User login
Hypertension worsened by commonly used prescription meds
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
FROM ACC 2021
Pediatric cancer survivors at risk for opioid misuse
Survivors of childhood cancers are at increased risk for prescription opioid misuse compared with their peers, a review of a claims database revealed.
Among more than 8,000 patients age 21 or younger who had completed treatment for hematologic, central nervous system, bone, or gonadal cancers, survivors were significantly more likely than were their peers to have an opioid prescription, longer duration of prescription, and higher daily doses of opioids, and to have opioid prescriptions overlapping for a week or more, reported Xu Ji, PhD, of Emory University in Atlanta.
Teenage and young adult patients were at higher risk than were patients younger than 12, and the risk was highest among patients who had been treated for bone malignancies, as well as those who had undergone any hematopoietic stem cell transplant.
“These findings suggest that health care providers who regularly see survivors should explore nonopioid options to help prevent opioid misuse, and screen for potential misuse in those who actually receive opioids,” she said in an oral abstract presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a really important topic, and something that’s probably been underinvestigated and underexplored in our patient population,” said session comoderator Sheri Spunt, MD, Endowed Professor of Pediatric Cancer at Stanford (Calif.) University.
Database review
Dr. Ji and colleagues used the IBM MarketScan Commercial Claims and Encounters database from 2009 to 2018 to examine prescription opioid use, potential misuse, and substance use disorders in pediatric cancer survivors in the first year after completion of therapy, and to identify factors associated with risk for misuse or substance use disorders. Specifically, the period of interest was the first year after completion of all treatments, including surgery, chemotherapy, radiation, and stem cell transplant (Abstract 2015).
They looked at deidentified records on any opioid prescription and for treatment of any opioid use or substance use disorder (alcohol, psychotherapeutic drugs, marijuana, or illicit drug use disorders).
They defined indicators of potential misuse as either prescriptions for long-acting or extended-release opioids for acute pain conditions; opioid and benzodiazepine prescriptions overlapping by a week or more; opioid prescriptions overlapping by a week or more; high daily opioid dosage (prescribed daily dose of 100 or greater morphine milligram equivalent [MME]; and/or opioid dose escalation (an increase of at least 50% in mean MMEs per month twice consecutively within 1 year).
They compared outcomes between a total of 8,635 survivors and 44,175 controls, matched on a 1:5 basis with survivors by age, sex, and region, and continuous enrollment during the 1-year posttherapy period.
In each of three age categories – 0 to 11 years, 12 to 17 years, and 18 years and older – survivors were significantly more likely to have received an opioid prescription, at 15% for the youngest survivors vs. 2% of controls, 25% vs. 8% for 12- to 17-year-olds, and 28% vs. 12% for those 18 and older (P < .01 for all three comparisons).
Survivors were also significantly more likely to have any indicator of potential misuse (1.6% vs. 0.1%, 4.6% vs. 0.5%, and 7.4% vs. 1.2%, respectively, P < .001 for all) and both the youngest and oldest groups (but not 12- to 17-year-olds) were significantly more like to have opioid or substance use disorder (0.4% vs. 0% for 0-11 years, 5.76% vs. 4.2% for 18 years and older, P < .001 for both).
Among patients with any opioid prescription, survivors were significantly more likely than were controls of any age to have indicators for potential misuse. For example, 13% of survivors aged 18 years and older had prescriptions for high opioid doses, compared with 5% of controls, and 12% had prescription overlap, vs. 2%.
Compared with patients with leukemia, patients treated for bone malignancies had a 6% greater risk for having any indicator of misuse, while patients with other malignancies were at slightly lower risk for misuse than those who completed leukemia therapy.
Patients who received any stem cell transplant had an 8.4% greater risk for misuse compared with patients who had surgery only.
Opioids pre- and posttreatment?
“Being someone who takes care of a lot of bone cancer patients, I do see patients with these issues,” Dr. Spunt said.
Audience member Jack H. Staddon, MD, PhD, of the Billings (Montana) Clinic, noted the possibility that opioid use during treatment may have been carried on into the posttreatment period, and asked whether use of narcotics during treatment was an independent risk factor for posttreatment narcotic use or misuse.
The researchers plan to investigate this question in future studies, Dr. Ji replied.
They did not report a study funding source. Dr. Ji and coauthors and Dr. Staddon reported no relevant disclosures.
Survivors of childhood cancers are at increased risk for prescription opioid misuse compared with their peers, a review of a claims database revealed.
Among more than 8,000 patients age 21 or younger who had completed treatment for hematologic, central nervous system, bone, or gonadal cancers, survivors were significantly more likely than were their peers to have an opioid prescription, longer duration of prescription, and higher daily doses of opioids, and to have opioid prescriptions overlapping for a week or more, reported Xu Ji, PhD, of Emory University in Atlanta.
Teenage and young adult patients were at higher risk than were patients younger than 12, and the risk was highest among patients who had been treated for bone malignancies, as well as those who had undergone any hematopoietic stem cell transplant.
“These findings suggest that health care providers who regularly see survivors should explore nonopioid options to help prevent opioid misuse, and screen for potential misuse in those who actually receive opioids,” she said in an oral abstract presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a really important topic, and something that’s probably been underinvestigated and underexplored in our patient population,” said session comoderator Sheri Spunt, MD, Endowed Professor of Pediatric Cancer at Stanford (Calif.) University.
Database review
Dr. Ji and colleagues used the IBM MarketScan Commercial Claims and Encounters database from 2009 to 2018 to examine prescription opioid use, potential misuse, and substance use disorders in pediatric cancer survivors in the first year after completion of therapy, and to identify factors associated with risk for misuse or substance use disorders. Specifically, the period of interest was the first year after completion of all treatments, including surgery, chemotherapy, radiation, and stem cell transplant (Abstract 2015).
They looked at deidentified records on any opioid prescription and for treatment of any opioid use or substance use disorder (alcohol, psychotherapeutic drugs, marijuana, or illicit drug use disorders).
They defined indicators of potential misuse as either prescriptions for long-acting or extended-release opioids for acute pain conditions; opioid and benzodiazepine prescriptions overlapping by a week or more; opioid prescriptions overlapping by a week or more; high daily opioid dosage (prescribed daily dose of 100 or greater morphine milligram equivalent [MME]; and/or opioid dose escalation (an increase of at least 50% in mean MMEs per month twice consecutively within 1 year).
They compared outcomes between a total of 8,635 survivors and 44,175 controls, matched on a 1:5 basis with survivors by age, sex, and region, and continuous enrollment during the 1-year posttherapy period.
In each of three age categories – 0 to 11 years, 12 to 17 years, and 18 years and older – survivors were significantly more likely to have received an opioid prescription, at 15% for the youngest survivors vs. 2% of controls, 25% vs. 8% for 12- to 17-year-olds, and 28% vs. 12% for those 18 and older (P < .01 for all three comparisons).
Survivors were also significantly more likely to have any indicator of potential misuse (1.6% vs. 0.1%, 4.6% vs. 0.5%, and 7.4% vs. 1.2%, respectively, P < .001 for all) and both the youngest and oldest groups (but not 12- to 17-year-olds) were significantly more like to have opioid or substance use disorder (0.4% vs. 0% for 0-11 years, 5.76% vs. 4.2% for 18 years and older, P < .001 for both).
Among patients with any opioid prescription, survivors were significantly more likely than were controls of any age to have indicators for potential misuse. For example, 13% of survivors aged 18 years and older had prescriptions for high opioid doses, compared with 5% of controls, and 12% had prescription overlap, vs. 2%.
Compared with patients with leukemia, patients treated for bone malignancies had a 6% greater risk for having any indicator of misuse, while patients with other malignancies were at slightly lower risk for misuse than those who completed leukemia therapy.
Patients who received any stem cell transplant had an 8.4% greater risk for misuse compared with patients who had surgery only.
Opioids pre- and posttreatment?
“Being someone who takes care of a lot of bone cancer patients, I do see patients with these issues,” Dr. Spunt said.
Audience member Jack H. Staddon, MD, PhD, of the Billings (Montana) Clinic, noted the possibility that opioid use during treatment may have been carried on into the posttreatment period, and asked whether use of narcotics during treatment was an independent risk factor for posttreatment narcotic use or misuse.
The researchers plan to investigate this question in future studies, Dr. Ji replied.
They did not report a study funding source. Dr. Ji and coauthors and Dr. Staddon reported no relevant disclosures.
Survivors of childhood cancers are at increased risk for prescription opioid misuse compared with their peers, a review of a claims database revealed.
Among more than 8,000 patients age 21 or younger who had completed treatment for hematologic, central nervous system, bone, or gonadal cancers, survivors were significantly more likely than were their peers to have an opioid prescription, longer duration of prescription, and higher daily doses of opioids, and to have opioid prescriptions overlapping for a week or more, reported Xu Ji, PhD, of Emory University in Atlanta.
Teenage and young adult patients were at higher risk than were patients younger than 12, and the risk was highest among patients who had been treated for bone malignancies, as well as those who had undergone any hematopoietic stem cell transplant.
“These findings suggest that health care providers who regularly see survivors should explore nonopioid options to help prevent opioid misuse, and screen for potential misuse in those who actually receive opioids,” she said in an oral abstract presented during the annual meeting of the American Society of Pediatric Hematology/Oncology.
“This is a really important topic, and something that’s probably been underinvestigated and underexplored in our patient population,” said session comoderator Sheri Spunt, MD, Endowed Professor of Pediatric Cancer at Stanford (Calif.) University.
Database review
Dr. Ji and colleagues used the IBM MarketScan Commercial Claims and Encounters database from 2009 to 2018 to examine prescription opioid use, potential misuse, and substance use disorders in pediatric cancer survivors in the first year after completion of therapy, and to identify factors associated with risk for misuse or substance use disorders. Specifically, the period of interest was the first year after completion of all treatments, including surgery, chemotherapy, radiation, and stem cell transplant (Abstract 2015).
They looked at deidentified records on any opioid prescription and for treatment of any opioid use or substance use disorder (alcohol, psychotherapeutic drugs, marijuana, or illicit drug use disorders).
They defined indicators of potential misuse as either prescriptions for long-acting or extended-release opioids for acute pain conditions; opioid and benzodiazepine prescriptions overlapping by a week or more; opioid prescriptions overlapping by a week or more; high daily opioid dosage (prescribed daily dose of 100 or greater morphine milligram equivalent [MME]; and/or opioid dose escalation (an increase of at least 50% in mean MMEs per month twice consecutively within 1 year).
They compared outcomes between a total of 8,635 survivors and 44,175 controls, matched on a 1:5 basis with survivors by age, sex, and region, and continuous enrollment during the 1-year posttherapy period.
In each of three age categories – 0 to 11 years, 12 to 17 years, and 18 years and older – survivors were significantly more likely to have received an opioid prescription, at 15% for the youngest survivors vs. 2% of controls, 25% vs. 8% for 12- to 17-year-olds, and 28% vs. 12% for those 18 and older (P < .01 for all three comparisons).
Survivors were also significantly more likely to have any indicator of potential misuse (1.6% vs. 0.1%, 4.6% vs. 0.5%, and 7.4% vs. 1.2%, respectively, P < .001 for all) and both the youngest and oldest groups (but not 12- to 17-year-olds) were significantly more like to have opioid or substance use disorder (0.4% vs. 0% for 0-11 years, 5.76% vs. 4.2% for 18 years and older, P < .001 for both).
Among patients with any opioid prescription, survivors were significantly more likely than were controls of any age to have indicators for potential misuse. For example, 13% of survivors aged 18 years and older had prescriptions for high opioid doses, compared with 5% of controls, and 12% had prescription overlap, vs. 2%.
Compared with patients with leukemia, patients treated for bone malignancies had a 6% greater risk for having any indicator of misuse, while patients with other malignancies were at slightly lower risk for misuse than those who completed leukemia therapy.
Patients who received any stem cell transplant had an 8.4% greater risk for misuse compared with patients who had surgery only.
Opioids pre- and posttreatment?
“Being someone who takes care of a lot of bone cancer patients, I do see patients with these issues,” Dr. Spunt said.
Audience member Jack H. Staddon, MD, PhD, of the Billings (Montana) Clinic, noted the possibility that opioid use during treatment may have been carried on into the posttreatment period, and asked whether use of narcotics during treatment was an independent risk factor for posttreatment narcotic use or misuse.
The researchers plan to investigate this question in future studies, Dr. Ji replied.
They did not report a study funding source. Dr. Ji and coauthors and Dr. Staddon reported no relevant disclosures.
FROM 2021 ASPHO CONFERENCE
High variability found in studies assessing hemophilia-related pain
Chronic pain is a common condition among people with hemophilia and is associated with joint deterioration because of repeated joint bleeds. This systematic review and meta-analysis aimed to determine the prevalence of chronic pain because of hemophilia and to analyze its interference in the lives of patients, according to Ana Cristina Paredes, a PhD student at the University of Minho, Braga, Portugal, and colleagues.
