User login
Cardiac issues twice as likely with COVID plus high troponin
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
New challenge for docs: End of COVID federal public health emergency
The Biden administration intends to end by May 11 certain COVID-19 emergency measures used to aid in the response to the pandemic, while many others will remain in place.
A separate declaration covers the Food and Drug Administration’s emergency use authorizations (EUAs) for COVID medicines and tests. That would not be affected by the May 11 deadline, the FDA said. In addition, Congress and state lawmakers have extended some COVID response measures.
The result is a patchwork of emergency COVID-19 measures with different end dates.
The American Medical Association and the American Academy of Family Physicians (AAFP) are assessing how best to advise their members about the end of the public health emergency.
Several waivers regarding copays and coverage and policies regarding controlled substances will expire, Claire Ernst, director of government affairs at the Medical Group Management Association, told this news organization.
The impact of the unwinding “will vary based on some factors, such as what state the practice resides in,” Ms. Ernst said. “Fortunately, Congress provided some predictability for practices by extending many of the telehealth waivers through the end of 2024.”
The AAFP told this news organization that it has joined several other groups in calling for the release of proposed Drug Enforcement Administration (DEA) regulations meant to permanently allow prescriptions of buprenorphine treatment for opioid use disorder via telehealth. The AAFP and other groups want to review these proposals and, if needed, urge the DEA to modify or finalize before there are any disruptions in access to medications for opioid use disorder.
Patients’ questions
Clinicians can expect to field patients’ questions about their insurance coverage and what they need to pay, said Nancy Foster, vice president for quality and patient safety policy at the American Hospital Association (AHA).
“Your doctor’s office, that clinic you typically get care at, that is the face of medicine to you,” Ms. Foster told this news organization. “Many doctors and their staff will be asked, ‘What’s happening with Medicaid?’ ‘What about my Medicare coverage?’ ‘Can I still access care in the same way that I did before?’ ”
Physicians will need to be ready to answers those question, or point patients to where they can get answers, Ms. Foster said.
For example, Medicaid will no longer cover postpartum care for some enrollees after giving birth, said Taylor Platt, health policy manager for the American College of Obstetricians and Gynecologists.
The federal response to the pandemic created “a de facto postpartum coverage extension for Medicaid enrollees,” which will be lost in some states, Ms. Platt told this news organization. However, 28 states and the District of Columbia have taken separate measures to extend postpartum coverage to 1 year.
“This coverage has been critical for postpartum individuals to address health needs like substance use and mental health treatment and chronic conditions,” Ms. Platt said.
States significantly changed Medicaid policy to expand access to care during the pandemic.
All 50 states and the District of Columbia, for example, expanded coverage or access to telehealth services in Medicaid during the pandemic, according to a Jan. 31 report from the Kaiser Family Foundation (KFF). These expansions expire under various deadlines, although most states have made or are planning to make some Medicaid telehealth flexibilities permanent, KFF said.
The KFF report notes that all states and the District of Columbia temporarily waived some aspects of state licensure requirements, so that clinicians with equivalent licenses in other states could practice via telehealth.
In some states, these waivers are still active and are tied to the end of the federal emergency declaration. In others, they expired, with some states allowing for long-term or permanent interstate telemedicine, KFF said. (The Federation of State Medical Boards has a detailed summary of these modifications.)
The end of free COVID vaccines, testing for some patients
The AAFP has also raised concerns about continued access to COVID-19 vaccines, particularly for uninsured adults. Ashish Jha, MD, MPH, the White House COVID-19 Response Coordinator, said in a tweet that this transition, however, wouldn’t happen until a few months after the public health emergency ends.
After those few months, there will be a transition from U.S. government–distributed vaccines and treatments to ones purchased through the regular health care system, the “way we do for every other vaccine and treatment,” Dr. Jha added.
But that raises the same kind of difficult questions that permeate U.S. health care, with a potential to keep COVID active, said Patricia Jackson, RN, president of the Association for Professionals in Infection Control and Epidemiology (APIC).
People who don’t have insurance may lose access to COVID testing and vaccines.
“Will that lead to increases in transmission? Who knows,” Ms. Jackson told this news organization. “We will have to see. There are some health equity issues that potentially arise.”
Future FDA actions
Biden’s May 11 deadline applies to emergency provisions made under a Section 319 declaration, which allow the Department of Health and Human Services to respond to crises.
But a separate flexibility, known as a Section 564 declaration, covers the FDA’s EUAs, which can remain in effect even as the other declarations end.
The best-known EUAs for the pandemic were used to bring COVID vaccines and treatments to market. Many of these have since been converted to normal approvals as companies presented more evidence to support the initial emergency approvals. In other cases, EUAs have been withdrawn owing to disappointing research results, changing virus strains, and evolving medical treatments.
The FDA also used many EUAs to cover new uses of ventilators and other hospital equipment and expand these supplies in response to the pandemic, said Mark Howell, AHA’s director of policy and patient safety.
The FDA should examine the EUAs issued during the pandemic to see what greater flexibilities might be used to deal with future serious shortages of critical supplies. International incidents such as the war in Ukraine show how fragile the supply chain can be. The FDA should consider its recent experience with EUAs to address this, Mr. Howell said.
“What do we do coming out of the pandemic? And how do we think about being more proactive in this space to ensure that our supply doesn’t bottleneck, that we continue to make sure that providers have access to supply that’s not only safe and effective, but that they can use?” Mr. Howell told this news organization.
Such planning might also help prepare the country for the next pandemic, which is a near certainty, APIC’s Ms. Jackson said. The nation needs a nimbler response to the next major outbreak of an infectious disease, she said.
“There is going to be a next time,” Ms. Jackson said. “We are going to have another pandemic.”
A version of this article first appeared on Medscape.com.
The Biden administration intends to end by May 11 certain COVID-19 emergency measures used to aid in the response to the pandemic, while many others will remain in place.
A separate declaration covers the Food and Drug Administration’s emergency use authorizations (EUAs) for COVID medicines and tests. That would not be affected by the May 11 deadline, the FDA said. In addition, Congress and state lawmakers have extended some COVID response measures.
