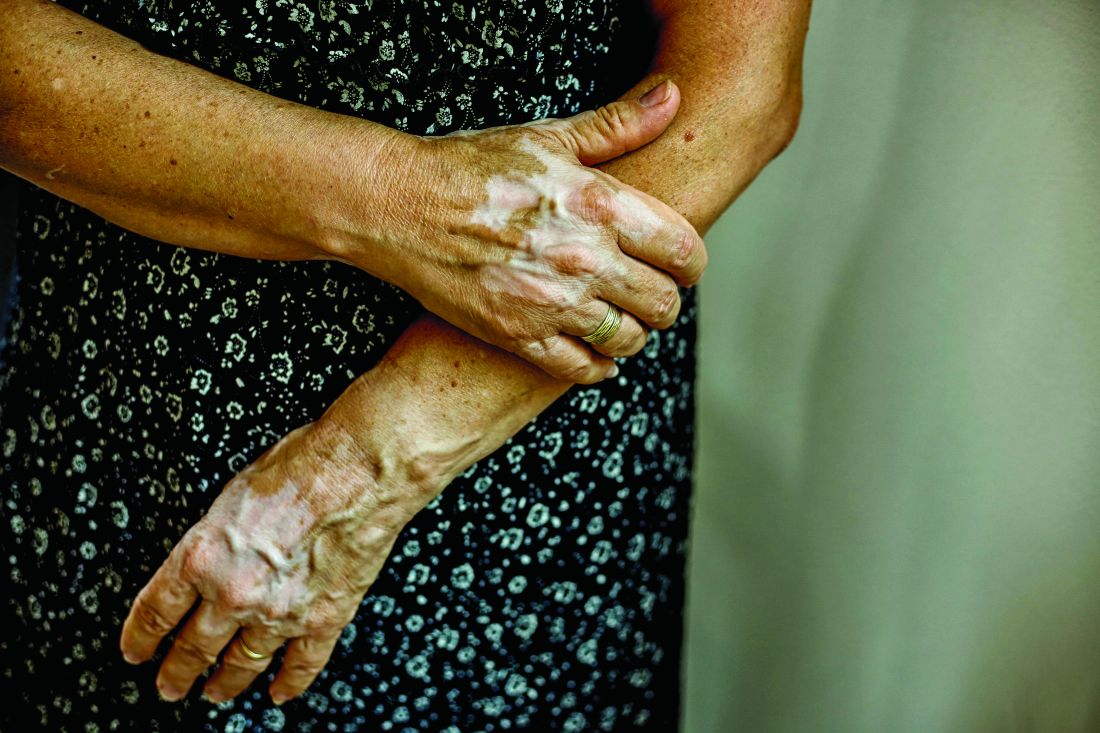
User login
Seborrheic Dermatitis
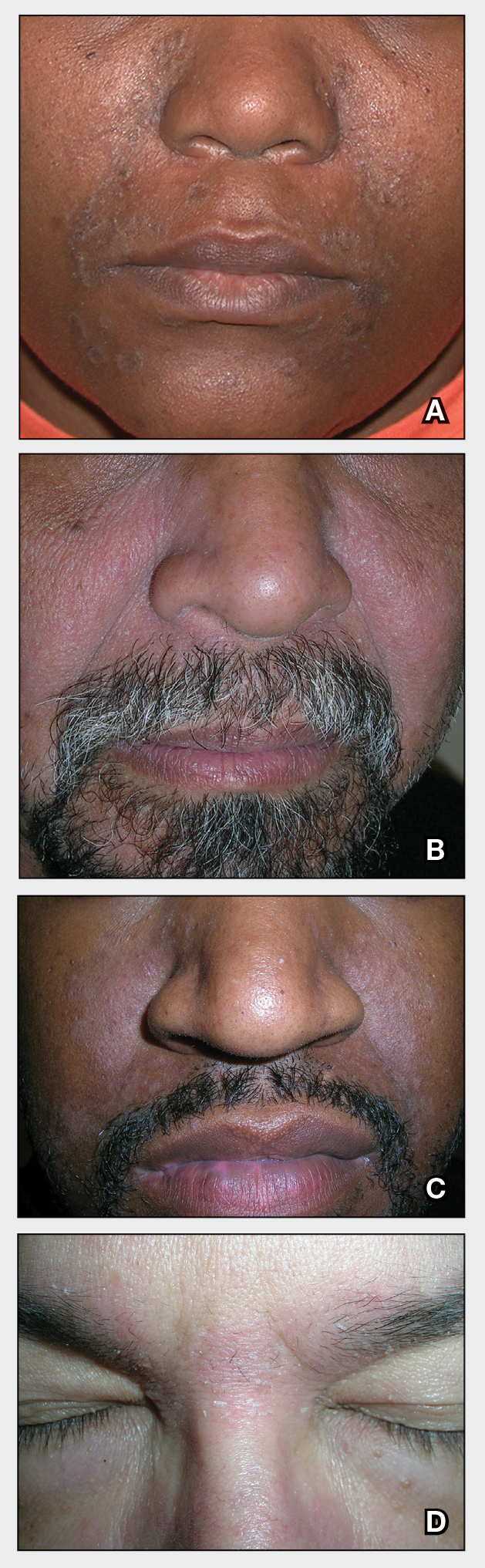
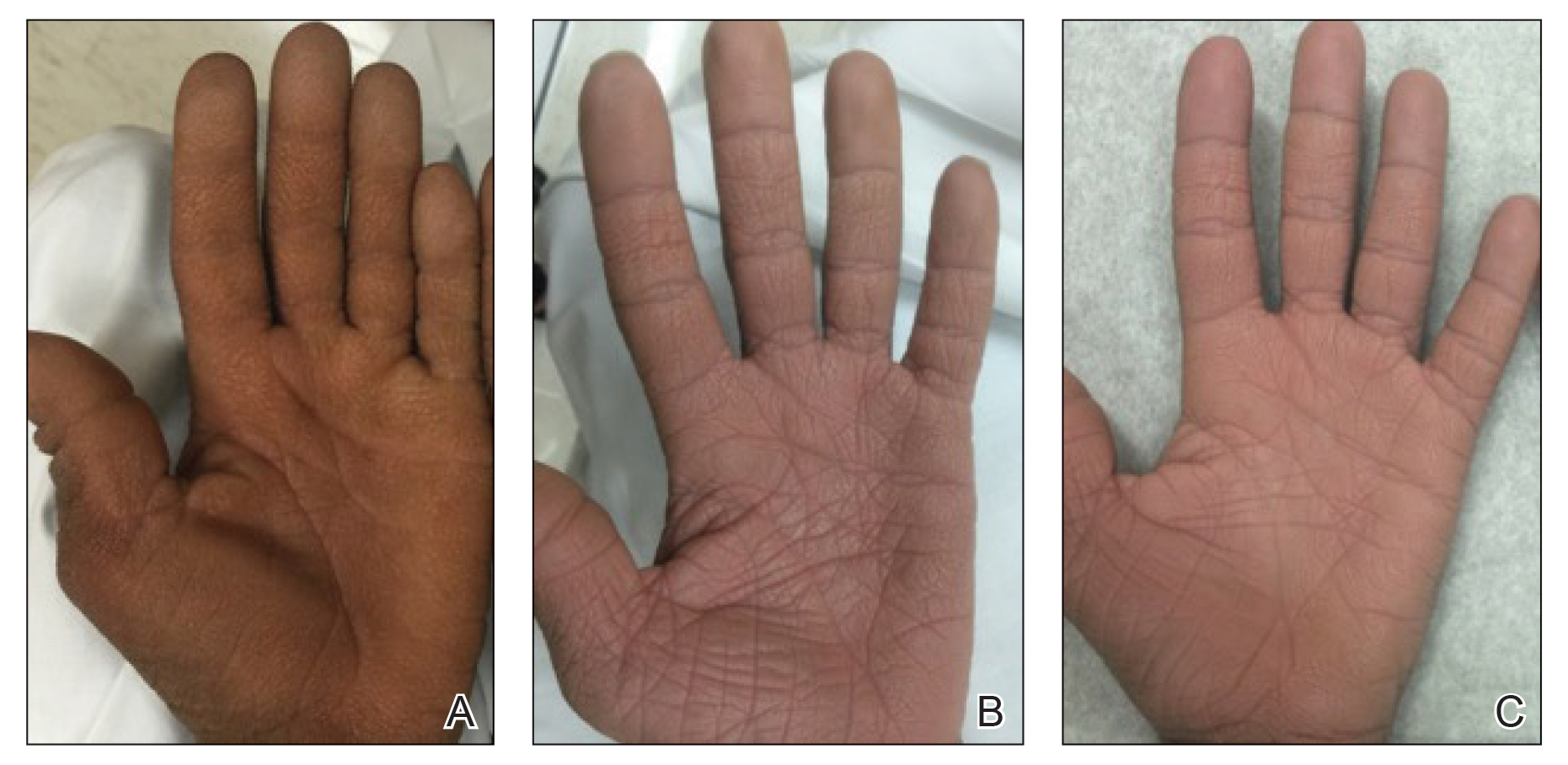
THE COMPARISON
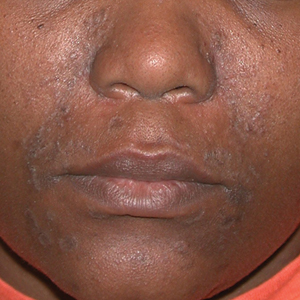
A Seborrheic dermatitis in a woman with brown-gray greasy scale as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
Seborrheic dermatitis affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petal–like) plaques may be present (Figure A). Also, hypopigmented patches and plaques may be prominent (Figures B and C). The classic description includes thin pink patches and plaques with white greasy scale on the face (Figure D).
