User login
Laser treatment of port wine stains in infancy found safe, effective
DALLAS – Laser treatment of port wine stains in infancy is both safe and effective, with no incidence of scarring or pigmentary changes, according to results from a single-center analysis.
“Early intervention allows for treatment without general anesthesia, with faster and more complete clearance than what has been reported for treatments begun at older ages,” Hana Jeon, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
A recent Food and Drug Administration Drug Safety Communication warned that “repeated or lengthy used of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains.” Dr. Jeon, a dermatologist in private practice in New York, noted that the FDA warning “places a greater importance on the already controversial topic of when to initiate port wine stain [PWS] treatments in pediatric patients, which requires repeated treatments and are often performed with general anesthesia. Without treatment, these lesions tend to get larger and thicker with time. Starting the treatment during infancy has the potential to limit the use of general anesthesia and to facilitate clearing.”
In what she said is the largest retrospective study of its kind to date, Dr. Jeon and her associates evaluated the success and safety of treating PWSs with a pulsed dye laser at the age of 1 year or younger in the office setting without general anesthesia. The patients received their first PWS treatment at their center during 2000-2017. They reviewed the charts of 197 patients to extract relevant data, including demographic information, age at the time of procedure, and treatment dates. The data cutoff was at 1 year following the initial treatment. Four physicians independently reviewed before and after photos and used the visual analogue scale to grade them.
The pulsed dye laser with dynamic cooling spray was used to minimize patient discomfort. No topical, local, or general anesthesia was used. Patients were immobilized by ancillary staff with parents present in most cases. Ocular shields were placed to allow treatment of periocular lesions.
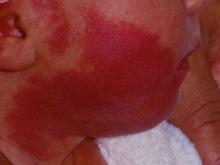
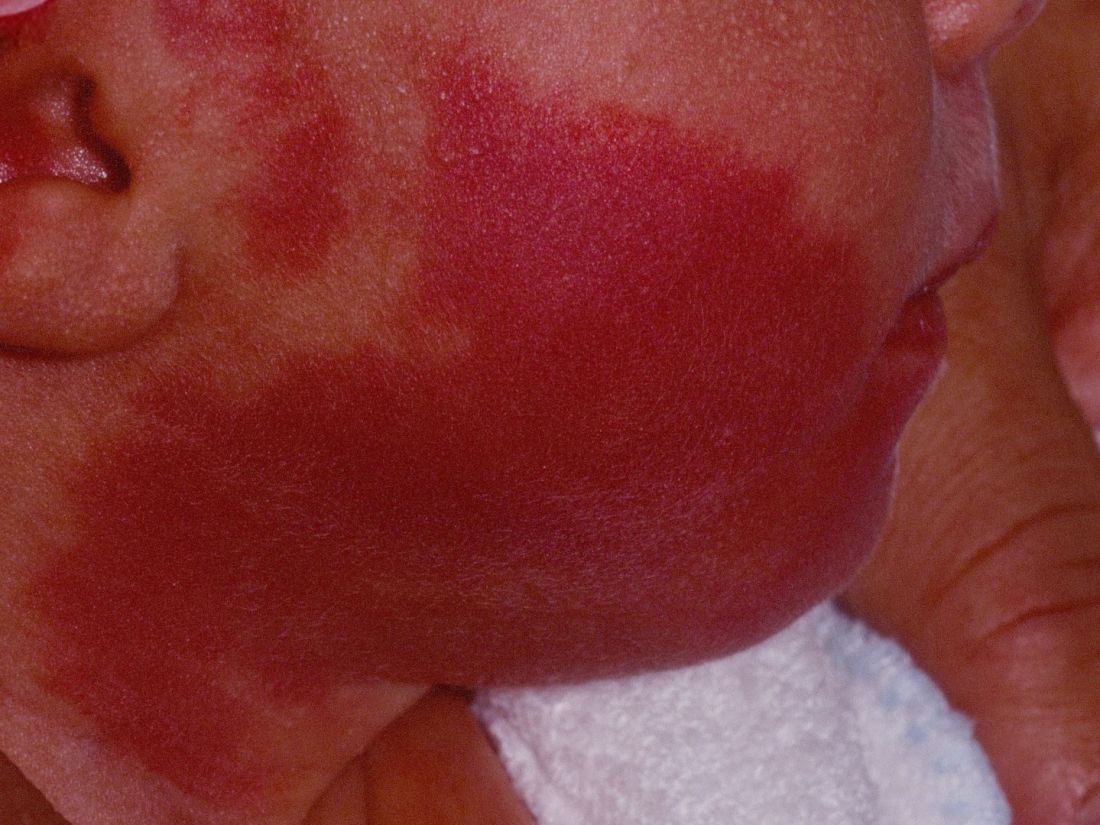
Of the 197 patients, 63% were female, 90.1% had Fitzpatrick skin types I-III, 8.1% had type IV skin, and the rest had type V-VI skin. Most of the lesions were facial (75.6%), and 41.1% had periocular involvement. The average lesion size was 61 cm2.
The treatment settings for the pulsed dye laser were a 10-12 mm spot size delivered at a fluence of 6.5-9 J/cm2 in a pulse duration of 0.45-1.5 milliseconds. The average age at the time of first treatment was 3.4 months (range, 5-355 days), and the average number of treatments was 9.8 (range, 2-23). Most of the patients (116) were aged 0-3 months at the time of first treatment, followed by 51 aged 3-6 months, 19 aged 6-9 months, and 11 aged 9-12 months.
According to the averaged scores on the visual analogue scale assigned by physicians, 27.4% showed a 100% clearance, 39.1% showed 76%-99% improvement, 15.1% showed 51%-75% improvement, 10.7% showed 26%-50% improvement, and 7.7% showed 0%-25% improvement. No scarring or pigmentary changes were observed.
An analysis of dermatomal distribution revealed that the presence of a V1 lesion was a statistically significant predictor of a higher clearance rate, while the presence of a V3 lesion was a statistically significant predictor of a low clearance rate. “Regardless of where the lesion was, all patients did well,” she said.
Given the small proportion of patients who received their first treatment between the ages of 6 and 12 months of age, Dr. Jeon said that “further studies would be needed to elucidate whether earlier intervention helps to achieve better results.”*
Advantages of early treatment, she said, include the ability to “treat lesions before they get larger and thicker, which also decreases the risk of spontaneous bleeding. Thinner skin allows for better penetration of the laser beam, and we can also avoid using general anesthesia. Psychologically, if we’re able to clear these lesions, it can result in improved quality of life and self-esteem; not just for the patient but for the family as well.”
Dr. Jeon acknowledged certain limitations of the study, including its retrospective design, variable follow-up times, and non-standardization of photographs.
She reported having no financial disclosures.
SOURCE: Jeon H et al. ASLMS 2018.
Correction, 4/18/18: An earlier version of this article misstated the age range of the study participants.
DALLAS – Laser treatment of port wine stains in infancy is both safe and effective, with no incidence of scarring or pigmentary changes, according to results from a single-center analysis.
“Early intervention allows for treatment without general anesthesia, with faster and more complete clearance than what has been reported for treatments begun at older ages,” Hana Jeon, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
A recent Food and Drug Administration Drug Safety Communication warned that “repeated or lengthy used of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains.” Dr. Jeon, a dermatologist in private practice in New York, noted that the FDA warning “places a greater importance on the already controversial topic of when to initiate port wine stain [PWS] treatments in pediatric patients, which requires repeated treatments and are often performed with general anesthesia. Without treatment, these lesions tend to get larger and thicker with time. Starting the treatment during infancy has the potential to limit the use of general anesthesia and to facilitate clearing.”
In what she said is the largest retrospective study of its kind to date, Dr. Jeon and her associates evaluated the success and safety of treating PWSs with a pulsed dye laser at the age of 1 year or younger in the office setting without general anesthesia. The patients received their first PWS treatment at their center during 2000-2017. They reviewed the charts of 197 patients to extract relevant data, including demographic information, age at the time of procedure, and treatment dates. The data cutoff was at 1 year following the initial treatment. Four physicians independently reviewed before and after photos and used the visual analogue scale to grade them.
The pulsed dye laser with dynamic cooling spray was used to minimize patient discomfort. No topical, local, or general anesthesia was used. Patients were immobilized by ancillary staff with parents present in most cases. Ocular shields were placed to allow treatment of periocular lesions.
Of the 197 patients, 63% were female, 90.1% had Fitzpatrick skin types I-III, 8.1% had type IV skin, and the rest had type V-VI skin. Most of the lesions were facial (75.6%), and 41.1% had periocular involvement. The average lesion size was 61 cm2.
The treatment settings for the pulsed dye laser were a 10-12 mm spot size delivered at a fluence of 6.5-9 J/cm2 in a pulse duration of 0.45-1.5 milliseconds. The average age at the time of first treatment was 3.4 months (range, 5-355 days), and the average number of treatments was 9.8 (range, 2-23). Most of the patients (116) were aged 0-3 months at the time of first treatment, followed by 51 aged 3-6 months, 19 aged 6-9 months, and 11 aged 9-12 months.
According to the averaged scores on the visual analogue scale assigned by physicians, 27.4% showed a 100% clearance, 39.1% showed 76%-99% improvement, 15.1% showed 51%-75% improvement, 10.7% showed 26%-50% improvement, and 7.7% showed 0%-25% improvement. No scarring or pigmentary changes were observed.
An analysis of dermatomal distribution revealed that the presence of a V1 lesion was a statistically significant predictor of a higher clearance rate, while the presence of a V3 lesion was a statistically significant predictor of a low clearance rate. “Regardless of where the lesion was, all patients did well,” she said.
Given the small proportion of patients who received their first treatment between the ages of 6 and 12 months of age, Dr. Jeon said that “further studies would be needed to elucidate whether earlier intervention helps to achieve better results.”*
Advantages of early treatment, she said, include the ability to “treat lesions before they get larger and thicker, which also decreases the risk of spontaneous bleeding. Thinner skin allows for better penetration of the laser beam, and we can also avoid using general anesthesia. Psychologically, if we’re able to clear these lesions, it can result in improved quality of life and self-esteem; not just for the patient but for the family as well.”
Dr. Jeon acknowledged certain limitations of the study, including its retrospective design, variable follow-up times, and non-standardization of photographs.
She reported having no financial disclosures.
SOURCE: Jeon H et al. ASLMS 2018.
Correction, 4/18/18: An earlier version of this article misstated the age range of the study participants.
DALLAS – Laser treatment of port wine stains in infancy is both safe and effective, with no incidence of scarring or pigmentary changes, according to results from a single-center analysis.
“Early intervention allows for treatment without general anesthesia, with faster and more complete clearance than what has been reported for treatments begun at older ages,” Hana Jeon, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
A recent Food and Drug Administration Drug Safety Communication warned that “repeated or lengthy used of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains.” Dr. Jeon, a dermatologist in private practice in New York, noted that the FDA warning “places a greater importance on the already controversial topic of when to initiate port wine stain [PWS] treatments in pediatric patients, which requires repeated treatments and are often performed with general anesthesia. Without treatment, these lesions tend to get larger and thicker with time. Starting the treatment during infancy has the potential to limit the use of general anesthesia and to facilitate clearing.”
In what she said is the largest retrospective study of its kind to date, Dr. Jeon and her associates evaluated the success and safety of treating PWSs with a pulsed dye laser at the age of 1 year or younger in the office setting without general anesthesia. The patients received their first PWS treatment at their center during 2000-2017. They reviewed the charts of 197 patients to extract relevant data, including demographic information, age at the time of procedure, and treatment dates. The data cutoff was at 1 year following the initial treatment. Four physicians independently reviewed before and after photos and used the visual analogue scale to grade them.
The pulsed dye laser with dynamic cooling spray was used to minimize patient discomfort. No topical, local, or general anesthesia was used. Patients were immobilized by ancillary staff with parents present in most cases. Ocular shields were placed to allow treatment of periocular lesions.
Of the 197 patients, 63% were female, 90.1% had Fitzpatrick skin types I-III, 8.1% had type IV skin, and the rest had type V-VI skin. Most of the lesions were facial (75.6%), and 41.1% had periocular involvement. The average lesion size was 61 cm2.
The treatment settings for the pulsed dye laser were a 10-12 mm spot size delivered at a fluence of 6.5-9 J/cm2 in a pulse duration of 0.45-1.5 milliseconds. The average age at the time of first treatment was 3.4 months (range, 5-355 days), and the average number of treatments was 9.8 (range, 2-23). Most of the patients (116) were aged 0-3 months at the time of first treatment, followed by 51 aged 3-6 months, 19 aged 6-9 months, and 11 aged 9-12 months.
According to the averaged scores on the visual analogue scale assigned by physicians, 27.4% showed a 100% clearance, 39.1% showed 76%-99% improvement, 15.1% showed 51%-75% improvement, 10.7% showed 26%-50% improvement, and 7.7% showed 0%-25% improvement. No scarring or pigmentary changes were observed.
An analysis of dermatomal distribution revealed that the presence of a V1 lesion was a statistically significant predictor of a higher clearance rate, while the presence of a V3 lesion was a statistically significant predictor of a low clearance rate. “Regardless of where the lesion was, all patients did well,” she said.
Given the small proportion of patients who received their first treatment between the ages of 6 and 12 months of age, Dr. Jeon said that “further studies would be needed to elucidate whether earlier intervention helps to achieve better results.”*
Advantages of early treatment, she said, include the ability to “treat lesions before they get larger and thicker, which also decreases the risk of spontaneous bleeding. Thinner skin allows for better penetration of the laser beam, and we can also avoid using general anesthesia. Psychologically, if we’re able to clear these lesions, it can result in improved quality of life and self-esteem; not just for the patient but for the family as well.”
Dr. Jeon acknowledged certain limitations of the study, including its retrospective design, variable follow-up times, and non-standardization of photographs.
She reported having no financial disclosures.
SOURCE: Jeon H et al. ASLMS 2018.
Correction, 4/18/18: An earlier version of this article misstated the age range of the study participants.
REPORTING FROM ASLMS 2018
Key clinical point:
Major finding: On the visual analogue scale, 27.4% of infants achieved complete clearance of their port wine lesion after treatment.
Study details: An analysis of 197 infants aged 1 year or younger with port wine stains who were treated with a pulsed dye laser during 2000-2017.
Disclosures: Dr. Jeon reported having no financial disclosures.
Source: Jeon H et al. ASLMS 2018.
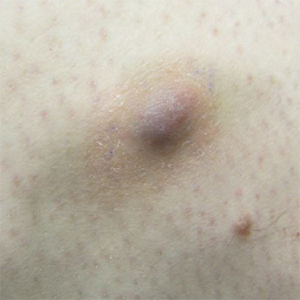
Painful Violaceous Nodule With Peripheral Hyperpigmentation
The Diagnosis: Aneurysmal Dermatofibroma
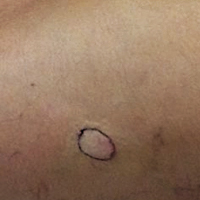
Biopsy of the lesion revealed a circumscribed dermal nodule comprised of storiform arrangements of enlarged, plump, fibrohistiocytic cells punctuated by variably sized clefts and large cystic spaces filled with blood that lacked an endothelial lining. No bizarre nuclear pleomorphism, atypical mitoses, or tumor necrosis were identified. The overlying epidermis exhibited mild acanthosis with broadening of the rete ridges. Proliferative spindled cells entrapped dermal collagen bundles at the periphery. Hemosiderin-laden macrophages were present throughout the proliferation and in the adjacent dermis (Figure). These findings supported the diagnosis of aneurysmal dermatofibroma (ADF).