The result is a patchwork of emergency COVID-19 measures with different end dates.
The American Medical Association and the American Academy of Family Physicians (AAFP) are assessing how best to advise their members about the end of the public health emergency.
Several waivers regarding copays and coverage and policies regarding controlled substances will expire, Claire Ernst, director of government affairs at the Medical Group Management Association, told this news organization.
The impact of the unwinding “will vary based on some factors, such as what state the practice resides in,” Ms. Ernst said. “Fortunately, Congress provided some predictability for practices by extending many of the telehealth waivers through the end of 2024.”
The AAFP told this news organization that it has joined several other groups in calling for the release of proposed Drug Enforcement Administration (DEA) regulations meant to permanently allow prescriptions of buprenorphine treatment for opioid use disorder via telehealth. The AAFP and other groups want to review these proposals and, if needed, urge the DEA to modify or finalize before there are any disruptions in access to medications for opioid use disorder.
Patients’ questions
Clinicians can expect to field patients’ questions about their insurance coverage and what they need to pay, said Nancy Foster, vice president for quality and patient safety policy at the American Hospital Association (AHA).
“Your doctor’s office, that clinic you typically get care at, that is the face of medicine to you,” Ms. Foster told this news organization. “Many doctors and their staff will be asked, ‘What’s happening with Medicaid?’ ‘What about my Medicare coverage?’ ‘Can I still access care in the same way that I did before?’ ”
Physicians will need to be ready to answers those question, or point patients to where they can get answers, Ms. Foster said.
For example, Medicaid will no longer cover postpartum care for some enrollees after giving birth, said Taylor Platt, health policy manager for the American College of Obstetricians and Gynecologists.
The federal response to the pandemic created “a de facto postpartum coverage extension for Medicaid enrollees,” which will be lost in some states, Ms. Platt told this news organization. However, 28 states and the District of Columbia have taken separate measures to extend postpartum coverage to 1 year.
“This coverage has been critical for postpartum individuals to address health needs like substance use and mental health treatment and chronic conditions,” Ms. Platt said.
States significantly changed Medicaid policy to expand access to care during the pandemic.
All 50 states and the District of Columbia, for example, expanded coverage or access to telehealth services in Medicaid during the pandemic, according to a Jan. 31 report from the Kaiser Family Foundation (KFF). These expansions expire under various deadlines, although most states have made or are planning to make some Medicaid telehealth flexibilities permanent, KFF said.
The KFF report notes that all states and the District of Columbia temporarily waived some aspects of state licensure requirements, so that clinicians with equivalent licenses in other states could practice via telehealth.
In some states, these waivers are still active and are tied to the end of the federal emergency declaration. In others, they expired, with some states allowing for long-term or permanent interstate telemedicine, KFF said. (The Federation of State Medical Boards has a detailed summary of these modifications.)
The end of free COVID vaccines, testing for some patients
The AAFP has also raised concerns about continued access to COVID-19 vaccines, particularly for uninsured adults. Ashish Jha, MD, MPH, the White House COVID-19 Response Coordinator, said in a tweet that this transition, however, wouldn’t happen until a few months after the public health emergency ends.
After those few months, there will be a transition from U.S. government–distributed vaccines and treatments to ones purchased through the regular health care system, the “way we do for every other vaccine and treatment,” Dr. Jha added.
But that raises the same kind of difficult questions that permeate U.S. health care, with a potential to keep COVID active, said Patricia Jackson, RN, president of the Association for Professionals in Infection Control and Epidemiology (APIC).
People who don’t have insurance may lose access to COVID testing and vaccines.
“Will that lead to increases in transmission? Who knows,” Ms. Jackson told this news organization. “We will have to see. There are some health equity issues that potentially arise.”
Future FDA actions
Biden’s May 11 deadline applies to emergency provisions made under a Section 319 declaration, which allow the Department of Health and Human Services to respond to crises.
But a separate flexibility, known as a Section 564 declaration, covers the FDA’s EUAs, which can remain in effect even as the other declarations end.
The best-known EUAs for the pandemic were used to bring COVID vaccines and treatments to market. Many of these have since been converted to normal approvals as companies presented more evidence to support the initial emergency approvals. In other cases, EUAs have been withdrawn owing to disappointing research results, changing virus strains, and evolving medical treatments.
The FDA also used many EUAs to cover new uses of ventilators and other hospital equipment and expand these supplies in response to the pandemic, said Mark Howell, AHA’s director of policy and patient safety.
The FDA should examine the EUAs issued during the pandemic to see what greater flexibilities might be used to deal with future serious shortages of critical supplies. International incidents such as the war in Ukraine show how fragile the supply chain can be. The FDA should consider its recent experience with EUAs to address this, Mr. Howell said.
“What do we do coming out of the pandemic? And how do we think about being more proactive in this space to ensure that our supply doesn’t bottleneck, that we continue to make sure that providers have access to supply that’s not only safe and effective, but that they can use?” Mr. Howell told this news organization.
Such planning might also help prepare the country for the next pandemic, which is a near certainty, APIC’s Ms. Jackson said. The nation needs a nimbler response to the next major outbreak of an infectious disease, she said.
“There is going to be a next time,” Ms. Jackson said. “We are going to have another pandemic.”
A version of this article first appeared on Medscape.com.
The Biden administration intends to end by May 11 certain COVID-19 emergency measures used to aid in the response to the pandemic, while many others will remain in place.
A separate declaration covers the Food and Drug Administration’s emergency use authorizations (EUAs) for COVID medicines and tests. That would not be affected by the May 11 deadline, the FDA said. In addition, Congress and state lawmakers have extended some COVID response measures.
The result is a patchwork of emergency COVID-19 measures with different end dates.
The American Medical Association and the American Academy of Family Physicians (AAFP) are assessing how best to advise their members about the end of the public health emergency.
Several waivers regarding copays and coverage and policies regarding controlled substances will expire, Claire Ernst, director of government affairs at the Medical Group Management Association, told this news organization.