- The scalp may have diffuse scale or isolated scaly plaques.
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school-aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
Seborrheic dermatitis is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. Seborrheic dermatitis of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/pde.14525
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j .jaad.2010.05.037
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018; 17:1032-1036.

THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
Seborrheic dermatitis affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petal–like) plaques may be present (Figure A). Also, hypopigmented patches and plaques may be prominent (Figures B and C). The classic description includes thin pink patches and plaques with white greasy scale on the face (Figure D).
- The scalp may have diffuse scale or isolated scaly plaques.
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school-aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
Seborrheic dermatitis is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. Seborrheic dermatitis of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.

THE COMPARISON
A Seborrheic dermatitis in a woman with brown-gray greasy scale as well as petaloid papules and plaques that are especially prominent in the nasolabial folds.
B Seborrheic dermatitis in a man with erythema, scale, and mild postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
C Seborrheic dermatitis in a man with erythema, faint scale, and postinflammatory hypopigmentation that are especially prominent in the nasolabial folds.
D Seborrheic dermatitis in a man with erythema and scale of the eyebrows and glabellar region.
Seborrheic dermatitis (SD) is an inflammatory condition that is thought to be part of a response to Malassezia yeast. The scalp and face are most commonly affected, particularly the nasolabial folds, eyebrows, ears, postauricular areas, and beard area. Men also may have SD on the mid upper chest in association with chest hair. In infants, the scalp and body skin folds often are affected.
Epidemiology
Seborrheic dermatitis affects patients of all ages: infants, adolescents, and adults. It is among the most common dermatologic diagnoses reported in Black patients in the United States.1
Key clinical features in darker skin tones
- In those with darker skin tones, arcuate, polycyclic, or petaloid (flower petal–like) plaques may be present (Figure A). Also, hypopigmented patches and plaques may be prominent (Figures B and C). The classic description includes thin pink patches and plaques with white greasy scale on the face (Figure D).
- The scalp may have diffuse scale or isolated scaly plaques.
Worth noting
- In those with tightly coiled hair, there is a predisposition for dry hair and increased risk for breakage.
- Treatment plans for patients with SD often include frequent hair washing. However, in those with tightly coiled hair, the treatment plan may need to be modified due to hair texture, tendency for dryness, and washing frequency preferences. Washing the scalp at least every 1 to 2 weeks may be a preferred approach for those with tightly coiled hair at increased risk for dryness/breakage vs washing daily.2 In a sample of 201 caregivers of Black girls, Rucker Wright et al3 found that washing the hair more than once per week was not correlated with a lower prevalence of SD.
- If tightly coiled hair is temporarily straightened with heat (eg, blow-dryer, flat iron), adding a liquid-based treatment such as clobetasol solution or fluocinonide solution will cause the hair to revert to its normal curl pattern.
- It is appropriate to ask patients for their vehicle preference for medications.2 For example, if clobetasol is the treatment selected for the patient, the vehicle can reflect patient preference for a liquid, foam, cream, or ointment.
- Some antifungal/antiyeast shampoos may cause further hair dryness and breakage.
- Treatment may be delayed because patients often use various topical pomades and ointments to cover up the scale and help with pruritus.
- Diffuse scale of tinea capitis in school-aged children can be mistaken for SD, which leads to delayed diagnosis and treatment.
- Clinicians should become comfortable with scalp examinations in patients with tightly coiled hair. Patients with chief concerns related to their hair and scalp expect their clinicians to touch these areas. Avoid leaning in to examine the patient without touching the patient’s hair and scalp.2,4
Health disparity highlight
Seborrheic dermatitis is among the most common cutaneous disorders diagnosed in patients with skin of color.1,5 Delay in recognition of SD in those with darker skin tones leads to delayed treatment. Seborrheic dermatitis of the face can cause notable postinflammatory pigmentation alteration. Pigmentation changes in the skin further impact quality of life.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/pde.14525
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j .jaad.2010.05.037
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018; 17:1032-1036.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. 2021;10.1111/pde.14525
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j .jaad.2010.05.037
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Gaulding JV, Gutierrez D, Bhatia BK, et al. Epidemiology of skin diseases in a diverse patient population. J Drugs Dermatol. 2018; 17:1032-1036.
Major increase seen in cosmeceutical alternatives to topical hydroquinone
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
FROM SOC 2021
Paraneoplastic Signs in Bladder Transitional Cell Carcinoma: An Unusual Presentation
To the Editor:
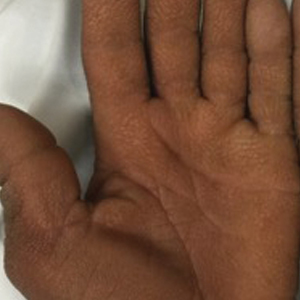
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.
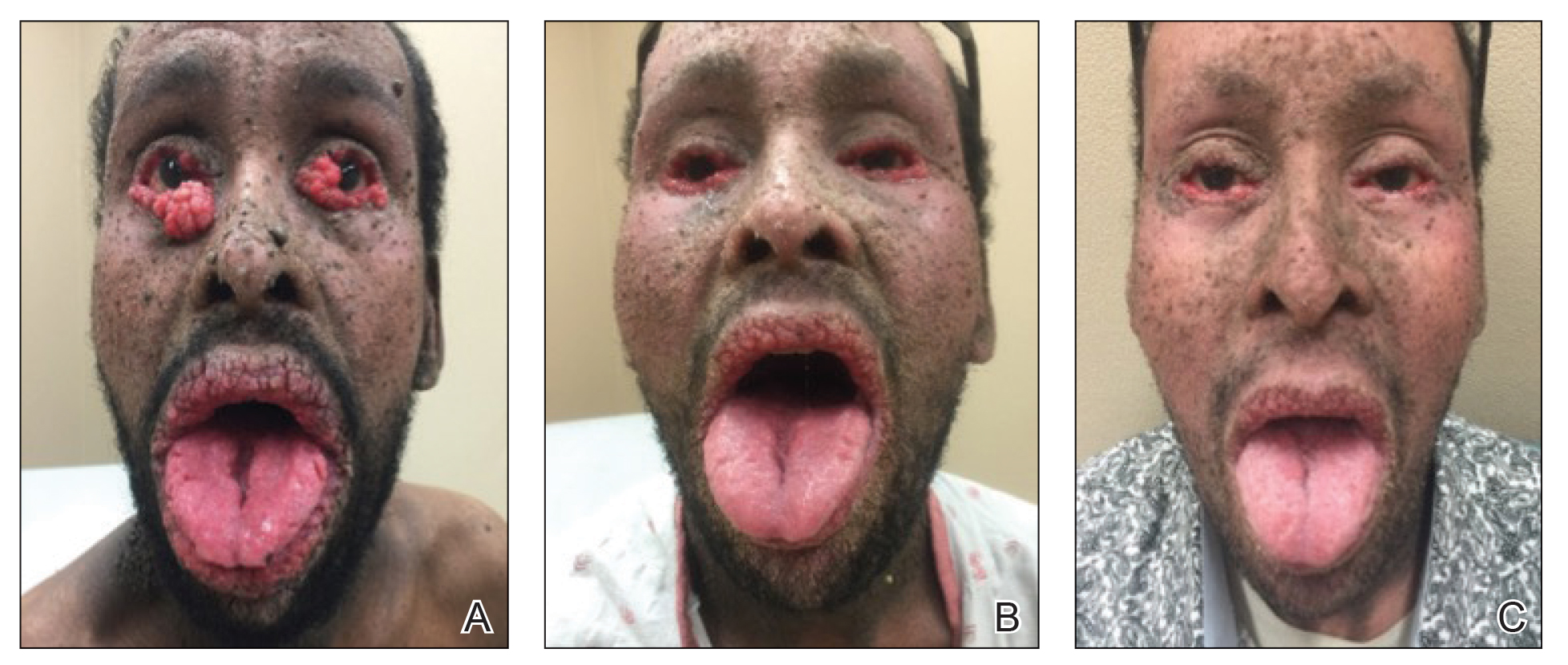
Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.
