User login
JAK Inhibitors for Vitiligo: Response Continues Over Time
SAN DIEGO — according to presentations at a late-breaking session at the annual meeting of the American Academy of Dermatology (AAD).
In one, the addition of narrow-band ultraviolet-B (NB-UVB) light therapy to ritlecitinib appears more effective than ritlecitinib alone. In the other study, the effectiveness of upadacitinib appears to improve over time.
Based on the ritlecitinib data, “if you have phototherapy in your office, it might be good to couple it with ritlecitinib for vitiligo patients,” said Emma Guttman-Yassky, MD, PhD, chair of the Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, who presented the findings.
However, because of the relatively small numbers in the extension study, Dr. Guttman-Yassky characterized the evidence as preliminary and in need of further investigation.
For vitiligo, the only approved JAK inhibitor is ruxolitinib, 1.5%, in a cream formulation. In June, ritlecitinib (Litfulo) was approved by the Food and Drug Administration (FDA) for alopecia areata. Phototherapy, which has been used for decades in the treatment of vitiligo, has an established efficacy and safety profile as a stand-alone vitiligo treatment. Upadacitinib has numerous indications for inflammatory diseases, such as rheumatoid arthritis, and was granted FDA approval for atopic dermatitis in 2022.
NB-UVB Arm Added in Ritlecitinib Extension
The ritlecitinib study population was drawn from patients with non-segmental vitiligo who initially participated in a 24-week dose-ranging period of a phase 2b trial published last year. In that study, 364 patients were randomized to doses of once-daily ritlecitinib ranging from 10 to 50 mg with or without a 4-week loading regimen. Higher doses were generally associated with greater efficacy on the primary endpoint of facial vitiligo area scoring index (F-VASI) but not with a greater risk for adverse events.
In the 24-week extension study, 187 patients received a 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib for the remaining 20 weeks. Another 43 patients were randomized to one of two arms: The same 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib or to 50-mg daily ritlecitinib without a loading dose but combined with NB-UVB delivered twice per week.
Important to interpretation of results, there was an additional twist. Patients in the randomized arm who had < 10% improvement in the total vitiligo area severity index (T-VASI) at week 12 of the extension were discontinued from the study.
The endpoints considered when comparing ritlecitinib with or without NB-UVB at the end of the extension study were F-VASI, T-VASI, patient global impression of change, and adverse events. Responses were assessed on the basis of both observed and last observation carried forward (LOCF).
Of the 43 people, who were randomized in the extension study, nine (21%) had < 10% improvement in T-VASI and were therefore discontinued from the study.
At the end of 24 weeks, both groups had a substantial response to their assigned therapy, but the addition of NB-UVB increased rates of response, although not always at a level of statistical significance, according to Dr. Guttman-Yassky.
For the percent improvement in F-VASI, specifically, the increase did not reach significance on the basis of LOCF (57.9% vs 51.5%; P = .158) but was highly significant on the basis of observed responses (69.6% vs 55.1%; P = .009). For T-VASI, differences for adjunctive NB-UVB over monotherapy did not reach significance for either observed or LOCF responses, but it was significant for observed responses in a patient global impression of change.
Small Numbers Limit Strength of Ritlecitinib, NB-UVB Evidence
However, Dr. Guttman-Yassky said it is important “to pay attention to the sample sizes” when noting the lack of significance.
The combination appeared safe, and there were no side effects associated with the addition of twice-weekly NB-UVB to ritlecitinib.
She acknowledged that the design of this analysis was “complicated” and that the number of randomized patients was small. She suggested the findings support the potential for benefit from the combination of a JAK inhibitor and NB-UVB, both of which have shown efficacy as monotherapy in previous studies. She indicated that a trial of this combination is reasonable while awaiting a more definitive study.
One of the questions that might be posed in a larger study is the timing of NB-UVB, such as whether it is best reserved for those with inadequate early response to a JAK inhibitor or if optimal results are achieved when a JAK inhibitor and NB-UVB are initiated simultaneously.
Upadacitinib Monotherapy Results
One rationale for initiating therapy with the combination of a JAK inhibitor and NB-UVB is the potential for a more rapid response, but extended results from a second phase 2b study with a different oral JAK inhibitor, upadacitinib, suggested responses on JAK inhibitor monotherapy improve steadily over time.
“The overall efficacy continued to improve without reaching a plateau at 1 year,” reported Thierry Passeron, MD, PhD, professor and chair, Department of Dermatology, Université Côte d’Azur, Nice, France. He spoke at the same AAD late-breaking session as Dr. Guttman-Yassky.
The 24-week dose-ranging data from the upadacitinib trial were previously reported at the 2023 annual meeting of the European Association of Dermatology and Venereology. In the placebo-controlled portion, which randomized 185 patients with extensive non-segmental vitiligo to 6 mg, 11 mg, or 22 mg, the two higher doses were significantly more effective than placebo.
In the extension, patients in the placebo group were randomized to 11 mg or 22 mg, while those in the higher dose groups remained on their assigned therapies.
F-VASI Almost Doubled in Extension Trial
From week 24 to week 52, there was nearly a doubling of the percent F-VASI reduction, climbing from 32% to 60.8% in the 11-mg group and from 38.7% to 64.9% in the 22-mg group, Dr. Passeron said. Placebo groups who were switched to active therapy at 24 weeks rapidly approached the rates of F-VASI response of those initiated on upadacitinib.
The percent reductions in T-VASI, although lower, followed the same pattern. For the 11-mg group, the reduction climbed from 16% at 24 weeks to 44.7% at 52 weeks. For the 22-mg group, the reduction climbed from 22.9% to 44.4%. Patients who were switched from placebo to 11 mg or to 22 mg also experienced improvements in T-VASI up to 52 weeks, although the level of improvement was lower than that in patients initially randomized to the higher doses of upadacitinib.
There were “no new safety signals” for upadacitinib, which is FDA-approved for multiple indications, according to Dr. Passeron. He said acne-like lesions were the most bothersome adverse event, and cases of herpes zoster were “rare.”
A version of these data was published in a British Journal of Dermatology supplement just prior to the AAD meeting.
Phase 3 vitiligo trials are planned for both ritlecitinib and upadacitinib.
Dr. Guttman-Yassky reported financial relationships with approximately 45 pharmaceutical companies, including Pfizer, which makes ritlecitinib and provided funding for the study she discussed. Dr. Passeron reported financial relationships with approximately 40 pharmaceutical companies, including AbbVie, which makes upadacitinib and provided funding for the study he discussed.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to presentations at a late-breaking session at the annual meeting of the American Academy of Dermatology (AAD).
In one, the addition of narrow-band ultraviolet-B (NB-UVB) light therapy to ritlecitinib appears more effective than ritlecitinib alone. In the other study, the effectiveness of upadacitinib appears to improve over time.
Based on the ritlecitinib data, “if you have phototherapy in your office, it might be good to couple it with ritlecitinib for vitiligo patients,” said Emma Guttman-Yassky, MD, PhD, chair of the Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, who presented the findings.
However, because of the relatively small numbers in the extension study, Dr. Guttman-Yassky characterized the evidence as preliminary and in need of further investigation.
For vitiligo, the only approved JAK inhibitor is ruxolitinib, 1.5%, in a cream formulation. In June, ritlecitinib (Litfulo) was approved by the Food and Drug Administration (FDA) for alopecia areata. Phototherapy, which has been used for decades in the treatment of vitiligo, has an established efficacy and safety profile as a stand-alone vitiligo treatment. Upadacitinib has numerous indications for inflammatory diseases, such as rheumatoid arthritis, and was granted FDA approval for atopic dermatitis in 2022.
NB-UVB Arm Added in Ritlecitinib Extension
The ritlecitinib study population was drawn from patients with non-segmental vitiligo who initially participated in a 24-week dose-ranging period of a phase 2b trial published last year. In that study, 364 patients were randomized to doses of once-daily ritlecitinib ranging from 10 to 50 mg with or without a 4-week loading regimen. Higher doses were generally associated with greater efficacy on the primary endpoint of facial vitiligo area scoring index (F-VASI) but not with a greater risk for adverse events.
