User login
Family placement better for deprived kids than institutions
SAN FRANCISCO – results of a new study suggest.
The study shows that sustained recovery is possible after severe, early-life adversity, study author Kathryn L. Humphreys, PhD, assistant professor, department of psychology and human development, Vanderbilt University, Nashville, Tenn., said in an interview.
“Given the strong evidence from the present study, I hope physicians will play a role in promoting family placements as an alternative to institutional care for children who have been orphaned,” she said.
The findings were presented at the annual meeting of the American Psychiatric Association and were published online in the American Journal of Psychiatry.
Millions of children around the world experience psychosocial deprivation while living in institutions, and many more are neglected in their families of origin. In addition, about 6.7 million children lost a parent or caregiver during the COVID-19 pandemic.
In particular, Romania has a history of institutionalizing children. Through decades of repressive policies from the Romanian dictator Nicolae Ceausescu, child abandonment became a national disaster. Families couldn’t afford to keep their children and were encouraged to turn them over to the state.
The current study was part of the Bucharest Early Intervention Project, initiated in 2001 to examine the impact of high-quality, family-based care on development. It included 136 Romanian children (mean age, about 22 months) who were abandoned at or shortly after birth and were placed in an institution.
Researchers randomly assigned each toddler to 1 of 56 foster families or to continue living in an institution (care as usual). The researchers had to create a foster care network, because such care was extremely limited at the start of the study.
Providing stimulating care
Foster parents in the study received regular support from social workers and U.S.-based psychologists. They were encouraged to “make a commitment to treat the child as if it was their own, providing sensitive, stimulating, and nurturing care, not just in the short term but for their whole life,” said Dr. Humphreys.
Foster care programs in the United States have been criticized for focusing on short-term care, she said. “It’s really just a bed to sleep on, clothes to wear, and food to eat rather than the psychological component we think is really important for child development.”
For the study, the researchers assessed the children across multiple developmental domains at baseline and at ages 30, 42, and 54 months. They conducted additional assessments when the kids were aged 8, 12, and 16-18 years.
The primary outcomes were cognitive functioning (IQ), physical growth (height, weight, head circumference), brain electrical activity (relative electroencephalography power in the alpha frequency band), and symptoms of five types of psychopathology (disinhibited social engagement disorder, reactive attachment disorder, ADHD symptoms, externalizing symptoms, and internalizing symptoms).
From over 7,000 observations analyzed across follow-ups, the investigators found that the intervention had an overall significant effect on cognitive, physical, and neural outcomes when considered collectively across waves (beta, 0.26; 95% confidence interval, 0.07-0.46; P = .012). Compared to children who received care as usual, those in foster homes had significantly higher average IQ scores (P < .001) and physical size (P = .008).
The intervention had an overall beneficial effect in regard to psychopathology. The greatest impact involved a reduction in symptoms of reactive attachment disorder (P < .001).
“There are a few forms of psychopathology that seem to almost entirely occur after severe neglect, including reactive attachment disorder; we think of these as disorders of social relatedness that derive from aberrant or insufficient early caregiving experiences,” said Dr. Humphreys. “Being placed in a family reduced the symptoms of reactive attachment disorder to pretty much nonexistent.”
To a lesser extent, the intervention reduced symptoms of disinhibited social engagement disorder. The foster care group also had significantly fewer internalizing symptoms than did children in the care-as-usual group.
But there was no significant overall effect of the intervention on symptoms of ADHD or externalizing problems.
Positive effects persisted
For the most part, the positive effects of the intervention on children’s functioning persisted during nearly 2 decades of follow-up. The impact of the intervention “can be described as rapidly apparent by age 30 months and sustained through late adolescence,” wrote the authors.
Regarding the impact of age at the time of placement, the study found that, compared with children placed into foster care later, those who entered foster care earlier (younger than 33 months) had significantly higher IQ scores and relative alpha power, but there was no difference in physical growth.
For some outcomes, the benefits of earlier placement were apparent in early childhood but faded by adolescence. But Dr. Humphreys noted all placements were early by most definitions.
The researchers also assessed stability of foster care placements. Children were considered “stable” if they remained with their original foster family; they were considered “disrupted” if they no longer resided with the family.
Here, the study found some “striking results,” said Dr. Humphreys. The effect of placement stability was largest in adolescence, when, overall, those who had remained with their original foster family had better cognitive and physical outcomes and less severe symptoms of psychopathology compared to those who experienced placement disruptions.
As for sex differences, “it’s a mixed bag,” said Dr. Humphreys, although overall, “we didn’t see strong evidence of sex differences” in terms of outcomes.
The investigators were unable to examine trajectories of children’s functioning, which would have provided important information on aspects such as rate of growth and the shape of growth curves. Specific features of the institutional or foster care environment in Bucharest during the study may limit the generalizability of the findings to other settings.
Absolutely unique project
The study examined an “absolutely unique project” and had “very exciting” results that should have “important clinical implications,” commented the American Journal of Psychiatry editor-in-chief Ned Kalin, MD, Hedberg Professor and chair, department of psychiatry, University of Wisconsin–Madison.
The findings are “pretty dramatic,” added Dr. Kalin. “This is probably the study to be thinking about when considering the future of treatment and interventions in children who have suffered from this type of neglect, which is unfortunately extremely common worldwide, including in the U.S.”
In particular, the findings regarding improved psychopathology “bode well for the future,” said Dr. Kalin. “We know these types of problems are risk factors for the later development of depression and anxiety disorders. It will be really interesting to find out, but my guess is these kids will be protected as they mature further.”
The study was supported by the NIH, the John D. and Catherine T. MacArthur Foundation, the Palix Foundation, and the Jacobs Foundation. Dr. Humphreys has received research funding from the Brain and Behavior Research Foundation, the Caplan Foundation, the Jacobs Foundation, the National Science Foundation, the NIH, the Vanderbilt Institute for Clinical and Translational Research, the Vanderbilt Kennedy Center, and Vanderbilt University; she has received honoraria from the Journal of Clinical Child and Adolescent Psychology Future Directions Forum, Learning Grove, the University of Iowa, the University of Texas at Austin, and ZERO TO THREE.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – results of a new study suggest.
The study shows that sustained recovery is possible after severe, early-life adversity, study author Kathryn L. Humphreys, PhD, assistant professor, department of psychology and human development, Vanderbilt University, Nashville, Tenn., said in an interview.
“Given the strong evidence from the present study, I hope physicians will play a role in promoting family placements as an alternative to institutional care for children who have been orphaned,” she said.
The findings were presented at the annual meeting of the American Psychiatric Association and were published online in the American Journal of Psychiatry.
Millions of children around the world experience psychosocial deprivation while living in institutions, and many more are neglected in their families of origin. In addition, about 6.7 million children lost a parent or caregiver during the COVID-19 pandemic.
In particular, Romania has a history of institutionalizing children. Through decades of repressive policies from the Romanian dictator Nicolae Ceausescu, child abandonment became a national disaster. Families couldn’t afford to keep their children and were encouraged to turn them over to the state.
The current study was part of the Bucharest Early Intervention Project, initiated in 2001 to examine the impact of high-quality, family-based care on development. It included 136 Romanian children (mean age, about 22 months) who were abandoned at or shortly after birth and were placed in an institution.
Researchers randomly assigned each toddler to 1 of 56 foster families or to continue living in an institution (care as usual). The researchers had to create a foster care network, because such care was extremely limited at the start of the study.
Providing stimulating care
Foster parents in the study received regular support from social workers and U.S.-based psychologists. They were encouraged to “make a commitment to treat the child as if it was their own, providing sensitive, stimulating, and nurturing care, not just in the short term but for their whole life,” said Dr. Humphreys.
