User login
Papulosquamous Dermatophytid Reaction in a Child With Tinea Capitis
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
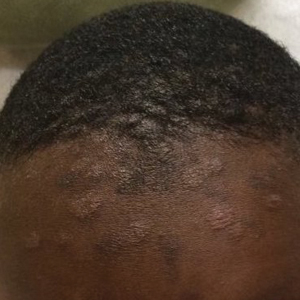
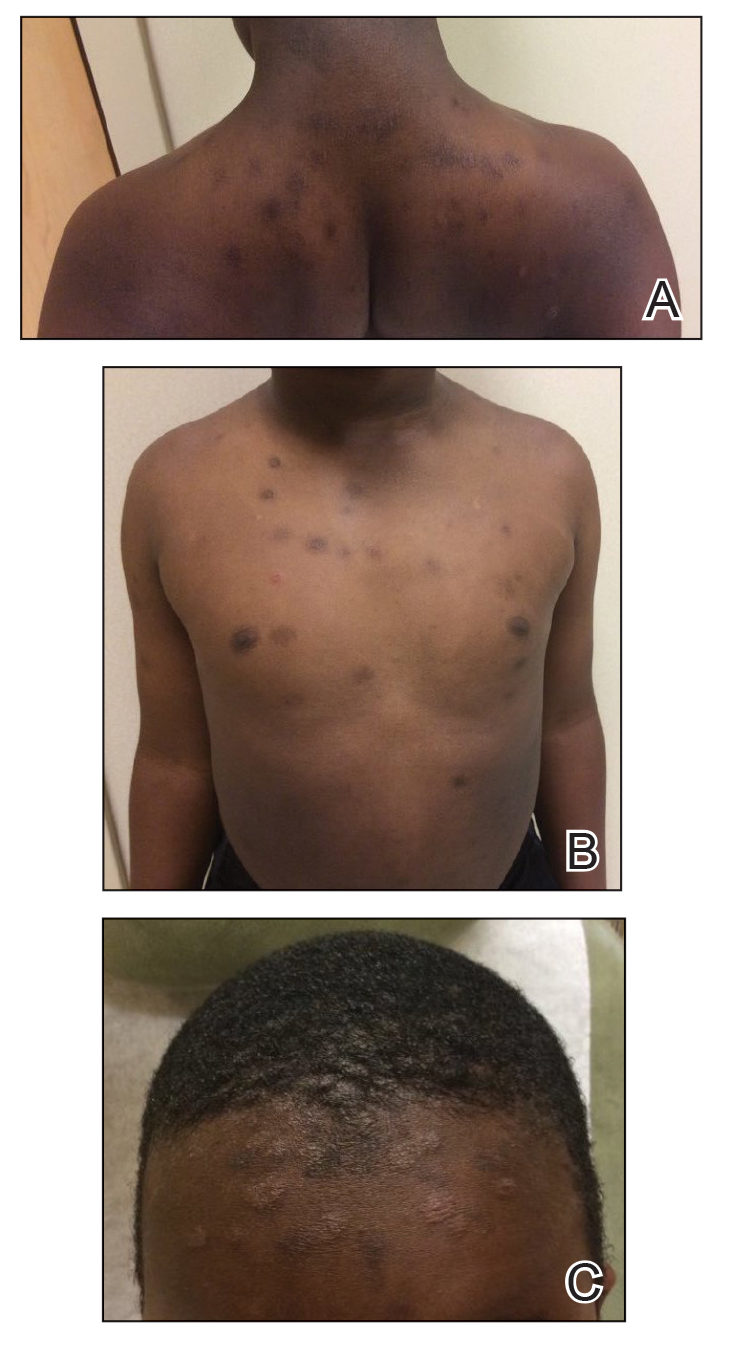
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.
Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.

Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.

Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
Practice Points
- Dermatophytid (id) reactions can manifest as papulosquamous eruptions after cutaneous infections or inflammatory skin conditions.
- High clinical suspicion for id reaction in patients of the appropriate age group and demographic—pediatric patients of African descent—is imperative for reaching the correct diagnosis.
- Repeat cultures of the scalp may be indicated in patients with high clinical probability for an id reaction despite a negative fungal culture or empiric systemic treatment.
How Abdominal Fibrogenesis Affects Adolescents With Obesity
TOPLINE:
Insulin resistance and obesity in adolescents may lead to increased abdominal fibrogenesis, impairing the capacity of the abdominal subcutaneous adipose tissue (SAT) to store lipids, which may cause fat accumulation in the visceral adipose tissue (VAT) depot and in other organs such as the liver.
METHODOLOGY:
- Abdominal fibrogenesis, but not adipose tissue expandability, is known to increase in adults with obesity and reduce insulin sensitivity; however, little is known about fibrogenesis in adolescents with obesity.
- In this study, researchers investigated if lipid dynamics, fibrogenesis, and abdominal and gluteal adipocyte turnover show dysregulation to a greater extent in insulin-resistant adolescents with obesity than in insulin-sensitive adolescents with obesity.
- They recruited 14 individuals between 12 and 20 years with a body mass index over 30 from the Yale Clinic, of whom seven participants were classified as insulin resistant.
- Deuterated water methodologies were used to study the indices of adipocyte turnover, lipid dynamics, and fibrogenesis in abdominal and gluteal fat deposits.
- A 3-hour oral glucose tolerance test and multisection MRI scan of the abdominal region were used to assess the indices of glucose metabolism, abdominal fat distribution patterns, and liver fat content.
TAKEAWAY:
- The abdominal and gluteal SAT turnover rate of lipid components (triglyceride production and breakdown as well as de novo lipogenesis contribution) was similar in insulin-resistant and insulin-sensitive adolescents with obesity.
- The insoluble collagen (type I, subunit alpha2) level was higher in the abdominal adipose tissue of insulin-resistant adolescents than in insulin-sensitive adolescents (difference in fractional synthesis rate, 0.611; P < .001), indicating increased abdominal fibrogenesis.
- Abdominal insoluble collagen I alpha2 was associated with higher fasting plasma insulin levels (correlation [r], 0.579; P = .015), a higher visceral to total adipose tissue ratio (r, 0.643; P = .007), and a lower whole-body insulin sensitivity index (r, -0.540; P = .023).
- There was no evidence of increased collagen production in the gluteal adipose tissue, and as a result, fibrogenesis was observed.
IN PRACTICE:
“The increased formation of insoluble collagen observed in insulin-resistant compared with insulin-sensitive individuals contributes to lipid spillover from SAT to VAT and, in turn, serves as a critically important mechanism involved in the complex sequelae of obesity-related metabolic and liver disease pathology,” the authors wrote.
SOURCE:
This study, led by Aaron L. Slusher, Department of Pediatrics, Yale School of Medicine, New Haven, Connecticut, was published online in Obesity.
LIMITATIONS:
The researchers did not measure hepatic collagen synthesis rates. The analysis was performed on a small study population. The authors were also unable to assess potential sex differences.
DISCLOSURES:
The study was funded by the Foundation for the National Institutes of Health and Clara Guthrie Patterson Trust Mentored Research Award. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Insulin resistance and obesity in adolescents may lead to increased abdominal fibrogenesis, impairing the capacity of the abdominal subcutaneous adipose tissue (SAT) to store lipids, which may cause fat accumulation in the visceral adipose tissue (VAT) depot and in other organs such as the liver.
METHODOLOGY:
- Abdominal fibrogenesis, but not adipose tissue expandability, is known to increase in adults with obesity and reduce insulin sensitivity; however, little is known about fibrogenesis in adolescents with obesity.
- In this study, researchers investigated if lipid dynamics, fibrogenesis, and abdominal and gluteal adipocyte turnover show dysregulation to a greater extent in insulin-resistant adolescents with obesity than in insulin-sensitive adolescents with obesity.
- They recruited 14 individuals between 12 and 20 years with a body mass index over 30 from the Yale Clinic, of whom seven participants were classified as insulin resistant.
- Deuterated water methodologies were used to study the indices of adipocyte turnover, lipid dynamics, and fibrogenesis in abdominal and gluteal fat deposits.
- A 3-hour oral glucose tolerance test and multisection MRI scan of the abdominal region were used to assess the indices of glucose metabolism, abdominal fat distribution patterns, and liver fat content.
TAKEAWAY:
- The abdominal and gluteal SAT turnover rate of lipid components (triglyceride production and breakdown as well as de novo lipogenesis contribution) was similar in insulin-resistant and insulin-sensitive adolescents with obesity.
- The insoluble collagen (type I, subunit alpha2) level was higher in the abdominal adipose tissue of insulin-resistant adolescents than in insulin-sensitive adolescents (difference in fractional synthesis rate, 0.611; P < .001), indicating increased abdominal fibrogenesis.
- Abdominal insoluble collagen I alpha2 was associated with higher fasting plasma insulin levels (correlation [r], 0.579; P = .015), a higher visceral to total adipose tissue ratio (r, 0.643; P = .007), and a lower whole-body insulin sensitivity index (r, -0.540; P = .023).
- There was no evidence of increased collagen production in the gluteal adipose tissue, and as a result, fibrogenesis was observed.
IN PRACTICE:
“The increased formation of insoluble collagen observed in insulin-resistant compared with insulin-sensitive individuals contributes to lipid spillover from SAT to VAT and, in turn, serves as a critically important mechanism involved in the complex sequelae of obesity-related metabolic and liver disease pathology,” the authors wrote.
SOURCE:
This study, led by Aaron L. Slusher, Department of Pediatrics, Yale School of Medicine, New Haven, Connecticut, was published online in Obesity.
LIMITATIONS:
The researchers did not measure hepatic collagen synthesis rates. The analysis was performed on a small study population. The authors were also unable to assess potential sex differences.
DISCLOSURES:
The study was funded by the Foundation for the National Institutes of Health and Clara Guthrie Patterson Trust Mentored Research Award. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Insulin resistance and obesity in adolescents may lead to increased abdominal fibrogenesis, impairing the capacity of the abdominal subcutaneous adipose tissue (SAT) to store lipids, which may cause fat accumulation in the visceral adipose tissue (VAT) depot and in other organs such as the liver.
METHODOLOGY:
- Abdominal fibrogenesis, but not adipose tissue expandability, is known to increase in adults with obesity and reduce insulin sensitivity; however, little is known about fibrogenesis in adolescents with obesity.
- In this study, researchers investigated if lipid dynamics, fibrogenesis, and abdominal and gluteal adipocyte turnover show dysregulation to a greater extent in insulin-resistant adolescents with obesity than in insulin-sensitive adolescents with obesity.
- They recruited 14 individuals between 12 and 20 years with a body mass index over 30 from the Yale Clinic, of whom seven participants were classified as insulin resistant.
- Deuterated water methodologies were used to study the indices of adipocyte turnover, lipid dynamics, and fibrogenesis in abdominal and gluteal fat deposits.
- A 3-hour oral glucose tolerance test and multisection MRI scan of the abdominal region were used to assess the indices of glucose metabolism, abdominal fat distribution patterns, and liver fat content.
TAKEAWAY:
- The abdominal and gluteal SAT turnover rate of lipid components (triglyceride production and breakdown as well as de novo lipogenesis contribution) was similar in insulin-resistant and insulin-sensitive adolescents with obesity.
- The insoluble collagen (type I, subunit alpha2) level was higher in the abdominal adipose tissue of insulin-resistant adolescents than in insulin-sensitive adolescents (difference in fractional synthesis rate, 0.611; P < .001), indicating increased abdominal fibrogenesis.
- Abdominal insoluble collagen I alpha2 was associated with higher fasting plasma insulin levels (correlation [r], 0.579; P = .015), a higher visceral to total adipose tissue ratio (r, 0.643; P = .007), and a lower whole-body insulin sensitivity index (r, -0.540; P = .023).
- There was no evidence of increased collagen production in the gluteal adipose tissue, and as a result, fibrogenesis was observed.
IN PRACTICE:
“The increased formation of insoluble collagen observed in insulin-resistant compared with insulin-sensitive individuals contributes to lipid spillover from SAT to VAT and, in turn, serves as a critically important mechanism involved in the complex sequelae of obesity-related metabolic and liver disease pathology,” the authors wrote.
SOURCE:
This study, led by Aaron L. Slusher, Department of Pediatrics, Yale School of Medicine, New Haven, Connecticut, was published online in Obesity.
LIMITATIONS:
The researchers did not measure hepatic collagen synthesis rates. The analysis was performed on a small study population. The authors were also unable to assess potential sex differences.
DISCLOSURES:
The study was funded by the Foundation for the National Institutes of Health and Clara Guthrie Patterson Trust Mentored Research Award. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Clinical Characteristics of Chronic Nonbacterial Osteomyelitis Can Predict Therapy Needs Over Time
CORRECTED April 7, 2024 // An earlier version of this article misstated the clinical factors of children with CNO that were significantly associated with the need for second-line treatment, as well as the scope of assessments of aspects of disease involvement and their relationship to total number of days on NSAID monotherapy and the odds of needing a second-line treatment.
Children with chronic nonbacterial osteomyelitis (CNO) who had symmetric bone lesions or multiple affected body regions were more likely to need second-line treatment than were patients without these features, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
CNO is an auto-inflammatory condition that results in sterile inflammatory bone lesions and most commonly affects the long bones of people who are skeletally immature. After a first-line treatment of nonsteroidal anti-inflammatory drugs (NSAIDs), second-line treatments per CARRA guidelines typically include methotrexate or sulfasalazine, tumor necrosis factor (TNF)–alpha inhibitors, and bisphosphonates.
“Since it’s common for there to be long delays before diagnosis of CNO, it is important to start an effective treatment promptly,” Katherine D. Nowicki, MD, of Children’s Hospital Colorado, Aurora, told attendees. “While we have guidance on which treatments to use, it remains unclear which patients are most likely to respond to NSAIDs and which patients will require a second-line treatment.”
Findings Helpful for Counseling
Melissa S. Oliver, MD, MS, assistant professor of clinical pediatrics in rheumatology at Riley Children’s Health at Indiana University Health, Indianapolis, who was not involved in the research, said the findings of this study are helpful in “counseling families and patients at that initial visit and having a lower threshold to start a second-line agent if NSAID monotherapy is not working well.”
There are no clinical trials on patients with CNO, Dr. Oliver said, so very little data exist for guiding clinicians on the best therapy to use and how long to keep patients on therapy while minimizing risk for flare when coming off therapy.
