User login
Alcohol dependence in teens tied to subsequent depression
TOPLINE
Alcohol dependence, but not consumption, at age 18 years increases the risk for depression at age 24 years.
METHODOLOGY
- The study included 3,902 mostly White adolescents, about 58% female, born in England from April 1991 to December 1992, who were part of the Avon Longitudinal Study of Parents and Children (ALSPAC) that examined genetic and environmental determinants of health and development.
- Participants completed the self-report Alcohol Use Disorders Identification Test (AUDIT) between the ages of 16 and 23 years, a period when average alcohol use increases rapidly.
- The primary outcome was probability for depression at age 24 years, using the Clinical Interview Schedule Revised (CIS-R), a self-administered computerized clinical assessment of common mental disorder symptoms during the past week.
- Researchers assessed frequency and quantity of alcohol consumption as well as alcohol dependence.
- Confounders included sex, housing type, maternal education and depressive symptoms, parents’ alcohol use, conduct problems at age 4 years, being bullied, and smoking status.
TAKEAWAYS
- After adjustments, alcohol dependence at age 18 years was associated with depression at age 24 years (unstandardized probit coefficient 0.13; 95% confidence interval, 0.02-0.25; P = .019)
- The relationship appeared to persist for alcohol dependence at each age of the growth curve (17-22 years).
- There was no evidence that frequency or quantity of alcohol consumption at age 18 was significantly associated with depression at age 24, suggesting these factors may not increase the risk for later depression unless there are also features of dependency.
IN PRACTICE
“Our findings suggest that preventing alcohol dependence during adolescence, or treating it early, could reduce the risk of depression,” which could have important public health implications, the researchers write.
STUDY DETAILS
The study was carried out by researchers at the University of Bristol; University College London; Critical Thinking Unit, Public Health Directorate, NHS; University of Nottingham, all in the United Kingdom. It was published online in Lancet Psychiatry
LIMITATIONS
There was substantial attrition in the ALSPAC cohort from birth to age 24 years. The sample was recruited from one U.K. region and most participants were White. Measures of alcohol consumption and dependence excluded some features of abuse. And as this is an observational study, the possibility of residual confounding can’t be excluded.
DISCLOSURES
The investigators report no relevant disclosures. The study received support from the UK Medical Research Council and Alcohol Research UK.
A version of this article first appeared on Medscape.com.
TOPLINE
Alcohol dependence, but not consumption, at age 18 years increases the risk for depression at age 24 years.
METHODOLOGY
- The study included 3,902 mostly White adolescents, about 58% female, born in England from April 1991 to December 1992, who were part of the Avon Longitudinal Study of Parents and Children (ALSPAC) that examined genetic and environmental determinants of health and development.
- Participants completed the self-report Alcohol Use Disorders Identification Test (AUDIT) between the ages of 16 and 23 years, a period when average alcohol use increases rapidly.
- The primary outcome was probability for depression at age 24 years, using the Clinical Interview Schedule Revised (CIS-R), a self-administered computerized clinical assessment of common mental disorder symptoms during the past week.
- Researchers assessed frequency and quantity of alcohol consumption as well as alcohol dependence.
- Confounders included sex, housing type, maternal education and depressive symptoms, parents’ alcohol use, conduct problems at age 4 years, being bullied, and smoking status.
TAKEAWAYS
- After adjustments, alcohol dependence at age 18 years was associated with depression at age 24 years (unstandardized probit coefficient 0.13; 95% confidence interval, 0.02-0.25; P = .019)
- The relationship appeared to persist for alcohol dependence at each age of the growth curve (17-22 years).
- There was no evidence that frequency or quantity of alcohol consumption at age 18 was significantly associated with depression at age 24, suggesting these factors may not increase the risk for later depression unless there are also features of dependency.
IN PRACTICE
“Our findings suggest that preventing alcohol dependence during adolescence, or treating it early, could reduce the risk of depression,” which could have important public health implications, the researchers write.
STUDY DETAILS
The study was carried out by researchers at the University of Bristol; University College London; Critical Thinking Unit, Public Health Directorate, NHS; University of Nottingham, all in the United Kingdom. It was published online in Lancet Psychiatry
LIMITATIONS
There was substantial attrition in the ALSPAC cohort from birth to age 24 years. The sample was recruited from one U.K. region and most participants were White. Measures of alcohol consumption and dependence excluded some features of abuse. And as this is an observational study, the possibility of residual confounding can’t be excluded.
DISCLOSURES
The investigators report no relevant disclosures. The study received support from the UK Medical Research Council and Alcohol Research UK.
A version of this article first appeared on Medscape.com.
TOPLINE
Alcohol dependence, but not consumption, at age 18 years increases the risk for depression at age 24 years.
METHODOLOGY
- The study included 3,902 mostly White adolescents, about 58% female, born in England from April 1991 to December 1992, who were part of the Avon Longitudinal Study of Parents and Children (ALSPAC) that examined genetic and environmental determinants of health and development.
- Participants completed the self-report Alcohol Use Disorders Identification Test (AUDIT) between the ages of 16 and 23 years, a period when average alcohol use increases rapidly.
- The primary outcome was probability for depression at age 24 years, using the Clinical Interview Schedule Revised (CIS-R), a self-administered computerized clinical assessment of common mental disorder symptoms during the past week.
- Researchers assessed frequency and quantity of alcohol consumption as well as alcohol dependence.
- Confounders included sex, housing type, maternal education and depressive symptoms, parents’ alcohol use, conduct problems at age 4 years, being bullied, and smoking status.
TAKEAWAYS
- After adjustments, alcohol dependence at age 18 years was associated with depression at age 24 years (unstandardized probit coefficient 0.13; 95% confidence interval, 0.02-0.25; P = .019)
- The relationship appeared to persist for alcohol dependence at each age of the growth curve (17-22 years).
- There was no evidence that frequency or quantity of alcohol consumption at age 18 was significantly associated with depression at age 24, suggesting these factors may not increase the risk for later depression unless there are also features of dependency.
IN PRACTICE
“Our findings suggest that preventing alcohol dependence during adolescence, or treating it early, could reduce the risk of depression,” which could have important public health implications, the researchers write.
STUDY DETAILS
The study was carried out by researchers at the University of Bristol; University College London; Critical Thinking Unit, Public Health Directorate, NHS; University of Nottingham, all in the United Kingdom. It was published online in Lancet Psychiatry
LIMITATIONS
There was substantial attrition in the ALSPAC cohort from birth to age 24 years. The sample was recruited from one U.K. region and most participants were White. Measures of alcohol consumption and dependence excluded some features of abuse. And as this is an observational study, the possibility of residual confounding can’t be excluded.
DISCLOSURES
The investigators report no relevant disclosures. The study received support from the UK Medical Research Council and Alcohol Research UK.
A version of this article first appeared on Medscape.com.
Cognitive decline risk in adult childhood cancer survivors
Among more than 2,300 adult survivors of childhood cancer and their siblings, who served as controls, new-onset memory impairment emerged more often in survivors decades later.
The increased risk was associated with the cancer treatment that was provided as well as modifiable health behaviors and chronic health conditions.
Even 35 years after being diagnosed, cancer survivors who never received chemotherapies or radiation therapies known to damage the brain reported far greater memory impairment than did their siblings, first author Nicholas Phillips, MD, told this news organization.
What the findings suggest is that “we need to educate oncologists and primary care providers on the risks our survivors face long after completion of therapy,” said Dr. Phillips, of the epidemiology and cancer control department at St. Jude Children’s Research Hospital, Memphis, Tenn.
The study was published online in JAMA Network Open.
Cancer survivors face an elevated risk for severe neurocognitive effects that can emerge 5-10 years following their diagnosis and treatment. However, it’s unclear whether new-onset neurocognitive problems can still develop a decade or more following diagnosis.
Over a long-term follow-up, Dr. Phillips and colleagues explored this question in 2,375 adult survivors of childhood cancer from the Childhood Cancer Survivor Study and 232 of their siblings.
Among the cancer cohort, 1,316 patients were survivors of acute lymphoblastic leukemia (ALL), 488 were survivors of central nervous system (CNS) tumors, and 571 had survived Hodgkin lymphoma.
The researchers determined the prevalence of new-onset neurocognitive impairment between baseline (23 years after diagnosis) and follow-up (35 years after diagnosis). New-onset neurocognitive impairment – present at follow-up but not at baseline – was defined as having a score in the worst 10% of the sibling cohort.
A higher proportion of survivors had new-onset memory impairment at follow-up compared with siblings. Specifically, about 8% of siblings had new-onset memory trouble, compared with 14% of ALL survivors treated with chemotherapy only, 26% of ALL survivors treated with cranial radiation, 35% of CNS tumor survivors, and 17% of Hodgkin lymphoma survivors.
New-onset memory impairment was associated with cranial radiation among CNS tumor survivors (relative risk [RR], 1.97) and alkylator chemotherapy at or above 8,000 mg/m2 among survivors of ALL who were treated without cranial radiation (RR, 2.80). The authors also found that smoking, low educational attainment, and low physical activity were associated with an elevated risk for new-onset memory impairment.
Dr. Phillips noted that current guidelines emphasize the importance of short-term monitoring of a survivor’s neurocognitive status on the basis of that person’s chemotherapy and radiation exposures.
However, “our study suggests that all survivors, regardless of their therapy, should be screened regularly for new-onset neurocognitive problems. And this screening should be done regularly for decades after diagnosis,” he said in an interview.
Dr. Phillips also noted the importance of communicating lifestyle modifications, such as not smoking and maintaining an active lifestyle.
“We need to start early and use the power of repetition when communicating with our survivors and their families,” Dr. Phillips said. “When our families and survivors hear the word ‘exercise,’ they think of gym memberships, lifting weights, and running on treadmills. But what we really want our survivors to do is stay active.”
What this means is engaging for about 2.5 hours a week in a range of activities, such as ballet, basketball, volleyball, bicycling, or swimming.
“And if our kids want to quit after 3 months, let them know that this is okay. They just need to replace that activity with another activity,” said Dr. Phillips. “We want them to find a fun hobby that they will enjoy that will keep them active.”
The study was supported by the National Cancer Institute. Dr. Phillips has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among more than 2,300 adult survivors of childhood cancer and their siblings, who served as controls, new-onset memory impairment emerged more often in survivors decades later.
The increased risk was associated with the cancer treatment that was provided as well as modifiable health behaviors and chronic health conditions.
Even 35 years after being diagnosed, cancer survivors who never received chemotherapies or radiation therapies known to damage the brain reported far greater memory impairment than did their siblings, first author Nicholas Phillips, MD, told this news organization.
What the findings suggest is that “we need to educate oncologists and primary care providers on the risks our survivors face long after completion of therapy,” said Dr. Phillips, of the epidemiology and cancer control department at St. Jude Children’s Research Hospital, Memphis, Tenn.
The study was published online in JAMA Network Open.
Cancer survivors face an elevated risk for severe neurocognitive effects that can emerge 5-10 years following their diagnosis and treatment. However, it’s unclear whether new-onset neurocognitive problems can still develop a decade or more following diagnosis.
Over a long-term follow-up, Dr. Phillips and colleagues explored this question in 2,375 adult survivors of childhood cancer from the Childhood Cancer Survivor Study and 232 of their siblings.
Among the cancer cohort, 1,316 patients were survivors of acute lymphoblastic leukemia (ALL), 488 were survivors of central nervous system (CNS) tumors, and 571 had survived Hodgkin lymphoma.