Foster care programs in the United States have been criticized for focusing on short-term care, she said. “It’s really just a bed to sleep on, clothes to wear, and food to eat rather than the psychological component we think is really important for child development.”
For the study, the researchers assessed the children across multiple developmental domains at baseline and at ages 30, 42, and 54 months. They conducted additional assessments when the kids were aged 8, 12, and 16-18 years.
The primary outcomes were cognitive functioning (IQ), physical growth (height, weight, head circumference), brain electrical activity (relative electroencephalography power in the alpha frequency band), and symptoms of five types of psychopathology (disinhibited social engagement disorder, reactive attachment disorder, ADHD symptoms, externalizing symptoms, and internalizing symptoms).
From over 7,000 observations analyzed across follow-ups, the investigators found that the intervention had an overall significant effect on cognitive, physical, and neural outcomes when considered collectively across waves (beta, 0.26; 95% confidence interval, 0.07-0.46; P = .012). Compared to children who received care as usual, those in foster homes had significantly higher average IQ scores (P < .001) and physical size (P = .008).
The intervention had an overall beneficial effect in regard to psychopathology. The greatest impact involved a reduction in symptoms of reactive attachment disorder (P < .001).
“There are a few forms of psychopathology that seem to almost entirely occur after severe neglect, including reactive attachment disorder; we think of these as disorders of social relatedness that derive from aberrant or insufficient early caregiving experiences,” said Dr. Humphreys. “Being placed in a family reduced the symptoms of reactive attachment disorder to pretty much nonexistent.”
To a lesser extent, the intervention reduced symptoms of disinhibited social engagement disorder. The foster care group also had significantly fewer internalizing symptoms than did children in the care-as-usual group.
But there was no significant overall effect of the intervention on symptoms of ADHD or externalizing problems.
Positive effects persisted
For the most part, the positive effects of the intervention on children’s functioning persisted during nearly 2 decades of follow-up. The impact of the intervention “can be described as rapidly apparent by age 30 months and sustained through late adolescence,” wrote the authors.
Regarding the impact of age at the time of placement, the study found that, compared with children placed into foster care later, those who entered foster care earlier (younger than 33 months) had significantly higher IQ scores and relative alpha power, but there was no difference in physical growth.
For some outcomes, the benefits of earlier placement were apparent in early childhood but faded by adolescence. But Dr. Humphreys noted all placements were early by most definitions.
The researchers also assessed stability of foster care placements. Children were considered “stable” if they remained with their original foster family; they were considered “disrupted” if they no longer resided with the family.
Here, the study found some “striking results,” said Dr. Humphreys. The effect of placement stability was largest in adolescence, when, overall, those who had remained with their original foster family had better cognitive and physical outcomes and less severe symptoms of psychopathology compared to those who experienced placement disruptions.
As for sex differences, “it’s a mixed bag,” said Dr. Humphreys, although overall, “we didn’t see strong evidence of sex differences” in terms of outcomes.
The investigators were unable to examine trajectories of children’s functioning, which would have provided important information on aspects such as rate of growth and the shape of growth curves. Specific features of the institutional or foster care environment in Bucharest during the study may limit the generalizability of the findings to other settings.
Absolutely unique project
The study examined an “absolutely unique project” and had “very exciting” results that should have “important clinical implications,” commented the American Journal of Psychiatry editor-in-chief Ned Kalin, MD, Hedberg Professor and chair, department of psychiatry, University of Wisconsin–Madison.
The findings are “pretty dramatic,” added Dr. Kalin. “This is probably the study to be thinking about when considering the future of treatment and interventions in children who have suffered from this type of neglect, which is unfortunately extremely common worldwide, including in the U.S.”
In particular, the findings regarding improved psychopathology “bode well for the future,” said Dr. Kalin. “We know these types of problems are risk factors for the later development of depression and anxiety disorders. It will be really interesting to find out, but my guess is these kids will be protected as they mature further.”
The study was supported by the NIH, the John D. and Catherine T. MacArthur Foundation, the Palix Foundation, and the Jacobs Foundation. Dr. Humphreys has received research funding from the Brain and Behavior Research Foundation, the Caplan Foundation, the Jacobs Foundation, the National Science Foundation, the NIH, the Vanderbilt Institute for Clinical and Translational Research, the Vanderbilt Kennedy Center, and Vanderbilt University; she has received honoraria from the Journal of Clinical Child and Adolescent Psychology Future Directions Forum, Learning Grove, the University of Iowa, the University of Texas at Austin, and ZERO TO THREE.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – results of a new study suggest.
The study shows that sustained recovery is possible after severe, early-life adversity, study author Kathryn L. Humphreys, PhD, assistant professor, department of psychology and human development, Vanderbilt University, Nashville, Tenn., said in an interview.
“Given the strong evidence from the present study, I hope physicians will play a role in promoting family placements as an alternative to institutional care for children who have been orphaned,” she said.
The findings were presented at the annual meeting of the American Psychiatric Association and were published online in the American Journal of Psychiatry.
Millions of children around the world experience psychosocial deprivation while living in institutions, and many more are neglected in their families of origin. In addition, about 6.7 million children lost a parent or caregiver during the COVID-19 pandemic.
In particular, Romania has a history of institutionalizing children. Through decades of repressive policies from the Romanian dictator Nicolae Ceausescu, child abandonment became a national disaster. Families couldn’t afford to keep their children and were encouraged to turn them over to the state.
The current study was part of the Bucharest Early Intervention Project, initiated in 2001 to examine the impact of high-quality, family-based care on development. It included 136 Romanian children (mean age, about 22 months) who were abandoned at or shortly after birth and were placed in an institution.
Researchers randomly assigned each toddler to 1 of 56 foster families or to continue living in an institution (care as usual). The researchers had to create a foster care network, because such care was extremely limited at the start of the study.
Providing stimulating care
Foster parents in the study received regular support from social workers and U.S.-based psychologists. They were encouraged to “make a commitment to treat the child as if it was their own, providing sensitive, stimulating, and nurturing care, not just in the short term but for their whole life,” said Dr. Humphreys.
Foster care programs in the United States have been criticized for focusing on short-term care, she said. “It’s really just a bed to sleep on, clothes to wear, and food to eat rather than the psychological component we think is really important for child development.”
For the study, the researchers assessed the children across multiple developmental domains at baseline and at ages 30, 42, and 54 months. They conducted additional assessments when the kids were aged 8, 12, and 16-18 years.
The primary outcomes were cognitive functioning (IQ), physical growth (height, weight, head circumference), brain electrical activity (relative electroencephalography power in the alpha frequency band), and symptoms of five types of psychopathology (disinhibited social engagement disorder, reactive attachment disorder, ADHD symptoms, externalizing symptoms, and internalizing symptoms).
From over 7,000 observations analyzed across follow-ups, the investigators found that the intervention had an overall significant effect on cognitive, physical, and neural outcomes when considered collectively across waves (beta, 0.26; 95% confidence interval, 0.07-0.46; P = .012). Compared to children who received care as usual, those in foster homes had significantly higher average IQ scores (P < .001) and physical size (P = .008).
The intervention had an overall beneficial effect in regard to psychopathology. The greatest impact involved a reduction in symptoms of reactive attachment disorder (P < .001).
“There are a few forms of psychopathology that seem to almost entirely occur after severe neglect, including reactive attachment disorder; we think of these as disorders of social relatedness that derive from aberrant or insufficient early caregiving experiences,” said Dr. Humphreys. “Being placed in a family reduced the symptoms of reactive attachment disorder to pretty much nonexistent.”
