User login
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Spending time in a war zone can lead to chronic mental and physical pain. Now, research points to a link between two common disorders that can leave service members struggling.
Published in the journal Arthritis Care & Research, a longitudinal cohort study of 1761 US military service members found that those who had posttraumatic stress disorder (PTSD) before deployment were nearly 3times more likely to develop fibromyalgia after returning home (odds ratio, 2.96; 95% CI, 2.08-4.22). Those with fibromyalgia before deployment had more than threefold greater likelihood of developing PTSD after deployment (odds ratio, 3.12; 95% CI, 1.63-5.95).
This is the largest prospective study to date linking the stress of combat deployment to the onset of fibromyalgia.
“We had the advantage of observing a large population before and after exposure to an environment that often involves significant stress,” said lead study author Jay Higgs, MD, a retired rheumatologist with Brooke Army Medical Center and the University of Texas Health Science Center at San Antonio.
Here’s what the team found and why it matters.
Significant Increase in Fibromyalgia After Development
Service members were checked for fibromyalgia using the 2011 questionnaire modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. They were assessed for PTSD using the PTSD Checklist Stressor-Specific Version.
Before deployment, service members had similar rates of fibromyalgia as the general population: 2.2% in men and 2.0% in women. After deployment, fibromyalgia rates increased significantly to 8.0% in men and 11.1% in women.
While fibromyalgia tends to be underreported in men, the findings suggest it should not be overlooked in this population. “Our results are consistent with the notion that there should be no gender bias when considering the possibility of fibromyalgia in an individual patient,” Higgs said.
Before deployment, 20.7% of men and 18.3% of women had PTSD symptoms. After deployment, the PTSD rate increased slightly to 22.7% in men and 25.5% in women.
The Link Between Fibromyalgia and PTSD
The researchers said the results suggest that PTSD and fibromyalgia might be linked through central nervous system mechanisms such as central sensitization, elevated hypothalamic-pituitary-adrenal axis activity, elevated cortisol, and proinflammatory cytokines. However, shared causation, associated risk factors, selection bias, or alternative mechanisms within the central and peripheral neuroendocrine and cytokine systems could also be part of the story.
“What we do not know is how much of what we see clinically represents central nervous system pathology, peripheral problems, or a combination of the 2,” Higgs said. “Neurotransmission in the central nervous system is highly complex, and may not only involve specific structures, but a web of communications between them.”
Loci in the midbrain appear especially important, he said.
Elizabeth Hoge, MD, professor and director of the Anxiety Disorders Research Program at Georgetown University School of Medicine, Washington, DC, said that patients with PTSD often have pain, headaches, sleep disturbances, and other symptoms that are part of the picture of fibromyalgia. It’s plausible that pain syndromes could be manifestations of PTSD or groupings of symptoms that suggest a subtype.
“Pain is one way that people experience distress, and we know that in PTSD, sometimes the trauma memories are encoded too strongly, more stressful and more alarming to the body system,” she said.
When patients have symptoms such as chronic pain, headaches, fatigue, or cognitive brain fog, clinicians should remember to ask about trauma exposure, Hoge said. You might be the first to broach the subject.
“I’ve certainly seen patients in clinic who never get asked about the exposure to trauma, including sexual trauma, so sometimes that can be the first pathway to helping people feel better is just to have their trauma recognized,” Hoge said.
If a patient has experienced or witnessed violence, consider a referral to a psychiatrist or psychologist to evaluate them for PTSD. Higgs said he collaborated closely with a psychologist to complement his treatment plans for active duty and retired military service members and families.
The US Department of Veterans Affairs and the Department of Defense (DoD) recommend trauma-focused psychotherapy as the first line of treatment for PTSD. This form of therapy deliberately focuses on bringing trauma memories into the open, Hoge said.
“When a person talks about their trauma, and it comes into direct consciousness, somehow it’s malleable, and so when it goes back down into the memory banks, it’s changed somewhat,” she said.
This study was supported by the DoD through awards from the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, and Psychological Health and Traumatic Brain Injury Research Program. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Higgs’s comments are his own and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, Carl R. Darnall Army Medical Center, the DoD, the Department of Veterans Affairs, or any agencies under the US government. Hoge had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Spending time in a war zone can lead to chronic mental and physical pain. Now, research points to a link between two common disorders that can leave service members struggling.
Published in the journal Arthritis Care & Research, a longitudinal cohort study of 1761 US military service members found that those who had posttraumatic stress disorder (PTSD) before deployment were nearly 3times more likely to develop fibromyalgia after returning home (odds ratio, 2.96; 95% CI, 2.08-4.22). Those with fibromyalgia before deployment had more than threefold greater likelihood of developing PTSD after deployment (odds ratio, 3.12; 95% CI, 1.63-5.95).
This is the largest prospective study to date linking the stress of combat deployment to the onset of fibromyalgia.
“We had the advantage of observing a large population before and after exposure to an environment that often involves significant stress,” said lead study author Jay Higgs, MD, a retired rheumatologist with Brooke Army Medical Center and the University of Texas Health Science Center at San Antonio.
Here’s what the team found and why it matters.
Significant Increase in Fibromyalgia After Development
Service members were checked for fibromyalgia using the 2011 questionnaire modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. They were assessed for PTSD using the PTSD Checklist Stressor-Specific Version.
Before deployment, service members had similar rates of fibromyalgia as the general population: 2.2% in men and 2.0% in women. After deployment, fibromyalgia rates increased significantly to 8.0% in men and 11.1% in women.
While fibromyalgia tends to be underreported in men, the findings suggest it should not be overlooked in this population. “Our results are consistent with the notion that there should be no gender bias when considering the possibility of fibromyalgia in an individual patient,” Higgs said.
Before deployment, 20.7% of men and 18.3% of women had PTSD symptoms. After deployment, the PTSD rate increased slightly to 22.7% in men and 25.5% in women.
The Link Between Fibromyalgia and PTSD
The researchers said the results suggest that PTSD and fibromyalgia might be linked through central nervous system mechanisms such as central sensitization, elevated hypothalamic-pituitary-adrenal axis activity, elevated cortisol, and proinflammatory cytokines. However, shared causation, associated risk factors, selection bias, or alternative mechanisms within the central and peripheral neuroendocrine and cytokine systems could also be part of the story.
“What we do not know is how much of what we see clinically represents central nervous system pathology, peripheral problems, or a combination of the 2,” Higgs said. “Neurotransmission in the central nervous system is highly complex, and may not only involve specific structures, but a web of communications between them.”
Loci in the midbrain appear especially important, he said.
Elizabeth Hoge, MD, professor and director of the Anxiety Disorders Research Program at Georgetown University School of Medicine, Washington, DC, said that patients with PTSD often have pain, headaches, sleep disturbances, and other symptoms that are part of the picture of fibromyalgia. It’s plausible that pain syndromes could be manifestations of PTSD or groupings of symptoms that suggest a subtype.
“Pain is one way that people experience distress, and we know that in PTSD, sometimes the trauma memories are encoded too strongly, more stressful and more alarming to the body system,” she said.
When patients have symptoms such as chronic pain, headaches, fatigue, or cognitive brain fog, clinicians should remember to ask about trauma exposure, Hoge said. You might be the first to broach the subject.
“I’ve certainly seen patients in clinic who never get asked about the exposure to trauma, including sexual trauma, so sometimes that can be the first pathway to helping people feel better is just to have their trauma recognized,” Hoge said.
If a patient has experienced or witnessed violence, consider a referral to a psychiatrist or psychologist to evaluate them for PTSD. Higgs said he collaborated closely with a psychologist to complement his treatment plans for active duty and retired military service members and families.
The US Department of Veterans Affairs and the Department of Defense (DoD) recommend trauma-focused psychotherapy as the first line of treatment for PTSD. This form of therapy deliberately focuses on bringing trauma memories into the open, Hoge said.
“When a person talks about their trauma, and it comes into direct consciousness, somehow it’s malleable, and so when it goes back down into the memory banks, it’s changed somewhat,” she said.
This study was supported by the DoD through awards from the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, and Psychological Health and Traumatic Brain Injury Research Program. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Higgs’s comments are his own and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, Carl R. Darnall Army Medical Center, the DoD, the Department of Veterans Affairs, or any agencies under the US government. Hoge had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Spending time in a war zone can lead to chronic mental and physical pain. Now, research points to a link between two common disorders that can leave service members struggling.
Published in the journal Arthritis Care & Research, a longitudinal cohort study of 1761 US military service members found that those who had posttraumatic stress disorder (PTSD) before deployment were nearly 3times more likely to develop fibromyalgia after returning home (odds ratio, 2.96; 95% CI, 2.08-4.22). Those with fibromyalgia before deployment had more than threefold greater likelihood of developing PTSD after deployment (odds ratio, 3.12; 95% CI, 1.63-5.95).
This is the largest prospective study to date linking the stress of combat deployment to the onset of fibromyalgia.
“We had the advantage of observing a large population before and after exposure to an environment that often involves significant stress,” said lead study author Jay Higgs, MD, a retired rheumatologist with Brooke Army Medical Center and the University of Texas Health Science Center at San Antonio.
Here’s what the team found and why it matters.
Significant Increase in Fibromyalgia After Development
Service members were checked for fibromyalgia using the 2011 questionnaire modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. They were assessed for PTSD using the PTSD Checklist Stressor-Specific Version.
Before deployment, service members had similar rates of fibromyalgia as the general population: 2.2% in men and 2.0% in women. After deployment, fibromyalgia rates increased significantly to 8.0% in men and 11.1% in women.
While fibromyalgia tends to be underreported in men, the findings suggest it should not be overlooked in this population. “Our results are consistent with the notion that there should be no gender bias when considering the possibility of fibromyalgia in an individual patient,” Higgs said.
Before deployment, 20.7% of men and 18.3% of women had PTSD symptoms. After deployment, the PTSD rate increased slightly to 22.7% in men and 25.5% in women.
The Link Between Fibromyalgia and PTSD
The researchers said the results suggest that PTSD and fibromyalgia might be linked through central nervous system mechanisms such as central sensitization, elevated hypothalamic-pituitary-adrenal axis activity, elevated cortisol, and proinflammatory cytokines. However, shared causation, associated risk factors, selection bias, or alternative mechanisms within the central and peripheral neuroendocrine and cytokine systems could also be part of the story.
“What we do not know is how much of what we see clinically represents central nervous system pathology, peripheral problems, or a combination of the 2,” Higgs said. “Neurotransmission in the central nervous system is highly complex, and may not only involve specific structures, but a web of communications between them.”
Loci in the midbrain appear especially important, he said.
Elizabeth Hoge, MD, professor and director of the Anxiety Disorders Research Program at Georgetown University School of Medicine, Washington, DC, said that patients with PTSD often have pain, headaches, sleep disturbances, and other symptoms that are part of the picture of fibromyalgia. It’s plausible that pain syndromes could be manifestations of PTSD or groupings of symptoms that suggest a subtype.
“Pain is one way that people experience distress, and we know that in PTSD, sometimes the trauma memories are encoded too strongly, more stressful and more alarming to the body system,” she said.
When patients have symptoms such as chronic pain, headaches, fatigue, or cognitive brain fog, clinicians should remember to ask about trauma exposure, Hoge said. You might be the first to broach the subject.
“I’ve certainly seen patients in clinic who never get asked about the exposure to trauma, including sexual trauma, so sometimes that can be the first pathway to helping people feel better is just to have their trauma recognized,” Hoge said.
If a patient has experienced or witnessed violence, consider a referral to a psychiatrist or psychologist to evaluate them for PTSD. Higgs said he collaborated closely with a psychologist to complement his treatment plans for active duty and retired military service members and families.
The US Department of Veterans Affairs and the Department of Defense (DoD) recommend trauma-focused psychotherapy as the first line of treatment for PTSD. This form of therapy deliberately focuses on bringing trauma memories into the open, Hoge said.
“When a person talks about their trauma, and it comes into direct consciousness, somehow it’s malleable, and so when it goes back down into the memory banks, it’s changed somewhat,” she said.
This study was supported by the DoD through awards from the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, and Psychological Health and Traumatic Brain Injury Research Program. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Higgs’s comments are his own and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, Carl R. Darnall Army Medical Center, the DoD, the Department of Veterans Affairs, or any agencies under the US government. Hoge had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Vasculitis Patients Need Multiple COVID Vaccine Boosters
People with vasculitis may need at least three or four vaccinations for COVID-19 before they start to show an immune response against SARS-CoV-2 infection, new research has suggested.
In a longitudinal retrospective study, serum antibody neutralization against the Omicron variant of the virus and its descendants was found to be “largely absent” after the first two doses of COVID-19 vaccine had been given to patients. But increasing neutralizing antibody titers were seen after both the third and fourth vaccine boosters had been administered.
