User login
Debate Arises Over Ovarian Tissue Transplants to Delay Menopause
The transplantation of ovarian tissue is often performed to extend fertility among women and adolescents with cancer. But some reproductive specialists believe the procedure may have another role to play with much wider application: delaying, or even preventing, menopause in healthy women.
Kutluk Oktay, MD, director of the Laboratory of Molecular Reproduction and Fertility Preservation at the Yale School of Medicine in New Haven, Connecticut, has used ovarian tissue transplantation (OTT) in his own practice — Innovation Fertility Preservation & IVF — for several years. He said the approach can reduce health risks associated with menopause, such as the loss of bone density and cardiovascular disease.
“We have started offering [ovarian tissue transplantation] in carefully selected candidates, but the pace will accelerate now that we have a way to better inform the candidates on the potential of the procedure,” Dr. Oktay said. To date, he said he has performed the procedure on approximately 20 patients.
But Dr. Oktay’s vision of the future for OTT remains on the fringe of reproductive medicine.
“I think there are ethical considerations to take into account here,” said Stephanie Faubion, MD, Medical Director for the North American Menopause Society. “You’re taking a perfectly healthy 25- to 30-year-old woman and putting her through surgery to take out a healthy organ. Let’s just think about that.”
The Promise and Risks of OTT
OTT involves removing part of the ovarian tissue, cryopreservation, and then transplanting it back into the body. The procedure has reversed early menopause in women who underwent cancer treatment and resulted in over 140 live births worldwide.
Dr. Oktay recently published a nonclinical study in the American Journal of Obstetrics & Gynecology using a mathematical model based on decades of clinical research on cancer patients and ovarian follicle counts in cadaver to forecast how OTT can delay the onset of menopause through restored ovarian function and hormonal shifts.
The model forecasts a delay in menopause of up to 47 years, depending on factors such as the age of tissue removal, a woman’s ovarian reserve, and an estimated number of primordial follicles — where tens to hundreds of thousands of undeveloped eggs can live — that survive the process of removal, freezing, and reimplantation.
OTT is currently associated with a survival rate of 40% for follicles, Dr. Oktay said. But technological advancements, including revascularization drugs and robotic surgery, are likely to extend the survival rate to 80% by the time reimplantation occurs, potentially 15-20 years after tissue removal, he said.
Prospective patients at Dr. Oktay’s practice can use an interactive tool to receive an estimate of their potential menopausal delay. Patients receive a clinical assessment, including tests for ovarian reserve markers, to determine their potential for the procedure.
The model predicted that harvesting tissue before age 30 could delay menopause significantly. A 25-year-old woman with an average ovarian reserve who preserved a quarter of one ovary would have a delay in menopause of 11.8 years if 40% of the follicles survived. Women around age 40, and especially those with a low ovarian reserve, would need a follicle survival rate of close to 100% to result in a delay significant enough to justify the procedure.
The procedure also comes with risks. Removing ovarian tissue can bring on early menopause, Dr. Oktay said. Removing part or all of the ovarian cortex — the outer part of the ovary that contains the follicles — can start menopause about 1.5 years earlier. But as long as the tissue is transplanted, a woman would gain many more years of fertility before menopause.
While potentially promising, some obstetrics and gynecology experts question the procedure, with no proven benefits.
“While theoretically possible, my biggest question is, how is this better than egg freezing in your 20s or 30s combined with hormone replacement for the aging benefits, given the risks associated with potentially multiple surgeries?” said Paula Amato, MD, professor of obstetrics and gynecology at Oregon Health & Science University in Portland, Oregon.
Any risks associated with receiving hormone therapy through OTT rather than traditional hormone replacement therapy are also unknown, Dr. Amato said.
A UK clinic, ProFam, based in Birmingham, also offered the procedure but faced criticism in 2020 for being unnecessary and experimental. This news organization could not confirm if the clinic is still in operation.
Why Delay Menopause?
While the procedure may extend fertility, the goal of the procedure is not to enable patients to become pregnant at ages that are not safe, Dr. Oktay said. Rather, he said postponing menopause is medically beneficial.
Some research shows that women who have late menopause have a lower risk for all-cause mortality and cardiovascular disease but a higher risk for breast, endometrial, and ovarian cancers.
Dr. Oktay said that delaying menopause could improve the quality of life for women by reducing menopausal symptoms like anxiety and depression. Clinicians could also use the procedure as preventive care for those who are at high risk for conditions associated with menopause, such as osteoporosis and dementia.
But Dr. Faubion is unconvinced that delaying menopause through OTT carries health benefits.
“Just because we can do this, should we?” she said. “And will it do the things that we think it will? Does preventing or delaying menopause delay the aging process? I think that’s what they’re trying to imply, and we don’t have evidence that that’s true.”
The study was funded by the National Science Foundation, U-Anschutz Department of Obstetrics and Gynecology Research Funds, SF Faculty Early Career Development Program, and the National Institutes of Health awards. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
The transplantation of ovarian tissue is often performed to extend fertility among women and adolescents with cancer. But some reproductive specialists believe the procedure may have another role to play with much wider application: delaying, or even preventing, menopause in healthy women.
Kutluk Oktay, MD, director of the Laboratory of Molecular Reproduction and Fertility Preservation at the Yale School of Medicine in New Haven, Connecticut, has used ovarian tissue transplantation (OTT) in his own practice — Innovation Fertility Preservation & IVF — for several years. He said the approach can reduce health risks associated with menopause, such as the loss of bone density and cardiovascular disease.
“We have started offering [ovarian tissue transplantation] in carefully selected candidates, but the pace will accelerate now that we have a way to better inform the candidates on the potential of the procedure,” Dr. Oktay said. To date, he said he has performed the procedure on approximately 20 patients.
But Dr. Oktay’s vision of the future for OTT remains on the fringe of reproductive medicine.
“I think there are ethical considerations to take into account here,” said Stephanie Faubion, MD, Medical Director for the North American Menopause Society. “You’re taking a perfectly healthy 25- to 30-year-old woman and putting her through surgery to take out a healthy organ. Let’s just think about that.”
The Promise and Risks of OTT
OTT involves removing part of the ovarian tissue, cryopreservation, and then transplanting it back into the body. The procedure has reversed early menopause in women who underwent cancer treatment and resulted in over 140 live births worldwide.
Dr. Oktay recently published a nonclinical study in the American Journal of Obstetrics & Gynecology using a mathematical model based on decades of clinical research on cancer patients and ovarian follicle counts in cadaver to forecast how OTT can delay the onset of menopause through restored ovarian function and hormonal shifts.
The model forecasts a delay in menopause of up to 47 years, depending on factors such as the age of tissue removal, a woman’s ovarian reserve, and an estimated number of primordial follicles — where tens to hundreds of thousands of undeveloped eggs can live — that survive the process of removal, freezing, and reimplantation.
OTT is currently associated with a survival rate of 40% for follicles, Dr. Oktay said. But technological advancements, including revascularization drugs and robotic surgery, are likely to extend the survival rate to 80% by the time reimplantation occurs, potentially 15-20 years after tissue removal, he said.
Prospective patients at Dr. Oktay’s practice can use an interactive tool to receive an estimate of their potential menopausal delay. Patients receive a clinical assessment, including tests for ovarian reserve markers, to determine their potential for the procedure.
The model predicted that harvesting tissue before age 30 could delay menopause significantly. A 25-year-old woman with an average ovarian reserve who preserved a quarter of one ovary would have a delay in menopause of 11.8 years if 40% of the follicles survived. Women around age 40, and especially those with a low ovarian reserve, would need a follicle survival rate of close to 100% to result in a delay significant enough to justify the procedure.
The procedure also comes with risks. Removing ovarian tissue can bring on early menopause, Dr. Oktay said. Removing part or all of the ovarian cortex — the outer part of the ovary that contains the follicles — can start menopause about 1.5 years earlier. But as long as the tissue is transplanted, a woman would gain many more years of fertility before menopause.
While potentially promising, some obstetrics and gynecology experts question the procedure, with no proven benefits.
“While theoretically possible, my biggest question is, how is this better than egg freezing in your 20s or 30s combined with hormone replacement for the aging benefits, given the risks associated with potentially multiple surgeries?” said Paula Amato, MD, professor of obstetrics and gynecology at Oregon Health & Science University in Portland, Oregon.
Any risks associated with receiving hormone therapy through OTT rather than traditional hormone replacement therapy are also unknown, Dr. Amato said.
A UK clinic, ProFam, based in Birmingham, also offered the procedure but faced criticism in 2020 for being unnecessary and experimental. This news organization could not confirm if the clinic is still in operation.
Why Delay Menopause?
While the procedure may extend fertility, the goal of the procedure is not to enable patients to become pregnant at ages that are not safe, Dr. Oktay said. Rather, he said postponing menopause is medically beneficial.
Some research shows that women who have late menopause have a lower risk for all-cause mortality and cardiovascular disease but a higher risk for breast, endometrial, and ovarian cancers.
Dr. Oktay said that delaying menopause could improve the quality of life for women by reducing menopausal symptoms like anxiety and depression. Clinicians could also use the procedure as preventive care for those who are at high risk for conditions associated with menopause, such as osteoporosis and dementia.
But Dr. Faubion is unconvinced that delaying menopause through OTT carries health benefits.
“Just because we can do this, should we?” she said. “And will it do the things that we think it will? Does preventing or delaying menopause delay the aging process? I think that’s what they’re trying to imply, and we don’t have evidence that that’s true.”
The study was funded by the National Science Foundation, U-Anschutz Department of Obstetrics and Gynecology Research Funds, SF Faculty Early Career Development Program, and the National Institutes of Health awards. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
The transplantation of ovarian tissue is often performed to extend fertility among women and adolescents with cancer. But some reproductive specialists believe the procedure may have another role to play with much wider application: delaying, or even preventing, menopause in healthy women.
Kutluk Oktay, MD, director of the Laboratory of Molecular Reproduction and Fertility Preservation at the Yale School of Medicine in New Haven, Connecticut, has used ovarian tissue transplantation (OTT) in his own practice — Innovation Fertility Preservation & IVF — for several years. He said the approach can reduce health risks associated with menopause, such as the loss of bone density and cardiovascular disease.
“We have started offering [ovarian tissue transplantation] in carefully selected candidates, but the pace will accelerate now that we have a way to better inform the candidates on the potential of the procedure,” Dr. Oktay said. To date, he said he has performed the procedure on approximately 20 patients.
But Dr. Oktay’s vision of the future for OTT remains on the fringe of reproductive medicine.
“I think there are ethical considerations to take into account here,” said Stephanie Faubion, MD, Medical Director for the North American Menopause Society. “You’re taking a perfectly healthy 25- to 30-year-old woman and putting her through surgery to take out a healthy organ. Let’s just think about that.”
The Promise and Risks of OTT
OTT involves removing part of the ovarian tissue, cryopreservation, and then transplanting it back into the body. The procedure has reversed early menopause in women who underwent cancer treatment and resulted in over 140 live births worldwide.
Dr. Oktay recently published a nonclinical study in the American Journal of Obstetrics & Gynecology using a mathematical model based on decades of clinical research on cancer patients and ovarian follicle counts in cadaver to forecast how OTT can delay the onset of menopause through restored ovarian function and hormonal shifts.
The model forecasts a delay in menopause of up to 47 years, depending on factors such as the age of tissue removal, a woman’s ovarian reserve, and an estimated number of primordial follicles — where tens to hundreds of thousands of undeveloped eggs can live — that survive the process of removal, freezing, and reimplantation.
OTT is currently associated with a survival rate of 40% for follicles, Dr. Oktay said. But technological advancements, including revascularization drugs and robotic surgery, are likely to extend the survival rate to 80% by the time reimplantation occurs, potentially 15-20 years after tissue removal, he said.
Prospective patients at Dr. Oktay’s practice can use an interactive tool to receive an estimate of their potential menopausal delay. Patients receive a clinical assessment, including tests for ovarian reserve markers, to determine their potential for the procedure.
The model predicted that harvesting tissue before age 30 could delay menopause significantly. A 25-year-old woman with an average ovarian reserve who preserved a quarter of one ovary would have a delay in menopause of 11.8 years if 40% of the follicles survived. Women around age 40, and especially those with a low ovarian reserve, would need a follicle survival rate of close to 100% to result in a delay significant enough to justify the procedure.
The procedure also comes with risks. Removing ovarian tissue can bring on early menopause, Dr. Oktay said. Removing part or all of the ovarian cortex — the outer part of the ovary that contains the follicles — can start menopause about 1.5 years earlier. But as long as the tissue is transplanted, a woman would gain many more years of fertility before menopause.
While potentially promising, some obstetrics and gynecology experts question the procedure, with no proven benefits.
“While theoretically possible, my biggest question is, how is this better than egg freezing in your 20s or 30s combined with hormone replacement for the aging benefits, given the risks associated with potentially multiple surgeries?” said Paula Amato, MD, professor of obstetrics and gynecology at Oregon Health & Science University in Portland, Oregon.
Any risks associated with receiving hormone therapy through OTT rather than traditional hormone replacement therapy are also unknown, Dr. Amato said.
A UK clinic, ProFam, based in Birmingham, also offered the procedure but faced criticism in 2020 for being unnecessary and experimental. This news organization could not confirm if the clinic is still in operation.
Why Delay Menopause?
While the procedure may extend fertility, the goal of the procedure is not to enable patients to become pregnant at ages that are not safe, Dr. Oktay said. Rather, he said postponing menopause is medically beneficial.
Some research shows that women who have late menopause have a lower risk for all-cause mortality and cardiovascular disease but a higher risk for breast, endometrial, and ovarian cancers.
Dr. Oktay said that delaying menopause could improve the quality of life for women by reducing menopausal symptoms like anxiety and depression. Clinicians could also use the procedure as preventive care for those who are at high risk for conditions associated with menopause, such as osteoporosis and dementia.
But Dr. Faubion is unconvinced that delaying menopause through OTT carries health benefits.
“Just because we can do this, should we?” she said. “And will it do the things that we think it will? Does preventing or delaying menopause delay the aging process? I think that’s what they’re trying to imply, and we don’t have evidence that that’s true.”