To a lesser extent, the intervention reduced symptoms of disinhibited social engagement disorder. The foster care group also had significantly fewer internalizing symptoms than did children in the care-as-usual group.
But there was no significant overall effect of the intervention on symptoms of ADHD or externalizing problems.
Positive effects persisted
For the most part, the positive effects of the intervention on children’s functioning persisted during nearly 2 decades of follow-up. The impact of the intervention “can be described as rapidly apparent by age 30 months and sustained through late adolescence,” wrote the authors.
Regarding the impact of age at the time of placement, the study found that, compared with children placed into foster care later, those who entered foster care earlier (younger than 33 months) had significantly higher IQ scores and relative alpha power, but there was no difference in physical growth.
For some outcomes, the benefits of earlier placement were apparent in early childhood but faded by adolescence. But Dr. Humphreys noted all placements were early by most definitions.
The researchers also assessed stability of foster care placements. Children were considered “stable” if they remained with their original foster family; they were considered “disrupted” if they no longer resided with the family.
Here, the study found some “striking results,” said Dr. Humphreys. The effect of placement stability was largest in adolescence, when, overall, those who had remained with their original foster family had better cognitive and physical outcomes and less severe symptoms of psychopathology compared to those who experienced placement disruptions.
As for sex differences, “it’s a mixed bag,” said Dr. Humphreys, although overall, “we didn’t see strong evidence of sex differences” in terms of outcomes.
The investigators were unable to examine trajectories of children’s functioning, which would have provided important information on aspects such as rate of growth and the shape of growth curves. Specific features of the institutional or foster care environment in Bucharest during the study may limit the generalizability of the findings to other settings.
Absolutely unique project
The study examined an “absolutely unique project” and had “very exciting” results that should have “important clinical implications,” commented the American Journal of Psychiatry editor-in-chief Ned Kalin, MD, Hedberg Professor and chair, department of psychiatry, University of Wisconsin–Madison.
The findings are “pretty dramatic,” added Dr. Kalin. “This is probably the study to be thinking about when considering the future of treatment and interventions in children who have suffered from this type of neglect, which is unfortunately extremely common worldwide, including in the U.S.”
In particular, the findings regarding improved psychopathology “bode well for the future,” said Dr. Kalin. “We know these types of problems are risk factors for the later development of depression and anxiety disorders. It will be really interesting to find out, but my guess is these kids will be protected as they mature further.”
The study was supported by the NIH, the John D. and Catherine T. MacArthur Foundation, the Palix Foundation, and the Jacobs Foundation. Dr. Humphreys has received research funding from the Brain and Behavior Research Foundation, the Caplan Foundation, the Jacobs Foundation, the National Science Foundation, the NIH, the Vanderbilt Institute for Clinical and Translational Research, the Vanderbilt Kennedy Center, and Vanderbilt University; she has received honoraria from the Journal of Clinical Child and Adolescent Psychology Future Directions Forum, Learning Grove, the University of Iowa, the University of Texas at Austin, and ZERO TO THREE.
A version of this article first appeared on Medscape.com.
AT APA 2023
Significant increase in vitamin D deficiency in kids with major depressive disorder
SAN FRANCISCO – , according to new findings that suggest spending more time indoors may have fueled this uptick.
“We suspect that this may be due to the COVID lockdowns and kids schooling from home and having less time outside,” study investigator Oluwatomiwa Babade, MD, MPH, with Virginia Tech Carilion School of Medicine, Roanoke, Va., said in an interview.
The study was presented at the annual meeting of the American Psychiatric Association.
Anecdotal observation confirmed
During the pandemic, investigators noticed an uptick in the number of children and adolescents attending their clinic for psychiatric hospitalization who had low vitamin D levels.
To investigate, they analyzed the records of all patients aged 6-17 years with psychiatric diagnoses and vitamin D level assessment who were admitted into the inpatient psychiatry unit from March 18, 2020, to June 30, 2021.
Among 599 unique patients, 275 (83% female) had a diagnosis of MDD and 226 of these patients were vitamin D deficient (< 30 ng/mL) – a prevalence rate of roughly 82%. Among 246 patients with psychiatric disorders other than MDD, the prevalence of vitamin D deficiency was 76%.
“This was very surprising and much higher than prior to the pandemic. Prior to COVID, the prevalence of vitamin D deficiency was around 14% in similar patients,” Dr. Babade said.
“Now that we are post-lockdown, it would be good to repeat the study. I think the prevalence should drop. That’s my guess,” he added.
Important research, no surprises
In a comment, Cemre Robinson, MD, director of the Mount Sinai Pediatric Bone Health and Calcium Metabolism Clinic, New York, said that although the study’s findings aren’t surprising, “it’s important to present such data in adolescents with major depression.”
“These findings reiterate the importance of screening for vitamin D deficiency in children and adolescents, with or without depression, particularly during winter, which is associated with less sun exposure,” Dr. Robinson, assistant professor of pediatrics, endocrinology, and diabetes at Icahn School of Medicine at Mount Sinai, said.
She noted that vitamin D deficiency is prevalent in the general population, and it can be easily corrected with supplementation.
“Vitamin D is important for bone growth, mineralization, and accretion as well as calcium absorption. Adolescence, in particular, is a period of rapid physical, cognitive, and psychosocial growth,” Dr. Robinson said.
“The requirement of all minerals and vitamins changes in this phase of life. Therefore, it is important to have sufficient vitamin D levels during adolescence for several health benefits,” she noted.
Dr. Robinson said that “more research is needed to validate the present findings in adolescents with major depression, and larger studies, including randomized control trials, are required to establish a causal association between MDD and vitamin D deficiency.”
The study had no specific funding. Dr. Babade and Dr. Robinson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , according to new findings that suggest spending more time indoors may have fueled this uptick.
“We suspect that this may be due to the COVID lockdowns and kids schooling from home and having less time outside,” study investigator Oluwatomiwa Babade, MD, MPH, with Virginia Tech Carilion School of Medicine, Roanoke, Va., said in an interview.
The study was presented at the annual meeting of the American Psychiatric Association.
Anecdotal observation confirmed
During the pandemic, investigators noticed an uptick in the number of children and adolescents attending their clinic for psychiatric hospitalization who had low vitamin D levels.
To investigate, they analyzed the records of all patients aged 6-17 years with psychiatric diagnoses and vitamin D level assessment who were admitted into the inpatient psychiatry unit from March 18, 2020, to June 30, 2021.
Among 599 unique patients, 275 (83% female) had a diagnosis of MDD and 226 of these patients were vitamin D deficient (< 30 ng/mL) – a prevalence rate of roughly 82%. Among 246 patients with psychiatric disorders other than MDD, the prevalence of vitamin D deficiency was 76%.
“This was very surprising and much higher than prior to the pandemic. Prior to COVID, the prevalence of vitamin D deficiency was around 14% in similar patients,” Dr. Babade said.
“Now that we are post-lockdown, it would be good to repeat the study. I think the prevalence should drop. That’s my guess,” he added.
Important research, no surprises
In a comment, Cemre Robinson, MD, director of the Mount Sinai Pediatric Bone Health and Calcium Metabolism Clinic, New York, said that although the study’s findings aren’t surprising, “it’s important to present such data in adolescents with major depression.”
“These findings reiterate the importance of screening for vitamin D deficiency in children and adolescents, with or without depression, particularly during winter, which is associated with less sun exposure,” Dr. Robinson, assistant professor of pediatrics, endocrinology, and diabetes at Icahn School of Medicine at Mount Sinai, said.
She noted that vitamin D deficiency is prevalent in the general population, and it can be easily corrected with supplementation.
“Vitamin D is important for bone growth, mineralization, and accretion as well as calcium absorption. Adolescence, in particular, is a period of rapid physical, cognitive, and psychosocial growth,” Dr. Robinson said.