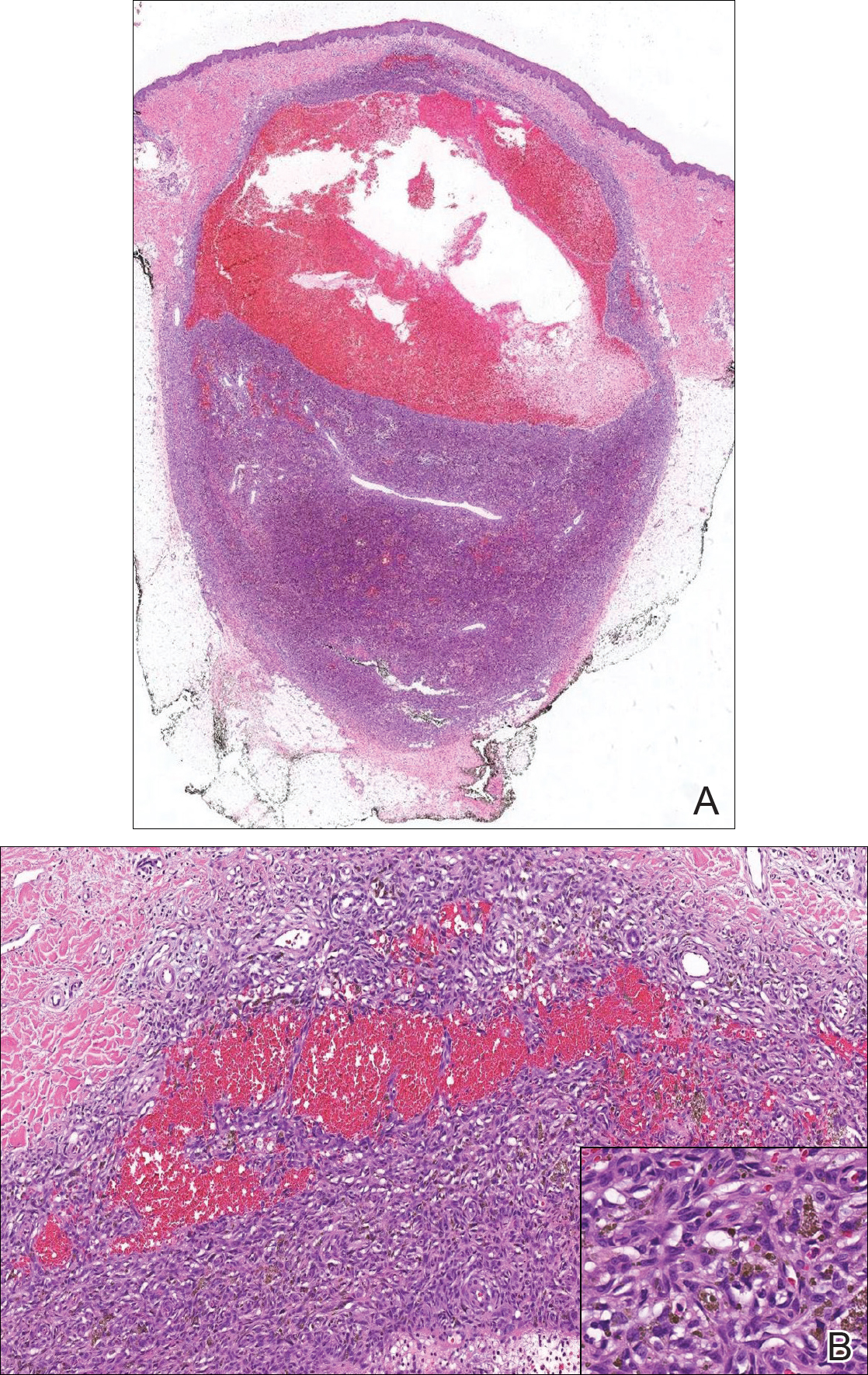
Aneurysmal dermatofibroma, also known as aneurysmal fibrous histiocytoma, is a rare variant of dermatofibroma that was described by Santa Cruz et al1 in 1981 and represents 2% to 6% of dermatofibromas.1,2 Aneurysmal dermatofibromas often lack the characteristic clinical and dermoscopic findings of conventional dermatofibromas, creating a diagnostic challenge for the clinician.3 Incomplete excision of this benign tumor was associated with a local recurrence rate of 19% (5/26) in one study,4 in contrast with the exceedingly low rate of local recurrence (<2%) attributed to conventional dermatofibromas.2,4
Clinically, ADFs commonly appear as blue-brown nodules on the arms and legs, often with a history of rapid and sometimes painful growth.1 Clinically, an ADF can have vascular, cystic, or melanocytic features that, in the context of lacking typical clinical findings of a dermatofibroma, can complicate clinical diagnosis; for example, ADFs can demonstrate several melanomalike features including atypical vessels, chrysalis structures, blue-white structures, a pinkish-white veil, irregular brown globulelike structures, an atypical pigment network, color variegation, a multicomponent pattern, and ulceration.3 Alternatively, ADFs can present with a vascular tumor-like pattern consisting of white areas and globular blue-red areas or a polymorphous vascular pattern with a peripheral collarette.
Our case illustrates the classic histologic appearance of an ADF. Large cavities and slitlike spaces filled with blood distinguish this entity from conventional dermatofibroma and other dermatofibroma variants; for example, cellular dermatofibroma is a benign variant of dermatofibroma that exhibits crowded fascicular architecture without an increase in vascular spaces. Aneurysmal dermatofibromas also should be distinguished from angiomatoid fibrous histiocytoma, which has intermediate malignant potential despite a similar-sounding name and a similar nodular appearance with large blood-filled spaces; however, many cases are located predominantly in the subcutis with epithelioid morphology, desmin immunohistochemical reactivity, and prominent tumor-associated lymphoid proliferation that can be mistaken for a lymph node.5 Furthermore, in contrast with vascular tumors, the blood-filled spaces of ADFs do not have an endothelial lining.
In summary, ADF is a rare dermatofibroma variant that has a variety of clinical presentations, often masquerading as a cyst, vascular tumor, or melanocytic neoplasm. The classic histopathologic features confirm the diagnosis. Although ADFs can be painful and have a tendency to recur, these lesions have a benign clinical course.
- Santa Cruz DJ, Kyriakos M. Aneurysmal ("angiomatoid") fibrous histiocytoma of the skin. Cancer. 1981;47:2053-2061.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Ferrari A, Argenziano G, Buccini P, et al. Typical and atypical dermoscopic presentations of dermatofibroma. J Eur Acad Dermatol Venereol. 2013;27:1375-1380.
- Calonje E, Fletcher CD. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours--an update. Histopathology. 2010;56:148-165.
The Diagnosis: Aneurysmal Dermatofibroma
Biopsy of the lesion revealed a circumscribed dermal nodule comprised of storiform arrangements of enlarged, plump, fibrohistiocytic cells punctuated by variably sized clefts and large cystic spaces filled with blood that lacked an endothelial lining. No bizarre nuclear pleomorphism, atypical mitoses, or tumor necrosis were identified. The overlying epidermis exhibited mild acanthosis with broadening of the rete ridges. Proliferative spindled cells entrapped dermal collagen bundles at the periphery. Hemosiderin-laden macrophages were present throughout the proliferation and in the adjacent dermis (Figure). These findings supported the diagnosis of aneurysmal dermatofibroma (ADF).

Aneurysmal dermatofibroma, also known as aneurysmal fibrous histiocytoma, is a rare variant of dermatofibroma that was described by Santa Cruz et al1 in 1981 and represents 2% to 6% of dermatofibromas.1,2 Aneurysmal dermatofibromas often lack the characteristic clinical and dermoscopic findings of conventional dermatofibromas, creating a diagnostic challenge for the clinician.3 Incomplete excision of this benign tumor was associated with a local recurrence rate of 19% (5/26) in one study,4 in contrast with the exceedingly low rate of local recurrence (<2%) attributed to conventional dermatofibromas.2,4
Clinically, ADFs commonly appear as blue-brown nodules on the arms and legs, often with a history of rapid and sometimes painful growth.1 Clinically, an ADF can have vascular, cystic, or melanocytic features that, in the context of lacking typical clinical findings of a dermatofibroma, can complicate clinical diagnosis; for example, ADFs can demonstrate several melanomalike features including atypical vessels, chrysalis structures, blue-white structures, a pinkish-white veil, irregular brown globulelike structures, an atypical pigment network, color variegation, a multicomponent pattern, and ulceration.3 Alternatively, ADFs can present with a vascular tumor-like pattern consisting of white areas and globular blue-red areas or a polymorphous vascular pattern with a peripheral collarette.
Our case illustrates the classic histologic appearance of an ADF. Large cavities and slitlike spaces filled with blood distinguish this entity from conventional dermatofibroma and other dermatofibroma variants; for example, cellular dermatofibroma is a benign variant of dermatofibroma that exhibits crowded fascicular architecture without an increase in vascular spaces. Aneurysmal dermatofibromas also should be distinguished from angiomatoid fibrous histiocytoma, which has intermediate malignant potential despite a similar-sounding name and a similar nodular appearance with large blood-filled spaces; however, many cases are located predominantly in the subcutis with epithelioid morphology, desmin immunohistochemical reactivity, and prominent tumor-associated lymphoid proliferation that can be mistaken for a lymph node.5 Furthermore, in contrast with vascular tumors, the blood-filled spaces of ADFs do not have an endothelial lining.
In summary, ADF is a rare dermatofibroma variant that has a variety of clinical presentations, often masquerading as a cyst, vascular tumor, or melanocytic neoplasm. The classic histopathologic features confirm the diagnosis. Although ADFs can be painful and have a tendency to recur, these lesions have a benign clinical course.
The Diagnosis: Aneurysmal Dermatofibroma
Biopsy of the lesion revealed a circumscribed dermal nodule comprised of storiform arrangements of enlarged, plump, fibrohistiocytic cells punctuated by variably sized clefts and large cystic spaces filled with blood that lacked an endothelial lining. No bizarre nuclear pleomorphism, atypical mitoses, or tumor necrosis were identified. The overlying epidermis exhibited mild acanthosis with broadening of the rete ridges. Proliferative spindled cells entrapped dermal collagen bundles at the periphery. Hemosiderin-laden macrophages were present throughout the proliferation and in the adjacent dermis (Figure). These findings supported the diagnosis of aneurysmal dermatofibroma (ADF).

Aneurysmal dermatofibroma, also known as aneurysmal fibrous histiocytoma, is a rare variant of dermatofibroma that was described by Santa Cruz et al1 in 1981 and represents 2% to 6% of dermatofibromas.1,2 Aneurysmal dermatofibromas often lack the characteristic clinical and dermoscopic findings of conventional dermatofibromas, creating a diagnostic challenge for the clinician.3 Incomplete excision of this benign tumor was associated with a local recurrence rate of 19% (5/26) in one study,4 in contrast with the exceedingly low rate of local recurrence (<2%) attributed to conventional dermatofibromas.2,4
Clinically, ADFs commonly appear as blue-brown nodules on the arms and legs, often with a history of rapid and sometimes painful growth.1 Clinically, an ADF can have vascular, cystic, or melanocytic features that, in the context of lacking typical clinical findings of a dermatofibroma, can complicate clinical diagnosis; for example, ADFs can demonstrate several melanomalike features including atypical vessels, chrysalis structures, blue-white structures, a pinkish-white veil, irregular brown globulelike structures, an atypical pigment network, color variegation, a multicomponent pattern, and ulceration.3 Alternatively, ADFs can present with a vascular tumor-like pattern consisting of white areas and globular blue-red areas or a polymorphous vascular pattern with a peripheral collarette.
Our case illustrates the classic histologic appearance of an ADF. Large cavities and slitlike spaces filled with blood distinguish this entity from conventional dermatofibroma and other dermatofibroma variants; for example, cellular dermatofibroma is a benign variant of dermatofibroma that exhibits crowded fascicular architecture without an increase in vascular spaces. Aneurysmal dermatofibromas also should be distinguished from angiomatoid fibrous histiocytoma, which has intermediate malignant potential despite a similar-sounding name and a similar nodular appearance with large blood-filled spaces; however, many cases are located predominantly in the subcutis with epithelioid morphology, desmin immunohistochemical reactivity, and prominent tumor-associated lymphoid proliferation that can be mistaken for a lymph node.5 Furthermore, in contrast with vascular tumors, the blood-filled spaces of ADFs do not have an endothelial lining.
In summary, ADF is a rare dermatofibroma variant that has a variety of clinical presentations, often masquerading as a cyst, vascular tumor, or melanocytic neoplasm. The classic histopathologic features confirm the diagnosis. Although ADFs can be painful and have a tendency to recur, these lesions have a benign clinical course.
- Santa Cruz DJ, Kyriakos M. Aneurysmal ("angiomatoid") fibrous histiocytoma of the skin. Cancer. 1981;47:2053-2061.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Ferrari A, Argenziano G, Buccini P, et al. Typical and atypical dermoscopic presentations of dermatofibroma. J Eur Acad Dermatol Venereol. 2013;27:1375-1380.
- Calonje E, Fletcher CD. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours--an update. Histopathology. 2010;56:148-165.
- Santa Cruz DJ, Kyriakos M. Aneurysmal ("angiomatoid") fibrous histiocytoma of the skin. Cancer. 1981;47:2053-2061.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Ferrari A, Argenziano G, Buccini P, et al. Typical and atypical dermoscopic presentations of dermatofibroma. J Eur Acad Dermatol Venereol. 2013;27:1375-1380.
- Calonje E, Fletcher CD. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours--an update. Histopathology. 2010;56:148-165.
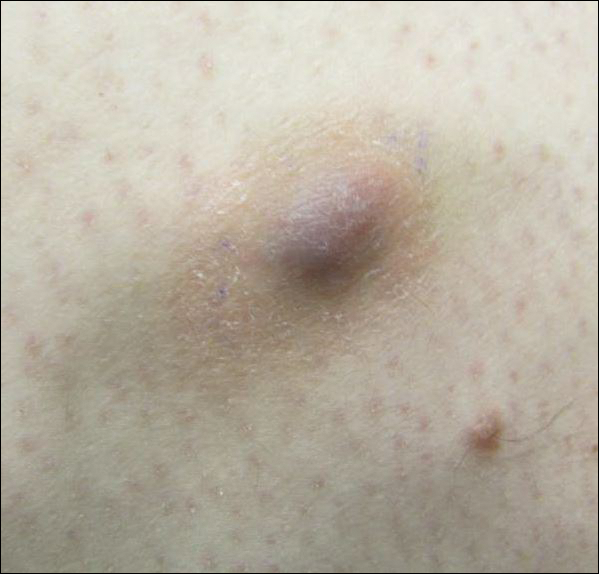
A 30-year-old man presented for evaluation of a painful lesion on the left thigh of 3 to 4 years' duration. Pain was exacerbated on physical exertion and was relieved by application of ice packs and use of over-the-counter analgesics. The patient denied any bleeding from the lesion. No other medical comorbidities were present. Physical examination demonstrated a pink, scaly, 3.2 ×2-cm patch with peripheral hyperpigmentation overlying a central, moderately firm, violaceous, 10.2 ×15-mm nodule on the left anteromedial thigh. The lesion was excised and sent to pathology.
Facial Involvement in Progressive Macular Hypomelanosis
Progressive macular hypomelanosis (PMH) is a noninflammatory skin disorder characterized by ill-defined, nummular, hypopigmented, and nonscaly macules. Historically, various names have been used to describe this entity. Several of these terms, including cutis trunci variata and nummular and confluent hypomelanosis of the trunk, reflected its predominantly truncal distribution.1,2 Less frequently, involvement on the neck, buttocks, and arms and legs has been noted.1,2 A lack of facial involvement previously has been highlighted as a key clinical feature of PMH.3
Progressive macular hypomelanosis is a diagnosis of exclusion. Hypopigmented diseases commonly considered in the differential include those caused by fungi and yeasts (eg, tinea versicolor, seborrheic dermatitis), inflammatory skin disorders (eg, pityriasis alba, postinflammatory dyschromia), and mycosis fungoides (MF) as well as leprosy.
The hypopigmented macules of PMH have nonspecific histopathologic findings; lesional skin often shows minimal alterations as compared to normal skin. A sparse perivascular lymphocytic infiltrate often is observed,4,5 and at times, a decrease in epidermal melanin content can be detected.1-3,6,7
We report 4 cases with considerable facial involvement of hypopigmented macules that were determined to be consistent with PMH. We propose that characteristic macules that are not clinically or histopathologically consistent with other disease entities are compatible with a diagnosis of PMH, regardless of the distribution. A diagnosis of PMH should be considered in the differential when there are suggestive facial lesions in addition to truncal lesions.
