User login
Getting tendinopathy treatment (and terminology) right
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
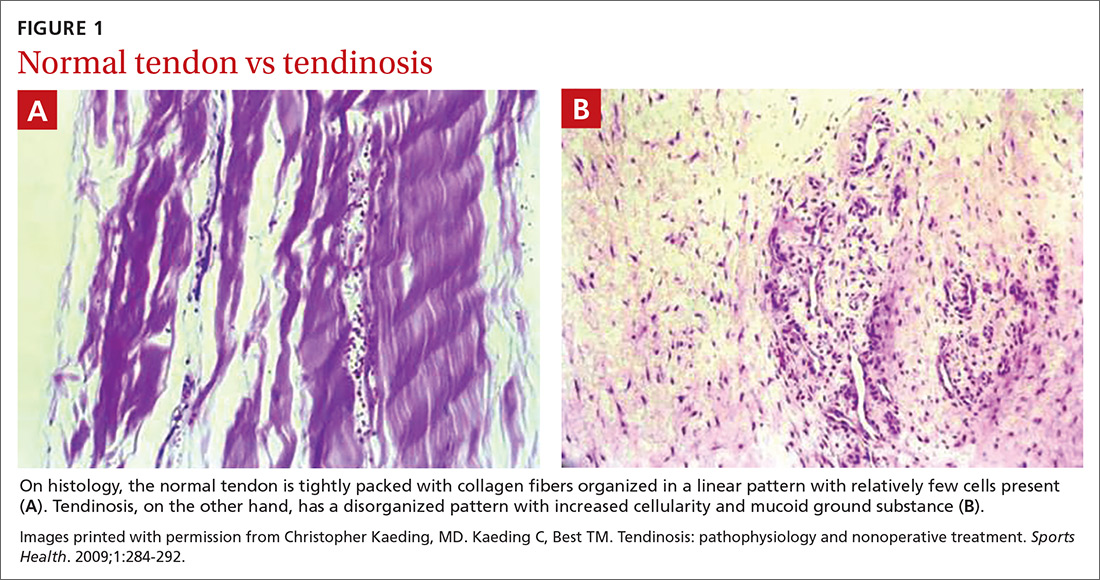
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5
Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
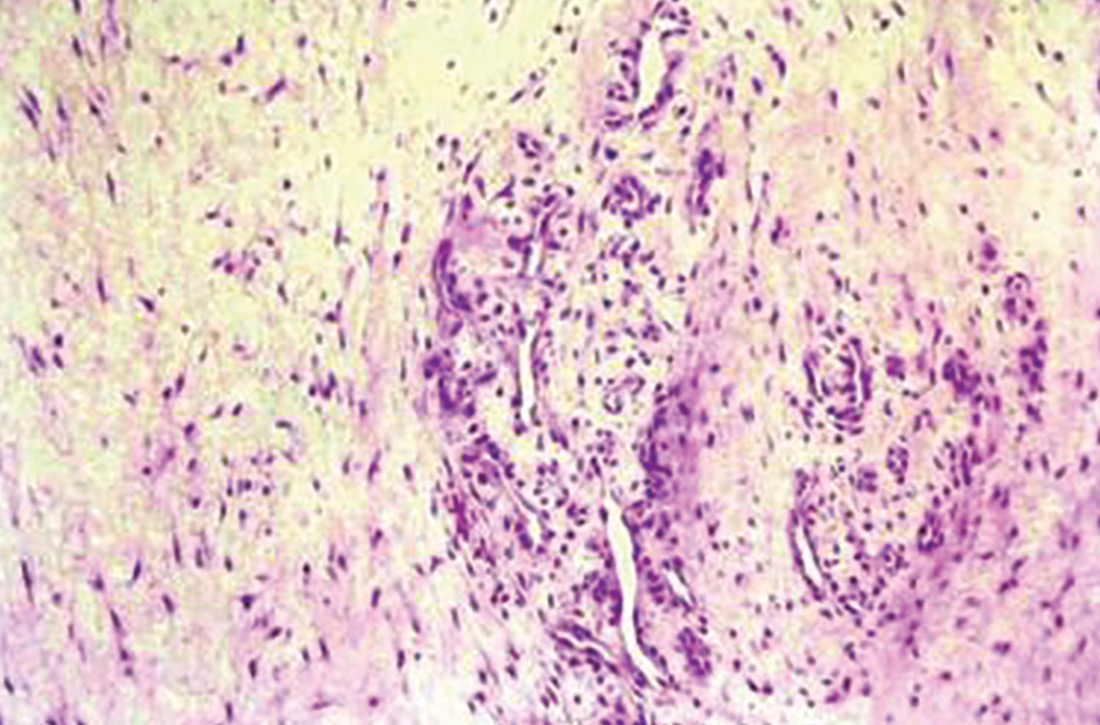
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
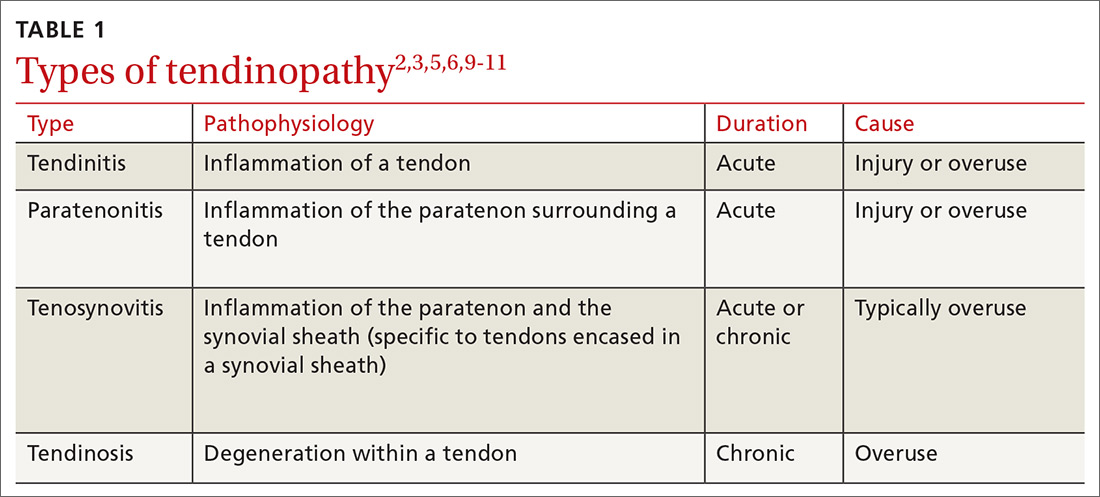
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5