“The requirement of all minerals and vitamins changes in this phase of life. Therefore, it is important to have sufficient vitamin D levels during adolescence for several health benefits,” she noted.
Dr. Robinson said that “more research is needed to validate the present findings in adolescents with major depression, and larger studies, including randomized control trials, are required to establish a causal association between MDD and vitamin D deficiency.”
The study had no specific funding. Dr. Babade and Dr. Robinson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , according to new findings that suggest spending more time indoors may have fueled this uptick.
“We suspect that this may be due to the COVID lockdowns and kids schooling from home and having less time outside,” study investigator Oluwatomiwa Babade, MD, MPH, with Virginia Tech Carilion School of Medicine, Roanoke, Va., said in an interview.
The study was presented at the annual meeting of the American Psychiatric Association.
Anecdotal observation confirmed
During the pandemic, investigators noticed an uptick in the number of children and adolescents attending their clinic for psychiatric hospitalization who had low vitamin D levels.
To investigate, they analyzed the records of all patients aged 6-17 years with psychiatric diagnoses and vitamin D level assessment who were admitted into the inpatient psychiatry unit from March 18, 2020, to June 30, 2021.
Among 599 unique patients, 275 (83% female) had a diagnosis of MDD and 226 of these patients were vitamin D deficient (< 30 ng/mL) – a prevalence rate of roughly 82%. Among 246 patients with psychiatric disorders other than MDD, the prevalence of vitamin D deficiency was 76%.
“This was very surprising and much higher than prior to the pandemic. Prior to COVID, the prevalence of vitamin D deficiency was around 14% in similar patients,” Dr. Babade said.
“Now that we are post-lockdown, it would be good to repeat the study. I think the prevalence should drop. That’s my guess,” he added.
Important research, no surprises
In a comment, Cemre Robinson, MD, director of the Mount Sinai Pediatric Bone Health and Calcium Metabolism Clinic, New York, said that although the study’s findings aren’t surprising, “it’s important to present such data in adolescents with major depression.”
“These findings reiterate the importance of screening for vitamin D deficiency in children and adolescents, with or without depression, particularly during winter, which is associated with less sun exposure,” Dr. Robinson, assistant professor of pediatrics, endocrinology, and diabetes at Icahn School of Medicine at Mount Sinai, said.
She noted that vitamin D deficiency is prevalent in the general population, and it can be easily corrected with supplementation.
“Vitamin D is important for bone growth, mineralization, and accretion as well as calcium absorption. Adolescence, in particular, is a period of rapid physical, cognitive, and psychosocial growth,” Dr. Robinson said.
“The requirement of all minerals and vitamins changes in this phase of life. Therefore, it is important to have sufficient vitamin D levels during adolescence for several health benefits,” she noted.
Dr. Robinson said that “more research is needed to validate the present findings in adolescents with major depression, and larger studies, including randomized control trials, are required to establish a causal association between MDD and vitamin D deficiency.”
The study had no specific funding. Dr. Babade and Dr. Robinson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT APA 2023
Child murder by parents: Toward prevention
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
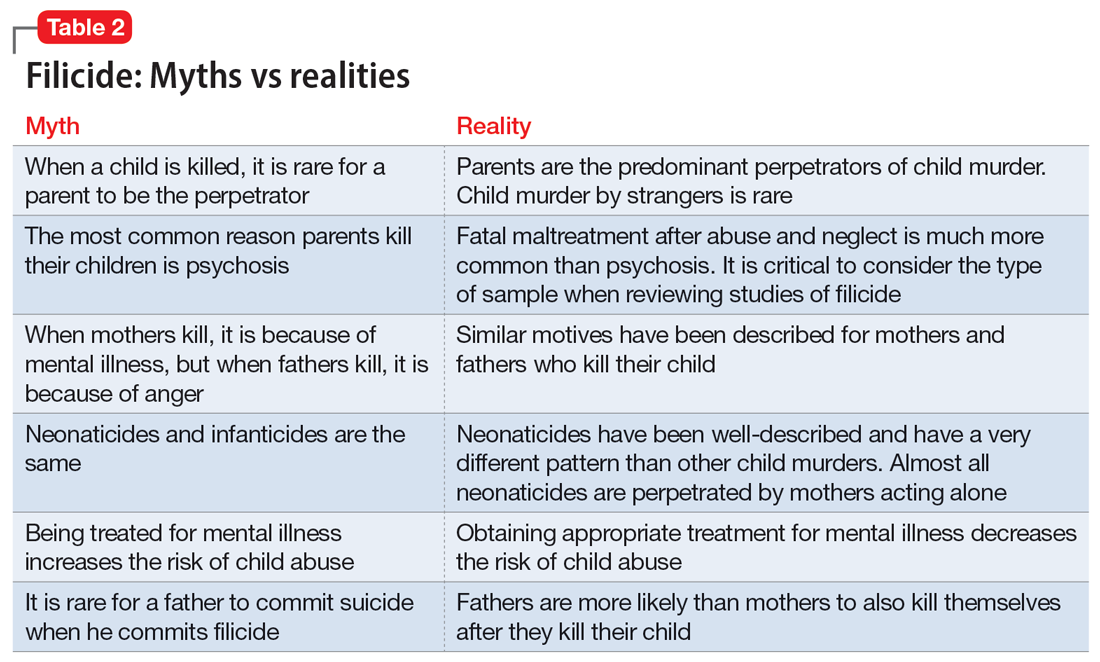
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21
Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
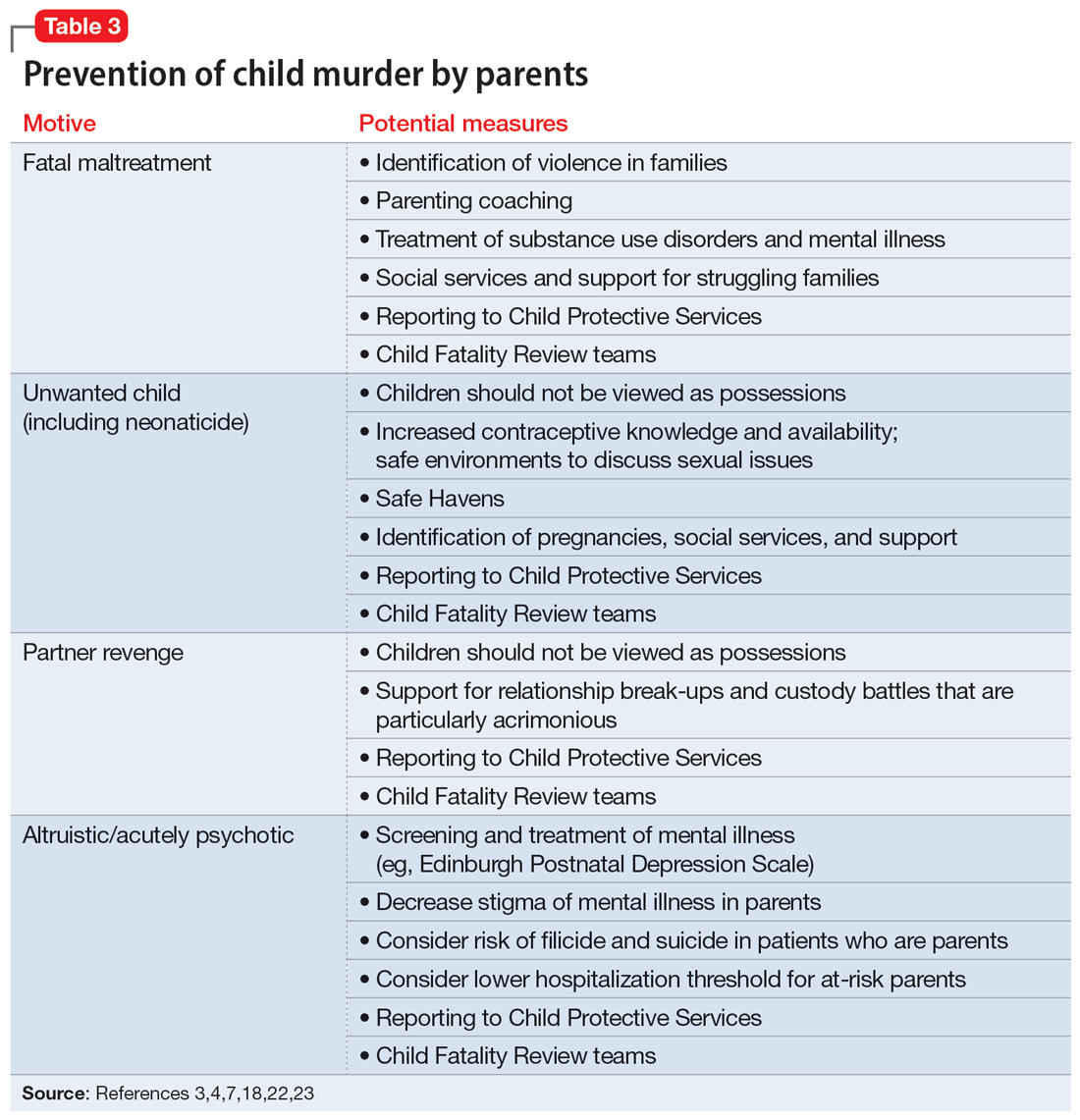
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.
Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21

Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.

Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21

Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.

Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
Is the contemporary mental health crisis among youth due to DMN disruption?
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
Pruritic Photosensitive Rash
The Diagnosis: Actinic Prurigo
Actinic prurigo is an idiopathic photodermatosis triggered by UV exposure that primarily affects sun-exposed areas of the skin.1,2 It typically presents as pruritic papules, plaques, and nodules, with most patients also experiencing oral tingling and pain.3 In more severe cases, it can progress to include conjunctival disease, scarring, and cheilitis.1 A study of ocular findings among children with actinic pruritus reported that photophobia was one of the most important features,4 which was present in our patient. The face, especially over the zygomatic arches, nasal bridge, and lower lip, commonly is affected.1 Secondary lichenification or eczematization may occur.5 In our patient, the combination of conjunctivitis, cheilitis, and an eruption on sun-exposed skin were crucial in making the diagnosis.
Most cases present in patients younger than 10 years. It most commonly is seen in American Indians in North America, Central America, and South America.2 After the diagnosis was considered in our patient, the family was asked about their ancestry and confirmed that both of the patient’s maternal and paternal grandparents were of American Indian descent. There also is a strong genetic component; the HLA-DR4 allele variant is present in 90% of cases, especially DRB1*0407, which is seen in 60% of cases.1,6 In our patient, testing revealed HLA-DR4, DRB1*04 positivity. We further hypothesized that his mother’s photosensitive rash may have been actinic prurigo as opposed to polymorphous light eruption, which could explain the lack of response to hydroxychloroquine.
The diagnosis of actinic prurigo usually is made clinically. A skin biopsy typically is not necessary but would show hyperkeratosis, spongiosis, and acanthosis with a lymphocytic perivascular infiltrate. Biopsies of the lip classically show lymphoid germinal centers in the lamina propria, which can help distinguish actinic prurigo from polymorphous light eruption.1
In our patient, the differential diagnosis included polymorphous light eruption, connective tissue disease such as lupus erythematosus or dermatomyositis, porphyria such as erythropoietic protoporphyria, and allergic contact dermatitis. Polymorphous light eruption was ruled out by the oral and ocular manifestations, which are not atypical for this diagnosis. The patient’s laboratory results displayed unremarkable antinuclear antibodies, creatine kinase, aldolase, and extractable nuclear antigens, which made connective tissue disease unlikely. Furthermore, a porphyria screen for total plasma porphyrins and whole blood protoporphyrin was negative, which helped rule out porphyria. Allergic contact dermatitis seemed less likely given the lack of improvement with topical steroids. Overall, the clinical presentation in a patient with relevant family ancestry and HLA-DR4 positivity supported a diagnosis of actinic prurigo.7
To manage the condition in our patient, strict photoprotection was recommended as well as the application of triamcinolone ointment 0.025% to the affected areas twice daily until the skin symptoms improved. For acute flares, other treatment considerations include topical tacrolimus, oral antihistamines, and oral corticosteroids. Some success has been reported with cyclosporine and azathioprine. For severe disease, thalidomide is the recommended treatment; it is effective in pediatric patients at dosages of 50 to 100 mg daily, but the dose has not yet been standardized for this age group.8,9 Many adult patients initially are controlled with 100 to 200 mg daily, which can be tapered down to a dosage of 25 to 50 mg weekly with few adverse effects; however, the overall substantial side effects of thalidomide limit its use in both pediatric and adult populations.1,2 Newer studies have suggested promising results with dupilumab, especially when actinic prurigo presents with high IgE levels or eosinophils on histology.7,10 In our patient, the IgE level was normal.
- Pile HD, Crane JS. Actinic prurigo. StatPearls. StatPearls Publishing; 2022.
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii.
- Vega Memije ME, Cuevas Gonzalez JC, Hojyo-Tomoka MT, et al. Actinic prurigo as a hypersensitivity reaction type 4. Int J Dermatol. 2017;56:E135-E136.
- Magaña M, Mendez Y, Rodriguez A, et al. The conjunctivitis of solar (actinic) prurigo. Pediatr Dermatol. 2000;17:432-435.
- Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272-275.
- Rodríguez-Carreón AA, Rodríguez-Lobato E, Rodríguez-Gutiérrez G, et al. Actinic prurigo. Skinmed. 2015;13:287-295.
- Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic protoporphyria, autosomal recessive. GeneReviews. University of Washington; 1993.
- Crouch RB, Foley PA, Ng JCH, et al. Thalidomide experience of a major Australian teaching hospital. Australas J Dermatol. 2002;43:278-284.
- Watts-Santos A, Martinez-Rico JC, Gomez-Flores M, et al. Thalidomide: an option for the pediatric patient with actinic prurigo. Pediatr Dermatol. 2020;37:362-365.
- Eickstaedt JB, Starke S, Krakora D, et al. Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-1178.
The Diagnosis: Actinic Prurigo
Actinic prurigo is an idiopathic photodermatosis triggered by UV exposure that primarily affects sun-exposed areas of the skin.1,2 It typically presents as pruritic papules, plaques, and nodules, with most patients also experiencing oral tingling and pain.3 In more severe cases, it can progress to include conjunctival disease, scarring, and cheilitis.1 A study of ocular findings among children with actinic pruritus reported that photophobia was one of the most important features,4 which was present in our patient. The face, especially over the zygomatic arches, nasal bridge, and lower lip, commonly is affected.1 Secondary lichenification or eczematization may occur.5 In our patient, the combination of conjunctivitis, cheilitis, and an eruption on sun-exposed skin were crucial in making the diagnosis.
Most cases present in patients younger than 10 years. It most commonly is seen in American Indians in North America, Central America, and South America.2 After the diagnosis was considered in our patient, the family was asked about their ancestry and confirmed that both of the patient’s maternal and paternal grandparents were of American Indian descent. There also is a strong genetic component; the HLA-DR4 allele variant is present in 90% of cases, especially DRB1*0407, which is seen in 60% of cases.1,6 In our patient, testing revealed HLA-DR4, DRB1*04 positivity. We further hypothesized that his mother’s photosensitive rash may have been actinic prurigo as opposed to polymorphous light eruption, which could explain the lack of response to hydroxychloroquine.