The researchers determined the prevalence of new-onset neurocognitive impairment between baseline (23 years after diagnosis) and follow-up (35 years after diagnosis). New-onset neurocognitive impairment – present at follow-up but not at baseline – was defined as having a score in the worst 10% of the sibling cohort.
A higher proportion of survivors had new-onset memory impairment at follow-up compared with siblings. Specifically, about 8% of siblings had new-onset memory trouble, compared with 14% of ALL survivors treated with chemotherapy only, 26% of ALL survivors treated with cranial radiation, 35% of CNS tumor survivors, and 17% of Hodgkin lymphoma survivors.
New-onset memory impairment was associated with cranial radiation among CNS tumor survivors (relative risk [RR], 1.97) and alkylator chemotherapy at or above 8,000 mg/m2 among survivors of ALL who were treated without cranial radiation (RR, 2.80). The authors also found that smoking, low educational attainment, and low physical activity were associated with an elevated risk for new-onset memory impairment.
Dr. Phillips noted that current guidelines emphasize the importance of short-term monitoring of a survivor’s neurocognitive status on the basis of that person’s chemotherapy and radiation exposures.
However, “our study suggests that all survivors, regardless of their therapy, should be screened regularly for new-onset neurocognitive problems. And this screening should be done regularly for decades after diagnosis,” he said in an interview.
Dr. Phillips also noted the importance of communicating lifestyle modifications, such as not smoking and maintaining an active lifestyle.
“We need to start early and use the power of repetition when communicating with our survivors and their families,” Dr. Phillips said. “When our families and survivors hear the word ‘exercise,’ they think of gym memberships, lifting weights, and running on treadmills. But what we really want our survivors to do is stay active.”
What this means is engaging for about 2.5 hours a week in a range of activities, such as ballet, basketball, volleyball, bicycling, or swimming.
“And if our kids want to quit after 3 months, let them know that this is okay. They just need to replace that activity with another activity,” said Dr. Phillips. “We want them to find a fun hobby that they will enjoy that will keep them active.”
The study was supported by the National Cancer Institute. Dr. Phillips has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among more than 2,300 adult survivors of childhood cancer and their siblings, who served as controls, new-onset memory impairment emerged more often in survivors decades later.
The increased risk was associated with the cancer treatment that was provided as well as modifiable health behaviors and chronic health conditions.
Even 35 years after being diagnosed, cancer survivors who never received chemotherapies or radiation therapies known to damage the brain reported far greater memory impairment than did their siblings, first author Nicholas Phillips, MD, told this news organization.
What the findings suggest is that “we need to educate oncologists and primary care providers on the risks our survivors face long after completion of therapy,” said Dr. Phillips, of the epidemiology and cancer control department at St. Jude Children’s Research Hospital, Memphis, Tenn.
The study was published online in JAMA Network Open.
Cancer survivors face an elevated risk for severe neurocognitive effects that can emerge 5-10 years following their diagnosis and treatment. However, it’s unclear whether new-onset neurocognitive problems can still develop a decade or more following diagnosis.
Over a long-term follow-up, Dr. Phillips and colleagues explored this question in 2,375 adult survivors of childhood cancer from the Childhood Cancer Survivor Study and 232 of their siblings.
Among the cancer cohort, 1,316 patients were survivors of acute lymphoblastic leukemia (ALL), 488 were survivors of central nervous system (CNS) tumors, and 571 had survived Hodgkin lymphoma.
The researchers determined the prevalence of new-onset neurocognitive impairment between baseline (23 years after diagnosis) and follow-up (35 years after diagnosis). New-onset neurocognitive impairment – present at follow-up but not at baseline – was defined as having a score in the worst 10% of the sibling cohort.
A higher proportion of survivors had new-onset memory impairment at follow-up compared with siblings. Specifically, about 8% of siblings had new-onset memory trouble, compared with 14% of ALL survivors treated with chemotherapy only, 26% of ALL survivors treated with cranial radiation, 35% of CNS tumor survivors, and 17% of Hodgkin lymphoma survivors.
New-onset memory impairment was associated with cranial radiation among CNS tumor survivors (relative risk [RR], 1.97) and alkylator chemotherapy at or above 8,000 mg/m2 among survivors of ALL who were treated without cranial radiation (RR, 2.80). The authors also found that smoking, low educational attainment, and low physical activity were associated with an elevated risk for new-onset memory impairment.
Dr. Phillips noted that current guidelines emphasize the importance of short-term monitoring of a survivor’s neurocognitive status on the basis of that person’s chemotherapy and radiation exposures.
However, “our study suggests that all survivors, regardless of their therapy, should be screened regularly for new-onset neurocognitive problems. And this screening should be done regularly for decades after diagnosis,” he said in an interview.
Dr. Phillips also noted the importance of communicating lifestyle modifications, such as not smoking and maintaining an active lifestyle.
“We need to start early and use the power of repetition when communicating with our survivors and their families,” Dr. Phillips said. “When our families and survivors hear the word ‘exercise,’ they think of gym memberships, lifting weights, and running on treadmills. But what we really want our survivors to do is stay active.”
What this means is engaging for about 2.5 hours a week in a range of activities, such as ballet, basketball, volleyball, bicycling, or swimming.
“And if our kids want to quit after 3 months, let them know that this is okay. They just need to replace that activity with another activity,” said Dr. Phillips. “We want them to find a fun hobby that they will enjoy that will keep them active.”
The study was supported by the National Cancer Institute. Dr. Phillips has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Review may help clinicians treat adolescents with depression
Depression is common among Canadian adolescents and often goes unnoticed. Many family physicians report feeling unprepared to identify and manage depression in these patients.
“Depression is an increasingly common but treatable condition among adolescents,” the authors wrote. “Primary care physicians and pediatricians are well positioned to support the assessment and first-line management of depression in this group, helping patients to regain their health and function.”
The article was published in CMAJ.
Distinct presentation
More than 40% of cases of depression begin during childhood. Onset at this life stage is associated with worse severity of depression in adulthood and worse social, occupational, and physical health outcomes.
Depression is influenced by genetic and environmental factors. Family history of depression is associated with a three- to fivefold increased risk of depression among older children. Genetic loci are known to be associated with depression, but exposure to parental depression, adverse childhood experiences, and family conflict are also linked to greater risk. Bullying and stigma are associated with greater risk among lesbian, gay, bisexual, and transgender youth.
Compared with adults, adolescents with depression are more likely to be irritable and to have a labile mood, rather than a low mood. Social withdrawal is also more common among adolescents than among adults. Unusual features, such as hypersomnia and increased appetite, may also be present. Anxiety, somatic symptoms, psychomotor agitation, and hallucinations are more common in adolescents than in younger persons with depression. It is vital to assess risk of suicidality and self-injury as well as support systems, and validated scales such as the Columbia Suicide Severity Rating Scale can be useful.
There is no consensus as to whether universal screening for depression is beneficial among adolescents. “Screening in this age group may be a reasonable approach, however, when implemented together with adequate systems that ensure accurate diagnosis and appropriate follow-up,” wrote the authors.
Management of depression in adolescents should begin with psychoeducation and may include lifestyle modification, psychotherapy, and medication. “Importantly, a suicide risk assessment must be done to ensure appropriateness of an outpatient management plan and facilitate safety planning,” the authors wrote.
Lifestyle interventions may target physical activity, diet, and sleep, since unhealthy patterns in all three are associated with heightened symptoms of depression in this population. Regular moderate to vigorous physical activity, and perhaps physical activity of short duration, can improve mood in adolescents. Reduced consumption of sugar-sweetened drinks, processed foods, and meats, along with greater consumption of fruits and legumes, has been shown to reduce depressive symptoms in randomized, controlled trials with adults.
Among psychotherapeutic approaches, cognitive-behavioral therapy has shown the most evidence of efficacy among adolescents with depression, though it is less effective for those with more severe symptoms, poor coping skills, and nonsuicidal self-injury. Some evidence supports interpersonal therapy, which focuses on relationships and social functioning. The involvement of caregivers may improve the response, compared with psychotherapy that only includes the adolescent.
The authors recommend antidepressant medications in more severe cases or when psychotherapy is ineffective or impossible. Guidelines generally support trials with at least two SSRIs before switching to another drug class, since efficacy data for them are sparser, and other drugs have worse side effect profiles.
About 2% of adolescents with depression experience an increase in suicidal ideation and behavior after exposure to antidepressants, usually within the first weeks of initiation, so this potential risk should be discussed with patients and caregivers.
Clinicians feel unprepared
Commenting on the review, Pierre-Paul Tellier, MD, an associate professor of family medicine at McGill University, Montreal, said that clinicians frequently report that they do not feel confident in their ability to manage and diagnose adolescent depression. “We did two systematic reviews to look at the continuing professional development of family physicians in adolescent health, and it turned out that there’s really a very large lack. When we looked at residents and the training that they were getting in adolescent medicine, it was very similar, so they felt unprepared to deal with issues around mental health.”
Medication can be effective, but it can be seen as “an easy way out,” Dr. Tellier added. “It’s not necessarily an ideal plan. What we need to do is to change the person’s way of thinking, the person’s way of responding to a variety of things which will occur throughout their lives. People will have other transition periods in their lives. It’s best if they learn a variety of techniques to deal with depression.”
These techniques include exercise, relaxation methods [which reduce anxiety], and wellness training. Through such techniques, patients “learn a healthier way of living with themselves and who they are, and then this is a lifelong way of learning,” said Dr. Tellier. “If I give you a pill, what I’m teaching is, yes, you can feel better. But you’re not dealing with the problem, you’re just dealing with the symptoms.”
He frequently refers his patients to YouTube videos that outline and explain various strategies. A favorite is a deep breathing exercise presented by Jeremy Howick.
The authors and Dr. Tellier disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Depression is common among Canadian adolescents and often goes unnoticed. Many family physicians report feeling unprepared to identify and manage depression in these patients.
“Depression is an increasingly common but treatable condition among adolescents,” the authors wrote. “Primary care physicians and pediatricians are well positioned to support the assessment and first-line management of depression in this group, helping patients to regain their health and function.”
The article was published in CMAJ.
Distinct presentation
More than 40% of cases of depression begin during childhood. Onset at this life stage is associated with worse severity of depression in adulthood and worse social, occupational, and physical health outcomes.
Depression is influenced by genetic and environmental factors. Family history of depression is associated with a three- to fivefold increased risk of depression among older children. Genetic loci are known to be associated with depression, but exposure to parental depression, adverse childhood experiences, and family conflict are also linked to greater risk. Bullying and stigma are associated with greater risk among lesbian, gay, bisexual, and transgender youth.
Compared with adults, adolescents with depression are more likely to be irritable and to have a labile mood, rather than a low mood. Social withdrawal is also more common among adolescents than among adults. Unusual features, such as hypersomnia and increased appetite, may also be present. Anxiety, somatic symptoms, psychomotor agitation, and hallucinations are more common in adolescents than in younger persons with depression. It is vital to assess risk of suicidality and self-injury as well as support systems, and validated scales such as the Columbia Suicide Severity Rating Scale can be useful.
There is no consensus as to whether universal screening for depression is beneficial among adolescents. “Screening in this age group may be a reasonable approach, however, when implemented together with adequate systems that ensure accurate diagnosis and appropriate follow-up,” wrote the authors.