Continue to: Treatment demands time and patience
Treatment demands time and patience
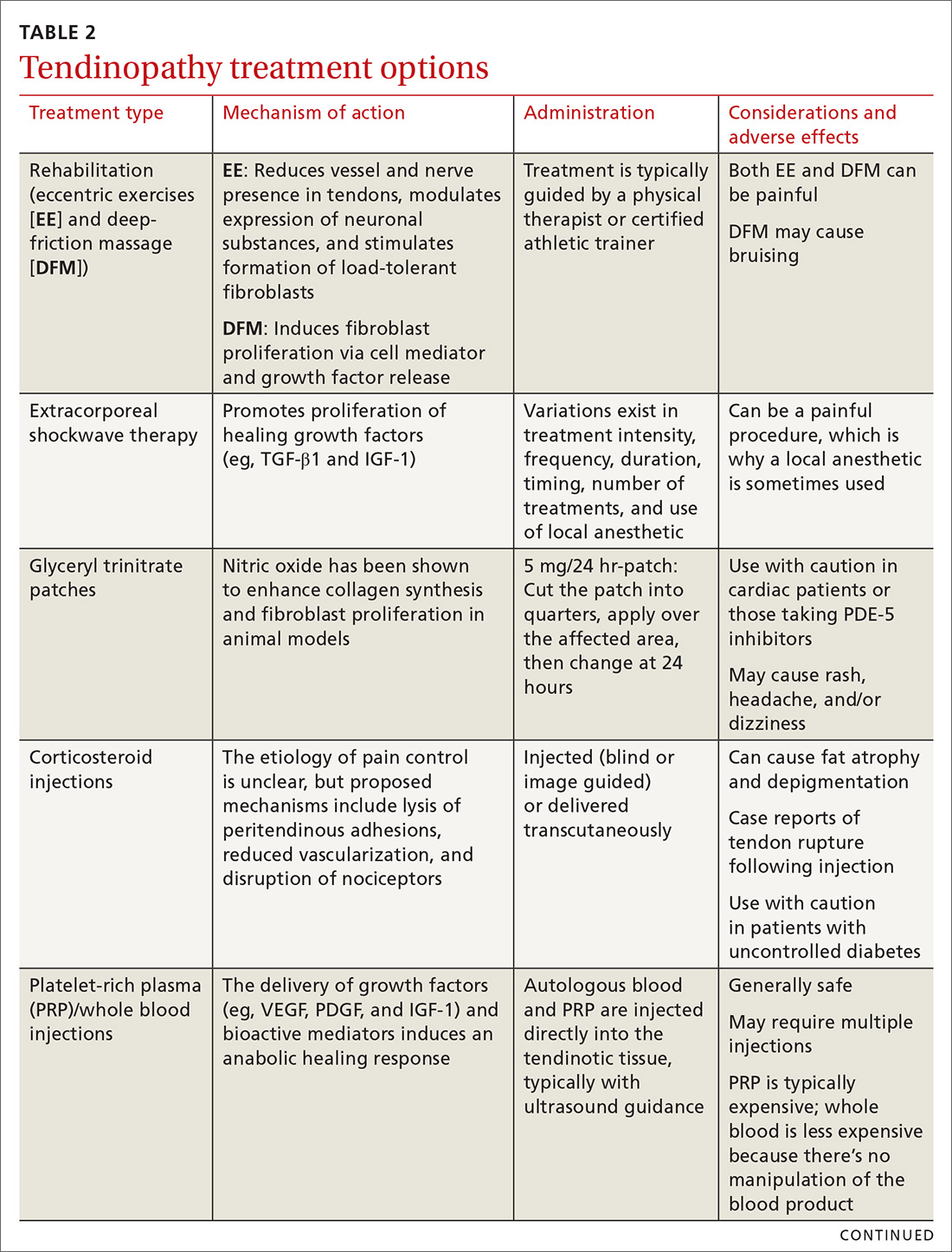
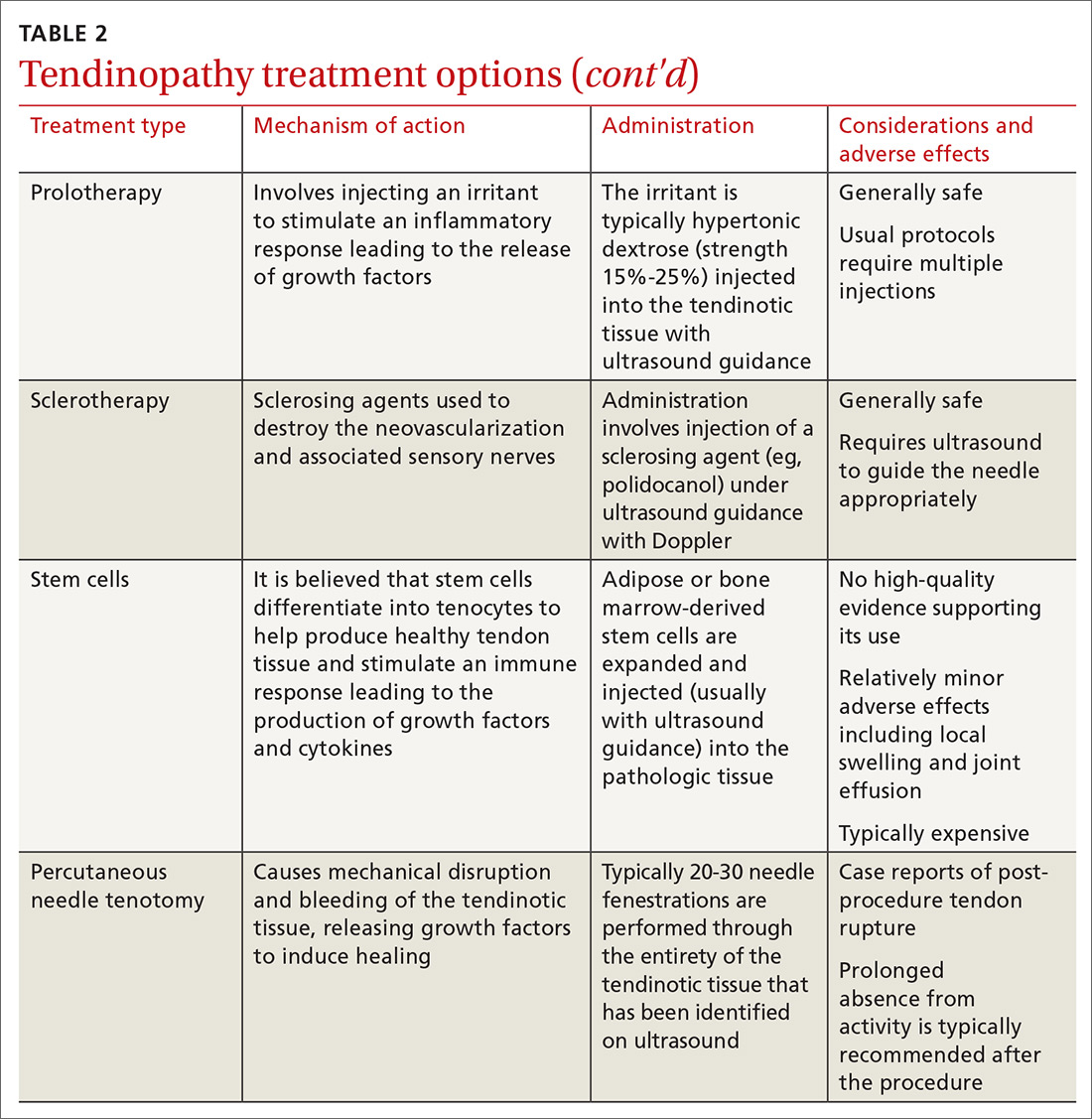
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.
First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14
Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; [email protected].
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5

Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5

Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.

First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14

Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; [email protected].
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5

Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5

Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.

First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14

Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; [email protected].
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
PRACTICE RECOMMENDATIONS
› Recommend eccentric exercises to treat patients with tendinosis; research has consistently shown them to be an effective and safe treatment for many types of this disorder. A
› Use corticosteroid injections with caution for tendinosis; pain relief is typically short lived, and good evidence exists for long-term relapse and worse outcomes including post-injection tendon rupture, especially in the lower extremity. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Elderly Americans carry heavier opioid burden
according to the Agency for Healthcare Quality and Research.
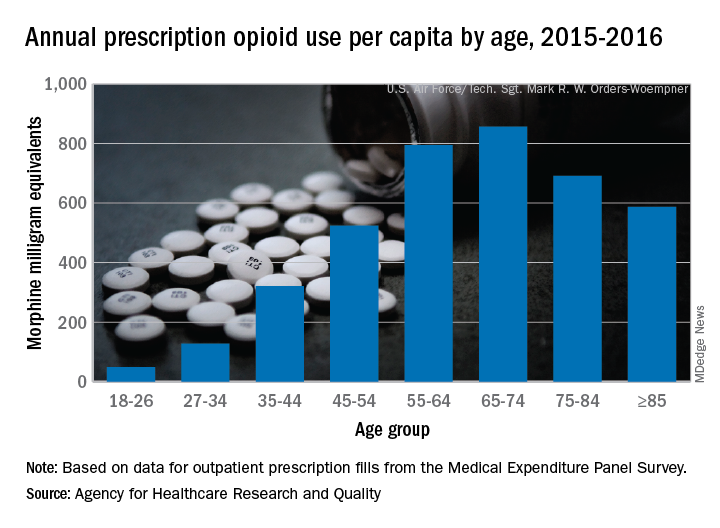
Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
American Headache Society updates guideline on neuroimaging for migraine
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
FROM HEADACHE
Implantable stimulator shows promise for chronic knee pain
NATIONAL HARBOR, MD. – Stimulation of the infrapatellar branch of the saphenous nerve with an implantable electrical device is a potentially effective treatment for chronic, intractable knee pain.
In a small case series consisting of five patients with chronic knee pain, pain intensity scores on the visual analog scale (VAS) dropped from an average of 8 out of 10 before the implant to 1.4 out of 10 when measured 6 months afterward.
Pain relief was also long lasting, with an average score at 2 years still significantly reduced from baseline, at 3 out of 10 on the VAS.
“We have a lot of patients with chronic knee pain, and unfortunately, our hands are tied in terms of what we can do for them,” lead author Kwo Wei David Ho, MD, PhD, Stanford University, California, told Medscape Medical News.
“They can use NSAIDs, physical therapy, some get steroid injections, or genicular nerve blocks, but they don’t work that well. Some have knee replacement surgery, and can still have persistent knee pain after the operation, so here we are using an alternative therapy called peripheral nerve stimulation of the saphenous nerve. This provides a way to relieve pain without nerve destruction or motor dysfunction,” Ho said.
The findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Patient Controlled
For the study, the investigators surgically implanted five patients with intractable knee pain with the StimRouter™ (Bioness, Inc).
The device takes about 15 to 30 minutes to implant, much like a pacemaker, and reduces pain by delivering gentle electrical stimulation directly to a target peripheral nerve, in this case the saphenous nerve, to interrupt the pain signal, Ho said.
“A thin, threadlike lead, or noodle, is implanted below the skin next to the target peripheral nerve responsible for the pain signal under ultrasound guidance, and then a patch or external pulse transmitter (EPT) is worn on top of the skin. This sends electric stimulation through the skin to the lead,” he explained.
The patient can then control the EPT and adjust stimulation with a wireless handheld programmer.
“Some patients turn it on at night for a couple of hours and then turn it off, some leave it on for the entire night, or the whole day if they prefer. What we’ve been noticing in our series is that after a while, patients are using less and less, and the pain gets better and better, and eventually they stop using it entirely because the pain completely resolves,” Ho said.
Good candidates for this treatment are post-knee replacement patients with residual pain, he added.
Durable Effect
Of the five patients in the case series, four had previous knee arthroplasty.
To determine the chances of a good response to the implant, study participants underwent a diagnostic saphenous nerve block, with the rationale that if the block successfully reduced knee pain by 50% or more in the short term, patients would likely respond well to the implant.
Before the peripheral nerve stimulation implant, the average pain intensity was 7.8 out of 10 on the VAS. After stimulator implantation, the average pain intensity was 1.4 at 6 months (P = .019, in 5 patients). At 1 year, the average pain intensity score was virtually the same, at 1.5 on the VAS, (P = .0032, in 4 patients). At 2 years, the average pain intensity score was 2.75 (P = .12, in 2 patients).
“This study provides preliminary evidence that stimulation at the saphenous nerve may be effective for selected patients with chronic knee pain,” Ho said.
Commenting on the findings for Medscape Medical News, Patrick Tighe, MD, MS, University of Florida, Gainesville, said that chronic knee pain continues to present “numerous diagnostic and therapeutic challenges for many patients.”
“It may be surprising, but there is still so much we don’t know about the innervation of the knee, and we are still learning about different ways to alter the behavior of those nerves,” said Tighe, who was not involved with the current study.
“This work points to some exciting opportunities to help patients suffering from chronic knee pain. We certainly need more research in this area to figure out the optimal approach to applying these findings more widely,” he said.
Ho and Tighe have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Stimulation of the infrapatellar branch of the saphenous nerve with an implantable electrical device is a potentially effective treatment for chronic, intractable knee pain.
In a small case series consisting of five patients with chronic knee pain, pain intensity scores on the visual analog scale (VAS) dropped from an average of 8 out of 10 before the implant to 1.4 out of 10 when measured 6 months afterward.
Pain relief was also long lasting, with an average score at 2 years still significantly reduced from baseline, at 3 out of 10 on the VAS.
“We have a lot of patients with chronic knee pain, and unfortunately, our hands are tied in terms of what we can do for them,” lead author Kwo Wei David Ho, MD, PhD, Stanford University, California, told Medscape Medical News.
“They can use NSAIDs, physical therapy, some get steroid injections, or genicular nerve blocks, but they don’t work that well. Some have knee replacement surgery, and can still have persistent knee pain after the operation, so here we are using an alternative therapy called peripheral nerve stimulation of the saphenous nerve. This provides a way to relieve pain without nerve destruction or motor dysfunction,” Ho said.
The findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Patient Controlled
For the study, the investigators surgically implanted five patients with intractable knee pain with the StimRouter™ (Bioness, Inc).
The device takes about 15 to 30 minutes to implant, much like a pacemaker, and reduces pain by delivering gentle electrical stimulation directly to a target peripheral nerve, in this case the saphenous nerve, to interrupt the pain signal, Ho said.
