User login
Climate Change, Climate Anxiety, Climate Hope
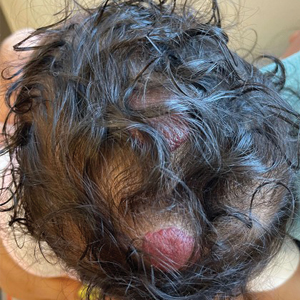
Clinical Case: Sol is a 10 year-old cisgender White girl who appears sad at her annual well visit. On further inquiry she describes that her father is angry that there is no snow, her mother keeps talking about the forests disappearing, and local flooding closed down her favorite family restaurant for good. She is worried “the planet is in trouble and there’s nothing we can do” so much that she gets stomachaches when she thinks about it.
Climate Anxiety
Climate change is a complex phenomenon that has been subject to decades of political disagreement. Lobbying by groups like the fossil fuel industry, state legislation to implement recycling, oil spills and pollution disasters, and outspoken icons like former US Vice President Al Gore and Swedish activist Greta Thunberg have kept the climate crisis a hot topic. What was once a slow burn has begun to boil as climate-related disasters occur — wildfires, droughts, floods, and increasingly powerful and frequent severe weather events — alongside increasing temperatures globally. With heroic efforts, the UN-convened Paris Agreement was adopted by 196 nations in 2015 with ambitious goals to reduce global greenhouse emissions and limit Earth’s rising temperature.1 Yet doomsday headlines on this topic remain a regular occurrence.
Between sensationalized news coverage, political controversy, and international disasters, it is no wonder some youth are overwhelmed. .2 Direct effects could include a family losing their home to flooding or wildfires, resulting in post-traumatic stress symptoms or an anxiety disorder. Indirect effects might include a drought that results in loss of agricultural income leading to a forced migration, family stress and/or separation, and disordered substance use.
Add to these direct and indirect effects the cultural and media pressures, such as frequent debate about the consequences of failure to reduce greenhouse gas emissions by 2030,3 and youth can encounter a sense of existential dread that intersects squarely with their developmental trajectory. “Climate anxiety,” also called eco-anxiety or solastalgia, refers to “distress about climate change and its impacts on the landscape and human existence.”4 Eco-anxiety is not a formal psychiatric diagnosis and is not found in the DSM-5-TR.
In practice, existential climate-centered fears range from worrying about what to do to help with the climate crisis all the way to being overwhelmed about humanity’s future to the point of dysfunction. Some argue that this is not pathological, but rather a practical response to real-world phenomena.5 An international survey of youth found 59% were “very or extremely” worried about climate change with a mix of associated emotions, and almost half described eco-anxiety as something that affects their daily functioning.6 The climate crisis often amplifies the inequities already experienced by youth from historically marginalized groups.
Managing Climate Anxiety
Climate anxiety presents with many of the typical features of other anxieties. These include worries that cycle repetitively and intrusively through the mind, somatic distress such as headaches or stomachaches, and avoidance of things that remind one of the uncertainty and distress associated with climate change. Because the climate crisis is so global and complex, hopelessness and fatigue are not uncommon.
However, climate anxiety can often be ameliorated with the typical approaches to treating anxiety. Borrowing from cognitive-behavioral and mindfulness-based interventions, many recommendations have been offered to help with eco-anxiety. External validation of youth’s concerns and fears is a starting point that might build a teen’s capacity to tolerate distressing emotions about global warming.
Once reactions to climate change are acknowledged and accepted, space is created for reflection. This might include a balance of hope and pragmatic action. For example, renewable energy sources have made up an increasing share of the market over time with the world adding 50% more renewable capacity in 2023.7 Seventy-two percent of Americans acknowledge global warming, 75% feel schools should teach about consequences and solutions for global warming, and 79% support investment in renewable energy.8
Climate activism itself has been shown to buffer climate anxiety, particularly when implemented collectively rather than individually.4 Nature connectedness, or cognitive and emotional connections with nature, not only has many direct mental health benefits, but is also associated with climate activism.9 Many other integrative interventions can improve well-being while reducing ecological harm. Nutrition, physical activity, mindfulness, and sleep are youth mental health interventions with a strong evidence base that also reduce the carbon footprint and pollution attributable to psychiatric pharmaceuticals. Moreover, these climate-friendly interventions can improve family-connectedness, thus boosting resilience.
Without needing to become eco-warriors, healthcare providers can model sustainable practices while caring for patients. This might include having more plants in the office, recycling and composting at work, adding solar panels to the rooftop, or joining local parks prescription programs (see mygreendoctor.org, a nonprofit owned by the Florida Medical Association).
Next Steps
Sol is relieved to hear that many kids her age share her family’s concerns. A conversation about how to manage distressing emotions and physical feelings leads to a referral for brief cognitive behavioral interventions. Her parents join your visit to hear her concerns. They want to begin a family plan for climate action. You recommend the books How to Change Everything: The Young Human’s Guide to Protecting the Planet and Each Other by Naomi Klein and The Parents’ Guide to Climate Revolution: 100 Ways to Build a Fossil-Free Future, Raise Empowered Kids, and Still Get a Good Night’s Sleep by Mary DeMocker.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington.
References
1. Maizland L. Global Climate Agreements: Successes and Failures. Council on Foreign Relations. https://www.cfr.org/backgrounder/paris-global-climate-change-agreements.
2. van Nieuwenhuizen A et al. The effects of climate change on child and adolescent mental health: Clinical considerations. Curr Psychiatry Rep. 2021 Dec 7;23(12):88. doi: 10.1007/s11920-021-01296-y.
3. Window to Reach Climate Goals ‘Rapidly Closing’, UN Report Warns. United Nations. https://news.un.org/en/story/2023/09/1140527.
4. Schwartz SEO et al. Climate change anxiety and mental health: Environmental activism as buffer. Curr Psychol. 2022 Feb 28:1-14. doi: 10.1007/s12144-022-02735-6.
5. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12:7836. doi: 10.3390/su12197836.
6. Hickman C et al. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet Health. 2021 Dec;5(12):e863-e873. doi: 10.1016/S2542-5196(21)00278-3.
7. IEA (2021), Global Energy Review 2021, IEA, Paris. https://www.iea.org/reports/global-energy-review-2021/renewables.
8. Marlon J et al. Yale Climate Opinion Maps 2023. https://climatecommunication.yale.edu/visualizations-data/ycom-us/.
9. Thomson EE, Roach SP. The Relationships Among Nature Connectedness, Climate Anxiety, Climate Action, Climate Knowledge, and Mental Health. Front Psychol. 2023 Nov 15:14:1241400. doi: 10.3389/fpsyg.2023.1241400.
Clinical Case: Sol is a 10 year-old cisgender White girl who appears sad at her annual well visit. On further inquiry she describes that her father is angry that there is no snow, her mother keeps talking about the forests disappearing, and local flooding closed down her favorite family restaurant for good. She is worried “the planet is in trouble and there’s nothing we can do” so much that she gets stomachaches when she thinks about it.
Climate Anxiety
Climate change is a complex phenomenon that has been subject to decades of political disagreement. Lobbying by groups like the fossil fuel industry, state legislation to implement recycling, oil spills and pollution disasters, and outspoken icons like former US Vice President Al Gore and Swedish activist Greta Thunberg have kept the climate crisis a hot topic. What was once a slow burn has begun to boil as climate-related disasters occur — wildfires, droughts, floods, and increasingly powerful and frequent severe weather events — alongside increasing temperatures globally. With heroic efforts, the UN-convened Paris Agreement was adopted by 196 nations in 2015 with ambitious goals to reduce global greenhouse emissions and limit Earth’s rising temperature.1 Yet doomsday headlines on this topic remain a regular occurrence.
Between sensationalized news coverage, political controversy, and international disasters, it is no wonder some youth are overwhelmed. .2 Direct effects could include a family losing their home to flooding or wildfires, resulting in post-traumatic stress symptoms or an anxiety disorder. Indirect effects might include a drought that results in loss of agricultural income leading to a forced migration, family stress and/or separation, and disordered substance use.
Add to these direct and indirect effects the cultural and media pressures, such as frequent debate about the consequences of failure to reduce greenhouse gas emissions by 2030,3 and youth can encounter a sense of existential dread that intersects squarely with their developmental trajectory. “Climate anxiety,” also called eco-anxiety or solastalgia, refers to “distress about climate change and its impacts on the landscape and human existence.”4 Eco-anxiety is not a formal psychiatric diagnosis and is not found in the DSM-5-TR.
In practice, existential climate-centered fears range from worrying about what to do to help with the climate crisis all the way to being overwhelmed about humanity’s future to the point of dysfunction. Some argue that this is not pathological, but rather a practical response to real-world phenomena.5 An international survey of youth found 59% were “very or extremely” worried about climate change with a mix of associated emotions, and almost half described eco-anxiety as something that affects their daily functioning.6 The climate crisis often amplifies the inequities already experienced by youth from historically marginalized groups.
