User login
Dermatology therapies evolve as disease knowledge and investment grow
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
AAD-NPF releases first guidelines for nonbiologic treatments of psoriasis
It’s been 11 years since monotherapy and suggest a framework for a number of off-label treatments.
The guidelines, issued jointly with the National Psoriasis Foundation (NPF), were published in the Journal of the American Academy of Dermatology.
“I think we are way behind,” Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, and cochair of the guideline writing committee, said in an interview. “Most other countries update their guidelines every 1 or 2 years; we were 10 years behind.” The guidelines for systemic nonbiologic drugs follow up psoriasis guidelines issued by the AAD and the NPF on pediatric patients issued earlier this year, and on phototherapy, biologic treatments, and management of comorbidities issued last year.
“A lot has happened in the last 10 years,” said cochair Craig Elmets, MD, professor of dermatology at the University of Alabama at Birmingham. “While much of the interest is on biologic agents, nonbiologics are still used quite frequently, and the guidelines for their appropriate use have changed. Use of the guidelines provides people in the health profession with the most up to date evidence-based information so they can give their patients the best care.”
The guidelines acknowledge that the medications it covers are still widely used, either by themselves or in combination with biologic agents; readily available; easy to use; and, in the case of older therapies, relatively cheap.
Methotrexate has been available since the 1970s. Given as an injection or taken orally, the guidelines recommend supplementation with folic acid to counteract methotrexate’s side effects, particularly GI upset. The guidelines note that folic acid is less expensive than folinic acid. Combination therapy with methotrexate and tumor necrosis factor (TNF) inhibitors is more effective than methotrexate monotherapy, with a similar side effect profile, the guidelines state.
Methotrexate is more widely used outside the United States, “but it is a very good, quick fix and it’s much safer in children and young people than it is in people with cardiovascular disease,” Dr. Menter noted. “It’s still the most commonly used drug worldwide because it’s cheap, and you do have to worry about the long-term toxicity which is related the liver issues.”
The guidelines say that subcutaneous administration of methotrexate “may be particularly useful” for patients on higher doses, which when taken orally, are associated with a higher risk of GI effects.
Dr. Menter referred to a 2017 study, which reported 41% of patients treated with subcutaneous methotrexate once a week achieved a Psoriasis Area and Severity Index 75 score of 41% after a year of treatment, compared with 10% of those on placebo (Lancet. 2017 Feb 4;389[10068]:528-37).
The guidelines rate strength of recommendation as class A for methotrexate for moderate to severe psoriasis in adults, recommend supplementation with folic or folinic acid to counteract GI complications and liver problems, and note that adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis. Class B recommendations for methotrexate and psoriasis include statements that patients should begin with a test dose, especially if they have impaired kidney function; methotrexate is effective for peripheral, but not axial, psoriatic arthritis (PsA); and TNF inhibitors are more effective than methotrexate for PsA.
Approved by the FDA in 2014 for psoriasis, apremilast, which inhibits phosphodiesterase-4, is the newest drug in the recommendations. The guidelines recommend its use for moderate to severe psoriasis in adults, with a class A recommendation. Patients should start on a low dose and then build up to the 30-mg, twice-daily dose over 6 days and should be counseled about the risk of depression before starting treatment. Routine laboratory testing can be considered on an individual basis.
The guidelines also lay out three recommendations (and strength of recommendation) for cyclosporine, a drug that’s been around since the 1990s: for severe, recalcitrant cases (class A); for erythrodermic, general pustular, and palmoplantar psoriasis (class B); and as short-term therapy for psoriasis flare in patients already on another drug (class C).
Acitretin is another longstanding therapy used mostly for palmar-plantar psoriasis, but it can also be used as monotherapy for plaque psoriasis as well as erythrodermic and pustular disease. It can also be used in combination with psoralens with UVA for psoriasis and combined with broadband UVB phototherapy for plaque psoriasis. The acitretin recommendations are class B.
The oral Janus kinase (JAK) inhibitor tofacitinib isn’t specifically approved for psoriasis, but it is approved for RA, PsA, and ulcerative colitis. The drug targets the JAK-STAT signaling pathway that causes inflammation. The guidelines state that tofacitinib can be considered for moderate to severe psoriasis, but lists no strength of recommendation. The recommended dose is either 5 or 10 mg orally twice a day, with a caveat that the higher dose carries a higher risk of adverse events. Patients should be evaluated for getting a zoster vaccine before they begin therapy.
“We thought that, because there was probably a small chance that it might get approved for psoriasis, that we would discuss it briefly,” Dr. Menter said of tofacitinib.
Another off-label use the guidelines address is for fumaric and acid esters, also known as fumarates, which are used to in Europe to treat moderate to severe psoriasis. Dimethyl fumarate is approved for relapsing forms of multiple sclerosis in the United States. The guidelines state that fumarates can be used for psoriasis, but offer no strength of recommendation. Side effects include gastrointestinal disturbance and flushing.
Other treatments that are also addressed in the guidelines include a host of systemic immunosuppressants and antimetabolites: azathioprine, hydroxyurea, leflunomide, mycophenolate mofetil, thioguanine, and tacrolimus, none of which are FDA approved for psoriasis. They’re rarely used for psoriasis, but may have value in selected cases, the guidelines state.
Dr. Menter said that apremilast is the only oral drug in the guidelines, but they are the wave of the future for treating psoriasis. “I think there’s a tremendous potential for new oral drugs – TK2 [thymidine kinase], the JAK inhibitors, and other drugs coming down the pipelines. The majority of patients, if you ask them their preference, would like to take an oral drug rather than an injectable drug. And it would be much easier for dermatologists, they wouldn’t have to train patients on how to do the injections.”
Dr. Menter and Dr. Elmets disclosed financial relationships with numerous pharmaceutical companies. Other authors/work group members also had disclosures related to pharmaceutical manufacturers, and several had no disclosures.
SOURCE: Menter A et al. J Am Acad Dermatol. 2020 Feb 28. doi: 10.1016/j.jaad.2020.02.044.
It’s been 11 years since monotherapy and suggest a framework for a number of off-label treatments.
The guidelines, issued jointly with the National Psoriasis Foundation (NPF), were published in the Journal of the American Academy of Dermatology.
“I think we are way behind,” Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, and cochair of the guideline writing committee, said in an interview. “Most other countries update their guidelines every 1 or 2 years; we were 10 years behind.” The guidelines for systemic nonbiologic drugs follow up psoriasis guidelines issued by the AAD and the NPF on pediatric patients issued earlier this year, and on phototherapy, biologic treatments, and management of comorbidities issued last year.
“A lot has happened in the last 10 years,” said cochair Craig Elmets, MD, professor of dermatology at the University of Alabama at Birmingham. “While much of the interest is on biologic agents, nonbiologics are still used quite frequently, and the guidelines for their appropriate use have changed. Use of the guidelines provides people in the health profession with the most up to date evidence-based information so they can give their patients the best care.”
The guidelines acknowledge that the medications it covers are still widely used, either by themselves or in combination with biologic agents; readily available; easy to use; and, in the case of older therapies, relatively cheap.
Methotrexate has been available since the 1970s. Given as an injection or taken orally, the guidelines recommend supplementation with folic acid to counteract methotrexate’s side effects, particularly GI upset. The guidelines note that folic acid is less expensive than folinic acid. Combination therapy with methotrexate and tumor necrosis factor (TNF) inhibitors is more effective than methotrexate monotherapy, with a similar side effect profile, the guidelines state.
Methotrexate is more widely used outside the United States, “but it is a very good, quick fix and it’s much safer in children and young people than it is in people with cardiovascular disease,” Dr. Menter noted. “It’s still the most commonly used drug worldwide because it’s cheap, and you do have to worry about the long-term toxicity which is related the liver issues.”
The guidelines say that subcutaneous administration of methotrexate “may be particularly useful” for patients on higher doses, which when taken orally, are associated with a higher risk of GI effects.
Dr. Menter referred to a 2017 study, which reported 41% of patients treated with subcutaneous methotrexate once a week achieved a Psoriasis Area and Severity Index 75 score of 41% after a year of treatment, compared with 10% of those on placebo (Lancet. 2017 Feb 4;389[10068]:528-37).
The guidelines rate strength of recommendation as class A for methotrexate for moderate to severe psoriasis in adults, recommend supplementation with folic or folinic acid to counteract GI complications and liver problems, and note that adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis. Class B recommendations for methotrexate and psoriasis include statements that patients should begin with a test dose, especially if they have impaired kidney function; methotrexate is effective for peripheral, but not axial, psoriatic arthritis (PsA); and TNF inhibitors are more effective than methotrexate for PsA.
Approved by the FDA in 2014 for psoriasis, apremilast, which inhibits phosphodiesterase-4, is the newest drug in the recommendations. The guidelines recommend its use for moderate to severe psoriasis in adults, with a class A recommendation. Patients should start on a low dose and then build up to the 30-mg, twice-daily dose over 6 days and should be counseled about the risk of depression before starting treatment. Routine laboratory testing can be considered on an individual basis.
The guidelines also lay out three recommendations (and strength of recommendation) for cyclosporine, a drug that’s been around since the 1990s: for severe, recalcitrant cases (class A); for erythrodermic, general pustular, and palmoplantar psoriasis (class B); and as short-term therapy for psoriasis flare in patients already on another drug (class C).
Acitretin is another longstanding therapy used mostly for palmar-plantar psoriasis, but it can also be used as monotherapy for plaque psoriasis as well as erythrodermic and pustular disease. It can also be used in combination with psoralens with UVA for psoriasis and combined with broadband UVB phototherapy for plaque psoriasis. The acitretin recommendations are class B.
The oral Janus kinase (JAK) inhibitor tofacitinib isn’t specifically approved for psoriasis, but it is approved for RA, PsA, and ulcerative colitis. The drug targets the JAK-STAT signaling pathway that causes inflammation. The guidelines state that tofacitinib can be considered for moderate to severe psoriasis, but lists no strength of recommendation. The recommended dose is either 5 or 10 mg orally twice a day, with a caveat that the higher dose carries a higher risk of adverse events. Patients should be evaluated for getting a zoster vaccine before they begin therapy.
“We thought that, because there was probably a small chance that it might get approved for psoriasis, that we would discuss it briefly,” Dr. Menter said of tofacitinib.
Another off-label use the guidelines address is for fumaric and acid esters, also known as fumarates, which are used to in Europe to treat moderate to severe psoriasis. Dimethyl fumarate is approved for relapsing forms of multiple sclerosis in the United States. The guidelines state that fumarates can be used for psoriasis, but offer no strength of recommendation. Side effects include gastrointestinal disturbance and flushing.
Other treatments that are also addressed in the guidelines include a host of systemic immunosuppressants and antimetabolites: azathioprine, hydroxyurea, leflunomide, mycophenolate mofetil, thioguanine, and tacrolimus, none of which are FDA approved for psoriasis. They’re rarely used for psoriasis, but may have value in selected cases, the guidelines state.
Dr. Menter said that apremilast is the only oral drug in the guidelines, but they are the wave of the future for treating psoriasis. “I think there’s a tremendous potential for new oral drugs – TK2 [thymidine kinase], the JAK inhibitors, and other drugs coming down the pipelines. The majority of patients, if you ask them their preference, would like to take an oral drug rather than an injectable drug. And it would be much easier for dermatologists, they wouldn’t have to train patients on how to do the injections.”
Dr. Menter and Dr. Elmets disclosed financial relationships with numerous pharmaceutical companies. Other authors/work group members also had disclosures related to pharmaceutical manufacturers, and several had no disclosures.
SOURCE: Menter A et al. J Am Acad Dermatol. 2020 Feb 28. doi: 10.1016/j.jaad.2020.02.044.
It’s been 11 years since monotherapy and suggest a framework for a number of off-label treatments.
The guidelines, issued jointly with the National Psoriasis Foundation (NPF), were published in the Journal of the American Academy of Dermatology.
“I think we are way behind,” Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, and cochair of the guideline writing committee, said in an interview. “Most other countries update their guidelines every 1 or 2 years; we were 10 years behind.” The guidelines for systemic nonbiologic drugs follow up psoriasis guidelines issued by the AAD and the NPF on pediatric patients issued earlier this year, and on phototherapy, biologic treatments, and management of comorbidities issued last year.
“A lot has happened in the last 10 years,” said cochair Craig Elmets, MD, professor of dermatology at the University of Alabama at Birmingham. “While much of the interest is on biologic agents, nonbiologics are still used quite frequently, and the guidelines for their appropriate use have changed. Use of the guidelines provides people in the health profession with the most up to date evidence-based information so they can give their patients the best care.”
The guidelines acknowledge that the medications it covers are still widely used, either by themselves or in combination with biologic agents; readily available; easy to use; and, in the case of older therapies, relatively cheap.
Methotrexate has been available since the 1970s. Given as an injection or taken orally, the guidelines recommend supplementation with folic acid to counteract methotrexate’s side effects, particularly GI upset. The guidelines note that folic acid is less expensive than folinic acid. Combination therapy with methotrexate and tumor necrosis factor (TNF) inhibitors is more effective than methotrexate monotherapy, with a similar side effect profile, the guidelines state.
Methotrexate is more widely used outside the United States, “but it is a very good, quick fix and it’s much safer in children and young people than it is in people with cardiovascular disease,” Dr. Menter noted. “It’s still the most commonly used drug worldwide because it’s cheap, and you do have to worry about the long-term toxicity which is related the liver issues.”
The guidelines say that subcutaneous administration of methotrexate “may be particularly useful” for patients on higher doses, which when taken orally, are associated with a higher risk of GI effects.
Dr. Menter referred to a 2017 study, which reported 41% of patients treated with subcutaneous methotrexate once a week achieved a Psoriasis Area and Severity Index 75 score of 41% after a year of treatment, compared with 10% of those on placebo (Lancet. 2017 Feb 4;389[10068]:528-37).
The guidelines rate strength of recommendation as class A for methotrexate for moderate to severe psoriasis in adults, recommend supplementation with folic or folinic acid to counteract GI complications and liver problems, and note that adalimumab and infliximab are more effective than methotrexate for cutaneous psoriasis. Class B recommendations for methotrexate and psoriasis include statements that patients should begin with a test dose, especially if they have impaired kidney function; methotrexate is effective for peripheral, but not axial, psoriatic arthritis (PsA); and TNF inhibitors are more effective than methotrexate for PsA.
Approved by the FDA in 2014 for psoriasis, apremilast, which inhibits phosphodiesterase-4, is the newest drug in the recommendations. The guidelines recommend its use for moderate to severe psoriasis in adults, with a class A recommendation. Patients should start on a low dose and then build up to the 30-mg, twice-daily dose over 6 days and should be counseled about the risk of depression before starting treatment. Routine laboratory testing can be considered on an individual basis.
The guidelines also lay out three recommendations (and strength of recommendation) for cyclosporine, a drug that’s been around since the 1990s: for severe, recalcitrant cases (class A); for erythrodermic, general pustular, and palmoplantar psoriasis (class B); and as short-term therapy for psoriasis flare in patients already on another drug (class C).
Acitretin is another longstanding therapy used mostly for palmar-plantar psoriasis, but it can also be used as monotherapy for plaque psoriasis as well as erythrodermic and pustular disease. It can also be used in combination with psoralens with UVA for psoriasis and combined with broadband UVB phototherapy for plaque psoriasis. The acitretin recommendations are class B.
The oral Janus kinase (JAK) inhibitor tofacitinib isn’t specifically approved for psoriasis, but it is approved for RA, PsA, and ulcerative colitis. The drug targets the JAK-STAT signaling pathway that causes inflammation. The guidelines state that tofacitinib can be considered for moderate to severe psoriasis, but lists no strength of recommendation. The recommended dose is either 5 or 10 mg orally twice a day, with a caveat that the higher dose carries a higher risk of adverse events. Patients should be evaluated for getting a zoster vaccine before they begin therapy.
“We thought that, because there was probably a small chance that it might get approved for psoriasis, that we would discuss it briefly,” Dr. Menter said of tofacitinib.
Another off-label use the guidelines address is for fumaric and acid esters, also known as fumarates, which are used to in Europe to treat moderate to severe psoriasis. Dimethyl fumarate is approved for relapsing forms of multiple sclerosis in the United States. The guidelines state that fumarates can be used for psoriasis, but offer no strength of recommendation. Side effects include gastrointestinal disturbance and flushing.
Other treatments that are also addressed in the guidelines include a host of systemic immunosuppressants and antimetabolites: azathioprine, hydroxyurea, leflunomide, mycophenolate mofetil, thioguanine, and tacrolimus, none of which are FDA approved for psoriasis. They’re rarely used for psoriasis, but may have value in selected cases, the guidelines state.
Dr. Menter said that apremilast is the only oral drug in the guidelines, but they are the wave of the future for treating psoriasis. “I think there’s a tremendous potential for new oral drugs – TK2 [thymidine kinase], the JAK inhibitors, and other drugs coming down the pipelines. The majority of patients, if you ask them their preference, would like to take an oral drug rather than an injectable drug. And it would be much easier for dermatologists, they wouldn’t have to train patients on how to do the injections.”
Dr. Menter and Dr. Elmets disclosed financial relationships with numerous pharmaceutical companies. Other authors/work group members also had disclosures related to pharmaceutical manufacturers, and several had no disclosures.
SOURCE: Menter A et al. J Am Acad Dermatol. 2020 Feb 28. doi: 10.1016/j.jaad.2020.02.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Human Immunodeficiency Virus Infection in a Hepatitis B Virus–Positive Psoriasis Patient Treated With Ustekinumab
To the Editor:
The incidence of psoriasis in human immunodeficiency virus (HIV)–infected patients is similar to the general population, but it usually becomes more severe as immunosuppression increases. Additionally, it tends to be more resistant to conventional therapies, and the incidence and severity of psoriatic arthropathy is increased. Psoriasis often worsens at the time of HIV primary infection.1 We describe a case of a patient with hepatitis B virus (HBV) whose severe plaque psoriasis was controlled on ustekinumab; he was later diagnosed with HIV infection.
A 42-year-old man with HBV treated with entecavir (HBV DNA viral load, <20 copies/mL [inactive carrier, <2000 copies/mL]) presented to our dermatology unit with severe plaque psoriasis (psoriasis area and severity index 23) that caused notable psychologic difficulties such as anxiety and depression. Treatment was attempted with cyclosporine; acitretin; psoralen plus UVA; infliximab; adalimumab; and eventually ustekinumab (45 mg every 3 months), which controlled the condition well (psoriasis area and severity index 0) in an almost completely sustained manner.
Serologic tests requested at one of his analytical control appointments 2 years after initiating treatment with ustekinumab revealed he was HIV positive. The patient reported unprotected sexual intercourse 4 months prior. He was referred to the infectious disease unit and was classified in subtype A1 of HIV infection (CD4 count, 583 cells/µL [reference range, 500-1200 cells/µL]; viral load, 159,268 copies/mL [rapid progression to AIDS, >100,000 copies/mL]). Treatment was initiated with raltegravir, ritonavir, darunavir, and abacavir; tolerance was suitable. Because of the patient’s history of severe psoriasis, treatment with ustekinumab was maintained. Normally, treatment with this drug would be contraindicated in patients with HIV, as it can lead to viral reactivation. Four years after his HIV diagnosis, neither the patient’s cutaneous nor HIV-associated condition had worsened.
For patients with HIV and mild or moderate psoriasis, topical therapies (eg, corticosteroids, vitamin D analogues, tazarotene) are recommended, similar to patients who are HIV negative. Human immunodeficiency virus–positive patients with severe psoriasis who do not respond to topical treatment should receive phototherapy (UVB or psoralen plus UVA) or acitretin along with their antiretroviral drugs.2 In refractory cases, immunosuppressants, including cyclosporine, methotrexate, or tumor necrosis factor α inhibitors, might be used, though experience with them is limited.3,4 Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in patients who receive such immunosuppressants, as is close monitoring of the viral load.
Ustekinumab is an IL-12/IL-23 monoclonal antibody indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, and inflammatory bowel disease. It is contraindicated in patients with clinically important active infections, such as HBV and hepatitis C virus infections.5 However, it was shown to be safe in a group of 18 patients with HBV who had received antiviral prophylaxis6; a degree of reactivation was observed in similar patients who received no such prophylaxis and in others with hepatitis C virus infection.7 The simultaneous use of methotrexate with ustekinumab in the treatment of psoriatic arthritis does not appear to affect the safety of the latter drug.8 Paparizos et al9 described a patient with HIV controlled with antiretroviral drugs who was treated with ustekinumab for psoriasis; no adverse effects were observed.
We report the case of a patient with HBV and psoriasis who was treated with ustekinumab and later became infected with HIV. His ustekinumab treatment was maintained without subsequent cutaneous or systemic complications.
- Menon K, Van Voorhees V, Bebo B, et al. Psoriasis in patients with HIV infection: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Chiricozzi A, Saraceno R, Cannizzaro MV. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy. Dermatology. 2012;225:333-337.
- Barco D, Puig L, Alomar A. Treatment of moderate-severe psoriasis with etanercept in patients with chronic human immunodeficiency virus infection. Actas Dermosifiliogr. 2010;101(suppl 1):77-81.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV positive patient. J Drugs Dermatol. 2014;13:869-871.
- Rustin MH. Long-term safety of biologics in the treatment of moderate to severe plaque psoriasis: review of the current data. Br J Dermatol. 2012;167(suppl 3):3-11.
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616.
- Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515-526.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399.
To the Editor:
The incidence of psoriasis in human immunodeficiency virus (HIV)–infected patients is similar to the general population, but it usually becomes more severe as immunosuppression increases. Additionally, it tends to be more resistant to conventional therapies, and the incidence and severity of psoriatic arthropathy is increased. Psoriasis often worsens at the time of HIV primary infection.1 We describe a case of a patient with hepatitis B virus (HBV) whose severe plaque psoriasis was controlled on ustekinumab; he was later diagnosed with HIV infection.
A 42-year-old man with HBV treated with entecavir (HBV DNA viral load, <20 copies/mL [inactive carrier, <2000 copies/mL]) presented to our dermatology unit with severe plaque psoriasis (psoriasis area and severity index 23) that caused notable psychologic difficulties such as anxiety and depression. Treatment was attempted with cyclosporine; acitretin; psoralen plus UVA; infliximab; adalimumab; and eventually ustekinumab (45 mg every 3 months), which controlled the condition well (psoriasis area and severity index 0) in an almost completely sustained manner.
Serologic tests requested at one of his analytical control appointments 2 years after initiating treatment with ustekinumab revealed he was HIV positive. The patient reported unprotected sexual intercourse 4 months prior. He was referred to the infectious disease unit and was classified in subtype A1 of HIV infection (CD4 count, 583 cells/µL [reference range, 500-1200 cells/µL]; viral load, 159,268 copies/mL [rapid progression to AIDS, >100,000 copies/mL]). Treatment was initiated with raltegravir, ritonavir, darunavir, and abacavir; tolerance was suitable. Because of the patient’s history of severe psoriasis, treatment with ustekinumab was maintained. Normally, treatment with this drug would be contraindicated in patients with HIV, as it can lead to viral reactivation. Four years after his HIV diagnosis, neither the patient’s cutaneous nor HIV-associated condition had worsened.
For patients with HIV and mild or moderate psoriasis, topical therapies (eg, corticosteroids, vitamin D analogues, tazarotene) are recommended, similar to patients who are HIV negative. Human immunodeficiency virus–positive patients with severe psoriasis who do not respond to topical treatment should receive phototherapy (UVB or psoralen plus UVA) or acitretin along with their antiretroviral drugs.2 In refractory cases, immunosuppressants, including cyclosporine, methotrexate, or tumor necrosis factor α inhibitors, might be used, though experience with them is limited.3,4 Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in patients who receive such immunosuppressants, as is close monitoring of the viral load.
Ustekinumab is an IL-12/IL-23 monoclonal antibody indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, and inflammatory bowel disease. It is contraindicated in patients with clinically important active infections, such as HBV and hepatitis C virus infections.5 However, it was shown to be safe in a group of 18 patients with HBV who had received antiviral prophylaxis6; a degree of reactivation was observed in similar patients who received no such prophylaxis and in others with hepatitis C virus infection.7 The simultaneous use of methotrexate with ustekinumab in the treatment of psoriatic arthritis does not appear to affect the safety of the latter drug.8 Paparizos et al9 described a patient with HIV controlled with antiretroviral drugs who was treated with ustekinumab for psoriasis; no adverse effects were observed.
We report the case of a patient with HBV and psoriasis who was treated with ustekinumab and later became infected with HIV. His ustekinumab treatment was maintained without subsequent cutaneous or systemic complications.
To the Editor:
The incidence of psoriasis in human immunodeficiency virus (HIV)–infected patients is similar to the general population, but it usually becomes more severe as immunosuppression increases. Additionally, it tends to be more resistant to conventional therapies, and the incidence and severity of psoriatic arthropathy is increased. Psoriasis often worsens at the time of HIV primary infection.1 We describe a case of a patient with hepatitis B virus (HBV) whose severe plaque psoriasis was controlled on ustekinumab; he was later diagnosed with HIV infection.
A 42-year-old man with HBV treated with entecavir (HBV DNA viral load, <20 copies/mL [inactive carrier, <2000 copies/mL]) presented to our dermatology unit with severe plaque psoriasis (psoriasis area and severity index 23) that caused notable psychologic difficulties such as anxiety and depression. Treatment was attempted with cyclosporine; acitretin; psoralen plus UVA; infliximab; adalimumab; and eventually ustekinumab (45 mg every 3 months), which controlled the condition well (psoriasis area and severity index 0) in an almost completely sustained manner.
Serologic tests requested at one of his analytical control appointments 2 years after initiating treatment with ustekinumab revealed he was HIV positive. The patient reported unprotected sexual intercourse 4 months prior. He was referred to the infectious disease unit and was classified in subtype A1 of HIV infection (CD4 count, 583 cells/µL [reference range, 500-1200 cells/µL]; viral load, 159,268 copies/mL [rapid progression to AIDS, >100,000 copies/mL]). Treatment was initiated with raltegravir, ritonavir, darunavir, and abacavir; tolerance was suitable. Because of the patient’s history of severe psoriasis, treatment with ustekinumab was maintained. Normally, treatment with this drug would be contraindicated in patients with HIV, as it can lead to viral reactivation. Four years after his HIV diagnosis, neither the patient’s cutaneous nor HIV-associated condition had worsened.
For patients with HIV and mild or moderate psoriasis, topical therapies (eg, corticosteroids, vitamin D analogues, tazarotene) are recommended, similar to patients who are HIV negative. Human immunodeficiency virus–positive patients with severe psoriasis who do not respond to topical treatment should receive phototherapy (UVB or psoralen plus UVA) or acitretin along with their antiretroviral drugs.2 In refractory cases, immunosuppressants, including cyclosporine, methotrexate, or tumor necrosis factor α inhibitors, might be used, though experience with them is limited.3,4 Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in patients who receive such immunosuppressants, as is close monitoring of the viral load.
Ustekinumab is an IL-12/IL-23 monoclonal antibody indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, and inflammatory bowel disease. It is contraindicated in patients with clinically important active infections, such as HBV and hepatitis C virus infections.5 However, it was shown to be safe in a group of 18 patients with HBV who had received antiviral prophylaxis6; a degree of reactivation was observed in similar patients who received no such prophylaxis and in others with hepatitis C virus infection.7 The simultaneous use of methotrexate with ustekinumab in the treatment of psoriatic arthritis does not appear to affect the safety of the latter drug.8 Paparizos et al9 described a patient with HIV controlled with antiretroviral drugs who was treated with ustekinumab for psoriasis; no adverse effects were observed.
We report the case of a patient with HBV and psoriasis who was treated with ustekinumab and later became infected with HIV. His ustekinumab treatment was maintained without subsequent cutaneous or systemic complications.
- Menon K, Van Voorhees V, Bebo B, et al. Psoriasis in patients with HIV infection: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Chiricozzi A, Saraceno R, Cannizzaro MV. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy. Dermatology. 2012;225:333-337.
- Barco D, Puig L, Alomar A. Treatment of moderate-severe psoriasis with etanercept in patients with chronic human immunodeficiency virus infection. Actas Dermosifiliogr. 2010;101(suppl 1):77-81.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV positive patient. J Drugs Dermatol. 2014;13:869-871.
- Rustin MH. Long-term safety of biologics in the treatment of moderate to severe plaque psoriasis: review of the current data. Br J Dermatol. 2012;167(suppl 3):3-11.
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616.
- Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515-526.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399.
- Menon K, Van Voorhees V, Bebo B, et al. Psoriasis in patients with HIV infection: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Chiricozzi A, Saraceno R, Cannizzaro MV. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy. Dermatology. 2012;225:333-337.
- Barco D, Puig L, Alomar A. Treatment of moderate-severe psoriasis with etanercept in patients with chronic human immunodeficiency virus infection. Actas Dermosifiliogr. 2010;101(suppl 1):77-81.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV positive patient. J Drugs Dermatol. 2014;13:869-871.
- Rustin MH. Long-term safety of biologics in the treatment of moderate to severe plaque psoriasis: review of the current data. Br J Dermatol. 2012;167(suppl 3):3-11.
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616.
- Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515-526.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399.
Practice Points
- Psoriasis in patients with human immunodeficiency virus (HIV) tends to be more resistant to conventional therapies.
- Experience is limited in the use of immunosuppressants and biologics to treat psoriasis in HIV patients.
- Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in HIV patients who receive biologics, as is close monitoring of the viral load.
Efficacy, Safety, and Tolerability of Halobetasol Propionate 0.01%–Tazarotene 0.045% Lotion for Moderate to Severe Plaque Psoriasis in the Hispanic Population: Post Hoc Analysis
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
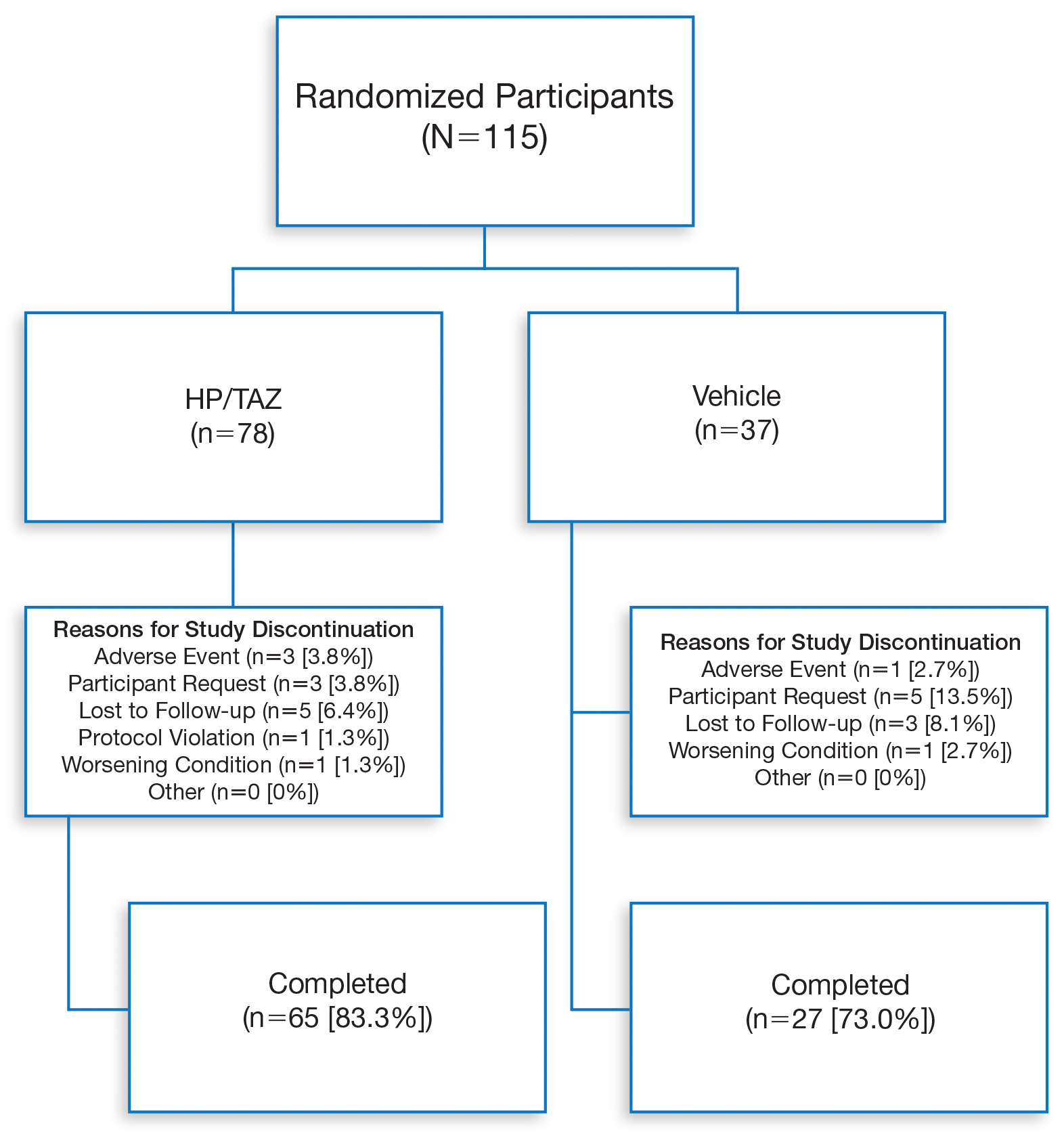
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
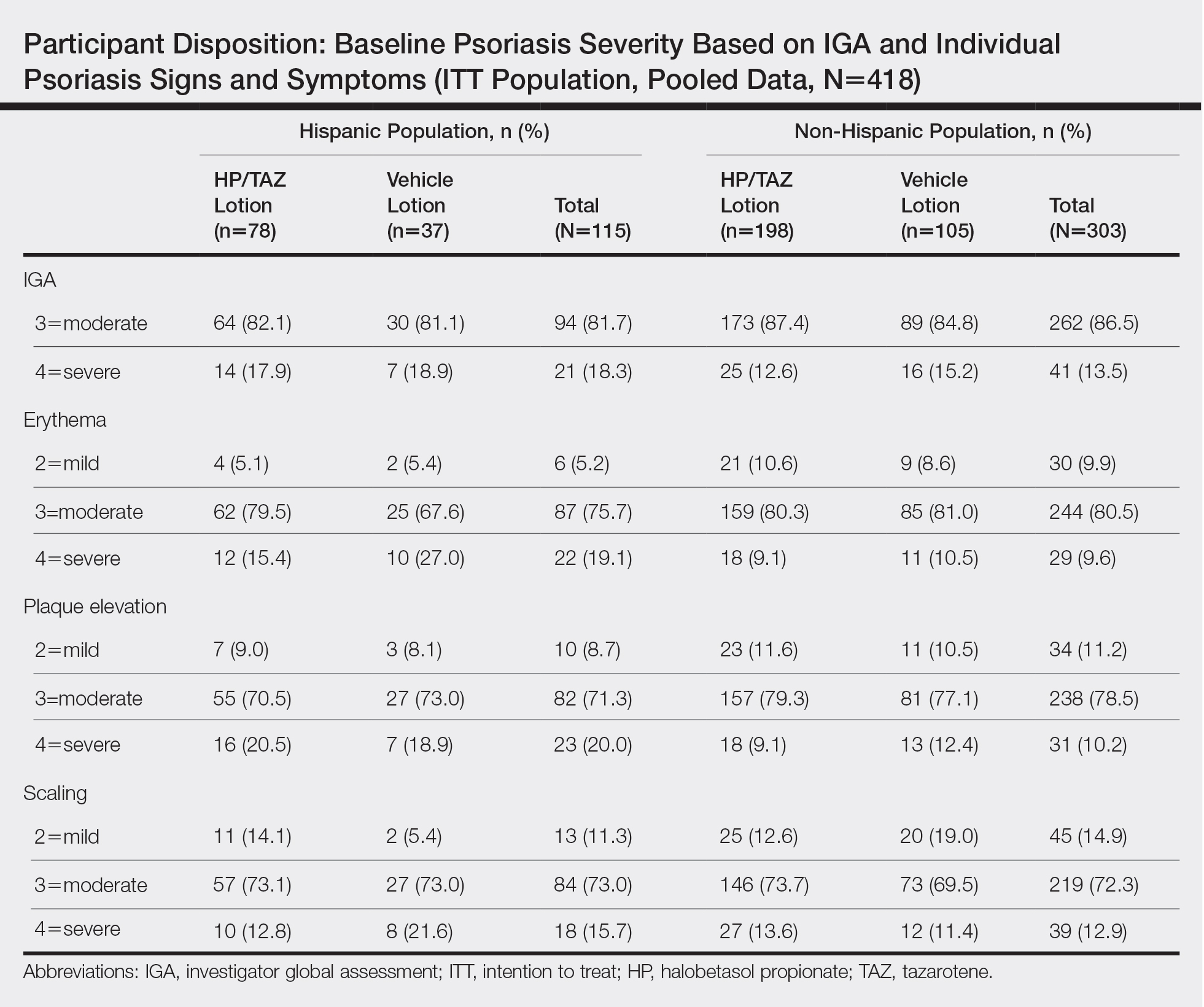
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
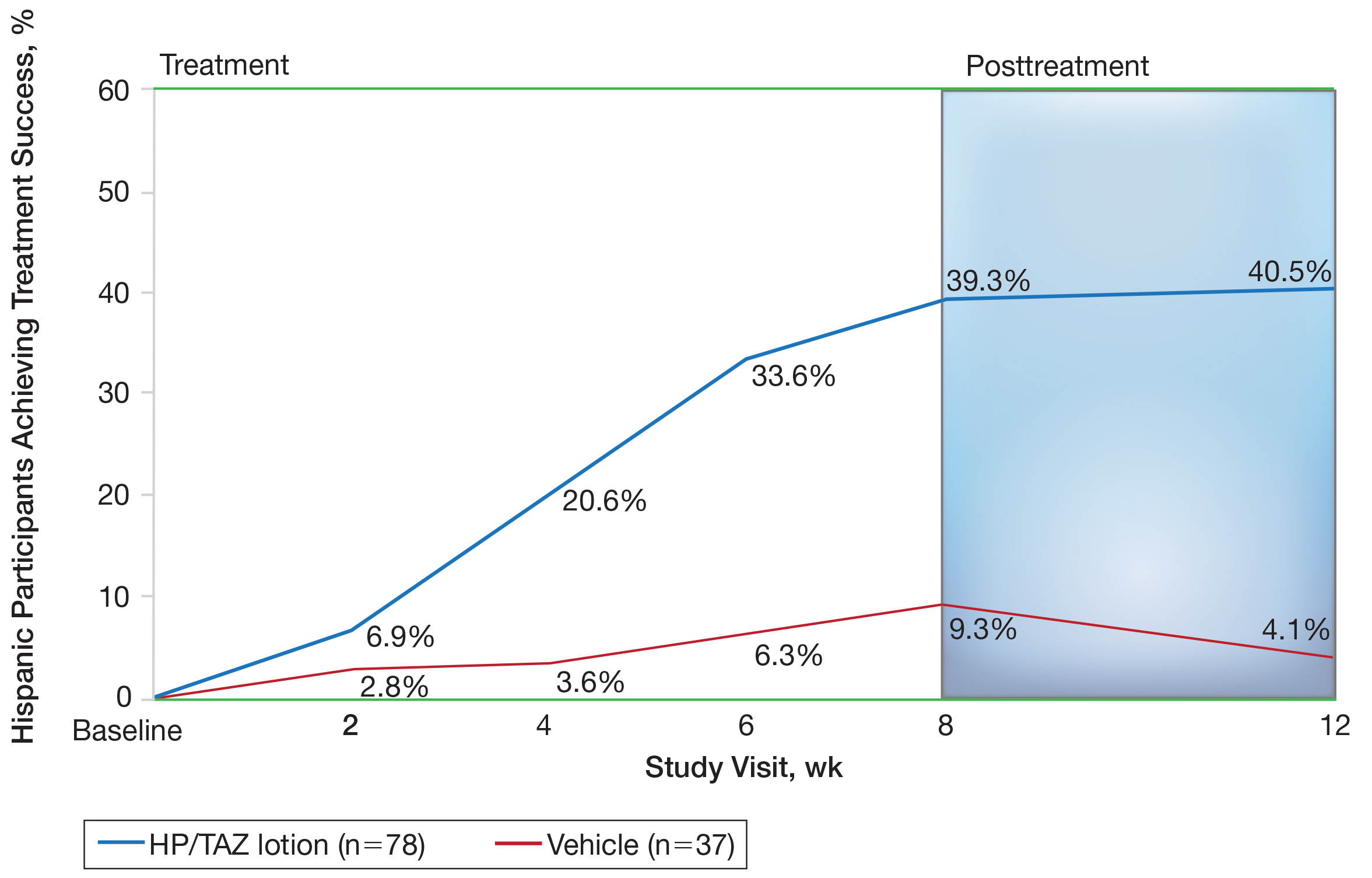
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
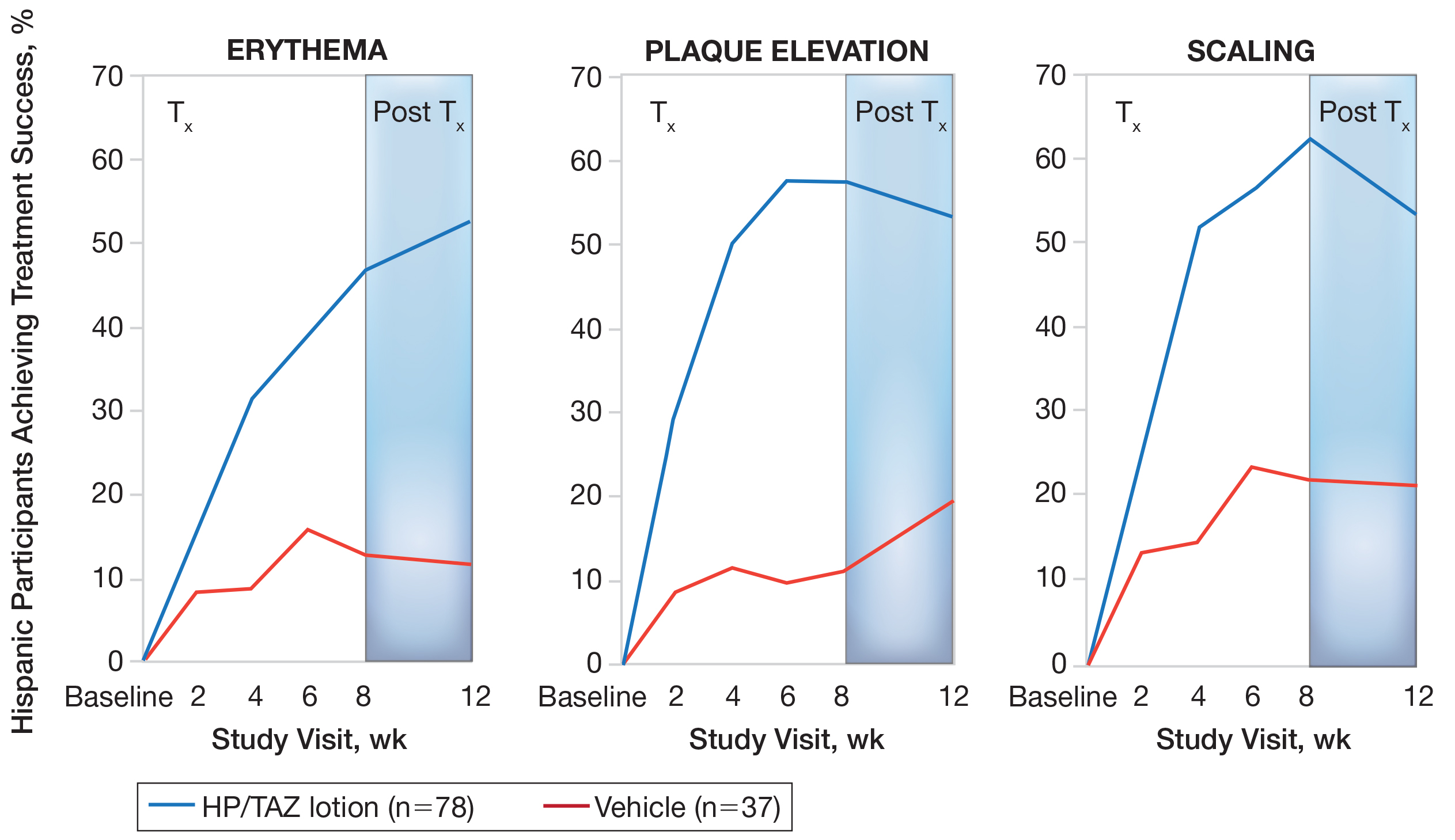
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
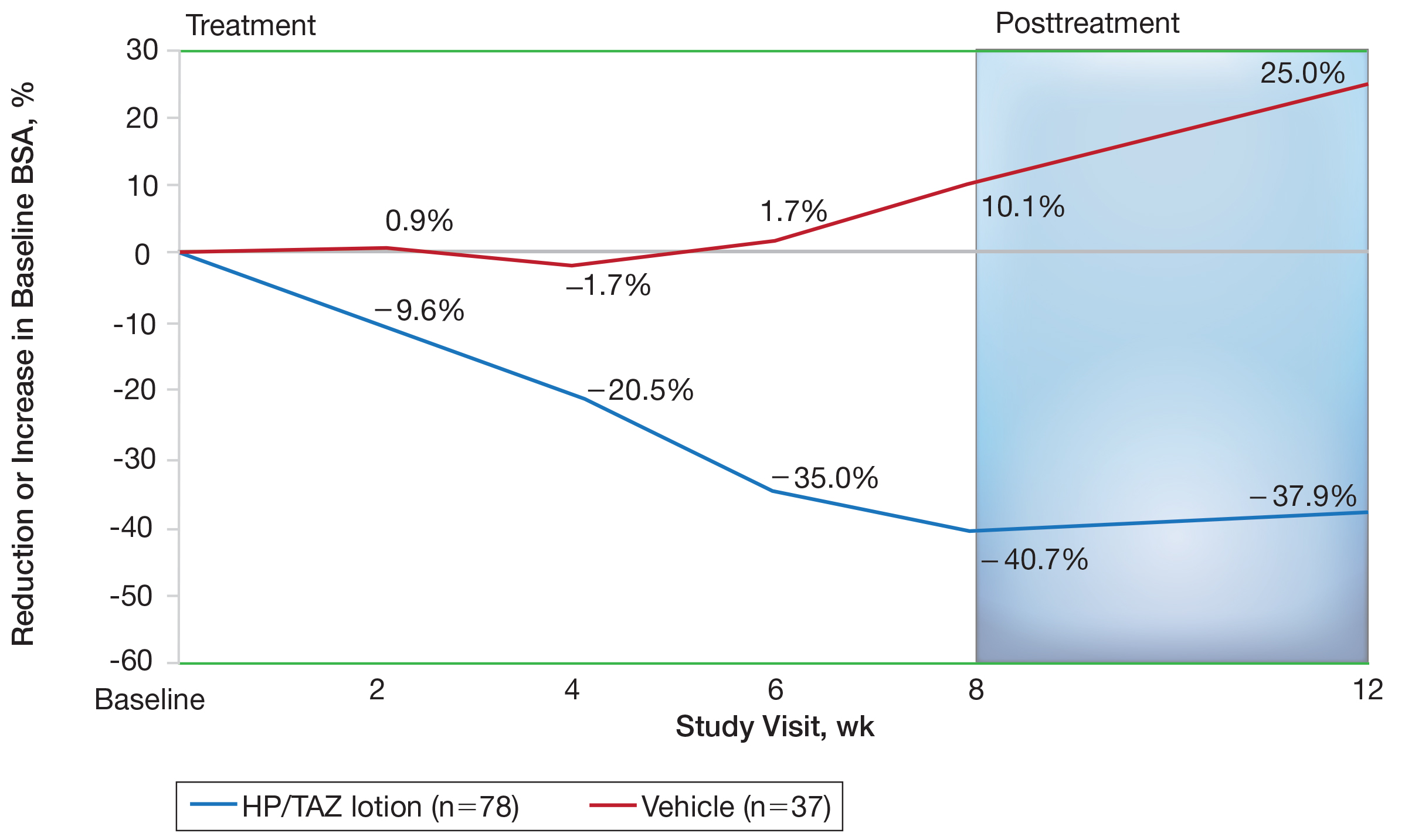
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
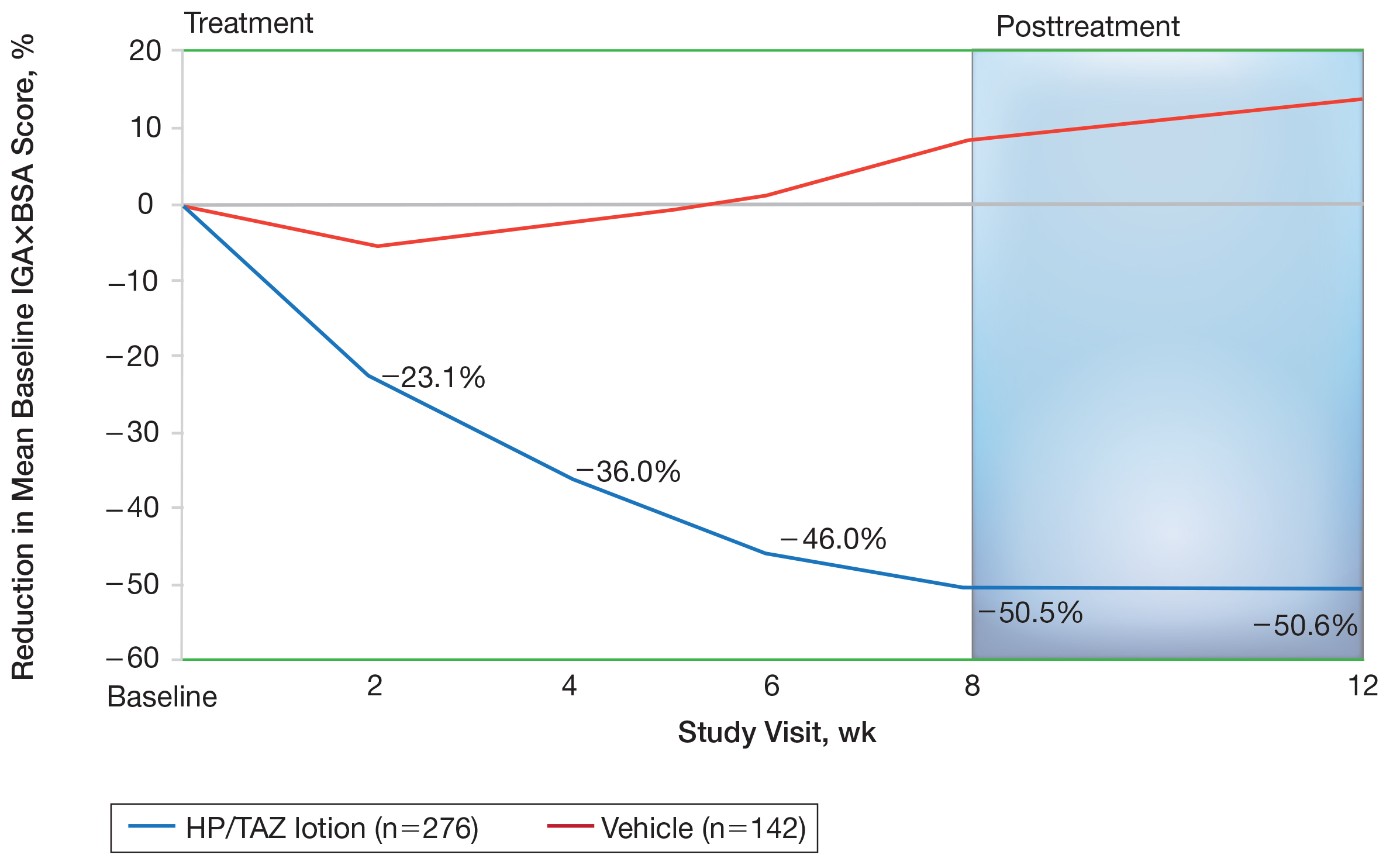
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
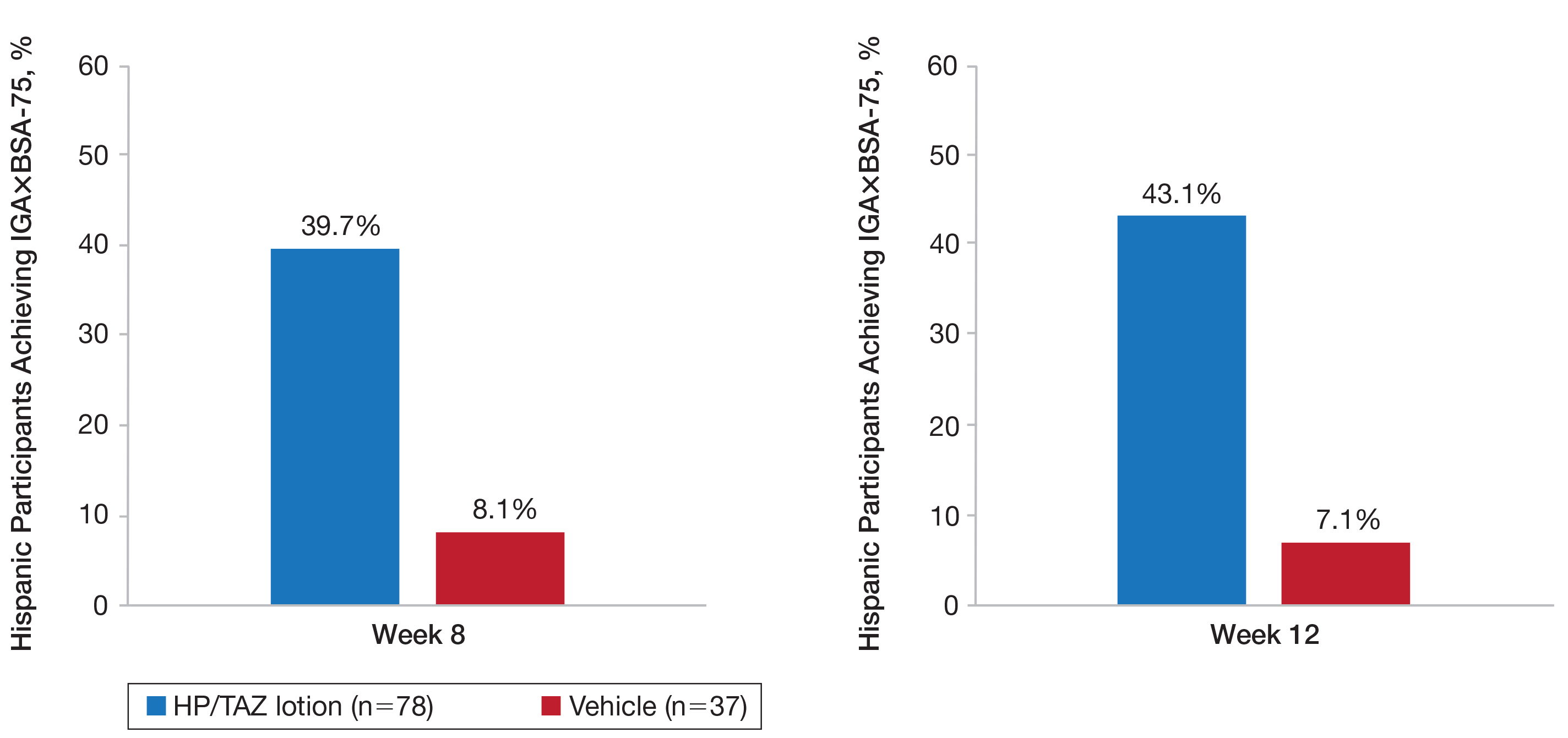
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
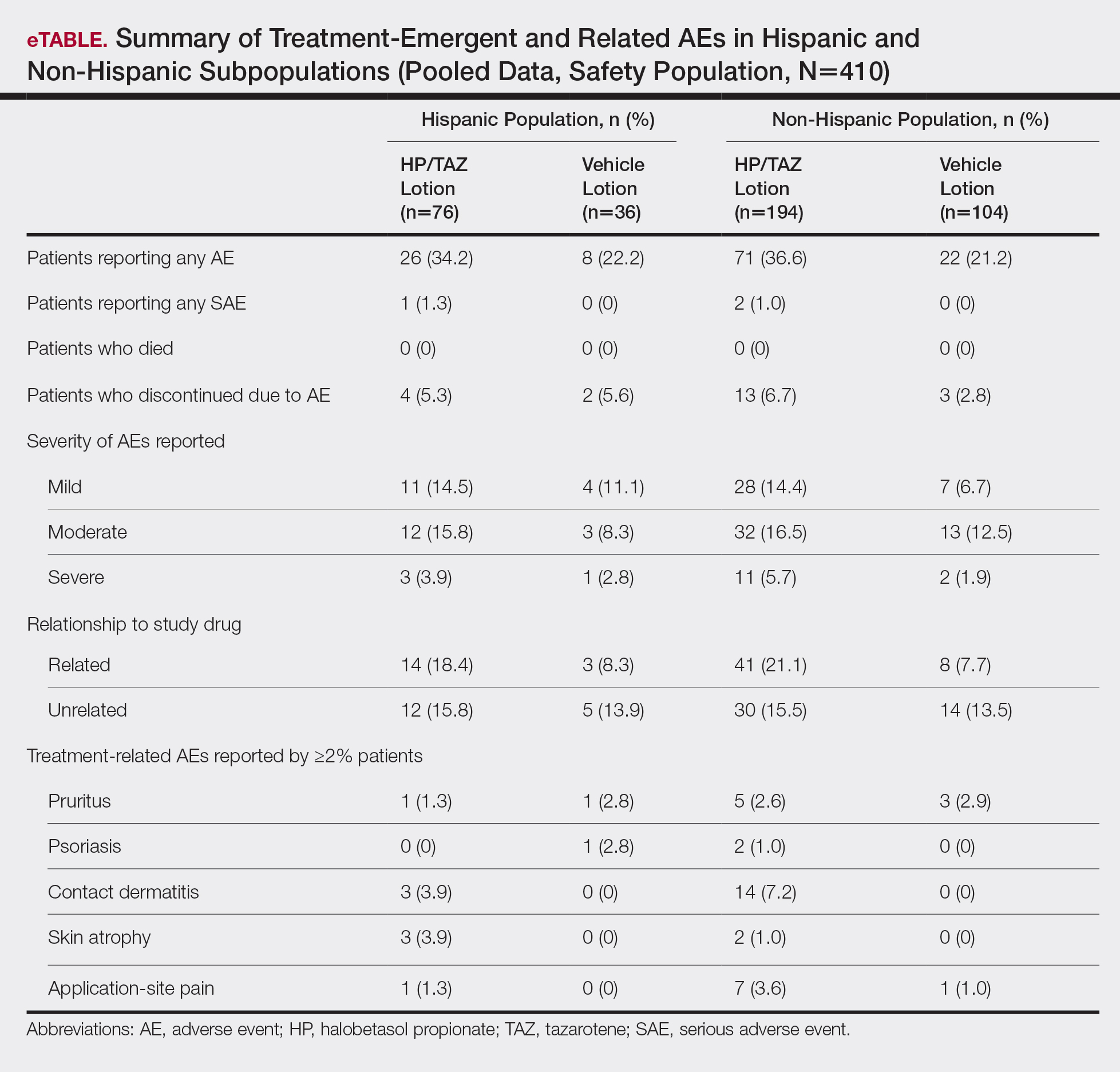
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).

Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).

Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).

Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.

Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).

Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10

A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).

For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.

Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).

Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).

Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).

Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.

Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).

Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10

A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).

For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.

Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Practice Points
- Although psoriasis is a common inflammatory disease, data in the Hispanic population are sparse and disease may be more severe.
- A recent clinical investigation with halobetasol propionate 0.01%–tazarotene 0.045% lotion included a number of Hispanic patients, affording an ideal opportunity to provide important data on this population.
- This fixed-combination therapy was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Keep your eye on tapinarof, a topical antipsoriatic therapy
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Specific markers detect psoriatic disease inflammation without elevated CRP
according to a cross-sectional study of patients and healthy controls.
“Different clinical subsets of psoriatic disease based on skin, entheseal, and joint involvement are characterized by specific inflammation marker profiles,” Maria V. Sokolova, MD, of Friedrich-Alexander University Erlangen-Nuremberg and University Clinic Erlangen (Germany) and colleagues reported in Arthritis Research & Therapy. “Treatment of psoriatic disease with cytokine inhibitors reduces these elevated levels of systemic inflammation markers.”
Quantifying systemic inflammation in psoriatic disease has been a challenge, Dr. Sokolova and colleagues wrote. Levels of C-reactive protein (CRP), a commonly used measure of systemic inflammation, “are often low or absent.” To examine other potential markers of systemic inflammation in psoriatic disease, the investigators conducted cross-sectional and longitudinal studies that included healthy controls and patients with psoriatic disease. Patients had isolated or combined manifestations of psoriatic disease, including the skin, the entheses, and the joints. The researchers grouped patients by isolated psoriatic skin disease; isolated enthesitis; isolated arthritis; psoriatic skin disease with enthesitis; psoriatic skin disease with arthritis; arthritis and enthesitis; and combined psoriatic skin disease, arthritis, and enthesitis.
Data from more than 100 patients
The researchers first assessed 10 potential markers using enzyme-linked immunosorbent assay: calprotectin, interleukin-22, IL-8, lipocalin 2, beta-defensin 2, IL-17, IL-23, vascular endothelial growth factors, LL37 (cathelicidin), and pentraxin 3. They measured the markers in 10 healthy controls and 10 patients with active polymorphic psoriatic arthritis. Five parameters – beta-defensin 2, lipocalin 2, IL-22, IL-8, and calprotectin – significantly differed between healthy controls and patients with psoriatic disease. Lipocalin 2, beta-defensin 2, and IL-22 are associated with IL-17/IL-23 activation, and calprotectin and IL-8 are associated with innate immune cell activation. The other markers did not significantly differ or were not detectable in enough participants.
To validate the signals, the researchers measured the five parameters as well as CRP in 105 controls and 105 patients with psoriatic disease, including 15 patients in each of the seven disease pattern groups. “As expected, CRP levels were normal in the majority of individuals,” the authors wrote. The proportion of patients with CRP greater than 5 mg/L was 0% in isolated psoriatic skin disease, 0% in isolated enthesitis; 20% in isolated arthritis; 7% in psoriatic skin disease with enthesitis; 33% in psoriatic skin disease with arthritis; 27% in arthritis with enthesitis; and 33% in combined psoriatic skin disease, arthritis, and enthesitis.
“Only a subset of patients with arthritis, but not patients with skin or entheseal disease show elevated CRP,” the researchers wrote. “In sharp contrast,” beta-defensin 2 and lipocalin 2 were elevated in a majority of patients with monomorphic skin and entheseal disease, but not in joint disease. “Both proteins were significantly correlated to the extent of skin disease and to a lesser extent also entheseal disease,” they added. Calprotectin and IL-8 were elevated in a majority of patients with joint disease and correlated with the extent of arthritis. “IL-22 was elevated ... in all three manifestations of psoriatic disease,” and the vast majority of patients with polymorphic disease had “widespread marker elevation,” the researchers wrote.
Effects of treatment
In a study of 20 patients with psoriatic arthritis, treatment with secukinumab or adalimumab significantly lowered all five markers. Compared with tumor necrosis factor inhibition with adalimumab, “IL-17 inhibition [with secukinumab] showed a more pronounced lowering of lipocalin 2 and beta-defensin 2 levels,” the investigators noted.
“These results confirm earlier data showing elevated beta-defensin levels in psoriasis patients and its association with the extent of skin involvement,” Dr. Sokolova and colleagues wrote. “Overall, these results offer a new possibility to measure systemic inflammation in psoriatic disease.”
The study was supported by the German Research Foundation and other grant and fellowship funding. The authors had no competing interests.
SOURCE: Sokolova MV et al. Arthritis Res Ther. 2020;22:26.
according to a cross-sectional study of patients and healthy controls.
“Different clinical subsets of psoriatic disease based on skin, entheseal, and joint involvement are characterized by specific inflammation marker profiles,” Maria V. Sokolova, MD, of Friedrich-Alexander University Erlangen-Nuremberg and University Clinic Erlangen (Germany) and colleagues reported in Arthritis Research & Therapy. “Treatment of psoriatic disease with cytokine inhibitors reduces these elevated levels of systemic inflammation markers.”
Quantifying systemic inflammation in psoriatic disease has been a challenge, Dr. Sokolova and colleagues wrote. Levels of C-reactive protein (CRP), a commonly used measure of systemic inflammation, “are often low or absent.” To examine other potential markers of systemic inflammation in psoriatic disease, the investigators conducted cross-sectional and longitudinal studies that included healthy controls and patients with psoriatic disease. Patients had isolated or combined manifestations of psoriatic disease, including the skin, the entheses, and the joints. The researchers grouped patients by isolated psoriatic skin disease; isolated enthesitis; isolated arthritis; psoriatic skin disease with enthesitis; psoriatic skin disease with arthritis; arthritis and enthesitis; and combined psoriatic skin disease, arthritis, and enthesitis.
Data from more than 100 patients
The researchers first assessed 10 potential markers using enzyme-linked immunosorbent assay: calprotectin, interleukin-22, IL-8, lipocalin 2, beta-defensin 2, IL-17, IL-23, vascular endothelial growth factors, LL37 (cathelicidin), and pentraxin 3. They measured the markers in 10 healthy controls and 10 patients with active polymorphic psoriatic arthritis. Five parameters – beta-defensin 2, lipocalin 2, IL-22, IL-8, and calprotectin – significantly differed between healthy controls and patients with psoriatic disease. Lipocalin 2, beta-defensin 2, and IL-22 are associated with IL-17/IL-23 activation, and calprotectin and IL-8 are associated with innate immune cell activation. The other markers did not significantly differ or were not detectable in enough participants.
To validate the signals, the researchers measured the five parameters as well as CRP in 105 controls and 105 patients with psoriatic disease, including 15 patients in each of the seven disease pattern groups. “As expected, CRP levels were normal in the majority of individuals,” the authors wrote. The proportion of patients with CRP greater than 5 mg/L was 0% in isolated psoriatic skin disease, 0% in isolated enthesitis; 20% in isolated arthritis; 7% in psoriatic skin disease with enthesitis; 33% in psoriatic skin disease with arthritis; 27% in arthritis with enthesitis; and 33% in combined psoriatic skin disease, arthritis, and enthesitis.
“Only a subset of patients with arthritis, but not patients with skin or entheseal disease show elevated CRP,” the researchers wrote. “In sharp contrast,” beta-defensin 2 and lipocalin 2 were elevated in a majority of patients with monomorphic skin and entheseal disease, but not in joint disease. “Both proteins were significantly correlated to the extent of skin disease and to a lesser extent also entheseal disease,” they added. Calprotectin and IL-8 were elevated in a majority of patients with joint disease and correlated with the extent of arthritis. “IL-22 was elevated ... in all three manifestations of psoriatic disease,” and the vast majority of patients with polymorphic disease had “widespread marker elevation,” the researchers wrote.
Effects of treatment
In a study of 20 patients with psoriatic arthritis, treatment with secukinumab or adalimumab significantly lowered all five markers. Compared with tumor necrosis factor inhibition with adalimumab, “IL-17 inhibition [with secukinumab] showed a more pronounced lowering of lipocalin 2 and beta-defensin 2 levels,” the investigators noted.
“These results confirm earlier data showing elevated beta-defensin levels in psoriasis patients and its association with the extent of skin involvement,” Dr. Sokolova and colleagues wrote. “Overall, these results offer a new possibility to measure systemic inflammation in psoriatic disease.”
The study was supported by the German Research Foundation and other grant and fellowship funding. The authors had no competing interests.
SOURCE: Sokolova MV et al. Arthritis Res Ther. 2020;22:26.
according to a cross-sectional study of patients and healthy controls.
“Different clinical subsets of psoriatic disease based on skin, entheseal, and joint involvement are characterized by specific inflammation marker profiles,” Maria V. Sokolova, MD, of Friedrich-Alexander University Erlangen-Nuremberg and University Clinic Erlangen (Germany) and colleagues reported in Arthritis Research & Therapy. “Treatment of psoriatic disease with cytokine inhibitors reduces these elevated levels of systemic inflammation markers.”
Quantifying systemic inflammation in psoriatic disease has been a challenge, Dr. Sokolova and colleagues wrote. Levels of C-reactive protein (CRP), a commonly used measure of systemic inflammation, “are often low or absent.” To examine other potential markers of systemic inflammation in psoriatic disease, the investigators conducted cross-sectional and longitudinal studies that included healthy controls and patients with psoriatic disease. Patients had isolated or combined manifestations of psoriatic disease, including the skin, the entheses, and the joints. The researchers grouped patients by isolated psoriatic skin disease; isolated enthesitis; isolated arthritis; psoriatic skin disease with enthesitis; psoriatic skin disease with arthritis; arthritis and enthesitis; and combined psoriatic skin disease, arthritis, and enthesitis.
Data from more than 100 patients
The researchers first assessed 10 potential markers using enzyme-linked immunosorbent assay: calprotectin, interleukin-22, IL-8, lipocalin 2, beta-defensin 2, IL-17, IL-23, vascular endothelial growth factors, LL37 (cathelicidin), and pentraxin 3. They measured the markers in 10 healthy controls and 10 patients with active polymorphic psoriatic arthritis. Five parameters – beta-defensin 2, lipocalin 2, IL-22, IL-8, and calprotectin – significantly differed between healthy controls and patients with psoriatic disease. Lipocalin 2, beta-defensin 2, and IL-22 are associated with IL-17/IL-23 activation, and calprotectin and IL-8 are associated with innate immune cell activation. The other markers did not significantly differ or were not detectable in enough participants.
To validate the signals, the researchers measured the five parameters as well as CRP in 105 controls and 105 patients with psoriatic disease, including 15 patients in each of the seven disease pattern groups. “As expected, CRP levels were normal in the majority of individuals,” the authors wrote. The proportion of patients with CRP greater than 5 mg/L was 0% in isolated psoriatic skin disease, 0% in isolated enthesitis; 20% in isolated arthritis; 7% in psoriatic skin disease with enthesitis; 33% in psoriatic skin disease with arthritis; 27% in arthritis with enthesitis; and 33% in combined psoriatic skin disease, arthritis, and enthesitis.
“Only a subset of patients with arthritis, but not patients with skin or entheseal disease show elevated CRP,” the researchers wrote. “In sharp contrast,” beta-defensin 2 and lipocalin 2 were elevated in a majority of patients with monomorphic skin and entheseal disease, but not in joint disease. “Both proteins were significantly correlated to the extent of skin disease and to a lesser extent also entheseal disease,” they added. Calprotectin and IL-8 were elevated in a majority of patients with joint disease and correlated with the extent of arthritis. “IL-22 was elevated ... in all three manifestations of psoriatic disease,” and the vast majority of patients with polymorphic disease had “widespread marker elevation,” the researchers wrote.
Effects of treatment
In a study of 20 patients with psoriatic arthritis, treatment with secukinumab or adalimumab significantly lowered all five markers. Compared with tumor necrosis factor inhibition with adalimumab, “IL-17 inhibition [with secukinumab] showed a more pronounced lowering of lipocalin 2 and beta-defensin 2 levels,” the investigators noted.
“These results confirm earlier data showing elevated beta-defensin levels in psoriasis patients and its association with the extent of skin involvement,” Dr. Sokolova and colleagues wrote. “Overall, these results offer a new possibility to measure systemic inflammation in psoriatic disease.”
The study was supported by the German Research Foundation and other grant and fellowship funding. The authors had no competing interests.
SOURCE: Sokolova MV et al. Arthritis Res Ther. 2020;22:26.
FROM ARTHRITIS RESEARCH & THERAPY
Psoriasis elevates cancer risk
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
FROM JAMA DERMATOLOGY
Nonspecific musculoskeletal symptoms might indicate early PsA
People with psoriatic arthritis can be symptomatic for years before the condition is diagnosed, according to two recent reports.
There are no reliable diagnostic biomarkers, and sometimes patients have vague symptoms with only minimal physical findings, which makes it hard for physicians to recognize the problem and refer to rheumatology.
In the meantime, the longer it takes to diagnose psoriatic arthritis (PsA) and treat it properly, the worse off patients are when it’s finally caught. They “present with a greater rate of clinical progression and worse physical function, compared with patients with an undelayed diagnosis,” and more radiographic joint damage, according to investigators led by rheumatologist Alexis Ogdie, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia.
Dr. Ogdie’s study in BMC Rheumatology, and a second one from Arthritis Care & Research, both described the early phase of psoriatic arthritis, before formal diagnosis, to help with early recognition.
Delay associated with misdiagnosis
Dr. Ogdie’s team surveyed 203 adults with PsA – average age of 52 years, mostly white, and over 80% women – about their diagnosis history. The time between seeking medical attention for PsA-related symptoms and receiving a diagnosis was less than 6 months for 69 participants, 6 months to 4 years for 68, and 5 years or more for 66.
Typical symptoms, like joint pain, swollen joints, reduced range of motion, and dactylitis, were associated with quicker diagnosis. Turning early to dermatologists and rheumatologists – instead of general practitioners, orthopedics, chiropractors, and others – sped diagnosis, as well. People diagnosed within 6 months also tended to be slightly older, were less likely to be disabled or unemployed, have more education, and were more likely to make $100,000 per year or more.
Vaguer symptoms, such as stiffness, fatigue, and enthesitis-associated foot pain, delayed diagnosis. The longer PsA went unrecognized, the more likely people were to be misdiagnosed with osteoarthritis, psychosomatic disorders, and other problems.
“Increased recognition of heterogeneous symptoms associated with PsA, as well as understanding existing diagnostic barriers, may lead to prompt diagnosis and initiation of appropriate treatment that may improve outcomes,” the investigators concluded.
A prodromal phase
In the Arthritis Care & Research study, investigators led by Lihi Eder, MD, PhD, codirector of the cardio-rheumatology program at Women’s College Hospital, Toronto, used health records and databases to compare primary care histories of 462 Canadian PsA patients in the 5 years before they were diagnosed with 2,310 age- and sex-matched controls without PsA and treated by the same family physicians. The mean age in the study was 54 years, and just over half the subjects were women. Socioeconomic status and rurality were similar between the two groups.
The mean time from the initial primary care visit for a musculoskeletal complaint to rheumatology referral was 513 days among PsA patients, “which was substantially longer than for other inflammatory arthritic conditions, such as rheumatoid arthritis,” Dr. Eder and associates noted.
PsA patients were more than twice as likely to visit primary care for nonspecific musculoskeletal issues in the year before their diagnosis, and more likely in the 5 years prior. The odds of visits to musculoskeletal specialists, joint injections, joint imaging, and ED visits, was also higher as early as 5 years before PsA recognition, and hinted at the impending diagnosis.
“Our study characterized a prediagnosis period in PsA and supports the notion that a prodromal PsA phase occurs in a significant proportion of patients. ... This pattern reveals some of the underlying causes of diagnosis delays of PsA and highlights the need for diagnostic strategies and novel reliable biomarkers to aid in early diagnosis of PsA,” the investigators concluded.
Dr. Ogdie and colleagues suggested that community case searches, public awareness programs, patient education, and referral guidelines for primary care providers might help. They also suggested greater use of validated screening tools, such as the Psoriasis Epidemiology Screening Tool, in primary care.
Dr. Eder had no disclosures, and her study was funded by the Canadian Rheumatology Association. Dr. Ogdie’s study was funded by Novartis, maker of secukinumab (Cosentyx), which is indicated for PsA. She is a consultant for Novartis and has received grant support from the company. One author is an employee.
SOURCES: Ogdie A et al. BMC Rheumatol. 2020 Jan 10. doi: 10.1186/s41927-019-0102-7; Eder L et al. Arthritis Care Res. 2020 Jan 21. doi: 10.1002/acr.24146.
People with psoriatic arthritis can be symptomatic for years before the condition is diagnosed, according to two recent reports.
There are no reliable diagnostic biomarkers, and sometimes patients have vague symptoms with only minimal physical findings, which makes it hard for physicians to recognize the problem and refer to rheumatology.
In the meantime, the longer it takes to diagnose psoriatic arthritis (PsA) and treat it properly, the worse off patients are when it’s finally caught. They “present with a greater rate of clinical progression and worse physical function, compared with patients with an undelayed diagnosis,” and more radiographic joint damage, according to investigators led by rheumatologist Alexis Ogdie, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia.
Dr. Ogdie’s study in BMC Rheumatology, and a second one from Arthritis Care & Research, both described the early phase of psoriatic arthritis, before formal diagnosis, to help with early recognition.
Delay associated with misdiagnosis
Dr. Ogdie’s team surveyed 203 adults with PsA – average age of 52 years, mostly white, and over 80% women – about their diagnosis history. The time between seeking medical attention for PsA-related symptoms and receiving a diagnosis was less than 6 months for 69 participants, 6 months to 4 years for 68, and 5 years or more for 66.
Typical symptoms, like joint pain, swollen joints, reduced range of motion, and dactylitis, were associated with quicker diagnosis. Turning early to dermatologists and rheumatologists – instead of general practitioners, orthopedics, chiropractors, and others – sped diagnosis, as well. People diagnosed within 6 months also tended to be slightly older, were less likely to be disabled or unemployed, have more education, and were more likely to make $100,000 per year or more.
Vaguer symptoms, such as stiffness, fatigue, and enthesitis-associated foot pain, delayed diagnosis. The longer PsA went unrecognized, the more likely people were to be misdiagnosed with osteoarthritis, psychosomatic disorders, and other problems.
“Increased recognition of heterogeneous symptoms associated with PsA, as well as understanding existing diagnostic barriers, may lead to prompt diagnosis and initiation of appropriate treatment that may improve outcomes,” the investigators concluded.
A prodromal phase
In the Arthritis Care & Research study, investigators led by Lihi Eder, MD, PhD, codirector of the cardio-rheumatology program at Women’s College Hospital, Toronto, used health records and databases to compare primary care histories of 462 Canadian PsA patients in the 5 years before they were diagnosed with 2,310 age- and sex-matched controls without PsA and treated by the same family physicians. The mean age in the study was 54 years, and just over half the subjects were women. Socioeconomic status and rurality were similar between the two groups.
The mean time from the initial primary care visit for a musculoskeletal complaint to rheumatology referral was 513 days among PsA patients, “which was substantially longer than for other inflammatory arthritic conditions, such as rheumatoid arthritis,” Dr. Eder and associates noted.
PsA patients were more than twice as likely to visit primary care for nonspecific musculoskeletal issues in the year before their diagnosis, and more likely in the 5 years prior. The odds of visits to musculoskeletal specialists, joint injections, joint imaging, and ED visits, was also higher as early as 5 years before PsA recognition, and hinted at the impending diagnosis.
“Our study characterized a prediagnosis period in PsA and supports the notion that a prodromal PsA phase occurs in a significant proportion of patients. ... This pattern reveals some of the underlying causes of diagnosis delays of PsA and highlights the need for diagnostic strategies and novel reliable biomarkers to aid in early diagnosis of PsA,” the investigators concluded.
Dr. Ogdie and colleagues suggested that community case searches, public awareness programs, patient education, and referral guidelines for primary care providers might help. They also suggested greater use of validated screening tools, such as the Psoriasis Epidemiology Screening Tool, in primary care.
Dr. Eder had no disclosures, and her study was funded by the Canadian Rheumatology Association. Dr. Ogdie’s study was funded by Novartis, maker of secukinumab (Cosentyx), which is indicated for PsA. She is a consultant for Novartis and has received grant support from the company. One author is an employee.
SOURCES: Ogdie A et al. BMC Rheumatol. 2020 Jan 10. doi: 10.1186/s41927-019-0102-7; Eder L et al. Arthritis Care Res. 2020 Jan 21. doi: 10.1002/acr.24146.
People with psoriatic arthritis can be symptomatic for years before the condition is diagnosed, according to two recent reports.
There are no reliable diagnostic biomarkers, and sometimes patients have vague symptoms with only minimal physical findings, which makes it hard for physicians to recognize the problem and refer to rheumatology.
In the meantime, the longer it takes to diagnose psoriatic arthritis (PsA) and treat it properly, the worse off patients are when it’s finally caught. They “present with a greater rate of clinical progression and worse physical function, compared with patients with an undelayed diagnosis,” and more radiographic joint damage, according to investigators led by rheumatologist Alexis Ogdie, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia.
Dr. Ogdie’s study in BMC Rheumatology, and a second one from Arthritis Care & Research, both described the early phase of psoriatic arthritis, before formal diagnosis, to help with early recognition.
Delay associated with misdiagnosis
Dr. Ogdie’s team surveyed 203 adults with PsA – average age of 52 years, mostly white, and over 80% women – about their diagnosis history. The time between seeking medical attention for PsA-related symptoms and receiving a diagnosis was less than 6 months for 69 participants, 6 months to 4 years for 68, and 5 years or more for 66.
Typical symptoms, like joint pain, swollen joints, reduced range of motion, and dactylitis, were associated with quicker diagnosis. Turning early to dermatologists and rheumatologists – instead of general practitioners, orthopedics, chiropractors, and others – sped diagnosis, as well. People diagnosed within 6 months also tended to be slightly older, were less likely to be disabled or unemployed, have more education, and were more likely to make $100,000 per year or more.
Vaguer symptoms, such as stiffness, fatigue, and enthesitis-associated foot pain, delayed diagnosis. The longer PsA went unrecognized, the more likely people were to be misdiagnosed with osteoarthritis, psychosomatic disorders, and other problems.
“Increased recognition of heterogeneous symptoms associated with PsA, as well as understanding existing diagnostic barriers, may lead to prompt diagnosis and initiation of appropriate treatment that may improve outcomes,” the investigators concluded.
A prodromal phase
In the Arthritis Care & Research study, investigators led by Lihi Eder, MD, PhD, codirector of the cardio-rheumatology program at Women’s College Hospital, Toronto, used health records and databases to compare primary care histories of 462 Canadian PsA patients in the 5 years before they were diagnosed with 2,310 age- and sex-matched controls without PsA and treated by the same family physicians. The mean age in the study was 54 years, and just over half the subjects were women. Socioeconomic status and rurality were similar between the two groups.
The mean time from the initial primary care visit for a musculoskeletal complaint to rheumatology referral was 513 days among PsA patients, “which was substantially longer than for other inflammatory arthritic conditions, such as rheumatoid arthritis,” Dr. Eder and associates noted.
PsA patients were more than twice as likely to visit primary care for nonspecific musculoskeletal issues in the year before their diagnosis, and more likely in the 5 years prior. The odds of visits to musculoskeletal specialists, joint injections, joint imaging, and ED visits, was also higher as early as 5 years before PsA recognition, and hinted at the impending diagnosis.
“Our study characterized a prediagnosis period in PsA and supports the notion that a prodromal PsA phase occurs in a significant proportion of patients. ... This pattern reveals some of the underlying causes of diagnosis delays of PsA and highlights the need for diagnostic strategies and novel reliable biomarkers to aid in early diagnosis of PsA,” the investigators concluded.
Dr. Ogdie and colleagues suggested that community case searches, public awareness programs, patient education, and referral guidelines for primary care providers might help. They also suggested greater use of validated screening tools, such as the Psoriasis Epidemiology Screening Tool, in primary care.
Dr. Eder had no disclosures, and her study was funded by the Canadian Rheumatology Association. Dr. Ogdie’s study was funded by Novartis, maker of secukinumab (Cosentyx), which is indicated for PsA. She is a consultant for Novartis and has received grant support from the company. One author is an employee.
SOURCES: Ogdie A et al. BMC Rheumatol. 2020 Jan 10. doi: 10.1186/s41927-019-0102-7; Eder L et al. Arthritis Care Res. 2020 Jan 21. doi: 10.1002/acr.24146.
FROM BMC RHEUMATOLOGY AND ARTHRITIS CARE & RESEARCH
Tildrakizumab signals safe for pregnant psoriasis patients
A post hoc analysis of .
“Although contraception in female patients of childbearing age was mandatory before initiation of and during tildrakizumab therapy, some pregnancies occurred during the tildrakizumab clinical development program as protocol violations,” wrote Kathleen Haycraft, MD, of Riverside Dermatology & Spa, Hannibal, Mo., and colleagues.
Tildrakizumab (Ilumya), an interleukin-23 antagonist, was approved in 2018 by the Food and Drug Administration for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Effects on birth outcomes or on neonates exposed during pregnancy have not been studied, the researchers said.
“Tildrakizumab plasma half-life after subcutaneous administration is approximately 25 days; therefore, tildrakizumab administered even in the first trimester may cross the placental barrier,” they noted.
In a research letter published in the British Journal of Dermatology, the investigators reviewed data from nine phase 1, 2, and 3 clinical trials and identified 528 women of childbearing age who received tildrakizumab. Fourteen pregnancies were reported among these women: six from a contraceptive failure, and eight for lack of contraception use. (One of the phase 1 trials was in patients with Crohn’s disease, which included one of the pregnancies; the rest were in patients with psoriasis.)
The 14 pregnancy outcomes included 2 spontaneous abortions (14.3%), 4 elective abortions (28.6%), and 8 live births (57.1%), which included 1 premature birth, with “no identifiable congenital anomalies,” the authors wrote. The longest duration of exposure to tildrakizumab in a pregnant woman was 1,196 days; this pregnancy resulted in a premature live birth at 36 weeks with no anomalies. The spontaneous abortion rate was similar to the rate in the general population, which is 12%-15%, the authors noted.
While the study “adds to the existing evidence on the outcomes of biologic treatment of psoriasis,” the findings were limited by several factors including the small number of pregnancies, short duration of exposure to tildrakizumab, variations in dosing, and lack of controls, the researchers noted. “Additional data from a larger population following tildrakizumab exposure are required to fully evaluate the safety and tolerability of tildrakizumab treatment during pregnancy,” they said. In the meantime, they advised women of childbearing age with psoriasis to continue to avoid pregnancy and follow practice guidelines for contraceptive use while taking the biologic therapy.
The studies were supported by Merck Sharp & Dohme, a Merck & Co. subsidiary; analyses were supported by Sun Pharmaceutical Industries. Lead author Dr. Haycraft disclosed relationships with companies including Sun, Celgene, Lilly, Novartis, Ortho-Derm, and Pfizer. Other authors disclosed relationships with Novartis, Celgene, Ortho Dermatologics, Janssen, and Merck; two authors are Sun employees.
SOURCE: Haycraft K et al. Br J Dermatol. 2020 Jan 29. doi: 10.1111/bjd.18897.
A post hoc analysis of .
“Although contraception in female patients of childbearing age was mandatory before initiation of and during tildrakizumab therapy, some pregnancies occurred during the tildrakizumab clinical development program as protocol violations,” wrote Kathleen Haycraft, MD, of Riverside Dermatology & Spa, Hannibal, Mo., and colleagues.
Tildrakizumab (Ilumya), an interleukin-23 antagonist, was approved in 2018 by the Food and Drug Administration for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Effects on birth outcomes or on neonates exposed during pregnancy have not been studied, the researchers said.
“Tildrakizumab plasma half-life after subcutaneous administration is approximately 25 days; therefore, tildrakizumab administered even in the first trimester may cross the placental barrier,” they noted.
In a research letter published in the British Journal of Dermatology, the investigators reviewed data from nine phase 1, 2, and 3 clinical trials and identified 528 women of childbearing age who received tildrakizumab. Fourteen pregnancies were reported among these women: six from a contraceptive failure, and eight for lack of contraception use. (One of the phase 1 trials was in patients with Crohn’s disease, which included one of the pregnancies; the rest were in patients with psoriasis.)
The 14 pregnancy outcomes included 2 spontaneous abortions (14.3%), 4 elective abortions (28.6%), and 8 live births (57.1%), which included 1 premature birth, with “no identifiable congenital anomalies,” the authors wrote. The longest duration of exposure to tildrakizumab in a pregnant woman was 1,196 days; this pregnancy resulted in a premature live birth at 36 weeks with no anomalies. The spontaneous abortion rate was similar to the rate in the general population, which is 12%-15%, the authors noted.
While the study “adds to the existing evidence on the outcomes of biologic treatment of psoriasis,” the findings were limited by several factors including the small number of pregnancies, short duration of exposure to tildrakizumab, variations in dosing, and lack of controls, the researchers noted. “Additional data from a larger population following tildrakizumab exposure are required to fully evaluate the safety and tolerability of tildrakizumab treatment during pregnancy,” they said. In the meantime, they advised women of childbearing age with psoriasis to continue to avoid pregnancy and follow practice guidelines for contraceptive use while taking the biologic therapy.
The studies were supported by Merck Sharp & Dohme, a Merck & Co. subsidiary; analyses were supported by Sun Pharmaceutical Industries. Lead author Dr. Haycraft disclosed relationships with companies including Sun, Celgene, Lilly, Novartis, Ortho-Derm, and Pfizer. Other authors disclosed relationships with Novartis, Celgene, Ortho Dermatologics, Janssen, and Merck; two authors are Sun employees.
SOURCE: Haycraft K et al. Br J Dermatol. 2020 Jan 29. doi: 10.1111/bjd.18897.
A post hoc analysis of .
“Although contraception in female patients of childbearing age was mandatory before initiation of and during tildrakizumab therapy, some pregnancies occurred during the tildrakizumab clinical development program as protocol violations,” wrote Kathleen Haycraft, MD, of Riverside Dermatology & Spa, Hannibal, Mo., and colleagues.
Tildrakizumab (Ilumya), an interleukin-23 antagonist, was approved in 2018 by the Food and Drug Administration for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Effects on birth outcomes or on neonates exposed during pregnancy have not been studied, the researchers said.
“Tildrakizumab plasma half-life after subcutaneous administration is approximately 25 days; therefore, tildrakizumab administered even in the first trimester may cross the placental barrier,” they noted.
In a research letter published in the British Journal of Dermatology, the investigators reviewed data from nine phase 1, 2, and 3 clinical trials and identified 528 women of childbearing age who received tildrakizumab. Fourteen pregnancies were reported among these women: six from a contraceptive failure, and eight for lack of contraception use. (One of the phase 1 trials was in patients with Crohn’s disease, which included one of the pregnancies; the rest were in patients with psoriasis.)
The 14 pregnancy outcomes included 2 spontaneous abortions (14.3%), 4 elective abortions (28.6%), and 8 live births (57.1%), which included 1 premature birth, with “no identifiable congenital anomalies,” the authors wrote. The longest duration of exposure to tildrakizumab in a pregnant woman was 1,196 days; this pregnancy resulted in a premature live birth at 36 weeks with no anomalies. The spontaneous abortion rate was similar to the rate in the general population, which is 12%-15%, the authors noted.
While the study “adds to the existing evidence on the outcomes of biologic treatment of psoriasis,” the findings were limited by several factors including the small number of pregnancies, short duration of exposure to tildrakizumab, variations in dosing, and lack of controls, the researchers noted. “Additional data from a larger population following tildrakizumab exposure are required to fully evaluate the safety and tolerability of tildrakizumab treatment during pregnancy,” they said. In the meantime, they advised women of childbearing age with psoriasis to continue to avoid pregnancy and follow practice guidelines for contraceptive use while taking the biologic therapy.
The studies were supported by Merck Sharp & Dohme, a Merck & Co. subsidiary; analyses were supported by Sun Pharmaceutical Industries. Lead author Dr. Haycraft disclosed relationships with companies including Sun, Celgene, Lilly, Novartis, Ortho-Derm, and Pfizer. Other authors disclosed relationships with Novartis, Celgene, Ortho Dermatologics, Janssen, and Merck; two authors are Sun employees.
SOURCE: Haycraft K et al. Br J Dermatol. 2020 Jan 29. doi: 10.1111/bjd.18897.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Effect of In-Office Samples on Dermatologists’ Prescribing Habits: A Retrospective Review
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).
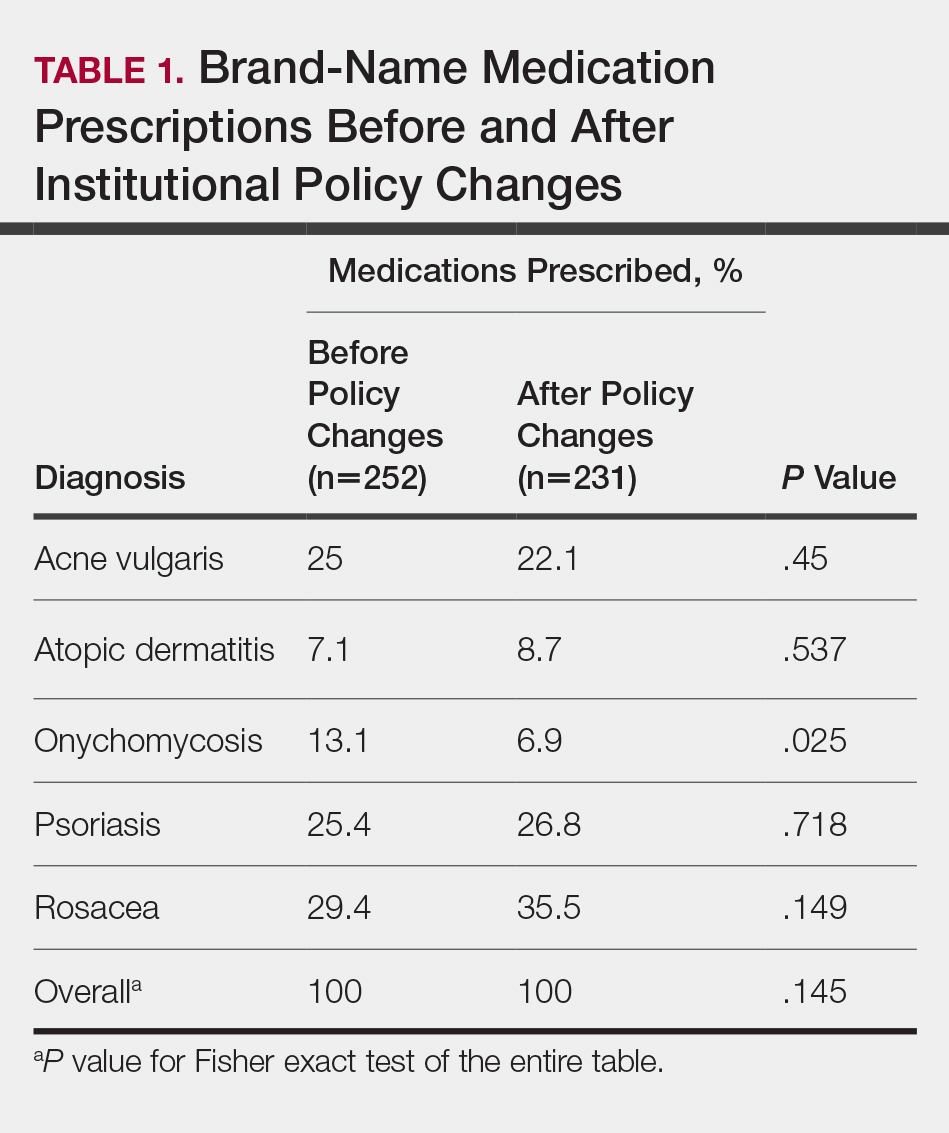