“A thin, threadlike lead, or noodle, is implanted below the skin next to the target peripheral nerve responsible for the pain signal under ultrasound guidance, and then a patch or external pulse transmitter (EPT) is worn on top of the skin. This sends electric stimulation through the skin to the lead,” he explained.
The patient can then control the EPT and adjust stimulation with a wireless handheld programmer.
“Some patients turn it on at night for a couple of hours and then turn it off, some leave it on for the entire night, or the whole day if they prefer. What we’ve been noticing in our series is that after a while, patients are using less and less, and the pain gets better and better, and eventually they stop using it entirely because the pain completely resolves,” Ho said.
Good candidates for this treatment are post-knee replacement patients with residual pain, he added.
Durable Effect
Of the five patients in the case series, four had previous knee arthroplasty.
To determine the chances of a good response to the implant, study participants underwent a diagnostic saphenous nerve block, with the rationale that if the block successfully reduced knee pain by 50% or more in the short term, patients would likely respond well to the implant.
Before the peripheral nerve stimulation implant, the average pain intensity was 7.8 out of 10 on the VAS. After stimulator implantation, the average pain intensity was 1.4 at 6 months (P = .019, in 5 patients). At 1 year, the average pain intensity score was virtually the same, at 1.5 on the VAS, (P = .0032, in 4 patients). At 2 years, the average pain intensity score was 2.75 (P = .12, in 2 patients).
“This study provides preliminary evidence that stimulation at the saphenous nerve may be effective for selected patients with chronic knee pain,” Ho said.
Commenting on the findings for Medscape Medical News, Patrick Tighe, MD, MS, University of Florida, Gainesville, said that chronic knee pain continues to present “numerous diagnostic and therapeutic challenges for many patients.”
“It may be surprising, but there is still so much we don’t know about the innervation of the knee, and we are still learning about different ways to alter the behavior of those nerves,” said Tighe, who was not involved with the current study.
“This work points to some exciting opportunities to help patients suffering from chronic knee pain. We certainly need more research in this area to figure out the optimal approach to applying these findings more widely,” he said.
Ho and Tighe have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Stimulation of the infrapatellar branch of the saphenous nerve with an implantable electrical device is a potentially effective treatment for chronic, intractable knee pain.
In a small case series consisting of five patients with chronic knee pain, pain intensity scores on the visual analog scale (VAS) dropped from an average of 8 out of 10 before the implant to 1.4 out of 10 when measured 6 months afterward.
Pain relief was also long lasting, with an average score at 2 years still significantly reduced from baseline, at 3 out of 10 on the VAS.
“We have a lot of patients with chronic knee pain, and unfortunately, our hands are tied in terms of what we can do for them,” lead author Kwo Wei David Ho, MD, PhD, Stanford University, California, told Medscape Medical News.
“They can use NSAIDs, physical therapy, some get steroid injections, or genicular nerve blocks, but they don’t work that well. Some have knee replacement surgery, and can still have persistent knee pain after the operation, so here we are using an alternative therapy called peripheral nerve stimulation of the saphenous nerve. This provides a way to relieve pain without nerve destruction or motor dysfunction,” Ho said.
The findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Patient Controlled
For the study, the investigators surgically implanted five patients with intractable knee pain with the StimRouter™ (Bioness, Inc).
The device takes about 15 to 30 minutes to implant, much like a pacemaker, and reduces pain by delivering gentle electrical stimulation directly to a target peripheral nerve, in this case the saphenous nerve, to interrupt the pain signal, Ho said.
“A thin, threadlike lead, or noodle, is implanted below the skin next to the target peripheral nerve responsible for the pain signal under ultrasound guidance, and then a patch or external pulse transmitter (EPT) is worn on top of the skin. This sends electric stimulation through the skin to the lead,” he explained.
The patient can then control the EPT and adjust stimulation with a wireless handheld programmer.
“Some patients turn it on at night for a couple of hours and then turn it off, some leave it on for the entire night, or the whole day if they prefer. What we’ve been noticing in our series is that after a while, patients are using less and less, and the pain gets better and better, and eventually they stop using it entirely because the pain completely resolves,” Ho said.
Good candidates for this treatment are post-knee replacement patients with residual pain, he added.
Durable Effect
Of the five patients in the case series, four had previous knee arthroplasty.
To determine the chances of a good response to the implant, study participants underwent a diagnostic saphenous nerve block, with the rationale that if the block successfully reduced knee pain by 50% or more in the short term, patients would likely respond well to the implant.
Before the peripheral nerve stimulation implant, the average pain intensity was 7.8 out of 10 on the VAS. After stimulator implantation, the average pain intensity was 1.4 at 6 months (P = .019, in 5 patients). At 1 year, the average pain intensity score was virtually the same, at 1.5 on the VAS, (P = .0032, in 4 patients). At 2 years, the average pain intensity score was 2.75 (P = .12, in 2 patients).
“This study provides preliminary evidence that stimulation at the saphenous nerve may be effective for selected patients with chronic knee pain,” Ho said.
Commenting on the findings for Medscape Medical News, Patrick Tighe, MD, MS, University of Florida, Gainesville, said that chronic knee pain continues to present “numerous diagnostic and therapeutic challenges for many patients.”
“It may be surprising, but there is still so much we don’t know about the innervation of the knee, and we are still learning about different ways to alter the behavior of those nerves,” said Tighe, who was not involved with the current study.
“This work points to some exciting opportunities to help patients suffering from chronic knee pain. We certainly need more research in this area to figure out the optimal approach to applying these findings more widely,” he said.
Ho and Tighe have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Chronic anterior knee pain
A 14-year-old girl with an unremarkable medical history presented to the family medicine clinic with a 6-month history of right knee pain (episodic locking and anterior pain). Physical examination of the knee ligaments revealed that the knee was stable and pain-free in the frontal and sagittal planes. There was no intra-articular effusion, the joint spaces were not painful, and range of motion was normal.
