User login
Antiphospholipid Syndrome in a Patient With Rheumatoid Arthritis
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
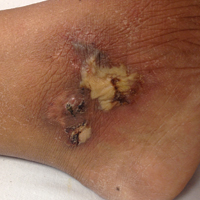
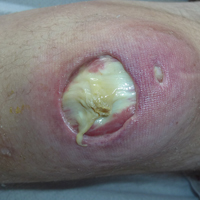
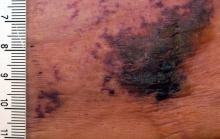
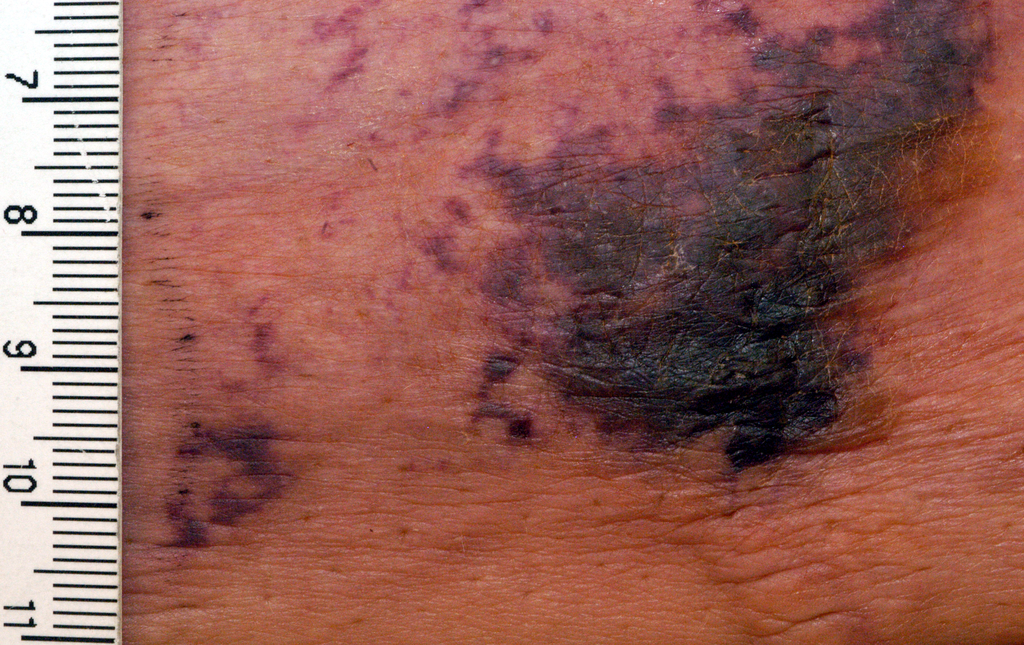
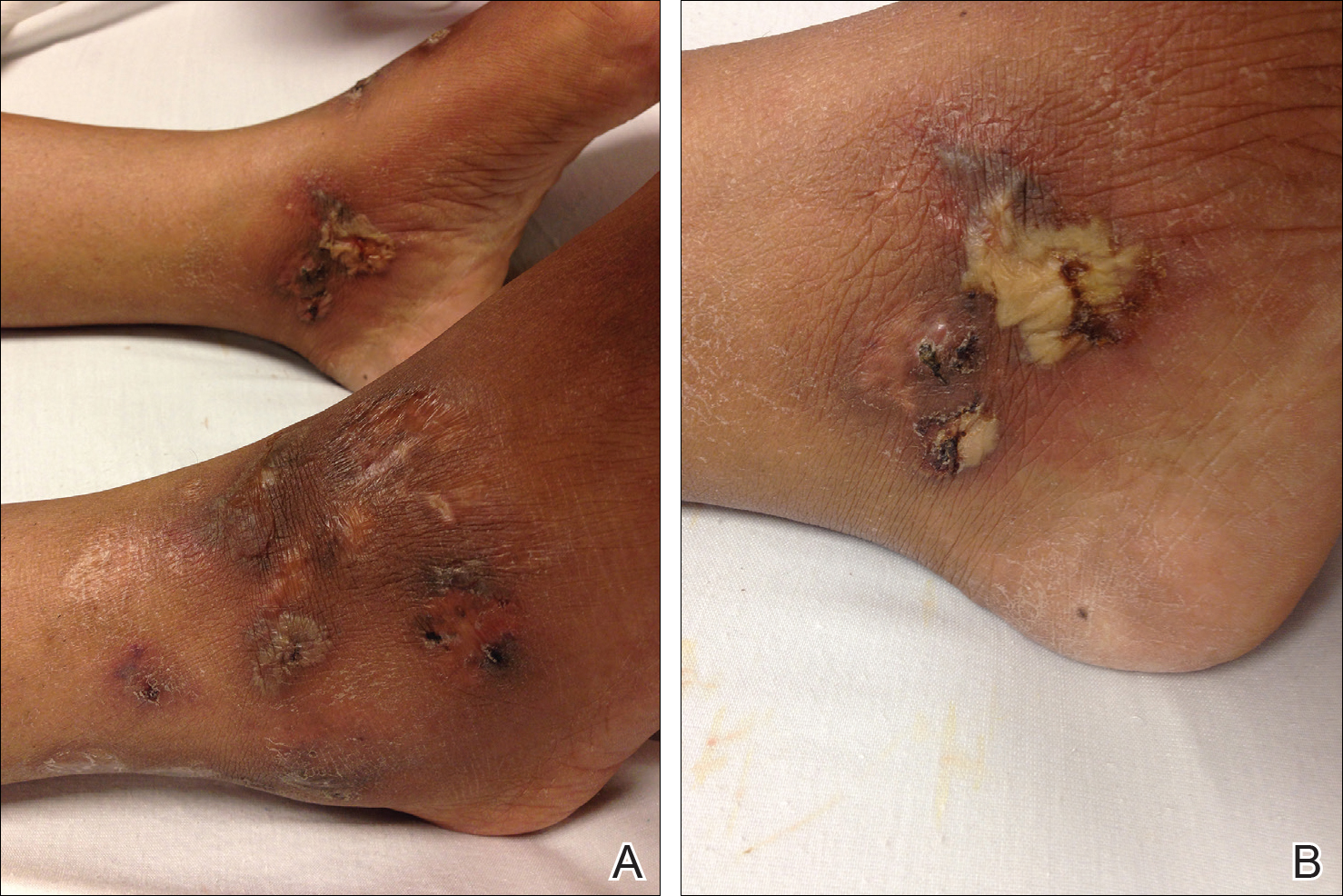
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.
Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.

Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.

Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
Practice Points
- Antiphospholipid syndrome (APS) is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated antiphospholipid antibody titers.
- Cutaneous findings of APS include livedo reticularis most commonly but also anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.
- The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.
New auto-grafting techniques could advance wound healing
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
Healing of Leg Ulcers Associated With Granulomatosis With Polyangiitis (Wegener Granulomatosis) After Rituximab Therapy
To the Editor:
A 52-year-old woman with a history of arthralgia, rhinitis, sinusitis, and episodic epistaxis was admitted to the hospital with multiple nonhealing severe leg ulcerations. She noticed subcutaneous nodules on the legs 6 months prior to the development of ulcers. The lesions progressed from subcutaneous nodules to red-black skin discoloration, blister formation, and eventually ulceration. Over a period of months, the ulcers were treated with several courses of antibiotics and wound care including a single surgical debridement of one of the ulcers on the dorsum of the right foot. These interventions did not make a remarkable impact on ulcer healing.
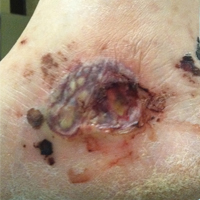
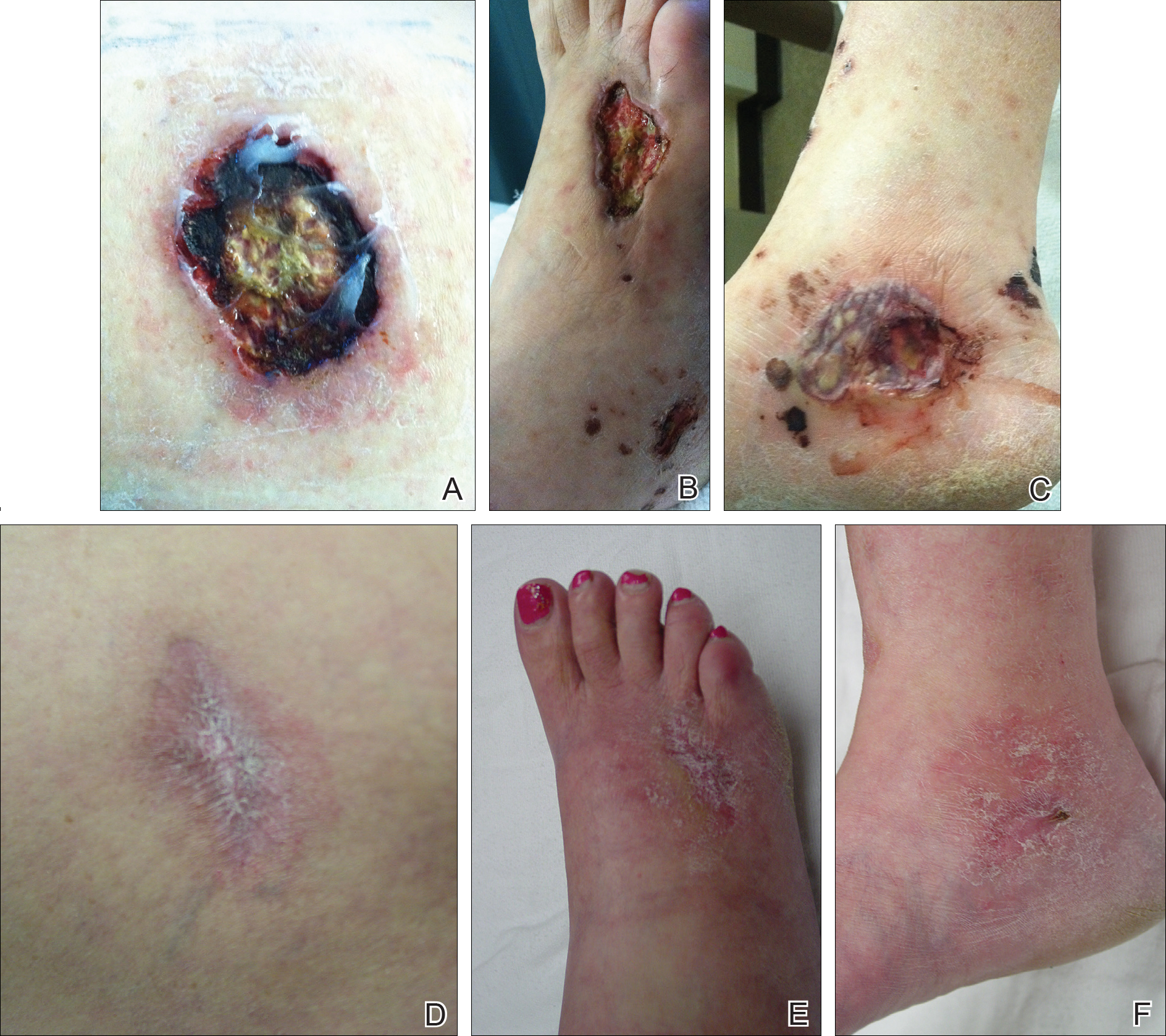
On physical examination, the patient had scattered 4- to 5-mm palpable purpura on the knees, elbows, and feet bilaterally. She had multiple 1- to 8-cm indurated purple ulcerations with friable surfaces and raised irregular borders on the feet, toes, and lower legs bilaterally (Figure, A–C). One notably larger ulcer was found on the anterior aspect of the left thigh (Figure, A). Scattered 5- to 15-mm eschars were present on the legs bilaterally. She also had multiple large, firm, nonerythematous dermal plaques on the thighs bilaterally that measured several centimeters. There were no oral mucosal lesions and no ulcerations above the waist.
Magnetic resonance imaging of the foot showed some surrounding cellulitis but no osteomyelitis. Chest radiograph and computed tomography revealed bilateral apical nodules. Proteinase 3–antineutrophil cytoplasmic antibody (PR3-ANCA) testing was positive. Serum complement levels were normal. An antinuclear antibody test and rheumatoid factor were both negative. Skin biopsies were obtained from the thigh ulcer, foot ulcer, and purpuric lesions on the right knee. The results demonstrated leukocytoclastic vasculitis and neutrophilic small vessel vasculitis with necrotizing neutrophilic dermatitis and panniculitis. Granulomatosis with polyangiitis (GPA) was diagnosed based on these findings.
Initial inpatient treatment included intravenous methylprednisolone (100 mg every 8 hours for 3 doses), followed by oral prednisone 60 mg daily. Two weeks later the ulcers were reevaluated and only mild improvement had occurred with the steroids. Therefore, rituximab (RTX) was initiated at 375 mg/m2 (700 mg) intravenously once weekly for 4 weeks. After 3 doses of RTX, the ulcerations were healing dramatically and the treatment was well tolerated. A rapid prednisone taper was started, and the patient received her fourth and final dose of RTX. Two months after the initial infusion, the thigh ulcer and most of the ulcerations on the feet and lower legs had almost completely resolved. Photographs were taken 5 months after initial RTX infusion (Figure, D–F). A chest radiograph 4 months after initial RTX infusion showed resolution of lung nodules. Two months after RTX induction therapy, azathioprine was added for maintenance but was stopped due to poor tolerance. Oral methotrexate 17.5 mg once weekly was added 5 months after RTX for maintenance and was well tolerated. At that time the prednisone dose was 10 mg daily and was successfully tapered to 5 mg by 9 months after RTX induction therapy.
Granulomatosis with polyangiitis (Wegener granulomatosis) is a granulomatous small- and medium-sized vessel vasculitis that traditionally affects the upper and lower respiratory tract and kidneys.1 Skin lesions also are quite common and include palpable purpura, ulcers, vesicles, papules, and subcutaneous nodules. Patients with active GPA also tend to have ANCAs directed against proteinase 3 (PR3-ANCA). Although GPA was once considered a fatal disease, treatment with cyclophosphamide combined with corticosteroids has been shown to substantially improve outcomes.1 Rituximab, a chimeric monoclonal anti-CD20 antibody, works by depleting B lymphocytes and has been used with success to treat diseases such as lymphoma and rheumatoid arthritis.2,3 The US Food and Drug Administration approved RTX for GPA and microscopic polyangiitis in 2011, with a number of trials supporting its efficacy.4
The success of RTX in treating GPA has been documented in case reports as well as several trials with extended follow-up. A single-center observational study of 53 patients showed that RTX was safe and effective for induction and maintenance of remission in patients with refractory GPA. This study also uncovered the potential for predicting relapse based on following B cell and ANCA levels and preventing relapse by initializing further treatment.5 Other small studies and case reports have shown similar success using RTX for refractory GPA.6-10 These studies included various combinations of concurrent therapies and various follow-up intervals. The Rituximab in ANCA-Associated Vasculitis (RAVE) trial compared RTX versus cyclophosphamide for ANCA-positive vasculitis.11 This multicenter, randomized, double-blind study found that RTX was as efficacious as cyclophosphamide for induction of remission in severe GPA.The data also suggested that RTX may be superior for relapsing disease.11 Another multicenter, open-label, randomized trial (RITUXVAS) compared RTX to cyclophosphamide in ANCA-associated renal vasculitis. This trial also found the 2 treatments to be similar in both efficacy in inducing remission and adverse events.12
Some conflicting reports have appeared on the effectiveness of using RTX for the granulomatous versus vasculitic manifestations of GPA. Aires et al13 showed failure of improvement in most patients with granulomatous manifestations of GPA in a study of 8 patients. A retrospective study including 59 patients who were treated with RTX also showed that complete remission was more common in patients with primarily vasculitic manifestations, not granulomatous manifestations.14 However, some case series that included patients with refractory ophthalmic GPA, a primarily granulomatous manifestation, have found success using RTX.15,16 More studies are needed to determine if there truly is a difference and whether this difference has an effect on when to use RTX. The skin lesions our patient demonstrated were due to the vasculitic component of the disease, and consequently, the rapid and complete response we observed would be consistent with the premise that the therapy works best for vasculitis.