Managing Climate Anxiety
Climate anxiety presents with many of the typical features of other anxieties. These include worries that cycle repetitively and intrusively through the mind, somatic distress such as headaches or stomachaches, and avoidance of things that remind one of the uncertainty and distress associated with climate change. Because the climate crisis is so global and complex, hopelessness and fatigue are not uncommon.
However, climate anxiety can often be ameliorated with the typical approaches to treating anxiety. Borrowing from cognitive-behavioral and mindfulness-based interventions, many recommendations have been offered to help with eco-anxiety. External validation of youth’s concerns and fears is a starting point that might build a teen’s capacity to tolerate distressing emotions about global warming.
Once reactions to climate change are acknowledged and accepted, space is created for reflection. This might include a balance of hope and pragmatic action. For example, renewable energy sources have made up an increasing share of the market over time with the world adding 50% more renewable capacity in 2023.7 Seventy-two percent of Americans acknowledge global warming, 75% feel schools should teach about consequences and solutions for global warming, and 79% support investment in renewable energy.8
Climate activism itself has been shown to buffer climate anxiety, particularly when implemented collectively rather than individually.4 Nature connectedness, or cognitive and emotional connections with nature, not only has many direct mental health benefits, but is also associated with climate activism.9 Many other integrative interventions can improve well-being while reducing ecological harm. Nutrition, physical activity, mindfulness, and sleep are youth mental health interventions with a strong evidence base that also reduce the carbon footprint and pollution attributable to psychiatric pharmaceuticals. Moreover, these climate-friendly interventions can improve family-connectedness, thus boosting resilience.
Without needing to become eco-warriors, healthcare providers can model sustainable practices while caring for patients. This might include having more plants in the office, recycling and composting at work, adding solar panels to the rooftop, or joining local parks prescription programs (see mygreendoctor.org, a nonprofit owned by the Florida Medical Association).
Next Steps
Sol is relieved to hear that many kids her age share her family’s concerns. A conversation about how to manage distressing emotions and physical feelings leads to a referral for brief cognitive behavioral interventions. Her parents join your visit to hear her concerns. They want to begin a family plan for climate action. You recommend the books How to Change Everything: The Young Human’s Guide to Protecting the Planet and Each Other by Naomi Klein and The Parents’ Guide to Climate Revolution: 100 Ways to Build a Fossil-Free Future, Raise Empowered Kids, and Still Get a Good Night’s Sleep by Mary DeMocker.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington.
References
1. Maizland L. Global Climate Agreements: Successes and Failures. Council on Foreign Relations. https://www.cfr.org/backgrounder/paris-global-climate-change-agreements.
2. van Nieuwenhuizen A et al. The effects of climate change on child and adolescent mental health: Clinical considerations. Curr Psychiatry Rep. 2021 Dec 7;23(12):88. doi: 10.1007/s11920-021-01296-y.
3. Window to Reach Climate Goals ‘Rapidly Closing’, UN Report Warns. United Nations. https://news.un.org/en/story/2023/09/1140527.
4. Schwartz SEO et al. Climate change anxiety and mental health: Environmental activism as buffer. Curr Psychol. 2022 Feb 28:1-14. doi: 10.1007/s12144-022-02735-6.
5. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12:7836. doi: 10.3390/su12197836.
6. Hickman C et al. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet Health. 2021 Dec;5(12):e863-e873. doi: 10.1016/S2542-5196(21)00278-3.
7. IEA (2021), Global Energy Review 2021, IEA, Paris. https://www.iea.org/reports/global-energy-review-2021/renewables.
8. Marlon J et al. Yale Climate Opinion Maps 2023. https://climatecommunication.yale.edu/visualizations-data/ycom-us/.
9. Thomson EE, Roach SP. The Relationships Among Nature Connectedness, Climate Anxiety, Climate Action, Climate Knowledge, and Mental Health. Front Psychol. 2023 Nov 15:14:1241400. doi: 10.3389/fpsyg.2023.1241400.
Clinical Case: Sol is a 10 year-old cisgender White girl who appears sad at her annual well visit. On further inquiry she describes that her father is angry that there is no snow, her mother keeps talking about the forests disappearing, and local flooding closed down her favorite family restaurant for good. She is worried “the planet is in trouble and there’s nothing we can do” so much that she gets stomachaches when she thinks about it.
Climate Anxiety
Climate change is a complex phenomenon that has been subject to decades of political disagreement. Lobbying by groups like the fossil fuel industry, state legislation to implement recycling, oil spills and pollution disasters, and outspoken icons like former US Vice President Al Gore and Swedish activist Greta Thunberg have kept the climate crisis a hot topic. What was once a slow burn has begun to boil as climate-related disasters occur — wildfires, droughts, floods, and increasingly powerful and frequent severe weather events — alongside increasing temperatures globally. With heroic efforts, the UN-convened Paris Agreement was adopted by 196 nations in 2015 with ambitious goals to reduce global greenhouse emissions and limit Earth’s rising temperature.1 Yet doomsday headlines on this topic remain a regular occurrence.
Between sensationalized news coverage, political controversy, and international disasters, it is no wonder some youth are overwhelmed. .2 Direct effects could include a family losing their home to flooding or wildfires, resulting in post-traumatic stress symptoms or an anxiety disorder. Indirect effects might include a drought that results in loss of agricultural income leading to a forced migration, family stress and/or separation, and disordered substance use.
Add to these direct and indirect effects the cultural and media pressures, such as frequent debate about the consequences of failure to reduce greenhouse gas emissions by 2030,3 and youth can encounter a sense of existential dread that intersects squarely with their developmental trajectory. “Climate anxiety,” also called eco-anxiety or solastalgia, refers to “distress about climate change and its impacts on the landscape and human existence.”4 Eco-anxiety is not a formal psychiatric diagnosis and is not found in the DSM-5-TR.
In practice, existential climate-centered fears range from worrying about what to do to help with the climate crisis all the way to being overwhelmed about humanity’s future to the point of dysfunction. Some argue that this is not pathological, but rather a practical response to real-world phenomena.5 An international survey of youth found 59% were “very or extremely” worried about climate change with a mix of associated emotions, and almost half described eco-anxiety as something that affects their daily functioning.6 The climate crisis often amplifies the inequities already experienced by youth from historically marginalized groups.
Managing Climate Anxiety
Climate anxiety presents with many of the typical features of other anxieties. These include worries that cycle repetitively and intrusively through the mind, somatic distress such as headaches or stomachaches, and avoidance of things that remind one of the uncertainty and distress associated with climate change. Because the climate crisis is so global and complex, hopelessness and fatigue are not uncommon.
However, climate anxiety can often be ameliorated with the typical approaches to treating anxiety. Borrowing from cognitive-behavioral and mindfulness-based interventions, many recommendations have been offered to help with eco-anxiety. External validation of youth’s concerns and fears is a starting point that might build a teen’s capacity to tolerate distressing emotions about global warming.
Once reactions to climate change are acknowledged and accepted, space is created for reflection. This might include a balance of hope and pragmatic action. For example, renewable energy sources have made up an increasing share of the market over time with the world adding 50% more renewable capacity in 2023.7 Seventy-two percent of Americans acknowledge global warming, 75% feel schools should teach about consequences and solutions for global warming, and 79% support investment in renewable energy.8
Climate activism itself has been shown to buffer climate anxiety, particularly when implemented collectively rather than individually.4 Nature connectedness, or cognitive and emotional connections with nature, not only has many direct mental health benefits, but is also associated with climate activism.9 Many other integrative interventions can improve well-being while reducing ecological harm. Nutrition, physical activity, mindfulness, and sleep are youth mental health interventions with a strong evidence base that also reduce the carbon footprint and pollution attributable to psychiatric pharmaceuticals. Moreover, these climate-friendly interventions can improve family-connectedness, thus boosting resilience.
Without needing to become eco-warriors, healthcare providers can model sustainable practices while caring for patients. This might include having more plants in the office, recycling and composting at work, adding solar panels to the rooftop, or joining local parks prescription programs (see mygreendoctor.org, a nonprofit owned by the Florida Medical Association).
Next Steps
Sol is relieved to hear that many kids her age share her family’s concerns. A conversation about how to manage distressing emotions and physical feelings leads to a referral for brief cognitive behavioral interventions. Her parents join your visit to hear her concerns. They want to begin a family plan for climate action. You recommend the books How to Change Everything: The Young Human’s Guide to Protecting the Planet and Each Other by Naomi Klein and The Parents’ Guide to Climate Revolution: 100 Ways to Build a Fossil-Free Future, Raise Empowered Kids, and Still Get a Good Night’s Sleep by Mary DeMocker.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington.
References
1. Maizland L. Global Climate Agreements: Successes and Failures. Council on Foreign Relations. https://www.cfr.org/backgrounder/paris-global-climate-change-agreements.