Palpation of the knee elicited pain, notably when the physician rolled his fingers over a “cord” above the internal parapatellar compartment. X-rays of the knee were normal. In light of the patient’s chronic pain, magnetic resonance imaging (MRI) was performed (FIGURE 1).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Synovial plica
The MRI with fat saturation revealed a symptomatic synovial plica between the patellar facet and the condyle (FIGURE 1, arrow). The normal x-ray findings had already ruled out osteochondritis dissecans of the femoral condyles, patellar abnormalities, and trochlear dysplasia; the MRI ruled out several additional items in the differential, such as damage to the meniscus, ligament, and/or cartilage.
The synovial plica is a normal structure that develops during the embryogenic phase; however, involution is incomplete in up to 50% of the population, resulting in persistent plicae.1 The plica is often located in a medial position but can occur lateral to, above, or below the knee cap. Although usually asymptomatic, the plica can become pathologic when irritation (eg, from repetitive motion) causes an inflammatory response.1
Synovial plica syndrome, as this condition is known, is a common cause of anterior knee pain in adolescents and athletes; incidence ranges from 3.8% to 5.5%.2 The patient often reports trauma (a direct impact to the knee) or participation in sports activities that require repeated flexion-extension of the knee.3
Presenting symptoms and MRI findings can unlock the diagnosis
The combination of anterior knee pain and a painful parapatellar “cord” on palpation is the most frequent diagnostic sign of synovial plica syndrome.1 Quadriceps wasting, intra-articular effusion, and reduced range of motion of the knee may also be observed.1,4 Some patients experience particularly disconcerting symptoms, such as knee locking, clicking, or instability.1
In most cases, MRI confirms the clinical diagnosis while ruling out other possible causes of the symptoms and associated pathologies.5 However, MRI may not reveal the plica if it is attached to the articular capsule or if there is no intra-articular effusion. Dynamic ultrasound might be of diagnostic value but is operator dependent.4
Continue to: If conservative treatment fails, consider surgical repair
If conservative treatment fails, consider surgical repair
Conservative treatment—a combination of analgesics, anti-inflammatories, and physiotherapy with vastus medialis strengthening and stretching—is the preferred first-line treatment, with a success rate of 40% to 60%.1 If conservative treatment fails, surgical treatment can be
Our patient underwent arthroscopic resection of the plica after 6 months of conservative treatment had failed (FIGURE 2). The patient was able to walk immediately after surgery. The outcome was favorable, since physiotherapy was no longer required 2 months after surgery.
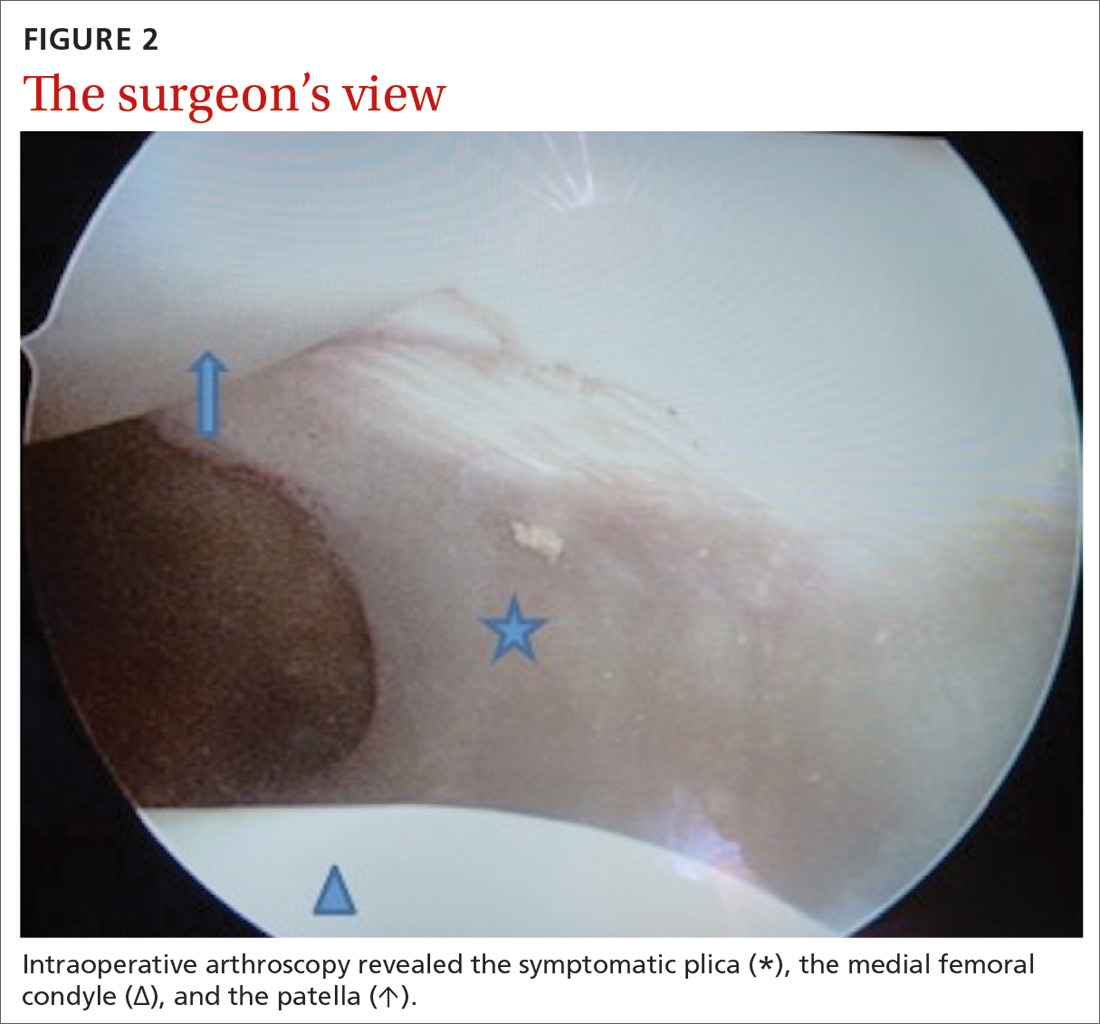
CORRESPONDENCE
Céline Klein, MD, Service d’Orthopédie Pédiatrique, CHU Amiens, Groupe Hospitalier Sud, F-80054 Amiens cedex 1, France; [email protected].
1. Camanho GL. Treatment of pathological synovial plicae of the knee. Clinics (Sao Paolo). 2010;65:247-250.
2. Ewing JW. Plica: pathologic or not? J Am Acad Orthop Surg. 1993;1:117-121.
3. Patel DR, Villalobos A. Evaluation and management of knee pain in young athletes: overuse injuries of the knee. Transl Pediatr. 2017;6:190-198.
4. Paczesny Ł, Kruczyński J. Medial plica syndrome of the knee: diagnosis with dynamic sonography. Radiology. 2009;251:439-446.
5. Samim M, Smitaman E, Lawrence D, et al. MRI of anterior knee pain. Skeletal Radiol. 2014;43:875-893.
6. Weckström M, Niva MH, Lamminen A, et al. Arthroscopic resection of medial plica of the knee in young adults. Knee. 2010;17:103-107.
7. Kan H, Arai Y, Nakagawa S, et al. Characteristics of medial plica syndrome complicated with cartilage damage. Int Orthop. 2015;39:2489-2494.
A 14-year-old girl with an unremarkable medical history presented to the family medicine clinic with a 6-month history of right knee pain (episodic locking and anterior pain). Physical examination of the knee ligaments revealed that the knee was stable and pain-free in the frontal and sagittal planes. There was no intra-articular effusion, the joint spaces were not painful, and range of motion was normal.
Palpation of the knee elicited pain, notably when the physician rolled his fingers over a “cord” above the internal parapatellar compartment. X-rays of the knee were normal. In light of the patient’s chronic pain, magnetic resonance imaging (MRI) was performed (FIGURE 1).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Synovial plica
The MRI with fat saturation revealed a symptomatic synovial plica between the patellar facet and the condyle (FIGURE 1, arrow). The normal x-ray findings had already ruled out osteochondritis dissecans of the femoral condyles, patellar abnormalities, and trochlear dysplasia; the MRI ruled out several additional items in the differential, such as damage to the meniscus, ligament, and/or cartilage.
The synovial plica is a normal structure that develops during the embryogenic phase; however, involution is incomplete in up to 50% of the population, resulting in persistent plicae.1 The plica is often located in a medial position but can occur lateral to, above, or below the knee cap. Although usually asymptomatic, the plica can become pathologic when irritation (eg, from repetitive motion) causes an inflammatory response.1
Synovial plica syndrome, as this condition is known, is a common cause of anterior knee pain in adolescents and athletes; incidence ranges from 3.8% to 5.5%.2 The patient often reports trauma (a direct impact to the knee) or participation in sports activities that require repeated flexion-extension of the knee.3
Presenting symptoms and MRI findings can unlock the diagnosis
The combination of anterior knee pain and a painful parapatellar “cord” on palpation is the most frequent diagnostic sign of synovial plica syndrome.1 Quadriceps wasting, intra-articular effusion, and reduced range of motion of the knee may also be observed.1,4 Some patients experience particularly disconcerting symptoms, such as knee locking, clicking, or instability.1
In most cases, MRI confirms the clinical diagnosis while ruling out other possible causes of the symptoms and associated pathologies.5 However, MRI may not reveal the plica if it is attached to the articular capsule or if there is no intra-articular effusion. Dynamic ultrasound might be of diagnostic value but is operator dependent.4
Continue to: If conservative treatment fails, consider surgical repair
If conservative treatment fails, consider surgical repair
Conservative treatment—a combination of analgesics, anti-inflammatories, and physiotherapy with vastus medialis strengthening and stretching—is the preferred first-line treatment, with a success rate of 40% to 60%.1 If conservative treatment fails, surgical treatment can be
Our patient underwent arthroscopic resection of the plica after 6 months of conservative treatment had failed (FIGURE 2). The patient was able to walk immediately after surgery. The outcome was favorable, since physiotherapy was no longer required 2 months after surgery.

