User login
Corticosteroid use in pregnancy linked to preterm birth
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
Pregnant women taking oral corticosteroids for rheumatoid arthritis may be at increased risk of preterm birth, according to research published online Sept. 30 in Rheumatology.
A study of 528 pregnant women with rheumatoid arthritis enrolled in the MotherToBaby Pregnancy Studies found that those taking a daily dose of 10 mg or more of prednisone equivalent – representing a mean cumulative dose of 2,208.6 mg over the first 139 days of pregnancy – had 4.77-fold higher odds of preterm birth, compared with those not taking oral corticosteroids. Women on medium doses – with a mean cumulative dose of 883 mg – had 81% higher odds of preterm birth, while those on low cumulative doses of 264.9 mg showed a nonsignificant 38% increase in preterm birth risk.
Women who did not use oral corticosteroids before day 140 of pregnancy had a 2.2% risk of early preterm birth. Among women with low use of oral corticosteroids, the risk was 3.4%, among those with medium use the risk was 3.3%, but among those with high use the risk was 26.7%.
After day 140 of gestation, there was a nonsignificant 64% increase in the risk for preterm birth with any use of oral corticosteroids, compared with no use. But among women taking 10 mg or more of prednisone equivalent per day, the risk was 2.45-fold higher, whereas those taking under 10 mg showed no significant increase in risk.
“Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and nonserious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of 10 mg of prednisone equivalent per day and greater,” wrote Kristin Palmsten, ScD, a research investigator with HealthPartners Institute in Minneapolis, Minn., and coauthors.
Given that intrauterine infection is believed to contribute to preterm birth, some have suggested that the immunosuppressive effects of oral corticosteroids could be associated with an increased risk of preterm birth because of subclinical intra-amniotic infection, they wrote.
However, they noted that there was a lack of information on the effect of dose and timing of oral corticosteroids during pregnancy on the risk of preterm birth.
The authors acknowledged that dosage of oral corticosteroids during pregnancy was linked to disease activity, which was itself associated with preterm birth risk. They adjusted for self-assessed rheumatoid arthritis severity at enrollment, which was generally during the first trimester, and found that this did attenuate the association with preterm birth.
“Ideally, we would have measures of disease severity at the time of every medication start, stop, or dose change to account for time-varying confounding later in pregnancy,” they wrote.
The study did not find any effect of biologic or nonbiologic disease-modifying antirheumatic drugs, either before or after the first 140 days of gestation.
The authors also looked at pregnancy outcomes among women with inflammatory bowel disease and asthma who were taking corticosteroids for those conditions.
While noting that these estimates were “imprecise,” they did see the suggestion of an increase in preterm birth among women taking oral corticosteroids for asthma, especially when used in the first half of pregnancy. There was also a suggestion of increased preterm birth risk associated with high oral corticosteroid use for inflammatory bowel disease, but these estimates were unadjusted, they noted.
“Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity,” they wrote.
The conclusions to be drawn from the study are limited by its small size, the investigators noted, as well as a lack of information on the type of rheumatoid arthritis and longitudinal disease severity. They added that while the hypothesized mechanism of action linking oral corticosteroid use to preterm birth was subclinical intrauterine infection, they did not have access to placental pathology to confirm this.
The study was supported by the National Institutes of Health, and the MotherToBaby Pregnancy Studies are supported by research grants from a number of pharmaceutical companies. No other conflicts of interest were declared.
SOURCE: Palmsten K et al. Rheumatology 2019 Sep 30. doi: 10.1093/rheumatology/kez405.
FROM RHEUMATOLOGY
Romosozumab benefits prevail, despite renal insufficiency
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
REPORTING FROM ASBMR 2019
Continuous NSAID use for ankylosing spondylitis may raise hypertension risk
Continuous use of NSAIDs could increase the risk of incident hypertension among patients with ankylosing spondylitis (AS), according to findings from a recent prospective study.
Against expectations, data also suggested that tumor necrosis factor inhibitor (TNFi) therapy could increase blood pressure, although this finding was not significant across all methods of analysis, reported lead author Jean W. Liew, MD, of the University of Washington, Seattle, and colleagues.
The investigators noted that patients with AS already have a greater risk of cardiovascular disease than that of the general population, making any added risks that much more concerning.
“[T]he evidence for increased cardiovascular disease burden and cardiovascular risk in patients with inflammatory rheumatic diseases is well recognized,” the investigators wrote in Arthritis Care & Research. “Multiple population-based studies have demonstrated increased cardiovascular events and cardiovascular-related mortality in AS. There is a high prevalence of cardiovascular risk factors among individuals with AS, particularly hypertension.”
Exacerbation of this risk by NSAIDs has been previously studied with mixed results, according to the investigators. Meta-analyses have suggested that NSAIDs increase blood pressure in normotensive and hypertensive individuals, but some data point to a cardioprotective effect among those with AS, possibly as a consequence of dampened inflammation, and/or improved physical activity, which could lead to a secondary CV benefit. Still, the relationship between NSAIDs and CV risk was unclear, prompting the current study.
The investigators enrolled 1,282 patients with AS at five centers in the United States and Australia. Using a combination of clinical evaluations and self-reporting, enrollees were monitored at regular intervals. Disease activity was tracked with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), while the Bath Ankylosing Spondylitis Functional Index (BASFI) was used for functional impairments. Patients were also checked for a variety of comorbidities such as hypertension, coronary artery disease, mental health conditions, and renal disorders. Medication data included type, dosage, frequency, duration, and number of missed doses.
Including only baseline normotensive patients with at least 1 year of follow-up, 628 participants were eligible for analysis, of whom 200 used NSAIDs continuously. After a median of 7 years follow-up, 129 out of 628 patients developed hypertension. This translated to a hazard ratio (HR) for incident hypertension of 1.12 (95% confidence interval, 1.04-1.20), compared with nonusers or those who took NSAIDs intermittently. This relationship did not differ across subgroups defined by age, disease activity, body mass index, or TNFi use. Multiple sensitivity analyses added support to the association between continuous NSAID use and hypertension.
“The association of NSAIDs and incident hypertension remains particularly concerning, as the early development of hypertension may portend a higher risk of premature CV events due to cumulative exposure,” the investigators wrote.
In contrast with NSAIDs, TNFi therapy was not associated with hypertension across all models; however, against expectations, two models of analysis pointed to an 8% increased risk.
“Although TNFi use did not reach statistical significance in the main model, the direction of association was opposite that hypothesized based on prior data, specifically that TNFi use reduces CV risk by suppressing chronic inflammation,” the investigators wrote.
Considering the present findings, and previous studies, which have reported conflicting associations between TNFi use and hypertension, the investigators suggested that more research is needed, and offered specific methods to approach the topic.
“The association of TNFi use and incident hypertension requires further clarification in future studies,” they wrote, “which may be done by applying a marginal structural modeling (MSM) framework and inverse probability of treatment weighting (IPTW) statistical analyses to account for the relationships between TNFi use, disease activity, and NSAID use.”
In their concluding remarks, the investigators further emphasized the current knowledge gap in this area.
“There is an unmet need to clarify how treatment choices, particularly the use of NSAIDs and TNFi, impact CV risk factors and CV events in AS,” they wrote. “Further studies are needed to focus on precision medicine and predicting risk and benefit for patients in whom continuous NSAIDs are being considered. These further studies can inform the revision of guidelines to address the management of CV risk factors and CV disease in AS and axial spondyloarthritis more broadly.”
The investigators reported funding from the National Institutes of Health, the Assessment of Spondyloarthritis International Society, the Spondylitis Association of America, and the Russel Engleman Rheumatology Research Center at the University of California, San Francisco. Some authors reported ties with Eli Lilly, Novartis, and other pharmaceutical companies..
SOURCE: Liew et al. Arthritis Care Res. 2019 Sep 17. doi: 10.1002/acr.24070.
Continuous use of NSAIDs could increase the risk of incident hypertension among patients with ankylosing spondylitis (AS), according to findings from a recent prospective study.
Against expectations, data also suggested that tumor necrosis factor inhibitor (TNFi) therapy could increase blood pressure, although this finding was not significant across all methods of analysis, reported lead author Jean W. Liew, MD, of the University of Washington, Seattle, and colleagues.
The investigators noted that patients with AS already have a greater risk of cardiovascular disease than that of the general population, making any added risks that much more concerning.
“[T]he evidence for increased cardiovascular disease burden and cardiovascular risk in patients with inflammatory rheumatic diseases is well recognized,” the investigators wrote in Arthritis Care & Research. “Multiple population-based studies have demonstrated increased cardiovascular events and cardiovascular-related mortality in AS. There is a high prevalence of cardiovascular risk factors among individuals with AS, particularly hypertension.”
Exacerbation of this risk by NSAIDs has been previously studied with mixed results, according to the investigators. Meta-analyses have suggested that NSAIDs increase blood pressure in normotensive and hypertensive individuals, but some data point to a cardioprotective effect among those with AS, possibly as a consequence of dampened inflammation, and/or improved physical activity, which could lead to a secondary CV benefit. Still, the relationship between NSAIDs and CV risk was unclear, prompting the current study.
The investigators enrolled 1,282 patients with AS at five centers in the United States and Australia. Using a combination of clinical evaluations and self-reporting, enrollees were monitored at regular intervals. Disease activity was tracked with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), while the Bath Ankylosing Spondylitis Functional Index (BASFI) was used for functional impairments. Patients were also checked for a variety of comorbidities such as hypertension, coronary artery disease, mental health conditions, and renal disorders. Medication data included type, dosage, frequency, duration, and number of missed doses.
Including only baseline normotensive patients with at least 1 year of follow-up, 628 participants were eligible for analysis, of whom 200 used NSAIDs continuously. After a median of 7 years follow-up, 129 out of 628 patients developed hypertension. This translated to a hazard ratio (HR) for incident hypertension of 1.12 (95% confidence interval, 1.04-1.20), compared with nonusers or those who took NSAIDs intermittently. This relationship did not differ across subgroups defined by age, disease activity, body mass index, or TNFi use. Multiple sensitivity analyses added support to the association between continuous NSAID use and hypertension.
“The association of NSAIDs and incident hypertension remains particularly concerning, as the early development of hypertension may portend a higher risk of premature CV events due to cumulative exposure,” the investigators wrote.
In contrast with NSAIDs, TNFi therapy was not associated with hypertension across all models; however, against expectations, two models of analysis pointed to an 8% increased risk.
“Although TNFi use did not reach statistical significance in the main model, the direction of association was opposite that hypothesized based on prior data, specifically that TNFi use reduces CV risk by suppressing chronic inflammation,” the investigators wrote.
Considering the present findings, and previous studies, which have reported conflicting associations between TNFi use and hypertension, the investigators suggested that more research is needed, and offered specific methods to approach the topic.
“The association of TNFi use and incident hypertension requires further clarification in future studies,” they wrote, “which may be done by applying a marginal structural modeling (MSM) framework and inverse probability of treatment weighting (IPTW) statistical analyses to account for the relationships between TNFi use, disease activity, and NSAID use.”
In their concluding remarks, the investigators further emphasized the current knowledge gap in this area.
“There is an unmet need to clarify how treatment choices, particularly the use of NSAIDs and TNFi, impact CV risk factors and CV events in AS,” they wrote. “Further studies are needed to focus on precision medicine and predicting risk and benefit for patients in whom continuous NSAIDs are being considered. These further studies can inform the revision of guidelines to address the management of CV risk factors and CV disease in AS and axial spondyloarthritis more broadly.”
The investigators reported funding from the National Institutes of Health, the Assessment of Spondyloarthritis International Society, the Spondylitis Association of America, and the Russel Engleman Rheumatology Research Center at the University of California, San Francisco. Some authors reported ties with Eli Lilly, Novartis, and other pharmaceutical companies..
SOURCE: Liew et al. Arthritis Care Res. 2019 Sep 17. doi: 10.1002/acr.24070.
Continuous use of NSAIDs could increase the risk of incident hypertension among patients with ankylosing spondylitis (AS), according to findings from a recent prospective study.