This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
To the Editor:
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.

Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.

This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
To the Editor:
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.

Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.

This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
Practice Points
- Paraneoplastic conditions may present secondary to urologic malignancy. Providers should perform thorough malignancy screening, including urologic cystoscopy, in patients presenting with paraneoplastic signs and no identified malignancy.
- Oral retinoids, such as acitretin, may be used as an adjuvant treatment to treat paraneoplastic cutaneous symptoms. The definitive treatment is malignancy management.
Chronic Hyperpigmented Patches on the Legs
The Diagnosis: Drug-Induced Hyperpigmentation
Additional history provided by the patient’s caretaker elucidated an extensive list of medications including chlorpromazine and minocycline, among several others. The caretaker revealed that the patient began treatment for acne vulgaris 2 years prior; despite the acne resolving, therapy was not discontinued. The blue-gray and brown pigmentation on our patient’s shins likely was attributed to a medication he was taking.
Both chlorpromazine and minocycline, among many other medications, are known to cause abnormal pigmentation of the skin.1 Minocycline is a tetracycline antibiotic prescribed for acne and other inflammatory cutaneous conditions. It is highly lipophilic, allowing it to reach high drug concentrations in the skin and nail unit.2 Patients taking minocycline long term and at high doses are at greatest risk for pigment deposition.3,4
Minocycline-induced hyperpigmentation is classified into 3 types. Type I describes blue-black deposition of pigment in acne scars and areas of inflammation, typically on facial skin.1,5 Histologically, type I stains positive for Perls Prussian blue, indicating an increased deposition of iron as hemosiderin,1 which likely occurs because minocycline is thought to play a role in defective clearance of hemosiderin from the dermis of injured tissue.5 Type II hyperpigmentation presents as bluegray pigment on the lower legs and occasionally the arms.6,7 Type II stains positive for both Perls Prussian blue and Fontana-Masson, demonstrating hemosiderin and melanin, respectively.6 The third form of hyperpigmentation results in diffuse, dark brown to gray pigmentation with a predilection for sun-exposed areas.8 Histology of type III shows increased pigment in the basal portion of the epidermis and brown-black pigment in macrophages of the dermis. Type III stains positive for Fontana-Masson and negative for Perls Prussian blue. The etiology of hyperpigmentation has been suspected to be caused by minocycline stimulating melanin production and/or deposition of minocycline-melanin complexes in dermal macrophages after a certain drug level; this largely is seen in patients receiving 100 to 200 mg daily as early as 1 year into treatment.8
Chlorpromazine is a typical antipsychotic that causes abnormal skin pigmentation in sun-exposed areas due to increased melanogenesis.9 Similar to type III minocyclineinduced hyperpigmentation, a histologic specimen may stain positive for Fontana-Masson yet negative for Perls Prussian blue. Lal et al10 demonstrated complete resolution of abnormal skin pigmentation within 5 years after stopping chlorpromazine. In contrast, minocyclineinduced hyperpigmentation may be permanent in some cases. There is substantial clinical and histologic overlap for drug-induced hyperpigmentation etiologies; it would behoove the clinician to focus on the most common locations affected and the generalized coloration.
Treatment of minocycline-induced hyperpigmentation includes the use of Q-switched lasers, specifically Q-switched ruby and Q-switched alexandrite.11 The use of the Q-switched Nd:YAG laser appears to be ineffective at clearing minocycline-induced pigmentation.7,11 In our patient, minocycline was discontinued immediately. Due to the patient’s critical condition, he deferred all other therapy. Erythema dyschromicum perstans, also referred to as ashy dermatosis, is an idiopathic form of hyperpigmentation.12 Lesions start as blue-gray to ashy gray macules, occasionally surrounded by a slightly erythematous, raised border.
Erythema dyschromicum perstans typically presents on the trunk, face, and arms of patients with Fitzpatrick skin types III and IV; it is considered a variant of lichen planus actinicus.12 Histologically, erythema dyschromicum perstans may mimic lichen planus pigmentosus (LPP); however, subtle differences exist to distinguish the 2 conditions. Erythema dyschromicum perstans demonstrates a mild lichenoid infiltrate, focal basal vacuolization at the dermoepidermal junction, and melanophage deposition.13 In contrast, LPP demonstrates pigmentary incontinence and a more severe inflammatory infiltrate. A perifollicular infiltrate and fibrosis also can be seen in LPP, which may explain the frontal fibrosing alopecia that often precedes LPP.13
Addison disease, also known as primary adrenal insufficiency, can cause diffuse hyperpigmentation in the skin, mucosae, and nail beds. The pigmentation is prominent in regions of naturally increased pigmentation, such as the flexural surfaces and intertriginous areas.14 Patients with adrenal insufficiency will have accompanying weight loss, hypotension, and fatigue, among other symptoms related to deficiency of cortisol and aldosterone. Skin biopsy shows acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, superficial perivascular lymphocytic infiltrate, basal melanin deposition, and superficial dermal macrophages.15
Confluent and reticulated papillomatosis is an uncommon dermatosis that presents with multiple hyperpigmented macules and papules that coalesce to form patches and plaques centrally with reticulation in the periphery.16 Confluent and reticulated papillomatosis commonly presents on the upper trunk, axillae, and neck, though involvement can include flexural surfaces as well as the lower trunk and legs.16,17 Biopsy demonstrates undulating hyperkeratosis, papillomatosis, acanthosis, and negative fungal staining.16
Pretibial myxedema most commonly is associated with Graves disease and presents as well-defined thickening and induration with overlying pink or purple-brown papules in the pretibial region.18 An acral surface and mucin deposition within the entire dermis may be appreciated on histology with staining for colloidal iron or Alcian blue.
- Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980;244:1103-1106. doi:10.1001/jama.1980.03310100021021
- Snodgrass A, Motaparthi K. Systemic antibacterial agents. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2020:69-98.
- Eisen D, Hakim MD. Minocycline-induced pigmentation. incidence, prevention and management. Drug Saf. 1998;18:431-440. doi:10.2165/00002018-199818060-00004
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
- Basler RS, Kohnen PW. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978;114:1695-1697.
- Ridgway HA, Sonnex TS, Kennedy CT, et al. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982;107:95-102. doi:10.1111/j.1365-2133.1982.tb00296.x
- Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
- Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3:244-247. doi:10.1016/s0190 -9622(80)80186-1
- Perry TL, Culling CF, Berry K, et al. 7-Hydroxychlorpromazine: potential toxic drug metabolite in psychiatric patients. Science. 1964;146:81-83. doi:10.1126/science.146.3640.81
- Lal S, Bloom D, Silver B, et al. Replacement of chlorpromazine with other neuroleptics: effect on abnormal skin pigmentation and ocular changes. J Psychiatry Neurosci. 1993;18:173-177.
- Tsao H, Busam K, Barnhill RL, et al. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250-1251.