Results also showed that the more recently people had been treated with the B cell–depleting therapy rituximab, the lower the levels of immunogenicity that were achieved, and thus protection against SARS-CoV-2.
“Our results have significant implications for individuals treated with rituximab in the post-Omicron era, highlighting the value of additive boosters in affirming increasing protection in clinically vulnerable populations,” the team behind the work at the University of Cambridge in England, has reported in Science Advances.
Moreover, because the use of rituximab reduced the neutralization of not just wild-type (WT) Omicron but also the Omicron-descendant variants BA.1, BA.2, BA.4, and XBB, this highlights “the urgent need for additional adjunctive strategies to enhance vaccine-induced immunity as well as preferential access for such patients to updated vaccines using spike from now circulating Omicron lineages,” the team added.
Studying Humoral Responses to SARS-CoV-2 Vaccines
Corresponding author Ravindra K. Gupta, BMBCh, MA, MPH, PhD, told this news organization that studying humoral responses to SARS-CoV-2 vaccines in immunocompromised individuals such as those with vasculitis was important for two main reasons.
“It is really important at individual level for their own health, of course, but also because we know that variants of concern have often evolved and developed within patients and can then spread in wider populations,” he said.
Gupta, who is professor of clinical microbiology at the Cambridge Institute for Therapeutic Immunology & Infectious Disease added: “We believe that the variants of concern that we’re having to deal with right now, including Omicron, have come from such [immunocompromised] individuals.”
Omicron “was a big shift,” Gupta noted. “It had a lot of new mutations on it, so it was almost like a new strain of the virus.” Few studies have looked at the longitudinal immunogenicity proffered by COVID vaccines in the post-Omicron era, particularly in those with vasculitis who are often treated with immunosuppressive drugs, including rituximab.
Two-Pronged Study Approach
For the study, a population of immunocompromised individuals diagnosed with vasculitis who had been treated with rituximab in the past 5 years was identified. Just over half (58%) had received adenovirus-based AZD1222/ChAdOx1 nCoV-19 (AstraZeneca-Oxford; AZN) and 37% BNT162b2 (Pfizer-BioNTech; mRNA) as their primary vaccines. Patients with antineutrophil cytoplasmic antibody–associated vasculitis comprised the majority of those who received rituximab (83%), compared with less than half of those who did not take rituximab (48%).
A two-pronged approach was taken with the researchers first measuring neutralizing antibody titers before and 30 days after four successive COVID vaccinations in a group of 32 individuals with available samples. They then performed a cross-sectional, case-control study in 95 individuals to look at neutralizing antibody titers and antibody-dependent cell-mediated cytotoxicity (ADCC) in individuals who had (n = 64) and had not (n = 31) been treated with rituximab in the past 5 years and had samples available after their third and fourth COVID vaccinations.
The first analysis was done to see how people were responding to vaccination over time. “That told us that there was a problem with the first two doses and that we got some response after doses three and four, but the response was uniformly quite poor against the new variants of concern,” Gupta said.
A human embryonic kidney cell model had been used to determine individuals’ neutralizing antibody titers in response to WT, BA.1, BA.2, BA.4, and XBB pseudotyped viruses. After the first and second COVID vaccinations, the geometric mean titer (GMT) against each variant barely increased from a baseline of 40.0. The greatest increases in GMT was seen with the WT virus, at 43.7, 90.7, 256.3, and 394.2, after the first, second, third and fourth doses, respectively. The lowest increases in GMT were seen with the XBB variant, with respective values of 40.0, 40.8, 45.7, and 53.9.
Incremental Benefit Offers Some ‘Reassurance’
Vasculitis specialist Rona Smith, MA, MB BChir, MD, who was one of the authors of the paper, told this news organization separately that the results showed there was “an incremental benefit of having COVID vaccinations,” which “offers a little bit of reassurance” that there can be an immune response in people with vasculitis.
Although results of the cross-sectional study showed that there was a significant dampening effect of rituximab treatment on the immune response, “I don’t think it’s an isolated effect in our [vasculitis] patients,” Smith suggested, adding the results were “probably still relevant to patients who receive routine dosing of rituximab for other conditions.”
Neutralizing antibody titers were consistently lower among individuals who had been treated with rituximab vs those who had not, with treatment in the past 18 months found to significantly impair immunogenicity.
The ADCC response was better preserved than the neutralizing antibody response, Gupta said, although it was still significantly lower in the rituximab-treated than in the non–rituximab-treated patients.
When to Vaccinate in Vasculitis?
Regarding when to give vaccines to people with vasculitis, Smith said: “Current recommendations are that patients should receive any vaccines that they’re offered routinely, whether that be COVID vaccines, flu vaccines, pneumococcal vaccines.”
As for the timing of those vaccinations, she observed that the current thinking was that vaccinations should “ideally be at least 1 month before a rituximab treatment, and ideally 3-4 [months] after their last dose. However, as many patients are on a 6-month dosing cycle, it can be difficult for some of them to find a suitable time window to have the COVID vaccine when it is offered.”
Additional precautions, such as wearing masks in crowded places and avoiding visits to acutely unwell friends or relatives, may still be prudent, Smith acknowledged, but he was clear that people should not be locking themselves away as they did during the COVID-19 pandemic.
When advising patients, “our general recommendation is that it is better to have a vaccine than not, but we can’t guarantee how well you will respond to it, but some response is better than none,” Smith said.
The study was independently supported. Gupta had no relevant financial relationships to disclose. Smith was a coauthor of the paper and has received research grant funding from Union Therapeutics, GlaxoSmithKline/Vir Biotechnology, Addenbrooke’s Charitable Trust, and Vasculitis UK. Another coauthor reported receiving research grants from CSL Vifor, Roche, and GlaxoSmithKline and advisory board, consultancy, and lecture fees from Roche and CSL Vifor.
A version of this article first appeared on Medscape.com.
People with vasculitis may need at least three or four vaccinations for COVID-19 before they start to show an immune response against SARS-CoV-2 infection, new research has suggested.
In a longitudinal retrospective study, serum antibody neutralization against the Omicron variant of the virus and its descendants was found to be “largely absent” after the first two doses of COVID-19 vaccine had been given to patients. But increasing neutralizing antibody titers were seen after both the third and fourth vaccine boosters had been administered.
Results also showed that the more recently people had been treated with the B cell–depleting therapy rituximab, the lower the levels of immunogenicity that were achieved, and thus protection against SARS-CoV-2.
“Our results have significant implications for individuals treated with rituximab in the post-Omicron era, highlighting the value of additive boosters in affirming increasing protection in clinically vulnerable populations,” the team behind the work at the University of Cambridge in England, has reported in Science Advances.
Moreover, because the use of rituximab reduced the neutralization of not just wild-type (WT) Omicron but also the Omicron-descendant variants BA.1, BA.2, BA.4, and XBB, this highlights “the urgent need for additional adjunctive strategies to enhance vaccine-induced immunity as well as preferential access for such patients to updated vaccines using spike from now circulating Omicron lineages,” the team added.
Studying Humoral Responses to SARS-CoV-2 Vaccines
Corresponding author Ravindra K. Gupta, BMBCh, MA, MPH, PhD, told this news organization that studying humoral responses to SARS-CoV-2 vaccines in immunocompromised individuals such as those with vasculitis was important for two main reasons.
“It is really important at individual level for their own health, of course, but also because we know that variants of concern have often evolved and developed within patients and can then spread in wider populations,” he said.
Gupta, who is professor of clinical microbiology at the Cambridge Institute for Therapeutic Immunology & Infectious Disease added: “We believe that the variants of concern that we’re having to deal with right now, including Omicron, have come from such [immunocompromised] individuals.”
Omicron “was a big shift,” Gupta noted. “It had a lot of new mutations on it, so it was almost like a new strain of the virus.” Few studies have looked at the longitudinal immunogenicity proffered by COVID vaccines in the post-Omicron era, particularly in those with vasculitis who are often treated with immunosuppressive drugs, including rituximab.
Two-Pronged Study Approach
For the study, a population of immunocompromised individuals diagnosed with vasculitis who had been treated with rituximab in the past 5 years was identified. Just over half (58%) had received adenovirus-based AZD1222/ChAdOx1 nCoV-19 (AstraZeneca-Oxford; AZN) and 37% BNT162b2 (Pfizer-BioNTech; mRNA) as their primary vaccines. Patients with antineutrophil cytoplasmic antibody–associated vasculitis comprised the majority of those who received rituximab (83%), compared with less than half of those who did not take rituximab (48%).
A two-pronged approach was taken with the researchers first measuring neutralizing antibody titers before and 30 days after four successive COVID vaccinations in a group of 32 individuals with available samples. They then performed a cross-sectional, case-control study in 95 individuals to look at neutralizing antibody titers and antibody-dependent cell-mediated cytotoxicity (ADCC) in individuals who had (n = 64) and had not (n = 31) been treated with rituximab in the past 5 years and had samples available after their third and fourth COVID vaccinations.
The first analysis was done to see how people were responding to vaccination over time. “That told us that there was a problem with the first two doses and that we got some response after doses three and four, but the response was uniformly quite poor against the new variants of concern,” Gupta said.
A human embryonic kidney cell model had been used to determine individuals’ neutralizing antibody titers in response to WT, BA.1, BA.2, BA.4, and XBB pseudotyped viruses. After the first and second COVID vaccinations, the geometric mean titer (GMT) against each variant barely increased from a baseline of 40.0. The greatest increases in GMT was seen with the WT virus, at 43.7, 90.7, 256.3, and 394.2, after the first, second, third and fourth doses, respectively. The lowest increases in GMT were seen with the XBB variant, with respective values of 40.0, 40.8, 45.7, and 53.9.
Incremental Benefit Offers Some ‘Reassurance’
Vasculitis specialist Rona Smith, MA, MB BChir, MD, who was one of the authors of the paper, told this news organization separately that the results showed there was “an incremental benefit of having COVID vaccinations,” which “offers a little bit of reassurance” that there can be an immune response in people with vasculitis.
Although results of the cross-sectional study showed that there was a significant dampening effect of rituximab treatment on the immune response, “I don’t think it’s an isolated effect in our [vasculitis] patients,” Smith suggested, adding the results were “probably still relevant to patients who receive routine dosing of rituximab for other conditions.”
Neutralizing antibody titers were consistently lower among individuals who had been treated with rituximab vs those who had not, with treatment in the past 18 months found to significantly impair immunogenicity.
The ADCC response was better preserved than the neutralizing antibody response, Gupta said, although it was still significantly lower in the rituximab-treated than in the non–rituximab-treated patients.
When to Vaccinate in Vasculitis?
Regarding when to give vaccines to people with vasculitis, Smith said: “Current recommendations are that patients should receive any vaccines that they’re offered routinely, whether that be COVID vaccines, flu vaccines, pneumococcal vaccines.”
As for the timing of those vaccinations, she observed that the current thinking was that vaccinations should “ideally be at least 1 month before a rituximab treatment, and ideally 3-4 [months] after their last dose. However, as many patients are on a 6-month dosing cycle, it can be difficult for some of them to find a suitable time window to have the COVID vaccine when it is offered.”
Additional precautions, such as wearing masks in crowded places and avoiding visits to acutely unwell friends or relatives, may still be prudent, Smith acknowledged, but he was clear that people should not be locking themselves away as they did during the COVID-19 pandemic.
When advising patients, “our general recommendation is that it is better to have a vaccine than not, but we can’t guarantee how well you will respond to it, but some response is better than none,” Smith said.
The study was independently supported. Gupta had no relevant financial relationships to disclose. Smith was a coauthor of the paper and has received research grant funding from Union Therapeutics, GlaxoSmithKline/Vir Biotechnology, Addenbrooke’s Charitable Trust, and Vasculitis UK. Another coauthor reported receiving research grants from CSL Vifor, Roche, and GlaxoSmithKline and advisory board, consultancy, and lecture fees from Roche and CSL Vifor.
A version of this article first appeared on Medscape.com.
People with vasculitis may need at least three or four vaccinations for COVID-19 before they start to show an immune response against SARS-CoV-2 infection, new research has suggested.
In a longitudinal retrospective study, serum antibody neutralization against the Omicron variant of the virus and its descendants was found to be “largely absent” after the first two doses of COVID-19 vaccine had been given to patients. But increasing neutralizing antibody titers were seen after both the third and fourth vaccine boosters had been administered.
Results also showed that the more recently people had been treated with the B cell–depleting therapy rituximab, the lower the levels of immunogenicity that were achieved, and thus protection against SARS-CoV-2.
“Our results have significant implications for individuals treated with rituximab in the post-Omicron era, highlighting the value of additive boosters in affirming increasing protection in clinically vulnerable populations,” the team behind the work at the University of Cambridge in England, has reported in Science Advances.
