User login
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
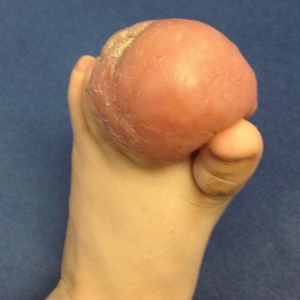
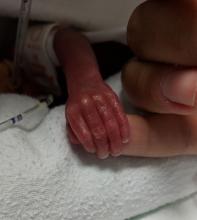
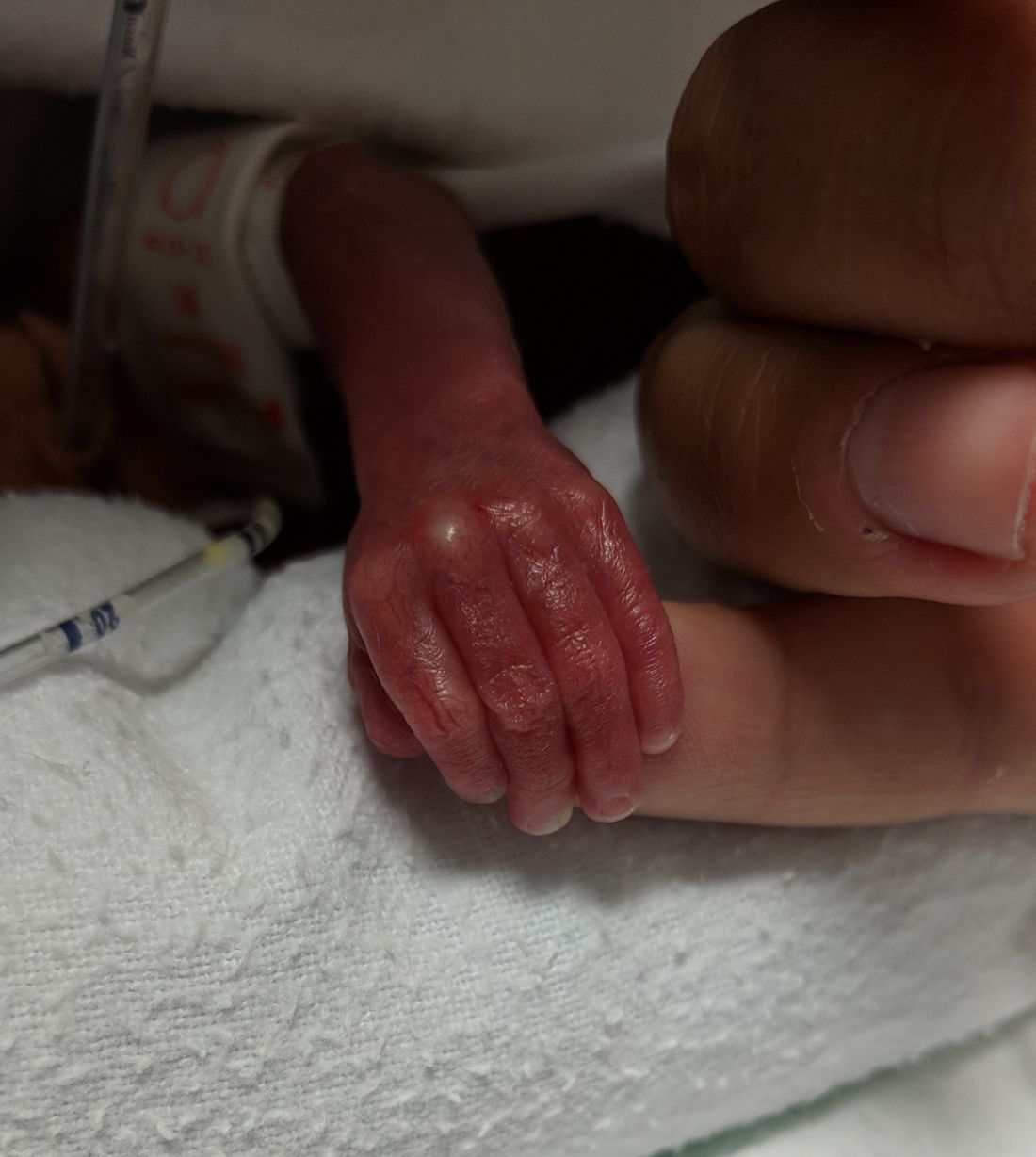
Firm Tumor Encasing the Left Second Toe of an Infant
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF), or recurring digital fibrous tumor of childhood, is a benign juvenile myofibromatosis that presents as a firm, flesh-colored or slightly erythematous, dome-shaped papule or nodule on the dorsolateral aspects of the digits, usually sparing the thumbs and great toes.1 The tumor appears most commonly at birth and in infants younger than 1 year. It grows slowly over the first month, then rapidly over the next 10 to 14 months.1,2
Although lesions usually regress spontaneously within a few years, excision may be necessary when functional impairment and joint deformity occur. Tumors, however, may recur locally.1,2
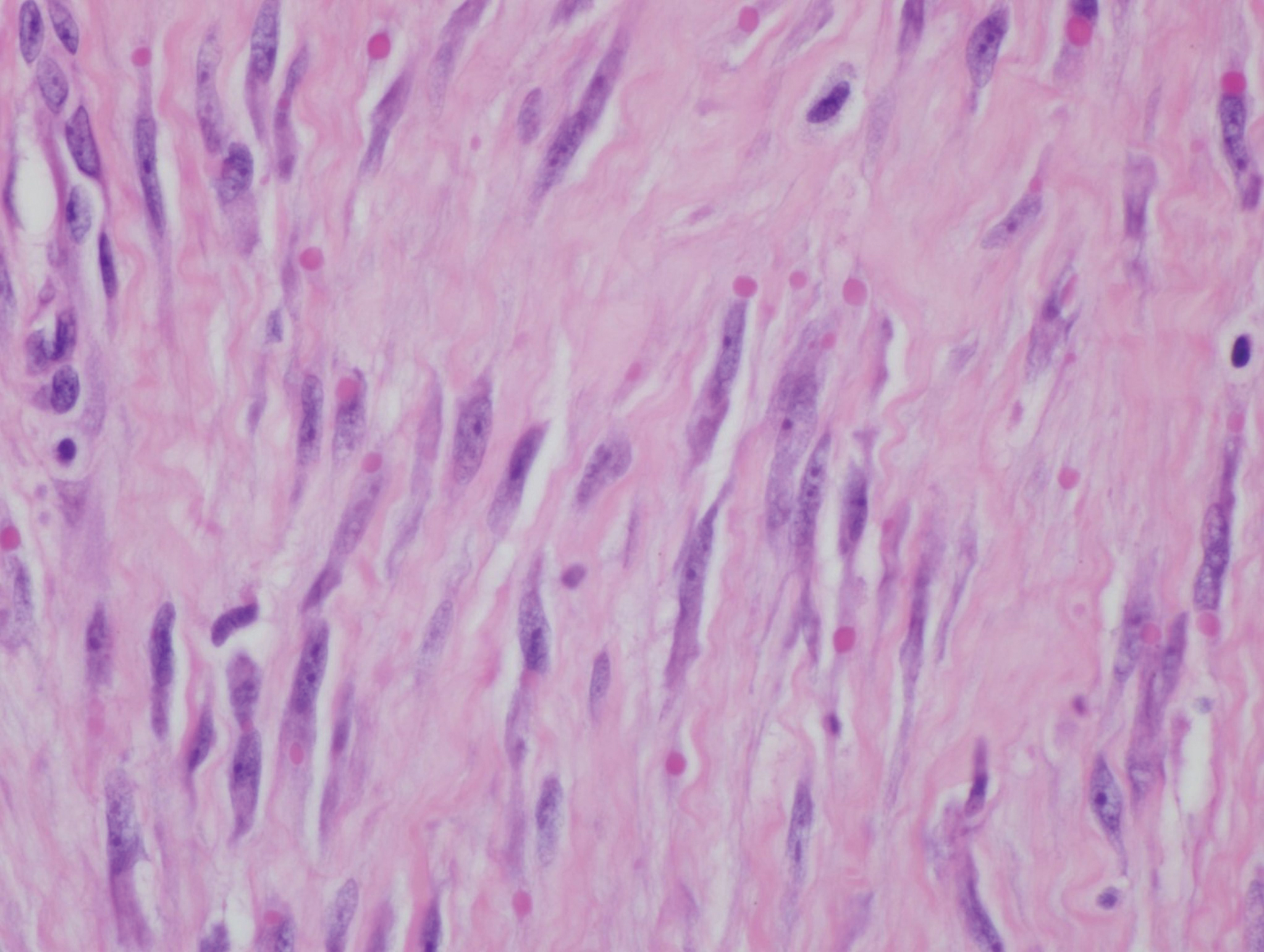
Histologically, IDFs are composed of spindled myofibroblasts with characteristic round eosinophilic intracytoplasmic inclusion bodies, which represent actin and vimentin filaments.1 In our case, histopathologic evaluation showed a proliferation of fibrous spindle cells with pathognomonic eosinophilic intracytoplasmic inclusions consistent with IDF (Figure).
Fibrosarcomas are high-grade and low-grade soft-tissue neoplasms comprised of atypical spindle cells in a herringbone pattern with mitotic figures on pathology.3 They typically present as a slowly growing subcutaneous tumor on the lower extremities of young to middle-aged adults that may progress to become a palpable tender nodule. Infantile hemangiomas, the most common benign soft-tissue tumors of childhood, are vascular proliferations more commonly seen in low-birth-weight female white infants of multiple gestation pregnancies. Superficial hemangiomas present as bright red and lobular nodules or plaques. Deep hemangiomas present as ill-defined, blue-violaceous nodules with no overlying skin changes. Mixed hemangiomas present with features of both superficial and deep hemangiomas. Infantile hemangiomas experience a proliferative phase until 9 to 12 months of age, followed by a gradual involutional phase ending with possible residual telangiectases or fibrofatty change. Unlike IDFs, infantile hemangiomas favor the head and neck over other areas of the body. Keloids are firm, smooth, variably colored papules or plaques of haphazardly arranged thick dermal collagen bundles. They usually develop within a year of skin injury and extend beyond the original injury margin into normal tissue. Supernumerary digits present as small fleshy papules or larger nodules with a vestigial nail, most commonly on the ulnar side of the fifth finger or the radial side of the thumb. Histologically, they are composed of fascicles of nerve fibers.3
Treatment in our patient included partial amputation of the left second toe and excision with local tissue rearrangement by a plastic surgeon. His postoperative course was complicated by a minor wound infection, which resolved with a 7-day course of cephalexin. Since then, the patient has healed well, with gradual toe tissue and nail growth. No recurrence was reported 11 months after surgery.
- Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
- Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia, PA: Elsevier Limited; 2018.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF), or recurring digital fibrous tumor of childhood, is a benign juvenile myofibromatosis that presents as a firm, flesh-colored or slightly erythematous, dome-shaped papule or nodule on the dorsolateral aspects of the digits, usually sparing the thumbs and great toes.1 The tumor appears most commonly at birth and in infants younger than 1 year. It grows slowly over the first month, then rapidly over the next 10 to 14 months.1,2
Although lesions usually regress spontaneously within a few years, excision may be necessary when functional impairment and joint deformity occur. Tumors, however, may recur locally.1,2
Histologically, IDFs are composed of spindled myofibroblasts with characteristic round eosinophilic intracytoplasmic inclusion bodies, which represent actin and vimentin filaments.1 In our case, histopathologic evaluation showed a proliferation of fibrous spindle cells with pathognomonic eosinophilic intracytoplasmic inclusions consistent with IDF (Figure).

Fibrosarcomas are high-grade and low-grade soft-tissue neoplasms comprised of atypical spindle cells in a herringbone pattern with mitotic figures on pathology.3 They typically present as a slowly growing subcutaneous tumor on the lower extremities of young to middle-aged adults that may progress to become a palpable tender nodule. Infantile hemangiomas, the most common benign soft-tissue tumors of childhood, are vascular proliferations more commonly seen in low-birth-weight female white infants of multiple gestation pregnancies. Superficial hemangiomas present as bright red and lobular nodules or plaques. Deep hemangiomas present as ill-defined, blue-violaceous nodules with no overlying skin changes. Mixed hemangiomas present with features of both superficial and deep hemangiomas. Infantile hemangiomas experience a proliferative phase until 9 to 12 months of age, followed by a gradual involutional phase ending with possible residual telangiectases or fibrofatty change. Unlike IDFs, infantile hemangiomas favor the head and neck over other areas of the body. Keloids are firm, smooth, variably colored papules or plaques of haphazardly arranged thick dermal collagen bundles. They usually develop within a year of skin injury and extend beyond the original injury margin into normal tissue. Supernumerary digits present as small fleshy papules or larger nodules with a vestigial nail, most commonly on the ulnar side of the fifth finger or the radial side of the thumb. Histologically, they are composed of fascicles of nerve fibers.3
Treatment in our patient included partial amputation of the left second toe and excision with local tissue rearrangement by a plastic surgeon. His postoperative course was complicated by a minor wound infection, which resolved with a 7-day course of cephalexin. Since then, the patient has healed well, with gradual toe tissue and nail growth. No recurrence was reported 11 months after surgery.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF), or recurring digital fibrous tumor of childhood, is a benign juvenile myofibromatosis that presents as a firm, flesh-colored or slightly erythematous, dome-shaped papule or nodule on the dorsolateral aspects of the digits, usually sparing the thumbs and great toes.1 The tumor appears most commonly at birth and in infants younger than 1 year. It grows slowly over the first month, then rapidly over the next 10 to 14 months.1,2
Although lesions usually regress spontaneously within a few years, excision may be necessary when functional impairment and joint deformity occur. Tumors, however, may recur locally.1,2
Histologically, IDFs are composed of spindled myofibroblasts with characteristic round eosinophilic intracytoplasmic inclusion bodies, which represent actin and vimentin filaments.1 In our case, histopathologic evaluation showed a proliferation of fibrous spindle cells with pathognomonic eosinophilic intracytoplasmic inclusions consistent with IDF (Figure).

Fibrosarcomas are high-grade and low-grade soft-tissue neoplasms comprised of atypical spindle cells in a herringbone pattern with mitotic figures on pathology.3 They typically present as a slowly growing subcutaneous tumor on the lower extremities of young to middle-aged adults that may progress to become a palpable tender nodule. Infantile hemangiomas, the most common benign soft-tissue tumors of childhood, are vascular proliferations more commonly seen in low-birth-weight female white infants of multiple gestation pregnancies. Superficial hemangiomas present as bright red and lobular nodules or plaques. Deep hemangiomas present as ill-defined, blue-violaceous nodules with no overlying skin changes. Mixed hemangiomas present with features of both superficial and deep hemangiomas. Infantile hemangiomas experience a proliferative phase until 9 to 12 months of age, followed by a gradual involutional phase ending with possible residual telangiectases or fibrofatty change. Unlike IDFs, infantile hemangiomas favor the head and neck over other areas of the body. Keloids are firm, smooth, variably colored papules or plaques of haphazardly arranged thick dermal collagen bundles. They usually develop within a year of skin injury and extend beyond the original injury margin into normal tissue. Supernumerary digits present as small fleshy papules or larger nodules with a vestigial nail, most commonly on the ulnar side of the fifth finger or the radial side of the thumb. Histologically, they are composed of fascicles of nerve fibers.3
Treatment in our patient included partial amputation of the left second toe and excision with local tissue rearrangement by a plastic surgeon. His postoperative course was complicated by a minor wound infection, which resolved with a 7-day course of cephalexin. Since then, the patient has healed well, with gradual toe tissue and nail growth. No recurrence was reported 11 months after surgery.
- Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
- Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia, PA: Elsevier Limited; 2018.
- Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
- Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia, PA: Elsevier Limited; 2018.
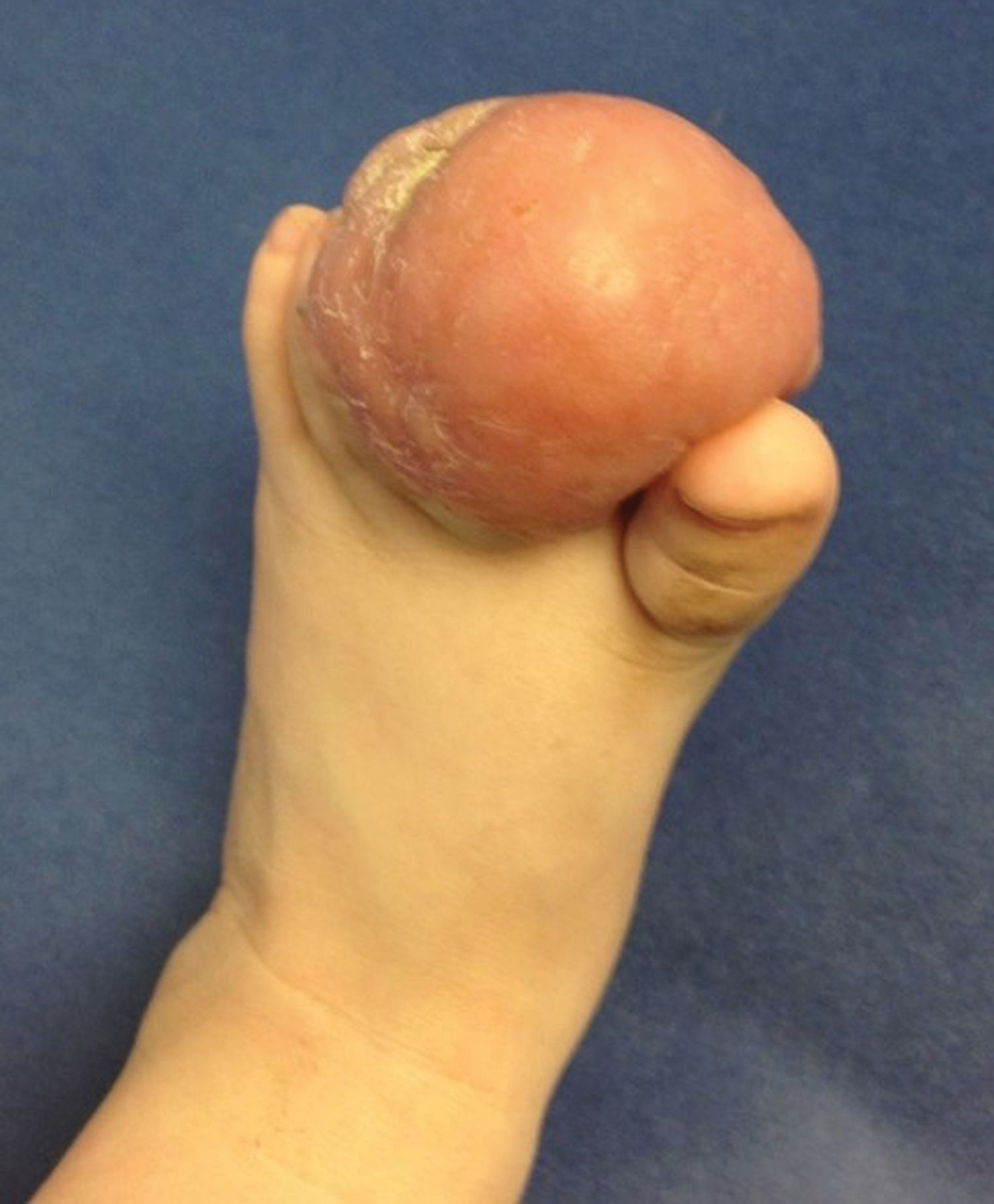
A 10-month-old infant boy presented to the dermatology clinic with a firm, nonpainful, 5.5.×5.6-cm tumor encasing the left second toe, with associated skin breakdown, gait impairment, and lateral displacement of the third toe. The lesion began as a small bump under the toenail at 2 months of age; it then grew rapidly without bleeding or ulceration. It was diagnosed as a hemangioma at 4 months of age, and oral propranolol was initiated for 3 months, without suppression of tumor growth. The patient was referred to the pediatric dermatology department where a clinical diagnosis was made, and the patient was subsequently referred to plastic surgery for excision.
Provide appropriate sexual, reproductive health care for transgender patients
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
FUEL trial: Post-Fontan udenafil shows mixed results
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM AHA 2019
Cognitive problems after extremely preterm birth persist
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
FROM PEDIATRICS
Adolescent alcohol, opioid misuse linked to risky behaviors
Binge drinking and misuse of opioids led to risky behavior during adolescence, two studies from the journal Pediatrics highlighted. And the binge drinking in high school may predict risky driving behaviors up to 4 years after high school.
Federico E. Vaca, MD, of the developmental neurocognitive driving simulation research center at Yale University, New Haven, Conn., and colleagues examined the associations between risky driving behaviors and binge drinking of 2,785 adolescents in the nationally representative, longitudinal NEXT Generation Health Study. The researchers studied the effects of binge drinking on driving while impaired (DWI), riding with an impaired driver (RWI), blackouts, extreme binge drinking, and risky driving.
The adolescents were studied across seven waves, with Wave 1 beginning in the 2009-2010 school year (10th grade; mean age, 16 years), and data extended up to 4 years after high school. Of all adolescents enrolled, 91% completed Wave 1, 88% completed Wave 2, 86% completed Wave 3 (12th grade), 78% completed Wave 4, 79% completed Wave 5, 84% completed Wave 6, and 83% completed Wave 7 (4 years after leaving high school) of the study.
High school binge drinking predicts later risky behavior
About one-quarter of adolescents reported binge drinking in Waves 1-3, with an incidence of 27% in Wave 1, 24% in Wave 2, and 27% in Wave 3. Adolescents who reported binge drinking in Wave 3 had a higher likelihood of DWI in subsequent waves, with nearly six times higher odds in Wave 5 and more than twice as likely in Wave 7, researchers said. Binge drinking in Wave 3 also was associated with greater than four times higher odds of RWI in Wave 4, and more than two and a half times higher odds of RWI in Wave 7. Among adolescents who reported binge drinking across 3 years in high school, there was a higher likelihood of extreme binge drinking in Wave 7, and higher likelihood of risky driving after graduating.
Impact of parental knowledge of drinking
in some waves. Father monitoring knowledge of drinking in Waves 1-3 lowered the odds of DWI by 30% in Wave 5 and 20% in Wave 6, while also lowering the odds of RWI in Wave 4 and Wave 7 by 20%.
Mother knowledge of drinking in Waves 1-3 was associated with 60% lower odds of DWI in Wave 4, but did not lower odds in any wave for RWI.
Overall, parental support for not drinking lowered odds for DWI by 40% in Waves 4 and 5, and by 30% in Wave 7 while also lowering odds of RWI in Wave 4 by 20%.
The results are consistent with other studies examining risky driving behavior and binge drinking in adolescent populations, but researchers noted that “to an important but limited extent, parental practices while the teenager is in high school may protect against DWI, RWI, and blackouts as adolescents move into early adulthood.”
“Our findings are relevant to prevention programs that seek to incorporate alcohol screening with intentional inquiry about binge drinking. Moreover, our results may be instructive to programs that seek to leverage facets of parental practices to reduce health-risk contexts for youth,” Dr. Vaca and colleagues concluded. “Such prevention activities coupled with strengthening of policies and practices reducing adolescents’ access to alcohol could reduce later major alcohol-related health-risk behaviors and their consequences.”
Opioid misuse and risky behavior
In a second study, Devika Bhatia, MD, of the University of Colorado at Denver, Aurora, and colleagues examined opioid misuse in a nationally-representative sample of 14,765 adolescents from the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Surveillance Survey. The researchers measured opioid misuse by categorizing adolescents into groups based on whether they had ever misused prescription opioids and whether they had engaged in risky driving behavior, violent behavior, risky sexual behavior, had a history of substance abuse, or attempted suicide.
Dr. Bhatia and colleagues found 14% of adolescents in the study reported misusing opioids, with an overrepresentation of 17-year-old and 18-year-old participants reporting opioid misuse (P less than .0001). there were no statistically significant difference between those who misused opioids and those who did not in terms of race, ethnicity, or sex.
Those adolescents who reported misusing opioids were 2.8 times more likely to not use a seatbelt; were 2.8 times more likely to have RWI; were 5.8 times more likely to have DWI; or 2.3 times more likely to have texted or emailed while driving. In each of these cases, P was less than .0001.
Adolescents who misused opioids also had significantly increased odds of engaging in risky sexual behaviors such as having sex before 13 years (3.9 times); having sex with four or more partners (4.8 times); using substances before sex (3.6 times); and not using a condom before sex (2.0 times). In each of these cases, P was less than .0001.
Additionally, adolescents in this category were between 5.4 times and 22.3 times more likely to use other substances (P less than .0001 for 10 variables); 4.9 times more likely to have attempted suicide (P less than .0001); or more likely to have engaged in violent behavior such as getting into physical fights (4.0 times), carrying a weapon (3.4 times) or a gun (5.1 times) within the last 30 days. In the four latter cases, P was less than .0001.
“With the ongoing opioid epidemic, pediatricians and child psychiatrists are likely to be more attuned to opioid misuse in their patients,” Dr. Bhatia and colleagues concluded. “If youth are screening positive for opioid misuse, pediatricians, nurses, social workers, child psychiatrists, and other providers assessing adolescents may have a new, broad range of other risky behaviors for which to screen regardless of the direction of the association.”
Substance use screening for treating substance use disorder traditionally has been is provided by a specialist, Jessica A. Kulak, PhD, MPH, said in an interview. “However, integration of care services may help to change societal norms around problematic substance use – both by decreasing stigma associated with substance use, as well as increasing clinicians’ preparedness, knowledge, and confidence in preventing and intervening on adolescents’ substance experimentation and use.” She recommended that clinicians in primary care improve their training by using the Substance Abuse and Mental Health Services Administration’s Screening, Brief Intervention, and Referral to Treatment program, which is available as a free online course.
Confidentiality is important in adolescent health, said Dr. Kulak, who is an assistant professor in the department of health, nutrition, and dietetics at State University of New York at Buffalo. “When discussing sensitive topics, such as binge drinking and opioid misuse, adolescents may fear that these or other risky activities may be disclosed to parents or law enforcement officials. Therefore, adolescent health providers should be aware of local, state, and federal laws pertaining to the confidentiality of minors.”
She added, “adolescents are often susceptible to others’ influences, so having open communication and support from a trusted adult – be it a parent or clinician – may also be protective against risky behaviors.”
The study by Vaca et al. was funded by the National Institutes of Health with support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Drug Abuse; and the Maternal and Child Health Bureau of the Health Resources and Services Administration. The study by Bhatia et al. had no external funding. The authors from both studies reported no relevant financial disclosures. Dr. Kulak said she had no financial disclosures or other conflicts of interest.
SOURCE: Vaca FE et al. Pediatrics. 2020; doi: 10.1542/peds.2018-4095. Bhatia D et al. Pediatrics. 2020; doi: 10.1542/peds.2019-2470.
These newly published reports indicate the high prevalence of risky behaviors and their associations – cross-sectionally and longitudinally – with major threats to adolescent health – so asking about alcohol use, opioid misuse, and associated health risks is truly “in the lane” of clinicians, school professionals, and parents who see and care about adolescents.
At this point, I think it’s incontrovertible that clinicians should screen adolescents to learn about their physical, emotional, and behavioral health. And they should seek opportunities for professional training, skills development, and expansion of their professional networks so they are able to address – individually or collaboratively via referrals – the behavioral and psychosocial health risks of their patients.
The good news is that there is growing awareness of the importance of using validated screening tools to identify patient behavioral health risks – including those pertaining to adolescent and young adult alcohol use and opioid misuse. “Best practice” dictates that screening approaches rely on asking questions using structured tools; intuition and “just winging it” are not effective or reliable for identifying patient behavior. Forward-looking clinics and practices could be asking patients to report about health behaviors in the waiting room (on a computer tablet, for example), or even remotely (using a secure app or data collection tool) in advance of a visit. Asking should be periodic – since behaviors can change fairly rapidly among young people. The benefit is that patient-reported information can be processed in advance to cue clinician follow-up and intervention. And youth tend to share more about their behaviors when they are asked electronically, rather than face to face. Intelligent screens can provide near real-time estimation of risk – to support in-office brief intervention tailored to the risk level of a young person or to trigger follow-up.
These studies indicate that binge alcohol use and misuse of prescription opioids among adolescents are real, pervasive, and deserving of our considered attention. There is no magic bullet. However busy clinicians may have a significant role to play in identifying and addressing these problems.
Elissa Weitzman, ScD, MSc, is an associate professor of pediatrics at Harvard Medical School, Boston, and an associate scientist based in adolescent/young adult medicine and the computational health informatics program at Boston Children’s Hospital. She was asked to comment on the articles by Vaca et al. and Bhatia et al. Dr. Weitzman said she had no relevant financial disclosures.
These newly published reports indicate the high prevalence of risky behaviors and their associations – cross-sectionally and longitudinally – with major threats to adolescent health – so asking about alcohol use, opioid misuse, and associated health risks is truly “in the lane” of clinicians, school professionals, and parents who see and care about adolescents.
At this point, I think it’s incontrovertible that clinicians should screen adolescents to learn about their physical, emotional, and behavioral health. And they should seek opportunities for professional training, skills development, and expansion of their professional networks so they are able to address – individually or collaboratively via referrals – the behavioral and psychosocial health risks of their patients.
The good news is that there is growing awareness of the importance of using validated screening tools to identify patient behavioral health risks – including those pertaining to adolescent and young adult alcohol use and opioid misuse. “Best practice” dictates that screening approaches rely on asking questions using structured tools; intuition and “just winging it” are not effective or reliable for identifying patient behavior. Forward-looking clinics and practices could be asking patients to report about health behaviors in the waiting room (on a computer tablet, for example), or even remotely (using a secure app or data collection tool) in advance of a visit. Asking should be periodic – since behaviors can change fairly rapidly among young people. The benefit is that patient-reported information can be processed in advance to cue clinician follow-up and intervention. And youth tend to share more about their behaviors when they are asked electronically, rather than face to face. Intelligent screens can provide near real-time estimation of risk – to support in-office brief intervention tailored to the risk level of a young person or to trigger follow-up.
These studies indicate that binge alcohol use and misuse of prescription opioids among adolescents are real, pervasive, and deserving of our considered attention. There is no magic bullet. However busy clinicians may have a significant role to play in identifying and addressing these problems.
Elissa Weitzman, ScD, MSc, is an associate professor of pediatrics at Harvard Medical School, Boston, and an associate scientist based in adolescent/young adult medicine and the computational health informatics program at Boston Children’s Hospital. She was asked to comment on the articles by Vaca et al. and Bhatia et al. Dr. Weitzman said she had no relevant financial disclosures.
These newly published reports indicate the high prevalence of risky behaviors and their associations – cross-sectionally and longitudinally – with major threats to adolescent health – so asking about alcohol use, opioid misuse, and associated health risks is truly “in the lane” of clinicians, school professionals, and parents who see and care about adolescents.
At this point, I think it’s incontrovertible that clinicians should screen adolescents to learn about their physical, emotional, and behavioral health. And they should seek opportunities for professional training, skills development, and expansion of their professional networks so they are able to address – individually or collaboratively via referrals – the behavioral and psychosocial health risks of their patients.
The good news is that there is growing awareness of the importance of using validated screening tools to identify patient behavioral health risks – including those pertaining to adolescent and young adult alcohol use and opioid misuse. “Best practice” dictates that screening approaches rely on asking questions using structured tools; intuition and “just winging it” are not effective or reliable for identifying patient behavior. Forward-looking clinics and practices could be asking patients to report about health behaviors in the waiting room (on a computer tablet, for example), or even remotely (using a secure app or data collection tool) in advance of a visit. Asking should be periodic – since behaviors can change fairly rapidly among young people. The benefit is that patient-reported information can be processed in advance to cue clinician follow-up and intervention. And youth tend to share more about their behaviors when they are asked electronically, rather than face to face. Intelligent screens can provide near real-time estimation of risk – to support in-office brief intervention tailored to the risk level of a young person or to trigger follow-up.
These studies indicate that binge alcohol use and misuse of prescription opioids among adolescents are real, pervasive, and deserving of our considered attention. There is no magic bullet. However busy clinicians may have a significant role to play in identifying and addressing these problems.
Elissa Weitzman, ScD, MSc, is an associate professor of pediatrics at Harvard Medical School, Boston, and an associate scientist based in adolescent/young adult medicine and the computational health informatics program at Boston Children’s Hospital. She was asked to comment on the articles by Vaca et al. and Bhatia et al. Dr. Weitzman said she had no relevant financial disclosures.
Binge drinking and misuse of opioids led to risky behavior during adolescence, two studies from the journal Pediatrics highlighted. And the binge drinking in high school may predict risky driving behaviors up to 4 years after high school.
Federico E. Vaca, MD, of the developmental neurocognitive driving simulation research center at Yale University, New Haven, Conn., and colleagues examined the associations between risky driving behaviors and binge drinking of 2,785 adolescents in the nationally representative, longitudinal NEXT Generation Health Study. The researchers studied the effects of binge drinking on driving while impaired (DWI), riding with an impaired driver (RWI), blackouts, extreme binge drinking, and risky driving.
The adolescents were studied across seven waves, with Wave 1 beginning in the 2009-2010 school year (10th grade; mean age, 16 years), and data extended up to 4 years after high school. Of all adolescents enrolled, 91% completed Wave 1, 88% completed Wave 2, 86% completed Wave 3 (12th grade), 78% completed Wave 4, 79% completed Wave 5, 84% completed Wave 6, and 83% completed Wave 7 (4 years after leaving high school) of the study.
High school binge drinking predicts later risky behavior
About one-quarter of adolescents reported binge drinking in Waves 1-3, with an incidence of 27% in Wave 1, 24% in Wave 2, and 27% in Wave 3. Adolescents who reported binge drinking in Wave 3 had a higher likelihood of DWI in subsequent waves, with nearly six times higher odds in Wave 5 and more than twice as likely in Wave 7, researchers said. Binge drinking in Wave 3 also was associated with greater than four times higher odds of RWI in Wave 4, and more than two and a half times higher odds of RWI in Wave 7. Among adolescents who reported binge drinking across 3 years in high school, there was a higher likelihood of extreme binge drinking in Wave 7, and higher likelihood of risky driving after graduating.
Impact of parental knowledge of drinking
in some waves. Father monitoring knowledge of drinking in Waves 1-3 lowered the odds of DWI by 30% in Wave 5 and 20% in Wave 6, while also lowering the odds of RWI in Wave 4 and Wave 7 by 20%.
Mother knowledge of drinking in Waves 1-3 was associated with 60% lower odds of DWI in Wave 4, but did not lower odds in any wave for RWI.
Overall, parental support for not drinking lowered odds for DWI by 40% in Waves 4 and 5, and by 30% in Wave 7 while also lowering odds of RWI in Wave 4 by 20%.
The results are consistent with other studies examining risky driving behavior and binge drinking in adolescent populations, but researchers noted that “to an important but limited extent, parental practices while the teenager is in high school may protect against DWI, RWI, and blackouts as adolescents move into early adulthood.”
“Our findings are relevant to prevention programs that seek to incorporate alcohol screening with intentional inquiry about binge drinking. Moreover, our results may be instructive to programs that seek to leverage facets of parental practices to reduce health-risk contexts for youth,” Dr. Vaca and colleagues concluded. “Such prevention activities coupled with strengthening of policies and practices reducing adolescents’ access to alcohol could reduce later major alcohol-related health-risk behaviors and their consequences.”
Opioid misuse and risky behavior
In a second study, Devika Bhatia, MD, of the University of Colorado at Denver, Aurora, and colleagues examined opioid misuse in a nationally-representative sample of 14,765 adolescents from the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Surveillance Survey. The researchers measured opioid misuse by categorizing adolescents into groups based on whether they had ever misused prescription opioids and whether they had engaged in risky driving behavior, violent behavior, risky sexual behavior, had a history of substance abuse, or attempted suicide.
Dr. Bhatia and colleagues found 14% of adolescents in the study reported misusing opioids, with an overrepresentation of 17-year-old and 18-year-old participants reporting opioid misuse (P less than .0001). there were no statistically significant difference between those who misused opioids and those who did not in terms of race, ethnicity, or sex.
Those adolescents who reported misusing opioids were 2.8 times more likely to not use a seatbelt; were 2.8 times more likely to have RWI; were 5.8 times more likely to have DWI; or 2.3 times more likely to have texted or emailed while driving. In each of these cases, P was less than .0001.
Adolescents who misused opioids also had significantly increased odds of engaging in risky sexual behaviors such as having sex before 13 years (3.9 times); having sex with four or more partners (4.8 times); using substances before sex (3.6 times); and not using a condom before sex (2.0 times). In each of these cases, P was less than .0001.
Additionally, adolescents in this category were between 5.4 times and 22.3 times more likely to use other substances (P less than .0001 for 10 variables); 4.9 times more likely to have attempted suicide (P less than .0001); or more likely to have engaged in violent behavior such as getting into physical fights (4.0 times), carrying a weapon (3.4 times) or a gun (5.1 times) within the last 30 days. In the four latter cases, P was less than .0001.
“With the ongoing opioid epidemic, pediatricians and child psychiatrists are likely to be more attuned to opioid misuse in their patients,” Dr. Bhatia and colleagues concluded. “If youth are screening positive for opioid misuse, pediatricians, nurses, social workers, child psychiatrists, and other providers assessing adolescents may have a new, broad range of other risky behaviors for which to screen regardless of the direction of the association.”
Substance use screening for treating substance use disorder traditionally has been is provided by a specialist, Jessica A. Kulak, PhD, MPH, said in an interview. “However, integration of care services may help to change societal norms around problematic substance use – both by decreasing stigma associated with substance use, as well as increasing clinicians’ preparedness, knowledge, and confidence in preventing and intervening on adolescents’ substance experimentation and use.” She recommended that clinicians in primary care improve their training by using the Substance Abuse and Mental Health Services Administration’s Screening, Brief Intervention, and Referral to Treatment program, which is available as a free online course.
Confidentiality is important in adolescent health, said Dr. Kulak, who is an assistant professor in the department of health, nutrition, and dietetics at State University of New York at Buffalo. “When discussing sensitive topics, such as binge drinking and opioid misuse, adolescents may fear that these or other risky activities may be disclosed to parents or law enforcement officials. Therefore, adolescent health providers should be aware of local, state, and federal laws pertaining to the confidentiality of minors.”
She added, “adolescents are often susceptible to others’ influences, so having open communication and support from a trusted adult – be it a parent or clinician – may also be protective against risky behaviors.”
The study by Vaca et al. was funded by the National Institutes of Health with support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Drug Abuse; and the Maternal and Child Health Bureau of the Health Resources and Services Administration. The study by Bhatia et al. had no external funding. The authors from both studies reported no relevant financial disclosures. Dr. Kulak said she had no financial disclosures or other conflicts of interest.
SOURCE: Vaca FE et al. Pediatrics. 2020; doi: 10.1542/peds.2018-4095. Bhatia D et al. Pediatrics. 2020; doi: 10.1542/peds.2019-2470.
Binge drinking and misuse of opioids led to risky behavior during adolescence, two studies from the journal Pediatrics highlighted. And the binge drinking in high school may predict risky driving behaviors up to 4 years after high school.
Federico E. Vaca, MD, of the developmental neurocognitive driving simulation research center at Yale University, New Haven, Conn., and colleagues examined the associations between risky driving behaviors and binge drinking of 2,785 adolescents in the nationally representative, longitudinal NEXT Generation Health Study. The researchers studied the effects of binge drinking on driving while impaired (DWI), riding with an impaired driver (RWI), blackouts, extreme binge drinking, and risky driving.
The adolescents were studied across seven waves, with Wave 1 beginning in the 2009-2010 school year (10th grade; mean age, 16 years), and data extended up to 4 years after high school. Of all adolescents enrolled, 91% completed Wave 1, 88% completed Wave 2, 86% completed Wave 3 (12th grade), 78% completed Wave 4, 79% completed Wave 5, 84% completed Wave 6, and 83% completed Wave 7 (4 years after leaving high school) of the study.
High school binge drinking predicts later risky behavior
About one-quarter of adolescents reported binge drinking in Waves 1-3, with an incidence of 27% in Wave 1, 24% in Wave 2, and 27% in Wave 3. Adolescents who reported binge drinking in Wave 3 had a higher likelihood of DWI in subsequent waves, with nearly six times higher odds in Wave 5 and more than twice as likely in Wave 7, researchers said. Binge drinking in Wave 3 also was associated with greater than four times higher odds of RWI in Wave 4, and more than two and a half times higher odds of RWI in Wave 7. Among adolescents who reported binge drinking across 3 years in high school, there was a higher likelihood of extreme binge drinking in Wave 7, and higher likelihood of risky driving after graduating.
Impact of parental knowledge of drinking
in some waves. Father monitoring knowledge of drinking in Waves 1-3 lowered the odds of DWI by 30% in Wave 5 and 20% in Wave 6, while also lowering the odds of RWI in Wave 4 and Wave 7 by 20%.
Mother knowledge of drinking in Waves 1-3 was associated with 60% lower odds of DWI in Wave 4, but did not lower odds in any wave for RWI.
Overall, parental support for not drinking lowered odds for DWI by 40% in Waves 4 and 5, and by 30% in Wave 7 while also lowering odds of RWI in Wave 4 by 20%.
The results are consistent with other studies examining risky driving behavior and binge drinking in adolescent populations, but researchers noted that “to an important but limited extent, parental practices while the teenager is in high school may protect against DWI, RWI, and blackouts as adolescents move into early adulthood.”
“Our findings are relevant to prevention programs that seek to incorporate alcohol screening with intentional inquiry about binge drinking. Moreover, our results may be instructive to programs that seek to leverage facets of parental practices to reduce health-risk contexts for youth,” Dr. Vaca and colleagues concluded. “Such prevention activities coupled with strengthening of policies and practices reducing adolescents’ access to alcohol could reduce later major alcohol-related health-risk behaviors and their consequences.”
Opioid misuse and risky behavior
In a second study, Devika Bhatia, MD, of the University of Colorado at Denver, Aurora, and colleagues examined opioid misuse in a nationally-representative sample of 14,765 adolescents from the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Surveillance Survey. The researchers measured opioid misuse by categorizing adolescents into groups based on whether they had ever misused prescription opioids and whether they had engaged in risky driving behavior, violent behavior, risky sexual behavior, had a history of substance abuse, or attempted suicide.
Dr. Bhatia and colleagues found 14% of adolescents in the study reported misusing opioids, with an overrepresentation of 17-year-old and 18-year-old participants reporting opioid misuse (P less than .0001). there were no statistically significant difference between those who misused opioids and those who did not in terms of race, ethnicity, or sex.
Those adolescents who reported misusing opioids were 2.8 times more likely to not use a seatbelt; were 2.8 times more likely to have RWI; were 5.8 times more likely to have DWI; or 2.3 times more likely to have texted or emailed while driving. In each of these cases, P was less than .0001.
Adolescents who misused opioids also had significantly increased odds of engaging in risky sexual behaviors such as having sex before 13 years (3.9 times); having sex with four or more partners (4.8 times); using substances before sex (3.6 times); and not using a condom before sex (2.0 times). In each of these cases, P was less than .0001.
Additionally, adolescents in this category were between 5.4 times and 22.3 times more likely to use other substances (P less than .0001 for 10 variables); 4.9 times more likely to have attempted suicide (P less than .0001); or more likely to have engaged in violent behavior such as getting into physical fights (4.0 times), carrying a weapon (3.4 times) or a gun (5.1 times) within the last 30 days. In the four latter cases, P was less than .0001.
“With the ongoing opioid epidemic, pediatricians and child psychiatrists are likely to be more attuned to opioid misuse in their patients,” Dr. Bhatia and colleagues concluded. “If youth are screening positive for opioid misuse, pediatricians, nurses, social workers, child psychiatrists, and other providers assessing adolescents may have a new, broad range of other risky behaviors for which to screen regardless of the direction of the association.”
Substance use screening for treating substance use disorder traditionally has been is provided by a specialist, Jessica A. Kulak, PhD, MPH, said in an interview. “However, integration of care services may help to change societal norms around problematic substance use – both by decreasing stigma associated with substance use, as well as increasing clinicians’ preparedness, knowledge, and confidence in preventing and intervening on adolescents’ substance experimentation and use.” She recommended that clinicians in primary care improve their training by using the Substance Abuse and Mental Health Services Administration’s Screening, Brief Intervention, and Referral to Treatment program, which is available as a free online course.
Confidentiality is important in adolescent health, said Dr. Kulak, who is an assistant professor in the department of health, nutrition, and dietetics at State University of New York at Buffalo. “When discussing sensitive topics, such as binge drinking and opioid misuse, adolescents may fear that these or other risky activities may be disclosed to parents or law enforcement officials. Therefore, adolescent health providers should be aware of local, state, and federal laws pertaining to the confidentiality of minors.”
She added, “adolescents are often susceptible to others’ influences, so having open communication and support from a trusted adult – be it a parent or clinician – may also be protective against risky behaviors.”
The study by Vaca et al. was funded by the National Institutes of Health with support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Drug Abuse; and the Maternal and Child Health Bureau of the Health Resources and Services Administration. The study by Bhatia et al. had no external funding. The authors from both studies reported no relevant financial disclosures. Dr. Kulak said she had no financial disclosures or other conflicts of interest.
SOURCE: Vaca FE et al. Pediatrics. 2020; doi: 10.1542/peds.2018-4095. Bhatia D et al. Pediatrics. 2020; doi: 10.1542/peds.2019-2470.
FROM PEDIATRICS
Adenotonsillectomy doesn’t improve cognitive function in preschoolers with OSA
according to a prospective study.
The study showed no significant difference in global IQ at 12 months between children who underwent adenotonsillectomy and those who did not. However, as expected, the adenotonsillectomy group did experience improvements in sleep.
Karen A. Waters, MBBS, PhD, of the Children’s Hospital at Westmead and the University of Sydney, and her colleagues reported these results in Pediatrics. There also was a related commentary.
The study enrolled 190 children (ages 3-5 years) with mild obstructive sleep apnea. Roughly half of patients (n = 99) were randomized to early adenotonsillectomy (within 2 months), and the other half (n = 91) were randomized to no adenotonsillectomy (12-month routine wait). There were 121 patients who had global IQ assessments at 12 months, as measured by the Woodcock Johnson III Brief Intellectual Ability (BIA) test. Of these patients, 61 were in the adenotonsillectomy group, and 60 were in the control group.
Both groups had improvements in BIA scores from baseline to 12 months, and the 12-month BIA score was not significantly different between the groups.
At baseline, the mean W score (task proficiency) for BIA was 448.36 in the adenotonsillectomy group and 451.3 in the control group. At 12 months, the scores were 465.46 and 463.12, respectively (P = .29).
“Intellectual ability scores improved in both groups over time with no effect attributable to the intervention [adenotonsillectomy],” Dr. Waters and her colleagues wrote.
However, patients in the adenotonsillectomy group did have greater improvements in sleep than patients in the control group, as assessed by polysomnogram and parent reports.
In the adenotonsillectomy group, the mean total sleep time was 469.2 minutes at baseline and 481.8 minutes at 12 months. In the control group, the mean total sleep time was 463.8 minutes at baseline and 475.3 minutes at 12 months. The adjusted mean difference was –2.12 (P less than .001).
According to parent reports, children in the adenotonsillectomy group were significantly less likely than those in the control group to have trouble sleeping at night at 12 months: 8% and 74%, respectively (P less than .001).
“Children randomly assigned to adenotonsillectomy did show greater improvement in polysomnography obstructive indices and parent-reported behavior but did not demonstrate a treatment-attributable improvement in cognitive function,” David O. Francis, MD, of University of Wisconsin–Madison, and Derek J. Lam, MD, of Oregon Health & Science University in Portland, wrote in a related commentary.
The commentators noted that these results are similar to those of the CHAT study, which showed no significant differences in Developmental Neuropsychological Assessment results between children (ages 5-9 years) who underwent adenotonsillectomy and those who did not (N Engl J Med. 2013 Jun 20;368[25]:2366-76).
The current study was funded by the National Health and Medical Research Council, Sydney University, The Garnett Passe and Rodney Williams Memorial Foundation, and The Golden Casket, Brisbane. Dr. Waters, her coauthors, and the commentary authors said they have no relevant conflicts of interest. The commentators received no external funding.
SOURCE: Waters KA et al. Pediatrics. 2020;145(2):e20191450; Francis DO and Lam DJ. Pediatrics. 2020;145(2):e20192479.
according to a prospective study.
The study showed no significant difference in global IQ at 12 months between children who underwent adenotonsillectomy and those who did not. However, as expected, the adenotonsillectomy group did experience improvements in sleep.
Karen A. Waters, MBBS, PhD, of the Children’s Hospital at Westmead and the University of Sydney, and her colleagues reported these results in Pediatrics. There also was a related commentary.
The study enrolled 190 children (ages 3-5 years) with mild obstructive sleep apnea. Roughly half of patients (n = 99) were randomized to early adenotonsillectomy (within 2 months), and the other half (n = 91) were randomized to no adenotonsillectomy (12-month routine wait). There were 121 patients who had global IQ assessments at 12 months, as measured by the Woodcock Johnson III Brief Intellectual Ability (BIA) test. Of these patients, 61 were in the adenotonsillectomy group, and 60 were in the control group.
Both groups had improvements in BIA scores from baseline to 12 months, and the 12-month BIA score was not significantly different between the groups.
At baseline, the mean W score (task proficiency) for BIA was 448.36 in the adenotonsillectomy group and 451.3 in the control group. At 12 months, the scores were 465.46 and 463.12, respectively (P = .29).
“Intellectual ability scores improved in both groups over time with no effect attributable to the intervention [adenotonsillectomy],” Dr. Waters and her colleagues wrote.
However, patients in the adenotonsillectomy group did have greater improvements in sleep than patients in the control group, as assessed by polysomnogram and parent reports.
In the adenotonsillectomy group, the mean total sleep time was 469.2 minutes at baseline and 481.8 minutes at 12 months. In the control group, the mean total sleep time was 463.8 minutes at baseline and 475.3 minutes at 12 months. The adjusted mean difference was –2.12 (P less than .001).
According to parent reports, children in the adenotonsillectomy group were significantly less likely than those in the control group to have trouble sleeping at night at 12 months: 8% and 74%, respectively (P less than .001).
“Children randomly assigned to adenotonsillectomy did show greater improvement in polysomnography obstructive indices and parent-reported behavior but did not demonstrate a treatment-attributable improvement in cognitive function,” David O. Francis, MD, of University of Wisconsin–Madison, and Derek J. Lam, MD, of Oregon Health & Science University in Portland, wrote in a related commentary.
The commentators noted that these results are similar to those of the CHAT study, which showed no significant differences in Developmental Neuropsychological Assessment results between children (ages 5-9 years) who underwent adenotonsillectomy and those who did not (N Engl J Med. 2013 Jun 20;368[25]:2366-76).
The current study was funded by the National Health and Medical Research Council, Sydney University, The Garnett Passe and Rodney Williams Memorial Foundation, and The Golden Casket, Brisbane. Dr. Waters, her coauthors, and the commentary authors said they have no relevant conflicts of interest. The commentators received no external funding.
SOURCE: Waters KA et al. Pediatrics. 2020;145(2):e20191450; Francis DO and Lam DJ. Pediatrics. 2020;145(2):e20192479.
according to a prospective study.
The study showed no significant difference in global IQ at 12 months between children who underwent adenotonsillectomy and those who did not. However, as expected, the adenotonsillectomy group did experience improvements in sleep.
Karen A. Waters, MBBS, PhD, of the Children’s Hospital at Westmead and the University of Sydney, and her colleagues reported these results in Pediatrics. There also was a related commentary.
The study enrolled 190 children (ages 3-5 years) with mild obstructive sleep apnea. Roughly half of patients (n = 99) were randomized to early adenotonsillectomy (within 2 months), and the other half (n = 91) were randomized to no adenotonsillectomy (12-month routine wait). There were 121 patients who had global IQ assessments at 12 months, as measured by the Woodcock Johnson III Brief Intellectual Ability (BIA) test. Of these patients, 61 were in the adenotonsillectomy group, and 60 were in the control group.
Both groups had improvements in BIA scores from baseline to 12 months, and the 12-month BIA score was not significantly different between the groups.
At baseline, the mean W score (task proficiency) for BIA was 448.36 in the adenotonsillectomy group and 451.3 in the control group. At 12 months, the scores were 465.46 and 463.12, respectively (P = .29).
“Intellectual ability scores improved in both groups over time with no effect attributable to the intervention [adenotonsillectomy],” Dr. Waters and her colleagues wrote.
However, patients in the adenotonsillectomy group did have greater improvements in sleep than patients in the control group, as assessed by polysomnogram and parent reports.
In the adenotonsillectomy group, the mean total sleep time was 469.2 minutes at baseline and 481.8 minutes at 12 months. In the control group, the mean total sleep time was 463.8 minutes at baseline and 475.3 minutes at 12 months. The adjusted mean difference was –2.12 (P less than .001).
According to parent reports, children in the adenotonsillectomy group were significantly less likely than those in the control group to have trouble sleeping at night at 12 months: 8% and 74%, respectively (P less than .001).
“Children randomly assigned to adenotonsillectomy did show greater improvement in polysomnography obstructive indices and parent-reported behavior but did not demonstrate a treatment-attributable improvement in cognitive function,” David O. Francis, MD, of University of Wisconsin–Madison, and Derek J. Lam, MD, of Oregon Health & Science University in Portland, wrote in a related commentary.
The commentators noted that these results are similar to those of the CHAT study, which showed no significant differences in Developmental Neuropsychological Assessment results between children (ages 5-9 years) who underwent adenotonsillectomy and those who did not (N Engl J Med. 2013 Jun 20;368[25]:2366-76).
The current study was funded by the National Health and Medical Research Council, Sydney University, The Garnett Passe and Rodney Williams Memorial Foundation, and The Golden Casket, Brisbane. Dr. Waters, her coauthors, and the commentary authors said they have no relevant conflicts of interest. The commentators received no external funding.
SOURCE: Waters KA et al. Pediatrics. 2020;145(2):e20191450; Francis DO and Lam DJ. Pediatrics. 2020;145(2):e20192479.
FROM PEDIATRICS
Fast-acting, mealtime insulin aspart is approved for kids
making it the first fast-acting mealtime insulin injection that does not come with a premeal dosing recommendation, according to a release.
The injection is now available in various dosing options for both adult and pediatric patients with diabetes. Fast-acting mealtime insulin was approved in September 2017 for adults with type 1 or 2 disease, and in October 2019, it was approved for use in insulin pumps for adults.
The most recent approval was based on findings from the onset 7 trial, a 26-week, phase 3b, partially double-blind, treat-to-target trial that included 777 patients aged 1-18 years and demonstrated noninferiority to ordinary, non–fast-acting insulin aspart (Diabetes Care. 2019 Jul;42[7]:1255-62).
Removal of the premeal dosing requirement could help better manage mealtime insulin needs in children, according to the release from Novo Nordisk.
Use of the mealtime insulin injection comes with concerns of serious side effects, such as hypoglycemia, hypokalemia, serious allergic reactions, and heart failure. Common side effects can include skin problems (such as rash, itching, and swelling), injection-site reactions, and weight gain.
making it the first fast-acting mealtime insulin injection that does not come with a premeal dosing recommendation, according to a release.
The injection is now available in various dosing options for both adult and pediatric patients with diabetes. Fast-acting mealtime insulin was approved in September 2017 for adults with type 1 or 2 disease, and in October 2019, it was approved for use in insulin pumps for adults.
The most recent approval was based on findings from the onset 7 trial, a 26-week, phase 3b, partially double-blind, treat-to-target trial that included 777 patients aged 1-18 years and demonstrated noninferiority to ordinary, non–fast-acting insulin aspart (Diabetes Care. 2019 Jul;42[7]:1255-62).
Removal of the premeal dosing requirement could help better manage mealtime insulin needs in children, according to the release from Novo Nordisk.
Use of the mealtime insulin injection comes with concerns of serious side effects, such as hypoglycemia, hypokalemia, serious allergic reactions, and heart failure. Common side effects can include skin problems (such as rash, itching, and swelling), injection-site reactions, and weight gain.
making it the first fast-acting mealtime insulin injection that does not come with a premeal dosing recommendation, according to a release.
The injection is now available in various dosing options for both adult and pediatric patients with diabetes. Fast-acting mealtime insulin was approved in September 2017 for adults with type 1 or 2 disease, and in October 2019, it was approved for use in insulin pumps for adults.
The most recent approval was based on findings from the onset 7 trial, a 26-week, phase 3b, partially double-blind, treat-to-target trial that included 777 patients aged 1-18 years and demonstrated noninferiority to ordinary, non–fast-acting insulin aspart (Diabetes Care. 2019 Jul;42[7]:1255-62).
Removal of the premeal dosing requirement could help better manage mealtime insulin needs in children, according to the release from Novo Nordisk.
Use of the mealtime insulin injection comes with concerns of serious side effects, such as hypoglycemia, hypokalemia, serious allergic reactions, and heart failure. Common side effects can include skin problems (such as rash, itching, and swelling), injection-site reactions, and weight gain.
Annual Skin Check: Examining the Dermatology Headlines of 2019
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
Resident Pearls
- Chemical sunscreen made headlines in 2019 due to concerns over coral reef toxicity and systemic absorption in humans.
- With a total of 654 cases, New York City’s largest measles outbreak in nearly 30 years ended in September 2019.
- From dupilumab for adolescent atopic dermatitis to apremilast for Behçet disease, the US Food and Drug Administration approved several therapies for pediatric dermatology and rare dermatologic conditions in 2019.
- Two popular skin care products—the Neutrogena Light Therapy Acne Mask and Johnson’s Baby Powder—were involved in recalls in 2019.
Flu records most active December since 2003
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.
For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.