Case Reports
Patient 1
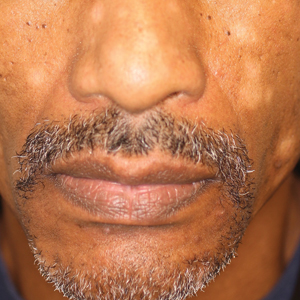
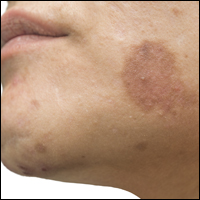
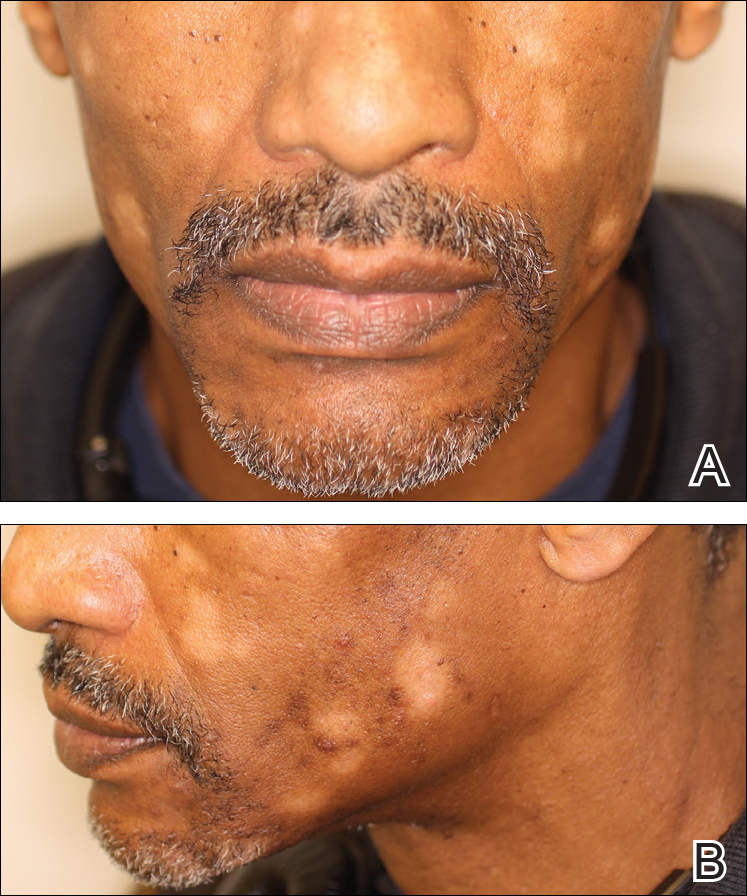
A 40-year-old man presented with hypopigmented macules on the face (Figure 1), trunk, chest, arms, and legs of 2 years’ duration. The lesions were asymptomatic and had started on the forehead as hypopigmented macules, then progressed to the trunk, arms, and legs. The patient denied any prior rash, injury, or hyperpigmentation associated with the distribution of the lesions.
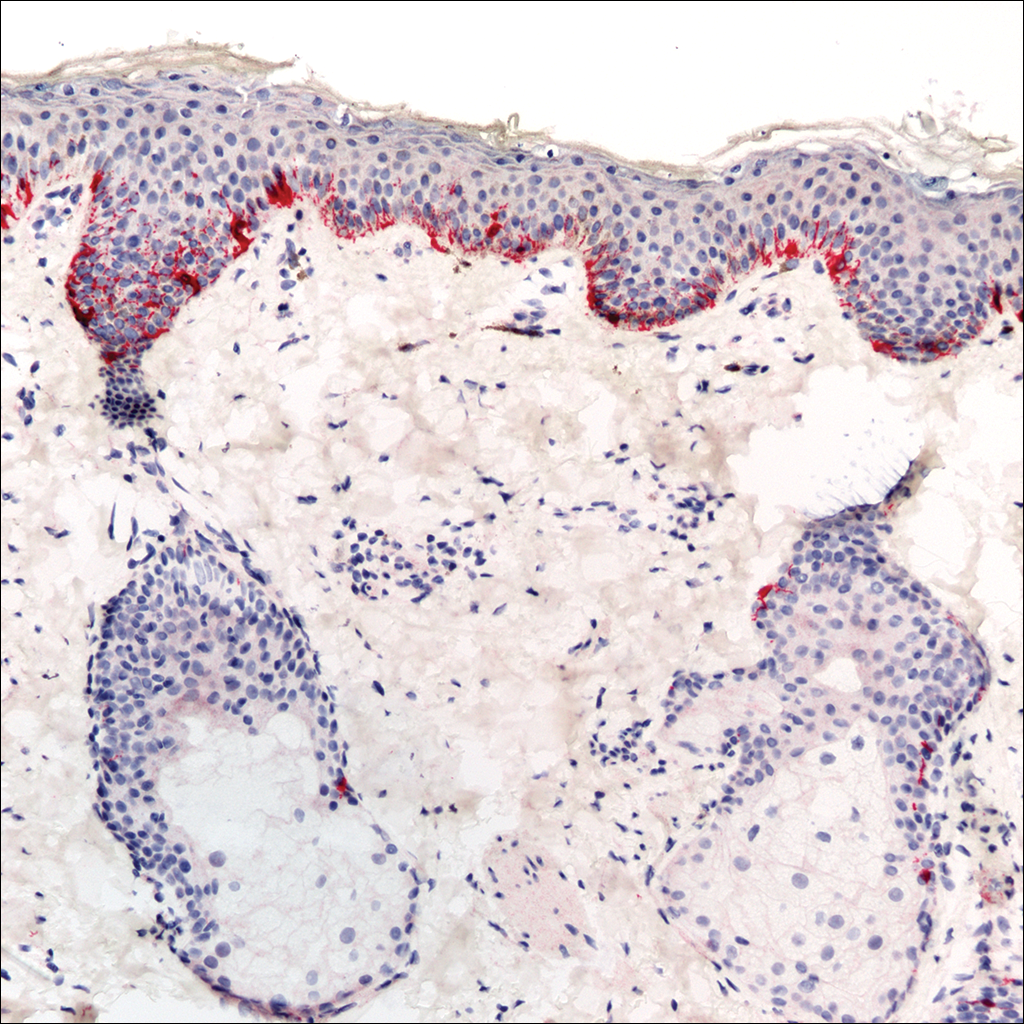
A rapid plasma reagin (RPR) test was conducted to rule out secondary syphilis and was nonreactive. During a series of clinical encounters over several months, a total of 5 biopsies of lesions on the face and back were performed. All specimens contained mild mononuclear perivascular inflammation (Figure 2). In some foci, staining for Melan-A revealed a decrease in epidermal melanocytes (Figure 3). Periodic acid–Schiff staining performed on one section revealed a few pityriasis spores but no hyphal elements, suggesting colonization rather than infection.

The patient initially was started on tacrolimus ointment 0.1% once daily and narrowband UVB phototherapy twice weekly for 3 months without benefit. A diagnosis of tinea versicolor was revisited and the patient was switched to ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing, and ketoconazole cream 2% was applied twice daily to the affected areas for 2 months without notable improvement. Once-weekly 150-mg pulse doses of oral fluconazole for 8 weeks were started but proved equally ineffective. Antibiotic therapy aimed at eradicating Propionibacterium acnes was considered following a provisional diagnosis of PMH after the patient failed 5 months of therapy for tinea versicolor.
Patient 2
A 54-year-old man presented with hypopigmented to depigmented nonscaly macules on the face, trunk, chest, and arms of several months’ duration. The patient initially noted hypopigmentation on the face that gradually spread to the rest of the body. The patient denied any prior rash or hyperpigmentation in the affected areas. At the initial visit to our clinic, a potassium hydroxide (KOH) preparation of the face and back was positive for tinea versicolor. The patient was treated with ketoconazole shampoo 1% two to 3 times weekly for several weeks on the scalp, face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and 2 total doses of oral fluconazole 150 mg taken 1 week apart.
Three months later the patient returned with no improvement of the existing lesions and with progression of the disease to previously uninvolved areas of the trunk, arms, and legs. Biopsy of a facial lesion was performed, and laboratory studies including RPR, thyroid-stimulating hormone, and antinuclear antibody tests were conducted to screen for possible systemic disease. Microscopic analysis of the biopsied facial lesion revealed a sparse perivascular infiltrate of lymphocytes and plasma cells but no evidence of yeast or hyphal elements. Melan-A staining did not reveal a decreased number of epidermal melanocytes. All laboratory studies were negative or within normal limits. Desonide ointment 0.05% was prescribed to relieve the patient’s occasional pruritus. Although the patient’s symptoms resolved, the hypopigmented macules continued to progress, making a diagnosis of PMH more likely given the lack of improvement on treatment for tinea versicolor. Pimecrolimus cream 1% was started with discontinuation of desonide for steroid-sparing therapy.
Patient 3
A 63-year-old man presented with progressive nonscaly and asymptomatic hypopigmented macules on the face, trunk, abdomen, and back of 5 years’ duration. He first noted lesions on the abdomen and they subsequently spread to the rest of the body. The patient denied any prior rash, hyperpigmentation, or other lesions in the involved areas.
One year prior to the current presentation, KOH scrapings from the lesions performed by an outside physician were negative. During his initial visit to our clinic, an abdominal biopsy was performed, and histopathologic analysis showed postinflammatory pigmentary alteration; however, the patient denied any prior history of rash or injury in the distribution of the lesions that would correlate with the histopathologic findings of postinflammatory pigmentation. Because the histopathologic findings showed postinflammatory pigmentary alteration, additional stains including Melan-A were not performed.
The patient was provisionally treated with ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and ketoconazole cream 2% twice daily to the affected areas. After several months on this regimen, the patient did not report any improvement. An abdominal skin biopsy was again performed and revealed similar histopathology. Periodic acid–Schiff staining was negative for fungus. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin lotion.
Patient 4
A 45-year-old woman presented with hypopigmented, nonscaly macules on the face, neck, chest, trunk, and back. She first noted the lesions on the face and trunk more than 8 years prior, and they subsequently progressed. Potassium hydroxide scrapings performed on the lesions at the current presentation were negative, and a skin biopsy from the neck revealed postinflammatory pigmentary alteration, although the patient had no history of rash or injury in the areas in which the lesions were distributed.
Fontana-Masson and Melan-A staining of the skin biopsy of the neck revealed a normal distribution of melanocytes and pigment at the dermoepidermal junction. An RPR test was nonreactive. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin phosphate lotion 1%.
Comment
The 4 cases of PMH reported here showed extensive facial involvement in addition to the characteristic hypopigmented lesions on the trunk, arms, and legs. It is unclear why the lesions in these patients had a predominantly facial distribution. Involvement of the face in PMH has not been commonly reported in the literature. Martínez-Martínez et al3 reported 12 PMH patients with lesions only presenting in lumbar and abdominal distributions. Kim et al8 presented a series of 23 PMH patients treated with narrowband UVB in whom 56% (9/16) saw repigmentation in 90% of the lesions following treatment. The most commonly affected area was the lower back, followed by the abdomen, upper back, chest, sacral region, flank, and shoulders, respectively.8 In a review by Relyveld et al,1 PMH is described as a predominantly truncal disease that can occasionally extend to the neck, face, and proximal arms and legs; however, no specific cases were reported.
Previous case series have reported PMH primarily in adolescents and young adults, with mean ages ranging from 26 to 30 years.1,3 The 4 patients reported here were older, ranging in age from 40 to 65 years. This discrepancy in age may contribute to the facial distribution encountered in this patient population; however, given the small number of patients in our case series, such extrapolation is premature. Most recently, Westerhof et al6 demonstrated a relationship between the presence of P acnes, a common skin commensal of the face, and the hypopigmented macules of PMH. The investigators suggested that some strains of P acnes produce a factor that is yet to be identified that interferes with melanogenesis. The response of PMH lesions to topical treatments such as benzoyl peroxide, clindamycin, and phototherapy has lent credence to the potential etiologic role of P acnes in this condition.9,10 The interplay between age, PMH distribution, and P acnes requires further investigation.
The biopsies in our 4 patients were consistent with the nonspecific histopathologic characteristics of PMH lesions. Biopsies in all 4 patients revealed a sparse perivascular lymphocytic infiltrate, and in 2 of the cases, postinflammatory pigmentary alteration was noted. Such changes often are described in PMH lesions.4,5 In other cases detailed in the literature, lesional and nonlesional skin often are indistinguishable on hematoxylin and eosin staining.11 In the 3 patients for whom we performed additional immunohistochemical studies, results were mixed: Melan-A staining revealed a decreased number of melanocytes in Patient 1 but not in Patients 2 or 4. Many reported cases in the literature have not demonstrated a decrease in melanocyte density but instead show a decrease in melanin content in lesional skin.1-3,6,7 Although additional stains performed in Patient 4 revealed neither a decrease in the number of melanocytes nor a decrease in the melanin content, such histopathologic findings of PMH often are subtle. Additional stains were not performed in Patient 3. More studies are needed to characterize the immunohistochemical staining patterns of lesional skin in patients with PMH.
Tinea versicolor, pityriasis alba, mycosis fungoides, sarcoidosis, leprosy, and syphilis typically are included in the differential diagnosis for PMH. Tinea versicolor traditionally is diagnosed based on the combination of irregular hypopigmented or hyperpigmented scaly macules and a KOH preparation that is positive for hyphae and spores. Similar to PMH, tinea versicolor is most often found on the trunk, but unusual cases have been reported involving the face.12
Patient 2 reflected how it can be difficult diagnostically to distinguish between tinea versicolor and PMH. Although this patient initially had a KOH scraping suggestive for tinea versicolor, adequate treatment with oral fluconazole and ketoconazole shampoo did not result in improvement. The hypopigmented lesions in this patient continued to progress despite therapy. Additionally, his hypopigmented to depigmented nonscaly macules were more clinically consistent with the characteristic description of lesion configuration in PMH than with the irregular, more sharply defined, asymmetric, and scaly spots of tinea versicolor. Furthermore, the inflammatory findings on biopsy favored a diagnosis of PMH.
Pityriasis alba, most frequently presents on the face in the form of hypopigmented, sometimes slightly scaly macules but also can occur on the body. It usually occurs in younger patients who often have an atopic diathesis. Histologic findings generally are nonspecific, but discrete eczematous changes can sometimes be appreciated in the epidermis and dermis. None of our patients had histories suggestive of an atopic diathesis or lesion distributions typical of pityriasis alba. Histologic findings also were more consistent with PMH than pityriasis alba.
A diagnosis of patch-stage hypopigmented MF should also be entertained in patients with hypopigmented macules, as it can appear similar to the lesions of PMH. Hypopigmented MF often is associated with subtle atrophy, scaling, poikiloderma, and erythema. These features were not present in the 4 cases presented here. Histologically, atypical lymphocytes with prominent epidermotropism and tagging of the epidermis by large lymphocytic infiltrates are seen in cases of hypopigmented MF. These findings were not present in biopsies from our patients.
Hypopigmented sarcoidosis, leprosy, and syphilis are other systemic diseases associated with hypopigmented lesions. Histologically, noncaseasting granulomas in the dermis or subcutaneous tissue would favor a diagnosis of sarcoidosis over PMH. In patients who live in endemic areas, a diagnosis of leprosy for an anesthetic hypopigmented lesion would be higher in the differential. Finally, it is important to rule out secondary syphilis when diagnosing PMH. Known as the great imitator, secondary syphilis may present in a patient in the form of hypopigmented macules. Patients 1, 2, and 4 had nonreactive RPR tests; unfortunately, RPR was not checked in Patient 3. He denied all risk factors for syphilis.
Various topical and oral treatments were prescribed for each patient, but so far none have been unequivocally effective. In the literature, there are reports supporting the efficacy of topical antimicrobial agents targeting P acnes.9,10 One case report noted improvement in a patient with PMH after isotretinoin use.13 Phototherapy also has been reported to improve PMH in several case reports4-8; however, consistent response to these therapies has not been documented. Unfortunately for patients with a diagnosis of PMH, a lack of effective treatment options often exists.
This series of 4 cases highlights the importance of considering PMH in the differential of hypopigmented macules, even when they appear predominantly on the face.
- Relyveld G, Menke H, Westerhof W. Progressive macular hypomelanosis: an overview. Am J Clin Dermatol. 2007;8:13-19.
- Hwang SW, Hong SK, Kim SH, et al. Progressive macular hypomelanosis in Korean patients: a clinicopathologic study. Ann Dermatol. 2009;21:261-267.
- Martinéz-Martinéz ML, Azaña-Defez JM, Rodríguez-Vázquez M, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29:460-462.
- Montero LC, Belinchonón I, Toledo F, et al. Progressive macular hypomelanosis, excellent response with narrow-band ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2011;27:162-163.
- Choi YJ, Hann SK. Two cases of progressive macular hypomelanosis of the trunk. Korean J Dermatol. 2000;38:655-658.
- Westerhof W, Rlyveld G, Kingswijk M, et al. Propionibacterium acnes and the pathogenesis of progressive macular hypomelanosis. Arch Dermatol. 2004;140:210-214.