Moreover, because the use of rituximab reduced the neutralization of not just wild-type (WT) Omicron but also the Omicron-descendant variants BA.1, BA.2, BA.4, and XBB, this highlights “the urgent need for additional adjunctive strategies to enhance vaccine-induced immunity as well as preferential access for such patients to updated vaccines using spike from now circulating Omicron lineages,” the team added.
Studying Humoral Responses to SARS-CoV-2 Vaccines
Corresponding author Ravindra K. Gupta, BMBCh, MA, MPH, PhD, told this news organization that studying humoral responses to SARS-CoV-2 vaccines in immunocompromised individuals such as those with vasculitis was important for two main reasons.
“It is really important at individual level for their own health, of course, but also because we know that variants of concern have often evolved and developed within patients and can then spread in wider populations,” he said.
Gupta, who is professor of clinical microbiology at the Cambridge Institute for Therapeutic Immunology & Infectious Disease added: “We believe that the variants of concern that we’re having to deal with right now, including Omicron, have come from such [immunocompromised] individuals.”
Omicron “was a big shift,” Gupta noted. “It had a lot of new mutations on it, so it was almost like a new strain of the virus.” Few studies have looked at the longitudinal immunogenicity proffered by COVID vaccines in the post-Omicron era, particularly in those with vasculitis who are often treated with immunosuppressive drugs, including rituximab.
Two-Pronged Study Approach
For the study, a population of immunocompromised individuals diagnosed with vasculitis who had been treated with rituximab in the past 5 years was identified. Just over half (58%) had received adenovirus-based AZD1222/ChAdOx1 nCoV-19 (AstraZeneca-Oxford; AZN) and 37% BNT162b2 (Pfizer-BioNTech; mRNA) as their primary vaccines. Patients with antineutrophil cytoplasmic antibody–associated vasculitis comprised the majority of those who received rituximab (83%), compared with less than half of those who did not take rituximab (48%).
A two-pronged approach was taken with the researchers first measuring neutralizing antibody titers before and 30 days after four successive COVID vaccinations in a group of 32 individuals with available samples. They then performed a cross-sectional, case-control study in 95 individuals to look at neutralizing antibody titers and antibody-dependent cell-mediated cytotoxicity (ADCC) in individuals who had (n = 64) and had not (n = 31) been treated with rituximab in the past 5 years and had samples available after their third and fourth COVID vaccinations.
The first analysis was done to see how people were responding to vaccination over time. “That told us that there was a problem with the first two doses and that we got some response after doses three and four, but the response was uniformly quite poor against the new variants of concern,” Gupta said.
A human embryonic kidney cell model had been used to determine individuals’ neutralizing antibody titers in response to WT, BA.1, BA.2, BA.4, and XBB pseudotyped viruses. After the first and second COVID vaccinations, the geometric mean titer (GMT) against each variant barely increased from a baseline of 40.0. The greatest increases in GMT was seen with the WT virus, at 43.7, 90.7, 256.3, and 394.2, after the first, second, third and fourth doses, respectively. The lowest increases in GMT were seen with the XBB variant, with respective values of 40.0, 40.8, 45.7, and 53.9.
Incremental Benefit Offers Some ‘Reassurance’
Vasculitis specialist Rona Smith, MA, MB BChir, MD, who was one of the authors of the paper, told this news organization separately that the results showed there was “an incremental benefit of having COVID vaccinations,” which “offers a little bit of reassurance” that there can be an immune response in people with vasculitis.
Although results of the cross-sectional study showed that there was a significant dampening effect of rituximab treatment on the immune response, “I don’t think it’s an isolated effect in our [vasculitis] patients,” Smith suggested, adding the results were “probably still relevant to patients who receive routine dosing of rituximab for other conditions.”
Neutralizing antibody titers were consistently lower among individuals who had been treated with rituximab vs those who had not, with treatment in the past 18 months found to significantly impair immunogenicity.
The ADCC response was better preserved than the neutralizing antibody response, Gupta said, although it was still significantly lower in the rituximab-treated than in the non–rituximab-treated patients.
When to Vaccinate in Vasculitis?
Regarding when to give vaccines to people with vasculitis, Smith said: “Current recommendations are that patients should receive any vaccines that they’re offered routinely, whether that be COVID vaccines, flu vaccines, pneumococcal vaccines.”
As for the timing of those vaccinations, she observed that the current thinking was that vaccinations should “ideally be at least 1 month before a rituximab treatment, and ideally 3-4 [months] after their last dose. However, as many patients are on a 6-month dosing cycle, it can be difficult for some of them to find a suitable time window to have the COVID vaccine when it is offered.”
Additional precautions, such as wearing masks in crowded places and avoiding visits to acutely unwell friends or relatives, may still be prudent, Smith acknowledged, but he was clear that people should not be locking themselves away as they did during the COVID-19 pandemic.
When advising patients, “our general recommendation is that it is better to have a vaccine than not, but we can’t guarantee how well you will respond to it, but some response is better than none,” Smith said.
The study was independently supported. Gupta had no relevant financial relationships to disclose. Smith was a coauthor of the paper and has received research grant funding from Union Therapeutics, GlaxoSmithKline/Vir Biotechnology, Addenbrooke’s Charitable Trust, and Vasculitis UK. Another coauthor reported receiving research grants from CSL Vifor, Roche, and GlaxoSmithKline and advisory board, consultancy, and lecture fees from Roche and CSL Vifor.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Around 5% of US Population Diagnosed With Autoimmune Disease
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TNF Inhibitors Show Comparable Safety With Non-TNF Inhibitors in US Veterans With RA-ILD
TOPLINE:
Tumor necrosis factor (TNF) inhibitors led to no significant difference in survival or respiratory-related hospitalizations, compared with non-TNF inhibitors, in patients with rheumatoid arthritis–associated interstitial lung disease (RA-ILD).
METHODOLOGY:
- Guidelines from the American College of Rheumatology and the American College of Chest Physicians conditionally advise against the use of TNF inhibitors for treating ILD in patients with RA-ILD, with persisting uncertainty about the safety of TNF inhibitors.
- Researchers conducted a retrospective cohort study using data from the US Department of Veterans Affairs, with a focus on comparing outcomes in patients with RA-ILD who initiated TNF or non-TNF inhibitors between 2006 and 2018.
- A total of 1047 US veterans with RA-ILD were included, with 237 who initiated TNF inhibitors propensity matched in a 1:1 ratio with 237 who initiated non-TNF inhibitors (mean age, 68 years; 92% men).
- The primary composite outcome was time to death or respiratory-related hospitalization over a follow-up period of up to 3 years.
- The secondary outcomes included all-cause mortality, respiratory-related mortality, and respiratory-related hospitalization, with additional assessments over a 1-year period.
TAKEAWAY:
- No significant difference was observed in the composite outcome of death or respiratory-related hospitalization between the TNF and non-TNF inhibitor groups (adjusted hazard ratio, 1.21; 95% CI, 0.92-1.58).
- No significant differences in the risk for respiratory-related hospitalization and all-cause or respiratory-related mortality were found between the TNF and non-TNF inhibitor groups. Similar findings were observed for all the outcomes during 1 year of follow-up.
- The mean duration of medication use prior to discontinuation, the time to discontinuation, and the mean predicted forced vital capacity percentage were similar for both groups.
- In a subgroup analysis of patients aged ≥ 65 years, those treated with non-TNF inhibitors had a higher risk for the composite outcome and all-cause and respiratory-related mortality than those treated with TNF inhibitors. No significant differences in outcomes were observed between the two treatment groups among patients aged < 65 years.
IN PRACTICE:
“Our results do not suggest that systematic avoidance of TNF inhibitors is required in all patients with rheumatoid arthritis–associated ILD. However, given disease heterogeneity and imprecision of our estimates, some subpopulations of patients with rheumatoid arthritis–associated ILD might benefit from specific biological or targeted synthetic DMARD [disease-modifying antirheumatic drug] treatment strategies,” the authors wrote.
SOURCE:
The study was led by Bryant R. England, MD, PhD, University of Nebraska Medical Center, Omaha It was published online on January 7, 2025, in The Lancet Rheumatology.
LIMITATIONS:
Administrative algorithms were used for identifying RA-ILD, potentially leading to misclassification and limiting phenotyping accuracy. Even with the use of propensity score methods, there might still be residual selection bias or unmeasured confounding. The study lacked comprehensive measures of posttreatment forced vital capacity and other indicators of ILD severity. The study population, predominantly men and those with a smoking history, may limit the generalizability of the findings to other groups.
DISCLOSURES:
The study was primarily funded by the US Department of Veterans Affairs. Some authors reported having financial relationships with pharmaceutical companies unrelated to the submitted work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Tumor necrosis factor (TNF) inhibitors led to no significant difference in survival or respiratory-related hospitalizations, compared with non-TNF inhibitors, in patients with rheumatoid arthritis–associated interstitial lung disease (RA-ILD).
METHODOLOGY:
- Guidelines from the American College of Rheumatology and the American College of Chest Physicians conditionally advise against the use of TNF inhibitors for treating ILD in patients with RA-ILD, with persisting uncertainty about the safety of TNF inhibitors.
- Researchers conducted a retrospective cohort study using data from the US Department of Veterans Affairs, with a focus on comparing outcomes in patients with RA-ILD who initiated TNF or non-TNF inhibitors between 2006 and 2018.
- A total of 1047 US veterans with RA-ILD were included, with 237 who initiated TNF inhibitors propensity matched in a 1:1 ratio with 237 who initiated non-TNF inhibitors (mean age, 68 years; 92% men).
- The primary composite outcome was time to death or respiratory-related hospitalization over a follow-up period of up to 3 years.
- The secondary outcomes included all-cause mortality, respiratory-related mortality, and respiratory-related hospitalization, with additional assessments over a 1-year period.
TAKEAWAY:
- No significant difference was observed in the composite outcome of death or respiratory-related hospitalization between the TNF and non-TNF inhibitor groups (adjusted hazard ratio, 1.21; 95% CI, 0.92-1.58).
- No significant differences in the risk for respiratory-related hospitalization and all-cause or respiratory-related mortality were found between the TNF and non-TNF inhibitor groups. Similar findings were observed for all the outcomes during 1 year of follow-up.
- The mean duration of medication use prior to discontinuation, the time to discontinuation, and the mean predicted forced vital capacity percentage were similar for both groups.
- In a subgroup analysis of patients aged ≥ 65 years, those treated with non-TNF inhibitors had a higher risk for the composite outcome and all-cause and respiratory-related mortality than those treated with TNF inhibitors. No significant differences in outcomes were observed between the two treatment groups among patients aged < 65 years.
IN PRACTICE:
“Our results do not suggest that systematic avoidance of TNF inhibitors is required in all patients with rheumatoid arthritis–associated ILD. However, given disease heterogeneity and imprecision of our estimates, some subpopulations of patients with rheumatoid arthritis–associated ILD might benefit from specific biological or targeted synthetic DMARD [disease-modifying antirheumatic drug] treatment strategies,” the authors wrote.
SOURCE:
The study was led by Bryant R. England, MD, PhD, University of Nebraska Medical Center, Omaha It was published online on January 7, 2025, in The Lancet Rheumatology.
LIMITATIONS:
Administrative algorithms were used for identifying RA-ILD, potentially leading to misclassification and limiting phenotyping accuracy. Even with the use of propensity score methods, there might still be residual selection bias or unmeasured confounding. The study lacked comprehensive measures of posttreatment forced vital capacity and other indicators of ILD severity. The study population, predominantly men and those with a smoking history, may limit the generalizability of the findings to other groups.
DISCLOSURES:
The study was primarily funded by the US Department of Veterans Affairs. Some authors reported having financial relationships with pharmaceutical companies unrelated to the submitted work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Tumor necrosis factor (TNF) inhibitors led to no significant difference in survival or respiratory-related hospitalizations, compared with non-TNF inhibitors, in patients with rheumatoid arthritis–associated interstitial lung disease (RA-ILD).
METHODOLOGY:
- Guidelines from the American College of Rheumatology and the American College of Chest Physicians conditionally advise against the use of TNF inhibitors for treating ILD in patients with RA-ILD, with persisting uncertainty about the safety of TNF inhibitors.
- Researchers conducted a retrospective cohort study using data from the US Department of Veterans Affairs, with a focus on comparing outcomes in patients with RA-ILD who initiated TNF or non-TNF inhibitors between 2006 and 2018.
- A total of 1047 US veterans with RA-ILD were included, with 237 who initiated TNF inhibitors propensity matched in a 1:1 ratio with 237 who initiated non-TNF inhibitors (mean age, 68 years; 92% men).
- The primary composite outcome was time to death or respiratory-related hospitalization over a follow-up period of up to 3 years.
- The secondary outcomes included all-cause mortality, respiratory-related mortality, and respiratory-related hospitalization, with additional assessments over a 1-year period.
TAKEAWAY:
- No significant difference was observed in the composite outcome of death or respiratory-related hospitalization between the TNF and non-TNF inhibitor groups (adjusted hazard ratio, 1.21; 95% CI, 0.92-1.58).
- No significant differences in the risk for respiratory-related hospitalization and all-cause or respiratory-related mortality were found between the TNF and non-TNF inhibitor groups. Similar findings were observed for all the outcomes during 1 year of follow-up.
- The mean duration of medication use prior to discontinuation, the time to discontinuation, and the mean predicted forced vital capacity percentage were similar for both groups.
- In a subgroup analysis of patients aged ≥ 65 years, those treated with non-TNF inhibitors had a higher risk for the composite outcome and all-cause and respiratory-related mortality than those treated with TNF inhibitors. No significant differences in outcomes were observed between the two treatment groups among patients aged < 65 years.
IN PRACTICE:
“Our results do not suggest that systematic avoidance of TNF inhibitors is required in all patients with rheumatoid arthritis–associated ILD. However, given disease heterogeneity and imprecision of our estimates, some subpopulations of patients with rheumatoid arthritis–associated ILD might benefit from specific biological or targeted synthetic DMARD [disease-modifying antirheumatic drug] treatment strategies,” the authors wrote.
SOURCE:
The study was led by Bryant R. England, MD, PhD, University of Nebraska Medical Center, Omaha It was published online on January 7, 2025, in The Lancet Rheumatology.
LIMITATIONS:
Administrative algorithms were used for identifying RA-ILD, potentially leading to misclassification and limiting phenotyping accuracy. Even with the use of propensity score methods, there might still be residual selection bias or unmeasured confounding. The study lacked comprehensive measures of posttreatment forced vital capacity and other indicators of ILD severity. The study population, predominantly men and those with a smoking history, may limit the generalizability of the findings to other groups.
DISCLOSURES:
The study was primarily funded by the US Department of Veterans Affairs. Some authors reported having financial relationships with pharmaceutical companies unrelated to the submitted work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Most Kids With COVID-Linked MIS-C Recover by 6 Months
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children who were severely ill with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19 infection appear to show excellent cardiovascular and noncardiovascular outcomes by 6 months, according to data published in JAMA Pediatrics.
MIS-C is a life-threatening complication of COVID-19 infection and data on outcomes are limited, wrote the authors, led by Dongngan T. Truong, MD, MSSI, with Children’s Healthcare of Atlanta Cardiology, Emory University School of Medicine in Atlanta, Georgia. These 6-month results are from the Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study, sponsored by the National Heart, Lung, and Blood Institute.
Researchers found in this cohort study of 1204 participants that by 6 months after hospital discharge, 99% had normalization of left ventricular systolic function, and 92.3% had normalized coronary artery dimensions. More than 95% reported being more than 90% back to baseline health.
Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health scores were at least equivalent to prepandemic population normative values. PROMIS Global Health parent/guardian proxy median T scores for fatigue, global health, and pain interference improved significantly from 2 weeks to 6 months: fatigue, 56.1 vs 48.9; global health, 48.8 vs 51.3; pain interference, 53.0 vs 43.3 (P < .001).
The most common symptoms reported at 2 weeks were fatigue (15.9%) and low stamina/energy (9.2%); both decreased to 3.4% and 3.3%, respectively, by 6 months. The most common cardiovascular symptom at 2 weeks was palpitations (1.5%), which decreased to 0.6%.
Chest Pain Increased Over Time
Reports of chest pain, however, reportedly increased over time, with 1.3% reporting chest pain at rest at 2 weeks and 2.2% at 6 months. Although gastrointestinal symptoms were common during the acute MIS-C, only 5.3% of respondents reported those symptoms at 2 weeks.
Children in the cohort had a median age of 9 years, and 60% were men. They self-identified with the following races and ethnicities: American Indian or Alaska Native (0.1%), Asian (3.3%), Black (27.0%), Hawaiian Native or Other Pacific Islander (0.2%), Hispanic or Latino (26.9%), multiracial (2.7%), White (31.2%), other (1.0%), and unknown or refused to specify (7.6%). Authors wrote that the cohort was followed-up to 2 years after illness onset and long-term results are not yet known.
Time to Exhale
David J. Goldberg, MD, with the Cardiac Center, Children’s Hospital of Philadelphia, Pennsylvania, and colleagues, wrote in an accompanying editorial that “the decreased frequency of the disease along (with) the reassuring reports on midterm outcomes can allow the pediatric community a moment of collective exhale.”
The editorialists note that of those who initially presented with myocardial dysfunction, all but one patient evaluated had a normal ejection fraction at follow-up. Energy, sleep, appetite, cognition, and mood also normalized by midterm.
“The results of the MUSIC study add to the emerging midterm outcomes data suggesting a near-complete cardiovascular recovery in the overwhelming majority of patients who develop MIS-C,” Goldberg and colleagues wrote. “Despite initial concerns, driven by the severity of acute presentation at diagnosis and longer-term questions that remain (for example, does coronary microvascular dysfunction persist even after normalization of coronary artery z score?), these data suggest an encouraging outlook for the long-term health of affected children.”
The Centers for Disease Control and Prevention and other agencies have reported a declining overall incidence of MIS-C and highlighted the protective value of vaccination.
The editorialists add, however, that while the drop in MIS-C cases is encouraging, cases are still reported, especially amid high viral activity periods, “and nearly half of affected children continue to require intensive care in the acute phase of illness.”
Truong reported grants from the National Institutes of Health and serving as coprincipal investigator for Pfizer for research on COVID-19 vaccine-associated myocarditis funded by Pfizer and occurring through the framework of the National Heart, Lung, and Blood Institute’s Pediatric Heart Network outside the submitted work. One coauthor reported grants from Pfizer and Boston Scientific outside the submitted work. One coauthor reported receiving grants from Additional Ventures Foundation outside the submitted work. One coauthor reported receiving consultant fees from Amryt Pharma, Chiesi, Esperion, and Ultragenyx outside the submitted work. A coauthor reported receiving consultant fees from Larimar Therapeutics for mitochondrial therapies outside the submitted work. One coauthor reported being an employee of Takeda Pharmaceuticals since July 2023. One editorialist reported grants from Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, Academy Health, and the Gordon and Betty Moore Foundation during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Sports Injuries of the Hip in Primary Care
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.
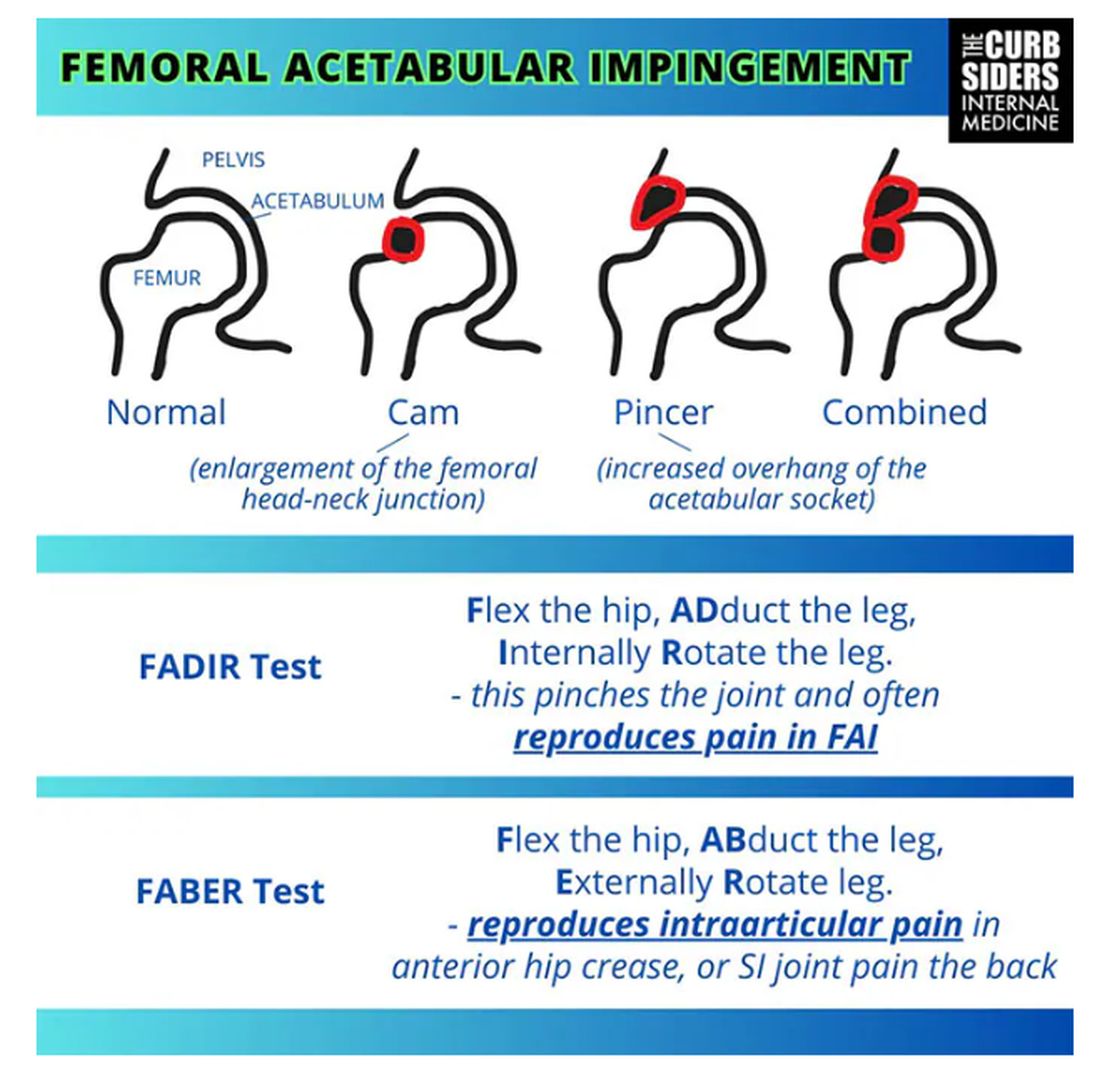
Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.
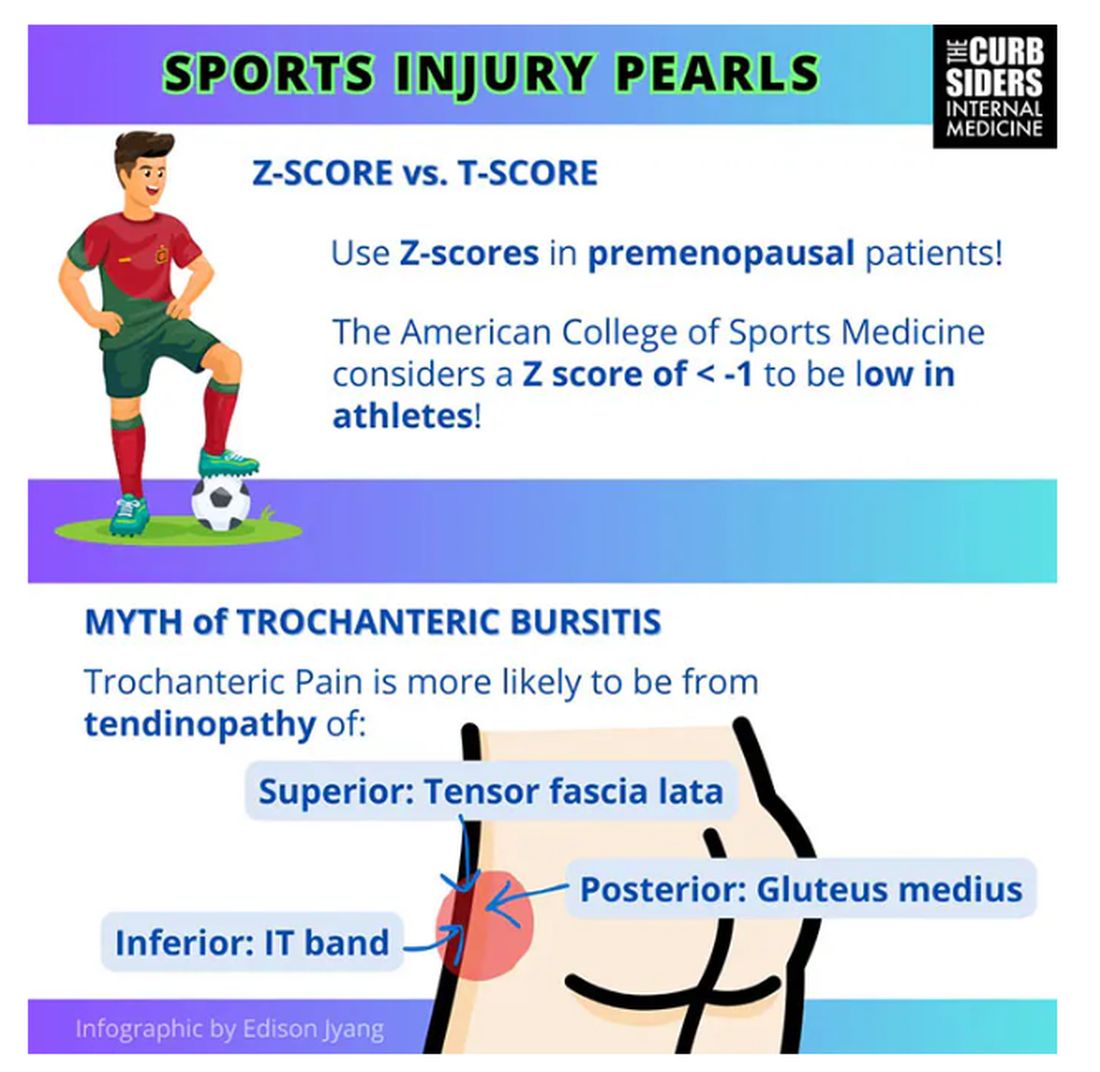
Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.

Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.

Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.

Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.

Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
Need for Biologic in Early RA Signals Lower Likelihood of Achieving Drug-Free Remission
TOPLINE:
Patients who require biologic disease-modifying antirheumatic drugs (DMARDs) for severe RA are less likely to achieve sustained DMARD-free remission than those not needing the medication.
METHODOLOGY:
- Patients with early RA from the Leiden Early Arthritis Clinic (EAC; n = 627) and the Rotterdam Early Arthritis Cohort (tREACH) trial (n = 425) were followed for 5 years and 3 years, respectively.
- Most patients in both the EAC (86%) and tREACH (64%) cohorts had never used a biologic DMARD during the follow-up period.
- The primary outcome measure was sustained DMARD-free remission, defined as the absence of clinical synovitis after discontinuation of DMARDs for at least 1 year.
TAKEAWAY:
- None of the EAC patients using a biologic DMARD achieved sustained DMARD-free remission, but 37% of those who never used the drug reached remission at 5 years (hazard ratio [HR], 0.02; P < .0001).
- No tREACH patients using a biologic DMARD reached sustained DMARD-free remission, but 15% of those who never used the drug achieved remission at 3 years (HR, 0.03; P < .0001).
- Sustained DMARD-free remission was higher in EAC patients who were negative for anti-citrullinated protein antibody (ACPA) than in those who were ACPA-positive at 5 years (56% vs 14%; P < .0001).
- During follow-up, some patients in both the EAC (9%) and tREACH (14%) cohorts experienced late flares after more than 1 year of discontinuing DMARDs.
IN PRACTICE:
“Sustained DMARD-free remission is unlikely in patients needing a biologic DMARD,” the authors said.
SOURCE:
Judith W. Heutz, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the study, published online on December 20, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Because both cohorts were defined during follow-up rather than at baseline, outcomes related to the use of DMARDs and remission status could have been misinterpreted. Although the study adjusted for ACPA status, other factors such as disease activity were not corrected, which could have potentially led to residual confounding. Sparse data bias was present, especially in the biologic DMARD user group, in which none of the patients reached sustained DMARD-free remission.
DISCLOSURES:
The EAC received funding from the Dutch Arthritis Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation program. The tREACH trial was supported by an unrestricted grant from Pfizer. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients who require biologic disease-modifying antirheumatic drugs (DMARDs) for severe RA are less likely to achieve sustained DMARD-free remission than those not needing the medication.
METHODOLOGY:
- Patients with early RA from the Leiden Early Arthritis Clinic (EAC; n = 627) and the Rotterdam Early Arthritis Cohort (tREACH) trial (n = 425) were followed for 5 years and 3 years, respectively.
- Most patients in both the EAC (86%) and tREACH (64%) cohorts had never used a biologic DMARD during the follow-up period.
- The primary outcome measure was sustained DMARD-free remission, defined as the absence of clinical synovitis after discontinuation of DMARDs for at least 1 year.
TAKEAWAY:
- None of the EAC patients using a biologic DMARD achieved sustained DMARD-free remission, but 37% of those who never used the drug reached remission at 5 years (hazard ratio [HR], 0.02; P < .0001).
- No tREACH patients using a biologic DMARD reached sustained DMARD-free remission, but 15% of those who never used the drug achieved remission at 3 years (HR, 0.03; P < .0001).
- Sustained DMARD-free remission was higher in EAC patients who were negative for anti-citrullinated protein antibody (ACPA) than in those who were ACPA-positive at 5 years (56% vs 14%; P < .0001).
- During follow-up, some patients in both the EAC (9%) and tREACH (14%) cohorts experienced late flares after more than 1 year of discontinuing DMARDs.
IN PRACTICE:
“Sustained DMARD-free remission is unlikely in patients needing a biologic DMARD,” the authors said.
SOURCE:
Judith W. Heutz, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the study, published online on December 20, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Because both cohorts were defined during follow-up rather than at baseline, outcomes related to the use of DMARDs and remission status could have been misinterpreted. Although the study adjusted for ACPA status, other factors such as disease activity were not corrected, which could have potentially led to residual confounding. Sparse data bias was present, especially in the biologic DMARD user group, in which none of the patients reached sustained DMARD-free remission.
DISCLOSURES:
The EAC received funding from the Dutch Arthritis Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation program. The tREACH trial was supported by an unrestricted grant from Pfizer. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients who require biologic disease-modifying antirheumatic drugs (DMARDs) for severe RA are less likely to achieve sustained DMARD-free remission than those not needing the medication.
METHODOLOGY:
- Patients with early RA from the Leiden Early Arthritis Clinic (EAC; n = 627) and the Rotterdam Early Arthritis Cohort (tREACH) trial (n = 425) were followed for 5 years and 3 years, respectively.
- Most patients in both the EAC (86%) and tREACH (64%) cohorts had never used a biologic DMARD during the follow-up period.
- The primary outcome measure was sustained DMARD-free remission, defined as the absence of clinical synovitis after discontinuation of DMARDs for at least 1 year.
TAKEAWAY:
- None of the EAC patients using a biologic DMARD achieved sustained DMARD-free remission, but 37% of those who never used the drug reached remission at 5 years (hazard ratio [HR], 0.02; P < .0001).
- No tREACH patients using a biologic DMARD reached sustained DMARD-free remission, but 15% of those who never used the drug achieved remission at 3 years (HR, 0.03; P < .0001).
- Sustained DMARD-free remission was higher in EAC patients who were negative for anti-citrullinated protein antibody (ACPA) than in those who were ACPA-positive at 5 years (56% vs 14%; P < .0001).
- During follow-up, some patients in both the EAC (9%) and tREACH (14%) cohorts experienced late flares after more than 1 year of discontinuing DMARDs.
IN PRACTICE:
“Sustained DMARD-free remission is unlikely in patients needing a biologic DMARD,” the authors said.
SOURCE:
Judith W. Heutz, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the study, published online on December 20, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Because both cohorts were defined during follow-up rather than at baseline, outcomes related to the use of DMARDs and remission status could have been misinterpreted. Although the study adjusted for ACPA status, other factors such as disease activity were not corrected, which could have potentially led to residual confounding. Sparse data bias was present, especially in the biologic DMARD user group, in which none of the patients reached sustained DMARD-free remission.
DISCLOSURES:
The EAC received funding from the Dutch Arthritis Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation program. The tREACH trial was supported by an unrestricted grant from Pfizer. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Colchicine Gout Flare Prophylaxis May Also Protect Against Cardiovascular Events
TOPLINE:
Gout patients who take colchicine at the start of urate-lowering therapy have a lower risk for cardiovascular events than those who do not receive prophylaxis.
METHODOLOGY:
- Retrospective cohort study of 99,800 patients (mean age, 62.8 years; 74.4% men; 85.1% White) newly diagnosed with gout between January 1997 and March 2021 who initiated urate-lowering therapy.
- Gout flare prophylaxis, defined as a colchicine prescription for 21 days or more, was prescribed to 16,028 patients for a mean duration of 47.3 days at a mean daily dose of 0.97 mg.
- Patients who received colchicine prophylaxis and 83,772 patients who did not receive prophylaxis were followed for a mean of 175.5 and 176.9 days, respectively, in the intention-to-treat analysis.
- The primary outcome was the occurrence of the first cardiovascular event (fatal or nonfatal myocardial infarction or stroke) within 180 days of initiation of urate-lowering therapy.
TAKEAWAY:
- The risk for cardiovascular events was significantly lower with colchicine prophylaxis than without it (weighted hazard ratio [HR], 0.82; 95% CI, 0.69-0.94).
- The risk for a first-ever cardiovascular event was significantly lower with colchicine prophylaxis than without it (adjusted HR, 0.80; 95% CI, 0.62-0.97).
- The findings were similar regardless of analytical approach, and the intention-to-treat analysis did not show an increased risk for diarrhea with colchicine.
IN PRACTICE:
“The findings support consideration for the use of colchicine in people with gout and cardiovascular diseases,” the authors wrote.
“The observed beneficial effect of colchicine concerns a huge group of patients worldwide. In addition, it is conceivable that, if a cardiovascular risk reduction is indeed confirmed, a strong argument arises to recommend the prescription of a course of colchicine to all [flaring] patients with gout, independently of their preference for urate-lowering therapy in general or urate-lowering therapy with or without colchicine prophylaxis more specifically,” experts wrote in a linked commentary.
SOURCE:
Edoardo Cipolletta, MD, Academic Rheumatology, School of Medicine, Nottingham City Hospital, University of Nottingham, England, led the study, which was published online in The Lancet Rheumatology.
LIMITATIONS:
Because of the retrospective nature of the data extraction from a prospective database, the study had variations in follow-up and data completeness. Potential surveillance bias could have been introduced because patients with prior cardiovascular events were included in the study, and patients’ adherence to prescribed medications could not be verified.
DISCLOSURES:
This study was funded by the Foundation for Research in Rheumatology. Some authors reported receiving consulting fees, lecturing fees, and travel grants from various pharmaceutical companies and other additional sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Gout patients who take colchicine at the start of urate-lowering therapy have a lower risk for cardiovascular events than those who do not receive prophylaxis.
METHODOLOGY:
- Retrospective cohort study of 99,800 patients (mean age, 62.8 years; 74.4% men; 85.1% White) newly diagnosed with gout between January 1997 and March 2021 who initiated urate-lowering therapy.
- Gout flare prophylaxis, defined as a colchicine prescription for 21 days or more, was prescribed to 16,028 patients for a mean duration of 47.3 days at a mean daily dose of 0.97 mg.
- Patients who received colchicine prophylaxis and 83,772 patients who did not receive prophylaxis were followed for a mean of 175.5 and 176.9 days, respectively, in the intention-to-treat analysis.
- The primary outcome was the occurrence of the first cardiovascular event (fatal or nonfatal myocardial infarction or stroke) within 180 days of initiation of urate-lowering therapy.
TAKEAWAY:
- The risk for cardiovascular events was significantly lower with colchicine prophylaxis than without it (weighted hazard ratio [HR], 0.82; 95% CI, 0.69-0.94).
- The risk for a first-ever cardiovascular event was significantly lower with colchicine prophylaxis than without it (adjusted HR, 0.80; 95% CI, 0.62-0.97).
- The findings were similar regardless of analytical approach, and the intention-to-treat analysis did not show an increased risk for diarrhea with colchicine.
IN PRACTICE:
“The findings support consideration for the use of colchicine in people with gout and cardiovascular diseases,” the authors wrote.
“The observed beneficial effect of colchicine concerns a huge group of patients worldwide. In addition, it is conceivable that, if a cardiovascular risk reduction is indeed confirmed, a strong argument arises to recommend the prescription of a course of colchicine to all [flaring] patients with gout, independently of their preference for urate-lowering therapy in general or urate-lowering therapy with or without colchicine prophylaxis more specifically,” experts wrote in a linked commentary.
SOURCE:
Edoardo Cipolletta, MD, Academic Rheumatology, School of Medicine, Nottingham City Hospital, University of Nottingham, England, led the study, which was published online in The Lancet Rheumatology.
LIMITATIONS:
Because of the retrospective nature of the data extraction from a prospective database, the study had variations in follow-up and data completeness. Potential surveillance bias could have been introduced because patients with prior cardiovascular events were included in the study, and patients’ adherence to prescribed medications could not be verified.