The impact of the unwinding “will vary based on some factors, such as what state the practice resides in,” Ms. Ernst said. “Fortunately, Congress provided some predictability for practices by extending many of the telehealth waivers through the end of 2024.”
The AAFP told this news organization that it has joined several other groups in calling for the release of proposed Drug Enforcement Administration (DEA) regulations meant to permanently allow prescriptions of buprenorphine treatment for opioid use disorder via telehealth. The AAFP and other groups want to review these proposals and, if needed, urge the DEA to modify or finalize before there are any disruptions in access to medications for opioid use disorder.
Patients’ questions
Clinicians can expect to field patients’ questions about their insurance coverage and what they need to pay, said Nancy Foster, vice president for quality and patient safety policy at the American Hospital Association (AHA).
“Your doctor’s office, that clinic you typically get care at, that is the face of medicine to you,” Ms. Foster told this news organization. “Many doctors and their staff will be asked, ‘What’s happening with Medicaid?’ ‘What about my Medicare coverage?’ ‘Can I still access care in the same way that I did before?’ ”
Physicians will need to be ready to answers those question, or point patients to where they can get answers, Ms. Foster said.
For example, Medicaid will no longer cover postpartum care for some enrollees after giving birth, said Taylor Platt, health policy manager for the American College of Obstetricians and Gynecologists.
The federal response to the pandemic created “a de facto postpartum coverage extension for Medicaid enrollees,” which will be lost in some states, Ms. Platt told this news organization. However, 28 states and the District of Columbia have taken separate measures to extend postpartum coverage to 1 year.
“This coverage has been critical for postpartum individuals to address health needs like substance use and mental health treatment and chronic conditions,” Ms. Platt said.
States significantly changed Medicaid policy to expand access to care during the pandemic.
All 50 states and the District of Columbia, for example, expanded coverage or access to telehealth services in Medicaid during the pandemic, according to a Jan. 31 report from the Kaiser Family Foundation (KFF). These expansions expire under various deadlines, although most states have made or are planning to make some Medicaid telehealth flexibilities permanent, KFF said.
The KFF report notes that all states and the District of Columbia temporarily waived some aspects of state licensure requirements, so that clinicians with equivalent licenses in other states could practice via telehealth.
In some states, these waivers are still active and are tied to the end of the federal emergency declaration. In others, they expired, with some states allowing for long-term or permanent interstate telemedicine, KFF said. (The Federation of State Medical Boards has a detailed summary of these modifications.)
The end of free COVID vaccines, testing for some patients
The AAFP has also raised concerns about continued access to COVID-19 vaccines, particularly for uninsured adults. Ashish Jha, MD, MPH, the White House COVID-19 Response Coordinator, said in a tweet that this transition, however, wouldn’t happen until a few months after the public health emergency ends.
After those few months, there will be a transition from U.S. government–distributed vaccines and treatments to ones purchased through the regular health care system, the “way we do for every other vaccine and treatment,” Dr. Jha added.
But that raises the same kind of difficult questions that permeate U.S. health care, with a potential to keep COVID active, said Patricia Jackson, RN, president of the Association for Professionals in Infection Control and Epidemiology (APIC).
People who don’t have insurance may lose access to COVID testing and vaccines.
“Will that lead to increases in transmission? Who knows,” Ms. Jackson told this news organization. “We will have to see. There are some health equity issues that potentially arise.”
Future FDA actions
Biden’s May 11 deadline applies to emergency provisions made under a Section 319 declaration, which allow the Department of Health and Human Services to respond to crises.
But a separate flexibility, known as a Section 564 declaration, covers the FDA’s EUAs, which can remain in effect even as the other declarations end.
The best-known EUAs for the pandemic were used to bring COVID vaccines and treatments to market. Many of these have since been converted to normal approvals as companies presented more evidence to support the initial emergency approvals. In other cases, EUAs have been withdrawn owing to disappointing research results, changing virus strains, and evolving medical treatments.
The FDA also used many EUAs to cover new uses of ventilators and other hospital equipment and expand these supplies in response to the pandemic, said Mark Howell, AHA’s director of policy and patient safety.
The FDA should examine the EUAs issued during the pandemic to see what greater flexibilities might be used to deal with future serious shortages of critical supplies. International incidents such as the war in Ukraine show how fragile the supply chain can be. The FDA should consider its recent experience with EUAs to address this, Mr. Howell said.
“What do we do coming out of the pandemic? And how do we think about being more proactive in this space to ensure that our supply doesn’t bottleneck, that we continue to make sure that providers have access to supply that’s not only safe and effective, but that they can use?” Mr. Howell told this news organization.
Such planning might also help prepare the country for the next pandemic, which is a near certainty, APIC’s Ms. Jackson said. The nation needs a nimbler response to the next major outbreak of an infectious disease, she said.
“There is going to be a next time,” Ms. Jackson said. “We are going to have another pandemic.”
A version of this article first appeared on Medscape.com.
Must-read acute care medicine articles from 2022
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The five biggest changes in the 2023 adult vaccine schedules
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
Less invasive NSCLC surgery does not compromise survival
suggest results from the CALGB 140503 trial, although strict patient selection remains key.
These new results contrast with those from a previous study from 1995, which found that local recurrence was three times higher and cancer mortality was twice as high with the less invasive procedure.
Those results from nearly 30 years ago established lobectomy as the standard of surgical care in this patient population, but since then advances in imaging and staging have allowed the detection of smaller and earlier tumors, which has “rekindled interest in sublobar resection,” the authors comment.
Hence, they conducted the new trial, which involved almost 700 U.S. patients with clinical T1aN0 NSCLC and a tumor size up to 2 cm, who were randomly assigned to lobar or sublobar tumor resection, and followed for 7 years.
The rates of both disease-free and overall survival were similar between the two groups, with no significant differences observed. There were also no substantial differences in rates of distant and locoregional recurrence.
In addition, there was a suggestion of less reduction in pulmonary function following the less invasive procedure.
