User login
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
GVHD raises vitiligo risk in transplant recipients
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
FROM JAMA DERMATOLOGY
Dietary supplements may play a role in managing vitiligo
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
FROM IDS 2023
Hyperpigmented Flexural Plaques, Hypohidrosis, and Hypotrichosis
The Diagnosis: Lelis Syndrome
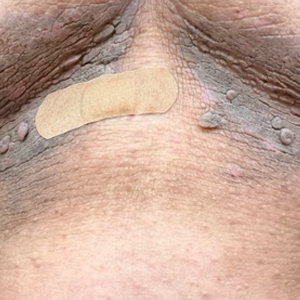
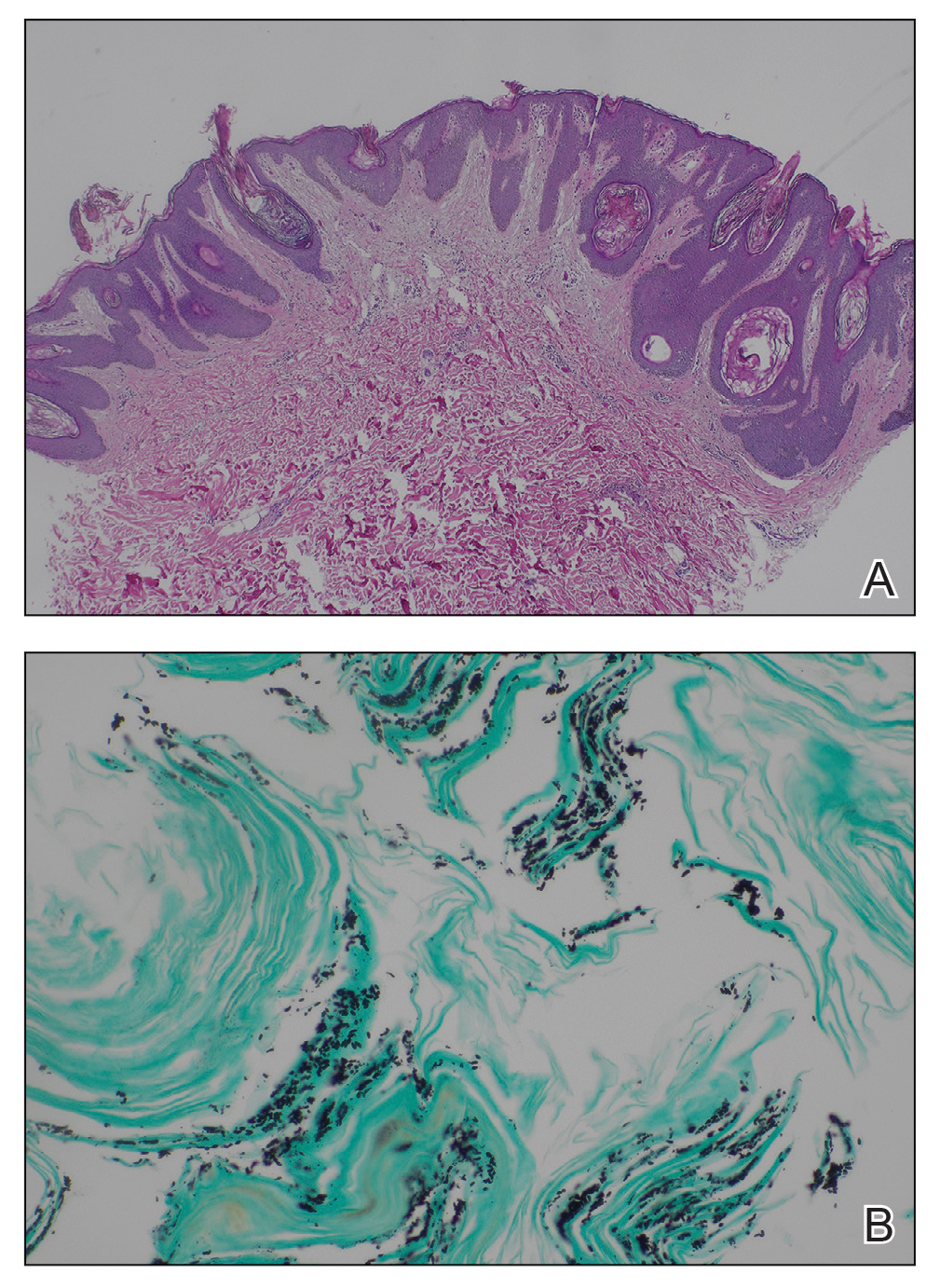
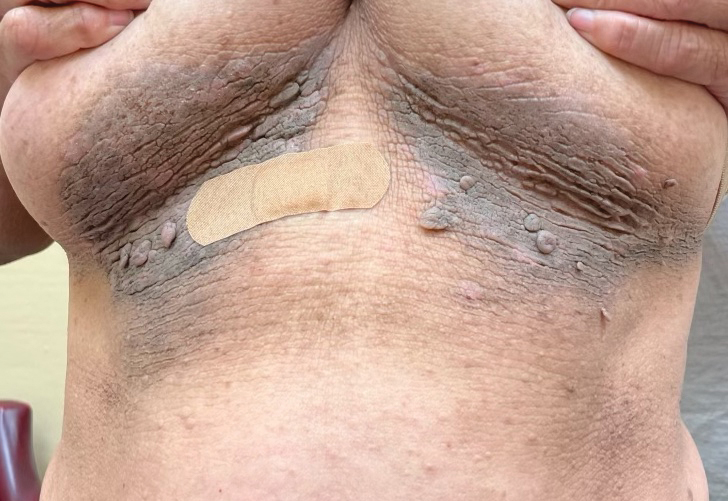
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.
Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.

Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.

Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
A 61-year-old woman with a history of hypohidrosis and deafness presented with a pruritic rash on the neck and antecubital fossae of several years’ duration. Prior treatment with topical corticosteroids failed to resolve the rash. Physical examination revealed thick, velvety, hyperpigmented plaques on the inframammary folds, axillae, groin, posterior neck, and antecubital fossae with lichenification of the latter 2 areas. Many pedunculated papules were seen on the face, chest, shoulders, and trunk, as well as diffuse hair thinning, particularly of the frontal and vertex scalp. Eyebrows, eyelashes, and axillary hair were absent. Two 5-mm punch biopsies of the antecubital fossa and inframammary fold were obtained for histopathologic analysis.
Parent concerns a factor when treating eczema in children with darker skin types
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
AT SOC 2023
Review finds no CV or VTE risk signal with use of JAK inhibitors for skin indications
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
FROM JAMA DERMATOLOGY
Cysteamine and melasma
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Suture Selection to Minimize Postoperative Postinflammatory Hyperpigmentation in Patients With Skin of Color During Mohs Micrographic Surgery
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
Treatment options for vitiligo reviewed
CARLSBAD, CALIF. – According to Delphine J. Lee, MD, PhD, some patients report that their dermatologists tell them there are no effective treatments for vitiligo.
However, this is not supported by the ongoing level of research on vitiligo, with more than 100 randomized controlled trials published over the last 5 years, Dr. Lee, chief of dermatology at Harbor-UCLA Medical Center, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery. And, in 2022, ruxolitinib cream became the first FDA-approved treatment for vitiligo. “There’s a lot of research happening now, and I’m pleased to say that despite the fact that some of these medications are not all brand new and exciting, they’re still new in that we have new evidence for them,” she said. “Of the 100 randomized, controlled trials, UV therapy remains a strong part of our armamentarium.”
Stabilizing disease
Dr. Lee underscored the importance of stabilizing existing vitiligo and arresting progressive disease, which may be indicated by four key signs: koebnerization; trichrome lesions; inflammation, which can appear as erythema, scaling, and pruritus; and confetti-like macules that are typically 1 mm to 5 mm in size. Key principles of vitiligo treatment are to stop immune destruction and to stimulate melanocyte differentiation, migration, and melanin production, which is “probably why phototherapy is so important and helpful,” she said.
Managing patients’ expectations is also important, added Dr. Lee, who shows patients photos from published clinical trials “so they can see what excellent repigmentation really means.”
Dexamethasone vs. mycophenolate
In a randomized, controlled trial published in 2021, researchers compared dexamethasone oral mini-pulse (OMP), 2.5 mg, on two successive days a week, with oral mycophenolate mofetil, 500 mg b.i.d., up to 2 g every day, for 180 days as a stabilizing treatment for patients with progressive, nonsegmental vitiligo, with 90 days of treatment-free follow-up. Assessments included the vitiligo disease activity (VIDA) score, the number of new lesions in the past 30 days, and the Vitiligo Area Scoring Index (VASI). Arrest of disease progression was defined as the absence of any new lesions in the previous 30 days.
Over the treatment and follow-up period, both groups showed a significant trend for reduction in VIDA and in the number of new lesions in the previous 30 days, compared with baseline (P < .001). The difference between VASI at baseline and VASI at 180 and at 270 days was not significant in both groups.
Adverse side effects reported with dexamethasone included acne, weight gain, headache, insomnia, and menstrual irregularity. “The misconception is that because we only give patients a tiny dose of steroids – 2.5 mg two days per week – that they aren’t going to have any side effects,” Dr. Lee commented. “But in fact, they do.” The most common side effects with mycophenolate were nausea and diarrhea. Two patients on mycophenolate discontinued treatment: one for leukopenia and one for transaminitis, but both conditions resolved after treatment was stopped.
The researchers concluded that both dexamethasone OMP and mycophenolate mofetil halt actively spreading vitiligo. “Relapse occurred earlier with mycophenolate, and the relapse rate was higher than with dexamethasone OMP, but this was not statistically significant,” said Dr. Lee, who also leads an immunology research team at The Lundquist Institute at Harbor-UCLA Medical Center.