In the 24-week extension study, 187 patients received a 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib for the remaining 20 weeks. Another 43 patients were randomized to one of two arms: The same 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib or to 50-mg daily ritlecitinib without a loading dose but combined with NB-UVB delivered twice per week.
Important to interpretation of results, there was an additional twist. Patients in the randomized arm who had < 10% improvement in the total vitiligo area severity index (T-VASI) at week 12 of the extension were discontinued from the study.
The endpoints considered when comparing ritlecitinib with or without NB-UVB at the end of the extension study were F-VASI, T-VASI, patient global impression of change, and adverse events. Responses were assessed on the basis of both observed and last observation carried forward (LOCF).
Of the 43 people, who were randomized in the extension study, nine (21%) had < 10% improvement in T-VASI and were therefore discontinued from the study.
At the end of 24 weeks, both groups had a substantial response to their assigned therapy, but the addition of NB-UVB increased rates of response, although not always at a level of statistical significance, according to Dr. Guttman-Yassky.
For the percent improvement in F-VASI, specifically, the increase did not reach significance on the basis of LOCF (57.9% vs 51.5%; P = .158) but was highly significant on the basis of observed responses (69.6% vs 55.1%; P = .009). For T-VASI, differences for adjunctive NB-UVB over monotherapy did not reach significance for either observed or LOCF responses, but it was significant for observed responses in a patient global impression of change.
Small Numbers Limit Strength of Ritlecitinib, NB-UVB Evidence
However, Dr. Guttman-Yassky said it is important “to pay attention to the sample sizes” when noting the lack of significance.
The combination appeared safe, and there were no side effects associated with the addition of twice-weekly NB-UVB to ritlecitinib.
She acknowledged that the design of this analysis was “complicated” and that the number of randomized patients was small. She suggested the findings support the potential for benefit from the combination of a JAK inhibitor and NB-UVB, both of which have shown efficacy as monotherapy in previous studies. She indicated that a trial of this combination is reasonable while awaiting a more definitive study.
One of the questions that might be posed in a larger study is the timing of NB-UVB, such as whether it is best reserved for those with inadequate early response to a JAK inhibitor or if optimal results are achieved when a JAK inhibitor and NB-UVB are initiated simultaneously.
Upadacitinib Monotherapy Results
One rationale for initiating therapy with the combination of a JAK inhibitor and NB-UVB is the potential for a more rapid response, but extended results from a second phase 2b study with a different oral JAK inhibitor, upadacitinib, suggested responses on JAK inhibitor monotherapy improve steadily over time.
“The overall efficacy continued to improve without reaching a plateau at 1 year,” reported Thierry Passeron, MD, PhD, professor and chair, Department of Dermatology, Université Côte d’Azur, Nice, France. He spoke at the same AAD late-breaking session as Dr. Guttman-Yassky.
The 24-week dose-ranging data from the upadacitinib trial were previously reported at the 2023 annual meeting of the European Association of Dermatology and Venereology. In the placebo-controlled portion, which randomized 185 patients with extensive non-segmental vitiligo to 6 mg, 11 mg, or 22 mg, the two higher doses were significantly more effective than placebo.
In the extension, patients in the placebo group were randomized to 11 mg or 22 mg, while those in the higher dose groups remained on their assigned therapies.
F-VASI Almost Doubled in Extension Trial
From week 24 to week 52, there was nearly a doubling of the percent F-VASI reduction, climbing from 32% to 60.8% in the 11-mg group and from 38.7% to 64.9% in the 22-mg group, Dr. Passeron said. Placebo groups who were switched to active therapy at 24 weeks rapidly approached the rates of F-VASI response of those initiated on upadacitinib.
The percent reductions in T-VASI, although lower, followed the same pattern. For the 11-mg group, the reduction climbed from 16% at 24 weeks to 44.7% at 52 weeks. For the 22-mg group, the reduction climbed from 22.9% to 44.4%. Patients who were switched from placebo to 11 mg or to 22 mg also experienced improvements in T-VASI up to 52 weeks, although the level of improvement was lower than that in patients initially randomized to the higher doses of upadacitinib.
There were “no new safety signals” for upadacitinib, which is FDA-approved for multiple indications, according to Dr. Passeron. He said acne-like lesions were the most bothersome adverse event, and cases of herpes zoster were “rare.”
A version of these data was published in a British Journal of Dermatology supplement just prior to the AAD meeting.
Phase 3 vitiligo trials are planned for both ritlecitinib and upadacitinib.
Dr. Guttman-Yassky reported financial relationships with approximately 45 pharmaceutical companies, including Pfizer, which makes ritlecitinib and provided funding for the study she discussed. Dr. Passeron reported financial relationships with approximately 40 pharmaceutical companies, including AbbVie, which makes upadacitinib and provided funding for the study he discussed.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to presentations at a late-breaking session at the annual meeting of the American Academy of Dermatology (AAD).
In one, the addition of narrow-band ultraviolet-B (NB-UVB) light therapy to ritlecitinib appears more effective than ritlecitinib alone. In the other study, the effectiveness of upadacitinib appears to improve over time.
Based on the ritlecitinib data, “if you have phototherapy in your office, it might be good to couple it with ritlecitinib for vitiligo patients,” said Emma Guttman-Yassky, MD, PhD, chair of the Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, who presented the findings.
However, because of the relatively small numbers in the extension study, Dr. Guttman-Yassky characterized the evidence as preliminary and in need of further investigation.
For vitiligo, the only approved JAK inhibitor is ruxolitinib, 1.5%, in a cream formulation. In June, ritlecitinib (Litfulo) was approved by the Food and Drug Administration (FDA) for alopecia areata. Phototherapy, which has been used for decades in the treatment of vitiligo, has an established efficacy and safety profile as a stand-alone vitiligo treatment. Upadacitinib has numerous indications for inflammatory diseases, such as rheumatoid arthritis, and was granted FDA approval for atopic dermatitis in 2022.
NB-UVB Arm Added in Ritlecitinib Extension
The ritlecitinib study population was drawn from patients with non-segmental vitiligo who initially participated in a 24-week dose-ranging period of a phase 2b trial published last year. In that study, 364 patients were randomized to doses of once-daily ritlecitinib ranging from 10 to 50 mg with or without a 4-week loading regimen. Higher doses were generally associated with greater efficacy on the primary endpoint of facial vitiligo area scoring index (F-VASI) but not with a greater risk for adverse events.
In the 24-week extension study, 187 patients received a 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib for the remaining 20 weeks. Another 43 patients were randomized to one of two arms: The same 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib or to 50-mg daily ritlecitinib without a loading dose but combined with NB-UVB delivered twice per week.
Important to interpretation of results, there was an additional twist. Patients in the randomized arm who had < 10% improvement in the total vitiligo area severity index (T-VASI) at week 12 of the extension were discontinued from the study.
The endpoints considered when comparing ritlecitinib with or without NB-UVB at the end of the extension study were F-VASI, T-VASI, patient global impression of change, and adverse events. Responses were assessed on the basis of both observed and last observation carried forward (LOCF).
Of the 43 people, who were randomized in the extension study, nine (21%) had < 10% improvement in T-VASI and were therefore discontinued from the study.
At the end of 24 weeks, both groups had a substantial response to their assigned therapy, but the addition of NB-UVB increased rates of response, although not always at a level of statistical significance, according to Dr. Guttman-Yassky.
For the percent improvement in F-VASI, specifically, the increase did not reach significance on the basis of LOCF (57.9% vs 51.5%; P = .158) but was highly significant on the basis of observed responses (69.6% vs 55.1%; P = .009). For T-VASI, differences for adjunctive NB-UVB over monotherapy did not reach significance for either observed or LOCF responses, but it was significant for observed responses in a patient global impression of change.
Small Numbers Limit Strength of Ritlecitinib, NB-UVB Evidence
However, Dr. Guttman-Yassky said it is important “to pay attention to the sample sizes” when noting the lack of significance.
The combination appeared safe, and there were no side effects associated with the addition of twice-weekly NB-UVB to ritlecitinib.