Most of the trials assessing the efficacy of RTX utilize a tool such as the Wegener granulomatosis-specific Birmingham Vasculitis Activity Score.17 This measure of treatment response does include a skin item, but it is part of the composite response score. Consequently, a specific statement regarding skin improvement cannot be made. Additionally, little is reported pertaining to the treatment of skin-related findings in GPA. One report did specifically address the treatment of dermatologic manifestations of GPA utilizing systemic tacrolimus with oral prednisone successfully in 1 patient with GPA and a history of recurrent lower extremity nodules and ulcers.18 The efficacy of RTX in limited GPA was good in a small study of 8 patients. However, the study had only 1 patient with purpura and 1 patient with a subcutaneous nodule.19 Several other case series and studies have included patients with various cutaneous findings associated with GPA.5-7,9,11 However, they did not comment specifically on skin response to treatment, and the focus appeared to be on other organ system involvement. One case series did report improvement of lower extremity gangrene with RTX therapy for ANCA-associated vasculitis.8 Our report demonstrates a case of severe skin disease that responded well to RTX. It is common to have various skin findings in GPA, and our patient presented with notable skin disease. Although skin findings may not be the more life-threatening manifestations of the disease, they can be quite debilitating, as shown in our case report.
Our patient with notable leg ulcerations required hospitalization due to GPA and received RTX in addition to corticosteroids for treatment. We observed a rapid and dramatic improvement in the skin findings, which seemed to exceed expectations from steroids alone. The other manifestations of the disease including lung nodules also improved. Although cyclophosphamide and corticosteroids have been quite successful in induction of remission, cyclophosphamide is not without serious adverse effects. There also are some patients who have contraindications to cyclophosphamide or do not see successful results. In our brief review of the literature, RTX, a B cell–depleting antibody, has shown to have success in treating refractory and severe GPA. There is little reported specifically about treating the skin manifestations of GPA. A few studies and case reports mention skin findings but do not comment on the success of RTX in treating them. Although the severity of other organ involvement in GPA may take precedence, the skin findings can be quite debilitating, as in our patient. Patients with GPA and notable skin findings may benefit from RTX, and it would be beneficial to include these results in future studies using RTX to treat GPA.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488-498.
- Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803-843.
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
- FDA approves Rituxan to treat two rare disorders [news release]. Silver Spring, MD: US Food and Drug Administration; April 19, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm251946.htm. Accessed January 6, 2017.
- Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum. 2012;64:3770-3778.
- Keogh KA, Ytterberg SR, Fervenza FC, et al. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180-187.
- Dalkilic E, Alkis N, Kamali S. Rituximab as a new therapeutic option in granulomatosis with polyangiitis: a report of two cases. Mod Rheumatol. 2012;22:463-466.
- Eriksson P. Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med. 2005;257:540-548.
- Oristrell J, Bejarano G, Jordana R, et al. Effectiveness of rituximab in severe Wegener’s granulomatosis: report of two cases and review of the literature. Open Respir Med J. 2009;3:94-99.
- Martinez Del Pero M, Chaudhry A, Jones RB, et al. B-cell depletion with rituximab for refractory head and neck Wegener’s granulomatosis: a cohort study. Clin Otolaryngol. 2009;34:328-335.
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221-232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211-220.
- Aries PM, Hellmich B, Voswinkel J, et al. Lack of efficacy of rituximab in Wegener’s granulomatosis with refractory granulomatous manifestations. Ann Rheum Dis. 2006;65:853-858.
- Holle JU, Dubrau C, Herlyn K, et al. Rituximab for refractory granulomatosis with polyangiitis (Wegener’s granulomatosis): comparison of efficacy in granulomatous versus vasculitic manifestations. Ann Rheum Dis. 2012;71:327-333.
- Taylor SR, Salama AD, Joshi L, et al. Rituximab is effective in the treatment of refractory ophthalmic Wegener’s granulomatosis. Arthritis Rheum. 2009;60:1540-1547.
- Joshi L, Lightman SL, Salama AD, et al. Rituximab in refractory ophthalmic Wegener’s granulomatosis: PR3 titers may predict relapse, but repeat treatment can be effective. Ophthalmol. 2011;118:2498-2503.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001;44:912-920.
- Wenzel J, Montag S, Wilsmann-Theis D, et al. Successful treatment of recalcitrant Wegener’s granulomatosis of the skin with tacrolimus (Prograf). Br J Dermatol. 2004;151:927-928.
- Seo P, Specks U, Keogh KA. Efficacy of rituximab in limited Wegener’s granulomatosis with refractory granulomatous manifestations. J Rheumatol. 2008;35:2017-2023.
To the Editor:
A 52-year-old woman with a history of arthralgia, rhinitis, sinusitis, and episodic epistaxis was admitted to the hospital with multiple nonhealing severe leg ulcerations. She noticed subcutaneous nodules on the legs 6 months prior to the development of ulcers. The lesions progressed from subcutaneous nodules to red-black skin discoloration, blister formation, and eventually ulceration. Over a period of months, the ulcers were treated with several courses of antibiotics and wound care including a single surgical debridement of one of the ulcers on the dorsum of the right foot. These interventions did not make a remarkable impact on ulcer healing.
On physical examination, the patient had scattered 4- to 5-mm palpable purpura on the knees, elbows, and feet bilaterally. She had multiple 1- to 8-cm indurated purple ulcerations with friable surfaces and raised irregular borders on the feet, toes, and lower legs bilaterally (Figure, A–C). One notably larger ulcer was found on the anterior aspect of the left thigh (Figure, A). Scattered 5- to 15-mm eschars were present on the legs bilaterally. She also had multiple large, firm, nonerythematous dermal plaques on the thighs bilaterally that measured several centimeters. There were no oral mucosal lesions and no ulcerations above the waist.

Magnetic resonance imaging of the foot showed some surrounding cellulitis but no osteomyelitis. Chest radiograph and computed tomography revealed bilateral apical nodules. Proteinase 3–antineutrophil cytoplasmic antibody (PR3-ANCA) testing was positive. Serum complement levels were normal. An antinuclear antibody test and rheumatoid factor were both negative. Skin biopsies were obtained from the thigh ulcer, foot ulcer, and purpuric lesions on the right knee. The results demonstrated leukocytoclastic vasculitis and neutrophilic small vessel vasculitis with necrotizing neutrophilic dermatitis and panniculitis. Granulomatosis with polyangiitis (GPA) was diagnosed based on these findings.
Initial inpatient treatment included intravenous methylprednisolone (100 mg every 8 hours for 3 doses), followed by oral prednisone 60 mg daily. Two weeks later the ulcers were reevaluated and only mild improvement had occurred with the steroids. Therefore, rituximab (RTX) was initiated at 375 mg/m2 (700 mg) intravenously once weekly for 4 weeks. After 3 doses of RTX, the ulcerations were healing dramatically and the treatment was well tolerated. A rapid prednisone taper was started, and the patient received her fourth and final dose of RTX. Two months after the initial infusion, the thigh ulcer and most of the ulcerations on the feet and lower legs had almost completely resolved. Photographs were taken 5 months after initial RTX infusion (Figure, D–F). A chest radiograph 4 months after initial RTX infusion showed resolution of lung nodules. Two months after RTX induction therapy, azathioprine was added for maintenance but was stopped due to poor tolerance. Oral methotrexate 17.5 mg once weekly was added 5 months after RTX for maintenance and was well tolerated. At that time the prednisone dose was 10 mg daily and was successfully tapered to 5 mg by 9 months after RTX induction therapy.
Granulomatosis with polyangiitis (Wegener granulomatosis) is a granulomatous small- and medium-sized vessel vasculitis that traditionally affects the upper and lower respiratory tract and kidneys.1 Skin lesions also are quite common and include palpable purpura, ulcers, vesicles, papules, and subcutaneous nodules. Patients with active GPA also tend to have ANCAs directed against proteinase 3 (PR3-ANCA). Although GPA was once considered a fatal disease, treatment with cyclophosphamide combined with corticosteroids has been shown to substantially improve outcomes.1 Rituximab, a chimeric monoclonal anti-CD20 antibody, works by depleting B lymphocytes and has been used with success to treat diseases such as lymphoma and rheumatoid arthritis.2,3 The US Food and Drug Administration approved RTX for GPA and microscopic polyangiitis in 2011, with a number of trials supporting its efficacy.4
The success of RTX in treating GPA has been documented in case reports as well as several trials with extended follow-up. A single-center observational study of 53 patients showed that RTX was safe and effective for induction and maintenance of remission in patients with refractory GPA. This study also uncovered the potential for predicting relapse based on following B cell and ANCA levels and preventing relapse by initializing further treatment.5 Other small studies and case reports have shown similar success using RTX for refractory GPA.6-10 These studies included various combinations of concurrent therapies and various follow-up intervals. The Rituximab in ANCA-Associated Vasculitis (RAVE) trial compared RTX versus cyclophosphamide for ANCA-positive vasculitis.11 This multicenter, randomized, double-blind study found that RTX was as efficacious as cyclophosphamide for induction of remission in severe GPA.The data also suggested that RTX may be superior for relapsing disease.11 Another multicenter, open-label, randomized trial (RITUXVAS) compared RTX to cyclophosphamide in ANCA-associated renal vasculitis. This trial also found the 2 treatments to be similar in both efficacy in inducing remission and adverse events.12
Some conflicting reports have appeared on the effectiveness of using RTX for the granulomatous versus vasculitic manifestations of GPA. Aires et al13 showed failure of improvement in most patients with granulomatous manifestations of GPA in a study of 8 patients. A retrospective study including 59 patients who were treated with RTX also showed that complete remission was more common in patients with primarily vasculitic manifestations, not granulomatous manifestations.14 However, some case series that included patients with refractory ophthalmic GPA, a primarily granulomatous manifestation, have found success using RTX.15,16 More studies are needed to determine if there truly is a difference and whether this difference has an effect on when to use RTX. The skin lesions our patient demonstrated were due to the vasculitic component of the disease, and consequently, the rapid and complete response we observed would be consistent with the premise that the therapy works best for vasculitis.
Most of the trials assessing the efficacy of RTX utilize a tool such as the Wegener granulomatosis-specific Birmingham Vasculitis Activity Score.17 This measure of treatment response does include a skin item, but it is part of the composite response score. Consequently, a specific statement regarding skin improvement cannot be made. Additionally, little is reported pertaining to the treatment of skin-related findings in GPA. One report did specifically address the treatment of dermatologic manifestations of GPA utilizing systemic tacrolimus with oral prednisone successfully in 1 patient with GPA and a history of recurrent lower extremity nodules and ulcers.18 The efficacy of RTX in limited GPA was good in a small study of 8 patients. However, the study had only 1 patient with purpura and 1 patient with a subcutaneous nodule.19 Several other case series and studies have included patients with various cutaneous findings associated with GPA.5-7,9,11 However, they did not comment specifically on skin response to treatment, and the focus appeared to be on other organ system involvement. One case series did report improvement of lower extremity gangrene with RTX therapy for ANCA-associated vasculitis.8 Our report demonstrates a case of severe skin disease that responded well to RTX. It is common to have various skin findings in GPA, and our patient presented with notable skin disease. Although skin findings may not be the more life-threatening manifestations of the disease, they can be quite debilitating, as shown in our case report.
Our patient with notable leg ulcerations required hospitalization due to GPA and received RTX in addition to corticosteroids for treatment. We observed a rapid and dramatic improvement in the skin findings, which seemed to exceed expectations from steroids alone. The other manifestations of the disease including lung nodules also improved. Although cyclophosphamide and corticosteroids have been quite successful in induction of remission, cyclophosphamide is not without serious adverse effects. There also are some patients who have contraindications to cyclophosphamide or do not see successful results. In our brief review of the literature, RTX, a B cell–depleting antibody, has shown to have success in treating refractory and severe GPA. There is little reported specifically about treating the skin manifestations of GPA. A few studies and case reports mention skin findings but do not comment on the success of RTX in treating them. Although the severity of other organ involvement in GPA may take precedence, the skin findings can be quite debilitating, as in our patient. Patients with GPA and notable skin findings may benefit from RTX, and it would be beneficial to include these results in future studies using RTX to treat GPA.
To the Editor:
A 52-year-old woman with a history of arthralgia, rhinitis, sinusitis, and episodic epistaxis was admitted to the hospital with multiple nonhealing severe leg ulcerations. She noticed subcutaneous nodules on the legs 6 months prior to the development of ulcers. The lesions progressed from subcutaneous nodules to red-black skin discoloration, blister formation, and eventually ulceration. Over a period of months, the ulcers were treated with several courses of antibiotics and wound care including a single surgical debridement of one of the ulcers on the dorsum of the right foot. These interventions did not make a remarkable impact on ulcer healing.
On physical examination, the patient had scattered 4- to 5-mm palpable purpura on the knees, elbows, and feet bilaterally. She had multiple 1- to 8-cm indurated purple ulcerations with friable surfaces and raised irregular borders on the feet, toes, and lower legs bilaterally (Figure, A–C). One notably larger ulcer was found on the anterior aspect of the left thigh (Figure, A). Scattered 5- to 15-mm eschars were present on the legs bilaterally. She also had multiple large, firm, nonerythematous dermal plaques on the thighs bilaterally that measured several centimeters. There were no oral mucosal lesions and no ulcerations above the waist.

Magnetic resonance imaging of the foot showed some surrounding cellulitis but no osteomyelitis. Chest radiograph and computed tomography revealed bilateral apical nodules. Proteinase 3–antineutrophil cytoplasmic antibody (PR3-ANCA) testing was positive. Serum complement levels were normal. An antinuclear antibody test and rheumatoid factor were both negative. Skin biopsies were obtained from the thigh ulcer, foot ulcer, and purpuric lesions on the right knee. The results demonstrated leukocytoclastic vasculitis and neutrophilic small vessel vasculitis with necrotizing neutrophilic dermatitis and panniculitis. Granulomatosis with polyangiitis (GPA) was diagnosed based on these findings.
Initial inpatient treatment included intravenous methylprednisolone (100 mg every 8 hours for 3 doses), followed by oral prednisone 60 mg daily. Two weeks later the ulcers were reevaluated and only mild improvement had occurred with the steroids. Therefore, rituximab (RTX) was initiated at 375 mg/m2 (700 mg) intravenously once weekly for 4 weeks. After 3 doses of RTX, the ulcerations were healing dramatically and the treatment was well tolerated. A rapid prednisone taper was started, and the patient received her fourth and final dose of RTX. Two months after the initial infusion, the thigh ulcer and most of the ulcerations on the feet and lower legs had almost completely resolved. Photographs were taken 5 months after initial RTX infusion (Figure, D–F). A chest radiograph 4 months after initial RTX infusion showed resolution of lung nodules. Two months after RTX induction therapy, azathioprine was added for maintenance but was stopped due to poor tolerance. Oral methotrexate 17.5 mg once weekly was added 5 months after RTX for maintenance and was well tolerated. At that time the prednisone dose was 10 mg daily and was successfully tapered to 5 mg by 9 months after RTX induction therapy.
Granulomatosis with polyangiitis (Wegener granulomatosis) is a granulomatous small- and medium-sized vessel vasculitis that traditionally affects the upper and lower respiratory tract and kidneys.1 Skin lesions also are quite common and include palpable purpura, ulcers, vesicles, papules, and subcutaneous nodules. Patients with active GPA also tend to have ANCAs directed against proteinase 3 (PR3-ANCA). Although GPA was once considered a fatal disease, treatment with cyclophosphamide combined with corticosteroids has been shown to substantially improve outcomes.1 Rituximab, a chimeric monoclonal anti-CD20 antibody, works by depleting B lymphocytes and has been used with success to treat diseases such as lymphoma and rheumatoid arthritis.2,3 The US Food and Drug Administration approved RTX for GPA and microscopic polyangiitis in 2011, with a number of trials supporting its efficacy.4
The success of RTX in treating GPA has been documented in case reports as well as several trials with extended follow-up. A single-center observational study of 53 patients showed that RTX was safe and effective for induction and maintenance of remission in patients with refractory GPA. This study also uncovered the potential for predicting relapse based on following B cell and ANCA levels and preventing relapse by initializing further treatment.5 Other small studies and case reports have shown similar success using RTX for refractory GPA.6-10 These studies included various combinations of concurrent therapies and various follow-up intervals. The Rituximab in ANCA-Associated Vasculitis (RAVE) trial compared RTX versus cyclophosphamide for ANCA-positive vasculitis.11 This multicenter, randomized, double-blind study found that RTX was as efficacious as cyclophosphamide for induction of remission in severe GPA.The data also suggested that RTX may be superior for relapsing disease.11 Another multicenter, open-label, randomized trial (RITUXVAS) compared RTX to cyclophosphamide in ANCA-associated renal vasculitis. This trial also found the 2 treatments to be similar in both efficacy in inducing remission and adverse events.12
Some conflicting reports have appeared on the effectiveness of using RTX for the granulomatous versus vasculitic manifestations of GPA. Aires et al13 showed failure of improvement in most patients with granulomatous manifestations of GPA in a study of 8 patients. A retrospective study including 59 patients who were treated with RTX also showed that complete remission was more common in patients with primarily vasculitic manifestations, not granulomatous manifestations.14 However, some case series that included patients with refractory ophthalmic GPA, a primarily granulomatous manifestation, have found success using RTX.15,16 More studies are needed to determine if there truly is a difference and whether this difference has an effect on when to use RTX. The skin lesions our patient demonstrated were due to the vasculitic component of the disease, and consequently, the rapid and complete response we observed would be consistent with the premise that the therapy works best for vasculitis.