Against expectations, data also suggested that tumor necrosis factor inhibitor (TNFi) therapy could increase blood pressure, although this finding was not significant across all methods of analysis, reported lead author Jean W. Liew, MD, of the University of Washington, Seattle, and colleagues.
The investigators noted that patients with AS already have a greater risk of cardiovascular disease than that of the general population, making any added risks that much more concerning.
“[T]he evidence for increased cardiovascular disease burden and cardiovascular risk in patients with inflammatory rheumatic diseases is well recognized,” the investigators wrote in Arthritis Care & Research. “Multiple population-based studies have demonstrated increased cardiovascular events and cardiovascular-related mortality in AS. There is a high prevalence of cardiovascular risk factors among individuals with AS, particularly hypertension.”
Exacerbation of this risk by NSAIDs has been previously studied with mixed results, according to the investigators. Meta-analyses have suggested that NSAIDs increase blood pressure in normotensive and hypertensive individuals, but some data point to a cardioprotective effect among those with AS, possibly as a consequence of dampened inflammation, and/or improved physical activity, which could lead to a secondary CV benefit. Still, the relationship between NSAIDs and CV risk was unclear, prompting the current study.
The investigators enrolled 1,282 patients with AS at five centers in the United States and Australia. Using a combination of clinical evaluations and self-reporting, enrollees were monitored at regular intervals. Disease activity was tracked with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), while the Bath Ankylosing Spondylitis Functional Index (BASFI) was used for functional impairments. Patients were also checked for a variety of comorbidities such as hypertension, coronary artery disease, mental health conditions, and renal disorders. Medication data included type, dosage, frequency, duration, and number of missed doses.
Including only baseline normotensive patients with at least 1 year of follow-up, 628 participants were eligible for analysis, of whom 200 used NSAIDs continuously. After a median of 7 years follow-up, 129 out of 628 patients developed hypertension. This translated to a hazard ratio (HR) for incident hypertension of 1.12 (95% confidence interval, 1.04-1.20), compared with nonusers or those who took NSAIDs intermittently. This relationship did not differ across subgroups defined by age, disease activity, body mass index, or TNFi use. Multiple sensitivity analyses added support to the association between continuous NSAID use and hypertension.
“The association of NSAIDs and incident hypertension remains particularly concerning, as the early development of hypertension may portend a higher risk of premature CV events due to cumulative exposure,” the investigators wrote.
In contrast with NSAIDs, TNFi therapy was not associated with hypertension across all models; however, against expectations, two models of analysis pointed to an 8% increased risk.
“Although TNFi use did not reach statistical significance in the main model, the direction of association was opposite that hypothesized based on prior data, specifically that TNFi use reduces CV risk by suppressing chronic inflammation,” the investigators wrote.
Considering the present findings, and previous studies, which have reported conflicting associations between TNFi use and hypertension, the investigators suggested that more research is needed, and offered specific methods to approach the topic.
“The association of TNFi use and incident hypertension requires further clarification in future studies,” they wrote, “which may be done by applying a marginal structural modeling (MSM) framework and inverse probability of treatment weighting (IPTW) statistical analyses to account for the relationships between TNFi use, disease activity, and NSAID use.”
In their concluding remarks, the investigators further emphasized the current knowledge gap in this area.
“There is an unmet need to clarify how treatment choices, particularly the use of NSAIDs and TNFi, impact CV risk factors and CV events in AS,” they wrote. “Further studies are needed to focus on precision medicine and predicting risk and benefit for patients in whom continuous NSAIDs are being considered. These further studies can inform the revision of guidelines to address the management of CV risk factors and CV disease in AS and axial spondyloarthritis more broadly.”
The investigators reported funding from the National Institutes of Health, the Assessment of Spondyloarthritis International Society, the Spondylitis Association of America, and the Russel Engleman Rheumatology Research Center at the University of California, San Francisco. Some authors reported ties with Eli Lilly, Novartis, and other pharmaceutical companies..
SOURCE: Liew et al. Arthritis Care Res. 2019 Sep 17. doi: 10.1002/acr.24070.
FROM ARTHRITIS CARE & RESEARCH
Consider cognitive reframing of osteoporosis to improve adherence
ORLANDO – Reframing the decision-making process for selecting osteoporosis treatment options may help increase adherence to medication, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“The language of this disease needs to be changed,” Deborah Gold, PhD, of Duke University, Durham, N.C., said in her presentation.
As medications for osteoporosis have changed over the decades – from estrogen in the 1940s and calcitonin in the 1970s to bisphosphonates in the 1990s – the delivery mechanisms of the drugs and the dosing intervals also have changed. However, long-term adherence to osteoporosis medication through various delivery mechanisms and doses have remained elusive, Dr. Gold said.
She cited the negative public and media response to osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) – two rare side effects of bisphosphonate treatment – as one reason why adherence to osteoporosis medications has changed.
While the more common side effects of osteoporosis medications are manageable, the emphasis in lay press has been on “rare and frightening side effects,” she said. A 2018 study found that reports in the media “strongly influence the level of awareness of osteoporosis and fracture risk” and that a gap exists between clinical recommendations and patient perceptions (J Endocrinol Invest. 2018;41[12]:1359-64. doi: 10.1007/s40618-018-0898-9). Over the same time period, another study found that 40.2% of high-risk patients hospitalized for a hip fracture were treated with osteoporosis medications in 2002, but that number declined to 20.5% in 2011 (J Bone Miner Res. 2014;29[9]:1929-37. doi: 10.1002/jbmr.2202).
Dr. Gold advised clinicians to counter negative patient perceptions about osteoporosis treatment by explaining the risks of treatment with medications as well as the risks of not undergoing treatment for osteoporosis. “A hip fracture is a lot more likely for nontreatment than ONJ or AFF are for treatment,” she said.
Dr. Gold also described the importance of listening to patients’ preferences for treatment as well as attempting to find an appropriate treatment they are likely to continue using. “This has been shown in the literature over and over again in other diseases,” she said. If “somebody says, ‘I can’t do needles,’ you can’t prescribe a medication that goes in through a needle.”
Assuring patients that they can visit again to address issues with treatment, change medications if needed, and discuss concerns about adverse outcomes such as ONJ and AFF is also relevant. “We need to promote osteoporosis understanding – not just osteoporosis – and we need to promote treatment to multiple sources,” she said.
When osteoporosis is characterized in terms of bone mineral density, T-scores, fragility fractures, appropriate exercises, and diet, there is plenty of opportunity for confusion or misunderstanding. Accurate, plain-language information, given both verbally and in handouts, works well , according to Dr. Gold.
“Health communication services – whether we’re talking about newspapers, magazines, radio shows, television shows, or even things that come out from organizations like the NOF [National Osteoporosis Foundation] and ASBMR – need to promote accurate information and not exaggerated negatives that we hear all the time,” she said.
“Cognitive reframing is not an easy thing; there’s no question,” Dr. Gold said. “I’m not standing up here telling you we can do it tomorrow. It will be difficult. But for those of us who know this disease and know how serious it is and know what the consequences are, we need to make a positive difference.”
Dr. Gold reported being a consultant for Amgen, Eli Lilly, and Radius Pharmaceuticals.
SOURCE: Gold D. ASBMR 2019. Adherence to Osteoporosis: A Conundrum of Significant Proportions.
ORLANDO – Reframing the decision-making process for selecting osteoporosis treatment options may help increase adherence to medication, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“The language of this disease needs to be changed,” Deborah Gold, PhD, of Duke University, Durham, N.C., said in her presentation.
As medications for osteoporosis have changed over the decades – from estrogen in the 1940s and calcitonin in the 1970s to bisphosphonates in the 1990s – the delivery mechanisms of the drugs and the dosing intervals also have changed. However, long-term adherence to osteoporosis medication through various delivery mechanisms and doses have remained elusive, Dr. Gold said.
She cited the negative public and media response to osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) – two rare side effects of bisphosphonate treatment – as one reason why adherence to osteoporosis medications has changed.
While the more common side effects of osteoporosis medications are manageable, the emphasis in lay press has been on “rare and frightening side effects,” she said. A 2018 study found that reports in the media “strongly influence the level of awareness of osteoporosis and fracture risk” and that a gap exists between clinical recommendations and patient perceptions (J Endocrinol Invest. 2018;41[12]:1359-64. doi: 10.1007/s40618-018-0898-9). Over the same time period, another study found that 40.2% of high-risk patients hospitalized for a hip fracture were treated with osteoporosis medications in 2002, but that number declined to 20.5% in 2011 (J Bone Miner Res. 2014;29[9]:1929-37. doi: 10.1002/jbmr.2202).
Dr. Gold advised clinicians to counter negative patient perceptions about osteoporosis treatment by explaining the risks of treatment with medications as well as the risks of not undergoing treatment for osteoporosis. “A hip fracture is a lot more likely for nontreatment than ONJ or AFF are for treatment,” she said.
Dr. Gold also described the importance of listening to patients’ preferences for treatment as well as attempting to find an appropriate treatment they are likely to continue using. “This has been shown in the literature over and over again in other diseases,” she said. If “somebody says, ‘I can’t do needles,’ you can’t prescribe a medication that goes in through a needle.”
Assuring patients that they can visit again to address issues with treatment, change medications if needed, and discuss concerns about adverse outcomes such as ONJ and AFF is also relevant. “We need to promote osteoporosis understanding – not just osteoporosis – and we need to promote treatment to multiple sources,” she said.
When osteoporosis is characterized in terms of bone mineral density, T-scores, fragility fractures, appropriate exercises, and diet, there is plenty of opportunity for confusion or misunderstanding. Accurate, plain-language information, given both verbally and in handouts, works well , according to Dr. Gold.
“Health communication services – whether we’re talking about newspapers, magazines, radio shows, television shows, or even things that come out from organizations like the NOF [National Osteoporosis Foundation] and ASBMR – need to promote accurate information and not exaggerated negatives that we hear all the time,” she said.
“Cognitive reframing is not an easy thing; there’s no question,” Dr. Gold said. “I’m not standing up here telling you we can do it tomorrow. It will be difficult. But for those of us who know this disease and know how serious it is and know what the consequences are, we need to make a positive difference.”
Dr. Gold reported being a consultant for Amgen, Eli Lilly, and Radius Pharmaceuticals.
SOURCE: Gold D. ASBMR 2019. Adherence to Osteoporosis: A Conundrum of Significant Proportions.
ORLANDO – Reframing the decision-making process for selecting osteoporosis treatment options may help increase adherence to medication, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“The language of this disease needs to be changed,” Deborah Gold, PhD, of Duke University, Durham, N.C., said in her presentation.
As medications for osteoporosis have changed over the decades – from estrogen in the 1940s and calcitonin in the 1970s to bisphosphonates in the 1990s – the delivery mechanisms of the drugs and the dosing intervals also have changed. However, long-term adherence to osteoporosis medication through various delivery mechanisms and doses have remained elusive, Dr. Gold said.
She cited the negative public and media response to osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) – two rare side effects of bisphosphonate treatment – as one reason why adherence to osteoporosis medications has changed.
While the more common side effects of osteoporosis medications are manageable, the emphasis in lay press has been on “rare and frightening side effects,” she said. A 2018 study found that reports in the media “strongly influence the level of awareness of osteoporosis and fracture risk” and that a gap exists between clinical recommendations and patient perceptions (J Endocrinol Invest. 2018;41[12]:1359-64. doi: 10.1007/s40618-018-0898-9). Over the same time period, another study found that 40.2% of high-risk patients hospitalized for a hip fracture were treated with osteoporosis medications in 2002, but that number declined to 20.5% in 2011 (J Bone Miner Res. 2014;29[9]:1929-37. doi: 10.1002/jbmr.2202).
Dr. Gold advised clinicians to counter negative patient perceptions about osteoporosis treatment by explaining the risks of treatment with medications as well as the risks of not undergoing treatment for osteoporosis. “A hip fracture is a lot more likely for nontreatment than ONJ or AFF are for treatment,” she said.
Dr. Gold also described the importance of listening to patients’ preferences for treatment as well as attempting to find an appropriate treatment they are likely to continue using. “This has been shown in the literature over and over again in other diseases,” she said. If “somebody says, ‘I can’t do needles,’ you can’t prescribe a medication that goes in through a needle.”
Assuring patients that they can visit again to address issues with treatment, change medications if needed, and discuss concerns about adverse outcomes such as ONJ and AFF is also relevant. “We need to promote osteoporosis understanding – not just osteoporosis – and we need to promote treatment to multiple sources,” she said.
When osteoporosis is characterized in terms of bone mineral density, T-scores, fragility fractures, appropriate exercises, and diet, there is plenty of opportunity for confusion or misunderstanding. Accurate, plain-language information, given both verbally and in handouts, works well , according to Dr. Gold.
“Health communication services – whether we’re talking about newspapers, magazines, radio shows, television shows, or even things that come out from organizations like the NOF [National Osteoporosis Foundation] and ASBMR – need to promote accurate information and not exaggerated negatives that we hear all the time,” she said.
“Cognitive reframing is not an easy thing; there’s no question,” Dr. Gold said. “I’m not standing up here telling you we can do it tomorrow. It will be difficult. But for those of us who know this disease and know how serious it is and know what the consequences are, we need to make a positive difference.”
Dr. Gold reported being a consultant for Amgen, Eli Lilly, and Radius Pharmaceuticals.
SOURCE: Gold D. ASBMR 2019. Adherence to Osteoporosis: A Conundrum of Significant Proportions.
REPORTING FROM ASBMR 2019
Osteoporosis remains a costly burden to older U.S. adults
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
REPORTING FROM ASBMR 2019
Cardiovascular complications of systemic sclerosis: What to look for
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
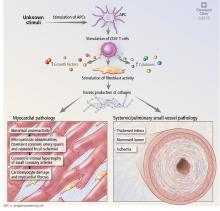
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
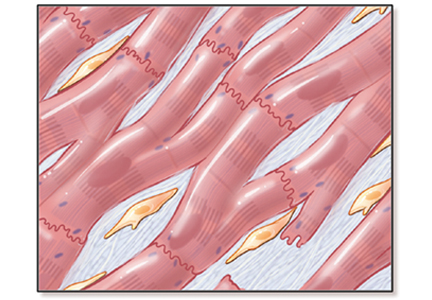
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
KEY POINTS
- Pulmonary hypertension is common in systemic sclerosis and carries a poor prognosis. Patients with systemic sclerosis should be screened regularly with echocardiography, followed, when necessary, by right heart catheterization to detect it early.
- Myocardial infarction and stroke are more common in patients with systemic sclerosis, and preventive measures are the same as for the general population.
- Right ventricular dysfunction secondary to pulmonary hypertension is common in systemic sclerosis; left ventricular dysfunction is less so. Routine echocardiography should include assessment of right and left ventricular function.
- Electrocardiography should be performed periodically, and urgently when indicated, to look for potentially dangerous arrhythmias.
A few pearls can help prepare the mind
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
Myopathy for the general internist: Statins and much more
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
KEY POINTS
- Inclusion body myositis affects older men more than women and is characterized by slowly progressive, asymmetric, distal and proximal weakness and atrophy.
- Statin-associated muscle complaints are common, whereas necrotizing myopathy, characterized by a very high CK plus weakness, is rare but must be recognized.
- Elevated CK does not necessarily indicate myositis, especially in African Americans or after heavy exercise.
- Dermatomyositis is characterized by muscle weakness and raised red or purple Gottron papules over the knuckles, elbows, or knees.
- Autoimmune interstitial lung disease may be caused by a variety of antibodies, the most common being anti-Jo-1 (directed against histidyl tRNA synthetase).
- The rarer non-Jo-1 antisynthetase autoantibodies may be associated with rapidly progressive interstitial lung disease, which is a challenge to recognize because associated rheumatologic symptoms may be minimal.
Heart disease raises risk of severe cutaneous adverse reactions to allopurinol
Researchers have found that patients with heart disease have an increased risk of hospitalization for severe cutaneous adverse reactions to allopurinol, with factors like chronic kidney disease and high initial dosage adding to that risk.
“Physicians who prescribe allopurinol should look for these risk factors so that they may consider initiating lower-dosage allopurinol and other precautions, which may prevent this rare but serious adverse reaction,” Chio Yokose, MD, of Massachusetts General Hospital in Boston and coauthors wrote in the Canadian Medical Association Journal.
To further investigate known associations between heart disease and severe cutaneous adverse reactions to allopurinol – including Stevens-Johnson syndrome and toxic epidermal necrolysis – the researchers used an administrative database known as Population Data BC to conduct a cohort study of allopurinol initiators in British Columbia between 1997 and 2015. Individuals with a history of severe cutaneous adverse reactions before starting allopurinol were excluded.
Of the 130,325 allopurinol users identified, 109 were hospitalized for allopurinol-associated severe cutaneous adverse reactions within 3 months of starting the drug. One in 655 allopurinol users with heart disease were admitted to the hospital for allopurinol-associated severe cutaneous adverse reaction (risk ratio = 1.53 per 1,000; 95% confidence interval, 1.10-2.06), compared with 1 in 1,548 allopurinol users without heart disease (risk ratio = 0.65 per 1,000; 95% CI, 0.50-0.82).
After multivariable analysis, other significant associations with hospital admission included chronic kidney disease (relative risk, 1.88; 95% CI, 1.17-3.02) and an initial allopurinol dosage greater than 100 mg/day (RR, 2.78; 95% CI, 1.75-4.43). In addition, patients with heart disease, chronic kidney disease, and an initial dosage greater than 100 mg/day had an 11-fold higher risk of hospital admission (RR, 11.13; 95% CI, 4.66-26.58).
The authors acknowledged their study’s limitations, including potential misclassification of reactions and comorbidities that can stem from a reliance on ICD codes. However, they also noted that “any misclassification is expected to be nondifferential” and bias results toward the null accordingly.
The study was funded by the Canadian Institutes of Health Research. One author reported receiving a grant from the National Institutes of Health and research support from AstraZeneca, along with consulting fees from Takeda, Selecta Biosciences, and Horizon. No other conflicts of interest were reported.
SOURCE: Yokose C et al. CMAJ. 2019 Sep 30. doi: 10.1503/cmaj.190339.
Researchers have found that patients with heart disease have an increased risk of hospitalization for severe cutaneous adverse reactions to allopurinol, with factors like chronic kidney disease and high initial dosage adding to that risk.
“Physicians who prescribe allopurinol should look for these risk factors so that they may consider initiating lower-dosage allopurinol and other precautions, which may prevent this rare but serious adverse reaction,” Chio Yokose, MD, of Massachusetts General Hospital in Boston and coauthors wrote in the Canadian Medical Association Journal.
To further investigate known associations between heart disease and severe cutaneous adverse reactions to allopurinol – including Stevens-Johnson syndrome and toxic epidermal necrolysis – the researchers used an administrative database known as Population Data BC to conduct a cohort study of allopurinol initiators in British Columbia between 1997 and 2015. Individuals with a history of severe cutaneous adverse reactions before starting allopurinol were excluded.
Of the 130,325 allopurinol users identified, 109 were hospitalized for allopurinol-associated severe cutaneous adverse reactions within 3 months of starting the drug. One in 655 allopurinol users with heart disease were admitted to the hospital for allopurinol-associated severe cutaneous adverse reaction (risk ratio = 1.53 per 1,000; 95% confidence interval, 1.10-2.06), compared with 1 in 1,548 allopurinol users without heart disease (risk ratio = 0.65 per 1,000; 95% CI, 0.50-0.82).
After multivariable analysis, other significant associations with hospital admission included chronic kidney disease (relative risk, 1.88; 95% CI, 1.17-3.02) and an initial allopurinol dosage greater than 100 mg/day (RR, 2.78; 95% CI, 1.75-4.43). In addition, patients with heart disease, chronic kidney disease, and an initial dosage greater than 100 mg/day had an 11-fold higher risk of hospital admission (RR, 11.13; 95% CI, 4.66-26.58).
The authors acknowledged their study’s limitations, including potential misclassification of reactions and comorbidities that can stem from a reliance on ICD codes. However, they also noted that “any misclassification is expected to be nondifferential” and bias results toward the null accordingly.
The study was funded by the Canadian Institutes of Health Research. One author reported receiving a grant from the National Institutes of Health and research support from AstraZeneca, along with consulting fees from Takeda, Selecta Biosciences, and Horizon. No other conflicts of interest were reported.
SOURCE: Yokose C et al. CMAJ. 2019 Sep 30. doi: 10.1503/cmaj.190339.
Researchers have found that patients with heart disease have an increased risk of hospitalization for severe cutaneous adverse reactions to allopurinol, with factors like chronic kidney disease and high initial dosage adding to that risk.
“Physicians who prescribe allopurinol should look for these risk factors so that they may consider initiating lower-dosage allopurinol and other precautions, which may prevent this rare but serious adverse reaction,” Chio Yokose, MD, of Massachusetts General Hospital in Boston and coauthors wrote in the Canadian Medical Association Journal.
To further investigate known associations between heart disease and severe cutaneous adverse reactions to allopurinol – including Stevens-Johnson syndrome and toxic epidermal necrolysis – the researchers used an administrative database known as Population Data BC to conduct a cohort study of allopurinol initiators in British Columbia between 1997 and 2015. Individuals with a history of severe cutaneous adverse reactions before starting allopurinol were excluded.
Of the 130,325 allopurinol users identified, 109 were hospitalized for allopurinol-associated severe cutaneous adverse reactions within 3 months of starting the drug. One in 655 allopurinol users with heart disease were admitted to the hospital for allopurinol-associated severe cutaneous adverse reaction (risk ratio = 1.53 per 1,000; 95% confidence interval, 1.10-2.06), compared with 1 in 1,548 allopurinol users without heart disease (risk ratio = 0.65 per 1,000; 95% CI, 0.50-0.82).
After multivariable analysis, other significant associations with hospital admission included chronic kidney disease (relative risk, 1.88; 95% CI, 1.17-3.02) and an initial allopurinol dosage greater than 100 mg/day (RR, 2.78; 95% CI, 1.75-4.43). In addition, patients with heart disease, chronic kidney disease, and an initial dosage greater than 100 mg/day had an 11-fold higher risk of hospital admission (RR, 11.13; 95% CI, 4.66-26.58).
The authors acknowledged their study’s limitations, including potential misclassification of reactions and comorbidities that can stem from a reliance on ICD codes. However, they also noted that “any misclassification is expected to be nondifferential” and bias results toward the null accordingly.
The study was funded by the Canadian Institutes of Health Research. One author reported receiving a grant from the National Institutes of Health and research support from AstraZeneca, along with consulting fees from Takeda, Selecta Biosciences, and Horizon. No other conflicts of interest were reported.
SOURCE: Yokose C et al. CMAJ. 2019 Sep 30. doi: 10.1503/cmaj.190339.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Older black women have worse outcomes after fragility fracture
ORLANDO – Older black women with postmenopausal osteoporosis had significantly higher mortality rates, were more likely to be placed in a long-term nursing facility, and were more likely to become newly eligible for Medicaid after a major fragility fracture event, compared with their white counterparts, according to a findings presented at the annual meeting of the American Society for Bone and Mineral Research.
Previous studies have examined racial differences in mortality and outcomes after fracture, but the data from those studies are older or limited to a certain region or health system, Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham, said in her presentation.
“To our knowledge, this is the first comprehensive evaluation of fractures and outcomes post fracture by race, particularly in black women,” she said.
Using Medicare data from between 2010 and 2016, Dr. Wright and colleagues performed a cohort-based, descriptive study of 400,479 white women and 11,563 black women with postmenopausal osteoporosis, who were covered by Medicare Parts A, B, C and D and had a hip, pelvis, femur, radius/ulna, humerus, or clinical vertebral fractures. Fractures were identified by way of a validated algorithm that used inpatient and outpatient claims, outpatient physical evaluations, and management claims, together with fracture repair codes (positive predictive value range, 90.9%-98.6%; from Wright N et al. J Bone Min Res. 2019 Jun 6. doi: 10.1002/jbmr.3807).
The groups had similar proportions of patients in each age group (65-75 years, 75-84 years, 85 years and older), with a slightly higher percentage of younger black patients than younger white patients (25.0% vs. 22.4%, respectively). Black patients were more likely than white patients to be from the South (58.6% vs. 39.7%) and have a Charlson Comorbidity Index score of 2 or higher (62.9% vs. 45.4%). White patients were more likely than black patients to have a Charlson score of 0 (42.0% vs. 24.1%).
The three identifying outcomes were: death/mortality, which was determined using the date in the Medicare vital status; debility, a term used for patients newly placed in a long-term nursing facility; and destitution, used to describe patients who became newly eligible for Medicaid after a major fragility fracture.
The results showed that the most common fracture types were hip and clinical vertebral fractures, with black women having a significantly lower rate of clinical vertebral fractures (29.0% vs. 34.1%, respectively) but a significantly higher rate of femur fractures (9.1% vs. 3.8%). Black women also had a significantly higher mortality rate after a fracture (19.6% vs. 15.4%), and a significantly higher composite outcome of all three identifying outcome measurements (24.6% vs. 20.2%). However, rates of debility and destitution were similar between the groups.
When measured by fracture type, black women had significantly different 1-year postfracture outcomes, compared with white women, with a 38.0% higher incidence of mortality, 40.2% higher rate of debility, 185.0% higher rate of destitution, and 35.4% higher composite outcome for hip fracture. For radius/ulna fractures, black women also had a 59.7% higher rate of death, 8.5% higher rate of debility, 164.7% higher rate of destitution, and 43.0% higher composite outcomes; and for clinical vertebral fractures, they had 11.4% higher rate of death, 10.8% higher rate of debility, 130.6% higher rate of destitution, and 13.6% higher composite outcome, compared with white women.
Overall, black women had higher incidence risk ratios for death (IRR, 1.24; 95% confidence interval, 1.15-1.33), debility (IRR, 1.19; 95% CI, 1.06-1.33), and destitution (IRR, 2.45; 95% CI, 2.20-2.73) for fractures of the hip; higher IRRs for death (IRR, 1.48; 95% CI, 1.33-1.66), debility (IRR, 1.02; 95% CI, 0.87-1.20), and destitution (IRR, 2.70; 95% CI, 2.33-3.13) for fractures of the radius or ulna; and higher IRRs for death (IRR, 1.07; 95% CI, 0.98-1.17) and destitution (IRR, 2.40; 95% CI, 2.15-2.67) for clinical vertebral fractures.
“These data show that we need to develop interventions and/or programs to mitigate and reduce disparities in fracture outcomes,” said Dr. Wright.
She noted that the study results were limited because of its observational nature, and results cannot be generalized beyond older women with postmenopausal osteoporosis with Medicare coverage. In addition, the algorithm used to determine fracture status also had a potentially low sensitivity, which may have affected the study results, she said.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer. Dr. Chen reported receiving grants from Amgen. Dr. Curtis reported receiving grants from, and is a consultant for, Amgen, Eli Lilly, and Radius. Dr. Saag reported receiving grants from Amgen and is a consultant for Gilead and Radius.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1125.
ORLANDO – Older black women with postmenopausal osteoporosis had significantly higher mortality rates, were more likely to be placed in a long-term nursing facility, and were more likely to become newly eligible for Medicaid after a major fragility fracture event, compared with their white counterparts, according to a findings presented at the annual meeting of the American Society for Bone and Mineral Research.
Previous studies have examined racial differences in mortality and outcomes after fracture, but the data from those studies are older or limited to a certain region or health system, Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham, said in her presentation.
“To our knowledge, this is the first comprehensive evaluation of fractures and outcomes post fracture by race, particularly in black women,” she said.
Using Medicare data from between 2010 and 2016, Dr. Wright and colleagues performed a cohort-based, descriptive study of 400,479 white women and 11,563 black women with postmenopausal osteoporosis, who were covered by Medicare Parts A, B, C and D and had a hip, pelvis, femur, radius/ulna, humerus, or clinical vertebral fractures. Fractures were identified by way of a validated algorithm that used inpatient and outpatient claims, outpatient physical evaluations, and management claims, together with fracture repair codes (positive predictive value range, 90.9%-98.6%; from Wright N et al. J Bone Min Res. 2019 Jun 6. doi: 10.1002/jbmr.3807).
The groups had similar proportions of patients in each age group (65-75 years, 75-84 years, 85 years and older), with a slightly higher percentage of younger black patients than younger white patients (25.0% vs. 22.4%, respectively). Black patients were more likely than white patients to be from the South (58.6% vs. 39.7%) and have a Charlson Comorbidity Index score of 2 or higher (62.9% vs. 45.4%). White patients were more likely than black patients to have a Charlson score of 0 (42.0% vs. 24.1%).
The three identifying outcomes were: death/mortality, which was determined using the date in the Medicare vital status; debility, a term used for patients newly placed in a long-term nursing facility; and destitution, used to describe patients who became newly eligible for Medicaid after a major fragility fracture.
The results showed that the most common fracture types were hip and clinical vertebral fractures, with black women having a significantly lower rate of clinical vertebral fractures (29.0% vs. 34.1%, respectively) but a significantly higher rate of femur fractures (9.1% vs. 3.8%). Black women also had a significantly higher mortality rate after a fracture (19.6% vs. 15.4%), and a significantly higher composite outcome of all three identifying outcome measurements (24.6% vs. 20.2%). However, rates of debility and destitution were similar between the groups.
When measured by fracture type, black women had significantly different 1-year postfracture outcomes, compared with white women, with a 38.0% higher incidence of mortality, 40.2% higher rate of debility, 185.0% higher rate of destitution, and 35.4% higher composite outcome for hip fracture. For radius/ulna fractures, black women also had a 59.7% higher rate of death, 8.5% higher rate of debility, 164.7% higher rate of destitution, and 43.0% higher composite outcomes; and for clinical vertebral fractures, they had 11.4% higher rate of death, 10.8% higher rate of debility, 130.6% higher rate of destitution, and 13.6% higher composite outcome, compared with white women.
Overall, black women had higher incidence risk ratios for death (IRR, 1.24; 95% confidence interval, 1.15-1.33), debility (IRR, 1.19; 95% CI, 1.06-1.33), and destitution (IRR, 2.45; 95% CI, 2.20-2.73) for fractures of the hip; higher IRRs for death (IRR, 1.48; 95% CI, 1.33-1.66), debility (IRR, 1.02; 95% CI, 0.87-1.20), and destitution (IRR, 2.70; 95% CI, 2.33-3.13) for fractures of the radius or ulna; and higher IRRs for death (IRR, 1.07; 95% CI, 0.98-1.17) and destitution (IRR, 2.40; 95% CI, 2.15-2.67) for clinical vertebral fractures.
“These data show that we need to develop interventions and/or programs to mitigate and reduce disparities in fracture outcomes,” said Dr. Wright.
She noted that the study results were limited because of its observational nature, and results cannot be generalized beyond older women with postmenopausal osteoporosis with Medicare coverage. In addition, the algorithm used to determine fracture status also had a potentially low sensitivity, which may have affected the study results, she said.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer. Dr. Chen reported receiving grants from Amgen. Dr. Curtis reported receiving grants from, and is a consultant for, Amgen, Eli Lilly, and Radius. Dr. Saag reported receiving grants from Amgen and is a consultant for Gilead and Radius.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1125.
ORLANDO – Older black women with postmenopausal osteoporosis had significantly higher mortality rates, were more likely to be placed in a long-term nursing facility, and were more likely to become newly eligible for Medicaid after a major fragility fracture event, compared with their white counterparts, according to a findings presented at the annual meeting of the American Society for Bone and Mineral Research.
Previous studies have examined racial differences in mortality and outcomes after fracture, but the data from those studies are older or limited to a certain region or health system, Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham, said in her presentation.
“To our knowledge, this is the first comprehensive evaluation of fractures and outcomes post fracture by race, particularly in black women,” she said.
Using Medicare data from between 2010 and 2016, Dr. Wright and colleagues performed a cohort-based, descriptive study of 400,479 white women and 11,563 black women with postmenopausal osteoporosis, who were covered by Medicare Parts A, B, C and D and had a hip, pelvis, femur, radius/ulna, humerus, or clinical vertebral fractures. Fractures were identified by way of a validated algorithm that used inpatient and outpatient claims, outpatient physical evaluations, and management claims, together with fracture repair codes (positive predictive value range, 90.9%-98.6%; from Wright N et al. J Bone Min Res. 2019 Jun 6. doi: 10.1002/jbmr.3807).
The groups had similar proportions of patients in each age group (65-75 years, 75-84 years, 85 years and older), with a slightly higher percentage of younger black patients than younger white patients (25.0% vs. 22.4%, respectively). Black patients were more likely than white patients to be from the South (58.6% vs. 39.7%) and have a Charlson Comorbidity Index score of 2 or higher (62.9% vs. 45.4%). White patients were more likely than black patients to have a Charlson score of 0 (42.0% vs. 24.1%).
The three identifying outcomes were: death/mortality, which was determined using the date in the Medicare vital status; debility, a term used for patients newly placed in a long-term nursing facility; and destitution, used to describe patients who became newly eligible for Medicaid after a major fragility fracture.
The results showed that the most common fracture types were hip and clinical vertebral fractures, with black women having a significantly lower rate of clinical vertebral fractures (29.0% vs. 34.1%, respectively) but a significantly higher rate of femur fractures (9.1% vs. 3.8%). Black women also had a significantly higher mortality rate after a fracture (19.6% vs. 15.4%), and a significantly higher composite outcome of all three identifying outcome measurements (24.6% vs. 20.2%). However, rates of debility and destitution were similar between the groups.
When measured by fracture type, black women had significantly different 1-year postfracture outcomes, compared with white women, with a 38.0% higher incidence of mortality, 40.2% higher rate of debility, 185.0% higher rate of destitution, and 35.4% higher composite outcome for hip fracture. For radius/ulna fractures, black women also had a 59.7% higher rate of death, 8.5% higher rate of debility, 164.7% higher rate of destitution, and 43.0% higher composite outcomes; and for clinical vertebral fractures, they had 11.4% higher rate of death, 10.8% higher rate of debility, 130.6% higher rate of destitution, and 13.6% higher composite outcome, compared with white women.
Overall, black women had higher incidence risk ratios for death (IRR, 1.24; 95% confidence interval, 1.15-1.33), debility (IRR, 1.19; 95% CI, 1.06-1.33), and destitution (IRR, 2.45; 95% CI, 2.20-2.73) for fractures of the hip; higher IRRs for death (IRR, 1.48; 95% CI, 1.33-1.66), debility (IRR, 1.02; 95% CI, 0.87-1.20), and destitution (IRR, 2.70; 95% CI, 2.33-3.13) for fractures of the radius or ulna; and higher IRRs for death (IRR, 1.07; 95% CI, 0.98-1.17) and destitution (IRR, 2.40; 95% CI, 2.15-2.67) for clinical vertebral fractures.
“These data show that we need to develop interventions and/or programs to mitigate and reduce disparities in fracture outcomes,” said Dr. Wright.
She noted that the study results were limited because of its observational nature, and results cannot be generalized beyond older women with postmenopausal osteoporosis with Medicare coverage. In addition, the algorithm used to determine fracture status also had a potentially low sensitivity, which may have affected the study results, she said.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer. Dr. Chen reported receiving grants from Amgen. Dr. Curtis reported receiving grants from, and is a consultant for, Amgen, Eli Lilly, and Radius. Dr. Saag reported receiving grants from Amgen and is a consultant for Gilead and Radius.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1125.
REPORTING FROM ASBMR 2019