She acknowledged that the design of this analysis was “complicated” and that the number of randomized patients was small. She suggested the findings support the potential for benefit from the combination of a JAK inhibitor and NB-UVB, both of which have shown efficacy as monotherapy in previous studies. She indicated that a trial of this combination is reasonable while awaiting a more definitive study.
One of the questions that might be posed in a larger study is the timing of NB-UVB, such as whether it is best reserved for those with inadequate early response to a JAK inhibitor or if optimal results are achieved when a JAK inhibitor and NB-UVB are initiated simultaneously.
Upadacitinib Monotherapy Results
One rationale for initiating therapy with the combination of a JAK inhibitor and NB-UVB is the potential for a more rapid response, but extended results from a second phase 2b study with a different oral JAK inhibitor, upadacitinib, suggested responses on JAK inhibitor monotherapy improve steadily over time.
“The overall efficacy continued to improve without reaching a plateau at 1 year,” reported Thierry Passeron, MD, PhD, professor and chair, Department of Dermatology, Université Côte d’Azur, Nice, France. He spoke at the same AAD late-breaking session as Dr. Guttman-Yassky.
The 24-week dose-ranging data from the upadacitinib trial were previously reported at the 2023 annual meeting of the European Association of Dermatology and Venereology. In the placebo-controlled portion, which randomized 185 patients with extensive non-segmental vitiligo to 6 mg, 11 mg, or 22 mg, the two higher doses were significantly more effective than placebo.
In the extension, patients in the placebo group were randomized to 11 mg or 22 mg, while those in the higher dose groups remained on their assigned therapies.
F-VASI Almost Doubled in Extension Trial
From week 24 to week 52, there was nearly a doubling of the percent F-VASI reduction, climbing from 32% to 60.8% in the 11-mg group and from 38.7% to 64.9% in the 22-mg group, Dr. Passeron said. Placebo groups who were switched to active therapy at 24 weeks rapidly approached the rates of F-VASI response of those initiated on upadacitinib.
The percent reductions in T-VASI, although lower, followed the same pattern. For the 11-mg group, the reduction climbed from 16% at 24 weeks to 44.7% at 52 weeks. For the 22-mg group, the reduction climbed from 22.9% to 44.4%. Patients who were switched from placebo to 11 mg or to 22 mg also experienced improvements in T-VASI up to 52 weeks, although the level of improvement was lower than that in patients initially randomized to the higher doses of upadacitinib.
There were “no new safety signals” for upadacitinib, which is FDA-approved for multiple indications, according to Dr. Passeron. He said acne-like lesions were the most bothersome adverse event, and cases of herpes zoster were “rare.”
A version of these data was published in a British Journal of Dermatology supplement just prior to the AAD meeting.
Phase 3 vitiligo trials are planned for both ritlecitinib and upadacitinib.
Dr. Guttman-Yassky reported financial relationships with approximately 45 pharmaceutical companies, including Pfizer, which makes ritlecitinib and provided funding for the study she discussed. Dr. Passeron reported financial relationships with approximately 40 pharmaceutical companies, including AbbVie, which makes upadacitinib and provided funding for the study he discussed.
A version of this article appeared on Medscape.com.
FROM AAD 2024
Advancements in Targeted Therapies for Vitiligo: Prioritizing Equity in Drug Development
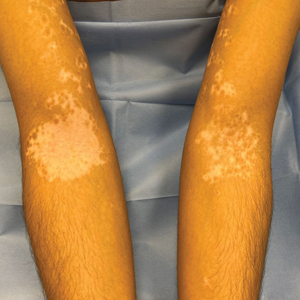
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
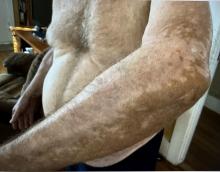
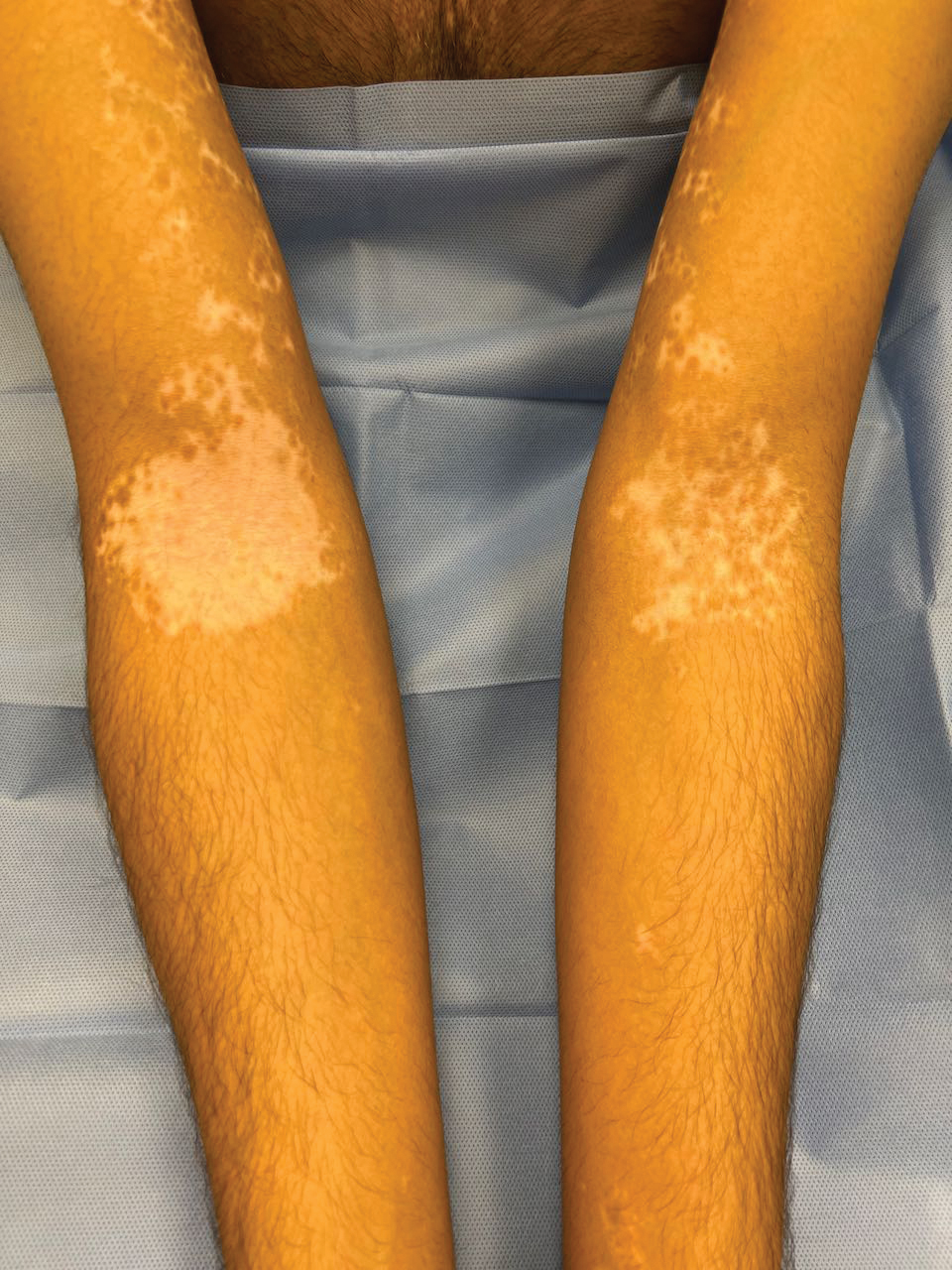
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).
Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
Vitiligo is a common acquired autoimmune disease that causes depigmented patches to develop throughout the skin , with descriptions dating back more than 3000 years to the earliest known Indian and Egyptian texts. Approximately 1.4% of the worldwide population has vitiligo,1 and onset follows a bimodal age distribution with an early-onset population (mean age at onset, 10.3 years) as well as an adult-onset population (mean age at onset, 34 years).2 Vitiligo manifests as well-defined, irregular, depigmented macules and patches surrounded by normal skin. The patches can vary in size from a few millimeters to several centimeters. There may be signs of inflammation, and the lesions can be itchy, but in most cases vitiligo is asymptomatic. In nonsegmental vitiligo, the depigmented patches are ymmetrical, can appear in any area of the body, and commonly progress slowly. In segmental vitiligo, the patches are unilateral, rarely cross the midline of the body, and are localized to one area. Segmental vitiligo commonly appears in childhood and progresses rapidly but stops abruptly within 6 to 12 months and remains stable, usually for life.3 Although the condition may be more apparent in patients with skin of color, vitiligo manifests at a similar rate in individuals of all races and ethnicities.4
Similar to most autoimmune diseases, vitiligo has a strong genetic predisposition. Although the overall prevalence of vitiligo is less than 2%, having a family history of vitiligo (ie, a first-degree relative with vitiligo) increases an individual’s risk to 6%, while concordance in identical twins is 23%.5 Beyond genetic predisposition, there is strong evidence that environmental exposures, such as hair dyes, contribute to risk for disease.6 Interestingly, vitiligo is associated with polyautoimmunity—the presence of multiple autoimmune diseases in a single patient,7 such as type 1 diabetes mellitus, rheumatoid arthritis, autoimmune thyroid disease, pernicious anemia, and Addison disease. Similar to vitiligo itself, polyautoimmunity likely is driven by a combination of genetic and environmental factors.5
We provide a brief overview of clinical trial results of Janus kinase (JAK) inhibitors for treating vitiligo and discuss the trial cohorts, with an emphasis on the impact of cohort demographic composition for individuals with skin of color. We recommend factors that investigators should consider to ensure equitable representation of individuals with skin of color in future clinical trials.
Autoimmune Pathogenesis and Treatment With JAK Inhibitors
Vitiligo is driven by autoreactive CD8+ T cells that target melanocytes and secrete IFN-g. Signaling of IFN-g occurs through the JAK–signal transducer and activator of transcription (JAK-STAT) pathway, leading to transcriptional changes that activate proinflammatory genes such as the chemokine CXCL10, which is required for the directed accumulation of melanocyte-specific CD8+ T cells at the epidermis where melanocytes reside.8 Once vitiligo has been initiated, the disease persists due to the presence of resident memory T cells that remain in the skin and destroy new melanocytes.9,10
Given the central role of IFN-g signaling in the pathogenesis of vitiligo, drugs that inhibit JAK signaling are appealing to treat the disease. These JAK inhibitors bind to the kinase domain of JAK to prevent its activation, thus preventing downstream signaling events including STAT phosphorylation and its translocation to the nucleus, which ultimately stops the upregulation of inflammatory gene transcription. This process attenuates the autoimmune response in the skin and results in repigmentation of vitiligo lesions. In 2022, the US Food and Drug Administration approved the topical JAK inhibitor ruxolitinib for the treatment of vitiligo. Additional clinical trials have been initiated to test oral JAK inhibitors—ritlecitinib (ClinicalTrials.gov identifiers NCT06163326, NCT06072183, NCT05583526), povorcitinib (NCT04818346, NCT06113445, NCT06113471), and upadacitinib (NCT04927975, NCT06118411)—with strong results reported so far.11
The effects of JAK inhibitors can be striking, as shown in the Figure. A patient of one of the authors (J.E.H.) used topical ruxolitinib on only the left arm for approximately 36 weeks and results were as expected—strong repigmentation of only the treated area, which is possible with JAK inhibitors. Indeed, 2 phase 3 studies—Topical Ruxolitinib Evaluation in Vitiligo (TRuE-V1 and TRuE-V2)—showed that approximately 30% of participants in TRuE-V1 (N=330) and 30.9% of participants in TRuE-V2 (N=344) achieved at least 75% improvement over baseline in the facial vitiligo area scoring index (VASI).12 In the oral ritlecitinib phase 2b study, 12.1% of the 187 participants on the highest tested dose of ritlecitinib (loading dose of 200 mg/d for 28 days, followed by 50 mg/d maintenance dose) achieved at least 75% improvement over baseline in the VASI at 24 weeks.11 Although this rate is lower than for topical ruxolitinib, this trial required all participants to have active disease (unlike the TRuE-V trials of ruxolitinib), which likely created a higher bar for repigmentation and thus resulted in fewer participants achieving the primary outcome at the early 6-month end point. Extension of treatment through 48 weeks demonstrated continued improvement over baseline without any evidence of plateau.11 Although treatment with JAK inhibitors can result in dramatic repigmentation of vitiligo patches, it falls short of providing a permanent cure, as stopping treatment results in relapse (ie, the return of depigmented lesions).

Racial Disparities in Clinical Trials
Even though vitiligo affects all skin types and races/ethnicities with similar prevalence and severity, the proportion of individuals with darker skin types enrolled in these clinical trials fails to match their representation in the population as a whole. A study examining the prevalence of vitiligo in the United States reported that Black or African American individuals represented 15.8% of vitiligo diagnoses in the United States4 even though they are only 12.7% of the total US population. However, Black or African American individuals comprised only 5% of the combined participants in the TRuE-V clinical trials for topical ruxolitinib12 and 2.7% of the participants in the phase 2b study of oral ritlecitinib.11 This lack of appropriate representation is not unique to JAK inhibitors or other vitiligo trials. Indeed, the US Food and Drug Administration reported that Black or African American individuals comprised only 8% of participants for all clinical trials in 2020.13
Efficacy Metrics Beyond Repigmentation
Disparities in quality-of-life (QOL) metrics in diseases affecting individuals with skin of color also exist. In vitiligo, the contrast between affected and unaffected skin is greater in patients with skin of color, which means that for a given VASI score, the visibility of depigmentation as well as repigmentation may be variable among patients. Additionally, there is evidence that QOL concerns vary between patients with skin of color and those with lighter skin types. Ezzedine et al14 found that QOL concerns in vitiligo patients with darker skin focused more on appearance, while concerns in vitiligo patients with lighter skin focused more on skin cancer risk. In addition to QOL differences among individuals with different skin types, there also are well-documented differences in attitudes to vitiligo among certain ethnic or cultural groups.15 For example, the Rigveda (an ancient Hindu text) indicates that individuals with vitiligo and their progeny are disqualified from marriage. Although the JAK inhibitor clinical trials for vitiligo did not appear to show differences in the degree of repigmentation among different skin types or races/ethnicities, QOL measures were not collected as a secondary end point in these studies—despite the fact that at least 1 study had documented that QOL measures were not uniform across patients when stratified by age and extent of disease.1,11,12 This same study also presented limited data suggestive of lower QOL in patients with the darkest skin phototype.1
Considerations for Future Clinical Trials
It is logical to assume that every clinical trialist in dermatology seeks equitable representation among a diverse set of races, ethnicities, and skin types, but achieving this goal remains elusive. Two recent publications16,17 outlined the challenges and examined solutions to address enrollment disparities, including several barriers to diversity among clinical trial participants: awareness of the clinical trials among minority populations; easy access to clinical trial sites; reluctance to participate because of prior experiences of discrimination, even if unrelated to clinical trials; and a lack of workforce diversity among the clinical trialist teams. To overcome these barriers, a multifaceted approach is needed that requires action at the level of the patient, provider, community, and institution. Once diverse representation is achieved, investigators should consider the need for QOL metrics as a secondary outcome in their trials, which will ensure that the intended clinical effect is matched by patient expectations across different races and ethnicities based on the potential differential impact that diseases such as vitiligo can have on patients with skin of color.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36:1831-1844.
- Jin Y, Roberts GHL, Ferrara TM, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class II HLA expression. Nat Commun. 2019;10:391.
- Rodrigues M, Ezzedine K, Hamzavi I, et al; Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50.
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol. 2021;141:265-73.
- Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151-161.
- Ahmed F, Moseley I, Ragi SD, et al. Vitiligo in underrepresented communities: an all of us database analysis. J Am Acad Dermatol. 2023;88:945-948.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621-648.
