Center, built specifically to study psychedelic-assisted therapies for psychological distress in people with cancer. The mission of the center is to help develop safe, FDA-approved psychedelic therapies for the mental health of cancer patients, and, once approved, provide a state-of-the-art facility and staff to administer those treatments.
A trip into the unknown
Back in the dosing room, Dr. Bansal was starting to feel the effects of the medication. As the psilocybin kicked in, spectacular images swirled.
“It was as if a million stained glass windows had suddenly come to life and were dancing in front of my vision,” Dr. Bansal said.
There were moving landscapes and intricate swirling patterns and massive stages in the sky where he saw orchestras playing the music he was hearing.
Dr. Bansal saw himself being crushed by a huge machine and buried, dead, in the Earth. He died and returned to life several times, glided over the top of New York City with the skyscrapers just below him, and took in the vision of the entire universe.
“I saw this expanse of the sky that was limitless. And there was this prehistoric reptile creature that spanned galaxies in the sky ahead of me who was dying. I said: ‘My God, the universe is dying,’ but then after a few moments, the universe came to life again in a burst of stars exploding.”
All the while, Dr. Bansal said, he was well aware that it was simply his mind creating these images, thoughts, and ideas. He knew he was in a safe room wearing eyeshades and headphones.
And yet, he says, it felt true. “The images and feelings are so powerful that you cannot help but believe they are in some way a part of reality.”
“At one point, I saw this giant Ferris wheel coming towards me and it was full of giant crabs, clicking and clacking their pincers. And my brain told me: ‘That’s my cancer!’ ”
Dr. Bansal was terrified. But he and his therapist had arranged a system of signals before the session. “If I was feeling afraid, I would hold his hand and if I had other issues, I would raise my hand. If I was feeling good, I would give him a thumbs up.”
Dr. Bansal reached out to his therapist and grasped his hand. “I said, ‘My cancer is coming at me!’ ”
His therapist was clear about what to do: Stand firm and walk toward it.
“That’s what they tell you: If you see anything frightening, you face it. And that’s the whole point of this exercise. And so, I stood and walked forward, and it just blew off in a puff of smoke.”
A state of peace
Around 3 hours into the experience, Dr. Bansal started to feel an immense sense of peace, happiness, and even comfort.
“I felt like I was watching a movie or a multidimensional slideshow. I was also a part of the movie. I felt like I could tell my mind what I wanted to see, and it would show it to me. It’s almost like you can mold your own visions. It was mystical.”
After about 8 hours, as the effects of the drug wore off, Dr. Bansal removed his eyeshades and headphones. He was completely drained.
“Even though I was lying down on my back for 7 hours, I felt like I had been run over by a truck. I was exhausted beyond belief physically and mentally.”
This was partly because of the fact that he hadn’t eaten much during the session. But mostly, said Dr. Bansal, it was because of the searing emotional intensity of the experience.
After the journey
It’s hard to put into words, said Dr. Bansal, what this treatment has done for his life. He feels as if he has stumbled onto something very precious that had been right in front of him all along. He wrote of his change in perspective almost obsessively in his journal in the days and weeks after treatment. One passage reads:
“It seems that, as time is passing on, I’m becoming more relaxed and hopeful, more calm, and at peace. Family has become even more important to me now. Money, politics, material gains, alcohol, seem less important.”
And yet there was nothing “easy” about the experience. In fact, in some ways the experience demanded more from him. “I feel I need to be more compassionate and considerate – less irritable and angry, more understanding of others’ needs. I feel I need to be a better human being, a better patient, a better father, and a better doctor for my patients.”
The experience, he said, gave him something far more important than mere ease. It gave him a sense of meaning.
From his journal:
“I died, and I was reborn. If I survived this, then I can face anything and anybody in the cosmic scheme. I can become part of it.
“How many sorrows in the universe? My cancer is nothing. Life does not end with the end of life. What was will be again. Eternally.”
That’s not an unusual response, according to the namesake of the Bill Richards Center for Healing. Bill Richards, PhD, has worked in the world of psychedelic-assisted psychotherapy since 1963.