Other vitiligo treatment options she discussed included the following:
Betamethasone OMP and oral azathioprine. In a comparative study, researchers compared betamethasone OMP with oral azathioprine in arresting disease progression and inducing repigmentation in adults with vitiligo. Significantly more patients in the betamethasone OMP group achieved arrest of progression at 2 months than those in the azathioprine group, but at 6 months the difference was not significant. At 6 months, of the 19 patients who completed 6 months of betamethasone OMP, 2, 2, and 9 patients had more than 20%, 10%-20%, and 5%-10% repigmentation, respectively; and of the 18 patients who completed 6 months of azathioprine, 2 patients had 10%-20% repigmentation, with the remaining patients having no repigmentation or less than 5% repigmentation.
One patient in the azathioprine group developed acute pancreatitis but none developed transaminitis or leukopenia. “Azathioprine is another agent to add to our toolbox,” Dr. Lee said of the study findings. “Both betamethasone OMP and daily azathioprine are effective” in halting disease progression.
Low-dose cyclosporine. In a comparative study, 50 patients with active vitiligo were randomized into two groups: 25 to dexamethasone OMP 2.5 mg on two consecutive days/week for 4 months, and 25 to cyclosporine 3 mg/kg per day for 4 months, stopped treatment, and were then followed up for another 2 months. After 6 months, 84% of patients in the dexamethasone OMP group and 88% of patients in the cyclosporine group achieved arrest of disease progression (P = 1.00), but the mean time to achieve that endpoint was shorter for those in the cyclosporine group, compared with those in the dexamethasone OMP group (a mean of 3.92 weeks vs. 4.12 weeks, respectively; P = .01).
The list of adverse side effects for cyclosporine was “quite lengthy compared to the usual you would expect for dexamethasone,” said Dr. Lee, who was not involved with the study. “This is something we want to take seriously and discuss with our patients. Still, I would say that low-dose cyclosporine is another possibility to add to our toolbox.”
Phototherapy combined with polypodium leucotomos. Dr. Lee highlighted a randomized, controlled trial in which 21 patients with generalized vitiligo received narrow band (NB)-UVB phototherapy plus polypodium leucotomos extract (480 mg b.i.d.) and 21 patients received NB-UVB phototherapy plus placebo. After 6 months of treatment, patients in the NB-UVB plus oral polypodium leucotomos extract group had a better response rate, compared with those in the NB-UVB plus placebo group (47.8% vs. 22%). “We know from studies of polypodium leucotomos that it seems to have an impact on adaptive immunity as well as helps to decrease oxidative stress, so that may help with melanocyte stability in vitiligo,” said Dr. Lee, who was not affiliated with the study. “As with all treatments, the head and neck is very responsive to this combination treatment. The next most responsive area would be the trunk, followed by the extremities, and hands, and feet.”
Topical treatments
What about topical options for vitiligo? In a randomized, double-blind, comparative study, researchers evaluated the efficacy and safety of combination treatment with 308-nm excimer light and topical calcipotriol or topical clobetasol ointment for acral vitiligo. Combination treatment (excimer light and topical medication) was applied in the first 12 weeks, followed by topical medication alone for 12 weeks. Calcipotriol 0.005% ointment was applied on one hand vs. clobetasol propionate 0.05% ointment on the other for 24 weeks.
Of the hands treated with excimer light and calcipotriol, 7.7% achieved excellent repigmentation at the end of the combination treatment period and 23% achieved good to excellent improvement after 12 weeks of calcipotriol monotherapy. More than 85% and 77% of the hands treated with calcipotriol-based and clobetasol-based regimens showed some repigmentation at the end of the study, respectively (P < .05). However, no significant difference was found between the two treatments. “The evaluation from study participants was similar in that they felt that there was clearly a difference from baseline, but there was no difference across the two-hand therapy,” Dr. Lee said.
Adverse side effects included the development of blisters in some of patients who received clobetasol. “The take-home here is that you get excellent repigmentation with calcipotriol, though it’s a small percentage, 7.7%,” Dr. Lee said. “No excellent repigmentation was observed with excimer light and topical clobetasol. These data support two possible topical regimens that could be added to phototherapy or excimer light therapy to improve results.”
In another study of 42 patients, researchers compared twice-daily tacrolimus 0.1% ointment with vehicle for facial vitiligo through 24 weeks of intervention and 24 weeks of follow-up. The researchers defined treatment success as a change of 75% or greater in repigmentation of the target lesion between baseline and week 24, as measured by computer imaging software.
They found that 65% of tacrolimus-treated patients achieved therapeutic success, compared with none of the vehicle-treated patients at week 24 (P < .0001). “Tacrolimus is thought to be an old drug, but it does deserve to have continued proper study based on much anecdotal evidence I hear,” Dr. Lee said. “There was also efficacy over vehicle during the 24 weeks of follow-up. I find that tacrolimus works very well on the face. I’ve had very good results in children.”
Another topical option is the cream formulation of the JAK inhibitor ruxolitinib (Opzelura), approved in 2022 for the treatment of nonsegmental vitiligo in patients ages 12 and older, the first FDA-approved treatment for vitiligo. “As with the tacrolimus study, there are patients who achieve 100% repigmentation [with ruxolitinib], but others who may not,” Dr. Lee said. In addition, she noted that the combination of JAK inhibitors with phototherapy is emerging as another possible treatment choice, referring to a recently published systematic review suggesting that concurrent UVB phototherapy appears to improve efficacy of JAK inhibitors for vitiligo.
Dr. Lee reported having no relevant financial disclosures.
CARLSBAD, CALIF. – According to Delphine J. Lee, MD, PhD, some patients report that their dermatologists tell them there are no effective treatments for vitiligo.
However, this is not supported by the ongoing level of research on vitiligo, with more than 100 randomized controlled trials published over the last 5 years, Dr. Lee, chief of dermatology at Harbor-UCLA Medical Center, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery. And, in 2022, ruxolitinib cream became the first FDA-approved treatment for vitiligo. “There’s a lot of research happening now, and I’m pleased to say that despite the fact that some of these medications are not all brand new and exciting, they’re still new in that we have new evidence for them,” she said. “Of the 100 randomized, controlled trials, UV therapy remains a strong part of our armamentarium.”
Stabilizing disease
Dr. Lee underscored the importance of stabilizing existing vitiligo and arresting progressive disease, which may be indicated by four key signs: koebnerization; trichrome lesions; inflammation, which can appear as erythema, scaling, and pruritus; and confetti-like macules that are typically 1 mm to 5 mm in size. Key principles of vitiligo treatment are to stop immune destruction and to stimulate melanocyte differentiation, migration, and melanin production, which is “probably why phototherapy is so important and helpful,” she said.
Managing patients’ expectations is also important, added Dr. Lee, who shows patients photos from published clinical trials “so they can see what excellent repigmentation really means.”
Dexamethasone vs. mycophenolate
In a randomized, controlled trial published in 2021, researchers compared dexamethasone oral mini-pulse (OMP), 2.5 mg, on two successive days a week, with oral mycophenolate mofetil, 500 mg b.i.d., up to 2 g every day, for 180 days as a stabilizing treatment for patients with progressive, nonsegmental vitiligo, with 90 days of treatment-free follow-up. Assessments included the vitiligo disease activity (VIDA) score, the number of new lesions in the past 30 days, and the Vitiligo Area Scoring Index (VASI). Arrest of disease progression was defined as the absence of any new lesions in the previous 30 days.
Over the treatment and follow-up period, both groups showed a significant trend for reduction in VIDA and in the number of new lesions in the previous 30 days, compared with baseline (P < .001). The difference between VASI at baseline and VASI at 180 and at 270 days was not significant in both groups.
Adverse side effects reported with dexamethasone included acne, weight gain, headache, insomnia, and menstrual irregularity. “The misconception is that because we only give patients a tiny dose of steroids – 2.5 mg two days per week – that they aren’t going to have any side effects,” Dr. Lee commented. “But in fact, they do.” The most common side effects with mycophenolate were nausea and diarrhea. Two patients on mycophenolate discontinued treatment: one for leukopenia and one for transaminitis, but both conditions resolved after treatment was stopped.
The researchers concluded that both dexamethasone OMP and mycophenolate mofetil halt actively spreading vitiligo. “Relapse occurred earlier with mycophenolate, and the relapse rate was higher than with dexamethasone OMP, but this was not statistically significant,” said Dr. Lee, who also leads an immunology research team at The Lundquist Institute at Harbor-UCLA Medical Center.
Other vitiligo treatment options she discussed included the following:
Betamethasone OMP and oral azathioprine. In a comparative study, researchers compared betamethasone OMP with oral azathioprine in arresting disease progression and inducing repigmentation in adults with vitiligo. Significantly more patients in the betamethasone OMP group achieved arrest of progression at 2 months than those in the azathioprine group, but at 6 months the difference was not significant. At 6 months, of the 19 patients who completed 6 months of betamethasone OMP, 2, 2, and 9 patients had more than 20%, 10%-20%, and 5%-10% repigmentation, respectively; and of the 18 patients who completed 6 months of azathioprine, 2 patients had 10%-20% repigmentation, with the remaining patients having no repigmentation or less than 5% repigmentation.
One patient in the azathioprine group developed acute pancreatitis but none developed transaminitis or leukopenia. “Azathioprine is another agent to add to our toolbox,” Dr. Lee said of the study findings. “Both betamethasone OMP and daily azathioprine are effective” in halting disease progression.
Low-dose cyclosporine. In a comparative study, 50 patients with active vitiligo were randomized into two groups: 25 to dexamethasone OMP 2.5 mg on two consecutive days/week for 4 months, and 25 to cyclosporine 3 mg/kg per day for 4 months, stopped treatment, and were then followed up for another 2 months. After 6 months, 84% of patients in the dexamethasone OMP group and 88% of patients in the cyclosporine group achieved arrest of disease progression (P = 1.00), but the mean time to achieve that endpoint was shorter for those in the cyclosporine group, compared with those in the dexamethasone OMP group (a mean of 3.92 weeks vs. 4.12 weeks, respectively; P = .01).
The list of adverse side effects for cyclosporine was “quite lengthy compared to the usual you would expect for dexamethasone,” said Dr. Lee, who was not involved with the study. “This is something we want to take seriously and discuss with our patients. Still, I would say that low-dose cyclosporine is another possibility to add to our toolbox.”
Phototherapy combined with polypodium leucotomos. Dr. Lee highlighted a randomized, controlled trial in which 21 patients with generalized vitiligo received narrow band (NB)-UVB phototherapy plus polypodium leucotomos extract (480 mg b.i.d.) and 21 patients received NB-UVB phototherapy plus placebo. After 6 months of treatment, patients in the NB-UVB plus oral polypodium leucotomos extract group had a better response rate, compared with those in the NB-UVB plus placebo group (47.8% vs. 22%). “We know from studies of polypodium leucotomos that it seems to have an impact on adaptive immunity as well as helps to decrease oxidative stress, so that may help with melanocyte stability in vitiligo,” said Dr. Lee, who was not affiliated with the study. “As with all treatments, the head and neck is very responsive to this combination treatment. The next most responsive area would be the trunk, followed by the extremities, and hands, and feet.”
Topical treatments
What about topical options for vitiligo? In a randomized, double-blind, comparative study, researchers evaluated the efficacy and safety of combination treatment with 308-nm excimer light and topical calcipotriol or topical clobetasol ointment for acral vitiligo. Combination treatment (excimer light and topical medication) was applied in the first 12 weeks, followed by topical medication alone for 12 weeks. Calcipotriol 0.005% ointment was applied on one hand vs. clobetasol propionate 0.05% ointment on the other for 24 weeks.
Of the hands treated with excimer light and calcipotriol, 7.7% achieved excellent repigmentation at the end of the combination treatment period and 23% achieved good to excellent improvement after 12 weeks of calcipotriol monotherapy. More than 85% and 77% of the hands treated with calcipotriol-based and clobetasol-based regimens showed some repigmentation at the end of the study, respectively (P < .05). However, no significant difference was found between the two treatments. “The evaluation from study participants was similar in that they felt that there was clearly a difference from baseline, but there was no difference across the two-hand therapy,” Dr. Lee said.
Adverse side effects included the development of blisters in some of patients who received clobetasol. “The take-home here is that you get excellent repigmentation with calcipotriol, though it’s a small percentage, 7.7%,” Dr. Lee said. “No excellent repigmentation was observed with excimer light and topical clobetasol. These data support two possible topical regimens that could be added to phototherapy or excimer light therapy to improve results.”
In another study of 42 patients, researchers compared twice-daily tacrolimus 0.1% ointment with vehicle for facial vitiligo through 24 weeks of intervention and 24 weeks of follow-up. The researchers defined treatment success as a change of 75% or greater in repigmentation of the target lesion between baseline and week 24, as measured by computer imaging software.
They found that 65% of tacrolimus-treated patients achieved therapeutic success, compared with none of the vehicle-treated patients at week 24 (P < .0001). “Tacrolimus is thought to be an old drug, but it does deserve to have continued proper study based on much anecdotal evidence I hear,” Dr. Lee said. “There was also efficacy over vehicle during the 24 weeks of follow-up. I find that tacrolimus works very well on the face. I’ve had very good results in children.”
Another topical option is the cream formulation of the JAK inhibitor ruxolitinib (Opzelura), approved in 2022 for the treatment of nonsegmental vitiligo in patients ages 12 and older, the first FDA-approved treatment for vitiligo. “As with the tacrolimus study, there are patients who achieve 100% repigmentation [with ruxolitinib], but others who may not,” Dr. Lee said. In addition, she noted that the combination of JAK inhibitors with phototherapy is emerging as another possible treatment choice, referring to a recently published systematic review suggesting that concurrent UVB phototherapy appears to improve efficacy of JAK inhibitors for vitiligo.
Dr. Lee reported having no relevant financial disclosures.
CARLSBAD, CALIF. – According to Delphine J. Lee, MD, PhD, some patients report that their dermatologists tell them there are no effective treatments for vitiligo.
However, this is not supported by the ongoing level of research on vitiligo, with more than 100 randomized controlled trials published over the last 5 years, Dr. Lee, chief of dermatology at Harbor-UCLA Medical Center, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery. And, in 2022, ruxolitinib cream became the first FDA-approved treatment for vitiligo. “There’s a lot of research happening now, and I’m pleased to say that despite the fact that some of these medications are not all brand new and exciting, they’re still new in that we have new evidence for them,” she said. “Of the 100 randomized, controlled trials, UV therapy remains a strong part of our armamentarium.”
Stabilizing disease
Dr. Lee underscored the importance of stabilizing existing vitiligo and arresting progressive disease, which may be indicated by four key signs: koebnerization; trichrome lesions; inflammation, which can appear as erythema, scaling, and pruritus; and confetti-like macules that are typically 1 mm to 5 mm in size. Key principles of vitiligo treatment are to stop immune destruction and to stimulate melanocyte differentiation, migration, and melanin production, which is “probably why phototherapy is so important and helpful,” she said.
Managing patients’ expectations is also important, added Dr. Lee, who shows patients photos from published clinical trials “so they can see what excellent repigmentation really means.”
Dexamethasone vs. mycophenolate
In a randomized, controlled trial published in 2021, researchers compared dexamethasone oral mini-pulse (OMP), 2.5 mg, on two successive days a week, with oral mycophenolate mofetil, 500 mg b.i.d., up to 2 g every day, for 180 days as a stabilizing treatment for patients with progressive, nonsegmental vitiligo, with 90 days of treatment-free follow-up. Assessments included the vitiligo disease activity (VIDA) score, the number of new lesions in the past 30 days, and the Vitiligo Area Scoring Index (VASI). Arrest of disease progression was defined as the absence of any new lesions in the previous 30 days.
Over the treatment and follow-up period, both groups showed a significant trend for reduction in VIDA and in the number of new lesions in the previous 30 days, compared with baseline (P < .001). The difference between VASI at baseline and VASI at 180 and at 270 days was not significant in both groups.
Adverse side effects reported with dexamethasone included acne, weight gain, headache, insomnia, and menstrual irregularity. “The misconception is that because we only give patients a tiny dose of steroids – 2.5 mg two days per week – that they aren’t going to have any side effects,” Dr. Lee commented. “But in fact, they do.” The most common side effects with mycophenolate were nausea and diarrhea. Two patients on mycophenolate discontinued treatment: one for leukopenia and one for transaminitis, but both conditions resolved after treatment was stopped.
The researchers concluded that both dexamethasone OMP and mycophenolate mofetil halt actively spreading vitiligo. “Relapse occurred earlier with mycophenolate, and the relapse rate was higher than with dexamethasone OMP, but this was not statistically significant,” said Dr. Lee, who also leads an immunology research team at The Lundquist Institute at Harbor-UCLA Medical Center.
Other vitiligo treatment options she discussed included the following:
Betamethasone OMP and oral azathioprine. In a comparative study, researchers compared betamethasone OMP with oral azathioprine in arresting disease progression and inducing repigmentation in adults with vitiligo. Significantly more patients in the betamethasone OMP group achieved arrest of progression at 2 months than those in the azathioprine group, but at 6 months the difference was not significant. At 6 months, of the 19 patients who completed 6 months of betamethasone OMP, 2, 2, and 9 patients had more than 20%, 10%-20%, and 5%-10% repigmentation, respectively; and of the 18 patients who completed 6 months of azathioprine, 2 patients had 10%-20% repigmentation, with the remaining patients having no repigmentation or less than 5% repigmentation.
One patient in the azathioprine group developed acute pancreatitis but none developed transaminitis or leukopenia. “Azathioprine is another agent to add to our toolbox,” Dr. Lee said of the study findings. “Both betamethasone OMP and daily azathioprine are effective” in halting disease progression.
Low-dose cyclosporine. In a comparative study, 50 patients with active vitiligo were randomized into two groups: 25 to dexamethasone OMP 2.5 mg on two consecutive days/week for 4 months, and 25 to cyclosporine 3 mg/kg per day for 4 months, stopped treatment, and were then followed up for another 2 months. After 6 months, 84% of patients in the dexamethasone OMP group and 88% of patients in the cyclosporine group achieved arrest of disease progression (P = 1.00), but the mean time to achieve that endpoint was shorter for those in the cyclosporine group, compared with those in the dexamethasone OMP group (a mean of 3.92 weeks vs. 4.12 weeks, respectively; P = .01).
The list of adverse side effects for cyclosporine was “quite lengthy compared to the usual you would expect for dexamethasone,” said Dr. Lee, who was not involved with the study. “This is something we want to take seriously and discuss with our patients. Still, I would say that low-dose cyclosporine is another possibility to add to our toolbox.”
Phototherapy combined with polypodium leucotomos. Dr. Lee highlighted a randomized, controlled trial in which 21 patients with generalized vitiligo received narrow band (NB)-UVB phototherapy plus polypodium leucotomos extract (480 mg b.i.d.) and 21 patients received NB-UVB phototherapy plus placebo. After 6 months of treatment, patients in the NB-UVB plus oral polypodium leucotomos extract group had a better response rate, compared with those in the NB-UVB plus placebo group (47.8% vs. 22%). “We know from studies of polypodium leucotomos that it seems to have an impact on adaptive immunity as well as helps to decrease oxidative stress, so that may help with melanocyte stability in vitiligo,” said Dr. Lee, who was not affiliated with the study. “As with all treatments, the head and neck is very responsive to this combination treatment. The next most responsive area would be the trunk, followed by the extremities, and hands, and feet.”