- Richmond JM, Strassner JP, Zapata L Jr, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10:eaam7710.
- Richmond JM, Strassner JP, Rashighi M, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol. 2019;139:769-778.
- Ezzedine K, Peeva E, Yamaguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403.
- Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455.
- Cavazzoni P, Anagnostiadis E, Lolic M. Drug trials snapshots summary report. US Food and Drug Administration website. Accessed March 19, 2024. https://www.fda.gov/media/145718/download
- Ezzedine K, Grimes PE, Meurant JM, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173:607-609.
- Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35:117-128.
- Kahn JM, Gray DM 2nd, Oliveri JM, et al. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128:216-221.
- Nolan TS, McKoy A, Gray DM 2nd, et al. Virtual community engagement for retention of black men in clinical research. Am J Mens Health. 2023;17:15579883221147767.
Practice Points
- Vitiligo is an autoimmune disease of the skin that affects all skin types but can be particularly disfiguring in those with skin of color.
- Ruxolitinib, a topical Janus kinase (JAK) inhibitor, is the only US Food and Drug Administration–approved treatment to repigment the skin in vitiligo and has shown efficacy for individuals with all skin phototypes.
- Individuals with skin of color are underrepresented in patient cohorts for JAK inhibitor clinical trials for vitiligo, mirroring a phenomenon seen in the majority of clinical trials. Ensuring diverse participant enrollment and measuring quality-of-life metrics will strengthen future clinical trials for treatment of vitiligo and other skin diseases impacting patients with skin of color.
Treating Pediatric Vitiligo: Consensus Statement Provides Recommendations
TOPLINE:
METHODOLOGY:
- While half of all vitiligo cases manifest within the initial two decades of life, no guidelines specifically address the management of vitiligo in children, adolescents, and young adults with vitiligo.
- A protocol was established to formulate consensus recommendations addressing questions related to pediatric vitiligo.
- Overall, 50 articles on topical corticosteroids and/or topical calcineurin inhibitors, five on topical Janus kinase inhibitors, and two each on pseudocatalase and microdermabrasion were included.
- The participants recorded their agreement levels with the formulated statements, using a 5-point Likert scale.
TAKEAWAY:
- TCIs, TCSs, JAK inhibitors, and phototherapy, specifically narrowband ultraviolet (UV)-B light therapy, are mainstay treatments; the combination of UV-B light and topical therapy may enhance initial repigmentation.
- Long-term monitoring for skin cancers is advised, and short outdoor UV exposure is suggested for pediatric patients.
- TCIs, such as tacrolimus and pimecrolimus, are recommended as first-line therapy, particularly on the face, applied twice daily for ≥ 3 months; continued use for 6-12 additional months is recommended if repigmentation is observed.
- The choice of TCS class depends on the site and planned usage duration. Short-term use or overlap with TCIs is recommended because of the risk for atrophy with long-term TCS use. Class 5-6 agents are another option.
- For areas with thin skin, TCSs can be considered second-line treatments.
- Topical JAK inhibitors, specifically topical 1.5% ruxolitinib cream, are recommended for patients aged ≥ 12 years, as first- or second-line therapy. Limitation to 10% body surface area is recommended to minimize systemic absorption. Limited evidence exists for children aged < 12 years.
IN PRACTICE:
“Effective therapy requires a focus on long-term therapeutic interventions to maximize the local gain and retention of pigmentation with a trial period of twice-weekly application. Counseling should include discussion of the chronicity of vitiligo and the need for long-term care,” the authors wrote.
LIMITATIONS:
Some of the recommendations were opinion-based because of the scarcity of evidence-based literature.
SOURCE:
The consensus statement was published on March 13 in JAMA Dermatology.
DISCLOSURES:
This work was supported by grants from Vitiligo Research Foundation and Incyte Pharmaceuticals. The majority of authors disclosed financial relationships outside this work; several reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- While half of all vitiligo cases manifest within the initial two decades of life, no guidelines specifically address the management of vitiligo in children, adolescents, and young adults with vitiligo.
- A protocol was established to formulate consensus recommendations addressing questions related to pediatric vitiligo.
- Overall, 50 articles on topical corticosteroids and/or topical calcineurin inhibitors, five on topical Janus kinase inhibitors, and two each on pseudocatalase and microdermabrasion were included.
- The participants recorded their agreement levels with the formulated statements, using a 5-point Likert scale.
TAKEAWAY:
- TCIs, TCSs, JAK inhibitors, and phototherapy, specifically narrowband ultraviolet (UV)-B light therapy, are mainstay treatments; the combination of UV-B light and topical therapy may enhance initial repigmentation.
- Long-term monitoring for skin cancers is advised, and short outdoor UV exposure is suggested for pediatric patients.
- TCIs, such as tacrolimus and pimecrolimus, are recommended as first-line therapy, particularly on the face, applied twice daily for ≥ 3 months; continued use for 6-12 additional months is recommended if repigmentation is observed.
- The choice of TCS class depends on the site and planned usage duration. Short-term use or overlap with TCIs is recommended because of the risk for atrophy with long-term TCS use. Class 5-6 agents are another option.
- For areas with thin skin, TCSs can be considered second-line treatments.
- Topical JAK inhibitors, specifically topical 1.5% ruxolitinib cream, are recommended for patients aged ≥ 12 years, as first- or second-line therapy. Limitation to 10% body surface area is recommended to minimize systemic absorption. Limited evidence exists for children aged < 12 years.
IN PRACTICE:
“Effective therapy requires a focus on long-term therapeutic interventions to maximize the local gain and retention of pigmentation with a trial period of twice-weekly application. Counseling should include discussion of the chronicity of vitiligo and the need for long-term care,” the authors wrote.
LIMITATIONS:
Some of the recommendations were opinion-based because of the scarcity of evidence-based literature.
SOURCE:
The consensus statement was published on March 13 in JAMA Dermatology.
DISCLOSURES:
This work was supported by grants from Vitiligo Research Foundation and Incyte Pharmaceuticals. The majority of authors disclosed financial relationships outside this work; several reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- While half of all vitiligo cases manifest within the initial two decades of life, no guidelines specifically address the management of vitiligo in children, adolescents, and young adults with vitiligo.
- A protocol was established to formulate consensus recommendations addressing questions related to pediatric vitiligo.
- Overall, 50 articles on topical corticosteroids and/or topical calcineurin inhibitors, five on topical Janus kinase inhibitors, and two each on pseudocatalase and microdermabrasion were included.
- The participants recorded their agreement levels with the formulated statements, using a 5-point Likert scale.
TAKEAWAY:
- TCIs, TCSs, JAK inhibitors, and phototherapy, specifically narrowband ultraviolet (UV)-B light therapy, are mainstay treatments; the combination of UV-B light and topical therapy may enhance initial repigmentation.
- Long-term monitoring for skin cancers is advised, and short outdoor UV exposure is suggested for pediatric patients.
- TCIs, such as tacrolimus and pimecrolimus, are recommended as first-line therapy, particularly on the face, applied twice daily for ≥ 3 months; continued use for 6-12 additional months is recommended if repigmentation is observed.
- The choice of TCS class depends on the site and planned usage duration. Short-term use or overlap with TCIs is recommended because of the risk for atrophy with long-term TCS use. Class 5-6 agents are another option.
- For areas with thin skin, TCSs can be considered second-line treatments.
- Topical JAK inhibitors, specifically topical 1.5% ruxolitinib cream, are recommended for patients aged ≥ 12 years, as first- or second-line therapy. Limitation to 10% body surface area is recommended to minimize systemic absorption. Limited evidence exists for children aged < 12 years.
IN PRACTICE:
“Effective therapy requires a focus on long-term therapeutic interventions to maximize the local gain and retention of pigmentation with a trial period of twice-weekly application. Counseling should include discussion of the chronicity of vitiligo and the need for long-term care,” the authors wrote.
LIMITATIONS:
Some of the recommendations were opinion-based because of the scarcity of evidence-based literature.
SOURCE:
The consensus statement was published on March 13 in JAMA Dermatology.