- Wu SG, Xu AE, Song XZ, et al. Clinical, pathologic, and ultrastructural studies of progressive macular hypomelanosis. Int J Dermatol. 2010;29:1127-1132.
- Kim MB, Kim GW, Cho HH, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66:598-605.
- Revlyveld GN, Menkie HE, Westerhof W. Benzoyl peroxide/clindamycin/UVA is more effective than fluticasone/UVA in progressive macular hypomelanosis: a randomized study. Am J Clin Dermatol. 2006;55:836-843.
- Santos JB, Almeida OL, Silva LM, et al. Efficacy of topical combination of benzoyl peroxide 5% and clindamcyin 1% for the treatment of progressive macular hypomelanosis: a randomized, doubleblind, placebo-controlled trial [in Portuguese]. An Bras Dermatol. 2011;86:50-54.
- Kumarasinghe SP, Tan SH, Thng S, et al. Progressive macular hypomelanosis in Singapore: a clinico-pathological study. Int J Dermatol. 2006;45:737-742.
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34:345-347.
- Kim YK, Lee DY, Lee, JY, et al. Progressive macular hypomelanosis showing excellent response to oral isotretinoin [published online June 23, 2012]. J Dermatol. 2012;39:937-938.
Progressive macular hypomelanosis (PMH) is a noninflammatory skin disorder characterized by ill-defined, nummular, hypopigmented, and nonscaly macules. Historically, various names have been used to describe this entity. Several of these terms, including cutis trunci variata and nummular and confluent hypomelanosis of the trunk, reflected its predominantly truncal distribution.1,2 Less frequently, involvement on the neck, buttocks, and arms and legs has been noted.1,2 A lack of facial involvement previously has been highlighted as a key clinical feature of PMH.3
Progressive macular hypomelanosis is a diagnosis of exclusion. Hypopigmented diseases commonly considered in the differential include those caused by fungi and yeasts (eg, tinea versicolor, seborrheic dermatitis), inflammatory skin disorders (eg, pityriasis alba, postinflammatory dyschromia), and mycosis fungoides (MF) as well as leprosy.
The hypopigmented macules of PMH have nonspecific histopathologic findings; lesional skin often shows minimal alterations as compared to normal skin. A sparse perivascular lymphocytic infiltrate often is observed,4,5 and at times, a decrease in epidermal melanin content can be detected.1-3,6,7
We report 4 cases with considerable facial involvement of hypopigmented macules that were determined to be consistent with PMH. We propose that characteristic macules that are not clinically or histopathologically consistent with other disease entities are compatible with a diagnosis of PMH, regardless of the distribution. A diagnosis of PMH should be considered in the differential when there are suggestive facial lesions in addition to truncal lesions.
Case Reports
Patient 1
A 40-year-old man presented with hypopigmented macules on the face (Figure 1), trunk, chest, arms, and legs of 2 years’ duration. The lesions were asymptomatic and had started on the forehead as hypopigmented macules, then progressed to the trunk, arms, and legs. The patient denied any prior rash, injury, or hyperpigmentation associated with the distribution of the lesions.

A rapid plasma reagin (RPR) test was conducted to rule out secondary syphilis and was nonreactive. During a series of clinical encounters over several months, a total of 5 biopsies of lesions on the face and back were performed. All specimens contained mild mononuclear perivascular inflammation (Figure 2). In some foci, staining for Melan-A revealed a decrease in epidermal melanocytes (Figure 3). Periodic acid–Schiff staining performed on one section revealed a few pityriasis spores but no hyphal elements, suggesting colonization rather than infection.


The patient initially was started on tacrolimus ointment 0.1% once daily and narrowband UVB phototherapy twice weekly for 3 months without benefit. A diagnosis of tinea versicolor was revisited and the patient was switched to ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing, and ketoconazole cream 2% was applied twice daily to the affected areas for 2 months without notable improvement. Once-weekly 150-mg pulse doses of oral fluconazole for 8 weeks were started but proved equally ineffective. Antibiotic therapy aimed at eradicating Propionibacterium acnes was considered following a provisional diagnosis of PMH after the patient failed 5 months of therapy for tinea versicolor.
Patient 2
A 54-year-old man presented with hypopigmented to depigmented nonscaly macules on the face, trunk, chest, and arms of several months’ duration. The patient initially noted hypopigmentation on the face that gradually spread to the rest of the body. The patient denied any prior rash or hyperpigmentation in the affected areas. At the initial visit to our clinic, a potassium hydroxide (KOH) preparation of the face and back was positive for tinea versicolor. The patient was treated with ketoconazole shampoo 1% two to 3 times weekly for several weeks on the scalp, face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and 2 total doses of oral fluconazole 150 mg taken 1 week apart.
Three months later the patient returned with no improvement of the existing lesions and with progression of the disease to previously uninvolved areas of the trunk, arms, and legs. Biopsy of a facial lesion was performed, and laboratory studies including RPR, thyroid-stimulating hormone, and antinuclear antibody tests were conducted to screen for possible systemic disease. Microscopic analysis of the biopsied facial lesion revealed a sparse perivascular infiltrate of lymphocytes and plasma cells but no evidence of yeast or hyphal elements. Melan-A staining did not reveal a decreased number of epidermal melanocytes. All laboratory studies were negative or within normal limits. Desonide ointment 0.05% was prescribed to relieve the patient’s occasional pruritus. Although the patient’s symptoms resolved, the hypopigmented macules continued to progress, making a diagnosis of PMH more likely given the lack of improvement on treatment for tinea versicolor. Pimecrolimus cream 1% was started with discontinuation of desonide for steroid-sparing therapy.
Patient 3
A 63-year-old man presented with progressive nonscaly and asymptomatic hypopigmented macules on the face, trunk, abdomen, and back of 5 years’ duration. He first noted lesions on the abdomen and they subsequently spread to the rest of the body. The patient denied any prior rash, hyperpigmentation, or other lesions in the involved areas.
One year prior to the current presentation, KOH scrapings from the lesions performed by an outside physician were negative. During his initial visit to our clinic, an abdominal biopsy was performed, and histopathologic analysis showed postinflammatory pigmentary alteration; however, the patient denied any prior history of rash or injury in the distribution of the lesions that would correlate with the histopathologic findings of postinflammatory pigmentation. Because the histopathologic findings showed postinflammatory pigmentary alteration, additional stains including Melan-A were not performed.
The patient was provisionally treated with ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and ketoconazole cream 2% twice daily to the affected areas. After several months on this regimen, the patient did not report any improvement. An abdominal skin biopsy was again performed and revealed similar histopathology. Periodic acid–Schiff staining was negative for fungus. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin lotion.
Patient 4
A 45-year-old woman presented with hypopigmented, nonscaly macules on the face, neck, chest, trunk, and back. She first noted the lesions on the face and trunk more than 8 years prior, and they subsequently progressed. Potassium hydroxide scrapings performed on the lesions at the current presentation were negative, and a skin biopsy from the neck revealed postinflammatory pigmentary alteration, although the patient had no history of rash or injury in the areas in which the lesions were distributed.
Fontana-Masson and Melan-A staining of the skin biopsy of the neck revealed a normal distribution of melanocytes and pigment at the dermoepidermal junction. An RPR test was nonreactive. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin phosphate lotion 1%.
Comment
The 4 cases of PMH reported here showed extensive facial involvement in addition to the characteristic hypopigmented lesions on the trunk, arms, and legs. It is unclear why the lesions in these patients had a predominantly facial distribution. Involvement of the face in PMH has not been commonly reported in the literature. Martínez-Martínez et al3 reported 12 PMH patients with lesions only presenting in lumbar and abdominal distributions. Kim et al8 presented a series of 23 PMH patients treated with narrowband UVB in whom 56% (9/16) saw repigmentation in 90% of the lesions following treatment. The most commonly affected area was the lower back, followed by the abdomen, upper back, chest, sacral region, flank, and shoulders, respectively.8 In a review by Relyveld et al,1 PMH is described as a predominantly truncal disease that can occasionally extend to the neck, face, and proximal arms and legs; however, no specific cases were reported.
Previous case series have reported PMH primarily in adolescents and young adults, with mean ages ranging from 26 to 30 years.1,3 The 4 patients reported here were older, ranging in age from 40 to 65 years. This discrepancy in age may contribute to the facial distribution encountered in this patient population; however, given the small number of patients in our case series, such extrapolation is premature. Most recently, Westerhof et al6 demonstrated a relationship between the presence of P acnes, a common skin commensal of the face, and the hypopigmented macules of PMH. The investigators suggested that some strains of P acnes produce a factor that is yet to be identified that interferes with melanogenesis. The response of PMH lesions to topical treatments such as benzoyl peroxide, clindamycin, and phototherapy has lent credence to the potential etiologic role of P acnes in this condition.9,10 The interplay between age, PMH distribution, and P acnes requires further investigation.
The biopsies in our 4 patients were consistent with the nonspecific histopathologic characteristics of PMH lesions. Biopsies in all 4 patients revealed a sparse perivascular lymphocytic infiltrate, and in 2 of the cases, postinflammatory pigmentary alteration was noted. Such changes often are described in PMH lesions.4,5 In other cases detailed in the literature, lesional and nonlesional skin often are indistinguishable on hematoxylin and eosin staining.11 In the 3 patients for whom we performed additional immunohistochemical studies, results were mixed: Melan-A staining revealed a decreased number of melanocytes in Patient 1 but not in Patients 2 or 4. Many reported cases in the literature have not demonstrated a decrease in melanocyte density but instead show a decrease in melanin content in lesional skin.1-3,6,7 Although additional stains performed in Patient 4 revealed neither a decrease in the number of melanocytes nor a decrease in the melanin content, such histopathologic findings of PMH often are subtle. Additional stains were not performed in Patient 3. More studies are needed to characterize the immunohistochemical staining patterns of lesional skin in patients with PMH.
Tinea versicolor, pityriasis alba, mycosis fungoides, sarcoidosis, leprosy, and syphilis typically are included in the differential diagnosis for PMH. Tinea versicolor traditionally is diagnosed based on the combination of irregular hypopigmented or hyperpigmented scaly macules and a KOH preparation that is positive for hyphae and spores. Similar to PMH, tinea versicolor is most often found on the trunk, but unusual cases have been reported involving the face.12
Patient 2 reflected how it can be difficult diagnostically to distinguish between tinea versicolor and PMH. Although this patient initially had a KOH scraping suggestive for tinea versicolor, adequate treatment with oral fluconazole and ketoconazole shampoo did not result in improvement. The hypopigmented lesions in this patient continued to progress despite therapy. Additionally, his hypopigmented to depigmented nonscaly macules were more clinically consistent with the characteristic description of lesion configuration in PMH than with the irregular, more sharply defined, asymmetric, and scaly spots of tinea versicolor. Furthermore, the inflammatory findings on biopsy favored a diagnosis of PMH.
Pityriasis alba, most frequently presents on the face in the form of hypopigmented, sometimes slightly scaly macules but also can occur on the body. It usually occurs in younger patients who often have an atopic diathesis. Histologic findings generally are nonspecific, but discrete eczematous changes can sometimes be appreciated in the epidermis and dermis. None of our patients had histories suggestive of an atopic diathesis or lesion distributions typical of pityriasis alba. Histologic findings also were more consistent with PMH than pityriasis alba.
A diagnosis of patch-stage hypopigmented MF should also be entertained in patients with hypopigmented macules, as it can appear similar to the lesions of PMH. Hypopigmented MF often is associated with subtle atrophy, scaling, poikiloderma, and erythema. These features were not present in the 4 cases presented here. Histologically, atypical lymphocytes with prominent epidermotropism and tagging of the epidermis by large lymphocytic infiltrates are seen in cases of hypopigmented MF. These findings were not present in biopsies from our patients.
Hypopigmented sarcoidosis, leprosy, and syphilis are other systemic diseases associated with hypopigmented lesions. Histologically, noncaseasting granulomas in the dermis or subcutaneous tissue would favor a diagnosis of sarcoidosis over PMH. In patients who live in endemic areas, a diagnosis of leprosy for an anesthetic hypopigmented lesion would be higher in the differential. Finally, it is important to rule out secondary syphilis when diagnosing PMH. Known as the great imitator, secondary syphilis may present in a patient in the form of hypopigmented macules. Patients 1, 2, and 4 had nonreactive RPR tests; unfortunately, RPR was not checked in Patient 3. He denied all risk factors for syphilis.
Various topical and oral treatments were prescribed for each patient, but so far none have been unequivocally effective. In the literature, there are reports supporting the efficacy of topical antimicrobial agents targeting P acnes.9,10 One case report noted improvement in a patient with PMH after isotretinoin use.13 Phototherapy also has been reported to improve PMH in several case reports4-8; however, consistent response to these therapies has not been documented. Unfortunately for patients with a diagnosis of PMH, a lack of effective treatment options often exists.
This series of 4 cases highlights the importance of considering PMH in the differential of hypopigmented macules, even when they appear predominantly on the face.
Progressive macular hypomelanosis (PMH) is a noninflammatory skin disorder characterized by ill-defined, nummular, hypopigmented, and nonscaly macules. Historically, various names have been used to describe this entity. Several of these terms, including cutis trunci variata and nummular and confluent hypomelanosis of the trunk, reflected its predominantly truncal distribution.1,2 Less frequently, involvement on the neck, buttocks, and arms and legs has been noted.1,2 A lack of facial involvement previously has been highlighted as a key clinical feature of PMH.3
Progressive macular hypomelanosis is a diagnosis of exclusion. Hypopigmented diseases commonly considered in the differential include those caused by fungi and yeasts (eg, tinea versicolor, seborrheic dermatitis), inflammatory skin disorders (eg, pityriasis alba, postinflammatory dyschromia), and mycosis fungoides (MF) as well as leprosy.
The hypopigmented macules of PMH have nonspecific histopathologic findings; lesional skin often shows minimal alterations as compared to normal skin. A sparse perivascular lymphocytic infiltrate often is observed,4,5 and at times, a decrease in epidermal melanin content can be detected.1-3,6,7
We report 4 cases with considerable facial involvement of hypopigmented macules that were determined to be consistent with PMH. We propose that characteristic macules that are not clinically or histopathologically consistent with other disease entities are compatible with a diagnosis of PMH, regardless of the distribution. A diagnosis of PMH should be considered in the differential when there are suggestive facial lesions in addition to truncal lesions.
Case Reports
Patient 1
A 40-year-old man presented with hypopigmented macules on the face (Figure 1), trunk, chest, arms, and legs of 2 years’ duration. The lesions were asymptomatic and had started on the forehead as hypopigmented macules, then progressed to the trunk, arms, and legs. The patient denied any prior rash, injury, or hyperpigmentation associated with the distribution of the lesions.

A rapid plasma reagin (RPR) test was conducted to rule out secondary syphilis and was nonreactive. During a series of clinical encounters over several months, a total of 5 biopsies of lesions on the face and back were performed. All specimens contained mild mononuclear perivascular inflammation (Figure 2). In some foci, staining for Melan-A revealed a decrease in epidermal melanocytes (Figure 3). Periodic acid–Schiff staining performed on one section revealed a few pityriasis spores but no hyphal elements, suggesting colonization rather than infection.


The patient initially was started on tacrolimus ointment 0.1% once daily and narrowband UVB phototherapy twice weekly for 3 months without benefit. A diagnosis of tinea versicolor was revisited and the patient was switched to ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing, and ketoconazole cream 2% was applied twice daily to the affected areas for 2 months without notable improvement. Once-weekly 150-mg pulse doses of oral fluconazole for 8 weeks were started but proved equally ineffective. Antibiotic therapy aimed at eradicating Propionibacterium acnes was considered following a provisional diagnosis of PMH after the patient failed 5 months of therapy for tinea versicolor.
Patient 2
A 54-year-old man presented with hypopigmented to depigmented nonscaly macules on the face, trunk, chest, and arms of several months’ duration. The patient initially noted hypopigmentation on the face that gradually spread to the rest of the body. The patient denied any prior rash or hyperpigmentation in the affected areas. At the initial visit to our clinic, a potassium hydroxide (KOH) preparation of the face and back was positive for tinea versicolor. The patient was treated with ketoconazole shampoo 1% two to 3 times weekly for several weeks on the scalp, face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and 2 total doses of oral fluconazole 150 mg taken 1 week apart.