Management of depression in adolescents should begin with psychoeducation and may include lifestyle modification, psychotherapy, and medication. “Importantly, a suicide risk assessment must be done to ensure appropriateness of an outpatient management plan and facilitate safety planning,” the authors wrote.
Lifestyle interventions may target physical activity, diet, and sleep, since unhealthy patterns in all three are associated with heightened symptoms of depression in this population. Regular moderate to vigorous physical activity, and perhaps physical activity of short duration, can improve mood in adolescents. Reduced consumption of sugar-sweetened drinks, processed foods, and meats, along with greater consumption of fruits and legumes, has been shown to reduce depressive symptoms in randomized, controlled trials with adults.
Among psychotherapeutic approaches, cognitive-behavioral therapy has shown the most evidence of efficacy among adolescents with depression, though it is less effective for those with more severe symptoms, poor coping skills, and nonsuicidal self-injury. Some evidence supports interpersonal therapy, which focuses on relationships and social functioning. The involvement of caregivers may improve the response, compared with psychotherapy that only includes the adolescent.
The authors recommend antidepressant medications in more severe cases or when psychotherapy is ineffective or impossible. Guidelines generally support trials with at least two SSRIs before switching to another drug class, since efficacy data for them are sparser, and other drugs have worse side effect profiles.
About 2% of adolescents with depression experience an increase in suicidal ideation and behavior after exposure to antidepressants, usually within the first weeks of initiation, so this potential risk should be discussed with patients and caregivers.
Clinicians feel unprepared
Commenting on the review, Pierre-Paul Tellier, MD, an associate professor of family medicine at McGill University, Montreal, said that clinicians frequently report that they do not feel confident in their ability to manage and diagnose adolescent depression. “We did two systematic reviews to look at the continuing professional development of family physicians in adolescent health, and it turned out that there’s really a very large lack. When we looked at residents and the training that they were getting in adolescent medicine, it was very similar, so they felt unprepared to deal with issues around mental health.”
Medication can be effective, but it can be seen as “an easy way out,” Dr. Tellier added. “It’s not necessarily an ideal plan. What we need to do is to change the person’s way of thinking, the person’s way of responding to a variety of things which will occur throughout their lives. People will have other transition periods in their lives. It’s best if they learn a variety of techniques to deal with depression.”
These techniques include exercise, relaxation methods [which reduce anxiety], and wellness training. Through such techniques, patients “learn a healthier way of living with themselves and who they are, and then this is a lifelong way of learning,” said Dr. Tellier. “If I give you a pill, what I’m teaching is, yes, you can feel better. But you’re not dealing with the problem, you’re just dealing with the symptoms.”
He frequently refers his patients to YouTube videos that outline and explain various strategies. A favorite is a deep breathing exercise presented by Jeremy Howick.
The authors and Dr. Tellier disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Depression is common among Canadian adolescents and often goes unnoticed. Many family physicians report feeling unprepared to identify and manage depression in these patients.
“Depression is an increasingly common but treatable condition among adolescents,” the authors wrote. “Primary care physicians and pediatricians are well positioned to support the assessment and first-line management of depression in this group, helping patients to regain their health and function.”
The article was published in CMAJ.
Distinct presentation
More than 40% of cases of depression begin during childhood. Onset at this life stage is associated with worse severity of depression in adulthood and worse social, occupational, and physical health outcomes.
Depression is influenced by genetic and environmental factors. Family history of depression is associated with a three- to fivefold increased risk of depression among older children. Genetic loci are known to be associated with depression, but exposure to parental depression, adverse childhood experiences, and family conflict are also linked to greater risk. Bullying and stigma are associated with greater risk among lesbian, gay, bisexual, and transgender youth.
Compared with adults, adolescents with depression are more likely to be irritable and to have a labile mood, rather than a low mood. Social withdrawal is also more common among adolescents than among adults. Unusual features, such as hypersomnia and increased appetite, may also be present. Anxiety, somatic symptoms, psychomotor agitation, and hallucinations are more common in adolescents than in younger persons with depression. It is vital to assess risk of suicidality and self-injury as well as support systems, and validated scales such as the Columbia Suicide Severity Rating Scale can be useful.
There is no consensus as to whether universal screening for depression is beneficial among adolescents. “Screening in this age group may be a reasonable approach, however, when implemented together with adequate systems that ensure accurate diagnosis and appropriate follow-up,” wrote the authors.
Management of depression in adolescents should begin with psychoeducation and may include lifestyle modification, psychotherapy, and medication. “Importantly, a suicide risk assessment must be done to ensure appropriateness of an outpatient management plan and facilitate safety planning,” the authors wrote.
Lifestyle interventions may target physical activity, diet, and sleep, since unhealthy patterns in all three are associated with heightened symptoms of depression in this population. Regular moderate to vigorous physical activity, and perhaps physical activity of short duration, can improve mood in adolescents. Reduced consumption of sugar-sweetened drinks, processed foods, and meats, along with greater consumption of fruits and legumes, has been shown to reduce depressive symptoms in randomized, controlled trials with adults.
Among psychotherapeutic approaches, cognitive-behavioral therapy has shown the most evidence of efficacy among adolescents with depression, though it is less effective for those with more severe symptoms, poor coping skills, and nonsuicidal self-injury. Some evidence supports interpersonal therapy, which focuses on relationships and social functioning. The involvement of caregivers may improve the response, compared with psychotherapy that only includes the adolescent.
The authors recommend antidepressant medications in more severe cases or when psychotherapy is ineffective or impossible. Guidelines generally support trials with at least two SSRIs before switching to another drug class, since efficacy data for them are sparser, and other drugs have worse side effect profiles.
About 2% of adolescents with depression experience an increase in suicidal ideation and behavior after exposure to antidepressants, usually within the first weeks of initiation, so this potential risk should be discussed with patients and caregivers.
Clinicians feel unprepared
Commenting on the review, Pierre-Paul Tellier, MD, an associate professor of family medicine at McGill University, Montreal, said that clinicians frequently report that they do not feel confident in their ability to manage and diagnose adolescent depression. “We did two systematic reviews to look at the continuing professional development of family physicians in adolescent health, and it turned out that there’s really a very large lack. When we looked at residents and the training that they were getting in adolescent medicine, it was very similar, so they felt unprepared to deal with issues around mental health.”
Medication can be effective, but it can be seen as “an easy way out,” Dr. Tellier added. “It’s not necessarily an ideal plan. What we need to do is to change the person’s way of thinking, the person’s way of responding to a variety of things which will occur throughout their lives. People will have other transition periods in their lives. It’s best if they learn a variety of techniques to deal with depression.”
These techniques include exercise, relaxation methods [which reduce anxiety], and wellness training. Through such techniques, patients “learn a healthier way of living with themselves and who they are, and then this is a lifelong way of learning,” said Dr. Tellier. “If I give you a pill, what I’m teaching is, yes, you can feel better. But you’re not dealing with the problem, you’re just dealing with the symptoms.”
He frequently refers his patients to YouTube videos that outline and explain various strategies. A favorite is a deep breathing exercise presented by Jeremy Howick.
The authors and Dr. Tellier disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMAJ
Suicidality risk in youth at highest at night
Investigators found that suicidal ideation and attempts were lowest in the mornings and highest in the evenings, particularly among youth with higher levels of self-critical rumination.
“These are preliminary findings, and there is a need for more data, but they signal potentially that there’s a need for support, particularly at nighttime, and that there might be a potential of targeting self-critical rumination in daily lives of youth,” said lead researcher Anastacia Kudinova, PhD, with the department of psychiatry and human behavior, Alpert Medical School of Brown University, Providence, R.I.
The findings were presented at the late-breaker session at the annual meeting of the Associated Professional Sleep Societies.
Urgent need
Suicidal ideation (SI) is a “robust” predictor of suicidal behavior and, “alarmingly,” both suicidal ideation and suicidal behavior have been increasing, Dr. Kudinova said.
“There is an urgent need to describe proximal time-period risk factors for suicide so that we can identify who is at a greater suicide risk on the time scale of weeks, days, or even hours,” she told attendees.
The researchers asked 165 psychiatrically hospitalized youth aged 11-18 (72% female) about the time of day of their most recent suicide attempt.
More than half (58%) said it occurred in the evenings and nights, followed by daytime (35%) and mornings (7%).
They also assessed the timing of suicidal ideation at home in 61 youth aged 12-15 (61% female) who were discharged after a partial hospitalization program.
They did this using ecological momentary assessments (EMAs) three times a day over 2 weeks. EMAs study people’s thoughts and behavior in their daily lives by repeatedly collecting data in an individual’s normal environment at or close to the time they carry out that behavior.
As in the other sample, youth in this sample also experienced significantly more frequent suicidal ideation later in the day (P < .01).
There was also a significant moderating effect of self-criticism (P < .01), such that more self-critical youth evidenced the highest levels of suicidal ideation later in the day.
True variation or mechanics?
Reached for comment, Paul Nestadt, MD, with Johns Hopkins Bloomberg School of Public Health, Baltimore, noted that EMA is becoming “an interesting way to track high-resolution temporal variation in suicidal ideation and other psych symptoms.”
Dr. Nestadt, who was not involved in the study, said that “it’s not surprising” that the majority of youth attempted suicide in evenings and nights, “as adolescents are generally being supervised in a school setting during daytime hours. It may not be the fluctuation in suicidality that impacts attempt timing so much as the mechanics – it is very hard to attempt suicide in math class.”
The same may be true for the youth in the second sample who were in the partial hospital program. “During the day, they were in therapy groups where feelings of suicidal ideation would have been solicited and addressed in real time,” Dr. Nestadt noted.
“Again, suicidal ideation later in the day may be a practical effect of how they are occupied in the partial hospital program, as opposed to some inherent suicidal ideation increase linked to something endogenous, such as circadian rhythm or cortisol level rises. That said, we do often see more attempts in the evenings in adults as well,” he added.
A vulnerable time
Also weighing in, Casey O’Brien, PsyD, a psychologist in the department of psychiatry at Columbia University Irving Medical Center, New York, said the findings in this study “track” with what she sees in the clinic.
Teens often report in session that the “unstructured time of night – especially the time when they usually should be getting to bed but are kind of staying up – tends to be a very vulnerable time for them,” Dr. O’Brien said in an interview.
“It’s really nice to have research confirm a lot of what we see reported anecdotally from the teens we work with,” said Dr. O’Brien.
Dr. O’Brien heads the intensive adolescent dialectical behavior therapy (DBT) program at Columbia for young people struggling with mental health issues.
“Within the DBT framework, we try to really focus on accepting that this is a vulnerable time and then planning ahead for what the strategies are that they can use to help them transition to bed quickly and smoothly,” Dr. O’Brien said.
These strategies may include spending time with their parents before bed, reading, or building into their bedtime routines things that they find soothing and comforting, like taking a longer shower or having comfortable pajamas to change into, she explained.
“We also work a lot on sleep hygiene strategies to help develop a regular bedtime and have a consistent sleep-wake cycle. We also will plan ahead for using distress tolerance skills during times of emotional vulnerability,” Dr. O’Brien said.
The Columbia DBT program also offers phone coaching “so teens can reach out to a therapist for help using skills outside of a therapeutic hour, and we do find that we get more coaching calls closer to around bedtime,” Dr. O’Brien said.
Support for the study was provided by the National Institute of Mental Health and Bradley Hospital COBRE Center. Dr. Kudinova, Dr. Nestadt, and Dr. O’Brien have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that suicidal ideation and attempts were lowest in the mornings and highest in the evenings, particularly among youth with higher levels of self-critical rumination.