DISCLOSURES:
This study was funded by the Foundation for Research in Rheumatology. Some authors reported receiving consulting fees, lecturing fees, and travel grants from various pharmaceutical companies and other additional sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Gout patients who take colchicine at the start of urate-lowering therapy have a lower risk for cardiovascular events than those who do not receive prophylaxis.
METHODOLOGY:
- Retrospective cohort study of 99,800 patients (mean age, 62.8 years; 74.4% men; 85.1% White) newly diagnosed with gout between January 1997 and March 2021 who initiated urate-lowering therapy.
- Gout flare prophylaxis, defined as a colchicine prescription for 21 days or more, was prescribed to 16,028 patients for a mean duration of 47.3 days at a mean daily dose of 0.97 mg.
- Patients who received colchicine prophylaxis and 83,772 patients who did not receive prophylaxis were followed for a mean of 175.5 and 176.9 days, respectively, in the intention-to-treat analysis.
- The primary outcome was the occurrence of the first cardiovascular event (fatal or nonfatal myocardial infarction or stroke) within 180 days of initiation of urate-lowering therapy.
TAKEAWAY:
- The risk for cardiovascular events was significantly lower with colchicine prophylaxis than without it (weighted hazard ratio [HR], 0.82; 95% CI, 0.69-0.94).
- The risk for a first-ever cardiovascular event was significantly lower with colchicine prophylaxis than without it (adjusted HR, 0.80; 95% CI, 0.62-0.97).
- The findings were similar regardless of analytical approach, and the intention-to-treat analysis did not show an increased risk for diarrhea with colchicine.
IN PRACTICE:
“The findings support consideration for the use of colchicine in people with gout and cardiovascular diseases,” the authors wrote.
“The observed beneficial effect of colchicine concerns a huge group of patients worldwide. In addition, it is conceivable that, if a cardiovascular risk reduction is indeed confirmed, a strong argument arises to recommend the prescription of a course of colchicine to all [flaring] patients with gout, independently of their preference for urate-lowering therapy in general or urate-lowering therapy with or without colchicine prophylaxis more specifically,” experts wrote in a linked commentary.
SOURCE:
Edoardo Cipolletta, MD, Academic Rheumatology, School of Medicine, Nottingham City Hospital, University of Nottingham, England, led the study, which was published online in The Lancet Rheumatology.
LIMITATIONS:
Because of the retrospective nature of the data extraction from a prospective database, the study had variations in follow-up and data completeness. Potential surveillance bias could have been introduced because patients with prior cardiovascular events were included in the study, and patients’ adherence to prescribed medications could not be verified.
DISCLOSURES:
This study was funded by the Foundation for Research in Rheumatology. Some authors reported receiving consulting fees, lecturing fees, and travel grants from various pharmaceutical companies and other additional sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Novel JAK1 Inhibitor Effective for RA in Phase 3 Study
TOPLINE:
(RA) who have an inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).
METHODOLOGY:
- This phase 3 trial, conducted across 59 sites in China, evaluated the efficacy and safety of ivarmacitinib in patients with moderate to severe active RA despite treatment with one or more csDMARDs.
- The patients were randomly assigned to receive either placebo (n = 188; mean age, 50.9 years; 85.6% women) or 4 mg ivarmacitinib (n = 189; mean age, 49.7 years; 91% women) or 8 mg ivarmacitinib (n = 189; mean age, 49.8 years; 83.6% women) once daily for 24 weeks, alongside background csDMARDs.
- After 24 weeks, the patients receiving placebo were switched to receive 4 mg ivarmacitinib for the additional 28-week extension period, whereas those receiving ivarmacitinib continued their initial dosage.
- Secondary endpoints included the proportion of patients achieving American College of Rheumatology (ACR) 50/70 responses and, Disease Activity Score 28-joint count C-reactive protein (DAS28(CRP)) score < 2.6 or ≤ 3.2, as well as measures of other patient-reported outcomes such as pain, physical function, and quality of life at 24 and 52 weeks.
TAKEAWAY:
- At 24 weeks, the proportion of patients achieving a 20% improvement in the ACR20 response — the primary endpoint — was higher among those receiving 4 mg ivarmacitinib (70.4%) or 8 mg ivarmacitinib (75.1%) than among those receiving placebo (40.4%; P < .0001 for both comparisons), with the efficacy either maintained or improved through 52 weeks.
- The proportion of patients achieving ACR50/70 responses or a DAS28(CRP) score < 2.6 or ≤ 3.2 was higher in the ivarmacitinib groups than in the placebo group (P < .0001 for all comparisons).
- Compared with the placebo group, both the ivarmacitinib groups showed improvements in patient-reported outcomes such as pain, physical function, quality of life, and duration and severity of morning stiffness.
- The overall rates of treatment discontinuation caused by adverse events were low across all the groups, with no deaths, tuberculosis or gastrointestinal perforations reported throughout the 52 weeks.
IN PRACTICE:
“Based on these findings, ivarmacitinib with background csDMARDs allowed, could be considered a treatment option in patients with moderate to severe active RA who have an inadequate response to csDMARDs,” the authors wrote.
SOURCE:
This study was led by Jinjing Liu and Xiaofeng Zeng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Beijing, China. It was published online on November 27, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
As the study was conducted in a Chinese population, the findings may have limited applicability across diverse global populations. Additionally, as the placebo-controlled period was limited to 24 weeks because of ethical concerns, comparisons between placebo and ivarmacitinib beyond that period were restricted. Lastly, this study was not powered to compare efficacy and safety between the two active dose regimens.
DISCLOSURES:
This study was funded by Jiangsu Hengrui Pharmaceuticals. Two authors declared being employees of the company. The other authors reported no competing interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
(RA) who have an inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).
METHODOLOGY:
- This phase 3 trial, conducted across 59 sites in China, evaluated the efficacy and safety of ivarmacitinib in patients with moderate to severe active RA despite treatment with one or more csDMARDs.
- The patients were randomly assigned to receive either placebo (n = 188; mean age, 50.9 years; 85.6% women) or 4 mg ivarmacitinib (n = 189; mean age, 49.7 years; 91% women) or 8 mg ivarmacitinib (n = 189; mean age, 49.8 years; 83.6% women) once daily for 24 weeks, alongside background csDMARDs.
- After 24 weeks, the patients receiving placebo were switched to receive 4 mg ivarmacitinib for the additional 28-week extension period, whereas those receiving ivarmacitinib continued their initial dosage.
- Secondary endpoints included the proportion of patients achieving American College of Rheumatology (ACR) 50/70 responses and, Disease Activity Score 28-joint count C-reactive protein (DAS28(CRP)) score < 2.6 or ≤ 3.2, as well as measures of other patient-reported outcomes such as pain, physical function, and quality of life at 24 and 52 weeks.
TAKEAWAY:
- At 24 weeks, the proportion of patients achieving a 20% improvement in the ACR20 response — the primary endpoint — was higher among those receiving 4 mg ivarmacitinib (70.4%) or 8 mg ivarmacitinib (75.1%) than among those receiving placebo (40.4%; P < .0001 for both comparisons), with the efficacy either maintained or improved through 52 weeks.
- The proportion of patients achieving ACR50/70 responses or a DAS28(CRP) score < 2.6 or ≤ 3.2 was higher in the ivarmacitinib groups than in the placebo group (P < .0001 for all comparisons).
- Compared with the placebo group, both the ivarmacitinib groups showed improvements in patient-reported outcomes such as pain, physical function, quality of life, and duration and severity of morning stiffness.
- The overall rates of treatment discontinuation caused by adverse events were low across all the groups, with no deaths, tuberculosis or gastrointestinal perforations reported throughout the 52 weeks.
IN PRACTICE:
“Based on these findings, ivarmacitinib with background csDMARDs allowed, could be considered a treatment option in patients with moderate to severe active RA who have an inadequate response to csDMARDs,” the authors wrote.
SOURCE:
This study was led by Jinjing Liu and Xiaofeng Zeng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Beijing, China. It was published online on November 27, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
As the study was conducted in a Chinese population, the findings may have limited applicability across diverse global populations. Additionally, as the placebo-controlled period was limited to 24 weeks because of ethical concerns, comparisons between placebo and ivarmacitinib beyond that period were restricted. Lastly, this study was not powered to compare efficacy and safety between the two active dose regimens.
DISCLOSURES:
This study was funded by Jiangsu Hengrui Pharmaceuticals. Two authors declared being employees of the company. The other authors reported no competing interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
(RA) who have an inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).
METHODOLOGY:
- This phase 3 trial, conducted across 59 sites in China, evaluated the efficacy and safety of ivarmacitinib in patients with moderate to severe active RA despite treatment with one or more csDMARDs.
- The patients were randomly assigned to receive either placebo (n = 188; mean age, 50.9 years; 85.6% women) or 4 mg ivarmacitinib (n = 189; mean age, 49.7 years; 91% women) or 8 mg ivarmacitinib (n = 189; mean age, 49.8 years; 83.6% women) once daily for 24 weeks, alongside background csDMARDs.
- After 24 weeks, the patients receiving placebo were switched to receive 4 mg ivarmacitinib for the additional 28-week extension period, whereas those receiving ivarmacitinib continued their initial dosage.
- Secondary endpoints included the proportion of patients achieving American College of Rheumatology (ACR) 50/70 responses and, Disease Activity Score 28-joint count C-reactive protein (DAS28(CRP)) score < 2.6 or ≤ 3.2, as well as measures of other patient-reported outcomes such as pain, physical function, and quality of life at 24 and 52 weeks.
TAKEAWAY:
- At 24 weeks, the proportion of patients achieving a 20% improvement in the ACR20 response — the primary endpoint — was higher among those receiving 4 mg ivarmacitinib (70.4%) or 8 mg ivarmacitinib (75.1%) than among those receiving placebo (40.4%; P < .0001 for both comparisons), with the efficacy either maintained or improved through 52 weeks.
- The proportion of patients achieving ACR50/70 responses or a DAS28(CRP) score < 2.6 or ≤ 3.2 was higher in the ivarmacitinib groups than in the placebo group (P < .0001 for all comparisons).
- Compared with the placebo group, both the ivarmacitinib groups showed improvements in patient-reported outcomes such as pain, physical function, quality of life, and duration and severity of morning stiffness.
- The overall rates of treatment discontinuation caused by adverse events were low across all the groups, with no deaths, tuberculosis or gastrointestinal perforations reported throughout the 52 weeks.
IN PRACTICE:
“Based on these findings, ivarmacitinib with background csDMARDs allowed, could be considered a treatment option in patients with moderate to severe active RA who have an inadequate response to csDMARDs,” the authors wrote.
SOURCE:
This study was led by Jinjing Liu and Xiaofeng Zeng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Beijing, China. It was published online on November 27, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
As the study was conducted in a Chinese population, the findings may have limited applicability across diverse global populations. Additionally, as the placebo-controlled period was limited to 24 weeks because of ethical concerns, comparisons between placebo and ivarmacitinib beyond that period were restricted. Lastly, this study was not powered to compare efficacy and safety between the two active dose regimens.
DISCLOSURES:
This study was funded by Jiangsu Hengrui Pharmaceuticals. Two authors declared being employees of the company. The other authors reported no competing interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Post-Exertional Malaise in Fatiguing Diseases: What to Know to Avoid Harmful Exercise
Identifying the phenomenon of post-exertional malaise (PEM) in patients with fatiguing conditions is critical because it necessitates a far more cautious approach to exercise, experts said.
PEM is a defining feature of the condition myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and it is present in many people with long COVID. It is characterized by a worsening of fatigue and of other symptoms after previously tolerated physical or mental exertion, typically emerging 24-72 hours after the exertion and lasting days or weeks thereafter. The experience is often called a “crash.”
In a study presented at the American College of Rheumatology (ACR) 2024 Annual Meeting, PEM was also identified in people with various rheumatologic conditions, ranging from 4% in those with osteoarthritis to 20% in those with fibromyalgia. The presence of PEM was also associated with worse pain, sleep, cognition, and other symptoms that are also characteristic of ME/CFS and many cases of long COVID.
“PEM assessment is becoming more important in those with long COVID, as we are assisting more of those with long durations of this condition. ... This is the first study we know of presenting PEM rates in a rheumatologic disease population,” Kaleb Michaud, PhD, director of FORWARD — The National Databank for Rheumatic Diseases and professor of rheumatology and immunology, University of Nebraska Medical Center, Omaha, said at the meeting.
During the discussion period, study investigator Leonard H. Calabrese, DO, head of the Section of Clinical Immunology, Cleveland Clinic, Ohio, commented, “PEM is seen with numerous post-acute infectious sequelae. It segregates with that population of patients who meet the diagnostic criteria for ME/CFS, of which 50%-70% of people will also meet criteria for fibromyalgia…This is a first step, but it has big ramifications regarding exercise.”
