User login
A technicality could keep RSV shots from kids in need
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Systemic sclerosis antibodies show link to interstitial lung disease in RA
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF TRANSLATIONAL AUTOIMMUNITY
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
COVID emergency orders ending: What’s next?
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
Washington medical board charges doctor with spreading COVID misinformation
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Managing respiratory symptoms in the ‘tripledemic’ era
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Six healthy lifestyle habits linked to slowed memory decline
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.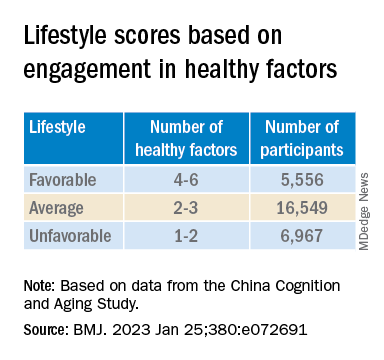
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.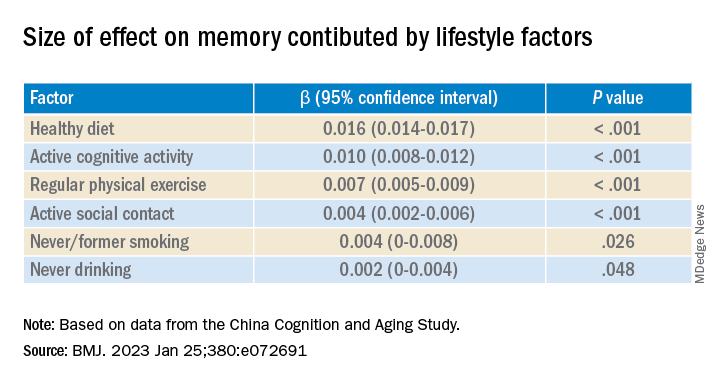
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Novel nomogram distinguishes pneumonias
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Long COVID affecting more than one-third of college students, faculty
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
FROM EMERGING INFECTIOUS DISEASES
Novel resuscitation for patients with nonshockable rhythms in cardiac arrest
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.