Topical treatments
What about topical options for vitiligo? In a randomized, double-blind, comparative study, researchers evaluated the efficacy and safety of combination treatment with 308-nm excimer light and topical calcipotriol or topical clobetasol ointment for acral vitiligo. Combination treatment (excimer light and topical medication) was applied in the first 12 weeks, followed by topical medication alone for 12 weeks. Calcipotriol 0.005% ointment was applied on one hand vs. clobetasol propionate 0.05% ointment on the other for 24 weeks.
Of the hands treated with excimer light and calcipotriol, 7.7% achieved excellent repigmentation at the end of the combination treatment period and 23% achieved good to excellent improvement after 12 weeks of calcipotriol monotherapy. More than 85% and 77% of the hands treated with calcipotriol-based and clobetasol-based regimens showed some repigmentation at the end of the study, respectively (P < .05). However, no significant difference was found between the two treatments. “The evaluation from study participants was similar in that they felt that there was clearly a difference from baseline, but there was no difference across the two-hand therapy,” Dr. Lee said.
Adverse side effects included the development of blisters in some of patients who received clobetasol. “The take-home here is that you get excellent repigmentation with calcipotriol, though it’s a small percentage, 7.7%,” Dr. Lee said. “No excellent repigmentation was observed with excimer light and topical clobetasol. These data support two possible topical regimens that could be added to phototherapy or excimer light therapy to improve results.”
In another study of 42 patients, researchers compared twice-daily tacrolimus 0.1% ointment with vehicle for facial vitiligo through 24 weeks of intervention and 24 weeks of follow-up. The researchers defined treatment success as a change of 75% or greater in repigmentation of the target lesion between baseline and week 24, as measured by computer imaging software.
They found that 65% of tacrolimus-treated patients achieved therapeutic success, compared with none of the vehicle-treated patients at week 24 (P < .0001). “Tacrolimus is thought to be an old drug, but it does deserve to have continued proper study based on much anecdotal evidence I hear,” Dr. Lee said. “There was also efficacy over vehicle during the 24 weeks of follow-up. I find that tacrolimus works very well on the face. I’ve had very good results in children.”
Another topical option is the cream formulation of the JAK inhibitor ruxolitinib (Opzelura), approved in 2022 for the treatment of nonsegmental vitiligo in patients ages 12 and older, the first FDA-approved treatment for vitiligo. “As with the tacrolimus study, there are patients who achieve 100% repigmentation [with ruxolitinib], but others who may not,” Dr. Lee said. In addition, she noted that the combination of JAK inhibitors with phototherapy is emerging as another possible treatment choice, referring to a recently published systematic review suggesting that concurrent UVB phototherapy appears to improve efficacy of JAK inhibitors for vitiligo.
Dr. Lee reported having no relevant financial disclosures.
AT CALDERM 2023
Analysis spotlights economic burden of vitiligo in the U.S.
TOPLINE:
METHODOLOGY:
- No published studies have quantified the medical costs and health care resource utilization (HCRU) among patients with vitiligo in the United States, compared with the general population.
- Drawing from the Merative MarketScan Commercial Claims and Encounters database, researchers reviewed the records of 49,512 patients diagnosed with vitiligo between Jan. 1, 2008, and Dec. 31, 2020, and those of 99,024 matched control persons who did not have vitiligo.
- Costs were in 2021 dollars during a 1-year postindex period. The student t test and chi square analysis were used to determine P values.
TAKEAWAY:
- In both cohorts, the median age of patients was 43 years, 79.2% were female, and most (39%) were from the southern region of the United States.
- All-cause total health care costs for patients with vitiligo were significantly higher than those of matched controls ($15,551 vs. $7,735; P < .0001).
- Similarly, medical costs for patients with vitiligo were significantly higher than those of control persons ($11,953 vs. $5,722), as were pharmacy costs ($3,598 vs. $2,014; P < .001 for both associations).
- A significantly greater proportion of patients with vitiligo had higher all-cause HCRU, compared with matched control persons. That included at least one ED visit (17.5% vs 13.4%), at least one inpatient visit (12.9% vs 6.8%), and at least one outpatient visit (99.8% vs. 88.3%; P < .0001 for all associations).
IN PRACTICE:
“These findings reveal an unmet need for cost-effective treatments and highlight the importance of fully identifying the drivers of economic burden for patients with vitiligo,” the authors concluded.
SOURCE:
Khaled Ezzedine, MD, PhD, of the department of dermatology at the Henri Mondor University Hospital, Créteil, France, led the study, which was published in the Journal of Investigative Dermatology.
LIMITATIONS:
The investigators did not evaluate indirect medical costs of vitiligo, such as work productivity, early retirement, and lost opportunities. Also, the results may not be generalizable to populations outside of the United States.
DISCLOSURES:
Dr. Ezzedine has received honoraria as a consultant for AbbVie, Incyte, La Roche–Posay, Pfizer, Pierre Fabre, Sanofi, and Viela Bio. One author is an investigator for Incyte and is a consultant for several pharmaceutical companies. Three authors are AbbVie employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- No published studies have quantified the medical costs and health care resource utilization (HCRU) among patients with vitiligo in the United States, compared with the general population.
- Drawing from the Merative MarketScan Commercial Claims and Encounters database, researchers reviewed the records of 49,512 patients diagnosed with vitiligo between Jan. 1, 2008, and Dec. 31, 2020, and those of 99,024 matched control persons who did not have vitiligo.
- Costs were in 2021 dollars during a 1-year postindex period. The student t test and chi square analysis were used to determine P values.
TAKEAWAY:
- In both cohorts, the median age of patients was 43 years, 79.2% were female, and most (39%) were from the southern region of the United States.
- All-cause total health care costs for patients with vitiligo were significantly higher than those of matched controls ($15,551 vs. $7,735; P < .0001).
- Similarly, medical costs for patients with vitiligo were significantly higher than those of control persons ($11,953 vs. $5,722), as were pharmacy costs ($3,598 vs. $2,014; P < .001 for both associations).
- A significantly greater proportion of patients with vitiligo had higher all-cause HCRU, compared with matched control persons. That included at least one ED visit (17.5% vs 13.4%), at least one inpatient visit (12.9% vs 6.8%), and at least one outpatient visit (99.8% vs. 88.3%; P < .0001 for all associations).
IN PRACTICE:
“These findings reveal an unmet need for cost-effective treatments and highlight the importance of fully identifying the drivers of economic burden for patients with vitiligo,” the authors concluded.
SOURCE:
Khaled Ezzedine, MD, PhD, of the department of dermatology at the Henri Mondor University Hospital, Créteil, France, led the study, which was published in the Journal of Investigative Dermatology.
LIMITATIONS:
The investigators did not evaluate indirect medical costs of vitiligo, such as work productivity, early retirement, and lost opportunities. Also, the results may not be generalizable to populations outside of the United States.
DISCLOSURES:
Dr. Ezzedine has received honoraria as a consultant for AbbVie, Incyte, La Roche–Posay, Pfizer, Pierre Fabre, Sanofi, and Viela Bio. One author is an investigator for Incyte and is a consultant for several pharmaceutical companies. Three authors are AbbVie employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- No published studies have quantified the medical costs and health care resource utilization (HCRU) among patients with vitiligo in the United States, compared with the general population.
- Drawing from the Merative MarketScan Commercial Claims and Encounters database, researchers reviewed the records of 49,512 patients diagnosed with vitiligo between Jan. 1, 2008, and Dec. 31, 2020, and those of 99,024 matched control persons who did not have vitiligo.
- Costs were in 2021 dollars during a 1-year postindex period. The student t test and chi square analysis were used to determine P values.
TAKEAWAY:
- In both cohorts, the median age of patients was 43 years, 79.2% were female, and most (39%) were from the southern region of the United States.
- All-cause total health care costs for patients with vitiligo were significantly higher than those of matched controls ($15,551 vs. $7,735; P < .0001).
- Similarly, medical costs for patients with vitiligo were significantly higher than those of control persons ($11,953 vs. $5,722), as were pharmacy costs ($3,598 vs. $2,014; P < .001 for both associations).
- A significantly greater proportion of patients with vitiligo had higher all-cause HCRU, compared with matched control persons. That included at least one ED visit (17.5% vs 13.4%), at least one inpatient visit (12.9% vs 6.8%), and at least one outpatient visit (99.8% vs. 88.3%; P < .0001 for all associations).
IN PRACTICE:
“These findings reveal an unmet need for cost-effective treatments and highlight the importance of fully identifying the drivers of economic burden for patients with vitiligo,” the authors concluded.
SOURCE:
Khaled Ezzedine, MD, PhD, of the department of dermatology at the Henri Mondor University Hospital, Créteil, France, led the study, which was published in the Journal of Investigative Dermatology.
LIMITATIONS:
The investigators did not evaluate indirect medical costs of vitiligo, such as work productivity, early retirement, and lost opportunities. Also, the results may not be generalizable to populations outside of the United States.
DISCLOSURES:
Dr. Ezzedine has received honoraria as a consultant for AbbVie, Incyte, La Roche–Posay, Pfizer, Pierre Fabre, Sanofi, and Viela Bio. One author is an investigator for Incyte and is a consultant for several pharmaceutical companies. Three authors are AbbVie employees.
A version of this article first appeared on Medscape.com.