User login
Tinted Sunscreens: Consumer Preferences Based on Light, Medium, and Dark Skin Tones
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
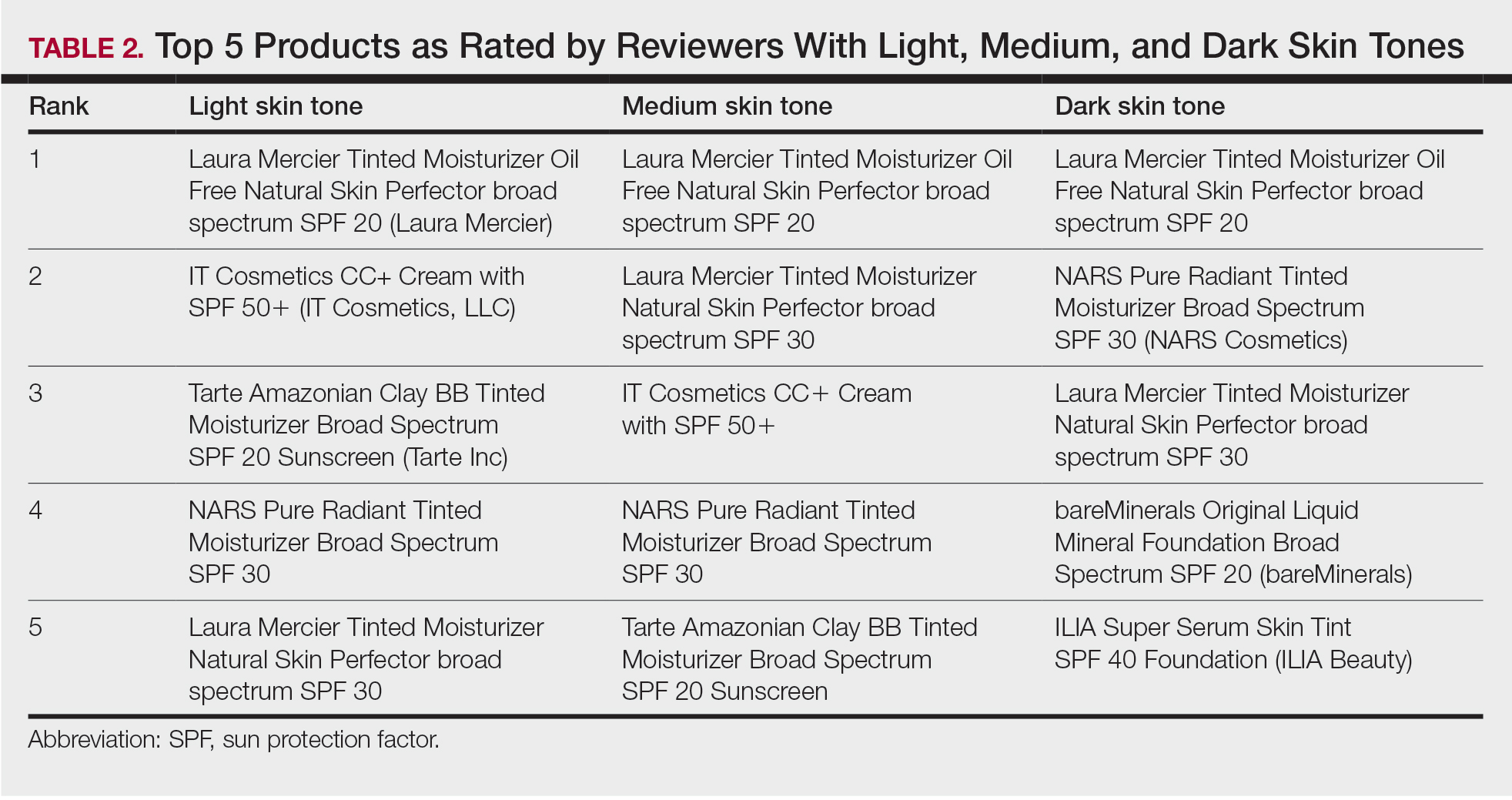
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.

Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.

Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Practice Points
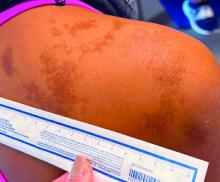
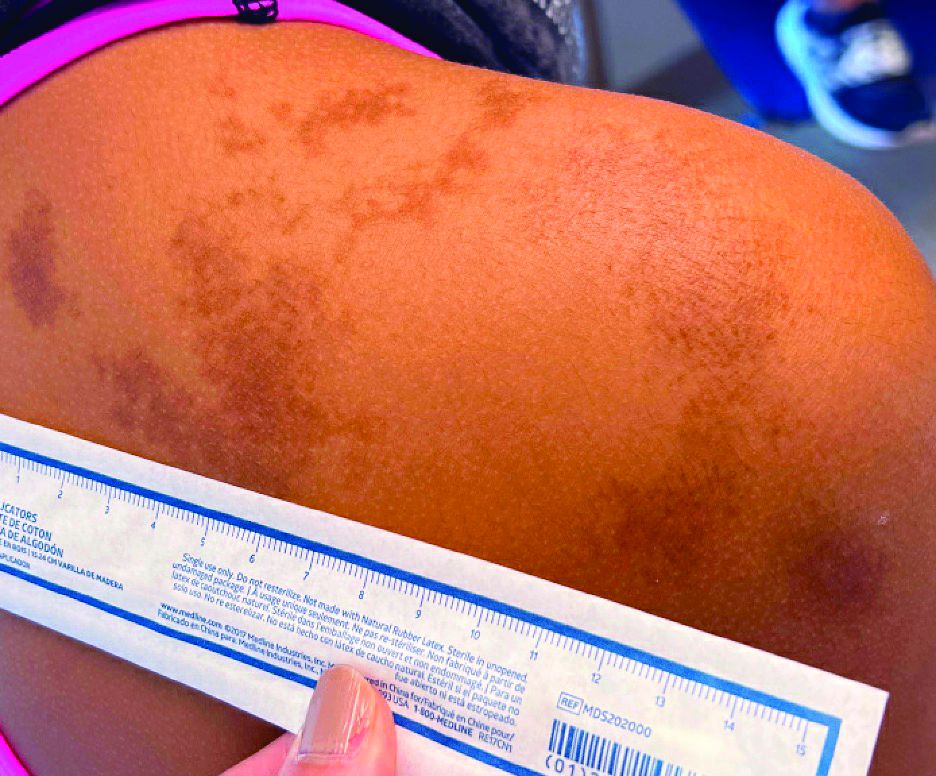
- Visible light has been shown to increase tyrosinase activity and induce immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.
- The formulation of sunscreens with iron oxides and pigmentary titanium dioxide are a safe and effective way to protect against high-energy visible light, especially when combined with zinc oxide.