The study was funded by the National Science Foundation, U-Anschutz Department of Obstetrics and Gynecology Research Funds, SF Faculty Early Career Development Program, and the National Institutes of Health awards. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
We Must Learn About Abortion as Primary Care Doctors
“No greater opportunity, responsibility, or obligation can fall to the lot of a human being than to become a physician. In the care of the suffering, [the physician] needs technical skill, scientific knowledge, and human understanding.”1 Internal medicine physicians have risen to this challenge for centuries. Today, it is time for us to use these skills to care for patients who need access to reproductive care — particularly medication abortion. Nationally accredited internal medicine training programs have not been required to provide abortion education, and this may evolve in the future.
However, considering the difficulty in people receiving contraception, the failure rate of contraception, the known risks from pregnancy, the increasing difficulty in accessing abortion, and the recent advocating to protect access to reproductive care by leadership of internal medicine and internal medicine subspecialty societies, we advocate that abortion must become a part of our education and practice.2
Most abortions are performed during the first trimester and can be managed with medications that are very safe.3 In fact, legal medication abortion is so safe that pregnancy in the United States has fourteen times the mortality risk as does legal medication abortion.4 Inability to access an abortion has widely documented negative health effects for women and their children.5,6
Within this context, it is important for internal medicine physicians to understand that the ability to access an abortion is the ability to access a life-saving procedure and there is no medical justification for restricting such a prescription any more than restricting any other standard medical therapy. Furthermore, the recent widespread criminalization of abortion gives new urgency to expanding the pool of physicians who understand this and are trained, able, and willing to prescribe medication abortion.
We understand that reproductive health care may not now be a component of clinical practice for some, but given the heterogeneity of internal medicine, we believe that some knowledge about medical abortion is an essential competency of foundational medical knowledge.7 The heterogeneity of practice in internal medicine lends itself to different levels of knowledge that should be embraced. Because of poor access to abortion, both ambulatory and hospital-based physicians will increasingly be required to care for patients who need abortion for medical or other reasons.
We advocate that all physicians — including those with internal medicine training — should understand counseling about choices and options (including an unbiased discussion of the options to continue or terminate the pregnancy), the safety of medication abortion in contrast to the risks from pregnancy, and where to refer someone seeking an abortion. In addition to this information, primary care physicians with a special interest in women’s health must have basic knowledge about mifepristone and misoprostol and how they work, the benefits and risks of these, and what the pregnant person seeking an abortion will experience.8
Lastly, physicians who wish to provide medication abortion — including in primary care, hospital medicine, and subspecialty care — should receive training and ongoing professional development. Such professional development should include counseling, indications, contraindications, medication regimens, navigating required documentation and reporting, and anticipating possible side effects and complications.
A major challenge to internal medicine and other primary care physicians, subspecialists, and hospitalists addressing abortion is the inadequate training in and knowledge about providing this care. However, the entire spectrum of medical education (undergraduate, graduate, and continuing education) should evolve to address this lack.
Integrating this education into medical conferences and journals is a meaningful start, possibly in partnership with medical societies that have been teaching these skills for decades. Partnering with other specialties can also help us stay current on the local legal landscape and engage in collaborative advocacy.
Specifically, some resources for training can be found at:
- www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/11/abortion-training-and-education
- https://prochoice.org/providers/continuing-medical-education/
- www.reproductiveaccess.org/medicationabortion/
Some may have concerns that managing the possible complications of medication abortion is a reason for internal medicine to not be involved in abortion care. However, medication abortions are safe and effective for pregnancy termination and internal medicine physicians can refer patients with complications to peers in gynecology, family medicine, and emergency medicine should complications arise.8 We have managed countless other conditions this way, including most recently during the pandemic.
We live in a country with increasing barriers to care – now with laws in many states that prevent basic health care for women. Internal medicine doctors increasingly may see patients who need care urgently, particularly those who practice in states that neighbor those that prevent this access. We are calling for all who practice internal medicine to educate themselves, optimizing their skills within the full scope of medical practice to provide possibly lifesaving care and thereby address increased needs for medical services.
We must continue to advocate for our patients. The COVID-19 pandemic has reinforced the fact that internal medicine–trained physicians are able to care for conditions that are new and, as a profession, we are capable of rapidly switching practices and learning new modalities of care. It is time for us to extend this competency to care for patients who constitute half the population and are at risk: women.
Dr. Barrett is an internal medicine hospitalist based in Albuquerque, New Mexico; she completed a medical justice in advocacy fellowship in 2022. Dr. Radhakrishnan is an internal medicine physician educator who completed an equity matters fellowship in 2022 and is based in Scottsdale, Arizona. Neither reports conflicts of interest.
References
1. Harrison’s Principles of Internal Medicine, 20e. Jameson J et al., eds. McGraw Hill; 2018. Accessed Sept. 27, 2023.
2. Serchen J et al. Reproductive Health Policy in the United States: An American College of Physicians Policy Brief. Ann Intern Med.2023;176:364-6. epub 28 Feb. 2023.
3. Jatlaoui TC et al. Abortion Surveillance — United States, 2016. MMWR Surveill Summ 2019;68(No. SS-11):1-41.
4. Raymond EG and Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215-9.
5. Ralph LJ et al. Self-reported Physical Health of Women Who Did and Did Not Terminate Pregnancy After Seeking Abortion Services: A Cohort Study. Ann Intern Med.2019;171:238-47. epub 11 June 2019.
6. Gerdts C et al. Side effects, physical health consequences, and mortality associated with abortion and birth after an unwanted pregnancy. Women’s Health Issues 2016;26:55-59.
7. Nobel K et al. Patient-reported experience with discussion of all options during pregnancy options counseling in the US south. Contraception. 2022;106:68-74.
8. Liu N and Ray JG. Short-Term Adverse Outcomes After Mifepristone–Misoprostol Versus Procedural Induced Abortion: A Population-Based Propensity-Weighted Study. Ann Intern Med.2023;176:145-53. epub 3 January 2023.
“No greater opportunity, responsibility, or obligation can fall to the lot of a human being than to become a physician. In the care of the suffering, [the physician] needs technical skill, scientific knowledge, and human understanding.”1 Internal medicine physicians have risen to this challenge for centuries. Today, it is time for us to use these skills to care for patients who need access to reproductive care — particularly medication abortion. Nationally accredited internal medicine training programs have not been required to provide abortion education, and this may evolve in the future.
However, considering the difficulty in people receiving contraception, the failure rate of contraception, the known risks from pregnancy, the increasing difficulty in accessing abortion, and the recent advocating to protect access to reproductive care by leadership of internal medicine and internal medicine subspecialty societies, we advocate that abortion must become a part of our education and practice.2
Most abortions are performed during the first trimester and can be managed with medications that are very safe.3 In fact, legal medication abortion is so safe that pregnancy in the United States has fourteen times the mortality risk as does legal medication abortion.4 Inability to access an abortion has widely documented negative health effects for women and their children.5,6
Within this context, it is important for internal medicine physicians to understand that the ability to access an abortion is the ability to access a life-saving procedure and there is no medical justification for restricting such a prescription any more than restricting any other standard medical therapy. Furthermore, the recent widespread criminalization of abortion gives new urgency to expanding the pool of physicians who understand this and are trained, able, and willing to prescribe medication abortion.
We understand that reproductive health care may not now be a component of clinical practice for some, but given the heterogeneity of internal medicine, we believe that some knowledge about medical abortion is an essential competency of foundational medical knowledge.7 The heterogeneity of practice in internal medicine lends itself to different levels of knowledge that should be embraced. Because of poor access to abortion, both ambulatory and hospital-based physicians will increasingly be required to care for patients who need abortion for medical or other reasons.
We advocate that all physicians — including those with internal medicine training — should understand counseling about choices and options (including an unbiased discussion of the options to continue or terminate the pregnancy), the safety of medication abortion in contrast to the risks from pregnancy, and where to refer someone seeking an abortion. In addition to this information, primary care physicians with a special interest in women’s health must have basic knowledge about mifepristone and misoprostol and how they work, the benefits and risks of these, and what the pregnant person seeking an abortion will experience.8
Lastly, physicians who wish to provide medication abortion — including in primary care, hospital medicine, and subspecialty care — should receive training and ongoing professional development. Such professional development should include counseling, indications, contraindications, medication regimens, navigating required documentation and reporting, and anticipating possible side effects and complications.
A major challenge to internal medicine and other primary care physicians, subspecialists, and hospitalists addressing abortion is the inadequate training in and knowledge about providing this care. However, the entire spectrum of medical education (undergraduate, graduate, and continuing education) should evolve to address this lack.
Integrating this education into medical conferences and journals is a meaningful start, possibly in partnership with medical societies that have been teaching these skills for decades. Partnering with other specialties can also help us stay current on the local legal landscape and engage in collaborative advocacy.
Specifically, some resources for training can be found at:
- www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/11/abortion-training-and-education
- https://prochoice.org/providers/continuing-medical-education/
- www.reproductiveaccess.org/medicationabortion/
Some may have concerns that managing the possible complications of medication abortion is a reason for internal medicine to not be involved in abortion care. However, medication abortions are safe and effective for pregnancy termination and internal medicine physicians can refer patients with complications to peers in gynecology, family medicine, and emergency medicine should complications arise.8 We have managed countless other conditions this way, including most recently during the pandemic.
We live in a country with increasing barriers to care – now with laws in many states that prevent basic health care for women. Internal medicine doctors increasingly may see patients who need care urgently, particularly those who practice in states that neighbor those that prevent this access. We are calling for all who practice internal medicine to educate themselves, optimizing their skills within the full scope of medical practice to provide possibly lifesaving care and thereby address increased needs for medical services.
We must continue to advocate for our patients. The COVID-19 pandemic has reinforced the fact that internal medicine–trained physicians are able to care for conditions that are new and, as a profession, we are capable of rapidly switching practices and learning new modalities of care. It is time for us to extend this competency to care for patients who constitute half the population and are at risk: women.
Dr. Barrett is an internal medicine hospitalist based in Albuquerque, New Mexico; she completed a medical justice in advocacy fellowship in 2022. Dr. Radhakrishnan is an internal medicine physician educator who completed an equity matters fellowship in 2022 and is based in Scottsdale, Arizona. Neither reports conflicts of interest.
References
1. Harrison’s Principles of Internal Medicine, 20e. Jameson J et al., eds. McGraw Hill; 2018. Accessed Sept. 27, 2023.
2. Serchen J et al. Reproductive Health Policy in the United States: An American College of Physicians Policy Brief. Ann Intern Med.2023;176:364-6. epub 28 Feb. 2023.
3. Jatlaoui TC et al. Abortion Surveillance — United States, 2016. MMWR Surveill Summ 2019;68(No. SS-11):1-41.
4. Raymond EG and Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215-9.
5. Ralph LJ et al. Self-reported Physical Health of Women Who Did and Did Not Terminate Pregnancy After Seeking Abortion Services: A Cohort Study. Ann Intern Med.2019;171:238-47. epub 11 June 2019.
6. Gerdts C et al. Side effects, physical health consequences, and mortality associated with abortion and birth after an unwanted pregnancy. Women’s Health Issues 2016;26:55-59.
7. Nobel K et al. Patient-reported experience with discussion of all options during pregnancy options counseling in the US south. Contraception. 2022;106:68-74.
8. Liu N and Ray JG. Short-Term Adverse Outcomes After Mifepristone–Misoprostol Versus Procedural Induced Abortion: A Population-Based Propensity-Weighted Study. Ann Intern Med.2023;176:145-53. epub 3 January 2023.
“No greater opportunity, responsibility, or obligation can fall to the lot of a human being than to become a physician. In the care of the suffering, [the physician] needs technical skill, scientific knowledge, and human understanding.”1 Internal medicine physicians have risen to this challenge for centuries. Today, it is time for us to use these skills to care for patients who need access to reproductive care — particularly medication abortion. Nationally accredited internal medicine training programs have not been required to provide abortion education, and this may evolve in the future.
However, considering the difficulty in people receiving contraception, the failure rate of contraception, the known risks from pregnancy, the increasing difficulty in accessing abortion, and the recent advocating to protect access to reproductive care by leadership of internal medicine and internal medicine subspecialty societies, we advocate that abortion must become a part of our education and practice.2
Most abortions are performed during the first trimester and can be managed with medications that are very safe.3 In fact, legal medication abortion is so safe that pregnancy in the United States has fourteen times the mortality risk as does legal medication abortion.4 Inability to access an abortion has widely documented negative health effects for women and their children.5,6
Within this context, it is important for internal medicine physicians to understand that the ability to access an abortion is the ability to access a life-saving procedure and there is no medical justification for restricting such a prescription any more than restricting any other standard medical therapy. Furthermore, the recent widespread criminalization of abortion gives new urgency to expanding the pool of physicians who understand this and are trained, able, and willing to prescribe medication abortion.
We understand that reproductive health care may not now be a component of clinical practice for some, but given the heterogeneity of internal medicine, we believe that some knowledge about medical abortion is an essential competency of foundational medical knowledge.7 The heterogeneity of practice in internal medicine lends itself to different levels of knowledge that should be embraced. Because of poor access to abortion, both ambulatory and hospital-based physicians will increasingly be required to care for patients who need abortion for medical or other reasons.
We advocate that all physicians — including those with internal medicine training — should understand counseling about choices and options (including an unbiased discussion of the options to continue or terminate the pregnancy), the safety of medication abortion in contrast to the risks from pregnancy, and where to refer someone seeking an abortion. In addition to this information, primary care physicians with a special interest in women’s health must have basic knowledge about mifepristone and misoprostol and how they work, the benefits and risks of these, and what the pregnant person seeking an abortion will experience.8
Lastly, physicians who wish to provide medication abortion — including in primary care, hospital medicine, and subspecialty care — should receive training and ongoing professional development. Such professional development should include counseling, indications, contraindications, medication regimens, navigating required documentation and reporting, and anticipating possible side effects and complications.
A major challenge to internal medicine and other primary care physicians, subspecialists, and hospitalists addressing abortion is the inadequate training in and knowledge about providing this care. However, the entire spectrum of medical education (undergraduate, graduate, and continuing education) should evolve to address this lack.
Integrating this education into medical conferences and journals is a meaningful start, possibly in partnership with medical societies that have been teaching these skills for decades. Partnering with other specialties can also help us stay current on the local legal landscape and engage in collaborative advocacy.
Specifically, some resources for training can be found at:
- www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/11/abortion-training-and-education
- https://prochoice.org/providers/continuing-medical-education/
- www.reproductiveaccess.org/medicationabortion/
Some may have concerns that managing the possible complications of medication abortion is a reason for internal medicine to not be involved in abortion care. However, medication abortions are safe and effective for pregnancy termination and internal medicine physicians can refer patients with complications to peers in gynecology, family medicine, and emergency medicine should complications arise.8 We have managed countless other conditions this way, including most recently during the pandemic.
We live in a country with increasing barriers to care – now with laws in many states that prevent basic health care for women. Internal medicine doctors increasingly may see patients who need care urgently, particularly those who practice in states that neighbor those that prevent this access. We are calling for all who practice internal medicine to educate themselves, optimizing their skills within the full scope of medical practice to provide possibly lifesaving care and thereby address increased needs for medical services.
We must continue to advocate for our patients. The COVID-19 pandemic has reinforced the fact that internal medicine–trained physicians are able to care for conditions that are new and, as a profession, we are capable of rapidly switching practices and learning new modalities of care. It is time for us to extend this competency to care for patients who constitute half the population and are at risk: women.
Dr. Barrett is an internal medicine hospitalist based in Albuquerque, New Mexico; she completed a medical justice in advocacy fellowship in 2022. Dr. Radhakrishnan is an internal medicine physician educator who completed an equity matters fellowship in 2022 and is based in Scottsdale, Arizona. Neither reports conflicts of interest.
References
1. Harrison’s Principles of Internal Medicine, 20e. Jameson J et al., eds. McGraw Hill; 2018. Accessed Sept. 27, 2023.
2. Serchen J et al. Reproductive Health Policy in the United States: An American College of Physicians Policy Brief. Ann Intern Med.2023;176:364-6. epub 28 Feb. 2023.
3. Jatlaoui TC et al. Abortion Surveillance — United States, 2016. MMWR Surveill Summ 2019;68(No. SS-11):1-41.
4. Raymond EG and Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215-9.
5. Ralph LJ et al. Self-reported Physical Health of Women Who Did and Did Not Terminate Pregnancy After Seeking Abortion Services: A Cohort Study. Ann Intern Med.2019;171:238-47. epub 11 June 2019.
6. Gerdts C et al. Side effects, physical health consequences, and mortality associated with abortion and birth after an unwanted pregnancy. Women’s Health Issues 2016;26:55-59.
7. Nobel K et al. Patient-reported experience with discussion of all options during pregnancy options counseling in the US south. Contraception. 2022;106:68-74.
8. Liu N and Ray JG. Short-Term Adverse Outcomes After Mifepristone–Misoprostol Versus Procedural Induced Abortion: A Population-Based Propensity-Weighted Study. Ann Intern Med.2023;176:145-53. epub 3 January 2023.
Paid Parental Leave: Impact on Maternal Mental Health and Child Wellbeing
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Web-Based Aid Educates Women on Tubal Sterilization
Although tubal sterilization is common, especially among those with lower income and education levels, misunderstandings persist about the reversibility of the procedure, and previous studies suggest that many pregnant individuals are not making well-informed decisions, wrote Sonya Borrero, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Network Open, the researchers randomized 350 pregnant individuals with Medicaid insurance to usual care or usual care plus a web-based decision aid in English or Spanish called MyDecision/MiDecisión that included written, audio, and video information about tubal sterilization. The tool also included an interactive table comparing tubal sterilization to other contraceptive options, exercises to clarify patients’ values, knowledge checks, and a final summary report.
The two primary outcomes were knowledge of tubal sterilization based on a 10-question true/false test and decisional conflict about contraceptive choices using the low-literacy Decision Conflict Scale. The participants ranged in age from 21 to 45 years, with a mean age of 29.7 years. Participants were randomized prior to 24 weeks’ gestation, and those in the intervention group completed the intervention immediately using a personal device or a university device in the clinical setting. Further assessments occurred by phone during the third trimester and at 3 months postpartum.
Participants in the decision aid group showed significantly greater knowledge of tubal sterilization compared with controls, with a mean of 76.5% correct responses to the knowledge questions, vs. 55.6% in the control group (P < .001). Decisional conflict scores also were significantly lower in the intervention group compared with controls (mean 12.7 vs. 18.7, P = .002).
The most dramatic knowledge gap related to permanence of tubal sterilization; 90.1% of participants in the intervention group answered correctly that the procedure is not easily reversible, compared to 39.3% of the controls. Similarly, 86.6% of the intervention group responded correctly that the tubes do not “come untied” spontaneously, vs. 33.7% of controls (P < .001 for both).
The findings were limited by several factors including the focus only on pregnant Medicaid patients, the presentation of the decision tool only at a point early in pregnancy, which may have been too soon for some participants to consider tubal sterilization, and a lack of data on long-term satisfaction or regret about tubal sterilization decisions, the researchers noted.
However, the knowledge differences between the intervention and control groups remained significant at the third trimester assessment, they said.
More research is needed in other populations and using other time points, but the current study results suggest that use of the MyDecision/MiDecisión tool in a real-world clinical setting at the actual time of decision-making could improve knowledge and inform patients’ choices, the researchers concluded. Improved patient education also could inform policy decisions about the potential elimination of the 30-day waiting period for sterilization procedures, they said.
The study was supported by the National Institute on Minority Health and Health Disparities. The researchers had no financial conflicts to disclose.
Although tubal sterilization is common, especially among those with lower income and education levels, misunderstandings persist about the reversibility of the procedure, and previous studies suggest that many pregnant individuals are not making well-informed decisions, wrote Sonya Borrero, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Network Open, the researchers randomized 350 pregnant individuals with Medicaid insurance to usual care or usual care plus a web-based decision aid in English or Spanish called MyDecision/MiDecisión that included written, audio, and video information about tubal sterilization. The tool also included an interactive table comparing tubal sterilization to other contraceptive options, exercises to clarify patients’ values, knowledge checks, and a final summary report.
The two primary outcomes were knowledge of tubal sterilization based on a 10-question true/false test and decisional conflict about contraceptive choices using the low-literacy Decision Conflict Scale. The participants ranged in age from 21 to 45 years, with a mean age of 29.7 years. Participants were randomized prior to 24 weeks’ gestation, and those in the intervention group completed the intervention immediately using a personal device or a university device in the clinical setting. Further assessments occurred by phone during the third trimester and at 3 months postpartum.
Participants in the decision aid group showed significantly greater knowledge of tubal sterilization compared with controls, with a mean of 76.5% correct responses to the knowledge questions, vs. 55.6% in the control group (P < .001). Decisional conflict scores also were significantly lower in the intervention group compared with controls (mean 12.7 vs. 18.7, P = .002).
The most dramatic knowledge gap related to permanence of tubal sterilization; 90.1% of participants in the intervention group answered correctly that the procedure is not easily reversible, compared to 39.3% of the controls. Similarly, 86.6% of the intervention group responded correctly that the tubes do not “come untied” spontaneously, vs. 33.7% of controls (P < .001 for both).
The findings were limited by several factors including the focus only on pregnant Medicaid patients, the presentation of the decision tool only at a point early in pregnancy, which may have been too soon for some participants to consider tubal sterilization, and a lack of data on long-term satisfaction or regret about tubal sterilization decisions, the researchers noted.
However, the knowledge differences between the intervention and control groups remained significant at the third trimester assessment, they said.
More research is needed in other populations and using other time points, but the current study results suggest that use of the MyDecision/MiDecisión tool in a real-world clinical setting at the actual time of decision-making could improve knowledge and inform patients’ choices, the researchers concluded. Improved patient education also could inform policy decisions about the potential elimination of the 30-day waiting period for sterilization procedures, they said.
The study was supported by the National Institute on Minority Health and Health Disparities. The researchers had no financial conflicts to disclose.
Although tubal sterilization is common, especially among those with lower income and education levels, misunderstandings persist about the reversibility of the procedure, and previous studies suggest that many pregnant individuals are not making well-informed decisions, wrote Sonya Borrero, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Network Open, the researchers randomized 350 pregnant individuals with Medicaid insurance to usual care or usual care plus a web-based decision aid in English or Spanish called MyDecision/MiDecisión that included written, audio, and video information about tubal sterilization. The tool also included an interactive table comparing tubal sterilization to other contraceptive options, exercises to clarify patients’ values, knowledge checks, and a final summary report.
The two primary outcomes were knowledge of tubal sterilization based on a 10-question true/false test and decisional conflict about contraceptive choices using the low-literacy Decision Conflict Scale. The participants ranged in age from 21 to 45 years, with a mean age of 29.7 years. Participants were randomized prior to 24 weeks’ gestation, and those in the intervention group completed the intervention immediately using a personal device or a university device in the clinical setting. Further assessments occurred by phone during the third trimester and at 3 months postpartum.
Participants in the decision aid group showed significantly greater knowledge of tubal sterilization compared with controls, with a mean of 76.5% correct responses to the knowledge questions, vs. 55.6% in the control group (P < .001). Decisional conflict scores also were significantly lower in the intervention group compared with controls (mean 12.7 vs. 18.7, P = .002).
The most dramatic knowledge gap related to permanence of tubal sterilization; 90.1% of participants in the intervention group answered correctly that the procedure is not easily reversible, compared to 39.3% of the controls. Similarly, 86.6% of the intervention group responded correctly that the tubes do not “come untied” spontaneously, vs. 33.7% of controls (P < .001 for both).
The findings were limited by several factors including the focus only on pregnant Medicaid patients, the presentation of the decision tool only at a point early in pregnancy, which may have been too soon for some participants to consider tubal sterilization, and a lack of data on long-term satisfaction or regret about tubal sterilization decisions, the researchers noted.
However, the knowledge differences between the intervention and control groups remained significant at the third trimester assessment, they said.
More research is needed in other populations and using other time points, but the current study results suggest that use of the MyDecision/MiDecisión tool in a real-world clinical setting at the actual time of decision-making could improve knowledge and inform patients’ choices, the researchers concluded. Improved patient education also could inform policy decisions about the potential elimination of the 30-day waiting period for sterilization procedures, they said.
The study was supported by the National Institute on Minority Health and Health Disparities. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Practicing Medicine in Canada’s Far North
In 2019, we interviewed Andrea Prince, MD, who was completing her internship in the Inuit village of Puvirnituq, a town of 2000 inhabitants located in Nunavik, in the Canadian Far North. Five years later, still in her position, what perspective does she have on her practice? Have the challenges of practicing medicine in a remote region within the Inuit community affected her vocation? Would she recommend this experience to young doctors?
Question: What position do you currently hold?
Dr. Prince: I am a full-time general practitioner at Puvirnituq Hospital. My responsibilities range from following up on hospitalized patients to those seen in outpatient clinics for chronic illnesses. Within our medical team, I receive patients in the emergency department (day and night shifts), and I travel to smaller dispensaries nearby, especially to the village of Akulivik. So, it’s quite a varied practice.
More recently, I have been involved in remote continuing medical education projects in collaboration with specialists based in Montreal. In this context, we are increasingly trying to collaborate with doctors from other indigenous communities, such as the Grand Council of the Cree, because our practices are quite similar.
Q: What is the patient volume you see?
Dr. Prince: We see approximately 20-30 patients per day in the clinic, plus about 10 by appointment, and dozens of calls from dispensaries, in addition to patients transferred from other villages. There are four daytime doctors (one at night) and about 15 nurses stationed full-time at Puvirnituq Hospital.
Our practice relies heavily on collaboration with the nursing team, which has an expanded role — they can manage certain patients according to the treatment plan established by the doctor and prescribe treatments (eg, antibiotics for uncomplicated otitis).
Q: Access to care in these isolated regions is considered difficult. Have you observed any improvement in the situation over the past 5 years? What about new material and human resources?
Dr. Prince: For the past year, we have had a Starlink internet connection at the hospital, which facilitates telemedicine exchanges with specialists; we can now send data and medical images to Montreal to obtain expertise much more easily. Previously, everything was done by phone or with significant delays. We do not yet have a cellular network, and all records are currently in paper format.
But the challenges remain numerous. Progress is very slow. Like everywhere in the country, we are experiencing a shortage of staff, particularly an insufficient number of nurses. But the impact is even more dramatic in these isolated territories. We have had to close dispensaries on the coast due to a lack of personnel and only offer emergency services. However, patients have no other options; they cannot drive to another hospital. In Nunavik, the road network is practically nonexistent, and travel to other regions is by plane (about a 2.5-hour medical evacuation trip).
So, sometimes, patients do not seek care in time, and when we finally see them, unfortunately, the issue can be quite advanced.
Q: What are the most pressing logistical needs?
Dr. Prince: We still do not have a scanner in the Far North. This has a significant impact on mortality, especially in the case of accidents and trauma, which are very common in these regions. “Residents of Nunavik are four times more likely to suffer trauma than the rest of Quebec’s population and 40 times more likely to die from it,” as recently reported in La Presse.
There has also been much discussion about cancer mortality, with a risk for death about 70% higher following a lung cancer diagnosis (reported by Medscape Medical News). We do not have a mammogram machine to diagnose breast cancer. Before the COVID-19 pandemic, equipped diagnostic teams sometimes traveled to the region, but this is no longer the case. Today, a patient needing a mammogram will have to travel to Montreal. The same goes for colonoscopies, but visits are becoming less frequent. Therefore, campaigns to screen for certain common types of cancer are practically nonexistent.
As for urgent surgeries (appendicitis, cesarean sections, trauma, etc.), patients must be transferred to Montreal by medical evacuation. We have a visiting surgeon twice a year.
Q: What improvement strategies do you foresee despite the lack of resources?
Dr. Prince: The saying “prevention is better than cure” makes perfect sense in such remote regions under extreme conditions (it is impossible to fly a medevac when it is too windy or during a snowstorm!). That’s why my colleagues and I believe that prevention should be the top priority in terms of healthcare intervention. It may seem obvious, but nothing is simple in the Far North.
Q: In which areas should prevention campaigns be prioritized in your opinion?
Dr. Prince: An example is wearing helmets. Practically no one wears this type of protection in the Far North. They use all-terrain vehicles that are dangerous and for which helmet use is crucial. But they are simply not available in stores. So, communication is difficult: We tell people, “you need a helmet for the ATV, another for the bike, for the snowmobile, for playing hockey, etc.” when it is difficult to obtain one. With traumatologists in Montreal, we had a project to create multifunctional helmets for children — to protect them but also to develop a culture of helmet use, which is not common practice in the community — but these are projects that take a lot of time and are more complex than they seem.
Villages still do not have running water. Therefore, it is difficult to give recommendations to patients as they live in sanitary conditions that are unseen elsewhere in Canada. Without clean water, we cannot ensure that wound care is done properly. Not to mention the occurrence of hepatitis A epidemics, like the one we had to face.
Residents also grapple with significant alcohol and smoking problems, but there is no detox center or dedicated psychological help on site. To follow a detox program, patients would have to leave, move away from their families, and that can be psychologically very destabilizing. I try, in my practice, to talk to my patients about this, especially pregnant women — because many continue to smoke or drink during their pregnancy — but we need more resources.
Q: What about women’s health in this region?
Dr. Prince: We are fortunate to have a team of midwives, several of whom are Inuit, who are of great help in accessing contraception, performing cervical cancer screening tests, etc. But some patients with high-risk pregnancies who should be transferred to Montreal refuse to give birth away from their families. Again, if we had the means to allow high-risk women — or those for whom continuous monitoring or a cesarean section may be necessary — to give birth here safely, it would be a big step. As for abortion, it is feasible but remains a very taboo subject in the community.
Regarding violence against women, I have not observed any particularly encouraging developments in the past 5 years, but recently, we met with the mayor about this, hoping that concrete actions will be taken to help victims of violence.
Q: What is the predominant feeling in your daily life in a situation that is slow to evolve?
Dr. Prince: I remain hopeful for my patients. We must continue to fight! Initiatives must also come from the communities themselves; they must be involved in developing solutions. Because patients, too, need to have hope. They have the right to be cared for like other inhabitants of Canada.
On my part, I try to find a balance between feeling good about my caregiving profession and not burning out professionally. But burnout is a subject that concerns many doctors around the world and is increasingly being discussed. We should all have psychological support when entering medicine!
Q: Would you recommend colleagues to come and work in the Far North? What would you tell them?
Dr. Prince: I would tell them they will have no regrets! Yes, it’s difficult, but it’s a unique type of practice and very rewarding on a human level.
Professionally, it is a general practice that is no longer seen in the city today. The spectrum is very broad, ranging from neonatology to geriatrics, from the simplest to the most complex. It’s very stimulating. Diagnostically, practice is also very different from what is done in the metropolis. Without a scanner, you really have to question and investigate to evaluate whether a patient should be evacuated by plane to Montreal or not. It’s not trivial. Decisions must be made judiciously and quickly.
The human experience is also unique. Inuit communities are little known, and the aspects relayed in the media are often negative due to their increased risk for addiction. However, they are cheerful and very warm people, with an extraordinary culture. I have learned a lot from them, including reconsidering the notion of time, reviewing my priorities, and approaching life one day at a time.
I am very grateful to them for accepting me. I am sometimes even greeted with a “Welcome home!” when I return from vacation...Being told that in Nunavik, I am also “at home,” touches me immensely. I have seen children grow up, adolescents become adults. A bond of trust has developed.
Of course, all of this comes with sacrifices like being away from family and loved ones. We miss birthdays, weddings, etc. But without hesitation, it’s worth it!
Q: What are the next steps in your career in Nunavik? Will you stay for a long time?
Dr. Prince: I take it one day at a time, especially since I am about to take maternity leave very soon. But if a full-time return to Nunavik is difficult with a newborn, I know that the Nunavummiuts [inhabitants of Nunavik] will always be part of my life and my practice.
I want to remain involved with these communities, whether on-site (by practicing there a few months a year) or in Montreal where many patients are transferred. Coming to be treated in a big city (Montreal, 1.7M inhabitants), in very large hospitals, can be very stressful for them. They express themselves much less verbally than Westerners, so we must know how to listen to them, dedicate the necessary time to them, consider their culture and beliefs. I would like to be the familiar face they will encounter when they are cared for away from home. It’s a bond I want to preserve.
This story was translated from Medscape France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In 2019, we interviewed Andrea Prince, MD, who was completing her internship in the Inuit village of Puvirnituq, a town of 2000 inhabitants located in Nunavik, in the Canadian Far North. Five years later, still in her position, what perspective does she have on her practice? Have the challenges of practicing medicine in a remote region within the Inuit community affected her vocation? Would she recommend this experience to young doctors?
Question: What position do you currently hold?
Dr. Prince: I am a full-time general practitioner at Puvirnituq Hospital. My responsibilities range from following up on hospitalized patients to those seen in outpatient clinics for chronic illnesses. Within our medical team, I receive patients in the emergency department (day and night shifts), and I travel to smaller dispensaries nearby, especially to the village of Akulivik. So, it’s quite a varied practice.
More recently, I have been involved in remote continuing medical education projects in collaboration with specialists based in Montreal. In this context, we are increasingly trying to collaborate with doctors from other indigenous communities, such as the Grand Council of the Cree, because our practices are quite similar.
Q: What is the patient volume you see?
Dr. Prince: We see approximately 20-30 patients per day in the clinic, plus about 10 by appointment, and dozens of calls from dispensaries, in addition to patients transferred from other villages. There are four daytime doctors (one at night) and about 15 nurses stationed full-time at Puvirnituq Hospital.
Our practice relies heavily on collaboration with the nursing team, which has an expanded role — they can manage certain patients according to the treatment plan established by the doctor and prescribe treatments (eg, antibiotics for uncomplicated otitis).
Q: Access to care in these isolated regions is considered difficult. Have you observed any improvement in the situation over the past 5 years? What about new material and human resources?
Dr. Prince: For the past year, we have had a Starlink internet connection at the hospital, which facilitates telemedicine exchanges with specialists; we can now send data and medical images to Montreal to obtain expertise much more easily. Previously, everything was done by phone or with significant delays. We do not yet have a cellular network, and all records are currently in paper format.
But the challenges remain numerous. Progress is very slow. Like everywhere in the country, we are experiencing a shortage of staff, particularly an insufficient number of nurses. But the impact is even more dramatic in these isolated territories. We have had to close dispensaries on the coast due to a lack of personnel and only offer emergency services. However, patients have no other options; they cannot drive to another hospital. In Nunavik, the road network is practically nonexistent, and travel to other regions is by plane (about a 2.5-hour medical evacuation trip).
So, sometimes, patients do not seek care in time, and when we finally see them, unfortunately, the issue can be quite advanced.
Q: What are the most pressing logistical needs?
Dr. Prince: We still do not have a scanner in the Far North. This has a significant impact on mortality, especially in the case of accidents and trauma, which are very common in these regions. “Residents of Nunavik are four times more likely to suffer trauma than the rest of Quebec’s population and 40 times more likely to die from it,” as recently reported in La Presse.
There has also been much discussion about cancer mortality, with a risk for death about 70% higher following a lung cancer diagnosis (reported by Medscape Medical News). We do not have a mammogram machine to diagnose breast cancer. Before the COVID-19 pandemic, equipped diagnostic teams sometimes traveled to the region, but this is no longer the case. Today, a patient needing a mammogram will have to travel to Montreal. The same goes for colonoscopies, but visits are becoming less frequent. Therefore, campaigns to screen for certain common types of cancer are practically nonexistent.
As for urgent surgeries (appendicitis, cesarean sections, trauma, etc.), patients must be transferred to Montreal by medical evacuation. We have a visiting surgeon twice a year.
Q: What improvement strategies do you foresee despite the lack of resources?
Dr. Prince: The saying “prevention is better than cure” makes perfect sense in such remote regions under extreme conditions (it is impossible to fly a medevac when it is too windy or during a snowstorm!). That’s why my colleagues and I believe that prevention should be the top priority in terms of healthcare intervention. It may seem obvious, but nothing is simple in the Far North.
Q: In which areas should prevention campaigns be prioritized in your opinion?
Dr. Prince: An example is wearing helmets. Practically no one wears this type of protection in the Far North. They use all-terrain vehicles that are dangerous and for which helmet use is crucial. But they are simply not available in stores. So, communication is difficult: We tell people, “you need a helmet for the ATV, another for the bike, for the snowmobile, for playing hockey, etc.” when it is difficult to obtain one. With traumatologists in Montreal, we had a project to create multifunctional helmets for children — to protect them but also to develop a culture of helmet use, which is not common practice in the community — but these are projects that take a lot of time and are more complex than they seem.
Villages still do not have running water. Therefore, it is difficult to give recommendations to patients as they live in sanitary conditions that are unseen elsewhere in Canada. Without clean water, we cannot ensure that wound care is done properly. Not to mention the occurrence of hepatitis A epidemics, like the one we had to face.
Residents also grapple with significant alcohol and smoking problems, but there is no detox center or dedicated psychological help on site. To follow a detox program, patients would have to leave, move away from their families, and that can be psychologically very destabilizing. I try, in my practice, to talk to my patients about this, especially pregnant women — because many continue to smoke or drink during their pregnancy — but we need more resources.
Q: What about women’s health in this region?
Dr. Prince: We are fortunate to have a team of midwives, several of whom are Inuit, who are of great help in accessing contraception, performing cervical cancer screening tests, etc. But some patients with high-risk pregnancies who should be transferred to Montreal refuse to give birth away from their families. Again, if we had the means to allow high-risk women — or those for whom continuous monitoring or a cesarean section may be necessary — to give birth here safely, it would be a big step. As for abortion, it is feasible but remains a very taboo subject in the community.
Regarding violence against women, I have not observed any particularly encouraging developments in the past 5 years, but recently, we met with the mayor about this, hoping that concrete actions will be taken to help victims of violence.
Q: What is the predominant feeling in your daily life in a situation that is slow to evolve?
Dr. Prince: I remain hopeful for my patients. We must continue to fight! Initiatives must also come from the communities themselves; they must be involved in developing solutions. Because patients, too, need to have hope. They have the right to be cared for like other inhabitants of Canada.
On my part, I try to find a balance between feeling good about my caregiving profession and not burning out professionally. But burnout is a subject that concerns many doctors around the world and is increasingly being discussed. We should all have psychological support when entering medicine!
Q: Would you recommend colleagues to come and work in the Far North? What would you tell them?
Dr. Prince: I would tell them they will have no regrets! Yes, it’s difficult, but it’s a unique type of practice and very rewarding on a human level.
Professionally, it is a general practice that is no longer seen in the city today. The spectrum is very broad, ranging from neonatology to geriatrics, from the simplest to the most complex. It’s very stimulating. Diagnostically, practice is also very different from what is done in the metropolis. Without a scanner, you really have to question and investigate to evaluate whether a patient should be evacuated by plane to Montreal or not. It’s not trivial. Decisions must be made judiciously and quickly.
The human experience is also unique. Inuit communities are little known, and the aspects relayed in the media are often negative due to their increased risk for addiction. However, they are cheerful and very warm people, with an extraordinary culture. I have learned a lot from them, including reconsidering the notion of time, reviewing my priorities, and approaching life one day at a time.
I am very grateful to them for accepting me. I am sometimes even greeted with a “Welcome home!” when I return from vacation...Being told that in Nunavik, I am also “at home,” touches me immensely. I have seen children grow up, adolescents become adults. A bond of trust has developed.
Of course, all of this comes with sacrifices like being away from family and loved ones. We miss birthdays, weddings, etc. But without hesitation, it’s worth it!
Q: What are the next steps in your career in Nunavik? Will you stay for a long time?
Dr. Prince: I take it one day at a time, especially since I am about to take maternity leave very soon. But if a full-time return to Nunavik is difficult with a newborn, I know that the Nunavummiuts [inhabitants of Nunavik] will always be part of my life and my practice.
I want to remain involved with these communities, whether on-site (by practicing there a few months a year) or in Montreal where many patients are transferred. Coming to be treated in a big city (Montreal, 1.7M inhabitants), in very large hospitals, can be very stressful for them. They express themselves much less verbally than Westerners, so we must know how to listen to them, dedicate the necessary time to them, consider their culture and beliefs. I would like to be the familiar face they will encounter when they are cared for away from home. It’s a bond I want to preserve.
This story was translated from Medscape France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In 2019, we interviewed Andrea Prince, MD, who was completing her internship in the Inuit village of Puvirnituq, a town of 2000 inhabitants located in Nunavik, in the Canadian Far North. Five years later, still in her position, what perspective does she have on her practice? Have the challenges of practicing medicine in a remote region within the Inuit community affected her vocation? Would she recommend this experience to young doctors?
Question: What position do you currently hold?
Dr. Prince: I am a full-time general practitioner at Puvirnituq Hospital. My responsibilities range from following up on hospitalized patients to those seen in outpatient clinics for chronic illnesses. Within our medical team, I receive patients in the emergency department (day and night shifts), and I travel to smaller dispensaries nearby, especially to the village of Akulivik. So, it’s quite a varied practice.
More recently, I have been involved in remote continuing medical education projects in collaboration with specialists based in Montreal. In this context, we are increasingly trying to collaborate with doctors from other indigenous communities, such as the Grand Council of the Cree, because our practices are quite similar.
Q: What is the patient volume you see?
Dr. Prince: We see approximately 20-30 patients per day in the clinic, plus about 10 by appointment, and dozens of calls from dispensaries, in addition to patients transferred from other villages. There are four daytime doctors (one at night) and about 15 nurses stationed full-time at Puvirnituq Hospital.
Our practice relies heavily on collaboration with the nursing team, which has an expanded role — they can manage certain patients according to the treatment plan established by the doctor and prescribe treatments (eg, antibiotics for uncomplicated otitis).
Q: Access to care in these isolated regions is considered difficult. Have you observed any improvement in the situation over the past 5 years? What about new material and human resources?
Dr. Prince: For the past year, we have had a Starlink internet connection at the hospital, which facilitates telemedicine exchanges with specialists; we can now send data and medical images to Montreal to obtain expertise much more easily. Previously, everything was done by phone or with significant delays. We do not yet have a cellular network, and all records are currently in paper format.
But the challenges remain numerous. Progress is very slow. Like everywhere in the country, we are experiencing a shortage of staff, particularly an insufficient number of nurses. But the impact is even more dramatic in these isolated territories. We have had to close dispensaries on the coast due to a lack of personnel and only offer emergency services. However, patients have no other options; they cannot drive to another hospital. In Nunavik, the road network is practically nonexistent, and travel to other regions is by plane (about a 2.5-hour medical evacuation trip).
So, sometimes, patients do not seek care in time, and when we finally see them, unfortunately, the issue can be quite advanced.
Q: What are the most pressing logistical needs?
Dr. Prince: We still do not have a scanner in the Far North. This has a significant impact on mortality, especially in the case of accidents and trauma, which are very common in these regions. “Residents of Nunavik are four times more likely to suffer trauma than the rest of Quebec’s population and 40 times more likely to die from it,” as recently reported in La Presse.
There has also been much discussion about cancer mortality, with a risk for death about 70% higher following a lung cancer diagnosis (reported by Medscape Medical News). We do not have a mammogram machine to diagnose breast cancer. Before the COVID-19 pandemic, equipped diagnostic teams sometimes traveled to the region, but this is no longer the case. Today, a patient needing a mammogram will have to travel to Montreal. The same goes for colonoscopies, but visits are becoming less frequent. Therefore, campaigns to screen for certain common types of cancer are practically nonexistent.
As for urgent surgeries (appendicitis, cesarean sections, trauma, etc.), patients must be transferred to Montreal by medical evacuation. We have a visiting surgeon twice a year.
Q: What improvement strategies do you foresee despite the lack of resources?
Dr. Prince: The saying “prevention is better than cure” makes perfect sense in such remote regions under extreme conditions (it is impossible to fly a medevac when it is too windy or during a snowstorm!). That’s why my colleagues and I believe that prevention should be the top priority in terms of healthcare intervention. It may seem obvious, but nothing is simple in the Far North.
Q: In which areas should prevention campaigns be prioritized in your opinion?
Dr. Prince: An example is wearing helmets. Practically no one wears this type of protection in the Far North. They use all-terrain vehicles that are dangerous and for which helmet use is crucial. But they are simply not available in stores. So, communication is difficult: We tell people, “you need a helmet for the ATV, another for the bike, for the snowmobile, for playing hockey, etc.” when it is difficult to obtain one. With traumatologists in Montreal, we had a project to create multifunctional helmets for children — to protect them but also to develop a culture of helmet use, which is not common practice in the community — but these are projects that take a lot of time and are more complex than they seem.
Villages still do not have running water. Therefore, it is difficult to give recommendations to patients as they live in sanitary conditions that are unseen elsewhere in Canada. Without clean water, we cannot ensure that wound care is done properly. Not to mention the occurrence of hepatitis A epidemics, like the one we had to face.
Residents also grapple with significant alcohol and smoking problems, but there is no detox center or dedicated psychological help on site. To follow a detox program, patients would have to leave, move away from their families, and that can be psychologically very destabilizing. I try, in my practice, to talk to my patients about this, especially pregnant women — because many continue to smoke or drink during their pregnancy — but we need more resources.
Q: What about women’s health in this region?
Dr. Prince: We are fortunate to have a team of midwives, several of whom are Inuit, who are of great help in accessing contraception, performing cervical cancer screening tests, etc. But some patients with high-risk pregnancies who should be transferred to Montreal refuse to give birth away from their families. Again, if we had the means to allow high-risk women — or those for whom continuous monitoring or a cesarean section may be necessary — to give birth here safely, it would be a big step. As for abortion, it is feasible but remains a very taboo subject in the community.
Regarding violence against women, I have not observed any particularly encouraging developments in the past 5 years, but recently, we met with the mayor about this, hoping that concrete actions will be taken to help victims of violence.
Q: What is the predominant feeling in your daily life in a situation that is slow to evolve?
Dr. Prince: I remain hopeful for my patients. We must continue to fight! Initiatives must also come from the communities themselves; they must be involved in developing solutions. Because patients, too, need to have hope. They have the right to be cared for like other inhabitants of Canada.
On my part, I try to find a balance between feeling good about my caregiving profession and not burning out professionally. But burnout is a subject that concerns many doctors around the world and is increasingly being discussed. We should all have psychological support when entering medicine!
Q: Would you recommend colleagues to come and work in the Far North? What would you tell them?
Dr. Prince: I would tell them they will have no regrets! Yes, it’s difficult, but it’s a unique type of practice and very rewarding on a human level.
Professionally, it is a general practice that is no longer seen in the city today. The spectrum is very broad, ranging from neonatology to geriatrics, from the simplest to the most complex. It’s very stimulating. Diagnostically, practice is also very different from what is done in the metropolis. Without a scanner, you really have to question and investigate to evaluate whether a patient should be evacuated by plane to Montreal or not. It’s not trivial. Decisions must be made judiciously and quickly.
The human experience is also unique. Inuit communities are little known, and the aspects relayed in the media are often negative due to their increased risk for addiction. However, they are cheerful and very warm people, with an extraordinary culture. I have learned a lot from them, including reconsidering the notion of time, reviewing my priorities, and approaching life one day at a time.
I am very grateful to them for accepting me. I am sometimes even greeted with a “Welcome home!” when I return from vacation...Being told that in Nunavik, I am also “at home,” touches me immensely. I have seen children grow up, adolescents become adults. A bond of trust has developed.
Of course, all of this comes with sacrifices like being away from family and loved ones. We miss birthdays, weddings, etc. But without hesitation, it’s worth it!
Q: What are the next steps in your career in Nunavik? Will you stay for a long time?
Dr. Prince: I take it one day at a time, especially since I am about to take maternity leave very soon. But if a full-time return to Nunavik is difficult with a newborn, I know that the Nunavummiuts [inhabitants of Nunavik] will always be part of my life and my practice.
I want to remain involved with these communities, whether on-site (by practicing there a few months a year) or in Montreal where many patients are transferred. Coming to be treated in a big city (Montreal, 1.7M inhabitants), in very large hospitals, can be very stressful for them. They express themselves much less verbally than Westerners, so we must know how to listen to them, dedicate the necessary time to them, consider their culture and beliefs. I would like to be the familiar face they will encounter when they are cared for away from home. It’s a bond I want to preserve.
This story was translated from Medscape France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
OTC Birth Control Pill Headed to US Pharmacies: What Your Patients Should Know
Primary care clinicians have largely welcomed the arrival of Opill, the first over-the-counter (OTC) birth control pill from Perrigo, which will reach US pharmacy shelves this month. Although the medicine has a long-track record of safe use, physicians and nurse practitioners may want to ready themselves to answer questions from patients about shifting to the option.
The switch to OTC status for the norgestrel-only contraceptive has the support of many physician groups, including the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG).
The end of the prescription-requirement removes a barrier to access for many women, especially those who lack insurance. But it also will take away a chief reason many women in their childbearing years make appointments with doctors, as they will no longer need prescriptions for birth control pills.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology at University of Washington School of Medicine, in Seattle, Washington, said she is also worried that the availability of an OTC pill will lead to missed opportunities to help patients avoid sexually transmitted diseases. For example, patients can get counseling about the need for testing for sexually transmitted diseases at the start of new relationships during a visit made to obtain a prescription for the pill.
“My hope is that they still follow our recommendations, which is to get tested with every partner,” said Dr. Oelschlager, who cares for many patients in their teens. “Adolescents are at a particularly high risk of infection compared to older ones.”
When clinicians do see patients, they may want to raise the issue of the OTC option and proper use. Patients will need to closely read materials provided for Opill, a step they might skip due to the ready access, according to Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, which scrutinizes the safety of medical products.
“When something is sold over the counter, it’s perceived by individuals as being safe,” Dr. Zuckerman told this news organization. “There’s less concern and a little less interest in reading the instructions and reading the warnings.”
Considerations for Safety
The US Food and Drug Administration (FDA) in July approved the sales of a daily 0.075 mg norgestrel tablet without prescription. Perrigo told this news organization that it spent the intervening months ensuring retailers and consumers will receive education on the drug.
One of the biggest challenges for people using Opill may be sticking with the dosing schedule, according to Dr. Oelschlager.
“There are going to be people that have a harder time remembering to take a pill every day,” at the same time, said Dr. Oelschlager, who is chair of ACOG’s Clinical Consensus Gynecology Committee. “We need to watch and see what happens as it becomes more widely available, and people start using it.”
Unexpected vaginal bleeding is the most common adverse event linked to this form of birth control, with over one fifth of participants from one study of the OTC drug reporting this side effect, according to an FDA memo.
“It is more likely to be a tolerability issue rather than a safety issue,” the FDA wrote.
Many prescription of birth control options contain estrogen, which is associated with venous thromboembolism (VTE). But Opill contains only norgestrel, a form of progestin, which is not associated with thrombosis. Patients may be more likely to overestimate their potential risks for VTE than to underestimate them, according to Kwuan Paruchabutr, DNP, president of National Association of Nurse Practitioners in Women’s Health and an assistant professor at Georgetown University in Washington, DC.
“This is a progesterone-only pill: The risk is relatively low” of VTE, Dr. Paruchabutr said.
Clinicians should also take special care with patients who are prescribed drugs for seizures, tuberculosis, HIV/AIDS, and pulmonary hypertension or who are taking supplements containing St John’s wort.
Patients in their childbearing years who take isotretinoin are already expected to use some form of birth control.
“All patients on isotretinoin must be registered in the iPLEDGE program, which mandates monthly contraception counseling and monthly pregnancy tests for persons of childbearing potential,” Terrence A. Cronin, Jr, MD, president of the American Academy of Dermatology, told news organization through email.
Dr. Oelschlager noted that many patients who take isotretinoin may benefit from taking a birth control pill containing estrogen, for which they will need a prescription. At least three pills have an FDA-approved indication for treating moderate acne, including Ortho Tri-Cyclen, Estrostep, and Yaz.
The FDA has posted consumer-friendly information about the OTC pill that clinicians can refer their patients to. For clinicians who want more information, ACOG released a practice advisory about the switch in status for this progestin-only pill.
The Cost
While federal laws mandate employer-based and Medicaid plans cover prescription birth control pills for free, the OTC version will carry a cost, according to A. Mark Fendrick, MD, director of the University of Michigan Center for Value-Based Insurance Design in Ann Arbor, Michigan.
Seven states, including New Mexico and New York, already have laws in effect that require health plans to cover certain OTC contraceptives without a prescription, according to a tally kept by the nonprofit research organization KFF.
Dr. Fendrick said it would be helpful for health plans to offer coverage for the OTC pill without copays even if they are not required to do so.
Priced at about $20 a month, Opill “is likely out of reach for many of the individuals who would most benefit from an OTC option,” Dr. Fendrick told this news organization in an email.
The new pill may be utilized most by those who do not have health insurance or have low incomes and cannot afford to see a doctor for a prescription, according to Sally Rafie, PharmD, a pharmacist specialist at University of California San Diego Health and founder of the Birth Control Pharmacist.
The manufacturer’s suggested retail prices will be $19.99 for a 1-month supply and $49.99 for a 3-month supply. Dublin-based Perrigo said it plans to offer a cost-assistance program for the drug in the coming weeks for people who have low incomes and lack insurance.
A version of this article appeared on Medscape.com.
Primary care clinicians have largely welcomed the arrival of Opill, the first over-the-counter (OTC) birth control pill from Perrigo, which will reach US pharmacy shelves this month. Although the medicine has a long-track record of safe use, physicians and nurse practitioners may want to ready themselves to answer questions from patients about shifting to the option.
The switch to OTC status for the norgestrel-only contraceptive has the support of many physician groups, including the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG).
The end of the prescription-requirement removes a barrier to access for many women, especially those who lack insurance. But it also will take away a chief reason many women in their childbearing years make appointments with doctors, as they will no longer need prescriptions for birth control pills.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology at University of Washington School of Medicine, in Seattle, Washington, said she is also worried that the availability of an OTC pill will lead to missed opportunities to help patients avoid sexually transmitted diseases. For example, patients can get counseling about the need for testing for sexually transmitted diseases at the start of new relationships during a visit made to obtain a prescription for the pill.
“My hope is that they still follow our recommendations, which is to get tested with every partner,” said Dr. Oelschlager, who cares for many patients in their teens. “Adolescents are at a particularly high risk of infection compared to older ones.”
When clinicians do see patients, they may want to raise the issue of the OTC option and proper use. Patients will need to closely read materials provided for Opill, a step they might skip due to the ready access, according to Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, which scrutinizes the safety of medical products.
“When something is sold over the counter, it’s perceived by individuals as being safe,” Dr. Zuckerman told this news organization. “There’s less concern and a little less interest in reading the instructions and reading the warnings.”
Considerations for Safety
The US Food and Drug Administration (FDA) in July approved the sales of a daily 0.075 mg norgestrel tablet without prescription. Perrigo told this news organization that it spent the intervening months ensuring retailers and consumers will receive education on the drug.
One of the biggest challenges for people using Opill may be sticking with the dosing schedule, according to Dr. Oelschlager.
“There are going to be people that have a harder time remembering to take a pill every day,” at the same time, said Dr. Oelschlager, who is chair of ACOG’s Clinical Consensus Gynecology Committee. “We need to watch and see what happens as it becomes more widely available, and people start using it.”
Unexpected vaginal bleeding is the most common adverse event linked to this form of birth control, with over one fifth of participants from one study of the OTC drug reporting this side effect, according to an FDA memo.
“It is more likely to be a tolerability issue rather than a safety issue,” the FDA wrote.
Many prescription of birth control options contain estrogen, which is associated with venous thromboembolism (VTE). But Opill contains only norgestrel, a form of progestin, which is not associated with thrombosis. Patients may be more likely to overestimate their potential risks for VTE than to underestimate them, according to Kwuan Paruchabutr, DNP, president of National Association of Nurse Practitioners in Women’s Health and an assistant professor at Georgetown University in Washington, DC.
“This is a progesterone-only pill: The risk is relatively low” of VTE, Dr. Paruchabutr said.
Clinicians should also take special care with patients who are prescribed drugs for seizures, tuberculosis, HIV/AIDS, and pulmonary hypertension or who are taking supplements containing St John’s wort.
Patients in their childbearing years who take isotretinoin are already expected to use some form of birth control.
“All patients on isotretinoin must be registered in the iPLEDGE program, which mandates monthly contraception counseling and monthly pregnancy tests for persons of childbearing potential,” Terrence A. Cronin, Jr, MD, president of the American Academy of Dermatology, told news organization through email.
Dr. Oelschlager noted that many patients who take isotretinoin may benefit from taking a birth control pill containing estrogen, for which they will need a prescription. At least three pills have an FDA-approved indication for treating moderate acne, including Ortho Tri-Cyclen, Estrostep, and Yaz.
The FDA has posted consumer-friendly information about the OTC pill that clinicians can refer their patients to. For clinicians who want more information, ACOG released a practice advisory about the switch in status for this progestin-only pill.
The Cost
While federal laws mandate employer-based and Medicaid plans cover prescription birth control pills for free, the OTC version will carry a cost, according to A. Mark Fendrick, MD, director of the University of Michigan Center for Value-Based Insurance Design in Ann Arbor, Michigan.
Seven states, including New Mexico and New York, already have laws in effect that require health plans to cover certain OTC contraceptives without a prescription, according to a tally kept by the nonprofit research organization KFF.
Dr. Fendrick said it would be helpful for health plans to offer coverage for the OTC pill without copays even if they are not required to do so.
Priced at about $20 a month, Opill “is likely out of reach for many of the individuals who would most benefit from an OTC option,” Dr. Fendrick told this news organization in an email.
The new pill may be utilized most by those who do not have health insurance or have low incomes and cannot afford to see a doctor for a prescription, according to Sally Rafie, PharmD, a pharmacist specialist at University of California San Diego Health and founder of the Birth Control Pharmacist.
The manufacturer’s suggested retail prices will be $19.99 for a 1-month supply and $49.99 for a 3-month supply. Dublin-based Perrigo said it plans to offer a cost-assistance program for the drug in the coming weeks for people who have low incomes and lack insurance.
A version of this article appeared on Medscape.com.
Primary care clinicians have largely welcomed the arrival of Opill, the first over-the-counter (OTC) birth control pill from Perrigo, which will reach US pharmacy shelves this month. Although the medicine has a long-track record of safe use, physicians and nurse practitioners may want to ready themselves to answer questions from patients about shifting to the option.
The switch to OTC status for the norgestrel-only contraceptive has the support of many physician groups, including the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG).
The end of the prescription-requirement removes a barrier to access for many women, especially those who lack insurance. But it also will take away a chief reason many women in their childbearing years make appointments with doctors, as they will no longer need prescriptions for birth control pills.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology at University of Washington School of Medicine, in Seattle, Washington, said she is also worried that the availability of an OTC pill will lead to missed opportunities to help patients avoid sexually transmitted diseases. For example, patients can get counseling about the need for testing for sexually transmitted diseases at the start of new relationships during a visit made to obtain a prescription for the pill.
“My hope is that they still follow our recommendations, which is to get tested with every partner,” said Dr. Oelschlager, who cares for many patients in their teens. “Adolescents are at a particularly high risk of infection compared to older ones.”
When clinicians do see patients, they may want to raise the issue of the OTC option and proper use. Patients will need to closely read materials provided for Opill, a step they might skip due to the ready access, according to Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, which scrutinizes the safety of medical products.
“When something is sold over the counter, it’s perceived by individuals as being safe,” Dr. Zuckerman told this news organization. “There’s less concern and a little less interest in reading the instructions and reading the warnings.”
Considerations for Safety
The US Food and Drug Administration (FDA) in July approved the sales of a daily 0.075 mg norgestrel tablet without prescription. Perrigo told this news organization that it spent the intervening months ensuring retailers and consumers will receive education on the drug.
One of the biggest challenges for people using Opill may be sticking with the dosing schedule, according to Dr. Oelschlager.
“There are going to be people that have a harder time remembering to take a pill every day,” at the same time, said Dr. Oelschlager, who is chair of ACOG’s Clinical Consensus Gynecology Committee. “We need to watch and see what happens as it becomes more widely available, and people start using it.”
Unexpected vaginal bleeding is the most common adverse event linked to this form of birth control, with over one fifth of participants from one study of the OTC drug reporting this side effect, according to an FDA memo.
“It is more likely to be a tolerability issue rather than a safety issue,” the FDA wrote.
Many prescription of birth control options contain estrogen, which is associated with venous thromboembolism (VTE). But Opill contains only norgestrel, a form of progestin, which is not associated with thrombosis. Patients may be more likely to overestimate their potential risks for VTE than to underestimate them, according to Kwuan Paruchabutr, DNP, president of National Association of Nurse Practitioners in Women’s Health and an assistant professor at Georgetown University in Washington, DC.
“This is a progesterone-only pill: The risk is relatively low” of VTE, Dr. Paruchabutr said.
Clinicians should also take special care with patients who are prescribed drugs for seizures, tuberculosis, HIV/AIDS, and pulmonary hypertension or who are taking supplements containing St John’s wort.
Patients in their childbearing years who take isotretinoin are already expected to use some form of birth control.
“All patients on isotretinoin must be registered in the iPLEDGE program, which mandates monthly contraception counseling and monthly pregnancy tests for persons of childbearing potential,” Terrence A. Cronin, Jr, MD, president of the American Academy of Dermatology, told news organization through email.
Dr. Oelschlager noted that many patients who take isotretinoin may benefit from taking a birth control pill containing estrogen, for which they will need a prescription. At least three pills have an FDA-approved indication for treating moderate acne, including Ortho Tri-Cyclen, Estrostep, and Yaz.
The FDA has posted consumer-friendly information about the OTC pill that clinicians can refer their patients to. For clinicians who want more information, ACOG released a practice advisory about the switch in status for this progestin-only pill.
The Cost
While federal laws mandate employer-based and Medicaid plans cover prescription birth control pills for free, the OTC version will carry a cost, according to A. Mark Fendrick, MD, director of the University of Michigan Center for Value-Based Insurance Design in Ann Arbor, Michigan.
Seven states, including New Mexico and New York, already have laws in effect that require health plans to cover certain OTC contraceptives without a prescription, according to a tally kept by the nonprofit research organization KFF.
Dr. Fendrick said it would be helpful for health plans to offer coverage for the OTC pill without copays even if they are not required to do so.
Priced at about $20 a month, Opill “is likely out of reach for many of the individuals who would most benefit from an OTC option,” Dr. Fendrick told this news organization in an email.
The new pill may be utilized most by those who do not have health insurance or have low incomes and cannot afford to see a doctor for a prescription, according to Sally Rafie, PharmD, a pharmacist specialist at University of California San Diego Health and founder of the Birth Control Pharmacist.
The manufacturer’s suggested retail prices will be $19.99 for a 1-month supply and $49.99 for a 3-month supply. Dublin-based Perrigo said it plans to offer a cost-assistance program for the drug in the coming weeks for people who have low incomes and lack insurance.
A version of this article appeared on Medscape.com.
Inexperience Diagnosing Syphilis Adding to Higher Rates
With rates of syphilis rising quickly in the United States and elsewhere, clinicians are having to up their game when it comes to diagnosing and treating an infection that they may not be paying enough attention to.
More than 200,000 cases of syphilis were reported in the United States in 2022, which is the highest number since 1950 and is a 17.3% increase over 2021, according to the latest figures from the Centers for Disease Control and Prevention (CDC). The rate of infection has increased almost every year since a historic low in 2001.
And the trend is not limited to the United States. Last year, the infection rate in the United Kingdom hit a 50-year high, said David Mabey, BCh, DM, from the London School of Hygiene and Tropical Medicine. Syphilis and other sexually transmitted infections are also a major problem in low- and middle-income countries, he added, although good data are not always available.
Many of today’s healthcare professionals have little experience with the disease, shared Ina Park, MD, a sexually transmitted infections specialist at the University of California at San Francisco. “An entire generation of physicians — including myself — did not see any cases until we were well out of our training,” Dr. Park reported. “We’re really playing catch-up.”
A Centuries-Old Ailment
Dr. Park offered some advice on the challenges of diagnosing what can be an elusive infection at the Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver. That advice boiled down to one simple rule: “Test, test, test.”
Because syphilis can mimic so many other conditions and can have long periods of latency, it can be easily missed or even misdiagnosed by experienced physicians, said Dr. Park. Clinicians need to keep it front of mind and have a lower threshold for testing, even if there are no obvious symptoms.
Following the CDC’s new recommendations for syphilis screening will help, she noted; every sexually active patient aged between 15 and 44 years who lives in a county with a syphilis infection rate of 4.6 per 100,000 people or higher should get the test. And clinicians should remain vigilant, even in areas with a lower prevalence. “If you can’t account for new symptoms in a sexually active patient, order a test,” said Dr. Park.
Complicated Cases
The lack of experience with syphilis affects not just diagnosis but also treatment, particularly for complex cases, said Khalil Ghanem, MD, PhD, from the Johns Hopkins University School of Medicine in Baltimore. “When you don’t have to deal with something for a while, you forget how to deal with it,” he added.
At CROI, Dr. Ghanem offered suggestions for how to navigate complicated cases of ocular syphilis, otic syphilis, and neurosyphilis, and how to interpret test results when a patient’s antigen titers are being “unruly.”
With potential ocular or otic syphilis, you shouldn’t wait for a specialist like an ophthalmologist to weigh in but instead refer the patient directly to the emergency department because of the risk that the symptoms may become irreversible and result in permanent blindness or deafness. “You don’t want to dilly-dally with those conditions,” Dr. Ghanem said.
Closely monitoring a patient’s rapid plasma regain and venereal disease research laboratory antigen levels is the only way to manage syphilis and to determine whether the infection is responding to treatment, he noted, but sometimes those titers “don’t do what you think they should be doing” and fail to decline or even go up after treatment.
“You don’t know if they went up because the patient was re-infected, or they developed neurosyphilis, or there was a problem at the lab,” he said. “It can be challenging to interpret.”
To decipher confusing test results, Dr. Ghanem recommended getting a detailed history to understand whether a patient is at risk for reinfection, whether there are signs of neurosyphilis or other complications, whether pregnancy is possible, and so on. “Based on the answers, you can determine what the most rational approach to treatment would be,” he shared.
Drug Shortages
Efforts to get the infection under control have become more complicated. Last summer, Pfizer announced that it had run out of penicillin G benzathine (Bicillin), an injectable, long-acting drug that is one of the main treatments for syphilis and the only one that can be given to pregnant people. Supplies for children ran out at the end of June 2023, and supplies for adults were gone by the end of September.
Because Pfizer is the only company that manufactures penicillin G benzathine, there is no one to pick up the slack in the short-term, so the shortage is expected to continue until at least the middle of 2024.
In response, the US Food and Drug Administration has temporarily allowed the use of benzylpenicillin benzathine (Extencilline), a French formulation that has not been approved in the United States, until supplies of penicillin G benzathine are stabilized.
The shortage has shone a spotlight on the important issue of a lack of alternatives for the treatment of syphilis during pregnancy, which increases the risk for congenital syphilis. “Hopefully, this pushes the National Institutes of Health and others to step up their game on studies for alternative drugs for use in pregnancy,” Dr. Ghanem said.
A version of this article appeared on Medscape.com.
With rates of syphilis rising quickly in the United States and elsewhere, clinicians are having to up their game when it comes to diagnosing and treating an infection that they may not be paying enough attention to.
More than 200,000 cases of syphilis were reported in the United States in 2022, which is the highest number since 1950 and is a 17.3% increase over 2021, according to the latest figures from the Centers for Disease Control and Prevention (CDC). The rate of infection has increased almost every year since a historic low in 2001.
And the trend is not limited to the United States. Last year, the infection rate in the United Kingdom hit a 50-year high, said David Mabey, BCh, DM, from the London School of Hygiene and Tropical Medicine. Syphilis and other sexually transmitted infections are also a major problem in low- and middle-income countries, he added, although good data are not always available.
Many of today’s healthcare professionals have little experience with the disease, shared Ina Park, MD, a sexually transmitted infections specialist at the University of California at San Francisco. “An entire generation of physicians — including myself — did not see any cases until we were well out of our training,” Dr. Park reported. “We’re really playing catch-up.”
A Centuries-Old Ailment
Dr. Park offered some advice on the challenges of diagnosing what can be an elusive infection at the Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver. That advice boiled down to one simple rule: “Test, test, test.”
Because syphilis can mimic so many other conditions and can have long periods of latency, it can be easily missed or even misdiagnosed by experienced physicians, said Dr. Park. Clinicians need to keep it front of mind and have a lower threshold for testing, even if there are no obvious symptoms.
Following the CDC’s new recommendations for syphilis screening will help, she noted; every sexually active patient aged between 15 and 44 years who lives in a county with a syphilis infection rate of 4.6 per 100,000 people or higher should get the test. And clinicians should remain vigilant, even in areas with a lower prevalence. “If you can’t account for new symptoms in a sexually active patient, order a test,” said Dr. Park.
Complicated Cases
The lack of experience with syphilis affects not just diagnosis but also treatment, particularly for complex cases, said Khalil Ghanem, MD, PhD, from the Johns Hopkins University School of Medicine in Baltimore. “When you don’t have to deal with something for a while, you forget how to deal with it,” he added.
At CROI, Dr. Ghanem offered suggestions for how to navigate complicated cases of ocular syphilis, otic syphilis, and neurosyphilis, and how to interpret test results when a patient’s antigen titers are being “unruly.”
With potential ocular or otic syphilis, you shouldn’t wait for a specialist like an ophthalmologist to weigh in but instead refer the patient directly to the emergency department because of the risk that the symptoms may become irreversible and result in permanent blindness or deafness. “You don’t want to dilly-dally with those conditions,” Dr. Ghanem said.
Closely monitoring a patient’s rapid plasma regain and venereal disease research laboratory antigen levels is the only way to manage syphilis and to determine whether the infection is responding to treatment, he noted, but sometimes those titers “don’t do what you think they should be doing” and fail to decline or even go up after treatment.
“You don’t know if they went up because the patient was re-infected, or they developed neurosyphilis, or there was a problem at the lab,” he said. “It can be challenging to interpret.”
To decipher confusing test results, Dr. Ghanem recommended getting a detailed history to understand whether a patient is at risk for reinfection, whether there are signs of neurosyphilis or other complications, whether pregnancy is possible, and so on. “Based on the answers, you can determine what the most rational approach to treatment would be,” he shared.
Drug Shortages
Efforts to get the infection under control have become more complicated. Last summer, Pfizer announced that it had run out of penicillin G benzathine (Bicillin), an injectable, long-acting drug that is one of the main treatments for syphilis and the only one that can be given to pregnant people. Supplies for children ran out at the end of June 2023, and supplies for adults were gone by the end of September.
Because Pfizer is the only company that manufactures penicillin G benzathine, there is no one to pick up the slack in the short-term, so the shortage is expected to continue until at least the middle of 2024.
In response, the US Food and Drug Administration has temporarily allowed the use of benzylpenicillin benzathine (Extencilline), a French formulation that has not been approved in the United States, until supplies of penicillin G benzathine are stabilized.
The shortage has shone a spotlight on the important issue of a lack of alternatives for the treatment of syphilis during pregnancy, which increases the risk for congenital syphilis. “Hopefully, this pushes the National Institutes of Health and others to step up their game on studies for alternative drugs for use in pregnancy,” Dr. Ghanem said.
A version of this article appeared on Medscape.com.
With rates of syphilis rising quickly in the United States and elsewhere, clinicians are having to up their game when it comes to diagnosing and treating an infection that they may not be paying enough attention to.
More than 200,000 cases of syphilis were reported in the United States in 2022, which is the highest number since 1950 and is a 17.3% increase over 2021, according to the latest figures from the Centers for Disease Control and Prevention (CDC). The rate of infection has increased almost every year since a historic low in 2001.
And the trend is not limited to the United States. Last year, the infection rate in the United Kingdom hit a 50-year high, said David Mabey, BCh, DM, from the London School of Hygiene and Tropical Medicine. Syphilis and other sexually transmitted infections are also a major problem in low- and middle-income countries, he added, although good data are not always available.
Many of today’s healthcare professionals have little experience with the disease, shared Ina Park, MD, a sexually transmitted infections specialist at the University of California at San Francisco. “An entire generation of physicians — including myself — did not see any cases until we were well out of our training,” Dr. Park reported. “We’re really playing catch-up.”
A Centuries-Old Ailment
Dr. Park offered some advice on the challenges of diagnosing what can be an elusive infection at the Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver. That advice boiled down to one simple rule: “Test, test, test.”
Because syphilis can mimic so many other conditions and can have long periods of latency, it can be easily missed or even misdiagnosed by experienced physicians, said Dr. Park. Clinicians need to keep it front of mind and have a lower threshold for testing, even if there are no obvious symptoms.
Following the CDC’s new recommendations for syphilis screening will help, she noted; every sexually active patient aged between 15 and 44 years who lives in a county with a syphilis infection rate of 4.6 per 100,000 people or higher should get the test. And clinicians should remain vigilant, even in areas with a lower prevalence. “If you can’t account for new symptoms in a sexually active patient, order a test,” said Dr. Park.
Complicated Cases
The lack of experience with syphilis affects not just diagnosis but also treatment, particularly for complex cases, said Khalil Ghanem, MD, PhD, from the Johns Hopkins University School of Medicine in Baltimore. “When you don’t have to deal with something for a while, you forget how to deal with it,” he added.
At CROI, Dr. Ghanem offered suggestions for how to navigate complicated cases of ocular syphilis, otic syphilis, and neurosyphilis, and how to interpret test results when a patient’s antigen titers are being “unruly.”
With potential ocular or otic syphilis, you shouldn’t wait for a specialist like an ophthalmologist to weigh in but instead refer the patient directly to the emergency department because of the risk that the symptoms may become irreversible and result in permanent blindness or deafness. “You don’t want to dilly-dally with those conditions,” Dr. Ghanem said.
Closely monitoring a patient’s rapid plasma regain and venereal disease research laboratory antigen levels is the only way to manage syphilis and to determine whether the infection is responding to treatment, he noted, but sometimes those titers “don’t do what you think they should be doing” and fail to decline or even go up after treatment.
“You don’t know if they went up because the patient was re-infected, or they developed neurosyphilis, or there was a problem at the lab,” he said. “It can be challenging to interpret.”
To decipher confusing test results, Dr. Ghanem recommended getting a detailed history to understand whether a patient is at risk for reinfection, whether there are signs of neurosyphilis or other complications, whether pregnancy is possible, and so on. “Based on the answers, you can determine what the most rational approach to treatment would be,” he shared.
Drug Shortages
Efforts to get the infection under control have become more complicated. Last summer, Pfizer announced that it had run out of penicillin G benzathine (Bicillin), an injectable, long-acting drug that is one of the main treatments for syphilis and the only one that can be given to pregnant people. Supplies for children ran out at the end of June 2023, and supplies for adults were gone by the end of September.
Because Pfizer is the only company that manufactures penicillin G benzathine, there is no one to pick up the slack in the short-term, so the shortage is expected to continue until at least the middle of 2024.
In response, the US Food and Drug Administration has temporarily allowed the use of benzylpenicillin benzathine (Extencilline), a French formulation that has not been approved in the United States, until supplies of penicillin G benzathine are stabilized.
The shortage has shone a spotlight on the important issue of a lack of alternatives for the treatment of syphilis during pregnancy, which increases the risk for congenital syphilis. “Hopefully, this pushes the National Institutes of Health and others to step up their game on studies for alternative drugs for use in pregnancy,” Dr. Ghanem said.
A version of this article appeared on Medscape.com.
Multimodal Treatment Found Effective for Overactive Bladder
TOPLINE:
A new study published in JAMA Network Open showed that an intervention including cognitive behavioral therapy improved the quality of life for women with overactive bladder (OAB).
METHODOLOGY:
- A total of 79 women with moderate to severe OAB were randomized to the control group or the intervention, which was composed of four 30-minute sessions using strategies including cognitive behavioral therapy (CBT).
- The first and second sessions provided education on OAB and CBT, lifestyle modifications such as limiting coffee intake, pelvic floor muscle training, and introduced exposure training.
- The third and fourth sessions continued exposure and pelvic floor muscle training and education on relapse prevention.
- Researchers assessed outcomes using the health-related quality of life (HRQOL), in which participants answered questions regarding their degree of distress, emotions, and physical and social limitations related to OAB symptoms.
TAKEAWAY:
- Participants who received the intervention on average improved in their HRQOL score by 12.6 points higher than those in the control group (usual care) from baseline to week 13 (between-group difference estimate, 12.6 [95% CI, 6.6-18.6] points; P < .001).
- The average age of participants was 63.5 years, and more than 87% of women in each group had moderate OAB.
- Patient-reported improvement and satisfaction scores were also more improved in the intervention group than in the control group; most participants in both groups had no change in the pharmacotherapy during the trial.
IN PRACTICE:
Urologists and other primary care clinicians who treat women with OAB may consider a multicomponent intervention that includes cognitive components and exposure-based bladder training or could refer to a cognitive behavioral therapist or pelvic floor physical therapist experienced in these techniques.
SOURCE:
Satoshi Funada, MD, PhD, and Takashi Kobayashi, MD, PhD, both with the Department of Urology at Kyoto University Graduate School of Medicine in Kyoto, Japan, are the corresponding authors. The study was published online in JAMA Network Open.
LIMITATIONS:
The trial was open label, and the use of a waiting list control group is known to produce greater differences between the two groups. The trial included patients both taking and not taking medication for OAB. The sample size was also relatively small, and the intervention was performed by a single clinician, possibly limiting the generalizability of results.
DISCLOSURES:
The study was funded by the Japan Society for the Promotion of Science (JSPS). Various study authors reported receiving grants from the Pfizer Health Research Foundation, AstraZeneca, and JSPS. Other study authors reported receiving personal fees from Eisai, Sawai Pharmaceutical, Statcom, and others. One author reported pending patents for intellectual properties for the Kokoro app licensed to Mitsubishi Tanabe Pharma.
A version of this article appeared on Medscape.com.
TOPLINE:
A new study published in JAMA Network Open showed that an intervention including cognitive behavioral therapy improved the quality of life for women with overactive bladder (OAB).
METHODOLOGY:
- A total of 79 women with moderate to severe OAB were randomized to the control group or the intervention, which was composed of four 30-minute sessions using strategies including cognitive behavioral therapy (CBT).
- The first and second sessions provided education on OAB and CBT, lifestyle modifications such as limiting coffee intake, pelvic floor muscle training, and introduced exposure training.
- The third and fourth sessions continued exposure and pelvic floor muscle training and education on relapse prevention.
- Researchers assessed outcomes using the health-related quality of life (HRQOL), in which participants answered questions regarding their degree of distress, emotions, and physical and social limitations related to OAB symptoms.
TAKEAWAY:
- Participants who received the intervention on average improved in their HRQOL score by 12.6 points higher than those in the control group (usual care) from baseline to week 13 (between-group difference estimate, 12.6 [95% CI, 6.6-18.6] points; P < .001).
- The average age of participants was 63.5 years, and more than 87% of women in each group had moderate OAB.
- Patient-reported improvement and satisfaction scores were also more improved in the intervention group than in the control group; most participants in both groups had no change in the pharmacotherapy during the trial.
IN PRACTICE:
Urologists and other primary care clinicians who treat women with OAB may consider a multicomponent intervention that includes cognitive components and exposure-based bladder training or could refer to a cognitive behavioral therapist or pelvic floor physical therapist experienced in these techniques.
SOURCE:
Satoshi Funada, MD, PhD, and Takashi Kobayashi, MD, PhD, both with the Department of Urology at Kyoto University Graduate School of Medicine in Kyoto, Japan, are the corresponding authors. The study was published online in JAMA Network Open.
LIMITATIONS:
The trial was open label, and the use of a waiting list control group is known to produce greater differences between the two groups. The trial included patients both taking and not taking medication for OAB. The sample size was also relatively small, and the intervention was performed by a single clinician, possibly limiting the generalizability of results.
DISCLOSURES:
The study was funded by the Japan Society for the Promotion of Science (JSPS). Various study authors reported receiving grants from the Pfizer Health Research Foundation, AstraZeneca, and JSPS. Other study authors reported receiving personal fees from Eisai, Sawai Pharmaceutical, Statcom, and others. One author reported pending patents for intellectual properties for the Kokoro app licensed to Mitsubishi Tanabe Pharma.
A version of this article appeared on Medscape.com.
TOPLINE:
A new study published in JAMA Network Open showed that an intervention including cognitive behavioral therapy improved the quality of life for women with overactive bladder (OAB).
METHODOLOGY:
- A total of 79 women with moderate to severe OAB were randomized to the control group or the intervention, which was composed of four 30-minute sessions using strategies including cognitive behavioral therapy (CBT).
- The first and second sessions provided education on OAB and CBT, lifestyle modifications such as limiting coffee intake, pelvic floor muscle training, and introduced exposure training.
- The third and fourth sessions continued exposure and pelvic floor muscle training and education on relapse prevention.
- Researchers assessed outcomes using the health-related quality of life (HRQOL), in which participants answered questions regarding their degree of distress, emotions, and physical and social limitations related to OAB symptoms.
TAKEAWAY:
- Participants who received the intervention on average improved in their HRQOL score by 12.6 points higher than those in the control group (usual care) from baseline to week 13 (between-group difference estimate, 12.6 [95% CI, 6.6-18.6] points; P < .001).
- The average age of participants was 63.5 years, and more than 87% of women in each group had moderate OAB.
- Patient-reported improvement and satisfaction scores were also more improved in the intervention group than in the control group; most participants in both groups had no change in the pharmacotherapy during the trial.
IN PRACTICE:
Urologists and other primary care clinicians who treat women with OAB may consider a multicomponent intervention that includes cognitive components and exposure-based bladder training or could refer to a cognitive behavioral therapist or pelvic floor physical therapist experienced in these techniques.
SOURCE:
Satoshi Funada, MD, PhD, and Takashi Kobayashi, MD, PhD, both with the Department of Urology at Kyoto University Graduate School of Medicine in Kyoto, Japan, are the corresponding authors. The study was published online in JAMA Network Open.
LIMITATIONS:
The trial was open label, and the use of a waiting list control group is known to produce greater differences between the two groups. The trial included patients both taking and not taking medication for OAB. The sample size was also relatively small, and the intervention was performed by a single clinician, possibly limiting the generalizability of results.
DISCLOSURES:
The study was funded by the Japan Society for the Promotion of Science (JSPS). Various study authors reported receiving grants from the Pfizer Health Research Foundation, AstraZeneca, and JSPS. Other study authors reported receiving personal fees from Eisai, Sawai Pharmaceutical, Statcom, and others. One author reported pending patents for intellectual properties for the Kokoro app licensed to Mitsubishi Tanabe Pharma.
A version of this article appeared on Medscape.com.
High Cesarean Rates Persist in Obesity Despite Standardized Protocols
NATIONAL HARBOR, MARYLAND — Implementation of a standardized induction of labor protocol had no significant effect on the rates of cesarean delivery in patients with obesity, based on data from more than 5000 individuals.
Previous research has shown that the risk for cesarean delivery increases by 5% with each 1-kg/m2 increase in body mass index (BMI) among nulliparous patients, said Melissa Riegel, MD, of the University of Pennsylvania, Philadelphia, in a presentation at the meeting sponsored by the Society for Maternal-Fetal Medicine. (abstract 82).
Research on the relationship between obesity and higher cesarean delivery rates “has been clouded by the inability to reduce variation in care,” Dr. Riegel said at the meeting sponsored by the Society for Maternal Fetal Medicine. Failed induction of labor (IOL) is a leading indicator for cesarean delivery, and cesarean delivery is 80% more likely in patients with obesity undergoing IOL than in normal-weight patients, Dr. Riegel said.
Possible explanations for these differences include provider factors such as variability in care management, conscious and unconscious biases, or physiologic differences in patients with obesity such as elevated hormones, differences in the labor curve, and higher doses of oxytocin and prostaglandins, Dr. Riegel said.
Dr. Riegel and colleagues hypothesized that differences in cesarean delivery rates would persist despite a standardized labor induction protocol, thereby supporting the effects of factors other than variations in care on increased cesarean delivery risk after IOL in patients with obesity.
The researchers reviewed data from two sites comparing 2-year periods before and after implementation of an IOL protocol from 2018 to 2022. The study population included nulliparous women with singleton pregnancies at term who underwent IOL with intact membranes and unfavorable cervices, and had a BMI of at least 30 kg/m2 at delivery. The preimplementation group (PRE) included 2480 individuals and the postimplementation group (POST) included 2651 individuals. Patients were divided into weight classes based on BMI: 30-34.9; 35-39.9; ≥40.
The standardized protocol consisted of active labor management with cervical exams, with an amniotomy by the time of the first exam with 4 cm or greater cervical dilation, and further intervention with medication such as oxytocin or an intrauterine pressure catheter if no cervical change was noted after 2 hours.
In a multivariate analysis, the overall cesarean delivery rate was 24.9% before the protocol implementation and 26.0% in the postimplementation group. There were no differences in the risk of cesarean delivery in any obesity class from the PRE to POST period.
In addition, no significant differences appeared in the secondary outcomes of duration of labor, maternal morbidity, or neonatal morbidity, Dr. Riegel said. Nonreassuring fetal heart rate tracing was the most common reason for cesarean delivery across all obesity classes and the PRE and POST groups.
Study limitations included the use of data from only two sites, but the results were strengthened by the large sample size, said Dr. Reigel. The results indicate that reducing variation in IOL management had no significant effect on the relationship between obesity and cesarean delivery and support underlying physiologic explanations, she said.
Making the Case for Physiology
“By standardizing induction practices, we were able to minimize differences in care and better answer why the increased cesarean delivery rate exists in this patient population,” Dr. Riegel said in an interview. The findings were in line with the primary hypothesis that standardized induction would not affect cesarean delivery rates in patients with obesity, she said. Instead, the findings support potential physiologic differences as “the driving force behind this relationship,” she added.
Looking ahead, “There is a role for translational work to investigate the specific biological changes in patients with obesity that might contribute to an increased risk of cesarean delivery and there is also a role for investigating the effectiveness of different labor induction interventions specifically in patients with obesity,” Dr. Riegel said.
Different Induction Protocols Needed for Obese Patients?
“Given that severe maternal morbidity and mortality are continuing to increase in the United States, this study is critical, as we know that both cesarean delivery and obesity are driving factors in increasing maternal morbidity,” said Marissa Platner, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, Georgia, in an interview.
However, the novel takeaway message from the current study is that patients with obesity were more likely to require cesarean delivery even with a protocol in which variation in labor induction techniques are minimized, said Dr. Platner, who was not involved in the study. “This leads to the question of [whether] we should have different standards or protocols for our patients with obesity, as well as a need for clear counseling for these patients early on in pregnancy,” she said.
As for further research, “It would be interesting to see if the risk of cesarean delivery changed based on class of obesity, and the primary drivers of cesarean delivery in this study,” Dr. Platner said. “Additionally, it would be helpful to know how much pitocin was needed for patients, based on their BMI category, to achieve successful vaginal delivery,” she noted.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Development. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
NATIONAL HARBOR, MARYLAND — Implementation of a standardized induction of labor protocol had no significant effect on the rates of cesarean delivery in patients with obesity, based on data from more than 5000 individuals.
Previous research has shown that the risk for cesarean delivery increases by 5% with each 1-kg/m2 increase in body mass index (BMI) among nulliparous patients, said Melissa Riegel, MD, of the University of Pennsylvania, Philadelphia, in a presentation at the meeting sponsored by the Society for Maternal-Fetal Medicine. (abstract 82).
Research on the relationship between obesity and higher cesarean delivery rates “has been clouded by the inability to reduce variation in care,” Dr. Riegel said at the meeting sponsored by the Society for Maternal Fetal Medicine. Failed induction of labor (IOL) is a leading indicator for cesarean delivery, and cesarean delivery is 80% more likely in patients with obesity undergoing IOL than in normal-weight patients, Dr. Riegel said.
Possible explanations for these differences include provider factors such as variability in care management, conscious and unconscious biases, or physiologic differences in patients with obesity such as elevated hormones, differences in the labor curve, and higher doses of oxytocin and prostaglandins, Dr. Riegel said.
Dr. Riegel and colleagues hypothesized that differences in cesarean delivery rates would persist despite a standardized labor induction protocol, thereby supporting the effects of factors other than variations in care on increased cesarean delivery risk after IOL in patients with obesity.
The researchers reviewed data from two sites comparing 2-year periods before and after implementation of an IOL protocol from 2018 to 2022. The study population included nulliparous women with singleton pregnancies at term who underwent IOL with intact membranes and unfavorable cervices, and had a BMI of at least 30 kg/m2 at delivery. The preimplementation group (PRE) included 2480 individuals and the postimplementation group (POST) included 2651 individuals. Patients were divided into weight classes based on BMI: 30-34.9; 35-39.9; ≥40.
The standardized protocol consisted of active labor management with cervical exams, with an amniotomy by the time of the first exam with 4 cm or greater cervical dilation, and further intervention with medication such as oxytocin or an intrauterine pressure catheter if no cervical change was noted after 2 hours.
In a multivariate analysis, the overall cesarean delivery rate was 24.9% before the protocol implementation and 26.0% in the postimplementation group. There were no differences in the risk of cesarean delivery in any obesity class from the PRE to POST period.
In addition, no significant differences appeared in the secondary outcomes of duration of labor, maternal morbidity, or neonatal morbidity, Dr. Riegel said. Nonreassuring fetal heart rate tracing was the most common reason for cesarean delivery across all obesity classes and the PRE and POST groups.
Study limitations included the use of data from only two sites, but the results were strengthened by the large sample size, said Dr. Reigel. The results indicate that reducing variation in IOL management had no significant effect on the relationship between obesity and cesarean delivery and support underlying physiologic explanations, she said.
Making the Case for Physiology
“By standardizing induction practices, we were able to minimize differences in care and better answer why the increased cesarean delivery rate exists in this patient population,” Dr. Riegel said in an interview. The findings were in line with the primary hypothesis that standardized induction would not affect cesarean delivery rates in patients with obesity, she said. Instead, the findings support potential physiologic differences as “the driving force behind this relationship,” she added.
Looking ahead, “There is a role for translational work to investigate the specific biological changes in patients with obesity that might contribute to an increased risk of cesarean delivery and there is also a role for investigating the effectiveness of different labor induction interventions specifically in patients with obesity,” Dr. Riegel said.
Different Induction Protocols Needed for Obese Patients?
“Given that severe maternal morbidity and mortality are continuing to increase in the United States, this study is critical, as we know that both cesarean delivery and obesity are driving factors in increasing maternal morbidity,” said Marissa Platner, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, Georgia, in an interview.
However, the novel takeaway message from the current study is that patients with obesity were more likely to require cesarean delivery even with a protocol in which variation in labor induction techniques are minimized, said Dr. Platner, who was not involved in the study. “This leads to the question of [whether] we should have different standards or protocols for our patients with obesity, as well as a need for clear counseling for these patients early on in pregnancy,” she said.
As for further research, “It would be interesting to see if the risk of cesarean delivery changed based on class of obesity, and the primary drivers of cesarean delivery in this study,” Dr. Platner said. “Additionally, it would be helpful to know how much pitocin was needed for patients, based on their BMI category, to achieve successful vaginal delivery,” she noted.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Development. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
NATIONAL HARBOR, MARYLAND — Implementation of a standardized induction of labor protocol had no significant effect on the rates of cesarean delivery in patients with obesity, based on data from more than 5000 individuals.
Previous research has shown that the risk for cesarean delivery increases by 5% with each 1-kg/m2 increase in body mass index (BMI) among nulliparous patients, said Melissa Riegel, MD, of the University of Pennsylvania, Philadelphia, in a presentation at the meeting sponsored by the Society for Maternal-Fetal Medicine. (abstract 82).
Research on the relationship between obesity and higher cesarean delivery rates “has been clouded by the inability to reduce variation in care,” Dr. Riegel said at the meeting sponsored by the Society for Maternal Fetal Medicine. Failed induction of labor (IOL) is a leading indicator for cesarean delivery, and cesarean delivery is 80% more likely in patients with obesity undergoing IOL than in normal-weight patients, Dr. Riegel said.
Possible explanations for these differences include provider factors such as variability in care management, conscious and unconscious biases, or physiologic differences in patients with obesity such as elevated hormones, differences in the labor curve, and higher doses of oxytocin and prostaglandins, Dr. Riegel said.
Dr. Riegel and colleagues hypothesized that differences in cesarean delivery rates would persist despite a standardized labor induction protocol, thereby supporting the effects of factors other than variations in care on increased cesarean delivery risk after IOL in patients with obesity.
The researchers reviewed data from two sites comparing 2-year periods before and after implementation of an IOL protocol from 2018 to 2022. The study population included nulliparous women with singleton pregnancies at term who underwent IOL with intact membranes and unfavorable cervices, and had a BMI of at least 30 kg/m2 at delivery. The preimplementation group (PRE) included 2480 individuals and the postimplementation group (POST) included 2651 individuals. Patients were divided into weight classes based on BMI: 30-34.9; 35-39.9; ≥40.
The standardized protocol consisted of active labor management with cervical exams, with an amniotomy by the time of the first exam with 4 cm or greater cervical dilation, and further intervention with medication such as oxytocin or an intrauterine pressure catheter if no cervical change was noted after 2 hours.
In a multivariate analysis, the overall cesarean delivery rate was 24.9% before the protocol implementation and 26.0% in the postimplementation group. There were no differences in the risk of cesarean delivery in any obesity class from the PRE to POST period.
In addition, no significant differences appeared in the secondary outcomes of duration of labor, maternal morbidity, or neonatal morbidity, Dr. Riegel said. Nonreassuring fetal heart rate tracing was the most common reason for cesarean delivery across all obesity classes and the PRE and POST groups.
Study limitations included the use of data from only two sites, but the results were strengthened by the large sample size, said Dr. Reigel. The results indicate that reducing variation in IOL management had no significant effect on the relationship between obesity and cesarean delivery and support underlying physiologic explanations, she said.
Making the Case for Physiology
“By standardizing induction practices, we were able to minimize differences in care and better answer why the increased cesarean delivery rate exists in this patient population,” Dr. Riegel said in an interview. The findings were in line with the primary hypothesis that standardized induction would not affect cesarean delivery rates in patients with obesity, she said. Instead, the findings support potential physiologic differences as “the driving force behind this relationship,” she added.
Looking ahead, “There is a role for translational work to investigate the specific biological changes in patients with obesity that might contribute to an increased risk of cesarean delivery and there is also a role for investigating the effectiveness of different labor induction interventions specifically in patients with obesity,” Dr. Riegel said.
Different Induction Protocols Needed for Obese Patients?
“Given that severe maternal morbidity and mortality are continuing to increase in the United States, this study is critical, as we know that both cesarean delivery and obesity are driving factors in increasing maternal morbidity,” said Marissa Platner, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, Georgia, in an interview.
However, the novel takeaway message from the current study is that patients with obesity were more likely to require cesarean delivery even with a protocol in which variation in labor induction techniques are minimized, said Dr. Platner, who was not involved in the study. “This leads to the question of [whether] we should have different standards or protocols for our patients with obesity, as well as a need for clear counseling for these patients early on in pregnancy,” she said.
As for further research, “It would be interesting to see if the risk of cesarean delivery changed based on class of obesity, and the primary drivers of cesarean delivery in this study,” Dr. Platner said. “Additionally, it would be helpful to know how much pitocin was needed for patients, based on their BMI category, to achieve successful vaginal delivery,” she noted.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Development. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
AT THE PREGNANCY MEETING
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.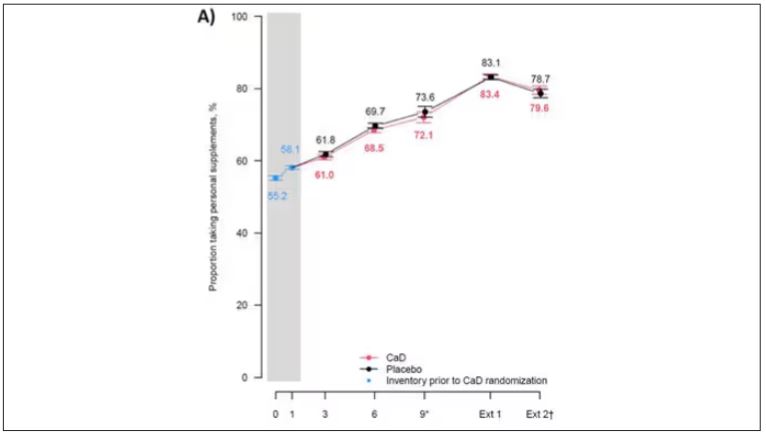
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.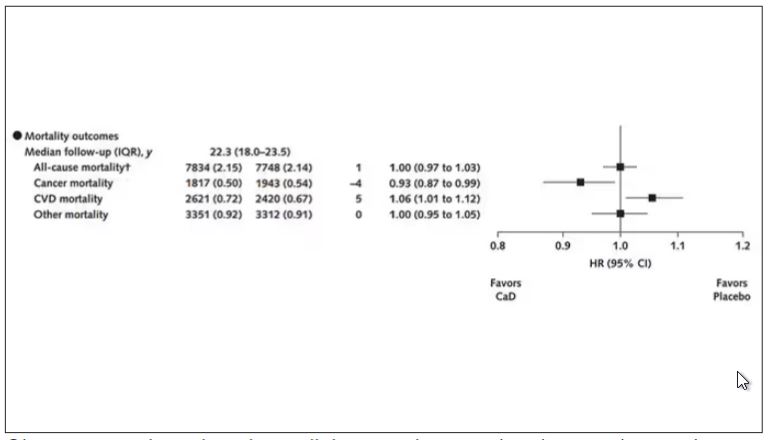
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.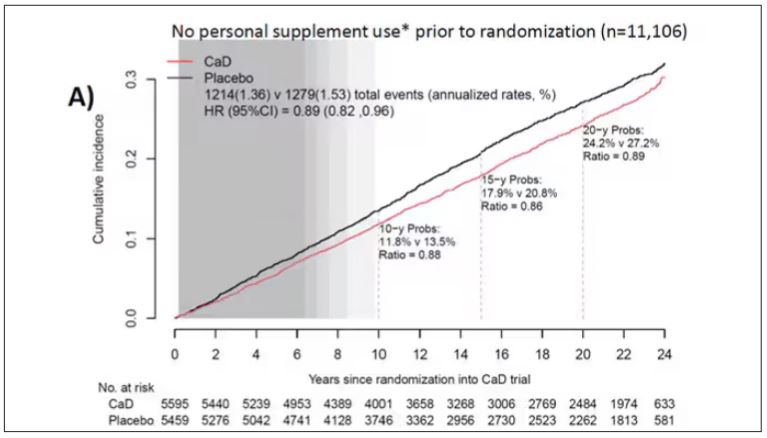
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.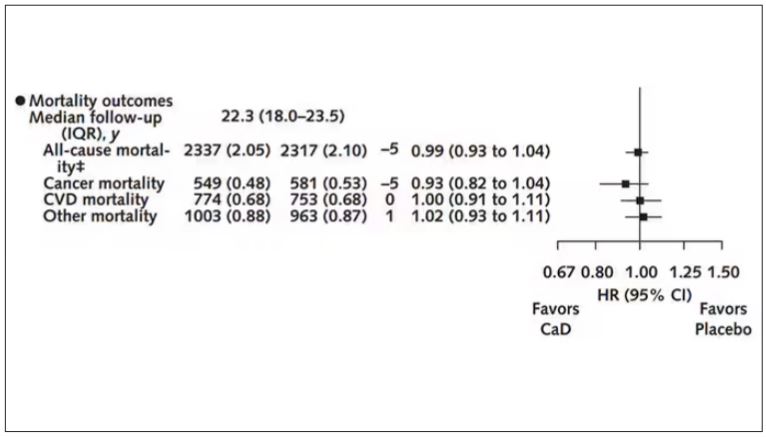
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.