The manuscripts included in the study, which was published online in the Journal of Pain, were mostly observational, cross-sectional studies and one prospective investigation, published between 2009 and 2019.
The issue of pain is particularly important among people with hemophilia, as many adult patients suffer from distinct degrees of arthropathy and associated chronic pain, due to the lifelong occurrence of hemarthrosis, the authors noted. In an important distinction, according to the authors, people with hemophilia may therefore experience both acute pain during bleeds and chronic pain caused by joint deterioration. Acute pain ceases with the resolution of the bleeding episode, but the chronic pain is significantly more challenging, since it persists in time and may trigger changes in the nervous system, leading to peripheral or central sensitization.
Data in the assessed studies were collected from a variety of sources: hemophilia centers, online surveys, by mail, or through a national database, with return rates ranging from 29.2% to 98%. Overall, these studies comprised 4,772 adults, with individual sample sizes ranging from 21 to 2,253 patients, the authors added.
Conflicting results
Overall, there was a widely varying prevalence of hemophilia-related chronic pain reported across studies. Additionally, methodologies and sample characteristics varied widely. The meta-analyses revealed high heterogeneity between studies, and, therefore, pooled prevalence estimates values must be interpreted with caution, the authors stated.
All of the 11 selected studies included for meta-analysis and review reported on the prevalence of chronic pain caused by hemophilia. Chronic pain was assessed using direct questions developed by the authors in eight studies and using the European Haemophilia Therapy Standardization Board definition in three studies. The prevalence for global samples ranged widely from 17% to 84%.
Although there was high heterogeneity, the random-effects meta-analysis including all studies demonstrated a pooled prevalence of 46% of patients reporting chronic pain. Subgroup analyses of studies including all disease severities (mild, moderate, and severe; seven studies) revealed a pooled prevalence of 48%, but also with high heterogeneity. Looking at severe patients only (six studies), the chronic pain prevalence ranged from 33% to 86.4%, with a pooled prevalence of 53% and high heterogeneity, the authors added.
The wide disparity of the chronic pain prevalence seen across the studies is likely because of the fact that some investigations inquired about pain without distinguishing between acute (hemarthrosis-related) or chronic (arthropathy-related) pain, and without clarifying if the only focus is pain caused by hemophilia, or including all causes of pain complaints, according to the researchers.
“Concerning hemophilia-related chronic pain interference, it is striking that the existing literature does not distinguish between the impact of acute or chronic pain. Such a distinction is needed and should be made in future studies to ensure accurate accounts of hemophilia-related pain and to fully understand its interference according to the type of pain (acute vs. chronic). This information is relevant to promote targeted and effective treatment approaches,” the researchers concluded.
The research was supported by a Novo Nordisk HERO Research Grant 2015, the Portuguese Foundation for Science and Technology, and the Foundation for Science and Technology in Portugal. The authors declared they had no conflicts of interest.
Chronic pain is a common condition among people with hemophilia and is associated with joint deterioration because of repeated joint bleeds. This systematic review and meta-analysis aimed to determine the prevalence of chronic pain because of hemophilia and to analyze its interference in the lives of patients, according to Ana Cristina Paredes, a PhD student at the University of Minho, Braga, Portugal, and colleagues.
The manuscripts included in the study, which was published online in the Journal of Pain, were mostly observational, cross-sectional studies and one prospective investigation, published between 2009 and 2019.
The issue of pain is particularly important among people with hemophilia, as many adult patients suffer from distinct degrees of arthropathy and associated chronic pain, due to the lifelong occurrence of hemarthrosis, the authors noted. In an important distinction, according to the authors, people with hemophilia may therefore experience both acute pain during bleeds and chronic pain caused by joint deterioration. Acute pain ceases with the resolution of the bleeding episode, but the chronic pain is significantly more challenging, since it persists in time and may trigger changes in the nervous system, leading to peripheral or central sensitization.
Data in the assessed studies were collected from a variety of sources: hemophilia centers, online surveys, by mail, or through a national database, with return rates ranging from 29.2% to 98%. Overall, these studies comprised 4,772 adults, with individual sample sizes ranging from 21 to 2,253 patients, the authors added.
Conflicting results
Overall, there was a widely varying prevalence of hemophilia-related chronic pain reported across studies. Additionally, methodologies and sample characteristics varied widely. The meta-analyses revealed high heterogeneity between studies, and, therefore, pooled prevalence estimates values must be interpreted with caution, the authors stated.
All of the 11 selected studies included for meta-analysis and review reported on the prevalence of chronic pain caused by hemophilia. Chronic pain was assessed using direct questions developed by the authors in eight studies and using the European Haemophilia Therapy Standardization Board definition in three studies. The prevalence for global samples ranged widely from 17% to 84%.
Although there was high heterogeneity, the random-effects meta-analysis including all studies demonstrated a pooled prevalence of 46% of patients reporting chronic pain. Subgroup analyses of studies including all disease severities (mild, moderate, and severe; seven studies) revealed a pooled prevalence of 48%, but also with high heterogeneity. Looking at severe patients only (six studies), the chronic pain prevalence ranged from 33% to 86.4%, with a pooled prevalence of 53% and high heterogeneity, the authors added.
The wide disparity of the chronic pain prevalence seen across the studies is likely because of the fact that some investigations inquired about pain without distinguishing between acute (hemarthrosis-related) or chronic (arthropathy-related) pain, and without clarifying if the only focus is pain caused by hemophilia, or including all causes of pain complaints, according to the researchers.
“Concerning hemophilia-related chronic pain interference, it is striking that the existing literature does not distinguish between the impact of acute or chronic pain. Such a distinction is needed and should be made in future studies to ensure accurate accounts of hemophilia-related pain and to fully understand its interference according to the type of pain (acute vs. chronic). This information is relevant to promote targeted and effective treatment approaches,” the researchers concluded.
The research was supported by a Novo Nordisk HERO Research Grant 2015, the Portuguese Foundation for Science and Technology, and the Foundation for Science and Technology in Portugal. The authors declared they had no conflicts of interest.
Chronic pain is a common condition among people with hemophilia and is associated with joint deterioration because of repeated joint bleeds. This systematic review and meta-analysis aimed to determine the prevalence of chronic pain because of hemophilia and to analyze its interference in the lives of patients, according to Ana Cristina Paredes, a PhD student at the University of Minho, Braga, Portugal, and colleagues.
The manuscripts included in the study, which was published online in the Journal of Pain, were mostly observational, cross-sectional studies and one prospective investigation, published between 2009 and 2019.
The issue of pain is particularly important among people with hemophilia, as many adult patients suffer from distinct degrees of arthropathy and associated chronic pain, due to the lifelong occurrence of hemarthrosis, the authors noted. In an important distinction, according to the authors, people with hemophilia may therefore experience both acute pain during bleeds and chronic pain caused by joint deterioration. Acute pain ceases with the resolution of the bleeding episode, but the chronic pain is significantly more challenging, since it persists in time and may trigger changes in the nervous system, leading to peripheral or central sensitization.
Data in the assessed studies were collected from a variety of sources: hemophilia centers, online surveys, by mail, or through a national database, with return rates ranging from 29.2% to 98%. Overall, these studies comprised 4,772 adults, with individual sample sizes ranging from 21 to 2,253 patients, the authors added.
Conflicting results
Overall, there was a widely varying prevalence of hemophilia-related chronic pain reported across studies. Additionally, methodologies and sample characteristics varied widely. The meta-analyses revealed high heterogeneity between studies, and, therefore, pooled prevalence estimates values must be interpreted with caution, the authors stated.
All of the 11 selected studies included for meta-analysis and review reported on the prevalence of chronic pain caused by hemophilia. Chronic pain was assessed using direct questions developed by the authors in eight studies and using the European Haemophilia Therapy Standardization Board definition in three studies. The prevalence for global samples ranged widely from 17% to 84%.
Although there was high heterogeneity, the random-effects meta-analysis including all studies demonstrated a pooled prevalence of 46% of patients reporting chronic pain. Subgroup analyses of studies including all disease severities (mild, moderate, and severe; seven studies) revealed a pooled prevalence of 48%, but also with high heterogeneity. Looking at severe patients only (six studies), the chronic pain prevalence ranged from 33% to 86.4%, with a pooled prevalence of 53% and high heterogeneity, the authors added.
The wide disparity of the chronic pain prevalence seen across the studies is likely because of the fact that some investigations inquired about pain without distinguishing between acute (hemarthrosis-related) or chronic (arthropathy-related) pain, and without clarifying if the only focus is pain caused by hemophilia, or including all causes of pain complaints, according to the researchers.
“Concerning hemophilia-related chronic pain interference, it is striking that the existing literature does not distinguish between the impact of acute or chronic pain. Such a distinction is needed and should be made in future studies to ensure accurate accounts of hemophilia-related pain and to fully understand its interference according to the type of pain (acute vs. chronic). This information is relevant to promote targeted and effective treatment approaches,” the researchers concluded.
The research was supported by a Novo Nordisk HERO Research Grant 2015, the Portuguese Foundation for Science and Technology, and the Foundation for Science and Technology in Portugal. The authors declared they had no conflicts of interest.
FROM THE JOURNAL OF PAIN
FDA OKs higher-dose naloxone nasal spray for opioid overdose
The Food and Drug Administration has approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
When administered quickly, naloxone can counter opioid overdose effects, usually within minutes. A higher dose of naloxone provides an additional option for the treatment of opioid overdoses, the FDA said in a news release.
“This approval meets another critical need in combating opioid overdose,” Patrizia Cavazzoni, MD, director, FDA Center for Drug Evaluation and Research, said in the release.
“Addressing the opioid crisis is a top priority for the FDA, and we will continue our efforts to increase access to naloxone and place this important medicine in the hands of those who need it most,” said Dr. Cavazzoni.
In a company news release announcing the approval, manufacturer Hikma Pharmaceuticals noted that a recent survey of community organizations in which the 4-mg naloxone nasal spray had been distributed showed that for 34% of attempted reversals, two or more doses of naloxone were used.
A separate study found that the percentage of overdose-related emergency medical service calls in the United States that led to the administration of multiple doses of naloxone increased to 21% during the period of 2013-2016, which represents a 43% increase over 4 years.
“The approval of Kloxxado is an important step in providing patients, friends, and family members – as well as the public health community – with an important new option for treating opioid overdose,” Brian Hoffmann, president of Hikma Generics, said in the release.
The FDA approved Kloxxado through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
When administered quickly, naloxone can counter opioid overdose effects, usually within minutes. A higher dose of naloxone provides an additional option for the treatment of opioid overdoses, the FDA said in a news release.
“This approval meets another critical need in combating opioid overdose,” Patrizia Cavazzoni, MD, director, FDA Center for Drug Evaluation and Research, said in the release.
“Addressing the opioid crisis is a top priority for the FDA, and we will continue our efforts to increase access to naloxone and place this important medicine in the hands of those who need it most,” said Dr. Cavazzoni.
In a company news release announcing the approval, manufacturer Hikma Pharmaceuticals noted that a recent survey of community organizations in which the 4-mg naloxone nasal spray had been distributed showed that for 34% of attempted reversals, two or more doses of naloxone were used.
A separate study found that the percentage of overdose-related emergency medical service calls in the United States that led to the administration of multiple doses of naloxone increased to 21% during the period of 2013-2016, which represents a 43% increase over 4 years.
“The approval of Kloxxado is an important step in providing patients, friends, and family members – as well as the public health community – with an important new option for treating opioid overdose,” Brian Hoffmann, president of Hikma Generics, said in the release.
The FDA approved Kloxxado through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
When administered quickly, naloxone can counter opioid overdose effects, usually within minutes. A higher dose of naloxone provides an additional option for the treatment of opioid overdoses, the FDA said in a news release.
“This approval meets another critical need in combating opioid overdose,” Patrizia Cavazzoni, MD, director, FDA Center for Drug Evaluation and Research, said in the release.
“Addressing the opioid crisis is a top priority for the FDA, and we will continue our efforts to increase access to naloxone and place this important medicine in the hands of those who need it most,” said Dr. Cavazzoni.
In a company news release announcing the approval, manufacturer Hikma Pharmaceuticals noted that a recent survey of community organizations in which the 4-mg naloxone nasal spray had been distributed showed that for 34% of attempted reversals, two or more doses of naloxone were used.
A separate study found that the percentage of overdose-related emergency medical service calls in the United States that led to the administration of multiple doses of naloxone increased to 21% during the period of 2013-2016, which represents a 43% increase over 4 years.
“The approval of Kloxxado is an important step in providing patients, friends, and family members – as well as the public health community – with an important new option for treating opioid overdose,” Brian Hoffmann, president of Hikma Generics, said in the release.