- Physicians should be aware of sunscreen characteristics that patients like and dislike to tailor recommendations that are appropriate for each individual to enhance adherence.
- Cosmetic elegance and tone compatibility are the most important criteria for individuals seeking tinted sunscreens.
Photoprotection strategies for melasma are increasing
BOSTON – Untinted chemical sunscreens on the market are not sufficient to protect the skin from the effects of visible light, complicating sun protection efforts for patients with melasma and other conditions aggravated by sun exposure, according to Henry W. Lim, MD.
A , Dr. Lim, former chair of the department of dermatology at Henry Ford Health, Detroit, said at the annual meeting of the American Academy of Dermatology. Tinted sunscreens contain iron oxides; some also contain pigmentary titanium dioxide.
“Black, red, and yellow iron oxide all reflect visible light,” he added, noting that currently, there are no regulations as to how tinted sunscreens are marketed, making it difficult for practicing clinicians to advise patients about what products to choose. However, he said, “unlike ‘SPF’ and ‘broad spectrum’ labeling, there is no specific guidance on tinted sunscreens. “ ‘Universal’ shade is a good start but might not be ideal for users with very fair or deep skin tones,” he noted.
In December 2021, a guide to tinted sunscreens, written by Dr. Lim and colleagues, was published, recommending that consumers choose a product that contains iron oxides, is labeled as broad spectrum, and has an SPF of at least 30.
A comprehensive list of 54 tinted sunscreens with an SPF of 30 or greater that contain iron oxide is also available . The authors of the guide contributed to this resource, which lists sunscreens by average price per ounce.
At the meeting, Dr. Lim highlighted tinted sunscreens that cost about $20 or less per ounce. They include Supergoop 100% Mineral CC Cream (SPF 50); Bare Republic Mineral Tinted Face Sunscreen Lotion (SPF 30); CeraVe Hydrating Sunscreen with Sheer Tint (SPF 30); Tizo Ultra Zinc Body & Face Sunscreen (SPF 40); Vichy Capital Soleil Tinted Face Mineral Sunscreen (SPF 60); EltaMD UV Elements Tinted (SPF 44); La Roche-Posay Anthelios Ultra-Light Tinted Mineral (SPF 50), SkinMedica Essential Defense Mineral Shield (SPF 32), ISDIN Eryfotona Ageless Ultralight Tinted Mineral Sunscreen (SPF 50), and SkinCeuticals Physical Fusion UV Defense (SPF 50).
Sunscreens with antioxidants
Sunscreens with biologically active antioxidants may be another option for patients with melasma. A proof-of-concept study that Dr. Lim and colleagues conducted in 20 patients found that application of a blend of topical antioxidants (2%) was associated with less erythema at the application sites among those with skin phototypes I-III and less pigmentation at the application sites among those with skin phototypes IV-VI after exposure to visible light and UVA-1, compared with controls.
Certain antioxidants have been added to sunscreens currently on the market, including niacinamide (vitamin B3), licochalcone A, carotenoids (beta-carotene), vitamin E, vitamin C, glycyrrhetinic acid, and diethylhexyl syringylidenemalonate.
A recently published paper on the role of antioxidants and free radical quenchers in protecting skin from visible light referred to unpublished data from Dr. Lim (the first author) and colleagues, which demonstrated a significant reduction in visual light–induced hyperpigmentation on skin with sunscreen that contained the antioxidants vitamin E, vitamin C, diethylhexyl syringylidenemalonate, licochalcone A, and a glycyrrhetinic acid, compared with sunscreen that had no antioxidants.
Novel filters
Another emerging option is sunscreen with new filters that cover UVA-1 and visible light. In a randomized, controlled trial of 19 patients, researchers evaluated the addition of methoxypropylamino cyclohexenylidene ethoxyethylcyanoacetate (MCE) absorber, a new UVA-1 filter known as Mexoryl 400, which has a peak absorption of 385 nm, to a sunscreen formulation.
“Currently, peak absorption in the U.S. is with avobenzone, which peaks at about 357 nm,” but MCE “covers a longer spectrum of UVA-1,” Dr. Lim said. The researchers found that the addition of MCE reduced UVA-1-induced dermal and epidermal alterations at cellular, biochemical, and molecular levels; and decreased UVA-1-induced pigmentation.
Another relatively new filter, phenylene bis-diphenyltriazine (also known as TriAsorB) not only protects against UVA but it extends into the blue light portion of visible light, according to a recently published paper. According to a press release from Pierre Fabre, which has developed the filter, studies have shown that TriAsorB is not toxic for three key species of marine biodiversity: a coral species, a phytoplankton species, and a zooplankton.
This filter and MCE are available in Europe but not in the United States.
Dr. Lim reported that he is an investigator for Incyte, L’Oréal, Pfizer, and the Patient-Centered Outcomes Research Institute.
BOSTON – Untinted chemical sunscreens on the market are not sufficient to protect the skin from the effects of visible light, complicating sun protection efforts for patients with melasma and other conditions aggravated by sun exposure, according to Henry W. Lim, MD.
A , Dr. Lim, former chair of the department of dermatology at Henry Ford Health, Detroit, said at the annual meeting of the American Academy of Dermatology. Tinted sunscreens contain iron oxides; some also contain pigmentary titanium dioxide.
“Black, red, and yellow iron oxide all reflect visible light,” he added, noting that currently, there are no regulations as to how tinted sunscreens are marketed, making it difficult for practicing clinicians to advise patients about what products to choose. However, he said, “unlike ‘SPF’ and ‘broad spectrum’ labeling, there is no specific guidance on tinted sunscreens. “ ‘Universal’ shade is a good start but might not be ideal for users with very fair or deep skin tones,” he noted.
In December 2021, a guide to tinted sunscreens, written by Dr. Lim and colleagues, was published, recommending that consumers choose a product that contains iron oxides, is labeled as broad spectrum, and has an SPF of at least 30.
A comprehensive list of 54 tinted sunscreens with an SPF of 30 or greater that contain iron oxide is also available . The authors of the guide contributed to this resource, which lists sunscreens by average price per ounce.
At the meeting, Dr. Lim highlighted tinted sunscreens that cost about $20 or less per ounce. They include Supergoop 100% Mineral CC Cream (SPF 50); Bare Republic Mineral Tinted Face Sunscreen Lotion (SPF 30); CeraVe Hydrating Sunscreen with Sheer Tint (SPF 30); Tizo Ultra Zinc Body & Face Sunscreen (SPF 40); Vichy Capital Soleil Tinted Face Mineral Sunscreen (SPF 60); EltaMD UV Elements Tinted (SPF 44); La Roche-Posay Anthelios Ultra-Light Tinted Mineral (SPF 50), SkinMedica Essential Defense Mineral Shield (SPF 32), ISDIN Eryfotona Ageless Ultralight Tinted Mineral Sunscreen (SPF 50), and SkinCeuticals Physical Fusion UV Defense (SPF 50).