Three months later the patient returned with no improvement of the existing lesions and with progression of the disease to previously uninvolved areas of the trunk, arms, and legs. Biopsy of a facial lesion was performed, and laboratory studies including RPR, thyroid-stimulating hormone, and antinuclear antibody tests were conducted to screen for possible systemic disease. Microscopic analysis of the biopsied facial lesion revealed a sparse perivascular infiltrate of lymphocytes and plasma cells but no evidence of yeast or hyphal elements. Melan-A staining did not reveal a decreased number of epidermal melanocytes. All laboratory studies were negative or within normal limits. Desonide ointment 0.05% was prescribed to relieve the patient’s occasional pruritus. Although the patient’s symptoms resolved, the hypopigmented macules continued to progress, making a diagnosis of PMH more likely given the lack of improvement on treatment for tinea versicolor. Pimecrolimus cream 1% was started with discontinuation of desonide for steroid-sparing therapy.
Patient 3
A 63-year-old man presented with progressive nonscaly and asymptomatic hypopigmented macules on the face, trunk, abdomen, and back of 5 years’ duration. He first noted lesions on the abdomen and they subsequently spread to the rest of the body. The patient denied any prior rash, hyperpigmentation, or other lesions in the involved areas.
One year prior to the current presentation, KOH scrapings from the lesions performed by an outside physician were negative. During his initial visit to our clinic, an abdominal biopsy was performed, and histopathologic analysis showed postinflammatory pigmentary alteration; however, the patient denied any prior history of rash or injury in the distribution of the lesions that would correlate with the histopathologic findings of postinflammatory pigmentation. Because the histopathologic findings showed postinflammatory pigmentary alteration, additional stains including Melan-A were not performed.
The patient was provisionally treated with ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and ketoconazole cream 2% twice daily to the affected areas. After several months on this regimen, the patient did not report any improvement. An abdominal skin biopsy was again performed and revealed similar histopathology. Periodic acid–Schiff staining was negative for fungus. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin lotion.
Patient 4
A 45-year-old woman presented with hypopigmented, nonscaly macules on the face, neck, chest, trunk, and back. She first noted the lesions on the face and trunk more than 8 years prior, and they subsequently progressed. Potassium hydroxide scrapings performed on the lesions at the current presentation were negative, and a skin biopsy from the neck revealed postinflammatory pigmentary alteration, although the patient had no history of rash or injury in the areas in which the lesions were distributed.
Fontana-Masson and Melan-A staining of the skin biopsy of the neck revealed a normal distribution of melanocytes and pigment at the dermoepidermal junction. An RPR test was nonreactive. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin phosphate lotion 1%.
Comment
The 4 cases of PMH reported here showed extensive facial involvement in addition to the characteristic hypopigmented lesions on the trunk, arms, and legs. It is unclear why the lesions in these patients had a predominantly facial distribution. Involvement of the face in PMH has not been commonly reported in the literature. Martínez-Martínez et al3 reported 12 PMH patients with lesions only presenting in lumbar and abdominal distributions. Kim et al8 presented a series of 23 PMH patients treated with narrowband UVB in whom 56% (9/16) saw repigmentation in 90% of the lesions following treatment. The most commonly affected area was the lower back, followed by the abdomen, upper back, chest, sacral region, flank, and shoulders, respectively.8 In a review by Relyveld et al,1 PMH is described as a predominantly truncal disease that can occasionally extend to the neck, face, and proximal arms and legs; however, no specific cases were reported.
Previous case series have reported PMH primarily in adolescents and young adults, with mean ages ranging from 26 to 30 years.1,3 The 4 patients reported here were older, ranging in age from 40 to 65 years. This discrepancy in age may contribute to the facial distribution encountered in this patient population; however, given the small number of patients in our case series, such extrapolation is premature. Most recently, Westerhof et al6 demonstrated a relationship between the presence of P acnes, a common skin commensal of the face, and the hypopigmented macules of PMH. The investigators suggested that some strains of P acnes produce a factor that is yet to be identified that interferes with melanogenesis. The response of PMH lesions to topical treatments such as benzoyl peroxide, clindamycin, and phototherapy has lent credence to the potential etiologic role of P acnes in this condition.9,10 The interplay between age, PMH distribution, and P acnes requires further investigation.
The biopsies in our 4 patients were consistent with the nonspecific histopathologic characteristics of PMH lesions. Biopsies in all 4 patients revealed a sparse perivascular lymphocytic infiltrate, and in 2 of the cases, postinflammatory pigmentary alteration was noted. Such changes often are described in PMH lesions.4,5 In other cases detailed in the literature, lesional and nonlesional skin often are indistinguishable on hematoxylin and eosin staining.11 In the 3 patients for whom we performed additional immunohistochemical studies, results were mixed: Melan-A staining revealed a decreased number of melanocytes in Patient 1 but not in Patients 2 or 4. Many reported cases in the literature have not demonstrated a decrease in melanocyte density but instead show a decrease in melanin content in lesional skin.1-3,6,7 Although additional stains performed in Patient 4 revealed neither a decrease in the number of melanocytes nor a decrease in the melanin content, such histopathologic findings of PMH often are subtle. Additional stains were not performed in Patient 3. More studies are needed to characterize the immunohistochemical staining patterns of lesional skin in patients with PMH.
Tinea versicolor, pityriasis alba, mycosis fungoides, sarcoidosis, leprosy, and syphilis typically are included in the differential diagnosis for PMH. Tinea versicolor traditionally is diagnosed based on the combination of irregular hypopigmented or hyperpigmented scaly macules and a KOH preparation that is positive for hyphae and spores. Similar to PMH, tinea versicolor is most often found on the trunk, but unusual cases have been reported involving the face.12
Patient 2 reflected how it can be difficult diagnostically to distinguish between tinea versicolor and PMH. Although this patient initially had a KOH scraping suggestive for tinea versicolor, adequate treatment with oral fluconazole and ketoconazole shampoo did not result in improvement. The hypopigmented lesions in this patient continued to progress despite therapy. Additionally, his hypopigmented to depigmented nonscaly macules were more clinically consistent with the characteristic description of lesion configuration in PMH than with the irregular, more sharply defined, asymmetric, and scaly spots of tinea versicolor. Furthermore, the inflammatory findings on biopsy favored a diagnosis of PMH.
Pityriasis alba, most frequently presents on the face in the form of hypopigmented, sometimes slightly scaly macules but also can occur on the body. It usually occurs in younger patients who often have an atopic diathesis. Histologic findings generally are nonspecific, but discrete eczematous changes can sometimes be appreciated in the epidermis and dermis. None of our patients had histories suggestive of an atopic diathesis or lesion distributions typical of pityriasis alba. Histologic findings also were more consistent with PMH than pityriasis alba.
A diagnosis of patch-stage hypopigmented MF should also be entertained in patients with hypopigmented macules, as it can appear similar to the lesions of PMH. Hypopigmented MF often is associated with subtle atrophy, scaling, poikiloderma, and erythema. These features were not present in the 4 cases presented here. Histologically, atypical lymphocytes with prominent epidermotropism and tagging of the epidermis by large lymphocytic infiltrates are seen in cases of hypopigmented MF. These findings were not present in biopsies from our patients.
Hypopigmented sarcoidosis, leprosy, and syphilis are other systemic diseases associated with hypopigmented lesions. Histologically, noncaseasting granulomas in the dermis or subcutaneous tissue would favor a diagnosis of sarcoidosis over PMH. In patients who live in endemic areas, a diagnosis of leprosy for an anesthetic hypopigmented lesion would be higher in the differential. Finally, it is important to rule out secondary syphilis when diagnosing PMH. Known as the great imitator, secondary syphilis may present in a patient in the form of hypopigmented macules. Patients 1, 2, and 4 had nonreactive RPR tests; unfortunately, RPR was not checked in Patient 3. He denied all risk factors for syphilis.
Various topical and oral treatments were prescribed for each patient, but so far none have been unequivocally effective. In the literature, there are reports supporting the efficacy of topical antimicrobial agents targeting P acnes.9,10 One case report noted improvement in a patient with PMH after isotretinoin use.13 Phototherapy also has been reported to improve PMH in several case reports4-8; however, consistent response to these therapies has not been documented. Unfortunately for patients with a diagnosis of PMH, a lack of effective treatment options often exists.
This series of 4 cases highlights the importance of considering PMH in the differential of hypopigmented macules, even when they appear predominantly on the face.
- Relyveld G, Menke H, Westerhof W. Progressive macular hypomelanosis: an overview. Am J Clin Dermatol. 2007;8:13-19.
- Hwang SW, Hong SK, Kim SH, et al. Progressive macular hypomelanosis in Korean patients: a clinicopathologic study. Ann Dermatol. 2009;21:261-267.
- Martinéz-Martinéz ML, Azaña-Defez JM, Rodríguez-Vázquez M, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29:460-462.
- Montero LC, Belinchonón I, Toledo F, et al. Progressive macular hypomelanosis, excellent response with narrow-band ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2011;27:162-163.
- Choi YJ, Hann SK. Two cases of progressive macular hypomelanosis of the trunk. Korean J Dermatol. 2000;38:655-658.
- Westerhof W, Rlyveld G, Kingswijk M, et al. Propionibacterium acnes and the pathogenesis of progressive macular hypomelanosis. Arch Dermatol. 2004;140:210-214.
- Wu SG, Xu AE, Song XZ, et al. Clinical, pathologic, and ultrastructural studies of progressive macular hypomelanosis. Int J Dermatol. 2010;29:1127-1132.
- Kim MB, Kim GW, Cho HH, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66:598-605.
- Revlyveld GN, Menkie HE, Westerhof W. Benzoyl peroxide/clindamycin/UVA is more effective than fluticasone/UVA in progressive macular hypomelanosis: a randomized study. Am J Clin Dermatol. 2006;55:836-843.
- Santos JB, Almeida OL, Silva LM, et al. Efficacy of topical combination of benzoyl peroxide 5% and clindamcyin 1% for the treatment of progressive macular hypomelanosis: a randomized, doubleblind, placebo-controlled trial [in Portuguese]. An Bras Dermatol. 2011;86:50-54.
- Kumarasinghe SP, Tan SH, Thng S, et al. Progressive macular hypomelanosis in Singapore: a clinico-pathological study. Int J Dermatol. 2006;45:737-742.
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34:345-347.
- Kim YK, Lee DY, Lee, JY, et al. Progressive macular hypomelanosis showing excellent response to oral isotretinoin [published online June 23, 2012]. J Dermatol. 2012;39:937-938.
- Relyveld G, Menke H, Westerhof W. Progressive macular hypomelanosis: an overview. Am J Clin Dermatol. 2007;8:13-19.
- Hwang SW, Hong SK, Kim SH, et al. Progressive macular hypomelanosis in Korean patients: a clinicopathologic study. Ann Dermatol. 2009;21:261-267.
- Martinéz-Martinéz ML, Azaña-Defez JM, Rodríguez-Vázquez M, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29:460-462.
- Montero LC, Belinchonón I, Toledo F, et al. Progressive macular hypomelanosis, excellent response with narrow-band ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2011;27:162-163.
- Choi YJ, Hann SK. Two cases of progressive macular hypomelanosis of the trunk. Korean J Dermatol. 2000;38:655-658.
- Westerhof W, Rlyveld G, Kingswijk M, et al. Propionibacterium acnes and the pathogenesis of progressive macular hypomelanosis. Arch Dermatol. 2004;140:210-214.
- Wu SG, Xu AE, Song XZ, et al. Clinical, pathologic, and ultrastructural studies of progressive macular hypomelanosis. Int J Dermatol. 2010;29:1127-1132.
- Kim MB, Kim GW, Cho HH, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66:598-605.
- Revlyveld GN, Menkie HE, Westerhof W. Benzoyl peroxide/clindamycin/UVA is more effective than fluticasone/UVA in progressive macular hypomelanosis: a randomized study. Am J Clin Dermatol. 2006;55:836-843.
- Santos JB, Almeida OL, Silva LM, et al. Efficacy of topical combination of benzoyl peroxide 5% and clindamcyin 1% for the treatment of progressive macular hypomelanosis: a randomized, doubleblind, placebo-controlled trial [in Portuguese]. An Bras Dermatol. 2011;86:50-54.
- Kumarasinghe SP, Tan SH, Thng S, et al. Progressive macular hypomelanosis in Singapore: a clinico-pathological study. Int J Dermatol. 2006;45:737-742.
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34:345-347.
- Kim YK, Lee DY, Lee, JY, et al. Progressive macular hypomelanosis showing excellent response to oral isotretinoin [published online June 23, 2012]. J Dermatol. 2012;39:937-938.
Practice Points
- Progressive macular hypomelanosis should be considered in the differential diagnosis for hypopigmented facial lesions.
- Progressive macular hypomelanosis proves to be a diagnosis of exclusion.