The FDA approved Kloxxado through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
A version of this article first appeared on Medscape.com.
The cloudy role of cannabis as a neuropsychiatric treatment
Although the healing properties of cannabis have been touted for millennia, research into its potential neuropsychiatric applications truly began to take off in the 1990s following the discovery of the cannabinoid system in the brain. This led to speculation that cannabis could play a therapeutic role in regulating dopamine, serotonin, and other neurotransmitters and offer a new means of treating various ailments.
At the same time, efforts to liberalize marijuana laws have successfully played out in several nations, including the United States, where, as of April 29, 36 states provide some access to cannabis. These dual tracks – medical and political – have made cannabis an increasingly accepted part of the cultural fabric.
Yet with this development has come a new quandary for clinicians. Medical cannabis has been made widely available to patients and has largely outpaced the clinical evidence, leaving it unclear how and for which indications it should be used.
The many forms of medical cannabis
Cannabis is a genus of plants that includes marijuana (Cannabis sativa) and hemp. These plants contain over 100 compounds, including terpenes, flavonoids, and – most importantly for medicinal applications – cannabinoids.
The most abundant cannabinoid in marijuana is the psychotropic delta-9-tetrahydrocannabinol (THC), which imparts the “high” sensation. The next most abundant cannabinoid is cannabidiol (CBD), which is the nonpsychotropic. THC and CBD are the most extensively studied cannabinoids, together and in isolation. Evidence suggests that other cannabinoids and terpenoids may also hold medical promise and that cannabis’ various compounds can work synergistically to produce a so-called entourage effect.
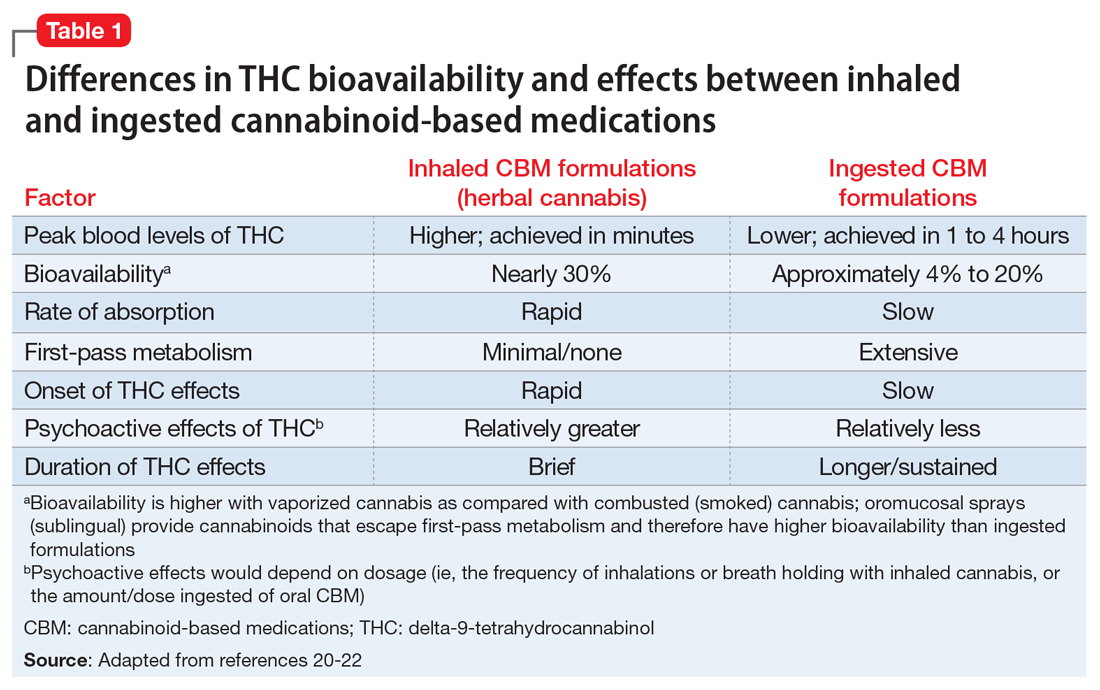
Patients walking into a typical medical cannabis dispensary will be faced with several plant-derived and synthetic options, which can differ considerably in terms of the ratios and amounts of THC and CBD they contain, as well in how they are consumed (i.e., via smoke, vapor, ingestion, topical administration, or oromucosal spray), all of which can alter their effects. Further complicating matters is the varying level of oversight each state and country has in how and whether they test for and accurately label products’ potency, cannabinoid content, and possible impurities.
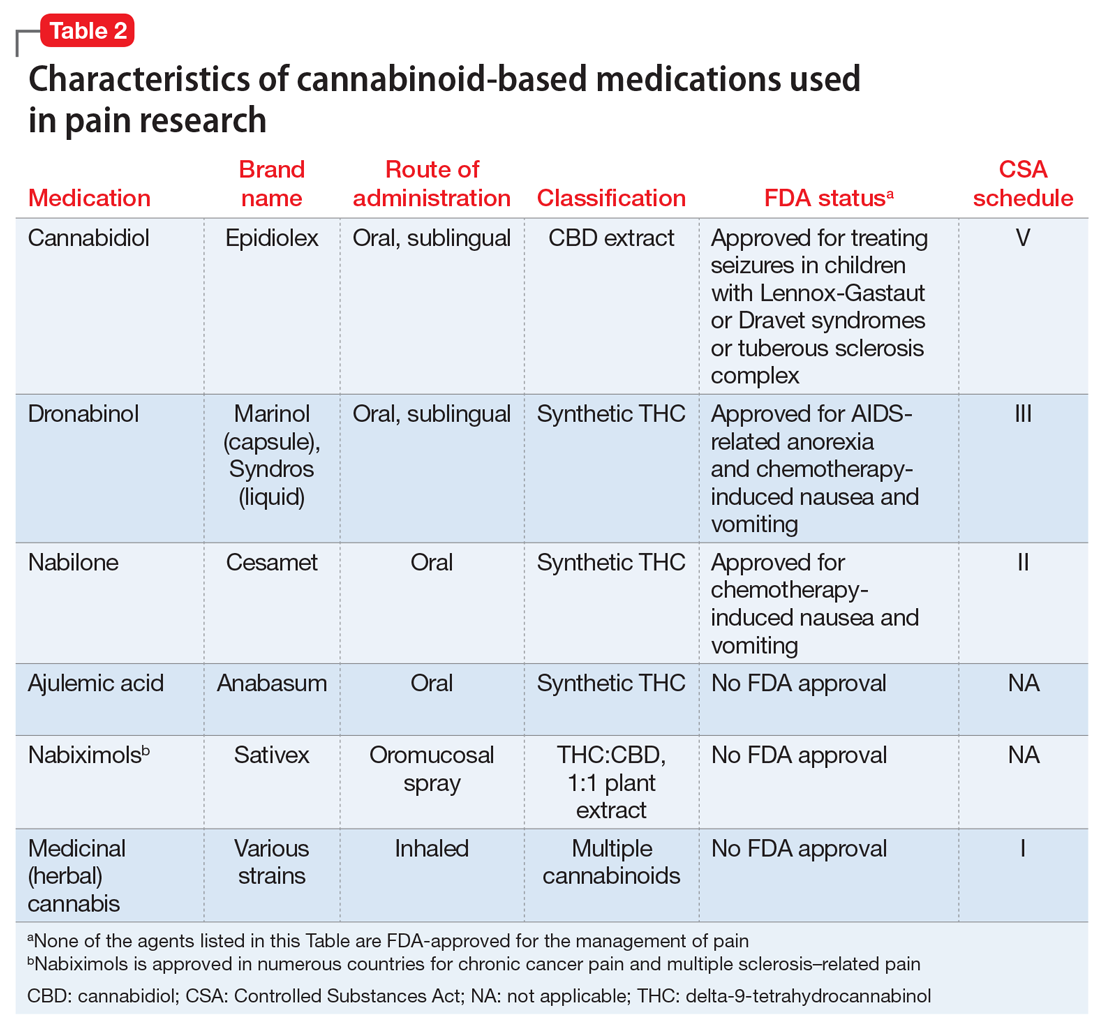
Medically authorized, prescription cannabis products go through an official regulatory review process, and indications/contraindications have been established for them. To date, the Food and Drug Administration has approved one cannabis-derived drug product – Epidiolex (purified CBD) – for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older. The FDA has also approved three synthetic cannabis-related drug products – Marinol, Syndros (or dronabinol, created from synthetic THC), and Cesamet (or nabilone, a synthetic cannabinoid similar to THC) – all of which are indicated for treatment-related nausea and anorexia associated with weight loss in AIDS patients.
Surveys of medical cannabis consumers indicate that most people cannot distinguish between THC and CBD, so the first role that physicians find themselves in when recommending this treatment may be in helping patients navigate the volume of options.
Promising treatment for pain
Chronic pain is the leading reason patients seek out medical cannabis. It is also the indication that most researchers agree has the strongest evidence to support its use.
“In my mind, the most promising immediate use for medical cannabis is with THC for pain,” Diana M. Martinez, MD, a professor of psychiatry at Columbia University, New York, who specializes in addiction research, said in a recent MDedge podcast. “THC could be added to the armamentarium of pain medications that we use today.”
In a 2015 systematic literature review, researchers assessed 28 randomized, controlled trials (RCTs) of the use of cannabinoids for chronic pain. They reported that a variety of formulations resulted in at least a 30% reduction in the odds of pain, compared with placebo. A meta-analysis of five RCTs involving patients with neuropathic pain found a 30% reduction in pain over placebo with inhaled, vaporized cannabis. Varying results have been reported in additional studies for this indication. The National Academies of Sciences, Engineering, and Medicine concluded that there was a substantial body of evidence that cannabis is an effective treatment for chronic pain in adults.
The ongoing opioid epidemic has lent these results additional relevance.
Seeing this firsthand has caused Mark Steven Wallace, MD, a pain management specialist and chair of the division of pain medicine at the University of California San Diego Health, to reconsider offering cannabis to his patients.
“I think it’s probably more efficacious, just from my personal experience, and it’s a much lower risk of abuse and dependence than the opioids,” he said.
Dr. Wallace advised that clinicians who treat pain consider the ratios of cannabinoids.
“This is anecdotal, but we do find that with the combination of the two, CBD reduces the psychoactive effects of the THC. The ratios we use during the daytime range around 20 mg of CBD to 1 mg of THC,” he said.
In a recent secondary analysis of an RCT involving patients with painful diabetic peripheral neuropathy, Dr. Wallace and colleagues showed that THC’s effects appear to reverse themselves at a certain level.
“As the THC level goes up, the pain reduces until you reach about 16 ng/mL; then it starts going in the opposite direction, and pain will start to increase,” he said. “Even recreational cannabis users have reported that they avoid high doses because it’s very aversive. Using cannabis is all about, start low and go slow.”
A mixed bag for neurologic indications
There are relatively limited data on the use of medical cannabis for other neurologic conditions, and results have varied. For uses other than pain management, the evidence that does exist is strongest regarding epilepsy, said Daniel Freedman, DO, assistant professor of neurology at the University of Texas at Austin. He noted “multiple high-quality RCTs showing that pharmaceutical-grade CBD can reduce seizures associated with two particular epilepsy syndromes: Dravet Syndrome and Lennox Gastaut.”