A psychologist with decades of experience, Dr. Richards and colleagues figure that, with few possible exceptions, he has helped treat more people with psychedelic therapies than anyone alive in Western medicine today. At Aquilino, he works directly with patients and oversees the therapy protocol that goes along with the psilocybin dosing sessions.
“It’s inspiring,” Dr. Richards said.
“You meet someone who’s very depressed and scared and isolating from family and having all kinds of physical complaints. And a few days later, you talk to the same person and they have a whole new lease on life.”
And the positive effects can extend deep into the family system, he said.
After psilocybin treatment, said Dr. Richards, the person with cancer can become a kind of social worker for the family. They’re often far better able to talk about death and loss and even money and family issues than their loved ones. It’s not uncommon after treatment to see the resolution of years-old resentments or grievances that have dogged a family for many years.
Plus, said Dr. Richards, the cancer patient often ends up as a kind model to other family members for how to approach death. “They can demonstrate how to live fully – right to the last breath – which is a real gift because those relatives and loved ones have to die someday too, you know.”
At 80 years old, Dr. Richards is still in active practice and hopes to spend the rest of his days working with people in end-of-life care.
After the experience
Psychedelic-assisted therapy does not end with the dosing session. Integration sessions, where you discuss what happened during the dosing session, are a key part of most treatments.
The goal is to help participants absorb and “integrate” their experience. It typically happens over two or more sessions of 60-90 minutes with a therapist. In some cases, the therapist may invite a significant other to join in the integration process.
Dr. Agrawal’s trial at the Bill Richards center added something new: group therapy. Not only did Dr. Bansal meet with his therapist, he also met with a group of three other people in the trial who had their dosing the same day.
The point, said Dr. Agrawal, is to try and determine the effect of the group on the therapy. After their private dosing sessions, they come back together to discuss their experiences.
“After the psilocybin, they feel like they’ve been to war together,” Dr. Agrawal said. “There is this profound openness and connection. They feel able to share things with each other that they wouldn’t with other people.”
It will take some time to figure out how the group affects the overall outcome, but Dr. Bansal thinks it was integral to the success of his treatment.
In fact, he continues to meet regularly with his therapy group, even though it’s long since past the requirements of the study.
Pradeep 2.0
Dr. Bansal still has tough days with his cancer. Recently, immunotherapy treatment for his bladder caused side effects – pain, bleeding, fever, and chills – for most of the night. He felt like he was “passing razor blades” when he peed.
“And yet it was somehow okay,” he said. “It was only pain.”
“It’s as if there is a part of me that is watching myself objectively, going through the painful process of treatments saying: ‘It’s all right. I will be with you through this journey, through this experience. Don’t worry.’”
Months after taking that one dose, Dr. Bansal still calls it as “the single most powerful experience of my life.”
The change in his mental outlook, Dr. Bansal said, was profound, particularly in regard to his cancer.
“I understood that I still had cancer and that it could kill me in a few weeks, or months, or years. But my perspective had shifted.”
Dr. Bansal was as surprised as anyone. “Had somebody told me going into this that I would come out a transformed being or a person with a completely different perspective on life, I would never have believed it.”
He even named his new outlook. “I call it Pradeep 2.0.”
A version of this article first appeared on WebMD.com.
Therapeutic aquatic exercise superior to physical therapy for back pain in study
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
FROM JAMA NETWORK OPEN
Duloxetine added to usual care doesn’t improve hip, knee OA pain
A small, open-label, randomized trial of patients with chronic pain from hip and knee osteoarthritis in the Netherlands shows that adding duloxetine to usual care doesn’t significantly improve clinical outcomes.
The results, published on Jan. 6 in Arthritis & Rheumatology, also showed duloxetine did not affect outcomes for a subgroup of patients who had symptoms of centrally sensitized pain, according to Jacoline J. van den Driest, MD, of the department of general practice at Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues.
The researchers acknowledged their findings contrast with other studies that showed a “small to moderate effect of duloxetine” for patients with chronic pain from hip and knee OA. There was also a higher rate of discontinuation of duloxetine around 3 months in the current trial, compared with previous studies, the authors said, which they attributed to the fact that clinicians were asked to discontinue treatment at 3 months if patients saw no effect or increased side effects.