“These are preliminary findings, and there is a need for more data, but they signal potentially that there’s a need for support, particularly at nighttime, and that there might be a potential of targeting self-critical rumination in daily lives of youth,” said lead researcher Anastacia Kudinova, PhD, with the department of psychiatry and human behavior, Alpert Medical School of Brown University, Providence, R.I.
The findings were presented at the late-breaker session at the annual meeting of the Associated Professional Sleep Societies.
Urgent need
Suicidal ideation (SI) is a “robust” predictor of suicidal behavior and, “alarmingly,” both suicidal ideation and suicidal behavior have been increasing, Dr. Kudinova said.
“There is an urgent need to describe proximal time-period risk factors for suicide so that we can identify who is at a greater suicide risk on the time scale of weeks, days, or even hours,” she told attendees.
The researchers asked 165 psychiatrically hospitalized youth aged 11-18 (72% female) about the time of day of their most recent suicide attempt.
More than half (58%) said it occurred in the evenings and nights, followed by daytime (35%) and mornings (7%).
They also assessed the timing of suicidal ideation at home in 61 youth aged 12-15 (61% female) who were discharged after a partial hospitalization program.
They did this using ecological momentary assessments (EMAs) three times a day over 2 weeks. EMAs study people’s thoughts and behavior in their daily lives by repeatedly collecting data in an individual’s normal environment at or close to the time they carry out that behavior.
As in the other sample, youth in this sample also experienced significantly more frequent suicidal ideation later in the day (P < .01).
There was also a significant moderating effect of self-criticism (P < .01), such that more self-critical youth evidenced the highest levels of suicidal ideation later in the day.
True variation or mechanics?
Reached for comment, Paul Nestadt, MD, with Johns Hopkins Bloomberg School of Public Health, Baltimore, noted that EMA is becoming “an interesting way to track high-resolution temporal variation in suicidal ideation and other psych symptoms.”
Dr. Nestadt, who was not involved in the study, said that “it’s not surprising” that the majority of youth attempted suicide in evenings and nights, “as adolescents are generally being supervised in a school setting during daytime hours. It may not be the fluctuation in suicidality that impacts attempt timing so much as the mechanics – it is very hard to attempt suicide in math class.”
The same may be true for the youth in the second sample who were in the partial hospital program. “During the day, they were in therapy groups where feelings of suicidal ideation would have been solicited and addressed in real time,” Dr. Nestadt noted.
“Again, suicidal ideation later in the day may be a practical effect of how they are occupied in the partial hospital program, as opposed to some inherent suicidal ideation increase linked to something endogenous, such as circadian rhythm or cortisol level rises. That said, we do often see more attempts in the evenings in adults as well,” he added.
A vulnerable time
Also weighing in, Casey O’Brien, PsyD, a psychologist in the department of psychiatry at Columbia University Irving Medical Center, New York, said the findings in this study “track” with what she sees in the clinic.
Teens often report in session that the “unstructured time of night – especially the time when they usually should be getting to bed but are kind of staying up – tends to be a very vulnerable time for them,” Dr. O’Brien said in an interview.
“It’s really nice to have research confirm a lot of what we see reported anecdotally from the teens we work with,” said Dr. O’Brien.
Dr. O’Brien heads the intensive adolescent dialectical behavior therapy (DBT) program at Columbia for young people struggling with mental health issues.
“Within the DBT framework, we try to really focus on accepting that this is a vulnerable time and then planning ahead for what the strategies are that they can use to help them transition to bed quickly and smoothly,” Dr. O’Brien said.
These strategies may include spending time with their parents before bed, reading, or building into their bedtime routines things that they find soothing and comforting, like taking a longer shower or having comfortable pajamas to change into, she explained.
“We also work a lot on sleep hygiene strategies to help develop a regular bedtime and have a consistent sleep-wake cycle. We also will plan ahead for using distress tolerance skills during times of emotional vulnerability,” Dr. O’Brien said.
The Columbia DBT program also offers phone coaching “so teens can reach out to a therapist for help using skills outside of a therapeutic hour, and we do find that we get more coaching calls closer to around bedtime,” Dr. O’Brien said.
Support for the study was provided by the National Institute of Mental Health and Bradley Hospital COBRE Center. Dr. Kudinova, Dr. Nestadt, and Dr. O’Brien have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that suicidal ideation and attempts were lowest in the mornings and highest in the evenings, particularly among youth with higher levels of self-critical rumination.
“These are preliminary findings, and there is a need for more data, but they signal potentially that there’s a need for support, particularly at nighttime, and that there might be a potential of targeting self-critical rumination in daily lives of youth,” said lead researcher Anastacia Kudinova, PhD, with the department of psychiatry and human behavior, Alpert Medical School of Brown University, Providence, R.I.
The findings were presented at the late-breaker session at the annual meeting of the Associated Professional Sleep Societies.
Urgent need
Suicidal ideation (SI) is a “robust” predictor of suicidal behavior and, “alarmingly,” both suicidal ideation and suicidal behavior have been increasing, Dr. Kudinova said.
“There is an urgent need to describe proximal time-period risk factors for suicide so that we can identify who is at a greater suicide risk on the time scale of weeks, days, or even hours,” she told attendees.
The researchers asked 165 psychiatrically hospitalized youth aged 11-18 (72% female) about the time of day of their most recent suicide attempt.
More than half (58%) said it occurred in the evenings and nights, followed by daytime (35%) and mornings (7%).
They also assessed the timing of suicidal ideation at home in 61 youth aged 12-15 (61% female) who were discharged after a partial hospitalization program.
They did this using ecological momentary assessments (EMAs) three times a day over 2 weeks. EMAs study people’s thoughts and behavior in their daily lives by repeatedly collecting data in an individual’s normal environment at or close to the time they carry out that behavior.
As in the other sample, youth in this sample also experienced significantly more frequent suicidal ideation later in the day (P < .01).
There was also a significant moderating effect of self-criticism (P < .01), such that more self-critical youth evidenced the highest levels of suicidal ideation later in the day.
True variation or mechanics?
Reached for comment, Paul Nestadt, MD, with Johns Hopkins Bloomberg School of Public Health, Baltimore, noted that EMA is becoming “an interesting way to track high-resolution temporal variation in suicidal ideation and other psych symptoms.”
Dr. Nestadt, who was not involved in the study, said that “it’s not surprising” that the majority of youth attempted suicide in evenings and nights, “as adolescents are generally being supervised in a school setting during daytime hours. It may not be the fluctuation in suicidality that impacts attempt timing so much as the mechanics – it is very hard to attempt suicide in math class.”
The same may be true for the youth in the second sample who were in the partial hospital program. “During the day, they were in therapy groups where feelings of suicidal ideation would have been solicited and addressed in real time,” Dr. Nestadt noted.
“Again, suicidal ideation later in the day may be a practical effect of how they are occupied in the partial hospital program, as opposed to some inherent suicidal ideation increase linked to something endogenous, such as circadian rhythm or cortisol level rises. That said, we do often see more attempts in the evenings in adults as well,” he added.
A vulnerable time
Also weighing in, Casey O’Brien, PsyD, a psychologist in the department of psychiatry at Columbia University Irving Medical Center, New York, said the findings in this study “track” with what she sees in the clinic.
Teens often report in session that the “unstructured time of night – especially the time when they usually should be getting to bed but are kind of staying up – tends to be a very vulnerable time for them,” Dr. O’Brien said in an interview.
“It’s really nice to have research confirm a lot of what we see reported anecdotally from the teens we work with,” said Dr. O’Brien.
Dr. O’Brien heads the intensive adolescent dialectical behavior therapy (DBT) program at Columbia for young people struggling with mental health issues.
“Within the DBT framework, we try to really focus on accepting that this is a vulnerable time and then planning ahead for what the strategies are that they can use to help them transition to bed quickly and smoothly,” Dr. O’Brien said.
These strategies may include spending time with their parents before bed, reading, or building into their bedtime routines things that they find soothing and comforting, like taking a longer shower or having comfortable pajamas to change into, she explained.
“We also work a lot on sleep hygiene strategies to help develop a regular bedtime and have a consistent sleep-wake cycle. We also will plan ahead for using distress tolerance skills during times of emotional vulnerability,” Dr. O’Brien said.
The Columbia DBT program also offers phone coaching “so teens can reach out to a therapist for help using skills outside of a therapeutic hour, and we do find that we get more coaching calls closer to around bedtime,” Dr. O’Brien said.
Support for the study was provided by the National Institute of Mental Health and Bradley Hospital COBRE Center. Dr. Kudinova, Dr. Nestadt, and Dr. O’Brien have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2023
Once-weekly growth hormone somapacitan approved for children
On May 26, the European Medicine Agency’s Committee for Medicinal Products for Human Use adopted a positive opinion, recommending the product for replacement of endogenous growth hormone in children aged 3 years and older.
That decision followed the Food and Drug Administration’s approval in April of the new indication for somapacitan injection in 5 mg, 10 mg, or 15 mg doses for children aged 2.5 years and older. The FDA approved the treatment for adults with growth hormone deficiency in September 2020.
Growth hormone deficiency is estimated to affect between 1 in 3,500 to 1 in 10,000 children. If left untreated, the condition can lead to shortened stature, reduced bone mineral density, and delayed appearance of teeth.
The European and American regulatory decisions were based on data from the phase 3 multinational REAL4 trial, published in the Journal of Clinical Endocrinology & Metabolism, in 200 prepubertal children with growth hormone deficiency randomly assigned 2:1 to weekly subcutaneous somapacitan or daily somatropin. At 52 weeks, height velocity was 11.2 cm/year with the once-weekly drug, compared with 11.7 cm/year with daily somatropin, a nonsignificant difference.
There were no major differences between the drugs in safety or tolerability. Adverse reactions in the REAL4 study that occurred in more than 5% of patients included nasopharyngitis, headache, pyrexia, extremity pain, and injection site reactions. A 3-year extension trial is ongoing.
The European Commission is expected to make a final decision in the coming months, and if approved somapacitan will be available in some European countries beginning in late 2023.
A version of this article originally appeared on Medscape.com.
On May 26, the European Medicine Agency’s Committee for Medicinal Products for Human Use adopted a positive opinion, recommending the product for replacement of endogenous growth hormone in children aged 3 years and older.
That decision followed the Food and Drug Administration’s approval in April of the new indication for somapacitan injection in 5 mg, 10 mg, or 15 mg doses for children aged 2.5 years and older. The FDA approved the treatment for adults with growth hormone deficiency in September 2020.
Growth hormone deficiency is estimated to affect between 1 in 3,500 to 1 in 10,000 children. If left untreated, the condition can lead to shortened stature, reduced bone mineral density, and delayed appearance of teeth.
The European and American regulatory decisions were based on data from the phase 3 multinational REAL4 trial, published in the Journal of Clinical Endocrinology & Metabolism, in 200 prepubertal children with growth hormone deficiency randomly assigned 2:1 to weekly subcutaneous somapacitan or daily somatropin. At 52 weeks, height velocity was 11.2 cm/year with the once-weekly drug, compared with 11.7 cm/year with daily somatropin, a nonsignificant difference.
There were no major differences between the drugs in safety or tolerability. Adverse reactions in the REAL4 study that occurred in more than 5% of patients included nasopharyngitis, headache, pyrexia, extremity pain, and injection site reactions. A 3-year extension trial is ongoing.