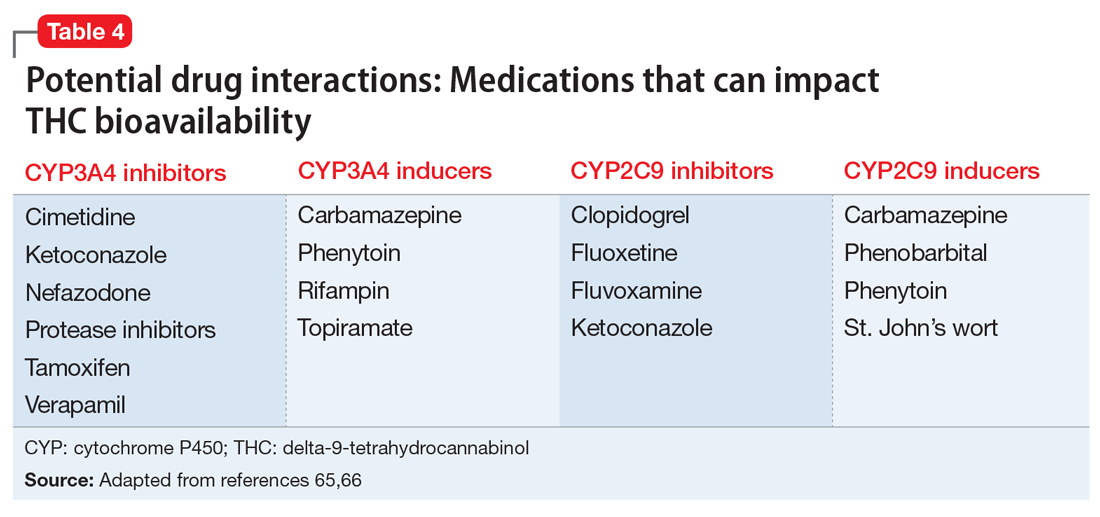
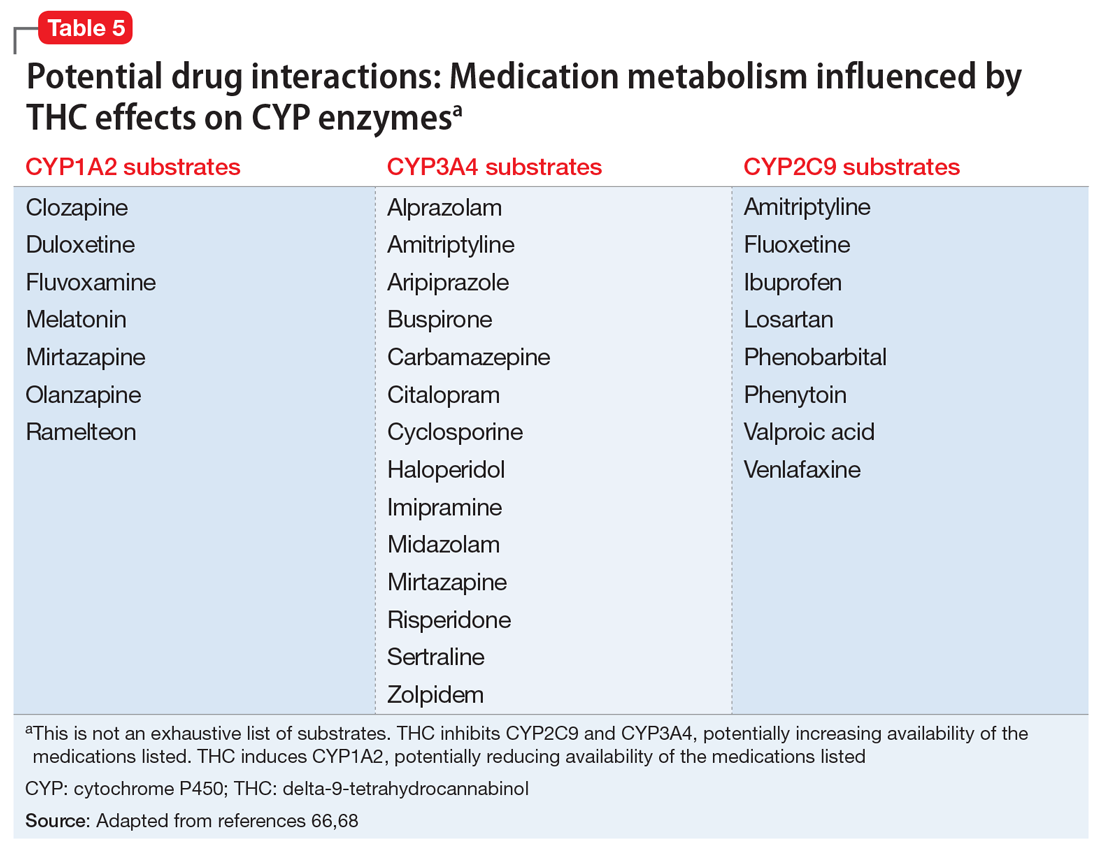
These findings led to the FDA’s 2018 approval of Epidiolex for these syndromes. In earlier years, interest in CBD for pediatric seizures was largely driven by anecdotal parental reports of its benefits. NASEM’s 2017 overview on medical cannabis found evidence from subsequent RCTs in this indication to be insufficient. Clinicians who prescribe CBD for this indication must be vigilant because it can interact with several commonly used antiepileptic drugs.
Cannabinoid treatments have also shown success in alleviating muscle spasticity resulting from multiple sclerosis, most prominently in the form of nabiximols (Sativex), a standardized oralmucosal spray containing approximately equal quantities of THC and CBD. Nabiximols is approved in Europe but not in the United States. Moderate evidence supports the efficacy of these and other treatments over placebo in reducing muscle spasticity. Patient ratings of its effects tend to be higher than clinician assessment.
Parkinson’s disease has not yet been approved as an indication for treatment with cannabis or cannabinoids, yet a growing body of preclinical data suggests these could influence the dopaminergic system, said Carsten Buhmann, MD, from the department of neurology at the University Medical Center Hamburg-Eppendorf (Germany).
“In general, cannabinoids modulate basal-ganglia function on two levels which are especially relevant in Parkinson’s disease, i.e., the glutamatergic/dopaminergic synaptic neurotransmission and the corticostriatal plasticity,” he said. “Furthermore, activation of the endocannabinoid system might induce neuroprotective effects related to direct receptor-independent mechanisms, activation of anti-inflammatory cascades in glial cells via the cannabinoid receptor type 2, and antiglutamatergic antiexcitotoxic properties.”
Dr. Buhmann said that currently, clinical evidence is scarce, consisting of only four double-blind, placebo-controlled RCTs involving 49 patients. Various cannabinoids and methods of administering treatment were employed. Improvement was only observed in one of these RCTs, which found that the cannabinoid receptor agonist nabilone significantly reduced levodopa-induced dyskinesia for patients with Parkinson’s disease. Subjective data support a beneficial effect. In a nationwide survey of 1,348 respondents conducted by Dr. Buhmann and colleagues, the majority of medical cannabis users reported that it improved their symptoms (54% with oral CBD and 68% with inhaled THC-containing cannabis).
NASEM concluded that there was insufficient evidence to support the efficacy of medical cannabis for other neurologic conditions, including Tourette syndrome, amyotrophic lateral sclerosis, Huntington disease, dystonia, or dementia. A 2020 position statement from the American Academy of Neurology cited the lack of sufficient peer-reviewed research as the reason it could not currently support the use of cannabis for neurologic disorders.
Yet, according to Dr. Freedman, who served as a coauthor of the AAN position statement, this hasn’t stymied research interest in the topic. He’s seen a substantial uptick in studies of CBD over the past 2 years. “The body of evidence grows, but I still see many claims being made without evidence. And no one seems to care about all the negative trials.”
Cannabis as a treatment for, and cause of, psychiatric disorders
Mental health problems – such as anxiety, depression, and PTSD – are among the most common reasons patients seek out medical cannabis. There is an understandable interest in using cannabis and cannabinoids to treat psychiatric disorders. Preclinical studies suggest that the endocannabinoid system plays a prominent role in modulating feelings of anxiety, mood, and fear. As with opioids and chronic pain management, there is hope that medical cannabis may provide a means of reducing prescription anxiolytics and their associated risks.
The authors of the first systematic review (BMC Psychiatry. 2020 Jan 16;20[1]:24) of the use of medical cannabis for major psychiatric disorders noted that the current evidence was “encouraging, albeit embryonic.”
Meta-analyses have indicated a small but positive association between cannabis use and anxiety, although this may reflect the fact that patients with anxiety sought out this treatment. Given the risks for substance use disorders among patients with anxiety, CBD may present a more viable option. Positive results have been shown as treatment for generalized social anxiety disorder.
Limited but encouraging results have also been reported regarding the alleviation of PTSD symptoms with both cannabis and CBD, although the body of high-quality evidence hasn’t notably progressed since 2017, when NASEM declared that the evidence was insufficient. Supportive evidence is similarly lacking regarding the treatment of depression. Longitudinal studies suggest that cannabis use, particularly heavy use, may increase the risk of developing this disorder. Because THC is psychoactive, it is advised that it be avoided by patients at risk for psychotic disorders. However, CBD has yielded limited benefits for patients with treatment-resistant schizophrenia and for young people at risk for psychosis.
The use of medical cannabis for psychiatric conditions requires a complex balancing act, inasmuch as these treatments may exacerbate the very problems they are intended to alleviate.
Marta Di Forti, MD, PhD, professor of psychiatric research at Kings College London, has been at the forefront of determining the mental health risks of continued cannabis use. In 2019, Dr. Di Forti developed the first and only Cannabis Clinic for Patients With Psychosis in London where she and her colleagues have continued to elucidate this connection.
Dr. Di Forti and colleagues have linked daily cannabis use to an increase in the risk of experiencing psychotic disorder, compared with never using it. That risk was further increased among users of high-potency cannabis (≥10% THC). The latter finding has troubling implications, because concentrations of THC have steadily risen since 1970. By contrast, CBD concentrations have remained generally stable. High-potency cannabis products are common in both recreational and medicinal settings.
“For somebody prescribing medicinal cannabis that has a ≥10% concentration of THC, I’d be particularly wary of the risk of psychosis,” said Dr. Di Forti. “If you’re expecting people to use a high content of THC daily to medicate pain or a chronic condition, you even more so need to be aware that this is a potential side effect.”
Dr. Di Forti noted that her findings come from a cohort of recreational users, most of whom were aged 18-35 years.
“There have actually not been studies developed from collecting data in this area from groups specifically using cannabis for medicinal rather than recreational purposes,” she said.
She added that she personally has no concerns about the use of medical cannabis but wants clinicians to be aware of the risk for psychosis, to structure their patient conversations to identify risk factors or family histories of psychosis, and to become knowledgeable in detecting the often subtle signs of its initial onset.
When cannabis-associated psychosis occurs, Dr. Di Forti said it is primarily treated with conventional means, such as antipsychotics and therapeutic interventions and by refraining from using cannabis. Achieving the latter goal can be a challenge for patients who are daily users of high-potency cannabis. Currently, there are no treatment options such as those offered to patients withdrawing from the use of alcohol or opioids. Dr. Di Forti and colleagues are currently researching a solution to that problem through the use of another medical cannabis, the oromucosal spray Sativex, which has been approved in the European Union.
The regulatory obstacles to clarifying cannabis’ role in medicine
That currently there is limited or no evidence to support the use of medical cannabis for the treatment of neuropsychiatric conditions points to the inherent difficulties in conducting high-level research in this area.
“There’s a tremendous shortage of reliable data, largely due to regulatory barriers,” said Dr. Martinez.
Since 1970, cannabis has been listed as a Schedule I drug that is illegal to prescribe (the Agriculture Improvement Act of 2018 removed hemp from such restrictions). The FDA has issued guidance for researchers who wish to investigate treatments using Cannabis sativa or its derivatives in which the THC content is greater than 0.3%. Such research requires regular interactions with several federal agencies, including the Drug Enforcement Administration.
“It’s impossible to do multicenter RCTs with large numbers of patients, because you can’t transport cannabis across state lines,” said Dr. Wallace.
Regulatory restrictions regarding medical cannabis vary considerably throughout the world (the European Monitoring Center for Drugs and Drug Addiction provides a useful breakdown of this on their website). The lack of consistency in regulatory oversight acts as an impediment for conducting large-scale international multicenter studies on the topic.
Dr. Buhmann noted that, in Germany, cannabis has been broadly approved for treatment-resistant conditions with severe symptoms that impair quality of life. In addition, it is easy to be reimbursed for the use of cannabis as a medical treatment. These factors serve as disincentives for the funding of high-quality studies.
“It’s likely that no pharmaceutical company will do an expensive RCT to get an approval for Parkinson’s disease because it is already possible to prescribe medical cannabis of any type of THC-containing cannabinoid, dose, or route of application,” Dr. Buhmann said.
In the face of such restrictions and barriers, researchers are turning to ambitious real-world data projects to better understand medical cannabis’ efficacy and safety. A notable example is ProjectTwenty21, which is supported by the Royal College of Psychiatrists. The project is collecting outcomes of the use of medical cannabis among 20,000 U.K. patients whose conventional treatments of chronic pain, anxiety disorder, epilepsy, multiple sclerosis, PTSD, substance use disorder, and Tourette syndrome failed.
Dr. Freedman noted that the continued lack of high-quality data creates a void that commercial interests fill with unfounded claims.
“The danger is that patients might abandon a medication or intervention backed by robust science in favor of something without any science or evidence behind it,” he said. “There is no reason not to expect the same level of data for claims about cannabis products as we would expect from pharmaceutical products.”
Getting to that point, however, will require that the authorities governing clinical trials begin to view cannabis as the research community does, as a possible treatment with potential value, rather than as an illicit drug that needs to be tamped down.
A version of this article first appeared on Medscape.com.
Although the healing properties of cannabis have been touted for millennia, research into its potential neuropsychiatric applications truly began to take off in the 1990s following the discovery of the cannabinoid system in the brain. This led to speculation that cannabis could play a therapeutic role in regulating dopamine, serotonin, and other neurotransmitters and offer a new means of treating various ailments.