- Knox JM, Dodge BG, Freeman RG. Erythema dyschromicum perstans. Arch Dermatol. 1968;97:262-272. doi:10.1001 /archderm.1968.01610090034006
- Rutnin S, Udompanich S, Pratumchart N, et al. Ashy dermatosis and lichen planus pigmentosus: the histopathological differences. Biomed Res Int. 2019;2019:5829185. doi:10.1155/2019/5829185
- Montgomery H, O’Leary PA. Pigmentation of the skin in Addison’s disease, acanthosis nigricans and hemochromatosis. Arch Derm Syphilol. 1930;21:970-984. doi:10.1001 /archderm.1930.01440120072005
- Fernandez-Flores A, Cassarino DS. Histopathologic findings of cutaneous hyperpigmentation in Addison disease and immunostain of the melanocytic population. Am J Dermatopathol. 2017;39:924-927. doi:10.1097/DAD.0000000000000937
- Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. a study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi:10.1111/j.1365-2133.2005.06955.x
- Jo S, Park HS, Cho S, et al. Updated diagnosis criteria for confluent and reticulated papillomatosis: a case report. Ann Dermatol. 2014; 26:409-410. doi:10.5021/ad.2014.26.3.409
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
The Diagnosis: Drug-Induced Hyperpigmentation
Additional history provided by the patient’s caretaker elucidated an extensive list of medications including chlorpromazine and minocycline, among several others. The caretaker revealed that the patient began treatment for acne vulgaris 2 years prior; despite the acne resolving, therapy was not discontinued. The blue-gray and brown pigmentation on our patient’s shins likely was attributed to a medication he was taking.
Both chlorpromazine and minocycline, among many other medications, are known to cause abnormal pigmentation of the skin.1 Minocycline is a tetracycline antibiotic prescribed for acne and other inflammatory cutaneous conditions. It is highly lipophilic, allowing it to reach high drug concentrations in the skin and nail unit.2 Patients taking minocycline long term and at high doses are at greatest risk for pigment deposition.3,4
Minocycline-induced hyperpigmentation is classified into 3 types. Type I describes blue-black deposition of pigment in acne scars and areas of inflammation, typically on facial skin.1,5 Histologically, type I stains positive for Perls Prussian blue, indicating an increased deposition of iron as hemosiderin,1 which likely occurs because minocycline is thought to play a role in defective clearance of hemosiderin from the dermis of injured tissue.5 Type II hyperpigmentation presents as bluegray pigment on the lower legs and occasionally the arms.6,7 Type II stains positive for both Perls Prussian blue and Fontana-Masson, demonstrating hemosiderin and melanin, respectively.6 The third form of hyperpigmentation results in diffuse, dark brown to gray pigmentation with a predilection for sun-exposed areas.8 Histology of type III shows increased pigment in the basal portion of the epidermis and brown-black pigment in macrophages of the dermis. Type III stains positive for Fontana-Masson and negative for Perls Prussian blue. The etiology of hyperpigmentation has been suspected to be caused by minocycline stimulating melanin production and/or deposition of minocycline-melanin complexes in dermal macrophages after a certain drug level; this largely is seen in patients receiving 100 to 200 mg daily as early as 1 year into treatment.8
Chlorpromazine is a typical antipsychotic that causes abnormal skin pigmentation in sun-exposed areas due to increased melanogenesis.9 Similar to type III minocyclineinduced hyperpigmentation, a histologic specimen may stain positive for Fontana-Masson yet negative for Perls Prussian blue. Lal et al10 demonstrated complete resolution of abnormal skin pigmentation within 5 years after stopping chlorpromazine. In contrast, minocyclineinduced hyperpigmentation may be permanent in some cases. There is substantial clinical and histologic overlap for drug-induced hyperpigmentation etiologies; it would behoove the clinician to focus on the most common locations affected and the generalized coloration.
Treatment of minocycline-induced hyperpigmentation includes the use of Q-switched lasers, specifically Q-switched ruby and Q-switched alexandrite.11 The use of the Q-switched Nd:YAG laser appears to be ineffective at clearing minocycline-induced pigmentation.7,11 In our patient, minocycline was discontinued immediately. Due to the patient’s critical condition, he deferred all other therapy. Erythema dyschromicum perstans, also referred to as ashy dermatosis, is an idiopathic form of hyperpigmentation.12 Lesions start as blue-gray to ashy gray macules, occasionally surrounded by a slightly erythematous, raised border.
Erythema dyschromicum perstans typically presents on the trunk, face, and arms of patients with Fitzpatrick skin types III and IV; it is considered a variant of lichen planus actinicus.12 Histologically, erythema dyschromicum perstans may mimic lichen planus pigmentosus (LPP); however, subtle differences exist to distinguish the 2 conditions. Erythema dyschromicum perstans demonstrates a mild lichenoid infiltrate, focal basal vacuolization at the dermoepidermal junction, and melanophage deposition.13 In contrast, LPP demonstrates pigmentary incontinence and a more severe inflammatory infiltrate. A perifollicular infiltrate and fibrosis also can be seen in LPP, which may explain the frontal fibrosing alopecia that often precedes LPP.13
Addison disease, also known as primary adrenal insufficiency, can cause diffuse hyperpigmentation in the skin, mucosae, and nail beds. The pigmentation is prominent in regions of naturally increased pigmentation, such as the flexural surfaces and intertriginous areas.14 Patients with adrenal insufficiency will have accompanying weight loss, hypotension, and fatigue, among other symptoms related to deficiency of cortisol and aldosterone. Skin biopsy shows acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, superficial perivascular lymphocytic infiltrate, basal melanin deposition, and superficial dermal macrophages.15
Confluent and reticulated papillomatosis is an uncommon dermatosis that presents with multiple hyperpigmented macules and papules that coalesce to form patches and plaques centrally with reticulation in the periphery.16 Confluent and reticulated papillomatosis commonly presents on the upper trunk, axillae, and neck, though involvement can include flexural surfaces as well as the lower trunk and legs.16,17 Biopsy demonstrates undulating hyperkeratosis, papillomatosis, acanthosis, and negative fungal staining.16
Pretibial myxedema most commonly is associated with Graves disease and presents as well-defined thickening and induration with overlying pink or purple-brown papules in the pretibial region.18 An acral surface and mucin deposition within the entire dermis may be appreciated on histology with staining for colloidal iron or Alcian blue.
The Diagnosis: Drug-Induced Hyperpigmentation
Additional history provided by the patient’s caretaker elucidated an extensive list of medications including chlorpromazine and minocycline, among several others. The caretaker revealed that the patient began treatment for acne vulgaris 2 years prior; despite the acne resolving, therapy was not discontinued. The blue-gray and brown pigmentation on our patient’s shins likely was attributed to a medication he was taking.
Both chlorpromazine and minocycline, among many other medications, are known to cause abnormal pigmentation of the skin.1 Minocycline is a tetracycline antibiotic prescribed for acne and other inflammatory cutaneous conditions. It is highly lipophilic, allowing it to reach high drug concentrations in the skin and nail unit.2 Patients taking minocycline long term and at high doses are at greatest risk for pigment deposition.3,4
Minocycline-induced hyperpigmentation is classified into 3 types. Type I describes blue-black deposition of pigment in acne scars and areas of inflammation, typically on facial skin.1,5 Histologically, type I stains positive for Perls Prussian blue, indicating an increased deposition of iron as hemosiderin,1 which likely occurs because minocycline is thought to play a role in defective clearance of hemosiderin from the dermis of injured tissue.5 Type II hyperpigmentation presents as bluegray pigment on the lower legs and occasionally the arms.6,7 Type II stains positive for both Perls Prussian blue and Fontana-Masson, demonstrating hemosiderin and melanin, respectively.6 The third form of hyperpigmentation results in diffuse, dark brown to gray pigmentation with a predilection for sun-exposed areas.8 Histology of type III shows increased pigment in the basal portion of the epidermis and brown-black pigment in macrophages of the dermis. Type III stains positive for Fontana-Masson and negative for Perls Prussian blue. The etiology of hyperpigmentation has been suspected to be caused by minocycline stimulating melanin production and/or deposition of minocycline-melanin complexes in dermal macrophages after a certain drug level; this largely is seen in patients receiving 100 to 200 mg daily as early as 1 year into treatment.8
Chlorpromazine is a typical antipsychotic that causes abnormal skin pigmentation in sun-exposed areas due to increased melanogenesis.9 Similar to type III minocyclineinduced hyperpigmentation, a histologic specimen may stain positive for Fontana-Masson yet negative for Perls Prussian blue. Lal et al10 demonstrated complete resolution of abnormal skin pigmentation within 5 years after stopping chlorpromazine. In contrast, minocyclineinduced hyperpigmentation may be permanent in some cases. There is substantial clinical and histologic overlap for drug-induced hyperpigmentation etiologies; it would behoove the clinician to focus on the most common locations affected and the generalized coloration.
Treatment of minocycline-induced hyperpigmentation includes the use of Q-switched lasers, specifically Q-switched ruby and Q-switched alexandrite.11 The use of the Q-switched Nd:YAG laser appears to be ineffective at clearing minocycline-induced pigmentation.7,11 In our patient, minocycline was discontinued immediately. Due to the patient’s critical condition, he deferred all other therapy. Erythema dyschromicum perstans, also referred to as ashy dermatosis, is an idiopathic form of hyperpigmentation.12 Lesions start as blue-gray to ashy gray macules, occasionally surrounded by a slightly erythematous, raised border.