Sunscreens with antioxidants
Sunscreens with biologically active antioxidants may be another option for patients with melasma. A proof-of-concept study that Dr. Lim and colleagues conducted in 20 patients found that application of a blend of topical antioxidants (2%) was associated with less erythema at the application sites among those with skin phototypes I-III and less pigmentation at the application sites among those with skin phototypes IV-VI after exposure to visible light and UVA-1, compared with controls.
Certain antioxidants have been added to sunscreens currently on the market, including niacinamide (vitamin B3), licochalcone A, carotenoids (beta-carotene), vitamin E, vitamin C, glycyrrhetinic acid, and diethylhexyl syringylidenemalonate.
A recently published paper on the role of antioxidants and free radical quenchers in protecting skin from visible light referred to unpublished data from Dr. Lim (the first author) and colleagues, which demonstrated a significant reduction in visual light–induced hyperpigmentation on skin with sunscreen that contained the antioxidants vitamin E, vitamin C, diethylhexyl syringylidenemalonate, licochalcone A, and a glycyrrhetinic acid, compared with sunscreen that had no antioxidants.
Novel filters
Another emerging option is sunscreen with new filters that cover UVA-1 and visible light. In a randomized, controlled trial of 19 patients, researchers evaluated the addition of methoxypropylamino cyclohexenylidene ethoxyethylcyanoacetate (MCE) absorber, a new UVA-1 filter known as Mexoryl 400, which has a peak absorption of 385 nm, to a sunscreen formulation.
“Currently, peak absorption in the U.S. is with avobenzone, which peaks at about 357 nm,” but MCE “covers a longer spectrum of UVA-1,” Dr. Lim said. The researchers found that the addition of MCE reduced UVA-1-induced dermal and epidermal alterations at cellular, biochemical, and molecular levels; and decreased UVA-1-induced pigmentation.
Another relatively new filter, phenylene bis-diphenyltriazine (also known as TriAsorB) not only protects against UVA but it extends into the blue light portion of visible light, according to a recently published paper. According to a press release from Pierre Fabre, which has developed the filter, studies have shown that TriAsorB is not toxic for three key species of marine biodiversity: a coral species, a phytoplankton species, and a zooplankton.
This filter and MCE are available in Europe but not in the United States.
Dr. Lim reported that he is an investigator for Incyte, L’Oréal, Pfizer, and the Patient-Centered Outcomes Research Institute.
BOSTON – Untinted chemical sunscreens on the market are not sufficient to protect the skin from the effects of visible light, complicating sun protection efforts for patients with melasma and other conditions aggravated by sun exposure, according to Henry W. Lim, MD.
A , Dr. Lim, former chair of the department of dermatology at Henry Ford Health, Detroit, said at the annual meeting of the American Academy of Dermatology. Tinted sunscreens contain iron oxides; some also contain pigmentary titanium dioxide.
“Black, red, and yellow iron oxide all reflect visible light,” he added, noting that currently, there are no regulations as to how tinted sunscreens are marketed, making it difficult for practicing clinicians to advise patients about what products to choose. However, he said, “unlike ‘SPF’ and ‘broad spectrum’ labeling, there is no specific guidance on tinted sunscreens. “ ‘Universal’ shade is a good start but might not be ideal for users with very fair or deep skin tones,” he noted.
In December 2021, a guide to tinted sunscreens, written by Dr. Lim and colleagues, was published, recommending that consumers choose a product that contains iron oxides, is labeled as broad spectrum, and has an SPF of at least 30.
A comprehensive list of 54 tinted sunscreens with an SPF of 30 or greater that contain iron oxide is also available . The authors of the guide contributed to this resource, which lists sunscreens by average price per ounce.
At the meeting, Dr. Lim highlighted tinted sunscreens that cost about $20 or less per ounce. They include Supergoop 100% Mineral CC Cream (SPF 50); Bare Republic Mineral Tinted Face Sunscreen Lotion (SPF 30); CeraVe Hydrating Sunscreen with Sheer Tint (SPF 30); Tizo Ultra Zinc Body & Face Sunscreen (SPF 40); Vichy Capital Soleil Tinted Face Mineral Sunscreen (SPF 60); EltaMD UV Elements Tinted (SPF 44); La Roche-Posay Anthelios Ultra-Light Tinted Mineral (SPF 50), SkinMedica Essential Defense Mineral Shield (SPF 32), ISDIN Eryfotona Ageless Ultralight Tinted Mineral Sunscreen (SPF 50), and SkinCeuticals Physical Fusion UV Defense (SPF 50).
Sunscreens with antioxidants
Sunscreens with biologically active antioxidants may be another option for patients with melasma. A proof-of-concept study that Dr. Lim and colleagues conducted in 20 patients found that application of a blend of topical antioxidants (2%) was associated with less erythema at the application sites among those with skin phototypes I-III and less pigmentation at the application sites among those with skin phototypes IV-VI after exposure to visible light and UVA-1, compared with controls.
Certain antioxidants have been added to sunscreens currently on the market, including niacinamide (vitamin B3), licochalcone A, carotenoids (beta-carotene), vitamin E, vitamin C, glycyrrhetinic acid, and diethylhexyl syringylidenemalonate.
A recently published paper on the role of antioxidants and free radical quenchers in protecting skin from visible light referred to unpublished data from Dr. Lim (the first author) and colleagues, which demonstrated a significant reduction in visual light–induced hyperpigmentation on skin with sunscreen that contained the antioxidants vitamin E, vitamin C, diethylhexyl syringylidenemalonate, licochalcone A, and a glycyrrhetinic acid, compared with sunscreen that had no antioxidants.
Novel filters
Another emerging option is sunscreen with new filters that cover UVA-1 and visible light. In a randomized, controlled trial of 19 patients, researchers evaluated the addition of methoxypropylamino cyclohexenylidene ethoxyethylcyanoacetate (MCE) absorber, a new UVA-1 filter known as Mexoryl 400, which has a peak absorption of 385 nm, to a sunscreen formulation.
“Currently, peak absorption in the U.S. is with avobenzone, which peaks at about 357 nm,” but MCE “covers a longer spectrum of UVA-1,” Dr. Lim said. The researchers found that the addition of MCE reduced UVA-1-induced dermal and epidermal alterations at cellular, biochemical, and molecular levels; and decreased UVA-1-induced pigmentation.
Another relatively new filter, phenylene bis-diphenyltriazine (also known as TriAsorB) not only protects against UVA but it extends into the blue light portion of visible light, according to a recently published paper. According to a press release from Pierre Fabre, which has developed the filter, studies have shown that TriAsorB is not toxic for three key species of marine biodiversity: a coral species, a phytoplankton species, and a zooplankton.