At the same time, efforts to liberalize marijuana laws have successfully played out in several nations, including the United States, where, as of April 29, 36 states provide some access to cannabis. These dual tracks – medical and political – have made cannabis an increasingly accepted part of the cultural fabric.
Yet with this development has come a new quandary for clinicians. Medical cannabis has been made widely available to patients and has largely outpaced the clinical evidence, leaving it unclear how and for which indications it should be used.
The many forms of medical cannabis
Cannabis is a genus of plants that includes marijuana (Cannabis sativa) and hemp. These plants contain over 100 compounds, including terpenes, flavonoids, and – most importantly for medicinal applications – cannabinoids.
The most abundant cannabinoid in marijuana is the psychotropic delta-9-tetrahydrocannabinol (THC), which imparts the “high” sensation. The next most abundant cannabinoid is cannabidiol (CBD), which is the nonpsychotropic. THC and CBD are the most extensively studied cannabinoids, together and in isolation. Evidence suggests that other cannabinoids and terpenoids may also hold medical promise and that cannabis’ various compounds can work synergistically to produce a so-called entourage effect.
Patients walking into a typical medical cannabis dispensary will be faced with several plant-derived and synthetic options, which can differ considerably in terms of the ratios and amounts of THC and CBD they contain, as well in how they are consumed (i.e., via smoke, vapor, ingestion, topical administration, or oromucosal spray), all of which can alter their effects. Further complicating matters is the varying level of oversight each state and country has in how and whether they test for and accurately label products’ potency, cannabinoid content, and possible impurities.
Medically authorized, prescription cannabis products go through an official regulatory review process, and indications/contraindications have been established for them. To date, the Food and Drug Administration has approved one cannabis-derived drug product – Epidiolex (purified CBD) – for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older. The FDA has also approved three synthetic cannabis-related drug products – Marinol, Syndros (or dronabinol, created from synthetic THC), and Cesamet (or nabilone, a synthetic cannabinoid similar to THC) – all of which are indicated for treatment-related nausea and anorexia associated with weight loss in AIDS patients.
Surveys of medical cannabis consumers indicate that most people cannot distinguish between THC and CBD, so the first role that physicians find themselves in when recommending this treatment may be in helping patients navigate the volume of options.
Promising treatment for pain
Chronic pain is the leading reason patients seek out medical cannabis. It is also the indication that most researchers agree has the strongest evidence to support its use.
“In my mind, the most promising immediate use for medical cannabis is with THC for pain,” Diana M. Martinez, MD, a professor of psychiatry at Columbia University, New York, who specializes in addiction research, said in a recent MDedge podcast. “THC could be added to the armamentarium of pain medications that we use today.”
In a 2015 systematic literature review, researchers assessed 28 randomized, controlled trials (RCTs) of the use of cannabinoids for chronic pain. They reported that a variety of formulations resulted in at least a 30% reduction in the odds of pain, compared with placebo. A meta-analysis of five RCTs involving patients with neuropathic pain found a 30% reduction in pain over placebo with inhaled, vaporized cannabis. Varying results have been reported in additional studies for this indication. The National Academies of Sciences, Engineering, and Medicine concluded that there was a substantial body of evidence that cannabis is an effective treatment for chronic pain in adults.
The ongoing opioid epidemic has lent these results additional relevance.
Seeing this firsthand has caused Mark Steven Wallace, MD, a pain management specialist and chair of the division of pain medicine at the University of California San Diego Health, to reconsider offering cannabis to his patients.
“I think it’s probably more efficacious, just from my personal experience, and it’s a much lower risk of abuse and dependence than the opioids,” he said.
Dr. Wallace advised that clinicians who treat pain consider the ratios of cannabinoids.
“This is anecdotal, but we do find that with the combination of the two, CBD reduces the psychoactive effects of the THC. The ratios we use during the daytime range around 20 mg of CBD to 1 mg of THC,” he said.
In a recent secondary analysis of an RCT involving patients with painful diabetic peripheral neuropathy, Dr. Wallace and colleagues showed that THC’s effects appear to reverse themselves at a certain level.
“As the THC level goes up, the pain reduces until you reach about 16 ng/mL; then it starts going in the opposite direction, and pain will start to increase,” he said. “Even recreational cannabis users have reported that they avoid high doses because it’s very aversive. Using cannabis is all about, start low and go slow.”
A mixed bag for neurologic indications
There are relatively limited data on the use of medical cannabis for other neurologic conditions, and results have varied. For uses other than pain management, the evidence that does exist is strongest regarding epilepsy, said Daniel Freedman, DO, assistant professor of neurology at the University of Texas at Austin. He noted “multiple high-quality RCTs showing that pharmaceutical-grade CBD can reduce seizures associated with two particular epilepsy syndromes: Dravet Syndrome and Lennox Gastaut.”
These findings led to the FDA’s 2018 approval of Epidiolex for these syndromes. In earlier years, interest in CBD for pediatric seizures was largely driven by anecdotal parental reports of its benefits. NASEM’s 2017 overview on medical cannabis found evidence from subsequent RCTs in this indication to be insufficient. Clinicians who prescribe CBD for this indication must be vigilant because it can interact with several commonly used antiepileptic drugs.
Cannabinoid treatments have also shown success in alleviating muscle spasticity resulting from multiple sclerosis, most prominently in the form of nabiximols (Sativex), a standardized oralmucosal spray containing approximately equal quantities of THC and CBD. Nabiximols is approved in Europe but not in the United States. Moderate evidence supports the efficacy of these and other treatments over placebo in reducing muscle spasticity. Patient ratings of its effects tend to be higher than clinician assessment.
Parkinson’s disease has not yet been approved as an indication for treatment with cannabis or cannabinoids, yet a growing body of preclinical data suggests these could influence the dopaminergic system, said Carsten Buhmann, MD, from the department of neurology at the University Medical Center Hamburg-Eppendorf (Germany).
“In general, cannabinoids modulate basal-ganglia function on two levels which are especially relevant in Parkinson’s disease, i.e., the glutamatergic/dopaminergic synaptic neurotransmission and the corticostriatal plasticity,” he said. “Furthermore, activation of the endocannabinoid system might induce neuroprotective effects related to direct receptor-independent mechanisms, activation of anti-inflammatory cascades in glial cells via the cannabinoid receptor type 2, and antiglutamatergic antiexcitotoxic properties.”
Dr. Buhmann said that currently, clinical evidence is scarce, consisting of only four double-blind, placebo-controlled RCTs involving 49 patients. Various cannabinoids and methods of administering treatment were employed. Improvement was only observed in one of these RCTs, which found that the cannabinoid receptor agonist nabilone significantly reduced levodopa-induced dyskinesia for patients with Parkinson’s disease. Subjective data support a beneficial effect. In a nationwide survey of 1,348 respondents conducted by Dr. Buhmann and colleagues, the majority of medical cannabis users reported that it improved their symptoms (54% with oral CBD and 68% with inhaled THC-containing cannabis).
NASEM concluded that there was insufficient evidence to support the efficacy of medical cannabis for other neurologic conditions, including Tourette syndrome, amyotrophic lateral sclerosis, Huntington disease, dystonia, or dementia. A 2020 position statement from the American Academy of Neurology cited the lack of sufficient peer-reviewed research as the reason it could not currently support the use of cannabis for neurologic disorders.
Yet, according to Dr. Freedman, who served as a coauthor of the AAN position statement, this hasn’t stymied research interest in the topic. He’s seen a substantial uptick in studies of CBD over the past 2 years. “The body of evidence grows, but I still see many claims being made without evidence. And no one seems to care about all the negative trials.”
Cannabis as a treatment for, and cause of, psychiatric disorders
Mental health problems – such as anxiety, depression, and PTSD – are among the most common reasons patients seek out medical cannabis. There is an understandable interest in using cannabis and cannabinoids to treat psychiatric disorders. Preclinical studies suggest that the endocannabinoid system plays a prominent role in modulating feelings of anxiety, mood, and fear. As with opioids and chronic pain management, there is hope that medical cannabis may provide a means of reducing prescription anxiolytics and their associated risks.
The authors of the first systematic review (BMC Psychiatry. 2020 Jan 16;20[1]:24) of the use of medical cannabis for major psychiatric disorders noted that the current evidence was “encouraging, albeit embryonic.”
Meta-analyses have indicated a small but positive association between cannabis use and anxiety, although this may reflect the fact that patients with anxiety sought out this treatment. Given the risks for substance use disorders among patients with anxiety, CBD may present a more viable option. Positive results have been shown as treatment for generalized social anxiety disorder.
Limited but encouraging results have also been reported regarding the alleviation of PTSD symptoms with both cannabis and CBD, although the body of high-quality evidence hasn’t notably progressed since 2017, when NASEM declared that the evidence was insufficient. Supportive evidence is similarly lacking regarding the treatment of depression. Longitudinal studies suggest that cannabis use, particularly heavy use, may increase the risk of developing this disorder. Because THC is psychoactive, it is advised that it be avoided by patients at risk for psychotic disorders. However, CBD has yielded limited benefits for patients with treatment-resistant schizophrenia and for young people at risk for psychosis.
The use of medical cannabis for psychiatric conditions requires a complex balancing act, inasmuch as these treatments may exacerbate the very problems they are intended to alleviate.
Marta Di Forti, MD, PhD, professor of psychiatric research at Kings College London, has been at the forefront of determining the mental health risks of continued cannabis use. In 2019, Dr. Di Forti developed the first and only Cannabis Clinic for Patients With Psychosis in London where she and her colleagues have continued to elucidate this connection.
Dr. Di Forti and colleagues have linked daily cannabis use to an increase in the risk of experiencing psychotic disorder, compared with never using it. That risk was further increased among users of high-potency cannabis (≥10% THC). The latter finding has troubling implications, because concentrations of THC have steadily risen since 1970. By contrast, CBD concentrations have remained generally stable. High-potency cannabis products are common in both recreational and medicinal settings.
“For somebody prescribing medicinal cannabis that has a ≥10% concentration of THC, I’d be particularly wary of the risk of psychosis,” said Dr. Di Forti. “If you’re expecting people to use a high content of THC daily to medicate pain or a chronic condition, you even more so need to be aware that this is a potential side effect.”
Dr. Di Forti noted that her findings come from a cohort of recreational users, most of whom were aged 18-35 years.
“There have actually not been studies developed from collecting data in this area from groups specifically using cannabis for medicinal rather than recreational purposes,” she said.
She added that she personally has no concerns about the use of medical cannabis but wants clinicians to be aware of the risk for psychosis, to structure their patient conversations to identify risk factors or family histories of psychosis, and to become knowledgeable in detecting the often subtle signs of its initial onset.
When cannabis-associated psychosis occurs, Dr. Di Forti said it is primarily treated with conventional means, such as antipsychotics and therapeutic interventions and by refraining from using cannabis. Achieving the latter goal can be a challenge for patients who are daily users of high-potency cannabis. Currently, there are no treatment options such as those offered to patients withdrawing from the use of alcohol or opioids. Dr. Di Forti and colleagues are currently researching a solution to that problem through the use of another medical cannabis, the oromucosal spray Sativex, which has been approved in the European Union.
The regulatory obstacles to clarifying cannabis’ role in medicine
That currently there is limited or no evidence to support the use of medical cannabis for the treatment of neuropsychiatric conditions points to the inherent difficulties in conducting high-level research in this area.
“There’s a tremendous shortage of reliable data, largely due to regulatory barriers,” said Dr. Martinez.
Since 1970, cannabis has been listed as a Schedule I drug that is illegal to prescribe (the Agriculture Improvement Act of 2018 removed hemp from such restrictions). The FDA has issued guidance for researchers who wish to investigate treatments using Cannabis sativa or its derivatives in which the THC content is greater than 0.3%. Such research requires regular interactions with several federal agencies, including the Drug Enforcement Administration.
“It’s impossible to do multicenter RCTs with large numbers of patients, because you can’t transport cannabis across state lines,” said Dr. Wallace.
Regulatory restrictions regarding medical cannabis vary considerably throughout the world (the European Monitoring Center for Drugs and Drug Addiction provides a useful breakdown of this on their website). The lack of consistency in regulatory oversight acts as an impediment for conducting large-scale international multicenter studies on the topic.
Dr. Buhmann noted that, in Germany, cannabis has been broadly approved for treatment-resistant conditions with severe symptoms that impair quality of life. In addition, it is easy to be reimbursed for the use of cannabis as a medical treatment. These factors serve as disincentives for the funding of high-quality studies.
“It’s likely that no pharmaceutical company will do an expensive RCT to get an approval for Parkinson’s disease because it is already possible to prescribe medical cannabis of any type of THC-containing cannabinoid, dose, or route of application,” Dr. Buhmann said.