The European Commission is expected to make a final decision in the coming months, and if approved somapacitan will be available in some European countries beginning in late 2023.
A version of this article originally appeared on Medscape.com.
On May 26, the European Medicine Agency’s Committee for Medicinal Products for Human Use adopted a positive opinion, recommending the product for replacement of endogenous growth hormone in children aged 3 years and older.
That decision followed the Food and Drug Administration’s approval in April of the new indication for somapacitan injection in 5 mg, 10 mg, or 15 mg doses for children aged 2.5 years and older. The FDA approved the treatment for adults with growth hormone deficiency in September 2020.
Growth hormone deficiency is estimated to affect between 1 in 3,500 to 1 in 10,000 children. If left untreated, the condition can lead to shortened stature, reduced bone mineral density, and delayed appearance of teeth.
The European and American regulatory decisions were based on data from the phase 3 multinational REAL4 trial, published in the Journal of Clinical Endocrinology & Metabolism, in 200 prepubertal children with growth hormone deficiency randomly assigned 2:1 to weekly subcutaneous somapacitan or daily somatropin. At 52 weeks, height velocity was 11.2 cm/year with the once-weekly drug, compared with 11.7 cm/year with daily somatropin, a nonsignificant difference.
There were no major differences between the drugs in safety or tolerability. Adverse reactions in the REAL4 study that occurred in more than 5% of patients included nasopharyngitis, headache, pyrexia, extremity pain, and injection site reactions. A 3-year extension trial is ongoing.
The European Commission is expected to make a final decision in the coming months, and if approved somapacitan will be available in some European countries beginning in late 2023.
A version of this article originally appeared on Medscape.com.
Extensive Erosions and Ulcerations on the Trunk and Extremities in a Neonate
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
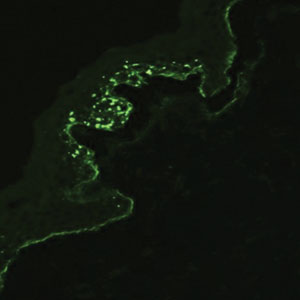
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5
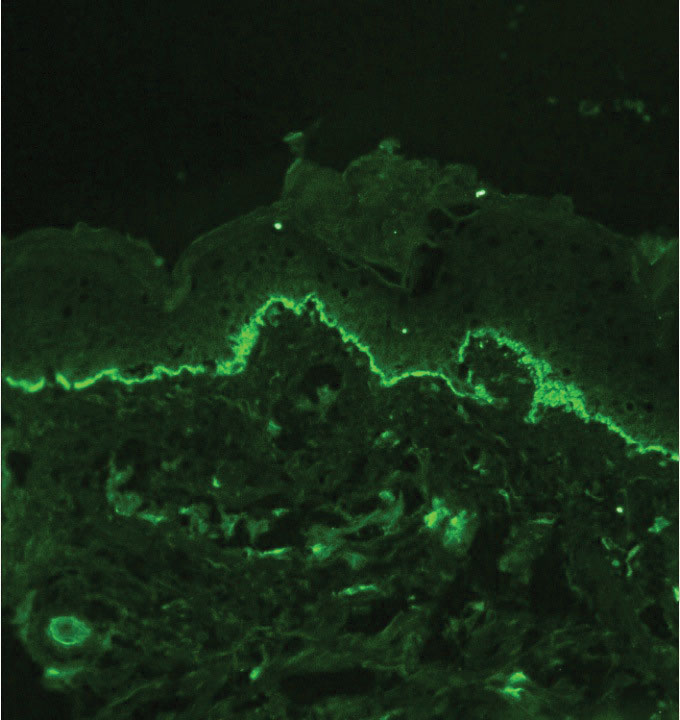
Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).
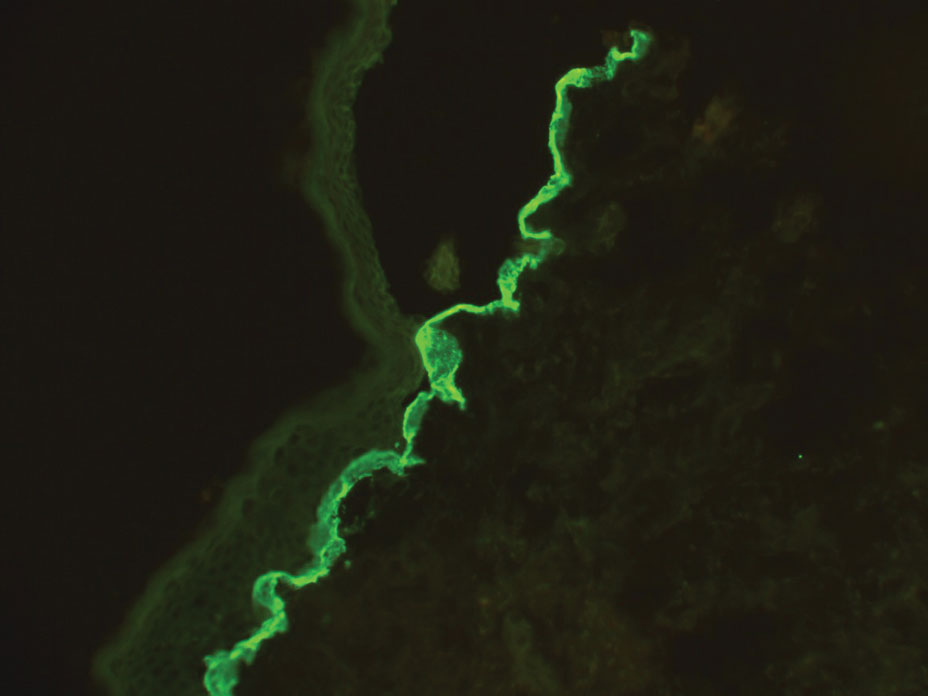
Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12
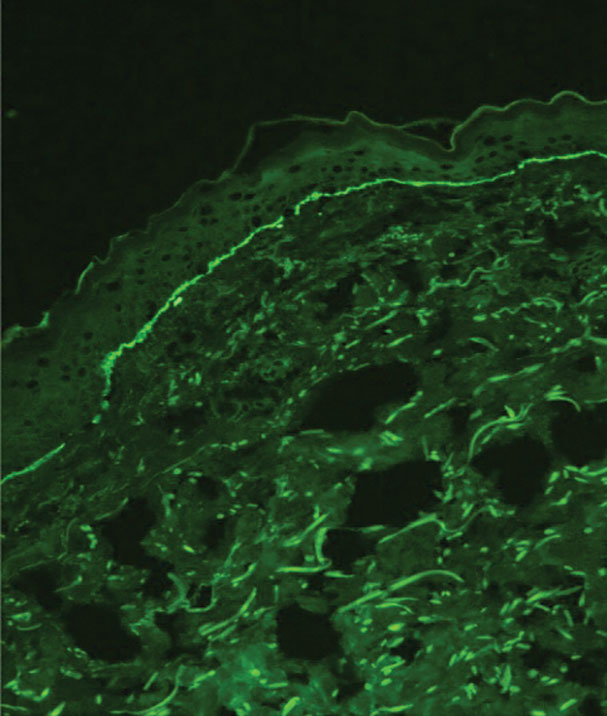
When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5

Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).

Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12

When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
The Diagnosis: Dominant Dystrophic Epidermolysis Bullosa
Blisters in a neonate may be caused by infectious, traumatic, autoimmune, or congenital etiologies. Biopsy findings correlated with clinical findings usually can establish a prompt diagnosis when the clinical diagnosis is uncertain. Direct immunofluorescence (DIF) as well as indirect immunofluorescence studies are useful when autoimmune blistering disease or congenital or heritable disorders of skin fragility are in the differential diagnosis. Many genetic abnormalities of skin fragility are associated with marked morbidity and mortality, and prompt diagnosis is essential to provide proper care. Our patient’s parents had no history of skin disorders, and there was no known family history of blistering disease or traumatic birth. A heritable disorder of skin fragility was still a top consideration because of the extensive blistering in the absence of any other symptoms.
Although dystrophic epidermolysis bullosa (DEB) is an uncommon cause of skin fragility in neonates, our patient’s presentation was typical because of the extensive blistering and increased fragility of the skin at pressure points. Dystrophic epidermolysis bullosa has both dominant and recessive presentations that span a spectrum from mild and focal skin blistering to extensive blistering with esophageal involvement.1 Early diagnosis and treatment can mitigate potential failure to thrive or premature death. Inherited mutations in the type VII collagen gene, COL7A1, are causative.2 Dominant DEB may be associated with dental caries, swallowing problems secondary to esophageal scarring, and constipation, as well as dystrophic or absent nails. Immunomapping studies of the skin often reveal type VII collagen cytoplasmic granules in the epidermis and weaker reaction in the roof of the subepidermal separation (quiz image).3 Abnormalities in type VII collagen impact the production of anchoring fibrils. Blister cleavage occurs in the sublamina densa with type VII collagen staining evident on the blister roof (quiz image).4 Patients with severe generalized recessive DEB may have barely detectable type VII collagen. In our patient, the cytoplasmic staining and weak staining in the epidermal roof of the separation confirmed the clinical impression of dominant DEB.
Autoimmune blistering disease should be considered in the histologic differential diagnosis, but it usually is associated with obvious disease in the mother. Direct immunofluorescence of pemphigoid gestationis reveals linear deposition of C3 at the basement membrane zone, which also can be associated with IgG (Figure 1). Neonates receiving passive transfer of antibodies may develop annular erythema, vesicles, and even dyshidroticlike changes on the soles.5

Suction blisters are subepithelial.6,7 When they occur in the neonatal period, they often are localized and are thought to be the result of vigorous sucking in utero.6 They quickly resolve without treatment and do not reveal abnormalities on DIF. If immunomapping is done for type VII collagen, it will be located at the floor of the suction blister (Figure 2).

Bullous pemphigoid is associated with deposition of linear IgG along the dermoepidermal junction—IgG4 is most common—and/or C3 (Figure 3). Direct immunofluorescence on split-skin biopsy reveals IgG on the epidermal side of the blister in bullous pemphigoid in contrast to epidermolysis bullosa acquisita, where the immune deposits are found on the dermal side of the split.8,9 Linear IgA bullous disease is associated with IgA deposition (Figure 4).10,11 Secretory IgA derived from breast milk can be causative.11 Neonatal linear IgA bullous disease is a serious condition associated with marked mucosal involvement that can eventuate in respiratory compromise. Prompt recognition is important; breastfeeding must be stopped and supportive therapy must be provided.

Other types of vesicular or pustular eruptions in the newborn usually are easily diagnosed by their typical clinical presentation without biopsy. Erythema toxicum neonatorum usually presents within 1 to 2 days of birth. It is self-limited and often resembles acne, but it also occurs on the trunk and extremities. Transient neonatal pustular melanosis may be present at birth and predominantly is seen in newborns with skin of color. Lesions easily rupture and usually resolve within 1 to 2 days. Infectious causes of blistering often can be identified on clinical examination and confirmed by culture. Herpes simplex virus infection is associated with characteristic multinucleated giant cells as well as steel grey nuclei evident on routine histologic evaluation. Bullous impetigo reveals superficial acantholysis and will have negative findings on DIF.12

When a neonate presents with widespread blistering, both genetic disorders of skin fragility as well as passive transfer of antibodies from maternal autoimmune disease need to be considered. Direct immunofluorescence and indirect immunofluorescence immunomapping findings can be useful in clarifying the diagnosis when heritable disorders of skin fragility or autoimmune blistering diseases are a clinical consideration.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627. doi:10.1111/bjd.18921
- Dang N, Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553-568. doi:10.1111/j.1600-0625.2008.00723.x
- Has C, He Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J Invest Dermatol. 2016;136:E65-E71. doi:10.1016/j.jid.2016.05.093
- Rao R, Mellerio J, Bhogal BS, et al. Immunofluorescence antigen mapping for hereditary epidermolysis bullosa. Indian J Dermatol Venereol Leprol. 2012;78:692-697.
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly followup of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168-1172. doi:10.1001/archderm.143.9.1168
- Afsar FS, Cun S, Seremet S. Neonatal sucking blister [published online November 15, 2019]. Dermatol Online J. 2019;25:13030 /qt33b1w59j.
- Yu WY, Wei ML. Suction blisters. JAMA Dermatol. 2019;155:237. doi:10.1001/jamadermatol.2018.3277
- Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69.
- Reis-Filho EG, Silva Tde A, Aguirre LH, et al. Bullous pemphigoid in a 3-month-old infant: case report and literature review of this dermatosis in childhood. An Bras Dermatol. 2013;88:961-965. doi:10.1590/abd1806-4841.20132378
- Hruza LL, Mallory SB, Fitzgibbons J, et al. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171-176. doi:10.1111/j.1525-1470
- Egami S, Suzuki C, Kurihara Y, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. 2021;157:1107-1111. doi:10.1001/jamadermatol.2021.2392
- Ligtenberg KG, Hu JK, Panse G, et al. Bullous impetigo masquerading as pemphigus foliaceus in an adult patient. JAAD Case Rep. 2020; 6:428-430. doi:10.1016/j.jdcr.2020.02.040
A neonate was born with extensive erosions and ulcerations on the trunk and extremities. The eroded areas had a beefy red appearance. A biopsy taken from a small blister was stained for type VII collagen by indirect immunofluorescence.
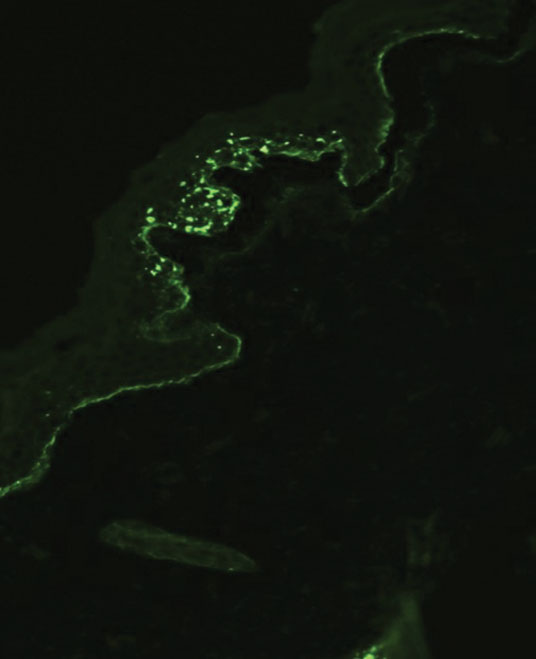
Dupilumab outcomes stable at end of open label atopic dermatitis study
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
AT RAD 2023
Cross-border U.S.-Mexican collaboration drives up ALL survival
A team from a hospital in San Diego combined a previously established training program from the World Health Organization with a new collaboration, which resulted in improvements in care standards and sustainability of care in a center in Tijuana, Mexico, just 23 miles away.
Implementation of the program in 2013 led to a significant 6% improvement in 5-year overall survival for children with ALL.
For patients at standard risk, 5-year overall survival increased from 73% to 100% after implementation of the program.
“This is really remarkable because this survival is the same as we have here in San Diego,” commented Paula Aristizabal, MD, MAS, a pediatric hematologist/oncologist at Rady Children’s Hospital, San Diego, at a press briefing before the annual meeting of the American Society of Clinical Oncology.
The findings show that “sustained improvements in cancer outcomes in low- and middle-income countries [LMICs] are feasible with innovative cross-border programs, particularly in borders that are shared” between a high- and low-income country, she commented. In other words, “it takes a village in both countries” to drive up standards.
Dr. Aristizabal also noted that the partnership will continue with a particularly focus on improving survival among patients with high-risk disease.
“We like to call it ‘twinning,’ because that means we are twins forever,” she said. “This is not a marriage that can be dissolved.”
‘Huge survival gap’
“The burden of childhood cancer has increased globally, but unfortunately, survival in low- and middle-income countries has not improved at the same level as in high-income countries,” Dr. Aristizabal commented.
This has resulted in a “huge survival gap” between high-income countries and the LMICs. ALL is now a leading cause of death among children in these countries, she commented.
“This study illustrates collaborative strategies that can be put into place today that could greatly improve outcomes for children with cancer globally,” commented Julie R. Gralow, MD, ASCO chief medical officer and executive vice president.
Speaking at the press conference, she added: “As I’ve heard Princess Dina Mired of Jordan say many times: ‘Your ZIP code should not determine if you survive cancer.’ ”
She said the differences in ALL survival between the United States and Mexico are an “example of children being so close in terms of proximity not having the same advantages.”
Also commenting, ASCO President Eric Winer, MD, from the Yale Cancer Center, New Haven, Conn., asked whether the proximity of the hospitals in San Diego and Tijuana “makes a difference, or do you think this is something that done ... at a distance?”
Dr. Aristizabal said that the proximity between the institutions “has been extremely helpful,” as they can go between hospitals in just 30 minutes.
However, “one of the things that we learned with COVID is that we can do a lot of things remotely,” she answered.
“Some of the projects that we started in Tijuana, through our collaboration with St. Jude Children’s Research Hospital, we have been able to implement in many other centers in Mexico,” she said.
Study details
Rady Children’s Hospital partnered with the public sector in Baja California, with the aim of improving outcomes in children’s cancer, she explained.
In 2008, the team collaborated with St. Jude Children’s Research Hospital, Memphis, to establish a training program in the Hospital General Tijuana in Tijuana that shared knowledge, technology, and organizational skills.
The team also consulted on clinical cases and set up education and research programs, all with the aim of building capacity and sustainability in Mexico.
“As the number of leukemia patients increased, we wanted to decrease depending on their international collaborators in the U.S. and ensure long-term sustainability,” Dr. Aristizabal explained.
This led in 2013 to the implementation of the WHO Framework for Action HSS training model, which has several components, including health service delivery.
Combined with the previously established model, the overall goals of the program were to improve health outcomes, systems efficiency, timely access to care, and social and financial risk protection.
Dr. Aristizabal said in an interview that this involved developing highly specific leukemia treatment guidelines, which have now gone through three iterations, as well as guidelines for supportive care.
Working with a local foundation, the team has also “focused on providing psychosocial support, nutritional support, a shelter for families that live 12-14 hours away from the pediatric cancer center, as well as food subsidies, trying to address financial toxicity and food insecurity in these families.”
Impact of the collaboration
To assess the impact of the WHO framework, the researchers conducted a study that involved 109 children with ALL who were treated at Hospital General Tijuana over the preimplementation phase in 2008-2012 and the postimplementation phase in 2013-2017.
The mean age of the patients was 7.04 years, and 50.4% were girls. The majority (67%) were classified as having high-risk disease.
Over the entire study period, the 5-year overall survival rate was 65%. Analysis revealed that between the pre- and postimplementation periods, 5-year overall survival increased from 59% to 65%, which Dr. Aristizabal described as “a significant improvement.”
Among high-risk patients, the improvement in 5-year survival between the pre- and postimplementation period went from 48% to 55%.
“This is an area for improvement,” Dr. Aristizabal said, “and we’re working on additional strategies to help improve survival for high-risk patients.
The study was funded by Rady Children’s Hospital, the Mexican Secretary of Health, and the Patronato Foundation. Dr. Aristizabal and coauthors reported no relevant financial relationships. Dr. Gralow reported relationships with Genentech and Roche. Dr. Winer reported relationships with Leap Therapeutics, Jounce Therapeutics, Carrick Therapeutics, and Genentech.
A version of this article first appeared on Medscape.com.
A team from a hospital in San Diego combined a previously established training program from the World Health Organization with a new collaboration, which resulted in improvements in care standards and sustainability of care in a center in Tijuana, Mexico, just 23 miles away.
Implementation of the program in 2013 led to a significant 6% improvement in 5-year overall survival for children with ALL.
For patients at standard risk, 5-year overall survival increased from 73% to 100% after implementation of the program.
“This is really remarkable because this survival is the same as we have here in San Diego,” commented Paula Aristizabal, MD, MAS, a pediatric hematologist/oncologist at Rady Children’s Hospital, San Diego, at a press briefing before the annual meeting of the American Society of Clinical Oncology.
The findings show that “sustained improvements in cancer outcomes in low- and middle-income countries [LMICs] are feasible with innovative cross-border programs, particularly in borders that are shared” between a high- and low-income country, she commented. In other words, “it takes a village in both countries” to drive up standards.
Dr. Aristizabal also noted that the partnership will continue with a particularly focus on improving survival among patients with high-risk disease.
“We like to call it ‘twinning,’ because that means we are twins forever,” she said. “This is not a marriage that can be dissolved.”
‘Huge survival gap’
“The burden of childhood cancer has increased globally, but unfortunately, survival in low- and middle-income countries has not improved at the same level as in high-income countries,” Dr. Aristizabal commented.
This has resulted in a “huge survival gap” between high-income countries and the LMICs. ALL is now a leading cause of death among children in these countries, she commented.
“This study illustrates collaborative strategies that can be put into place today that could greatly improve outcomes for children with cancer globally,” commented Julie R. Gralow, MD, ASCO chief medical officer and executive vice president.
Speaking at the press conference, she added: “As I’ve heard Princess Dina Mired of Jordan say many times: ‘Your ZIP code should not determine if you survive cancer.’ ”
She said the differences in ALL survival between the United States and Mexico are an “example of children being so close in terms of proximity not having the same advantages.”
Also commenting, ASCO President Eric Winer, MD, from the Yale Cancer Center, New Haven, Conn., asked whether the proximity of the hospitals in San Diego and Tijuana “makes a difference, or do you think this is something that done ... at a distance?”
Dr. Aristizabal said that the proximity between the institutions “has been extremely helpful,” as they can go between hospitals in just 30 minutes.
However, “one of the things that we learned with COVID is that we can do a lot of things remotely,” she answered.
“Some of the projects that we started in Tijuana, through our collaboration with St. Jude Children’s Research Hospital, we have been able to implement in many other centers in Mexico,” she said.
Study details
Rady Children’s Hospital partnered with the public sector in Baja California, with the aim of improving outcomes in children’s cancer, she explained.
In 2008, the team collaborated with St. Jude Children’s Research Hospital, Memphis, to establish a training program in the Hospital General Tijuana in Tijuana that shared knowledge, technology, and organizational skills.
The team also consulted on clinical cases and set up education and research programs, all with the aim of building capacity and sustainability in Mexico.
“As the number of leukemia patients increased, we wanted to decrease depending on their international collaborators in the U.S. and ensure long-term sustainability,” Dr. Aristizabal explained.
This led in 2013 to the implementation of the WHO Framework for Action HSS training model, which has several components, including health service delivery.