This filter and MCE are available in Europe but not in the United States.
Dr. Lim reported that he is an investigator for Incyte, L’Oréal, Pfizer, and the Patient-Centered Outcomes Research Institute.
AT AAD 22
Topical options for treating melasma continue to expand
BOSTON – In the opinion of Seemal R. Desai, MD, dermatologists are obligated to tell their patients with melasma that their condition is a chronic disease with no cure.
“We have to set expectations upfront, because you all know the history,” Dr. Desai, founder and medical director of Innovative Dermatology in Dallas, said at the annual meeting of the American Academy of Dermatology. “You get someone better, their melasma gets lighter, and then they’re lost to follow-up for a year. Then they’re back to your office after that beach vacation because their melasma has come back with a vengeance because they were out in the sun too much. We have to tell our patients that melasma therapy is a journey of skin lightening but it’s not going to be a one-stop shop of getting it completely cured.”
As for treatment of melasma, “hydroquinone is still our workhorse, our gold standard.” Dr. Desai said. “I tell patients, ‘I’m going to keep you on it for 16 weeks. Then you’re going to come back. I’m going to see where you are, and we’ll move into the nonhydroquinone therapies once your disease is under control.’ ”
However, new therapies for melasma are needed because long-term use of hydroquinone can lead to complications such as ochronosis, nail discoloration, conjunctival melanosis, and corneal degeneration.
Emerging treatments
. Dr. Desai described azelaic acid as his “go to” nonhydroquinone option for skin lightening. In one study, 20% azelaic acid was used twice daily in 155 patients with facial melasma. Of these, 73% showed improvement after 6 months of therapy. Side effects were minimal and included erythema, pruritus, and burning.
Another option is topically compounded methimazole, a potent peroxidase inhibitor that causes morphologic change in melanocytes. “You can get it compounded as a 5% cream,” he said of the antithyroid agent. “It’s not that expensive, and even high concentrations are not melanocytotoxic. There’s minimal systemic absorption because the molecule is large, so there really is not any effect on TSH [thyroid-stimulating hormone] or T4 levels.”
Kojic acid dipalmitate, an antibiotic produced by many species of Aspergillus and Penicillium, can also be used as a second-line melasma treatment. Unlike kojic acid, kojic acid dipalmitate is more stable to light, heat, pH, and oxidation, and is also compatible with most organic sunscreens. It works by inhibiting tyrosinase. “It’s already available overseas and will soon be available in the U.S. as a derivative of kojic acid,” he said.
There is also vitamin C serum, which reduces tyrosinase activity via an antioxidant effect. “When you combine it with azelaic acid or sunscreen, vitamin C helps to augment the response,” Dr. Desai said. In one study that compared 5% ascorbic acid with 4% hydroquinone, 62.5% vs. 93% of patients improved, respectively, but side effects were more prominent in those who received 4% hydroquinone (68.7% vs. 6.2%).
An additional off-label option for melasma is oral tranexamic acid, which controls pigmentation by inhibiting the release of inflammatory mediators, specifically prostaglandins and arachidonic acid, which are involved in melanogenesis.
Dr. Desai often uses a dose of 325 mg twice daily. “Think of tranexamic acid as an anti-inflammatory,” he said. Tranexamic acid is contraindicated in patients who are currently taking or have previously taken anticoagulant medications; those who are pregnant or breastfeeding, or are smokers; and in those with renal, cardiac, and/or pulmonary disease. It has a half-life of about 7.5 hours, so the twice daily dosing “is quite effective,” he said.
“Do I leave my patients on this for years at a time to see if it’s going to work? No. When this works in treating melasma it works very quickly. I tell patients they’re going to see results in the first 8-12 weeks. That’s the beauty of using this orally.”
Another emerging therapy is Rubus occidentalis (black raspberry), a botanical-based ingredient in a 3% topical suspension that was compared with 4% hydroquinone in a randomized placebo-controlled trial. In the study, efficacy of Rubus occidentalis was considered comparable to that of hydroquinone. “This not only blocks melanogenesis, it also helps to block melanosome transfer,” said Dr. Desai, who is a past president of the Skin of Color Society.
Another natural option for melasma patients is topical cysteamine, which is the simplest aminothiol physiologically produced in human cells from the essential amino acid cysteine. “This is great for patients with recalcitrant disease, or for patients who, after 12-16 weeks of hydroquinone, you want them to have a break. I use it as a 5% concentration, and it works nicely,” he said. Cysteamine is also highly concentrated in human milk.
Dr. Desai disclosed that he performs clinical trials and consulting for many companies including L’Oréal, Galderma, Allergan, and AbbVie.
BOSTON – In the opinion of Seemal R. Desai, MD, dermatologists are obligated to tell their patients with melasma that their condition is a chronic disease with no cure.
“We have to set expectations upfront, because you all know the history,” Dr. Desai, founder and medical director of Innovative Dermatology in Dallas, said at the annual meeting of the American Academy of Dermatology. “You get someone better, their melasma gets lighter, and then they’re lost to follow-up for a year. Then they’re back to your office after that beach vacation because their melasma has come back with a vengeance because they were out in the sun too much. We have to tell our patients that melasma therapy is a journey of skin lightening but it’s not going to be a one-stop shop of getting it completely cured.”
As for treatment of melasma, “hydroquinone is still our workhorse, our gold standard.” Dr. Desai said. “I tell patients, ‘I’m going to keep you on it for 16 weeks. Then you’re going to come back. I’m going to see where you are, and we’ll move into the nonhydroquinone therapies once your disease is under control.’ ”
However, new therapies for melasma are needed because long-term use of hydroquinone can lead to complications such as ochronosis, nail discoloration, conjunctival melanosis, and corneal degeneration.
Emerging treatments
. Dr. Desai described azelaic acid as his “go to” nonhydroquinone option for skin lightening. In one study, 20% azelaic acid was used twice daily in 155 patients with facial melasma. Of these, 73% showed improvement after 6 months of therapy. Side effects were minimal and included erythema, pruritus, and burning.
Another option is topically compounded methimazole, a potent peroxidase inhibitor that causes morphologic change in melanocytes. “You can get it compounded as a 5% cream,” he said of the antithyroid agent. “It’s not that expensive, and even high concentrations are not melanocytotoxic. There’s minimal systemic absorption because the molecule is large, so there really is not any effect on TSH [thyroid-stimulating hormone] or T4 levels.”
Kojic acid dipalmitate, an antibiotic produced by many species of Aspergillus and Penicillium, can also be used as a second-line melasma treatment. Unlike kojic acid, kojic acid dipalmitate is more stable to light, heat, pH, and oxidation, and is also compatible with most organic sunscreens. It works by inhibiting tyrosinase. “It’s already available overseas and will soon be available in the U.S. as a derivative of kojic acid,” he said.