In the face of such restrictions and barriers, researchers are turning to ambitious real-world data projects to better understand medical cannabis’ efficacy and safety. A notable example is ProjectTwenty21, which is supported by the Royal College of Psychiatrists. The project is collecting outcomes of the use of medical cannabis among 20,000 U.K. patients whose conventional treatments of chronic pain, anxiety disorder, epilepsy, multiple sclerosis, PTSD, substance use disorder, and Tourette syndrome failed.
Dr. Freedman noted that the continued lack of high-quality data creates a void that commercial interests fill with unfounded claims.
“The danger is that patients might abandon a medication or intervention backed by robust science in favor of something without any science or evidence behind it,” he said. “There is no reason not to expect the same level of data for claims about cannabis products as we would expect from pharmaceutical products.”
Getting to that point, however, will require that the authorities governing clinical trials begin to view cannabis as the research community does, as a possible treatment with potential value, rather than as an illicit drug that needs to be tamped down.
A version of this article first appeared on Medscape.com.
Although the healing properties of cannabis have been touted for millennia, research into its potential neuropsychiatric applications truly began to take off in the 1990s following the discovery of the cannabinoid system in the brain. This led to speculation that cannabis could play a therapeutic role in regulating dopamine, serotonin, and other neurotransmitters and offer a new means of treating various ailments.
At the same time, efforts to liberalize marijuana laws have successfully played out in several nations, including the United States, where, as of April 29, 36 states provide some access to cannabis. These dual tracks – medical and political – have made cannabis an increasingly accepted part of the cultural fabric.
Yet with this development has come a new quandary for clinicians. Medical cannabis has been made widely available to patients and has largely outpaced the clinical evidence, leaving it unclear how and for which indications it should be used.
The many forms of medical cannabis
Cannabis is a genus of plants that includes marijuana (Cannabis sativa) and hemp. These plants contain over 100 compounds, including terpenes, flavonoids, and – most importantly for medicinal applications – cannabinoids.
The most abundant cannabinoid in marijuana is the psychotropic delta-9-tetrahydrocannabinol (THC), which imparts the “high” sensation. The next most abundant cannabinoid is cannabidiol (CBD), which is the nonpsychotropic. THC and CBD are the most extensively studied cannabinoids, together and in isolation. Evidence suggests that other cannabinoids and terpenoids may also hold medical promise and that cannabis’ various compounds can work synergistically to produce a so-called entourage effect.
Patients walking into a typical medical cannabis dispensary will be faced with several plant-derived and synthetic options, which can differ considerably in terms of the ratios and amounts of THC and CBD they contain, as well in how they are consumed (i.e., via smoke, vapor, ingestion, topical administration, or oromucosal spray), all of which can alter their effects. Further complicating matters is the varying level of oversight each state and country has in how and whether they test for and accurately label products’ potency, cannabinoid content, and possible impurities.
Medically authorized, prescription cannabis products go through an official regulatory review process, and indications/contraindications have been established for them. To date, the Food and Drug Administration has approved one cannabis-derived drug product – Epidiolex (purified CBD) – for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older. The FDA has also approved three synthetic cannabis-related drug products – Marinol, Syndros (or dronabinol, created from synthetic THC), and Cesamet (or nabilone, a synthetic cannabinoid similar to THC) – all of which are indicated for treatment-related nausea and anorexia associated with weight loss in AIDS patients.
Surveys of medical cannabis consumers indicate that most people cannot distinguish between THC and CBD, so the first role that physicians find themselves in when recommending this treatment may be in helping patients navigate the volume of options.
Promising treatment for pain
Chronic pain is the leading reason patients seek out medical cannabis. It is also the indication that most researchers agree has the strongest evidence to support its use.
“In my mind, the most promising immediate use for medical cannabis is with THC for pain,” Diana M. Martinez, MD, a professor of psychiatry at Columbia University, New York, who specializes in addiction research, said in a recent MDedge podcast. “THC could be added to the armamentarium of pain medications that we use today.”
In a 2015 systematic literature review, researchers assessed 28 randomized, controlled trials (RCTs) of the use of cannabinoids for chronic pain. They reported that a variety of formulations resulted in at least a 30% reduction in the odds of pain, compared with placebo. A meta-analysis of five RCTs involving patients with neuropathic pain found a 30% reduction in pain over placebo with inhaled, vaporized cannabis. Varying results have been reported in additional studies for this indication. The National Academies of Sciences, Engineering, and Medicine concluded that there was a substantial body of evidence that cannabis is an effective treatment for chronic pain in adults.
The ongoing opioid epidemic has lent these results additional relevance.
Seeing this firsthand has caused Mark Steven Wallace, MD, a pain management specialist and chair of the division of pain medicine at the University of California San Diego Health, to reconsider offering cannabis to his patients.
“I think it’s probably more efficacious, just from my personal experience, and it’s a much lower risk of abuse and dependence than the opioids,” he said.
Dr. Wallace advised that clinicians who treat pain consider the ratios of cannabinoids.
“This is anecdotal, but we do find that with the combination of the two, CBD reduces the psychoactive effects of the THC. The ratios we use during the daytime range around 20 mg of CBD to 1 mg of THC,” he said.
In a recent secondary analysis of an RCT involving patients with painful diabetic peripheral neuropathy, Dr. Wallace and colleagues showed that THC’s effects appear to reverse themselves at a certain level.
“As the THC level goes up, the pain reduces until you reach about 16 ng/mL; then it starts going in the opposite direction, and pain will start to increase,” he said. “Even recreational cannabis users have reported that they avoid high doses because it’s very aversive. Using cannabis is all about, start low and go slow.”
A mixed bag for neurologic indications
There are relatively limited data on the use of medical cannabis for other neurologic conditions, and results have varied. For uses other than pain management, the evidence that does exist is strongest regarding epilepsy, said Daniel Freedman, DO, assistant professor of neurology at the University of Texas at Austin. He noted “multiple high-quality RCTs showing that pharmaceutical-grade CBD can reduce seizures associated with two particular epilepsy syndromes: Dravet Syndrome and Lennox Gastaut.”
These findings led to the FDA’s 2018 approval of Epidiolex for these syndromes. In earlier years, interest in CBD for pediatric seizures was largely driven by anecdotal parental reports of its benefits. NASEM’s 2017 overview on medical cannabis found evidence from subsequent RCTs in this indication to be insufficient. Clinicians who prescribe CBD for this indication must be vigilant because it can interact with several commonly used antiepileptic drugs.
Cannabinoid treatments have also shown success in alleviating muscle spasticity resulting from multiple sclerosis, most prominently in the form of nabiximols (Sativex), a standardized oralmucosal spray containing approximately equal quantities of THC and CBD. Nabiximols is approved in Europe but not in the United States. Moderate evidence supports the efficacy of these and other treatments over placebo in reducing muscle spasticity. Patient ratings of its effects tend to be higher than clinician assessment.
Parkinson’s disease has not yet been approved as an indication for treatment with cannabis or cannabinoids, yet a growing body of preclinical data suggests these could influence the dopaminergic system, said Carsten Buhmann, MD, from the department of neurology at the University Medical Center Hamburg-Eppendorf (Germany).
“In general, cannabinoids modulate basal-ganglia function on two levels which are especially relevant in Parkinson’s disease, i.e., the glutamatergic/dopaminergic synaptic neurotransmission and the corticostriatal plasticity,” he said. “Furthermore, activation of the endocannabinoid system might induce neuroprotective effects related to direct receptor-independent mechanisms, activation of anti-inflammatory cascades in glial cells via the cannabinoid receptor type 2, and antiglutamatergic antiexcitotoxic properties.”
Dr. Buhmann said that currently, clinical evidence is scarce, consisting of only four double-blind, placebo-controlled RCTs involving 49 patients. Various cannabinoids and methods of administering treatment were employed. Improvement was only observed in one of these RCTs, which found that the cannabinoid receptor agonist nabilone significantly reduced levodopa-induced dyskinesia for patients with Parkinson’s disease. Subjective data support a beneficial effect. In a nationwide survey of 1,348 respondents conducted by Dr. Buhmann and colleagues, the majority of medical cannabis users reported that it improved their symptoms (54% with oral CBD and 68% with inhaled THC-containing cannabis).
NASEM concluded that there was insufficient evidence to support the efficacy of medical cannabis for other neurologic conditions, including Tourette syndrome, amyotrophic lateral sclerosis, Huntington disease, dystonia, or dementia. A 2020 position statement from the American Academy of Neurology cited the lack of sufficient peer-reviewed research as the reason it could not currently support the use of cannabis for neurologic disorders.
Yet, according to Dr. Freedman, who served as a coauthor of the AAN position statement, this hasn’t stymied research interest in the topic. He’s seen a substantial uptick in studies of CBD over the past 2 years. “The body of evidence grows, but I still see many claims being made without evidence. And no one seems to care about all the negative trials.”
Cannabis as a treatment for, and cause of, psychiatric disorders
Mental health problems – such as anxiety, depression, and PTSD – are among the most common reasons patients seek out medical cannabis. There is an understandable interest in using cannabis and cannabinoids to treat psychiatric disorders. Preclinical studies suggest that the endocannabinoid system plays a prominent role in modulating feelings of anxiety, mood, and fear. As with opioids and chronic pain management, there is hope that medical cannabis may provide a means of reducing prescription anxiolytics and their associated risks.
The authors of the first systematic review (BMC Psychiatry. 2020 Jan 16;20[1]:24) of the use of medical cannabis for major psychiatric disorders noted that the current evidence was “encouraging, albeit embryonic.”
Meta-analyses have indicated a small but positive association between cannabis use and anxiety, although this may reflect the fact that patients with anxiety sought out this treatment. Given the risks for substance use disorders among patients with anxiety, CBD may present a more viable option. Positive results have been shown as treatment for generalized social anxiety disorder.
Limited but encouraging results have also been reported regarding the alleviation of PTSD symptoms with both cannabis and CBD, although the body of high-quality evidence hasn’t notably progressed since 2017, when NASEM declared that the evidence was insufficient. Supportive evidence is similarly lacking regarding the treatment of depression. Longitudinal studies suggest that cannabis use, particularly heavy use, may increase the risk of developing this disorder. Because THC is psychoactive, it is advised that it be avoided by patients at risk for psychotic disorders. However, CBD has yielded limited benefits for patients with treatment-resistant schizophrenia and for young people at risk for psychosis.
The use of medical cannabis for psychiatric conditions requires a complex balancing act, inasmuch as these treatments may exacerbate the very problems they are intended to alleviate.
Marta Di Forti, MD, PhD, professor of psychiatric research at Kings College London, has been at the forefront of determining the mental health risks of continued cannabis use. In 2019, Dr. Di Forti developed the first and only Cannabis Clinic for Patients With Psychosis in London where she and her colleagues have continued to elucidate this connection.
Dr. Di Forti and colleagues have linked daily cannabis use to an increase in the risk of experiencing psychotic disorder, compared with never using it. That risk was further increased among users of high-potency cannabis (≥10% THC). The latter finding has troubling implications, because concentrations of THC have steadily risen since 1970. By contrast, CBD concentrations have remained generally stable. High-potency cannabis products are common in both recreational and medicinal settings.
“For somebody prescribing medicinal cannabis that has a ≥10% concentration of THC, I’d be particularly wary of the risk of psychosis,” said Dr. Di Forti. “If you’re expecting people to use a high content of THC daily to medicate pain or a chronic condition, you even more so need to be aware that this is a potential side effect.”
Dr. Di Forti noted that her findings come from a cohort of recreational users, most of whom were aged 18-35 years.
“There have actually not been studies developed from collecting data in this area from groups specifically using cannabis for medicinal rather than recreational purposes,” she said.
She added that she personally has no concerns about the use of medical cannabis but wants clinicians to be aware of the risk for psychosis, to structure their patient conversations to identify risk factors or family histories of psychosis, and to become knowledgeable in detecting the often subtle signs of its initial onset.
When cannabis-associated psychosis occurs, Dr. Di Forti said it is primarily treated with conventional means, such as antipsychotics and therapeutic interventions and by refraining from using cannabis. Achieving the latter goal can be a challenge for patients who are daily users of high-potency cannabis. Currently, there are no treatment options such as those offered to patients withdrawing from the use of alcohol or opioids. Dr. Di Forti and colleagues are currently researching a solution to that problem through the use of another medical cannabis, the oromucosal spray Sativex, which has been approved in the European Union.
The regulatory obstacles to clarifying cannabis’ role in medicine
That currently there is limited or no evidence to support the use of medical cannabis for the treatment of neuropsychiatric conditions points to the inherent difficulties in conducting high-level research in this area.
“There’s a tremendous shortage of reliable data, largely due to regulatory barriers,” said Dr. Martinez.
Since 1970, cannabis has been listed as a Schedule I drug that is illegal to prescribe (the Agriculture Improvement Act of 2018 removed hemp from such restrictions). The FDA has issued guidance for researchers who wish to investigate treatments using Cannabis sativa or its derivatives in which the THC content is greater than 0.3%. Such research requires regular interactions with several federal agencies, including the Drug Enforcement Administration.