Erythema dyschromicum perstans typically presents on the trunk, face, and arms of patients with Fitzpatrick skin types III and IV; it is considered a variant of lichen planus actinicus.12 Histologically, erythema dyschromicum perstans may mimic lichen planus pigmentosus (LPP); however, subtle differences exist to distinguish the 2 conditions. Erythema dyschromicum perstans demonstrates a mild lichenoid infiltrate, focal basal vacuolization at the dermoepidermal junction, and melanophage deposition.13 In contrast, LPP demonstrates pigmentary incontinence and a more severe inflammatory infiltrate. A perifollicular infiltrate and fibrosis also can be seen in LPP, which may explain the frontal fibrosing alopecia that often precedes LPP.13
Addison disease, also known as primary adrenal insufficiency, can cause diffuse hyperpigmentation in the skin, mucosae, and nail beds. The pigmentation is prominent in regions of naturally increased pigmentation, such as the flexural surfaces and intertriginous areas.14 Patients with adrenal insufficiency will have accompanying weight loss, hypotension, and fatigue, among other symptoms related to deficiency of cortisol and aldosterone. Skin biopsy shows acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, superficial perivascular lymphocytic infiltrate, basal melanin deposition, and superficial dermal macrophages.15
Confluent and reticulated papillomatosis is an uncommon dermatosis that presents with multiple hyperpigmented macules and papules that coalesce to form patches and plaques centrally with reticulation in the periphery.16 Confluent and reticulated papillomatosis commonly presents on the upper trunk, axillae, and neck, though involvement can include flexural surfaces as well as the lower trunk and legs.16,17 Biopsy demonstrates undulating hyperkeratosis, papillomatosis, acanthosis, and negative fungal staining.16
Pretibial myxedema most commonly is associated with Graves disease and presents as well-defined thickening and induration with overlying pink or purple-brown papules in the pretibial region.18 An acral surface and mucin deposition within the entire dermis may be appreciated on histology with staining for colloidal iron or Alcian blue.
- Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980;244:1103-1106. doi:10.1001/jama.1980.03310100021021
- Snodgrass A, Motaparthi K. Systemic antibacterial agents. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2020:69-98.
- Eisen D, Hakim MD. Minocycline-induced pigmentation. incidence, prevention and management. Drug Saf. 1998;18:431-440. doi:10.2165/00002018-199818060-00004
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
- Basler RS, Kohnen PW. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978;114:1695-1697.
- Ridgway HA, Sonnex TS, Kennedy CT, et al. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982;107:95-102. doi:10.1111/j.1365-2133.1982.tb00296.x
- Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
- Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3:244-247. doi:10.1016/s0190 -9622(80)80186-1
- Perry TL, Culling CF, Berry K, et al. 7-Hydroxychlorpromazine: potential toxic drug metabolite in psychiatric patients. Science. 1964;146:81-83. doi:10.1126/science.146.3640.81
- Lal S, Bloom D, Silver B, et al. Replacement of chlorpromazine with other neuroleptics: effect on abnormal skin pigmentation and ocular changes. J Psychiatry Neurosci. 1993;18:173-177.
- Tsao H, Busam K, Barnhill RL, et al. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250-1251.
- Knox JM, Dodge BG, Freeman RG. Erythema dyschromicum perstans. Arch Dermatol. 1968;97:262-272. doi:10.1001 /archderm.1968.01610090034006
- Rutnin S, Udompanich S, Pratumchart N, et al. Ashy dermatosis and lichen planus pigmentosus: the histopathological differences. Biomed Res Int. 2019;2019:5829185. doi:10.1155/2019/5829185
- Montgomery H, O’Leary PA. Pigmentation of the skin in Addison’s disease, acanthosis nigricans and hemochromatosis. Arch Derm Syphilol. 1930;21:970-984. doi:10.1001 /archderm.1930.01440120072005
- Fernandez-Flores A, Cassarino DS. Histopathologic findings of cutaneous hyperpigmentation in Addison disease and immunostain of the melanocytic population. Am J Dermatopathol. 2017;39:924-927. doi:10.1097/DAD.0000000000000937
- Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. a study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi:10.1111/j.1365-2133.2005.06955.x
- Jo S, Park HS, Cho S, et al. Updated diagnosis criteria for confluent and reticulated papillomatosis: a case report. Ann Dermatol. 2014; 26:409-410. doi:10.5021/ad.2014.26.3.409
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
- Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980;244:1103-1106. doi:10.1001/jama.1980.03310100021021
- Snodgrass A, Motaparthi K. Systemic antibacterial agents. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2020:69-98.
- Eisen D, Hakim MD. Minocycline-induced pigmentation. incidence, prevention and management. Drug Saf. 1998;18:431-440. doi:10.2165/00002018-199818060-00004
- Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693-695. doi:10.1111/j.1365-2133.1996.tb06972.x
- Basler RS, Kohnen PW. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978;114:1695-1697.
- Ridgway HA, Sonnex TS, Kennedy CT, et al. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982;107:95-102. doi:10.1111/j.1365-2133.1982.tb00296.x
- Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162. doi:10.2147/CCID.S42166
- Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3:244-247. doi:10.1016/s0190 -9622(80)80186-1
- Perry TL, Culling CF, Berry K, et al. 7-Hydroxychlorpromazine: potential toxic drug metabolite in psychiatric patients. Science. 1964;146:81-83. doi:10.1126/science.146.3640.81
- Lal S, Bloom D, Silver B, et al. Replacement of chlorpromazine with other neuroleptics: effect on abnormal skin pigmentation and ocular changes. J Psychiatry Neurosci. 1993;18:173-177.
- Tsao H, Busam K, Barnhill RL, et al. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250-1251.
- Knox JM, Dodge BG, Freeman RG. Erythema dyschromicum perstans. Arch Dermatol. 1968;97:262-272. doi:10.1001 /archderm.1968.01610090034006
- Rutnin S, Udompanich S, Pratumchart N, et al. Ashy dermatosis and lichen planus pigmentosus: the histopathological differences. Biomed Res Int. 2019;2019:5829185. doi:10.1155/2019/5829185
- Montgomery H, O’Leary PA. Pigmentation of the skin in Addison’s disease, acanthosis nigricans and hemochromatosis. Arch Derm Syphilol. 1930;21:970-984. doi:10.1001 /archderm.1930.01440120072005
- Fernandez-Flores A, Cassarino DS. Histopathologic findings of cutaneous hyperpigmentation in Addison disease and immunostain of the melanocytic population. Am J Dermatopathol. 2017;39:924-927. doi:10.1097/DAD.0000000000000937
- Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. a study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287-293. doi:10.1111/j.1365-2133.2005.06955.x
- Jo S, Park HS, Cho S, et al. Updated diagnosis criteria for confluent and reticulated papillomatosis: a case report. Ann Dermatol. 2014; 26:409-410. doi:10.5021/ad.2014.26.3.409
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312. doi:10.21037 /tp.2017.09.08
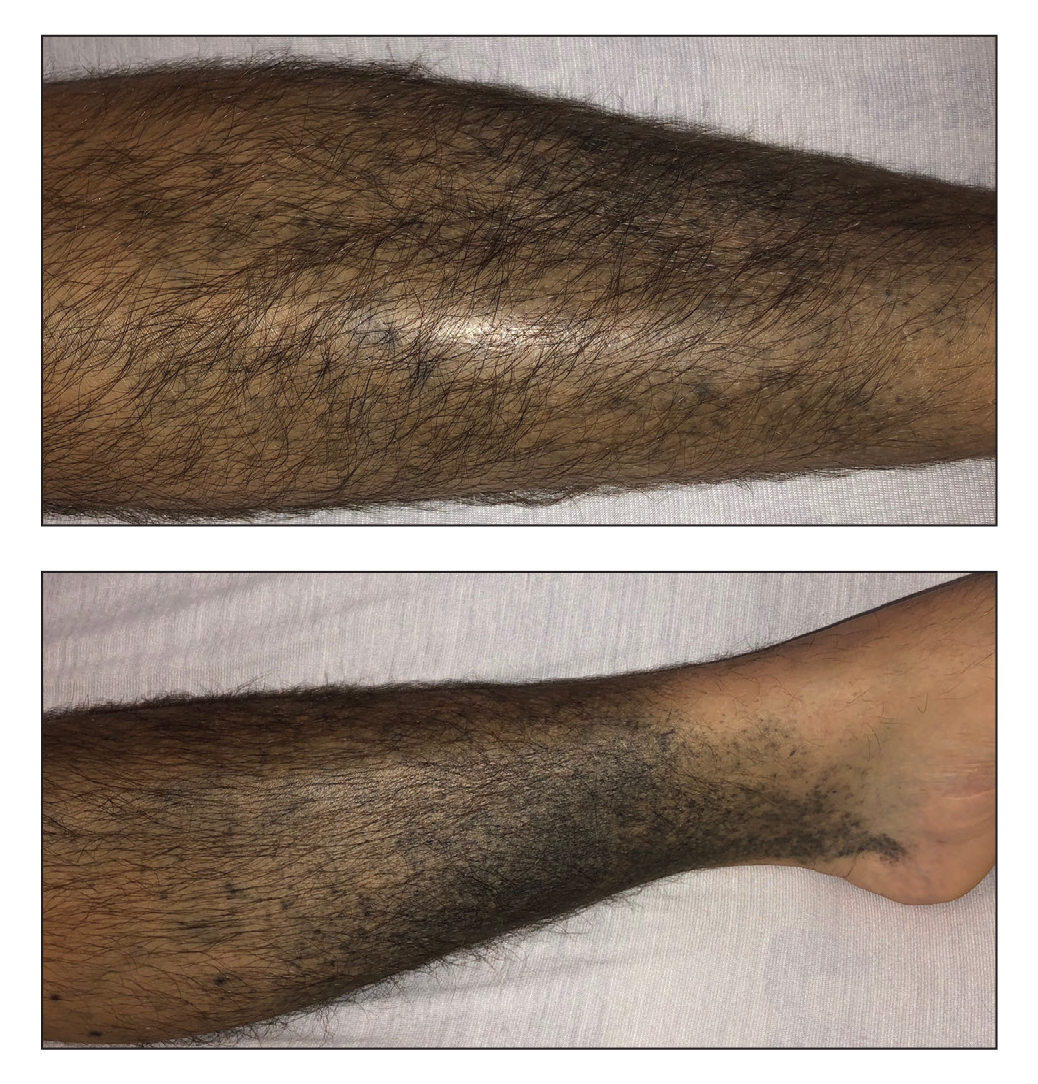
A 37-year-old man with a history of cerebral palsy, bipolar disorder, and impulse control disorder presented to the emergency department with breathing difficulty and worsening malaise. The patient subsequently was intubated due to hypoxic respiratory failure and was found to be positive for SARS-CoV-2. He was admitted to the intensive care unit, and dermatology was consulted due to concern that the cutaneous findings were demonstrative of a vasculitic process. Physical examination revealed diffuse, symmetric, dark brown to blue-gray macules coalescing into patches on the anterior tibia (top) and covering the entire lower leg (bottom). The patches were mottled and did not blanch with pressure. According to the patient’s caretaker, the leg hyperpigmentation had been present for 2 years.
Ruxolitinib cream meets primary endpoints in phase 3 vitiligo trial
presented together at the annual meeting of the European Academy of Dermatology and Venereology.
On the primary endpoint of F-VASI 75 (75% improvement in the Facial and Vitiligo Scoring Index), rates were nearly four times higher at 24 weeks in one trial (29.9% vs. 7.5%; P < .0001) and more than twice as great in the other (29.9% vs. 12.9%; P < .01).
“The larger phase 3 trials confirm the previous phase 2 findings,” reported David Rosmarin, MD, vice chairman for research and education, department of dermatology, Tufts Medical Center, Boston. These findings not only include substantial clinical efficacy but good tolerability with “no serious treatment-related adverse events,” he noted.
600 patients randomized
In one of the trials, called TRuE-V1, 330 patients with vitiligo were randomly assigned in a 2:1 ratio to 1.5% ruxolitinib or vehicle applied twice daily. In the other trial, called TRuE-V2, 344 patients were randomly assigned. The participating centers were in Europe and North America.
Patients aged 12 years or older with nonsegmental vitiligo and depigmentation covering no more than 10% of the total body surface area were eligible. The mean baseline F-VASI values were 1.0. The mean total VASI (T-FASI) values were 6.5. On those enrolled, half were female, 11% were adolescents, and 73% had Fitzpatrick skin phototypes III-VI.
Ruxolitinib cream provided near-complete vitiligo clearance (F-VASI 90) on the face at 24 weeks in only about 15% of patients, but this was several times higher than the 2% achieved on vehicle in the TRuE-V1 (P < .01) and the TRuE-V2 trials (P < .05), respectively.
F-VASI 50 response rates greater than 50%
For F-VASI 50, the response rate with ruxolitinib in both studies was approximately 51%. Relative to the 17.2% response on vehicle in TRuE-v1 and 23.4% in TRuE-V2 (both P < .0001 vs. active therapy), the advantage of the topical JAK inhibitor was considered to be a clinically meaningful, not just significant from a statistical standpoint.
In fact, improvement on the 5-point Vitiligo Noticeability Scale “also supported a clinically meaningful benefit,” Dr. Rosmarin reported. When those achieving a score of 4 (much less noticeable) or 5 (no longer noticeable), the response rates at 24 weeks were 24.5% and 21.6% in the TRuE-V1 and TRuE-V2 trials, respectively. Again, these response rates were several times greater than the 3.3% (P < .001) and 6.6% (P < .01) observed in the vehicle arms of TRuE-V1 and TRuE-V2 (P < .01), respectively.
Treatment-related adverse events were infrequent. The most common were acne at the application site, which occurred in about 5% of patients receiving ruxolitinib (vs. 2% or fewer of those receiving vehicle) and pruritus, which also occurred in about 5% of patients. However, the rates of pruritus among those on placebo reached 4% in TRuE-V1 and 2% in TRuE-V2 trials.
In vitiligo, where there has been recent progress in understanding the pathophysiology, loss of melanocytes in immune dysregulation has been linked to activation of the JAK signaling pathway, according to Dr. Rosmarin. In the 52-week phase 2 trial with 205 patients, ruxolitinib was associated with a sustained response and no serious treatment-related adverse events.
52-week data might show more benefit
Patients are continuing to be followed in the TRuE-V1 and TRuE-V-2 trials. Based on the phase 2 data and on the progressive improvement still being observed at the end of 24 weeks in the phase 3 trials, Dr. Rosmarin expects 52-week results be valuable in understanding the clinical role of ruxolitinib.
“We will be looking for further improvement in response as we follow these patients out to 1 year,” he said.
This further follow-up is important, agreed Iltefat Hamzavi, MD, senior staff physician, department of dermatology, Henry Ford Hospital, Detroit.
Despite the promise of perhaps other JAK inhibitors, “we still need to understand how long it will take for the drug to offer optimal results. We already know that is more than 24 weeks,” said Dr. Hamzavi, who has been involved in the clinical trials with this drug but was not involved with the TRuE-V1 or -V2 trials.
He also said more follow-up is needed to understand the duration of effect. He is, however, optimistic about the clinical role of this mechanism for treatment of vitiligo.
“I do think that JAK inhibitors show a lot of promise [in vitiligo] for certain locations of the body,” he said.
Given the limited treatment options for effective and prolonged improvement in vitiligo, both Dr. Hamzavi and Dr. Rosmarin indicated an effective topical cream is likely to be considered by physicians and patients to be a substantial advance.
On Sept. 21, ruxolitinib (Opzelura) 1.5% cream was approved by the Food and Drug Administration for the short-term treatment of mild to moderate atopic dermatitis in children and adults ages 12 years and older – the first FDA approval of this product.
Dr. Rosmarin reported financial relationships with more than 20 pharmaceutical companies, including Incyte, which provided funding for the TRuE-V1 and -V2 trials. Dr. Hamzavi reported financial relationships with more than 15 companies with pharmaceutical or cosmetic products, including Incyte.
A version of this article first appeared on Medscape.com.
presented together at the annual meeting of the European Academy of Dermatology and Venereology.
On the primary endpoint of F-VASI 75 (75% improvement in the Facial and Vitiligo Scoring Index), rates were nearly four times higher at 24 weeks in one trial (29.9% vs. 7.5%; P < .0001) and more than twice as great in the other (29.9% vs. 12.9%; P < .01).