2. van Nieuwenhuizen A et al. The effects of climate change on child and adolescent mental health: Clinical considerations. Curr Psychiatry Rep. 2021 Dec 7;23(12):88. doi: 10.1007/s11920-021-01296-y.
3. Window to Reach Climate Goals ‘Rapidly Closing’, UN Report Warns. United Nations. https://news.un.org/en/story/2023/09/1140527.
4. Schwartz SEO et al. Climate change anxiety and mental health: Environmental activism as buffer. Curr Psychol. 2022 Feb 28:1-14. doi: 10.1007/s12144-022-02735-6.
5. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12:7836. doi: 10.3390/su12197836.
6. Hickman C et al. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet Health. 2021 Dec;5(12):e863-e873. doi: 10.1016/S2542-5196(21)00278-3.
7. IEA (2021), Global Energy Review 2021, IEA, Paris. https://www.iea.org/reports/global-energy-review-2021/renewables.
8. Marlon J et al. Yale Climate Opinion Maps 2023. https://climatecommunication.yale.edu/visualizations-data/ycom-us/.
9. Thomson EE, Roach SP. The Relationships Among Nature Connectedness, Climate Anxiety, Climate Action, Climate Knowledge, and Mental Health. Front Psychol. 2023 Nov 15:14:1241400. doi: 10.3389/fpsyg.2023.1241400.
Similar Outcomes With Labetalol, Nifedipine for Chronic Hypertension in Pregnancy
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
FROM OBSTETRICS & GYNECOLOGY
Which Surgeries Drive the Most Opioid Prescriptions in Youth?
TOPLINE:
according to a new study.
METHODOLOGY:
- The researchers analyzed national commercial and Medicaid claims from December 2020 to November 2021 in children aged 0-21 years.
- More than 200,000 procedures were included in the study.
- For each type of surgery, researchers calculated the total amount of opioids given within 3 days of discharge, measured in morphine milligram equivalents (MMEs).
TAKEAWAY:
- In children up to age 11 years, three procedures accounted for 59.1% of MMEs: Tonsillectomy and/or adenoidectomy (50.3%), open treatment of upper extremity fracture (5.3%), and removal of deep implants (3.5%).
- In patients aged 12-21 years, three procedures accounted for 33.1% of MMEs: Tonsillectomy and/or adenoidectomy (12.7%), knee arthroscopy (12.6%), and analgesia after cesarean delivery (7.8%).
- Refill rates for children were all 1% or less.
- Refill rates for adolescents ranged from 2.3% to 9.6%.
IN PRACTICE:
“Targeting these procedures in opioid stewardship initiatives could help minimize the risks of opioid prescribing while maintaining effective postoperative pain control,” the researchers wrote.
SOURCE:
The study was led by Kao-Ping Chua, MD, PhD, of the Department of Pediatrics at the University of Michigan Medical School, Ann Arbor, and was published in Pediatrics
LIMITATIONS:
The researchers analyzed opioids prescribed only after major surgeries. The sources of data used in the analysis may not fully represent all pediatric patients.
DISCLOSURES:
Dr. Chua reported consulting fees from the US Department of Justice and the Benter Foundation outside the submitted work. Other authors reported a variety of financial interests, including consulting for the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
TOPLINE:
according to a new study.
METHODOLOGY:
- The researchers analyzed national commercial and Medicaid claims from December 2020 to November 2021 in children aged 0-21 years.
- More than 200,000 procedures were included in the study.
- For each type of surgery, researchers calculated the total amount of opioids given within 3 days of discharge, measured in morphine milligram equivalents (MMEs).
TAKEAWAY:
- In children up to age 11 years, three procedures accounted for 59.1% of MMEs: Tonsillectomy and/or adenoidectomy (50.3%), open treatment of upper extremity fracture (5.3%), and removal of deep implants (3.5%).
- In patients aged 12-21 years, three procedures accounted for 33.1% of MMEs: Tonsillectomy and/or adenoidectomy (12.7%), knee arthroscopy (12.6%), and analgesia after cesarean delivery (7.8%).
- Refill rates for children were all 1% or less.
- Refill rates for adolescents ranged from 2.3% to 9.6%.
IN PRACTICE:
“Targeting these procedures in opioid stewardship initiatives could help minimize the risks of opioid prescribing while maintaining effective postoperative pain control,” the researchers wrote.
SOURCE:
The study was led by Kao-Ping Chua, MD, PhD, of the Department of Pediatrics at the University of Michigan Medical School, Ann Arbor, and was published in Pediatrics
LIMITATIONS:
The researchers analyzed opioids prescribed only after major surgeries. The sources of data used in the analysis may not fully represent all pediatric patients.
DISCLOSURES:
Dr. Chua reported consulting fees from the US Department of Justice and the Benter Foundation outside the submitted work. Other authors reported a variety of financial interests, including consulting for the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
TOPLINE:
according to a new study.
METHODOLOGY:
- The researchers analyzed national commercial and Medicaid claims from December 2020 to November 2021 in children aged 0-21 years.
- More than 200,000 procedures were included in the study.
- For each type of surgery, researchers calculated the total amount of opioids given within 3 days of discharge, measured in morphine milligram equivalents (MMEs).
TAKEAWAY:
- In children up to age 11 years, three procedures accounted for 59.1% of MMEs: Tonsillectomy and/or adenoidectomy (50.3%), open treatment of upper extremity fracture (5.3%), and removal of deep implants (3.5%).
- In patients aged 12-21 years, three procedures accounted for 33.1% of MMEs: Tonsillectomy and/or adenoidectomy (12.7%), knee arthroscopy (12.6%), and analgesia after cesarean delivery (7.8%).
- Refill rates for children were all 1% or less.
- Refill rates for adolescents ranged from 2.3% to 9.6%.
IN PRACTICE:
“Targeting these procedures in opioid stewardship initiatives could help minimize the risks of opioid prescribing while maintaining effective postoperative pain control,” the researchers wrote.
SOURCE:
The study was led by Kao-Ping Chua, MD, PhD, of the Department of Pediatrics at the University of Michigan Medical School, Ann Arbor, and was published in Pediatrics
LIMITATIONS:
The researchers analyzed opioids prescribed only after major surgeries. The sources of data used in the analysis may not fully represent all pediatric patients.
DISCLOSURES:
Dr. Chua reported consulting fees from the US Department of Justice and the Benter Foundation outside the submitted work. Other authors reported a variety of financial interests, including consulting for the pharmaceutical industry.
A version of this article first appeared on Medscape.com.
Neurofilament Light Chain Detects Early Chemotherapy-Related Neurotoxicity
Investigators found Nfl levels increased in cancer patients following a first infusion of the medication paclitaxel and corresponded to neuropathy severity 6-12 months post-treatment, suggesting the blood protein may provide an early CIPN biomarker.
“Nfl after a single cycle could detect axonal degeneration,” said lead investigator Masarra Joda, a researcher and PhD candidate at the University of Sydney in Australia. She added that “quantification of Nfl may provide a clinically useful marker of emerging neurotoxicity in patients vulnerable to CIPN.”
The findings were presented at the Peripheral Nerve Society (PNS) 2024 annual meeting.
Common, Burdensome Side Effect
A common side effect of chemotherapy, CIPN manifests as sensory neuropathy and causes degeneration of the peripheral axons. A protein biomarker of axonal degeneration, Nfl has previously been investigated as a way of identifying patients at risk of CIPN.
The goal of the current study was to identify the potential link between Nfl with neurophysiological markers of axon degeneration in patients receiving the neurotoxin chemotherapy paclitaxel.
The study included 93 cancer patients. All were assessed at the beginning, middle, and end of treatment. CIPN was assessed using blood samples of Nfl and the Total Neuropathy Score (TNS), the Common Terminology Criteria for Adverse Events (CTCAE) neuropathy scale, and patient-reported measures using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (EORTC-CIPN20).
Axonal degeneration was measured with neurophysiological tests including sural nerve compound sensory action potential (CSAP) for the lower limbs, and sensory median nerve CSAP, as well as stimulus threshold testing, for the upper limbs.
Almost all of study participants (97%) were female. The majority (66%) had breast cancer and 30% had gynecological cancer. Most (73%) were receiving a weekly regimen of paclitaxel, and the remainder were treated with taxanes plus platinum once every 3 weeks. By the end of treatment, 82% of the patients had developed CIPN, which was mild in 44% and moderate/severe in 38%.
Nfl levels increased significantly from baseline to after the first dose of chemotherapy (P < .001), “highlighting that nerve damage occurs from the very beginning of treatment,” senior investigator Susanna Park, PhD, told this news organization.
In addition, “patients with higher Nfl levels after a single paclitaxel treatment had greater neuropathy at the end of treatment (higher EORTC scores [P ≤ .026], and higher TNS scores [P ≤ .00]),” added Dr. Park, who is associate professor at the University of Sydney.
“Importantly, we also looked at long-term outcomes beyond the end of chemotherapy, because chronic neuropathy produces a significant burden in cancer survivors,” said Dr. Park.