Combined with the previously established model, the overall goals of the program were to improve health outcomes, systems efficiency, timely access to care, and social and financial risk protection.
Dr. Aristizabal said in an interview that this involved developing highly specific leukemia treatment guidelines, which have now gone through three iterations, as well as guidelines for supportive care.
Working with a local foundation, the team has also “focused on providing psychosocial support, nutritional support, a shelter for families that live 12-14 hours away from the pediatric cancer center, as well as food subsidies, trying to address financial toxicity and food insecurity in these families.”
Impact of the collaboration
To assess the impact of the WHO framework, the researchers conducted a study that involved 109 children with ALL who were treated at Hospital General Tijuana over the preimplementation phase in 2008-2012 and the postimplementation phase in 2013-2017.
The mean age of the patients was 7.04 years, and 50.4% were girls. The majority (67%) were classified as having high-risk disease.
Over the entire study period, the 5-year overall survival rate was 65%. Analysis revealed that between the pre- and postimplementation periods, 5-year overall survival increased from 59% to 65%, which Dr. Aristizabal described as “a significant improvement.”
Among high-risk patients, the improvement in 5-year survival between the pre- and postimplementation period went from 48% to 55%.
“This is an area for improvement,” Dr. Aristizabal said, “and we’re working on additional strategies to help improve survival for high-risk patients.
The study was funded by Rady Children’s Hospital, the Mexican Secretary of Health, and the Patronato Foundation. Dr. Aristizabal and coauthors reported no relevant financial relationships. Dr. Gralow reported relationships with Genentech and Roche. Dr. Winer reported relationships with Leap Therapeutics, Jounce Therapeutics, Carrick Therapeutics, and Genentech.
A version of this article first appeared on Medscape.com.
A team from a hospital in San Diego combined a previously established training program from the World Health Organization with a new collaboration, which resulted in improvements in care standards and sustainability of care in a center in Tijuana, Mexico, just 23 miles away.
Implementation of the program in 2013 led to a significant 6% improvement in 5-year overall survival for children with ALL.
For patients at standard risk, 5-year overall survival increased from 73% to 100% after implementation of the program.
“This is really remarkable because this survival is the same as we have here in San Diego,” commented Paula Aristizabal, MD, MAS, a pediatric hematologist/oncologist at Rady Children’s Hospital, San Diego, at a press briefing before the annual meeting of the American Society of Clinical Oncology.
The findings show that “sustained improvements in cancer outcomes in low- and middle-income countries [LMICs] are feasible with innovative cross-border programs, particularly in borders that are shared” between a high- and low-income country, she commented. In other words, “it takes a village in both countries” to drive up standards.
Dr. Aristizabal also noted that the partnership will continue with a particularly focus on improving survival among patients with high-risk disease.
“We like to call it ‘twinning,’ because that means we are twins forever,” she said. “This is not a marriage that can be dissolved.”
‘Huge survival gap’
“The burden of childhood cancer has increased globally, but unfortunately, survival in low- and middle-income countries has not improved at the same level as in high-income countries,” Dr. Aristizabal commented.
This has resulted in a “huge survival gap” between high-income countries and the LMICs. ALL is now a leading cause of death among children in these countries, she commented.
“This study illustrates collaborative strategies that can be put into place today that could greatly improve outcomes for children with cancer globally,” commented Julie R. Gralow, MD, ASCO chief medical officer and executive vice president.
Speaking at the press conference, she added: “As I’ve heard Princess Dina Mired of Jordan say many times: ‘Your ZIP code should not determine if you survive cancer.’ ”
She said the differences in ALL survival between the United States and Mexico are an “example of children being so close in terms of proximity not having the same advantages.”
Also commenting, ASCO President Eric Winer, MD, from the Yale Cancer Center, New Haven, Conn., asked whether the proximity of the hospitals in San Diego and Tijuana “makes a difference, or do you think this is something that done ... at a distance?”
Dr. Aristizabal said that the proximity between the institutions “has been extremely helpful,” as they can go between hospitals in just 30 minutes.
However, “one of the things that we learned with COVID is that we can do a lot of things remotely,” she answered.
“Some of the projects that we started in Tijuana, through our collaboration with St. Jude Children’s Research Hospital, we have been able to implement in many other centers in Mexico,” she said.
Study details
Rady Children’s Hospital partnered with the public sector in Baja California, with the aim of improving outcomes in children’s cancer, she explained.
In 2008, the team collaborated with St. Jude Children’s Research Hospital, Memphis, to establish a training program in the Hospital General Tijuana in Tijuana that shared knowledge, technology, and organizational skills.
The team also consulted on clinical cases and set up education and research programs, all with the aim of building capacity and sustainability in Mexico.
“As the number of leukemia patients increased, we wanted to decrease depending on their international collaborators in the U.S. and ensure long-term sustainability,” Dr. Aristizabal explained.
This led in 2013 to the implementation of the WHO Framework for Action HSS training model, which has several components, including health service delivery.
Combined with the previously established model, the overall goals of the program were to improve health outcomes, systems efficiency, timely access to care, and social and financial risk protection.
Dr. Aristizabal said in an interview that this involved developing highly specific leukemia treatment guidelines, which have now gone through three iterations, as well as guidelines for supportive care.
Working with a local foundation, the team has also “focused on providing psychosocial support, nutritional support, a shelter for families that live 12-14 hours away from the pediatric cancer center, as well as food subsidies, trying to address financial toxicity and food insecurity in these families.”
Impact of the collaboration
To assess the impact of the WHO framework, the researchers conducted a study that involved 109 children with ALL who were treated at Hospital General Tijuana over the preimplementation phase in 2008-2012 and the postimplementation phase in 2013-2017.
The mean age of the patients was 7.04 years, and 50.4% were girls. The majority (67%) were classified as having high-risk disease.
Over the entire study period, the 5-year overall survival rate was 65%. Analysis revealed that between the pre- and postimplementation periods, 5-year overall survival increased from 59% to 65%, which Dr. Aristizabal described as “a significant improvement.”
Among high-risk patients, the improvement in 5-year survival between the pre- and postimplementation period went from 48% to 55%.
“This is an area for improvement,” Dr. Aristizabal said, “and we’re working on additional strategies to help improve survival for high-risk patients.
The study was funded by Rady Children’s Hospital, the Mexican Secretary of Health, and the Patronato Foundation. Dr. Aristizabal and coauthors reported no relevant financial relationships. Dr. Gralow reported relationships with Genentech and Roche. Dr. Winer reported relationships with Leap Therapeutics, Jounce Therapeutics, Carrick Therapeutics, and Genentech.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023
SPF is only the start when recommending sunscreens
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
Lomitapide shows promise in pediatric homozygous FH
MANNHEIM, Germany – Lomitapide, which reduces lipoprotein production in the liver, could help manage pediatric homozygous familial hypercholesterolemia (HoFH), suggest results of a trial that showed large reductions in circulating lipids.
The research was presented May 23 at the 91st European Atherosclerosis Society Congress.
Lomitapide inhibits microsomal triglyceride transfer protein, which plays a key role in apolipoprotein B-containing lipoprotein assembly and secretion in the liver and intestines. Crucially, the drug acts independently of the LDL cholesterol receptor.
It was approved in December 2012 by the U.S. Food and Drug Administration for use in adults with HoFH, sold under the name Juxtapid, and by the European Medicines Agency, where the brand name is Lojuxta.
The current trial involved more than 40 children and teenagers with HoFH aged 5-17 years; they were treated with the drug for 24 weeks, resulting in reductions of low density lipoprotein cholesterol of almost 54%, with nearly 42% reaching target levels.
The drug was also associated with marked reductions in other key lipids of at least 50%. However, 67% of patients also experienced gastrointestinal adverse events, and around 25% saw their levels of liver enzymes increase.
Early diagnosis ‘imperative’
The findings show that the “early diagnosis and treatment of HoFH is imperative,” said study presenter Luis Masana, MD, PhD, director of the Vascular Medicine and Metabolism Unit at Sant Joan de Reus University Hospital, Tarragona, Spain.
“I think that, with these results, we are bringing a new hope for this group of patients,” he continued. “I also think we will increase the quality of life, not just of the patients but also all the families involved in [managing] this problem.”
Session co-chair Andreas Zirlik, MD, PhD, head of the department of cardiology and chairman of the University Heart Center Graz, LKH-University Hospital, and Medical University of Graz (Austria), was more circumspect in his appraisal of the results.
He told this news organization that it is “always very difficult to establish therapy in pediatrics,” and believes that the drug “will give us an additional option” in managing HoFH.
However, Dr. Zirlik warned that he is a “little bit concerned” about lomitapide’s adverse event profile, and “would need to see a little bit deeper into the safety data.”
Highlighting the elevations in liver enzymes of around 25%, he asked: “What does it mean?” And how will it “play out in the long run?”
Beyond lomitapide, Dr. Zirlik pointed out that there are other drugs that have shown potential in managing HoFH and could potentially be used in the pediatric population, such as angiopoietin-like 3 protein (ANGPTL3) inhibitors and small interfering RNA (siRNA) compounds that target upstream production. “So, let’s see how they pan out,” he said.
Life-limiting condition
HoFH is an “ultra-rare, life-limiting condition,” with an estimated prevalence of approximately 3 per 1 million people, and a life expectancy in untreated patients of just 18 years, Dr. Masana said during his presentation.
Case series of lomitapide use in pediatric HoFH patients have shown encouraging results that are consistent with those seen in adults, he noted, with many able to achieve their LDL cholesterol target and stop or reduce apheresis.
To investigate further, a phase 3, single arm, open-label study was conducted. Following screening, 46 children and teenagers with HoFH underwent a 6- to 12-week run-in period, during which they were put on a low-fat diet with nutritional supplements.
“As you can imagine,” Dr. Masana said, “we are reducing the capacity for fat absorption with lomitapide, so the supplements and low-fat diet are necessary.”
Of these, 43 participants then entered a 24-week treatment period in which they were started on one of three doses, before undergoing dose escalation to the maximally tolerated dose. This was followed by an 80-week open-label safety phase, in which they continued on the maximally tolerated dose, then a follow-up period.
For the current presentation, Dr. Masana focused on the efficacy phase, showing that the mean age of participants was 10.7 years and that 55.8% were female. The HoFH diagnosis was confirmed genetically in 88.4% of cases.
Results showed that lomitapide was associated with a significant reduction in LDL cholesterol levels, from 435.8 mg/dL at baseline to 176.5 mg/dL at Week 24, which corresponded to a 53.5% overall reduction (P < .0001).
This meant that 41.9% of patients achieved their EAS LDL cholesterol target of less than 135 mg/dL at some point during the 24-week treatment period.
Stratifying by age, the reduction between baseline and week 24 was 538.5 mg/dL to 207.2 mg/dL, or 56.5%, in the 20 children aged 5-10 years, and 346.5 mg/dL to 149.9 mg/L, or 50.9%, in the 23 patients aged 11-17 years.
Dr. Masana explained that the results were “a little bit better in the younger group because they were receiving less treatment at this stage of the disease” than the older group.
He showed that lomitapide was associated with significant reductions in other lipid markers, including a 53.9% reduction in non–HDL cholesterol (P < .0001), a 50.1% drop in total cholesterol (P < .0001), and a 50.2% fall in very-low-density lipoprotein cholesterol (P < .0001).
Results showed 93% of patients experienced treatment-related adverse events, with 11.6% having serious events and 4.7% having events that led to study discontinuation. There was one (2.3%) major adverse cardiac event but no deaths.