Most of the trials assessing the efficacy of RTX utilize a tool such as the Wegener granulomatosis-specific Birmingham Vasculitis Activity Score.17 This measure of treatment response does include a skin item, but it is part of the composite response score. Consequently, a specific statement regarding skin improvement cannot be made. Additionally, little is reported pertaining to the treatment of skin-related findings in GPA. One report did specifically address the treatment of dermatologic manifestations of GPA utilizing systemic tacrolimus with oral prednisone successfully in 1 patient with GPA and a history of recurrent lower extremity nodules and ulcers.18 The efficacy of RTX in limited GPA was good in a small study of 8 patients. However, the study had only 1 patient with purpura and 1 patient with a subcutaneous nodule.19 Several other case series and studies have included patients with various cutaneous findings associated with GPA.5-7,9,11 However, they did not comment specifically on skin response to treatment, and the focus appeared to be on other organ system involvement. One case series did report improvement of lower extremity gangrene with RTX therapy for ANCA-associated vasculitis.8 Our report demonstrates a case of severe skin disease that responded well to RTX. It is common to have various skin findings in GPA, and our patient presented with notable skin disease. Although skin findings may not be the more life-threatening manifestations of the disease, they can be quite debilitating, as shown in our case report.
Our patient with notable leg ulcerations required hospitalization due to GPA and received RTX in addition to corticosteroids for treatment. We observed a rapid and dramatic improvement in the skin findings, which seemed to exceed expectations from steroids alone. The other manifestations of the disease including lung nodules also improved. Although cyclophosphamide and corticosteroids have been quite successful in induction of remission, cyclophosphamide is not without serious adverse effects. There also are some patients who have contraindications to cyclophosphamide or do not see successful results. In our brief review of the literature, RTX, a B cell–depleting antibody, has shown to have success in treating refractory and severe GPA. There is little reported specifically about treating the skin manifestations of GPA. A few studies and case reports mention skin findings but do not comment on the success of RTX in treating them. Although the severity of other organ involvement in GPA may take precedence, the skin findings can be quite debilitating, as in our patient. Patients with GPA and notable skin findings may benefit from RTX, and it would be beneficial to include these results in future studies using RTX to treat GPA.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488-498.
- Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803-843.
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
- FDA approves Rituxan to treat two rare disorders [news release]. Silver Spring, MD: US Food and Drug Administration; April 19, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm251946.htm. Accessed January 6, 2017.
- Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum. 2012;64:3770-3778.
- Keogh KA, Ytterberg SR, Fervenza FC, et al. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180-187.
- Dalkilic E, Alkis N, Kamali S. Rituximab as a new therapeutic option in granulomatosis with polyangiitis: a report of two cases. Mod Rheumatol. 2012;22:463-466.
- Eriksson P. Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med. 2005;257:540-548.
- Oristrell J, Bejarano G, Jordana R, et al. Effectiveness of rituximab in severe Wegener’s granulomatosis: report of two cases and review of the literature. Open Respir Med J. 2009;3:94-99.
- Martinez Del Pero M, Chaudhry A, Jones RB, et al. B-cell depletion with rituximab for refractory head and neck Wegener’s granulomatosis: a cohort study. Clin Otolaryngol. 2009;34:328-335.
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221-232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211-220.
- Aries PM, Hellmich B, Voswinkel J, et al. Lack of efficacy of rituximab in Wegener’s granulomatosis with refractory granulomatous manifestations. Ann Rheum Dis. 2006;65:853-858.
- Holle JU, Dubrau C, Herlyn K, et al. Rituximab for refractory granulomatosis with polyangiitis (Wegener’s granulomatosis): comparison of efficacy in granulomatous versus vasculitic manifestations. Ann Rheum Dis. 2012;71:327-333.
- Taylor SR, Salama AD, Joshi L, et al. Rituximab is effective in the treatment of refractory ophthalmic Wegener’s granulomatosis. Arthritis Rheum. 2009;60:1540-1547.
- Joshi L, Lightman SL, Salama AD, et al. Rituximab in refractory ophthalmic Wegener’s granulomatosis: PR3 titers may predict relapse, but repeat treatment can be effective. Ophthalmol. 2011;118:2498-2503.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001;44:912-920.
- Wenzel J, Montag S, Wilsmann-Theis D, et al. Successful treatment of recalcitrant Wegener’s granulomatosis of the skin with tacrolimus (Prograf). Br J Dermatol. 2004;151:927-928.
- Seo P, Specks U, Keogh KA. Efficacy of rituximab in limited Wegener’s granulomatosis with refractory granulomatous manifestations. J Rheumatol. 2008;35:2017-2023.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488-498.
- Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803-843.
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
- FDA approves Rituxan to treat two rare disorders [news release]. Silver Spring, MD: US Food and Drug Administration; April 19, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm251946.htm. Accessed January 6, 2017.
- Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum. 2012;64:3770-3778.
- Keogh KA, Ytterberg SR, Fervenza FC, et al. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180-187.
- Dalkilic E, Alkis N, Kamali S. Rituximab as a new therapeutic option in granulomatosis with polyangiitis: a report of two cases. Mod Rheumatol. 2012;22:463-466.
- Eriksson P. Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med. 2005;257:540-548.
- Oristrell J, Bejarano G, Jordana R, et al. Effectiveness of rituximab in severe Wegener’s granulomatosis: report of two cases and review of the literature. Open Respir Med J. 2009;3:94-99.
- Martinez Del Pero M, Chaudhry A, Jones RB, et al. B-cell depletion with rituximab for refractory head and neck Wegener’s granulomatosis: a cohort study. Clin Otolaryngol. 2009;34:328-335.
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221-232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211-220.
- Aries PM, Hellmich B, Voswinkel J, et al. Lack of efficacy of rituximab in Wegener’s granulomatosis with refractory granulomatous manifestations. Ann Rheum Dis. 2006;65:853-858.
- Holle JU, Dubrau C, Herlyn K, et al. Rituximab for refractory granulomatosis with polyangiitis (Wegener’s granulomatosis): comparison of efficacy in granulomatous versus vasculitic manifestations. Ann Rheum Dis. 2012;71:327-333.
- Taylor SR, Salama AD, Joshi L, et al. Rituximab is effective in the treatment of refractory ophthalmic Wegener’s granulomatosis. Arthritis Rheum. 2009;60:1540-1547.
- Joshi L, Lightman SL, Salama AD, et al. Rituximab in refractory ophthalmic Wegener’s granulomatosis: PR3 titers may predict relapse, but repeat treatment can be effective. Ophthalmol. 2011;118:2498-2503.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001;44:912-920.
- Wenzel J, Montag S, Wilsmann-Theis D, et al. Successful treatment of recalcitrant Wegener’s granulomatosis of the skin with tacrolimus (Prograf). Br J Dermatol. 2004;151:927-928.
- Seo P, Specks U, Keogh KA. Efficacy of rituximab in limited Wegener’s granulomatosis with refractory granulomatous manifestations. J Rheumatol. 2008;35:2017-2023.
Practice Points
- Recognition of the dermatologic manifestations of granulomatosis with polyangiitis (GPA) may aid in an earlier diagnosis and appropriate treatment.
- Rituximab combined with corticosteroids may be a rapid and effective therapy for severe cutaneous ulcers related to GPA.
Shea butter
Indigenous to Africa, Vitellaria paradoxa, better known as the shea or shi tree, is a member of the Sapotaceae family. It has long been used in traditional medicine in sub-Saharan West Africa (as far west as Mali) as well as parts of East Africa (as far east as Uganda and Ethiopia) for its anti-inflammatory and analgesic properties.1,2
Some indications in traditional Nigerian medicine include nasal congestion, scabies, and ulcers.2 In addition, anecdotal success in treating keloids has been reported in association with traditional African remedies, including shea butter and boa constrictor oil.3 Antioxidant activities have also been linked to V. paradoxa.4 Given such purported properties, it is not surprising that demand for shea kernels and butter has steadily increased in recent years for various purposes, including use as food (particularly as a cocoa butter additive in chocolate) and in medical and cosmetic products.2,4 The use of shea butter in skin care is attributed to its hydrating qualities and reputed effectiveness in softening scars.3
Constituents
Shea butter contains fatty acids that have been shown to improve the skin barrier. These include palmitic, stearic, and linoleic acid. It also contains the fatty acids oleic and arachidic. Shea butter also has phenolic components that function as antioxidants.
Anti-inflammatory effects
In 2010, Akihisa et al. evaluated the inhibitory effects of four triterpene acetates and four triterpene cinnamates isolated from the kernel fat of V. paradoxa against 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice. All of the tested compounds showed considerable anti-inflammatory activity (ID50 values ranged from 0.15 to 0.75 micromol/ear). Lupeol cinnamate displayed the greatest anti-inflammatory activity, on carrageenan-induced edema on rat hind paws. All eight substances also exhibited moderate inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) in Raji cells as a primary screening test for tumor promoter inhibitors. Using 7,12-dimethylbenz[a]anthracene (DMBA) as an initiator and TPA as a promoter in a two-stage carcinogenesis model in mice, the investigators also found that lupeol cinnamate inhibited skin tumor promotion. They concluded that the triterpenes and triterpene esters found in shea nuts and shea butter are significant anti-inflammatory and antitumor-promoting agents.2
The next year, Akihisa et al. determined the triacylglycerol and triterpene ester fraction composition of the kernel fats of the shea tree from 36 samples from Cote d’Ivoire, Ghana, Nigeria, Cameroon, Chad, Sudan, and Uganda. There were no significant differences in the composition of the triterpene ester fractions between West African and East African plants. Generally, though, West African shea kernel fats contained higher levels of high-melting triacylglycerols (e.g., stearic-oleic-stearic) and triterpene esters.6
Also that year, Olaitan et al. found that shea butter (as well as boa constrictor oil) was effective in suppressing the in vitro growth of normal and keloid fibroblasts.3
In 2012, Verma et al. used the lipopolysaccharide (LPS)-induced murine macrophage cell line J774 to investigate the anti-inflammatory properties of the methanolic extract of shea butter. They found that shea butter extract dose-dependently reduced, to a significant degree, the levels of nitric oxide, tumor necrosis factor (TNF)–alpha, as well as interleukin (IL)-1beta and IL-12 in the culture supernatants. In addition, the botanical extract suppressed IkappaB phosphorylation and NF-kappaB nuclear translocation as well as the expression of pro-inflammatory enzymes, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2. The investigators attributed the anti-inflammatory activity of the extract to its inhibitory impact on LPS-induced iNOS, COX-2, TNF-alpha, IL-1beta, and IL-12 mRNA expression.1
In 2014, Honfo et al. conducted a literature review indicating that shea pulp is laden with vitamin C and the kernels contain copious fat (butter), which is used in food, drugs, and cosmetics.4
Notably, shea butter is also an ingredient in the topical nonsteroidal anti-inflammatory drug (NSAID) atopiclair, which has shown efficacy in alleviating pruritus in adults with mild to-moderate atopic dermatitis.7
Conclusion
Shea butter has long been incorporated into traditional medical practice in West and East Africa based on observed anti-inflammatory and analgesic characteristics. Such uses are compelling and often the basis for systematic scientific investigation. That said, there remains a dearth of experimental and clinical research on the potential cutaneous benefits of topically applied shea butter. Current data and traditional applications provide ample reason for continued research into this popular botanical agent.
References
1. J Complement Integr Med. 2012;9:Article 4.
2. J Oleo Sci. 2010;59[6]:273-80)
3. Wounds. 2011;23[4]:97-106.
4. Crit Rev Food Sci Nutr. 2014;54[5]:673-86.
5. J Agric Food Chem. 2003;51[21]:6268-73.
6. J Oleo Sci. 2011;60[8]:385-91.
7. J Drugs Dermatol. 2009 Jun;8[6]:537-9.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Indigenous to Africa, Vitellaria paradoxa, better known as the shea or shi tree, is a member of the Sapotaceae family. It has long been used in traditional medicine in sub-Saharan West Africa (as far west as Mali) as well as parts of East Africa (as far east as Uganda and Ethiopia) for its anti-inflammatory and analgesic properties.1,2
Some indications in traditional Nigerian medicine include nasal congestion, scabies, and ulcers.2 In addition, anecdotal success in treating keloids has been reported in association with traditional African remedies, including shea butter and boa constrictor oil.3 Antioxidant activities have also been linked to V. paradoxa.4 Given such purported properties, it is not surprising that demand for shea kernels and butter has steadily increased in recent years for various purposes, including use as food (particularly as a cocoa butter additive in chocolate) and in medical and cosmetic products.2,4 The use of shea butter in skin care is attributed to its hydrating qualities and reputed effectiveness in softening scars.3
Constituents
Shea butter contains fatty acids that have been shown to improve the skin barrier. These include palmitic, stearic, and linoleic acid. It also contains the fatty acids oleic and arachidic. Shea butter also has phenolic components that function as antioxidants.
Anti-inflammatory effects
In 2010, Akihisa et al. evaluated the inhibitory effects of four triterpene acetates and four triterpene cinnamates isolated from the kernel fat of V. paradoxa against 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice. All of the tested compounds showed considerable anti-inflammatory activity (ID50 values ranged from 0.15 to 0.75 micromol/ear). Lupeol cinnamate displayed the greatest anti-inflammatory activity, on carrageenan-induced edema on rat hind paws. All eight substances also exhibited moderate inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) in Raji cells as a primary screening test for tumor promoter inhibitors. Using 7,12-dimethylbenz[a]anthracene (DMBA) as an initiator and TPA as a promoter in a two-stage carcinogenesis model in mice, the investigators also found that lupeol cinnamate inhibited skin tumor promotion. They concluded that the triterpenes and triterpene esters found in shea nuts and shea butter are significant anti-inflammatory and antitumor-promoting agents.2
The next year, Akihisa et al. determined the triacylglycerol and triterpene ester fraction composition of the kernel fats of the shea tree from 36 samples from Cote d’Ivoire, Ghana, Nigeria, Cameroon, Chad, Sudan, and Uganda. There were no significant differences in the composition of the triterpene ester fractions between West African and East African plants. Generally, though, West African shea kernel fats contained higher levels of high-melting triacylglycerols (e.g., stearic-oleic-stearic) and triterpene esters.6
Also that year, Olaitan et al. found that shea butter (as well as boa constrictor oil) was effective in suppressing the in vitro growth of normal and keloid fibroblasts.3
In 2012, Verma et al. used the lipopolysaccharide (LPS)-induced murine macrophage cell line J774 to investigate the anti-inflammatory properties of the methanolic extract of shea butter. They found that shea butter extract dose-dependently reduced, to a significant degree, the levels of nitric oxide, tumor necrosis factor (TNF)–alpha, as well as interleukin (IL)-1beta and IL-12 in the culture supernatants. In addition, the botanical extract suppressed IkappaB phosphorylation and NF-kappaB nuclear translocation as well as the expression of pro-inflammatory enzymes, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2. The investigators attributed the anti-inflammatory activity of the extract to its inhibitory impact on LPS-induced iNOS, COX-2, TNF-alpha, IL-1beta, and IL-12 mRNA expression.1
In 2014, Honfo et al. conducted a literature review indicating that shea pulp is laden with vitamin C and the kernels contain copious fat (butter), which is used in food, drugs, and cosmetics.4
Notably, shea butter is also an ingredient in the topical nonsteroidal anti-inflammatory drug (NSAID) atopiclair, which has shown efficacy in alleviating pruritus in adults with mild to-moderate atopic dermatitis.7
Conclusion
Shea butter has long been incorporated into traditional medical practice in West and East Africa based on observed anti-inflammatory and analgesic characteristics. Such uses are compelling and often the basis for systematic scientific investigation. That said, there remains a dearth of experimental and clinical research on the potential cutaneous benefits of topically applied shea butter. Current data and traditional applications provide ample reason for continued research into this popular botanical agent.