A key clinical takeaway for clinicians is being able to tell patients with unifocal disease that they may not need to be on NSAIDs for a long period and can still do well, Dr. Oliver said. For patients with multifocal disease with symmetric bone lesions or multiple regions involved with CNO, “pediatric rheumatologists should have a lower threshold to start a second-line therapy for these patients,” she said.
To better understand how different clinical characteristics predict treatment needs, the researchers conducted a retrospective chart review of 234 patients who received a CNO diagnosis before age 18 and who established care in the Children’s Hospital Colorado’s CNO multidisciplinary clinic between January 2005 and July 2021. After excluding 70 patients, primarily due to inadequate follow-up for assessing treatment response, the researchers included 164 patients whose records they reviewed through January 2022.
The researchers assessed how multiple aspects of disease involvement, including unifocal or multifocal at diagnosis, ever having symmetric bone lesions, number of regions ever affected by CNO, complications, and disease activity at most recent follow-up, to determine their relationship to the total number of days on NSAID monotherapy and the odds of needing a second-line treatment.
Among the 164 patients in the study, 32 had a short course of NSAIDs (3-7 months), 62 had a long course of NSAIDs (7 or more months), and 70 received second-line treatment.
Findings From Largest Single-Center Cohort in North America
Their topline findings revealed that patients with unifocal disease at diagnosis required 47% fewer total days of NSAID monotherapy treatment than those with multifocal disease at diagnosis, Dr. Nowicki told attendees. Having symmetric bone lesions increased the likelihood of needing a second-line therapy by 6.86 times compared with those without symmetric bone lesions, and for each additional region affected by CNO, the odds of needing a second-line therapy increased by a factor of 1.94, she said.
There were no significant differences in patient ages or sex or in mean interval from symptom onset to treatment onset across treatment groups. However, patients who received second-line treatment did have a significantly longer average time from symptom onset to diagnosis (324 days) than those who had a short course (119 days) or long course (270 days) of NSAIDs (P = .023). Mean follow-up was also significantly longer for patients with second-line treatment (3.8 years) or long-course NSAIDs (2.7 years) than for those with short-course NSAIDs (1.2 years; P < .001).
Mean erythrocyte sedimentation rate or C-reactive protein did not differ across treatment groups nor did presence of a CNO lesion on x-rays at presentation. But significantly more patients in the second-line group had a biopsy (94%) than in the long-course (74%) or short-course (69%) NSAID groups (P = .0025). They were also more likely to have one or more whole-body MRIs. Most of the patients on short-course (88%) and long-course (82%) NSAIDs did not undergo a whole-body MRI, whereas most patients (59%) on a second-line treatment underwent at least one and 24% underwent three or more MRIs (P < .001).
More patients on short-course NSAIDs had unifocal disease at diagnosis (72%) than those on long-course NSAIDs (47%) or a second-line treatment (41%; P = .015). Patients on a second-line treatment were also more likely to have symmetric involvement in the same bone (73% vs 16% short-course and 23% long-course NSAIDs) and to have more regions of the body affected (P < .001).
There were significant differences in mean days on NSAID monotherapy and number of NSAIDs trialed. Patients on a second-line treatment had a mean 441 days of NSAID monotherapy compared with 175 days for patients on short-course NSAIDs and 725 for patients on long-course NSAIDs (P < .001). Nearly all the short-course patients (94%) trialed a single NSAID, while more than half the long-course and second-line patients trialed two or more (P < .001).
None of the patients on short-course NSAIDs had complications. More patients on second-line treatments had vertebral height loss (20%) or amplified pain (14%) than long-course patients (13% and 5%, respectively; P = .02).
At the study’s end date, nearly all the patients on short-course NSAIDs were in remission (94%) compared with 71% of patients on long-course NSAIDs and only half of patients (51%) on the second-line treatment (P < .001). None of the patients on short-course NSAIDs had active disease compared with 11% of patients on long-course NSAIDs and 20% of patients on second-line treatments (P = .02).
This study included the largest single-center cohort of patients with CNO in North America, all treated at a multidisciplinary clinic with a protocolized treatment approach, but it remains limited by its retrospective nature and the missing data for 70 patients, Dr. Nowicki said. She noted that whole-body MRI was not systematically performed on all patients, so it was possible patients without a whole-body MRI had undetected asymptomatic lesions.
Despite these limitations, Dr. Oliver said retrospective studies like these can help pediatric rheumatologists get an idea of reasonable therapies to start, how long to keep patients on them, and when to escalate to the next step.
“I hope one day our CNO research will be able to tell us about which is the optimal second-line therapy for patients, such as bisphosphonates vs TNF inhibitors vs DMARDs [disease-modifying antirheumatic drugs],” Dr. Oliver said.
Dr. Nowicki and Dr. Oliver reported no disclosures. Information on study funding was not provided.
A version of this article appeared on Medscape.com .
CORRECTED April 7, 2024 // An earlier version of this article misstated the clinical factors of children with CNO that were significantly associated with the need for second-line treatment, as well as the scope of assessments of aspects of disease involvement and their relationship to total number of days on NSAID monotherapy and the odds of needing a second-line treatment.
Children with chronic nonbacterial osteomyelitis (CNO) who had symmetric bone lesions or multiple affected body regions were more likely to need second-line treatment than were patients without these features, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
CNO is an auto-inflammatory condition that results in sterile inflammatory bone lesions and most commonly affects the long bones of people who are skeletally immature. After a first-line treatment of nonsteroidal anti-inflammatory drugs (NSAIDs), second-line treatments per CARRA guidelines typically include methotrexate or sulfasalazine, tumor necrosis factor (TNF)–alpha inhibitors, and bisphosphonates.
“Since it’s common for there to be long delays before diagnosis of CNO, it is important to start an effective treatment promptly,” Katherine D. Nowicki, MD, of Children’s Hospital Colorado, Aurora, told attendees. “While we have guidance on which treatments to use, it remains unclear which patients are most likely to respond to NSAIDs and which patients will require a second-line treatment.”
Findings Helpful for Counseling
Melissa S. Oliver, MD, MS, assistant professor of clinical pediatrics in rheumatology at Riley Children’s Health at Indiana University Health, Indianapolis, who was not involved in the research, said the findings of this study are helpful in “counseling families and patients at that initial visit and having a lower threshold to start a second-line agent if NSAID monotherapy is not working well.”
There are no clinical trials on patients with CNO, Dr. Oliver said, so very little data exist for guiding clinicians on the best therapy to use and how long to keep patients on therapy while minimizing risk for flare when coming off therapy.
A key clinical takeaway for clinicians is being able to tell patients with unifocal disease that they may not need to be on NSAIDs for a long period and can still do well, Dr. Oliver said. For patients with multifocal disease with symmetric bone lesions or multiple regions involved with CNO, “pediatric rheumatologists should have a lower threshold to start a second-line therapy for these patients,” she said.
To better understand how different clinical characteristics predict treatment needs, the researchers conducted a retrospective chart review of 234 patients who received a CNO diagnosis before age 18 and who established care in the Children’s Hospital Colorado’s CNO multidisciplinary clinic between January 2005 and July 2021. After excluding 70 patients, primarily due to inadequate follow-up for assessing treatment response, the researchers included 164 patients whose records they reviewed through January 2022.
The researchers assessed how multiple aspects of disease involvement, including unifocal or multifocal at diagnosis, ever having symmetric bone lesions, number of regions ever affected by CNO, complications, and disease activity at most recent follow-up, to determine their relationship to the total number of days on NSAID monotherapy and the odds of needing a second-line treatment.
Among the 164 patients in the study, 32 had a short course of NSAIDs (3-7 months), 62 had a long course of NSAIDs (7 or more months), and 70 received second-line treatment.
Findings From Largest Single-Center Cohort in North America
Their topline findings revealed that patients with unifocal disease at diagnosis required 47% fewer total days of NSAID monotherapy treatment than those with multifocal disease at diagnosis, Dr. Nowicki told attendees. Having symmetric bone lesions increased the likelihood of needing a second-line therapy by 6.86 times compared with those without symmetric bone lesions, and for each additional region affected by CNO, the odds of needing a second-line therapy increased by a factor of 1.94, she said.
There were no significant differences in patient ages or sex or in mean interval from symptom onset to treatment onset across treatment groups. However, patients who received second-line treatment did have a significantly longer average time from symptom onset to diagnosis (324 days) than those who had a short course (119 days) or long course (270 days) of NSAIDs (P = .023). Mean follow-up was also significantly longer for patients with second-line treatment (3.8 years) or long-course NSAIDs (2.7 years) than for those with short-course NSAIDs (1.2 years; P < .001).
Mean erythrocyte sedimentation rate or C-reactive protein did not differ across treatment groups nor did presence of a CNO lesion on x-rays at presentation. But significantly more patients in the second-line group had a biopsy (94%) than in the long-course (74%) or short-course (69%) NSAID groups (P = .0025). They were also more likely to have one or more whole-body MRIs. Most of the patients on short-course (88%) and long-course (82%) NSAIDs did not undergo a whole-body MRI, whereas most patients (59%) on a second-line treatment underwent at least one and 24% underwent three or more MRIs (P < .001).
More patients on short-course NSAIDs had unifocal disease at diagnosis (72%) than those on long-course NSAIDs (47%) or a second-line treatment (41%; P = .015). Patients on a second-line treatment were also more likely to have symmetric involvement in the same bone (73% vs 16% short-course and 23% long-course NSAIDs) and to have more regions of the body affected (P < .001).
There were significant differences in mean days on NSAID monotherapy and number of NSAIDs trialed. Patients on a second-line treatment had a mean 441 days of NSAID monotherapy compared with 175 days for patients on short-course NSAIDs and 725 for patients on long-course NSAIDs (P < .001). Nearly all the short-course patients (94%) trialed a single NSAID, while more than half the long-course and second-line patients trialed two or more (P < .001).
None of the patients on short-course NSAIDs had complications. More patients on second-line treatments had vertebral height loss (20%) or amplified pain (14%) than long-course patients (13% and 5%, respectively; P = .02).
At the study’s end date, nearly all the patients on short-course NSAIDs were in remission (94%) compared with 71% of patients on long-course NSAIDs and only half of patients (51%) on the second-line treatment (P < .001). None of the patients on short-course NSAIDs had active disease compared with 11% of patients on long-course NSAIDs and 20% of patients on second-line treatments (P = .02).
This study included the largest single-center cohort of patients with CNO in North America, all treated at a multidisciplinary clinic with a protocolized treatment approach, but it remains limited by its retrospective nature and the missing data for 70 patients, Dr. Nowicki said. She noted that whole-body MRI was not systematically performed on all patients, so it was possible patients without a whole-body MRI had undetected asymptomatic lesions.
Despite these limitations, Dr. Oliver said retrospective studies like these can help pediatric rheumatologists get an idea of reasonable therapies to start, how long to keep patients on them, and when to escalate to the next step.
“I hope one day our CNO research will be able to tell us about which is the optimal second-line therapy for patients, such as bisphosphonates vs TNF inhibitors vs DMARDs [disease-modifying antirheumatic drugs],” Dr. Oliver said.
Dr. Nowicki and Dr. Oliver reported no disclosures. Information on study funding was not provided.
A version of this article appeared on Medscape.com .
CORRECTED April 7, 2024 // An earlier version of this article misstated the clinical factors of children with CNO that were significantly associated with the need for second-line treatment, as well as the scope of assessments of aspects of disease involvement and their relationship to total number of days on NSAID monotherapy and the odds of needing a second-line treatment.
Children with chronic nonbacterial osteomyelitis (CNO) who had symmetric bone lesions or multiple affected body regions were more likely to need second-line treatment than were patients without these features, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
CNO is an auto-inflammatory condition that results in sterile inflammatory bone lesions and most commonly affects the long bones of people who are skeletally immature. After a first-line treatment of nonsteroidal anti-inflammatory drugs (NSAIDs), second-line treatments per CARRA guidelines typically include methotrexate or sulfasalazine, tumor necrosis factor (TNF)–alpha inhibitors, and bisphosphonates.
“Since it’s common for there to be long delays before diagnosis of CNO, it is important to start an effective treatment promptly,” Katherine D. Nowicki, MD, of Children’s Hospital Colorado, Aurora, told attendees. “While we have guidance on which treatments to use, it remains unclear which patients are most likely to respond to NSAIDs and which patients will require a second-line treatment.”
Findings Helpful for Counseling
Melissa S. Oliver, MD, MS, assistant professor of clinical pediatrics in rheumatology at Riley Children’s Health at Indiana University Health, Indianapolis, who was not involved in the research, said the findings of this study are helpful in “counseling families and patients at that initial visit and having a lower threshold to start a second-line agent if NSAID monotherapy is not working well.”
There are no clinical trials on patients with CNO, Dr. Oliver said, so very little data exist for guiding clinicians on the best therapy to use and how long to keep patients on therapy while minimizing risk for flare when coming off therapy.
A key clinical takeaway for clinicians is being able to tell patients with unifocal disease that they may not need to be on NSAIDs for a long period and can still do well, Dr. Oliver said. For patients with multifocal disease with symmetric bone lesions or multiple regions involved with CNO, “pediatric rheumatologists should have a lower threshold to start a second-line therapy for these patients,” she said.
To better understand how different clinical characteristics predict treatment needs, the researchers conducted a retrospective chart review of 234 patients who received a CNO diagnosis before age 18 and who established care in the Children’s Hospital Colorado’s CNO multidisciplinary clinic between January 2005 and July 2021. After excluding 70 patients, primarily due to inadequate follow-up for assessing treatment response, the researchers included 164 patients whose records they reviewed through January 2022.
The researchers assessed how multiple aspects of disease involvement, including unifocal or multifocal at diagnosis, ever having symmetric bone lesions, number of regions ever affected by CNO, complications, and disease activity at most recent follow-up, to determine their relationship to the total number of days on NSAID monotherapy and the odds of needing a second-line treatment.
Among the 164 patients in the study, 32 had a short course of NSAIDs (3-7 months), 62 had a long course of NSAIDs (7 or more months), and 70 received second-line treatment.
Findings From Largest Single-Center Cohort in North America
Their topline findings revealed that patients with unifocal disease at diagnosis required 47% fewer total days of NSAID monotherapy treatment than those with multifocal disease at diagnosis, Dr. Nowicki told attendees. Having symmetric bone lesions increased the likelihood of needing a second-line therapy by 6.86 times compared with those without symmetric bone lesions, and for each additional region affected by CNO, the odds of needing a second-line therapy increased by a factor of 1.94, she said.