The diagnosis of actinic prurigo usually is made clinically. A skin biopsy typically is not necessary but would show hyperkeratosis, spongiosis, and acanthosis with a lymphocytic perivascular infiltrate. Biopsies of the lip classically show lymphoid germinal centers in the lamina propria, which can help distinguish actinic prurigo from polymorphous light eruption.1
In our patient, the differential diagnosis included polymorphous light eruption, connective tissue disease such as lupus erythematosus or dermatomyositis, porphyria such as erythropoietic protoporphyria, and allergic contact dermatitis. Polymorphous light eruption was ruled out by the oral and ocular manifestations, which are not atypical for this diagnosis. The patient’s laboratory results displayed unremarkable antinuclear antibodies, creatine kinase, aldolase, and extractable nuclear antigens, which made connective tissue disease unlikely. Furthermore, a porphyria screen for total plasma porphyrins and whole blood protoporphyrin was negative, which helped rule out porphyria. Allergic contact dermatitis seemed less likely given the lack of improvement with topical steroids. Overall, the clinical presentation in a patient with relevant family ancestry and HLA-DR4 positivity supported a diagnosis of actinic prurigo.7
To manage the condition in our patient, strict photoprotection was recommended as well as the application of triamcinolone ointment 0.025% to the affected areas twice daily until the skin symptoms improved. For acute flares, other treatment considerations include topical tacrolimus, oral antihistamines, and oral corticosteroids. Some success has been reported with cyclosporine and azathioprine. For severe disease, thalidomide is the recommended treatment; it is effective in pediatric patients at dosages of 50 to 100 mg daily, but the dose has not yet been standardized for this age group.8,9 Many adult patients initially are controlled with 100 to 200 mg daily, which can be tapered down to a dosage of 25 to 50 mg weekly with few adverse effects; however, the overall substantial side effects of thalidomide limit its use in both pediatric and adult populations.1,2 Newer studies have suggested promising results with dupilumab, especially when actinic prurigo presents with high IgE levels or eosinophils on histology.7,10 In our patient, the IgE level was normal.
The Diagnosis: Actinic Prurigo
Actinic prurigo is an idiopathic photodermatosis triggered by UV exposure that primarily affects sun-exposed areas of the skin.1,2 It typically presents as pruritic papules, plaques, and nodules, with most patients also experiencing oral tingling and pain.3 In more severe cases, it can progress to include conjunctival disease, scarring, and cheilitis.1 A study of ocular findings among children with actinic pruritus reported that photophobia was one of the most important features,4 which was present in our patient. The face, especially over the zygomatic arches, nasal bridge, and lower lip, commonly is affected.1 Secondary lichenification or eczematization may occur.5 In our patient, the combination of conjunctivitis, cheilitis, and an eruption on sun-exposed skin were crucial in making the diagnosis.
Most cases present in patients younger than 10 years. It most commonly is seen in American Indians in North America, Central America, and South America.2 After the diagnosis was considered in our patient, the family was asked about their ancestry and confirmed that both of the patient’s maternal and paternal grandparents were of American Indian descent. There also is a strong genetic component; the HLA-DR4 allele variant is present in 90% of cases, especially DRB1*0407, which is seen in 60% of cases.1,6 In our patient, testing revealed HLA-DR4, DRB1*04 positivity. We further hypothesized that his mother’s photosensitive rash may have been actinic prurigo as opposed to polymorphous light eruption, which could explain the lack of response to hydroxychloroquine.
The diagnosis of actinic prurigo usually is made clinically. A skin biopsy typically is not necessary but would show hyperkeratosis, spongiosis, and acanthosis with a lymphocytic perivascular infiltrate. Biopsies of the lip classically show lymphoid germinal centers in the lamina propria, which can help distinguish actinic prurigo from polymorphous light eruption.1
In our patient, the differential diagnosis included polymorphous light eruption, connective tissue disease such as lupus erythematosus or dermatomyositis, porphyria such as erythropoietic protoporphyria, and allergic contact dermatitis. Polymorphous light eruption was ruled out by the oral and ocular manifestations, which are not atypical for this diagnosis. The patient’s laboratory results displayed unremarkable antinuclear antibodies, creatine kinase, aldolase, and extractable nuclear antigens, which made connective tissue disease unlikely. Furthermore, a porphyria screen for total plasma porphyrins and whole blood protoporphyrin was negative, which helped rule out porphyria. Allergic contact dermatitis seemed less likely given the lack of improvement with topical steroids. Overall, the clinical presentation in a patient with relevant family ancestry and HLA-DR4 positivity supported a diagnosis of actinic prurigo.7
To manage the condition in our patient, strict photoprotection was recommended as well as the application of triamcinolone ointment 0.025% to the affected areas twice daily until the skin symptoms improved. For acute flares, other treatment considerations include topical tacrolimus, oral antihistamines, and oral corticosteroids. Some success has been reported with cyclosporine and azathioprine. For severe disease, thalidomide is the recommended treatment; it is effective in pediatric patients at dosages of 50 to 100 mg daily, but the dose has not yet been standardized for this age group.8,9 Many adult patients initially are controlled with 100 to 200 mg daily, which can be tapered down to a dosage of 25 to 50 mg weekly with few adverse effects; however, the overall substantial side effects of thalidomide limit its use in both pediatric and adult populations.1,2 Newer studies have suggested promising results with dupilumab, especially when actinic prurigo presents with high IgE levels or eosinophils on histology.7,10 In our patient, the IgE level was normal.
- Pile HD, Crane JS. Actinic prurigo. StatPearls. StatPearls Publishing; 2022.
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii.
- Vega Memije ME, Cuevas Gonzalez JC, Hojyo-Tomoka MT, et al. Actinic prurigo as a hypersensitivity reaction type 4. Int J Dermatol. 2017;56:E135-E136.
- Magaña M, Mendez Y, Rodriguez A, et al. The conjunctivitis of solar (actinic) prurigo. Pediatr Dermatol. 2000;17:432-435.
- Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272-275.
- Rodríguez-Carreón AA, Rodríguez-Lobato E, Rodríguez-Gutiérrez G, et al. Actinic prurigo. Skinmed. 2015;13:287-295.
- Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic protoporphyria, autosomal recessive. GeneReviews. University of Washington; 1993.
- Crouch RB, Foley PA, Ng JCH, et al. Thalidomide experience of a major Australian teaching hospital. Australas J Dermatol. 2002;43:278-284.
- Watts-Santos A, Martinez-Rico JC, Gomez-Flores M, et al. Thalidomide: an option for the pediatric patient with actinic prurigo. Pediatr Dermatol. 2020;37:362-365.
- Eickstaedt JB, Starke S, Krakora D, et al. Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-1178.
- Pile HD, Crane JS. Actinic prurigo. StatPearls. StatPearls Publishing; 2022.
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii.
- Vega Memije ME, Cuevas Gonzalez JC, Hojyo-Tomoka MT, et al. Actinic prurigo as a hypersensitivity reaction type 4. Int J Dermatol. 2017;56:E135-E136.
- Magaña M, Mendez Y, Rodriguez A, et al. The conjunctivitis of solar (actinic) prurigo. Pediatr Dermatol. 2000;17:432-435.
- Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272-275.
- Rodríguez-Carreón AA, Rodríguez-Lobato E, Rodríguez-Gutiérrez G, et al. Actinic prurigo. Skinmed. 2015;13:287-295.
- Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic protoporphyria, autosomal recessive. GeneReviews. University of Washington; 1993.
- Crouch RB, Foley PA, Ng JCH, et al. Thalidomide experience of a major Australian teaching hospital. Australas J Dermatol. 2002;43:278-284.
- Watts-Santos A, Martinez-Rico JC, Gomez-Flores M, et al. Thalidomide: an option for the pediatric patient with actinic prurigo. Pediatr Dermatol. 2020;37:362-365.
- Eickstaedt JB, Starke S, Krakora D, et al. Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-1178.
A 6-year-old boy presented via telemedicine for evaluation of a recurring rash that first presented on the face 9 months prior to presentation and waxed and waned throughout the fall and winter seasons for about 5 months. His mother noted that on a warm and sunny day 5 months after its first appearance, the patient was at a dog park and developed the rash on the face and hands—the only areas that had been exposed to the sun—later that evening. The patient reported pruritus but no associated burning or stinging. He was evaluated by an allergist 1 month later and was treated with oral cefazolin and hydrocortisone ointment 2.5% for suspected impetiginized dermatitis without improvement. The rash persisted until evaluation by our clinic 2 months later. Photographs showed erythematous scaly plaques and papules scattered on the cheeks, nose, upper and lower lips, and vermilion borders, as well as the dorsal aspect of the hands. He also had conjunctival erythema, which his mother reported was particularly worse in the summer months and associated with photophobia. His mother also noted increased tear production when in the sun. There was no mucosal involvement. The patient had no notable medical history and was not taking any medications. His mother had a history of polymorphous light eruption that recently was treated with hydroxychloroquine but without benefit.
Researchers discover brain abnormalities in babies who had SIDS
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF NEUROPATHY & EXPERIMENTAL NEUROLOGY
JAK-inhibitor safety in adolescents with AD: Long-term analyses reported
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
AT RAD 2023
Talking tobacco with youth? Ask the right questions
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
Youth-led sexual health program improves teen knowledge, autonomy
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
AT ACOG 2023
Serious mental illness not a factor in most mass school shootings
Mass shootings, often on school campuses, have become a regular and sad reality in the United States.
The statistics are grim. Every day 12 children die from gun violence in America and another 32 are shot and injured. Since the Columbine High School shooting in 1999, more than 338,000 students in the United States have experienced school gun violence, according to the nonprofit organization Sandy Hook Promise.
A new analysis from the Columbia Mass Murder Database (CMMD) sheds fresh light on the debate over whether mental illness or easy access to guns is the key driver of mass shootings.
The findings, which are published in the Journal of Forensic Sciences, show that most perpetrators of mass school shootings are young, White men without serious mental illness.
A ‘straw man’
Mental health is often used as a “straw man” in debates about mass shootings, lead investigator Ragy Girgis, MD, told this news organization.
“There are many factors that contribute to the mass shooting epidemic, including gun access, criminality, substance use and misuse, and many others. Mental illness is incidental in the vast majority of cases,” said Dr. Girgis, with Columbia University Irving Medical Center, New York, and the New York State Psychiatric Institute.
“People with serious mental illness constitute only a small portion of the perpetrators of gun violence in this country,” coinvestigator Paul Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, told this news organization.
Using the CMMD, the researchers examined 82 incidents of mass murder perpetrated in academic settings including schools, colleges, and universities. The average number of victims of these incidents was eight. More than half (60%) of mass school shootings involved at least one semi- or fully automatic firearm.
All 82 incidents were initiated by men (mean age, 28), and 67% were White. About two-thirds (63%) involved guns.
More than three-quarters (77%) of all perpetrators of mass murders in academic settings had no recorded history of psychotic symptoms.
Despite the absence of serious mental illness, almost half (46%) of the mass school shooters took their own lives at the scene, suggesting that they viewed themselves as engaging in some form of “final act,” the researchers note.
“The major difference between mass shooters in school settings and elsewhere is the higher rate of suicide by the perpetrators in school settings. That suggests that the shootings are often part of a preexisting intent to die on the part of the shooter,” said Dr. Appelbaum.
Epidemic of emptiness
He noted that the typical profile of a mass school shooter is that of “a young male with anger problems, often as a result of bullying or abuse, frequently described as a loner, who has signaled a desire to kill other people.”
“If we only focus on mental illness, we will miss the warning signs in the majority of cases associated with victimization (such as bullying) and consequent anger,” Dr. Appelbaum said.
Dr. Girgis said there is a need to deal with the “epidemic of emptiness, narcissism, anger, and societal rejection felt by many young men/boys who, when combined with a desire to take their own lives and a great need for notoriety, feel that perpetrating a mass school shooting is their only option.”
“We also need to understand why it is so easy for so many mass school shooters to obtain firearms that are not theirs – either illegally or from someone else who themselves may have obtained the firearm legally,” Dr. Girgis said.
“All countries have people with mental illness,” Dr. Appelbaum said, “but among developed countries the U.S. is unique in the easy availability of weapons and in our disproportionate rate of murders.”
He also noted that school shootings are not a problem that clinicians are going to solve.
“Although they can be alert to signals from their patients of an intent to harm people in a school (or other) setting, the vast majority of shooters are not receiving treatment for a mental disorder,” Dr. Appelbaum said.
“This is a problem that can only be substantially diminished by reducing access to firearms, which includes requirements for safe storage, universal background checks, waiting periods to purchase firearms, and similar means-oriented interventions,” he added.
Need for regular mental health checks
Thea Gallagher, PsyD, who was not involved in the study, noted that mass school shooters may not have a psychotic illness, but with mental health there is a “spectrum, and obviously, that individual is struggling to some extent, most likely, mentally, if they are at a place where they are willing to take the lives of others and themselves.”
“We need to understand more about how people get to this place and the issues people are struggling with. We need to push for yearly mental health checks just like the yearly physical,” Dr. Gallagher, with the department of psychiatry at NYU Langone Health, New York, told this news organization.
“The more that we create conversation and moments to talk about how people are feeling internally, the better chance we have to give people who are struggling healthy coping strategies and the opportunity to process their emotions and not bury them,” Dr. Gallagher said.
Support for the study was provided in part by the New York State Office of Mental Hygiene, and the Elizabeth K. Dollard Charitable Trust. Dr. Girgis has received royalties and/or advances from books on mental health published by Wipf and Stock, and Routledge/Taylor and Francis. He has consulted for Noble Insights, IMS Expert Services, and Fowler White Burnett. Dr. Appelbaum and Dr. Gallagher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mass shootings, often on school campuses, have become a regular and sad reality in the United States.
The statistics are grim. Every day 12 children die from gun violence in America and another 32 are shot and injured. Since the Columbine High School shooting in 1999, more than 338,000 students in the United States have experienced school gun violence, according to the nonprofit organization Sandy Hook Promise.
A new analysis from the Columbia Mass Murder Database (CMMD) sheds fresh light on the debate over whether mental illness or easy access to guns is the key driver of mass shootings.
The findings, which are published in the Journal of Forensic Sciences, show that most perpetrators of mass school shootings are young, White men without serious mental illness.
A ‘straw man’
Mental health is often used as a “straw man” in debates about mass shootings, lead investigator Ragy Girgis, MD, told this news organization.
“There are many factors that contribute to the mass shooting epidemic, including gun access, criminality, substance use and misuse, and many others. Mental illness is incidental in the vast majority of cases,” said Dr. Girgis, with Columbia University Irving Medical Center, New York, and the New York State Psychiatric Institute.
“People with serious mental illness constitute only a small portion of the perpetrators of gun violence in this country,” coinvestigator Paul Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, told this news organization.