“Among a total of 44 patients who completed the 6- to 12-month post-treatment follow-up, NfL levels after a single treatment were linked to severity of nerve damage quantified with neurophysiological tests, and greater Nfl levels at mid-treatment were correlated with worse patient and neurologically graded neuropathy at 6-12 months.”
Dr. Park said the results suggest that NfL may provide a biomarker of long-term axon damage and that Nfl assays “may enable clinicians to evaluate the risk of long-term toxicity early during paclitaxel treatment to hopefully provide clinically significant information to guide better treatment titration.”
Currently, she said, CIPN is a prominent cause of dose reduction and early chemotherapy cessation.
“For example, in early breast cancer around 25% of patients experience a dose reduction due to the severity of neuropathy symptoms.” But, she said, “there is no standardized way of identifying which patients are at risk of long-term neuropathy and therefore, may benefit more from dose reduction. In this setting, a biomarker such as Nfl could provide oncologists with more information about the risk of long-term toxicity and take that into account in dose decision-making.”
For some cancers, she added, there are multiple potential therapy options.
“A biomarker such as NfL could assist in determining risk-benefit profile in terms of switching to alternate therapies. However, further studies will be needed to fully define the utility of NfL as a biomarker of paclitaxel neuropathy.”
Promising Research
Commenting on the research for this news organization, Maryam Lustberg, MD, associate professor, director of the Center for Breast Cancer at Smilow Cancer Hospital and Yale Cancer Center, and chief of Breast Medical Oncology at Yale Cancer Center, in New Haven, Connecticut, said the study “builds on a body of work previously reported by others showing that neurofilament light chains as detected in the blood can be associated with early signs of neurotoxic injury.”
She added that the research “is promising, since existing clinical and patient-reported measures tend to under-detect chemotherapy-induced neuropathy until more permanent injury might have occurred.”
Dr. Lustberg, who is immediate past president of the Multinational Association of Supportive Care in Cancer, said future studies are needed before Nfl testing can be implemented in routine practice, but that “early detection will allow earlier initiation of supportive care strategies such as physical therapy and exercise, as well as dose modifications, which may be helpful for preventing permanent damage and improving quality of life.”
The investigators and Dr. Lustberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Investigators found Nfl levels increased in cancer patients following a first infusion of the medication paclitaxel and corresponded to neuropathy severity 6-12 months post-treatment, suggesting the blood protein may provide an early CIPN biomarker.
“Nfl after a single cycle could detect axonal degeneration,” said lead investigator Masarra Joda, a researcher and PhD candidate at the University of Sydney in Australia. She added that “quantification of Nfl may provide a clinically useful marker of emerging neurotoxicity in patients vulnerable to CIPN.”
The findings were presented at the Peripheral Nerve Society (PNS) 2024 annual meeting.
Common, Burdensome Side Effect
A common side effect of chemotherapy, CIPN manifests as sensory neuropathy and causes degeneration of the peripheral axons. A protein biomarker of axonal degeneration, Nfl has previously been investigated as a way of identifying patients at risk of CIPN.
The goal of the current study was to identify the potential link between Nfl with neurophysiological markers of axon degeneration in patients receiving the neurotoxin chemotherapy paclitaxel.
The study included 93 cancer patients. All were assessed at the beginning, middle, and end of treatment. CIPN was assessed using blood samples of Nfl and the Total Neuropathy Score (TNS), the Common Terminology Criteria for Adverse Events (CTCAE) neuropathy scale, and patient-reported measures using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (EORTC-CIPN20).
Axonal degeneration was measured with neurophysiological tests including sural nerve compound sensory action potential (CSAP) for the lower limbs, and sensory median nerve CSAP, as well as stimulus threshold testing, for the upper limbs.
Almost all of study participants (97%) were female. The majority (66%) had breast cancer and 30% had gynecological cancer. Most (73%) were receiving a weekly regimen of paclitaxel, and the remainder were treated with taxanes plus platinum once every 3 weeks. By the end of treatment, 82% of the patients had developed CIPN, which was mild in 44% and moderate/severe in 38%.
Nfl levels increased significantly from baseline to after the first dose of chemotherapy (P < .001), “highlighting that nerve damage occurs from the very beginning of treatment,” senior investigator Susanna Park, PhD, told this news organization.
In addition, “patients with higher Nfl levels after a single paclitaxel treatment had greater neuropathy at the end of treatment (higher EORTC scores [P ≤ .026], and higher TNS scores [P ≤ .00]),” added Dr. Park, who is associate professor at the University of Sydney.
“Importantly, we also looked at long-term outcomes beyond the end of chemotherapy, because chronic neuropathy produces a significant burden in cancer survivors,” said Dr. Park.
“Among a total of 44 patients who completed the 6- to 12-month post-treatment follow-up, NfL levels after a single treatment were linked to severity of nerve damage quantified with neurophysiological tests, and greater Nfl levels at mid-treatment were correlated with worse patient and neurologically graded neuropathy at 6-12 months.”
Dr. Park said the results suggest that NfL may provide a biomarker of long-term axon damage and that Nfl assays “may enable clinicians to evaluate the risk of long-term toxicity early during paclitaxel treatment to hopefully provide clinically significant information to guide better treatment titration.”
Currently, she said, CIPN is a prominent cause of dose reduction and early chemotherapy cessation.
“For example, in early breast cancer around 25% of patients experience a dose reduction due to the severity of neuropathy symptoms.” But, she said, “there is no standardized way of identifying which patients are at risk of long-term neuropathy and therefore, may benefit more from dose reduction. In this setting, a biomarker such as Nfl could provide oncologists with more information about the risk of long-term toxicity and take that into account in dose decision-making.”
For some cancers, she added, there are multiple potential therapy options.
“A biomarker such as NfL could assist in determining risk-benefit profile in terms of switching to alternate therapies. However, further studies will be needed to fully define the utility of NfL as a biomarker of paclitaxel neuropathy.”
Promising Research
Commenting on the research for this news organization, Maryam Lustberg, MD, associate professor, director of the Center for Breast Cancer at Smilow Cancer Hospital and Yale Cancer Center, and chief of Breast Medical Oncology at Yale Cancer Center, in New Haven, Connecticut, said the study “builds on a body of work previously reported by others showing that neurofilament light chains as detected in the blood can be associated with early signs of neurotoxic injury.”
She added that the research “is promising, since existing clinical and patient-reported measures tend to under-detect chemotherapy-induced neuropathy until more permanent injury might have occurred.”
Dr. Lustberg, who is immediate past president of the Multinational Association of Supportive Care in Cancer, said future studies are needed before Nfl testing can be implemented in routine practice, but that “early detection will allow earlier initiation of supportive care strategies such as physical therapy and exercise, as well as dose modifications, which may be helpful for preventing permanent damage and improving quality of life.”
The investigators and Dr. Lustberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Investigators found Nfl levels increased in cancer patients following a first infusion of the medication paclitaxel and corresponded to neuropathy severity 6-12 months post-treatment, suggesting the blood protein may provide an early CIPN biomarker.
“Nfl after a single cycle could detect axonal degeneration,” said lead investigator Masarra Joda, a researcher and PhD candidate at the University of Sydney in Australia. She added that “quantification of Nfl may provide a clinically useful marker of emerging neurotoxicity in patients vulnerable to CIPN.”
The findings were presented at the Peripheral Nerve Society (PNS) 2024 annual meeting.
Common, Burdensome Side Effect
A common side effect of chemotherapy, CIPN manifests as sensory neuropathy and causes degeneration of the peripheral axons. A protein biomarker of axonal degeneration, Nfl has previously been investigated as a way of identifying patients at risk of CIPN.
The goal of the current study was to identify the potential link between Nfl with neurophysiological markers of axon degeneration in patients receiving the neurotoxin chemotherapy paclitaxel.
The study included 93 cancer patients. All were assessed at the beginning, middle, and end of treatment. CIPN was assessed using blood samples of Nfl and the Total Neuropathy Score (TNS), the Common Terminology Criteria for Adverse Events (CTCAE) neuropathy scale, and patient-reported measures using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (EORTC-CIPN20).
Axonal degeneration was measured with neurophysiological tests including sural nerve compound sensory action potential (CSAP) for the lower limbs, and sensory median nerve CSAP, as well as stimulus threshold testing, for the upper limbs.
Almost all of study participants (97%) were female. The majority (66%) had breast cancer and 30% had gynecological cancer. Most (73%) were receiving a weekly regimen of paclitaxel, and the remainder were treated with taxanes plus platinum once every 3 weeks. By the end of treatment, 82% of the patients had developed CIPN, which was mild in 44% and moderate/severe in 38%.
Nfl levels increased significantly from baseline to after the first dose of chemotherapy (P < .001), “highlighting that nerve damage occurs from the very beginning of treatment,” senior investigator Susanna Park, PhD, told this news organization.