References
1. J Complement Integr Med. 2012;9:Article 4.
2. J Oleo Sci. 2010;59[6]:273-80)
3. Wounds. 2011;23[4]:97-106.
4. Crit Rev Food Sci Nutr. 2014;54[5]:673-86.
5. J Agric Food Chem. 2003;51[21]:6268-73.
6. J Oleo Sci. 2011;60[8]:385-91.
7. J Drugs Dermatol. 2009 Jun;8[6]:537-9.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Indigenous to Africa, Vitellaria paradoxa, better known as the shea or shi tree, is a member of the Sapotaceae family. It has long been used in traditional medicine in sub-Saharan West Africa (as far west as Mali) as well as parts of East Africa (as far east as Uganda and Ethiopia) for its anti-inflammatory and analgesic properties.1,2
Some indications in traditional Nigerian medicine include nasal congestion, scabies, and ulcers.2 In addition, anecdotal success in treating keloids has been reported in association with traditional African remedies, including shea butter and boa constrictor oil.3 Antioxidant activities have also been linked to V. paradoxa.4 Given such purported properties, it is not surprising that demand for shea kernels and butter has steadily increased in recent years for various purposes, including use as food (particularly as a cocoa butter additive in chocolate) and in medical and cosmetic products.2,4 The use of shea butter in skin care is attributed to its hydrating qualities and reputed effectiveness in softening scars.3
Constituents
Shea butter contains fatty acids that have been shown to improve the skin barrier. These include palmitic, stearic, and linoleic acid. It also contains the fatty acids oleic and arachidic. Shea butter also has phenolic components that function as antioxidants.
Anti-inflammatory effects
In 2010, Akihisa et al. evaluated the inhibitory effects of four triterpene acetates and four triterpene cinnamates isolated from the kernel fat of V. paradoxa against 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice. All of the tested compounds showed considerable anti-inflammatory activity (ID50 values ranged from 0.15 to 0.75 micromol/ear). Lupeol cinnamate displayed the greatest anti-inflammatory activity, on carrageenan-induced edema on rat hind paws. All eight substances also exhibited moderate inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) in Raji cells as a primary screening test for tumor promoter inhibitors. Using 7,12-dimethylbenz[a]anthracene (DMBA) as an initiator and TPA as a promoter in a two-stage carcinogenesis model in mice, the investigators also found that lupeol cinnamate inhibited skin tumor promotion. They concluded that the triterpenes and triterpene esters found in shea nuts and shea butter are significant anti-inflammatory and antitumor-promoting agents.2
The next year, Akihisa et al. determined the triacylglycerol and triterpene ester fraction composition of the kernel fats of the shea tree from 36 samples from Cote d’Ivoire, Ghana, Nigeria, Cameroon, Chad, Sudan, and Uganda. There were no significant differences in the composition of the triterpene ester fractions between West African and East African plants. Generally, though, West African shea kernel fats contained higher levels of high-melting triacylglycerols (e.g., stearic-oleic-stearic) and triterpene esters.6
Also that year, Olaitan et al. found that shea butter (as well as boa constrictor oil) was effective in suppressing the in vitro growth of normal and keloid fibroblasts.3
In 2012, Verma et al. used the lipopolysaccharide (LPS)-induced murine macrophage cell line J774 to investigate the anti-inflammatory properties of the methanolic extract of shea butter. They found that shea butter extract dose-dependently reduced, to a significant degree, the levels of nitric oxide, tumor necrosis factor (TNF)–alpha, as well as interleukin (IL)-1beta and IL-12 in the culture supernatants. In addition, the botanical extract suppressed IkappaB phosphorylation and NF-kappaB nuclear translocation as well as the expression of pro-inflammatory enzymes, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2. The investigators attributed the anti-inflammatory activity of the extract to its inhibitory impact on LPS-induced iNOS, COX-2, TNF-alpha, IL-1beta, and IL-12 mRNA expression.1
In 2014, Honfo et al. conducted a literature review indicating that shea pulp is laden with vitamin C and the kernels contain copious fat (butter), which is used in food, drugs, and cosmetics.4
Notably, shea butter is also an ingredient in the topical nonsteroidal anti-inflammatory drug (NSAID) atopiclair, which has shown efficacy in alleviating pruritus in adults with mild to-moderate atopic dermatitis.7
Conclusion
Shea butter has long been incorporated into traditional medical practice in West and East Africa based on observed anti-inflammatory and analgesic characteristics. Such uses are compelling and often the basis for systematic scientific investigation. That said, there remains a dearth of experimental and clinical research on the potential cutaneous benefits of topically applied shea butter. Current data and traditional applications provide ample reason for continued research into this popular botanical agent.
References
1. J Complement Integr Med. 2012;9:Article 4.
2. J Oleo Sci. 2010;59[6]:273-80)
3. Wounds. 2011;23[4]:97-106.
4. Crit Rev Food Sci Nutr. 2014;54[5]:673-86.
5. J Agric Food Chem. 2003;51[21]:6268-73.
6. J Oleo Sci. 2011;60[8]:385-91.
7. J Drugs Dermatol. 2009 Jun;8[6]:537-9.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Genetically corrected skin grafts explored in dystrophic EB
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
FROM JAMA
Key clinical point: Genetically corrected autologous skin grafts produced wound healing in a phase I study of four patients with recessive dystrophic epidermolysis bullosa.
Major finding: All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up; none of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
Data source: A single-center open-label phase I study involving four men followed for 1 year.
Disclosures: This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Discharging Nodule on the Jaw
The Diagnosis: Dental Sinus Secondary to Osteonecrosis of the Jaw
Cone beam computed tomography revealed an area of lucency measuring 40×20 mm in the body of the right mandible (Figure). The patient subsequently underwent curettage of the wound with sequestrectomy of the involved area.
Osteonecrosis of the jaw is a form of avascular necrosis. It is an uncommon but potentially serious side effect of bisphosphonate use.1 Bisphosphonates commonly are used as first-line therapy for osteoporosis, with proven efficacy to reduce fracture risk by exerting an antiresorptive effect on bones.2 Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is defined by the American Association of Oral and Maxillofacial Surgeons as the presence of exposed necrotic bone in the maxillofacial region that has persisted for more than 8 weeks, with current or prior treatment with a bisphosphonate and absence of prior radiation therapy to the jaw.3 Bisphosphonate-related osteonecrosis of the jaw can be associated with infections, pathologic fractures, extraoral fistulae, or osteolysis extending to the inferior border.
Our patient had a dental sinus that resulted from the underlying BRONJ. The jawbones, unlike the long bones, are in a special environment in that both acute and chronic infections occur often within the bone, and surgical procedures as well as masticatory trauma expose the bone to a bacteria-laden environment.4 Infection around the root apex of a tooth results in a dental abscess and a sinus tract can develop from the abscess, draining either intraorally or extraorally.5 Facial sinus tracts can be either odontogenic or nonodontogenic, and sometimes the lesions of dental origin may be confused with dermatological lesions.
Bisphosphonates inhibit osteoclasts, which are responsible for bone resorption. Antiangiogenetic effects also have been reported in bisphosphonates, resulting in devitalized bone.6 The potent and prolonged inhibition of bone remodeling likely plays an important role in BRONJ. The more frequently occurring microdamage inflicted on the lower jawbone with mastication also may represent a contributory factor.7
Bisphosphonate-related osteonecrosis of the jaw more often is associated with the use of high-dose intravenous (IV) bisphosphonate in cancer-related hypercalcemia and less so with oral bisphosphonates, which are generally used to treat osteoporosis.3 In a Swedish study conducted from 2003 to 2010, 55 cases of BRONJ were documented in a population of 1.2 million individuals. The prevalence of BRONJ in patients on oral bisphosphonates and IV bisphosphonates was estimated to be 0.024% and 2.8%, respectively.8
Bisphosphonates are widely used worldwide as the main treatment of osteoporosis. The association between osteonecrosis of the jaw and oral bisphosphonates is contentious among the osteoporosis population, as most studies focus on IV bisphosphonate use in cancer patients.9 Bisphosphonate-related osteonecrosis of the jaw adversely affects the patient's quality of life, producing notable morbidity in afflicted patients. Thus, a complete dental assessment and treatment is recommended before the initiation of bisphosphonate treatment. The risk for developing BRONJ associated with oral bisphosphonates increases when the duration of therapy exceeds 3 years.3 It has been reported that antifracture efficacy would persist for 1 to 2 years following discontinuation of alendronate or risedronate that had been taken for 3 to 5 years, but patients with low bone mineral density at the femoral neck (T-score below -2.5) after 3 to 5 years of treatment of bisphosphonates are at the highest risk for vertebral fractures and therefore appear to benefit most from continuation of therapy.10 For dental procedures, the American Association of Oral and Maxillofacial Surgeons suggests that if systemic conditions persist, the clinician might consider discontinuation of oral bisphosphonates for a 3-month period before and after elective invasive dental surgery to lower the risk for BRONJ.3 When possible, invasive dentoalveolar procedures such as extractions should be avoided; conservative endodontic treatment is preferable.
Bisphosphonate-related osteonecrosis of the jaw is a devastating condition that is difficult to treat and manage, thus the focus should be on prevention through dental clearance prior to starting bisphosphonates. It also is crucial to have a high index of suspicion for BRONJ in patients presenting with orofacial lesions so that they can be treated expediently.
- Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1166-1172.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126:13-20.
- Ruggiero SL, Dodson TB, Assael LA, et al; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67(5 suppl):2-12.
- Mawardi H, Treister N, Richardson P, et al. Sinus tracts--an early sign of bisphosphonate-associated osteonecrosis of the jaws? J Oral Maxillofac Surg. 2009;67:593-601.
- Sammut S, Malden N, Lopes V. Facial cutaneous sinuses of dental origin--a diagnostic challenge. Br Dent J. 2013;215:555-558.
- Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055-1061.
- Hoefert S, Schmitz I, Tannapfel A, et al. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: a possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin Oral Investig. 2010;14:271-284.
- Hallmer F, Bjørnland T, Nicklasson A, et al. Osteonecrosis of the jaw in patients treated with oral and intravenous bisphosphonates: experience in Sweden. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:202-208.
- Lin TC, Yang CY, Kao Yang YH, et al. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population [published online February 11, 2014]. Osteoporos Int. 2014;25:1503-1511.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555-1565.
The Diagnosis: Dental Sinus Secondary to Osteonecrosis of the Jaw
Cone beam computed tomography revealed an area of lucency measuring 40×20 mm in the body of the right mandible (Figure). The patient subsequently underwent curettage of the wound with sequestrectomy of the involved area.

Osteonecrosis of the jaw is a form of avascular necrosis. It is an uncommon but potentially serious side effect of bisphosphonate use.1 Bisphosphonates commonly are used as first-line therapy for osteoporosis, with proven efficacy to reduce fracture risk by exerting an antiresorptive effect on bones.2 Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is defined by the American Association of Oral and Maxillofacial Surgeons as the presence of exposed necrotic bone in the maxillofacial region that has persisted for more than 8 weeks, with current or prior treatment with a bisphosphonate and absence of prior radiation therapy to the jaw.3 Bisphosphonate-related osteonecrosis of the jaw can be associated with infections, pathologic fractures, extraoral fistulae, or osteolysis extending to the inferior border.
Our patient had a dental sinus that resulted from the underlying BRONJ. The jawbones, unlike the long bones, are in a special environment in that both acute and chronic infections occur often within the bone, and surgical procedures as well as masticatory trauma expose the bone to a bacteria-laden environment.4 Infection around the root apex of a tooth results in a dental abscess and a sinus tract can develop from the abscess, draining either intraorally or extraorally.5 Facial sinus tracts can be either odontogenic or nonodontogenic, and sometimes the lesions of dental origin may be confused with dermatological lesions.
Bisphosphonates inhibit osteoclasts, which are responsible for bone resorption. Antiangiogenetic effects also have been reported in bisphosphonates, resulting in devitalized bone.6 The potent and prolonged inhibition of bone remodeling likely plays an important role in BRONJ. The more frequently occurring microdamage inflicted on the lower jawbone with mastication also may represent a contributory factor.7
Bisphosphonate-related osteonecrosis of the jaw more often is associated with the use of high-dose intravenous (IV) bisphosphonate in cancer-related hypercalcemia and less so with oral bisphosphonates, which are generally used to treat osteoporosis.3 In a Swedish study conducted from 2003 to 2010, 55 cases of BRONJ were documented in a population of 1.2 million individuals. The prevalence of BRONJ in patients on oral bisphosphonates and IV bisphosphonates was estimated to be 0.024% and 2.8%, respectively.8
Bisphosphonates are widely used worldwide as the main treatment of osteoporosis. The association between osteonecrosis of the jaw and oral bisphosphonates is contentious among the osteoporosis population, as most studies focus on IV bisphosphonate use in cancer patients.9 Bisphosphonate-related osteonecrosis of the jaw adversely affects the patient's quality of life, producing notable morbidity in afflicted patients. Thus, a complete dental assessment and treatment is recommended before the initiation of bisphosphonate treatment. The risk for developing BRONJ associated with oral bisphosphonates increases when the duration of therapy exceeds 3 years.3 It has been reported that antifracture efficacy would persist for 1 to 2 years following discontinuation of alendronate or risedronate that had been taken for 3 to 5 years, but patients with low bone mineral density at the femoral neck (T-score below -2.5) after 3 to 5 years of treatment of bisphosphonates are at the highest risk for vertebral fractures and therefore appear to benefit most from continuation of therapy.10 For dental procedures, the American Association of Oral and Maxillofacial Surgeons suggests that if systemic conditions persist, the clinician might consider discontinuation of oral bisphosphonates for a 3-month period before and after elective invasive dental surgery to lower the risk for BRONJ.3 When possible, invasive dentoalveolar procedures such as extractions should be avoided; conservative endodontic treatment is preferable.
Bisphosphonate-related osteonecrosis of the jaw is a devastating condition that is difficult to treat and manage, thus the focus should be on prevention through dental clearance prior to starting bisphosphonates. It also is crucial to have a high index of suspicion for BRONJ in patients presenting with orofacial lesions so that they can be treated expediently.
The Diagnosis: Dental Sinus Secondary to Osteonecrosis of the Jaw
Cone beam computed tomography revealed an area of lucency measuring 40×20 mm in the body of the right mandible (Figure). The patient subsequently underwent curettage of the wound with sequestrectomy of the involved area.

Osteonecrosis of the jaw is a form of avascular necrosis. It is an uncommon but potentially serious side effect of bisphosphonate use.1 Bisphosphonates commonly are used as first-line therapy for osteoporosis, with proven efficacy to reduce fracture risk by exerting an antiresorptive effect on bones.2 Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is defined by the American Association of Oral and Maxillofacial Surgeons as the presence of exposed necrotic bone in the maxillofacial region that has persisted for more than 8 weeks, with current or prior treatment with a bisphosphonate and absence of prior radiation therapy to the jaw.3 Bisphosphonate-related osteonecrosis of the jaw can be associated with infections, pathologic fractures, extraoral fistulae, or osteolysis extending to the inferior border.
Our patient had a dental sinus that resulted from the underlying BRONJ. The jawbones, unlike the long bones, are in a special environment in that both acute and chronic infections occur often within the bone, and surgical procedures as well as masticatory trauma expose the bone to a bacteria-laden environment.4 Infection around the root apex of a tooth results in a dental abscess and a sinus tract can develop from the abscess, draining either intraorally or extraorally.5 Facial sinus tracts can be either odontogenic or nonodontogenic, and sometimes the lesions of dental origin may be confused with dermatological lesions.
Bisphosphonates inhibit osteoclasts, which are responsible for bone resorption. Antiangiogenetic effects also have been reported in bisphosphonates, resulting in devitalized bone.6 The potent and prolonged inhibition of bone remodeling likely plays an important role in BRONJ. The more frequently occurring microdamage inflicted on the lower jawbone with mastication also may represent a contributory factor.7
Bisphosphonate-related osteonecrosis of the jaw more often is associated with the use of high-dose intravenous (IV) bisphosphonate in cancer-related hypercalcemia and less so with oral bisphosphonates, which are generally used to treat osteoporosis.3 In a Swedish study conducted from 2003 to 2010, 55 cases of BRONJ were documented in a population of 1.2 million individuals. The prevalence of BRONJ in patients on oral bisphosphonates and IV bisphosphonates was estimated to be 0.024% and 2.8%, respectively.8
Bisphosphonates are widely used worldwide as the main treatment of osteoporosis. The association between osteonecrosis of the jaw and oral bisphosphonates is contentious among the osteoporosis population, as most studies focus on IV bisphosphonate use in cancer patients.9 Bisphosphonate-related osteonecrosis of the jaw adversely affects the patient's quality of life, producing notable morbidity in afflicted patients. Thus, a complete dental assessment and treatment is recommended before the initiation of bisphosphonate treatment. The risk for developing BRONJ associated with oral bisphosphonates increases when the duration of therapy exceeds 3 years.3 It has been reported that antifracture efficacy would persist for 1 to 2 years following discontinuation of alendronate or risedronate that had been taken for 3 to 5 years, but patients with low bone mineral density at the femoral neck (T-score below -2.5) after 3 to 5 years of treatment of bisphosphonates are at the highest risk for vertebral fractures and therefore appear to benefit most from continuation of therapy.10 For dental procedures, the American Association of Oral and Maxillofacial Surgeons suggests that if systemic conditions persist, the clinician might consider discontinuation of oral bisphosphonates for a 3-month period before and after elective invasive dental surgery to lower the risk for BRONJ.3 When possible, invasive dentoalveolar procedures such as extractions should be avoided; conservative endodontic treatment is preferable.
Bisphosphonate-related osteonecrosis of the jaw is a devastating condition that is difficult to treat and manage, thus the focus should be on prevention through dental clearance prior to starting bisphosphonates. It also is crucial to have a high index of suspicion for BRONJ in patients presenting with orofacial lesions so that they can be treated expediently.
- Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1166-1172.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126:13-20.
- Ruggiero SL, Dodson TB, Assael LA, et al; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67(5 suppl):2-12.
- Mawardi H, Treister N, Richardson P, et al. Sinus tracts--an early sign of bisphosphonate-associated osteonecrosis of the jaws? J Oral Maxillofac Surg. 2009;67:593-601.
- Sammut S, Malden N, Lopes V. Facial cutaneous sinuses of dental origin--a diagnostic challenge. Br Dent J. 2013;215:555-558.
- Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055-1061.
- Hoefert S, Schmitz I, Tannapfel A, et al. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: a possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin Oral Investig. 2010;14:271-284.
- Hallmer F, Bjørnland T, Nicklasson A, et al. Osteonecrosis of the jaw in patients treated with oral and intravenous bisphosphonates: experience in Sweden. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:202-208.
- Lin TC, Yang CY, Kao Yang YH, et al. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population [published online February 11, 2014]. Osteoporos Int. 2014;25:1503-1511.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555-1565.
- Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1166-1172.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126:13-20.
- Ruggiero SL, Dodson TB, Assael LA, et al; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67(5 suppl):2-12.
- Mawardi H, Treister N, Richardson P, et al. Sinus tracts--an early sign of bisphosphonate-associated osteonecrosis of the jaws? J Oral Maxillofac Surg. 2009;67:593-601.
- Sammut S, Malden N, Lopes V. Facial cutaneous sinuses of dental origin--a diagnostic challenge. Br Dent J. 2013;215:555-558.
- Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055-1061.
- Hoefert S, Schmitz I, Tannapfel A, et al. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: a possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin Oral Investig. 2010;14:271-284.
- Hallmer F, Bjørnland T, Nicklasson A, et al. Osteonecrosis of the jaw in patients treated with oral and intravenous bisphosphonates: experience in Sweden. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:202-208.
- Lin TC, Yang CY, Kao Yang YH, et al. Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population [published online February 11, 2014]. Osteoporos Int. 2014;25:1503-1511.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555-1565.
An 83-year-old woman presented with a painless, discharging, swollen nodule on the right side of the jaw of 6 months' duration. She had a history of osteoporosis diagnosed 3 years prior for which she was taking alendronate and cholecalciferol. Bone mineral density test scores were -3.93 (spine) and -2.81 (hip)(reference range, -2 and above). She also had hypertension that was treated with amlodipine. On examination there was fetor oris and a discharging sinus with purulent discharge at the jaw. The lower jaw was edentulous. A 5-mm area of red beefy granulation tissue was attached to underlying bone. An exposed sequestrum was seen intraorally with a 3-cm opening at the mandible. There also was submandibular lymphadenopathy.
Aquatic Antagonists: Cutaneous Sea Urchin Spine Injury
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.
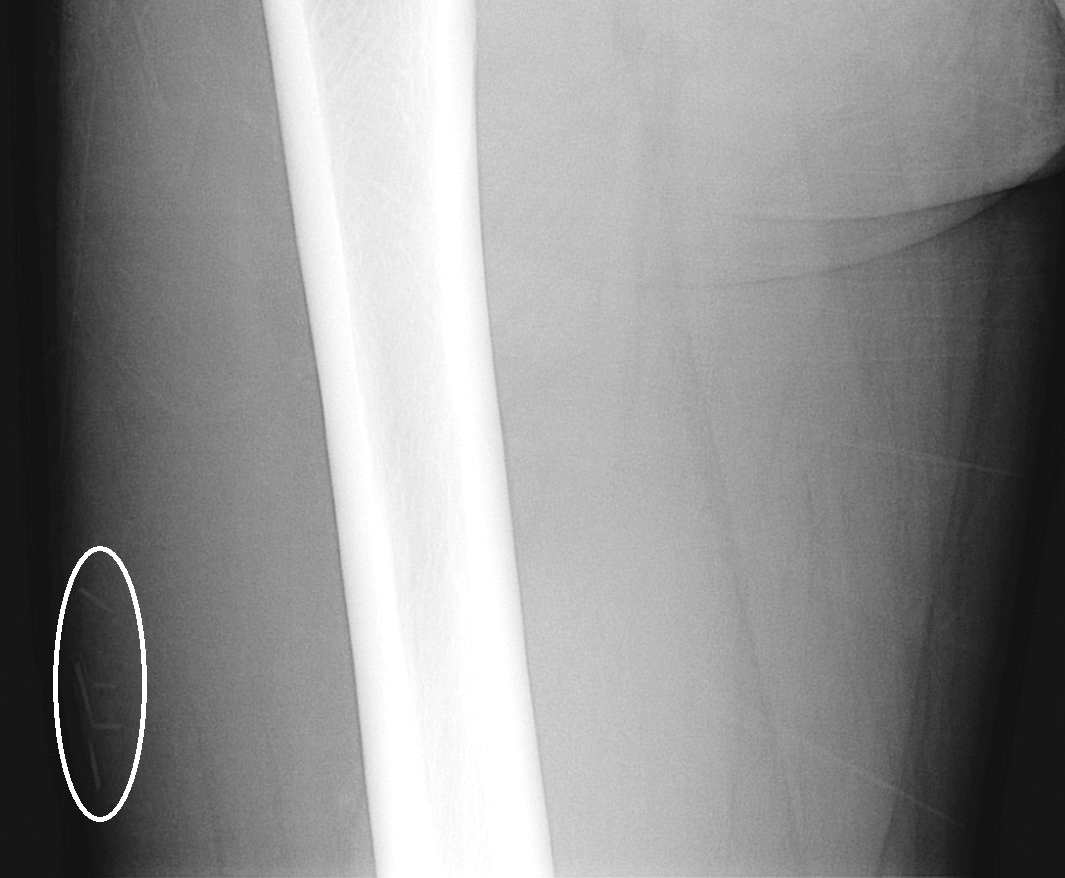
At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.
Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.
At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Practice Points
- Radiographic imaging may aid in the identification of sea urchin spines, especially if there are no visible or palpable skin nodules.
- Treatment of sea urchin spine injuries typically involves surgical removal of the spines with local anesthesia and cutaneous extraction.
- Prompt extraction of sea urchin spines can improve pain symptoms and decrease the likelihood of granuloma formation, infection, and extracutaneous complications.
Abnormal Wound Healing Related to High-Dose Systemic Corticosteroid Therapy in a Patient With Ehlers-Danlos Syndrome Benign Hypermobility Type
The process of wound healing has been well characterized. Immediately following injury, neutrophils arrive at the site in response to chemotactic factors produced by the coagulation cascade. Monocytes follow 24 to 36 hours later; transform into macrophages; and begin to phagocytose tissue debris, organisms, and any remaining neutrophils. In turn, macrophages release chemotactic factors such as basic fibroblast growth factor to attract fibroblasts to the wound, which then begin the process of synthesizing collagen and ground substance. Fibroblasts then take over as the dominant cell type, with collagen synthesis continuing for approximately 6 weeks. Keratinocytes and endothelial cells also proliferate during this time. After approximately 6 weeks, collagen remodeling begins. Tensile strength of the wound may continue to increase up to one year after the injury.1,2
Corticosteroids inhibit wound healing in several ways. Notably, they decrease the number of circulating monocytes, leading to fewer macrophages in the tissue at the site of injury, which then leads to impaired phagocytosis and reduced release of chemotactic factors that attract fibroblasts. Additionally, corticosteroids can inhibit collagen synthesis and remodeling, leading to delayed wound healing and decreased tensile strength of the wound as well as impacting capillary proliferation.3
The subtypes of EDS were reclassified in 1998 by Beighton et al,4 and the benign hypermobility type (EDS-BHT)(formerly type III) is considered the least severe. There is some controversy as to whether this subtype constitutes a separate diagnosis from the benign familial joint hypermobility syndrome. It is characterized by hypermobility of the joints (objectively measured with the Beighton scale) and mild hyperextensibility of the skin, and patients often have a history of joint subluxations and dislocations with resultant degenerative joint disease and chronic pain. Manifestations of fragile skin and soft tissue (eg, abnormal wound healing or scarring; spontaneous tearing of the skin, ligaments, tendons, or organs) are notably absent from the findings in this syndrome.5 The genetic basis for EDS is unknown in the majority of patients, although a deficiency in tenascin X (secondary to defects in the tenascin XB gene [TNXB]) has been identified in a small subset (<5%) of patients, leading to elastic fiber abnormalities, reduced collagen deposition, and impaired cross-linking of collagen.6,7 Inheritance usually is autosomal dominant but also can be autosomal recessive. In contrast, the classic type of EDS (formerly types I and II) is associated with atrophic scarring and tissue fragility, in addition to joint hypermobility and skin hyperextensibility. Type V collagen mutations are found in more than half of patients with this disorder.8
We present the case of a patient with EDS-BHT who developed large nonhealing cutaneous ulcerations with initiation of high-dose systemic corticosteroids for treatment of dermatomyositis. This case provides a dramatic illustration of the effects of the use of chronic systemic corticosteroids on skin fragility and wound healing in patients with an underlying inherited defect in collagen or connective tissue.
Case Report
A 23-year-old man with an unremarkable medical history was admitted to our inpatient cardiology service with palpitations attributable to new-onset atrial fibrillation. Dermatology was consulted to evaluate a rash of approximately 4 months’ duration that started on the dorsal aspect of the hands, then progressed to involve the extensor elbows and knees. The rash also was associated with fatigue, arthralgia, and proximal muscle weakness. A taper of prednisone that was prescribed approximately 2 months prior to admission by a rheumatologist for presumed dermatomyositis improved his symptoms, but they recurred with discontinuation of the medication.
Physical examination revealed reddish, violaceous and hyperpigmented patches on the dorsal aspect of the hands and digits and the extensor aspect of the knees and elbows. A skin biopsy from the right elbow showed a mild interface reaction and nonspecific direct immunofluorescence, consistent with a diagnosis of dermatomyositis. Autoimmune serologies were negative, including antinuclear, anti–Jo-1, anti–Mi-2, anti–Sjögren syndrome antigen A, anti–Sjögren syndrome antigen B, anti-Smith, and antiribonucleoprotein antibodies. Creatine kinase and rheumatoid factor levels were within reference range. Electromyogram was supportive of the diagnosis of dermatomyositis, showing an irritable myopathy. Cardiac magnetic resonance imaging showed an acute inflammatory process of the myocardium, and a transthoracic echocardiogram revealed a depressed left ventricular ejection fraction of 35% to 40% (reference range, 55%–70%). His cardiac disease also was attributed to dermatomyositis, and he was managed by cardiology with anangiotensin-converting enzyme inhibitor and antiarrhythmic therapy. Rheumatology was consulted and prednisone 60 mg once daily was started, with the patient reporting improvement in his muscle weakness and the rash.
Interestingly, the patient also noted a history of joint hypermobility, and a genetics consultation was obtained during the current hospitalization. He denied a history of abnormal scarring or skin problems, but he did note dislocation of the patella on 2 occasions and an umbilical hernia repair at 3 years of age. A paternal uncle had a history of similar joint hypermobility. His Beighton score was noted to be 8/8 (bending at the waist was unable to be tested due to recent lumbar puncture obtained during this hospitalization). The patient was diagnosed with EDS-BHT, and no further workup was recommended.
Subsequent to his hospitalization for several days, the patient’s prednisone was slowly tapered down from 60 mg once daily to 12.5 mg once daily, and azathioprine was started and titrated up to 150 mg once daily. Approximately 6 months after his initial hospitalization, he was readmitted due to increased pain of the right knee with concern for osteomyelitis. Dermatology was again consulted, and at this time, the patient reported a 4-month history of nonhealing ulcers to the knees and elbows (Figure 1). He stated that the ulcers were initially about the size of a pencil eraser and had started approximately 2 months after the prednisone was started, with subsequent slow enlargement. He noted a stinging sensation with enlargement of the ulcers, but otherwise they were not painful. He denied major trauma to the areas. He noted that his prior rash from the dermatomyositis seemed to have resolved, along with his muscle weakness, and he reported weight gain and improvement in his energy levels. Physical examination at this time revealed several stigmata of chronic systemic corticosteroids, including fatty deposits in the face (moon facies) and between the shoulders (buffalo hump), facial acne, and numerous erythematous striae on the trunk and proximal extremities (Figure 2). Multiple noninflammatory ulcers with punched-out borders ranging in size from 0.5 to 6 cm were seen at sites overlying bony prominences, including the bilateral extensor elbows and knees and the right plantar foot. Similar ulcers were noted on the trunk within the striae. Some of the ulcers were covered with a thick hyperkeratotic crust. A biopsy from the edge of an ulcer on the right side of the flank showed only dermal fibrosis. Workup by orthopedic surgery was felt to be inconsistent with osteomyelitis, and plastic surgery was consulted to consider surgical options for repair. Consequently, the patient was taken to the operating room for primary closure of the ulcers to the bilateral knees and right elbow. He has been followed closely by plastic surgery, with the use of joint immobilization to promote wound healing.
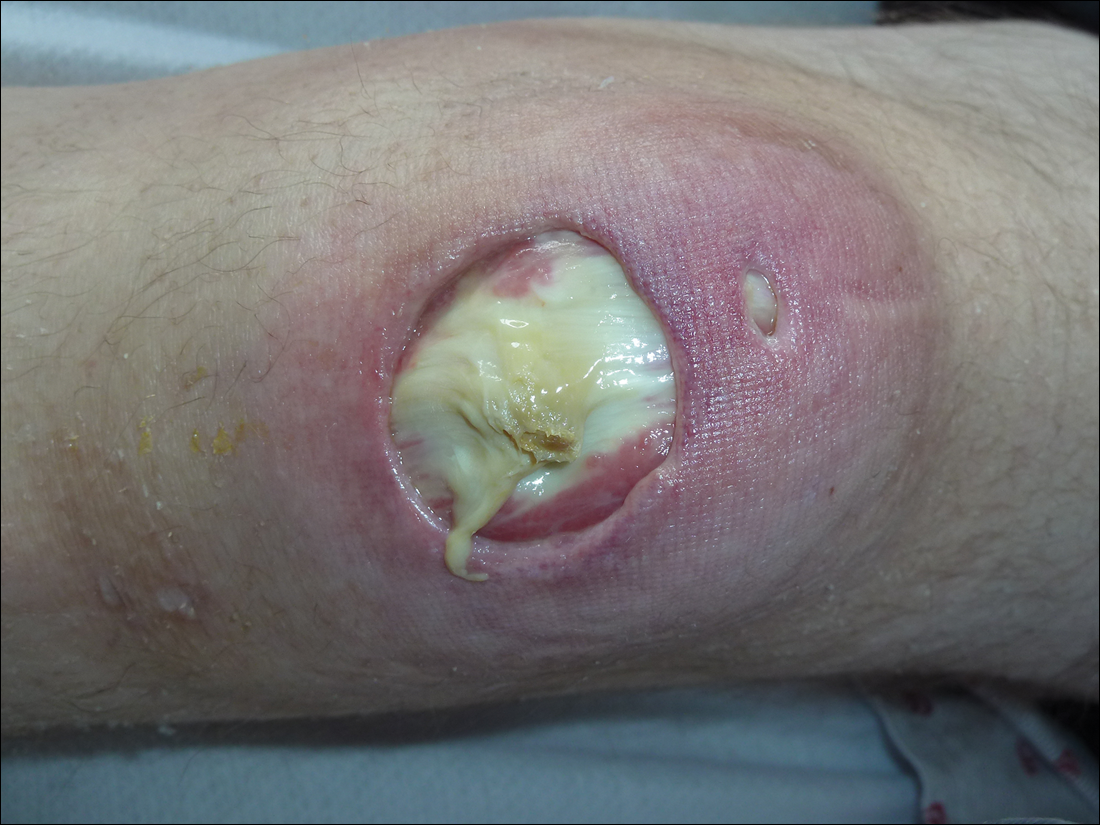
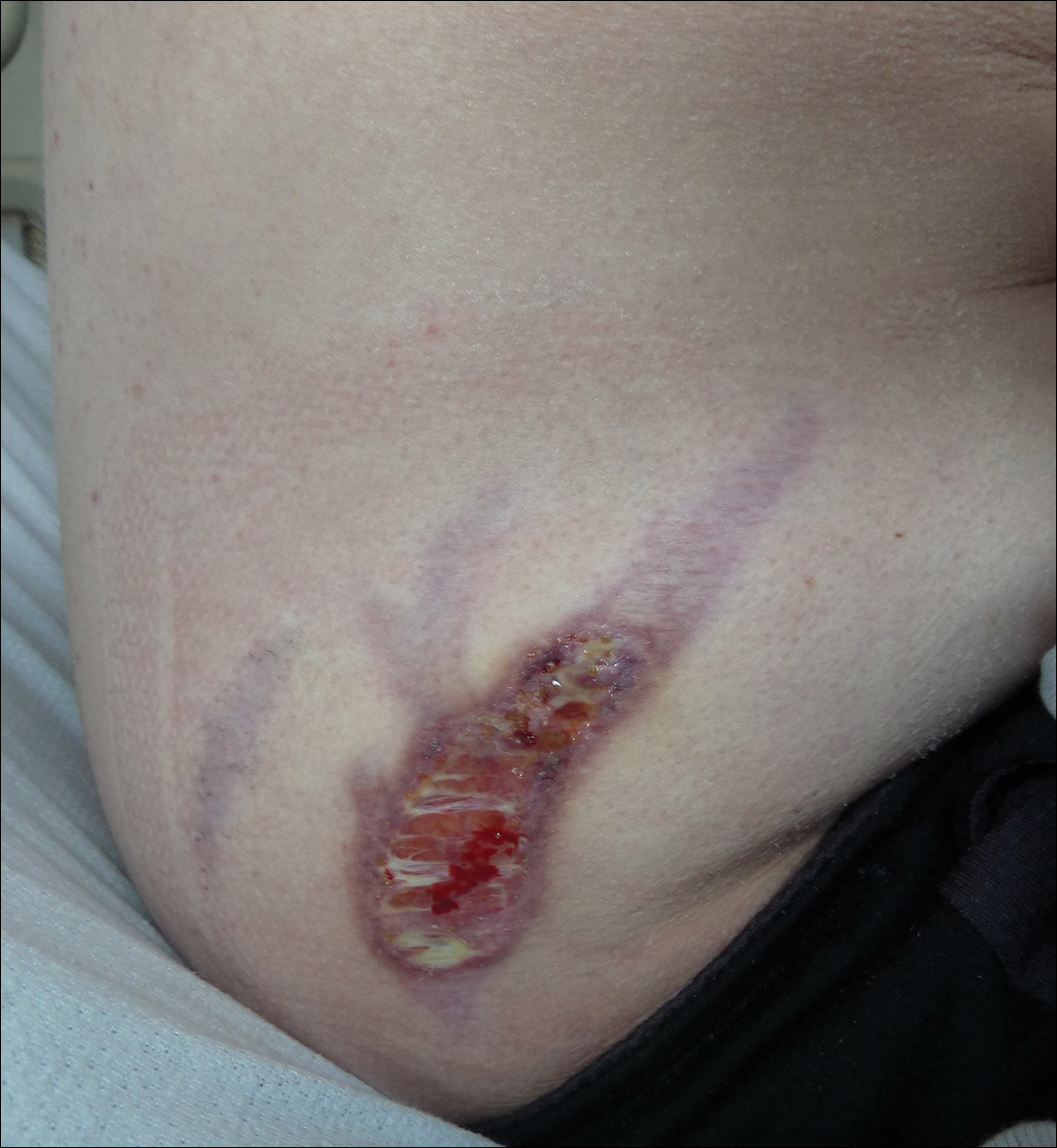
Comment
This case represents a dramatic illustration of the effects of chronic systemic corticosteroids on skin fragility and wound healing in a patient with an underlying genetic defect in the connective tissue. The ulcers were all located within striae or overlying bony prominences where the skin was subjected to increased tension; however, the patient reported no problems with wound healing or scarring at these sites prior to the initiation of corticosteroids, suggesting that the addition of this medication was disruptive to the cutaneous wound healing mechanisms. This case is unique because abnormal wound healing in an EDS patient was so clearly linked to the initiation of systemic steroids.
The exact pathogenesis of the patient’s ulcers is unclear. The diagnosis of EDS was primarily clinical, and without genetic testing, we cannot state with certainty the underlying molecular problem in this patient. Although tenascin X deficiency has been found in a few patients, a genetic defect remains uncharacterized in most patients with EDS-BHT, and in most situations, EDS-BHT remains a clinical diagnosis. In 2001, Schalkwijk et al9 first described the association of tenascin X deficiency and EDS in 5 patients, and they noted delayed wound healing in 1 patient who had received systemic corticosteroids for congenital adrenal hyperplasia. The authors remarked that it was not clear whether the abnormality was linked to the patient’s EDS or to his treatment with systemic corticosteroids.9 Furthermore, it is possible that our patient in fact has a milder variant of classic type EDS and that the manifestations of tissue fragility remained subclinical until the addition of systemic corticosteroids. It also is interesting to note that muscle weakness can be a symptom of EDS, both classic and BHT of EDS, but our patient’s muscle weakness improved with immunosuppression, supporting an underlying autoimmune disease as the cause for it.10 Skin ulcerations have been reported as a rare manifestation of dermatomyositis, but it is remarkable that his ulcers progressed as his other dermatomyositis symptoms improved with therapy, suggesting that his autoimmune disease was not the underlying cause for the ulcers.11-13 This case points to the need to thoughtfully consider the adverse effects of corticosteroids on wound healing in patients with an inherited disorder of collagen or connective tissue such as EDS.
- Bolognia JL, Jorizzo JL, Rapini RP, et al. Dermatology. 2nd ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryng Clin N Am. 2010;43:753-768.
- Beighton P, De Paepe A, Steinmann B, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31-37.
- Levy HP. Ehlers-Danlos syndrome, hypermobility type. In: Pagon RA, Bird TD, Dolan CR, et al, es. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1279/. Accessed August 5, 2015.
- Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214-217.
- Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Spor. 2009;19:511-519.
- Malfait F, Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. In: Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews. Seattle,WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1244/. Accessed August 5, 2015.
- Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin X deficiency. N Engl J Med. 2001;345:1167-1175.
- Voermans NC, Alfen NV, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687-697.
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed. 2006;5:94-96.
- Tomb R, Stephan F. Perforating skin ulcers occurring in an adult with dermatomyositis [in French]. Ann Dermatol Venerol. 2002;129:1383-1385.
- Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol. 1998;11:48-50.
The process of wound healing has been well characterized. Immediately following injury, neutrophils arrive at the site in response to chemotactic factors produced by the coagulation cascade. Monocytes follow 24 to 36 hours later; transform into macrophages; and begin to phagocytose tissue debris, organisms, and any remaining neutrophils. In turn, macrophages release chemotactic factors such as basic fibroblast growth factor to attract fibroblasts to the wound, which then begin the process of synthesizing collagen and ground substance. Fibroblasts then take over as the dominant cell type, with collagen synthesis continuing for approximately 6 weeks. Keratinocytes and endothelial cells also proliferate during this time. After approximately 6 weeks, collagen remodeling begins. Tensile strength of the wound may continue to increase up to one year after the injury.1,2
Corticosteroids inhibit wound healing in several ways. Notably, they decrease the number of circulating monocytes, leading to fewer macrophages in the tissue at the site of injury, which then leads to impaired phagocytosis and reduced release of chemotactic factors that attract fibroblasts. Additionally, corticosteroids can inhibit collagen synthesis and remodeling, leading to delayed wound healing and decreased tensile strength of the wound as well as impacting capillary proliferation.3
The subtypes of EDS were reclassified in 1998 by Beighton et al,4 and the benign hypermobility type (EDS-BHT)(formerly type III) is considered the least severe. There is some controversy as to whether this subtype constitutes a separate diagnosis from the benign familial joint hypermobility syndrome. It is characterized by hypermobility of the joints (objectively measured with the Beighton scale) and mild hyperextensibility of the skin, and patients often have a history of joint subluxations and dislocations with resultant degenerative joint disease and chronic pain. Manifestations of fragile skin and soft tissue (eg, abnormal wound healing or scarring; spontaneous tearing of the skin, ligaments, tendons, or organs) are notably absent from the findings in this syndrome.5 The genetic basis for EDS is unknown in the majority of patients, although a deficiency in tenascin X (secondary to defects in the tenascin XB gene [TNXB]) has been identified in a small subset (<5%) of patients, leading to elastic fiber abnormalities, reduced collagen deposition, and impaired cross-linking of collagen.6,7 Inheritance usually is autosomal dominant but also can be autosomal recessive. In contrast, the classic type of EDS (formerly types I and II) is associated with atrophic scarring and tissue fragility, in addition to joint hypermobility and skin hyperextensibility. Type V collagen mutations are found in more than half of patients with this disorder.8
We present the case of a patient with EDS-BHT who developed large nonhealing cutaneous ulcerations with initiation of high-dose systemic corticosteroids for treatment of dermatomyositis. This case provides a dramatic illustration of the effects of the use of chronic systemic corticosteroids on skin fragility and wound healing in patients with an underlying inherited defect in collagen or connective tissue.
Case Report
A 23-year-old man with an unremarkable medical history was admitted to our inpatient cardiology service with palpitations attributable to new-onset atrial fibrillation. Dermatology was consulted to evaluate a rash of approximately 4 months’ duration that started on the dorsal aspect of the hands, then progressed to involve the extensor elbows and knees. The rash also was associated with fatigue, arthralgia, and proximal muscle weakness. A taper of prednisone that was prescribed approximately 2 months prior to admission by a rheumatologist for presumed dermatomyositis improved his symptoms, but they recurred with discontinuation of the medication.
Physical examination revealed reddish, violaceous and hyperpigmented patches on the dorsal aspect of the hands and digits and the extensor aspect of the knees and elbows. A skin biopsy from the right elbow showed a mild interface reaction and nonspecific direct immunofluorescence, consistent with a diagnosis of dermatomyositis. Autoimmune serologies were negative, including antinuclear, anti–Jo-1, anti–Mi-2, anti–Sjögren syndrome antigen A, anti–Sjögren syndrome antigen B, anti-Smith, and antiribonucleoprotein antibodies. Creatine kinase and rheumatoid factor levels were within reference range. Electromyogram was supportive of the diagnosis of dermatomyositis, showing an irritable myopathy. Cardiac magnetic resonance imaging showed an acute inflammatory process of the myocardium, and a transthoracic echocardiogram revealed a depressed left ventricular ejection fraction of 35% to 40% (reference range, 55%–70%). His cardiac disease also was attributed to dermatomyositis, and he was managed by cardiology with anangiotensin-converting enzyme inhibitor and antiarrhythmic therapy. Rheumatology was consulted and prednisone 60 mg once daily was started, with the patient reporting improvement in his muscle weakness and the rash.
Interestingly, the patient also noted a history of joint hypermobility, and a genetics consultation was obtained during the current hospitalization. He denied a history of abnormal scarring or skin problems, but he did note dislocation of the patella on 2 occasions and an umbilical hernia repair at 3 years of age. A paternal uncle had a history of similar joint hypermobility. His Beighton score was noted to be 8/8 (bending at the waist was unable to be tested due to recent lumbar puncture obtained during this hospitalization). The patient was diagnosed with EDS-BHT, and no further workup was recommended.
Subsequent to his hospitalization for several days, the patient’s prednisone was slowly tapered down from 60 mg once daily to 12.5 mg once daily, and azathioprine was started and titrated up to 150 mg once daily. Approximately 6 months after his initial hospitalization, he was readmitted due to increased pain of the right knee with concern for osteomyelitis. Dermatology was again consulted, and at this time, the patient reported a 4-month history of nonhealing ulcers to the knees and elbows (Figure 1). He stated that the ulcers were initially about the size of a pencil eraser and had started approximately 2 months after the prednisone was started, with subsequent slow enlargement. He noted a stinging sensation with enlargement of the ulcers, but otherwise they were not painful. He denied major trauma to the areas. He noted that his prior rash from the dermatomyositis seemed to have resolved, along with his muscle weakness, and he reported weight gain and improvement in his energy levels. Physical examination at this time revealed several stigmata of chronic systemic corticosteroids, including fatty deposits in the face (moon facies) and between the shoulders (buffalo hump), facial acne, and numerous erythematous striae on the trunk and proximal extremities (Figure 2). Multiple noninflammatory ulcers with punched-out borders ranging in size from 0.5 to 6 cm were seen at sites overlying bony prominences, including the bilateral extensor elbows and knees and the right plantar foot. Similar ulcers were noted on the trunk within the striae. Some of the ulcers were covered with a thick hyperkeratotic crust. A biopsy from the edge of an ulcer on the right side of the flank showed only dermal fibrosis. Workup by orthopedic surgery was felt to be inconsistent with osteomyelitis, and plastic surgery was consulted to consider surgical options for repair. Consequently, the patient was taken to the operating room for primary closure of the ulcers to the bilateral knees and right elbow. He has been followed closely by plastic surgery, with the use of joint immobilization to promote wound healing.


Comment
This case represents a dramatic illustration of the effects of chronic systemic corticosteroids on skin fragility and wound healing in a patient with an underlying genetic defect in the connective tissue. The ulcers were all located within striae or overlying bony prominences where the skin was subjected to increased tension; however, the patient reported no problems with wound healing or scarring at these sites prior to the initiation of corticosteroids, suggesting that the addition of this medication was disruptive to the cutaneous wound healing mechanisms. This case is unique because abnormal wound healing in an EDS patient was so clearly linked to the initiation of systemic steroids.
The exact pathogenesis of the patient’s ulcers is unclear. The diagnosis of EDS was primarily clinical, and without genetic testing, we cannot state with certainty the underlying molecular problem in this patient. Although tenascin X deficiency has been found in a few patients, a genetic defect remains uncharacterized in most patients with EDS-BHT, and in most situations, EDS-BHT remains a clinical diagnosis. In 2001, Schalkwijk et al9 first described the association of tenascin X deficiency and EDS in 5 patients, and they noted delayed wound healing in 1 patient who had received systemic corticosteroids for congenital adrenal hyperplasia. The authors remarked that it was not clear whether the abnormality was linked to the patient’s EDS or to his treatment with systemic corticosteroids.9 Furthermore, it is possible that our patient in fact has a milder variant of classic type EDS and that the manifestations of tissue fragility remained subclinical until the addition of systemic corticosteroids. It also is interesting to note that muscle weakness can be a symptom of EDS, both classic and BHT of EDS, but our patient’s muscle weakness improved with immunosuppression, supporting an underlying autoimmune disease as the cause for it.10 Skin ulcerations have been reported as a rare manifestation of dermatomyositis, but it is remarkable that his ulcers progressed as his other dermatomyositis symptoms improved with therapy, suggesting that his autoimmune disease was not the underlying cause for the ulcers.11-13 This case points to the need to thoughtfully consider the adverse effects of corticosteroids on wound healing in patients with an inherited disorder of collagen or connective tissue such as EDS.
The process of wound healing has been well characterized. Immediately following injury, neutrophils arrive at the site in response to chemotactic factors produced by the coagulation cascade. Monocytes follow 24 to 36 hours later; transform into macrophages; and begin to phagocytose tissue debris, organisms, and any remaining neutrophils. In turn, macrophages release chemotactic factors such as basic fibroblast growth factor to attract fibroblasts to the wound, which then begin the process of synthesizing collagen and ground substance. Fibroblasts then take over as the dominant cell type, with collagen synthesis continuing for approximately 6 weeks. Keratinocytes and endothelial cells also proliferate during this time. After approximately 6 weeks, collagen remodeling begins. Tensile strength of the wound may continue to increase up to one year after the injury.1,2
Corticosteroids inhibit wound healing in several ways. Notably, they decrease the number of circulating monocytes, leading to fewer macrophages in the tissue at the site of injury, which then leads to impaired phagocytosis and reduced release of chemotactic factors that attract fibroblasts. Additionally, corticosteroids can inhibit collagen synthesis and remodeling, leading to delayed wound healing and decreased tensile strength of the wound as well as impacting capillary proliferation.3
The subtypes of EDS were reclassified in 1998 by Beighton et al,4 and the benign hypermobility type (EDS-BHT)(formerly type III) is considered the least severe. There is some controversy as to whether this subtype constitutes a separate diagnosis from the benign familial joint hypermobility syndrome. It is characterized by hypermobility of the joints (objectively measured with the Beighton scale) and mild hyperextensibility of the skin, and patients often have a history of joint subluxations and dislocations with resultant degenerative joint disease and chronic pain. Manifestations of fragile skin and soft tissue (eg, abnormal wound healing or scarring; spontaneous tearing of the skin, ligaments, tendons, or organs) are notably absent from the findings in this syndrome.5 The genetic basis for EDS is unknown in the majority of patients, although a deficiency in tenascin X (secondary to defects in the tenascin XB gene [TNXB]) has been identified in a small subset (<5%) of patients, leading to elastic fiber abnormalities, reduced collagen deposition, and impaired cross-linking of collagen.6,7 Inheritance usually is autosomal dominant but also can be autosomal recessive. In contrast, the classic type of EDS (formerly types I and II) is associated with atrophic scarring and tissue fragility, in addition to joint hypermobility and skin hyperextensibility. Type V collagen mutations are found in more than half of patients with this disorder.8
We present the case of a patient with EDS-BHT who developed large nonhealing cutaneous ulcerations with initiation of high-dose systemic corticosteroids for treatment of dermatomyositis. This case provides a dramatic illustration of the effects of the use of chronic systemic corticosteroids on skin fragility and wound healing in patients with an underlying inherited defect in collagen or connective tissue.
Case Report
A 23-year-old man with an unremarkable medical history was admitted to our inpatient cardiology service with palpitations attributable to new-onset atrial fibrillation. Dermatology was consulted to evaluate a rash of approximately 4 months’ duration that started on the dorsal aspect of the hands, then progressed to involve the extensor elbows and knees. The rash also was associated with fatigue, arthralgia, and proximal muscle weakness. A taper of prednisone that was prescribed approximately 2 months prior to admission by a rheumatologist for presumed dermatomyositis improved his symptoms, but they recurred with discontinuation of the medication.
Physical examination revealed reddish, violaceous and hyperpigmented patches on the dorsal aspect of the hands and digits and the extensor aspect of the knees and elbows. A skin biopsy from the right elbow showed a mild interface reaction and nonspecific direct immunofluorescence, consistent with a diagnosis of dermatomyositis. Autoimmune serologies were negative, including antinuclear, anti–Jo-1, anti–Mi-2, anti–Sjögren syndrome antigen A, anti–Sjögren syndrome antigen B, anti-Smith, and antiribonucleoprotein antibodies. Creatine kinase and rheumatoid factor levels were within reference range. Electromyogram was supportive of the diagnosis of dermatomyositis, showing an irritable myopathy. Cardiac magnetic resonance imaging showed an acute inflammatory process of the myocardium, and a transthoracic echocardiogram revealed a depressed left ventricular ejection fraction of 35% to 40% (reference range, 55%–70%). His cardiac disease also was attributed to dermatomyositis, and he was managed by cardiology with anangiotensin-converting enzyme inhibitor and antiarrhythmic therapy. Rheumatology was consulted and prednisone 60 mg once daily was started, with the patient reporting improvement in his muscle weakness and the rash.
Interestingly, the patient also noted a history of joint hypermobility, and a genetics consultation was obtained during the current hospitalization. He denied a history of abnormal scarring or skin problems, but he did note dislocation of the patella on 2 occasions and an umbilical hernia repair at 3 years of age. A paternal uncle had a history of similar joint hypermobility. His Beighton score was noted to be 8/8 (bending at the waist was unable to be tested due to recent lumbar puncture obtained during this hospitalization). The patient was diagnosed with EDS-BHT, and no further workup was recommended.
Subsequent to his hospitalization for several days, the patient’s prednisone was slowly tapered down from 60 mg once daily to 12.5 mg once daily, and azathioprine was started and titrated up to 150 mg once daily. Approximately 6 months after his initial hospitalization, he was readmitted due to increased pain of the right knee with concern for osteomyelitis. Dermatology was again consulted, and at this time, the patient reported a 4-month history of nonhealing ulcers to the knees and elbows (Figure 1). He stated that the ulcers were initially about the size of a pencil eraser and had started approximately 2 months after the prednisone was started, with subsequent slow enlargement. He noted a stinging sensation with enlargement of the ulcers, but otherwise they were not painful. He denied major trauma to the areas. He noted that his prior rash from the dermatomyositis seemed to have resolved, along with his muscle weakness, and he reported weight gain and improvement in his energy levels. Physical examination at this time revealed several stigmata of chronic systemic corticosteroids, including fatty deposits in the face (moon facies) and between the shoulders (buffalo hump), facial acne, and numerous erythematous striae on the trunk and proximal extremities (Figure 2). Multiple noninflammatory ulcers with punched-out borders ranging in size from 0.5 to 6 cm were seen at sites overlying bony prominences, including the bilateral extensor elbows and knees and the right plantar foot. Similar ulcers were noted on the trunk within the striae. Some of the ulcers were covered with a thick hyperkeratotic crust. A biopsy from the edge of an ulcer on the right side of the flank showed only dermal fibrosis. Workup by orthopedic surgery was felt to be inconsistent with osteomyelitis, and plastic surgery was consulted to consider surgical options for repair. Consequently, the patient was taken to the operating room for primary closure of the ulcers to the bilateral knees and right elbow. He has been followed closely by plastic surgery, with the use of joint immobilization to promote wound healing.


Comment
This case represents a dramatic illustration of the effects of chronic systemic corticosteroids on skin fragility and wound healing in a patient with an underlying genetic defect in the connective tissue. The ulcers were all located within striae or overlying bony prominences where the skin was subjected to increased tension; however, the patient reported no problems with wound healing or scarring at these sites prior to the initiation of corticosteroids, suggesting that the addition of this medication was disruptive to the cutaneous wound healing mechanisms. This case is unique because abnormal wound healing in an EDS patient was so clearly linked to the initiation of systemic steroids.
The exact pathogenesis of the patient’s ulcers is unclear. The diagnosis of EDS was primarily clinical, and without genetic testing, we cannot state with certainty the underlying molecular problem in this patient. Although tenascin X deficiency has been found in a few patients, a genetic defect remains uncharacterized in most patients with EDS-BHT, and in most situations, EDS-BHT remains a clinical diagnosis. In 2001, Schalkwijk et al9 first described the association of tenascin X deficiency and EDS in 5 patients, and they noted delayed wound healing in 1 patient who had received systemic corticosteroids for congenital adrenal hyperplasia. The authors remarked that it was not clear whether the abnormality was linked to the patient’s EDS or to his treatment with systemic corticosteroids.9 Furthermore, it is possible that our patient in fact has a milder variant of classic type EDS and that the manifestations of tissue fragility remained subclinical until the addition of systemic corticosteroids. It also is interesting to note that muscle weakness can be a symptom of EDS, both classic and BHT of EDS, but our patient’s muscle weakness improved with immunosuppression, supporting an underlying autoimmune disease as the cause for it.10 Skin ulcerations have been reported as a rare manifestation of dermatomyositis, but it is remarkable that his ulcers progressed as his other dermatomyositis symptoms improved with therapy, suggesting that his autoimmune disease was not the underlying cause for the ulcers.11-13 This case points to the need to thoughtfully consider the adverse effects of corticosteroids on wound healing in patients with an inherited disorder of collagen or connective tissue such as EDS.
- Bolognia JL, Jorizzo JL, Rapini RP, et al. Dermatology. 2nd ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryng Clin N Am. 2010;43:753-768.
- Beighton P, De Paepe A, Steinmann B, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31-37.
- Levy HP. Ehlers-Danlos syndrome, hypermobility type. In: Pagon RA, Bird TD, Dolan CR, et al, es. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1279/. Accessed August 5, 2015.
- Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214-217.
- Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Spor. 2009;19:511-519.
- Malfait F, Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. In: Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews. Seattle,WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1244/. Accessed August 5, 2015.
- Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin X deficiency. N Engl J Med. 2001;345:1167-1175.
- Voermans NC, Alfen NV, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687-697.
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed. 2006;5:94-96.
- Tomb R, Stephan F. Perforating skin ulcers occurring in an adult with dermatomyositis [in French]. Ann Dermatol Venerol. 2002;129:1383-1385.
- Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol. 1998;11:48-50.
- Bolognia JL, Jorizzo JL, Rapini RP, et al. Dermatology. 2nd ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryng Clin N Am. 2010;43:753-768.
- Beighton P, De Paepe A, Steinmann B, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31-37.
- Levy HP. Ehlers-Danlos syndrome, hypermobility type. In: Pagon RA, Bird TD, Dolan CR, et al, es. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1279/. Accessed August 5, 2015.
- Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214-217.
- Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Spor. 2009;19:511-519.
- Malfait F, Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. In: Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews. Seattle,WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1244/. Accessed August 5, 2015.
- Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin X deficiency. N Engl J Med. 2001;345:1167-1175.
- Voermans NC, Alfen NV, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687-697.
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed. 2006;5:94-96.
- Tomb R, Stephan F. Perforating skin ulcers occurring in an adult with dermatomyositis [in French]. Ann Dermatol Venerol. 2002;129:1383-1385.
- Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol. 1998;11:48-50.
Practice Points
- Chronic corticosteroids have profound effects on the wound-healing process, and their detrimental effects may be amplified in patients with underlying connective tissue defects.
- Although genetic testing is available, the diagnosis of Ehlers-Danlos syndrome benign hypermobility type usually is made clinically.
NOACs show benefit in calciphylaxis
VIENNA – The novel oral anticoagulants may provide effective adjunctive therapy in patients with calciphylaxis, Brian J. King, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a retrospective case series of 16 patients with a confirmed diagnosis of calciphylaxis who were treated with NOACs at the Mayo Clinic in Rochester, Minn., where he is a dermatology resident. The results were impressive, particularly given that the estimated 1-year survival following diagnosis of calciphylaxis is only 45%.
At a mean followup of 418 days, 9 of 16 patients were still alive. More remarkably, five of those nine experienced complete resolution of their clinical lesions and remained alive at a mean followup of 775 days.
Calciphylaxis is a cutaneous manifestation of arteriolar thrombosis. It is classically associated with end-stage renal disease, hyperparathyroidism, a variety of hypercoagulable states, diabetes, and/or obesity. Fifteen of the 16 patients in the Mayo series were women. Fourteen patients had proximal involvement. The lesions occurred most often in fatty tissue on the hips, abdomen, thighs, breasts, and buttocks.
“It’s important to know that this is a deep, incredibly painful process and should not be confused with superficial crusted ulcerations,” Dr. King said.
A variety of treatments have been utilized for calciphylaxis, including sodium thiosulfate, debridement, advanced wound care, hyperbaric oxygen, and parathyroidectomy. But they are often ineffective.
Why not simply use warfarin instead of a costlier NOAC in addressing the problem? Because warfarin has actually been implicated as a cause of the vascular calcification that leads to thrombosis of dermal and pannicular arterioles. Indeed, 12 of the 16 patients in this series were on warfarin at the time of diagnosis of calciphylaxis, either for deep venous thrombosis, pulmonary embolism, or stroke prevention in atrial fibrillation. All were transitioned to a NOAC.
One group of Belgian investigators has provided evidence that strongly suggests the mechanism by which warfarin causes vascular calcification is via inhibition of vitamin K-dependent activation of matrix GLA 1, an enzyme which prevents calcification of vascular endothelial cells (BMC Nephrol. 2014 Sep 4;15:145).
“It is possible and even likely that the vessel calcification we see in patients on warfarin predisposes to thrombosis,” according to Dr. King.
The pathologic diagnostic criteria for calciphylaxis utilized at the Mayo Clinic require skin biopsy evidence of medial calcification and intimal fibroplasia of pannicular arterioles with cutaneous necrosis. Extravascular calcium deposition or thrombosis of pannicular or dermal arterioles is also typically present.
The major clinical criteria are necrotic cutaneous ulcers over indurated plaques, or indurated plaques without ulceration in adipose-rich tissue. The minor criteria are livedo racemosa, hemorrhagic bullae, or hemorrhagic plaques.
Asked if the NOACs can be used interchangeably for treatment of calciphylaxis, Dr. King said the direct factor Xa inhibitor apixaban is the NOAC of choice for this condition at the Mayo Clinic because unlike rivaroxaban (Xarelto) it doesn’t require dosing adjustment in the setting of renal impairment, which is extremely common in patients with calciphylaxis. The direct thrombin inhibitor dabigatran (Pradaxa) is contraindicated in chronic renal failure. Edoxaban (Savaysa) is not on the Mayo Clinic’s formulary, but it is contraindicated in patients with a creatinine clearance of 95 mL/min or more.
Dr. King said he and his coinvestigators recognize that a retrospective case series such as this must be considered hypothesis-generating and nondefinitive. They have already begun a larger prospective comparative outcomes study.
Dr. King reported having no financial conflicts of interest.
VIENNA – The novel oral anticoagulants may provide effective adjunctive therapy in patients with calciphylaxis, Brian J. King, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a retrospective case series of 16 patients with a confirmed diagnosis of calciphylaxis who were treated with NOACs at the Mayo Clinic in Rochester, Minn., where he is a dermatology resident. The results were impressive, particularly given that the estimated 1-year survival following diagnosis of calciphylaxis is only 45%.
At a mean followup of 418 days, 9 of 16 patients were still alive. More remarkably, five of those nine experienced complete resolution of their clinical lesions and remained alive at a mean followup of 775 days.
Calciphylaxis is a cutaneous manifestation of arteriolar thrombosis. It is classically associated with end-stage renal disease, hyperparathyroidism, a variety of hypercoagulable states, diabetes, and/or obesity. Fifteen of the 16 patients in the Mayo series were women. Fourteen patients had proximal involvement. The lesions occurred most often in fatty tissue on the hips, abdomen, thighs, breasts, and buttocks.
“It’s important to know that this is a deep, incredibly painful process and should not be confused with superficial crusted ulcerations,” Dr. King said.
A variety of treatments have been utilized for calciphylaxis, including sodium thiosulfate, debridement, advanced wound care, hyperbaric oxygen, and parathyroidectomy. But they are often ineffective.
Why not simply use warfarin instead of a costlier NOAC in addressing the problem? Because warfarin has actually been implicated as a cause of the vascular calcification that leads to thrombosis of dermal and pannicular arterioles. Indeed, 12 of the 16 patients in this series were on warfarin at the time of diagnosis of calciphylaxis, either for deep venous thrombosis, pulmonary embolism, or stroke prevention in atrial fibrillation. All were transitioned to a NOAC.
One group of Belgian investigators has provided evidence that strongly suggests the mechanism by which warfarin causes vascular calcification is via inhibition of vitamin K-dependent activation of matrix GLA 1, an enzyme which prevents calcification of vascular endothelial cells (BMC Nephrol. 2014 Sep 4;15:145).
“It is possible and even likely that the vessel calcification we see in patients on warfarin predisposes to thrombosis,” according to Dr. King.
The pathologic diagnostic criteria for calciphylaxis utilized at the Mayo Clinic require skin biopsy evidence of medial calcification and intimal fibroplasia of pannicular arterioles with cutaneous necrosis. Extravascular calcium deposition or thrombosis of pannicular or dermal arterioles is also typically present.
The major clinical criteria are necrotic cutaneous ulcers over indurated plaques, or indurated plaques without ulceration in adipose-rich tissue. The minor criteria are livedo racemosa, hemorrhagic bullae, or hemorrhagic plaques.
Asked if the NOACs can be used interchangeably for treatment of calciphylaxis, Dr. King said the direct factor Xa inhibitor apixaban is the NOAC of choice for this condition at the Mayo Clinic because unlike rivaroxaban (Xarelto) it doesn’t require dosing adjustment in the setting of renal impairment, which is extremely common in patients with calciphylaxis. The direct thrombin inhibitor dabigatran (Pradaxa) is contraindicated in chronic renal failure. Edoxaban (Savaysa) is not on the Mayo Clinic’s formulary, but it is contraindicated in patients with a creatinine clearance of 95 mL/min or more.
Dr. King said he and his coinvestigators recognize that a retrospective case series such as this must be considered hypothesis-generating and nondefinitive. They have already begun a larger prospective comparative outcomes study.
Dr. King reported having no financial conflicts of interest.
VIENNA – The novel oral anticoagulants may provide effective adjunctive therapy in patients with calciphylaxis, Brian J. King, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a retrospective case series of 16 patients with a confirmed diagnosis of calciphylaxis who were treated with NOACs at the Mayo Clinic in Rochester, Minn., where he is a dermatology resident. The results were impressive, particularly given that the estimated 1-year survival following diagnosis of calciphylaxis is only 45%.
At a mean followup of 418 days, 9 of 16 patients were still alive. More remarkably, five of those nine experienced complete resolution of their clinical lesions and remained alive at a mean followup of 775 days.
Calciphylaxis is a cutaneous manifestation of arteriolar thrombosis. It is classically associated with end-stage renal disease, hyperparathyroidism, a variety of hypercoagulable states, diabetes, and/or obesity. Fifteen of the 16 patients in the Mayo series were women. Fourteen patients had proximal involvement. The lesions occurred most often in fatty tissue on the hips, abdomen, thighs, breasts, and buttocks.
“It’s important to know that this is a deep, incredibly painful process and should not be confused with superficial crusted ulcerations,” Dr. King said.
A variety of treatments have been utilized for calciphylaxis, including sodium thiosulfate, debridement, advanced wound care, hyperbaric oxygen, and parathyroidectomy. But they are often ineffective.
Why not simply use warfarin instead of a costlier NOAC in addressing the problem? Because warfarin has actually been implicated as a cause of the vascular calcification that leads to thrombosis of dermal and pannicular arterioles. Indeed, 12 of the 16 patients in this series were on warfarin at the time of diagnosis of calciphylaxis, either for deep venous thrombosis, pulmonary embolism, or stroke prevention in atrial fibrillation. All were transitioned to a NOAC.
One group of Belgian investigators has provided evidence that strongly suggests the mechanism by which warfarin causes vascular calcification is via inhibition of vitamin K-dependent activation of matrix GLA 1, an enzyme which prevents calcification of vascular endothelial cells (BMC Nephrol. 2014 Sep 4;15:145).
“It is possible and even likely that the vessel calcification we see in patients on warfarin predisposes to thrombosis,” according to Dr. King.
The pathologic diagnostic criteria for calciphylaxis utilized at the Mayo Clinic require skin biopsy evidence of medial calcification and intimal fibroplasia of pannicular arterioles with cutaneous necrosis. Extravascular calcium deposition or thrombosis of pannicular or dermal arterioles is also typically present.
The major clinical criteria are necrotic cutaneous ulcers over indurated plaques, or indurated plaques without ulceration in adipose-rich tissue. The minor criteria are livedo racemosa, hemorrhagic bullae, or hemorrhagic plaques.
Asked if the NOACs can be used interchangeably for treatment of calciphylaxis, Dr. King said the direct factor Xa inhibitor apixaban is the NOAC of choice for this condition at the Mayo Clinic because unlike rivaroxaban (Xarelto) it doesn’t require dosing adjustment in the setting of renal impairment, which is extremely common in patients with calciphylaxis. The direct thrombin inhibitor dabigatran (Pradaxa) is contraindicated in chronic renal failure. Edoxaban (Savaysa) is not on the Mayo Clinic’s formulary, but it is contraindicated in patients with a creatinine clearance of 95 mL/min or more.
Dr. King said he and his coinvestigators recognize that a retrospective case series such as this must be considered hypothesis-generating and nondefinitive. They have already begun a larger prospective comparative outcomes study.
Dr. King reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Nine of 16 patients with calciphylaxis who were placed on adjunctive therapy with a novel oral anticoagulant responded with significant improvement in their cutaneous disease, four experienced disease stabilization, and three had progressive disease.
Data source: This was a retrospective case study of 16 patients with biopsy-confirmed calciphylaxis.
Disclosures: The study presenter reported having no financial conflicts of interest.
Severe foot pathology predicts death in diabetes
MUNICH – When it comes to predicting mortality in diabetes patients, the foot may trump the heart.
Over a 5-year period, patients who had the most severe foot pathology were almost four times more likely to die than those who had a constellation of cardiovascular risk factors. The association with foot pathology stayed strong and, in a multivariate analysis, was the only factor that remained significantly associated with death in the cohort, Dragan Tesic, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“On average, our patients with severe foot pathology had a 5-year shortening of their lifespan,” said Dr. Tesic of the University of Novi Sad, Serbia. This finding was even more pronounced in younger patients: Those aged 55 years and younger with severe foot pathology died a median of 14 years earlier than their normal expected lifespan would have ended.
Dr. Tesic and his colleagues conducted a 5-year mortality analysis of 244 patients with diabetes, 12% of whom had type 1 disease. The analysis included cardiovascular risk factors (hypertension, triglycerides, cholesterol levels, fibrinogen, proteinuria, and smoking); diabetes duration; coronary artery and cerebrovascular disease; and peripheral artery disease.
Foot pathology was determined according the recently published clinical guidelines by the International Working Group on the Diabetic Foot (IWGDF) (Diab Met Res Rev. 2016. doi: 10.1002/dmrr.2694).
The document parses the diabetic foot into four severity categories:
• 0: No peripheral neuropathy.
• 1: Peripheral neuropathy.
• 2: Peripheral neuropathy with peripheral artery disease and/or a foot deformity.
• 3: Peripheral neuropathy and a history of a foot ulcer or a lower-extremity amputation.
At baseline, patients were a median of 68 years old, though they ranged in age from 36 to 83 years. The mean duration of diabetes was 17 years, and the mean HbA1c was 8.9%. About half had retinopathy; no one was on hemodialysis.
By 5 years, 53 patients (22%) had died. Their median age was 70 years – 5 years short of the Serbian national life expectancy. However, deaths were evenly distributed among the age groups, Dr. Tesic said: 30% of deceased patients were aged 40-64, 41% aged 65-74, and 28% aged 74 and older.
The causes of death were sudden cardiac death (38%), acute coronary syndrome (32%), stroke (11%), cancer (13%), and sepsis (6%).
There were no significant between-group differences in the type of diabetes; any lipid parameter; diabetic retinopathy; proteinuria; or cardiovascular or cerebrovascular disease. However, patients who died had significantly more severe foot pathology, with 71% scoring either a 2 or 3 on the IWGDF scale compared with 36% of those who were still alive. This level of foot pathology was seen in every age group of the deceased patients: 75% of those in the youngest, 54% of those in the middle group, and 73% of those in the oldest group.
Those who died had developed their foot lesions earlier (66 years vs. 69 years). They had poorer ankle reflexes, and worse results on the Neuropad, a visual indicator test for human diabetic neuropathy. Those who died were older (70 years vs. 66 years), had a longer diabetes duration (20 years vs. 17 years), and more hypertension (79% vs. 61%).
But in a multivariate analysis, only the IWGDF score remained a significant predictor of mortality (odds ratio, 3.78). All of the cardiovascular risk factors, as well as age, diabetes duration, and glucose measures, had no effect on survivalk in that analysis.
The study underscores the importance of preventing and treating diabetic neuropathy and foot complications, Dr. Tesic stressed.
“I like to say that I see an opportunity in every difficulty. The examination of the diabetic foot may be time-consuming, but without that we are not delivering adequate or appropriate care to our patients.”
He had no financial disclosures.
MUNICH – When it comes to predicting mortality in diabetes patients, the foot may trump the heart.
Over a 5-year period, patients who had the most severe foot pathology were almost four times more likely to die than those who had a constellation of cardiovascular risk factors. The association with foot pathology stayed strong and, in a multivariate analysis, was the only factor that remained significantly associated with death in the cohort, Dragan Tesic, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“On average, our patients with severe foot pathology had a 5-year shortening of their lifespan,” said Dr. Tesic of the University of Novi Sad, Serbia. This finding was even more pronounced in younger patients: Those aged 55 years and younger with severe foot pathology died a median of 14 years earlier than their normal expected lifespan would have ended.
Dr. Tesic and his colleagues conducted a 5-year mortality analysis of 244 patients with diabetes, 12% of whom had type 1 disease. The analysis included cardiovascular risk factors (hypertension, triglycerides, cholesterol levels, fibrinogen, proteinuria, and smoking); diabetes duration; coronary artery and cerebrovascular disease; and peripheral artery disease.
Foot pathology was determined according the recently published clinical guidelines by the International Working Group on the Diabetic Foot (IWGDF) (Diab Met Res Rev. 2016. doi: 10.1002/dmrr.2694).
The document parses the diabetic foot into four severity categories:
• 0: No peripheral neuropathy.
• 1: Peripheral neuropathy.
• 2: Peripheral neuropathy with peripheral artery disease and/or a foot deformity.
• 3: Peripheral neuropathy and a history of a foot ulcer or a lower-extremity amputation.
At baseline, patients were a median of 68 years old, though they ranged in age from 36 to 83 years. The mean duration of diabetes was 17 years, and the mean HbA1c was 8.9%. About half had retinopathy; no one was on hemodialysis.
By 5 years, 53 patients (22%) had died. Their median age was 70 years – 5 years short of the Serbian national life expectancy. However, deaths were evenly distributed among the age groups, Dr. Tesic said: 30% of deceased patients were aged 40-64, 41% aged 65-74, and 28% aged 74 and older.
The causes of death were sudden cardiac death (38%), acute coronary syndrome (32%), stroke (11%), cancer (13%), and sepsis (6%).
There were no significant between-group differences in the type of diabetes; any lipid parameter; diabetic retinopathy; proteinuria; or cardiovascular or cerebrovascular disease. However, patients who died had significantly more severe foot pathology, with 71% scoring either a 2 or 3 on the IWGDF scale compared with 36% of those who were still alive. This level of foot pathology was seen in every age group of the deceased patients: 75% of those in the youngest, 54% of those in the middle group, and 73% of those in the oldest group.
Those who died had developed their foot lesions earlier (66 years vs. 69 years). They had poorer ankle reflexes, and worse results on the Neuropad, a visual indicator test for human diabetic neuropathy. Those who died were older (70 years vs. 66 years), had a longer diabetes duration (20 years vs. 17 years), and more hypertension (79% vs. 61%).
But in a multivariate analysis, only the IWGDF score remained a significant predictor of mortality (odds ratio, 3.78). All of the cardiovascular risk factors, as well as age, diabetes duration, and glucose measures, had no effect on survivalk in that analysis.
The study underscores the importance of preventing and treating diabetic neuropathy and foot complications, Dr. Tesic stressed.
“I like to say that I see an opportunity in every difficulty. The examination of the diabetic foot may be time-consuming, but without that we are not delivering adequate or appropriate care to our patients.”
He had no financial disclosures.
MUNICH – When it comes to predicting mortality in diabetes patients, the foot may trump the heart.
Over a 5-year period, patients who had the most severe foot pathology were almost four times more likely to die than those who had a constellation of cardiovascular risk factors. The association with foot pathology stayed strong and, in a multivariate analysis, was the only factor that remained significantly associated with death in the cohort, Dragan Tesic, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“On average, our patients with severe foot pathology had a 5-year shortening of their lifespan,” said Dr. Tesic of the University of Novi Sad, Serbia. This finding was even more pronounced in younger patients: Those aged 55 years and younger with severe foot pathology died a median of 14 years earlier than their normal expected lifespan would have ended.
Dr. Tesic and his colleagues conducted a 5-year mortality analysis of 244 patients with diabetes, 12% of whom had type 1 disease. The analysis included cardiovascular risk factors (hypertension, triglycerides, cholesterol levels, fibrinogen, proteinuria, and smoking); diabetes duration; coronary artery and cerebrovascular disease; and peripheral artery disease.
Foot pathology was determined according the recently published clinical guidelines by the International Working Group on the Diabetic Foot (IWGDF) (Diab Met Res Rev. 2016. doi: 10.1002/dmrr.2694).
The document parses the diabetic foot into four severity categories:
• 0: No peripheral neuropathy.
• 1: Peripheral neuropathy.
• 2: Peripheral neuropathy with peripheral artery disease and/or a foot deformity.
• 3: Peripheral neuropathy and a history of a foot ulcer or a lower-extremity amputation.
At baseline, patients were a median of 68 years old, though they ranged in age from 36 to 83 years. The mean duration of diabetes was 17 years, and the mean HbA1c was 8.9%. About half had retinopathy; no one was on hemodialysis.
By 5 years, 53 patients (22%) had died. Their median age was 70 years – 5 years short of the Serbian national life expectancy. However, deaths were evenly distributed among the age groups, Dr. Tesic said: 30% of deceased patients were aged 40-64, 41% aged 65-74, and 28% aged 74 and older.
The causes of death were sudden cardiac death (38%), acute coronary syndrome (32%), stroke (11%), cancer (13%), and sepsis (6%).
There were no significant between-group differences in the type of diabetes; any lipid parameter; diabetic retinopathy; proteinuria; or cardiovascular or cerebrovascular disease. However, patients who died had significantly more severe foot pathology, with 71% scoring either a 2 or 3 on the IWGDF scale compared with 36% of those who were still alive. This level of foot pathology was seen in every age group of the deceased patients: 75% of those in the youngest, 54% of those in the middle group, and 73% of those in the oldest group.
Those who died had developed their foot lesions earlier (66 years vs. 69 years). They had poorer ankle reflexes, and worse results on the Neuropad, a visual indicator test for human diabetic neuropathy. Those who died were older (70 years vs. 66 years), had a longer diabetes duration (20 years vs. 17 years), and more hypertension (79% vs. 61%).
But in a multivariate analysis, only the IWGDF score remained a significant predictor of mortality (odds ratio, 3.78). All of the cardiovascular risk factors, as well as age, diabetes duration, and glucose measures, had no effect on survivalk in that analysis.
The study underscores the importance of preventing and treating diabetic neuropathy and foot complications, Dr. Tesic stressed.
“I like to say that I see an opportunity in every difficulty. The examination of the diabetic foot may be time-consuming, but without that we are not delivering adequate or appropriate care to our patients.”
He had no financial disclosures.
AT EASD 2016
Key clinical point: Severe foot pathology was a better predictor of mortality than cardiovascular risk factors.
Major finding: Patients with severe foot pathology were almost four times more likely to die over 5 years.
Data source: The study comprised 244 patients with types 1 and 2 diabetes.
Disclosures: Dr. Tesic had no financial disclosures.