Linear Terra Firma–Forme Dermatosis of the Midline Back
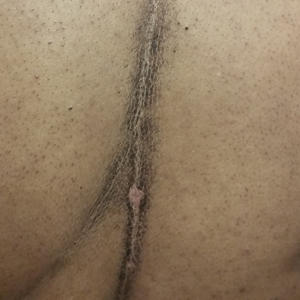
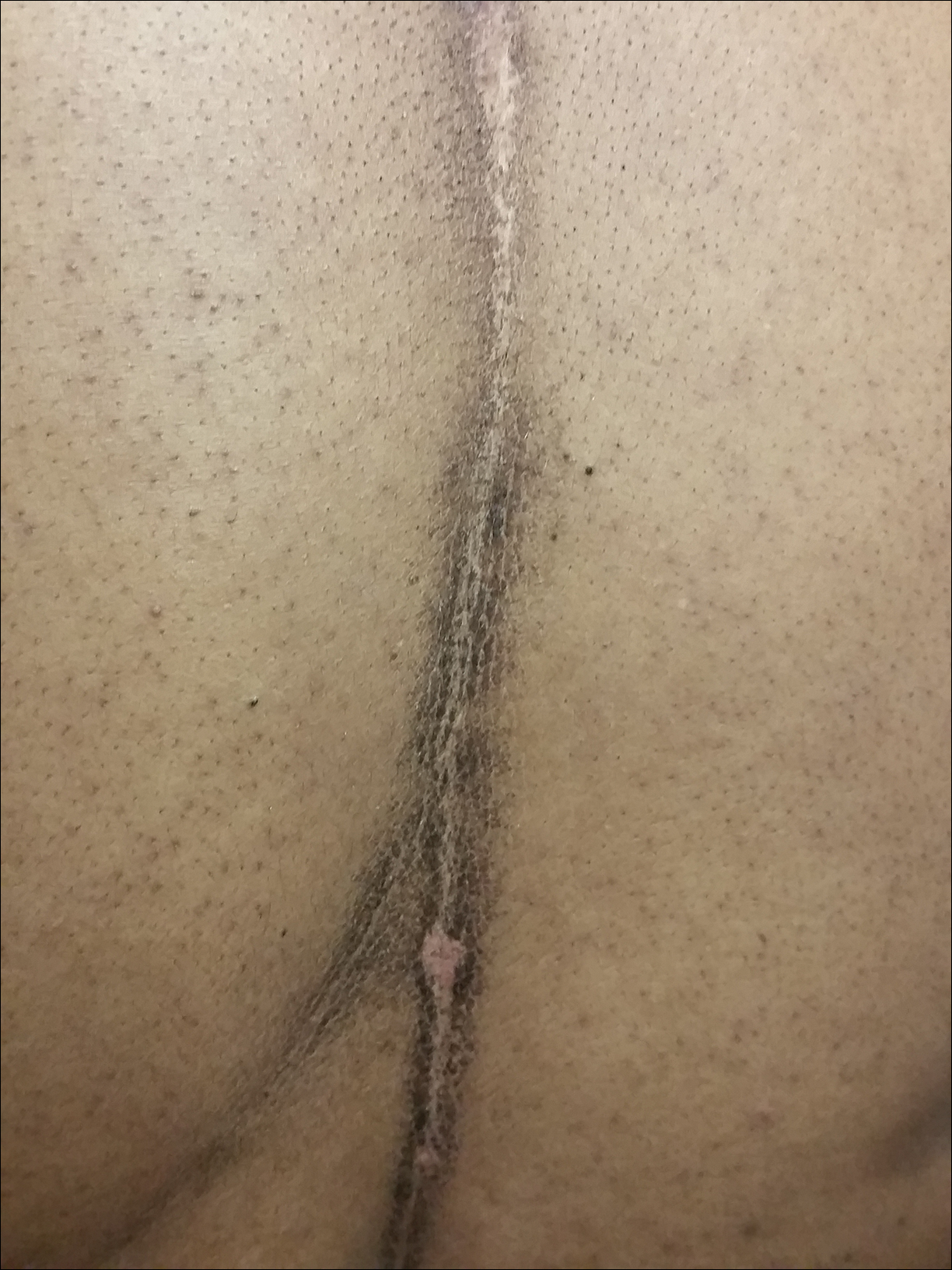
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
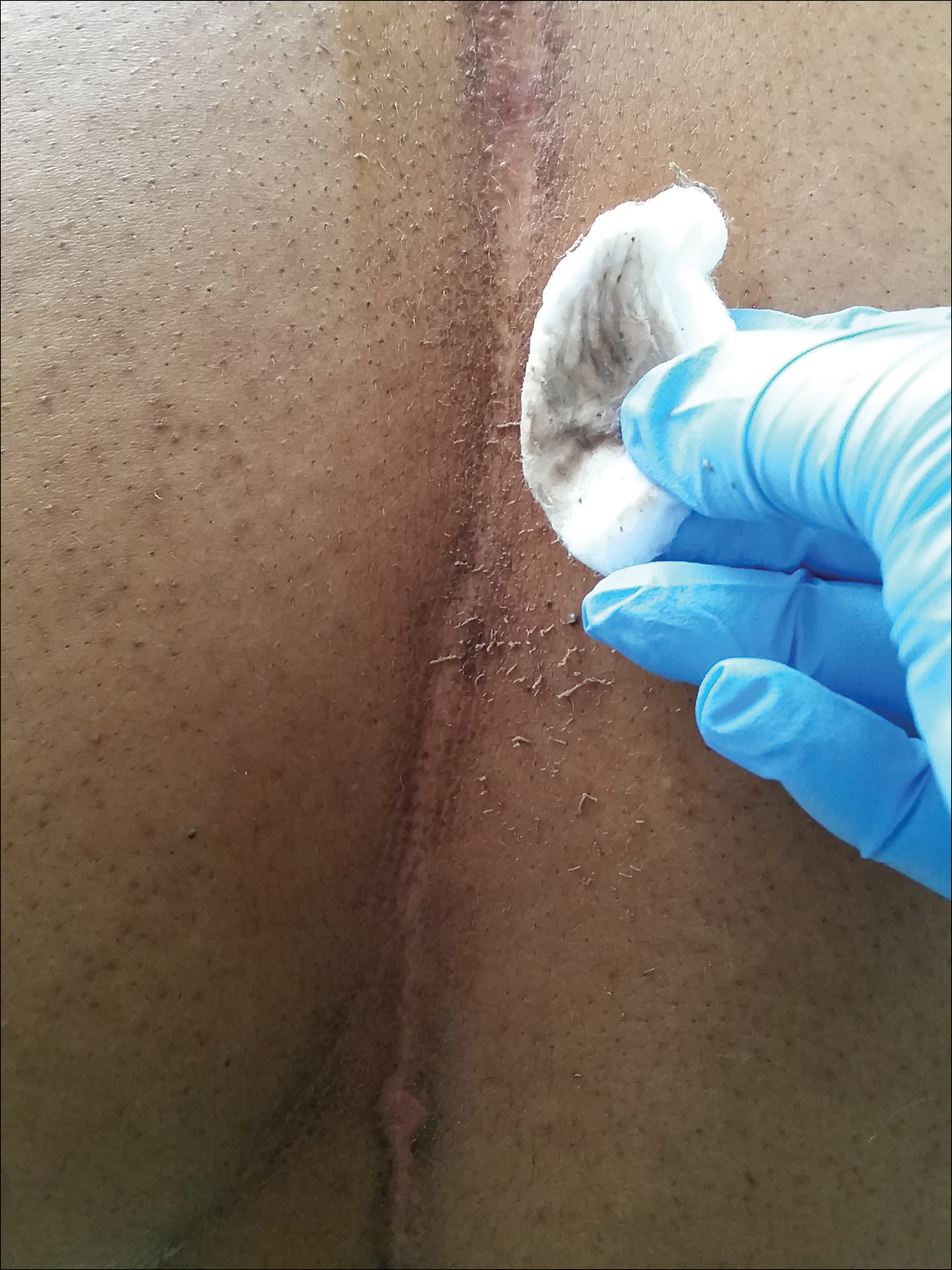
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.
Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.


Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.


Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
Practice Points
- Terra firma-forme dermatosis (TFFD) is an idiopathic condition characterized by asymptomatic hyperpigmented and hyperkeratotic plaques that are resistant to removal with soap and water.
- Diagnosis and cure of TFFD can be achieved through removal by rubbing with isopropyl alcohol.
- Increased awareness of the clinical presentation and treatment of TFFD may help patients avoid unnecessary treatment and workup and leads to immediate resolution of the condition.
When to worry about congenital melanocytic nevi
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Treatment of Melasma Using Tranexamic Acid: What’s Known and What’s Next
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.1
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light–induced plasminogen activator and plasmin activity.2 UV radiation induces the synthesis of plasminogen activator by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.3 By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization. In treated guinea pig skin, application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.4,5
The largest study on the use of oral tranexamic acid for treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore.6 More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.6
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.1,2,6-8
Challenges to the use of tranexamic acid for melasma treatment in the United States include the medicolegal environment, specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States. Tranexamic acid is available for off-label use in the United States with a prescription in the form of 650-mg tablets that can be split by patients to approximate twice-daily 325 mg dosing. This cosmetic indication poses an out-of-pocket cost to patients of over $110 per month or as low as $48 per month with a coupon at the time of publication.9
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interest in formulating and evaluating topical tranexamic acid for cosmetic indications.10-13 Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, microneedling, and fractionated CO2 laser.12-14 Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit use of this medication in the United States.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser–treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedurally to reduce the risk for postinflammatory hyperpigmentation in prone skin types.15 Oral and topical tranexamic acid also have been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization.3,16,17 Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
- Higashi N. Treatment of melasma with oral tranexamic acid. Skin Res. 1988;30:676-680.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12:57-66.
- Sundbeck A, Karlsson L, Lilja J, et al. Inhibition of tumour vascularization by tranexamic acid. experimental studies on possible mechanisms. Anticancer Res. 1981;1:299-304.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136-141.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20:289-292.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75:385-392.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18:373-381.
- Tranexamic acid. GoodRx website. https://www.goodrx.com/tranexamic-acid. Accessed February 2, 2018.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96(19):e6897.
- Hsiao CY, Sung HC, Hu S, et al. Fractional CO2 laser treatment to enhance skin permeation of tranexamic acid with minimal skin disruption. Dermatology (Basel). 2015;230:269-275.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial [published online November 9, 2017]. J Dermatolog Treat. doi:10.1080/09546634.2017.1392476.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27:250-256.
- Kim MS, Chang SE, Haw S, et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40:70-71.
- Kwon HJ, Suh JH, Ko EJ, et al. Combination treatment of propranolol, minocycline, and tranexamic acid for effective control of rosacea [published online November 26, 2017]. Dermatol Ther. doi:10.1111/dth.12439.
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.1
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light–induced plasminogen activator and plasmin activity.2 UV radiation induces the synthesis of plasminogen activator by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.3 By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization. In treated guinea pig skin, application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.4,5
The largest study on the use of oral tranexamic acid for treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore.6 More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.6
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.1,2,6-8
Challenges to the use of tranexamic acid for melasma treatment in the United States include the medicolegal environment, specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States. Tranexamic acid is available for off-label use in the United States with a prescription in the form of 650-mg tablets that can be split by patients to approximate twice-daily 325 mg dosing. This cosmetic indication poses an out-of-pocket cost to patients of over $110 per month or as low as $48 per month with a coupon at the time of publication.9
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interest in formulating and evaluating topical tranexamic acid for cosmetic indications.10-13 Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, microneedling, and fractionated CO2 laser.12-14 Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit use of this medication in the United States.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser–treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedurally to reduce the risk for postinflammatory hyperpigmentation in prone skin types.15 Oral and topical tranexamic acid also have been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization.3,16,17 Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.1
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light–induced plasminogen activator and plasmin activity.2 UV radiation induces the synthesis of plasminogen activator by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.3 By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization. In treated guinea pig skin, application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.4,5
The largest study on the use of oral tranexamic acid for treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore.6 More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.6
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.1,2,6-8
Challenges to the use of tranexamic acid for melasma treatment in the United States include the medicolegal environment, specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States. Tranexamic acid is available for off-label use in the United States with a prescription in the form of 650-mg tablets that can be split by patients to approximate twice-daily 325 mg dosing. This cosmetic indication poses an out-of-pocket cost to patients of over $110 per month or as low as $48 per month with a coupon at the time of publication.9
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interest in formulating and evaluating topical tranexamic acid for cosmetic indications.10-13 Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, microneedling, and fractionated CO2 laser.12-14 Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit use of this medication in the United States.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser–treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedurally to reduce the risk for postinflammatory hyperpigmentation in prone skin types.15 Oral and topical tranexamic acid also have been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization.3,16,17 Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
- Higashi N. Treatment of melasma with oral tranexamic acid. Skin Res. 1988;30:676-680.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12:57-66.
- Sundbeck A, Karlsson L, Lilja J, et al. Inhibition of tumour vascularization by tranexamic acid. experimental studies on possible mechanisms. Anticancer Res. 1981;1:299-304.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136-141.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20:289-292.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75:385-392.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18:373-381.
- Tranexamic acid. GoodRx website. https://www.goodrx.com/tranexamic-acid. Accessed February 2, 2018.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96(19):e6897.
- Hsiao CY, Sung HC, Hu S, et al. Fractional CO2 laser treatment to enhance skin permeation of tranexamic acid with minimal skin disruption. Dermatology (Basel). 2015;230:269-275.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial [published online November 9, 2017]. J Dermatolog Treat. doi:10.1080/09546634.2017.1392476.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27:250-256.
- Kim MS, Chang SE, Haw S, et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40:70-71.
- Kwon HJ, Suh JH, Ko EJ, et al. Combination treatment of propranolol, minocycline, and tranexamic acid for effective control of rosacea [published online November 26, 2017]. Dermatol Ther. doi:10.1111/dth.12439.
- Higashi N. Treatment of melasma with oral tranexamic acid. Skin Res. 1988;30:676-680.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12:57-66.
- Sundbeck A, Karlsson L, Lilja J, et al. Inhibition of tumour vascularization by tranexamic acid. experimental studies on possible mechanisms. Anticancer Res. 1981;1:299-304.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136-141.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20:289-292.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75:385-392.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18:373-381.
- Tranexamic acid. GoodRx website. https://www.goodrx.com/tranexamic-acid. Accessed February 2, 2018.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96(19):e6897.
- Hsiao CY, Sung HC, Hu S, et al. Fractional CO2 laser treatment to enhance skin permeation of tranexamic acid with minimal skin disruption. Dermatology (Basel). 2015;230:269-275.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial [published online November 9, 2017]. J Dermatolog Treat. doi:10.1080/09546634.2017.1392476.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27:250-256.
- Kim MS, Chang SE, Haw S, et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40:70-71.
- Kwon HJ, Suh JH, Ko EJ, et al. Combination treatment of propranolol, minocycline, and tranexamic acid for effective control of rosacea [published online November 26, 2017]. Dermatol Ther. doi:10.1111/dth.12439.
Resident Pearl
- Oral tranexamic acid is an antifibrinolytic agent that can be used off-label for the treatment of melasma.
Hypopigmented Discoloration on the Thigh
The Diagnosis: Hypopigmented Mycosis Fungoides
The patient was started on clobetasol dipropionate cream 0.05% twice daily, which she did not tolerate due to a burning sensation on application. She then was started on narrowband UVB phototherapy 2 to 3 times weekly, and the hypopigmented areas began to improve. Narrowband UVB phototherapy was discontinued after 7 weeks due to the high cost to the patient, but the hypopigmented patches on the left thigh appeared to remit, and the patient did not return to the clinic for 6 months. She returned when the areas on the left thigh reappeared, along with new areas on the right buttock and right medial upper arm. Serial biopsies of the new patches also revealed a CD8+ atypical lymphocytic infiltrate consistent with hypopigmented patch-stage mycosis fungoides (MF). She was started on halobetasol ointment 0.05% twice daily to affected areas, which she tolerated well. Complete blood count and peripheral blood smear were unremarkable, and the patient continued to deny systemic symptoms. Over the next year, the patient's cutaneous findings continued to wax and wane with topical treatment, and she was referred to a regional cancer treatment center for a second opinion from a hematopathologist. Hematopathologic and dermatopathologic review of the case, including hematoxylin and eosin and immunohistochemical staining, was highly consistent with hypopigmented MF (Figures 1-3).
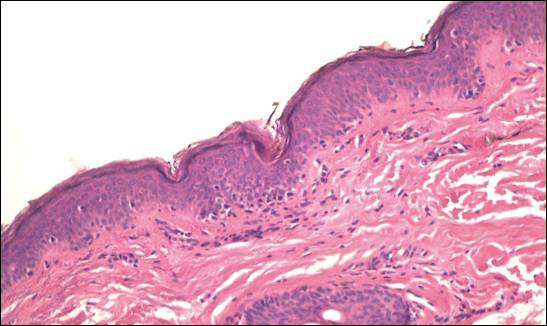
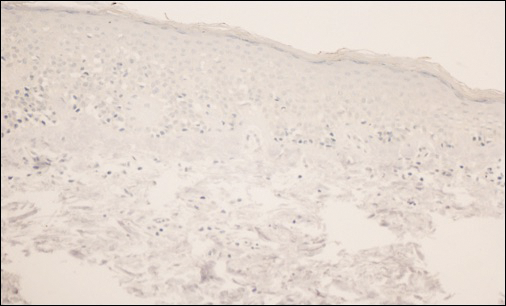
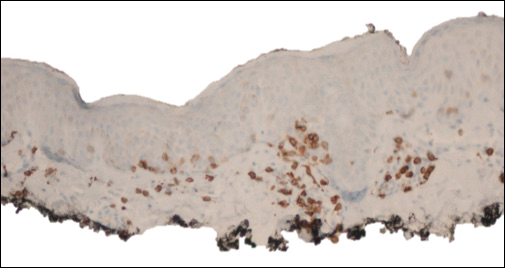
Mycosis fungoides is an uncommon disease characterized by atypical clonal T cells exhibiting epidermotropism. Most commonly, MF is characterized by a CD4+ lymphocytic infiltrate. Mycosis fungoides can be difficult to diagnose in its early stages, as it may resemble benign inflammatory conditions (eg, chronic atopic dermatitis, nummular eczema) and often requires biopsy and additional studies, such as immunohistochemistry, to secure a diagnosis. Hypopigmented MF is regarded as a subtype of MF, as it can exhibit different clinical and pathologic characteristics from classical MF. In particular, the lymphocytic phenotype in hypopigmented MF is more likely to be CD8+.
In general, the progression of MF is characterized as stage IA (patches or plaques involving less than 10% body surface area [BSA]), IB (patches or plaques involving ≥10% BSA without lymph node or visceral involvement), IIA (patches or plaques of any percentage of BSA with lymph node involvement), IIB (cutaneous tumors with or without lymph node involvement), III (erythroderma with low blood tumor burden), or IV (erythroderma with high blood tumor burden with or without visceral involvement). Hypopigmented MF generally presents in early patch stage and rarely progresses past stage IB, and thus generally has a favorable prognosis.1,2 Kim et al3 demonstrated that evolution from patch to plaque stage MF is accompanied by a shift in lymphocytes from the T helper 1 (Th1) to T helper 2 phenotype; therefore the Th1 phenotype, CD8+ T cells are associated with lower risk for disease progression. Other investigators also have hypothesized that predominance of Th1 phenotype, CD8+ T cells may have an immunoregulatory effect, thus preventing evolution of disease from patch to plaque stage and explaining why hypopigmented MF, with a predominantly CD8+ phenotype, confers better prognosis with less chance for disease progression than classical MF.4,5 The patch- or plaque-stage lesions of classical MF have a predilection for non-sun exposed areas (eg, buttocks, medial thighs, breasts),2 whereas hypopigmented MF tends to present with hypopigmented or depigmented lesions mainly distributed on the trunk, arms, and legs. These lesions may become more visible following sun exposure.1 The size of the hypopigmented lesions can vary, and patients may complain of pruritus with variable intensity.