“This difference in outcome can be due to the fact that we studied the effectiveness of duloxetine in primary care, while the other studies examined the efficacy in placebo-controlled trials in secondary care,” the researchers wrote. Patients in the current trial were also older, had more comorbidities, and had been living with OA symptoms “for a longer time” than patients in other trials, they explained.
“It is known that, in these more ‘real-life’ primary care populations and in effectiveness studies, smaller effects are found than in highly controlled efficacy trials,” they noted.
Dr. van den Driest and colleagues evaluated 132 patients with hip or knee OA between January 2016 and February 2019 who were cluster randomized at 66 general practitioner practice sites to receive duloxetine (30 mg/day in the first week, 60 mg/day in the second week and beyond) in addition to usual care that consisted of analgesics, physiotherapy, patient education, diet, and lifestyle advice. Patients were included in the study if they were at least 18 years old, met the American College of Rheumatology criteria for hip or knee OA, and experienced chronic pain for “most days” over 3 months that was not improved through use of NSAIDs or acetaminophen or were unable to use NSAIDs because of contraindications or adverse effects. They were excluded if taking duloxetine was contraindicated for them, if they were taking an antidepressant or neuropathic pain medication, and if they had rheumatoid arthritis or were scheduled for total hip or total knee replacement.
The researchers assessed patients’ Western Ontario McMaster Universities (WOMAC) Osteoarthritis Index pain scores at 3 months, compared with baseline, as a primary outcome, with secondary outcomes of WOMAC pain and function at 1 year, and cost-effectiveness as measured by the EQ-5D-5L. A modified painDETECT questionnaire was also used at baseline to identify a subset of patients with presence of centralized pain, which was defined as a score >12.
At 12 months, 80.3% of patients in both groups completed follow-up. Patient characteristics differed in duloxetine and usual-care groups, with the duloxetine group being younger (63.2 years vs. 65.4 years) and having fewer women (59.1% vs. 75.8%). The duloxetine group also had a lower percentage of patients with knee OA (77.3% vs. 86.4%) and a lower percentage of patients with two or more comorbidities (15.2% vs. 33.2%).
Duloxetine led to a nonsignificant improvement in WOMAC-measured pain at 3 months, compared with usual care (adjusted difference, –0.58; 95% confidence interval, –1.80 to 0.63), and at 12 months (adjusted difference, –0.26; 95% CI, –1.86 to 1.34). Among a subgroup of patients with central sensitization symptoms, there was a nonsignificant improvement in WOMAC-measured pain at 3 months (adjusted difference, –0.32; 95% CI, –2.32 to 1.67) and 12 months (adjusted difference, 1.02; 95% CI, –1.22 to 3.27).
Duloxetine also did not significantly improve WOMAC-measured function at 3 months (adjusted difference, –2.10; 95% CI, –6.39 to 2.20) or 12 months (adjusted difference, –1.79; 95% CI, –7.22 to 3.64).
For other secondary outcomes of quality of life, patient satisfaction, and Outcome Measures in Rheumatology (OMERACT)-Osteoarthritis Research Society International (OARSI) responder criteria, Dr. van den Driest and colleagues noted that “none of the differences between the two groups were clinically relevant or statistically significant.”
Some patients may likely still benefit from duloxetine
Commenting on the results, Joshua F. Baker, MD, MSCE, associate professor of rheumatology and epidemiology at the University of Pennsylvania and Philadelphia VA Medical Center, said the study by van den Driest and colleagues is pragmatic and demonstrates the “ ‘real-world’ benefits of trying duloxetine” – one of the study’s strengths.
“As we would probably expect, the benefits are small, and somewhat smaller in this setting than what was observed in more standard clinical trials evaluating this question,” he said, noting that the study is limited by a small sample size and loss to follow-up, as well as its open-label design and the fact that most patients stopped treatment during follow-up.
Dr. Baker also explained that while patients on average did not have a meaningful effect after taking duloxetine, “that doesn’t mean that the therapy didn’t have a meaningful effect for some people.”
“In fact, though most people didn’t receive a meaningful benefit in this study, some did,” he said. “[A]ccording to these data, treating 8 people would be expected to result in 1 person achieving an [OMERACT-OARSI] response. That’s pretty good for a disease with few things that work.”