Using the CMMD, the researchers examined 82 incidents of mass murder perpetrated in academic settings including schools, colleges, and universities. The average number of victims of these incidents was eight. More than half (60%) of mass school shootings involved at least one semi- or fully automatic firearm.
All 82 incidents were initiated by men (mean age, 28), and 67% were White. About two-thirds (63%) involved guns.
More than three-quarters (77%) of all perpetrators of mass murders in academic settings had no recorded history of psychotic symptoms.
Despite the absence of serious mental illness, almost half (46%) of the mass school shooters took their own lives at the scene, suggesting that they viewed themselves as engaging in some form of “final act,” the researchers note.
“The major difference between mass shooters in school settings and elsewhere is the higher rate of suicide by the perpetrators in school settings. That suggests that the shootings are often part of a preexisting intent to die on the part of the shooter,” said Dr. Appelbaum.
Epidemic of emptiness
He noted that the typical profile of a mass school shooter is that of “a young male with anger problems, often as a result of bullying or abuse, frequently described as a loner, who has signaled a desire to kill other people.”
“If we only focus on mental illness, we will miss the warning signs in the majority of cases associated with victimization (such as bullying) and consequent anger,” Dr. Appelbaum said.
Dr. Girgis said there is a need to deal with the “epidemic of emptiness, narcissism, anger, and societal rejection felt by many young men/boys who, when combined with a desire to take their own lives and a great need for notoriety, feel that perpetrating a mass school shooting is their only option.”
“We also need to understand why it is so easy for so many mass school shooters to obtain firearms that are not theirs – either illegally or from someone else who themselves may have obtained the firearm legally,” Dr. Girgis said.
“All countries have people with mental illness,” Dr. Appelbaum said, “but among developed countries the U.S. is unique in the easy availability of weapons and in our disproportionate rate of murders.”
He also noted that school shootings are not a problem that clinicians are going to solve.
“Although they can be alert to signals from their patients of an intent to harm people in a school (or other) setting, the vast majority of shooters are not receiving treatment for a mental disorder,” Dr. Appelbaum said.
“This is a problem that can only be substantially diminished by reducing access to firearms, which includes requirements for safe storage, universal background checks, waiting periods to purchase firearms, and similar means-oriented interventions,” he added.
Need for regular mental health checks
Thea Gallagher, PsyD, who was not involved in the study, noted that mass school shooters may not have a psychotic illness, but with mental health there is a “spectrum, and obviously, that individual is struggling to some extent, most likely, mentally, if they are at a place where they are willing to take the lives of others and themselves.”
“We need to understand more about how people get to this place and the issues people are struggling with. We need to push for yearly mental health checks just like the yearly physical,” Dr. Gallagher, with the department of psychiatry at NYU Langone Health, New York, told this news organization.
“The more that we create conversation and moments to talk about how people are feeling internally, the better chance we have to give people who are struggling healthy coping strategies and the opportunity to process their emotions and not bury them,” Dr. Gallagher said.
Support for the study was provided in part by the New York State Office of Mental Hygiene, and the Elizabeth K. Dollard Charitable Trust. Dr. Girgis has received royalties and/or advances from books on mental health published by Wipf and Stock, and Routledge/Taylor and Francis. He has consulted for Noble Insights, IMS Expert Services, and Fowler White Burnett. Dr. Appelbaum and Dr. Gallagher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mass shootings, often on school campuses, have become a regular and sad reality in the United States.
The statistics are grim. Every day 12 children die from gun violence in America and another 32 are shot and injured. Since the Columbine High School shooting in 1999, more than 338,000 students in the United States have experienced school gun violence, according to the nonprofit organization Sandy Hook Promise.
A new analysis from the Columbia Mass Murder Database (CMMD) sheds fresh light on the debate over whether mental illness or easy access to guns is the key driver of mass shootings.
The findings, which are published in the Journal of Forensic Sciences, show that most perpetrators of mass school shootings are young, White men without serious mental illness.
A ‘straw man’
Mental health is often used as a “straw man” in debates about mass shootings, lead investigator Ragy Girgis, MD, told this news organization.
“There are many factors that contribute to the mass shooting epidemic, including gun access, criminality, substance use and misuse, and many others. Mental illness is incidental in the vast majority of cases,” said Dr. Girgis, with Columbia University Irving Medical Center, New York, and the New York State Psychiatric Institute.
“People with serious mental illness constitute only a small portion of the perpetrators of gun violence in this country,” coinvestigator Paul Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, told this news organization.
Using the CMMD, the researchers examined 82 incidents of mass murder perpetrated in academic settings including schools, colleges, and universities. The average number of victims of these incidents was eight. More than half (60%) of mass school shootings involved at least one semi- or fully automatic firearm.
All 82 incidents were initiated by men (mean age, 28), and 67% were White. About two-thirds (63%) involved guns.
More than three-quarters (77%) of all perpetrators of mass murders in academic settings had no recorded history of psychotic symptoms.
Despite the absence of serious mental illness, almost half (46%) of the mass school shooters took their own lives at the scene, suggesting that they viewed themselves as engaging in some form of “final act,” the researchers note.
“The major difference between mass shooters in school settings and elsewhere is the higher rate of suicide by the perpetrators in school settings. That suggests that the shootings are often part of a preexisting intent to die on the part of the shooter,” said Dr. Appelbaum.
Epidemic of emptiness
He noted that the typical profile of a mass school shooter is that of “a young male with anger problems, often as a result of bullying or abuse, frequently described as a loner, who has signaled a desire to kill other people.”
“If we only focus on mental illness, we will miss the warning signs in the majority of cases associated with victimization (such as bullying) and consequent anger,” Dr. Appelbaum said.
Dr. Girgis said there is a need to deal with the “epidemic of emptiness, narcissism, anger, and societal rejection felt by many young men/boys who, when combined with a desire to take their own lives and a great need for notoriety, feel that perpetrating a mass school shooting is their only option.”
“We also need to understand why it is so easy for so many mass school shooters to obtain firearms that are not theirs – either illegally or from someone else who themselves may have obtained the firearm legally,” Dr. Girgis said.
“All countries have people with mental illness,” Dr. Appelbaum said, “but among developed countries the U.S. is unique in the easy availability of weapons and in our disproportionate rate of murders.”
He also noted that school shootings are not a problem that clinicians are going to solve.
“Although they can be alert to signals from their patients of an intent to harm people in a school (or other) setting, the vast majority of shooters are not receiving treatment for a mental disorder,” Dr. Appelbaum said.
“This is a problem that can only be substantially diminished by reducing access to firearms, which includes requirements for safe storage, universal background checks, waiting periods to purchase firearms, and similar means-oriented interventions,” he added.
Need for regular mental health checks
Thea Gallagher, PsyD, who was not involved in the study, noted that mass school shooters may not have a psychotic illness, but with mental health there is a “spectrum, and obviously, that individual is struggling to some extent, most likely, mentally, if they are at a place where they are willing to take the lives of others and themselves.”
“We need to understand more about how people get to this place and the issues people are struggling with. We need to push for yearly mental health checks just like the yearly physical,” Dr. Gallagher, with the department of psychiatry at NYU Langone Health, New York, told this news organization.
“The more that we create conversation and moments to talk about how people are feeling internally, the better chance we have to give people who are struggling healthy coping strategies and the opportunity to process their emotions and not bury them,” Dr. Gallagher said.
Support for the study was provided in part by the New York State Office of Mental Hygiene, and the Elizabeth K. Dollard Charitable Trust. Dr. Girgis has received royalties and/or advances from books on mental health published by Wipf and Stock, and Routledge/Taylor and Francis. He has consulted for Noble Insights, IMS Expert Services, and Fowler White Burnett. Dr. Appelbaum and Dr. Gallagher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.