There were no significant differences in patient ages or sex or in mean interval from symptom onset to treatment onset across treatment groups. However, patients who received second-line treatment did have a significantly longer average time from symptom onset to diagnosis (324 days) than those who had a short course (119 days) or long course (270 days) of NSAIDs (P = .023). Mean follow-up was also significantly longer for patients with second-line treatment (3.8 years) or long-course NSAIDs (2.7 years) than for those with short-course NSAIDs (1.2 years; P < .001).
Mean erythrocyte sedimentation rate or C-reactive protein did not differ across treatment groups nor did presence of a CNO lesion on x-rays at presentation. But significantly more patients in the second-line group had a biopsy (94%) than in the long-course (74%) or short-course (69%) NSAID groups (P = .0025). They were also more likely to have one or more whole-body MRIs. Most of the patients on short-course (88%) and long-course (82%) NSAIDs did not undergo a whole-body MRI, whereas most patients (59%) on a second-line treatment underwent at least one and 24% underwent three or more MRIs (P < .001).
More patients on short-course NSAIDs had unifocal disease at diagnosis (72%) than those on long-course NSAIDs (47%) or a second-line treatment (41%; P = .015). Patients on a second-line treatment were also more likely to have symmetric involvement in the same bone (73% vs 16% short-course and 23% long-course NSAIDs) and to have more regions of the body affected (P < .001).
There were significant differences in mean days on NSAID monotherapy and number of NSAIDs trialed. Patients on a second-line treatment had a mean 441 days of NSAID monotherapy compared with 175 days for patients on short-course NSAIDs and 725 for patients on long-course NSAIDs (P < .001). Nearly all the short-course patients (94%) trialed a single NSAID, while more than half the long-course and second-line patients trialed two or more (P < .001).
None of the patients on short-course NSAIDs had complications. More patients on second-line treatments had vertebral height loss (20%) or amplified pain (14%) than long-course patients (13% and 5%, respectively; P = .02).
At the study’s end date, nearly all the patients on short-course NSAIDs were in remission (94%) compared with 71% of patients on long-course NSAIDs and only half of patients (51%) on the second-line treatment (P < .001). None of the patients on short-course NSAIDs had active disease compared with 11% of patients on long-course NSAIDs and 20% of patients on second-line treatments (P = .02).
This study included the largest single-center cohort of patients with CNO in North America, all treated at a multidisciplinary clinic with a protocolized treatment approach, but it remains limited by its retrospective nature and the missing data for 70 patients, Dr. Nowicki said. She noted that whole-body MRI was not systematically performed on all patients, so it was possible patients without a whole-body MRI had undetected asymptomatic lesions.
Despite these limitations, Dr. Oliver said retrospective studies like these can help pediatric rheumatologists get an idea of reasonable therapies to start, how long to keep patients on them, and when to escalate to the next step.
“I hope one day our CNO research will be able to tell us about which is the optimal second-line therapy for patients, such as bisphosphonates vs TNF inhibitors vs DMARDs [disease-modifying antirheumatic drugs],” Dr. Oliver said.
Dr. Nowicki and Dr. Oliver reported no disclosures. Information on study funding was not provided.
A version of this article appeared on Medscape.com .
FROM CARRA 2024
What We’ve Learned About Remote Learning
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Visionary Surgery Saved Pitcher’s Arm. Now Even Children Get It
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
In 1974, Tommy John of the Los Angeles Dodgers was 31 and a 12-year veteran of Major League Baseball when he became the unwitting vanguard of a revolution in baseball and orthopedics. Fifty years later, Mr. John might not be a candidate for the latest advances to a procedure that bears his name.
The southpaw pitcher had faced the abrupt end of his career when, after one fateful delivery, he found himself unable to throw to home. So he took a gamble on the surgical equivalent of a Hail Mary: the reconstruction of a torn ligament in his pitching elbow.
The experiment was a wild success. Mr. John pitched— and better than he had before — for another 14 seasons, retiring in 1989 at the age of 46. How much better? After the surgery, he tallied three 20-win seasons compared with none before the operation, and he finished among the top five vote-getters for the annual Cy Young Award three times. He was named an All-Star once before the surgery and three times after.
The triumph notwithstanding, Tommy John now cautions against Tommy John surgery. What’s given him and clinicians pause is a trend in recent years of ever-younger athletes who undergo the procedure.
Along with the surgical improvements in repairing a torn ulnar collateral ligament (UCL) is a demographic shift toward school-aged athletes who get it. By 2014, one study concluded that 67.4% of UCL reconstruction surgeries were performed on athletes between 16 and 20 years of age. Some patients are still in Little League when they undergo the procedure.
Experts say these athletes have weakened their UCLs through overuse. They disagree on whether to call it an “epidemic,” but if it is, “the vaccine is awareness” against throwing too hard and too often, said Eric Makhni, MD, an orthopedic surgeon at Henry Ford Health in Detroit.
From Career-Ending to Routine
Mr. John’s entry into baseball and orthopedic lore was initially slow, but the trickle turned into a tide. After Frank Jobe, MD, swapped a healthy tendon from John’s right wrist for his worn and torn left UCL on September 25, 1974, he didn’t perform his second surgery for another 1194 days. By the time “Tommy John surgery” became a recognized phrase, Mr. John was still active but only 14 professional baseball players had undergone the operation.
Prior to the start of spring training this year, an oft-cited database listed 366 pro players who’d undergone the operation.
“Before Tommy John, that was a career-ending injury,” said Grant E. Garrigues, MD, an orthopedic surgeon at Midwest Orthopaedics at RUSH in Chicago, who called Mr. John “a pure revolutionary.”
Tommy John surgery is “the only one that I can think of that is named after the patient rather than the doctor who first did it,” said Patrick McCulloch, MD, an orthopedic surgeon in Houston and a team physician for the Astros.
Dr. McCulloch, who performs about 25 UCL repairs a year, said that by recent estimates, one-third of pro pitchers had had some sort of surgical repair. He hesitated to call the increasing number of operations an epidemic but acknowledged that the ingredients exist for more elbow trauma among baseball players.
“More people are playing more often, and people are bigger and stronger and throwing harder,” he said.
Either way, Dr. McCulloch said, “the procedure is a victim of its own success” because it is “just done phenomenally well.”
The surgery is now commonplace — perhaps too commonplace, said David W. Altchek, MD, attending surgeon and co-chief emeritus at Hospital for Special Surgery in New York City.
Dr. Altchek played a key role in the popularity of the operation. Twenty-two years after Mr. John’s surgery, he helped develop a variation of the procedure called the docking technique.
Whereas Dr. Jobe sutured Mr. John’s replacement graft to itself, “we developed a different way of tying it over a bone bridge, which was more secure and more easy to tension,” Dr. Altchek explained.
The advance meant less drilling into bone and enabled surgeons to avoid moving a problem-free ulnar nerve or removing the flexor-pronator muscle that protects the elbow from stress. “The trauma of the surgery is significantly less,” he said. “We just made it a lot easier very quickly,” cutting the surgery time from 2 hours to 30-40 minutes.
Maybe the surgery became too easy, said Dr. Altchek, who estimates he has done 2000 of them over the past 30 years. “I don’t want to condemn my colleagues, but there are a lot of people doing the surgery,” he said. “And not a lot of people are doing a lot of them, and they don’t know the nuances of doing the surgery.”
The older procedures are known as the “full Tommy John”; each has a 12- to 18-month healing process, with a success rate of 80%-85%. Pitchers typically sit out a season while recovering.
Brandon Erickson, MD, an orthopedic surgeon at Rothman Orthopaedic Institute in New York City, said that in younger patients he has recently turned more often to the suture of the future: an internal brace that provides a repair rather than reconstruction.
The procedure, pioneered by Felix H. Savoie III, MD, the Ray J. Haddad Professor of Orthopaedics at Tulane University School of Medicine in New Orleans, and Jeffrey R. Dugas, MD, of Andrews Sports Medicine & Orthopaedic Center in Birmingham, Alabama, uses collagen-coated tape that looks like a shoelace and provides a scaffold that Dr. McCulloch said “is inductive to healing and growth of ligament tissue.”
The brace is intended for an “overhead” athlete (mostly baseball players but also javelin throwers and gymnasts) whose UCL is torn on only one side but is otherwise in good shape. In a pitcher the same age as Mr. John was when Dr. Jobe performed the first procedure, “that ligament may not be of very good quality,” Dr. McCulloch said. “It may have thickened. It may have calcifications.” But for a high-school junior with aspirations to pitch in college or beyond without “way too many miles on the elbow,” the approach is a good fit. The healing process is as little as 6 months.
“The ones who have a good ligament are very likely to do well,” said Dr. Erickson, an assistant team doctor for the Philadelphia Phillies.
“If the patient’s ligament is generally ‘good’ with only a tear, the InternalBrace procedure may be used to repair the native ligament. On the other end of the spectrum, if the patient’s ligament is torn and degenerative the surgeon may opt to do a UCL reconstruction using an auto or allograft — ie, Tommy John surgery,” Allen Holowecky, senior product manager of Arthrex of Naples, Florida, the maker of the InternalBrace, told this news organization. “Before UCL repair, Tommy John surgery was the only real treatment option. We tend to see repairs done on younger patients since their ligament hasn’t seen years of use-damage.”
Calls for Caution
Tommy John III wanted to play baseball like his dad until near-fatal complications from shoulder surgery altered his path. He was drawn to chiropractic and consults on injury prevention. “All surgeries and all medical interventions are cut first, ask questions later,” he said. “I was born with that.”
He saw his dad’s slow, heroic comeback from the surgery and described him as the perfect candidate for Dr. Jobe’s experiment. Tommy John spent his recovery time squeezing Silly Putty and throwing tennis balls. “He was willing to do anything necessary. He wanted to throw. That was his brush.” When the son was recovering from his own injury, “he said, ‘Learn the knuckleball.’ I said, ‘I don’t want to. I’ve reached my point.’ ”
He said he tells young patients with UCL injuries to rest. But instead “we have year-round sports with the promise that the more you play, the better,” he said. “They’re over-activitied.”
According to the American Academy of Orthopaedic Surgeons, 6.4 million children and adolescents in the United States played organized baseball in 2022, down from 11.5 million in 2014. Nearly half of pitchers played in a league with no maximum pitch counts, and 43.5% pitched on consecutive days, the group said.
How many UCL repair or reconstruction surgeries are performed on youth athletes each year is unclear. A 2019 study, however, found that although baseball injuries decreased between 2006 and 2016, the elbow was “the only location of injury that saw an increase.”
Dr. Garrigues said some parents of throwing athletes have asked about prophylactic Tommy John surgery for their children. He said it shouldn’t apply to pitchers.
“People have taken it a little too far,” he said. Dr. Garrigues and others argue against children throwing weighted balls when coming back from surgery. Instead, “we’re shutting them down,” he said.
Throwing any pitch is an act of violence on the body, Dr. Garrigues said, with the elbow taking the final brunt of the force. “These pitchers are functioning at the absolute limits of what the human body can take,” he said. “There’s only so many bullets in a gun,” which is why pitchers often feel the twinge of a torn UCL on a routine pitch.
Dr. Makhni suggested cross-training for pitchers in the off-season instead of playing baseball year-round. “If you play soccer, your footwork is going to be better,” he said.
“Kids shouldn’t be doing this all year round,” said Rebecca Carl, MD, associate professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago. “We are recommending that kids take 2 or 3 months off.” In the off-season, she urges them to strengthen their backs and cores.
Such advice can “feel like a bombshell,” said Dr. Carl, who chairs the Council on Sports Medicine and Fitness for the American Academy of Pediatrics. ‘Some started at a very young age. They go to camps. If I say to a teenager, ‘If you do this, I can keep you from getting injured,’ they think, ‘I won’t be injured.’” Most parents, however, understand the risk of “doing too much, too soon.”
Justin Orenduff, a former pitching prospect until his arm blew out, has made a career teaching head-to-toe pitching mechanics. He founded DVS Baseball, which uses software to teach pitchers how to properly use every muscle, starting with the orientation of the back foot. He, too, argues against pitching year-round. “Everyone on that travel team expects to get their fair share of playing time,” he said. “It just never stops.”
Organized baseball is paying attention. It has come up with the Pitch Smart program that gives maximum pitch counts for young players, but experts said children often get around that by belonging to several leagues.
Dr. Altchek said some surgeons have added platelet-rich plasma, stem cells, and bone marrow during surgery to quicken the slow healing time from UCL replacement. But he said, “it has to heal. Can you speed up biology?”
Dr. McCulloch said that, all the advances in Tommy John surgery aside, “the next frontier is really trying to crack the code on prevention.”
A version of this article first appeared on Medscape.com.
Maternal Lifestyle Interventions Boost Babies’ Heart Health
Infants born to women with obesity showed improved measures of cardiovascular health when their mothers adopted healthier lifestyles before and during pregnancy, based on data from a systematic review presented at the annual meeting of the Society for Reproductive Investigation.
Previous research has shown that children born to mothers with a high body mass index (BMI) are more likely to die from cardiovascular disease in later life, said presenting author Samuel J. Burden, PhD, in an interview.
“Surprisingly, early signs of these heart issues can start before birth and continue into childhood,” said Dr. Burden, a research associate in the Department of Women & Children’s Health, School of Life Course & Population Sciences, King’s College London, London, United Kingdom.
To examine the effect of interventions such as a healthy diet and exercise in pregnant women with obesity on the heart health of their infants, Dr. Burden and colleagues reviewed data from eight randomized, controlled trials involving diet and exercise for pregnant women with obesity. Of these, two used antenatal exercise, two used diet and physical activity, and one used preconception diet and physical activity. The studies ranged in size from 18 to 404 participants. Two studies included infants younger than 2 months of age, and four studies included children aged 3-7 years.
Overall, lifestyle interventions before conception and before birth were associated with significant changes in cardiac remodeling, specifically reduced interventricular septal wall thickness.
In addition, one of three studies of cardiac diastolic function and four of five studies of systolic function showed significant improvements. The five studies of cardiac systolic function and three studies of diastolic function also showed improvement in systolic and diastolic blood pressure in infants of mothers who took part in the interventions. The studies were limited mainly by large attrition rates, the researchers wrote in their presentation. However, more studies in larger populations that also include older children could confirm the findings and inform public health strategies to promote healthy lifestyles for pregnant women, they noted.