In addition, “patients with higher Nfl levels after a single paclitaxel treatment had greater neuropathy at the end of treatment (higher EORTC scores [P ≤ .026], and higher TNS scores [P ≤ .00]),” added Dr. Park, who is associate professor at the University of Sydney.
“Importantly, we also looked at long-term outcomes beyond the end of chemotherapy, because chronic neuropathy produces a significant burden in cancer survivors,” said Dr. Park.
“Among a total of 44 patients who completed the 6- to 12-month post-treatment follow-up, NfL levels after a single treatment were linked to severity of nerve damage quantified with neurophysiological tests, and greater Nfl levels at mid-treatment were correlated with worse patient and neurologically graded neuropathy at 6-12 months.”
Dr. Park said the results suggest that NfL may provide a biomarker of long-term axon damage and that Nfl assays “may enable clinicians to evaluate the risk of long-term toxicity early during paclitaxel treatment to hopefully provide clinically significant information to guide better treatment titration.”
Currently, she said, CIPN is a prominent cause of dose reduction and early chemotherapy cessation.
“For example, in early breast cancer around 25% of patients experience a dose reduction due to the severity of neuropathy symptoms.” But, she said, “there is no standardized way of identifying which patients are at risk of long-term neuropathy and therefore, may benefit more from dose reduction. In this setting, a biomarker such as Nfl could provide oncologists with more information about the risk of long-term toxicity and take that into account in dose decision-making.”
For some cancers, she added, there are multiple potential therapy options.
“A biomarker such as NfL could assist in determining risk-benefit profile in terms of switching to alternate therapies. However, further studies will be needed to fully define the utility of NfL as a biomarker of paclitaxel neuropathy.”
Promising Research
Commenting on the research for this news organization, Maryam Lustberg, MD, associate professor, director of the Center for Breast Cancer at Smilow Cancer Hospital and Yale Cancer Center, and chief of Breast Medical Oncology at Yale Cancer Center, in New Haven, Connecticut, said the study “builds on a body of work previously reported by others showing that neurofilament light chains as detected in the blood can be associated with early signs of neurotoxic injury.”
She added that the research “is promising, since existing clinical and patient-reported measures tend to under-detect chemotherapy-induced neuropathy until more permanent injury might have occurred.”
Dr. Lustberg, who is immediate past president of the Multinational Association of Supportive Care in Cancer, said future studies are needed before Nfl testing can be implemented in routine practice, but that “early detection will allow earlier initiation of supportive care strategies such as physical therapy and exercise, as well as dose modifications, which may be helpful for preventing permanent damage and improving quality of life.”
The investigators and Dr. Lustberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT PNS 2024
First-line Canakinumab Without Steroids Shows Effectiveness for Systemic Juvenile Idiopathic Arthritis
VIENNA — The interleukin-1 receptor antagonist (IL-1RA) canakinumab provided control of systemic juvenile idiopathic arthritis (sJIA) without the use of glucocorticoids for up to a year in most study participants after three monthly injections.
In this study of 20 patients with newly diagnosed sJIA treated off glucocorticoids, fever was controlled after a single injection in all patients, and 16 patients reached the primary outcome of remission after three injections, said Gerd Horneff, MD, PhD, Asklepios Children’s Hospital, Sankt Augustin, Germany.
Results of this open-label study, called CANAKINUMAB FIRST, were presented as late-breaking findings at the European Alliance of Associations for Rheumatology (EULAR) 2024 Annual Meeting.
“Steroid-free, first-line treatment with canakinumab led to sustained responses in most patients, with a considerable number achieving remission,” said Dr. Horneff, adding that the observation in this group is ongoing.
Building on Earlier Data
The efficacy of canakinumab was previously reported in anecdotal experiences and one small patient series published 10 years ago. Dr. Horneff noted that he has offered this drug off label to patients with challenging cases.
The objective was to evaluate canakinumab as a first-line monotherapy administered in the absence of glucocorticoids. The study was open to children aged 2-18 years with active sJIA/juvenile Still disease confirmed with published criteria. All were naive to biologic or nonbiologic disease-modifying antirheumatic drugs as well as steroids.
The median age of the children was 8.4 years. A total of 60% were men. The median disease duration at the time of entry was 1.2 months. Most had fever (95%) and rash (80%) with high levels of inflammatory markers at baseline. The mean number of painful joints was 3.1, and the mean number of systemic manifestations was 2.8. No patient was without any systemic involvement, but four of the patients did not have any painful joints.
At enrollment, patients were scheduled to receive three injections of canakinumab at monthly intervals during an active treatment phase, after which they entered an observation phase lasting 40 weeks. In the event of nonresponse or flares in either phase, they were transitioned to usual care.
Symptoms Resolve After Single Injection
After the first injection, active joint disease and all systemic manifestations resolved in 16 (80%) of the 20 patients. Joint activity and systemic manifestations also remained controlled after the second and third injections in 16 of the 20 patients.
One patient in this series achieved inactive disease after a single injection but developed what appeared to be a treatment-related allergic reaction. He received no further treatment and was excluded from the study, although he is being followed separately.
“According to sJADAS [systemic JIA Disease Activity Score] criteria at month 3, 14 had inactive disease, three had minimal disease activity, and one patient had moderate disease activity,” Dr. Horneff said.
At week 24, or 3 months after the last injection, there was still no joint activity in 16 patients. Systemic manifestations remained controlled in 13 patients, but 1 patient by this point had a flare. Another flare occurred after this point, and other patients have not yet completed the 52-week observation period.
“Of the 10 patients who remained in the study and have completed the 52-week observation period, eight have had a drug-free remission,” Dr. Horneff said.
MAS Event Observed in One Patient
In addition to the allergic skin reaction, which was considered probably related to the study drug, there were three flares, one of which was a macrophage activation syndrome (MAS) event. The MAS occurred 8 weeks after the last injection, but it was managed successfully.
Of 30 infections that developed during the observation period, 18 involved the upper airway. All were treated successfully. There were also two injection-site reactions and one case of cytopenia.
Among the studies planned for follow-up, investigators will examine genomic and gene activation in relation to disease activity and the effect of canakinumab.
Comoderator of the abstract session and chair of the EULAR 2024 Abstract Selection Committee, Christian Dejaco, MD, PhD, a consultant rheumatologist and associate professor at the Medical University of Graz in Graz, Austria, suggested that these are highly encouraging data for a disease that does not currently have any approved therapies. Clearly, larger studies with a longer follow-up period are needed, but he pointed out that phase 3 trials in a rare disease like sJIA are challenging.
Because of the limited number of cases, “it will be difficult to conduct a placebo-controlled trial,” he pointed out. However, he hopes this study will provide the basis for larger studies and sufficient data to lead to an indication for this therapy.
In the meantime, he also believes that these data are likely to support empirical use in a difficult disease, even in advance of formal regulatory approval.
“We heard that canakinumab is already being used off label in JIA, and these data might encourage more of that,” he said.
Dr. Horneff reported financial relationships with AbbVie, Boehringer Ingelheim, Celgene, Chugai, GlaxoSmithKline, Janssen, Merck Sharpe & Dohme, Novartis, Pfizer, Roche, Sanofi, and Sobe. Dr. Dejaco reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
VIENNA — The interleukin-1 receptor antagonist (IL-1RA) canakinumab provided control of systemic juvenile idiopathic arthritis (sJIA) without the use of glucocorticoids for up to a year in most study participants after three monthly injections.
In this study of 20 patients with newly diagnosed sJIA treated off glucocorticoids, fever was controlled after a single injection in all patients, and 16 patients reached the primary outcome of remission after three injections, said Gerd Horneff, MD, PhD, Asklepios Children’s Hospital, Sankt Augustin, Germany.
Results of this open-label study, called CANAKINUMAB FIRST, were presented as late-breaking findings at the European Alliance of Associations for Rheumatology (EULAR) 2024 Annual Meeting.
“Steroid-free, first-line treatment with canakinumab led to sustained responses in most patients, with a considerable number achieving remission,” said Dr. Horneff, adding that the observation in this group is ongoing.
Building on Earlier Data
The efficacy of canakinumab was previously reported in anecdotal experiences and one small patient series published 10 years ago. Dr. Horneff noted that he has offered this drug off label to patients with challenging cases.
The objective was to evaluate canakinumab as a first-line monotherapy administered in the absence of glucocorticoids. The study was open to children aged 2-18 years with active sJIA/juvenile Still disease confirmed with published criteria. All were naive to biologic or nonbiologic disease-modifying antirheumatic drugs as well as steroids.
The median age of the children was 8.4 years. A total of 60% were men. The median disease duration at the time of entry was 1.2 months. Most had fever (95%) and rash (80%) with high levels of inflammatory markers at baseline. The mean number of painful joints was 3.1, and the mean number of systemic manifestations was 2.8. No patient was without any systemic involvement, but four of the patients did not have any painful joints.
At enrollment, patients were scheduled to receive three injections of canakinumab at monthly intervals during an active treatment phase, after which they entered an observation phase lasting 40 weeks. In the event of nonresponse or flares in either phase, they were transitioned to usual care.
Symptoms Resolve After Single Injection
After the first injection, active joint disease and all systemic manifestations resolved in 16 (80%) of the 20 patients. Joint activity and systemic manifestations also remained controlled after the second and third injections in 16 of the 20 patients.
One patient in this series achieved inactive disease after a single injection but developed what appeared to be a treatment-related allergic reaction. He received no further treatment and was excluded from the study, although he is being followed separately.
“According to sJADAS [systemic JIA Disease Activity Score] criteria at month 3, 14 had inactive disease, three had minimal disease activity, and one patient had moderate disease activity,” Dr. Horneff said.
At week 24, or 3 months after the last injection, there was still no joint activity in 16 patients. Systemic manifestations remained controlled in 13 patients, but 1 patient by this point had a flare. Another flare occurred after this point, and other patients have not yet completed the 52-week observation period.
“Of the 10 patients who remained in the study and have completed the 52-week observation period, eight have had a drug-free remission,” Dr. Horneff said.
MAS Event Observed in One Patient
In addition to the allergic skin reaction, which was considered probably related to the study drug, there were three flares, one of which was a macrophage activation syndrome (MAS) event. The MAS occurred 8 weeks after the last injection, but it was managed successfully.
Of 30 infections that developed during the observation period, 18 involved the upper airway. All were treated successfully. There were also two injection-site reactions and one case of cytopenia.
Among the studies planned for follow-up, investigators will examine genomic and gene activation in relation to disease activity and the effect of canakinumab.
Comoderator of the abstract session and chair of the EULAR 2024 Abstract Selection Committee, Christian Dejaco, MD, PhD, a consultant rheumatologist and associate professor at the Medical University of Graz in Graz, Austria, suggested that these are highly encouraging data for a disease that does not currently have any approved therapies. Clearly, larger studies with a longer follow-up period are needed, but he pointed out that phase 3 trials in a rare disease like sJIA are challenging.
Because of the limited number of cases, “it will be difficult to conduct a placebo-controlled trial,” he pointed out. However, he hopes this study will provide the basis for larger studies and sufficient data to lead to an indication for this therapy.
In the meantime, he also believes that these data are likely to support empirical use in a difficult disease, even in advance of formal regulatory approval.
“We heard that canakinumab is already being used off label in JIA, and these data might encourage more of that,” he said.
Dr. Horneff reported financial relationships with AbbVie, Boehringer Ingelheim, Celgene, Chugai, GlaxoSmithKline, Janssen, Merck Sharpe & Dohme, Novartis, Pfizer, Roche, Sanofi, and Sobe. Dr. Dejaco reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
VIENNA — The interleukin-1 receptor antagonist (IL-1RA) canakinumab provided control of systemic juvenile idiopathic arthritis (sJIA) without the use of glucocorticoids for up to a year in most study participants after three monthly injections.
In this study of 20 patients with newly diagnosed sJIA treated off glucocorticoids, fever was controlled after a single injection in all patients, and 16 patients reached the primary outcome of remission after three injections, said Gerd Horneff, MD, PhD, Asklepios Children’s Hospital, Sankt Augustin, Germany.
Results of this open-label study, called CANAKINUMAB FIRST, were presented as late-breaking findings at the European Alliance of Associations for Rheumatology (EULAR) 2024 Annual Meeting.
“Steroid-free, first-line treatment with canakinumab led to sustained responses in most patients, with a considerable number achieving remission,” said Dr. Horneff, adding that the observation in this group is ongoing.
Building on Earlier Data
The efficacy of canakinumab was previously reported in anecdotal experiences and one small patient series published 10 years ago. Dr. Horneff noted that he has offered this drug off label to patients with challenging cases.
The objective was to evaluate canakinumab as a first-line monotherapy administered in the absence of glucocorticoids. The study was open to children aged 2-18 years with active sJIA/juvenile Still disease confirmed with published criteria. All were naive to biologic or nonbiologic disease-modifying antirheumatic drugs as well as steroids.
The median age of the children was 8.4 years. A total of 60% were men. The median disease duration at the time of entry was 1.2 months. Most had fever (95%) and rash (80%) with high levels of inflammatory markers at baseline. The mean number of painful joints was 3.1, and the mean number of systemic manifestations was 2.8. No patient was without any systemic involvement, but four of the patients did not have any painful joints.
At enrollment, patients were scheduled to receive three injections of canakinumab at monthly intervals during an active treatment phase, after which they entered an observation phase lasting 40 weeks. In the event of nonresponse or flares in either phase, they were transitioned to usual care.
Symptoms Resolve After Single Injection
After the first injection, active joint disease and all systemic manifestations resolved in 16 (80%) of the 20 patients. Joint activity and systemic manifestations also remained controlled after the second and third injections in 16 of the 20 patients.
One patient in this series achieved inactive disease after a single injection but developed what appeared to be a treatment-related allergic reaction. He received no further treatment and was excluded from the study, although he is being followed separately.
“According to sJADAS [systemic JIA Disease Activity Score] criteria at month 3, 14 had inactive disease, three had minimal disease activity, and one patient had moderate disease activity,” Dr. Horneff said.
At week 24, or 3 months after the last injection, there was still no joint activity in 16 patients. Systemic manifestations remained controlled in 13 patients, but 1 patient by this point had a flare. Another flare occurred after this point, and other patients have not yet completed the 52-week observation period.
“Of the 10 patients who remained in the study and have completed the 52-week observation period, eight have had a drug-free remission,” Dr. Horneff said.
MAS Event Observed in One Patient
In addition to the allergic skin reaction, which was considered probably related to the study drug, there were three flares, one of which was a macrophage activation syndrome (MAS) event. The MAS occurred 8 weeks after the last injection, but it was managed successfully.
Of 30 infections that developed during the observation period, 18 involved the upper airway. All were treated successfully. There were also two injection-site reactions and one case of cytopenia.
Among the studies planned for follow-up, investigators will examine genomic and gene activation in relation to disease activity and the effect of canakinumab.
Comoderator of the abstract session and chair of the EULAR 2024 Abstract Selection Committee, Christian Dejaco, MD, PhD, a consultant rheumatologist and associate professor at the Medical University of Graz in Graz, Austria, suggested that these are highly encouraging data for a disease that does not currently have any approved therapies. Clearly, larger studies with a longer follow-up period are needed, but he pointed out that phase 3 trials in a rare disease like sJIA are challenging.
Because of the limited number of cases, “it will be difficult to conduct a placebo-controlled trial,” he pointed out. However, he hopes this study will provide the basis for larger studies and sufficient data to lead to an indication for this therapy.
In the meantime, he also believes that these data are likely to support empirical use in a difficult disease, even in advance of formal regulatory approval.
“We heard that canakinumab is already being used off label in JIA, and these data might encourage more of that,” he said.
Dr. Horneff reported financial relationships with AbbVie, Boehringer Ingelheim, Celgene, Chugai, GlaxoSmithKline, Janssen, Merck Sharpe & Dohme, Novartis, Pfizer, Roche, Sanofi, and Sobe. Dr. Dejaco reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM EULAR 2024
Oncology Mergers Are on the Rise. How Can Independent Practices Survive?
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
Treatment of Infantile Hemangiomas in Concomitant Tuberous Sclerosis Complex Should Prompt Evaluation for Cardiac Rhabdomyomas Prior to Initiation of Propranolol
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.
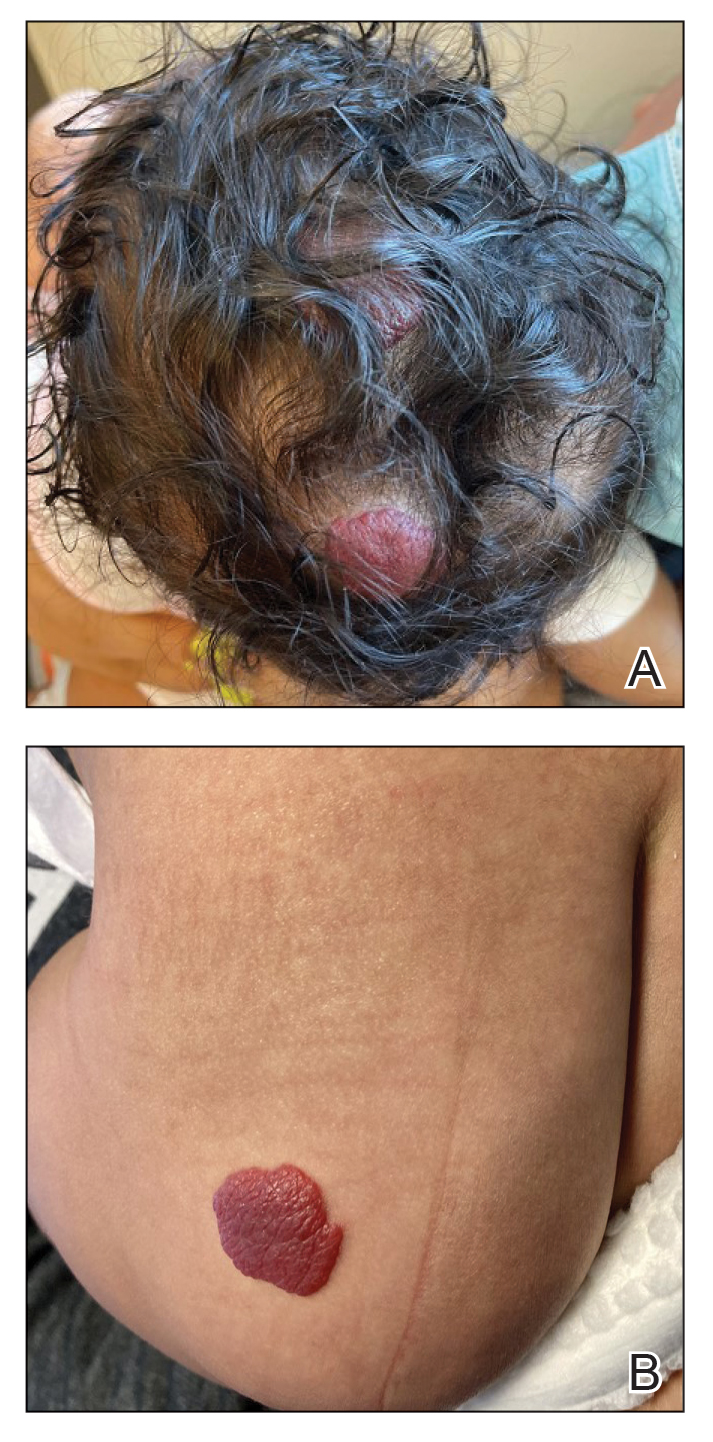
Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.
Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
Practice Points
- Dermatologists may see patients with infantile hemangiomas (IHs) and tuberous sclerosis complex (TSC); therefore, they should be familiar with TSC diagnostic criteria to reach a prompt diagnosis and make appropriate referrals.
- Cardiologic evaluation is not routinely required prior to systemic treatment of IH, but knowledge of cardiac findings in TSC should prompt cardiologic clearance prior to β-blocker initiation.
FDA Approves Topical Anticholinergic for Axillary Hyperhidrosis
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
Ixekizumab Met Phase 3 Trial Endpoint in Juvenile PsA, Enthesitis-Related Arthritis
VIENNA — Ixekizumab (Taltz), an interleukin-17A inhibitor that’s already approved for the treatment of psoriatic arthritis and axial spondyloarthritis in adults appears likely to be granted the same corresponding indications for children, based on initial results from an open-label, phase 3 trial that employed adalimumab as a reference.
With a safety profile comparable with that seen in adult patients, ixekizumab “met the prespecified criterion for success at 16 weeks,” reported Athimalaipet V. Ramanan, MD, PhD, of Bristol Royal Hospital for Children and Translational Health Sciences, Bristol, England.
In this multicenter, randomized, open-label trial called COSPIRIT-JIA, which is still ongoing, investigators enrolled 101 children with active juvenile PsA (JPsA) or enthesitis-related arthritis (ERA), which is akin to spondyloarthritis in adults.
The efficacy and safety data at 16 weeks were presented as a late-breaking abstract at the annual European Congress of Rheumatology. Dr. Ramanan said that the open-label extension to 104 weeks is underway and further follow-up out to 264 weeks is planned.
Nearly 90% Achieve ACR30
The trial had an adaptive design in which the first 40 patients without biologics experience were randomized to ixekizumab or adalimumab, stratified by JPsA or ERA diagnosis, and the following 61 patients with either no biologic experience or an inadequate response or intolerance to biologics all received ixekizumab. The drugs were dosed according to weight. Dr. Ramanan explained that a placebo-controlled trial was considered unethical because of the strong evidence of benefit from biologics for JPsA and ERA.
The trial easily met its predefined threshold for success, which required ≥ 80% probability, based on Bayesian analysis, that ≥ 50% of patients would have 30% improvement in American College of Rheumatology response criteria (ACR30) at week 16. ACR30 was achieved in 88.9% of those treated with ixekizumab overall vs 95.0% of those treated with adalimumab, but the trial was not designed as a head-to-head comparison. Rather, adalimumab served as a reference.
When compared for the distinct diseases, the ACR30 rates were also numerically lower for ixekizumab relative to adalimumab for both ERA (88.9% vs 93.8%) and JPsA (88.9% vs 100%), but all of the adalimumab patients were naive to biologics. In comparison, about 75% of patients receiving ixekizumab were biologic-therapy naive.
Response rates to ixekizumab overall were numerically higher for patients without previous biologic experience than for those with experience (90.0% vs 85.7%), and this was also the case for patients with ERA (92.5% vs 78.6%). However, in the JPsA group, biologic-experienced patients had higher numerical response rates to ixekizumab (100% vs 85.0%).
An ACR30 is not a clinical goal that satisfies most patients and clinicians, Dr. Ramanan conceded, but he noted that ACR50 was reached with ixekizumab by 81.5% with ERA and 74.1% with JPsA, and ACR70 was reached by 68.5% and 55.6%, respectively. The highest responses of ACR90 (27.8% and 33.3%) and ACR100 (14.8% and 25.9%) were lower but still substantial in the ERA and JPsA groups, respectively.
Through week 16, 58.0% of those treated with ixekizumab had an adverse event considered treatment-related. Nearly half were of mild severity, and the remainder were moderate. Only 3.7% were considered serious. No patient discontinued study treatment because of an adverse event.
In this study, the presence of at least three active peripheral joints was an inclusion criterion. The median age was about 13 years in the biologic-naive adalimumab and ixekizumab groups and 14 years in the ixekizumab biologic-experienced group. The youngest patient in the study was aged 5 years, and the oldest was aged 18 years. Although about 40% of patients were women in the two biologic-naive subgroups, it was 60% in the biologic-experienced group.
On average, patients in the biologic-naive group were entered about 1 year after diagnosis. In the experienced patients, the average duration of disease at entry was nearly 4 years. About 45% of patients remained on conventional synthetic disease-modifying antirheumatic drugs while receiving ixekizumab. The proportion was 35% in the adalimumab reference arm.
Ixekizumab Might Fulfill Need for More Options
There are several biologics that have received regulatory approval or are already widely used for the treatment of JPsA or ERA, but more options are needed, according to Dr. Ramanan and the chair of the abstract session in which these data were reported. According to Caroline Ospelt, MD, PhD, a researcher at the Center for Experimental Rheumatology, University Hospital Zurich, Switzerland, regulatory approval of ixekizumab will depend on sustained efficacy and safety in longer follow-up from the COSPIRIT-JIA trial, but this trial supports continued development.
Despite a novel mechanism of action, “the data so far suggest a level of efficacy similar to that of anti-TNF [anti-tumor necrosis factor] biologics,” said Dr. Ospelt, who, in addition to moderating the late-breaking session, served as Scientific Program Chair of EULAR 2024.
While Dr. Ospelt emphasized that she is a researcher involved in translational rheumatology studies and not a clinician, she said there was consensus within the program committee to select this abstract from other high-quality latebreaker submissions on the basis of its potential clinical significance.
Dr. Ramanan reported financial relationships with AbbVie, AstraZeneca, Novartis, Pfizer, Roche, SOBI, UCB, and Eli Lilly, which provided funding for this study. Dr. Ospelt reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
VIENNA — Ixekizumab (Taltz), an interleukin-17A inhibitor that’s already approved for the treatment of psoriatic arthritis and axial spondyloarthritis in adults appears likely to be granted the same corresponding indications for children, based on initial results from an open-label, phase 3 trial that employed adalimumab as a reference.
With a safety profile comparable with that seen in adult patients, ixekizumab “met the prespecified criterion for success at 16 weeks,” reported Athimalaipet V. Ramanan, MD, PhD, of Bristol Royal Hospital for Children and Translational Health Sciences, Bristol, England.
In this multicenter, randomized, open-label trial called COSPIRIT-JIA, which is still ongoing, investigators enrolled 101 children with active juvenile PsA (JPsA) or enthesitis-related arthritis (ERA), which is akin to spondyloarthritis in adults.
The efficacy and safety data at 16 weeks were presented as a late-breaking abstract at the annual European Congress of Rheumatology. Dr. Ramanan said that the open-label extension to 104 weeks is underway and further follow-up out to 264 weeks is planned.
Nearly 90% Achieve ACR30
The trial had an adaptive design in which the first 40 patients without biologics experience were randomized to ixekizumab or adalimumab, stratified by JPsA or ERA diagnosis, and the following 61 patients with either no biologic experience or an inadequate response or intolerance to biologics all received ixekizumab. The drugs were dosed according to weight. Dr. Ramanan explained that a placebo-controlled trial was considered unethical because of the strong evidence of benefit from biologics for JPsA and ERA.
The trial easily met its predefined threshold for success, which required ≥ 80% probability, based on Bayesian analysis, that ≥ 50% of patients would have 30% improvement in American College of Rheumatology response criteria (ACR30) at week 16. ACR30 was achieved in 88.9% of those treated with ixekizumab overall vs 95.0% of those treated with adalimumab, but the trial was not designed as a head-to-head comparison. Rather, adalimumab served as a reference.
When compared for the distinct diseases, the ACR30 rates were also numerically lower for ixekizumab relative to adalimumab for both ERA (88.9% vs 93.8%) and JPsA (88.9% vs 100%), but all of the adalimumab patients were naive to biologics. In comparison, about 75% of patients receiving ixekizumab were biologic-therapy naive.
Response rates to ixekizumab overall were numerically higher for patients without previous biologic experience than for those with experience (90.0% vs 85.7%), and this was also the case for patients with ERA (92.5% vs 78.6%). However, in the JPsA group, biologic-experienced patients had higher numerical response rates to ixekizumab (100% vs 85.0%).
An ACR30 is not a clinical goal that satisfies most patients and clinicians, Dr. Ramanan conceded, but he noted that ACR50 was reached with ixekizumab by 81.5% with ERA and 74.1% with JPsA, and ACR70 was reached by 68.5% and 55.6%, respectively. The highest responses of ACR90 (27.8% and 33.3%) and ACR100 (14.8% and 25.9%) were lower but still substantial in the ERA and JPsA groups, respectively.
Through week 16, 58.0% of those treated with ixekizumab had an adverse event considered treatment-related. Nearly half were of mild severity, and the remainder were moderate. Only 3.7% were considered serious. No patient discontinued study treatment because of an adverse event.
In this study, the presence of at least three active peripheral joints was an inclusion criterion. The median age was about 13 years in the biologic-naive adalimumab and ixekizumab groups and 14 years in the ixekizumab biologic-experienced group. The youngest patient in the study was aged 5 years, and the oldest was aged 18 years. Although about 40% of patients were women in the two biologic-naive subgroups, it was 60% in the biologic-experienced group.
On average, patients in the biologic-naive group were entered about 1 year after diagnosis. In the experienced patients, the average duration of disease at entry was nearly 4 years. About 45% of patients remained on conventional synthetic disease-modifying antirheumatic drugs while receiving ixekizumab. The proportion was 35% in the adalimumab reference arm.
Ixekizumab Might Fulfill Need for More Options
There are several biologics that have received regulatory approval or are already widely used for the treatment of JPsA or ERA, but more options are needed, according to Dr. Ramanan and the chair of the abstract session in which these data were reported. According to Caroline Ospelt, MD, PhD, a researcher at the Center for Experimental Rheumatology, University Hospital Zurich, Switzerland, regulatory approval of ixekizumab will depend on sustained efficacy and safety in longer follow-up from the COSPIRIT-JIA trial, but this trial supports continued development.
Despite a novel mechanism of action, “the data so far suggest a level of efficacy similar to that of anti-TNF [anti-tumor necrosis factor] biologics,” said Dr. Ospelt, who, in addition to moderating the late-breaking session, served as Scientific Program Chair of EULAR 2024.
While Dr. Ospelt emphasized that she is a researcher involved in translational rheumatology studies and not a clinician, she said there was consensus within the program committee to select this abstract from other high-quality latebreaker submissions on the basis of its potential clinical significance.
Dr. Ramanan reported financial relationships with AbbVie, AstraZeneca, Novartis, Pfizer, Roche, SOBI, UCB, and Eli Lilly, which provided funding for this study. Dr. Ospelt reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
VIENNA — Ixekizumab (Taltz), an interleukin-17A inhibitor that’s already approved for the treatment of psoriatic arthritis and axial spondyloarthritis in adults appears likely to be granted the same corresponding indications for children, based on initial results from an open-label, phase 3 trial that employed adalimumab as a reference.
With a safety profile comparable with that seen in adult patients, ixekizumab “met the prespecified criterion for success at 16 weeks,” reported Athimalaipet V. Ramanan, MD, PhD, of Bristol Royal Hospital for Children and Translational Health Sciences, Bristol, England.
In this multicenter, randomized, open-label trial called COSPIRIT-JIA, which is still ongoing, investigators enrolled 101 children with active juvenile PsA (JPsA) or enthesitis-related arthritis (ERA), which is akin to spondyloarthritis in adults.
The efficacy and safety data at 16 weeks were presented as a late-breaking abstract at the annual European Congress of Rheumatology. Dr. Ramanan said that the open-label extension to 104 weeks is underway and further follow-up out to 264 weeks is planned.
Nearly 90% Achieve ACR30
The trial had an adaptive design in which the first 40 patients without biologics experience were randomized to ixekizumab or adalimumab, stratified by JPsA or ERA diagnosis, and the following 61 patients with either no biologic experience or an inadequate response or intolerance to biologics all received ixekizumab. The drugs were dosed according to weight. Dr. Ramanan explained that a placebo-controlled trial was considered unethical because of the strong evidence of benefit from biologics for JPsA and ERA.
The trial easily met its predefined threshold for success, which required ≥ 80% probability, based on Bayesian analysis, that ≥ 50% of patients would have 30% improvement in American College of Rheumatology response criteria (ACR30) at week 16. ACR30 was achieved in 88.9% of those treated with ixekizumab overall vs 95.0% of those treated with adalimumab, but the trial was not designed as a head-to-head comparison. Rather, adalimumab served as a reference.
When compared for the distinct diseases, the ACR30 rates were also numerically lower for ixekizumab relative to adalimumab for both ERA (88.9% vs 93.8%) and JPsA (88.9% vs 100%), but all of the adalimumab patients were naive to biologics. In comparison, about 75% of patients receiving ixekizumab were biologic-therapy naive.
Response rates to ixekizumab overall were numerically higher for patients without previous biologic experience than for those with experience (90.0% vs 85.7%), and this was also the case for patients with ERA (92.5% vs 78.6%). However, in the JPsA group, biologic-experienced patients had higher numerical response rates to ixekizumab (100% vs 85.0%).
An ACR30 is not a clinical goal that satisfies most patients and clinicians, Dr. Ramanan conceded, but he noted that ACR50 was reached with ixekizumab by 81.5% with ERA and 74.1% with JPsA, and ACR70 was reached by 68.5% and 55.6%, respectively. The highest responses of ACR90 (27.8% and 33.3%) and ACR100 (14.8% and 25.9%) were lower but still substantial in the ERA and JPsA groups, respectively.
Through week 16, 58.0% of those treated with ixekizumab had an adverse event considered treatment-related. Nearly half were of mild severity, and the remainder were moderate. Only 3.7% were considered serious. No patient discontinued study treatment because of an adverse event.
In this study, the presence of at least three active peripheral joints was an inclusion criterion. The median age was about 13 years in the biologic-naive adalimumab and ixekizumab groups and 14 years in the ixekizumab biologic-experienced group. The youngest patient in the study was aged 5 years, and the oldest was aged 18 years. Although about 40% of patients were women in the two biologic-naive subgroups, it was 60% in the biologic-experienced group.
On average, patients in the biologic-naive group were entered about 1 year after diagnosis. In the experienced patients, the average duration of disease at entry was nearly 4 years. About 45% of patients remained on conventional synthetic disease-modifying antirheumatic drugs while receiving ixekizumab. The proportion was 35% in the adalimumab reference arm.
Ixekizumab Might Fulfill Need for More Options
There are several biologics that have received regulatory approval or are already widely used for the treatment of JPsA or ERA, but more options are needed, according to Dr. Ramanan and the chair of the abstract session in which these data were reported. According to Caroline Ospelt, MD, PhD, a researcher at the Center for Experimental Rheumatology, University Hospital Zurich, Switzerland, regulatory approval of ixekizumab will depend on sustained efficacy and safety in longer follow-up from the COSPIRIT-JIA trial, but this trial supports continued development.
Despite a novel mechanism of action, “the data so far suggest a level of efficacy similar to that of anti-TNF [anti-tumor necrosis factor] biologics,” said Dr. Ospelt, who, in addition to moderating the late-breaking session, served as Scientific Program Chair of EULAR 2024.
While Dr. Ospelt emphasized that she is a researcher involved in translational rheumatology studies and not a clinician, she said there was consensus within the program committee to select this abstract from other high-quality latebreaker submissions on the basis of its potential clinical significance.
Dr. Ramanan reported financial relationships with AbbVie, AstraZeneca, Novartis, Pfizer, Roche, SOBI, UCB, and Eli Lilly, which provided funding for this study. Dr. Ospelt reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM EULAR 2024
Atopic Dermatitis: Study Compares Prevalence by Gender, Age, and Ethnic Background
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .