User login
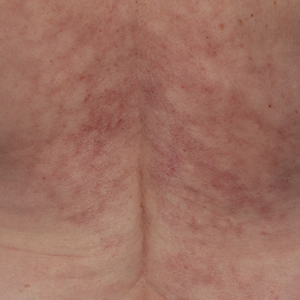
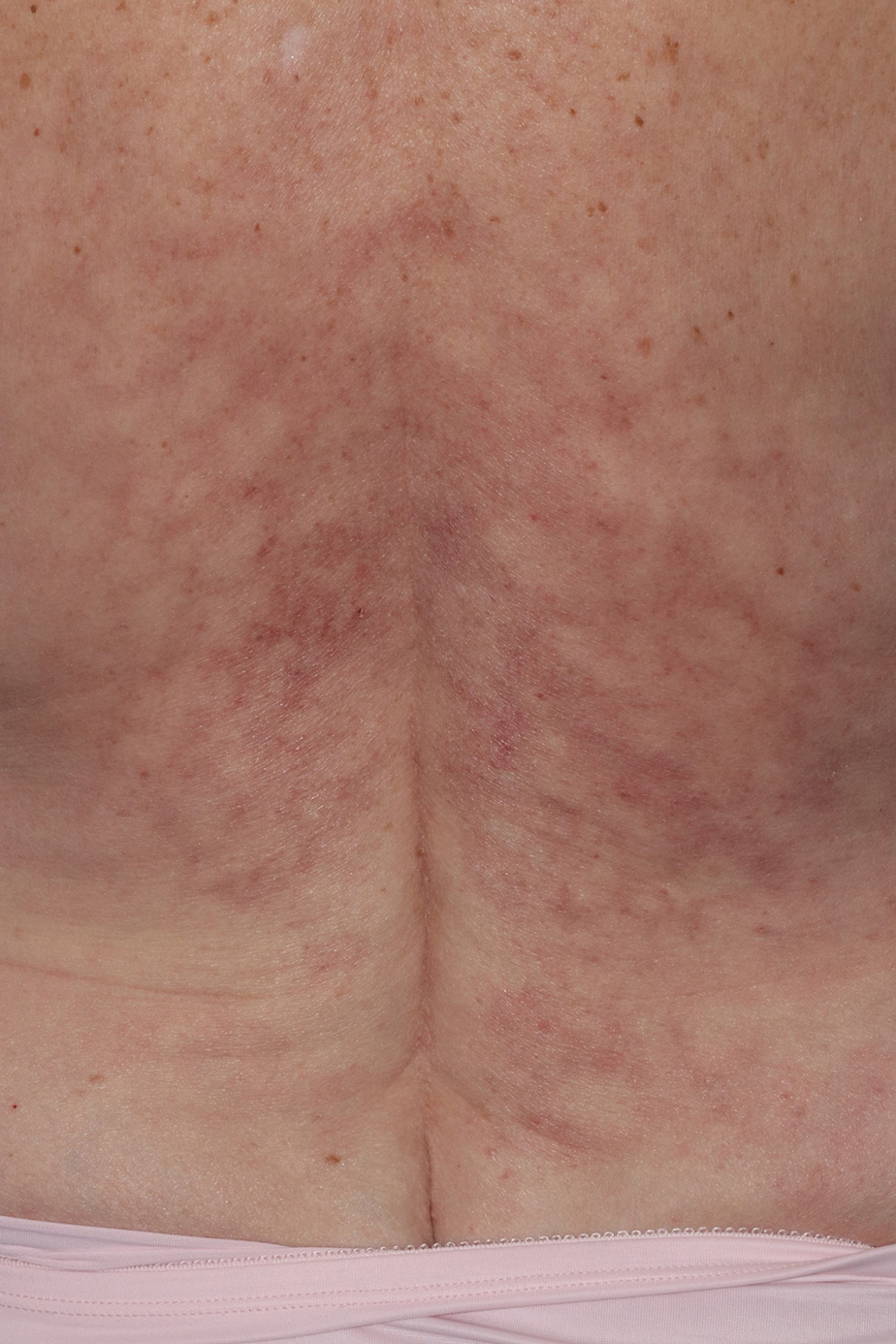
Reticulated Brownish Erythema on the Lower Back
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
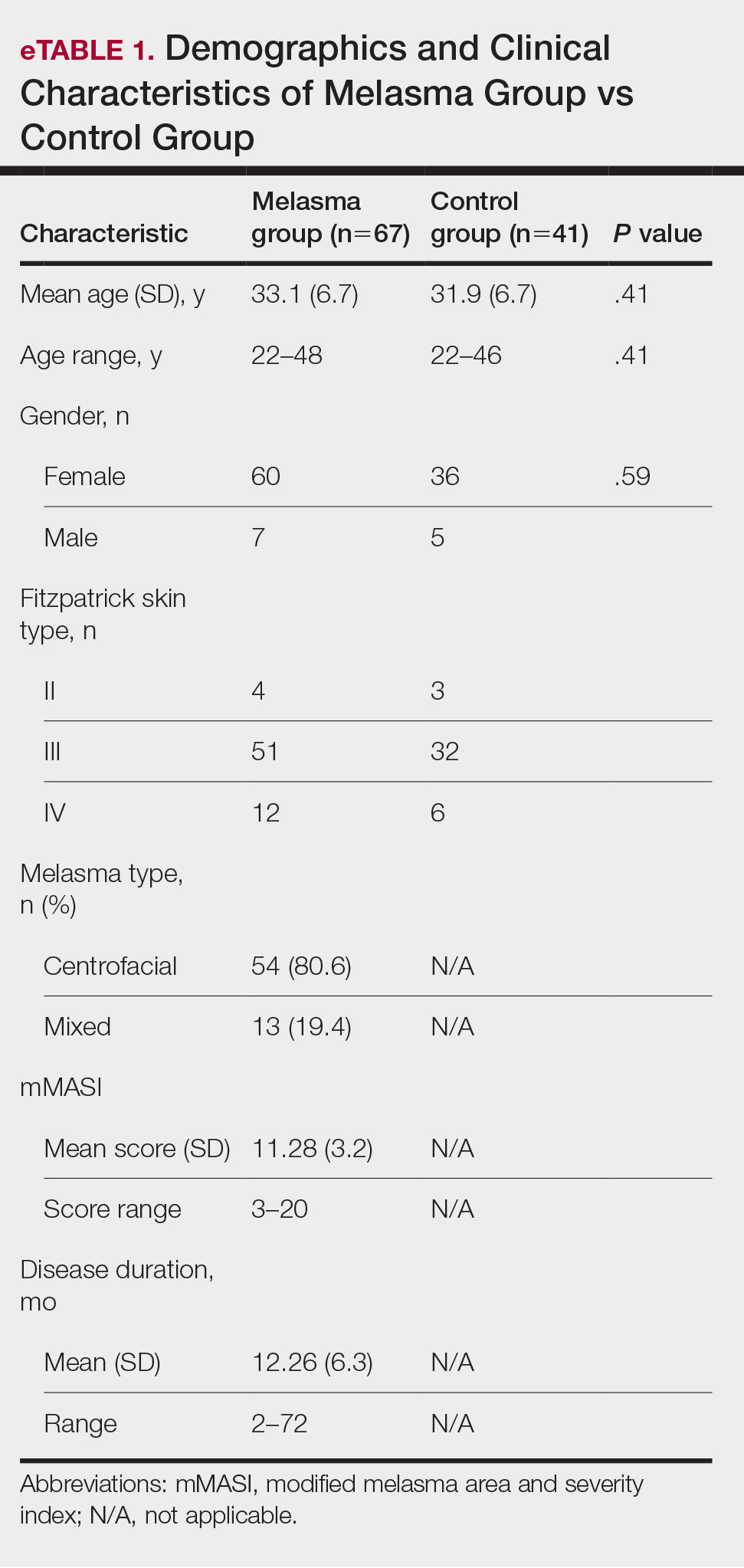
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
A 42-year-old woman presented with an asymptomatic, erythematous, lacelike rash on the lower back of 8 months’ duration that was first noticed by her husband. The patient had a long-standing history of chronic fatigue and lower back pain treated with acetaminophen, diclofenac gel, and heating pads. Physical examination revealed reticulated brownish erythema confined to the lower back. Laboratory findings were unremarkable.
Plantar Hyperpigmentation
The Comparison
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
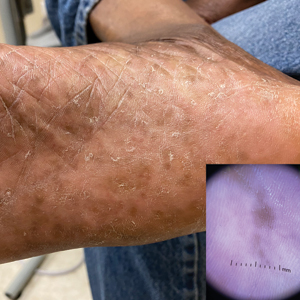
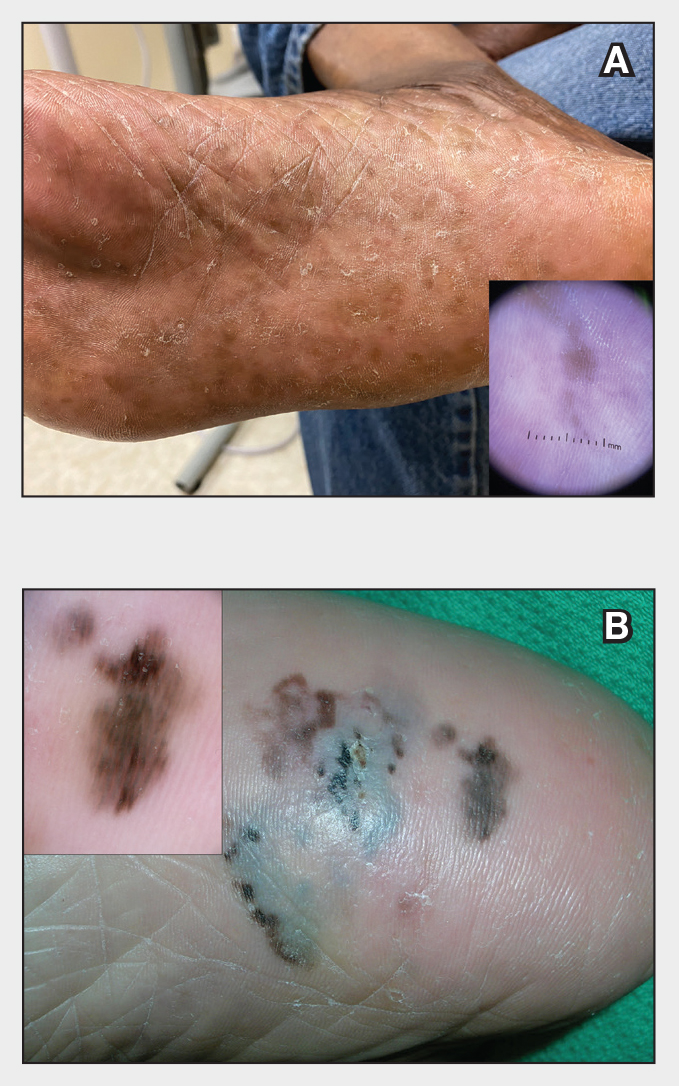
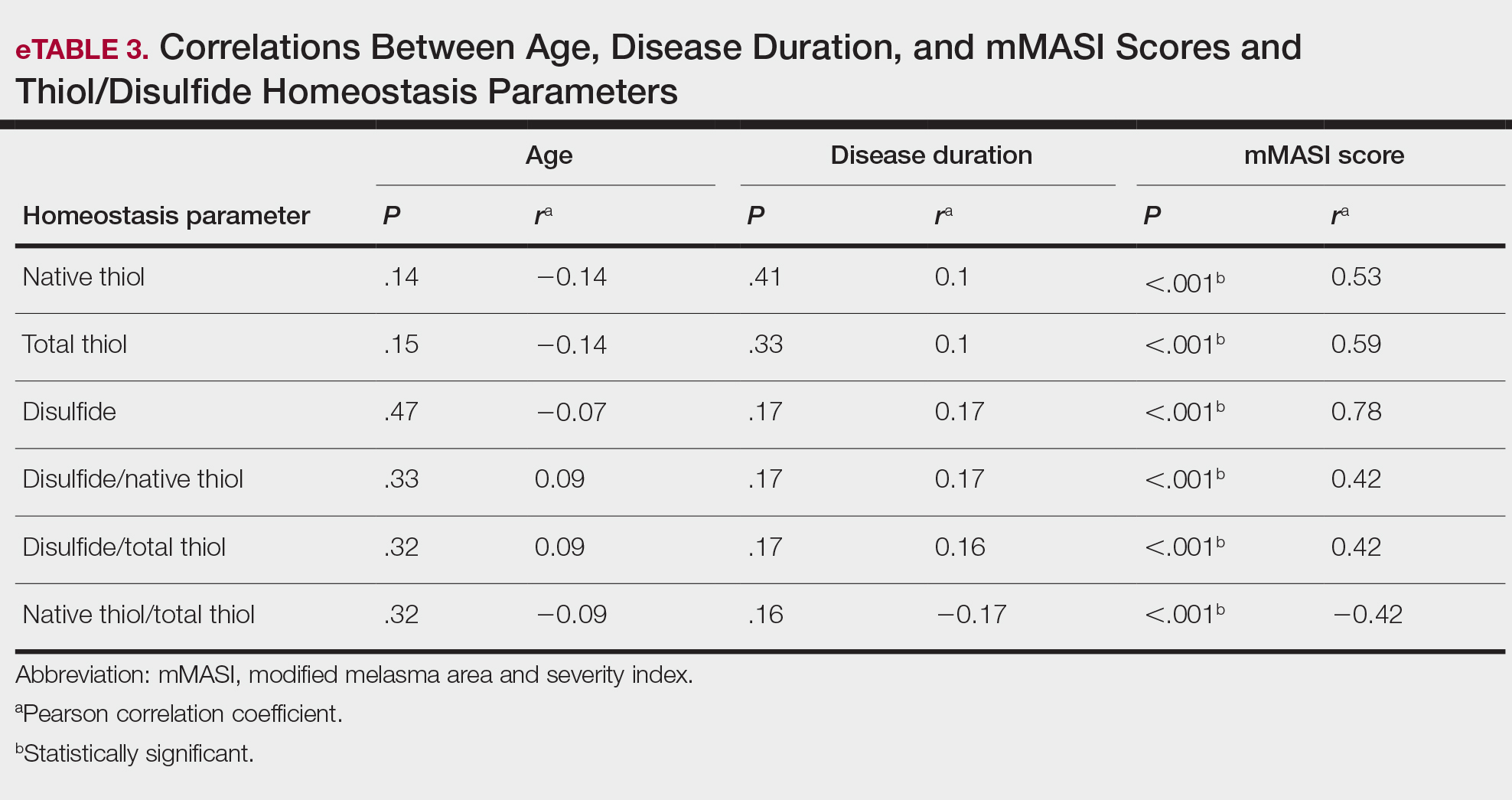
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Oxidative Stress in Patients With Melasma: An Evaluation of the Correlation of the Thiol/Disulfide Homeostasis Parameters and Modified MASI Score
Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
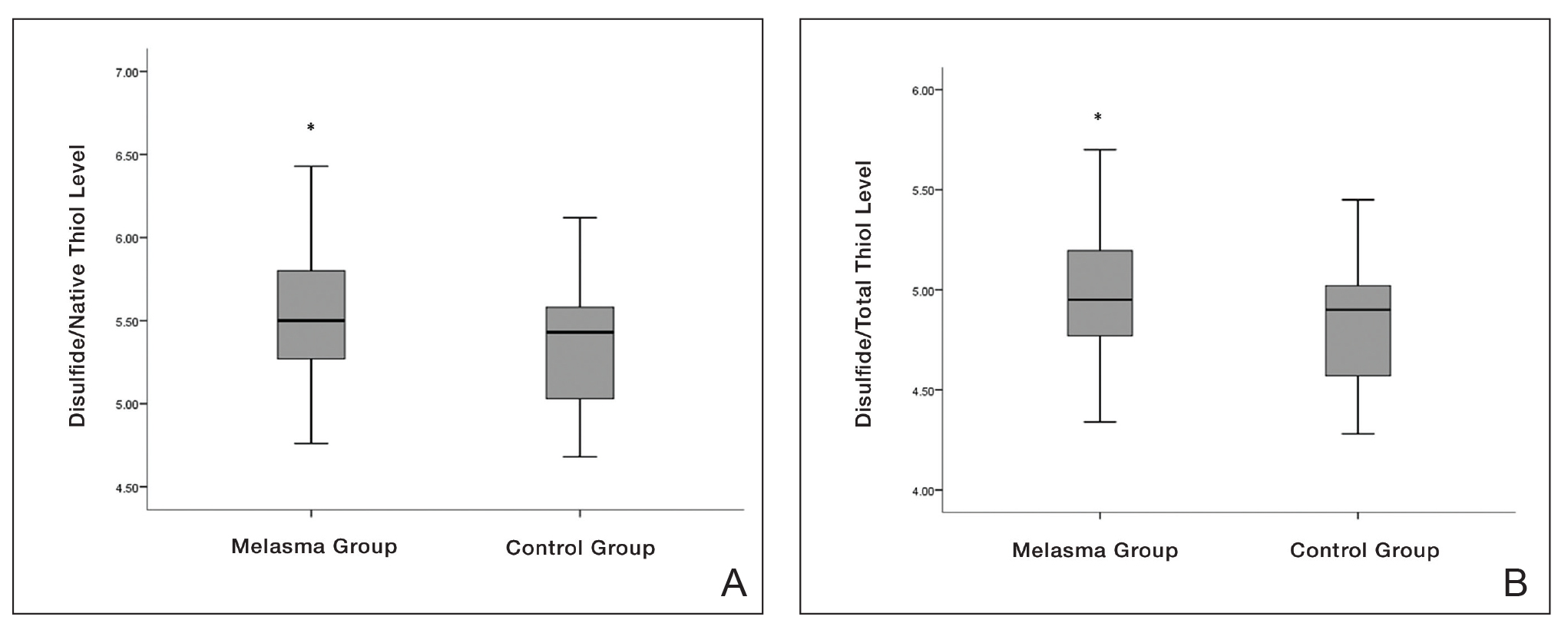
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4

Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reports in the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.

- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4


Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reports in the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.



Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4


Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reports in the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.



- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
Practice Points
- Melasma is a common pigmentation disorder that causes brown or grayish patches on the skin.
- Disulfide/native thiol and disulfide/total thiol ratios were higher in patients with melasma compared with controls, which indicated the presence of oxidative stress in melasma.
- The evaluation of modified melasma area and severity index score with disulfide/native thiol and disulfide/total thiol values suggests that oxidative stress is correlated with melasma disease severity.
Need a Wood Lamp Alternative? Grab Your Smartphone
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).
Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
Hypopigmented Cutaneous Langerhans Cell Histiocytosis in a Hispanic Infant
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.
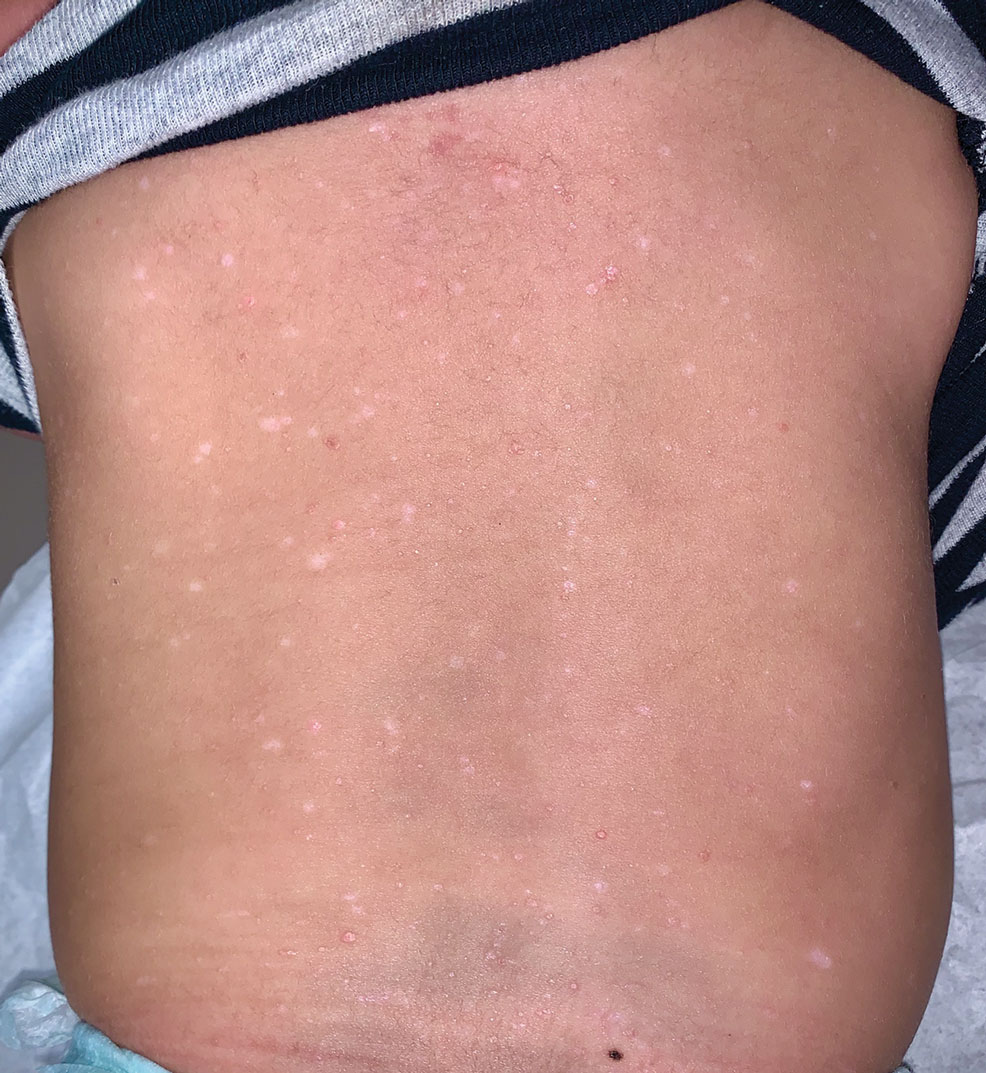
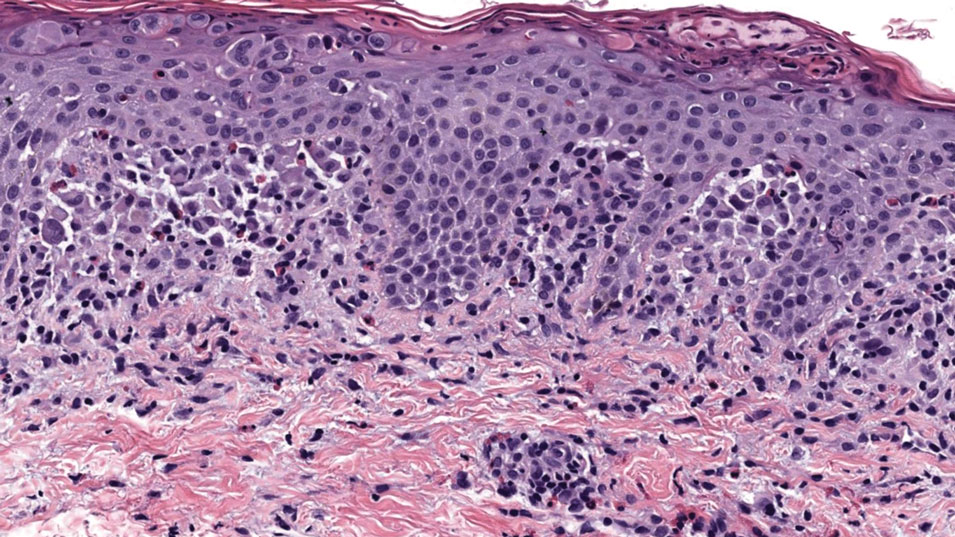
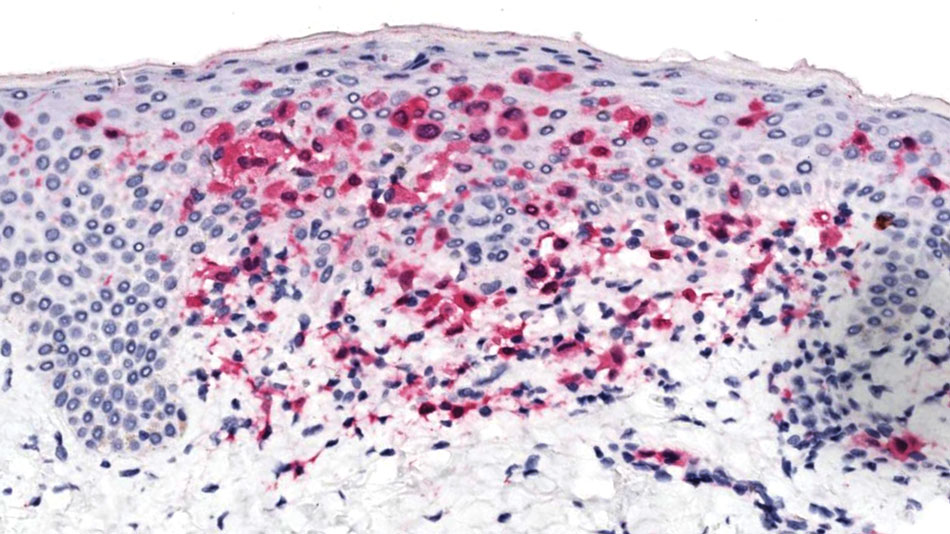
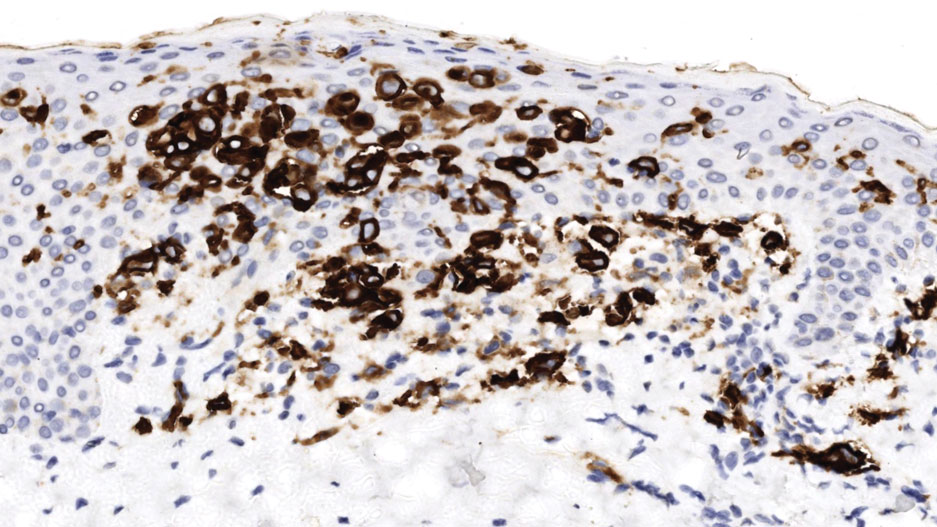
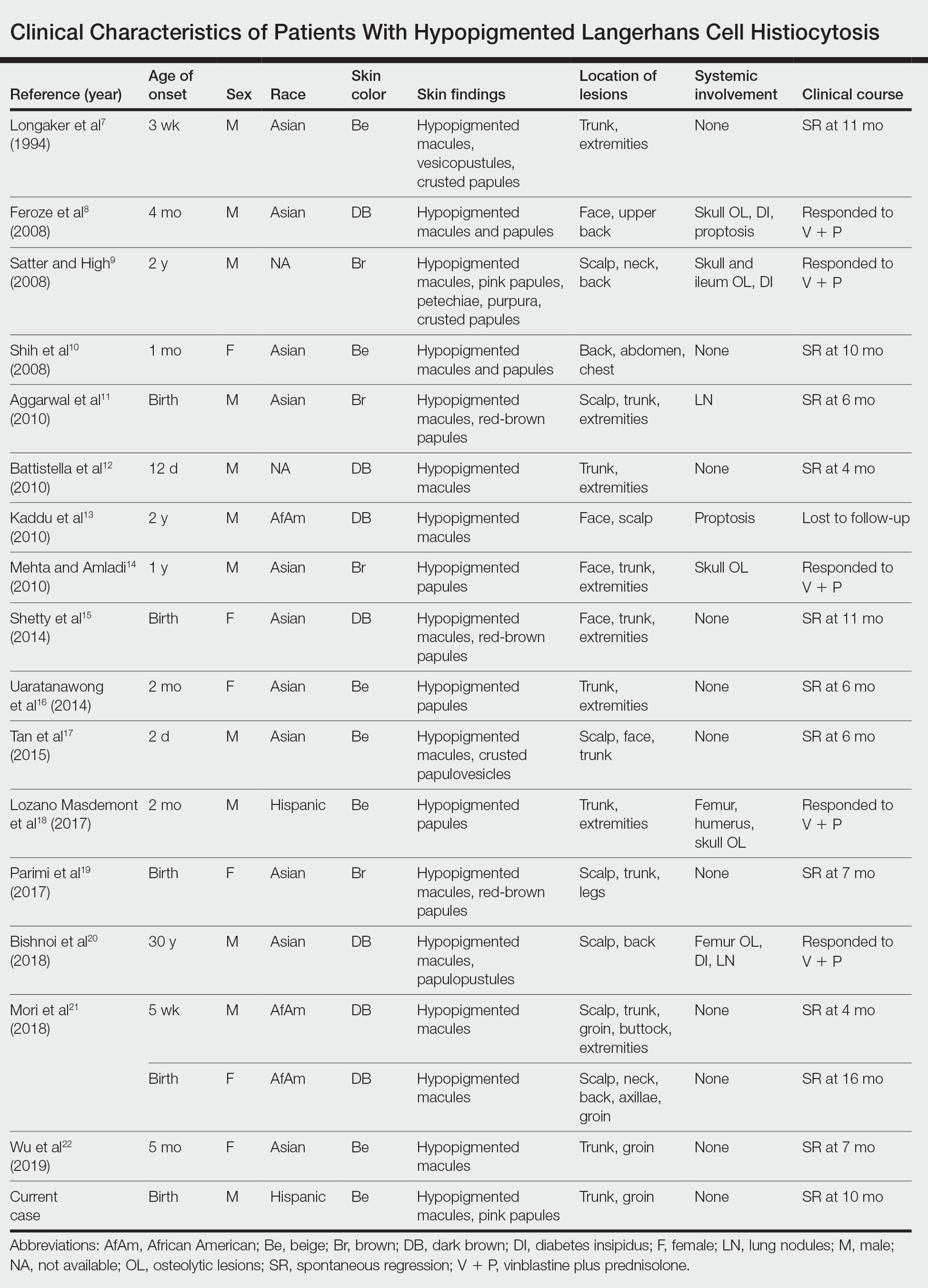
The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
Practice Points
- Dermatologists should be aware of the hypopigmented variant of cutaneous Langerhans cell histiocytosis (LCH), which has been reported exclusively in patients with skin of color.
- Langerhans cell histiocytosis should be included in the differential diagnosis of hypopigmented macules, which may be the only cutaneous manifestation or may coincide with typical lesions of LCH.
- Hypopigmented cutaneous LCH may be more common in newborns and associated with a better prognosis.
Exploring Skin Pigmentation Adaptation: A Systematic Review on the Vitamin D Adaptation Hypothesis
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).
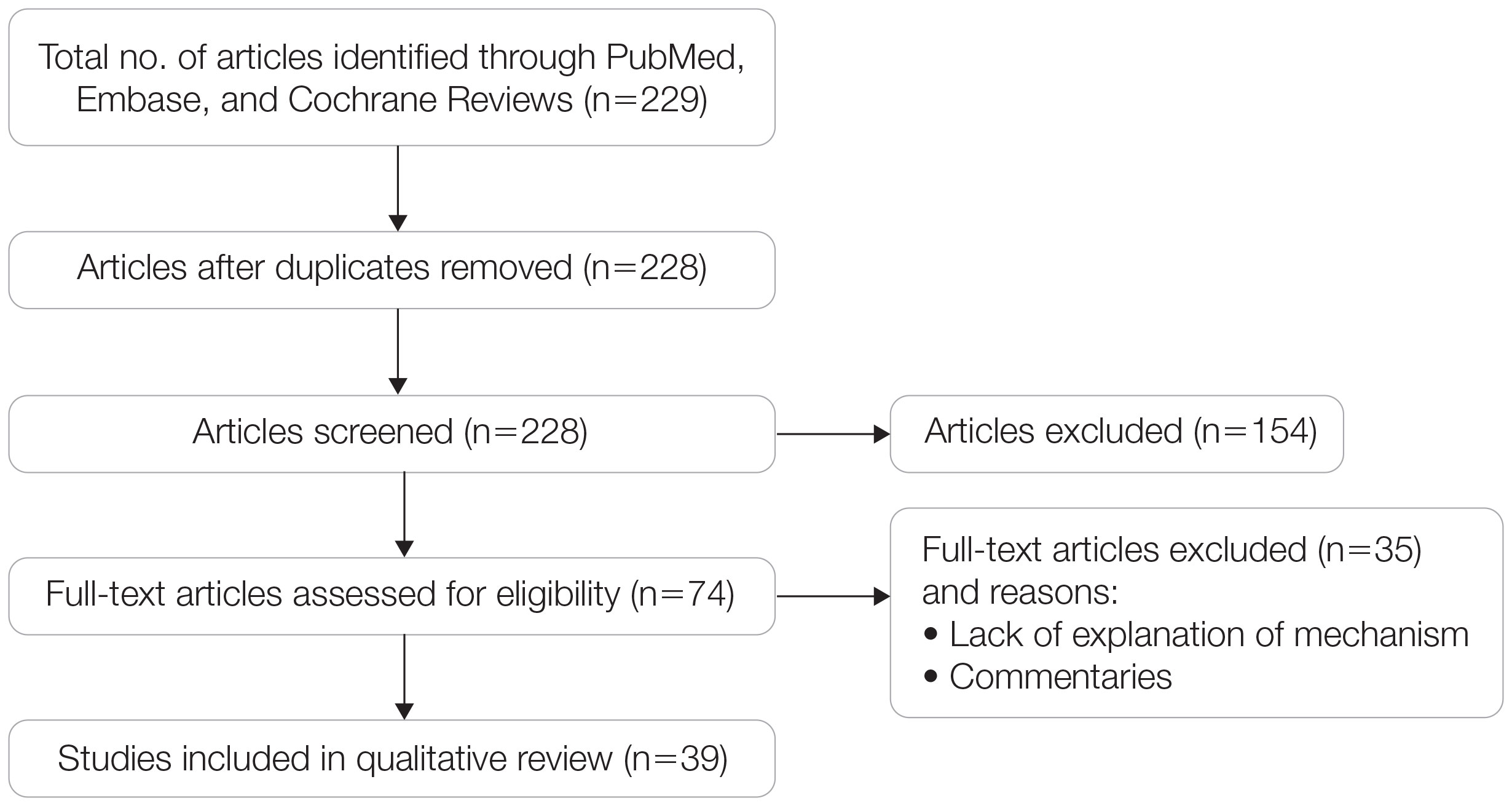
Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6
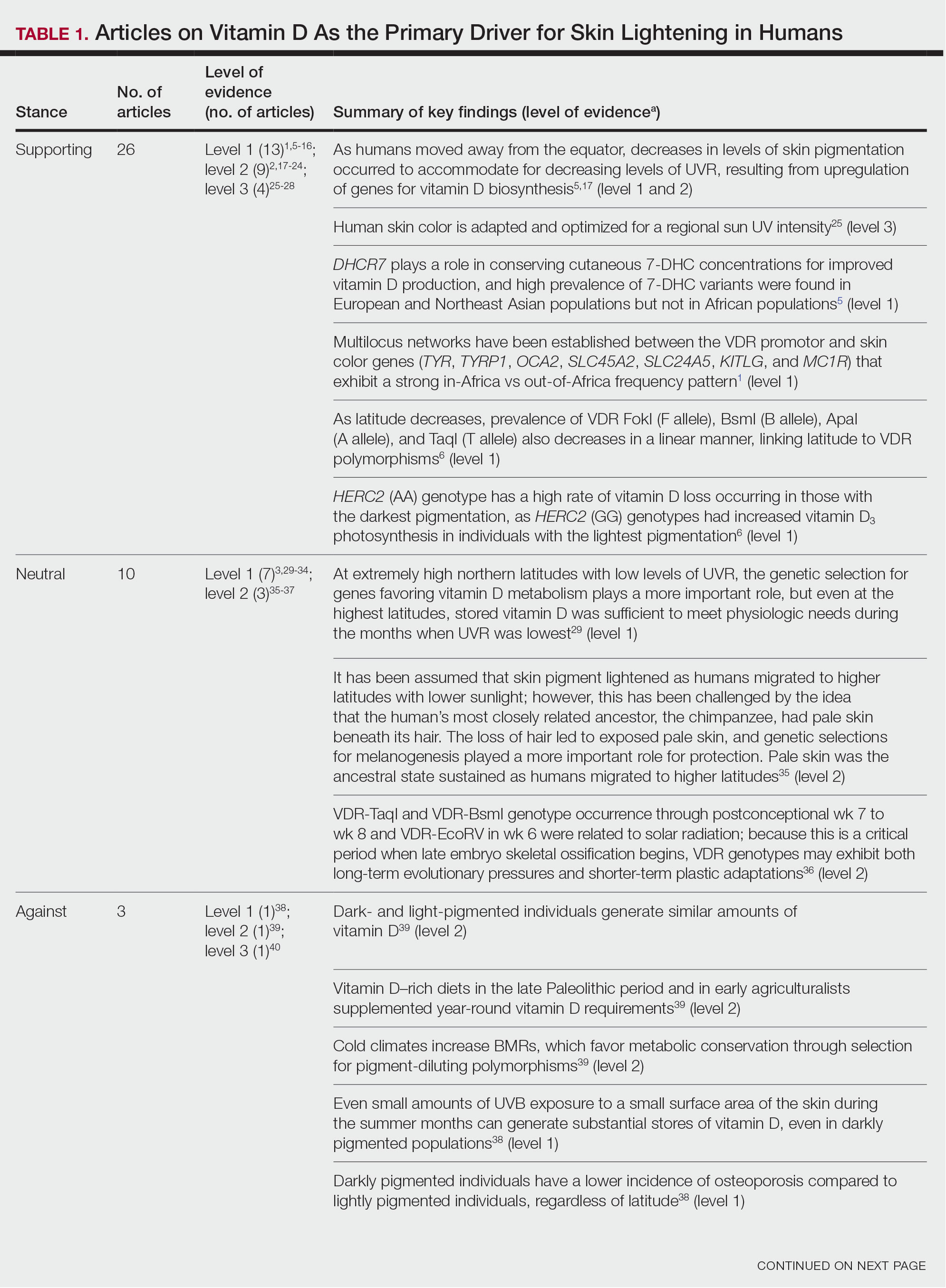
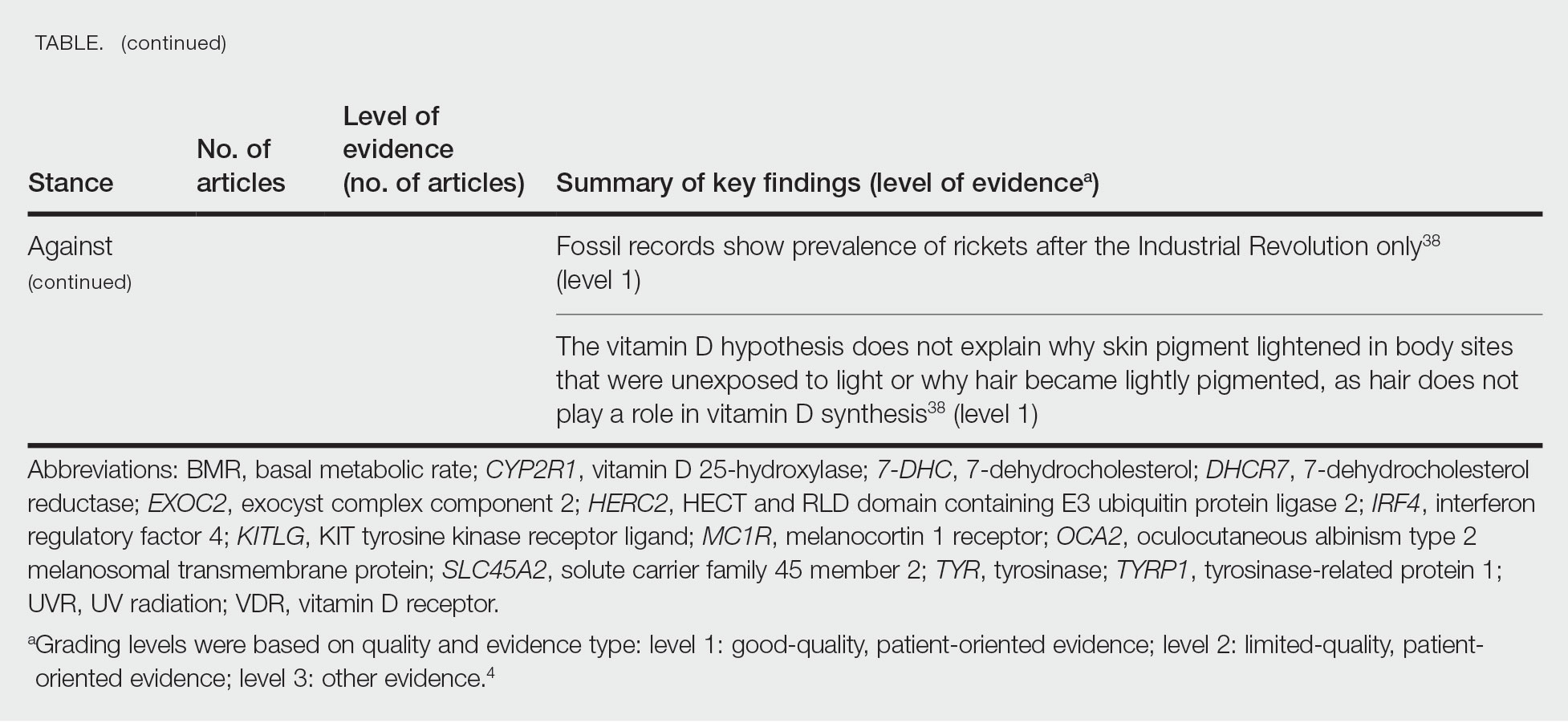
Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.
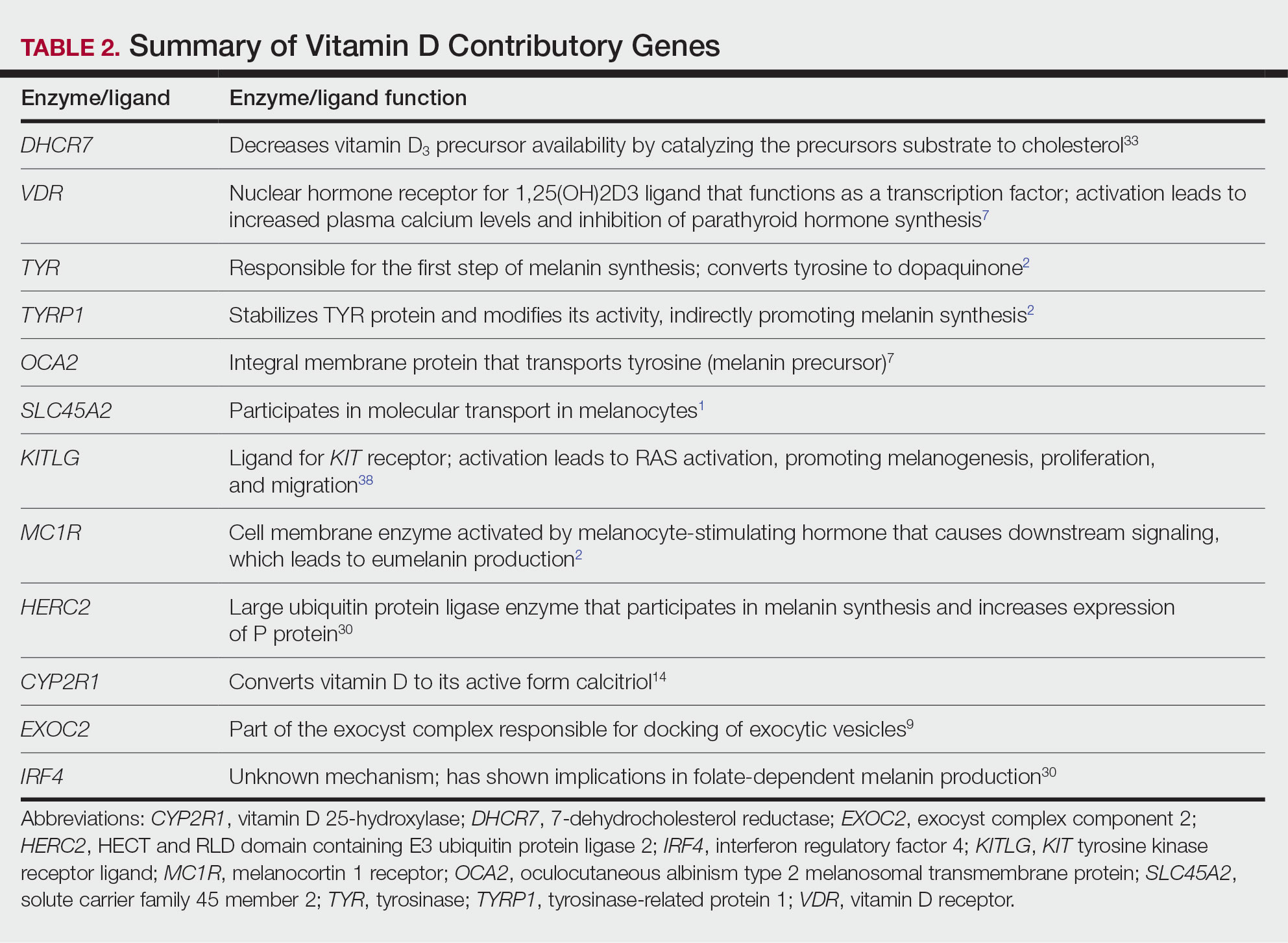
Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
Practice Points
- Sufficient UV radiation exposure is required to synthesize vitamin D, but excess exposure increases skin cancer risk.
- Genes associated with vitamin D production and melanin synthesis form an interconnected network that explains skin tone polymorphisms and their influence on healthy sun behaviors.
- Adaptations in genetics of skin pigmentation and vitamin D metabolism due to anthropologic patterns of migration to northern latitudes may help explain predisposition to dermatologic diseases such as skin cancer.
Periorbital Changes Induced by Prostaglandin Eye Drops
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.
The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
PRACTICE POINTS
- Ask patients to provide photographs taken prior to noticed changes to assess progression if they are new to your practice.
- Take photographs of patients in good light against a solid-colored background to have a baseline. It may be helpful to update patient images annually.
- Discuss with patients the aesthetic changes that may occur with the use of prescription medications. Provide pamphlets with images to educate them on what to expect.
Risk for COVID-19 Infection in Patients With Vitiligo
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).
Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.
Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.
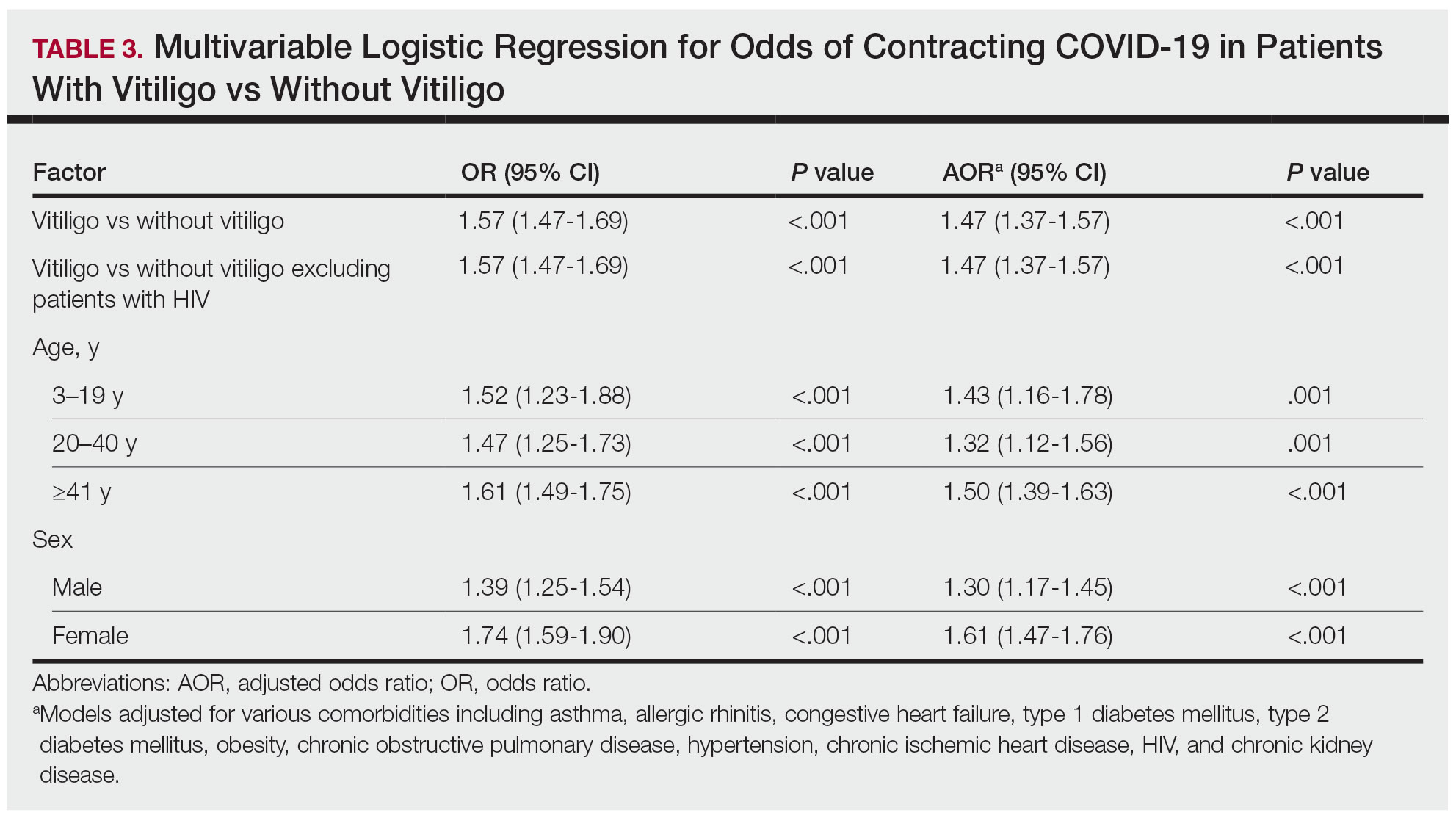
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
Practice Points
- The underlying autoimmune process in vitiligo can result in various changes to the immune system.
- A diagnosis of vitiligo may alter the body’s immune response to COVID-19 infection.
Port-Wine Birthmarks: Shorter Interval Laser Treatments Show Promise in Infants
TOPLINE:
METHODOLOGY:
- Early intervention of PWB in infants can significantly improve outcomes, and some studies suggest shorter intervals between laser treatments may be more effective. While laser treatment with PDL is the gold standard, the optimal treatment interval has not been determined.
- Researchers evaluated the records of 10 infants with PWB who received weekly PDL treatments from 2022 to 2023 at a single center. Treatment was initiated when the infants were 6 months old or younger, with the median age at the first treatment being 4 weeks. Of the 10 infants, eight had Fitzpatrick skin types I-III and two had skin type IV.
- Two dermatologists assessed photographs taken before and after laser treatment, and the primary outcome was the percentage improvement of PWB.
TAKEAWAY:
- Of the 10 patients, six achieved near-total (76%-95%) clearance, and one achieved total (96%-100%) clearance of PWB at a mean of 2 months after the first treatment.
- Marked improvement (51%-75%) in PWB was observed in the remaining three patients, who achieved near-total clearance with additional treatments.
- The median duration of treatment was 2 months (range, 0.2-5.1), and a median of eight treatments (range, 2-20) were needed to achieve near total or total clearance.
- No adverse events were reported, including pigmentary changes, scarring, burns, erosions, or infections.
IN PRACTICE:
The outcomes in the case series, the authors concluded, “are compelling and warrant attention and further investigation into the possibility that this novel and decreased treatment interval of 1 week ... is associated with potential improvement in outcomes and shorter overall treatment duration.”
SOURCE:
This study was led by Shirin Bajaj, MD, of the Laser & Skin Surgery Center of New York, where the infants were treated, and was published online on April 17, 2024, in JAMA Dermatology.
LIMITATIONS:
A small sample size and the lack of a comparison arm limited the ability to draw any conclusions or make treatment recommendations based on the results.
DISCLOSURES:
The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early intervention of PWB in infants can significantly improve outcomes, and some studies suggest shorter intervals between laser treatments may be more effective. While laser treatment with PDL is the gold standard, the optimal treatment interval has not been determined.
- Researchers evaluated the records of 10 infants with PWB who received weekly PDL treatments from 2022 to 2023 at a single center. Treatment was initiated when the infants were 6 months old or younger, with the median age at the first treatment being 4 weeks. Of the 10 infants, eight had Fitzpatrick skin types I-III and two had skin type IV.
- Two dermatologists assessed photographs taken before and after laser treatment, and the primary outcome was the percentage improvement of PWB.
TAKEAWAY:
- Of the 10 patients, six achieved near-total (76%-95%) clearance, and one achieved total (96%-100%) clearance of PWB at a mean of 2 months after the first treatment.
- Marked improvement (51%-75%) in PWB was observed in the remaining three patients, who achieved near-total clearance with additional treatments.
- The median duration of treatment was 2 months (range, 0.2-5.1), and a median of eight treatments (range, 2-20) were needed to achieve near total or total clearance.
- No adverse events were reported, including pigmentary changes, scarring, burns, erosions, or infections.
IN PRACTICE:
The outcomes in the case series, the authors concluded, “are compelling and warrant attention and further investigation into the possibility that this novel and decreased treatment interval of 1 week ... is associated with potential improvement in outcomes and shorter overall treatment duration.”
SOURCE:
This study was led by Shirin Bajaj, MD, of the Laser & Skin Surgery Center of New York, where the infants were treated, and was published online on April 17, 2024, in JAMA Dermatology.
LIMITATIONS:
A small sample size and the lack of a comparison arm limited the ability to draw any conclusions or make treatment recommendations based on the results.
DISCLOSURES:
The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early intervention of PWB in infants can significantly improve outcomes, and some studies suggest shorter intervals between laser treatments may be more effective. While laser treatment with PDL is the gold standard, the optimal treatment interval has not been determined.
- Researchers evaluated the records of 10 infants with PWB who received weekly PDL treatments from 2022 to 2023 at a single center. Treatment was initiated when the infants were 6 months old or younger, with the median age at the first treatment being 4 weeks. Of the 10 infants, eight had Fitzpatrick skin types I-III and two had skin type IV.
- Two dermatologists assessed photographs taken before and after laser treatment, and the primary outcome was the percentage improvement of PWB.
TAKEAWAY:
- Of the 10 patients, six achieved near-total (76%-95%) clearance, and one achieved total (96%-100%) clearance of PWB at a mean of 2 months after the first treatment.
- Marked improvement (51%-75%) in PWB was observed in the remaining three patients, who achieved near-total clearance with additional treatments.
- The median duration of treatment was 2 months (range, 0.2-5.1), and a median of eight treatments (range, 2-20) were needed to achieve near total or total clearance.
- No adverse events were reported, including pigmentary changes, scarring, burns, erosions, or infections.
IN PRACTICE:
The outcomes in the case series, the authors concluded, “are compelling and warrant attention and further investigation into the possibility that this novel and decreased treatment interval of 1 week ... is associated with potential improvement in outcomes and shorter overall treatment duration.”
SOURCE:
This study was led by Shirin Bajaj, MD, of the Laser & Skin Surgery Center of New York, where the infants were treated, and was published online on April 17, 2024, in JAMA Dermatology.
LIMITATIONS:
A small sample size and the lack of a comparison arm limited the ability to draw any conclusions or make treatment recommendations based on the results.
DISCLOSURES:
The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Analysis Finds Low Malignancy Rate in Pediatric Longitudinal Melanonychia
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.