Encourage Healthy Lifestyle Before and During Pregnancy
The evidence supports the findings from animal studies showing that an offspring’s health is influenced by maternal lifestyle before and during pregnancy, Dr. Burden said in an interview. The data suggest that healthcare providers should encourage women with a high BMI who want to become pregnant to eat healthfully and become more active as a way to enhance the future cardiovascular health of their children, he said.
The full results of the current study are soon to be published, but more work is needed, said Dr. Burden. “While we observed a protective effect from these lifestyle programs, there is a need for more extensive studies involving a larger number of women (and their children) who were part of the initial research,” he said. “Additionally, it will be crucial to track these children into adulthood to determine whether these antenatal lifestyle interventions persist in lowering the risk of future cardiovascular disease.”
Beginning healthy lifestyle programs prior to pregnancy might yield the best results for promoting infant cardiovascular health, and more prepregnancy interventions for women with obesity are needed, Dr. Burden added.
The current study adds to the growing body of evidence that the in utero environment can have lifelong effects on offspring, Joseph R. Biggio Jr, MD, system chair of maternal fetal medicine at Ochsner Health, New Orleans, Louisiana, said in an interview.
“Several studies have previously shown that the children of mothers with diabetes, hypertension, or obesity are at increased risk for developing signs of metabolic syndrome and cardiovascular changes during childhood or adolescence,” said Dr. Biggio.
The data from this systematic review support the potential value of interventions aimed at improving maternal weight gain and cardiovascular performance before and during pregnancy that may result in reduced cardiovascular remodeling and myocardial thickening in infants, he said.
The study was supported by a British Heart Foundation Special Project Grant. The researchers had no financial conflicts to disclose. Dr. Biggio had no financial conflicts to disclose.
Infants born to women with obesity showed improved measures of cardiovascular health when their mothers adopted healthier lifestyles before and during pregnancy, based on data from a systematic review presented at the annual meeting of the Society for Reproductive Investigation.
Previous research has shown that children born to mothers with a high body mass index (BMI) are more likely to die from cardiovascular disease in later life, said presenting author Samuel J. Burden, PhD, in an interview.
“Surprisingly, early signs of these heart issues can start before birth and continue into childhood,” said Dr. Burden, a research associate in the Department of Women & Children’s Health, School of Life Course & Population Sciences, King’s College London, London, United Kingdom.
To examine the effect of interventions such as a healthy diet and exercise in pregnant women with obesity on the heart health of their infants, Dr. Burden and colleagues reviewed data from eight randomized, controlled trials involving diet and exercise for pregnant women with obesity. Of these, two used antenatal exercise, two used diet and physical activity, and one used preconception diet and physical activity. The studies ranged in size from 18 to 404 participants. Two studies included infants younger than 2 months of age, and four studies included children aged 3-7 years.
Overall, lifestyle interventions before conception and before birth were associated with significant changes in cardiac remodeling, specifically reduced interventricular septal wall thickness.
In addition, one of three studies of cardiac diastolic function and four of five studies of systolic function showed significant improvements. The five studies of cardiac systolic function and three studies of diastolic function also showed improvement in systolic and diastolic blood pressure in infants of mothers who took part in the interventions. The studies were limited mainly by large attrition rates, the researchers wrote in their presentation. However, more studies in larger populations that also include older children could confirm the findings and inform public health strategies to promote healthy lifestyles for pregnant women, they noted.
Encourage Healthy Lifestyle Before and During Pregnancy
The evidence supports the findings from animal studies showing that an offspring’s health is influenced by maternal lifestyle before and during pregnancy, Dr. Burden said in an interview. The data suggest that healthcare providers should encourage women with a high BMI who want to become pregnant to eat healthfully and become more active as a way to enhance the future cardiovascular health of their children, he said.
The full results of the current study are soon to be published, but more work is needed, said Dr. Burden. “While we observed a protective effect from these lifestyle programs, there is a need for more extensive studies involving a larger number of women (and their children) who were part of the initial research,” he said. “Additionally, it will be crucial to track these children into adulthood to determine whether these antenatal lifestyle interventions persist in lowering the risk of future cardiovascular disease.”
Beginning healthy lifestyle programs prior to pregnancy might yield the best results for promoting infant cardiovascular health, and more prepregnancy interventions for women with obesity are needed, Dr. Burden added.
The current study adds to the growing body of evidence that the in utero environment can have lifelong effects on offspring, Joseph R. Biggio Jr, MD, system chair of maternal fetal medicine at Ochsner Health, New Orleans, Louisiana, said in an interview.
“Several studies have previously shown that the children of mothers with diabetes, hypertension, or obesity are at increased risk for developing signs of metabolic syndrome and cardiovascular changes during childhood or adolescence,” said Dr. Biggio.
The data from this systematic review support the potential value of interventions aimed at improving maternal weight gain and cardiovascular performance before and during pregnancy that may result in reduced cardiovascular remodeling and myocardial thickening in infants, he said.
The study was supported by a British Heart Foundation Special Project Grant. The researchers had no financial conflicts to disclose. Dr. Biggio had no financial conflicts to disclose.
Infants born to women with obesity showed improved measures of cardiovascular health when their mothers adopted healthier lifestyles before and during pregnancy, based on data from a systematic review presented at the annual meeting of the Society for Reproductive Investigation.
Previous research has shown that children born to mothers with a high body mass index (BMI) are more likely to die from cardiovascular disease in later life, said presenting author Samuel J. Burden, PhD, in an interview.
“Surprisingly, early signs of these heart issues can start before birth and continue into childhood,” said Dr. Burden, a research associate in the Department of Women & Children’s Health, School of Life Course & Population Sciences, King’s College London, London, United Kingdom.
To examine the effect of interventions such as a healthy diet and exercise in pregnant women with obesity on the heart health of their infants, Dr. Burden and colleagues reviewed data from eight randomized, controlled trials involving diet and exercise for pregnant women with obesity. Of these, two used antenatal exercise, two used diet and physical activity, and one used preconception diet and physical activity. The studies ranged in size from 18 to 404 participants. Two studies included infants younger than 2 months of age, and four studies included children aged 3-7 years.
Overall, lifestyle interventions before conception and before birth were associated with significant changes in cardiac remodeling, specifically reduced interventricular septal wall thickness.
In addition, one of three studies of cardiac diastolic function and four of five studies of systolic function showed significant improvements. The five studies of cardiac systolic function and three studies of diastolic function also showed improvement in systolic and diastolic blood pressure in infants of mothers who took part in the interventions. The studies were limited mainly by large attrition rates, the researchers wrote in their presentation. However, more studies in larger populations that also include older children could confirm the findings and inform public health strategies to promote healthy lifestyles for pregnant women, they noted.
Encourage Healthy Lifestyle Before and During Pregnancy
The evidence supports the findings from animal studies showing that an offspring’s health is influenced by maternal lifestyle before and during pregnancy, Dr. Burden said in an interview. The data suggest that healthcare providers should encourage women with a high BMI who want to become pregnant to eat healthfully and become more active as a way to enhance the future cardiovascular health of their children, he said.
The full results of the current study are soon to be published, but more work is needed, said Dr. Burden. “While we observed a protective effect from these lifestyle programs, there is a need for more extensive studies involving a larger number of women (and their children) who were part of the initial research,” he said. “Additionally, it will be crucial to track these children into adulthood to determine whether these antenatal lifestyle interventions persist in lowering the risk of future cardiovascular disease.”
Beginning healthy lifestyle programs prior to pregnancy might yield the best results for promoting infant cardiovascular health, and more prepregnancy interventions for women with obesity are needed, Dr. Burden added.
The current study adds to the growing body of evidence that the in utero environment can have lifelong effects on offspring, Joseph R. Biggio Jr, MD, system chair of maternal fetal medicine at Ochsner Health, New Orleans, Louisiana, said in an interview.
“Several studies have previously shown that the children of mothers with diabetes, hypertension, or obesity are at increased risk for developing signs of metabolic syndrome and cardiovascular changes during childhood or adolescence,” said Dr. Biggio.
The data from this systematic review support the potential value of interventions aimed at improving maternal weight gain and cardiovascular performance before and during pregnancy that may result in reduced cardiovascular remodeling and myocardial thickening in infants, he said.
The study was supported by a British Heart Foundation Special Project Grant. The researchers had no financial conflicts to disclose. Dr. Biggio had no financial conflicts to disclose.
Teen Pregnancy Linked With Risk for Premature Death
Teen pregnancy is associated with a higher risk for premature mortality, both among those who carry the pregnancies to term and those who miscarry, according to a new study.
Among 2.2 million female teenagers in Ontario, Canada, the risk for premature death by age 31 years was 1.5 times higher among those who had one teen pregnancy and 2.1 times higher among those with two or more teen pregnancies.
“No person should die during childhood or early adulthood. Such deaths, unexpected and tragic, are often from preventable causes, including intentional injury,” lead author Joel Ray, MD, an obstetric medicine specialist and epidemiologist at St. Michael’s Hospital in Toronto, told this news organization.
“Women who experience teen pregnancy appear more vulnerable, often having experienced a history of adverse experiences in childhood, including abuse and economic challenges,” he said.
The study was published online in JAMA Network Open.
Analyzing Pregnancy Associations
The investigators conducted a population-based cohort study of all girls who were alive at age 12 years from April 1991 to March 2021 in Ontario. They evaluated the risk for all-cause mortality from age 12 years onward in association with the number of teen pregnancies between ages 12 and 19 years and the age at first pregnancy. The investigators adjusted the hazard ratios for year of birth, comorbidities at ages 9-11 years, area-level education, income level, and rural status.
Among more than 2.2 million teens, 163,124 (7.3%) had a pregnancy at a median age of 18 years, including 121,276 (74.3%) who had one pregnancy and 41,848 (25.6%) who had two or more. These teens were more likely to live in the lowest neighborhood income quintile and in an area with a lower rate of high school completion. They also had a higher prevalence of self-harm history between ages 12 and 19 years but not a higher prevalence of physical or mental comorbidities.
Among all teens who had a pregnancy, 60,037 (36.8%) ended in a birth, including 59,485 (99.1%) live births. A further 106,135 (65.1%) ended in induced abortion, and 17,945 (11%) ended in miscarriage or ectopic pregnancy.
Overall, there were 6030 premature deaths among those without a teen pregnancy, or 1.9 per 10,000 person-years. There were 701 deaths among those with one teen pregnancy (4.1 per 10,000 person-years) and 345 deaths among those with two or more teen pregnancies (6.1 per 10,000 person-years).
The adjusted hazard ratios (AHRs) for mortality were 1.51 for those with one pregnancy and 2.14 for those with two or more pregnancies. Compared with no teen pregnancy, the AHRs for premature death were 1.41 if the first teen pregnancy ended in an induced abortion and 2.10 if it ended in a miscarriage or birth.
Comparing those with a teen pregnancy and those without, the AHRs for premature death were 1.25 from noninjury, 2.06 from unintentional injury, and 2.02 from intentional injury. Among patients with teen pregnancy, noninjury-related premature mortality was more common, at 2.0 per 10,000 person-years, than unintentional and intentional injuries, at 1.0 per 10,000 person-years and 0.4 per 10,000 person-years, respectively.
A teen pregnancy before age 16 years entailed the highest associated risk for premature death, with an AHR of 2.00.
Next Research Steps
“We were not surprised by our findings, but it was new to us to see that the risk for premature death was higher for women who had an induced abortion in their teen years,” said Dr. Ray. “It was even higher in those whose pregnancy ended in a birth or miscarriage.”
The investigators plan to evaluate whether the future risk for premature death after teen pregnancy differs by the type of induced abortion, such as procedural or pharmaceutical, or by whether the pregnancy ended in a live birth, stillbirth, or miscarriage. Among those with a live birth, the researchers will also analyze the risk for premature death in relation to whether the newborn was taken into custody by child protection services in Canada.
Other factors associated with teen pregnancy and overall mortality, particularly adverse childhood experiences, may point to the reasons for premature mortality and should be studied further, the authors wrote. Structural and systems-related factors should be considered as well.
Stigmatization and Isolation
“Some teens choose to become pregnant, but most teen pregnancies are unintended, which exposes shortcomings in the systems that exist to educate, guide, and support young people,” said Elizabeth Cook, a research scientist at Child Trends in Rockville, Maryland.
Dr. Cook, who wasn’t involved with this study, wrote an accompanying editorial in JAMA Network Open. She conducts studies of sexual and reproductive health for Child Trends.
“Teens who become pregnant often experience stigmatization and isolation that can make it more difficult to thrive in adulthood, especially if they lack the necessary support to navigate such a significant decision,” she said. “Fortunately, the systems that youths encounter, such as healthcare, education, and child welfare, are taking on a larger role in prevention efforts than they have in the past.”
These systems are shifting the burden of unintended teen pregnancy from the teens themselves and their behaviors to the health and education systems, Dr. Cook noted, though more work is needed around local policies and lack of access to healthcare facilities.
“Teen pregnancy may offer an opportunity to intervene in the lives of people at higher risk for premature death, but knowing how best to offer support requires an understanding of the context of their lives,” she said. “As a starting point, we must celebrate and listen to all pregnant young people so they can tell us what they need to live long, fulfilled lives.”
The study was funded by grants from the PSI Foundation and the Canadian Institutes of Health Research, as well as ICES, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Ray and Dr. Cook reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Teen pregnancy is associated with a higher risk for premature mortality, both among those who carry the pregnancies to term and those who miscarry, according to a new study.
Among 2.2 million female teenagers in Ontario, Canada, the risk for premature death by age 31 years was 1.5 times higher among those who had one teen pregnancy and 2.1 times higher among those with two or more teen pregnancies.
“No person should die during childhood or early adulthood. Such deaths, unexpected and tragic, are often from preventable causes, including intentional injury,” lead author Joel Ray, MD, an obstetric medicine specialist and epidemiologist at St. Michael’s Hospital in Toronto, told this news organization.
“Women who experience teen pregnancy appear more vulnerable, often having experienced a history of adverse experiences in childhood, including abuse and economic challenges,” he said.
The study was published online in JAMA Network Open.