CORRESPONDENCE
Céline Klein, MD, Service d’Orthopédie Pédiatrique, CHU Amiens, Groupe Hospitalier Sud, F-80054 Amiens cedex 1, France; [email protected].
A 14-year-old girl with an unremarkable medical history presented to the family medicine clinic with a 6-month history of right knee pain (episodic locking and anterior pain). Physical examination of the knee ligaments revealed that the knee was stable and pain-free in the frontal and sagittal planes. There was no intra-articular effusion, the joint spaces were not painful, and range of motion was normal.
Palpation of the knee elicited pain, notably when the physician rolled his fingers over a “cord” above the internal parapatellar compartment. X-rays of the knee were normal. In light of the patient’s chronic pain, magnetic resonance imaging (MRI) was performed (FIGURE 1).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Synovial plica
The MRI with fat saturation revealed a symptomatic synovial plica between the patellar facet and the condyle (FIGURE 1, arrow). The normal x-ray findings had already ruled out osteochondritis dissecans of the femoral condyles, patellar abnormalities, and trochlear dysplasia; the MRI ruled out several additional items in the differential, such as damage to the meniscus, ligament, and/or cartilage.
The synovial plica is a normal structure that develops during the embryogenic phase; however, involution is incomplete in up to 50% of the population, resulting in persistent plicae.1 The plica is often located in a medial position but can occur lateral to, above, or below the knee cap. Although usually asymptomatic, the plica can become pathologic when irritation (eg, from repetitive motion) causes an inflammatory response.1
Synovial plica syndrome, as this condition is known, is a common cause of anterior knee pain in adolescents and athletes; incidence ranges from 3.8% to 5.5%.2 The patient often reports trauma (a direct impact to the knee) or participation in sports activities that require repeated flexion-extension of the knee.3
Presenting symptoms and MRI findings can unlock the diagnosis
The combination of anterior knee pain and a painful parapatellar “cord” on palpation is the most frequent diagnostic sign of synovial plica syndrome.1 Quadriceps wasting, intra-articular effusion, and reduced range of motion of the knee may also be observed.1,4 Some patients experience particularly disconcerting symptoms, such as knee locking, clicking, or instability.1
In most cases, MRI confirms the clinical diagnosis while ruling out other possible causes of the symptoms and associated pathologies.5 However, MRI may not reveal the plica if it is attached to the articular capsule or if there is no intra-articular effusion. Dynamic ultrasound might be of diagnostic value but is operator dependent.4
Continue to: If conservative treatment fails, consider surgical repair
If conservative treatment fails, consider surgical repair
Conservative treatment—a combination of analgesics, anti-inflammatories, and physiotherapy with vastus medialis strengthening and stretching—is the preferred first-line treatment, with a success rate of 40% to 60%.1 If conservative treatment fails, surgical treatment can be
Our patient underwent arthroscopic resection of the plica after 6 months of conservative treatment had failed (FIGURE 2). The patient was able to walk immediately after surgery. The outcome was favorable, since physiotherapy was no longer required 2 months after surgery.