DISCLOSURES:
This work was supported by grants from Vitiligo Research Foundation and Incyte Pharmaceuticals. The majority of authors disclosed financial relationships outside this work; several reported no disclosures.
A version of this article appeared on Medscape.com.
Lichen Sclerosus: The Silent Genital Health Concern Often Missed
Ashley Winter, MD, remembers the first time she Googled the skin condition lichen sclerosus. Most of the websites listed the autoimmune condition as a rare disease.
In the realm of genital health, some conditions remain shrouded in silence and consequently are more likely to go undercounted and underdiagnosed, said Dr. Winter, a urologist based in Los Angeles.
“I truly believe that we just miss the diagnosis a vast majority of the time because there isn’t enough training on [detecting] it,” said Dr. Winter.
, according to the US National Institutes of Health. The condition also more commonly occurs among women, and symptoms include hypopigmentation, itching, pain, changes in skin appearance, and skin atrophy.
“Most cases [affect the] genital [area] only, so often patients don’t bring it up because they don’t want to be examined,” said Sarah Lonowski, MD, assistant professor of dermatology and codirector of the Multidisciplinary Autoimmune Skin Disease/Derm-Rheum Program at the University of Nebraska–Lincoln. “It’s a sensitive area, it’s an uncomfortable area to have examined, so it comes with a lot of emotional burden,” for patients, Dr. Lonowski said.
Receiving a lichen sclerosis diagnosis can take 5 years or longer, in part because the condition’s symptoms can lead clinicians to first make a diagnosis of a yeast infection or bacterial vaginosis, according to Christina Kraus, MD, assistant professor of dermatology at UCI Health in Irvine, California.
“There is still limited information on this condition in medical education, and it is not uncommon for clinicians who are not in dermatology or gynecology to be unfamiliar with this diagnosis,” Dr. Kraus said.
Because no medical tests are available to confirm lichen sclerosus, clinicians diagnose the condition based on the skin’s appearance and symptoms. In some cases, a skin biopsy may help differentiate it from similar rashes that occur in the genital area.
Prepubescent children and postmenopausal women are most likely to develop genital lichen sclerosis, so pediatricians and primary care physicians may be the first to see possible cases, Dr. Lonowski said.
Patients “may not mention it unless they’re asked,” Dr. Lonowski said, adding clinicians can inquire with patients about genital health, examine bothersome areas, “and refer if you’re not sure.”
Clinicians may also miss the condition during physical exams if they do not examine the vulvar skin, she said. The exact cause also remains elusive, but researchers believe genetic and hormonal factors, as well as an overactive immune response, may contribute to development of the condition.
Watch Out for Presentation
While lichen sclerosus more frequently occurs in women, men are also affected by the condition. Benjamin N. Breyer, MD, professor and chair of urology at the University of California San Francisco, said lichen sclerosus is one of the most common skin conditions he treats in his male patients.
“Advanced cases can cause urethral narrowing, which is a condition I treat commonly,” said Dr. Breyer. “Lichen sclerosus is often an underrecognized cause of pain or tearing with erections and sex in men.”
Similar to women, lichen sclerosus presents as white color changes on the skin. For men, the condition can also result in fusion of the shaft skin to the head of the penis and burying or concealment of the penis, Dr. Breyer said.
“This leads to challenges with intimacy and urination and can have extensive impacts on quality of life,” said Dr. Breyer.
For women, the skin changes often extend to the perianal area and can cause scarring, said Dr. Kraus.
“Early scarring may present as adherence of the labia minora to the labia majora or inability to fully retract the clitoral hood from the clitoris,” said Dr. Kraus.
In both men and women, lichen sclerosus and another autoimmune condition known as morphea, characterized by skin hardening and discoloration, often present together, said Dr. Lonowski.
“If you have a patient with known morphea, it’s important to ask about genital symptoms,” said Dr. Lonowski. “The association between the two is fairly strong.”
Circumcision is often the first step to help prevent chronic inflammation among male patients, said Dr. Breyer. Because lichen sclerosus is associated with an increased risk for penile cancer, “it is important to biopsy suspicious lesions,” Dr. Breyer added.
Increasing awareness of lichen sclerosus is crucial for early detection and timely intervention, said Dr. Lonowski. The first-line treatment of genital lichen sclerosus is strong topical steroid ointments to reduce inflammation. Clinicians might prescribe this treatment for use twice daily for 2-3 months and then assesses the patient on their response.
“It is fairly responsive to treatment in most cases,” said Dr. Lonowski.
Once symptoms have improved, Dr. Lonowski transitions patients to a maintenance regimen, which might include using the same steroid but only three times a week, switching to a topical with a less potent steroid dosage, or using a combination of a topical steroid and a nonsteroidal anti-inflammatory cream. Despite the prolonged use of the drug, she said patients with lichen sclerosus usually do not present with side effects like discoloration or thinning of skin.
“You may achieve control or remission, but we don’t stop treatment completely because we know the natural history of the disease is to have flares and recurrence.”
If left untreated, the condition can lead to atrophy, scarring, and distortion of the genital anatomy and, in some cases, develop into squamous cell carcinoma.
“The fact that you can do a topical cream intervention and prevent cancer is huge,” said Dr. Winter.
She said open discussions surrounding genital health led by primary care providers can destigmatize conditions like lichen sclerosus and promote early detection and management.
“We need to foster an environment where individuals feel comfortable discussing their symptoms openly,” Dr. Winter said. “Increased awareness can pave the way for early detection, which is crucial for managing the condition effectively.”
The experts included in the story reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Ashley Winter, MD, remembers the first time she Googled the skin condition lichen sclerosus. Most of the websites listed the autoimmune condition as a rare disease.
In the realm of genital health, some conditions remain shrouded in silence and consequently are more likely to go undercounted and underdiagnosed, said Dr. Winter, a urologist based in Los Angeles.
“I truly believe that we just miss the diagnosis a vast majority of the time because there isn’t enough training on [detecting] it,” said Dr. Winter.
, according to the US National Institutes of Health. The condition also more commonly occurs among women, and symptoms include hypopigmentation, itching, pain, changes in skin appearance, and skin atrophy.
“Most cases [affect the] genital [area] only, so often patients don’t bring it up because they don’t want to be examined,” said Sarah Lonowski, MD, assistant professor of dermatology and codirector of the Multidisciplinary Autoimmune Skin Disease/Derm-Rheum Program at the University of Nebraska–Lincoln. “It’s a sensitive area, it’s an uncomfortable area to have examined, so it comes with a lot of emotional burden,” for patients, Dr. Lonowski said.
Receiving a lichen sclerosis diagnosis can take 5 years or longer, in part because the condition’s symptoms can lead clinicians to first make a diagnosis of a yeast infection or bacterial vaginosis, according to Christina Kraus, MD, assistant professor of dermatology at UCI Health in Irvine, California.
“There is still limited information on this condition in medical education, and it is not uncommon for clinicians who are not in dermatology or gynecology to be unfamiliar with this diagnosis,” Dr. Kraus said.
Because no medical tests are available to confirm lichen sclerosus, clinicians diagnose the condition based on the skin’s appearance and symptoms. In some cases, a skin biopsy may help differentiate it from similar rashes that occur in the genital area.
Prepubescent children and postmenopausal women are most likely to develop genital lichen sclerosis, so pediatricians and primary care physicians may be the first to see possible cases, Dr. Lonowski said.
Patients “may not mention it unless they’re asked,” Dr. Lonowski said, adding clinicians can inquire with patients about genital health, examine bothersome areas, “and refer if you’re not sure.”
Clinicians may also miss the condition during physical exams if they do not examine the vulvar skin, she said. The exact cause also remains elusive, but researchers believe genetic and hormonal factors, as well as an overactive immune response, may contribute to development of the condition.
Watch Out for Presentation
While lichen sclerosus more frequently occurs in women, men are also affected by the condition. Benjamin N. Breyer, MD, professor and chair of urology at the University of California San Francisco, said lichen sclerosus is one of the most common skin conditions he treats in his male patients.
“Advanced cases can cause urethral narrowing, which is a condition I treat commonly,” said Dr. Breyer. “Lichen sclerosus is often an underrecognized cause of pain or tearing with erections and sex in men.”
Similar to women, lichen sclerosus presents as white color changes on the skin. For men, the condition can also result in fusion of the shaft skin to the head of the penis and burying or concealment of the penis, Dr. Breyer said.
“This leads to challenges with intimacy and urination and can have extensive impacts on quality of life,” said Dr. Breyer.
For women, the skin changes often extend to the perianal area and can cause scarring, said Dr. Kraus.
“Early scarring may present as adherence of the labia minora to the labia majora or inability to fully retract the clitoral hood from the clitoris,” said Dr. Kraus.
In both men and women, lichen sclerosus and another autoimmune condition known as morphea, characterized by skin hardening and discoloration, often present together, said Dr. Lonowski.
“If you have a patient with known morphea, it’s important to ask about genital symptoms,” said Dr. Lonowski. “The association between the two is fairly strong.”
Circumcision is often the first step to help prevent chronic inflammation among male patients, said Dr. Breyer. Because lichen sclerosus is associated with an increased risk for penile cancer, “it is important to biopsy suspicious lesions,” Dr. Breyer added.
Increasing awareness of lichen sclerosus is crucial for early detection and timely intervention, said Dr. Lonowski. The first-line treatment of genital lichen sclerosus is strong topical steroid ointments to reduce inflammation. Clinicians might prescribe this treatment for use twice daily for 2-3 months and then assesses the patient on their response.
“It is fairly responsive to treatment in most cases,” said Dr. Lonowski.
Once symptoms have improved, Dr. Lonowski transitions patients to a maintenance regimen, which might include using the same steroid but only three times a week, switching to a topical with a less potent steroid dosage, or using a combination of a topical steroid and a nonsteroidal anti-inflammatory cream. Despite the prolonged use of the drug, she said patients with lichen sclerosus usually do not present with side effects like discoloration or thinning of skin.
“You may achieve control or remission, but we don’t stop treatment completely because we know the natural history of the disease is to have flares and recurrence.”
If left untreated, the condition can lead to atrophy, scarring, and distortion of the genital anatomy and, in some cases, develop into squamous cell carcinoma.
“The fact that you can do a topical cream intervention and prevent cancer is huge,” said Dr. Winter.
She said open discussions surrounding genital health led by primary care providers can destigmatize conditions like lichen sclerosus and promote early detection and management.
“We need to foster an environment where individuals feel comfortable discussing their symptoms openly,” Dr. Winter said. “Increased awareness can pave the way for early detection, which is crucial for managing the condition effectively.”
The experts included in the story reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Ashley Winter, MD, remembers the first time she Googled the skin condition lichen sclerosus. Most of the websites listed the autoimmune condition as a rare disease.
In the realm of genital health, some conditions remain shrouded in silence and consequently are more likely to go undercounted and underdiagnosed, said Dr. Winter, a urologist based in Los Angeles.
“I truly believe that we just miss the diagnosis a vast majority of the time because there isn’t enough training on [detecting] it,” said Dr. Winter.
, according to the US National Institutes of Health. The condition also more commonly occurs among women, and symptoms include hypopigmentation, itching, pain, changes in skin appearance, and skin atrophy.
“Most cases [affect the] genital [area] only, so often patients don’t bring it up because they don’t want to be examined,” said Sarah Lonowski, MD, assistant professor of dermatology and codirector of the Multidisciplinary Autoimmune Skin Disease/Derm-Rheum Program at the University of Nebraska–Lincoln. “It’s a sensitive area, it’s an uncomfortable area to have examined, so it comes with a lot of emotional burden,” for patients, Dr. Lonowski said.
Receiving a lichen sclerosis diagnosis can take 5 years or longer, in part because the condition’s symptoms can lead clinicians to first make a diagnosis of a yeast infection or bacterial vaginosis, according to Christina Kraus, MD, assistant professor of dermatology at UCI Health in Irvine, California.
“There is still limited information on this condition in medical education, and it is not uncommon for clinicians who are not in dermatology or gynecology to be unfamiliar with this diagnosis,” Dr. Kraus said.
Because no medical tests are available to confirm lichen sclerosus, clinicians diagnose the condition based on the skin’s appearance and symptoms. In some cases, a skin biopsy may help differentiate it from similar rashes that occur in the genital area.
Prepubescent children and postmenopausal women are most likely to develop genital lichen sclerosis, so pediatricians and primary care physicians may be the first to see possible cases, Dr. Lonowski said.
Patients “may not mention it unless they’re asked,” Dr. Lonowski said, adding clinicians can inquire with patients about genital health, examine bothersome areas, “and refer if you’re not sure.”
Clinicians may also miss the condition during physical exams if they do not examine the vulvar skin, she said. The exact cause also remains elusive, but researchers believe genetic and hormonal factors, as well as an overactive immune response, may contribute to development of the condition.
Watch Out for Presentation
While lichen sclerosus more frequently occurs in women, men are also affected by the condition. Benjamin N. Breyer, MD, professor and chair of urology at the University of California San Francisco, said lichen sclerosus is one of the most common skin conditions he treats in his male patients.
“Advanced cases can cause urethral narrowing, which is a condition I treat commonly,” said Dr. Breyer. “Lichen sclerosus is often an underrecognized cause of pain or tearing with erections and sex in men.”
Similar to women, lichen sclerosus presents as white color changes on the skin. For men, the condition can also result in fusion of the shaft skin to the head of the penis and burying or concealment of the penis, Dr. Breyer said.
“This leads to challenges with intimacy and urination and can have extensive impacts on quality of life,” said Dr. Breyer.
For women, the skin changes often extend to the perianal area and can cause scarring, said Dr. Kraus.
“Early scarring may present as adherence of the labia minora to the labia majora or inability to fully retract the clitoral hood from the clitoris,” said Dr. Kraus.
In both men and women, lichen sclerosus and another autoimmune condition known as morphea, characterized by skin hardening and discoloration, often present together, said Dr. Lonowski.
“If you have a patient with known morphea, it’s important to ask about genital symptoms,” said Dr. Lonowski. “The association between the two is fairly strong.”
Circumcision is often the first step to help prevent chronic inflammation among male patients, said Dr. Breyer. Because lichen sclerosus is associated with an increased risk for penile cancer, “it is important to biopsy suspicious lesions,” Dr. Breyer added.
Increasing awareness of lichen sclerosus is crucial for early detection and timely intervention, said Dr. Lonowski. The first-line treatment of genital lichen sclerosus is strong topical steroid ointments to reduce inflammation. Clinicians might prescribe this treatment for use twice daily for 2-3 months and then assesses the patient on their response.
“It is fairly responsive to treatment in most cases,” said Dr. Lonowski.
Once symptoms have improved, Dr. Lonowski transitions patients to a maintenance regimen, which might include using the same steroid but only three times a week, switching to a topical with a less potent steroid dosage, or using a combination of a topical steroid and a nonsteroidal anti-inflammatory cream. Despite the prolonged use of the drug, she said patients with lichen sclerosus usually do not present with side effects like discoloration or thinning of skin.
“You may achieve control or remission, but we don’t stop treatment completely because we know the natural history of the disease is to have flares and recurrence.”
If left untreated, the condition can lead to atrophy, scarring, and distortion of the genital anatomy and, in some cases, develop into squamous cell carcinoma.
“The fact that you can do a topical cream intervention and prevent cancer is huge,” said Dr. Winter.
She said open discussions surrounding genital health led by primary care providers can destigmatize conditions like lichen sclerosus and promote early detection and management.
“We need to foster an environment where individuals feel comfortable discussing their symptoms openly,” Dr. Winter said. “Increased awareness can pave the way for early detection, which is crucial for managing the condition effectively.”
The experts included in the story reported no relevant disclosures.
A version of this article appeared on Medscape.com.
An Ethical Analysis of Treatment of an Active-Duty Service Member With Limited Follow-up
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
PRACTICE POINTS
- Dermatologic conditions are among the most common concerns reported by active-duty service members.
- The unique considerations of deployments are important for dermatologists to consider in the treatment of skin disease.