Analyzing Pregnancy Associations
The investigators conducted a population-based cohort study of all girls who were alive at age 12 years from April 1991 to March 2021 in Ontario. They evaluated the risk for all-cause mortality from age 12 years onward in association with the number of teen pregnancies between ages 12 and 19 years and the age at first pregnancy. The investigators adjusted the hazard ratios for year of birth, comorbidities at ages 9-11 years, area-level education, income level, and rural status.
Among more than 2.2 million teens, 163,124 (7.3%) had a pregnancy at a median age of 18 years, including 121,276 (74.3%) who had one pregnancy and 41,848 (25.6%) who had two or more. These teens were more likely to live in the lowest neighborhood income quintile and in an area with a lower rate of high school completion. They also had a higher prevalence of self-harm history between ages 12 and 19 years but not a higher prevalence of physical or mental comorbidities.
Among all teens who had a pregnancy, 60,037 (36.8%) ended in a birth, including 59,485 (99.1%) live births. A further 106,135 (65.1%) ended in induced abortion, and 17,945 (11%) ended in miscarriage or ectopic pregnancy.
Overall, there were 6030 premature deaths among those without a teen pregnancy, or 1.9 per 10,000 person-years. There were 701 deaths among those with one teen pregnancy (4.1 per 10,000 person-years) and 345 deaths among those with two or more teen pregnancies (6.1 per 10,000 person-years).
The adjusted hazard ratios (AHRs) for mortality were 1.51 for those with one pregnancy and 2.14 for those with two or more pregnancies. Compared with no teen pregnancy, the AHRs for premature death were 1.41 if the first teen pregnancy ended in an induced abortion and 2.10 if it ended in a miscarriage or birth.
Comparing those with a teen pregnancy and those without, the AHRs for premature death were 1.25 from noninjury, 2.06 from unintentional injury, and 2.02 from intentional injury. Among patients with teen pregnancy, noninjury-related premature mortality was more common, at 2.0 per 10,000 person-years, than unintentional and intentional injuries, at 1.0 per 10,000 person-years and 0.4 per 10,000 person-years, respectively.
A teen pregnancy before age 16 years entailed the highest associated risk for premature death, with an AHR of 2.00.
Next Research Steps
“We were not surprised by our findings, but it was new to us to see that the risk for premature death was higher for women who had an induced abortion in their teen years,” said Dr. Ray. “It was even higher in those whose pregnancy ended in a birth or miscarriage.”
The investigators plan to evaluate whether the future risk for premature death after teen pregnancy differs by the type of induced abortion, such as procedural or pharmaceutical, or by whether the pregnancy ended in a live birth, stillbirth, or miscarriage. Among those with a live birth, the researchers will also analyze the risk for premature death in relation to whether the newborn was taken into custody by child protection services in Canada.
Other factors associated with teen pregnancy and overall mortality, particularly adverse childhood experiences, may point to the reasons for premature mortality and should be studied further, the authors wrote. Structural and systems-related factors should be considered as well.
Stigmatization and Isolation
“Some teens choose to become pregnant, but most teen pregnancies are unintended, which exposes shortcomings in the systems that exist to educate, guide, and support young people,” said Elizabeth Cook, a research scientist at Child Trends in Rockville, Maryland.
Dr. Cook, who wasn’t involved with this study, wrote an accompanying editorial in JAMA Network Open. She conducts studies of sexual and reproductive health for Child Trends.
“Teens who become pregnant often experience stigmatization and isolation that can make it more difficult to thrive in adulthood, especially if they lack the necessary support to navigate such a significant decision,” she said. “Fortunately, the systems that youths encounter, such as healthcare, education, and child welfare, are taking on a larger role in prevention efforts than they have in the past.”
These systems are shifting the burden of unintended teen pregnancy from the teens themselves and their behaviors to the health and education systems, Dr. Cook noted, though more work is needed around local policies and lack of access to healthcare facilities.
“Teen pregnancy may offer an opportunity to intervene in the lives of people at higher risk for premature death, but knowing how best to offer support requires an understanding of the context of their lives,” she said. “As a starting point, we must celebrate and listen to all pregnant young people so they can tell us what they need to live long, fulfilled lives.”
The study was funded by grants from the PSI Foundation and the Canadian Institutes of Health Research, as well as ICES, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Ray and Dr. Cook reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Teen pregnancy is associated with a higher risk for premature mortality, both among those who carry the pregnancies to term and those who miscarry, according to a new study.
Among 2.2 million female teenagers in Ontario, Canada, the risk for premature death by age 31 years was 1.5 times higher among those who had one teen pregnancy and 2.1 times higher among those with two or more teen pregnancies.
“No person should die during childhood or early adulthood. Such deaths, unexpected and tragic, are often from preventable causes, including intentional injury,” lead author Joel Ray, MD, an obstetric medicine specialist and epidemiologist at St. Michael’s Hospital in Toronto, told this news organization.
“Women who experience teen pregnancy appear more vulnerable, often having experienced a history of adverse experiences in childhood, including abuse and economic challenges,” he said.
The study was published online in JAMA Network Open.
Analyzing Pregnancy Associations
The investigators conducted a population-based cohort study of all girls who were alive at age 12 years from April 1991 to March 2021 in Ontario. They evaluated the risk for all-cause mortality from age 12 years onward in association with the number of teen pregnancies between ages 12 and 19 years and the age at first pregnancy. The investigators adjusted the hazard ratios for year of birth, comorbidities at ages 9-11 years, area-level education, income level, and rural status.
Among more than 2.2 million teens, 163,124 (7.3%) had a pregnancy at a median age of 18 years, including 121,276 (74.3%) who had one pregnancy and 41,848 (25.6%) who had two or more. These teens were more likely to live in the lowest neighborhood income quintile and in an area with a lower rate of high school completion. They also had a higher prevalence of self-harm history between ages 12 and 19 years but not a higher prevalence of physical or mental comorbidities.
Among all teens who had a pregnancy, 60,037 (36.8%) ended in a birth, including 59,485 (99.1%) live births. A further 106,135 (65.1%) ended in induced abortion, and 17,945 (11%) ended in miscarriage or ectopic pregnancy.
Overall, there were 6030 premature deaths among those without a teen pregnancy, or 1.9 per 10,000 person-years. There were 701 deaths among those with one teen pregnancy (4.1 per 10,000 person-years) and 345 deaths among those with two or more teen pregnancies (6.1 per 10,000 person-years).
The adjusted hazard ratios (AHRs) for mortality were 1.51 for those with one pregnancy and 2.14 for those with two or more pregnancies. Compared with no teen pregnancy, the AHRs for premature death were 1.41 if the first teen pregnancy ended in an induced abortion and 2.10 if it ended in a miscarriage or birth.
Comparing those with a teen pregnancy and those without, the AHRs for premature death were 1.25 from noninjury, 2.06 from unintentional injury, and 2.02 from intentional injury. Among patients with teen pregnancy, noninjury-related premature mortality was more common, at 2.0 per 10,000 person-years, than unintentional and intentional injuries, at 1.0 per 10,000 person-years and 0.4 per 10,000 person-years, respectively.
A teen pregnancy before age 16 years entailed the highest associated risk for premature death, with an AHR of 2.00.
Next Research Steps
“We were not surprised by our findings, but it was new to us to see that the risk for premature death was higher for women who had an induced abortion in their teen years,” said Dr. Ray. “It was even higher in those whose pregnancy ended in a birth or miscarriage.”
The investigators plan to evaluate whether the future risk for premature death after teen pregnancy differs by the type of induced abortion, such as procedural or pharmaceutical, or by whether the pregnancy ended in a live birth, stillbirth, or miscarriage. Among those with a live birth, the researchers will also analyze the risk for premature death in relation to whether the newborn was taken into custody by child protection services in Canada.
Other factors associated with teen pregnancy and overall mortality, particularly adverse childhood experiences, may point to the reasons for premature mortality and should be studied further, the authors wrote. Structural and systems-related factors should be considered as well.
Stigmatization and Isolation
“Some teens choose to become pregnant, but most teen pregnancies are unintended, which exposes shortcomings in the systems that exist to educate, guide, and support young people,” said Elizabeth Cook, a research scientist at Child Trends in Rockville, Maryland.
Dr. Cook, who wasn’t involved with this study, wrote an accompanying editorial in JAMA Network Open. She conducts studies of sexual and reproductive health for Child Trends.
“Teens who become pregnant often experience stigmatization and isolation that can make it more difficult to thrive in adulthood, especially if they lack the necessary support to navigate such a significant decision,” she said. “Fortunately, the systems that youths encounter, such as healthcare, education, and child welfare, are taking on a larger role in prevention efforts than they have in the past.”
These systems are shifting the burden of unintended teen pregnancy from the teens themselves and their behaviors to the health and education systems, Dr. Cook noted, though more work is needed around local policies and lack of access to healthcare facilities.
“Teen pregnancy may offer an opportunity to intervene in the lives of people at higher risk for premature death, but knowing how best to offer support requires an understanding of the context of their lives,” she said. “As a starting point, we must celebrate and listen to all pregnant young people so they can tell us what they need to live long, fulfilled lives.”
The study was funded by grants from the PSI Foundation and the Canadian Institutes of Health Research, as well as ICES, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Ray and Dr. Cook reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Think Beyond the ‘Go-Tos’ for Wart Management, Expert Advises
SAN DIEGO — When Jennifer Adams, MD, recently entered the search term “warts” on the ClinicalTrials.gov web site, nearly 240 results popped up.
“There is a lot of research activity around this topic,” Dr. Adams, vice chair of the department of dermatology at the University of Nebraska Medical Center, said at the annual meeting of the American Academy of Dermatology. “We just don’t have fantastic, well-run trials on many of the currently available treatments.”
In a 2012 Cochrane review on the topical treatment of non-genital cutaneous warts, authors drew from 85 trials involving 8,815 randomized patients. They found that most warts spontaneously resolved, and the authors determined salicylic acid to be safe and modestly beneficial. Specifically, trials of salicylic acid (SA) versus placebo showed that the former significantly increased the chance of clearance of warts at all sites (risk ratio, 1.56, 95% confidence interval [CI], 1.20-2.03). A meta-analysis of cryotherapy versus placebo for warts at all sites favored neither intervention nor control (RR, 1.45, 95% CI, 0.65-3.23).
“The authors determined that there is less evidence for cryotherapy but stated that it may work when salicylic acid does not, or in combination with salicylic acid,” Dr. Adams said. “However, salicylic acid and cryotherapy don’t do enough for our patients [with warts]. There are a lot of situations where we need to reach further into the toolbox.”
A 2021 review article listed many options for managing difficult-to-treat warts, including intralesional Candida antigen, intralesional measles-mumps-rubella (MMR), intralesional HPV vaccine, intralesional vitamin D, intralesional cidofovir, intralesional bleomycin, and intralesional 5-FU injections, and topical vitamin D, topical cidofovir, and topical bleomycin. According to Dr. Adams, clinical data exist for cidofovir and vitamin D but studies evaluated different formulations, doses, sites of administration, and limited randomized controlled trials.
“Intralesional cidofovir is more effective than the topical form, but intralesional cidofovir can be painful and both forms are expensive,” she said. “Topical vitamin D is less likely to cause dyspigmentation compared to other available treatments, so it’s a great option in skin of color, but it has been less effective compared to some of our other topical treatments.”
Newer Options Promising
On the horizon, berdazimer gel was approved in January of 2024 for the treatment of molluscum but results from trials of its use for extragenital warts are encouraging. Another promising option is topical ionic contraviral therapy (ICVT) with digoxin and furosemide combined, which inhibits cellular potassium influx. A phase 2a randomized controlled trial of 80 adults found a statistically significant reduction in the diameter of cutaneous warts among those who received ICVT compared with those who received placebo (P = .002). “It’s cheap and well tolerated,” Dr. Adams added.
Intralesional approaches to treating warts offer another alternative. A 2020 review of 43 articles concluded that intralesional treatments for warts have equal or superior efficacy to first-line salicylic acid or cryotherapy.
Dr. Adams said that she considers intralesional treatments such as vitamin D, MMR vaccine antigen, and Candida antigen for refractory, numerous, or distant site warts. “Injecting the MMR vaccine into the largest wart every 2 weeks has been found to lead to complete clearance in 60%-68% of cases in one study,” she said. “The benefit is that it’s $21 per dose, which is nice, but as with any vaccination, patients can develop flu-like symptoms as side effects.”
Use of the HPV vaccine for treating cutaneous warts remains controversial, she continued, but it seems to work better in younger patients. In one open-label study that evaluated the HPV vaccine for the treatment of multiple recalcitrant warts, with doses administered at 0. 2, and 6 months, the response rate 3 months after the third dose was 55% among those older than age 26, compared with 84% among those ages 9-26 years.
Another option, intralesional cidofovir, has been shown to be especially effective for refractory warts. “It has also been shown to work for warts in immunocompetent and immunocompromised patients,” Dr. Adams said.
In the realm of adjuvant treatments, microneedling has been found to have similar efficacy to needling, Dr. Adams said, but with minimal pain. “When we combine it with topical treatments like 5-FU, it’s even more efficacious,” she said.
One study found that combining microneedling with topical 5-FU had clearance similar to that of intralesional 5-FU or microneedling alone, but involved fewer treatment sessions and less pain in the combination group.
Autoinoculation has been used to stimulate an immune response in patients with warts, leading to clearance rates of 4% (mild clearance) to 66% (complete clearance) in one study. “We would expect this to work better in immunocompetent patients, but it’s something to keep in mind if you’re limited in the medications you can get for a patient,” Dr. Adams said. Also, results from a systematic review and meta-analysis suggest that systemic retinoids combined with intralesional immunotherapy leads to higher clearance rates and lower rates of recurrence of warts. The top performer among those tested was acitretin plus Candida antigen.
Dr. Adams advised dermatologists who try alternatives to salicylic acid and cryotherapy for warts to be “wary of a lack of high-level evidence” for their use. “They can be helpful for patients who have failed traditional therapies or have a contraindication to the usual go-tos.”
She reported having no relevant financial disclosures.
SAN DIEGO — When Jennifer Adams, MD, recently entered the search term “warts” on the ClinicalTrials.gov web site, nearly 240 results popped up.
“There is a lot of research activity around this topic,” Dr. Adams, vice chair of the department of dermatology at the University of Nebraska Medical Center, said at the annual meeting of the American Academy of Dermatology. “We just don’t have fantastic, well-run trials on many of the currently available treatments.”