There is also vitamin C serum, which reduces tyrosinase activity via an antioxidant effect. “When you combine it with azelaic acid or sunscreen, vitamin C helps to augment the response,” Dr. Desai said. In one study that compared 5% ascorbic acid with 4% hydroquinone, 62.5% vs. 93% of patients improved, respectively, but side effects were more prominent in those who received 4% hydroquinone (68.7% vs. 6.2%).
An additional off-label option for melasma is oral tranexamic acid, which controls pigmentation by inhibiting the release of inflammatory mediators, specifically prostaglandins and arachidonic acid, which are involved in melanogenesis.
Dr. Desai often uses a dose of 325 mg twice daily. “Think of tranexamic acid as an anti-inflammatory,” he said. Tranexamic acid is contraindicated in patients who are currently taking or have previously taken anticoagulant medications; those who are pregnant or breastfeeding, or are smokers; and in those with renal, cardiac, and/or pulmonary disease. It has a half-life of about 7.5 hours, so the twice daily dosing “is quite effective,” he said.
“Do I leave my patients on this for years at a time to see if it’s going to work? No. When this works in treating melasma it works very quickly. I tell patients they’re going to see results in the first 8-12 weeks. That’s the beauty of using this orally.”
Another emerging therapy is Rubus occidentalis (black raspberry), a botanical-based ingredient in a 3% topical suspension that was compared with 4% hydroquinone in a randomized placebo-controlled trial. In the study, efficacy of Rubus occidentalis was considered comparable to that of hydroquinone. “This not only blocks melanogenesis, it also helps to block melanosome transfer,” said Dr. Desai, who is a past president of the Skin of Color Society.
Another natural option for melasma patients is topical cysteamine, which is the simplest aminothiol physiologically produced in human cells from the essential amino acid cysteine. “This is great for patients with recalcitrant disease, or for patients who, after 12-16 weeks of hydroquinone, you want them to have a break. I use it as a 5% concentration, and it works nicely,” he said. Cysteamine is also highly concentrated in human milk.
Dr. Desai disclosed that he performs clinical trials and consulting for many companies including L’Oréal, Galderma, Allergan, and AbbVie.
BOSTON – In the opinion of Seemal R. Desai, MD, dermatologists are obligated to tell their patients with melasma that their condition is a chronic disease with no cure.
“We have to set expectations upfront, because you all know the history,” Dr. Desai, founder and medical director of Innovative Dermatology in Dallas, said at the annual meeting of the American Academy of Dermatology. “You get someone better, their melasma gets lighter, and then they’re lost to follow-up for a year. Then they’re back to your office after that beach vacation because their melasma has come back with a vengeance because they were out in the sun too much. We have to tell our patients that melasma therapy is a journey of skin lightening but it’s not going to be a one-stop shop of getting it completely cured.”
As for treatment of melasma, “hydroquinone is still our workhorse, our gold standard.” Dr. Desai said. “I tell patients, ‘I’m going to keep you on it for 16 weeks. Then you’re going to come back. I’m going to see where you are, and we’ll move into the nonhydroquinone therapies once your disease is under control.’ ”
However, new therapies for melasma are needed because long-term use of hydroquinone can lead to complications such as ochronosis, nail discoloration, conjunctival melanosis, and corneal degeneration.
Emerging treatments
. Dr. Desai described azelaic acid as his “go to” nonhydroquinone option for skin lightening. In one study, 20% azelaic acid was used twice daily in 155 patients with facial melasma. Of these, 73% showed improvement after 6 months of therapy. Side effects were minimal and included erythema, pruritus, and burning.
Another option is topically compounded methimazole, a potent peroxidase inhibitor that causes morphologic change in melanocytes. “You can get it compounded as a 5% cream,” he said of the antithyroid agent. “It’s not that expensive, and even high concentrations are not melanocytotoxic. There’s minimal systemic absorption because the molecule is large, so there really is not any effect on TSH [thyroid-stimulating hormone] or T4 levels.”
Kojic acid dipalmitate, an antibiotic produced by many species of Aspergillus and Penicillium, can also be used as a second-line melasma treatment. Unlike kojic acid, kojic acid dipalmitate is more stable to light, heat, pH, and oxidation, and is also compatible with most organic sunscreens. It works by inhibiting tyrosinase. “It’s already available overseas and will soon be available in the U.S. as a derivative of kojic acid,” he said.
There is also vitamin C serum, which reduces tyrosinase activity via an antioxidant effect. “When you combine it with azelaic acid or sunscreen, vitamin C helps to augment the response,” Dr. Desai said. In one study that compared 5% ascorbic acid with 4% hydroquinone, 62.5% vs. 93% of patients improved, respectively, but side effects were more prominent in those who received 4% hydroquinone (68.7% vs. 6.2%).
An additional off-label option for melasma is oral tranexamic acid, which controls pigmentation by inhibiting the release of inflammatory mediators, specifically prostaglandins and arachidonic acid, which are involved in melanogenesis.
Dr. Desai often uses a dose of 325 mg twice daily. “Think of tranexamic acid as an anti-inflammatory,” he said. Tranexamic acid is contraindicated in patients who are currently taking or have previously taken anticoagulant medications; those who are pregnant or breastfeeding, or are smokers; and in those with renal, cardiac, and/or pulmonary disease. It has a half-life of about 7.5 hours, so the twice daily dosing “is quite effective,” he said.
“Do I leave my patients on this for years at a time to see if it’s going to work? No. When this works in treating melasma it works very quickly. I tell patients they’re going to see results in the first 8-12 weeks. That’s the beauty of using this orally.”
Another emerging therapy is Rubus occidentalis (black raspberry), a botanical-based ingredient in a 3% topical suspension that was compared with 4% hydroquinone in a randomized placebo-controlled trial. In the study, efficacy of Rubus occidentalis was considered comparable to that of hydroquinone. “This not only blocks melanogenesis, it also helps to block melanosome transfer,” said Dr. Desai, who is a past president of the Skin of Color Society.
Another natural option for melasma patients is topical cysteamine, which is the simplest aminothiol physiologically produced in human cells from the essential amino acid cysteine. “This is great for patients with recalcitrant disease, or for patients who, after 12-16 weeks of hydroquinone, you want them to have a break. I use it as a 5% concentration, and it works nicely,” he said. Cysteamine is also highly concentrated in human milk.
Dr. Desai disclosed that he performs clinical trials and consulting for many companies including L’Oréal, Galderma, Allergan, and AbbVie.
AT AAD 2022
Mercury and other risks of cosmetic skin lighteners
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
An 11-year-old female presented with skin discoloration on her back
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Necrotic Ulcerations After the Use of an Over-the-counter Mole and Skin Tag Removal Product
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.
The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
Practice Point
- Self-administered mole and skin tag removal products are rising in popularity, but unregulated ingredients in over-the-counter products that are not approved by the US Food and Drug Administration may mask underlying transformation of atypical nevi.
At-Home Treatment of Pigmented Lesions With a Zinc Chloride Preparation
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
Practice Points
- Zinc chloride preparations are readily available over the counter and unregulated.
- Patients may attempt to self-treat pigmented lesions based on claims they see online.
- When asking patients about prior treatments, it may be prudent to specifically ask about over-the-counter products and their ingredients.
Lower Leg Hyperpigmentation in MYH9-Related Disorder
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.
Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.

Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.

Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
Practice Points
- MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.
- Leg hyperpigmentation can occur secondary to hemosiderin deposition from MYH9-related disorder.
- The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder.
- Lasers and intense pulsed light therapy are modalities to consider for the hyperpigmentation of MYH9- related disorder.
Views and Beliefs of Vitiligo Patients in Online Discussion Forums: A Qualitative Study
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25
Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.
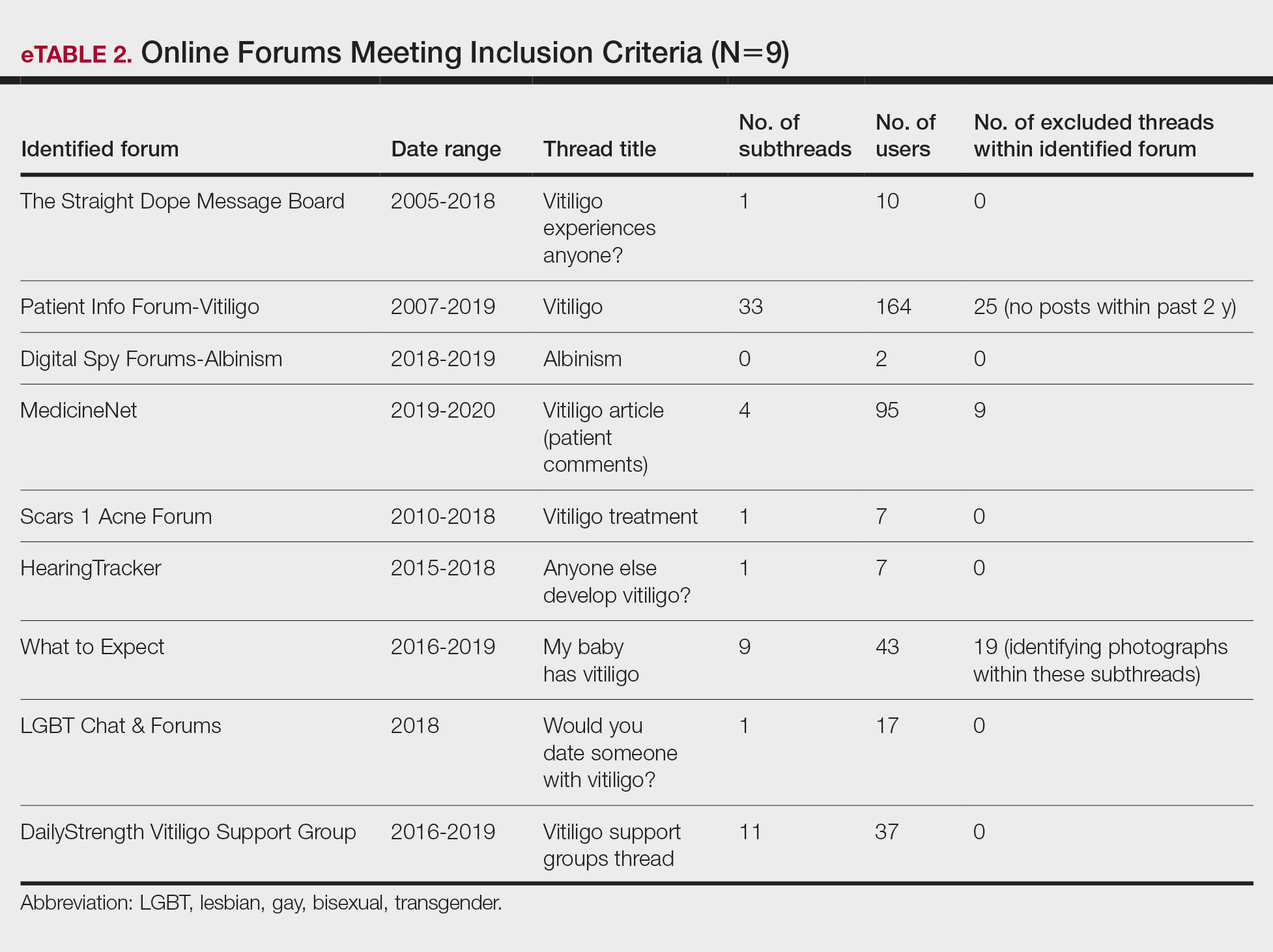
An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25

Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.

An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25

Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.

An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Practice Points
- Online forums provide invaluable insight on vitiligo disease management, psychosocial impact, and burden on quality of life. Patient care can be improved by inquiring where patients seek information and whether online forums are utilized.
- Commonly discussed topics in online forums were cosmetic concealment of vitiligo lesions and homeopathy or “cure” discussions. Health care providers can engage in honest conversations about evidence-based medical treatments for vitiligo. The interest in cosmetic management highlights a relevant research area in this field.
- Health care providers can better serve patients with vitiligo by providing online resources that are reputable and can help guide patients to credible internet sources such as the Global Vitiligo Foundation.
Review finds microneedling an effective add-on to topical melasma therapies
, results from a combined systematic review and meta-analysis suggest.
“Microneedling has a similar efficacy to other drug delivery methods, such as CO2 laser or intradermal microinjections, for the treatment of melasma,” presenting author Marcus G. Tan, MD, said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “When used in combination with topical depigmenting therapies, microneedling also demonstrated superior efficacy and a more favorable safety profile compared to oral tranexamic acid.”
For the study, Dr. Tan, a 5-year dermatology resident at the University of Ottawa, and colleagues searched MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials using the keywords “melasma” and “microneedling.” They limited their analysis to prospective, comparative studies incorporating the use of microneedling in the treatment of melasma and excluded those involving radiofrequency. The primary outcome was improvement in melasma severity, evaluated through the Melasma Area and Severity Index (MASI). The secondary outcomes were improvement in patient satisfaction, quality of life, and any reported adverse events.
Twelve studies involving 459 patients from seven countries were included in the final analysis. Of these, seven were randomized controlled studies and five were nonrandomized split-face studies. Topical treatments used in the studies included tranexamic acid (TXA), vitamin C, platelet-rich plasma, and hydroquinone-based depigmenting serums such as rucinol, sophora-alpha, and N-acetyl glucosamine. Of the 12 studies, 4 used mechanical microneedling and 8 used electric repeating microneedling. The most common needle length used was 1.5 mm, with a range from 0.1 to 1.5 mm, depending on the anatomic site treated. Topical anesthesia was applied 30-60 minutes prior to treatment. Treatment intervals were 2-4 weeks apart.
Their analysis found that microneedling alone resulted in a 23%-29% improvement in MASI. “Across all studies, adding topical therapies resulted in greater improvements in melasma severity, with a moderate effect at 8 weeks and a large effect at 12-16 weeks,” Dr. Tan said. “This also translated to higher patient satisfaction scores and improved patient-reported quality of life.”
A split-face study in the analysis, which compared topical TXA with microneedling to topical TXA with fractional CO2 laser, found that both approaches had similar efficacy and rates of adverse events. Another split-face study that evaluated recalcitrant melasma found that adding vitamin C with microneedling to a nonablative Q-switched Nd:YAG laser resulted in a further 38.3% greater improvement in MASI and a 12.5% lower recurrence rate at 6 months.
In two other studies, researchers compared microneedling to intradermal microinjections to deliver platelet-rich plasma or topical TXA. Both modalities were found to have similar efficacy. “However, microneedling was found to be better tolerated and had higher patient satisfaction as a result,” Dr. Tan said.
A separate analysis found that Tri-Luma (fluocinolone acetonide, hydroquinone, and tretinoin) cream with microneedling outperformed Tri-Luma plus oral TXA in terms of efficacy, patient satisfaction, and tolerability. “Interestingly, adding oral TXA to Tri-Luma with microneedling did not lead to further improvements,” Dr. Tan said.
The researchers found that microneedling was well tolerated in all 12 studies. Overall, no scarring or serious adverse events were reported. Mild-transient dyspigmentation occurred in 5%-12% of cases and herpes simplex virus reactivation was seen in a minority of patients.
Dr. Tan commented on three proposed mechanisms of action, which support the efficacy of microneedling for the treatment of melasma. “First, microneedling assists in the transcutaneous delivery of topical agents through the micropores,” he said. “Second, microneedling also assists in the transcutaneous elimination of melanin and other skin debris through the micropores. Third, the microinjuries stimulate the wound healing response, resulting in neocollagenesis, neoelastogenesis, and epidermal thickening.”
In an interview, Dr. Tan acknowledged certain limitations of the study, including the pooling of randomized and nonrandomized studies in the final meta-analysis, the heterogeneity in the treatment protocols and devices used, as well as the inclusion of studies with a moderate risk of bias. “Nonetheless, these limitations do not affect the conclusion that microneedling is a useful and safe adjuvant to topical therapies for melasma,” he said.
Catherine M. DiGiorgio, MD, who was asked to comment on the study, noted that melasma is a notoriously difficult condition to treat. “Many energy-based device treatments as well as other therapies have been proposed for treatment over the years. However, none have shown reliable, reproducible, and most importantly long-lasting results,” said Dr. DiGiorgio, a laser and cosmetic dermatologist at The Boston Center for Facial Rejuvenation. “Caution should be employed regarding the true efficacy of treatments for other than, at best, temporary results.”
The review included numerous studies without a clear definition of the strengths or methodologies of the studies, she added, noting that randomized controlled split-face studies with long-term follow up are the best way to assess the efficacy of treatments. “Further, regarding drug delivery, microneedling is the least effective method of delivery of drugs to the skin and laser-assisted drug delivery using ablative fractional lasers is the most effective. As with all melasma treatments, healthy skepticism is never a bad approach.”
Dr. Tan reported having no financial disclosures. Dr. DiGiorgio disclosed that she conducts research for Quthero Inc., and holds stock in the company.
, results from a combined systematic review and meta-analysis suggest.
“Microneedling has a similar efficacy to other drug delivery methods, such as CO2 laser or intradermal microinjections, for the treatment of melasma,” presenting author Marcus G. Tan, MD, said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “When used in combination with topical depigmenting therapies, microneedling also demonstrated superior efficacy and a more favorable safety profile compared to oral tranexamic acid.”
For the study, Dr. Tan, a 5-year dermatology resident at the University of Ottawa, and colleagues searched MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials using the keywords “melasma” and “microneedling.” They limited their analysis to prospective, comparative studies incorporating the use of microneedling in the treatment of melasma and excluded those involving radiofrequency. The primary outcome was improvement in melasma severity, evaluated through the Melasma Area and Severity Index (MASI). The secondary outcomes were improvement in patient satisfaction, quality of life, and any reported adverse events.
Twelve studies involving 459 patients from seven countries were included in the final analysis. Of these, seven were randomized controlled studies and five were nonrandomized split-face studies. Topical treatments used in the studies included tranexamic acid (TXA), vitamin C, platelet-rich plasma, and hydroquinone-based depigmenting serums such as rucinol, sophora-alpha, and N-acetyl glucosamine. Of the 12 studies, 4 used mechanical microneedling and 8 used electric repeating microneedling. The most common needle length used was 1.5 mm, with a range from 0.1 to 1.5 mm, depending on the anatomic site treated. Topical anesthesia was applied 30-60 minutes prior to treatment. Treatment intervals were 2-4 weeks apart.
Their analysis found that microneedling alone resulted in a 23%-29% improvement in MASI. “Across all studies, adding topical therapies resulted in greater improvements in melasma severity, with a moderate effect at 8 weeks and a large effect at 12-16 weeks,” Dr. Tan said. “This also translated to higher patient satisfaction scores and improved patient-reported quality of life.”
A split-face study in the analysis, which compared topical TXA with microneedling to topical TXA with fractional CO2 laser, found that both approaches had similar efficacy and rates of adverse events. Another split-face study that evaluated recalcitrant melasma found that adding vitamin C with microneedling to a nonablative Q-switched Nd:YAG laser resulted in a further 38.3% greater improvement in MASI and a 12.5% lower recurrence rate at 6 months.
In two other studies, researchers compared microneedling to intradermal microinjections to deliver platelet-rich plasma or topical TXA. Both modalities were found to have similar efficacy. “However, microneedling was found to be better tolerated and had higher patient satisfaction as a result,” Dr. Tan said.
A separate analysis found that Tri-Luma (fluocinolone acetonide, hydroquinone, and tretinoin) cream with microneedling outperformed Tri-Luma plus oral TXA in terms of efficacy, patient satisfaction, and tolerability. “Interestingly, adding oral TXA to Tri-Luma with microneedling did not lead to further improvements,” Dr. Tan said.
The researchers found that microneedling was well tolerated in all 12 studies. Overall, no scarring or serious adverse events were reported. Mild-transient dyspigmentation occurred in 5%-12% of cases and herpes simplex virus reactivation was seen in a minority of patients.