“It’s impossible to do multicenter RCTs with large numbers of patients, because you can’t transport cannabis across state lines,” said Dr. Wallace.
Regulatory restrictions regarding medical cannabis vary considerably throughout the world (the European Monitoring Center for Drugs and Drug Addiction provides a useful breakdown of this on their website). The lack of consistency in regulatory oversight acts as an impediment for conducting large-scale international multicenter studies on the topic.
Dr. Buhmann noted that, in Germany, cannabis has been broadly approved for treatment-resistant conditions with severe symptoms that impair quality of life. In addition, it is easy to be reimbursed for the use of cannabis as a medical treatment. These factors serve as disincentives for the funding of high-quality studies.
“It’s likely that no pharmaceutical company will do an expensive RCT to get an approval for Parkinson’s disease because it is already possible to prescribe medical cannabis of any type of THC-containing cannabinoid, dose, or route of application,” Dr. Buhmann said.
In the face of such restrictions and barriers, researchers are turning to ambitious real-world data projects to better understand medical cannabis’ efficacy and safety. A notable example is ProjectTwenty21, which is supported by the Royal College of Psychiatrists. The project is collecting outcomes of the use of medical cannabis among 20,000 U.K. patients whose conventional treatments of chronic pain, anxiety disorder, epilepsy, multiple sclerosis, PTSD, substance use disorder, and Tourette syndrome failed.
Dr. Freedman noted that the continued lack of high-quality data creates a void that commercial interests fill with unfounded claims.
“The danger is that patients might abandon a medication or intervention backed by robust science in favor of something without any science or evidence behind it,” he said. “There is no reason not to expect the same level of data for claims about cannabis products as we would expect from pharmaceutical products.”
Getting to that point, however, will require that the authorities governing clinical trials begin to view cannabis as the research community does, as a possible treatment with potential value, rather than as an illicit drug that needs to be tamped down.
A version of this article first appeared on Medscape.com.
Cannabinoid-based medications for pain
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.
In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.
CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67
Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70
Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.
In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.
CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67
Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70
Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
Against the backdrop of an increasing opioid use epidemic and a marked acceleration of prescription opioid–related deaths,1,2 there has been an impetus to explore the usefulness of alternative and co-analgesic agents to assist patients with chronic pain. Preclinical studies employing animal-based models of human pain syndromes have demonstrated that cannabis and chemicals derived from cannabis extracts may mitigate several pain conditions.3
Because there are significant comorbidities between psychiatric disorders and chronic pain, psychiatrists are likely to care for patients with chronic pain. As the availability of and interest in cannabinoid-based medications (CBM) increases, psychiatrists will need to be apprised of the utility, adverse effects, and potential drug interactions of these agents.
The endocannabinoid system and cannabis receptors
The endogenous cannabinoid (endocannabinoid) system is abundantly present within the peripheral and central nervous systems. The first identified, and best studied, endocannabinoids are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG).4 Unlike typical neurotransmitters, AEA and 2-AG are not stored within vesicles within presynaptic neuron axons. Instead, they are lipophilic molecules produced on demand, synthesized from phospholipids (ie, arachidonic acid derivatives) at the membranes of post-synaptic neurons, and released into the synapse directly.5
Acting as retrograde messengers, the endocannabinoids traverse the synapse, binding to receptors located on the axons of the presynaptic neuron. Two receptors—CB1 and CB2—have been most extensively studied and characterized.6,7 These receptors couple to Gi/o-proteins to inhibit adenylate cyclase, decreasing Ca2+ conductance and increasing K+ conductance.8 Once activated, cannabinoid receptors modulate neurotransmitter release from presynaptic axon terminals. Evidence points to a similar retrograde signaling between neurons and glial cells. Shortly after receptor activation, the endocannabinoids are deactivated by the actions of a transporter mechanism and enzyme degradation.9,10
The endocannabinoid system and pain transmission
Cannabinoid receptors are present in pain transmission circuits spanning from the peripheral sensory nerve endings (from which pain signals originate) to the spinal cord and supraspinal regions within the brain.11-14 CB1 receptors are abundantly present within the CNS, including regions involved in pain transmission. Binding to CB1 receptors, endocannabinoids modulate neurotransmission that impacts pain transmission centrally. Endocannabinoids can also indirectly modulate opiate and N-methyl-
By contrast, CB2 receptors are predominantly localized to peripheral tissues and immune cells, although there has been some discovery of their presence within the CNS (eg, on microglia). Endocannabinoid activation of CB2 receptors is thought to modulate the activity of peripheral afferent pain fibers and immune-mediated neuroinflammatory processes—such as inhibition of prostaglandin synthesis and mast cell degranulation—that can precipitate and maintain chronic pain states.16-18
Evidence garnered from preclinical (animal) studies points to the role of the endocannabinoid system in modulating normal pain transmission (see Manzanares et al3 for details). These studies offer a putative basis for understanding how exogenous cannabinoid congeners might serve to ameliorate pain transmission in pathophysiologic states, including chronic pain.
Continue to: Cannabinoid-based medications
Cannabinoid-based medications
Marijuana contains multiple components (cannabinoids). The most extensively studied are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because it predominantly binds CB1 receptors centrally, THC is the major psychoactive component of cannabis; it promotes sleep and appetite, influences anxiety, and produces the “high” associated with cannabis use. By contrast, CBD weakly binds CB1 and thus exerts minimal or no psychoactive effects.19
Cannabinoid absorption, metabolism, bioavailability, and clinical effects vary depending on the formulation and method of administration (Table 1).20-22 THC and CBD content and potency in inhaled cannabis can vary significantly depending on the strains of the cannabis plant and manner of cultivation.23 To standardize approaches for administering cannabinoids in clinical trials and for clinical use, researchers have developed pharmaceutical analogs that contain extracted chemicals or synthetic chemicals similar to THC and/or CBD.
In this article, CBM refers to smoked/vaporized herbal cannabis as well as pharmaceutical cannabis analogs. Table 2 summarizes the characteristics of CBM commonly used in studies investigating their use for managing pain conditions.
CBM for chronic pain
The literature base examining the role of CBM for managing chronic nonmalignant and malignant pain of varying etiologies is rapidly expanding. Randomized controlled trials (RCTs) have focused on inhaled/smoked products and related cannabinoid medications, some of which are FDA-approved (Table 2).
A multitude of other cannabinoid-based products are currently commercially available to consumers, including tincture and oil-based products; over-the-counter CBD products; and several other formulations of CBM (eg, edible and suppository products). Because such products are not standardized or quality-controlled,24 RCTs have not assessed their efficacy for mitigating pain. Consequently, the findings summarized in this article do not address the utility of these agents.
Continue to: CBM for non-cancer pain
CBM for non-cancer pain
Neuropathic pain. Randomized controlled trials have assessed the pain-mitigating effects of various CBM, including inhaled cannabis, synthetic THC, plant-extracted CBD, and a THC/CBD spray. Studies have shown that inhaled/vaporized cannabis can produce short-term pain reduction in patients with chronic neuropathic pain of diverse etiologies, including diabetes mellitus-, HIV-, trauma-, and medication-induced neuropathies.22,25,26 Similar beneficial effects have been observed with the use of cannabis analogues (eg, nabiximols).25,26-29
Meta-analyses and systematic reviews have determined that most of these RCTs were of low-to-moderate quality.26,30 Meta-analyses have revealed divergent and conflicting results because of differences in the inclusion and exclusion criteria used to select RCTs for analysis and differences in the standards with which the quality of evidence were determined.25,30
Overall, the benefit of CBM for mitigating neuropathic pain is promising, but the effectiveness may not be robust.30,31 Several noteworthy caveats limit the interpretation of the results of these RCTs:
- due to the small sample sizes and brief durations of study, questions remain regarding the extent to which effects are generalizable, whether the benefits are sustained, and whether adverse effects emerge over time with continued use
- most RCTs evaluated inhaled (herbal) cannabis and nabiximols; there is little data on the effectiveness of other CBM formulations25,26,30
- the pain-mitigating effects of CBM were usually compared with those of placebo; the comparative efficacy against agents commonly used to treat neuropathic pain remains largely unexamined
- these RCTs typically compared mean pain severity score differences between cannabis-treated and placebo groups using standard subjective rating scales of pain intensity, such as the Numerical Rating Scale or Visual Analogue Scale. Customarily, the pain literature has used a 30% or 50% reduction in pain severity from baseline as an indicator of significant clinical improvement.32,33 The RCTs of CBM for neuropathic pain rarely used this standard, which makes it unclear whether CBM results in clinically significant pain reductions30
- indirect measures of effectiveness (ie, whether using CBM reduces the need for opioids or other analgesics to manage pain) were seldom reported in these RCTs.
Due to these limitations, clinical guidelines and systematic reviews consider CBM as a third- or fourth-line therapy for patients experiencing chronic neuropathic pain for whom conventional agents such as anticonvulsants and antidepressants have failed.34,35
Spasticity in multiple sclerosis (MS). Several RCTs have assessed the use of CBM for MS-related spasticity, although few were deemed to be high quality. Nabiximols and synthetic THC were effective in managing spasticity and reducing pain severity associated with muscle spasms.36 Generally, investigations revealed that CBM were associated with improvements in subjective measures of spasticity, but these were not born out in clinical, objective measures.26,37 The efficacy of smoked cannabis was uncertain.37 The existing literature on CBM for MS-related spasticity does not address dosing, duration of effects, tolerability, or comparative effectiveness against conventional anti-spasm medications.36,37
Continue to: Other chronic pain conditions
Other chronic pain conditions. CBM have also been studied for their usefulness in several other noncancer chronic conditions, including Crohn’s disease, inflammatory bowel disease, fibromyalgia, and other rheumatologic pain conditions.22,31,38-40 However, a solid foundation of empirical work to inform their utility for managing pain in these conditions is lacking.
CBM for cancer pain
Anecdotal evidence suggests that inhaled cannabis has promising pain-mitigating effects in patients with advanced cancer.41-43 There is a dearth of high-quality RCTs assessing the utility of CBM in patients with cancer pain.43-45 The types of CBM used and dosing strategies varied across RCTs, which makes it difficult to infer how best to treat patients with cancer pain. The agents studied included nabiximols, THC spray, and synthetic THC capsules.43-45 Although some studies have demonstrated that synthetic THC and nabiximols have potential for reducing subjective pain ratings compared with placebo,46,47 these results were inconsistent.46,48 Oromucosal nabiximols did not appear to confer any additional analgesic benefit in patients who were already prescribed opioids.31,45
The benefit of CBM for mitigating cancer pain is promising, but it remains difficult to know how to position the use of CBM in managing cancer pain. Limitations in the cancer literature include:
- the RCTs addressing CBM use for cancer pain were often brief, which raises questions about the long-term effectiveness and adverse effects of these agents
- tolerability and dosing limits encountered due to adverse effects were seldom reported43,45
- the types of cancer pain that patients had were often quite diverse. The small sample sizes and the heterogeneity of conditions included in these RCTs limit the ability to determine whether pain-mitigating effects might vary according to type of cancer-related pain.31,45
Despite these limitations, some clinical guidelines and systematic reviews have suggested that CBM have some role in addressing refractory malignant pain conditions.49
Psychiatric considerations related to CBM
As of November 2020, 36 states had legalized the use of cannabis for medical purposes, typically for painful conditions, despite the fact that empirical evidence to support their efficacy is mixed.50 In light of recent changes in both the legal and popular attitudes regarding cannabis, the implications of legalizing CBM remains to be seen. For example, some research suggests that adults with pain are vulnerable to frequent nonmedical cannabis use and/or cannabis use disorder.51 Although well-intended, the legalization of CBM use might represent society’s next misstep in the quest to address the suffering of patients with chronic pain. Some evidence shows that cannabis use and cannabis use disorders increase in states that have legalized medical marijuana.52,53 Psychiatrists will be on the front lines of addressing any potential consequences arising from the use of CBM for treating pain.
Continue to: Psychiatric disorders and CBM
Psychiatric disorders and CBM. The psychological impact of CBM use among patients enduring chronic pain can include sedation, cognitive/attention disturbance, and fatigue. These adverse effects can limit the utility of such agents.22,29,45
Contraindications for CBM use, and conditions for which CBM ought to be used with caution, are listed in Table 354,55.The safety of CBM, particularly in patients with chronic pain and psychiatric disorders, has not been examined. Patients with psychiatric disorders may be poor candidates for medical cannabis. Epidemiologic data suggest that recreational cannabis use is positively associated both cross-sectionally and prospectively with psychotic spectrum disorders, depressive symptoms, and anxiety symptoms, including panic disorder.56 Psychotic reactions have also been associated with CBM (dronabinol and nabilone).57 Cannabis use also has been associated with an earlier onset of, and lower remission rates of, symptoms associated with bipolar disorder.58,59 Consequently, patients who have been diagnosed with or are at risk for developing any of the aforementioned conditions may not be suitable candidates for CBM. If CBM are used, patients should be closely monitored for the emergence/exacerbation of psychiatric symptoms. The frequency and extent of follow-up is not clear, however. Because of its reduced propensity to produce psychoactive effects, CBD may be safer than THC for managing pain in individuals who have or are vulnerable to developing psychiatric disorders.