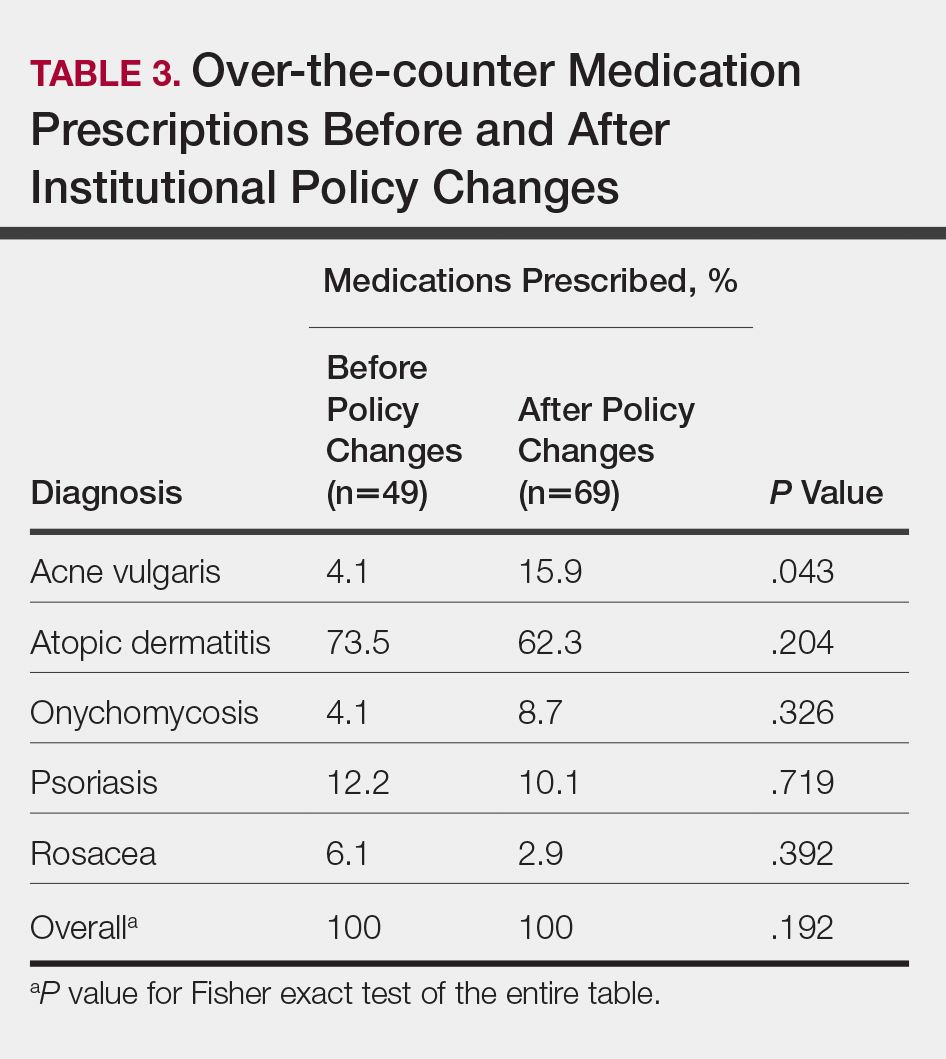
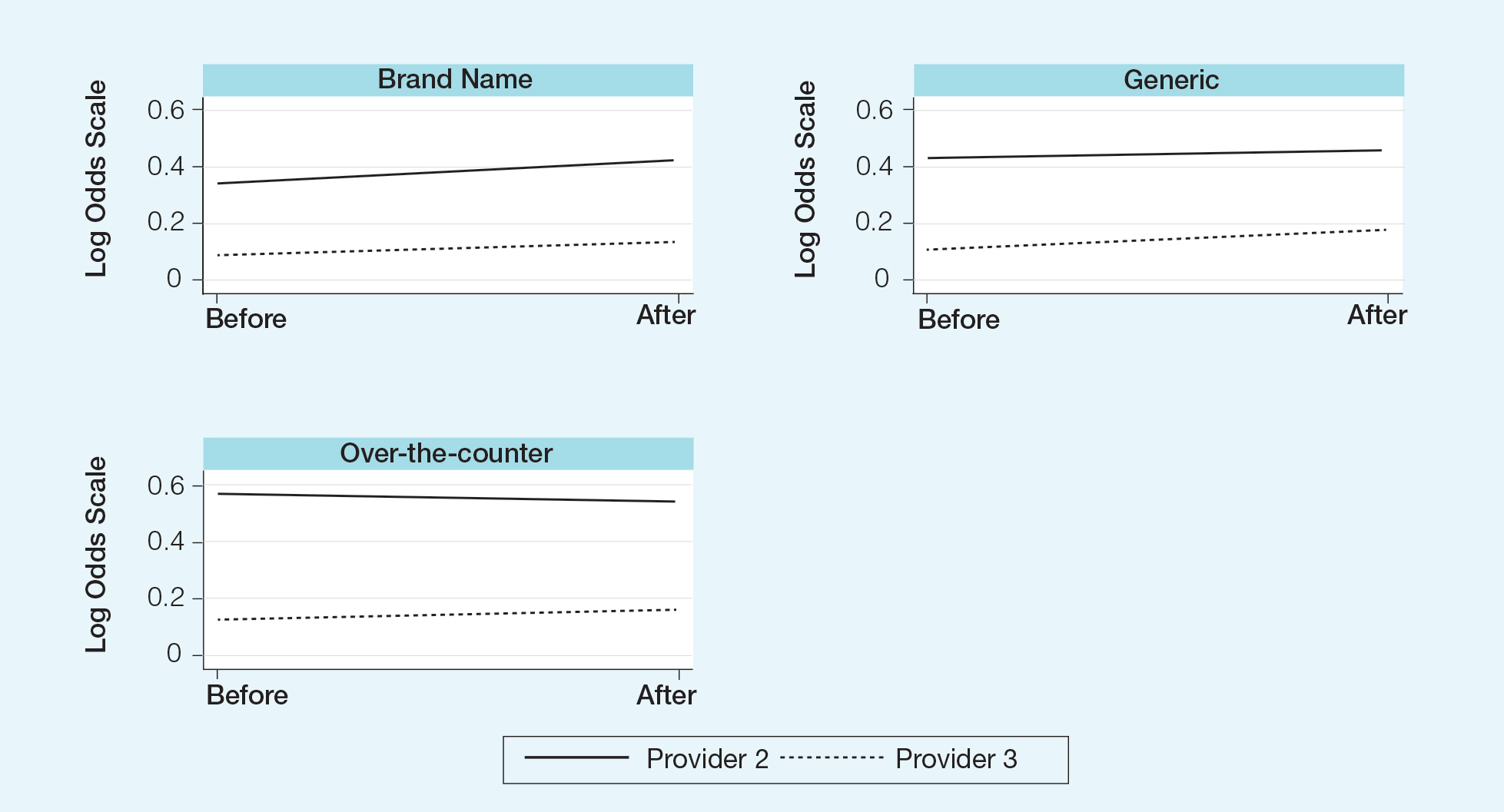
Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).




Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).




Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
Practice Points
- There has been growing concern that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.
- Many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians.
- This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after the medical school implemented new policies that banned in-office samples.