Hypopigmented MF presents more commonly in younger populations, in contrast to classical MF.6-8 However, like classical MF, hypopigmented MF appears to more frequently affect individuals with darker Fitzpatrick skin types.1,9,10 Although it generally is accepted that hypopigmented MF does not favor either sex, some studies suggest that hypopigmented MF has a female predominance.6,10
Classical MF is characterized by an epidermotropic infiltrate of CD4+ T helper cells,10 whereas CD8+ epidermotropism is considered hallmark in hypopigmented MF.10-12 The other typical histopathologic features of hypopigmented MF generally are identical to those of classical MF, with solitary or small groups of atypical haloed lymphocytes within the basal layer, exocytosis of lymphocytes out of proportion to spongiosis, and papillary dermal fibrosis. Immunohistochemistry generally is helpful in distinguishing between classical MF and hypopigmented MF.
The clinical differential diagnosis for hypopigmented MF includes the early (inflammatory) stage of vitiligo, postinflammatory hypopigmentation, lichen sclerosus, pityriasis alba, and leprosy.
First-line treatment for hypopigmented MF consists of phototherapy/photochemotherapy and topical steroids.9,13 Narrowband UVB phototherapy has been used with good success in pediatric patients.14 However, narrowband UVB may not be as effective in darker-skinned individuals; it has been hypothesized that this lack of efficacy could be due to the protective effects of increased melanin in the skin.1 Other topical therapies may include topical carmustine and topical nitrogen mustard.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978-1988.
- Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798-812.
- Stone ML, Styles AR, Cockerell CJ, et al. Hypopigmented report of 7 cases and review of the literature. Cutis. 2001;67:133-138.
- Volkenandt M, Soyer HP, Cerroni L, et al. Molecular detection of clone-specific DNA in hypopigmented lesions of a patient with early evolving mycosis fungoides. Br J Dermatol. 1993;128:423-428.
- Furlan FC, Pereira BA, Sotto MN, et al. Hypopigmented mycosis fungoides versus mycosis fungoides with concomitant hypopigmented lesions: same disease or different variants of mycosis fungoides? Dermatology. 2014;229:271-274.
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270.
- Boulos S, Vaid R, Aladily TN, et al. Clinical presentation, immunopathology, and treatment of juvenile-onset mycosis fungoides: a case series of 34 patients. J Am Acad Dermatol. 2014;71:1117-1126.
- Lambroza E, Cohen SR, Phelps R, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, et al. Hypopigmented mycosis fungoides: Frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450-457.
- Furlan FC, de Paula Pereira BA, da Silva LF, et al. Loss of melanocytes in hypopigmented mycosis fungoides: a study of 18 patients. J Cutan Pathol. 2014;41:101-107.
- Tolkachjov SN, Comfere NI. Hypopigmented mycosis fungoides: a clinical mimicker of vitiligo. J Drugs Dermatol. 2015;14:193-194.
- Duarte I, Bedrikow, R, Aoki S. Mycosis fungoides: epidemiologic study of 17 cases and evaluation of PUVA photochemotherapy. An Bras Dermatol. 2006;81:40-45.
- Onsun N, Kural Y, Su O, et al. Hypopigmented mycosis fungoides associated with atopy in two children. Pediatr Dermatol. 2006;23:493-496.
The Diagnosis: Hypopigmented Mycosis Fungoides
The patient was started on clobetasol dipropionate cream 0.05% twice daily, which she did not tolerate due to a burning sensation on application. She then was started on narrowband UVB phototherapy 2 to 3 times weekly, and the hypopigmented areas began to improve. Narrowband UVB phototherapy was discontinued after 7 weeks due to the high cost to the patient, but the hypopigmented patches on the left thigh appeared to remit, and the patient did not return to the clinic for 6 months. She returned when the areas on the left thigh reappeared, along with new areas on the right buttock and right medial upper arm. Serial biopsies of the new patches also revealed a CD8+ atypical lymphocytic infiltrate consistent with hypopigmented patch-stage mycosis fungoides (MF). She was started on halobetasol ointment 0.05% twice daily to affected areas, which she tolerated well. Complete blood count and peripheral blood smear were unremarkable, and the patient continued to deny systemic symptoms. Over the next year, the patient's cutaneous findings continued to wax and wane with topical treatment, and she was referred to a regional cancer treatment center for a second opinion from a hematopathologist. Hematopathologic and dermatopathologic review of the case, including hematoxylin and eosin and immunohistochemical staining, was highly consistent with hypopigmented MF (Figures 1-3).



Mycosis fungoides is an uncommon disease characterized by atypical clonal T cells exhibiting epidermotropism. Most commonly, MF is characterized by a CD4+ lymphocytic infiltrate. Mycosis fungoides can be difficult to diagnose in its early stages, as it may resemble benign inflammatory conditions (eg, chronic atopic dermatitis, nummular eczema) and often requires biopsy and additional studies, such as immunohistochemistry, to secure a diagnosis. Hypopigmented MF is regarded as a subtype of MF, as it can exhibit different clinical and pathologic characteristics from classical MF. In particular, the lymphocytic phenotype in hypopigmented MF is more likely to be CD8+.
In general, the progression of MF is characterized as stage IA (patches or plaques involving less than 10% body surface area [BSA]), IB (patches or plaques involving ≥10% BSA without lymph node or visceral involvement), IIA (patches or plaques of any percentage of BSA with lymph node involvement), IIB (cutaneous tumors with or without lymph node involvement), III (erythroderma with low blood tumor burden), or IV (erythroderma with high blood tumor burden with or without visceral involvement). Hypopigmented MF generally presents in early patch stage and rarely progresses past stage IB, and thus generally has a favorable prognosis.1,2 Kim et al3 demonstrated that evolution from patch to plaque stage MF is accompanied by a shift in lymphocytes from the T helper 1 (Th1) to T helper 2 phenotype; therefore the Th1 phenotype, CD8+ T cells are associated with lower risk for disease progression. Other investigators also have hypothesized that predominance of Th1 phenotype, CD8+ T cells may have an immunoregulatory effect, thus preventing evolution of disease from patch to plaque stage and explaining why hypopigmented MF, with a predominantly CD8+ phenotype, confers better prognosis with less chance for disease progression than classical MF.4,5 The patch- or plaque-stage lesions of classical MF have a predilection for non-sun exposed areas (eg, buttocks, medial thighs, breasts),2 whereas hypopigmented MF tends to present with hypopigmented or depigmented lesions mainly distributed on the trunk, arms, and legs. These lesions may become more visible following sun exposure.1 The size of the hypopigmented lesions can vary, and patients may complain of pruritus with variable intensity.
Hypopigmented MF presents more commonly in younger populations, in contrast to classical MF.6-8 However, like classical MF, hypopigmented MF appears to more frequently affect individuals with darker Fitzpatrick skin types.1,9,10 Although it generally is accepted that hypopigmented MF does not favor either sex, some studies suggest that hypopigmented MF has a female predominance.6,10
Classical MF is characterized by an epidermotropic infiltrate of CD4+ T helper cells,10 whereas CD8+ epidermotropism is considered hallmark in hypopigmented MF.10-12 The other typical histopathologic features of hypopigmented MF generally are identical to those of classical MF, with solitary or small groups of atypical haloed lymphocytes within the basal layer, exocytosis of lymphocytes out of proportion to spongiosis, and papillary dermal fibrosis. Immunohistochemistry generally is helpful in distinguishing between classical MF and hypopigmented MF.
The clinical differential diagnosis for hypopigmented MF includes the early (inflammatory) stage of vitiligo, postinflammatory hypopigmentation, lichen sclerosus, pityriasis alba, and leprosy.
First-line treatment for hypopigmented MF consists of phototherapy/photochemotherapy and topical steroids.9,13 Narrowband UVB phototherapy has been used with good success in pediatric patients.14 However, narrowband UVB may not be as effective in darker-skinned individuals; it has been hypothesized that this lack of efficacy could be due to the protective effects of increased melanin in the skin.1 Other topical therapies may include topical carmustine and topical nitrogen mustard.
The Diagnosis: Hypopigmented Mycosis Fungoides
The patient was started on clobetasol dipropionate cream 0.05% twice daily, which she did not tolerate due to a burning sensation on application. She then was started on narrowband UVB phototherapy 2 to 3 times weekly, and the hypopigmented areas began to improve. Narrowband UVB phototherapy was discontinued after 7 weeks due to the high cost to the patient, but the hypopigmented patches on the left thigh appeared to remit, and the patient did not return to the clinic for 6 months. She returned when the areas on the left thigh reappeared, along with new areas on the right buttock and right medial upper arm. Serial biopsies of the new patches also revealed a CD8+ atypical lymphocytic infiltrate consistent with hypopigmented patch-stage mycosis fungoides (MF). She was started on halobetasol ointment 0.05% twice daily to affected areas, which she tolerated well. Complete blood count and peripheral blood smear were unremarkable, and the patient continued to deny systemic symptoms. Over the next year, the patient's cutaneous findings continued to wax and wane with topical treatment, and she was referred to a regional cancer treatment center for a second opinion from a hematopathologist. Hematopathologic and dermatopathologic review of the case, including hematoxylin and eosin and immunohistochemical staining, was highly consistent with hypopigmented MF (Figures 1-3).



Mycosis fungoides is an uncommon disease characterized by atypical clonal T cells exhibiting epidermotropism. Most commonly, MF is characterized by a CD4+ lymphocytic infiltrate. Mycosis fungoides can be difficult to diagnose in its early stages, as it may resemble benign inflammatory conditions (eg, chronic atopic dermatitis, nummular eczema) and often requires biopsy and additional studies, such as immunohistochemistry, to secure a diagnosis. Hypopigmented MF is regarded as a subtype of MF, as it can exhibit different clinical and pathologic characteristics from classical MF. In particular, the lymphocytic phenotype in hypopigmented MF is more likely to be CD8+.
In general, the progression of MF is characterized as stage IA (patches or plaques involving less than 10% body surface area [BSA]), IB (patches or plaques involving ≥10% BSA without lymph node or visceral involvement), IIA (patches or plaques of any percentage of BSA with lymph node involvement), IIB (cutaneous tumors with or without lymph node involvement), III (erythroderma with low blood tumor burden), or IV (erythroderma with high blood tumor burden with or without visceral involvement). Hypopigmented MF generally presents in early patch stage and rarely progresses past stage IB, and thus generally has a favorable prognosis.1,2 Kim et al3 demonstrated that evolution from patch to plaque stage MF is accompanied by a shift in lymphocytes from the T helper 1 (Th1) to T helper 2 phenotype; therefore the Th1 phenotype, CD8+ T cells are associated with lower risk for disease progression. Other investigators also have hypothesized that predominance of Th1 phenotype, CD8+ T cells may have an immunoregulatory effect, thus preventing evolution of disease from patch to plaque stage and explaining why hypopigmented MF, with a predominantly CD8+ phenotype, confers better prognosis with less chance for disease progression than classical MF.4,5 The patch- or plaque-stage lesions of classical MF have a predilection for non-sun exposed areas (eg, buttocks, medial thighs, breasts),2 whereas hypopigmented MF tends to present with hypopigmented or depigmented lesions mainly distributed on the trunk, arms, and legs. These lesions may become more visible following sun exposure.1 The size of the hypopigmented lesions can vary, and patients may complain of pruritus with variable intensity.
Hypopigmented MF presents more commonly in younger populations, in contrast to classical MF.6-8 However, like classical MF, hypopigmented MF appears to more frequently affect individuals with darker Fitzpatrick skin types.1,9,10 Although it generally is accepted that hypopigmented MF does not favor either sex, some studies suggest that hypopigmented MF has a female predominance.6,10
Classical MF is characterized by an epidermotropic infiltrate of CD4+ T helper cells,10 whereas CD8+ epidermotropism is considered hallmark in hypopigmented MF.10-12 The other typical histopathologic features of hypopigmented MF generally are identical to those of classical MF, with solitary or small groups of atypical haloed lymphocytes within the basal layer, exocytosis of lymphocytes out of proportion to spongiosis, and papillary dermal fibrosis. Immunohistochemistry generally is helpful in distinguishing between classical MF and hypopigmented MF.
The clinical differential diagnosis for hypopigmented MF includes the early (inflammatory) stage of vitiligo, postinflammatory hypopigmentation, lichen sclerosus, pityriasis alba, and leprosy.
First-line treatment for hypopigmented MF consists of phototherapy/photochemotherapy and topical steroids.9,13 Narrowband UVB phototherapy has been used with good success in pediatric patients.14 However, narrowband UVB may not be as effective in darker-skinned individuals; it has been hypothesized that this lack of efficacy could be due to the protective effects of increased melanin in the skin.1 Other topical therapies may include topical carmustine and topical nitrogen mustard.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978-1988.
- Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798-812.
- Stone ML, Styles AR, Cockerell CJ, et al. Hypopigmented report of 7 cases and review of the literature. Cutis. 2001;67:133-138.
- Volkenandt M, Soyer HP, Cerroni L, et al. Molecular detection of clone-specific DNA in hypopigmented lesions of a patient with early evolving mycosis fungoides. Br J Dermatol. 1993;128:423-428.
- Furlan FC, Pereira BA, Sotto MN, et al. Hypopigmented mycosis fungoides versus mycosis fungoides with concomitant hypopigmented lesions: same disease or different variants of mycosis fungoides? Dermatology. 2014;229:271-274.
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270.
- Boulos S, Vaid R, Aladily TN, et al. Clinical presentation, immunopathology, and treatment of juvenile-onset mycosis fungoides: a case series of 34 patients. J Am Acad Dermatol. 2014;71:1117-1126.
- Lambroza E, Cohen SR, Phelps R, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, et al. Hypopigmented mycosis fungoides: Frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450-457.
- Furlan FC, de Paula Pereira BA, da Silva LF, et al. Loss of melanocytes in hypopigmented mycosis fungoides: a study of 18 patients. J Cutan Pathol. 2014;41:101-107.
- Tolkachjov SN, Comfere NI. Hypopigmented mycosis fungoides: a clinical mimicker of vitiligo. J Drugs Dermatol. 2015;14:193-194.
- Duarte I, Bedrikow, R, Aoki S. Mycosis fungoides: epidemiologic study of 17 cases and evaluation of PUVA photochemotherapy. An Bras Dermatol. 2006;81:40-45.
- Onsun N, Kural Y, Su O, et al. Hypopigmented mycosis fungoides associated with atopy in two children. Pediatr Dermatol. 2006;23:493-496.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978-1988.
- Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798-812.
- Stone ML, Styles AR, Cockerell CJ, et al. Hypopigmented report of 7 cases and review of the literature. Cutis. 2001;67:133-138.
- Volkenandt M, Soyer HP, Cerroni L, et al. Molecular detection of clone-specific DNA in hypopigmented lesions of a patient with early evolving mycosis fungoides. Br J Dermatol. 1993;128:423-428.
- Furlan FC, Pereira BA, Sotto MN, et al. Hypopigmented mycosis fungoides versus mycosis fungoides with concomitant hypopigmented lesions: same disease or different variants of mycosis fungoides? Dermatology. 2014;229:271-274.
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270.
- Boulos S, Vaid R, Aladily TN, et al. Clinical presentation, immunopathology, and treatment of juvenile-onset mycosis fungoides: a case series of 34 patients. J Am Acad Dermatol. 2014;71:1117-1126.
- Lambroza E, Cohen SR, Phelps R, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, et al. Hypopigmented mycosis fungoides: Frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450-457.
- Furlan FC, de Paula Pereira BA, da Silva LF, et al. Loss of melanocytes in hypopigmented mycosis fungoides: a study of 18 patients. J Cutan Pathol. 2014;41:101-107.
- Tolkachjov SN, Comfere NI. Hypopigmented mycosis fungoides: a clinical mimicker of vitiligo. J Drugs Dermatol. 2015;14:193-194.
- Duarte I, Bedrikow, R, Aoki S. Mycosis fungoides: epidemiologic study of 17 cases and evaluation of PUVA photochemotherapy. An Bras Dermatol. 2006;81:40-45.
- Onsun N, Kural Y, Su O, et al. Hypopigmented mycosis fungoides associated with atopy in two children. Pediatr Dermatol. 2006;23:493-496.
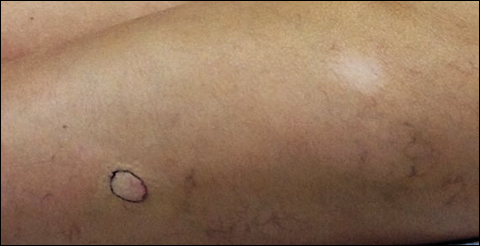
A 39-year-old woman presented with 2 areas of hypopigmented discoloration on the left thigh of 6 months' duration. The hypopigmentation was more visible following sun exposure because the areas did not tan. The patient had not sought prior treatment for the discoloration and denied any previous rash or trauma to the area. Her medical history was remarkable for hypothyroidism associated with mild and transient alopecia, acne, and xerosis. Her daily medications included oral contraceptive pills (norgestimate/ethinyl estradiol), oral levothyroxine/liothyronine, and sulfacetamide lotion 10%. She denied any allergies, and the remainder of her medical, surgical, social, and family history was unremarkable. A review of systems was negative for enlarged lymph nodes, fever, night sweats, and fatigue. Physical examination revealed 2 subtle hypopigmented patches with fine, atrophic, cigarette paper-like wrinkling distributed on the left medial and posterior upper thigh. Initial biopsy of the hypopigmented patches revealed a CD8+ lymphocytic infiltrate with an atypical interface.