A 74-year-old White male presented with a 1-year history of depigmented patches on the hands, arms, and face, as well as white eyelashes and eyebrows
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
Botanical Briefs: Neem Oil (Azadirachta indica)
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.
Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Practice Points
- Neem is a traditional herb with various bioactivities, such as melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.
- Neem should be used with caution as a remedy because of its skin-lightening properties, which are attributed to melanogenesis-inhibitory activity via tyrosinase inhibition.
- Chemical leukoderma should be included in the differential diagnosis when a patient presents with a hypopigmented rash after topical use of neem products.
Analysis of Online Diet Recommendations for Vitiligo
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.
From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).
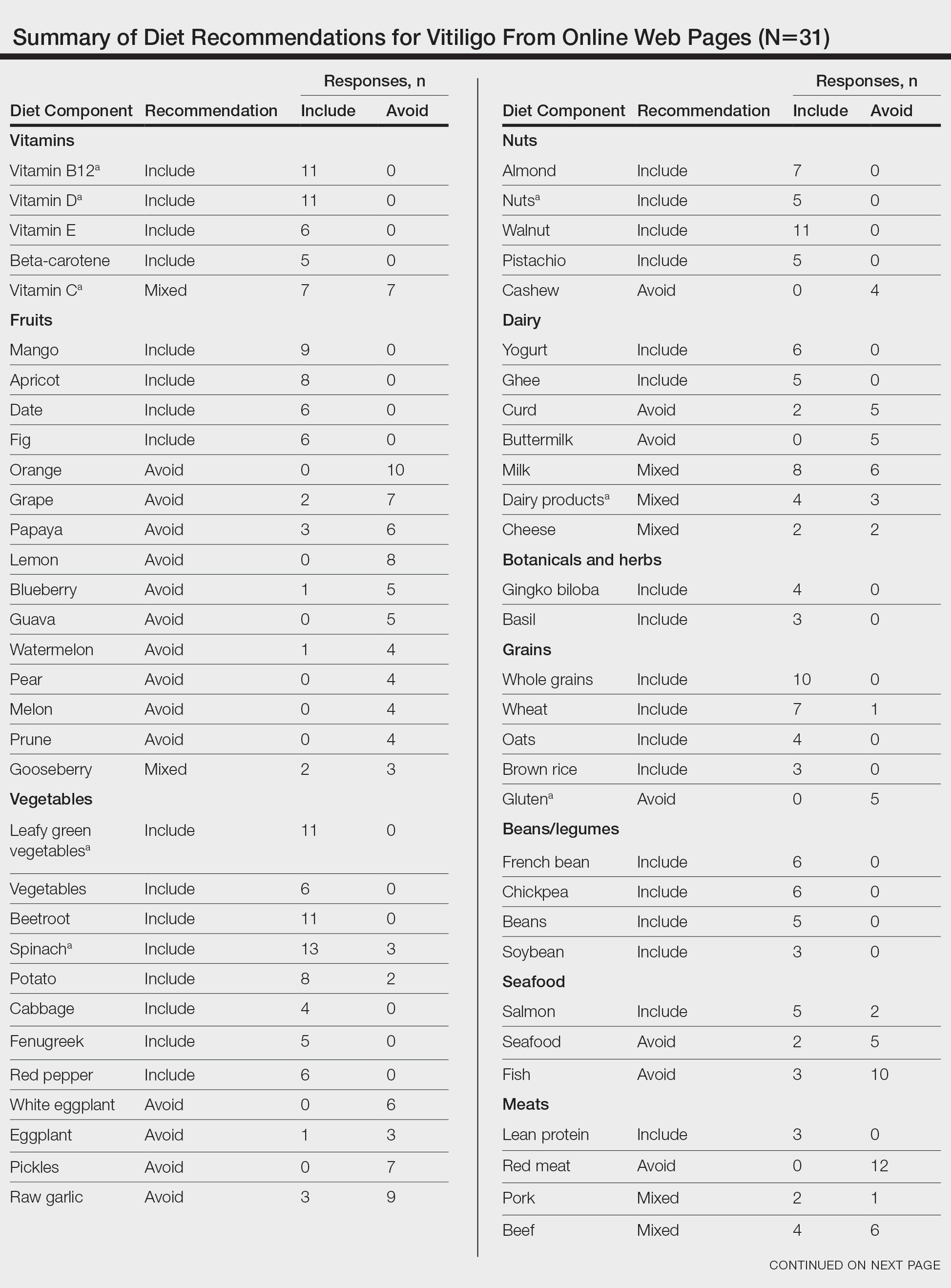
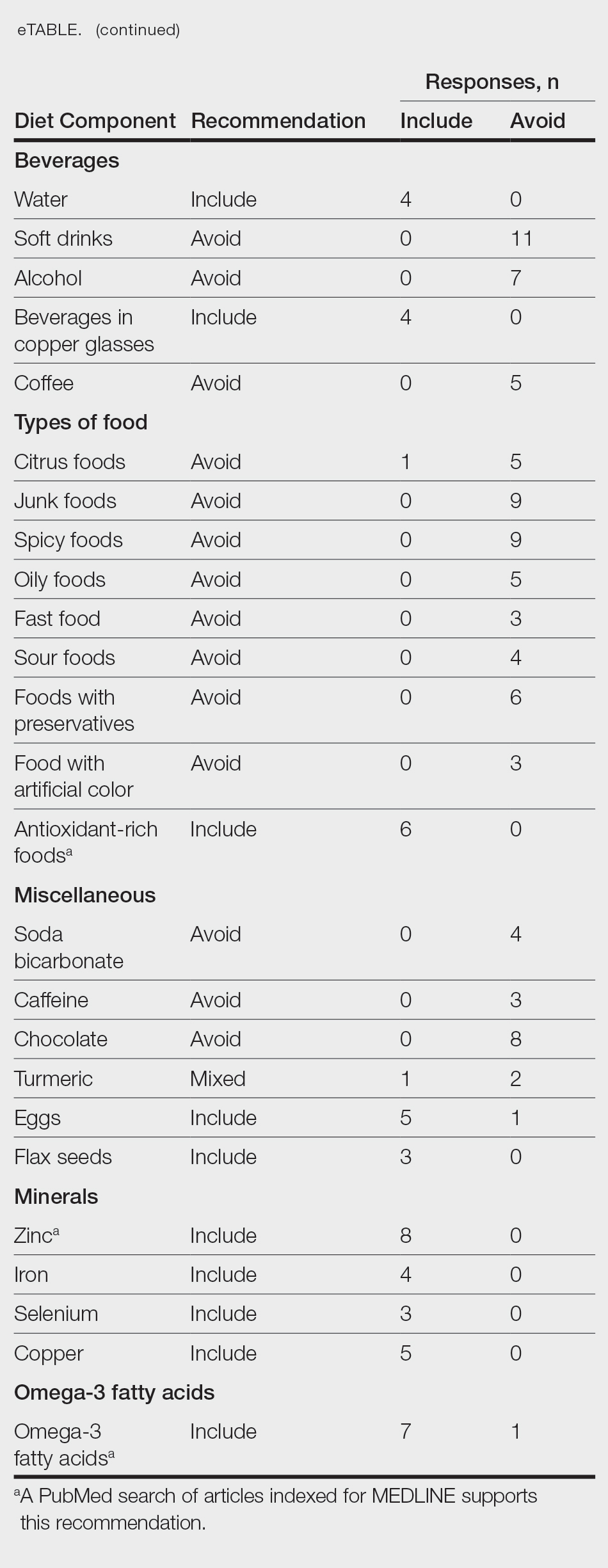
For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Practice Points
- There are numerous online dietary and supplement recommendations that claim to impact vitiligo but most are not authored by medical professionals or dermatologists.
- Scientific evidence supporting specific dietary and supplement recommendations for vitiligo is limited.
- Current preliminary data support the potential recommendation for dietary supplementation with vitamin D, vitamin B12, zinc, and omega-3 fatty acids.
US Dermatologic Drug Approvals Rose Between 2012 and 2022
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.