CORRESPONDENCE
Céline Klein, MD, Service d’Orthopédie Pédiatrique, CHU Amiens, Groupe Hospitalier Sud, F-80054 Amiens cedex 1, France; [email protected].
1. Camanho GL. Treatment of pathological synovial plicae of the knee. Clinics (Sao Paolo). 2010;65:247-250.
2. Ewing JW. Plica: pathologic or not? J Am Acad Orthop Surg. 1993;1:117-121.
3. Patel DR, Villalobos A. Evaluation and management of knee pain in young athletes: overuse injuries of the knee. Transl Pediatr. 2017;6:190-198.
4. Paczesny Ł, Kruczyński J. Medial plica syndrome of the knee: diagnosis with dynamic sonography. Radiology. 2009;251:439-446.
5. Samim M, Smitaman E, Lawrence D, et al. MRI of anterior knee pain. Skeletal Radiol. 2014;43:875-893.
6. Weckström M, Niva MH, Lamminen A, et al. Arthroscopic resection of medial plica of the knee in young adults. Knee. 2010;17:103-107.
7. Kan H, Arai Y, Nakagawa S, et al. Characteristics of medial plica syndrome complicated with cartilage damage. Int Orthop. 2015;39:2489-2494.
1. Camanho GL. Treatment of pathological synovial plicae of the knee. Clinics (Sao Paolo). 2010;65:247-250.
2. Ewing JW. Plica: pathologic or not? J Am Acad Orthop Surg. 1993;1:117-121.
3. Patel DR, Villalobos A. Evaluation and management of knee pain in young athletes: overuse injuries of the knee. Transl Pediatr. 2017;6:190-198.
4. Paczesny Ł, Kruczyński J. Medial plica syndrome of the knee: diagnosis with dynamic sonography. Radiology. 2009;251:439-446.
5. Samim M, Smitaman E, Lawrence D, et al. MRI of anterior knee pain. Skeletal Radiol. 2014;43:875-893.
6. Weckström M, Niva MH, Lamminen A, et al. Arthroscopic resection of medial plica of the knee in young adults. Knee. 2010;17:103-107.
7. Kan H, Arai Y, Nakagawa S, et al. Characteristics of medial plica syndrome complicated with cartilage damage. Int Orthop. 2015;39:2489-2494.
Surgery for shoulder pain? Think twice
Shoulder pain is a very common presenting complaint in family physicians’ offices. Typically, a patient will have had minor trauma, such as a fall, or overuse from work or a recreational activity. Most of these patients have rotator cuff injuries, so we refer them to physical therapy or we prescribe a self-directed home exercise program and the problem gradually resolves. If the patient does not improve, however, should s(he) be referred for arthroscopic surgery? This answer, of course, is “it depends.”
In this issue of JFP, Onks et al provide an excellent review of conservative vs surgical management of rotator cuff tears. For complete or near complete tears in young people—especially athletes—arthroscopic surgery is the preferred approach. For partial tears, chronic tears, and for older folks like me, nonoperative management is the preferred approach. Surgery is reserved for those who do not improve with prolonged conservative management.
But what approach is best for the majority of people in whom shoulder pain is due to impingement syndrome, with or without a small rotator cuff tear? This question has been studied extensively and summarized in a recent Cochrane meta-analysis.1
The meta-analysis included 8 trials, with a total of 1062 participants with rotator cuff disease, all with subacromial impingement. “Compared with placebo, high-certainty evidence indicates that subacromial decompression provides no improvement in pain, shoulder function, or health-related quality of life up to one year, and probably no improvement in global success (moderate-certainty evidence).”1
A recently published guideline developed by doctors and patients for the treatment of shoulder pain gives a strong recommendation to avoid surgery for chronic shoulder pain due to impingement syndrome.2
Interestingly, research has shown that arthroscopic surgery for knee osteoarthritis and chronic meniscus tears is no better that conservative therapy.3,4 Similarly, surgery for chronic back pain due to degenerative disease (in the absence of spondylolisthesis) provides minimal, if any, improvement in pain and function.5 I see a pattern here.
When we talk to our patients who are contemplating these surgical procedures for these indications (except complete rotator cuff tears), we should advise them to have limited expectations or to avoid surgery altogether.
1. Karjalainen TV, Jain NB, Page CM, et al. Subacromial decompression surgery for rotator cuff disease. Cochrane Database Syst Rev. 2019;(1):CD005619. Epub January 17, 2019.
2. Vandvik PO, Lahdeoja T, Ardern C, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ. 2019;364:1294.
3. Monk P, Garfjeld Roberts P, Palmer AJ, et al. The urgent need for evidence in arthroscopic meniscal surgery. Am J Sports Med. 2017;45:965-973.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
5. Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80:701-715.
Shoulder pain is a very common presenting complaint in family physicians’ offices. Typically, a patient will have had minor trauma, such as a fall, or overuse from work or a recreational activity. Most of these patients have rotator cuff injuries, so we refer them to physical therapy or we prescribe a self-directed home exercise program and the problem gradually resolves. If the patient does not improve, however, should s(he) be referred for arthroscopic surgery? This answer, of course, is “it depends.”
In this issue of JFP, Onks et al provide an excellent review of conservative vs surgical management of rotator cuff tears. For complete or near complete tears in young people—especially athletes—arthroscopic surgery is the preferred approach. For partial tears, chronic tears, and for older folks like me, nonoperative management is the preferred approach. Surgery is reserved for those who do not improve with prolonged conservative management.
But what approach is best for the majority of people in whom shoulder pain is due to impingement syndrome, with or without a small rotator cuff tear? This question has been studied extensively and summarized in a recent Cochrane meta-analysis.1
The meta-analysis included 8 trials, with a total of 1062 participants with rotator cuff disease, all with subacromial impingement. “Compared with placebo, high-certainty evidence indicates that subacromial decompression provides no improvement in pain, shoulder function, or health-related quality of life up to one year, and probably no improvement in global success (moderate-certainty evidence).”1
A recently published guideline developed by doctors and patients for the treatment of shoulder pain gives a strong recommendation to avoid surgery for chronic shoulder pain due to impingement syndrome.2
Interestingly, research has shown that arthroscopic surgery for knee osteoarthritis and chronic meniscus tears is no better that conservative therapy.3,4 Similarly, surgery for chronic back pain due to degenerative disease (in the absence of spondylolisthesis) provides minimal, if any, improvement in pain and function.5 I see a pattern here.
When we talk to our patients who are contemplating these surgical procedures for these indications (except complete rotator cuff tears), we should advise them to have limited expectations or to avoid surgery altogether.
Shoulder pain is a very common presenting complaint in family physicians’ offices. Typically, a patient will have had minor trauma, such as a fall, or overuse from work or a recreational activity. Most of these patients have rotator cuff injuries, so we refer them to physical therapy or we prescribe a self-directed home exercise program and the problem gradually resolves. If the patient does not improve, however, should s(he) be referred for arthroscopic surgery? This answer, of course, is “it depends.”
In this issue of JFP, Onks et al provide an excellent review of conservative vs surgical management of rotator cuff tears. For complete or near complete tears in young people—especially athletes—arthroscopic surgery is the preferred approach. For partial tears, chronic tears, and for older folks like me, nonoperative management is the preferred approach. Surgery is reserved for those who do not improve with prolonged conservative management.
But what approach is best for the majority of people in whom shoulder pain is due to impingement syndrome, with or without a small rotator cuff tear? This question has been studied extensively and summarized in a recent Cochrane meta-analysis.1
The meta-analysis included 8 trials, with a total of 1062 participants with rotator cuff disease, all with subacromial impingement. “Compared with placebo, high-certainty evidence indicates that subacromial decompression provides no improvement in pain, shoulder function, or health-related quality of life up to one year, and probably no improvement in global success (moderate-certainty evidence).”1
A recently published guideline developed by doctors and patients for the treatment of shoulder pain gives a strong recommendation to avoid surgery for chronic shoulder pain due to impingement syndrome.2
Interestingly, research has shown that arthroscopic surgery for knee osteoarthritis and chronic meniscus tears is no better that conservative therapy.3,4 Similarly, surgery for chronic back pain due to degenerative disease (in the absence of spondylolisthesis) provides minimal, if any, improvement in pain and function.5 I see a pattern here.
When we talk to our patients who are contemplating these surgical procedures for these indications (except complete rotator cuff tears), we should advise them to have limited expectations or to avoid surgery altogether.
1. Karjalainen TV, Jain NB, Page CM, et al. Subacromial decompression surgery for rotator cuff disease. Cochrane Database Syst Rev. 2019;(1):CD005619. Epub January 17, 2019.
2. Vandvik PO, Lahdeoja T, Ardern C, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ. 2019;364:1294.
3. Monk P, Garfjeld Roberts P, Palmer AJ, et al. The urgent need for evidence in arthroscopic meniscal surgery. Am J Sports Med. 2017;45:965-973.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
5. Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80:701-715.
1. Karjalainen TV, Jain NB, Page CM, et al. Subacromial decompression surgery for rotator cuff disease. Cochrane Database Syst Rev. 2019;(1):CD005619. Epub January 17, 2019.
2. Vandvik PO, Lahdeoja T, Ardern C, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ. 2019;364:1294.
3. Monk P, Garfjeld Roberts P, Palmer AJ, et al. The urgent need for evidence in arthroscopic meniscal surgery. Am J Sports Med. 2017;45:965-973.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097-1107.
5. Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80:701-715.
Borderline personality disorder common in chronic pain patients
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
REPORTING FROM THE AAPM 2020 ANNUAL MEETING
Fever, abdominal pain, and adnexal mass
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
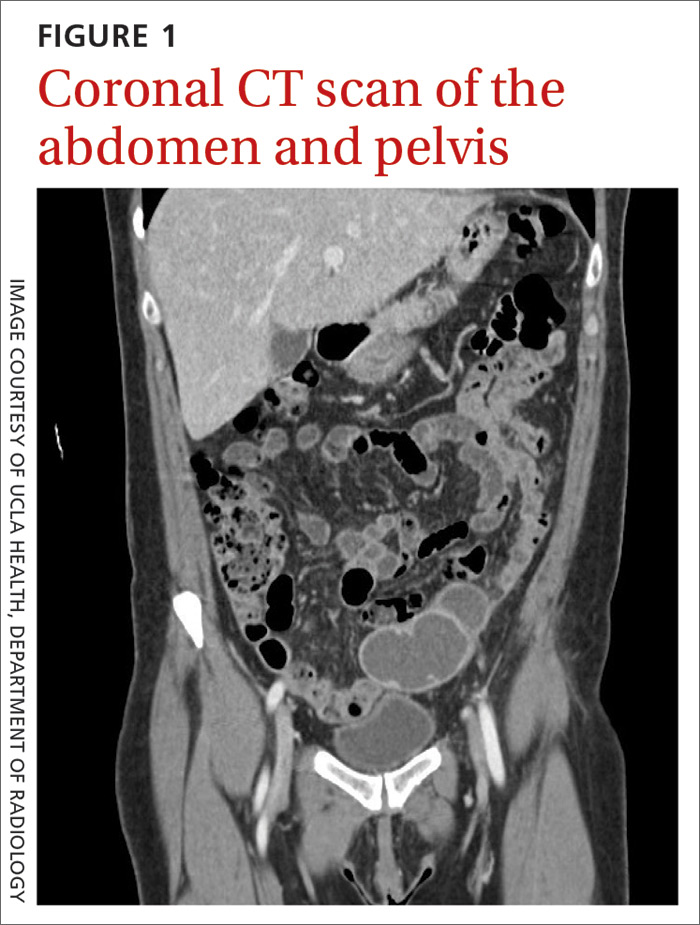
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
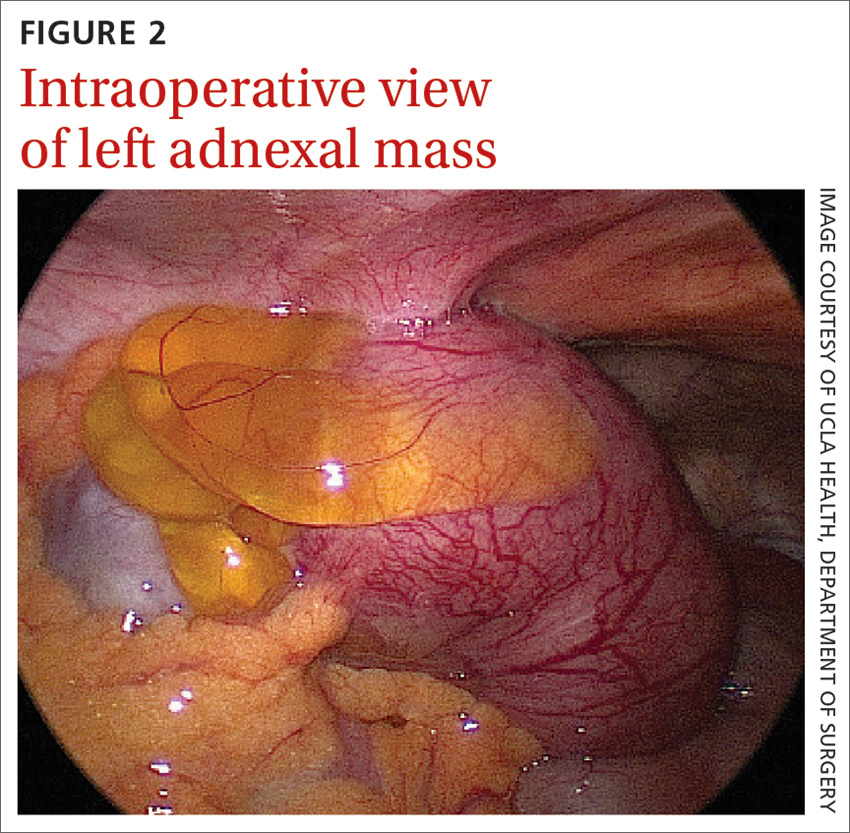
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; [email protected]
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.

Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; [email protected]
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.

Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; [email protected]
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
Conservative care or surgery for rotator cuff tears?
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
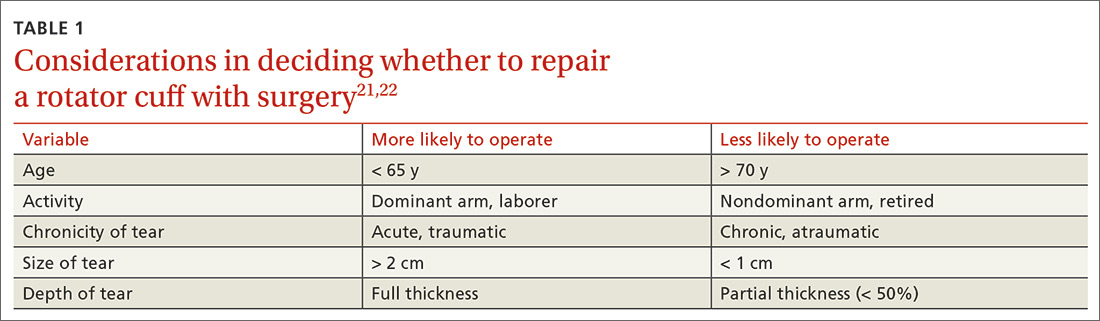
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected].
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.

What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26

Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49

Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected].
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.

What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26

Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49

Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected].
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
PRACTICE RECOMMENDATIONS
› Offer a trial of conservative management to patients with chronic, nontraumatic, or partial-thickness rotator cuff injury and to those who are poor surgical candidates. B
› Counsel patients that the rate of surgical complications is low and outcomes are favorable in properly selected patients for operative repair of rotator cuff tear. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA OKs first orally disintegrating agent for rapid migraine relief
In clinical testing, a single 75-mg dose of rimegepant provided rapid migraine pain relief with patients returning to normal activities within 1 hour, with sustained benefit lasting up to 2 days in many patients. The majority of patients (86%) treated with a single dose did not need a migraine rescue medication within 24 hours.
“I see many patients in my practice whose lives are disrupted by migraine, afraid to go about everyday life in case of a migraine attack,” Peter Goadsby, MD, PhD, professor of neurology and director of the King’s Clinical Research Facility, King’s College Hospital, London, UK, said in a news release from Biohaven.
“Many feel unsure if their acute treatment will work and if they can manage the side effects. With the FDA approval of Nurtec ODT, there is renewed hope for people living with migraine that they can get back to living their lives without fear of the next attack,” said Goadsby.
More than 3100 patients have been treated with rimegepant with more than 113,000 doses administered in clinical trials, including a 1-year long-term safety study, the company said.
In the phase 3 trial, rimegepant achieved statistical significance on the co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) 2 hours after administration compared with placebo.
Rimegepant also showed statistical superiority at 1 hour for pain relief (reduction of moderate or severe pain to no pain or mild pain) and return to normal function.
In many patients, the benefits of pain freedom, pain relief, return to normal function, and freedom from MBS with a single dose lasted up to 48 hours.
Rimegepant was generally well tolerated. The most common adverse reaction was nausea (2%) in patients who received rimegepant compared with 0.4% of patients who received placebo.
“Everyone knows someone living with migraine, yet it remains an invisible disease that is often overlooked and misunderstood,” Mary Franklin, executive director of the National Headache Foundation, commented in the news release.
“The approval of Nurtec ODT is exciting for people with migraine as it provides a new treatment option to help people regain control of their attacks and their lives,” said Franklin.
Nurtec ODT will be available in pharmacies in early March in packs of eight tablets. Each eight-tablet pack covers treatment of eight migraine attacks with one dose, as needed, up to once daily. Sample packs containing two tablets will also be made available to healthcare providers.
Rimegepant is not indicated for the preventive treatment of migraine. The company expects to report top-line results from its prevention of migraine trial later this quarter.
This story first appeared on Medscape.com.
In clinical testing, a single 75-mg dose of rimegepant provided rapid migraine pain relief with patients returning to normal activities within 1 hour, with sustained benefit lasting up to 2 days in many patients. The majority of patients (86%) treated with a single dose did not need a migraine rescue medication within 24 hours.
“I see many patients in my practice whose lives are disrupted by migraine, afraid to go about everyday life in case of a migraine attack,” Peter Goadsby, MD, PhD, professor of neurology and director of the King’s Clinical Research Facility, King’s College Hospital, London, UK, said in a news release from Biohaven.
“Many feel unsure if their acute treatment will work and if they can manage the side effects. With the FDA approval of Nurtec ODT, there is renewed hope for people living with migraine that they can get back to living their lives without fear of the next attack,” said Goadsby.
More than 3100 patients have been treated with rimegepant with more than 113,000 doses administered in clinical trials, including a 1-year long-term safety study, the company said.
In the phase 3 trial, rimegepant achieved statistical significance on the co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) 2 hours after administration compared with placebo.
Rimegepant also showed statistical superiority at 1 hour for pain relief (reduction of moderate or severe pain to no pain or mild pain) and return to normal function.
In many patients, the benefits of pain freedom, pain relief, return to normal function, and freedom from MBS with a single dose lasted up to 48 hours.
Rimegepant was generally well tolerated. The most common adverse reaction was nausea (2%) in patients who received rimegepant compared with 0.4% of patients who received placebo.
“Everyone knows someone living with migraine, yet it remains an invisible disease that is often overlooked and misunderstood,” Mary Franklin, executive director of the National Headache Foundation, commented in the news release.
“The approval of Nurtec ODT is exciting for people with migraine as it provides a new treatment option to help people regain control of their attacks and their lives,” said Franklin.
Nurtec ODT will be available in pharmacies in early March in packs of eight tablets. Each eight-tablet pack covers treatment of eight migraine attacks with one dose, as needed, up to once daily. Sample packs containing two tablets will also be made available to healthcare providers.
Rimegepant is not indicated for the preventive treatment of migraine. The company expects to report top-line results from its prevention of migraine trial later this quarter.
This story first appeared on Medscape.com.
In clinical testing, a single 75-mg dose of rimegepant provided rapid migraine pain relief with patients returning to normal activities within 1 hour, with sustained benefit lasting up to 2 days in many patients. The majority of patients (86%) treated with a single dose did not need a migraine rescue medication within 24 hours.
“I see many patients in my practice whose lives are disrupted by migraine, afraid to go about everyday life in case of a migraine attack,” Peter Goadsby, MD, PhD, professor of neurology and director of the King’s Clinical Research Facility, King’s College Hospital, London, UK, said in a news release from Biohaven.
“Many feel unsure if their acute treatment will work and if they can manage the side effects. With the FDA approval of Nurtec ODT, there is renewed hope for people living with migraine that they can get back to living their lives without fear of the next attack,” said Goadsby.
More than 3100 patients have been treated with rimegepant with more than 113,000 doses administered in clinical trials, including a 1-year long-term safety study, the company said.
In the phase 3 trial, rimegepant achieved statistical significance on the co-primary endpoints of pain freedom and freedom from most bothersome symptom (MBS) 2 hours after administration compared with placebo.
Rimegepant also showed statistical superiority at 1 hour for pain relief (reduction of moderate or severe pain to no pain or mild pain) and return to normal function.
In many patients, the benefits of pain freedom, pain relief, return to normal function, and freedom from MBS with a single dose lasted up to 48 hours.
Rimegepant was generally well tolerated. The most common adverse reaction was nausea (2%) in patients who received rimegepant compared with 0.4% of patients who received placebo.
“Everyone knows someone living with migraine, yet it remains an invisible disease that is often overlooked and misunderstood,” Mary Franklin, executive director of the National Headache Foundation, commented in the news release.
“The approval of Nurtec ODT is exciting for people with migraine as it provides a new treatment option to help people regain control of their attacks and their lives,” said Franklin.
Nurtec ODT will be available in pharmacies in early March in packs of eight tablets. Each eight-tablet pack covers treatment of eight migraine attacks with one dose, as needed, up to once daily. Sample packs containing two tablets will also be made available to healthcare providers.
Rimegepant is not indicated for the preventive treatment of migraine. The company expects to report top-line results from its prevention of migraine trial later this quarter.
This story first appeared on Medscape.com.