“The larger phase 3 trials confirm the previous phase 2 findings,” reported David Rosmarin, MD, vice chairman for research and education, department of dermatology, Tufts Medical Center, Boston. These findings not only include substantial clinical efficacy but good tolerability with “no serious treatment-related adverse events,” he noted.
600 patients randomized
In one of the trials, called TRuE-V1, 330 patients with vitiligo were randomly assigned in a 2:1 ratio to 1.5% ruxolitinib or vehicle applied twice daily. In the other trial, called TRuE-V2, 344 patients were randomly assigned. The participating centers were in Europe and North America.
Patients aged 12 years or older with nonsegmental vitiligo and depigmentation covering no more than 10% of the total body surface area were eligible. The mean baseline F-VASI values were 1.0. The mean total VASI (T-FASI) values were 6.5. On those enrolled, half were female, 11% were adolescents, and 73% had Fitzpatrick skin phototypes III-VI.
Ruxolitinib cream provided near-complete vitiligo clearance (F-VASI 90) on the face at 24 weeks in only about 15% of patients, but this was several times higher than the 2% achieved on vehicle in the TRuE-V1 (P < .01) and the TRuE-V2 trials (P < .05), respectively.
F-VASI 50 response rates greater than 50%
For F-VASI 50, the response rate with ruxolitinib in both studies was approximately 51%. Relative to the 17.2% response on vehicle in TRuE-v1 and 23.4% in TRuE-V2 (both P < .0001 vs. active therapy), the advantage of the topical JAK inhibitor was considered to be a clinically meaningful, not just significant from a statistical standpoint.
In fact, improvement on the 5-point Vitiligo Noticeability Scale “also supported a clinically meaningful benefit,” Dr. Rosmarin reported. When those achieving a score of 4 (much less noticeable) or 5 (no longer noticeable), the response rates at 24 weeks were 24.5% and 21.6% in the TRuE-V1 and TRuE-V2 trials, respectively. Again, these response rates were several times greater than the 3.3% (P < .001) and 6.6% (P < .01) observed in the vehicle arms of TRuE-V1 and TRuE-V2 (P < .01), respectively.
Treatment-related adverse events were infrequent. The most common were acne at the application site, which occurred in about 5% of patients receiving ruxolitinib (vs. 2% or fewer of those receiving vehicle) and pruritus, which also occurred in about 5% of patients. However, the rates of pruritus among those on placebo reached 4% in TRuE-V1 and 2% in TRuE-V2 trials.
In vitiligo, where there has been recent progress in understanding the pathophysiology, loss of melanocytes in immune dysregulation has been linked to activation of the JAK signaling pathway, according to Dr. Rosmarin. In the 52-week phase 2 trial with 205 patients, ruxolitinib was associated with a sustained response and no serious treatment-related adverse events.
52-week data might show more benefit
Patients are continuing to be followed in the TRuE-V1 and TRuE-V-2 trials. Based on the phase 2 data and on the progressive improvement still being observed at the end of 24 weeks in the phase 3 trials, Dr. Rosmarin expects 52-week results be valuable in understanding the clinical role of ruxolitinib.
“We will be looking for further improvement in response as we follow these patients out to 1 year,” he said.
This further follow-up is important, agreed Iltefat Hamzavi, MD, senior staff physician, department of dermatology, Henry Ford Hospital, Detroit.
Despite the promise of perhaps other JAK inhibitors, “we still need to understand how long it will take for the drug to offer optimal results. We already know that is more than 24 weeks,” said Dr. Hamzavi, who has been involved in the clinical trials with this drug but was not involved with the TRuE-V1 or -V2 trials.
He also said more follow-up is needed to understand the duration of effect. He is, however, optimistic about the clinical role of this mechanism for treatment of vitiligo.
“I do think that JAK inhibitors show a lot of promise [in vitiligo] for certain locations of the body,” he said.
Given the limited treatment options for effective and prolonged improvement in vitiligo, both Dr. Hamzavi and Dr. Rosmarin indicated an effective topical cream is likely to be considered by physicians and patients to be a substantial advance.
On Sept. 21, ruxolitinib (Opzelura) 1.5% cream was approved by the Food and Drug Administration for the short-term treatment of mild to moderate atopic dermatitis in children and adults ages 12 years and older – the first FDA approval of this product.
Dr. Rosmarin reported financial relationships with more than 20 pharmaceutical companies, including Incyte, which provided funding for the TRuE-V1 and -V2 trials. Dr. Hamzavi reported financial relationships with more than 15 companies with pharmaceutical or cosmetic products, including Incyte.
A version of this article first appeared on Medscape.com.
presented together at the annual meeting of the European Academy of Dermatology and Venereology.
On the primary endpoint of F-VASI 75 (75% improvement in the Facial and Vitiligo Scoring Index), rates were nearly four times higher at 24 weeks in one trial (29.9% vs. 7.5%; P < .0001) and more than twice as great in the other (29.9% vs. 12.9%; P < .01).
“The larger phase 3 trials confirm the previous phase 2 findings,” reported David Rosmarin, MD, vice chairman for research and education, department of dermatology, Tufts Medical Center, Boston. These findings not only include substantial clinical efficacy but good tolerability with “no serious treatment-related adverse events,” he noted.
600 patients randomized
In one of the trials, called TRuE-V1, 330 patients with vitiligo were randomly assigned in a 2:1 ratio to 1.5% ruxolitinib or vehicle applied twice daily. In the other trial, called TRuE-V2, 344 patients were randomly assigned. The participating centers were in Europe and North America.
Patients aged 12 years or older with nonsegmental vitiligo and depigmentation covering no more than 10% of the total body surface area were eligible. The mean baseline F-VASI values were 1.0. The mean total VASI (T-FASI) values were 6.5. On those enrolled, half were female, 11% were adolescents, and 73% had Fitzpatrick skin phototypes III-VI.
Ruxolitinib cream provided near-complete vitiligo clearance (F-VASI 90) on the face at 24 weeks in only about 15% of patients, but this was several times higher than the 2% achieved on vehicle in the TRuE-V1 (P < .01) and the TRuE-V2 trials (P < .05), respectively.
F-VASI 50 response rates greater than 50%
For F-VASI 50, the response rate with ruxolitinib in both studies was approximately 51%. Relative to the 17.2% response on vehicle in TRuE-v1 and 23.4% in TRuE-V2 (both P < .0001 vs. active therapy), the advantage of the topical JAK inhibitor was considered to be a clinically meaningful, not just significant from a statistical standpoint.
In fact, improvement on the 5-point Vitiligo Noticeability Scale “also supported a clinically meaningful benefit,” Dr. Rosmarin reported. When those achieving a score of 4 (much less noticeable) or 5 (no longer noticeable), the response rates at 24 weeks were 24.5% and 21.6% in the TRuE-V1 and TRuE-V2 trials, respectively. Again, these response rates were several times greater than the 3.3% (P < .001) and 6.6% (P < .01) observed in the vehicle arms of TRuE-V1 and TRuE-V2 (P < .01), respectively.
Treatment-related adverse events were infrequent. The most common were acne at the application site, which occurred in about 5% of patients receiving ruxolitinib (vs. 2% or fewer of those receiving vehicle) and pruritus, which also occurred in about 5% of patients. However, the rates of pruritus among those on placebo reached 4% in TRuE-V1 and 2% in TRuE-V2 trials.
In vitiligo, where there has been recent progress in understanding the pathophysiology, loss of melanocytes in immune dysregulation has been linked to activation of the JAK signaling pathway, according to Dr. Rosmarin. In the 52-week phase 2 trial with 205 patients, ruxolitinib was associated with a sustained response and no serious treatment-related adverse events.
52-week data might show more benefit
Patients are continuing to be followed in the TRuE-V1 and TRuE-V-2 trials. Based on the phase 2 data and on the progressive improvement still being observed at the end of 24 weeks in the phase 3 trials, Dr. Rosmarin expects 52-week results be valuable in understanding the clinical role of ruxolitinib.
“We will be looking for further improvement in response as we follow these patients out to 1 year,” he said.
This further follow-up is important, agreed Iltefat Hamzavi, MD, senior staff physician, department of dermatology, Henry Ford Hospital, Detroit.
Despite the promise of perhaps other JAK inhibitors, “we still need to understand how long it will take for the drug to offer optimal results. We already know that is more than 24 weeks,” said Dr. Hamzavi, who has been involved in the clinical trials with this drug but was not involved with the TRuE-V1 or -V2 trials.
He also said more follow-up is needed to understand the duration of effect. He is, however, optimistic about the clinical role of this mechanism for treatment of vitiligo.