He said that, despite these figures, the adverse events were “mostly mild or moderate.”
The majority (67%) of patients nevertheless had gastrointestinal adverse events, which were, “in general, associated with a lack of adherence to the low-fat diet.”
Aspartate aminotransferase levels were elevated in 23% of patients, while 28% had elevations in alanine aminotransferase, which were described by Dr. Masana as “moderate.”
The study was sponsored by Amryt Pharma. Dr. Masana declares relationships with Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Servier, Servier, and Viatrix.
A version of this article first appeared on Medscape.com.
MANNHEIM, Germany – Lomitapide, which reduces lipoprotein production in the liver, could help manage pediatric homozygous familial hypercholesterolemia (HoFH), suggest results of a trial that showed large reductions in circulating lipids.
The research was presented May 23 at the 91st European Atherosclerosis Society Congress.
Lomitapide inhibits microsomal triglyceride transfer protein, which plays a key role in apolipoprotein B-containing lipoprotein assembly and secretion in the liver and intestines. Crucially, the drug acts independently of the LDL cholesterol receptor.
It was approved in December 2012 by the U.S. Food and Drug Administration for use in adults with HoFH, sold under the name Juxtapid, and by the European Medicines Agency, where the brand name is Lojuxta.
The current trial involved more than 40 children and teenagers with HoFH aged 5-17 years; they were treated with the drug for 24 weeks, resulting in reductions of low density lipoprotein cholesterol of almost 54%, with nearly 42% reaching target levels.
The drug was also associated with marked reductions in other key lipids of at least 50%. However, 67% of patients also experienced gastrointestinal adverse events, and around 25% saw their levels of liver enzymes increase.
Early diagnosis ‘imperative’
The findings show that the “early diagnosis and treatment of HoFH is imperative,” said study presenter Luis Masana, MD, PhD, director of the Vascular Medicine and Metabolism Unit at Sant Joan de Reus University Hospital, Tarragona, Spain.
“I think that, with these results, we are bringing a new hope for this group of patients,” he continued. “I also think we will increase the quality of life, not just of the patients but also all the families involved in [managing] this problem.”
Session co-chair Andreas Zirlik, MD, PhD, head of the department of cardiology and chairman of the University Heart Center Graz, LKH-University Hospital, and Medical University of Graz (Austria), was more circumspect in his appraisal of the results.
He told this news organization that it is “always very difficult to establish therapy in pediatrics,” and believes that the drug “will give us an additional option” in managing HoFH.
However, Dr. Zirlik warned that he is a “little bit concerned” about lomitapide’s adverse event profile, and “would need to see a little bit deeper into the safety data.”
Highlighting the elevations in liver enzymes of around 25%, he asked: “What does it mean?” And how will it “play out in the long run?”
Beyond lomitapide, Dr. Zirlik pointed out that there are other drugs that have shown potential in managing HoFH and could potentially be used in the pediatric population, such as angiopoietin-like 3 protein (ANGPTL3) inhibitors and small interfering RNA (siRNA) compounds that target upstream production. “So, let’s see how they pan out,” he said.
Life-limiting condition
HoFH is an “ultra-rare, life-limiting condition,” with an estimated prevalence of approximately 3 per 1 million people, and a life expectancy in untreated patients of just 18 years, Dr. Masana said during his presentation.
Case series of lomitapide use in pediatric HoFH patients have shown encouraging results that are consistent with those seen in adults, he noted, with many able to achieve their LDL cholesterol target and stop or reduce apheresis.
To investigate further, a phase 3, single arm, open-label study was conducted. Following screening, 46 children and teenagers with HoFH underwent a 6- to 12-week run-in period, during which they were put on a low-fat diet with nutritional supplements.
“As you can imagine,” Dr. Masana said, “we are reducing the capacity for fat absorption with lomitapide, so the supplements and low-fat diet are necessary.”
Of these, 43 participants then entered a 24-week treatment period in which they were started on one of three doses, before undergoing dose escalation to the maximally tolerated dose. This was followed by an 80-week open-label safety phase, in which they continued on the maximally tolerated dose, then a follow-up period.
For the current presentation, Dr. Masana focused on the efficacy phase, showing that the mean age of participants was 10.7 years and that 55.8% were female. The HoFH diagnosis was confirmed genetically in 88.4% of cases.
Results showed that lomitapide was associated with a significant reduction in LDL cholesterol levels, from 435.8 mg/dL at baseline to 176.5 mg/dL at Week 24, which corresponded to a 53.5% overall reduction (P < .0001).
This meant that 41.9% of patients achieved their EAS LDL cholesterol target of less than 135 mg/dL at some point during the 24-week treatment period.
Stratifying by age, the reduction between baseline and week 24 was 538.5 mg/dL to 207.2 mg/dL, or 56.5%, in the 20 children aged 5-10 years, and 346.5 mg/dL to 149.9 mg/L, or 50.9%, in the 23 patients aged 11-17 years.
Dr. Masana explained that the results were “a little bit better in the younger group because they were receiving less treatment at this stage of the disease” than the older group.
He showed that lomitapide was associated with significant reductions in other lipid markers, including a 53.9% reduction in non–HDL cholesterol (P < .0001), a 50.1% drop in total cholesterol (P < .0001), and a 50.2% fall in very-low-density lipoprotein cholesterol (P < .0001).
Results showed 93% of patients experienced treatment-related adverse events, with 11.6% having serious events and 4.7% having events that led to study discontinuation. There was one (2.3%) major adverse cardiac event but no deaths.
He said that, despite these figures, the adverse events were “mostly mild or moderate.”
The majority (67%) of patients nevertheless had gastrointestinal adverse events, which were, “in general, associated with a lack of adherence to the low-fat diet.”
Aspartate aminotransferase levels were elevated in 23% of patients, while 28% had elevations in alanine aminotransferase, which were described by Dr. Masana as “moderate.”
The study was sponsored by Amryt Pharma. Dr. Masana declares relationships with Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Servier, Servier, and Viatrix.
A version of this article first appeared on Medscape.com.
MANNHEIM, Germany – Lomitapide, which reduces lipoprotein production in the liver, could help manage pediatric homozygous familial hypercholesterolemia (HoFH), suggest results of a trial that showed large reductions in circulating lipids.
The research was presented May 23 at the 91st European Atherosclerosis Society Congress.
Lomitapide inhibits microsomal triglyceride transfer protein, which plays a key role in apolipoprotein B-containing lipoprotein assembly and secretion in the liver and intestines. Crucially, the drug acts independently of the LDL cholesterol receptor.
It was approved in December 2012 by the U.S. Food and Drug Administration for use in adults with HoFH, sold under the name Juxtapid, and by the European Medicines Agency, where the brand name is Lojuxta.
The current trial involved more than 40 children and teenagers with HoFH aged 5-17 years; they were treated with the drug for 24 weeks, resulting in reductions of low density lipoprotein cholesterol of almost 54%, with nearly 42% reaching target levels.
The drug was also associated with marked reductions in other key lipids of at least 50%. However, 67% of patients also experienced gastrointestinal adverse events, and around 25% saw their levels of liver enzymes increase.
Early diagnosis ‘imperative’
The findings show that the “early diagnosis and treatment of HoFH is imperative,” said study presenter Luis Masana, MD, PhD, director of the Vascular Medicine and Metabolism Unit at Sant Joan de Reus University Hospital, Tarragona, Spain.
“I think that, with these results, we are bringing a new hope for this group of patients,” he continued. “I also think we will increase the quality of life, not just of the patients but also all the families involved in [managing] this problem.”
Session co-chair Andreas Zirlik, MD, PhD, head of the department of cardiology and chairman of the University Heart Center Graz, LKH-University Hospital, and Medical University of Graz (Austria), was more circumspect in his appraisal of the results.
He told this news organization that it is “always very difficult to establish therapy in pediatrics,” and believes that the drug “will give us an additional option” in managing HoFH.
However, Dr. Zirlik warned that he is a “little bit concerned” about lomitapide’s adverse event profile, and “would need to see a little bit deeper into the safety data.”
Highlighting the elevations in liver enzymes of around 25%, he asked: “What does it mean?” And how will it “play out in the long run?”
Beyond lomitapide, Dr. Zirlik pointed out that there are other drugs that have shown potential in managing HoFH and could potentially be used in the pediatric population, such as angiopoietin-like 3 protein (ANGPTL3) inhibitors and small interfering RNA (siRNA) compounds that target upstream production. “So, let’s see how they pan out,” he said.
Life-limiting condition
HoFH is an “ultra-rare, life-limiting condition,” with an estimated prevalence of approximately 3 per 1 million people, and a life expectancy in untreated patients of just 18 years, Dr. Masana said during his presentation.
Case series of lomitapide use in pediatric HoFH patients have shown encouraging results that are consistent with those seen in adults, he noted, with many able to achieve their LDL cholesterol target and stop or reduce apheresis.
To investigate further, a phase 3, single arm, open-label study was conducted. Following screening, 46 children and teenagers with HoFH underwent a 6- to 12-week run-in period, during which they were put on a low-fat diet with nutritional supplements.
“As you can imagine,” Dr. Masana said, “we are reducing the capacity for fat absorption with lomitapide, so the supplements and low-fat diet are necessary.”
Of these, 43 participants then entered a 24-week treatment period in which they were started on one of three doses, before undergoing dose escalation to the maximally tolerated dose. This was followed by an 80-week open-label safety phase, in which they continued on the maximally tolerated dose, then a follow-up period.
For the current presentation, Dr. Masana focused on the efficacy phase, showing that the mean age of participants was 10.7 years and that 55.8% were female. The HoFH diagnosis was confirmed genetically in 88.4% of cases.
Results showed that lomitapide was associated with a significant reduction in LDL cholesterol levels, from 435.8 mg/dL at baseline to 176.5 mg/dL at Week 24, which corresponded to a 53.5% overall reduction (P < .0001).
This meant that 41.9% of patients achieved their EAS LDL cholesterol target of less than 135 mg/dL at some point during the 24-week treatment period.
Stratifying by age, the reduction between baseline and week 24 was 538.5 mg/dL to 207.2 mg/dL, or 56.5%, in the 20 children aged 5-10 years, and 346.5 mg/dL to 149.9 mg/L, or 50.9%, in the 23 patients aged 11-17 years.
Dr. Masana explained that the results were “a little bit better in the younger group because they were receiving less treatment at this stage of the disease” than the older group.
He showed that lomitapide was associated with significant reductions in other lipid markers, including a 53.9% reduction in non–HDL cholesterol (P < .0001), a 50.1% drop in total cholesterol (P < .0001), and a 50.2% fall in very-low-density lipoprotein cholesterol (P < .0001).
Results showed 93% of patients experienced treatment-related adverse events, with 11.6% having serious events and 4.7% having events that led to study discontinuation. There was one (2.3%) major adverse cardiac event but no deaths.
He said that, despite these figures, the adverse events were “mostly mild or moderate.”
The majority (67%) of patients nevertheless had gastrointestinal adverse events, which were, “in general, associated with a lack of adherence to the low-fat diet.”
Aspartate aminotransferase levels were elevated in 23% of patients, while 28% had elevations in alanine aminotransferase, which were described by Dr. Masana as “moderate.”
The study was sponsored by Amryt Pharma. Dr. Masana declares relationships with Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Servier, Servier, and Viatrix.
A version of this article first appeared on Medscape.com.