“These findings affirm that sublobar resection ... is an effective management approach for this subgroup of patients with NSCLC,” says lead author Nasser Altorki, MD, Weill Cornell Medicine, NewYork–Presbyterian Hospital, New York.
“It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial, he emphasizes. “Specifically, the results are applicable only to a highly selected group of patients ... in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed.”
Nevertheless, Dr. Altorki said that “these results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening, and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.”
The study was published online in the New England Journal of Medicine.
In an accompanying editorial, Valerie W. Rusch, MD, Thoracic Service, Memorial Sloan Kettering Cancer Center, New York, agrees. “As CT screening becomes more widespread, this patient population will increase in clinical practice,” she explains.
However, Dr. Rusch also urges caution around patient selection, underlining that the results do not “provide a license for suboptimal surgical care.”
She says that “safeguards” such as the meticulous and strict patient criteria used in the trial “must be preserved in routine practice.”
“Thoracic surgeons will need to expand their expertise in sublobar resections, especially complex segmentectomies, and will need to collaborate closely with pathologists in assessing margins of resection, adequacy of lymph-node staging, and tumor characteristics that may predict recurrence.”
While emphasizing that lobectomy should still be performed when appropriate, Dr. Rusch nevertheless says: “The era of ‘precision’ surgery for NSCLC has arrived.”
Consistent with Japanese results
The investigators also point out that their findings are “consistent” with those of a recent Japanese study that compared lobectomy with anatomical segmentectomy, which found that the 5-year overall survival was 91.1% for lobectomy and 94.3% for segmentectomy.
The authors suggest that the difference in overall survival rates between the two trials might be due to anatomical segmentectomy being “considered by most surgeons to be more oncologically sound than wedge resection.”
In the current trial, wedge resection was allowed, however, “because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a ‘real world’ setting.”
Another important difference could be that more than 90% of the patients in the Japanese trial had adenocarcinoma, 45% with an associated ground-glass component, which is associated with better survival than a completely solid adenocarcinoma.
Dr. Rusch agrees that there are likely to be various factors related to the survival differences between the two trials, including patient selection, intraoperative management, and tumor characteristics.
“However, these two landmark trials are practice-changing because they establish sublobar resection as the standard of care for a select group of patients with NSCLC,” Dr. Rusch concluded.
Study details
Dr. Altorki and colleagues conducted the multicenter, international, randomized, noninferiority, phase 3 trial in patients with clinically staged T1aN0 NSCLC from 83 academic and community-based institutions in the United States, Canada, and Australia.
Patients were required to have a peripheral lung nodule with a solid component of up to 2 cm on preoperative CT, a tumor center in the outer third of the lung, and a tumor location amenable to sublobar resection, whether wedge or segment, or lobar resection, among other criteria.
In all, 697 patients were randomly assigned to undergo either lobar resection or sublobar resection, of whom 59.1% had wedge resection and 37.9% anatomical segmental resection. The median age was 67.9 years, and 57.4% were female. The vast majority (90%) were White.
After a median follow-up of 7 years, the 5-year disease-free survival was 63.6% with sublobar resection and 64.1% following lobar resection.
The team found that sublobar resection was not inferior to lobectomy for disease-free survival, at a hazard ratio for disease recurrence or death of 1.01 (90% confidence interval, 0.83-1.24), which adjusted to 0.99 after taking into account the site where the patient was treated.
The 5-year overall survival rate was 80.3% after sublobar resection, and 78.9% following lobar resection, at a hazard ratio for death of 0.95 (95% CI, 0.72-1.26).
The results were “generally consistent” when accounting for factors such as age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance status, the team says.
Turning to recurrence, they showed that, among 687 patients eligible for assessment, 30.4% of those in the sublobar resection group and 29.3% of those assigned to lobar resection experienced disease recurrence, with 13.4% and 10%, respectively, having locoregional recurrence.
An exploratory analysis indicated that 5-year recurrence-free survival was similar in the two groups, at 70.2% vs. 71.2% or a hazard ratio for recurrence of 1.05 (95% CI, 0.80-1.39). The cumulative incidence of death was also similar.
It was also notable that reduction in predictive forced expiratory volume in 1 second from baseline was lower with sublobar than lobar resection, at –4.0 vs. –6.0, as was the reduction in predicted forced vital capacity, at –3.0 vs. –5.0.
“Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions,” the team writes, “it may be more clinically relevant in patients with compromised pulmonary functions, or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function.”
Dr. Rusch suggests that “more sensitive or functional assessments” of pulmonary function might include “diffusion capacity and 6-minute walk tests,” although she noted that even short-term differences in pulmonary function “may affect perioperative and functional outcomes, especially for tumors in the lower lobe.”
The study was supported by the National Cancer Institute of the National Institutes of Health, including via grants to the Alliance for Clinical Trials in Oncology and the Canadian Cancer Trials Group, and supported in part by Covidien and Ethicon.
Dr. Altorki reports relationships with AstraZeneca, Genentech, Johnson & Johnson, and Regeneron. Dr. Rusch reports relationships with Cancer Research UK, Genentech, and the National Cancer Institute.
A version of this article first appeared on Medscape.com.
suggest results from the CALGB 140503 trial, although strict patient selection remains key.
These new results contrast with those from a previous study from 1995, which found that local recurrence was three times higher and cancer mortality was twice as high with the less invasive procedure.
Those results from nearly 30 years ago established lobectomy as the standard of surgical care in this patient population, but since then advances in imaging and staging have allowed the detection of smaller and earlier tumors, which has “rekindled interest in sublobar resection,” the authors comment.
Hence, they conducted the new trial, which involved almost 700 U.S. patients with clinical T1aN0 NSCLC and a tumor size up to 2 cm, who were randomly assigned to lobar or sublobar tumor resection, and followed for 7 years.
The rates of both disease-free and overall survival were similar between the two groups, with no significant differences observed. There were also no substantial differences in rates of distant and locoregional recurrence.
In addition, there was a suggestion of less reduction in pulmonary function following the less invasive procedure.