Cases link vitiligoid lichen sclerosus and darker skin
, said Margaret H. Dennin, of the University of Chicago, and her associates.
Vitiligoid lichen sclerosus is a superficial variant of lichen sclerosus (LS), in which the lesion clinically appears to be vitiligo, but histologically is consistent with LS.
Seven dark-skinned girls aged 3-9 years had symptomatic (pruritus, pain, bleeding, constipation) depigmented patches of the vulvar or perianal region; three had purpuric lesions. None of the patients had atrophy or scarring, and they had no depigmentation anywhere else on their bodies. Follow-up was an average 2 years (range 3 months to 4 years).
Treatment with high-potency topical steroids, calcineurin inhibitors, or both resulted in improvement or resolution of their symptoms in all cases, but there was mild or no improvement in the depigmentation. Biopsies were not performed because of the patients’ young age and the location of the lesions, the investigators said.
The term vitiligoid lichen sclerosus was first coined in 1961 by Borda et al. when depigmented patches, as seen in both conditions, constituted the clinical appearance, but lacked the inflammation, atrophy, and sclerosis of typical LS. Histologically, these lesions were like LS, “based on the presence of a thin band of papillary dermal sclerosis,” Ms. Dennin and her associates said. Borda et al. suggested that vitiligoid lichen sclerosus might be limited to dark-skinned people, and recent reports support this. Alternatively, it may be that the depigmentation simply is more obvious on dark-skinned people, and asymptomatic cases go unnoticed on lighter-skinned people, the investigators surmised.
Both vitiligo and LS are autoimmune cutaneous disorders, and they both often affect the anogenital region. The conditions “may be linked through a common autoimmune response from exposed intracellular or altered cell surface antigens on damaged melanocytes,” the investigators said. “Histologic evidence demonstrates that development of vitiligo involves a preceding lichenoid inflammatory reaction that may trigger an autoimmune reaction to melanocytes, decreasing their number. Evolving vitiligo with a lichenoid reaction may result in epitope spreading and the development of LS.”
The study is limited by its retrospective nature, small sample size, and lack of biopsies, the researchers noted. Larger studies are needed to look at the overlap of the conditions, and “understand the true prevalence of vitiligoid lichen sclerosus,” Ms. Dennin and her associates said.
Read more in Pediatric Dermatology (2018. doi: 10.1111/pde.13399).
, said Margaret H. Dennin, of the University of Chicago, and her associates.
Vitiligoid lichen sclerosus is a superficial variant of lichen sclerosus (LS), in which the lesion clinically appears to be vitiligo, but histologically is consistent with LS.
Seven dark-skinned girls aged 3-9 years had symptomatic (pruritus, pain, bleeding, constipation) depigmented patches of the vulvar or perianal region; three had purpuric lesions. None of the patients had atrophy or scarring, and they had no depigmentation anywhere else on their bodies. Follow-up was an average 2 years (range 3 months to 4 years).
Treatment with high-potency topical steroids, calcineurin inhibitors, or both resulted in improvement or resolution of their symptoms in all cases, but there was mild or no improvement in the depigmentation. Biopsies were not performed because of the patients’ young age and the location of the lesions, the investigators said.
The term vitiligoid lichen sclerosus was first coined in 1961 by Borda et al. when depigmented patches, as seen in both conditions, constituted the clinical appearance, but lacked the inflammation, atrophy, and sclerosis of typical LS. Histologically, these lesions were like LS, “based on the presence of a thin band of papillary dermal sclerosis,” Ms. Dennin and her associates said. Borda et al. suggested that vitiligoid lichen sclerosus might be limited to dark-skinned people, and recent reports support this. Alternatively, it may be that the depigmentation simply is more obvious on dark-skinned people, and asymptomatic cases go unnoticed on lighter-skinned people, the investigators surmised.
Both vitiligo and LS are autoimmune cutaneous disorders, and they both often affect the anogenital region. The conditions “may be linked through a common autoimmune response from exposed intracellular or altered cell surface antigens on damaged melanocytes,” the investigators said. “Histologic evidence demonstrates that development of vitiligo involves a preceding lichenoid inflammatory reaction that may trigger an autoimmune reaction to melanocytes, decreasing their number. Evolving vitiligo with a lichenoid reaction may result in epitope spreading and the development of LS.”
The study is limited by its retrospective nature, small sample size, and lack of biopsies, the researchers noted. Larger studies are needed to look at the overlap of the conditions, and “understand the true prevalence of vitiligoid lichen sclerosus,” Ms. Dennin and her associates said.
Read more in Pediatric Dermatology (2018. doi: 10.1111/pde.13399).
, said Margaret H. Dennin, of the University of Chicago, and her associates.
Vitiligoid lichen sclerosus is a superficial variant of lichen sclerosus (LS), in which the lesion clinically appears to be vitiligo, but histologically is consistent with LS.
Seven dark-skinned girls aged 3-9 years had symptomatic (pruritus, pain, bleeding, constipation) depigmented patches of the vulvar or perianal region; three had purpuric lesions. None of the patients had atrophy or scarring, and they had no depigmentation anywhere else on their bodies. Follow-up was an average 2 years (range 3 months to 4 years).
Treatment with high-potency topical steroids, calcineurin inhibitors, or both resulted in improvement or resolution of their symptoms in all cases, but there was mild or no improvement in the depigmentation. Biopsies were not performed because of the patients’ young age and the location of the lesions, the investigators said.
The term vitiligoid lichen sclerosus was first coined in 1961 by Borda et al. when depigmented patches, as seen in both conditions, constituted the clinical appearance, but lacked the inflammation, atrophy, and sclerosis of typical LS. Histologically, these lesions were like LS, “based on the presence of a thin band of papillary dermal sclerosis,” Ms. Dennin and her associates said. Borda et al. suggested that vitiligoid lichen sclerosus might be limited to dark-skinned people, and recent reports support this. Alternatively, it may be that the depigmentation simply is more obvious on dark-skinned people, and asymptomatic cases go unnoticed on lighter-skinned people, the investigators surmised.
Both vitiligo and LS are autoimmune cutaneous disorders, and they both often affect the anogenital region. The conditions “may be linked through a common autoimmune response from exposed intracellular or altered cell surface antigens on damaged melanocytes,” the investigators said. “Histologic evidence demonstrates that development of vitiligo involves a preceding lichenoid inflammatory reaction that may trigger an autoimmune reaction to melanocytes, decreasing their number. Evolving vitiligo with a lichenoid reaction may result in epitope spreading and the development of LS.”
The study is limited by its retrospective nature, small sample size, and lack of biopsies, the researchers noted. Larger studies are needed to look at the overlap of the conditions, and “understand the true prevalence of vitiligoid lichen sclerosus,” Ms. Dennin and her associates said.
Read more in Pediatric Dermatology (2018. doi: 10.1111/pde.13399).
FROM PEDIATRIC DERMATOLOGY
Local Depigmentation of a Tattoo
The Diagnosis: Dermatofibroma
On dermoscopy, a central stellate, white, scarlike patch was seen (Figure). On both legs the patient had several additional brown 5- to 7-mm papules with similar dermoscopic features.
Dermatofibromas are common benign fibrosing tumors that appear as firm papules or plaques with variable color, commonly on the legs. Typically, lateral compression of a dermatofibroma causes downward displacement, called a positive dimple sign. On histology, fibroblasts and myofibroblasts can be seen as short intersecting fascicles with variable inflammatory cells and induction of adjacent structure hyperplasia. The etiology of dermatofibromas is unclear, though some are thought to be secondary to trauma or arthropod bites.1 Because these tumors are benign, the correct diagnosis can avoid unnecessary biopsies or other procedures.
The dermoscopic features of dermatofibromas have been well established.2 As perhaps the most easily identified structure, scarlike patches were seen in as many as 92% (22/24) of dermatofibromas in one study by Ferarri et al,3 while pigment networks also are commonly seen.2 In our case, given the surrounding dense tattoo deposition, it was difficult to ascertain any pigment network. However, the scarlike central patch was clearly apparent by dermoscopy.
Because dermatofibromas are hypothesized to be secondary to trauma, presumably applying tattoos also may cause dermatofibromas. Limited cases have described dermatofibromas arising in tattoos applied several months to years prior.4-6 No prior cases utilized dermoscopy. In our case, clinical examination and dermoscopy clearly demonstrated features consistent with a dermatofibroma, and the patient had more characteristic dermatofibromas scattered elsewhere on both legs. The patient was reassured that the lesions were benign and that the depigmentation was likely secondary to the process of dermatofibroma growth. She declined any treatment.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Zaballos P, Puig S, Llambrich A, et al. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75-83.
- Ferrari A, Soyer HP, Peris K, et al. Central white scarlike patch: a dermatoscopic clue for the diagnosis of dermatofibroma. J Am Acad Dermatol. 2000;43:1123-1125.
- Kluger N, Cotten H, Magana C, et al. Dermatofibroma occurring within a tattoo: report of two cases. J Cutan Pathol. 2008;35:696-698.
- Lobato-Berezo A, Churruca-Grijelmo M, Martínez-Pérez M, et al. Dermatofibroma arising within a black tattoo [published online September 23, 2014]. Case Rep Dermatol Med. 2014;2014:745304.
- Bittencourt Mde J, Miranda MF, Parijós AM, et al. Dermatofibroma in a black tattoo: report of a case. An Bras Dermatol. 2013;88:614-616.
The Diagnosis: Dermatofibroma
On dermoscopy, a central stellate, white, scarlike patch was seen (Figure). On both legs the patient had several additional brown 5- to 7-mm papules with similar dermoscopic features.

Dermatofibromas are common benign fibrosing tumors that appear as firm papules or plaques with variable color, commonly on the legs. Typically, lateral compression of a dermatofibroma causes downward displacement, called a positive dimple sign. On histology, fibroblasts and myofibroblasts can be seen as short intersecting fascicles with variable inflammatory cells and induction of adjacent structure hyperplasia. The etiology of dermatofibromas is unclear, though some are thought to be secondary to trauma or arthropod bites.1 Because these tumors are benign, the correct diagnosis can avoid unnecessary biopsies or other procedures.
The dermoscopic features of dermatofibromas have been well established.2 As perhaps the most easily identified structure, scarlike patches were seen in as many as 92% (22/24) of dermatofibromas in one study by Ferarri et al,3 while pigment networks also are commonly seen.2 In our case, given the surrounding dense tattoo deposition, it was difficult to ascertain any pigment network. However, the scarlike central patch was clearly apparent by dermoscopy.
Because dermatofibromas are hypothesized to be secondary to trauma, presumably applying tattoos also may cause dermatofibromas. Limited cases have described dermatofibromas arising in tattoos applied several months to years prior.4-6 No prior cases utilized dermoscopy. In our case, clinical examination and dermoscopy clearly demonstrated features consistent with a dermatofibroma, and the patient had more characteristic dermatofibromas scattered elsewhere on both legs. The patient was reassured that the lesions were benign and that the depigmentation was likely secondary to the process of dermatofibroma growth. She declined any treatment.
The Diagnosis: Dermatofibroma
On dermoscopy, a central stellate, white, scarlike patch was seen (Figure). On both legs the patient had several additional brown 5- to 7-mm papules with similar dermoscopic features.

Dermatofibromas are common benign fibrosing tumors that appear as firm papules or plaques with variable color, commonly on the legs. Typically, lateral compression of a dermatofibroma causes downward displacement, called a positive dimple sign. On histology, fibroblasts and myofibroblasts can be seen as short intersecting fascicles with variable inflammatory cells and induction of adjacent structure hyperplasia. The etiology of dermatofibromas is unclear, though some are thought to be secondary to trauma or arthropod bites.1 Because these tumors are benign, the correct diagnosis can avoid unnecessary biopsies or other procedures.
The dermoscopic features of dermatofibromas have been well established.2 As perhaps the most easily identified structure, scarlike patches were seen in as many as 92% (22/24) of dermatofibromas in one study by Ferarri et al,3 while pigment networks also are commonly seen.2 In our case, given the surrounding dense tattoo deposition, it was difficult to ascertain any pigment network. However, the scarlike central patch was clearly apparent by dermoscopy.
Because dermatofibromas are hypothesized to be secondary to trauma, presumably applying tattoos also may cause dermatofibromas. Limited cases have described dermatofibromas arising in tattoos applied several months to years prior.4-6 No prior cases utilized dermoscopy. In our case, clinical examination and dermoscopy clearly demonstrated features consistent with a dermatofibroma, and the patient had more characteristic dermatofibromas scattered elsewhere on both legs. The patient was reassured that the lesions were benign and that the depigmentation was likely secondary to the process of dermatofibroma growth. She declined any treatment.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Zaballos P, Puig S, Llambrich A, et al. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75-83.
- Ferrari A, Soyer HP, Peris K, et al. Central white scarlike patch: a dermatoscopic clue for the diagnosis of dermatofibroma. J Am Acad Dermatol. 2000;43:1123-1125.
- Kluger N, Cotten H, Magana C, et al. Dermatofibroma occurring within a tattoo: report of two cases. J Cutan Pathol. 2008;35:696-698.
- Lobato-Berezo A, Churruca-Grijelmo M, Martínez-Pérez M, et al. Dermatofibroma arising within a black tattoo [published online September 23, 2014]. Case Rep Dermatol Med. 2014;2014:745304.
- Bittencourt Mde J, Miranda MF, Parijós AM, et al. Dermatofibroma in a black tattoo: report of a case. An Bras Dermatol. 2013;88:614-616.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Zaballos P, Puig S, Llambrich A, et al. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75-83.
- Ferrari A, Soyer HP, Peris K, et al. Central white scarlike patch: a dermatoscopic clue for the diagnosis of dermatofibroma. J Am Acad Dermatol. 2000;43:1123-1125.
- Kluger N, Cotten H, Magana C, et al. Dermatofibroma occurring within a tattoo: report of two cases. J Cutan Pathol. 2008;35:696-698.
- Lobato-Berezo A, Churruca-Grijelmo M, Martínez-Pérez M, et al. Dermatofibroma arising within a black tattoo [published online September 23, 2014]. Case Rep Dermatol Med. 2014;2014:745304.
- Bittencourt Mde J, Miranda MF, Parijós AM, et al. Dermatofibroma in a black tattoo: report of a case. An Bras Dermatol. 2013;88:614-616.
A 41-year-old woman presented with loss of pigment in a tattoo on the left ankle. The tattoo was initially placed several years prior to presentation. For an uncertain amount of time, she had noticed a small palpable whitish area with loss of tattoo pigment. There was no corresponding pain, pruritis, or other symptoms. Her dermatologic history was notable only for keratosis pilaris. Physical examination showed an approximately 7-mm whitish firm papule on the lateral aspect of the left ankle, clearly visible in an otherwise green-black area of the tattoo (arrow). The lesion displaced downward with lateral compression.
Cosmetic Corner: Dermatologists Weigh in on Pigment Correctors
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on pigment correctors. Consideration must be given to:
- dEp Patch Full Face Mask
Activaderm, Inc
“This product uses microcurrent to push vitamin C into the skin. Vitamin C, a known antioxidant that usually has a difficult time passing through the stratum corneum, corrects pigmentary abnormalities. The product also comes with a botanical pigment corrector.”—Gary Goldenberg, MD, New York, New York
- De-Spot Skin Brightening Corrector
Peter Thomas Roth Labs LLC
“This product is a useful over-the-counter adjunct to prescription-strength hydroquinone, with niacinamide as one of the active ingredients.”—Shari Lipner, MD, PhD, New York, New York
- Glytone Dark Spot Corrector
Pierre Fabre Laboratories
“With 2% hydroquinone, glycolic acid, and kojic acid, you have a highly effective combination of ingredients that work synergistically to lighten areas of skin discoloration.”—Jeannette Graf, MD, Great Neck, New York
Cutis invites readers to send us their recommendations. Bar soap, lip plumper, and night cream will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.