In an interview with this news organization, Calabrese said, “We recommend exercise to virtually everyone with fibromyalgia who doesn’t have ME/CFS,” but that the assessment tool used in the study, the 5-item DePaul Symptoms Questionnaire, isn’t adequate for assessing true PEM that would preclude exercise, despite being validated. “That instrument is inexact and lacks specificity. ... It just shows where the field is. We need better biomarkers.”
In Those With PEM, Exercise May Harm
Asked to comment, Brayden P. Yellman, MD, a rheumatologist at the Bateman Horne Center, Salt Lake City, Utah, told this news organization, “if there is an infection-associated chronic condition that meets criteria for what we would call ME/CFS or long COVID, and if there’s true post-exertional malaise, any graded exercise that ultimately leads to post-exertional malaise is harmful. ... There is a subset of people who have milder disease, who can sometimes do very mild exercise that does not trigger PEM, and they do see benefits over time very slowly with really carefully curated, carefully monitored exercise. But we have to be really careful.”
For the majority, however, the approach is to teach patients to pace their activities in order to avoid PEM, also referred to as staying within their “energy envelope.” Clinician resources are available on the Bateman Horne Center’s website.
This isn’t typically included in rheumatology training, Yellman noted. “Having completed an entire rheumatology fellowship and working in rheumatology, I was not taught at all about [then-termed] chronic fatigue syndrome. It was lumped under fibromyalgia. And of course, they teach about fibromyalgia because it’s a great mimic of a lot of inflammatory, rheumatologic conditions, but the idea of [PEM], that pathognomonic feature that we see in infection-associated chronic conditions, was not once mentioned when I trained, in 2014 to 2016.”
Nonetheless, he added, “rheumatologists are definitely seeing this in their fibromyalgia patients and some of their other patients at a high rate, and I’m sure that they’re missing it, along with other comorbidities like orthostatic hypotension.”
Another expert asked to weigh in, Todd Davenport, PT, DPT, PhD, professor and chair of the Department of Physical Therapy at the University of the Pacific, Stockton, California, told this news organization: “Our experience is that the body’s responses to short bouts of exercise are abnormal, and graded exercise is unsuccessful and makes people worse. ... Clinicians should be particularly on the lookout for PEM in patients who are already reporting fatigue, such as with fibromyalgia and rheumatologic conditions that can have some diagnostic overlaps with ME/CFS, because you can get fooled into thinking that your well-meaning exercise program intended to help give them a little more juice during their daily activities actually might be harmful.”
There are several lines of evidence for abnormal responses to exercise in people with PEM, Davenport said. These include muscle worsening, cardiac preload failure and impaired systemic oxygen extraction, metabolic dysregulation, and abnormal immunologic and neurologic changes.
Several studies show impaired recovery after 2-day cardiopulmonary exercise testing, with the largest to date published in July 2024. Patients with PEM have also reported harm from prescribed exercise.
Yellman commented: “We think of PEM like an injury, where you need to recover. If you keep stacking injuries on top of it, that injury is never going to heal the same way again…We are still trying to understand the pathophysiology of ME/CFS in general, and of PEM. But if you think of it as a neuroinflammatory injury, and there’s some evidence suggesting neuroinflammation, you can kind of understand the approach of needing to heal and to recover.”
How Prevalent Is PEM in Rheumatologic Conditions?
For the study presented at the ACR meeting, data of people with confirmed rheumatic diseases were taken from the ongoing longitudinal US-based research database FORWARD. Participants completed biannual self-reported questionnaires during January–June 2024 that included the 5-item PEM subscale from the validated DePaul Symptoms Questionnaire.
Questions relate to frequency and severity of each of the five items: “Dead, heavy feeling after starting to exercise,” “next-day soreness or fatigue after nonstrenuous, everyday activities,” “mentally tired after the slightest effort,” “minimum exercise makes you physically tired,” and “physically drained or sick after mild activity.” Participants are asked to rate each item on a scale from 0 if not present to 1 (mild/a little of the time) up to 4 (very severe/all of the time).
A positive PEM result was defined as a frequency of at least two and simultaneous severity of at least two on any survey item. Additional questions asked about recent and previous SARS-CoV-2 infections, long COVID diagnoses, and comorbidities.
Of 1158 individuals who completed the PEM questionnaire, 7.5% overall met PEM criteria. By individual condition, the proportions were 4.4% with osteoarthritis, 7.4% with rheumatoid arthritis, 12.2% with systemic lupus erythematosus, 13.8% with fibromyalgia diagnosed by rheumatologists, and 20.3% with fibromyalgia based on the 2016 revised ACR criteria.
The overall PEM prevalence was 8.3% among those reporting ever having COVID-19 and 9.5% among those who had COVID-19 during July–December 2023. The PEM prevalence increased more dramatically with more severe COVID-19 — 17.2% among those who had been hospitalized for COVID-19, 22.0% of those ever diagnosed with long COVID, and 28.1% with a long COVID diagnosis in January 2024.
By diagnosis, 50% of individuals who met the ACR’s 2016 fibromyalgia criteria and currently had long COVID scored positively for PEM.
Measures of pain, fatigue, sleep, patient global assessment, activity score, polysymptomatic distress, disability, depression, anxiety, and other functional scores were all significantly worse among those scoring positive for PEM (P < .001), Michaud reported.
Better Tools Are Available
The developer of the DePaul questionnaire, Leonard Jason, PhD, director of the Center for Community Research and professor of psychology at DePaul College of Science and Health, Chicago, Illinois, told this news organization that an updated 10-item screening tool specifically designed to screen for PEM adds some important elements missing from the 5-item version.
Here, patients are initially asked two questions: “Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?” and “Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?” If they answer “yes” to either, the next question is “If you feel worse after activities, how long does this last?” Answers are coded from 0 to 6 (24 hours or more).
The fourth additional question then asks how quickly patients recover, while a fifth question asks whether the person is avoiding activity because it makes them feel worse (thereby potentially creating a false negative).
For those scoring positive on the 10-item screen, a more comprehensive measure could be used, such as this online screening tool, Jason said.
Yellman said that the Bateman Horne Center uses a “good day, bad day” questionnaire to tease out some of the same information. In addition, he noted that it’s important to capture the timeframe between the exertion and the onset of symptoms because PEM doesn’t start during or immediately after activity. “If somebody is mowing the lawn and they start feeling symptoms immediately, they’re probably, at least in ME/CFS, experiencing orthostatic intolerance. Post-exertional malaise occurs 12-72 hours later, when their function is severely reduced as compared to baseline.”
And of course, Davenport noted, listening to patients is key. “Patients will tell you wildly unusual responses to activity before you even do the work of trying to figure out what the activity was. They’ll tell you things like they can’t think as well, that they have to be in bed for 3 days to a week to 2 weeks, depending on the level of exertion.”
Yellman, Davenport, and several other colleagues are currently working on a paper that will explain the differences between pacing and graded exercise, define PEM, and provide guidelines. They aim to submit it in time for publication early in 2025. In the meantime, the Bateman Horne Center’s website provides numerous resources for healthcare professionals and patients.
Yellman is also working to define minimum quality of care standards for infection-associated chronic conditions for state medical boards and to provide continuing medical education for clinicians on those standards. These would include recognizing and evaluating patients for PEM, as well as orthostatic intolerance, cognitive impairment, and other associated comorbidities.
Importantly, he said, the standards will include the principles of teaching people with PEM how to pace and will emphasize not prescribing them graded exercise as first- or even second-line therapy. “We need people to do some basic things. And the first thing is do no harm.”
None of the individuals quoted for this article had relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Identifying the phenomenon of post-exertional malaise (PEM) in patients with fatiguing conditions is critical because it necessitates a far more cautious approach to exercise, experts said.
PEM is a defining feature of the condition myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and it is present in many people with long COVID. It is characterized by a worsening of fatigue and of other symptoms after previously tolerated physical or mental exertion, typically emerging 24-72 hours after the exertion and lasting days or weeks thereafter. The experience is often called a “crash.”
In a study presented at the American College of Rheumatology (ACR) 2024 Annual Meeting, PEM was also identified in people with various rheumatologic conditions, ranging from 4% in those with osteoarthritis to 20% in those with fibromyalgia. The presence of PEM was also associated with worse pain, sleep, cognition, and other symptoms that are also characteristic of ME/CFS and many cases of long COVID.
“PEM assessment is becoming more important in those with long COVID, as we are assisting more of those with long durations of this condition. ... This is the first study we know of presenting PEM rates in a rheumatologic disease population,” Kaleb Michaud, PhD, director of FORWARD — The National Databank for Rheumatic Diseases and professor of rheumatology and immunology, University of Nebraska Medical Center, Omaha, said at the meeting.
During the discussion period, study investigator Leonard H. Calabrese, DO, head of the Section of Clinical Immunology, Cleveland Clinic, Ohio, commented, “PEM is seen with numerous post-acute infectious sequelae. It segregates with that population of patients who meet the diagnostic criteria for ME/CFS, of which 50%-70% of people will also meet criteria for fibromyalgia…This is a first step, but it has big ramifications regarding exercise.”
In an interview with this news organization, Calabrese said, “We recommend exercise to virtually everyone with fibromyalgia who doesn’t have ME/CFS,” but that the assessment tool used in the study, the 5-item DePaul Symptoms Questionnaire, isn’t adequate for assessing true PEM that would preclude exercise, despite being validated. “That instrument is inexact and lacks specificity. ... It just shows where the field is. We need better biomarkers.”
In Those With PEM, Exercise May Harm
Asked to comment, Brayden P. Yellman, MD, a rheumatologist at the Bateman Horne Center, Salt Lake City, Utah, told this news organization, “if there is an infection-associated chronic condition that meets criteria for what we would call ME/CFS or long COVID, and if there’s true post-exertional malaise, any graded exercise that ultimately leads to post-exertional malaise is harmful. ... There is a subset of people who have milder disease, who can sometimes do very mild exercise that does not trigger PEM, and they do see benefits over time very slowly with really carefully curated, carefully monitored exercise. But we have to be really careful.”
For the majority, however, the approach is to teach patients to pace their activities in order to avoid PEM, also referred to as staying within their “energy envelope.” Clinician resources are available on the Bateman Horne Center’s website.
This isn’t typically included in rheumatology training, Yellman noted. “Having completed an entire rheumatology fellowship and working in rheumatology, I was not taught at all about [then-termed] chronic fatigue syndrome. It was lumped under fibromyalgia. And of course, they teach about fibromyalgia because it’s a great mimic of a lot of inflammatory, rheumatologic conditions, but the idea of [PEM], that pathognomonic feature that we see in infection-associated chronic conditions, was not once mentioned when I trained, in 2014 to 2016.”
Nonetheless, he added, “rheumatologists are definitely seeing this in their fibromyalgia patients and some of their other patients at a high rate, and I’m sure that they’re missing it, along with other comorbidities like orthostatic hypotension.”
Another expert asked to weigh in, Todd Davenport, PT, DPT, PhD, professor and chair of the Department of Physical Therapy at the University of the Pacific, Stockton, California, told this news organization: “Our experience is that the body’s responses to short bouts of exercise are abnormal, and graded exercise is unsuccessful and makes people worse. ... Clinicians should be particularly on the lookout for PEM in patients who are already reporting fatigue, such as with fibromyalgia and rheumatologic conditions that can have some diagnostic overlaps with ME/CFS, because you can get fooled into thinking that your well-meaning exercise program intended to help give them a little more juice during their daily activities actually might be harmful.”
There are several lines of evidence for abnormal responses to exercise in people with PEM, Davenport said. These include muscle worsening, cardiac preload failure and impaired systemic oxygen extraction, metabolic dysregulation, and abnormal immunologic and neurologic changes.
Several studies show impaired recovery after 2-day cardiopulmonary exercise testing, with the largest to date published in July 2024. Patients with PEM have also reported harm from prescribed exercise.
Yellman commented: “We think of PEM like an injury, where you need to recover. If you keep stacking injuries on top of it, that injury is never going to heal the same way again…We are still trying to understand the pathophysiology of ME/CFS in general, and of PEM. But if you think of it as a neuroinflammatory injury, and there’s some evidence suggesting neuroinflammation, you can kind of understand the approach of needing to heal and to recover.”
How Prevalent Is PEM in Rheumatologic Conditions?
For the study presented at the ACR meeting, data of people with confirmed rheumatic diseases were taken from the ongoing longitudinal US-based research database FORWARD. Participants completed biannual self-reported questionnaires during January–June 2024 that included the 5-item PEM subscale from the validated DePaul Symptoms Questionnaire.
Questions relate to frequency and severity of each of the five items: “Dead, heavy feeling after starting to exercise,” “next-day soreness or fatigue after nonstrenuous, everyday activities,” “mentally tired after the slightest effort,” “minimum exercise makes you physically tired,” and “physically drained or sick after mild activity.” Participants are asked to rate each item on a scale from 0 if not present to 1 (mild/a little of the time) up to 4 (very severe/all of the time).