“I do think that JAK inhibitors show a lot of promise [in vitiligo] for certain locations of the body,” he said.
Given the limited treatment options for effective and prolonged improvement in vitiligo, both Dr. Hamzavi and Dr. Rosmarin indicated an effective topical cream is likely to be considered by physicians and patients to be a substantial advance.
On Sept. 21, ruxolitinib (Opzelura) 1.5% cream was approved by the Food and Drug Administration for the short-term treatment of mild to moderate atopic dermatitis in children and adults ages 12 years and older – the first FDA approval of this product.
Dr. Rosmarin reported financial relationships with more than 20 pharmaceutical companies, including Incyte, which provided funding for the TRuE-V1 and -V2 trials. Dr. Hamzavi reported financial relationships with more than 15 companies with pharmaceutical or cosmetic products, including Incyte.
A version of this article first appeared on Medscape.com.
Acid series: Lactic acid
One of the most commonly used organic acids used on the skin, lactic acid, has been used for over 3 decades. Originally derived from milk or plant-derived sugars, this gentle exfoliating acid can be used in peels, serums, masks, and toners, and has the additional benefit of hydrating the skin. Lactic acid is formulated in concentrations from 2% to 50%; however, because of its large molecular size, it doesn’t penetrate the deeper layers of the dermis to the same extent as the other alpha-hydroxy acids (AHAs), such as glycolic acid. Thus, it is one of the gentler exfoliants and one that can be used in sensitive skin or darker skin types.
Despite its mild peeling effects, lactic acid is best used to treat xerotic skin because of its function as a humectant, drawing moisture into the stratum corneum. Similar to the other AHAs, lactic acid has also been shown to decrease melanogenesis and is a gentle treatment for skin hyperpigmentation, particularly in skin of color. Side effects include peeling, stinging, erythema, photosensitivity, and hyperpigmentation when improperly used.
Very little clinical research has been reported in the last 20 years as to the uses and benefits of lactic acid in skincare. In my clinical experience, daily use of lactic acid is more effective and has more long-term benefits for hydration and rejuvenation of the skin than the other AHAs. Concentrations of 10%-15% used daily on the skin as a mild exfoliant and humectant have shown to improve texture, decrease pigmentation and improve fine lines – without thinning of the skin seen with the deeper dermal penetrating acids.
Confusion in the market has also risen as many over-the-counter brands have included ammonium lactate in their portfolio of moisturizers. Ammonium lactate is a combination of ammonium hydroxide and lactic acid, or the salt of lactic acid. A comparative study evaluating the difference between 5% lactic acid and 12% ammonium lactate for the treatment of xerosis showed that ammonium lactate was significantly more effective at reducing xerosis. It is widely used in the treatment of keratosis pilaris, calluses, xerosis, and ichthyosis.
Widespread use of lactic acid has not gotten as much glory as that of glycolic acid. However, in clinical practice, its functions are more widespread. It is a much safer acid to use, and its added benefit of increasing hydration of the skin is crucial in its long-term use for both photoaging and the prevention of wrinkles. With any acid, the exfoliating properties must be treated with adequate hydration and barrier repair.
The intrinsic moisturizing effect of lactic acid makes it a much more well-rounded acid and that can be used for longer periods of time in a broader spectrum of patients.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
One of the most commonly used organic acids used on the skin, lactic acid, has been used for over 3 decades. Originally derived from milk or plant-derived sugars, this gentle exfoliating acid can be used in peels, serums, masks, and toners, and has the additional benefit of hydrating the skin. Lactic acid is formulated in concentrations from 2% to 50%; however, because of its large molecular size, it doesn’t penetrate the deeper layers of the dermis to the same extent as the other alpha-hydroxy acids (AHAs), such as glycolic acid. Thus, it is one of the gentler exfoliants and one that can be used in sensitive skin or darker skin types.
Despite its mild peeling effects, lactic acid is best used to treat xerotic skin because of its function as a humectant, drawing moisture into the stratum corneum. Similar to the other AHAs, lactic acid has also been shown to decrease melanogenesis and is a gentle treatment for skin hyperpigmentation, particularly in skin of color. Side effects include peeling, stinging, erythema, photosensitivity, and hyperpigmentation when improperly used.
Very little clinical research has been reported in the last 20 years as to the uses and benefits of lactic acid in skincare. In my clinical experience, daily use of lactic acid is more effective and has more long-term benefits for hydration and rejuvenation of the skin than the other AHAs. Concentrations of 10%-15% used daily on the skin as a mild exfoliant and humectant have shown to improve texture, decrease pigmentation and improve fine lines – without thinning of the skin seen with the deeper dermal penetrating acids.
Confusion in the market has also risen as many over-the-counter brands have included ammonium lactate in their portfolio of moisturizers. Ammonium lactate is a combination of ammonium hydroxide and lactic acid, or the salt of lactic acid. A comparative study evaluating the difference between 5% lactic acid and 12% ammonium lactate for the treatment of xerosis showed that ammonium lactate was significantly more effective at reducing xerosis. It is widely used in the treatment of keratosis pilaris, calluses, xerosis, and ichthyosis.
Widespread use of lactic acid has not gotten as much glory as that of glycolic acid. However, in clinical practice, its functions are more widespread. It is a much safer acid to use, and its added benefit of increasing hydration of the skin is crucial in its long-term use for both photoaging and the prevention of wrinkles. With any acid, the exfoliating properties must be treated with adequate hydration and barrier repair.
The intrinsic moisturizing effect of lactic acid makes it a much more well-rounded acid and that can be used for longer periods of time in a broader spectrum of patients.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
One of the most commonly used organic acids used on the skin, lactic acid, has been used for over 3 decades. Originally derived from milk or plant-derived sugars, this gentle exfoliating acid can be used in peels, serums, masks, and toners, and has the additional benefit of hydrating the skin. Lactic acid is formulated in concentrations from 2% to 50%; however, because of its large molecular size, it doesn’t penetrate the deeper layers of the dermis to the same extent as the other alpha-hydroxy acids (AHAs), such as glycolic acid. Thus, it is one of the gentler exfoliants and one that can be used in sensitive skin or darker skin types.
Despite its mild peeling effects, lactic acid is best used to treat xerotic skin because of its function as a humectant, drawing moisture into the stratum corneum. Similar to the other AHAs, lactic acid has also been shown to decrease melanogenesis and is a gentle treatment for skin hyperpigmentation, particularly in skin of color. Side effects include peeling, stinging, erythema, photosensitivity, and hyperpigmentation when improperly used.
Very little clinical research has been reported in the last 20 years as to the uses and benefits of lactic acid in skincare. In my clinical experience, daily use of lactic acid is more effective and has more long-term benefits for hydration and rejuvenation of the skin than the other AHAs. Concentrations of 10%-15% used daily on the skin as a mild exfoliant and humectant have shown to improve texture, decrease pigmentation and improve fine lines – without thinning of the skin seen with the deeper dermal penetrating acids.
Confusion in the market has also risen as many over-the-counter brands have included ammonium lactate in their portfolio of moisturizers. Ammonium lactate is a combination of ammonium hydroxide and lactic acid, or the salt of lactic acid. A comparative study evaluating the difference between 5% lactic acid and 12% ammonium lactate for the treatment of xerosis showed that ammonium lactate was significantly more effective at reducing xerosis. It is widely used in the treatment of keratosis pilaris, calluses, xerosis, and ichthyosis.
Widespread use of lactic acid has not gotten as much glory as that of glycolic acid. However, in clinical practice, its functions are more widespread. It is a much safer acid to use, and its added benefit of increasing hydration of the skin is crucial in its long-term use for both photoaging and the prevention of wrinkles. With any acid, the exfoliating properties must be treated with adequate hydration and barrier repair.
The intrinsic moisturizing effect of lactic acid makes it a much more well-rounded acid and that can be used for longer periods of time in a broader spectrum of patients.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Insurance coverage for vitiligo varies widely in the U.S., analysis finds
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
FROM PEDIATRIC DERMATOLOGY
When is MRI useful in the management of congenital melanocytic nevi?
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
FROM SPD 2021
Nadolol bests propranolol for infantile hemangioma treatment out to 52 weeks
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
FROM SPD 2021
Pigmented Basal Cell Carcinoma With Annular Leukoderma
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.
The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.


The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.


The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
Practice Points
- Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that more commonly is associated with benign processes such as halo nevi.
- The halo phenomenon may accompany malignant processes, such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.