“These findings affirm that sublobar resection ... is an effective management approach for this subgroup of patients with NSCLC,” says lead author Nasser Altorki, MD, Weill Cornell Medicine, NewYork–Presbyterian Hospital, New York.
“It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial, he emphasizes. “Specifically, the results are applicable only to a highly selected group of patients ... in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed.”
Nevertheless, Dr. Altorki said that “these results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening, and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.”
The study was published online in the New England Journal of Medicine.
In an accompanying editorial, Valerie W. Rusch, MD, Thoracic Service, Memorial Sloan Kettering Cancer Center, New York, agrees. “As CT screening becomes more widespread, this patient population will increase in clinical practice,” she explains.
However, Dr. Rusch also urges caution around patient selection, underlining that the results do not “provide a license for suboptimal surgical care.”
She says that “safeguards” such as the meticulous and strict patient criteria used in the trial “must be preserved in routine practice.”
“Thoracic surgeons will need to expand their expertise in sublobar resections, especially complex segmentectomies, and will need to collaborate closely with pathologists in assessing margins of resection, adequacy of lymph-node staging, and tumor characteristics that may predict recurrence.”
While emphasizing that lobectomy should still be performed when appropriate, Dr. Rusch nevertheless says: “The era of ‘precision’ surgery for NSCLC has arrived.”
Consistent with Japanese results
The investigators also point out that their findings are “consistent” with those of a recent Japanese study that compared lobectomy with anatomical segmentectomy, which found that the 5-year overall survival was 91.1% for lobectomy and 94.3% for segmentectomy.
The authors suggest that the difference in overall survival rates between the two trials might be due to anatomical segmentectomy being “considered by most surgeons to be more oncologically sound than wedge resection.”
In the current trial, wedge resection was allowed, however, “because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a ‘real world’ setting.”
Another important difference could be that more than 90% of the patients in the Japanese trial had adenocarcinoma, 45% with an associated ground-glass component, which is associated with better survival than a completely solid adenocarcinoma.
Dr. Rusch agrees that there are likely to be various factors related to the survival differences between the two trials, including patient selection, intraoperative management, and tumor characteristics.
“However, these two landmark trials are practice-changing because they establish sublobar resection as the standard of care for a select group of patients with NSCLC,” Dr. Rusch concluded.
Study details
Dr. Altorki and colleagues conducted the multicenter, international, randomized, noninferiority, phase 3 trial in patients with clinically staged T1aN0 NSCLC from 83 academic and community-based institutions in the United States, Canada, and Australia.
Patients were required to have a peripheral lung nodule with a solid component of up to 2 cm on preoperative CT, a tumor center in the outer third of the lung, and a tumor location amenable to sublobar resection, whether wedge or segment, or lobar resection, among other criteria.
In all, 697 patients were randomly assigned to undergo either lobar resection or sublobar resection, of whom 59.1% had wedge resection and 37.9% anatomical segmental resection. The median age was 67.9 years, and 57.4% were female. The vast majority (90%) were White.
After a median follow-up of 7 years, the 5-year disease-free survival was 63.6% with sublobar resection and 64.1% following lobar resection.
The team found that sublobar resection was not inferior to lobectomy for disease-free survival, at a hazard ratio for disease recurrence or death of 1.01 (90% confidence interval, 0.83-1.24), which adjusted to 0.99 after taking into account the site where the patient was treated.
The 5-year overall survival rate was 80.3% after sublobar resection, and 78.9% following lobar resection, at a hazard ratio for death of 0.95 (95% CI, 0.72-1.26).
The results were “generally consistent” when accounting for factors such as age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance status, the team says.
Turning to recurrence, they showed that, among 687 patients eligible for assessment, 30.4% of those in the sublobar resection group and 29.3% of those assigned to lobar resection experienced disease recurrence, with 13.4% and 10%, respectively, having locoregional recurrence.
An exploratory analysis indicated that 5-year recurrence-free survival was similar in the two groups, at 70.2% vs. 71.2% or a hazard ratio for recurrence of 1.05 (95% CI, 0.80-1.39). The cumulative incidence of death was also similar.
It was also notable that reduction in predictive forced expiratory volume in 1 second from baseline was lower with sublobar than lobar resection, at –4.0 vs. –6.0, as was the reduction in predicted forced vital capacity, at –3.0 vs. –5.0.
“Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions,” the team writes, “it may be more clinically relevant in patients with compromised pulmonary functions, or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function.”
Dr. Rusch suggests that “more sensitive or functional assessments” of pulmonary function might include “diffusion capacity and 6-minute walk tests,” although she noted that even short-term differences in pulmonary function “may affect perioperative and functional outcomes, especially for tumors in the lower lobe.”
The study was supported by the National Cancer Institute of the National Institutes of Health, including via grants to the Alliance for Clinical Trials in Oncology and the Canadian Cancer Trials Group, and supported in part by Covidien and Ethicon.
Dr. Altorki reports relationships with AstraZeneca, Genentech, Johnson & Johnson, and Regeneron. Dr. Rusch reports relationships with Cancer Research UK, Genentech, and the National Cancer Institute.
A version of this article first appeared on Medscape.com.
suggest results from the CALGB 140503 trial, although strict patient selection remains key.
These new results contrast with those from a previous study from 1995, which found that local recurrence was three times higher and cancer mortality was twice as high with the less invasive procedure.
Those results from nearly 30 years ago established lobectomy as the standard of surgical care in this patient population, but since then advances in imaging and staging have allowed the detection of smaller and earlier tumors, which has “rekindled interest in sublobar resection,” the authors comment.
Hence, they conducted the new trial, which involved almost 700 U.S. patients with clinical T1aN0 NSCLC and a tumor size up to 2 cm, who were randomly assigned to lobar or sublobar tumor resection, and followed for 7 years.
The rates of both disease-free and overall survival were similar between the two groups, with no significant differences observed. There were also no substantial differences in rates of distant and locoregional recurrence.
In addition, there was a suggestion of less reduction in pulmonary function following the less invasive procedure.