A positive PEM result was defined as a frequency of at least two and simultaneous severity of at least two on any survey item. Additional questions asked about recent and previous SARS-CoV-2 infections, long COVID diagnoses, and comorbidities.
Of 1158 individuals who completed the PEM questionnaire, 7.5% overall met PEM criteria. By individual condition, the proportions were 4.4% with osteoarthritis, 7.4% with rheumatoid arthritis, 12.2% with systemic lupus erythematosus, 13.8% with fibromyalgia diagnosed by rheumatologists, and 20.3% with fibromyalgia based on the 2016 revised ACR criteria.
The overall PEM prevalence was 8.3% among those reporting ever having COVID-19 and 9.5% among those who had COVID-19 during July–December 2023. The PEM prevalence increased more dramatically with more severe COVID-19 — 17.2% among those who had been hospitalized for COVID-19, 22.0% of those ever diagnosed with long COVID, and 28.1% with a long COVID diagnosis in January 2024.
By diagnosis, 50% of individuals who met the ACR’s 2016 fibromyalgia criteria and currently had long COVID scored positively for PEM.
Measures of pain, fatigue, sleep, patient global assessment, activity score, polysymptomatic distress, disability, depression, anxiety, and other functional scores were all significantly worse among those scoring positive for PEM (P < .001), Michaud reported.
Better Tools Are Available
The developer of the DePaul questionnaire, Leonard Jason, PhD, director of the Center for Community Research and professor of psychology at DePaul College of Science and Health, Chicago, Illinois, told this news organization that an updated 10-item screening tool specifically designed to screen for PEM adds some important elements missing from the 5-item version.
Here, patients are initially asked two questions: “Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?” and “Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?” If they answer “yes” to either, the next question is “If you feel worse after activities, how long does this last?” Answers are coded from 0 to 6 (24 hours or more).
The fourth additional question then asks how quickly patients recover, while a fifth question asks whether the person is avoiding activity because it makes them feel worse (thereby potentially creating a false negative).
For those scoring positive on the 10-item screen, a more comprehensive measure could be used, such as this online screening tool, Jason said.
Yellman said that the Bateman Horne Center uses a “good day, bad day” questionnaire to tease out some of the same information. In addition, he noted that it’s important to capture the timeframe between the exertion and the onset of symptoms because PEM doesn’t start during or immediately after activity. “If somebody is mowing the lawn and they start feeling symptoms immediately, they’re probably, at least in ME/CFS, experiencing orthostatic intolerance. Post-exertional malaise occurs 12-72 hours later, when their function is severely reduced as compared to baseline.”
And of course, Davenport noted, listening to patients is key. “Patients will tell you wildly unusual responses to activity before you even do the work of trying to figure out what the activity was. They’ll tell you things like they can’t think as well, that they have to be in bed for 3 days to a week to 2 weeks, depending on the level of exertion.”
Yellman, Davenport, and several other colleagues are currently working on a paper that will explain the differences between pacing and graded exercise, define PEM, and provide guidelines. They aim to submit it in time for publication early in 2025. In the meantime, the Bateman Horne Center’s website provides numerous resources for healthcare professionals and patients.
Yellman is also working to define minimum quality of care standards for infection-associated chronic conditions for state medical boards and to provide continuing medical education for clinicians on those standards. These would include recognizing and evaluating patients for PEM, as well as orthostatic intolerance, cognitive impairment, and other associated comorbidities.
Importantly, he said, the standards will include the principles of teaching people with PEM how to pace and will emphasize not prescribing them graded exercise as first- or even second-line therapy. “We need people to do some basic things. And the first thing is do no harm.”
None of the individuals quoted for this article had relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Identifying the phenomenon of post-exertional malaise (PEM) in patients with fatiguing conditions is critical because it necessitates a far more cautious approach to exercise, experts said.
PEM is a defining feature of the condition myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and it is present in many people with long COVID. It is characterized by a worsening of fatigue and of other symptoms after previously tolerated physical or mental exertion, typically emerging 24-72 hours after the exertion and lasting days or weeks thereafter. The experience is often called a “crash.”
In a study presented at the American College of Rheumatology (ACR) 2024 Annual Meeting, PEM was also identified in people with various rheumatologic conditions, ranging from 4% in those with osteoarthritis to 20% in those with fibromyalgia. The presence of PEM was also associated with worse pain, sleep, cognition, and other symptoms that are also characteristic of ME/CFS and many cases of long COVID.
“PEM assessment is becoming more important in those with long COVID, as we are assisting more of those with long durations of this condition. ... This is the first study we know of presenting PEM rates in a rheumatologic disease population,” Kaleb Michaud, PhD, director of FORWARD — The National Databank for Rheumatic Diseases and professor of rheumatology and immunology, University of Nebraska Medical Center, Omaha, said at the meeting.
During the discussion period, study investigator Leonard H. Calabrese, DO, head of the Section of Clinical Immunology, Cleveland Clinic, Ohio, commented, “PEM is seen with numerous post-acute infectious sequelae. It segregates with that population of patients who meet the diagnostic criteria for ME/CFS, of which 50%-70% of people will also meet criteria for fibromyalgia…This is a first step, but it has big ramifications regarding exercise.”
In an interview with this news organization, Calabrese said, “We recommend exercise to virtually everyone with fibromyalgia who doesn’t have ME/CFS,” but that the assessment tool used in the study, the 5-item DePaul Symptoms Questionnaire, isn’t adequate for assessing true PEM that would preclude exercise, despite being validated. “That instrument is inexact and lacks specificity. ... It just shows where the field is. We need better biomarkers.”
In Those With PEM, Exercise May Harm
Asked to comment, Brayden P. Yellman, MD, a rheumatologist at the Bateman Horne Center, Salt Lake City, Utah, told this news organization, “if there is an infection-associated chronic condition that meets criteria for what we would call ME/CFS or long COVID, and if there’s true post-exertional malaise, any graded exercise that ultimately leads to post-exertional malaise is harmful. ... There is a subset of people who have milder disease, who can sometimes do very mild exercise that does not trigger PEM, and they do see benefits over time very slowly with really carefully curated, carefully monitored exercise. But we have to be really careful.”
For the majority, however, the approach is to teach patients to pace their activities in order to avoid PEM, also referred to as staying within their “energy envelope.” Clinician resources are available on the Bateman Horne Center’s website.
This isn’t typically included in rheumatology training, Yellman noted. “Having completed an entire rheumatology fellowship and working in rheumatology, I was not taught at all about [then-termed] chronic fatigue syndrome. It was lumped under fibromyalgia. And of course, they teach about fibromyalgia because it’s a great mimic of a lot of inflammatory, rheumatologic conditions, but the idea of [PEM], that pathognomonic feature that we see in infection-associated chronic conditions, was not once mentioned when I trained, in 2014 to 2016.”
Nonetheless, he added, “rheumatologists are definitely seeing this in their fibromyalgia patients and some of their other patients at a high rate, and I’m sure that they’re missing it, along with other comorbidities like orthostatic hypotension.”
Another expert asked to weigh in, Todd Davenport, PT, DPT, PhD, professor and chair of the Department of Physical Therapy at the University of the Pacific, Stockton, California, told this news organization: “Our experience is that the body’s responses to short bouts of exercise are abnormal, and graded exercise is unsuccessful and makes people worse. ... Clinicians should be particularly on the lookout for PEM in patients who are already reporting fatigue, such as with fibromyalgia and rheumatologic conditions that can have some diagnostic overlaps with ME/CFS, because you can get fooled into thinking that your well-meaning exercise program intended to help give them a little more juice during their daily activities actually might be harmful.”
There are several lines of evidence for abnormal responses to exercise in people with PEM, Davenport said. These include muscle worsening, cardiac preload failure and impaired systemic oxygen extraction, metabolic dysregulation, and abnormal immunologic and neurologic changes.
Several studies show impaired recovery after 2-day cardiopulmonary exercise testing, with the largest to date published in July 2024. Patients with PEM have also reported harm from prescribed exercise.
Yellman commented: “We think of PEM like an injury, where you need to recover. If you keep stacking injuries on top of it, that injury is never going to heal the same way again…We are still trying to understand the pathophysiology of ME/CFS in general, and of PEM. But if you think of it as a neuroinflammatory injury, and there’s some evidence suggesting neuroinflammation, you can kind of understand the approach of needing to heal and to recover.”
How Prevalent Is PEM in Rheumatologic Conditions?
For the study presented at the ACR meeting, data of people with confirmed rheumatic diseases were taken from the ongoing longitudinal US-based research database FORWARD. Participants completed biannual self-reported questionnaires during January–June 2024 that included the 5-item PEM subscale from the validated DePaul Symptoms Questionnaire.
Questions relate to frequency and severity of each of the five items: “Dead, heavy feeling after starting to exercise,” “next-day soreness or fatigue after nonstrenuous, everyday activities,” “mentally tired after the slightest effort,” “minimum exercise makes you physically tired,” and “physically drained or sick after mild activity.” Participants are asked to rate each item on a scale from 0 if not present to 1 (mild/a little of the time) up to 4 (very severe/all of the time).
A positive PEM result was defined as a frequency of at least two and simultaneous severity of at least two on any survey item. Additional questions asked about recent and previous SARS-CoV-2 infections, long COVID diagnoses, and comorbidities.
Of 1158 individuals who completed the PEM questionnaire, 7.5% overall met PEM criteria. By individual condition, the proportions were 4.4% with osteoarthritis, 7.4% with rheumatoid arthritis, 12.2% with systemic lupus erythematosus, 13.8% with fibromyalgia diagnosed by rheumatologists, and 20.3% with fibromyalgia based on the 2016 revised ACR criteria.
The overall PEM prevalence was 8.3% among those reporting ever having COVID-19 and 9.5% among those who had COVID-19 during July–December 2023. The PEM prevalence increased more dramatically with more severe COVID-19 — 17.2% among those who had been hospitalized for COVID-19, 22.0% of those ever diagnosed with long COVID, and 28.1% with a long COVID diagnosis in January 2024.
By diagnosis, 50% of individuals who met the ACR’s 2016 fibromyalgia criteria and currently had long COVID scored positively for PEM.
Measures of pain, fatigue, sleep, patient global assessment, activity score, polysymptomatic distress, disability, depression, anxiety, and other functional scores were all significantly worse among those scoring positive for PEM (P < .001), Michaud reported.
Better Tools Are Available
The developer of the DePaul questionnaire, Leonard Jason, PhD, director of the Center for Community Research and professor of psychology at DePaul College of Science and Health, Chicago, Illinois, told this news organization that an updated 10-item screening tool specifically designed to screen for PEM adds some important elements missing from the 5-item version.
Here, patients are initially asked two questions: “Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?” and “Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?” If they answer “yes” to either, the next question is “If you feel worse after activities, how long does this last?” Answers are coded from 0 to 6 (24 hours or more).
The fourth additional question then asks how quickly patients recover, while a fifth question asks whether the person is avoiding activity because it makes them feel worse (thereby potentially creating a false negative).
For those scoring positive on the 10-item screen, a more comprehensive measure could be used, such as this online screening tool, Jason said.
Yellman said that the Bateman Horne Center uses a “good day, bad day” questionnaire to tease out some of the same information. In addition, he noted that it’s important to capture the timeframe between the exertion and the onset of symptoms because PEM doesn’t start during or immediately after activity. “If somebody is mowing the lawn and they start feeling symptoms immediately, they’re probably, at least in ME/CFS, experiencing orthostatic intolerance. Post-exertional malaise occurs 12-72 hours later, when their function is severely reduced as compared to baseline.”
And of course, Davenport noted, listening to patients is key. “Patients will tell you wildly unusual responses to activity before you even do the work of trying to figure out what the activity was. They’ll tell you things like they can’t think as well, that they have to be in bed for 3 days to a week to 2 weeks, depending on the level of exertion.”
Yellman, Davenport, and several other colleagues are currently working on a paper that will explain the differences between pacing and graded exercise, define PEM, and provide guidelines. They aim to submit it in time for publication early in 2025. In the meantime, the Bateman Horne Center’s website provides numerous resources for healthcare professionals and patients.
Yellman is also working to define minimum quality of care standards for infection-associated chronic conditions for state medical boards and to provide continuing medical education for clinicians on those standards. These would include recognizing and evaluating patients for PEM, as well as orthostatic intolerance, cognitive impairment, and other associated comorbidities.
Importantly, he said, the standards will include the principles of teaching people with PEM how to pace and will emphasize not prescribing them graded exercise as first- or even second-line therapy. “We need people to do some basic things. And the first thing is do no harm.”
None of the individuals quoted for this article had relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM ACR 2024