In a 2012 Cochrane review on the topical treatment of non-genital cutaneous warts, authors drew from 85 trials involving 8,815 randomized patients. They found that most warts spontaneously resolved, and the authors determined salicylic acid to be safe and modestly beneficial. Specifically, trials of salicylic acid (SA) versus placebo showed that the former significantly increased the chance of clearance of warts at all sites (risk ratio, 1.56, 95% confidence interval [CI], 1.20-2.03). A meta-analysis of cryotherapy versus placebo for warts at all sites favored neither intervention nor control (RR, 1.45, 95% CI, 0.65-3.23).
“The authors determined that there is less evidence for cryotherapy but stated that it may work when salicylic acid does not, or in combination with salicylic acid,” Dr. Adams said. “However, salicylic acid and cryotherapy don’t do enough for our patients [with warts]. There are a lot of situations where we need to reach further into the toolbox.”
A 2021 review article listed many options for managing difficult-to-treat warts, including intralesional Candida antigen, intralesional measles-mumps-rubella (MMR), intralesional HPV vaccine, intralesional vitamin D, intralesional cidofovir, intralesional bleomycin, and intralesional 5-FU injections, and topical vitamin D, topical cidofovir, and topical bleomycin. According to Dr. Adams, clinical data exist for cidofovir and vitamin D but studies evaluated different formulations, doses, sites of administration, and limited randomized controlled trials.
“Intralesional cidofovir is more effective than the topical form, but intralesional cidofovir can be painful and both forms are expensive,” she said. “Topical vitamin D is less likely to cause dyspigmentation compared to other available treatments, so it’s a great option in skin of color, but it has been less effective compared to some of our other topical treatments.”
Newer Options Promising
On the horizon, berdazimer gel was approved in January of 2024 for the treatment of molluscum but results from trials of its use for extragenital warts are encouraging. Another promising option is topical ionic contraviral therapy (ICVT) with digoxin and furosemide combined, which inhibits cellular potassium influx. A phase 2a randomized controlled trial of 80 adults found a statistically significant reduction in the diameter of cutaneous warts among those who received ICVT compared with those who received placebo (P = .002). “It’s cheap and well tolerated,” Dr. Adams added.
Intralesional approaches to treating warts offer another alternative. A 2020 review of 43 articles concluded that intralesional treatments for warts have equal or superior efficacy to first-line salicylic acid or cryotherapy.
Dr. Adams said that she considers intralesional treatments such as vitamin D, MMR vaccine antigen, and Candida antigen for refractory, numerous, or distant site warts. “Injecting the MMR vaccine into the largest wart every 2 weeks has been found to lead to complete clearance in 60%-68% of cases in one study,” she said. “The benefit is that it’s $21 per dose, which is nice, but as with any vaccination, patients can develop flu-like symptoms as side effects.”
Use of the HPV vaccine for treating cutaneous warts remains controversial, she continued, but it seems to work better in younger patients. In one open-label study that evaluated the HPV vaccine for the treatment of multiple recalcitrant warts, with doses administered at 0. 2, and 6 months, the response rate 3 months after the third dose was 55% among those older than age 26, compared with 84% among those ages 9-26 years.
Another option, intralesional cidofovir, has been shown to be especially effective for refractory warts. “It has also been shown to work for warts in immunocompetent and immunocompromised patients,” Dr. Adams said.
In the realm of adjuvant treatments, microneedling has been found to have similar efficacy to needling, Dr. Adams said, but with minimal pain. “When we combine it with topical treatments like 5-FU, it’s even more efficacious,” she said.
One study found that combining microneedling with topical 5-FU had clearance similar to that of intralesional 5-FU or microneedling alone, but involved fewer treatment sessions and less pain in the combination group.
Autoinoculation has been used to stimulate an immune response in patients with warts, leading to clearance rates of 4% (mild clearance) to 66% (complete clearance) in one study. “We would expect this to work better in immunocompetent patients, but it’s something to keep in mind if you’re limited in the medications you can get for a patient,” Dr. Adams said. Also, results from a systematic review and meta-analysis suggest that systemic retinoids combined with intralesional immunotherapy leads to higher clearance rates and lower rates of recurrence of warts. The top performer among those tested was acitretin plus Candida antigen.
Dr. Adams advised dermatologists who try alternatives to salicylic acid and cryotherapy for warts to be “wary of a lack of high-level evidence” for their use. “They can be helpful for patients who have failed traditional therapies or have a contraindication to the usual go-tos.”
She reported having no relevant financial disclosures.
SAN DIEGO — When Jennifer Adams, MD, recently entered the search term “warts” on the ClinicalTrials.gov web site, nearly 240 results popped up.
“There is a lot of research activity around this topic,” Dr. Adams, vice chair of the department of dermatology at the University of Nebraska Medical Center, said at the annual meeting of the American Academy of Dermatology. “We just don’t have fantastic, well-run trials on many of the currently available treatments.”
In a 2012 Cochrane review on the topical treatment of non-genital cutaneous warts, authors drew from 85 trials involving 8,815 randomized patients. They found that most warts spontaneously resolved, and the authors determined salicylic acid to be safe and modestly beneficial. Specifically, trials of salicylic acid (SA) versus placebo showed that the former significantly increased the chance of clearance of warts at all sites (risk ratio, 1.56, 95% confidence interval [CI], 1.20-2.03). A meta-analysis of cryotherapy versus placebo for warts at all sites favored neither intervention nor control (RR, 1.45, 95% CI, 0.65-3.23).
“The authors determined that there is less evidence for cryotherapy but stated that it may work when salicylic acid does not, or in combination with salicylic acid,” Dr. Adams said. “However, salicylic acid and cryotherapy don’t do enough for our patients [with warts]. There are a lot of situations where we need to reach further into the toolbox.”
A 2021 review article listed many options for managing difficult-to-treat warts, including intralesional Candida antigen, intralesional measles-mumps-rubella (MMR), intralesional HPV vaccine, intralesional vitamin D, intralesional cidofovir, intralesional bleomycin, and intralesional 5-FU injections, and topical vitamin D, topical cidofovir, and topical bleomycin. According to Dr. Adams, clinical data exist for cidofovir and vitamin D but studies evaluated different formulations, doses, sites of administration, and limited randomized controlled trials.
“Intralesional cidofovir is more effective than the topical form, but intralesional cidofovir can be painful and both forms are expensive,” she said. “Topical vitamin D is less likely to cause dyspigmentation compared to other available treatments, so it’s a great option in skin of color, but it has been less effective compared to some of our other topical treatments.”
Newer Options Promising
On the horizon, berdazimer gel was approved in January of 2024 for the treatment of molluscum but results from trials of its use for extragenital warts are encouraging. Another promising option is topical ionic contraviral therapy (ICVT) with digoxin and furosemide combined, which inhibits cellular potassium influx. A phase 2a randomized controlled trial of 80 adults found a statistically significant reduction in the diameter of cutaneous warts among those who received ICVT compared with those who received placebo (P = .002). “It’s cheap and well tolerated,” Dr. Adams added.
Intralesional approaches to treating warts offer another alternative. A 2020 review of 43 articles concluded that intralesional treatments for warts have equal or superior efficacy to first-line salicylic acid or cryotherapy.
Dr. Adams said that she considers intralesional treatments such as vitamin D, MMR vaccine antigen, and Candida antigen for refractory, numerous, or distant site warts. “Injecting the MMR vaccine into the largest wart every 2 weeks has been found to lead to complete clearance in 60%-68% of cases in one study,” she said. “The benefit is that it’s $21 per dose, which is nice, but as with any vaccination, patients can develop flu-like symptoms as side effects.”
Use of the HPV vaccine for treating cutaneous warts remains controversial, she continued, but it seems to work better in younger patients. In one open-label study that evaluated the HPV vaccine for the treatment of multiple recalcitrant warts, with doses administered at 0. 2, and 6 months, the response rate 3 months after the third dose was 55% among those older than age 26, compared with 84% among those ages 9-26 years.
Another option, intralesional cidofovir, has been shown to be especially effective for refractory warts. “It has also been shown to work for warts in immunocompetent and immunocompromised patients,” Dr. Adams said.
In the realm of adjuvant treatments, microneedling has been found to have similar efficacy to needling, Dr. Adams said, but with minimal pain. “When we combine it with topical treatments like 5-FU, it’s even more efficacious,” she said.
One study found that combining microneedling with topical 5-FU had clearance similar to that of intralesional 5-FU or microneedling alone, but involved fewer treatment sessions and less pain in the combination group.
Autoinoculation has been used to stimulate an immune response in patients with warts, leading to clearance rates of 4% (mild clearance) to 66% (complete clearance) in one study. “We would expect this to work better in immunocompetent patients, but it’s something to keep in mind if you’re limited in the medications you can get for a patient,” Dr. Adams said. Also, results from a systematic review and meta-analysis suggest that systemic retinoids combined with intralesional immunotherapy leads to higher clearance rates and lower rates of recurrence of warts. The top performer among those tested was acitretin plus Candida antigen.
Dr. Adams advised dermatologists who try alternatives to salicylic acid and cryotherapy for warts to be “wary of a lack of high-level evidence” for their use. “They can be helpful for patients who have failed traditional therapies or have a contraindication to the usual go-tos.”
She reported having no relevant financial disclosures.
FROM AAD 2024
Early Biologic Initiation Linked to Rapid Improvement of JIA, Sustained Remission
CORRECTED April 16, 2024 // An earlier version of this article stated incorrect percentages of patients who never received any biologics during the study's 3-year period but improved rapidly or moderately.
Early initiation of biologics — within the first 2 months of symptom presentation — appears to have a significant impact on how rapidly patients with juvenile idiopathic arthritis (JIA) improve, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Our study provides evidence that early use of biologics can significantly alter the disease trajectory of patients with JIA,” Mei-Sing Ong, PhD, of Harvard Medical School, Boston, told attendees. At the same time, however, not all patients who improved rapidly during a 3-year follow-up period needed biologics, a finding that Ong said the researchers are continuing to investigate.
Marinka Twilt, MD, MScE, PhD, chair of CARRA’s JIA Research Committee and a pediatric rheumatologist and clinician scientist at Alberta Children’s Hospital in Calgary, Canada, was not involved in the research but said the continued sustained remission in patients who improved rapidly is very reassuring.
“We always wonder if initial response will be sustained or if patients tend to flare after the initial treatment,” Dr. Twilt told this news organization. “To see the sustained response up to 3 years is fantastic.” She added that it would be enlightening to see more information about patients who rapidly improved over 3 years, including whether they were still taking a [conventional disease-modifying antirheumatic drug (DMARD)] and/or biologic.
“A new diagnosis can be overwhelming for families, and this sometimes leads to step-up therapy to not overwhelm them more with information on new drugs,” Dr. Twilt said. “This study shows that an earlier start is beneficial, and this should be discussed with families early on so there is less delay in early treatment.”
Canada and many US states currently require 3 months of conventional DMARD treatment before patients can start a biologic, Dr. Twilt said, yet “this study shows the additive benefit of using a biologic within 2 months of starting a DMARD, which hopefully will lead to insurance companies adopting this threshold.”
The STOP-JIA study is a prospective observational study that compares the effectiveness of three different treatment plans for JIA. A Step-Up cohort of 257 patients received conventional antirheumatic monotherapy initially, with a biologic added at 3 months or later as needed. The Early Combination cohort of 100 patients received conventional antirheumatic therapy with a biologic from the start. The Biologic First cohort of 43 patients began taking a biologic as a first-line therapy.
In previously reported results of the study at 12 months’ follow-up, there was no significant difference between the Step-Up and Biologic First groups, but there were significant differences between the Step-Up and Early Combination groups. Significantly more patients in the Early Combination group (58.8%) than in the Step-Up group (42.8%) had inactive disease, based on the clinical Juvenile Arthritis Disease Activity Score 10 (cJADAS-10) (P = .03). Similarly, 81% of Early Combination patients achieved the American College of Rheumatology 70% improvement criteria, compared with 62% of the Step-Up patients (P = .01).
To learn whether the timing of starting a biologic influenced the disease trajectory over time, the researchers compared subgroups of patients with similar trajectories.
“Assessing treatment outcomes at a single point in time does not give us a complete picture of the effects of treatment on disease trajectory, which is an important outcome given that JIA is characterized by a relapsing-remitting course,” Dr. Ong told attendees.
Patients were sorted in the slow, moderate, or rapid improvement trajectories. In previously reported data at 12 months’ follow-up, patients’ odds of achieving rapid improvement were 3.6 times greater if they had started a biologic within 3 months.
This study compared patients’ trajectories over 3 years in the 259 patients (65% of the original cohort) who had at least one cJADAS-10 assessment in each year of follow-up. Most patients (66.8%) were in the rapid improvement class, with 25.9% in the moderate improvement class and 7.3% in the slow improvement class.
Patients in the rapid improvement group achieved inactive disease (cJADAS-10 of 2.5 or less) within 1 year and maintained inactive disease through the second and third years. The moderate and low improvement groups both had higher disease activity at baseline, but the moderate group continued to improve in years 2 and 3, with minimal disease by year 3, on the basis of the cJADAS-10 scores of 2.5-5. The slow group continued to experience moderate disease activity during years 2 and 3.
The findings also revealed that the earlier patients began a biologic, the more likely they were to be in the rapid improvement group than the slow improvement group. Participants who started a biologic in the first month had more than five times greater odds of being in the rapid improvement group than in the slow improvement group (odds ratio [OR], 5.33; P = .017).
Those who started a biologic in the second month were also more likely to be in the rapid improvement group (OR, 2.67; P = .032). For those who began a biologic by the third month, the odds of improving rapidly were not statistically significant, though Ong noted that could have been because of the small sample size. There was also no significant difference between those who improved moderately vs slowly based on when a biologic was initiated.
It would be helpful to learn whether any of the patients in the rapid improvement group were able to stop medications or whether they all continued treatment during the 3 years of follow-up, Dr. Twilt said. “Does early treatment with biologics not only lead to early remission after initiation but also to the possibility of stopping treatment earlier and remaining in remission?” she asked.
The researchers also found that not all patients needed biologics to end up in the rapid improvement group. Among patients who never received any biologics during the 3-year period, 83% improved rapidly and 17% improved moderately. Yet the researchers identified no significant differences in demographics or clinical factors between patients who received biologics and those who did not.
“The fact that there is a group of patients in the rapid response group who never need a biologic is of great interest, as we always want to treat patients early with the medications they need, but we also want to avoid overtreating patients,” Dr. Twilt said. It’s important to find out what differentiates those patients and whether it is possible to predict which patients do not need biologics early on, she said.