Future study of duloxetine should focus on who is most likely to benefit from treatment “since while most probably don’t benefit a lot, some probably do,” he said.
Dr. Baker also called attention to the questions surrounding use of antidepressants. “Use of antidepressants has been questioned by some, since the average clinical benefit is low, even for conditions like depression,” he explained. “However, some would argue that even small benefits may be important since there are few things that do work very well, and because a multimodal approach that provides multiple small benefits to patients can add up to a meaningful benefit.”
This study was funded by The Netherlands Organization for Health Research and Development. One author reported receiving grants from The Netherlands Organization for Health Research and Development, the European Union, FOREUM, and the Dutch Arthritis Association, as well as personal fees from OARSI and Pfizer. The other authors reported no relevant financial disclosures.
* This story was updated 1/6/22.
A small, open-label, randomized trial of patients with chronic pain from hip and knee osteoarthritis in the Netherlands shows that adding duloxetine to usual care doesn’t significantly improve clinical outcomes.
The results, published on Jan. 6 in Arthritis & Rheumatology, also showed duloxetine did not affect outcomes for a subgroup of patients who had symptoms of centrally sensitized pain, according to Jacoline J. van den Driest, MD, of the department of general practice at Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues.
The researchers acknowledged their findings contrast with other studies that showed a “small to moderate effect of duloxetine” for patients with chronic pain from hip and knee OA. There was also a higher rate of discontinuation of duloxetine around 3 months in the current trial, compared with previous studies, the authors said, which they attributed to the fact that clinicians were asked to discontinue treatment at 3 months if patients saw no effect or increased side effects.
“This difference in outcome can be due to the fact that we studied the effectiveness of duloxetine in primary care, while the other studies examined the efficacy in placebo-controlled trials in secondary care,” the researchers wrote. Patients in the current trial were also older, had more comorbidities, and had been living with OA symptoms “for a longer time” than patients in other trials, they explained.
“It is known that, in these more ‘real-life’ primary care populations and in effectiveness studies, smaller effects are found than in highly controlled efficacy trials,” they noted.
Dr. van den Driest and colleagues evaluated 132 patients with hip or knee OA between January 2016 and February 2019 who were cluster randomized at 66 general practitioner practice sites to receive duloxetine (30 mg/day in the first week, 60 mg/day in the second week and beyond) in addition to usual care that consisted of analgesics, physiotherapy, patient education, diet, and lifestyle advice. Patients were included in the study if they were at least 18 years old, met the American College of Rheumatology criteria for hip or knee OA, and experienced chronic pain for “most days” over 3 months that was not improved through use of NSAIDs or acetaminophen or were unable to use NSAIDs because of contraindications or adverse effects. They were excluded if taking duloxetine was contraindicated for them, if they were taking an antidepressant or neuropathic pain medication, and if they had rheumatoid arthritis or were scheduled for total hip or total knee replacement.
The researchers assessed patients’ Western Ontario McMaster Universities (WOMAC) Osteoarthritis Index pain scores at 3 months, compared with baseline, as a primary outcome, with secondary outcomes of WOMAC pain and function at 1 year, and cost-effectiveness as measured by the EQ-5D-5L. A modified painDETECT questionnaire was also used at baseline to identify a subset of patients with presence of centralized pain, which was defined as a score >12.
At 12 months, 80.3% of patients in both groups completed follow-up. Patient characteristics differed in duloxetine and usual-care groups, with the duloxetine group being younger (63.2 years vs. 65.4 years) and having fewer women (59.1% vs. 75.8%). The duloxetine group also had a lower percentage of patients with knee OA (77.3% vs. 86.4%) and a lower percentage of patients with two or more comorbidities (15.2% vs. 33.2%).
Duloxetine led to a nonsignificant improvement in WOMAC-measured pain at 3 months, compared with usual care (adjusted difference, –0.58; 95% confidence interval, –1.80 to 0.63), and at 12 months (adjusted difference, –0.26; 95% CI, –1.86 to 1.34). Among a subgroup of patients with central sensitization symptoms, there was a nonsignificant improvement in WOMAC-measured pain at 3 months (adjusted difference, –0.32; 95% CI, –2.32 to 1.67) and 12 months (adjusted difference, 1.02; 95% CI, –1.22 to 3.27).