“These findings affirm that sublobar resection ... is an effective management approach for this subgroup of patients with NSCLC,” says lead author Nasser Altorki, MD, Weill Cornell Medicine, NewYork–Presbyterian Hospital, New York.
“It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial, he emphasizes. “Specifically, the results are applicable only to a highly selected group of patients ... in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed.”
Nevertheless, Dr. Altorki said that “these results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening, and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.”
The study was published online in the New England Journal of Medicine.
In an accompanying editorial, Valerie W. Rusch, MD, Thoracic Service, Memorial Sloan Kettering Cancer Center, New York, agrees. “As CT screening becomes more widespread, this patient population will increase in clinical practice,” she explains.
However, Dr. Rusch also urges caution around patient selection, underlining that the results do not “provide a license for suboptimal surgical care.”
She says that “safeguards” such as the meticulous and strict patient criteria used in the trial “must be preserved in routine practice.”
“Thoracic surgeons will need to expand their expertise in sublobar resections, especially complex segmentectomies, and will need to collaborate closely with pathologists in assessing margins of resection, adequacy of lymph-node staging, and tumor characteristics that may predict recurrence.”
While emphasizing that lobectomy should still be performed when appropriate, Dr. Rusch nevertheless says: “The era of ‘precision’ surgery for NSCLC has arrived.”
Consistent with Japanese results
The investigators also point out that their findings are “consistent” with those of a recent Japanese study that compared lobectomy with anatomical segmentectomy, which found that the 5-year overall survival was 91.1% for lobectomy and 94.3% for segmentectomy.
The authors suggest that the difference in overall survival rates between the two trials might be due to anatomical segmentectomy being “considered by most surgeons to be more oncologically sound than wedge resection.”
In the current trial, wedge resection was allowed, however, “because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a ‘real world’ setting.”
Another important difference could be that more than 90% of the patients in the Japanese trial had adenocarcinoma, 45% with an associated ground-glass component, which is associated with better survival than a completely solid adenocarcinoma.
Dr. Rusch agrees that there are likely to be various factors related to the survival differences between the two trials, including patient selection, intraoperative management, and tumor characteristics.
“However, these two landmark trials are practice-changing because they establish sublobar resection as the standard of care for a select group of patients with NSCLC,” Dr. Rusch concluded.
Study details
Dr. Altorki and colleagues conducted the multicenter, international, randomized, noninferiority, phase 3 trial in patients with clinically staged T1aN0 NSCLC from 83 academic and community-based institutions in the United States, Canada, and Australia.
Patients were required to have a peripheral lung nodule with a solid component of up to 2 cm on preoperative CT, a tumor center in the outer third of the lung, and a tumor location amenable to sublobar resection, whether wedge or segment, or lobar resection, among other criteria.
In all, 697 patients were randomly assigned to undergo either lobar resection or sublobar resection, of whom 59.1% had wedge resection and 37.9% anatomical segmental resection. The median age was 67.9 years, and 57.4% were female. The vast majority (90%) were White.
After a median follow-up of 7 years, the 5-year disease-free survival was 63.6% with sublobar resection and 64.1% following lobar resection.
The team found that sublobar resection was not inferior to lobectomy for disease-free survival, at a hazard ratio for disease recurrence or death of 1.01 (90% confidence interval, 0.83-1.24), which adjusted to 0.99 after taking into account the site where the patient was treated.
The 5-year overall survival rate was 80.3% after sublobar resection, and 78.9% following lobar resection, at a hazard ratio for death of 0.95 (95% CI, 0.72-1.26).
The results were “generally consistent” when accounting for factors such as age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance status, the team says.
Turning to recurrence, they showed that, among 687 patients eligible for assessment, 30.4% of those in the sublobar resection group and 29.3% of those assigned to lobar resection experienced disease recurrence, with 13.4% and 10%, respectively, having locoregional recurrence.
An exploratory analysis indicated that 5-year recurrence-free survival was similar in the two groups, at 70.2% vs. 71.2% or a hazard ratio for recurrence of 1.05 (95% CI, 0.80-1.39). The cumulative incidence of death was also similar.
It was also notable that reduction in predictive forced expiratory volume in 1 second from baseline was lower with sublobar than lobar resection, at –4.0 vs. –6.0, as was the reduction in predicted forced vital capacity, at –3.0 vs. –5.0.
“Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions,” the team writes, “it may be more clinically relevant in patients with compromised pulmonary functions, or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function.”
Dr. Rusch suggests that “more sensitive or functional assessments” of pulmonary function might include “diffusion capacity and 6-minute walk tests,” although she noted that even short-term differences in pulmonary function “may affect perioperative and functional outcomes, especially for tumors in the lower lobe.”
The study was supported by the National Cancer Institute of the National Institutes of Health, including via grants to the Alliance for Clinical Trials in Oncology and the Canadian Cancer Trials Group, and supported in part by Covidien and Ethicon.
Dr. Altorki reports relationships with AstraZeneca, Genentech, Johnson & Johnson, and Regeneron. Dr. Rusch reports relationships with Cancer Research UK, Genentech, and the National Cancer Institute.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
A doctor intervenes in a fiery car crash
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
A new (old) drug joins the COVID fray, and guess what? It works
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
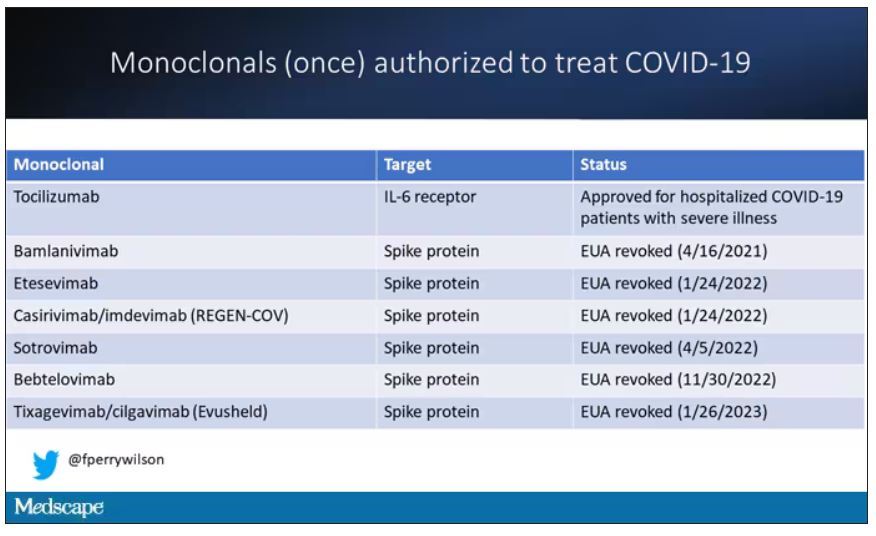
At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.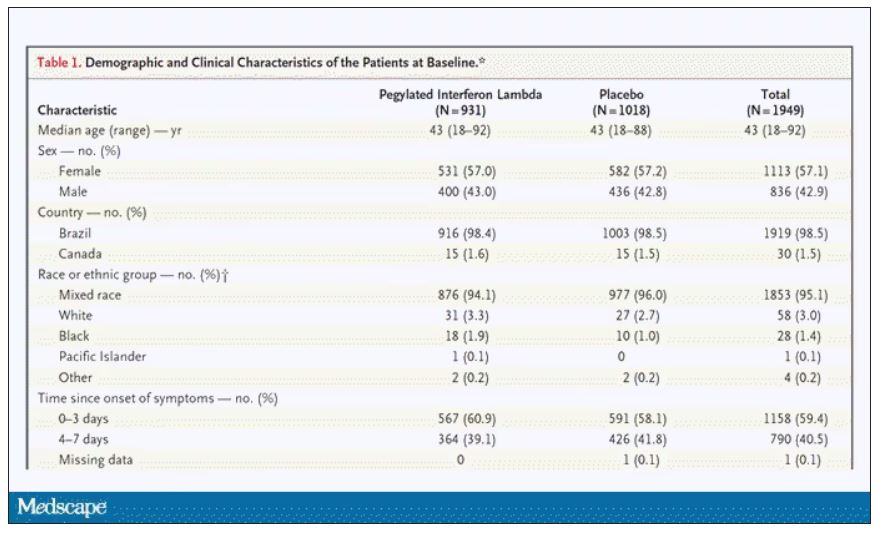
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.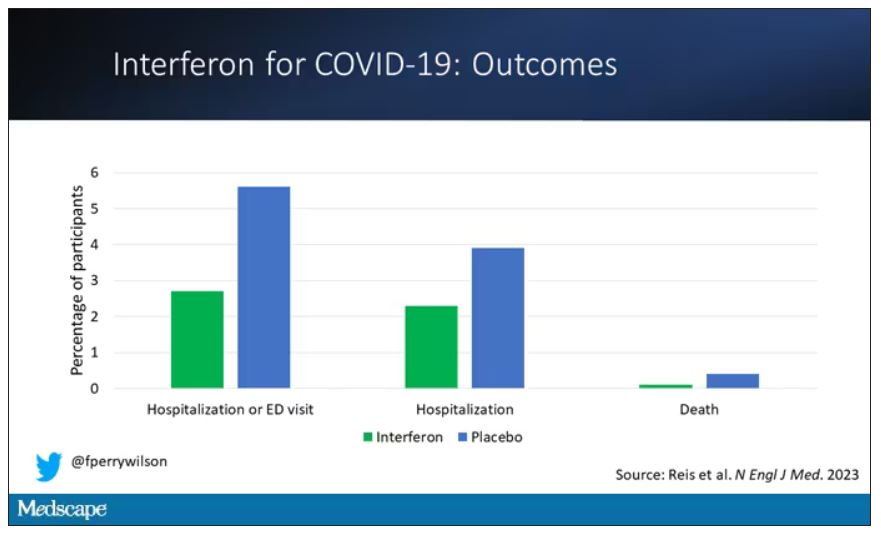
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.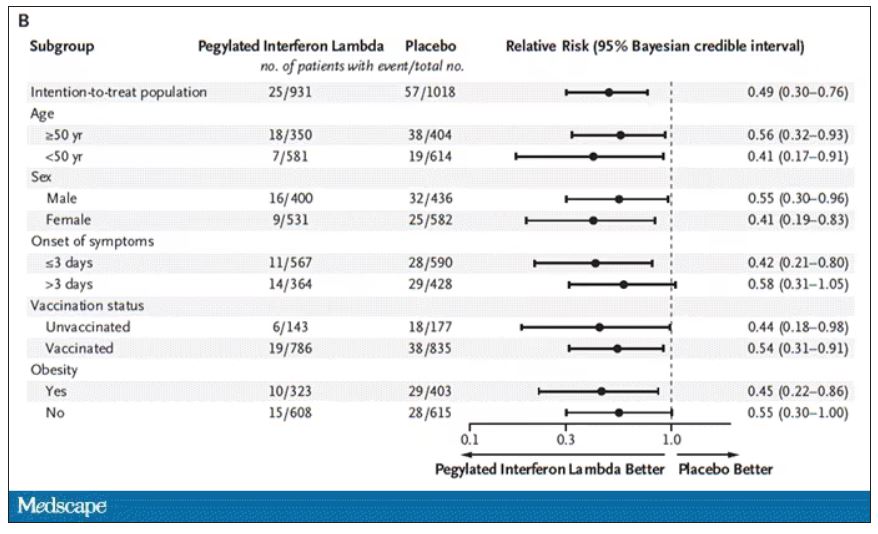
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.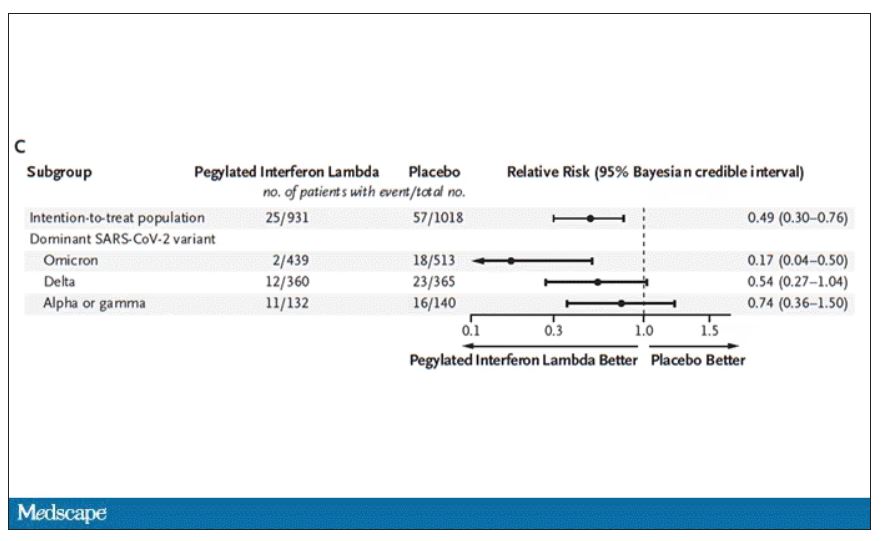
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.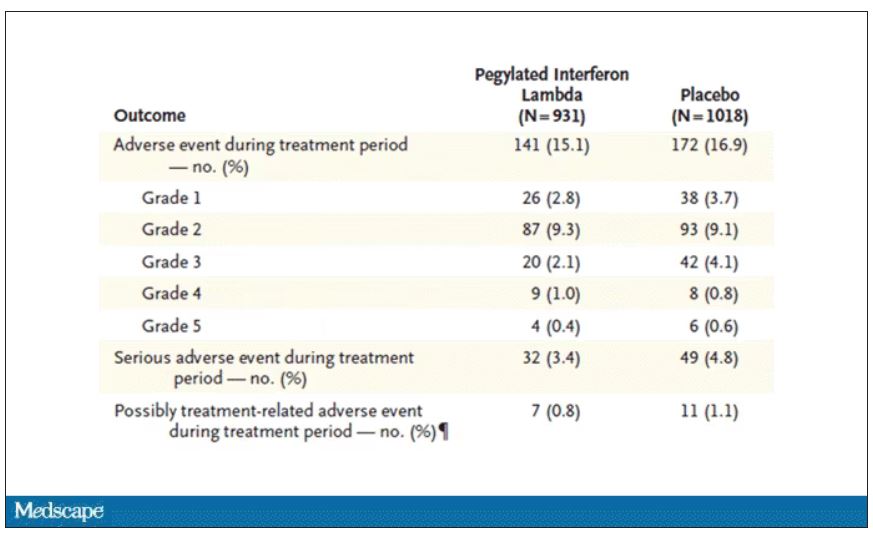
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.

At this point, with the monoclonals found to be essentially useless, we are left with remdesivir with its modest efficacy and Paxlovid, which, for some reason, people don’t seem to be taking.
Part of the reason the monoclonals have failed lately is because of their specificity; they are homogeneous antibodies targeted toward a very specific epitope that may change from variant to variant. We need a broader therapeutic, one that has activity across all variants — maybe even one that has activity against all viruses? We’ve got one. Interferon.
The first mention of interferon as a potential COVID therapy was at the very start of the pandemic, so I’m sort of surprised that the first large, randomized trial is only being reported now in the New England Journal of Medicine.
Before we dig into the results, let’s talk mechanism. This is a trial of interferon-lambda, also known as interleukin-29.
The lambda interferons were only discovered in 2003. They differ from the more familiar interferons only in their cellular receptors; the downstream effects seem quite similar. As opposed to the cellular receptors for interferon alfa, which are widely expressed, the receptors for lambda are restricted to epithelial tissues. This makes it a good choice as a COVID treatment, since the virus also preferentially targets those epithelial cells.
In this study, 1,951 participants from Brazil and Canada, but mostly Brazil, with new COVID infections who were not yet hospitalized were randomized to receive 180 mcg of interferon lambda or placebo.
This was a relatively current COVID trial, as you can see from the participant characteristics. The majority had been vaccinated, and nearly half of the infections were during the Omicron phase of the pandemic.
If you just want to cut to the chase, interferon worked.
The primary outcome – hospitalization or a prolonged emergency room visit for COVID – was 50% lower in the interferon group.
Key secondary outcomes, including death from COVID, were lower in the interferon group as well. These effects persisted across most of the subgroups I was looking out for.
Interferon seemed to help those who were already vaccinated and those who were unvaccinated. There’s a hint that it works better within the first few days of symptoms, which isn’t surprising; we’ve seen this for many of the therapeutics, including Paxlovid. Time is of the essence. Encouragingly, the effect was a bit more pronounced among those infected with Omicron.
Of course, if you have any experience with interferon, you know that the side effects can be pretty rough. In the bad old days when we treated hepatitis C infection with interferon, patients would get their injections on Friday in anticipation of being essentially out of commission with flu-like symptoms through the weekend. But we don’t see much evidence of adverse events in this trial, maybe due to the greater specificity of interferon lambda.
Putting it all together, the state of play for interferons in COVID may be changing. To date, the FDA has not recommended the use of interferon alfa or -beta for COVID-19, citing some data that they are ineffective or even harmful in hospitalized patients with COVID. Interferon lambda is not FDA approved and thus not even available in the United States. But the reason it has not been approved is that there has not been a large, well-conducted interferon lambda trial. Now there is. Will this study be enough to prompt an emergency use authorization? The elephant in the room, of course, is Paxlovid, which at this point has a longer safety track record and, importantly, is oral. I’d love to see a head-to-head trial. Short of that, I tend to be in favor of having more options on the table.
Dr. Perry Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Maternal COVID-19 vaccine curbs infant infection
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
FROM THE BMJ
Acute cardiac events common during COVID hospitalization
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
What is the psychological cost of performing CPR?
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.