Dr. Ong said the research team is working to develop machine learning methods to improve risk stratification in hopes of addressing that question.
Dr. Ong and Dr. Twilt reported no disclosures. The research was funded by CARRA and the Patient-Centered Outcomes Research Institute.
A version of this article appeared on Medscape.com .
CORRECTED April 16, 2024 // An earlier version of this article stated incorrect percentages of patients who never received any biologics during the study's 3-year period but improved rapidly or moderately.
Early initiation of biologics — within the first 2 months of symptom presentation — appears to have a significant impact on how rapidly patients with juvenile idiopathic arthritis (JIA) improve, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Our study provides evidence that early use of biologics can significantly alter the disease trajectory of patients with JIA,” Mei-Sing Ong, PhD, of Harvard Medical School, Boston, told attendees. At the same time, however, not all patients who improved rapidly during a 3-year follow-up period needed biologics, a finding that Ong said the researchers are continuing to investigate.
Marinka Twilt, MD, MScE, PhD, chair of CARRA’s JIA Research Committee and a pediatric rheumatologist and clinician scientist at Alberta Children’s Hospital in Calgary, Canada, was not involved in the research but said the continued sustained remission in patients who improved rapidly is very reassuring.
“We always wonder if initial response will be sustained or if patients tend to flare after the initial treatment,” Dr. Twilt told this news organization. “To see the sustained response up to 3 years is fantastic.” She added that it would be enlightening to see more information about patients who rapidly improved over 3 years, including whether they were still taking a [conventional disease-modifying antirheumatic drug (DMARD)] and/or biologic.
“A new diagnosis can be overwhelming for families, and this sometimes leads to step-up therapy to not overwhelm them more with information on new drugs,” Dr. Twilt said. “This study shows that an earlier start is beneficial, and this should be discussed with families early on so there is less delay in early treatment.”
Canada and many US states currently require 3 months of conventional DMARD treatment before patients can start a biologic, Dr. Twilt said, yet “this study shows the additive benefit of using a biologic within 2 months of starting a DMARD, which hopefully will lead to insurance companies adopting this threshold.”
The STOP-JIA study is a prospective observational study that compares the effectiveness of three different treatment plans for JIA. A Step-Up cohort of 257 patients received conventional antirheumatic monotherapy initially, with a biologic added at 3 months or later as needed. The Early Combination cohort of 100 patients received conventional antirheumatic therapy with a biologic from the start. The Biologic First cohort of 43 patients began taking a biologic as a first-line therapy.
In previously reported results of the study at 12 months’ follow-up, there was no significant difference between the Step-Up and Biologic First groups, but there were significant differences between the Step-Up and Early Combination groups. Significantly more patients in the Early Combination group (58.8%) than in the Step-Up group (42.8%) had inactive disease, based on the clinical Juvenile Arthritis Disease Activity Score 10 (cJADAS-10) (P = .03). Similarly, 81% of Early Combination patients achieved the American College of Rheumatology 70% improvement criteria, compared with 62% of the Step-Up patients (P = .01).
To learn whether the timing of starting a biologic influenced the disease trajectory over time, the researchers compared subgroups of patients with similar trajectories.
“Assessing treatment outcomes at a single point in time does not give us a complete picture of the effects of treatment on disease trajectory, which is an important outcome given that JIA is characterized by a relapsing-remitting course,” Dr. Ong told attendees.
Patients were sorted in the slow, moderate, or rapid improvement trajectories. In previously reported data at 12 months’ follow-up, patients’ odds of achieving rapid improvement were 3.6 times greater if they had started a biologic within 3 months.
This study compared patients’ trajectories over 3 years in the 259 patients (65% of the original cohort) who had at least one cJADAS-10 assessment in each year of follow-up. Most patients (66.8%) were in the rapid improvement class, with 25.9% in the moderate improvement class and 7.3% in the slow improvement class.
Patients in the rapid improvement group achieved inactive disease (cJADAS-10 of 2.5 or less) within 1 year and maintained inactive disease through the second and third years. The moderate and low improvement groups both had higher disease activity at baseline, but the moderate group continued to improve in years 2 and 3, with minimal disease by year 3, on the basis of the cJADAS-10 scores of 2.5-5. The slow group continued to experience moderate disease activity during years 2 and 3.
The findings also revealed that the earlier patients began a biologic, the more likely they were to be in the rapid improvement group than the slow improvement group. Participants who started a biologic in the first month had more than five times greater odds of being in the rapid improvement group than in the slow improvement group (odds ratio [OR], 5.33; P = .017).
Those who started a biologic in the second month were also more likely to be in the rapid improvement group (OR, 2.67; P = .032). For those who began a biologic by the third month, the odds of improving rapidly were not statistically significant, though Ong noted that could have been because of the small sample size. There was also no significant difference between those who improved moderately vs slowly based on when a biologic was initiated.
It would be helpful to learn whether any of the patients in the rapid improvement group were able to stop medications or whether they all continued treatment during the 3 years of follow-up, Dr. Twilt said. “Does early treatment with biologics not only lead to early remission after initiation but also to the possibility of stopping treatment earlier and remaining in remission?” she asked.
The researchers also found that not all patients needed biologics to end up in the rapid improvement group. Among patients who never received any biologics during the 3-year period, 83% improved rapidly and 17% improved moderately. Yet the researchers identified no significant differences in demographics or clinical factors between patients who received biologics and those who did not.
“The fact that there is a group of patients in the rapid response group who never need a biologic is of great interest, as we always want to treat patients early with the medications they need, but we also want to avoid overtreating patients,” Dr. Twilt said. It’s important to find out what differentiates those patients and whether it is possible to predict which patients do not need biologics early on, she said.
Dr. Ong said the research team is working to develop machine learning methods to improve risk stratification in hopes of addressing that question.
Dr. Ong and Dr. Twilt reported no disclosures. The research was funded by CARRA and the Patient-Centered Outcomes Research Institute.
A version of this article appeared on Medscape.com .
CORRECTED April 16, 2024 // An earlier version of this article stated incorrect percentages of patients who never received any biologics during the study's 3-year period but improved rapidly or moderately.
Early initiation of biologics — within the first 2 months of symptom presentation — appears to have a significant impact on how rapidly patients with juvenile idiopathic arthritis (JIA) improve, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Our study provides evidence that early use of biologics can significantly alter the disease trajectory of patients with JIA,” Mei-Sing Ong, PhD, of Harvard Medical School, Boston, told attendees. At the same time, however, not all patients who improved rapidly during a 3-year follow-up period needed biologics, a finding that Ong said the researchers are continuing to investigate.
Marinka Twilt, MD, MScE, PhD, chair of CARRA’s JIA Research Committee and a pediatric rheumatologist and clinician scientist at Alberta Children’s Hospital in Calgary, Canada, was not involved in the research but said the continued sustained remission in patients who improved rapidly is very reassuring.
“We always wonder if initial response will be sustained or if patients tend to flare after the initial treatment,” Dr. Twilt told this news organization. “To see the sustained response up to 3 years is fantastic.” She added that it would be enlightening to see more information about patients who rapidly improved over 3 years, including whether they were still taking a [conventional disease-modifying antirheumatic drug (DMARD)] and/or biologic.
“A new diagnosis can be overwhelming for families, and this sometimes leads to step-up therapy to not overwhelm them more with information on new drugs,” Dr. Twilt said. “This study shows that an earlier start is beneficial, and this should be discussed with families early on so there is less delay in early treatment.”
Canada and many US states currently require 3 months of conventional DMARD treatment before patients can start a biologic, Dr. Twilt said, yet “this study shows the additive benefit of using a biologic within 2 months of starting a DMARD, which hopefully will lead to insurance companies adopting this threshold.”
The STOP-JIA study is a prospective observational study that compares the effectiveness of three different treatment plans for JIA. A Step-Up cohort of 257 patients received conventional antirheumatic monotherapy initially, with a biologic added at 3 months or later as needed. The Early Combination cohort of 100 patients received conventional antirheumatic therapy with a biologic from the start. The Biologic First cohort of 43 patients began taking a biologic as a first-line therapy.
In previously reported results of the study at 12 months’ follow-up, there was no significant difference between the Step-Up and Biologic First groups, but there were significant differences between the Step-Up and Early Combination groups. Significantly more patients in the Early Combination group (58.8%) than in the Step-Up group (42.8%) had inactive disease, based on the clinical Juvenile Arthritis Disease Activity Score 10 (cJADAS-10) (P = .03). Similarly, 81% of Early Combination patients achieved the American College of Rheumatology 70% improvement criteria, compared with 62% of the Step-Up patients (P = .01).
To learn whether the timing of starting a biologic influenced the disease trajectory over time, the researchers compared subgroups of patients with similar trajectories.
“Assessing treatment outcomes at a single point in time does not give us a complete picture of the effects of treatment on disease trajectory, which is an important outcome given that JIA is characterized by a relapsing-remitting course,” Dr. Ong told attendees.
Patients were sorted in the slow, moderate, or rapid improvement trajectories. In previously reported data at 12 months’ follow-up, patients’ odds of achieving rapid improvement were 3.6 times greater if they had started a biologic within 3 months.
This study compared patients’ trajectories over 3 years in the 259 patients (65% of the original cohort) who had at least one cJADAS-10 assessment in each year of follow-up. Most patients (66.8%) were in the rapid improvement class, with 25.9% in the moderate improvement class and 7.3% in the slow improvement class.
Patients in the rapid improvement group achieved inactive disease (cJADAS-10 of 2.5 or less) within 1 year and maintained inactive disease through the second and third years. The moderate and low improvement groups both had higher disease activity at baseline, but the moderate group continued to improve in years 2 and 3, with minimal disease by year 3, on the basis of the cJADAS-10 scores of 2.5-5. The slow group continued to experience moderate disease activity during years 2 and 3.
The findings also revealed that the earlier patients began a biologic, the more likely they were to be in the rapid improvement group than the slow improvement group. Participants who started a biologic in the first month had more than five times greater odds of being in the rapid improvement group than in the slow improvement group (odds ratio [OR], 5.33; P = .017).
Those who started a biologic in the second month were also more likely to be in the rapid improvement group (OR, 2.67; P = .032). For those who began a biologic by the third month, the odds of improving rapidly were not statistically significant, though Ong noted that could have been because of the small sample size. There was also no significant difference between those who improved moderately vs slowly based on when a biologic was initiated.
It would be helpful to learn whether any of the patients in the rapid improvement group were able to stop medications or whether they all continued treatment during the 3 years of follow-up, Dr. Twilt said. “Does early treatment with biologics not only lead to early remission after initiation but also to the possibility of stopping treatment earlier and remaining in remission?” she asked.
The researchers also found that not all patients needed biologics to end up in the rapid improvement group. Among patients who never received any biologics during the 3-year period, 83% improved rapidly and 17% improved moderately. Yet the researchers identified no significant differences in demographics or clinical factors between patients who received biologics and those who did not.
“The fact that there is a group of patients in the rapid response group who never need a biologic is of great interest, as we always want to treat patients early with the medications they need, but we also want to avoid overtreating patients,” Dr. Twilt said. It’s important to find out what differentiates those patients and whether it is possible to predict which patients do not need biologics early on, she said.
Dr. Ong said the research team is working to develop machine learning methods to improve risk stratification in hopes of addressing that question.
Dr. Ong and Dr. Twilt reported no disclosures. The research was funded by CARRA and the Patient-Centered Outcomes Research Institute.
A version of this article appeared on Medscape.com .
FROM CARRA 2024
Linaclotide Succeeds for Functional Constipation in Children
, according to data from 330 individuals.
“Functional constipation is prevalent in pediatrics and is associated with chronic burdensome symptoms and impaired quality of life with an unmet need for treatment options for this age group,” corresponding study author Julie Khlevner, MD, AGAF, a pediatric gastroenterologist at Columbia University Vagelos College of Physicians and Surgeons, New York, said in an interview.
“Linaclotide has been approved for adults with chronic idiopathic constipation and irritable bowel syndrome with constipation, but its efficacy and safety in pediatric patients were unknown. Therefore, evaluating its use in this population was crucial to provide evidence-based treatment option,” she said.
In a study published in The Lancet Gastroenterology & Hepatology, the researchers randomized 166 pediatric patients with functional constipation to 72 micrograms of linaclotide once daily for 12 weeks and 164 to a placebo. The study was conducted at 64 clinic or hospital sites across 7 countries between October 1, 2019, and March 21, 2022. Approximately half (55%) of the patients were female.
The primary outcome was a change from baseline to 12 weeks in the frequency of spontaneous bowel movements (SBMs) per week, with no rescue medication on the day of or before the bowel movement. The secondary endpoint was change in stool consistency from baseline to 12 weeks. The mean frequency for SBMs at baseline was 1.16 per week in patients randomized to linaclotide and 1.28 for those randomized to placebo; these rates increased to 3.41 and 2.29, respectively, over the study period. The linaclotide patients showed a significantly greater improvement over placebo patients based on least-squares mean change from baseline (2.22 vs. 1.05, P = .0001).
In a subgroup analysis by age, the response was stronger in younger patients aged 6-11 years than in those aged 12-17 years, the researchers noted. This difference might stem from different pathophysiological mechanisms between older and younger ages, such as withholding behavior, they added.
Linaclotide was well tolerated overall; the most frequently reported treatment-emergent events were diarrhea (seven linaclotide patients and three placebo patients). In addition, five linaclotide patients and four placebo patients developed COVID-19 during treatment. No deaths occurred during the study, but one serious adverse event involving severe diarrhea, dehydration, and hospitalization, occurred in a 17-year-old female patient, but resolved after administration of intravenous fluids, the researchers noted.
Clinical Implications and Next Steps
The study findings reflect previous research on linaclotide in adults, Dr. Khlevner said. “The significant improvement in spontaneous bowel movements frequency and stool consistency with linaclotide compared to placebo is consistent with its mechanism of action as a guanylate cyclase C agonist,” she noted.
In clinical practice, barriers to the use of linaclotide may include lack of awareness of linaclotide’s safety and efficacy profile, and of its Food and Drug Administration approval for use in children aged 6-17 years with functional constipation, said Dr. Khlevner. “Additionally, access to the medication and insurance coverage may be potential barriers for some patients.” However, “some of these barriers can be overcome through education and training of healthcare providers regarding the appropriate use of linaclotide in pediatric patients with functional constipation,” she added.