Duloxetine also did not significantly improve WOMAC-measured function at 3 months (adjusted difference, –2.10; 95% CI, –6.39 to 2.20) or 12 months (adjusted difference, –1.79; 95% CI, –7.22 to 3.64).
For other secondary outcomes of quality of life, patient satisfaction, and Outcome Measures in Rheumatology (OMERACT)-Osteoarthritis Research Society International (OARSI) responder criteria, Dr. van den Driest and colleagues noted that “none of the differences between the two groups were clinically relevant or statistically significant.”
Some patients may likely still benefit from duloxetine
Commenting on the results, Joshua F. Baker, MD, MSCE, associate professor of rheumatology and epidemiology at the University of Pennsylvania and Philadelphia VA Medical Center, said the study by van den Driest and colleagues is pragmatic and demonstrates the “ ‘real-world’ benefits of trying duloxetine” – one of the study’s strengths.
“As we would probably expect, the benefits are small, and somewhat smaller in this setting than what was observed in more standard clinical trials evaluating this question,” he said, noting that the study is limited by a small sample size and loss to follow-up, as well as its open-label design and the fact that most patients stopped treatment during follow-up.
Dr. Baker also explained that while patients on average did not have a meaningful effect after taking duloxetine, “that doesn’t mean that the therapy didn’t have a meaningful effect for some people.”
“In fact, though most people didn’t receive a meaningful benefit in this study, some did,” he said. “[A]ccording to these data, treating 8 people would be expected to result in 1 person achieving an [OMERACT-OARSI] response. That’s pretty good for a disease with few things that work.”
Future study of duloxetine should focus on who is most likely to benefit from treatment “since while most probably don’t benefit a lot, some probably do,” he said.
Dr. Baker also called attention to the questions surrounding use of antidepressants. “Use of antidepressants has been questioned by some, since the average clinical benefit is low, even for conditions like depression,” he explained. “However, some would argue that even small benefits may be important since there are few things that do work very well, and because a multimodal approach that provides multiple small benefits to patients can add up to a meaningful benefit.”
This study was funded by The Netherlands Organization for Health Research and Development. One author reported receiving grants from The Netherlands Organization for Health Research and Development, the European Union, FOREUM, and the Dutch Arthritis Association, as well as personal fees from OARSI and Pfizer. The other authors reported no relevant financial disclosures.
* This story was updated 1/6/22.
A small, open-label, randomized trial of patients with chronic pain from hip and knee osteoarthritis in the Netherlands shows that adding duloxetine to usual care doesn’t significantly improve clinical outcomes.
The results, published on Jan. 6 in Arthritis & Rheumatology, also showed duloxetine did not affect outcomes for a subgroup of patients who had symptoms of centrally sensitized pain, according to Jacoline J. van den Driest, MD, of the department of general practice at Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues.
The researchers acknowledged their findings contrast with other studies that showed a “small to moderate effect of duloxetine” for patients with chronic pain from hip and knee OA. There was also a higher rate of discontinuation of duloxetine around 3 months in the current trial, compared with previous studies, the authors said, which they attributed to the fact that clinicians were asked to discontinue treatment at 3 months if patients saw no effect or increased side effects.
“This difference in outcome can be due to the fact that we studied the effectiveness of duloxetine in primary care, while the other studies examined the efficacy in placebo-controlled trials in secondary care,” the researchers wrote. Patients in the current trial were also older, had more comorbidities, and had been living with OA symptoms “for a longer time” than patients in other trials, they explained.
“It is known that, in these more ‘real-life’ primary care populations and in effectiveness studies, smaller effects are found than in highly controlled efficacy trials,” they noted.
Dr. van den Driest and colleagues evaluated 132 patients with hip or knee OA between January 2016 and February 2019 who were cluster randomized at 66 general practitioner practice sites to receive duloxetine (30 mg/day in the first week, 60 mg/day in the second week and beyond) in addition to usual care that consisted of analgesics, physiotherapy, patient education, diet, and lifestyle advice. Patients were included in the study if they were at least 18 years old, met the American College of Rheumatology criteria for hip or knee OA, and experienced chronic pain for “most days” over 3 months that was not improved through use of NSAIDs or acetaminophen or were unable to use NSAIDs because of contraindications or adverse effects. They were excluded if taking duloxetine was contraindicated for them, if they were taking an antidepressant or neuropathic pain medication, and if they had rheumatoid arthritis or were scheduled for total hip or total knee replacement.