Dr. Tan commented on three proposed mechanisms of action, which support the efficacy of microneedling for the treatment of melasma. “First, microneedling assists in the transcutaneous delivery of topical agents through the micropores,” he said. “Second, microneedling also assists in the transcutaneous elimination of melanin and other skin debris through the micropores. Third, the microinjuries stimulate the wound healing response, resulting in neocollagenesis, neoelastogenesis, and epidermal thickening.”
In an interview, Dr. Tan acknowledged certain limitations of the study, including the pooling of randomized and nonrandomized studies in the final meta-analysis, the heterogeneity in the treatment protocols and devices used, as well as the inclusion of studies with a moderate risk of bias. “Nonetheless, these limitations do not affect the conclusion that microneedling is a useful and safe adjuvant to topical therapies for melasma,” he said.
Catherine M. DiGiorgio, MD, who was asked to comment on the study, noted that melasma is a notoriously difficult condition to treat. “Many energy-based device treatments as well as other therapies have been proposed for treatment over the years. However, none have shown reliable, reproducible, and most importantly long-lasting results,” said Dr. DiGiorgio, a laser and cosmetic dermatologist at The Boston Center for Facial Rejuvenation. “Caution should be employed regarding the true efficacy of treatments for other than, at best, temporary results.”
The review included numerous studies without a clear definition of the strengths or methodologies of the studies, she added, noting that randomized controlled split-face studies with long-term follow up are the best way to assess the efficacy of treatments. “Further, regarding drug delivery, microneedling is the least effective method of delivery of drugs to the skin and laser-assisted drug delivery using ablative fractional lasers is the most effective. As with all melasma treatments, healthy skepticism is never a bad approach.”
Dr. Tan reported having no financial disclosures. Dr. DiGiorgio disclosed that she conducts research for Quthero Inc., and holds stock in the company.
, results from a combined systematic review and meta-analysis suggest.
“Microneedling has a similar efficacy to other drug delivery methods, such as CO2 laser or intradermal microinjections, for the treatment of melasma,” presenting author Marcus G. Tan, MD, said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “When used in combination with topical depigmenting therapies, microneedling also demonstrated superior efficacy and a more favorable safety profile compared to oral tranexamic acid.”
For the study, Dr. Tan, a 5-year dermatology resident at the University of Ottawa, and colleagues searched MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials using the keywords “melasma” and “microneedling.” They limited their analysis to prospective, comparative studies incorporating the use of microneedling in the treatment of melasma and excluded those involving radiofrequency. The primary outcome was improvement in melasma severity, evaluated through the Melasma Area and Severity Index (MASI). The secondary outcomes were improvement in patient satisfaction, quality of life, and any reported adverse events.
Twelve studies involving 459 patients from seven countries were included in the final analysis. Of these, seven were randomized controlled studies and five were nonrandomized split-face studies. Topical treatments used in the studies included tranexamic acid (TXA), vitamin C, platelet-rich plasma, and hydroquinone-based depigmenting serums such as rucinol, sophora-alpha, and N-acetyl glucosamine. Of the 12 studies, 4 used mechanical microneedling and 8 used electric repeating microneedling. The most common needle length used was 1.5 mm, with a range from 0.1 to 1.5 mm, depending on the anatomic site treated. Topical anesthesia was applied 30-60 minutes prior to treatment. Treatment intervals were 2-4 weeks apart.
Their analysis found that microneedling alone resulted in a 23%-29% improvement in MASI. “Across all studies, adding topical therapies resulted in greater improvements in melasma severity, with a moderate effect at 8 weeks and a large effect at 12-16 weeks,” Dr. Tan said. “This also translated to higher patient satisfaction scores and improved patient-reported quality of life.”
A split-face study in the analysis, which compared topical TXA with microneedling to topical TXA with fractional CO2 laser, found that both approaches had similar efficacy and rates of adverse events. Another split-face study that evaluated recalcitrant melasma found that adding vitamin C with microneedling to a nonablative Q-switched Nd:YAG laser resulted in a further 38.3% greater improvement in MASI and a 12.5% lower recurrence rate at 6 months.
In two other studies, researchers compared microneedling to intradermal microinjections to deliver platelet-rich plasma or topical TXA. Both modalities were found to have similar efficacy. “However, microneedling was found to be better tolerated and had higher patient satisfaction as a result,” Dr. Tan said.
A separate analysis found that Tri-Luma (fluocinolone acetonide, hydroquinone, and tretinoin) cream with microneedling outperformed Tri-Luma plus oral TXA in terms of efficacy, patient satisfaction, and tolerability. “Interestingly, adding oral TXA to Tri-Luma with microneedling did not lead to further improvements,” Dr. Tan said.
The researchers found that microneedling was well tolerated in all 12 studies. Overall, no scarring or serious adverse events were reported. Mild-transient dyspigmentation occurred in 5%-12% of cases and herpes simplex virus reactivation was seen in a minority of patients.
Dr. Tan commented on three proposed mechanisms of action, which support the efficacy of microneedling for the treatment of melasma. “First, microneedling assists in the transcutaneous delivery of topical agents through the micropores,” he said. “Second, microneedling also assists in the transcutaneous elimination of melanin and other skin debris through the micropores. Third, the microinjuries stimulate the wound healing response, resulting in neocollagenesis, neoelastogenesis, and epidermal thickening.”
In an interview, Dr. Tan acknowledged certain limitations of the study, including the pooling of randomized and nonrandomized studies in the final meta-analysis, the heterogeneity in the treatment protocols and devices used, as well as the inclusion of studies with a moderate risk of bias. “Nonetheless, these limitations do not affect the conclusion that microneedling is a useful and safe adjuvant to topical therapies for melasma,” he said.
Catherine M. DiGiorgio, MD, who was asked to comment on the study, noted that melasma is a notoriously difficult condition to treat. “Many energy-based device treatments as well as other therapies have been proposed for treatment over the years. However, none have shown reliable, reproducible, and most importantly long-lasting results,” said Dr. DiGiorgio, a laser and cosmetic dermatologist at The Boston Center for Facial Rejuvenation. “Caution should be employed regarding the true efficacy of treatments for other than, at best, temporary results.”
The review included numerous studies without a clear definition of the strengths or methodologies of the studies, she added, noting that randomized controlled split-face studies with long-term follow up are the best way to assess the efficacy of treatments. “Further, regarding drug delivery, microneedling is the least effective method of delivery of drugs to the skin and laser-assisted drug delivery using ablative fractional lasers is the most effective. As with all melasma treatments, healthy skepticism is never a bad approach.”
Dr. Tan reported having no financial disclosures. Dr. DiGiorgio disclosed that she conducts research for Quthero Inc., and holds stock in the company.
FROM ASDS 2021