There is a lack of evidence to support the use of CBM for treating primary depressive disorders, general anxiety disorder, posttraumatic stress disorder, or psychosis.60,61 Very low-quality evidence suggests that CBM could lead to a small improvement in anxiety among individuals with noncancer pain and MS.60 However, interpreting causality is complicated. It is plausible that, for some patients, subjective improvement in pain severity may be related to reduced anxiety.62 Conversely, it is equally plausible that reductions in emotional distress may reduce the propensity to attend to, and thus magnify, pain severity. In the latter case, the indirect impact of reducing pain by modifying emotional distress can be impacted by the type and dose of CBM used. For example, low concentrations of THC produce anxiolytic effects, but high concentrations may be anxiety-provoking.63,64
Several potential pharmacokinetic drug interactions may arise between herbal cannabis or CBM and other medications (Table 465,66). THC and CBD are both metabolized by cytochrome P450 (CYP) 2C19 and 3A4.65,66 In addition, THC is also metabolized by CYP2C9. Medications that inhibit or induce these enzymes can increase or decrease the bioavailability of THC and CBD.67
Simultaneously, cannabinoids can impact the bioavailability of co-prescribed medications (Table 566,68). Although such CYP enzyme interactions remain a theoretical possibility, it is uncertain whether significant perturbations in plasma concentrations (and clinical effects) have been encountered with prescription medications when co-administered with CBM.69 Nonetheless, patients receiving CBM should be closely monitored for their response to prescribed medications.70
Continue to: Potential CYP enzyme interactions...
Potential CYP enzyme interactions aside, clinicians need to consider the additive effects that may occur when CBM are combined with sympathomimetic agents (eg, tachycardia, hypertension); CNS depressants such as alcohol, benzodiazepines, and opioids (eg, drowsiness, ataxia); or anticholinergics (eg, tachycardia, confusion).71 Inhaled herbal cannabis contains mutagens and can result in lung damage, exacerbations of chronic bronchitis, and certain types of cancer.54,72 Co-prescribing benzodiazepines may be contraindicated in light of their effects on respiratory rate and effort.
The THC contained in CBM produces hormonal effects (ie, significantly increases plasma levels of ghrelin and leptin and decreases peptide YY levels)73 that affect appetite and can produce weight gain. This may be problematic for patients receiving psychoactive medications associated with increased risk of weight gain and dyslipidemia. Because of the association between cannabis use and motor vehicle accidents, patients whose jobs require them to drive or operate industrial equipment may not be ideal candidates for CBM, especially if such patients also consume alcohol or are prescribed benzodiazepines and/or sedative hypnotics.74 Lastly, due to their lipophilicity, cannabinoids cross the placental barrier and can be found in breast milk75 and therefore can affect pregnancy outcomes and neurodevelopment.
Bottom Line
The popularity of cannabinoid-based medications (CBM) for the treatment of chronic pain conditions is growing, but the interest in their use may be outpacing the evidence supporting their analgesic benefits. High-quality, well-controlled randomized controlled trials are needed to decipher whether, and to what extent, these agents can be positioned in chronic pain management. Because psychiatrists are likely to encounter patients considering, or receiving, CBM, they must be aware of the potential benefits, risks, and adverse effects of such treatments.
Related Resources
- Joshi KG. Cannabis-derived compounds: what you need to know. Current Psychiatry. 2020;19(10):64-65. doi:10.12788/ cp.0050
- Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018; 17(1):34-41.
Drug Brand Names
Ajulemic acid • Anabasum
Alprazolam • Xanax
Amitriptyline • Elavil
Aripiprazole • Abilify, Abilify Maintena
Buspirone • BuSpar
Cannabidiol • Epidiolex
Carbamazepine • Tegretol, Equetro
Cimetidine • Tagamet HB
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Cyclosporine • Neoral, Sandimmune
Dronabinol • Marinol, Syndros
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Imipramine • Tofranil
Ketoconazole • Nizoral AD
Losartan • Cozaar
Midazolam • Versed
Mirtazapine • Remeron
Nabilone • Cesamet
Nabiximols • Sativex
Nefazodone • Serzone
Olanzapine • Zyprexa
Phenobarbital • Solfoton
Phenytoin • Dilantin
Ramelteon • Rozerem
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Topiramate • Topamax
Valproic acid • Depakote, Depakene
Venlafaxine • Effexor
Verapamil • Verelan
Zolpidem • Ambien
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
1. Okie S. A floor of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985. doi:10.1056/NEJMp1011512
2. Powell D, Pacula RL, Taylor E. How increasing medical access to opioids contributes to the opioid epidemic: evidence from Medicare Part D. J Health Econ. 2020;71:102286. doi: 10.1016/j.jhealeco.2019.102286
3. Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239-257. doi: 10.2174/157015906778019527
4. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833
5. Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899-2903. doi:10.3892/mmr.2016.5585
6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83-90. doi:10.1016/0006-2952(95)00109-d
7. Walker JM, Krey JF, Chu CJ, et al. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121(1-2):159-172. doi: 10.1016/s0009-3084(02)00152-4
8. Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142(8):1209-1218. doi: 10.1038/sj.bjp.0705881
9. Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7-14.
10. Piomelli D, Beltramo M, Giuffrida A, et al. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5(6 Pt B):462-473. doi: 10.1006/nbdi.1998.0221
11. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175-191. doi: 10.1093/cercor/bhj136
12. Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534(Pt 3):805-812. doi: 10.1111/j.1469-7793.2001.00805.x
13. Vaughan CW, Connor M, Bagley EE, et al. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57(2):288-295.
14. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127(4):935-940. doi: 10.1038/sj.bjp.0702636
15. Raichlen DA, Foster AD, Gerdeman GI, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high.” J Exp Biol. 2012;215(Pt 8):1331-1336. doi: 10.1242/jeb.063677
16. Beltrano M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Chem. 2009;234:253-254.
17. Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-10533. doi: 10.1073/pnas.1834309100
18. Valenzano KJ, Tafessem L, Lee G, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658-672.
19. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588-631. doi: 10.1124/pr.110.003004
20. Carter GT, Weydt P, Kyashna-Tocha M, et al. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;7(5):464-470.
21. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
22. Johal H, Devji T, Chang Y, et al. cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461. doi: 10.1177/1179544120906461
23. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91(6):966-975. doi: 10.3732/ajb.91.6.966
24. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. doi: 10.1080/02791072.2013.805976
25. Andreae MH, Carter GM, Shaparin N, et al. inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221-1232. doi: 10.1016/j.jpain.2015.07.009
26. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358
27. Boychuk DG, Goddard G, Mauro G, et al. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7-14. doi: 10.11607/ofph.1274
28. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735-744. doi: 10.1111/j.1365-2125.2011.03970.x
29. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932-1954. doi: 10.1097/j.pain.0000000000001293
30. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2
31. Häuser W, Fitzcharles MA, Radbruch L, et al. Cannabinoids in pain management and palliative medicine. Dtsch Arztebl Int. 2017;114(38):627-634. doi: 10.3238/arztebl.2017.0627
32. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005
33. Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30(5):974-985. doi: 10.1016/j.clinthera.2008.05.011
34. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-335. doi: 10.1155/2014/754693
35. Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62-88. doi: 10.1007/s00482-015-0089-y
36. Rice J, Cameron M. Cannabinoids for treatment of MS symptoms: state of the evidence. Curr Neurol Neurosci Rep. 2018;18(8):50. doi: 10.1007/s11910-018-0859-x
37. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563. doi: 10.1212/WNL.0000000000000363
38. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn’s disease and ulcerative colitis: evidence from Cochrane Reviews. Inflamm Bowel Dis. 2020;26(4):502-509. doi: 10.1093/ibd/izz233
39. Katz-Talmor D, Katz I, Porat-Katz BS, et al. Cannabinoids for the treatment of rheumatic diseases - where do we stand? Nat Rev Rheumatol. 2018;14(8):488-498. doi: 10.1038/s41584-018-0025-5
40. Walitt B, Klose P, Fitzcharles MA, et al. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2
41. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37‐43. doi: 10.1016/j.ejim.2018.01.023
42. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705‐713. doi:10.1200/EDBK_180469
43. Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):5215-5222. doi: 10.21037/apm.2017.08.05
44. Aviram J, Samuelly-Lechtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755-E796.
45. Häuser W, Welsch P, Klose P, et al. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: a systematic review with meta-analysis of randomised controlled trials. Schmerz. 2019;33(5):424-436. doi: 10.1007/s00482-019-0373-3
46. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39:167-179.
47. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438-449. doi: 10.1016/j.jpain.2012.01.003
48. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166-173. doi: 10.1016/j.jpainsymman.2013.02.018
49. Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11:1758835919866362. doi: 10.1177/1758835919866362
50. National Conference of State Legislatures (ncsl.org). State Medical Marijuana Laws. Accessed April 5, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
51. Hasin DS, Shmulewitz D, Cerda M, et al. US adults with pain, a group increasingly vulnerable to nonmedical cannabis use and cannabis use disorder: 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(7):611-618. doi: 10.1176/appi.ajp.2019.19030284
52. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74(6):579-588. doi: 10.1001/jamapsychiatry.2017.0724
53. National Institute on Drug Abuse. Illicit cannabis use and use disorders increase in states with medical marijuana laws. April 26, 2017. Accessed October 24, 2020. https://archives.drugabuse.gov/news-events/news-releases/2017/04/illicit-cannabis-use-use-disorders-increase-in-states-medical-marijuana-laws
54. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press; 2017. https://doi.org/10.17226/24625
55. Stanford M. Physician recommended marijuana: contraindications & standards of care. A review of the literature. Accessed July 7, 2020. http://drneurosci.com/MedicalMarijuanaStandardsofCare.pdf
56. Repp K, Raich A. Marijuana and health: a comprehensive review of 20 years of research. Washington County Oregon Department of Health and Human Services. 2014. Accessed April 8, 2021. https://www.co.washington.or.us/CAO/upload/HHSmarijuana-review.pdf
57. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638-654. doi: 10.1016/j.sapharm.2015.09.002.
58. Leite RT, Nogueira Sde O, do Nascimento JP, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/43412
59. Kim SW, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. doi: 10.4306/pi.2015.12.3.349
60. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995-1010.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. doi: 10.4088/JCP.15r10036.
62. Woolf CJ, American College of Physicians. American Physiological Society Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
63. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515‐523. doi: 10.1002/hup.1048
64. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735‐746. doi: 10.1007/s13311-015-0380-8
65. Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;2;192:E206. doi: 10.1503/cmaj.191097
66. Brown JD. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug-drug interactions. J Clin Med. 2020;9(4):919. doi: 10.3390/jcm9040919.
67. Maida V, Daeninck P. A user’s guide to cannabinoid therapy in oncology. Curr Oncol. 2016;23(6):398-406. doi: http://dx.doi.org/10.3747/co.23.3487
68. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95. doi: 10.3109/03602532.2013.849268
69. Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol. 2016;23(52):S8-S14.
70. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018;6(1):3. doi: 10.3390/medicines6010003
71. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477-2482.
72. Ghasemiesfe M, Barrow B, Leonard S, et al. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(11):e1916318. doi: 10.1001/jamanetworkopen.2019.16318
73. Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46-52. doi: 10.1016/j.brainres.2011.11.001
74. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536
75. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42(2):194-204.
Doctors more likely to prescribe opioids to COVID ‘long-haulers,’ raising addiction fears
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Sarcoidosis: An FP’s primer on an enigmatic disease
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
PRACTICE RECOMMENDATIONS
› Consider biopsy to aid in diagnosing sarcoidosis; it may be avoided with a high clinical suspicion for sarcoidosis (eg, Löfgren syndrome, lupus pernio, or Heerfordt syndrome). C
› Rule out alternative diagnoses such as infection, malignancy, collagen vascular disease, and vasculitis. C
› Identify extra-pulmonary organ involvement, as clinically indicated, by screening with a baseline eye examination; complete blood count; creatinine, alkaline phosphatase, and calcium levels; electrocardiogram, and other organ-specific studies. C
› Make a patient-centered decision whether to begin antiinflammatory treatment based on symptomatology and risk of organ failure or death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A thoughtful approach to drug screening and addiction
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
Opioid Management in Older Adults: Lessons Learned From a Geriatric Patient-Centered Medical Home
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.