The researchers assessed patients’ Western Ontario McMaster Universities (WOMAC) Osteoarthritis Index pain scores at 3 months, compared with baseline, as a primary outcome, with secondary outcomes of WOMAC pain and function at 1 year, and cost-effectiveness as measured by the EQ-5D-5L. A modified painDETECT questionnaire was also used at baseline to identify a subset of patients with presence of centralized pain, which was defined as a score >12.
At 12 months, 80.3% of patients in both groups completed follow-up. Patient characteristics differed in duloxetine and usual-care groups, with the duloxetine group being younger (63.2 years vs. 65.4 years) and having fewer women (59.1% vs. 75.8%). The duloxetine group also had a lower percentage of patients with knee OA (77.3% vs. 86.4%) and a lower percentage of patients with two or more comorbidities (15.2% vs. 33.2%).
Duloxetine led to a nonsignificant improvement in WOMAC-measured pain at 3 months, compared with usual care (adjusted difference, –0.58; 95% confidence interval, –1.80 to 0.63), and at 12 months (adjusted difference, –0.26; 95% CI, –1.86 to 1.34). Among a subgroup of patients with central sensitization symptoms, there was a nonsignificant improvement in WOMAC-measured pain at 3 months (adjusted difference, –0.32; 95% CI, –2.32 to 1.67) and 12 months (adjusted difference, 1.02; 95% CI, –1.22 to 3.27).
Duloxetine also did not significantly improve WOMAC-measured function at 3 months (adjusted difference, –2.10; 95% CI, –6.39 to 2.20) or 12 months (adjusted difference, –1.79; 95% CI, –7.22 to 3.64).
For other secondary outcomes of quality of life, patient satisfaction, and Outcome Measures in Rheumatology (OMERACT)-Osteoarthritis Research Society International (OARSI) responder criteria, Dr. van den Driest and colleagues noted that “none of the differences between the two groups were clinically relevant or statistically significant.”
Some patients may likely still benefit from duloxetine
Commenting on the results, Joshua F. Baker, MD, MSCE, associate professor of rheumatology and epidemiology at the University of Pennsylvania and Philadelphia VA Medical Center, said the study by van den Driest and colleagues is pragmatic and demonstrates the “ ‘real-world’ benefits of trying duloxetine” – one of the study’s strengths.
“As we would probably expect, the benefits are small, and somewhat smaller in this setting than what was observed in more standard clinical trials evaluating this question,” he said, noting that the study is limited by a small sample size and loss to follow-up, as well as its open-label design and the fact that most patients stopped treatment during follow-up.
Dr. Baker also explained that while patients on average did not have a meaningful effect after taking duloxetine, “that doesn’t mean that the therapy didn’t have a meaningful effect for some people.”
“In fact, though most people didn’t receive a meaningful benefit in this study, some did,” he said. “[A]ccording to these data, treating 8 people would be expected to result in 1 person achieving an [OMERACT-OARSI] response. That’s pretty good for a disease with few things that work.”
Future study of duloxetine should focus on who is most likely to benefit from treatment “since while most probably don’t benefit a lot, some probably do,” he said.
Dr. Baker also called attention to the questions surrounding use of antidepressants. “Use of antidepressants has been questioned by some, since the average clinical benefit is low, even for conditions like depression,” he explained. “However, some would argue that even small benefits may be important since there are few things that do work very well, and because a multimodal approach that provides multiple small benefits to patients can add up to a meaningful benefit.”
This study was funded by The Netherlands Organization for Health Research and Development. One author reported receiving grants from The Netherlands Organization for Health Research and Development, the European Union, FOREUM, and the Dutch Arthritis Association, as well as personal fees from OARSI and Pfizer. The other authors reported no relevant financial disclosures.
* This story was updated 1/6/22.
FROM ARTHRITIS & RHEUMATOLOGY