The findings were limited by several factors including potential measurement bias and selection bias, lack of assessment of lifestyle modifications as confounding factors, and lack of quality-of-life assessment, the researchers noted. Other limitations included the relatively short 12-week treatment duration, which may not fully capture long-term safety and efficacy, and the focus on patients aged 6-17 years, Dr. Khlevner told this news organization.
“Future research could address these limitations through longer-term studies with broader age ranges and incorporating patient-reported outcomes in real world situations to assess the overall impact of linaclotide treatment on pediatric patients with functional constipation,” she said.
Study Supports Noninvasive Treatment Option
An alternative medication for children with functional constipation who do not respond to current therapies could prevent the use of more invasive interventions such as frequent enemas or antegrade enemas, Stephen M. Borowitz, MD, professor of pediatrics at the University of Virginia, Charlottesville, said in an interview.
Dr. Borowitz said he was not surprised by study findings. “Given the mechanism of action of the drug, I would expect the majority of children with functional constipation to respond in the sense of having more frequent and softer stools,” he said. “The bigger question, which wasn’t answered, is whether children who fail more conservative therapies respond to linaclotide,” said Dr. Borowitz, who was not involved in the study. “This was a phase 3 trial of otherwise healthy children with functional constipation and we know the majority of these children will respond to aggressive management with osmotic stool softeners, plus or minus a stimulant like senna coupled with lifestyle modifications (such as drinking more fluid, regular toileting, and appropriate toileting behaviors),” he said.
The greatest short-term barrier to the expanded use of linaclotide in clinical practice will likely be cost, and whether insurance will cover the drug, Dr. Borowitz told this news organization. Insurance coverage may not be an option until the child has failed more conservative, less expensive therapies, he said.
Also, the current study was a placebo-controlled trial, and not a comparison between linaclotide and polyethylene glycol, plus or minus senna, with other routine interventions, he said.
Looking ahead, “now that we know linaclotide is better than placebo, we need to know if it is as good, better, or worse than other proven interventions, and perhaps even more importantly, is it effective among children who have failed more conservative management,” Dr. Borowitz said. “We also need to know long-term risks, and given that the majority of childhood constipation develops before age 6 years, whether the drug can be used in younger children,” he emphasized. If so, studies need to examine whether linaclotide alters the natural history of the problem, he added. Previous studies suggest that the longer the symptom goes on, the harder it is to undo the secondary behaviors that result, such as withholding, pelvic floor dysfunction, and toileting refusal, he noted.
The study was supported by AbbVie and Ironwood Pharmaceuticals. The lead author, Carlo Di Lorenzo, MD, disclosed consulting fees from AbbVie, Ironwood Pharmaceuticals, Mallinckrodt, NeurAxis, QOL Medical, and Takeda. Dr. Khlevner disclosed honoraria from Abbott Pediatric Nutrition and participation on a data safety monitoring board and advisory board for AbbVie. Dr. Borowitz had no financial conflicts to disclose.
, according to data from 330 individuals.
“Functional constipation is prevalent in pediatrics and is associated with chronic burdensome symptoms and impaired quality of life with an unmet need for treatment options for this age group,” corresponding study author Julie Khlevner, MD, AGAF, a pediatric gastroenterologist at Columbia University Vagelos College of Physicians and Surgeons, New York, said in an interview.
“Linaclotide has been approved for adults with chronic idiopathic constipation and irritable bowel syndrome with constipation, but its efficacy and safety in pediatric patients were unknown. Therefore, evaluating its use in this population was crucial to provide evidence-based treatment option,” she said.
In a study published in The Lancet Gastroenterology & Hepatology, the researchers randomized 166 pediatric patients with functional constipation to 72 micrograms of linaclotide once daily for 12 weeks and 164 to a placebo. The study was conducted at 64 clinic or hospital sites across 7 countries between October 1, 2019, and March 21, 2022. Approximately half (55%) of the patients were female.
The primary outcome was a change from baseline to 12 weeks in the frequency of spontaneous bowel movements (SBMs) per week, with no rescue medication on the day of or before the bowel movement. The secondary endpoint was change in stool consistency from baseline to 12 weeks. The mean frequency for SBMs at baseline was 1.16 per week in patients randomized to linaclotide and 1.28 for those randomized to placebo; these rates increased to 3.41 and 2.29, respectively, over the study period. The linaclotide patients showed a significantly greater improvement over placebo patients based on least-squares mean change from baseline (2.22 vs. 1.05, P = .0001).
In a subgroup analysis by age, the response was stronger in younger patients aged 6-11 years than in those aged 12-17 years, the researchers noted. This difference might stem from different pathophysiological mechanisms between older and younger ages, such as withholding behavior, they added.
Linaclotide was well tolerated overall; the most frequently reported treatment-emergent events were diarrhea (seven linaclotide patients and three placebo patients). In addition, five linaclotide patients and four placebo patients developed COVID-19 during treatment. No deaths occurred during the study, but one serious adverse event involving severe diarrhea, dehydration, and hospitalization, occurred in a 17-year-old female patient, but resolved after administration of intravenous fluids, the researchers noted.
Clinical Implications and Next Steps
The study findings reflect previous research on linaclotide in adults, Dr. Khlevner said. “The significant improvement in spontaneous bowel movements frequency and stool consistency with linaclotide compared to placebo is consistent with its mechanism of action as a guanylate cyclase C agonist,” she noted.
In clinical practice, barriers to the use of linaclotide may include lack of awareness of linaclotide’s safety and efficacy profile, and of its Food and Drug Administration approval for use in children aged 6-17 years with functional constipation, said Dr. Khlevner. “Additionally, access to the medication and insurance coverage may be potential barriers for some patients.” However, “some of these barriers can be overcome through education and training of healthcare providers regarding the appropriate use of linaclotide in pediatric patients with functional constipation,” she added.
The findings were limited by several factors including potential measurement bias and selection bias, lack of assessment of lifestyle modifications as confounding factors, and lack of quality-of-life assessment, the researchers noted. Other limitations included the relatively short 12-week treatment duration, which may not fully capture long-term safety and efficacy, and the focus on patients aged 6-17 years, Dr. Khlevner told this news organization.
“Future research could address these limitations through longer-term studies with broader age ranges and incorporating patient-reported outcomes in real world situations to assess the overall impact of linaclotide treatment on pediatric patients with functional constipation,” she said.
Study Supports Noninvasive Treatment Option
An alternative medication for children with functional constipation who do not respond to current therapies could prevent the use of more invasive interventions such as frequent enemas or antegrade enemas, Stephen M. Borowitz, MD, professor of pediatrics at the University of Virginia, Charlottesville, said in an interview.
Dr. Borowitz said he was not surprised by study findings. “Given the mechanism of action of the drug, I would expect the majority of children with functional constipation to respond in the sense of having more frequent and softer stools,” he said. “The bigger question, which wasn’t answered, is whether children who fail more conservative therapies respond to linaclotide,” said Dr. Borowitz, who was not involved in the study. “This was a phase 3 trial of otherwise healthy children with functional constipation and we know the majority of these children will respond to aggressive management with osmotic stool softeners, plus or minus a stimulant like senna coupled with lifestyle modifications (such as drinking more fluid, regular toileting, and appropriate toileting behaviors),” he said.
The greatest short-term barrier to the expanded use of linaclotide in clinical practice will likely be cost, and whether insurance will cover the drug, Dr. Borowitz told this news organization. Insurance coverage may not be an option until the child has failed more conservative, less expensive therapies, he said.
Also, the current study was a placebo-controlled trial, and not a comparison between linaclotide and polyethylene glycol, plus or minus senna, with other routine interventions, he said.
Looking ahead, “now that we know linaclotide is better than placebo, we need to know if it is as good, better, or worse than other proven interventions, and perhaps even more importantly, is it effective among children who have failed more conservative management,” Dr. Borowitz said. “We also need to know long-term risks, and given that the majority of childhood constipation develops before age 6 years, whether the drug can be used in younger children,” he emphasized. If so, studies need to examine whether linaclotide alters the natural history of the problem, he added. Previous studies suggest that the longer the symptom goes on, the harder it is to undo the secondary behaviors that result, such as withholding, pelvic floor dysfunction, and toileting refusal, he noted.
The study was supported by AbbVie and Ironwood Pharmaceuticals. The lead author, Carlo Di Lorenzo, MD, disclosed consulting fees from AbbVie, Ironwood Pharmaceuticals, Mallinckrodt, NeurAxis, QOL Medical, and Takeda. Dr. Khlevner disclosed honoraria from Abbott Pediatric Nutrition and participation on a data safety monitoring board and advisory board for AbbVie. Dr. Borowitz had no financial conflicts to disclose.
, according to data from 330 individuals.
“Functional constipation is prevalent in pediatrics and is associated with chronic burdensome symptoms and impaired quality of life with an unmet need for treatment options for this age group,” corresponding study author Julie Khlevner, MD, AGAF, a pediatric gastroenterologist at Columbia University Vagelos College of Physicians and Surgeons, New York, said in an interview.
“Linaclotide has been approved for adults with chronic idiopathic constipation and irritable bowel syndrome with constipation, but its efficacy and safety in pediatric patients were unknown. Therefore, evaluating its use in this population was crucial to provide evidence-based treatment option,” she said.
In a study published in The Lancet Gastroenterology & Hepatology, the researchers randomized 166 pediatric patients with functional constipation to 72 micrograms of linaclotide once daily for 12 weeks and 164 to a placebo. The study was conducted at 64 clinic or hospital sites across 7 countries between October 1, 2019, and March 21, 2022. Approximately half (55%) of the patients were female.
The primary outcome was a change from baseline to 12 weeks in the frequency of spontaneous bowel movements (SBMs) per week, with no rescue medication on the day of or before the bowel movement. The secondary endpoint was change in stool consistency from baseline to 12 weeks. The mean frequency for SBMs at baseline was 1.16 per week in patients randomized to linaclotide and 1.28 for those randomized to placebo; these rates increased to 3.41 and 2.29, respectively, over the study period. The linaclotide patients showed a significantly greater improvement over placebo patients based on least-squares mean change from baseline (2.22 vs. 1.05, P = .0001).
In a subgroup analysis by age, the response was stronger in younger patients aged 6-11 years than in those aged 12-17 years, the researchers noted. This difference might stem from different pathophysiological mechanisms between older and younger ages, such as withholding behavior, they added.
Linaclotide was well tolerated overall; the most frequently reported treatment-emergent events were diarrhea (seven linaclotide patients and three placebo patients). In addition, five linaclotide patients and four placebo patients developed COVID-19 during treatment. No deaths occurred during the study, but one serious adverse event involving severe diarrhea, dehydration, and hospitalization, occurred in a 17-year-old female patient, but resolved after administration of intravenous fluids, the researchers noted.
Clinical Implications and Next Steps
The study findings reflect previous research on linaclotide in adults, Dr. Khlevner said. “The significant improvement in spontaneous bowel movements frequency and stool consistency with linaclotide compared to placebo is consistent with its mechanism of action as a guanylate cyclase C agonist,” she noted.
In clinical practice, barriers to the use of linaclotide may include lack of awareness of linaclotide’s safety and efficacy profile, and of its Food and Drug Administration approval for use in children aged 6-17 years with functional constipation, said Dr. Khlevner. “Additionally, access to the medication and insurance coverage may be potential barriers for some patients.” However, “some of these barriers can be overcome through education and training of healthcare providers regarding the appropriate use of linaclotide in pediatric patients with functional constipation,” she added.
The findings were limited by several factors including potential measurement bias and selection bias, lack of assessment of lifestyle modifications as confounding factors, and lack of quality-of-life assessment, the researchers noted. Other limitations included the relatively short 12-week treatment duration, which may not fully capture long-term safety and efficacy, and the focus on patients aged 6-17 years, Dr. Khlevner told this news organization.
“Future research could address these limitations through longer-term studies with broader age ranges and incorporating patient-reported outcomes in real world situations to assess the overall impact of linaclotide treatment on pediatric patients with functional constipation,” she said.
Study Supports Noninvasive Treatment Option
An alternative medication for children with functional constipation who do not respond to current therapies could prevent the use of more invasive interventions such as frequent enemas or antegrade enemas, Stephen M. Borowitz, MD, professor of pediatrics at the University of Virginia, Charlottesville, said in an interview.
Dr. Borowitz said he was not surprised by study findings. “Given the mechanism of action of the drug, I would expect the majority of children with functional constipation to respond in the sense of having more frequent and softer stools,” he said. “The bigger question, which wasn’t answered, is whether children who fail more conservative therapies respond to linaclotide,” said Dr. Borowitz, who was not involved in the study. “This was a phase 3 trial of otherwise healthy children with functional constipation and we know the majority of these children will respond to aggressive management with osmotic stool softeners, plus or minus a stimulant like senna coupled with lifestyle modifications (such as drinking more fluid, regular toileting, and appropriate toileting behaviors),” he said.
The greatest short-term barrier to the expanded use of linaclotide in clinical practice will likely be cost, and whether insurance will cover the drug, Dr. Borowitz told this news organization. Insurance coverage may not be an option until the child has failed more conservative, less expensive therapies, he said.
Also, the current study was a placebo-controlled trial, and not a comparison between linaclotide and polyethylene glycol, plus or minus senna, with other routine interventions, he said.
Looking ahead, “now that we know linaclotide is better than placebo, we need to know if it is as good, better, or worse than other proven interventions, and perhaps even more importantly, is it effective among children who have failed more conservative management,” Dr. Borowitz said. “We also need to know long-term risks, and given that the majority of childhood constipation develops before age 6 years, whether the drug can be used in younger children,” he emphasized. If so, studies need to examine whether linaclotide alters the natural history of the problem, he added. Previous studies suggest that the longer the symptom goes on, the harder it is to undo the secondary behaviors that result, such as withholding, pelvic floor dysfunction, and toileting refusal, he noted.
The study was supported by AbbVie and Ironwood Pharmaceuticals. The lead author, Carlo Di Lorenzo, MD, disclosed consulting fees from AbbVie, Ironwood Pharmaceuticals, Mallinckrodt, NeurAxis, QOL Medical, and Takeda. Dr. Khlevner disclosed honoraria from Abbott Pediatric Nutrition and participation on a data safety monitoring board and advisory board for AbbVie. Dr. Borowitz had no financial conflicts to disclose.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY